 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photocatalytic valorisation of real-world substrates
Kathryn
Ralphs
 *a,
Junhong
Liu
a,
Lan
Lan
b,
Christopher
Hardacre
*a,
Junhong
Liu
a,
Lan
Lan
b,
Christopher
Hardacre
 b,
Nathan
Skillen
c and
Peter K. J.
Robertson
b,
Nathan
Skillen
c and
Peter K. J.
Robertson
 a
a
aSchool of Chemistry and Chemical Engineering, Queen's University Belfast, Belfast, BT95AG, UK. E-mail: k.ralphs@qub.ac.uk
bDepartment of Chemical Engineering, School of Engineering, The University of Manchester, Manchester, M139PL, UK
cInternational Centre for Brewing & Distilling, School of Engineering and Physical Sciences, Heriot Watt University, Edinburgh, EH144AS, Scotland
First published on 19th March 2025
Abstract
There are several key environmental sustainability challenges that the world needs to address over the next thirty years, particularly against the backdrop of achieving global net carbon zero emission this century. In addition to reducing global carbon emissions, the provision of clean “green” energy, reduction of water pollution and production of high value chemicals in a sustainable manner are clear priorities for sustainable economic growth. The photocatalytic valorisation of real-world substrates (waste biomass, plastic pollution and wastewater) is an opportunity to contribute significantly towards tackling water pollution, cutting CO2 emissions and contributing to sustainably producing value added chemicals and hydrogen from waste materials and water contaminants/pollutants. To date, however, research is critically lagging in terms of the utilization of actual real-world substrates and instead concentrates on much simpler model compounds such as sugars, monomers, dyes and individual pollutants. Lack of progress in this field is further exacerbated by the general lack of scaling up of photocatalytic technology. Nevertheless, there are some pioneers who have explored the photocatalytic valorization of real-world waste materials which have been highlighted in this review. This review considers the application of semiconductor photocatalysis for such applications with a particular focus on valorisation of waste biomass (e.g. cardboard, grass, wood), plastic pollution (e.g. plastic bottles) and wastewater effluents (e.g. from juice processing factories) to produce hydrogen and value-added chemicals. Current engineering aspects are reviewed and discussed. A perspective of the role of photocatalysis in the circular economy is also discussed and an overall perspective and future outlook is presented.
Sustainability spotlightTransitioning to a net carbon zero world is a monumental task for humankind. It is a very complex multifaceted ambition that requires substantial innovation and investment. The hydrogen economy is considered to be one of the most promising options to decarbonise the energy and transport sectors. The circular economy can also play a major role in reaching net zero and reducing carbon emissions. Therefore, this review has been prepared to assess the potential of the photocatalytic valorization of real-world waste products such as food waste, wastewater and plastic pollution to generate value added chemicals and hydrogen. The research detailed in the review clearly aligns with SDG7, SDG12 and SDG13. |
1. Introduction
1.1 Current state of play
It is indisputable that human activity has had a devastating impact on the planet. Human activity results in around 51 billion tonnes of greenhouse gases added to the atmosphere every year.1 From fossil fuel use alone, we emit over 34 billion tonnes of CO2 per year,2 burn 929 million tonnes of coal each year,3 consume 94 million barrels of oil per day, and mine 1.3 million tonnes of precious metals, 207 million tons of industrial metals and over 3 billion tonnes of iron ore per year.4 Each year our plastic production rises with just under 460 million tonnes of plastic being produced in 2019.5 It is estimated we cut down 10 million hectares of forest per year,6 the size of animal populations for which data is available has declined by 69% from 1970.7 Since the rise of humans, wild mammals have declined by 85%.8 Livestock activities contribute 18% (made up of 9% total CO2, 34% CH4 and 65% NOx) of the total greenhouse gas emissions, which is more than the road transport sector.9With an ever-increasing global population, the catastrophic costs for the planet are only going to worsen. Population is the multiplier to everything we as humans consume and the damage we do to the planet.10,11 On 15th November 2022 the world's population reached 8 billion people. By 2050 it is projected to reach 8.5 billion and to increase further to 9.7 billion in 2050. By 2100 the UN estimates the global population will reach 10.4 billion people.12 Population and economic growth are the driving factors towards increased greenhouse gas emissions.13–20 Despite the evidence,21 however, this is often overlooked by policy makers.
The planetary boundaries framework22 is based upon Earth system science and identifies boundaries that are vital for maintaining the stability and resilience of the planet. As of 2023 nine boundaries have been identified and currently all nine are heavily negatively affected by human activity (Fig. 1).23 According to the framework, transgressing these boundaries will increase the risk of irreversible climate change with consequences that are detrimental or even catastrophic for large parts of the world.
 | ||
| Fig. 1 The 2023 planetary boundaries. Reproduced from Stockholm Resilience centre, based on analysis in ref. 23. Licensed under CC BY-NC-ND 3.0. Credit: “Azote for Stockholm Resilience Centre”. | ||
The effects of climate change are ubiquitous and impossible to ignore. Earth's temperature has risen by an average of 0.08 °C per decade since 1880. Since 1981 the rate of warming has more than doubled to 0.18 °C per decade (Fig. 2). The 10 warmest years since recording began, have occurred since 2010. In 2022 the surface temperature was 1.06 °C warmer than the pre-industrial period (1880–1900).27 This rapidly increasing warming of the Earth's surface has a myriad of cataclysmic consequences. The ice sheets are melting,28 sea levels are rising,29 and oceans are becoming more acidic.30 Forest fires are increasing in occurrence and ferocity,31,32 rainfall patterns are shifting, leading to flooding33 and droughts,34 biodiversity is being lost,35 and food36 and water security37 is being threatened. Ultimately more of the world's population will find themselves living in various types of hostile environments.
 | ||
| Fig. 2 Graph showing global CO2 emissions,24 world population25 and global temperature with respect to 20th century average temperature.26 | ||
1.2 Net zero
Net zero, the new buzz word of recent times, was first popularized by the Paris Agreement 2015 to limit the impact of greenhouse gas emissions.38 The United Nations definition of net zero is “cutting greenhouse gas emissions to as close to zero as possible with any remaining emissions reabsorbed from the atmosphere by oceans and forests for instance”.Net zero has been met with enthusiasm around the globe with a growing coalition of countries, business and other institutes pledging to net zero emissions. More than 70 countries, including China, US and EU have set net zero targets. In 2019, the UK was one of the first major economies to pass laws that aligned with these targets by requiring all greenhouse gas emissions to be net zero (in comparison to levels in 1990) by 2050.39,40
The Paris Agreement is a binding agreement that brings all nations together to combat climate control with the aim of keeping the increase in the global average temperature below 2 °C (above pre-industrial levels). More recently IPCC has stressed that the average global temperature increase must be kept below a 1.5 °C rise to prevent devastating climate related consequences. To achieve this 1.5 °C goal, the UN climate science panel have stated that global CO2 emissions must fall by 43% by 2030, and to net zero by 2050.41
Net zero is a very ambitious target and is an excellent idea in theory to alleviate CO2 levels; however in practice it could possibly end up being nothing more than Schlimmbesserung, by depending on the use of carbon offsets to reach net zero targets. Carbon offsets are tradable “rights” or certificates linked to activities that lower the amount of CO2 in the atmosphere.42 Carbon offsets are widely used by individuals, corporations, and governments to mitigate their greenhouse gas emissions on the assumption that offsets reflect equivalent climate benefits achieved elsewhere. By purchasing carbon credits, a person/company can pay to have their carbon emissions offset rather than taking any actions to lower their emissions. Unfortunately, many countries are yet to set out detailed plans to achieve their net zero pledges and are opaque about the role of carbon offsetting. The Paris Agreement left it up to each country to define their own emissions pathways and their plans to contribute to global net zero. There is no official method to quantify the adequacy, ambition, or fairness of a country's global net zero contributions.43
Net zero, done right, is an extremely ambitious and not a straightforward target to achieve. It is becoming evident, however, that net zero is probably almost certainly not enough by itself to prevent global temperatures increasing beyond the limits set out in the Paris Agreement.44 Nevertheless, in order to achieve or come to close to achieving net zero, a rapid global transformation with huge investment is needed. It is estimated $125 trillion of climate investment is needed by 2050 to meet net zero.45 In 2020 the UK government set out a 10-point plan to accelerate the UK's path to net zero.46 The plan is to mobilise £12 billion of government investment, and potentially 3 times as much from the private sector, to create and support up to 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 green jobs. The 10-point plan focuses on low carbon hydrogen, offshore wind, nuclear power, zero emission vehicles and carbon capture as well as protecting the environment, working towards “jet zero”, greener buildings and green public transport.47
000 green jobs. The 10-point plan focuses on low carbon hydrogen, offshore wind, nuclear power, zero emission vehicles and carbon capture as well as protecting the environment, working towards “jet zero”, greener buildings and green public transport.47
1.3 Hydrogen
Green hydrogen, i.e., hydrogen produced via water electrolysis using renewable electricity, is thought to be one of the most promising options to decarbonize the energy and transport sector.48,49 Hydrogen is largely regarded as a potential clean, cost effective, reliable, and potentially sustainable energy vector. “Energy vector” refers to “an energy-rich substance that facilitates the translocation and/or the storage of energy with the intention of using it at a distance in time and/or space from the primary production site.50 Hydrogen is not a primary energy source but an energy carrier, like electricity.51 Energy obtained from renewable sources, such as solar, wind and wave can be stored as chemical potential energy in hydrogen and hydrogen-rich materials (ammonia, urea, formate, etc.) and liberated by oxidation, generating water as the by-product. It is also possible to produce sustainable hydrogen through photocatalysis using renewable sources such as biomass or waste streams which is discussed in Section 2.Hydrogen is the lightest element and the most abundant gas in the universe. Despite the abundance of hydrogen, obtaining this molecule in its elemental form is not facile. Industrially, hydrogen is produced via hydrocarbon reforming and pyrolysis. Steam reforming of methane is the most widely used method for large scale hydrogen production and has a conversion efficiency between 74 and 85%. Steam and natural gas are reacted over a nickel catalyst at temperatures of 850–900 °C (grey hydrogen). It is estimated this method produces 0.3–0.4 m3 CO2 per m3 H2.52 By 2021, 47% of the global hydrogen production was obtained from natural gas, 27% from coal, 22% from oil and 4% from electrolysis.53
Despite the immense potential of green hydrogen to decarbonize the world's economy, the economics are challenging.54 The cost of producing green hydrogen varies widely by location and availability of renewable resources. For example, in parts of the Middle East, green hydrogen can be produced for 3–5 euro per kg. However, in Europe, the cost of production can vary from 3–8 euro per kg.55 In the USA, grey hydrogen costs just $2 per kg due to the low cost of fracked natural gas, while due to increasing natural gas prices in Europe because of the ongoing war in Ukraine, the cost to produce grey hydrogen is $6–8 per kg.56 With grey hydrogen now costing more than green hydrogen in some regions, this could significantly accelerate the ramping up of the hydrogen economy.57 It is estimated that by 2030 a UK-wide hydrogen economy could be worth £900 million and create over 9000 high quality jobs. By 2050 this is estimated to increase to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 jobs and a hydrogen economy worth up to £13 billion.58 In addition, modelling data suggests that 41 Mt of CO2e could be saved between 2023 and 2032 as a result.59
000 jobs and a hydrogen economy worth up to £13 billion.58 In addition, modelling data suggests that 41 Mt of CO2e could be saved between 2023 and 2032 as a result.59
As well as use as an energy vector, hydrogen as a replacement for fossil fuels is a significant component of many decarbonization strategies.60 Hydrogen can be utilized as a fuel either via combustion or an electrochemical fuel cell. A fuel cell generates electricity from hydrogen with only water as the by-product. It is assumed, however, in the UK that most hydrogen will be utilized as a fuel by combustion. Hydrogen is already blended into streams of natural gas across the globe.61–64 However, one of the major issues of combusting hydrogen, which is frequently overlooked, is that it can lead to the formation of NOx at a higher quantity than produced by the combustion of fossil fuels.65,66 Another area of concern is the transport and storage of hydrogen. If steel is exposed to hydrogen at high temperatures, it can cause the steel to become brittle resulting in leaks and subsequent explosions. Consequently, current infrastructure cannot be used to store and transport hydrogen. Significant investment is required to upgrade current infrastructure to utilize hydrogen as fuel.
1.4 Photocatalysis
Photocatalysis harnesses light energy using a semiconductor material i.e., a photocatalyst, to drive chemical reactions. Upon the absorption of light of an appropriate wavelength an electron hole pair is generated, in which initially the photogenerated hole is created in the valence band and the photogenerated electron is located in the conduction band. The difference in energy between the two bands is called a band gap and the light energy absorbed must be equal to or greater than this band gap in order to promote the electron.67 The generation of an electron hole pair initiates a series of complex processes and mechanisms that yield oxidized and reduced species.68 Photogenerated electrons can react with oxygen to form a superoxide radical. The photogenerated holes can be transferred to the target molecule leading to its oxidation or can react with water to generate hydroxyl radicals. These hydroxyl radicals can then go on to oxidize the target molecules. The actual mechanism is very complex and not easy to elucidate. Numerous publications have discussed the mechanism in detail and can be found elsewhere.69–74 A simplified summary of the potential mechanism is given in Fig. 3.Photocatalysis holds significant importance due to its potential to address various environmental and energy challenges. It is an extremely active and constantly developing research area ranging from pollution degradation,75,76 water electrolysis for clean hydrogen production,77 solar energy conversion,78 water79 and air purification,80 development of photonic materials and devices and sustainable chemistry and synthesis and energy.81
Semiconductor photocatalysis is clearly a very versatile technology that could have huge potential scope in providing a route to low carbon fuels such as hydrogen via reduction of protons by conduction band electrons. A further potential advantage of this process is that the corresponding valence band oxidation reaction can be simultaneously used for other environmentally beneficial processes such as water treatment or for generation of high value chemicals through oxidation of waste materials such as microplastics or cellulosic biomass. This review considers the utilisation of photocatalysis as a process for the valorisation of a range of such “real-world substrates” and considers the potential of this process as part of future circular economy and net carbon zero energy and chemical production. Specifically, examples of real-life substrates which are considered in this review include biomass, waste plastics and contaminated wastewater.
2. Substrates
2.1 Biomass
More recently photocatalytic reforming of biomass has become one of the most rapidly evolving applications in the field with enormous potential to contribute to a more sustainable circular economy.82–84 Biomass can be converted by use of a suitable semiconductor photocatalyst and light energy, generating value added chemicals, sustainable hydrogen and fuels. The first report of biomass reforming via photocatalysis was by Kawai and Sakata in 1980;85 however it is only in the last decade that publications in this area have begun to increase, which is thought to be due to the expansion of the bioenergy sector and the demand for sustainable hydrogen production. The concept of a biorefinery focuses on the use of biomass in place of fossil fuels86 to generate both bioenergy, value-added chemicals and green hydrogen. The integration of the photocatalytic reforming of biomass with the biorefinery concept and the bioenergy sector holds much promise for advancing a sustainable society and contributes vastly to a decarbonized economy.87As the largest biomass energy resource, lignocellulosic biomass, which is the major component of trees, grasses, and straws, has drawn considerable attention in recent years. Lignocellulose is a functionalized biopolymer consisting of three major components, which are lignin (25–30%), cellulose (40–45%) and hemicellulose (30–35%)88 (Fig. 4). Cellulose and hemicellulose, also known as polysaccharides, are biopolymers composed of β-D-glucose and C5, C6 sugars and sugar acids respectively. These units are linked by β-1,4-glycosidic bonds and their degrees of polymerization can reach 800 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.89 Compared to the components above, lignin, the largest natural large scale of aromatic sources,90 is a complicated three-dimensional amorphous polymer, consisting of three aromatic main units: syringyl, guaiacyl, and p-hydroxyphenyl units, as well as other molecules.91 As these main units all contain a phenyl and a propyl side chain, the typical aromatic unit in lignin is generally referred to as the phenyl propane unit (ppu). The number of methoxyl groups varies from unit to unit, with sinapyl containing two methoxy groups, coniferyl alcohol with one, and p-coumaryl alcohol with none. Also, the amount of each unit in lignin is related to the plant species. For instance, the lignin of softwoods has a high content of guaiacyl monomers, that of hardwoods is a mixture of guaiacyl and syringyl, and that of grasses presents a mixture of all three monomers.92
000.89 Compared to the components above, lignin, the largest natural large scale of aromatic sources,90 is a complicated three-dimensional amorphous polymer, consisting of three aromatic main units: syringyl, guaiacyl, and p-hydroxyphenyl units, as well as other molecules.91 As these main units all contain a phenyl and a propyl side chain, the typical aromatic unit in lignin is generally referred to as the phenyl propane unit (ppu). The number of methoxyl groups varies from unit to unit, with sinapyl containing two methoxy groups, coniferyl alcohol with one, and p-coumaryl alcohol with none. Also, the amount of each unit in lignin is related to the plant species. For instance, the lignin of softwoods has a high content of guaiacyl monomers, that of hardwoods is a mixture of guaiacyl and syringyl, and that of grasses presents a mixture of all three monomers.92
 | ||
| Fig. 4 Proportion of hemicellulose (30–35%), cellulose (40–45%) and lignin (25–30%) as lignocellulose components. | ||
The utilization of biomass for bioenergy production can have negative repercussions on food security. Providing adequate food and nutrition to an increasing population while conserving natural resources is a key aspect of the United Nations sustainable development goals.93 Globally, two billion people have nutritional deficiencies and around 800 million still suffer from hunger.94 A recent review of the literature found that 56% of studies on the effects of bioenergy on edible and inedible feedstocks reported a negative impact on food security regardless of feedstock source. It is vital to maintain food security while expanding the bioenergy sector. One way to do this is to utilize waste as a feedstock for bioenergy/chemicals/sustainable hydrogen production.95 Agricultural residues account for 21% of the total biomass resources and are associated with manure from livestock, vegetable/fruit waste96 and lignocellulosic biomass such as rice straw, wheat straw, maize straw, etc.97,98 Nevertheless, these resources are ineffectively utilized, whereby 75% of straw is combusted or dumped and only 0.5% is used for biogas production.99
Biomass such as agricultural residues and waste, plastics, sludge and industrial effluent are the most viable options of waste streams for energy production and valorisation via photocatalysis. Each feedstock will be discussed in detail in this review. There are numerous benefits to employing waste as a feedstock; for example, agricultural waste is renewable and abundant while using plastic waste as a feedstock can offer a potential solution to waste management and adding value to waste materials. However, until recently the majority of research has focused on the photocatalytic conversion of simple molecules, such as glucose100 or cellulose101–103 as well as cleavage of β-O-4 ether bonds found in lignin using model lignin compounds.104,105 However more recently vast progress has been made in using much more complex lignin model compounds.106,107 It is imperative that research using real-world substrates as well as reactor design and scale up is radically ramped up. The Technology Readiness Level (TRL) of photocatalysis techniques needs to be at a much higher level to reach its full potential. This review aims to analyse the current state of research and provide a prospective moving forward with respect to utilizing real word feedstocks as photocatalytic substrates and potential future technology barriers as the field develops. In the following section, the photocatalytic valorisation of real-world lignocellulosic biomass feedstocks will be discussed. In context these reports will be classified by products such as hydrogen and value-added chemicals such as formic acid, hydrocarbons, and aldehydes.
In 1980, Sakata et al.109 utilized various types of biomasses as substrates to test their feasibility for photocatalytic hydrogen production, including cherry wood, Dutch clover, golden-rod, water hyacinth and rice plant. Using a 4% Pt/TiO2 photocatalyst, up to 54 μmol of hydrogen was produced after 10 h irradiation under a 500 W Xe lamp from Dutch clover. The hydrogen yields under the same conditions ranged from 12 to 25 μmol for the other biomass feedstocks. It's worth noting that each of the feedstocks displayed 2–7 times higher hydrogen yield following pre-treatment with 10 M NaOH.
Speltini et al.110 also utilized a Pt/TiO2, with a Pt loading of 0.5%, catalyst for solar photocatalytic hydrogen production from water suspended cellulose (CLS). It was reported that under 366 nm (UV-A) irradiation, CLS greatly enhanced water splitting, with yields up to ten-fold higher than those observed in neat water. At the same time 5-hydroxymethylfurfural (HMF) was identified as the result of polysaccharide depolymerization. Encouraged by this result, rice husk and alfalfa (Medicago sativa) stems as raw cellulosic biomass were evaluated, with reaction yields threefold higher than those observed in the water splitting process in pure water.
In other research,111 2% Pt/TiO2 was utilized in photoreforming of alkali-pre-treated bamboo, rice straw, and silver grass saccharification and fermentation solution. The transformation was reported in three steps. The first step started with treatment with NaOH solution at 95 °C for 1 h. Subsequently, the pre-treated lignocellulose was converted into a suspension of ethanol and xylose, xylanase, and S. cerevisiae by a simultaneous saccharification and fermentation process (SSF) using cellulase and xylanase in acetate buffer at 34 °C. The yeast suspension converted cellulose to ethanol and xylose. The xylose was subsequently photoreformed to ethanol and hydrogen using a Pt/TiO2 photocatalyst. The hydrogen yield demonstrated a combustion energy of 73.4–91.1% compared to that of the alkali-pre-treated lignocelluloses (holocellulose).
Over a 1% Pt/TiO2 photocatalyst, alkaline pre-treated corn stover and its waste liquid produced 212.3 and 205.4 μmol gcat−1 h−1 H2, respectively, under the irradiation of a 300 W Xe lamp.112 The concentration of NaOH and pre-treatment temperature were found to greatly influence H2 production. Alkali treatment on corn stover resulted in changes in morphology, structural composition, and functional groups due to the removal of lignin and hemicellulose.113 The measured and calculated crystallinity (CrI) of corn stover increased from 39.04% to 51.06% after alkali treatment, which was attributed to the partial removal of hemicellulose and lignin under alkaline conditions, dissociation of hydrogen bonds between lignin, hemicellulose and cellulose, and destruction of amorphous cellulose due to swelling. Hydrogen production increased sequentially after the alkaline pre-treatment process. When fescue grass was used as a substrate114, after washing with water and then methanol at 55 °C and then drying in an oven at 120 °C overnight, 0.6 ml hydrogen was generated following irradiation in the presence of a Pt/TiO2 photocatalyst. Jaswal et al. investigated dilute acid hydrolysis of pinewood for the production of hydrogen using a 1% Pt/TiO2 catalyst and activated carbon treatment. After 8 consecutive photocatalytic cycles of activated carbon/acid treated material a cumulative yield of 19.9 ml H2 per h pinewood was achieved.115
The use of photocatalysts such as TiO2 modified with precious metals for H2 production from biomass is well documented as detailed above. The use of precious metals, however, increases the cost, and cheaper alternatives need to be developed for the photocatalytic biomass conversion process. The following reports of photocatalysts developed for hydrogen production were reviewed.
Cu, In doped ZnS derived from ZIF-8 showed high activity in biomass photoreforming for hydrogen production.116 Grass, paper and wood were chosen as real-world substrates. Under simulated solar (Air Mass 1.5 spectrum, AM 1.5) irradiation in 10 M NaOH solution at 70 °C, 31.7, 60.2 and 73.9 μmol per h per g H2 were produced respectively. To investigate the contribution of various components of biomass to hydrogen production, model reagents such as glucose, cellobiose, formate, and cellulose were tested for hydrogen production respectively. Notably the hydrogen production rate was low when lignin was used as substrate, which indicates that most of the generated hydrogen was contributed by the cellulosic portion of raw biomass.
Thermal radiative Pt/SiC/CdS117 performed well as a material for the photocatalytic generation of hydrogen from biomass. In an alkaline pre-treated grass solution (10 M NaOH), 259 μmol per h per g H2 yield was achieved under irradiation with a 300 W Xe lamp light source at 343 K. In addition to grass, wood and paper were also used as raw biomass to investigate the efficiency of hydrogen production under this catalyst. The hydrogen production yield decreased in the order of grass, wood and paper, which was not further investigated in this work.
Typical non-metal photocatalyst carbon nitride (CNx) was also investigated. Kasap et al.118 reported cyanamide-functionalized carbon nitride (NCNCNx) allowed visible light-driven photoreforming of raw biomass. All of the raw biomass examined, including sawdust, paper, cardboard, bagasse and wooden branches, successfully resulted in H2 formation. Sawdust produced 202 μmol h−1 g−1 with NiP and NCNCNx materials under simulated solar light (AM 1.5 G, 100 mW cm−2). It is noteworthy that lignin and its components were generally poor at producing hydrogen under these conditions.
In addition to raw biomass, raw biomass waste was also used as a substrate for hydrogen production. Pulp and black liquor, as one of the most abundant fibrous biomass wastes, has great potential to be used as a substrate for hydrogen production. The pulp and paper industry ranks sixth among the world's most polluting industries, creating toxic wastewater on a large scale after the paper is processed.119 Pulp and paper are manufactured from cellulose fibres and other plant materials. During the manufacturing process, deposits of cellulose, lignin, phenols, fatty acids, tannins, and resins are released from the pulp to form an alkaline, sticky, dark black waste called black liquor. The production of pulp and paper generates large quantities of pollutants, mainly in the form of high concentrations of suspended solids (SS), chemical oxygen demand (COD), toxicity and biochemical oxygen demand (BOD).120 Toxic effects on various fish species due to exposure to pulp and paper mill effluents have been reported.121–125 Therefore, the oxidative degradation of lignocellulosic biomass from waste pulp and black liquor with simultaneous hydrogen production is an attractive research direction.
Zhong et al.126 designed and synthesized a three-dimensional ordered macroporous (3DOM) structure TiO2–Au–CdS for biomass photoreforming for hydrogen production (Fig. 5). Cellulose, cellobiose and dissolved pulp were tested. As the complexity of structures grew, the hydrogen production rates gradually decreased from 273.9 μmol h−1 g−1 (cellobiose) to 79.7 μmol h−1 g−1 (dissolved pulp). It is worth noting that other gaseous components such as CO and CH4 were also produced as part of the photocatalytic process.
 | ||
| Fig. 5 Schematic illustration of the 3DOM TiO2–Au–CdS preparation process reproduced from ref. 126 with permission from MDPI. | ||
Zou et al.127 combined photoreforming and acid hydrolysis to achieve simultaneous biomass conversion and hydrogen production. Paper pulp as a representative raw feedstock resulted in 89% H2 yields, based on the number of monosaccharides in pulp. At 403 K the efficient and stable hydrogen generation could reach 1320 μmol per h per g cat. in 0.6 M H2SO4. The products of the pulp conversion were, however, not described.
In addition to the paper industry, waste and wastewater in the food industry are also potential hydrogen-producing biomass waste. Low-toxicity food industry wastewater usually contains high concentrations of organic matter (e.g., sugars, proteins, etc.).128 Therefore, if it is discharged without treatment, it can easily lead to eutrophication of the water body thus creating an anoxic environment, which ultimately makes it toxic for fish and other aquatic organisms to survive.129 Organic matter in wastewater can act as a sacrificial and proton donor for oxidative degradation along with hydrogen production130 and will be discussed in detail in Section 2.3.
In 1995, Greenbaum et al.137 investigated the photocatalytic conversion of woody biomass to volatile hydrocarbons. It was reported that the wood samples were converted into a wide range of hydrocarbons from C4 to C20 hydrocarbons in a flowing photoreactor over a ZnO photocatalyst irradiated with a Xe lamp. This research demonstrated the feasibility of photoreforming lignocellulosic biomass into value added chemicals.
Nguyen and co-workers reported a photocatalytic method for redox-neutral depolymerization of raw lignin biomass. Raw lignin biomass i.e. grand fir, ponderosa, cedar and larch which were extracted from the corresponding sawdust were investigated with [Ir(dFCF3ppy)2-(5,5′-dCF3bpy)]PF6 (ppy; polypyrrole, bpy; 2,2′-bipyridine) as a catalyst under blue LED irradiation for 24 h.138 3,4-Dimethoxybenzaldehyde, vanillin and 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde were detected after photocatalytic depolymerization. These products suggested that native lignin could be converted into monomers by β-ether bond cleavage.
To fully utilize the entire lignocellulosic biomass, a lignin-first approach was reported by Wu et al.139 In this approach native lignin in lignocellulosic biomass was converted into value added chemicals by cadmium sulfide quantum dots with MPA ligand (CdS QDs-MPA) while cellulose and hemicellulose remained almost fully intact. Furthermore, the colloidal character of quantum dots enabled their facile separation and recycling by a reversible aggregation–colloidization strategy. With birch woodmeal as substrate the major products were syringyl- and guaiacyl-derived ketones due to β-O-4 bond cleavage and the total yield of monomeric aromatics generated was 26.7 wt%. Lu et al.140 described the photocatalytic depolymerization of rice husk over TiO2 in an aqueous H2O2 (30%) solution with a 500 W mercury lamp. GC-MS confirmed the presence of 172 organic species in the product extract. Alkanes, phthalates and ketones were the largest groups of products.
In summary, research on photoconversion of lignocellulosic biomass has been conducted since the 1980s. The huge differences between raw biomass and model compounds have led to the fact that research is still trying to expand from model compounds to real-world biomass. From a feedstock perspective, real-world lignocellulosic biomass is derived from crops and wood, with a recalcitrant structure of cellulose, hemicellulose, and lignin crosslinked with each other meaning existing photocatalytic materials perform poorly. Moreover, the water-insoluble nature further reduces the efficiency of conventional photocatalytic suspension systems, and the use of organic solvents increases the cost and may generate pollution as well. To increase the hydrogen yields, most studies have reported the use alkali pre-treatment of biomass, thus further increasing the potential cost of the process. The substrate remaining after the photocatalytic hydrogen production reaction has also not been considered in detail and the remaining organic components may cause secondary pollution if discharged without additional treatments. Many high-value chemicals generated during photoreforming require subsequent separation and purification before utilization. From a material point of view, the most widely used material for hydrogen production is Pt/TiO2, which is expensive due to the scarcity of precious metals and it is still challenging to recover and reuse the photocatalyst materials from the reaction system. Other low-cost photocatalytic materials have not yet reached the level of commercial production of titanium dioxide and are therefore still far from practical application.
Table 1 presents an overview of the valorisation of a range of “real-world” biomass materials which have been investigated using semiconductor photocatalysis.
| Feedstock details | Catalyst | Experimental conditions | Product details | Reference |
|---|---|---|---|---|
| Cherry wood | 4% Pt/TiO2 | 500 W Xe lamp | Cotton: 200 μmol H2 | 109 |
| Dutch clover | 5 M NaOH pre-treatment | Lignin: 77 μmol H2 | ||
| Golden-rod | Sweet potato: 378 μmol H2 | |||
| Water hyacinth | Fatty oil: 212 μmol H2 | |||
| Rice plant | Cherry wood: 148 μmol H2 | |||
| Seaweed | Dutch clover: 142 μmol H2 | |||
| Algae | Golden rod: 55 μmol H2 | |||
| Oil | Water hyacinth: 130 μmol H2 | |||
| Cotton | Rice plant: 175 μmol H2 | |||
| Sweet potato | Chlorella algae: 270 μmol H2 | |||
| Seaweed: 166 μmol H2 | ||||
| Laver seaweed: 332 μmol H2 | ||||
| Additional products included ammonia, short chain hydrocarbons, ethanol, and methanol | ||||
| Rice husk | 0.5% Pt/TiO2 | 366 nm UV light | Rice husk 18 L H2 per m3 under UV-light | 110 |
| Alfalfa stems | Rice husk 15 L H2 per m3 under natural solar light | |||
| Bamboo | 2% Pt/TiO2 | 1% NaOH pre-treatment followed by conversion to ethanol and xylose by SSF using enzymes | Bamboo 74% yield H2 per 10 g bamboo following pre-treatment and SSF | 111 |
| Rice straw | Hg lamp | Rice straw 96% yield H2 10 g rice straw following pre-treatment and SSF | ||
| Silvergrass | Silvergrass 97% yield per 10 g silvergrass following pre-treatment and SSF | |||
| Corn stover | 1% Pt/TiO2 | NaOH pre-treatment | Corn stover: 25.84 μmol per h H2 | 112 |
| 300 W Xe lamp | ||||
| Fescue grass | 0.2% Pt/TiO2 | Grass washed with methanol at 55 °C in an ultrasonic bath then dried overnight | Fescue grass: 494 μmol per h per g per gcatal H2 | 114 |
| 150 W Xe lamp | ||||
| Grass | Cu and In doped ZnS derived from ZIF-8 | No pre-treatment | Grass: 31.7 μmol per h per g H2 | 116 |
| Wood | Simulated solar (AM 1.5) irradiation | Wood: 73.9 μmol per h per g H2 | ||
| Paper | Paper: 60.2 μmol per h per g H2 | |||
| Lignin | 0.5% Pt/SiC/CdS | 300 W Xe lamp | Lignin: 11 μmol per h per g H2 | 117 |
| Grass | 10 M NaOH reaction media | Grass: 259 μmol per h per g H2 | ||
| Wood | Wood: 131.7 μmol per h per g H2 | |||
| Paper | Paper: 60.7 μmol per h per g H2 | |||
| Sawdust | Activated NCNCNx and NiP catalyst | Solar simulator Xe lamp | Sawdust: 202 μmol H2 per (g CNx) per h H2 | 118 |
| Paper | CNx was activated by ultra-sonication in a potassium phosphate solution | Reactions carried out in KPi at a pH of 4.5 | Paper: 42.7 μmol H2 per (g CNx) per h H2 | |
| Cardboard | Cardboard: 46.9 μmol H2 per (g CNx) per h H2 | |||
| Bagasse | Bagasse: 34.8 μmol H2 per (g CNx) per h H2 | |||
| Wooden branch | Wooden branch: 35.7 μmol H2 per (g CNx) per h H2 | |||
| Pulp | 3DOM TiO2–Au–CdS | Pre-treated but not specified how | Pulp: 79.7 H2 mol per h per g H2 | 126 |
| Pine wood | 1% Pt/TiO2 | Acid hydrolysis pre-treatment (1% HCL at 180 °C for 120 min) | Pine wood: 19.9 ml H2 per g pinewood after 8 consecutive cycles | 115 |
| Xenon lamp AM 1.5 G | ||||
| Paper pulp | 0.5% Pt/TiO2 | 250 W iron doped halide lamp | Paper pulp: H2 1320 μmol per h per g cat. | 127 |
| 403 K reaction temperature in acidic reaction media (0.6 M H2SO4) | ||||
| Poplar wood | ZnO | Treatment with either 3 M ferric chloride or nitrate solution or ZnO at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi with a lab press 000 psi with a lab press |
Poplar wood (ferric chloride treated): short chain alcohols, furan, methyl acetate, furfural | 137 |
| Pine wood | Canrad-Hanovia compact xenon-cathode tip lamp | Poplar wood (ZnO treated): C4–C20 alcohols, long chain hydrocarbons, aldehydes | ||
| Grand fir | [Ir(dF(CF3)ppy)2(5,5′-d(CF3)bpy)]PF6 | Blue LEDs | Grand fir: 2.1% vanillin, 1.3% 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde. B-Ether bond cleavage: 41% | 138 |
| Ponderosa | PBu4OP(O)(OtBu)2, 3,4-diflurorthiophenol, dioxane | Ponderosa: 2.3% vanillin, 1.4% 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde, B-ether bond cleavage: 34% | ||
| Cedar | Cedar: 3% vanillin, 1.8% 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde, B-ether bond cleavage: 42% | |||
| Larch | Larch: 3.2% vanillin, 1.9% 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde, B-ether bond cleavage: 22% | |||
| Birch woodmeal | Cadmium sulfide quantum dots with MPA ligand (CdS QDs-MPA) | 300 W Xe lamp with an ultraviolet (UV) cut-of filter (λ ≥ 420 nm) | Birch woodmeal: syringyl-derived ketones (17.3%) | 139 |
| Guaiacyl-derived ketones (7.4%) | ||||
| Rice husk | TiO2 in aqueous 30% H2O2 solution | 500 W low-pressure mercury lamp (365 nm) | 172 organic compounds, alkanes, alkenes, arenes, non-substituted alkanols, substituted alkanols, alkenols, phenols, alkanals, alkenals, benzaldehydes, ketones, carboxylic acids, alkanoates, phthalates, nitrogen-containing organic compounds, sulfur-containing organic compounds and other species | 140 |
2.2 Waste plastics
For the past century plastics have been an inherent part of our daily lives with the development and production of new plastic products really gaining momentum after WWII. Plastics are extremely versatile, and they have revolutionized almost every aspect of our lives, from medicine to cutting food waste and conserving energy.141 They are cheap, convenient, light weight, durable and have an almost indestructible morphology. It is these highly desirable properties that make plastic the ideal material for such a wide range of applications that has resulted in the current environmental plague of plastic pollution. Single use plastics, such as plastic bottles can take up to 450 years to decompose and 500 years for a plastic toothbrush to decompose.142 Globally only 9% of plastic waste is recycled, 19% is incinerated, 50% ends up in landfill and 22% evades waste management.143 While landfill and incineration are low cost and simple disposal methods, they are extremely detrimental to the environment.144 Since 1950, over 8 billion tons of plastic have been produced, over 80% of which has become either waste in landfills or in the natural environment145,146 and it has been estimated that ca. 8 million tons of plastic enters the oceans every year. In 2016 alone, it has been estimated that up to 23 million tonnes of plastics entered aquatic ecosystems.147,148In the Pacific Ocean there is a “garbage patch” of floating plastic denoted as the 7th continent.149 At a size of 1.6 million square kilometres, it is more than 3 times the size of France and 2 times the size of Texas. The “garbage patch” is estimated to contain 80 thousand tons of plastic and 1.8 trillion pieces of plastic (microplastics constitute 94% of the patch) which has been exponentially increasing since the 1970's. As larger pieces of plastic making up the patch such as discarded fishing gear, bottle caps, crates and containers eventually break down they have the potential to increase the levels of microplastics by approximately 30-fold to around 50 trillion particles.150 The great pacific garbage patch can be seen in Fig. 6.
 | ||
| Fig. 6 The mass concentration model, pictured above, shows how the concentration levels gradually decrease by orders of magnitude towards the outside boundaries of the GPGP (great pacific garbage patch). The centre concentration levels contain the highest density, reaching 100 s of kg km−2 while decreasing down to 10 kg km−2 in the outermost region. Reproduced from ref. 150 with permission from the Ocean Cleanup. | ||
Very recently microplastics (ranging from 5 to 10 μm in size) were detected in human placenta samples.151 The microplastics were identified as pigmented polypropylenes which are used in paints, coatings, adhesives, plasters, polymers, dyes, cosmetics, and personal care products.152 It is thought that microplastics access the bloodstream from either the maternal gastrointestinal tract or respiratory system and then enter the placenta. Microplastics were found in both the maternal and foetal sides of the placenta as well as the chiromantic membranes. This may cause a range of detrimental effects on a developing foetus including triggering an immune response and the release of toxic contaminants that could lead to adverse pregnancy outcomes. With microplastics found in placenta, it is no surprise they have also been found in human blood,153 faeces,154,155 gastrointestinal tract,156 lungs,157 liver tissue, brain,158 spleen and kidney tissues.159,160 Humans are exposed to microplastics and by a variety of different methods, such as the consumption of contaminated marine animals,161 drinking water (tap and bottled),162 and foodstuff,163–165 such as honey, salt sugar, beer, vegetables, fruits,166 dairy,167 meat,168 soft drinks, tea,169 and cereals, as well as in agricultural soil.170 Additionally, exposure comes from use of cosmetics, toothpaste, and pharmaceuticals.171–175.
Plastic waste is a persistent, abundant, cheap feedstock with both enormous challenges and potential. Plastic waste stores a significant amount of chemical energy which can be recovered to meet energy demand. The valorisation of plastic waste presents a significantly untapped opportunity to address environmental issues while also creating an economic push for a circular economy. While there has been significant research using pure control samples of e.g., PVC and polystyrene, it is absolutely crucial to investigate real-world plastic samples, as they typically contain additional filters, cross-linkers or antioxidants that could make photoreforming more challenging. This section outlines the current literature on the photocatalytic reforming of plastic waste.
More recently, Reisner et al.200 investigated the photoreforming of 3 commonly produced polymers: polyethylene terephthalate (PET), polylactic acid and polyurethane over toxic yet inexpensive CdS/CdOx quantum dots. The process operated under ambient conditions using visible light to produce pure H2 and organic products such as formate, pyruvate, and acetate. Activity of the photoreforming system for PET and PU was found to be 12.4 and 3.22 mmol H2 per gCds per h respectively. Following an alkaline hydrolysis, monomers were released into solution which could be reused. Real-world applicability has been demonstrated by photoreforming a PET water bottle to produce H2 using CdS/CdOx. Over the course of 6 days of photocatalytic reaction, there was continuous generation of H2, with 4.13 mmol H2 per g CdSs per h generated, with an external quantum yield of 2.17% and a conversion efficiency of 5.15% (Fig. 7).
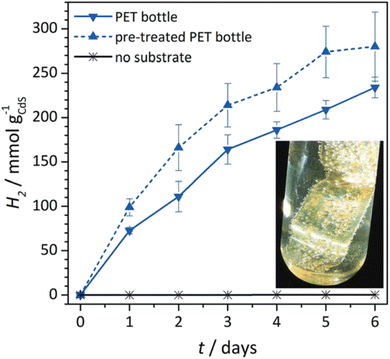 | ||
| Fig. 7 Long-term photoreforming of a PET bottle to H2 using CdS/CdOx QDs (1 nmol) under simulated sunlight (6 days, AM 1.5 G, 100 mW cm−2, 25 °C). Conditions: ground PET bottle (25 mg ml−1) freshly prepared or pre-treated in 10 M aq. NaOH (2 ml). Inset: photograph of a PET bottle sample; H2 bubbles are visible on the plastic surface. Reproduced from ref. 200 with permission from the Royal Society of Chemistry. | ||
The Reisner group has also investigated the photoreforming of plastic using a non-toxic and non-precious metal containing carbon nitride with nickel phosphide cocatalyst CNx/Ni2P.201 Initial tests were carried out to convert PET and PLA to H2 and chemicals including acetate, formate and glyoxal as well as hydrogen. Following the successful conversion of PET and PLA, real-world substrates including polyester fibres and an oil contaminated PET and a non-contaminated PET water bottle were also successfully photoreformed. After 5 days of irradiation in the presence of the photocatalyst, the hydrogen yields of 104, 22 and 11.4 μmol H2 per g sub. were obtained for polyester fibres, PET bottle and the oil contaminated PET bottle respectively. As the reaction progressed, the surface area of the fibres exposed during the reaction increased, which resulted in an increasing rate of reaction over time. The oil contaminated PET bottle had a low yield due to the oil preventing access to the PET water bottle by the reactive oxygen species. Additional fillers and lower solubility of the real-world substrates were thought to result in the poorer performance of these materials compared to pure samples. The photocatalytic process was scaled up successfully from 2 ml to a 120 ml system.
The Reisner group202 have recently shown a visible light-driven reaction to deconstruct real-world polystyrene into benzoic acid and other monomeric aromatic products at 50 °C under ambient pressure. A range of fluorenone photocatalysts (e.g. 9-fluorenone, 2-bromo-9-fluorenone and 2, 7-dicholoro-9-fluorenone) were tested and irradiated under blue LED light with 1 equivalent of H2SO4 and oxygen supplied in a balloon. It was determined that a fluorenone loading of 20 mol% was required for the optimum benzoic acid yield (38% after 48 h over 9-fluorenone). The reaction was then scaled up to the gram scale to investigate the deconstruction of pure polystyrene and real-world polystyrene foam. In the case of pure polystyrene, after 64 h photocatalysis the yield of all aromatic compounds was 60 ± 2% (including 36 ± 4% benzoic acid). Conversion of the real-world polystyrene foam produced a similar product distribution with 60 ± 4% aromatic compound yield and 36 ± 2% yield of benzoic acid after 72 h of photocatalysis. The reaction was believed to proceed via a C–H bond oxidation pathway over all catalysts and allowed valuable products to be generated from real-world waste plastic.
Li et al.203 prepared a series of MoS2/CdxZn1−xS photocatalysts for solar H2 production coupled with the degradation of plastics. 4.3 wt% MoS2 integrated Cd0.5Zn0.5S exhibited the highest H2 evolution rate of 15.90 mmol h−1 g−1 and H2 yield of 80 mmol g−1 from a PET based aqueous alkaline solution. This enhanced H2 production over this material was attributed to the synergetic effects in terms of charge separation efficiency, light absorption capability and suitable oxidation potential. Various loadings of MoS2 were investigated. The presence of any loading of MoS2 compared to no loading was found to enhance the H2 evolution compared to no loading, which was attributed to the MoS2 material providing more active sites to facilitate proton reduction and accelerate photoexcited carrier separation. A loading above 4.3 wt% MoS2 was found to be detrimental to photocatalytic activity. 4.3 w% MoS2 was determined to be the optimum loading resulting in the best H2 yield and production rate. This study was further extended to a PET plastic bottle, with a H2 evolution rate of 14.9 mmol h−1 g−1 and an H2 yield of 70 mmol g−1. The eventual products of the PET bottle's photocatalytic reforming were formate, methanol, acetate and ethanol. PET/pieces of PET bottle were stirred in 10 M NaOH for 24 h at 40 °C, then the mixture was centrifuged, and the supernatant was used as the photocatalytic substrate. SEM and TEM images of the catalysts used in this study can be found in Fig. 8.
 | ||
| Fig. 8 (a and b) SEM and (c and d) TEM images of the MoS2/CdS and (e) MoS2/CdS interface convinced by HRTEM and (f) corresponding theoretical model; (g) element mapping of MoS2/CdS. Reproduced from ref. 203 with permission from Wiley. | ||
Du et al.204 described the synthesis of MoS2 tipped CdS nanorods for the photocatalytic reforming of plastic waste into commodity chemicals and hydrogen. The CdS nanorod behaved as the light absorber and a hole acceptor while MoS2 acted as an electron collector (Fig. 8). The spatial separation of these two components enabled fast charge migration to achieve a long-lived charge carrier. Like other studies the plastics were subjected to a pre-treatment step with 10 M KOH or HNO3 depending on the plastic. Using PLA as the substrate it was found that a higher rate of H2 was produced (3–6.2 mmol g−1 h−1) as the concentration of KOH was increased from 1–10 ml over a 21.8 wt% MoS2/CdS catalyst respectively. This enhanced activity was attributed to the higher concentration of lactate in solution as the alkalinity of the solution was increased. As the MoS2 loading was increased the H2 formation increased up to a loading of 21.8 wt%. A further increase in the loading of MoS2 to 46.2 wt% resulted in a decrease in H2 formation. After photocatalysis over a 21.8 wt% MoS2/CdS catalyst for 25 h, the total H2 evolved was 95 mmol g−1 from pre-treated PET. Encouragingly the total H2 evolved from a pre-treated PET bottle was 85 mmol g−1 after 25 h. Using pre-treated PLA as the substrate resulted in ca 160 mmol per g H2 produced after 25 h of photocatalysis.
Xie et al.205 developed a universal photoinduced C–C cleavage and coupling pathway for converting waste plastics into high-energy-density C2 fuels under atmospheric conditions. Using the developed pathway Xie and co-workers were able to selectively photoconvert real-world plastics such as plastic bags, disposable food containers, food wrap films and their main components of polyethylene, polypropylene and polyvinylchloride into C2 fuels using single-unit-cell thick NbO2 layers and sunlight. In the case of polyethylene, polypropylene and polyvinylchloride, 100% of these materials were photocatalytically degraded to CO2 gas within 40, 60 and 90 h, respectively. The CO2 was then photoreduced to acetic acid via the same catalyst with formation rates of ca. 47.4, 40.6 and 39.5 μmol gcat−1 h−1 respectively being reported.
A novel floating carbon nitride composite was developed for the solar reforming of waste plastics and biomass in a very recent study by Reisner et al.206 CNx/Pt and CN/NiP were immobilised on hollow glass microspheres (HGM) which acted as supports enabling the catalytic material to float and overcome limitations such as turbid waste streams (Fig. 9) Not surprisingly, solar reforming activity was highest over Pt (0.91%) deposited on CN with a specific activity of 78.2 ± 7.5 μmolH2 gCNx−1 h−1 for KOH pretreated PET. The specific activity was found to increase to 99.9 ± 2.6 μmolH2 gCNx−1 h−1 using the same catalyst following reaction scale up and recycling.
 | ||
Fig. 9 SEM images showing the morphology of floating CNx composites differentiated by their HGM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) melamine ratio. (A) 1 melamine ratio. (A) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (B) 1 1, (B) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (C) 1 2, (C) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (D) 1 3, (D) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (scale bar shared by panels A–D), (E) coloured EDX overlay showing Ni, N, and Si of 1 4 (scale bar shared by panels A–D), (E) coloured EDX overlay showing Ni, N, and Si of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with a Ni2P co-catalyst, (F) coloured EDX overlay showing Pt, N and Si of 1 3 with a Ni2P co-catalyst, (F) coloured EDX overlay showing Pt, N and Si of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with a Pt co-catalyst, (G) Im30K glass bubbles, (H) STEM-HAADF of HGM/CNx|Pt (1 3 with a Pt co-catalyst, (G) Im30K glass bubbles, (H) STEM-HAADF of HGM/CNx|Pt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) showing elemental contrast, (I) EDX overlay on STEM-HAADF of HGM/CNx|Pt (1 3) showing elemental contrast, (I) EDX overlay on STEM-HAADF of HGM/CNx|Pt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) showing Pt, N and Si. Reproduced from ref. 206 with permission from Wiley. 3) showing Pt, N and Si. Reproduced from ref. 206 with permission from Wiley. | ||
Photoreforming of plastic waste has huge potential to combat plastic pollution as well as generating value added chemicals and hydrogen, thus contributing to a circular economy and helping towards achieving net zero targets. However, adding value to plastic is not without its challenges. Currently the plastics undergo some sort of undesirable pre-treatment such as alkaline hydrolysis and the use of real-world contaminated plastic remains a further challenge. Table 2 provides an overview of some of the key research undertaken to date on photocatalytic reforming of plastic materials.
| Feedstock details | Catalyst | Experimental conditions | Product details | Reference |
|---|---|---|---|---|
| PVC | 5% Pt/TiO2 | 500 W Xe lamp | PVC: 45 μmol H2 | 199 |
| PE | Alkaline hydrolysis pretreatment | PE: 93 μmol H2 | ||
| PVA | PVA: 86 μmol H2 | |||
| PET | CdS/CdOx | Ambient conditions and visible light | PET: 12.4 mmol H2 per gCds per h | 200 |
| PLA | Alkaline hydrolysis pretreatment | PU: 3.22 mmol H2 per gCds per h | ||
| PU | PET water bottle: 4.13 mmol H2 per gCds per h external quantum yield; 2.17% conversion; 5.15% | |||
| PET water bottle | ||||
| PET | CN/Ni2P | Alkaline hydrolysis pretreatment | PET: acetate 190 nmol, formate 190 nmol, glyoxal, 9300 nmol | 201 |
| PLA | Visible light | PLA: acetate 100 nmol, formate 95 nmol | ||
| Polyester fibres | Fibres: 104 H2 per g sub. | |||
| PET water bottle oil contaminated PET water bottle | PET water bottle: 22 H2 per g sub. | |||
| Contaminated PET water bottle: 11.4 μmol H2 per g sub. | ||||
| PS pure | 20 mol% fluorenone | 50 °C | Pure PS: 60% aromatic compounds | 202 |
| PS foam | Blue LED | PS foam: 60% aromatic compounds | ||
| 1 eq. of H2SO4 | ||||
| O2 balloon | ||||
| PET | MoS2/CdxZn1−xS | Alkaline hydrolysis pretreatment | PET: 80 mmol per g H2 | 203 |
| PET water bottle | PET water bottle: 70 mmol per g H2 plus additional products such as formate, methanol, acetate, ethanol | |||
| PLA | 21.8% MoS2 tipped CdS nanorods | Alkaline/acid hydrolysis pretreatment | PLA: 160 mmol per g H2 | 204 |
| PET | 300 W Xe lamp illumination equipped with an AM 1.5 solar simulator | PET: 95 mmol per g H2 | ||
| PET water bottle | PET water bottle: 85 mmol per g H2 | |||
| Pure PE | Nb2O5 | 300 W Xe lamp illumination equipped with an AM 1.5 solar simulator | Evolution rate of acetic acid | 205 |
| Pure PP | O2 atmosphere | Pure PE: 42.6 μg gcat−1 h−1 | ||
| Pure PVC | Pure PP: 39.6 μg gcat−1 h−1 | |||
| Food container | Pure PVC: 37.8 μg gcat−1 h−1 | |||
| Food wrap film | Food container: 20.8 μg gcat−1 h−1 | |||
| Single use bag | Food wrap film: 19 μg gcat−1 h−1 | |||
| Single use bag: 24.6 μg gcat−1 h−1 | ||||
| PET | Floating CNx, CNx|Pt, CNx|Ni2P | Vertical simulated solar irradiation (AM 1.5 G) | PET: 78.2 ± 7.5 umolH2 per gCNx per h H2 | 206 |
| Alkaline hydrolysis pretreatment |
2.3 Wastewater
Industrial wastewater or effluents is one of the most frequently exposed waste resources in our daily life.207 Industrial wastewater can be resource-rich in nutrients and chemical compounds.208 It can contain a broad range of contaminants including heavy metals, organic matter, inorganic particles, toxins, pharmaceuticals, agrichemicals, and microplastics. Chemical oxygen demand (COD) is a measure of the capacity of water to consume oxygen during the decomposition of organic matter in the water.209 For example, an average COD of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 is generated from paper and pulp industries every year,210 and the value is 16
000 mg L−1 is generated from paper and pulp industries every year,210 and the value is 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 for palm oil mill effluent.211 The organic matter in industrial wastewater is potentially the most valuable part which could be reformed through various technologies generating value-added products and energy, especially through a sustainable process such as photocatalysis.212–214 Based on the basics of photocatalytic process in the system of water, the generated reactive species, i.e., hydroxyl radicals (˙OH) and superoxide radicals (˙O2−), can provide the redox potential of the reactions of organic matter adsorbed on the photocatalysts,215 which leads to the reforming/degradation of organic matter and production of H2 energy. The following considers the current state-of-the-art research on the photocatalytic process of a real-world feedstock: industrial wastewater effluents.
000 mg L−1 for palm oil mill effluent.211 The organic matter in industrial wastewater is potentially the most valuable part which could be reformed through various technologies generating value-added products and energy, especially through a sustainable process such as photocatalysis.212–214 Based on the basics of photocatalytic process in the system of water, the generated reactive species, i.e., hydroxyl radicals (˙OH) and superoxide radicals (˙O2−), can provide the redox potential of the reactions of organic matter adsorbed on the photocatalysts,215 which leads to the reforming/degradation of organic matter and production of H2 energy. The following considers the current state-of-the-art research on the photocatalytic process of a real-world feedstock: industrial wastewater effluents.
Effluents from the food industry (i.e., juice,216,217 olive oil218,219) were studied mostly as a feedstock for photocatalytic H2 production. H2 yields of 115 μmol gcatalyst−1 h−1 were reported using an Au/TiO2 material for the photocatalytic reforming of wastewater from juice manufacture. This was significantly higher than yields of hydrogen from the photoreforming of municipal wastewater in the same reactor. The relatively higher production rate of H2 was potentially due to the significant level of saccharides contained in the wastewater from the juice manufacturing process.216 It was reported, however, the wastewater from fruit bottling plants with higher initial DOC content (600 mg L−1 in fruit bottling wastewater vs. 100 mg L−1 in municipal wastewater) resulted in lower H2 production compared to that produced from municipal wastewater following THE photocatalysis of the effluent.
Therefore, the concentration of organic matter in the effluents is not the only consideration affecting the reactivity of H2 production from industrial wastewater. Badawy et al.218 investigated the effect of the pH of olive oil industrial wastewater on its photoreforming reactivity over a mesoporous TiO2 photocatalyst. According to their study, an acidic pH was favourable for the photocatalytic degradation of organic compounds due to the improved adsorption of organic compounds onto the TiO2 surface and therefore facilitated the photocatalytic degradation process. An acidic pH value was also reported to benefit the H2 production from the photocatalytic reforming of olive oil industrial wastewater. This positive influence was believed to be from:
(i) the positions of the valence-band and conduction-band levels of the semiconductor with respect to those of redox couples in solution,
(ii) the charging behaviour of the TiO2 surface, and
(iii) the competitive adsorption of organic pollutants on the TiO2 surface.
Moreover, it was reported by Speltini et al.219 that the H2 yield could be significantly enhanced (an increase of the yields to 81%) by performing a simple clean-up (magnesium silicate as a clean-up sorbent) of the raw olive oil wastewater which resulted in the removal of species which could potentially foul the photocatalyst surface, for example lipidic compounds. Partial deactivation of Pt/TiO2 for H2 production was observed from the presence of oil and grease compounds, and inorganics (F−, Cl−, PO43− and SO42−) in the olive oil wastewater effluent.219
The photocatalytic degradation of the organic matter present in effluents from the paper and cellulose industry was reported by Machado et al.220 The pH, the use of additives, and the morphology of the photocatalyst were reported to influence the degradation efficiency considerably. Acidic pH (<4) was beneficial to the degradation efficiency observed for effluents containing lignin and cellulose pulp. The use of a P25 TiO2 photocatalyst resulted in a 60% higher degradation rate than that obtained when an anatase TiO2 material was used for the photocatalytic degradation of that effluent. The addition of hydrogen peroxide in the reaction system over P25 TiO2 increased the efficiency of the photocatalytic process by over 170%.
Other effluents such as municipal wastewater were also investigated as real-world feedstocks for photocatalytic H2 production.216,217 H2 production was limited over H,DPCu/TiO2 and H,DPAu/TiO2 photocatalysts as reported by Imizcoz et al.216 The efficiency of H2 production (21 μmol H2 per L wastewater) and organic pollutant removal (15% of dissolved organic carbon, DOC) via photocatalysis over Au/TiO2 in municipal wastewater at pilot-plant scale (5 h irradiation under direct solar light in a Compound Parabolic Collector reactor, CPC reactor, accumulated radiation energy of 110 kJ L−1) was reported by Salgado et al.217 Based on their studies, the chemical nature and the concentration of organic compounds present in the real wastewater effluents significantly influenced the photocatalytic reactivity. Furthermore, the presence of high concentrations of Cl− and SO42− ions was found to reduce the photocatalytic activity for H2 generation.217
Secondary effluent from a municipal wastewater treatment plant was also investigated as feedstock for H2 production over P–TiO2/Pt microspheres.221 In this study, the dissolved organic matter and inorganic ions in secondary effluent were isolated into four fractions, i.e., hydrophobic acids (HOA), hydrophobic bases (HOB), hydrophobic neutrals (HON), and hydrophilic substances (HIS). Among those species, the decomposition of humic/fulvic acid-like substances in the HIS fraction played a role in accelerating hydrogen production and removal of estrogenic substances. This was proposed to be a result of the electron donors (i.e., formaldehyde, acetaldehyde, acetate, and formate) being generated from the decomposition of humic/fulvic acid-like substances by reactive oxygen species (ROS, i.e., h+, ˙OH, and ˙O2−) in the aerobic reaction system, which could significantly improve the efficiency of H2 production.
Based on the reports for photocatalytic reforming/degradation of real-world effluents, the concentration of organic matter in the effluents and the pH value of the effluents could significantly influence the efficiency of photocatalytic reforming/degradation processes. The chemical composition of the effluents was also important, for example, the lipidic compounds, oil and grease, as well as the inorganics such as F−, Cl−, PO43− and SO42− could potentially deactivate the photocatalysts in the reaction systems. Therefore, pre-treatments such as precipitation and filtration, and pH adjustment of the effluents were required to obtain an efficient photocatalytic treatment process. TiO2-based noble metal photocatalysts have been frequently investigated for their ability for use in photocatalytic reforming/degradation of wastewater effluents. To further improve the efficiency of the photocatalytic reforming/degradation of real-world effluents, cheaper and highly efficient photocatalysts will need to be developed. The inorganic and metal content present in the industrial wastewater could also be considered as sources of materials for the preparation of photocatalysts.
Table 3 provides an overview of a range of industrial wastewater effluents that have been used for photocatalytic reforming processes.
| Feedstock details | Catalyst | Experimental conditions | Product details | Reference |
|---|---|---|---|---|
| Juice production wastewater | H,DPAu/TiO2 and H,DPCu/TiO2 25 mg | Simulated solar light (AM 1.5 G, 1.0 kW m−2) | Juice production wastewater | 216 |
| Municipal wastewater | 25 °C Ar atmosphere | H,DPAu/TiO2: <0.1 μmol per gcat per h H2, 15.8 μmol per gcat per h CO2 | ||
| No pre-treatment | H,DPCu/TiO2: 0.1 μmol per gcat per h H2, 18.2 μmol per gcat per h CO2 | |||
| Municipal wastewater | ||||
| H,DPAu/TiO2: 115.1 μmol per gcat per h H2, 306.9 μmol per gcat per h CO2 | ||||
| H,DPCu/TiO2: 10.7 μmol per gcat per h H2, 291.2 μmol per gcat per h CO2 | ||||
| Juice production wastewater | 0.5 wt% Au/TiO2 | Irradiation of natural sunlight for 5 h, pH = 3 | Methanol: 0.5 × 103 μmol per L H2 | 217 |
| Municipal wastewater | Glycerol: 4.5 × 103 μmol per L H2 | |||
| Formic acid: 6.5 × 103 μmol per L H2 | ||||
| Juice production wastewater: 5.2 μmol per L H2 | ||||
| Municipal wastewater: 17.5 μmol per L H2 | ||||
| Olive mill wastewater | Mesoporous TiO2 | UV mercury lamp (150 W, 1.32 × 10 −5 einstein per s), pH = 5.4 | Olive mill wastewater: initial H2 production rate (first 120 min): 0.18 mmol per min H2 | 218 |
| Olive mill wastewater: H2 production rate (after 240 min): 0.104 mmol per min H2 | ||||
| Olive mill wastewater | 0.3 wt% Pt/TiO2 | Irradiation of a UV-A lamp (flux 5.8 × 10−7 mol photons per s) | Under UV-A irradiation for 4 h | 219 |
| Eosin Y/Pt/TiO2 | pH = 3 | Olive mill wastewater: 44 μmol and 80 μmol (pre-treated) of H2 | ||
| Pre-treatment with magnesium silicate (125 g L−1, 6 h) | Consecutive irradiation cycles 4 × 4 h under UV-A | |||
| Under irradiation of a solar light simulator (250 W m−2) | Olive mill wastewater: 280 μmol of H2 | |||
| Glucose: 343 μmol of H2 | ||||
| Under irradiation of solar light simulator | ||||
| Olive mill wastewater: 66% H2 with respect to that obtained by UV-A irradiation | ||||
| Effluent from a paper and cellulose mill | TiO2 | 400 W mercury lamp (1100 W m−2), pH = 3 | COD reduction/removal in 180 min: lignosulphonate: 15% | 220 |
| Pre-degradation treatment of lignosulphonate | Pre-degraded lignosulphonate: 62% | |||
| Effluent: 78% | ||||
| Secondary effluent from a municipal wastewater treatment plant | P–TiO2/Pt microspheres | 450 W solar-light simulator (400–750 nm, 4.6 × 10−7 einstein per s) | H2 production from each group of compounds in the effluent | 221 |
| 5 h irradiation | De-aerated system: 0.2 μmol of H2 (HIS), 6.8 μmol of H2 (HOA), 1.2 μmol of H2 (HOB), 2.2 μmol of H2 (HON) | |||
| Dissolved oxygen-decreasing system | ||||
| 9.3 μmol of H2 (HIS), 1.7 μmol of H2 (HOA), 0.3 μmol of H2 (HOB), 1.6 μmol of H2 (HON) | ||||
| Hydrophobic neutrals (HON), hydrophobic bases (HOB), hydrophobic acids (HOA), hydrophilic substances (HIS) |
3. Comparison of different reforming methods for H2 production
Among all the reforming methods for H2 production (Table 4), steam reforming showed a relatively high technology readiness level (TRL), for example, a value of 9 for steam methane reforming (SMR) which has been commercialised. In the process of SMR, H2 was produced with CO and a small amount of CO2 through the reaction of methane and steam. Partial oxidation (POX) of the feed by limited oxygen can release heat during the oxidation, which is normally integrated with SMR. This process is called autothermal reforming (ATR). Apart from methane, propane, gasoline, coal and natural gas can also be used as feed in the ATR process for H2 production with a high efficiency of 60–75%.223| Reforming technology | Input energy source | Feed/fuels | TRL |
|---|---|---|---|
| Steam reforming (auto thermal reforming) | Heat | Methane, propane, gasoline, coal, natural gas | High |
| Aqueous phase reforming | Heat | Alcohols, polyalcohol, oxygenated organic compounds (OOCs) from biomass | Medium |
| Dry reforming | Heat | Carbon dioxide, methane | Medium |
| Plasma-assisted reforming | Electricity | Hydrocarbons, diesel, biomass, gasoline, oil, jet fuel, natural gas | Low |
| Electro-reforming | Electricity | Methanol, formic acid, OOCs from biomass222 | Low |
| Photo-reforming | Solar/electricity | OOCs from biomass, plastics, waste streams | Low |
H2 generation from aqueous phase reforming (APR) and dry reforming (DR) has medium TRL levels. In the process of APR, hydrocarbons can react with water at mild temperature between 220 and 270 °C and high pressure (30–60 bar).224 The efficiency of H2 production from APR can reach more than 55% with the challenges of activating catalysts in short time that requires a large-scale reactor.225 Alcohols (methanol, ethanol), polyalcohol (ethylene glycol, glycerol, xylitol, sorbitol), and complex matrixes such as glucose, xylose, and woody biomass can all be used as the feed in the procedure of APR for H2 generation. DR is the process during which CO2 reacts with methane to form syngas. The highest energy efficiency of 70% can be reached at 1000 K accompanied by a conversion of 83%.226 Coking and sintering of the catalyst at high temperature are the two major problems related to a loss in activity of DR.
Other reforming technologies for H2 generation such as electro-reforming (ER) and plasma-assisted reforming (PR) have relatively low TRL values, which require a long term (>10 years) to achieve commercial maturity.227 The process of ER can be seen as a reaction of proton reduction in acidic media and water reduction in alkaline media at the cathode with the oxidation of oxygenated organic compounds (OOCs) at the anode of an electrolysis cell. It is less mature than H2 production from other electro-technologies such as water electrolysis (e.g., TRL 9 for alkaline electrolysis and TRL 5–7 for proton exchange membrane electrolysis).228 However, the ER process has important advantages such as clean H2 production without downstream purification processes and the possibility of coproduction of value-added compounds at the anode of the electrolysis cell. PR for H2 production is developed from the conventional reforming method and it showed many benefits such as broad range of feed/fuels, fast response, and high conversion efficiency of up to 85%.229 In the procedure of PR, diesel, biomass, gasoline, oil, jet fuel, and natural gas can be used as feed.
H2 generation from photocatalytic reforming as an “emerging” technology has a low TRL (<4) compared to conventional technologies such as SMR (or ATR, TRL = 9) and DR (TRL = 5). Compared to other “emerging” technologies such as ER and PR, photoreforming technology also showed a broad acceptance of feeds, including from simple molecules to complex matrixes. Additionally, the requirement of energy input from solar or electricity (i.e., artificial light) makes it more sustainable and flexible in terms of energy consumption. It is promising to generate H2 with formation of value-added chemicals under mild conditions, although the photoreforming technology still has a very low efficiency of conversion and energy use compared to other reforming technologies.
4. Perspective: the role of photocatalysis within a circular economy
Photocatalysis is frequently described as an “emerging” technology and one which is capable of being deployed across multiple applications. These applications are often considered under two broad areas of environmental remediation and energy production. The former is more advanced in relation to a Technology Readiness Level (TRL), while the latter is predicted to achieve substantial impact despite its current lower TRL status. Regardless of the TRL status or predicted impact, however, there are a limited number of examples demonstrating activity or efficiency at increased or industry level capacity. In more recent years, this point has been highlighted for both photocatalytic environmental remediation230 and energy production.218Transitioning photocatalysis towards pilot scale and even industry level deployment is challenging and dependent on several parameters. If the TRL scale is used as a metric for evaluating the technology, this complexity is clearly highlighted. For example, progression from a TRL of 4 to 5 represents a photocatalytic unit operating in a ‘relevant environment’, rather than a lab environment, which crucially is underpinned by industrial stakeholder engagement. Given that most of the photocatalytic research is typically focused on materials synthesis and improving fundamental understanding, achieving that transition will not be achieved quickly. Previous papers have also warned that if this trend continues, the technology transfer challenges are neglected which can be determinantal to the research field. Specifically, Loeb et al. stated that such a trend “ignores the immense technology transfer problems, perpetuating a widening gap between academic advocation and industrial application”.231 The technology transfer challenges are those that are represented by advancing the TRL of photocatalytic technology, specifically into the region of 4–6. Despite the challenges, however, it is possible to view evidence across multiple platforms which suggests the photocatalytic transition is beginning:
• The role of the literature: an increase in the number of perspectives, roadmaps and articles which focus on holistic system analysis represents an awareness of both the challenges and opportunities with advancing photocatalytic technology.
• Net zero emission targets and national strategies: legal binding targets have been set which have changed the global energy landscape and accelerated the need for renewable energy vectors such as hydrogen.
The first point refers to the publication of articles which are beyond research advancements and address a holistic view of photocatalysis. The paper by Loeb et al.231 is an excellent example of this as it adopts a critical perspective of photocatalysis to highlight the key issues associated with the technology horizon. Furthermore, it also presents a way forward for the technology by suggesting key targets to be achieved. Our own previous article considered a similar approach but in relation to photocatalytic biomass reforming by coupling a review with a critical evaluation of the technology. The work by Skillen et al. concluded with a proposed roadmap and strategy for achieving technology deployment, which included four key points ranging from catalyst development that facilitates technology growth and not academic hype through to even distribution of research priorities232 In relation to the latter point, there is evidence of this occurring with specific examples including the work by Rumayor et al.,233 Bahnemann et al.,234 and Nishiyama et al.235 A recent lifecycle assessment (LCA) based study not only emphasized the potential for photocatalytic hydrogen production but highlighted the need for additional research in key areas. Rumayor and colleagues233 demonstrated that photocatalytic waste (glycerol) reforming was within the portfolio of sustainable hydrogen production technologies based on kg CO2 eq. In January 2023, a roadmap article was published by Bahnemann et al. for the Journal of Physics: Energy, which was a collection of articles that explored photocatalytic and photoelectrocatalytic hydrogen formation.236 The collection considered low-carbon hydrogen generation from the perspectives of basic processes, materials science through to reactor engineering and applications for biomass reforming. The roadmap article was developed with a view towards the role that alternative or low-carbon technologies can play within the transition to net zero carbon economies. This key point was also reflected in the work by Welfle et al., in 2023,87 who considered photocatalysis alongside other conversion technologies when mapping the risk and benefits of future bioenergy systems. The research applied a Bioeconomy Sustainability Indicator Model (BSIM) to “map and analyse the performance of bioenergy across 126 sustainability issues”. In order to achieve this, the authors considered 16 bioenergy case study scenarios which included:
• Hydrogen from biomass wastewater via photocatalysis.
• Hydrogen from miscanthus via photocatalysis.
In both case study scenarios, there was an assumed increase in the TRL status of photocatalysis to better evaluate the sustainability risks and benefits. The result of the modelling work (Fig. 10 and Table 5) highlighted that photocatalytic technology contributed towards several sustainability indicators, specifically highlighting ‘innovation’, ‘replaced fuels’ and ‘water use and efficiency’ as benefits. In contrast, ‘techno-economics’ was determined to be a sustainability risk in both scenarios. Given that an assumed increase in TRL status was applied for this study, it is expected that techno-economics would be a risk due to limited data to validate the feasibility of large-scale deployment. An interesting point worth noting, however, was that both case studies utilized a sustainable (miscanthus) or waste (wastewater) feedstock for hydrogen generation. This point is key as such an approach not only contributes towards net zero emission targets, but it also supports the role that photocatalysis can play in relation to enhancing and maximizing sustainable resource use. As a result, it demonstrates that photocatalysis, as an emerging technology, can be a viable option within a circular economy.
 | ||
| Fig. 10 BSIM results for photocatalytic hydrogen production within a bioenergy system highlighting sustainability risks and opportunities based on (a) photocatalytic hydrogen from biomass wastewater and (b) photocatalytic hydrogen from miscanthus reforming. Figure reproduced from ref. 87 with permission from Elsevier. | ||
The second driver behind a photocatalytic transition is countries establishing net zero emission targets coupled with appropriate legislation to support them. This has subsequently led to the development of national hydrogen strategies for the first time, which set out capacity targets for low-carbon hydrogen production. Both points are crucial as they have substantially and irreversibly altered the global energy landscape by accelerating the need for renewable energy systems to be deployed. As a result, there is increasing focus on current and emerging low-carbon technologies with the capabilities of producing renewable or ‘green’ fuels such as hydrogen. While electrolysis is expected to be the primary contributor towards renewable hydrogen targets as we approach 2030, it is becoming apparent that additional technologies will be needed. Net zero by 2050 is estimated to require approx. 4–5 TW of renewable hydrogen capacity; however, global production is currently only 0.7 GW. In order to reach such an ambitious target, a diverse collection of technologies will be required that are capable of being deployed across a broad range of sectors and geographies. The ability of these technologies to integrate into a circular economy will be a crucial consideration as reaching net zero is dependent on more than increased renewable energy production. There will be a substantial need to implement improved sustainable practices which include technology development. The emergence of biohydrogen and artificial photosynthesis (including photocatalysis) will be fundamental to that process.
At present there is of course a substantial gap between the current technology readiness status of photocatalysis and the status needed to contribute towards net zero. A specific challenge is the cost at which photocatalytic hydrogen could be produced. While the target for renewable hydrogen is $1.5 per kg H2, to date an accurate costing for photocatalytic hydrogen is yet to be established. A relative comparison, however, would be to biohydrogen production methods. A recent study by Ganeshan et al.237 conducted a techno-economic analysis (TEA) and lifecycle assessment (LCA) of biohydrogen supply chains to determine production costs and their carbon footprint. The TEA determined a range of $1.2–14.9 per kg H2 based on biomass or wastewater feedstocks, while the LCA showed −1.6–12.2 kg CO2 eq. per kg H2 based on holistic systems analysis. The lowest production costs, which would be competitive with fossil-fuel derived hydrogen, were achieved for anaerobic digestion (AD) and biomass gasification at $1.2 per kg H2 and $1.25 per kg H2 respectively. Given that both technologies are already mature (TRL 9) and well established, a low production cost is to be expected. It is, however, worth nothing that microbial electrolysis cells (MEC) were estimated to produce hydrogen at ∼$2 kg per H2. MECs have a TRL of ∼3 and therefore represent a more emerging technology that is capable of achieving a desirable H2 production cost.
Bridging the gap for photocatalysis can however be achieved through increased investment, collaborative research and development activity with a primary objective being the development of demonstrator plants. While this is ambitious given the prohibitive costs, the recent announcement of the European Hydrogen Bank (EHB) is a leading example of the level of support emerging for renewable hydrogen production. The EHB will offer producers a fixed premium cap of €4.5 per kg H2 as a subsidy for a maximum of 10 years in an attempt to establish a renewable hydrogen market. The EU hopes this will bridge the gap between fossil-fuel derived hydrogen and renewable hydrogen as they attempt to reach their ambitious targets of 10 Mt of domestic renewable hydrogen production by 2030. The EHB is the first example of a dedicated financial framework that supports renewable hydrogen generation; moreover, it has been entirely driven by the net zero transition. As that transition progresses, however, more investment and support will be needed which is inclusive of a wider range of renewable technologies, especially for those that support a circular economy such as biohydrogen and photocatalysis. At its core, the EHB is an example of financial support for technologies towards achieving larger scale deployment. It's interesting to note that such an investment was not discussed prior to net zero Emissions laws being passed, which further emphasizes the global energy transition occurring.
The significant advantage of photocatalysis is its ability to contribute towards pollutant removal and waste valorisation while simultaneously generating low-carbon hydrogen. This article has highlighted the excellent work being conducted on photocatalytic valorisation of real-world substrates which demonstrates the potential for the technology. While technology barriers still exist, there is increasing evidence and drivers that suggest photocatalysis is undergoing a transition. In doing so, it has promoted how the technology can contribute towards a circular economy and subsequently support the net zero transition.
5. Current engineering aspects
As mentioned in Section 4, photocatalysis is often described as an “emerging” technology. The majority of photocatalytic research has focused on niche material synthesis and mechanistic investigation using simple model substrates. On the lab scale, photocatalysis is a flourishing research area; however, actual industrial application is somewhat disappointing. In addition to the limited number of literature examples exploring the use of real-world substrates, there are very few examples of photocatalytic technology at industrial level application particularly for energy production. The use of non-sustainable pre-treatment steps, basic reactor design that shows little promise for scale up (e.g. a test tube), use of niche materials that are not practical for scale up and the use of platinum metal as a co-catalyst highlight some of the limitations for the potential scale up of these processes. In this section we will discuss the main issues in reactor design for photocatalytic scale up and review some of the current technology. Most of the examples in this section involving reactor design focus on environmental remediation and synthesis, rather than energy production.The main limitations for photocatalytic scale up are photon transfer limitations to the photocatalyst surface and mass transfer limitations within the reactor. Photons are obtained from either natural sunlight or artificial sources. Natural solar illumination is obviously a highly desirable renewable source of photons; however, it is location dependent and the variability of sunlight is a major drawback. Sunlight reaching the Earth's surface is composed of 55% infra-red (>700 nm), 42–43% visible light (400–700 nm) and 3–5% UV (<400 nm). Because of this there has been a huge drive in research to develop visible light activated photocatalysts. However, the development of more efficient and scalable technologies to be deployed on large scales such as industrial and pilot plants also need to be prioritised over material synthesis.
There has been a number of different technologies developed to enhance and focus light energy and also to reduce loss of light due to scattering or absorption such as solar concentrators and optical fibres. While most of the examples given in this section focus on environmental remediation, there is potential to utilize these designs and applications for photocatalytic energy production.
Solar concentrators are systems that utilize mirrors or lenses to focus a large area of sunlight to a small area, typically used for electricity production.238 However, they have found use in the harvesting of solar energy to promote light-driven photochemical transformations. A recent paper by Zondag et al.239 describes the development of a luminescent solar concentrator-based photo-microreactor and its application in the solar-driven synthesis of organic molecules. The use of this photo-microreactor allowed for transforming solar energy to a concentrated and wavelength shifted irradiation which matches the absorption characteristics of the photocatalyst. To intensify photochemical reactions, various organic dyes can be introduced to the device to target specific wavelengths through down-conversion of incident light.
Recently Zhang et al.240 described the development of a novel solar energy controllable linear Fresnel photoreactor (LFP) for wastewater treatment using real-world conditions. The reactor was composed of a 6-column mirror set up (Fig. 11 and 12). The reactor was able to track and control solar light and adjust the temperature during a photocatalytic reaction under real weather conditions. For example, under sunny conditions the reactor could maintain the optimal light irradiance and temperature, while under overcast conditions it could provide the highest possible light irradiance and temperature as a control; the performance of LFP was compared to the degradation of common pollutants such as amoxicillin, E. coli and rhodamine B, using an inclined plate reactor.
 | ||
| Fig. 11 The flow chart of the LFP control system. Reproduced from ref. 240 with permission from MDPI. | ||
 | ||
| Fig. 12 Blueprint of LFP. Reproduced from ref. 240 with permission from MDPI. | ||
Using the LFP reactor the rhodamine B degradation efficiencies were 2.1, 1.5 and 2.28 fold higher than when using an inclined plate reactor under overcast, slightly overcast and sunny conditions respectively. The degradation of amoxicillin and E. coli was also greater using the LFP reactor compared to the inclined plate control reactor under sunny conditions (2 and 1.37 fold greater respectively). LFP could achieve effective adjustment of sunlight by flexibly controlling 6 mirrors according to solar position and weather conditions. The reactor has promising potential as a real sunlight track and control reactor for practical photocatalysis. While this technology has been described for wastewater treatment, it also has the potential for application in photocatalytic energy production.
The use of optical fibres has been employed over the past few decades to reduce the loss of light due to scattering or absorption by the reaction medium. Walko et al.241 have described a scalable optical fibre reactor to produce H2 photocatalytically from water splitting. The optical fibres were coated with 5% Cu/TiO2. The photons are transmitted to the catalyst from within the light conducting medium in optical fibres. Under conventional powdered catalyst conditions, it was found that as the quantities of catalyst increased from 50 mg to 1 g the activity reduced exponentially. The scale up in the optical fibre systems was implemented by simply increasing the number of optical fibres. It was found that upon increasing the quantity of optical fibres from 1000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, activity also increases from 1 μmol to 120 μmol indicating a linear increase in activity relative to catalyst amount. Hydrogen evolution was more than doubled when the reaction was scaled up to 700 mg of catalyst to 222 μmol. The activity of each system was also tested in highly turbid non-potable water; the optical fibre systems retained ca. 70% activity compared to the powdered system which showed complete reduction in activity by 99.99%.
000, activity also increases from 1 μmol to 120 μmol indicating a linear increase in activity relative to catalyst amount. Hydrogen evolution was more than doubled when the reaction was scaled up to 700 mg of catalyst to 222 μmol. The activity of each system was also tested in highly turbid non-potable water; the optical fibre systems retained ca. 70% activity compared to the powdered system which showed complete reduction in activity by 99.99%.
Mass transfer limitations play a vital role in the rate of photochemical reactions. In a homogeneous catalytic system, the catalyst, substrates and products are all in the same phase, hence the effect of mass transfer between phases is negligible. However, in a heterogeneous system, the catalyst is typically in a different phase from the reactants. Consequently, the reaction rate can depend on the mass transfer between or diffusion between these phases.242 To overcome mass transfer limitations several types of reactors have been developed, including slurry reactors and fixed bed photoreactors. These main types of photoreactor can be further divided into many different reactor types, for example, thin film photoreactors, spinning disk, slurry and monolith, Taylor vortex and fibre optic and membrane photoreactors.243
Adams et al.244 developed a novel thin film multi-tubular photoreactor for use with liquid, vapour or gas phase media. The immobilised film design enabled utilization of the full catalyst coating and thus reduced mass transfer limitations by enabling the catalyst and the substrate to be in very close contact. The immobilized catalyst improved the efficiently of the process as a separation step was not required. The design allows for scale up by increasing the length of the glass columns.
O'Neill et al.67 recently compared the inactivation of E. coli using a slurry reactor set up and a spinning disk reactor. A spinning disk reactor employs a horizontally aligned rotating disk to create high centrifugal acceleration. When a liquid is fed from the centre onto the reactor the centrifugal forces cause it to spread out into a highly sheared and thin film to improve mass and heat transfer. The thin film also allows higher light penetration. O'Neill et al. determined ZnO was the more efficient photocatalyst for E. coli removal in both reactor set-ups.
An excellent paper by Khan et al.245 outlines recent advancements in engineering for the design of photoreactors to produce renewable fuels via reduction of CO2. The authors reviewed the pros and cons for typical photoreactors, including slurry, fixed bed and membrane reactors and concluded the fabrication of a hybrid type photoreactor which utilizes positives from each type of reactor would be highly beneficial in the photocatalytic generation of fuels.
The scale up of photochemical reactors presents additional challenges to those associated with the scale up of chemical reactions due to the attenuation effect of photon transport which is directly related to the dimension of reactors. Heterogeneous catalytic systems, which are typically preferred in industry, are challenging as the penetration of light though the catalyst is limited. As mentioned before, due to the lack of examples of reactor design and scale up for energy production we are including examples of the more widely studied area of environmental remediation. However, despite the level of research in this area over the last five decades it has been difficult to find examples of commercialized processes. A recent paper by Mills et al.246 detailed seven companies involved in the commercialization of photocatalytic technology for water treatment, with some listed as having “no obvious products”. For those listed with their technologies it proved difficult to find out any additional information. Nevertheless, an industrial scale photocatalytic water purification technology by “Purifics Photo-Cat” which is described as the largest photocatalytic AOP system in the world (Fig. 13) has been developed. The technology uses a TiO2 slurry based photocatalytic system for water purification.247
 | ||
| Fig. 13 Reactor technology from Purfics reproduced from ref. 247 with permission from Purifics. | ||
Imoberdorf et al.248 designed a scaled up multi-annular photocatalytic reactor for the photocatalytic oxidation of perchloroethylene. In this work the authors investigated six annular photocatalytic wall reactor configurations. For validation purposes a multi-annular reactor with interconnected channels and uniform TiO2 thickness due to good performance for perchloroethylene oxidation was chosen. A complete reactor model was developed and solved. It was possible to predict reactor behaviour based on this model based on chemical reaction fundamentals, reactor engineering and radiation transport theory. Reactor volume ranged from 633 cm3 to 2178 cm3 and photocatalytic active area ranged from 1266 cm2 to 5208 cm2 for the 6 reactors investigated in this study.
The scale up of photochemical reactions has also been investigated using continuous flow systems. Typically, the work has focused on photochemical synthesis of organic compounds. For example, Beaver et al.249 developed a high-throughput (>5 kg per day) photochemical flow process to generate >250 kg of the target molecule, cyclobutene. Details of the continuous flow set up can be seen in Fig. 14. The reaction was scaled up from lab scale using a 500 ml volumetric flask, to demo scale that utilized a 50 L flask to production scale that employed a 3000 L stainless steel reactor. A continuous flow set up was found to be significantly safer and more efficient to operate than a batch system at the same scale (Fig. 14 and 15).
 | ||
| Fig. 14 Modifications to the skid to support safe implementation in the production setting. Reproduced from ref. 249 with permission from American Chemical Society. | ||
 | ||
| Fig. 15 Pictures of the production-scale photochemical skid. Reproduced from ref. 249 with permission from American Chemical Society. | ||
Microreactor technology has a wide range of advantages over conventional batch technology and has found use in scaling up of chemical processes. Microreactor technology that utilizes continuous flow operation has enhanced heat and mass transfer rates and large specific surface areas. Microreactors have extremely small dimensions which ensures excellent light irradiation. Throughput can be increased by increasing the number of microreactors. Microreactors also offer increased safety for processing at larger scales compared to batch reactors at a similar scale. Su et al.250 used a numbering up approach in a capillary multiphase flow photoreactor to scale up a photocatalytic oxidation reaction of thiols to disulfides. The yield obtained in the numbered up photoreactors (Fig. 16) was comparable to the yield obtained in a single photo-microreactor device and hence demonstrated the efficiency of the system.
 | ||
| Fig. 16 (a) Schematic overview of the 8-capillary microreactor system, (b) a picture of the 8-capillary microreactor system with a gas–liquid photocatalytic transformation running. Reproduced from ref. 250 with permission from Chemistry Europe. | ||
The scale up of any chemical process has its challenges, add in light which is required for photochemical processes and the difficulty significantly increases. Photocatalysis has much potential in a net zero landscape as a renewable technology. As the strive for net zero intensifies it is envisaged that scaling up photochemical processes will ramp up and gain the momentum that is needed to play their part in a net zero future.
6. Overall perspective and future outlook
As with all emerging technologies, it can be challenging to accurately predict the role photocatalysis will play within future energy systems and a stable circular economy. This is further complicated when considering the rapidly changing renewable and sustainable energy landscape associated with the net zero transition. That transition is multi-faceted and complex, and as a result requires solutions which are not just interdisciplinary in nature but capable of achieving an impact across social, economic, environmental and technological platforms. The role and impact of technologies such as photocatalysis will have within that will always be debated, as an emerging technology by its nature requires support to achieve further development and deployment. It's crucial that support facilitates growth, rather than perpetuating a level of academic stagnation or a continued hype cycle which is often associated with trying to develop the ‘perfect’ material. Moreover, this support must be contextualised in relation to other emerging technologies, as well as those at higher TRLs, which face manufacturing and deployment challenges. Put simply, there may not be enough resources to support all emerging technologies which can contribute towards our low-carbon and net zero targets. It is therefore vital that technologies such as photocatalysis can be evaluated and monitored with a view towards developing a strategy for TRL advancement. This includes assessing the current status, which is frequently done within the literature, and coupling that with identifying research priorities to support growth.The core advantages of photocatalysis are well known: ambient operating conditions, a low-carbon technology and the generation of highly oxidising reactive species. Leveraging those advantages within future systems is crucial and as demonstrated in this article, can be targeted towards the use of real-world substrates and feedstocks. Therefore, deployment and operation ‘beyond the lab’ should be a primary objective for photocatalytic technology. Achieving that requires commercial scale availability of new materials, modular reactor design that supports scale up and holistic system level analysis to support industry engagement. In view of the discussion provided in this article, there are key areas which the authors feel should be considered and addressed by the research community in future work. Firstly, availability and sustainability of photocatalytic feedstocks to ensure efficient remediation and valorisation towards valuable products. While photocatalysis can be non-selective towards substrate oxidation, the composition of feedstocks should be carefully considered to maximise the yield of desirable products, while ensuring no harmful by-products are generated. This means photocatalysis should be deployed with appropriate feedstocks, and where necessary pre-treatment should also be used. In addition, it's important that comparisons are made to alternative treatment and conversion processes to ensure feedstocks are correctly coupled with appropriate technologies. This approach can also facilitate integrated and hybrid systems comprised of multiple technologies that promote synergistic effects. This was demonstrated in our previous work to show coupling photocatalysis with acoustic cavitation improved diisobutyl phthalate removal.251 This approach can also include adopting ‘best practices’ or ‘lessons learned’ from other technologies in materials design and reactor engineering e.g. achieving stable electron–hole charge separation is a fundamental requirement in photovoltaics and also necessary for photocatalytic hydrogen generation. It should be noted, however, that materials with high charge separation and transfer don't always support ROS generation. Therefore, repurposing materials from other applications or disciplines may not always be viable. In relation to reactor engineering, electrolysers and fuel cells are scalable due to a modular design, which should be adopted by photocatalytic units also. While challenges such as mass transfer limitations and efficient photon delivery will still require consideration, modularity should be a key priority in initial reactor design.
In addition to feedstock considerations, holistic systems analysis is a valuable tool often used to evaluate and compare conversion processes and technologies. It's use with photocatalytic systems, however, is very limited with only a few examples reported in the literature.252–258 It's important to note that tools such as LCA and TEA are reliant on data availability and for many photocatalytic applications, there is often insufficient data to ensure accurate results from systems analysis are generated. This, however, should not be seen as a reason to disregard such methodologies but instead as an opportunity to assess how additional data can be generated, analysed and interpreted. In the first instance, that can be achieved by utilising metrics that encompass a broader range of operating parameters. Reaction rates (often as a function of irradiation time) along with photonic efficiencies and apparent quantum yields are useful metrics for benchmarking; however, they do not account for other key parameters such as power consumption, environmental impact, cost or scale. Perhaps a key example of this is in relation to environmental remediation and the use of the Electrical Energy per Order (EEO) value which is a tool used for reporting the efficiency of AOPs for organic contamination removal. While this is a standardised metric, which allows for accurate comparison between AOPs, it's rarely reported in the literature for photocatalysis. This reflects the need for a shift and transition within photocatalytic (and photoelectrochemical) research towards studies and work that can be assessed across a broader range of metrics to determine the real-world impact.
While limited, it is encouraging to see an increasing number of examples in the literature of LCA and environmental impact assessments being conducted for photocatalysis. Furthermore, these examples include both energy production and environmental remediation applications, along with consideration towards key parameters such as the sustainability impact of metal-doping on TiO2,255 pilot scale operation254 and comparison to other conversion technologies.259 Observing these trends in the literature is important as its evidence of analysing parameters which support photocatalysis technology advancement. Moreover, it addresses key topics such as the sustainability of larger scale operation and the environmental impact of photocatalytic materials development e.g. synthesis routes, the use of critical elements, and end-of-life assessments. While these are complex research areas to address for photocatalysis, it's crucial they are seen as priorities and, where possible, incorporated into on-going research. Examples which highlight this in recent literature include the work of Maurya et al.257 in 2023 who performed LCA of earth abundant materials for photocatalytic hydrogen generation. As such materials are often utilised in either synthesis routes or as co-catalysts which facilitate the reduction of protons to H2, their use and subsequent impact is a crucial parameter when performing systems analysis. This is also a factor when considering catalyst recovery and reuse. The study developed a cradle-to-grave methodology to assess the carbon footprint of TiO2 and C3N4 based photomaterials. The framework encompassed synthesis (e.g. material extraction and production), system operation (e.g. reactor assembly, H2 production and storage) and end-of-life (e.g. decommissioning and recycling) to generate data which is reflective of full system operation. The data showed for all the catalysts considered (TiO2 nanorods, fluorine-doped C3N4 quantum dots, g-C3N4 sheets and C3N4/BiOI composites) that materials extraction contributed the largest portion of life cycle GHG emissions based on kg CO2 eq. per kg H2. As shown in Fig. 17a, the data ranged from 0.43 for the composite material to 2.08 kg CO2 eq. per kg H2 for g-C3N4 sheets. In each case, however, materials extraction accounted for >80% of emissions. In addition, the study also demonstrated that KOH and Ag generated the largest GHG emissions based on the elemental contribution for material extraction (Fig. 17b). Interestingly, this data also underpins the importance of catalyst stability over prolonged use, which should encourage the research community to consider catalyst recycling, recovery and any reconditioning steps required to ensure activity is maintained over multiple years. This work and others in the literature highlight the importance of full systems analysis to identify not just strengths and weaknesses in photocatalysis, but also risks and opportunities. It is therefore vital that careful consideration is given towards both materials synthesis routes and reactor design along with end-of-life processes to determine an accurate assessment of environmental impact. In doing so, this will establish a strong rhetoric for supporting the role of photocatalysis as a low-carbon technology capable of contributing towards net zero targets.
 | ||
| Fig. 17 (a) Life cycle GHG Emissions as a function of catalyst and (b) elemental contribution in material extraction. Reproduced from ref. 257 and with permission from Elsevier. | ||
7. Conclusion
This review has considered the application of semiconductor photocatalysis to the valorisation of “real-world” wastes as opposed to “model” compounds. From this review a comprehensive range of substrates are clearly amenable to valorisation using this process and there is clear potential to not only process recalcitrant waste materials/chemicals but also simultaneously generate renewable hydrogen and convert the waste to high value chemicals. This clearly demonstrates the applicability of the technology for circular economy applications. While the initial laboratory studies have shown some very promising results, there are several issues and challenges that need to be addressed before this technology will be truly applicable. Firstly, for many of the processes, relatively harsh operating conditions are required utilising strong acids or bases to maximise product yields, and these solvents have their own environmental challenges. Even when the reactions are conducted in strong acids or bases, the product yields are still relatively low, compared to other valorisation processes. For example, the H2 yields are typically in the μmol gcat−1 h−1 to mmol gcat−1 h−1 range. Typically, this means that ml quantities of gas are generated rather than m3 that would be required for a commercially practical process. Furthermore, many of the processes also require photocatalysts that have been modified with precious metals such as platinum. This not only increases the cost of the process, but it also has issues with respect to the demand for globally scarce materials. Many of the most effective photocatalysts reported to date also require UV light to activate them. In cases where visible light active materials have been successfully demonstrated, these have mostly involved the use of photocatalysts such as cadmium sulfide which is known to photo-corrode with prolonged irradiation and hence there is a risk of toxic cadmium ions leaching into the environment. As with other semiconductor photocatalytic processes there is a need for cheap, efficient, stable, visible light active photocatalysts if photocatalytic valorisation processes are to be realised on any practical scale. Moving forward there also needs to be significant research effort put into reactor development where the process can process m3 quantities of the “real-world” wastes and move the technology on from the lab scale as reported in much of the research. While challenges such as mass transfer limitations and efficient photon delivery will still require consideration, modularity should be a key priority in initial reactor design. While there are clearly significant challenges that still need to be addressed if semiconductor photocatalysis is going to be realised as a practical process for valorisation of “real-world” substrates, the research that has been reported in recent years is very promising, having proved the principle and provided a firm foundation for future development.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We would like to acknowledge the funding provided by the Department of Agriculture, Environment and Rural Affairs for supporting this research (contract number 21/R/475 – ValiD – Valorisation of Digestate using Photocatalysis and Hydrodynamic Cavitation).References
- https://www.gov.uk/government/speeches/more-than-zero-why-net-zero-alone-wont-save-the-planet-and-what-will, accessed Oct 2023.
- https://ourworldindata.org/co2-emissions, accessed Oct 2023.
- https://iea.blob.core.windows.net/assets/91982b4e-26dc-41d5-88b1-4c47ea436882/Coal2022.pdf, accessed Oct 2023.
- https://www2.bgs.ac.uk/mineralsuk/download/world_statistics/2010s/WMP_2015_2019.pdf. accessed Oct 2023.
- https://ourworldindata.org/grapher/global-plastics-production, accessed Oct 2023.
- https://www.fao.org/documents/card/en/c/ca8753en, accessed Oct 2023.
- https://ourworldindata.org/, accessed Oct 2023.
- https://ourworldindata.org/biodiversity?insight=wild-mammals-have-declined-by-85-since-the-rise-of-humans#key-insights-on-biodiversity. accessed Oct 2023.
- https://web.archive.org/web/20080625012113/http://www.virtualcentre.org/en/library/key_pub/longshad/A0701E00.pdf, accessed Oct 2023.
- J. C. Dodson, P. Dérer, P. Cafaro and F. Götmark, Sci. Total Environ., 2020, 748, 141346–141356 CrossRef CAS PubMed.
- Life on the Brink: Environmentalists Confront Overpopulation, ed. Philip Cafaro and Eileen Crsit, 2012 Search PubMed.
- https://www.un.org/en/global-issues/population, accessed Oct 2023.
- B. C. O'Neill, B. Liddle, L. Jiang, K. R. Smith, S. Pachauri, M. Dalton and R. Fuchs, Lancet, 2012, 380, 157–164 CrossRef PubMed.
- B. C. O'Neill, M. Dalton, R. Fuchs and K. Zigova, Proc. Natl. Acad. Sci. U.S.A., 2010, 107, 17521–17526 CrossRef PubMed.
- S. R. Gaffin and B. C. O'Neill, Popul. Environ., 1997, 18, 389–413 CrossRef.
- J. C. Dodson, P. Dérer, P. Cafaro and F. Götmark, Sci. Total Environ., 2020, 748, 141346–141356 CrossRef CAS PubMed.
- J. Bongaarts and B. C. O'Neill, Science, 2018, 361(6403), 650–652 CrossRef CAS PubMed.
- P. D. Habumuremyi and M. Zenawi, Lancet, 2012, 380, 78–80 CrossRef PubMed.
- J. Cleland, A. C-Agudelo, H. Peterson, J. Ross and A. Tsui, Lancet, 2012, 380, 149–156 CrossRef PubMed.
- Climate Change 2014: Mitigation of Climate Change, Working Group 3 Contribution to the IPCC Fifth Assessment Report, Cambridge University Press, 2015, accessed October 2023 Search PubMed.
- https://population.un.org/wpp/Methodology. accessed October 2023.
- https://www.nature.com/articles/461472a .
- K. Richardson, W. Steffen, W. Lucht, J. Bendtsen, S. E. Cornell, J. F. Donges, M. Druke, I. Fetzer, G. Bala, W. Bloh, G. Feulner, S. Fiedler, D. Gerten, T. Gleeson, M. Hofmann, W. Huiskamp, M. Kummu, C. Mohan, D. Bravo, S. Petri, M. Porkka, S. Rahmstorf, S. Schaphoff, K. Thonicke, A. Tobian, V. Virkki, L. Wang-Erlandsson, L. Weber and J. Rockstrom, Sci. Adv., 2023, 9, eadh2458 CrossRef PubMed.
- https://ourworldindata.org/contributed-most-global-co2, accessed Jan 2024.
- https://ourworldindata.org/explorers/population-and-demography, accessed Jan 2024.
- https://www.statista.com/topics/11239/global-warming/#topicOverview, accessed Jan 2024.
- https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature, accessed October 2023.
- N. R. Golledge, E. D. Keller, N. Gomez, K. Naughten, J. Bernales, L. D. Trusel and T. L. Edwards, Nature, 2019, 566, 65–72 CrossRef CAS PubMed.
- M. Zemp, M. Huss, E. Thibert, N. Eckert, R. McNabb, M. Barandun, H. Machguth, S. U. Nuddbaumer, I. Garner-Roer, L. Thompson, F. Paul, F. Maussion, S. Kutuzov and J. G. Cogley, Nature, 2019, 568, 382–386 CrossRef CAS PubMed.
- E. V. Kennedy, C. T. Perry, P. R. Halloran, R. Iglesias-Prieto, C. H. L. Schonberg, M. Wisshak, A. U. Form, J. P. Carricart-Ganivet, M. Fine, C. M. Eakin and P. J. Mumby, Curr. Biol., 2013, 23(10), 912–918 CrossRef CAS PubMed.
- N. J. Abram, B. J. Henley, A. S. Gupta, T. J. R. Lippmann, H. Clarke, A. J. Dowdy, J. J. Sharples, R. H. Nolan, T. Zhang, M. J. Wooster, J. B. Wurtzel, K. J. Meissner, A. J. Pittman, A. M. Ukkola, B. P. Murphy, N. J. Tapper and M. M. Boer, Commun. Earth Environ., 2021, 2, 8 CrossRef.
- V. Varerla, D. Vlachogiannis, A. Sfetsos, S. Karozis, N. Politi and F. Giroud, Sustainability, 2019, 11(16), 4284–4297 CrossRef.
- E. Bevacqua, M. I. Vousdoukas, G. Zappa, K. Hodges, T. G. Shepard, D. Maraun, L. Mentaschi and L. Feyen, Commun. Earth Environ., 2020, 1(47), 2020 Search PubMed.
- M. G. Grillakis, Sci. Total Environ., 2019, 660(10), 1245–1255 CrossRef CAS PubMed.
- P. H. Raven and D. L. Wagner, Proc. Natl. Acad. Sci. U.S.A., 2021, 118(2), e2002548117 CrossRef CAS PubMed.
- R. Mukhopadhyay, B. Sarkar, H. S. Jat, R. C. Sharma and N. S. Bolan, J. Environ. Manag., 2021, 280, 111736–111750 CrossRef CAS PubMed.
- L. C. Stringer, A. Mirzabaev, T. A. Benjaminsen, R. M. B. Harris, M. Jafari, T. K. Lissner, N. Stevens and C. Tirado-von der Pahlen, One Earth, 2021, 4(6), 851–864 CrossRef.
- https://unfccc.int/process-and-meetings/the-paris-agreement. accessed October 2023.
- UK Government, The Ten-point Plan for a Green Industrial Revolution, London, 2020, accessed October 2023 Search PubMed.
- UK Government, Net Zero Strategy: Build Back Greener, London, 2021, accessed October 2023 Search PubMed.
- https://unfccc.int/, accessed October 2023.
- https://climate.mit.edu/explainers/carbon-offsets, accessed October 2023.
- S. Fankhauser, S. M. Smith, M. Allen, K. Axelsson, T. Hale, C. Hepburn, J. M. Kendall, R. Khosla, J. Lezaun, E. Mitchell-Larson, M. Obersteiner, L. Rajamani, R. Rickaby, N. Seddon and T. Wetzer, Nat. Clim. Change, 2022, 12, 15–21 CrossRef.
- https://www.gov.uk/government/speeches/more-than-zero-why-net-zero-alone-wont-save-the-planet-and-what-will, accessed Oct 2023.
- https://climatechampions.unfccc.int/whats-the-cost-of-net-zero-2/, accessed Oct 2023.
- https://www.gov.uk/government/publications/the-ten-point-plan-for-a-green-industrial-revolution, accessed Oct 2023.
- https://www.gov.uk/government/publications/the-ten-point-plan-for-a-green-industrial-revolution, accessed Oct 2023.
- R. Dickson, M. Akhtar, A. Abbas, E. Park and J. Liu, Green Chem., 2022, 24, 8484–8493 RSC.
- K. de Kleijne, H. de Coninck, R. van Zelm, M. A. J. Huijbregts and S. V. Hanssen, Sustain. Energy Fuels, 2022, 6, 4383–4387 RSC.
- P. Falcone, M. Hiete and A. Sapio, Curr. Opin. Green Sustainable Chem., 2021, 31, 100506–100514 CrossRef CAS.
- A. Sartbaeva, V. L. Kuznetsov, S. A. Wells and P. P. Edwards, Energy Environ. Sci., 2008, 1, 79–85 RSC.
- P. Nikolaidis and A. Poullikkas, Renew. Sustain. Energy Rev., 2017, 67, 597–611 CrossRef CAS.
- https://www.irena.org/Energy-Transition/Technology/Hydrogen, accessed November 2023.
- https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf, accessed November 2023.
- https://www.pwc.com/gx/en/industries/energy-utilities-resources/future-energy/green-hydrogen-cost.html, accessed November 2023.
- https://www.sgh2energy.com/economics, accessed November 2023.
- https://www.cleanenergywire.org/news/rising-gas-prices-make-green-hydrogen-cheaper-gray-hydrogen, accessed November 2023.
- https://www.gov.uk/government/news/uk-government-launches-plan-for-a-world-leading-hydrogen-economy, accessed November 2023.
- R. Taylor, J. Howes, E. Cotton, E. Raphael, P. Kiri, L. Liu, S. Haye, T. Houghton, A. Bauen, M. Altmann and P. Schmidt, Options for a UK Low Carbon Hydrogen Standard—Final Report, London, 2021, accessed November 2023 Search PubMed.
- A. C. Lewis, Environ. Sci.: Atmos., 2021, 1, 201–207 CAS.
- https://www.gie.eu/blended-hydrogen-for-decarbonisation/, accessed November 2023.
- https://www.gie.eu/blended-hydrogen-for-decarbonisation/, accessed November 2023.
- https://www.rechargenews.com/energy-transition/green-light-given-for-uks-first-hydrogen-blend-in-public-natural-gas-network/2-1-1045075, accessed November 2023.
- https://www.nationalgrid.com/stories/journey-to-net-zero-stories/hygrid-green-hydrogen-blending-project-launches, accessed November 2023.
- M. S. Cellek and A. Pinarbasi, Int. J. Hydrogen Energy, 2018, 43(2), 1194–1207 CrossRef CAS.
- https://www.cleanegroup.org/wp-content/uploads/Five-Reasons-to-be-Concerned-About-Green-Hydrogen.pdf, accessed November 2023.
- S. O'Neill, J. M. C. Robertson, V. Hequet, F. Chazarenc, X. Pang, K. Ralphs, N. Skillen and P. J. K. Robertson, Ind. Eng. Chem. Res., 2023, 62(45), 18952–18959 Search PubMed.
- J. Scneider, M. Matsuoka, M. Takwuchi, J. Zhanf, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef PubMed.
- Photocatalysis: Fundamentals and Perspectives, ed. J. Schneider, D. Bahnemann, J. Ye, G. Li Puma, D. D. Dionysiou, J. Schneider, D. D. Dionysiou, et al., The Royal Society of Chemistry, 2016, pp. P001–P004 Search PubMed.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS.
- D. F. Ollis, E. Pelizzetti, N. Serpone, Photocatalysis: Fundamentals and Applications, 1989 Search PubMed.
- M. A. Fox and M. T. Dulay, Chem. Rev., 1993, 93, 341–357 CrossRef CAS.
- P. K. J. Robertson, J. Cleaner Prod., 1996, 4, 203–212 CrossRef.
- Y. Nosaka and A. Y. Nosaka, Chem. Rev., 2017, 117, 11302–11336 CrossRef CAS PubMed.
- W. J. McCormick., D. McCrudden, N. Skillen and P. K. J. Robertson, Appl. Catal., A, 2023, 660, 1119201–1119210 CrossRef.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng, R. Zhang, L. Wang, H. Liu, Y. Liu and R. Ruan, J. Clean. Prod., 2020, 268, 121725–121740 CrossRef CAS.
- C. Acar, I. Dincer and G. F. Naterer, Int. J. Energy Res., 2016, 40, 1449–1473 CrossRef CAS.
- J. Gong, C. Li and M. R. Wasilewski, Chem. Soc. Rev., 2019, 48, 1862–1864 RSC.
- J. Bedia, V. Muelas-Ramos, M. Penas-Garzon, A. Gomez-Aviles, J. J. Rodriguez and C. Belver, Catalysts, 2019, 9(1), 52 CrossRef.
- A. Mamaghani, F. Haghighat and C. Lee, Appl. Catal. B Environ., 2017, 203, 247–269 CrossRef CAS.
- D. Robert and A. Laghzizil, Environ. Sci. Pollut. Res., 2023, 30, 81616–81618 CrossRef PubMed.
- J. Ma, K. Liu, X. Yang, D. Jin, Y. Li, G. Jiao, J. Zhou and R. Sun, ChemSusChem, 2021, 14(22), 4903–4922 CrossRef CAS PubMed.
- V. Rao, T. T. Malu, K. Cheralathan, M. Sakar, S. Pitchaimuthu, V. Rodriguez-Gonzalez, M. M. Kumari and M. V. Shankar, J. Environ. Manage., 2021, 284, 111983–111998 CrossRef PubMed.
- S. Xu, X. Huang and H. Lu, Fuel Process. Technol., 2024, 255, 108057–108075 CrossRef CAS.
- T. Kawai and T. Sakata, Nature, 1980, 286, 474 CrossRef CAS.
- F. Cherubini, Energy Convers. Manage., 2010, 51(7), 1412–1421 CrossRef CAS.
- A. J. Welfle, A. Alemena, M. N. Arshad, S. W. Banks, I. Butnar, K. J. Chong, S. J. G. Cooper, H. Daly, S. G. Freites, F. Gulec, C. Hardacre, r. Holland, L. Lan, C. S. Lee, P. K. J. Roberston, R. Rowe, A. Shepard, N. Skillen, S. Tedesco, P. Thornley, P. V. Barbara, I. Watson, O. S. A. Williams and M. Roder, Biomass Bioenergy, 2023, 177, 1106919–1106938 CrossRef.
- A. M. Ruppert, K. Weinberg and R. Palkovits, Angew. Chem., Int. Ed., 2012, 51(11), 2564–2601 CrossRef CAS PubMed.
- K. Tekin, S. Karagoz and S. Bektas, Renewable Sustainable Energy Rev., 2014, 40, 673–687 CrossRef CAS.
- C. Xu, R. A. Arancon and J. Labidi, Chem. Soc. Rev., 2014, 43(22), 7485–7500 RSC.
- J. Wen, B. Xue and F. Xu, Ind. Crops Prod., 2013, 42, 332–343 CrossRef CAS.
- F. G. Calvo-Flores and J. A. Dobado, ChemSusChem, 2010, 3(11), 1227–1235 CrossRef CAS PubMed.
- S. Ahmed, T. Warne, E. Smith, H. Goemann, G. Linse, M. G. J. Kedziora, M. Snapp, D. Kraner, K. Roemer, J. H. Haggerty, M. Jarchow, D. Swanson, B. Poulter and P. C. Stoy, npj Sci. Food., 2021, 5, 9 CrossRef PubMed.
- C. Chen, A. Chaudhary and A. Mathys, Resour. Conserv. Recycl., 2020, 160, 104912–104924 CrossRef.
- J. Ruane, A. Sonnino and A. Agostini A, J. Biomass Bioenergy, 2010, 34(10), 1427–1439 CrossRef.
- H. Bouallagui, H. Lahdheb and E. B. Romdan, J. Environ. Manage., 2009, 90(5), 1844–1849 CrossRef CAS PubMed.
- L. C. Y. Yongjie and W. Chuangzhi, Biomass Bioenergy, 2004,(27), 111–117 Search PubMed.
- P. Weiland, Appl. Microbiol. Biotechnol., 2010, 85(4), 849–860 CrossRef CAS PubMed.
- H. Liu, G. M. Jiang and H. Y. Zhuang, Renewable and Sus. Ener. Rev., 2008, 12(5), 1402–1418 CrossRef.
- L. Lan, H. Daly, R. Sung, F. Tuna, N. Skillen, P. K. J. Roberston, C. Hardacre and X. Fan, ACS Catal., 2023, 13(13), 8574–8587 CrossRef CAS PubMed.
- A. Speltini, M. Sturini, D. Dondi, E. Annovazzi, F. Maraschi, V. Caratto, A. Profumo and A. Buttafava, Photochem. Photobiol. Sci., 2014, 13, 1410 CrossRef CAS PubMed.
- C. Chang, N. Skillen, S. Nagarajan, K. Ralphs, J. T. S. Irvine, L. Lawton and P. K. J. Robertson, Energy Fuels, 2019, 3, 1971–1975 CAS.
- A. Caravaca, W. Jones, C. Hardacre and M. Bowker, Proc. R. Soc. A, 2016, 472, 54–75 CrossRef PubMed.
- C. Chen, P. Liu, H. Xia, M. Zhou, J. Zhao, B. K. Sharma and J. Jiang, Molecules, 2020, 25(9), 2109–2123 CrossRef CAS PubMed.
- X. Xu, L. Shi, S. Zhang, Z. Ao, J. Zhang and H. Sun, Chem. Eng. J., 2023, 469, 143972–143990 CrossRef CAS.
- C. W. J. Murnaghan, N. Skillen, C. Hardacre, J. Bruce, G. N. Sheldrake and P. K. J. Robertson, J. Phys.: Energy, 2021, 3, 035002 CAS.
- C. W. J. Murnaghan, N. Skillen, B. Hackett, J. Lafferty, P. K. J. Robertson and G. N. Sheldrake, ACS Sustainable Chem. Eng., 2022, 10(37), 12107–12116 CrossRef CAS PubMed.
- S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2(10), 1–17 CrossRef.
- T. Sakata and T. Kawai, J. Synth. Org. Chem., Jpn., 1981, 39(7), 589–602 CrossRef CAS.
- A. Speltini, M. Sturini and D. Dondi, Photochem. Photobiol. Sci., 2014, 13(10), 1410–1419 CrossRef CAS PubMed.
- M. Yasuda, R. Kurogi and H. Tsumagari, Energies, 2014, 7(7), 4087–4097 CrossRef.
- Y. Zhou, X. Ye and D. Lin, Int. J. Energy Res., 2020, 44(6), 4616–4628 CrossRef CAS.
- W. Yuan, Z. Gong and G. Wang, Biores. Tech., 2018, 265, 464–470 CrossRef CAS PubMed.
- A. Caravaca, W. Jones and C. Hardacre, Proc. R. Soc. A, 2016, 472(2191), 20160054 CrossRef CAS PubMed.
- R. Jaswal, R. Shende, A. Shende and W. Nan, Int. J. Hydrogen Energy, 2017, 42(5), 2839–2848 CrossRef CAS.
- H. Nagakawa and M. Nagata, Adv. Mater. Interfac., 2022, 9(2), 2101581 CrossRef CAS.
- H. Nagakawa and M. Nagata, ACS Appl. Energy Mater., 2020, 4(2), 1059–1062 CrossRef.
- H. Kasap, D. A. Achilleos and A. Huang, J. Am. Chem. Soc., 2018, 140(37), 11604–11607 CrossRef CAS PubMed.
- M. Uğurlu, A. Gürses and Ç. Doğar, J. Environ. Manag., 2008, 87(3), 20–428 Search PubMed.
- D. Pokhrel and T. Viraraghavan, Sci. Total Environ., 2004, 333(1–3), 37–58 CrossRef CAS PubMed.
- J. W. Owens, S. M. Wanson and D. A. Birkholz, Chemosphere, 1994, 29(1), 89–109 CrossRef CAS PubMed.
- K. K. Vass, M. K. Mukhopadhyay and K. Mitra, Environ. Ecol., 1996, 14(4), 895–897 Search PubMed.
- A. Schnell, P. V. Hodson and P. Steel, Water Res., 2000, 34(2), 501–509 CrossRef CAS.
- P. Lindstrom-Seppa, S. Huuskonen and S. Kotelevtsev, Mar. Environ. Res., 1998, 46(1), 273–278 CrossRef CAS.
- H. Leppänen and A. Oikari, Environ. Toxicol. Chem., 1999, 18(7), 1498–1505 CrossRef.
- N. Zhong, X. Yu and H. Zhao, Catalysts, 2022, 12(8), 819 CrossRef CAS.
- J. Zou, G. Zhang and X. Xu, Appl. Catal., A, 2018, 563, 73–79 CrossRef CAS.
- M. P. Shah, Advanced Oxidation Processes for Effluent Treatment Plants, Elsevier, 2020 Search PubMed.
- R. K. Goswami, K. Agrawal and P. Verma, J. Basic Microbiol., 2022, 62(3–4), 279–295 CrossRef CAS PubMed.
- A. Speltini, F. Gualco and F. Maraschi, Int. J. Hydrogen Energy, 2019, 44(8), 4072–4078 CrossRef CAS.
- A. Speltini, M. Sturini and D. Dondi, Photochem. Photobiol. Sci., 2014, 13(10), 1410–1419 CrossRef CAS PubMed.
- S. T. Nguyen, P. R. D. Murray and R. R. Knowles, ACS Catal., 2020, 10(1), 800–805 CrossRef CAS.
- L. Lan, H. Daly and R. Sung, ACS Catal., 2023, 13, 8574–8587 CrossRef CAS PubMed.
- S. Xu, P. Zhou and Z. Zhang, J. Am. Chem. Soc., 2017, 139(41), 14775–14782 CrossRef CAS PubMed.
- P. Zhou and Z. Zhang, Catal. Sci. Technol., 2016, 6(11), 3694–3712 RSC.
- H. Priefert, J. Rabenhorst and A. Steinbüchel, Appl. Microbiol. Biotechnol., 2001, 56(3), 296–314 CrossRef CAS PubMed.
- E. Greenbaum, C. V. Tevault and C. Y. Ma, Energy Fuels, 1995, 9(1), 163–167 CrossRef CAS.
- S. T. Nguyen, P. R. D. Murray and R. R. Knowles, ACS Catal., 2020, 10(1), 800–805 CrossRef CAS.
- X. Wu, X. Fan and S. Xie, Nat. Catal., 2018, 1(10), 772–780 CrossRef CAS.
- Y. Lu, X. Wei and Z. Wen, Fuel Process. Technol., 2014, 117, 8–16 CrossRef CAS.
- https://www.bpf.co.uk/industry/Benefits_of_Plastics.aspx, accessed November 2023.
- https://www.wwf.org.au/news/blogs/the-lifecycle-of-plastics, accessed November 2023.
- https://www.oecd.org/environment/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm, accessed November 2023.
- D. C. Ashworth, P. Elliot and M. B. Toledano, Environ. Int., 2014, 69, 120 CrossRef PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3(No), e1700782 CrossRef PubMed.
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed.
- S. B. Borrelle, J. Ringma, K. L. Law, C. C. Monnahan, L. Lebreton, A. McGivern, E. Murphy, J. Jambeck, G. H. Leonard and M. A. Hilleary, Science, 2020, 369(6510), 1515–1518 CrossRef CAS PubMed.
- C. J. Rhodes, Sci. Prog., 2018, 101(3), 207–260 CrossRef PubMed.
- L. Lebreton, B. Slat, F. Ferrari, B. Sainte-Rose, J. Aitken, R. Marthouse, S. Hajbane, S. Cunsolo, A. Schwarz and A. Levivier, Sci. Rep., 2018, 8, 46666 Search PubMed.
- https://theoceancleanup.com/updates/the-exponential-increase-of-the-great-pacific-garbage-patch/, accessed November 2023.
- A. Ragusa, A. Svelatoa, C. Santacroce, P. Catalano, V. Notarstefano, O. Carnevali, F. Papa, M. Antonio, R. F. Baiocco, S. Draghi, E. D'Amore, D. Rinaldo, M. Matta and E. Giorgini, Environ. Int., 2021, 146, 106274–106282 CrossRef CAS PubMed.
- G. Kutralam-Muniasamy, V. C. Shruti, F. Perez-Guevara and P. D. Roy, Sci. Total Environ., 2023, 856, 156164–156175 CrossRef PubMed.
- H. A. Leslie, M. J. M. van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Environ. Int., 2022, 163, 107199–107207 CrossRef CAS PubMed.
- P. Schwabl, S. Koppel, P. Konigsofer, T. Bucsics, M. Trauner, T. Reilberger and B. Liebmann, Ann. Intern. Med., 2019, 171(7), 453–457 CrossRef PubMed.
- N. Zhang, Y. B. Li, H. R. He, J. F. Zhang and G. S. Ma, Sci. Total Environ., 2021, 767, 144345–144352 CrossRef CAS PubMed.
- Y. S. Ibrahim, S. T. Anuar, A. A. Azmi, W. M. A. Khalik, S. Lehata, S. R. H. D. Ismail, Z. F. Ma, A. Dzulkarnaen, Z. Zakaria, N. Mustaffa, S. E. T. Sharif and Y. Y. Lee, JGH Open, 2020, 5(1), 116–121 CrossRef PubMed.
- L. C. Jenner, J. M. Rotchell, R. T. Bennett, M. Cowen, V. Tentzeris and l. R. Sadofsky, Sci. Total Environ., 2022, 831, 154907–154917 CrossRef CAS PubMed.
- G. Sorci and C. Loiseau, EBioMedicine, 2022, 82, 1041911–1041913 CrossRef PubMed.
- https://www.theguardian.com/environment/2020/aug/17/microplastic-particles-discovered-in-human-organs, accessed November 2023.
- M. Prust, J. Meijer and R. H. S. Westerink, Part. Fibre Toxicol., 2020, 17, 24 CrossRef PubMed.
- M. Carbery, W. O'Connor and P. Thavamani, Environ. Int., 2018, 115, 400–409 CrossRef PubMed.
- D. Eerkes-Medrano, H. A. Leslie and B. Quinn, Curr. Opin Environ. Sci. Health, 2019, 7, 69–75 CrossRef.
- J. Kwon, J. Kim, T. D. Pham, A. Tarafdar, S. Hong, S. Chun, S. Lee, D. Kang, J. Kim and S. Kim, Int. J. Environ. Res. Publ. Health, 2020, 17, 6710 CrossRef CAS PubMed.
- C. Bai, L. Liu, Y. Hu, E. Y. Zeng and Y. Guo, Sci. Total Environ., 2022, 806, 150263 CrossRef CAS PubMed.
- C. Rubio-Armendáriz, S. Alejandro-Vega, S. Paz-Montelongo, Á. J. Gutiérrez-Fernández, C. J. Carrascosa-Iruzubieta and A. Hardisson-de la Torr, Int. J. Environ. Res. Public Health, 2022, 19, 1174–1188 CrossRef PubMed.
- O. G. Conti, M. Ferrante, M. Banni, C. Favara, I. Nicolosi, A. Cristaldi, M. Fiore and P. Zuccarello, Environ. Res., 2020, 187, 109677 CrossRef PubMed.
- G. utralam-Muniasamy, F. Pérez-Guevara, I. Elizalde-Martínez and V. C. Shruti, Sci. Total Environ., 2020, 714, 6823 Search PubMed.
- Y. Huang, J. Chapman, Y. Deng and D. Cozzolino, Food Control, 2020, 113, 107187 CrossRef CAS.
- V. C. Hruti, F. Pérez-Guevara, I. Elizalde-Martínez and G. Kutralam-Muniasamy, Sci. Total Environ., 2020, 726, 138580 CrossRef PubMed.
- C. Campanale, S. Galafassi, I. Savino, C. Massarelli, V. Ancona, P. Volta and V. F. Uricchio, Sci. Total Environ., 2022, 805, 150431 CrossRef CAS PubMed.
- K. S. Basri, A. Daud, R. D. P. Astuti and K. Basri, Maced. J. Med. Sci., 2021, 9, 275–280 CrossRef.
- P. Soni and S. Joseph, Pharma Technol. J., 2021, 45(1), 40–43 Search PubMed.
- D. Materić, Environ. Pollut., 2021, 288, 117697 CrossRef PubMed.
- J. Brahney, M. Hallerud, M. Hahneberger and S. Sujumaran, Science, 2020, 368(6496), 1257–1260 CrossRef CAS PubMed.
- M. Aeschlimann, G. Li, Z. A. Kanji and D. M. Mitrano, Nat. Geosci., 2022, 15, 967–975 CrossRef CAS PubMed.
- G. Ren, H. Han, Y. Wang, S. Liu, J. Zhao, X. Meng and Z. Li, Nanomaterials, 2021, 11(7), 1804–1826 CrossRef CAS PubMed.
- A. Kushniarou, G. Navarro and S. Navarro, Chemosphere, 2019, 214, 839–845 CrossRef CAS PubMed.
- N. Vela, M. Calín and M. J. Yáñez-Gascón, J. Photochem. Photobiol., A, 2018, 353, 271–278 CrossRef CAS.
- H. D. Burrows, L. M. Canle, J. A. Santaballa and S. Steenken, J. Photochem. Photobiol. B Biol., 2002, 67, 71–108 CrossRef CAS PubMed.
- W. J. McCormick, D. McCrudden, N. Skillen and P. K. J. Robertson, Appl. Catal., A, 2023, 660, 1119201–1119210 CrossRef.
- H. N. Haslina, N. M. Hafiz and R. M Syamim, MATEC Web Conf., 2016, 47, 1–6 CrossRef.
- W. Li, Y. Shi and L. Gao, Sci. Total Environ., 2013, 445–446, 306–313 CrossRef CAS PubMed.
- A. R. Khataee and M. B. Kasiri, J. Mol. Catal. A:Chem., 2010, 328, 8–26 CrossRef CAS.
- J. Šíma and P. Hasal, Chem. Eng. Trans., 2013, 32, 79–84 Search PubMed.
- E. A. Emam and N. A. K. Aboul-Gheit, Energy Sources, Part A, 2014, 36, 1123–1133 CrossRef CAS.
- C. J. Pestana, J. Hui, D. Camacho-Munoz, C. Edwards, P. K. J. Roberston, J. T. S. Irvine and L. A. Lawton, Chemosphere, 2023, 310, 136828–136838 CrossRef CAS PubMed.
- X. Pang, V. P. Sarvothaman, N. Skillen, Z. Wang, D. W. Rooney, V. V. Ranade and P. K. J. Roberston, Chem. Eng. J., 2022, 136494–136506 CrossRef CAS.
- Z. Ouyang, Y. Yang, C. Zhang, S. Zhu, L. Qin, W. Wang, D. He, Y. Zhou, H. Luo and F. Qin, J. Mater. Chem. A, 2021, 9, 13402 RSC.
- Q. Y. Lee and H. Li, Micromachines, 2021, 12, 907 CrossRef PubMed.
- M. Malhotra, L. Pisharody, A. V. Karim and S. Krishnan, Mater. Res. Found., 2021, 99, 163 CAS.
- Q. Xu, Q. S. Huang, T. Y. Luo, R. L. Wu, W. Wei and B. J. Ni, Chem. Eng. J., 2021, 416, 129123 CrossRef CAS.
- P. Ebrahimbabaie, K. Yousefi and J. Pichtel, Sci. Total Environ., 2022, 806, 150603 CrossRef CAS PubMed.
- S. Horikoshi, N. Serpone, Y. Hisamatsu and H. Hidaka, Environ. Sci. Technol., 1998, 32, 4010–4016 CrossRef CAS.
- B. Ohtani, S. Adzuma, S. Nishimoto and T. Kagiya, Polym. Degrad. Stab., 1992, 35, 53–60 CrossRef CAS.
- T. S. Tofa, K. L. Kunjali, S. Paul and J. Dutta, Environ. Chem. Lett., 2019, 17, 1341–1346 CrossRef CAS.
- D. Castilla-Caballero, O. Sadak, J. Martínez-Díaz, V. Martínez-Castro, J. Colina-Márquez, F. Machuca-Martínez, A. Hernandez-Ramirez, S. Vazquez-Rodriguez and S. Gunasekaran, Mater. Sci. Semicond. Process., 2022, 149, 106890–106911 CrossRef CAS.
- A. Chen, M. Yang, S. Wang and Q. Qian, Frontal Nanotechnol., 2021, 3, 723120 CrossRef.
- Z. Ouyang, Y. Yang, C. Zhnag, S. Zhu, L. Qin, W. Wang, D. He, Y. Zhou, H. Luo and F. Qin, J. Mater. Chem. A, 2021, 9, 13402–13441 RSC.
- T. Kawai and T. Sakata, Chem. Lett., 1981, 10(1), 81–84 CrossRef.
- T. Uekert, M. F. Kuehnel, D. W. Wakerley and E. Reisner, Energy Environ. Sci., 2018, 11, 2853–2857 RSC.
- T. Uekert, H. Kasap and E. Reisner, J. Am. Chem. Soc., 2019, 141(38), 15201–15210 CrossRef CAS PubMed.
- T. Li, A. Vijeta, C. Casadevall, A. S. Gentleman, T. Euser and E. Reisner, ACS Catal., 2022, 12(14), 8155–8163 CrossRef CAS PubMed.
- Y. Li, S. Wan, L. Gao, Y. Lu, L. Wang and K. Zhang, Sol. RRL, 2020, 5, 2000427–2000434 CrossRef.
- M. Du, Y. Zhang, S. Kang, X. Guo, Y. Ma, M. Xing, Y. Zhu, Y. Chai and B. Qiu, ACS Catal., 2022, 12(20), 12823–12832 CrossRef CAS.
- X. Jiao, K. Zheng, Q. Chen, X. Li, Y. Li, W. Shao, J. Xu, J. Zhu, Y. Pan, Y. Sun and Y. Xie, Angew. Chem., Int. Ed., 2020, 132, 15627–15631 CrossRef.
- S. Linley and E. Reisner, Adv. Sci., 2023, 10, 2207314–2207325 CrossRef CAS PubMed.
- S. Hanchang, Encyclopedia of life support systems, Point Sources of Pollution: Local Effects and Control, 2009, vol. 1, pp. 191–203 Search PubMed.
- S. M. Abdelbasir and A. E. Shalan, Korean J. Chem. Eng., 2019, 36, 1209–1225 CrossRef CAS.
- R. B. Geerdink, R. S. van den Hurk and O. J. Epema, Anal. Chim. Acta, 2017, 961, 1–11 CrossRef CAS PubMed.
- W. H. Saputera, A. F. Amri, R. Daiyan and D. Sasongko, Materials, 2021, 14(11), 2846–2881 CrossRef CAS PubMed.
- A. Moses, J. Komandur, D. Maarisetty, P. Mohapatra and S. S. Baral, Biomass Convers. Biorefin., 2022, 14, 3135–3159 CrossRef.
- S. Li, S. Zhao, S. Yan, Y. Qiu, C. Song, Y. Li and Y. Kitamura, Chin. J. Chem. Eng., 2019, 27(12), 2845–2856 CrossRef CAS.
- S. Varjani, P. Rakholiya, T. Shindhal, A. V. Shah and H. H. Ngo, J. Water Process Eng., 2021, 39, 101734 CrossRef.
- S. S. Tak, O. Shetye, O. Muley, H. Jaiswal and S. N. Malik, Int. J. Hydrogen Energy, 2022, 47(88), 37282–37301 CrossRef CAS.
- N. Skillen, H. Daly, L. Lan, M. Aljohani, C. W. J. Murnaghan, X. Fan, C. Hardacre, G. N. Sheldrake and P. K. J. Robertson, Top. Curr. Chem., 2022, 380(5), 33 CrossRef CAS PubMed.
- M. Imizcoz and A. V. Puga, Catalysts, 2019, 9(7), 584 CrossRef CAS.
- S. Y. Arzate Salgado, R. M. Ramírez Zamora, R. Zanella, J. Peral, S. Malato and M. I. Maldonado, Int. J. Hydrogen Energy, 2016, 41(28), 11933–11940 CrossRef CAS.
- M. I. Badawy, M. Y. Ghaly and M. E. M. Ali, Desalination, 2011, 267(2–3), 250–255 CrossRef CAS.
- A. Speltini, M. Sturini, F. Maraschi, D. Dondi, G. Fisogni, E. Annovazzi, A. Profumo and A. Buttafava, Int. J. Hydrogen Energy, 2015, 40(12), 4303–4310 CrossRef CAS.
- A. E. H. Machado, J. A. de Miranda, R. F. de Freitas, E. T. F. Duarte, L. F. Ferreira, Y. D. Albuquerque, R. Ruggiero, C. Sattler and L. de Oliveira, J. Photochem. Photobiol., A, 2003, 155(1–3), 231–241 CrossRef CAS.
- W. Zhang, Y. Li, C. Wang, P. Wang, Q. Wang and D. Wang, Water Res., 2013, 47(9), 3173–3182 CrossRef CAS PubMed.
- W. Cheng, N. Singh, J. A. Maciá-Agulló, G. D. Stucky, E. W. McFarland and J. Baltrusaitis, Int. J. Hydrogen Energy, 2012, 37, 13304–13313 CrossRef CAS.
- S. N. Khan, Z. Yang, W. Dong and M. Zhao, Sustain. Energy Fuels, 2022, 6, 4357–4374 RSC.
- G. Pipitone, G. Zoppi, R. Pirone and S. Bensaid, Int. J. Hydrogen Energy, 2022, 47, 151–180 CrossRef CAS.
- A. Chanthakett, M. T. Arif, M. M. K. Khan and A. M. T. Oo, in Bioenergy Resources and Technologies, 2021, pp. 219–247 Search PubMed.
- I. Michielsen, Plasma Catalysis: Study of Packing Materials on CO2 Reforming in a DBD Reactor, University of Antwerp, 2019 Search PubMed.
- S. N. Khan, Z. Yang, W. Dong and M. Zhao, Sustain. Energy Fuels, 2022, 6, 4357–4374 RSC.
- C. Dolle, N. Neha and C. Coutanceau, Curr. Opin. Electrochem., 2022, 31, 100841–100847 CrossRef CAS.
- L. Bromberg, D. R. Cohn and A. Rabinovich, Int. J. Hydrogen Energy, 1997, 22, 83–94 CrossRef CAS.
- S. K. Loeb, P. J. J. Alvarez, P. J. J. Alvarez, J. A. Brame, E. L. Cates, W. Choi, J. Crittenden, D. D. Dionysiou, Q. Li, G. Li-Puma, X. Quan, D. L. Sedlak, T. D. Waite, P. Westerhoff and J. H. Kim, Environ. Sci. Technol., 2019, 53(6), 2937–2947 CrossRef CAS PubMed.
- S. K. Loeb, P. J. J. Alvarez, J. A. Brame, E. L. Cates, W. Choi, J. Crittenden, D. D. Dionysiou, Q. Li, G. Li-Puma, X. Quan, D. L. Sedlak, T. David Waite, P. Westerhoff and J.-H. Kim, Environ. Sci. Technol., 2019, 53(6), 2937–2947 CrossRef CAS PubMed.
- N. Skillen, H. Daly, L. Lan, M. Aljohani, C. W. J. Murnaghan, X. Fan, C. Hardacre, G. N. Sheldrake and P. K. J. Robertson, Top. Curr. Chem., 2022, 380, 33 CrossRef CAS PubMed.
- M. Rumayor, J. Corredor, M. J. Rivero and I. Ortiz, J. Clean. Prod., 2022, 336, 130430–130441 CrossRef CAS.
- D. Bahnemann, P. K. J. Roberston, C. Wang, W. Choi, H. Daly, M. Danish, H. de Lasa, S. Escobedo, C. Hardacre, T. H. Jeon, B. Kim, H. Kisch, W. Li, M. Long, M. Muneerm, N. Skillen and J. Zhnag, J. Phys.: Energy, 2023, 5, 012004–012032 Search PubMed.
- H. Nishiyama, T. Yamda, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takate, T. Hisatomi and K. Domen, Nature, 2021, 598, 304–318 CrossRef CAS PubMed.
- D. Bahnemann, P. K. J. Robertson, C. Wang, W. Choi, H. Daly, M. Danish, H. Lasa, S. Escobedo, C. Hardacre, T. Jeon, B. Kim, H. Kosch, W. Li, M. Long, M. Muneer, N. Skillen and J. Zhang, J. Phys.: Energy, 2023, 5, 012004 Search PubMed.
- P. Ganeshan, V. S. Vigneswaran, S. C. Gowd, D. Kondusamy, C. S. Kuma, N. Kirshnanmoorthy, D. Kumar, A. Juneja, B. Paramasivan, N. N. Raju, K. Rajendran and A. Pugazhendhi, Fuel, 2023, 341, 127601–127619 CrossRef CAS.
- C. Zhan, N. Li and G. An, Energies, 2024, 17(2), 463 CrossRef.
- S. D. A. Zondag, T. M. Masson, M. G. Debije and T. Noel, Photochem. Photobiol. Sci., 2022, 21, 705–717 CrossRef CAS PubMed.
- C. Zhang, N. Liu, J. Ming, A. Sharma, Q. Ma, Z. Liu, G. Chen and Y. Yang, Water Res., 2022, 208, 117880 CrossRef CAS PubMed.
- P. S. Walko and R. N. Devi, Int. J. Hydrogen Energy, 2023, 48, 17086–17096 CrossRef CAS.
- R. Klaewkla, M. Arend and W. F. Hoelderich, A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems, Mass Transfer – Advanced Aspects, ed. H. Nakajima, InTech, 2011, ISBN: 978-953-307-636-2 Search PubMed.
- C. McCullagh, N. Skillen, M. Adams and P. K. J. Robertson, J. Chem. Technol. Biotechnol., 2011, 86, 1002–1017 CrossRef CAS.
- M. Adams, N. Skillen, C. McCullagh and P. K. J. Robertson, Appl. Catal. B Environ., 2013, 130–131, 99–105 CrossRef CAS.
- A. A. Khan and M. Tahir, J. CO2 Util., 2019, 29, 205–239 CrossRef CAS.
- A. Mills and S. K. Lee, J. Photochem. Photobiol., A, 2002, 152, 233–247 CrossRef CAS.
- https://www.purifics.com/photo-cat, accessed June 2024.
- G. E. Imoberdorf, A. E. Cassano, H. A. Irazoqui and O. M. Alfano, Catal. Today, 2007, 129, 118–126 CrossRef CAS.
- M. G. Beaver, E. Zhang, S. Zheng, B. Wang, J. Lu, J. Tao, M. Gonzalez, S. Jones and J. S. Tedrow, Org. Process Res. Dev., 2020, 24(10), 2139–2146 CrossRef CAS.
- Y. Su, N. J. W. Straathof, V. Hessel and T. Noel, Chem.–Eur. J., 2014, 20, 10562–10589 CrossRef CAS PubMed.
- X. Pang, V. P. Sarvothaman, N. Skillen, Z. Wang, D. W. Rooney, V. V. Ranade and P. K. J. Robertson, Chem. Eng. J., 2022, 444, 136494–136506 CrossRef CAS.
- J. F. J. R. Pesqueira, M. F. R. Pereira and A. M. T. Silva, J. Clean. Prod., 2024, 444, 140845–140855 CrossRef CAS.
- M. A. Tony and M. M. Eltabey, Appl. Water Sci., 2022, 12, 21–38 CrossRef CAS.
- I. Munoz, J. Peral, J. A. Ayllon, S. Malato, P. Passarinho and X. Domenech, Water Res., 2006, 40(19), 3533–3540 CrossRef CAS PubMed.
- S. Fernandes, J. C. G. Esteves da Silva and L. Pinto da Silva, Material, 2020, 25(13), 1487–1501 CrossRef PubMed.
- M. Rumayor, J. Corredor, M. J. Rivero and I. Ortiz, J. Clean. Prod., 2022, 336(15), 130430–130441 CrossRef CAS.
- J. Maurya, E. Gemechu and A. Kumar, Int. J. Hydrogen Energy, 2023, 48(52), 20077–20095 CrossRef CAS.
- A. Quintavalla, D. Carboni and M. Lombardo, ChemCatChem, 2024, 16, e202301225 CrossRef CAS.
- J. Dufour, D. P. Serrano, J. L. Galvez, A. Gonzalez, E. Soria and J. L. G. Fierro, Int. J. Hydrogen Energy, 2012, 37(2), 1173–1183 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


