 Open Access Article
Open Access ArticleMOF electrocatalysts in CO2 conversion: critical analysis of research trends, challenges and prospects
Oriyomi
Ogunbanjo
 a,
Paramaconi
Rodríguez
a,
Paramaconi
Rodríguez
 *bc and
Paul
Anderson
*bc and
Paul
Anderson
 *a
*a
aSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK. E-mail: p.a.anderson@bham.ac.uk
bCentre for Cooperative Research on Alternative Energies (CICenergiGUNE), Basque Research and Technology Alliance (BRTA), Alava Technology Park, 01510 Vitoria-Gasteiz, Spain. E-mail: Prodriguez@cicenergigune.com
cIKERBASQUE, Basque Foundation for Science, Plaza de Euskadi 5, 48009 Bilbao, Spain
First published on 15th April 2025
Abstract
Achieving sustainable energy and a clean environment is a strong driving force behind the exploration of the electrocatalytic potential of MOFs for CO2 conversion. The growing interest in the application of MOFs as electrocatalysts for CO2RR has been attributed to their high surface area and excellent catalytic properties. MOFs have been deployed in their pristine form as catalysts, as porous cavity supports for the incorporation of catalytic active material, or as precursors to obtain single-atom catalysts, showing that they can reduce CO2 into CO, formic acid and even hydrocarbons and alcohols. Despite these advantages and promising early results, they still have several challenges to overcome, such as poor electrical conductivity, stability and selectivity, and high overpotential, which would limit their practical application as electrocatalysts for CO2 reduction. In this review, various strategies to improve the electrocatalytic performance of MOFs are highlighted and directions that future studies in this field may take identified.
1. Introduction
Right from the start of industrialization to the present time, global energy demand has skyrocketed due to the attendant increase in the human population.1,2 An effort to sustain this ever-increasing population and economic growth has resulted in an over-dependence on hydrocarbon-based fossil fuels such as petroleum, natural gas and coal which still account for over 80% of world energy consumption.3 This is in addition to the fact that the long-term availability of fossil fuels may not be guaranteed which could plunge the world into an energy crisis. However, the use of fossil fuels has been associated with global climate change which has been identified as the root cause of various environmental disasters ravaging the world in recent times.4 Pertinent among these environmental problems are increased atmospheric CO2 level which is being projected to reach 450 ppm by 2050 and a rising global average temperature which is promoting extreme weather events and negatively impacting our ecosystem.5 The concern of continuous environmental disaster and the anticipated energy crisis has necessitated the urgent need to revolutionize technologies aimed at developing sustainable, clean and renewable energy to replace the use of fossil fuels.To tackle this problem, various CO2 capture and utilization technologies have been implemented.6 However, CO2 capture technology does not just consume a great deal of energy and financial resource, but its sustainability over a long period of time cannot be guaranteed. For this reason, the electrochemical CO2 reduction reaction (CO2RR) has been suggested as a promising CO2 utilization technology to convert waste CO2 streams into useful chemicals.7 Unlike other chemical processes, electrochemical CO2 reduction can be performed at ambient temperature using green renewable energy sources and water as a source of the protons making the overall operation a carbon neutral process.8 However, so far the electrochemical reduction of CO2 still suffers from high overpotential (large energy requirement) and poor product selectivity, making the practical application of electrochemical CO2 reduction still unfeasible from the industrial point of view. The high overpotential and poor product selectivity are the result of non-ideal adsorption energies of key reaction intermediates.9 The low faradaic efficiencies are due to the competition with the hydrogen evolution reaction (HER), which takes place in the same potential window as CO2 reduction.10
Materials such as transition metals, noble metals, nanoporous carbon, oxides, sulfides and phosphides are notable state-of-the-art electrocatalysts for various electrochemical systems.11–13 Despite the exceptional catalytic performance of these materials in some electrochemical systems, high cost, coupled with the scarcity of noble metals and the propensity to be poisoned of precious metal catalysts on exposure to some chemical compounds, has severely hindered their large-scale commercialization.14 Therefore, the focus of 21st-century researchers is to develop nanostructured porous electrocatalysts featuring, high activities, low cost, ease of synthesis, and widespread availability. In the past decades, electrocatalytic porous functional materials such as zeolitic materials, mesoporous silica, metal–organic frameworks, etc., have been the subject of intense research in the literature.14–16 Among the numerous porous materials available in the literature, metal–organic frameworks (MOFs) as electrocatalysts have captured the attention of a wide variety of research scientists.
MOFs are a specific class of materials constructed by joining metal-containing units, termed secondary building units (SBUs), with organic linkers using strong bonds to create open crystalline frameworks with permanent porosity.17 The early foundations of metal–organic frameworks (MOFs) can be traced back to the establishment of coordination chemistry by Swiss chemist Alfred Werner in 1893.18 Werner proposed the arrangement of coordination complexes as structures comprising a central metal atom surrounded by organic or inorganic ligands, laying the groundwork for future developments. Over the following decades, research into porous supramolecular architectures gradually progressed, eventually leading to the creation of novel hybrid porous crystalline structures in the 1990s.19 These structures, formed by the coordination of inorganic metal centres with organic linkers, marked a significant milestone. The term “metal–organic framework” (MOF) was introduced in 1995 by Yaghi's group, whose pioneering work on the topic was first published in Nature.20 This was followed by the synthesis of MOF-5, the first known porous MOF with permanent porosity in 1999 which was also reported in Nature.21 Since these important discoveries, the number of known MOF structures and published papers concerning MOFs has increased astronomically, attributed to the ever-expanding potential applications resulting from their unique properties and ease of synthesis.22
In this review, our discussion starts by investigating the numerous opportunities and challenges in the electrocatalytic application of metal–organic frameworks (MOFs) in CO2 reduction. Attention is focused toward addressing the conductivity and stability problems, and how MOF electrode preparation methods can affect the electrocatalytic performance of MOFs in respect of the CO2RR. We include a critical view on the lack of consensus on the normalization of catalytic activity—and some practices that can be considered to be incorrect—and the repetitive lack of information regarding post-mortem analysis of the MOFs. Finally, recent progress in utilizing MOFs as electrocatalysts in CO2 reduction is explored, and we briefly summarize the findings and proposed future outlook for this research field. It is anticipated that this review will serve as useful reference for scientists in chemistry and materials science and interdisciplinary researchers who are interested in electrocatalytic application of MOFs in CO2 reduction.
2. MOFS as electrocatalysts for CO2 reduction
The growing interest in the application of MOFs as homogeneous and heterogeneous electrocatalysts in the CO2 reduction reaction has been attributed to their promising catalytic properties.23–26 The exceptionally high surface area of MOFs provides plentiful catalytic active sites for surface-related reactions such as adsorption/desorption, insertion/de-insertion, surface redox reactions and heterogeneous catalysis.27 The large pore volume commonly exhibited by MOFs facilitates mass transport of selected substrates to the active sites as well as providing a platform for loading guest species.27 Catalytically active sites, and an understanding of where they originate from, are essential in MOF catalytic applications (Fig. 1). For pristine MOFs, catalytically active sites are often associated with coordinatively unsaturated metal sites, which act as Lewis acids, and dangling acid/base sites from the organic linkers.28 | ||
| Fig. 1 Metal–organic framework catalyst structure where: (A) is the connecting site for metal clusters and linkers in pristine MOFs, (B) is a site for grafting metal/non-metals in MOFs by modification, (C) is a site for encapsulated species in MOF composites and (D) represents sites generated in MOF derivatives upon thermal/chemical conversion.28 Copyright 2019, Elsevier. | ||
Secondly, the porous nature of MOFs makes it possible in practice to modify them post-synthetically with various catalytically active species, such as molecular catalysts, metal-based nanoparticles (NPs), enzymes, etc., which can create structural defects within the MOFs for efficient catalysis. Thirdly, the catalytically active sites of MOFs can be exposed to chemical/thermal treatment to obtain porous materials, including metal-based compounds, porous carbons and their composites. These MOF derivatives are promising candidates for catalytic application because of their exceptionally large surface area, tunable structures/composites and highly dispersed active sites. An interesting attribute of MOF-derived materials is their ability to retain, to a certain degree, the morphological architecture of the pristine MOF, which upon doping with heteroatoms, can improve the conductivity and the degree of graphitization, resulting in better performance in electrocatalytic applications.28–30 Based on these merits—tunable porosity, high surface area and modifiable active sites—the electrocatalytic application of MOF-based nanocomposites has become the subject of intense discussion in recent years.31–35 However, their role as electrocatalysts remains limited by critical challenges that hinder industrial viability. While MOFs provide well-defined catalytic sites, including coordinatively unsaturated metal centres and redox-active ligands, their inherently poor electrical conductivity severely limits charge transfer, requiring additional modifications such as guest-molecule doping or hybridization with conductive supports.
Stability is another major limitation. Many MOFs degrade in electrochemical environments, particularly in aqueous bicarbonate electrolytes, leading to metal leaching or framework collapse. Strategies such as robust metal–ligand combinations and post-synthetic modifications improve durability but add complexity and cost. Moreover, while 2D MOFs, nanocages and defect engineering enhance active site accessibility, these modifications do not at present fully resolve conductivity and stability issues.
Despite their theoretical promise, MOFs in the CO2RR largely serve as precursors or support materials rather than standalone catalysts. The field must shift toward MOF-derived materials or radically rethink MOF design to address fundamental limitations. Without breakthroughs in conductivity and stability, MOFs will remain an academic curiosity rather than a scalable CO2RR solution.
2.1. Addressing conductivity challenges in MOFs
Based on the literature reports so far, conductivity in MOFs can range from 10−10 to 103 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36,37 which is still, unfortunately, far below the electrical conductivity requirement for industrial application in an electrochemical system. The poor electrical conductivity of MOFs has been linked to: (i) the insulating character of the organic ligands, attributable to the lack of free charge carriers; and (ii) poor overlap between π orbitals of the organic ligands and the d orbitals of the metal ions, which present a barrier for charge transfer.8 Conductivity has become an important indicator to measure the suitability of MOFs for electrochemical application.38 Since electrical conductivity (σ) in MOFs is dictated by the amount of charge density (n) and mobility (μ) of electrons (e) and holes (h), it is essential to maximize both charge density and mobility of charge carriers (eqn (I)) to achieve high conductivity.
36,37 which is still, unfortunately, far below the electrical conductivity requirement for industrial application in an electrochemical system. The poor electrical conductivity of MOFs has been linked to: (i) the insulating character of the organic ligands, attributable to the lack of free charge carriers; and (ii) poor overlap between π orbitals of the organic ligands and the d orbitals of the metal ions, which present a barrier for charge transfer.8 Conductivity has become an important indicator to measure the suitability of MOFs for electrochemical application.38 Since electrical conductivity (σ) in MOFs is dictated by the amount of charge density (n) and mobility (μ) of electrons (e) and holes (h), it is essential to maximize both charge density and mobility of charge carriers (eqn (I)) to achieve high conductivity.| σ = e(μene + μhnh) | (I) |
In redox hopping, the charge carriers (such as redox centres) are usually localized at specific sites within the framework which often results in charge hopping between neighbouring sites in MOFs. However, in order to maintain electroneutrality during charge hopping, a counter ion usually must diffuse through the MOF structure, thereby limiting the charge transport.39 Therefore, charge transport via redox hopping is evaluated based on diffusion phenomena where the measurable diffusion coefficient De (cm2 s−1) for an electron-hopping mechanism in MOFs is related to total redox-active linker concentration C0P, the electron-exchange rate constant ke (s−1) and average hopping distance d via the following equation:40
 | (II) |
 | ||
| Fig. 2 (a) Fabrication of electrically conductive TCNQ@Cu3(BTC)2via post-synthetic incorporation (b) I–V curve showing the electrical conductivity of pristine Cu3(BTC)2 (red), and after infiltration with TCNQ (green), F4-TCNQ (gold) and H4-TCNQ (purple). Reproduced with permission from Talin et al.44 Copyright from The American Association for the Advancement of Science, 2014. | ||
The second and more efficient process that can be used in obtaining high charge transport and enhance conductivity in MOFs is through band transport.38 This is achieved by increasing the extent of delocalization between the framework components by introducing mixed valent states in the node/linker, donor–acceptor type interactions, π–π stacking or π-conjugation into the framework which could lead to improved charge propagation.47 Carefully selecting atoms with diffuse frontier orbitals, such as softer transition metal ions (Fe, Mn, Co, Ni and Cu) and linkers containing nitrogen or sulfur can result in construction of highly delocalized MOF frameworks.48 Sun et al., demonstrated that when a linker 2,5-dihydroxybenzene-1,4-dicarboxylic acid was replaced with 2,5-disulfhydrylbenzene-1,4-dicarboxylic acid in the construction of a Mn-MOF, infinite 1D metal–sulfur chains were created within the framework, prompting charge delocalization and resulting in a dramatic increase in conductivity.49 The presence of extended π–π-stacking or π-conjugation in MOFs destined for electrocatalysis have been shown to create a pathway for the efficient delocalization of charge carriers within the framework.50 The strategy of extended π–π-stacking was implemented by some researchers to design MOFs with functionalized TTF (tetrathiafulvalene) derivatives, with the π–π stacking of TTF moieties resulting in the formation of highly conductive charge transport columns within the framework, leading to improved conductivity.51–53 In a similar strategy, incorporation of in-plane π-conjugation into MOF frameworks has been discovered to induce charge delocalization in 2D MOFs.54–56 Of particular interest is the work of Zhu et al. where they reported that 2D MOF Cu-BHT (BHT = benzenehexathiol) displays great charge delocalization due to extended 2D π-conjugation, prompting conductivity as high as 1580 S cm−1 at room temperature (Fig. 3).36 Overall, findings have shown that π-conjugation and π–π stacking strategies have resulted in the largest increase in conductivity.37,57
 | ||
| Fig. 3 (a) Electrically conductive Cu-BHT 2D MOFs designed by π-conjugation (b) electrical conductivity measurement of Cu-BHT film as a function of temperature ranging from 2 to 300 K. Reproduced with permission from Zhu et al.36 Copyright Springer Nature, 2015. | ||
The reports on conductive MOFs for electrocatalysis have thus been encouraging so far. However, opportunities still abound in developing conductive MOFs with optimal electrocatalytic performance by carefully selecting ligand, metal centre and guest molecule that will promote redox hopping and/or band transport within the MOFs. It is important to notice that the vast majority of the reports, if not all, have provided information on the conductivity of pristine or post-synthetic modified MOF catalysts, however, information on the conductivity of the catalyst after the electrochemical experiments is missing. Such information is key to understanding the changes in MOFs under operating conditions, in particular with regard to stability and changes in the product selectivity.
It is important to note that the correlation between the conductivity and stability of metal–organic frameworks (MOFs) and their performance in the CO2 reduction reaction (CO2RR) is a critical aspect for their practical application as electrocatalysts. Conductivity in MOFs, whether achieved via redox hopping, band transport or guest-promoted pathways, is essential for efficient electron transfer during electrocatalysis. Enhanced electrical conductivity reduces charge transfer resistance, facilitating the activation of CO2 and stabilizing key reaction intermediates, thus improving faradaic efficiency and product selectivity. Stability, on the other hand, ensures that MOFs maintain their structural integrity and catalytic activity over extended operational periods. However, many MOFs suffer from degradation under electrochemical conditions due to framework dissolution, ligand hydrolysis, or metal node leaching. It is also important to mention that strategies such as post-synthetic modification, incorporation of conductive linkers, and hybridization with conductive supports have been explored to enhance simultaneously conductivity and stability. The challenge remains in designing MOFs that balance high electrical conductivity with robust chemical stability, ensuring their long-term viability for CO2RR applications.
2.2. Addressing chemical stability challenges in MOFs
Stability of MOFs during electrochemical operation is another crucial factor for their use in electrocatalytic applications.58 During electrocatalysis, the voltage applied induces diffusion of ions/molecules onto the MOF's surface to establish an electrical double layer (EDL) (Fig. 4(a)). This charged MOF surface creates a concentration gradient of ions (H+ in this case), causing the local pH in the EDL to drift to higher or low values resulting in complete or partial destruction of the MOF structure (Fig. 4(b) and (c)).59 Therefore, one of the main challenges to overcome in the CO2RR on MOFs is to deliver materials that are stable in neutral and strongly alkaline media. Additionally, during potential cycling (Fig. 4(d)) chemical gradients of cation/anion at the MOFs electrochemical interface can result in degradation of the metal nodes and organic linkers (Fig. 4(e)) resulting on transformation of the MOFs into metal oxides, hydroxides or oxyhydroxides during catalysis.58,60,61 | ||
| Fig. 4 (a) Established EDL structure on the surface of a MOF particle. (b) Diffusion of ions/molecules into a MOF framework in the established EDL. (c) Cross-sectional illustration of a MOF-coated electrode in neutral electrolyte. (d) EDL formation and ion diffusion on the surface or inside the pores of MOFs (right) due to the dynamic potential sweeping (left). (e) Structural destruction of MOFs due to the redox reaction of metal nodes and/or organic linkers. Reproduced with permission from Zheng et al.62 Copyright American Chemical Society, 2021. | ||
While it might be challenging to address both the chemical and electrochemical stability of MOFs without some form of trade off, research has progressed over the years on how to improve MOF stability in electrocatalytic applications.63,64 There remain questions which are still the subject of discussion regarding the role of MOFs during electrocatalysis: are MOFs the real catalysts? Or are the MOFs merely the precursors to active phases produced during electrochemical treatment? It is therefore important to understand the reactivity of MOFs in the chemical and electrochemical environment, and confirm, either in situ or post mortem, their chemical and structural integrity in order to design and construct stable MOFs for electrocatalytic application. The chemical environment of MOFs during electrochemical reaction comprises water, counter ions, reactants and products from electrocatalysis, as well a flow of electrons owing to the applied potential. Generally, water has the tendency to interact with metal-linker coordination sites, prompting the decomposition of the framework, producing hydrated/hydroxide metal species (in basic electrolytes) and/or protonated linkers (in acidic electrolytes) that further diffuse into the electrolyte.65,66 Aside from hydrolysis of MOFs, the effect of counter ions has also been documented to be destructive. For example, phosphate-buffered (PB) solution contains PO43− anions (hard Lewis base) which can strongly interact with high valency metal ions. An example of this effect has been found when using the UiO-66 framework, which disintegrates within a few minutes in PB solution as a result of the strong interaction of PO43− anions with the Zr4+ nodes of the UiO-66 framework.67 Zeolitic imidazolate frameworks (ZIFs) are a class of porous materials characterized by their zeolite-like topology and imidazolate linkers. Mirsaidov's group demonstrated the lack of stability of ZIF-8 in Co2+-containing solution resulting in the formation of ZnCo(OH)x.68 The products from electrocatalysis have not been given much attention as a possible cause of instability of MOFs. For example, it has been shown that nanobubbles can be generated at the active sites during gas evolution reactions which can pose both mechanical and chemical challenges to the structural integrity of MOFs.69,70 Other products, such as formate/CH3OH from the CO2 reduction reaction (CO2RR) and NH4+ from the nitrogen reduction reaction (NRR), may pose a similar threat.62
Due to the poor stability, some MOFs could show poor performance upon exposure to moisture which can attack the metal centre within the framework, causing decomposition and phase transformation of the MOF. Some reports have anticipated that the bond strength between the metal cation and the organic ligands is one of the main criteria that influences stability.71–74 Therefore, the chemical stability of a MOF can be intrinsically improved by judicious selection of metal nodes and design of linkers.75De novo synthesis of MOFs by linking carboxylate-based ligands (hard bases) with high-valent metal ions (hard acids) has been explored in search of MOFs with improved stability. Based on the Hard–Soft Acid–Base Principle (HSAB) principle, high-valent metals with high charge density (hard acids), such as Zr4+, Cr3+, Al3+, Fe3+, etc., tend to form strong coordination bonds with O donor ligands (hard bases) (Fig. 5) thereby presenting MOFs with good chemical stability.76–79 Interestingly, the strong coordination bonds and the low pKa of carboxylate linkers endow the high-valent metal ion-containing MOFs with a good level of stability in acid.
 | ||
| Fig. 5 Some representative O donor ligands, with their pKas. Reproduced with permission from Ding et al.63 Copyright Royal Society of Chemistry, 2019. | ||
On the other hand, low-valent metal ions, including Zn2+, Co2+, Ni2+, Fe2+ and Ag+, which are considered as soft acids can form strong coordination bonds with suitable N-containing linkers (soft bases) (Fig. 6) producing a highly stable MOFs.80–82 The high pKa of azoles and the strong coordination bonds usually bestows remarkable stability in basic solutions on these MOFs. Generally speaking, MOFs built from high-valent metal ions and carboxylate ligands tend to be stable in acids and decompose in basic solutions while MOFs constructed using low-valent metals and azolate linkers tend to be stable under alkaline conditions while being readily hydrolysed by acids.83 The introduction of hydrophobic/rigid ligands, and phosphonate/carboxyphosphonate ligands into MOF synthesis has also been suggested as a way to improve MOF stability.84,85 Surface hydrophobicity prevents the adsorption of water into pores and/or the condensation of water around the metal clusters, which enhances the MOF stability in the presence of water. Phosphonate/carboxyphosphonate MOFs have been shown to exhibit robust structural tolerance in harsh chemical environments,86 which has been attributed to the stronger metal–ligand bonds formed when the three oxygen atoms from phosphonic acid coordinate to metal ions compared to the corresponding carboxylate MOFs.
 | ||
| Fig. 6 Some representative N donor ligands, with their pKas. Reproduced with permission from Ding et al.63 Copyright Royal Society of Chemistry, 2019. | ||
The challenge of electrochemical stability can be approached by optimizing the various electrocatalyst parameters. For example non-aqueous electrolytes such as tetrabutylammonium hexafluorophosphate (TBAPF6) in acetonitrile or DMF, have been used as substitutes in studying the catalytic activity of MOFs that are unstable in aqueous electrolytes.87 The implementation of organic solvents as electrolytes can also be linked to their ability to increase the solubility of CO2 which can, as a result, reduce mass transport limitations.88 In addition, the low proton concentration inhibits the competing process of the hydrogen evolution reaction which is prevalent in aqueous electrolyte. Nevertheless, aqueous electrolytes remain the electrolytes of choice as water is a green and abundant solvent that can be used in large scale processes.89 Therefore, the use of catalytically active MOFs stable in the presence of bicarbonate ions is necessary for future applications. MOFs constructed with nitrogen-containing linkers such as ZIFs have been found to be highly stable in water over a wide pH range and this family of MOFs has consistently shown promising activity towards the CO2RR in aqueous electrolytes.80,90,91 On a final note, MOFs under electrochemical conditions must meet two additional stability requirements: (i) maintaining physical attachment to the electrode surface, and (ii) remaining structurally intact under reducing and/or oxidizing conditions during electron flow through the framework.83 The likelihood of meeting the first stability challenge—the potential for delamination or generally loss of MOF from an electrode surface—depends to a large degree on how the electrode is prepared. The second stability issue of resisting degradation under electron flow while in the presence of substrate is a much more MOF-specific challenge. MOFs constructed from ligands that are able to give reversible electrochemical responses are often considered to be stable under electrochemical conditions. However, a significant number of reports have not performed adequate and exhaustive characterization of the MOF after electrochemical experiments. It should be expected and common practice to evaluate the structural and compositional integrity of the MOF catalyst after electrochemical experiments including: high-resolution scanning transmission electron microscopy (HR-STEM), energy-dispersive X-ray spectroscopy (EDX) and, if possible, in situ X-ray absorption spectroscopy XAS experiments to confirm the oxidation state and coordination of the metal centres. Numerous electrochemically stable MOFs have been successfully built from ligands based on a naphthalene diimide core,92–94 a moiety that seems to be stable when reduced. For more in-depth discussion, readers are directed to a recent review by Liu et al.95 Future studies focusing on the structural evolution of MOFs under CO2RR conditions are fundamental to establish structure–activity correlations and to understand the different effects that contribute to the stability of MOF-based catalysts.
2.3. Addressing the product selectivity in MOFs
Achieving precise control over product selectivity in the CO2 reduction reaction (CO2RR) on MOF-based electrodes is essential for optimizing their practical applications in carbon-neutral fuel and chemicals production. While MOFs inherently offer tunable catalytic environments, their effectiveness in selectively generating desired products depends on a combination of structural modifications, electronic tuning and reaction environment optimization. However, as observed in other CO2RR catalysts, product selectivity is highly sensitive to mass transport phenomena, including CO2 diffusion, intermediate adsorption, and proton availability at the electrode interface.96–98 The interplay between catalyst structure and reaction conditions must be carefully controlled to mitigate transport limitations that could otherwise lead to competing side reactions, such as hydrogen evolution. Additionally, the intrinsic porosity of MOFs plays a crucial role in governing mass transport, as pore size and connectivity dictate the diffusion of CO2 molecules and reaction intermediates to active sites. While highly porous MOFs offer increased surface area and active site accessibility, excessive porosity or narrow pore channels can hinder CO2 diffusion, leading to local variations in reactant concentration and altering selectivity.Regulating the selectivity of products in the CO2RR process on MOF electrodes requires a combination of material design and reaction environment optimization. One of the most effective strategies involves tailoring the organic ligands that coordinate with the metal centres. The choice of ligands influences the electronic properties and coordination environment, which in turn affects the binding strength of CO2 and its reaction intermediates.2 For example, 2-methylimidazole coordinated to Zn2+ in ZIFs has been shown to favor CO2 reduction to CO, while other ligands may steer the reaction toward formate or hydrocarbons.91
Another powerful approach is doping MOFs with electron-donating molecules, such as 1,10-phenanthroline or metallocenes.90 These additives enhance CO2 adsorption and reduce the Gibbs free energy barrier for key reaction steps, making it easier to direct the reaction pathway toward a specific product. Similarly, structural modifications to MOFs can significantly impact selectivity. By reducing MOFs to 2D nanosheets, a larger proportion of active metal sites are exposed, improving electron transfer and influencing reaction intermediates.99,100 Introducing strain or defects further modifies the electronic structure, fine-tuning the catalytic behaviour and favouring certain products over others.
The way MOFs are integrated into electrodes also plays a crucial role in selectivity. Growing MOFs directly on conductive substrates improves charge transfer efficiency, ensuring more effective electron flow to the catalytic sites. Additionally, combining MOFs with conductive additives such as carbon black or graphene oxide further enhances charge transport and helps control product distribution. However, mass transport limitations in porous MOFs must be carefully managed, as restricted CO2 diffusion within the framework can lead to selectivity shifts, favouring hydrogen evolution over CO2RR. Pore engineering strategies, such as optimizing pore size distribution or introducing hierarchical porosity, can help balance CO2 diffusion rates and reactant accessibility, preventing local reactant depletion or oversaturation, both of which affect selectivity. Studies have shown that using imidazolium-based ionic liquids in electrolytes can promote the formation of methane,101 while other electrolytes may favor CO or formic acid production.102–104
Finally, the construction of heterogeneous MOF structures, such as MOF heterostructures or composites with metal oxides, introduces synergistic effects that influence reaction pathways. By carefully designing these hybrid systems, it is possible to steer CO2 reduction toward higher-value products like hydrocarbons or alcohols. Together, these strategies offer a comprehensive approach to improving product selectivity in CO2 reduction on MOF electrodes, opening new possibilities for efficient and sustainable carbon conversion.
3. Assessing the performance of various methods of MOF electrocatalyst preparation
Electrochemical evaluation of MOFs requires interfacing the MOF with an electrode. Finding an effective method of preparing MOF-modified electrodes play an important role in the attachment of the MOF catalyst to the electrode and by extension determines the MOF electrochemical response, specifically regarding the rate of electron transfer between the electrode and the first MOF layer as well as how much of the bulk MOF is electrochemically accessible.83 The type of MOF-electrode attachment can also have a large impact on the strength of mechanical adhesion, with poor adhesion resulting in delamination.83,105 Two approaches have generally been adopted in preparing MOF-modified electrodes for the CO2RR and other applications. The first entails immobilizing the MOF on a conductive substrate via physical interactions or through the formation of chemical bonds between the substrate and the MOF. The second method involves synthesizing MOFs directly on the electrodes. MOFs for electrochemical studies are generally prepared by four methods: drop-casting, direct solvothermal growth on a bare electrode surface, self-assembled monolayer deposition on the electrode surface or electrochemical methods. Each of these methods has different characteristics of mechanical stability, preparation time, and ease of electron transfer between the MOF and conductive electrode substrate. Despite the diversity of synthetic approaches used for preparing MOF-modified electrodes, some of them, like direct growth on conductive substrates are yet to be fully explored for preparing MOF electrodes for the CO2RR unlike other electrochemical reactions such as the OER and ORR.1063.1. Drop casting
Drop casting is one of the facile methods for immobilizing MOFs on a conductive electrode for electrochemical studies. In this case, a catalyst ink is usually prepared by dispersing (typically by sonication) MOF powder into a solvent or solvent mixture. The electrode is either left to dry in open air or oven dried at 60 °C, leaving behind a thin film of the MOF dispersed on the electrode for electrochemical analysis (Fig. 7(a)). Additives such as carbon black and graphite can be added to the catalyst ink to increase the MOF electrical conductivity,107–109 while polymeric binders such as Nafion can be added to improve catalyst immobilization on the electrode.110 However, it is also possible for the electrochemical system to be contaminated by these additives, thereby increasing resistance and possibly blocking some MOF active sites, leading to a decrease of the desired catalytic activity or even change in selectivity.111 Common working electrode substrates, where the drop casting takes place, include glassy carbon (GC), carbon paper and fluorine-doped tin oxide (FTO).90,100,110,112–114 However, despite the simplicity of this method of preparing MOF electrodes, controlling the resulting films’ thickness and morphology is challenging.83 As a result of their poor stability, MOF films prepared by drop casting can only be used as electrodes for fundamental studies and are not suitable for use in electrolysers. Alternatively, MOF-based gas diffusion electrodes (GDEs) prepared by airbrushing catalyst ink on porous carbon paper have been proposed to overcome the above challenges (Fig. 7(b)).115,116 In GDEs, since CO2 gas is fed directly on one side of the electrode, the possible hindrance caused by the low solubility of CO2 in the electrolyte is reduced. The work of Albo et al.117 and Sikdar et al.115 have used MOF-based GDEs in the CO2RR with some promising results in terms of high selectivity towards hydrocarbon formation.3.2. Direct solvothermal synthesis
In this method, direct growth of MOFs on conductive substrates is achieved by placing the electrode in a solvothermal reactor with the standard MOF precursors and heating for the reaction to take place (Fig. 8).83,118 The direct solvothermal synthesis method has a significant advantage over drop casting which is that the resulting MOF is directly attached to the electrode surface without the need for extra additives that might interfere with the electrochemical performance. During synthesis, the rugosity of the surface of the electrode itself provides nucleation sites for MOF crystallization. To achieve effective MOF binding, the electrode is usually pre-treated in dilute linker, creating a monolayer, which provides the binding sites for the first secondary building unit (SBU) layer and subsequently initiate further growth.119 Similarly to the drop casting method, GC, FTO glass and metal foams are commonly used as electrode substrates.Before a MOF is grown on a carbon electrode, the electrode is usually pre-treated to create functional groups such as carboxylate groups that facilitate nucleation and subsequent MOF growth. As for FTO and ITO substrates, the metal oxides of the electrode provide centres for the nucleation of MOF precursors, facilitating MOF growth on the FTO and ITO, resulting in a uniform MOF thin film of nanocrystals.120 The main drawback of these substrates, apart from the cost, is their reduced conductivity when compared with carbon electrodes.8
3.3. Liquid phase epitaxy (LPE)
The liquid phase epitaxy method for the preparation of MOF electrodes was first proposed by Shekhah et al.121 The first step of the method involves the pre-coating of the substrate with self-assembled monolayers (SAMs) of organic molecules bearing carboxylate or pyridine moieties, which later will serve as nucleation sites.122 Then the substrate is immersed sequentially into different solutions of MOF precursors in a layer-by-layer fashion, producing a highly orientated and homogeneous MOF thin film directly over the support (Fig. 9).118 This strategy is also known as SURMOF (surface-bound metal–organic framework) in the literature.Although this technique has useful advantages like control of orientation, tunable thickness and strong adhesion to the substrate surface,123,124 the resulting SAM could also constitute an insulating layer between the electrode surface and the MOF, resulting in an increase of the electrical resistance, thus diminishing the electrochemical performance of the MOF-modified electrode.125 Since its inception over a decade ago, there has been extensive research work on surface-mounted metal–organic frameworks (SURMOFs) and related epitaxial growth methods,126,127 however, few SURMOFs prepared by LPE have been tested as electrocatalysts.124 This, however, is a synthesis method that could be further explored since the strong bond between the MOF and the substrate could be advantageous for long-term electrolysis. It may also be possible to leverage the highly oriented nature of SURMOFs to tune their morphology and thickness in order to promote mass and charge transport through the MOF and possibly enhance catalytic performance.
3.4. Electrochemical synthesis
Two approaches have been identified for preparing MOF thin films on substrates for electrochemical application: direct and indirect electrosynthesis. With direct electrosynthesis, the process can be anodic or cathodic. On the other hand, electrophoretic deposition is a popular indirect electrosynthesis method used in depositing MOF thin films on conductive substrates (Fig. 10). The characteristics of these methods are summarized in Table 1. | ||
| Fig. 10 MOF-based electrode construction by electrophoretic deposition. Reproduced from Liu et al.128 Copyright 2021, Wiley-VCH. | ||
| Direct electrosynthesis | Indirect electrosynthesis | |
|---|---|---|
| Anodic electrosynthesis | Cathodic electrosynthesis | Electrophoretic deposition |
| • Anode electrode is immersed in an electrolyte which contains the ligand | • Anode and cathode electrodes are immersed in an electrolyte which contains MOF precursors | • Two similar electrodes acting as the anode and the cathode are immersed in a cell containing MOF suspension and supporting electrolyte |
| • As potential is applied, electrode (metal) is oxidized to metal ions | • MOFs are formed directly on the cathode electrode surface by an electrochemical reaction129 | • After applying a potential, the MOF diffuses into the electrolyte and becomes deposited onto the oppositely biased electrode surface depending on the partial charges on the MOF (Fig. 13)130 |
| • Metal ions react with ligand to form a thin-film MOF on the anode electrode surface | • The electrodes merely act as a source of electrons and surface for nucleation and MOF growth | |
| • MOF properties are independent of the metal precursor since no metal salts are used131 | • MOFs are generated at lower potential (1.5–10 V) and within few minutes at room temperature | • Higher applied potential and longer time (typically 50–100 V during 1–3 h) are required to produce MOF films |
| • Different metal oxidation states can be generated at different potentials which lead to a better control over the MOF properties129 | • Two different electrodes act as anode and cathode | • Longer deposition time is advantageous as it allows control of MOF electrode properties e.g. MOF film thickness and particle–particle connectivity at FTO surfaces130 |
| • MOFs are generated at high potential (12–19 V) and longer time | ||
The first MOF synthesized by anodic dissolution was HKUST-1. In this method, a potential of 12–19 V was applied for 150 min to a Cu anode which oxidized to Cu2+ ions in a BTC linker containing solution (Fig. 10). The chemical reaction between the Cu ions and the BTC linker resulted in the formation of an HKUST-1 layer on the electrode surface.129 Since its first deployment for HKUST-1 synthesis, many other MOFs have been produced using electrochemical synthesis.132–136 Dinca et al. reported the first MOF synthesized by cathodic deposition producing Zn4O(BDC)3 (MOF-5).137 The synthesis was carried out by immersing FTO electrode in MOF-5 precursors and (NBu4)PF6 as conducting electrolyte (Fig. 11). As a constant cathodic potential of −1.6 V was applied for 15 min at a current density of −6.6 mA cm−2, the probase (P) became reduced to a base equivalent (B) which increased the local pH near the electrode surface.138 This change in pH induced deprotonation of the ligand (H2BDC), which further coordinated with metal ions and eventually triggered precipitation of MOF-5 crystals.
 | ||
| Fig. 11 (a) General scheme for the cathodically induced electrochemical deposition of MOFs, involving the reduction of probase (P), the generation of base equivalents (B), the deprotonation of ligands (H2BDC), and MOF crystallization from BDC2− and metal ions (Zn2+). (b) Schematic illustration of the formation of a biphasic mixed film. Reproduced from Li et al.138 Copyright 2014, Royal Society of Chemistry. | ||
It has been proposed that electrochemical synthesis has the potential to produce more CO2RR efficient MOF-modified electrodes. For example, Kang et al.139 synthesized MFM-300 on indium foil electrodes by immersing in a solution containing the organic linker and supporting electrolyte, and the MOF-modified electrode system was found to be catalytically active for the CO2RR, exhibiting a current density of 46.1 mA cm−2 at applied potential of −2.15 V vs. Ag/Ag+, demonstrating an faradaic efficiency of 99.1% for the formation of formic acid. Similarly, Hod et al.125 deployed the electrophoretic deposition method to obtain a Fe–porphyrin MOF thin film on an FTO electrode which showed unprecedented catalytic activity towards the CO2RR, generating a mixture of CO and H2 at 100% faradaic efficiency. However, despite these promising results, not many MOF catalysts for the CO2RR prepared by electrochemical synthesis have been reported, and this is an apparently promising path that remains largely unexplored.
Table 2 provides a concise summary of the various MOF-based electrode preparation methods discussed in the manuscript. It highlights their key characteristics and associated remarks, offering a comparative perspective on their advantages and limitations for CO2RR applications.
| Method | Characteristics | Remarks |
|---|---|---|
| Drop casting | Simple, fast, limited control over film thickness | Prone to delamination, poor stability |
| Gas diffusion electrode (GDE) | Improved CO2 mass transport, better selectivity | Improved dispersion on the support |
| Solvothermal growth on electrode | Strong adhesion, high crystallinity | Ensures better electron transfer and durability |
| Electrophoretic deposition | Uniform coverage, good mechanical stability | Enables precise control over film thickness, enhanced conductivity |
| Thermal treatment (MOF-derived catalysts) | Generates defect-rich, conductive structures | Improved conductivity, but may alter active sites |
| Electrochemical synthesis | Direct deposition, controlled morphology | Improved stability |
4. Critical review of activity metrics for benchmarking electrocatalyst performance
The unprecedented growth witnessed in the field of MOFs has resulted in a rise in papers directly using MOFs in electrocatalysis.140,141 Despite the increasing number of reports, a standardized method for determining the electrocatalytic performance and true electrochemical surface area of MOFs has not been fully established.142 Many researchers on MOF electrocatalysts summarize and compare their results, however, the comparison of different experimental data from different laboratories remains problematic owing to a lack of standardization in the measurement procedure and reference system.143,144 Therefore, a correct and convenient activity metric that can reasonably normalize the electrocatalytic performance is needed, to provide a basis for comparing the performance of different catalysts.Several activity metrics have been utilized to report selectivity and electrocatalytic activity. One commonly used metric for selectivity is faradaic efficiency, which is defined as the fraction of faradaic charge utilized to produce a given product. Despite its utility in describing selectivity, faradaic efficiency is problematic when comparing catalysts with different activities. As an example, one might conclude that a catalyst that produces a specific product more selectively is also more active in producing that product. However, it is possible for selectivity toward a product to increase without increase in production rates of that product. Therefore, true differences between two catalysts can be obscured if they are only compared on the basis of faradaic efficiencies.145 The rate of product generation, which is proportional to its partial current density, is a much less ambiguous descriptor of catalytic activity.
As for electrocatalytic activity, the most important activity metric used to benchmark the performance of a catalyst is exchange current density (j0).146 However, this must be normalized to provide a common ground for comparing the performance of catalysts. Normalized current densities may be described as: (i) geometric activity i.e. the current density normalized to the geometric area of electrode (mA cmgeo−2), (ii) specific area activity i.e. the current per surface area of electrocatalyst (mA cmcatalyst−2) and (iii) mass activity i.e. the current per loading mass of electrocatalysts (A gcatalyst−1).
In geometric normalization, the current is normalized to the geometric area of electrode. However, geometric activity does not necessarily reflect a catalyst's intrinsic activity. The geometric activity fails to consider that the electrocatalytic reaction is a surface process, where only the surface active sites participate in the reaction.147 On the other hand, the geometric activity is largely dominated by the loading mass of catalysts. It has been commonly observed that with more catalyst loading, the overpotential to reach the given geometric surface area normalized current density (igeo) certainly becomes smaller.148,149 Most electrocatalysts studied these days are nanostructured and have a large surface area with very high roughness factors, thus current normalization based on the geometrical area of the electrode is not appropriate and should ideally be avoided.150
Since electrocatalysis is a surface reaction where the adsorption/desorption of reactants/products take place only at the surface of the catalyst, the intrinsic electrocatalytic activity is best described using specific activity which is defined as the current divided by the surface area of the catalyst (mA cm−2 catalyst).151 Specific activity is estimated by normalizing the current density by either the number of catalyst active sites or electrochemically active surface area.147 While normalization to the number of active sites is the preferable metric,142,152,153 in heterogeneous systems such as MOFs, it can be difficult to identify what actually the active site is. Extensive and detailed in situ spectroscopic studies will be required for such electrocatalysts. Therefore, normalizing the measured activity by the electrochemically active surface area is a more rational way to normalize catalytic activity.144 Previous reports have proposed that the electrochemically active surface area (ECSA) of the catalyst can be determined by measuring the double-layer capacitance of the electrode–electrolyte interface. The double layer capacitance can be measured by conducting cyclic voltammetry (CV) in a potential range where no faradaic processes occur, typically a 100 mV window centred at the open circuit potential (OCP). In this potential region, any measured current can be ascribed to the non-faradaic process of charging the electrochemical double layer. The obtained Cdl is divided by the specific capacitance (Cs) of the material studied, which is not usually determined as part of the same experiment but taken from literature sources.142 Unfortunately, poor practice of this method has led to misuse of the specific capacitance of the material. To determine the specific capacitance of a material, the Cdl of the material should be measured on an electrode with a well defined geometrical area in order to obtain a value of C cm−2. As this is not usually the case, researchers are commonly tempted to use a generic value of Cs which inevitably causes a major error in the calculation of the ECSA.
Mass activity may serve as a reasonable parameter for evaluating intrinsic performance of different electrode materials in bulk chemical processes, such as lithium ion battery reactions, where the Li+ diffuses deeply into the material and the original micro-structure cracks on charge/discharge cycles.154 However, for the surface chemistry processes such as electrocatalysis, where the reaction occurs only at/near the surface of the electrode, the mass activity does not represent the intrinsic activity.151 The definition of mass activity assumes that all atoms within each particle are electrocatalytic active sites, which is in conflict with the fact that the bulk atoms do not contribute to the electrocatalytic process. As a result, the mass activity is largely dependent on the particle size (or equivalently, surface area of catalyst), which reflects the fraction of surface atoms. Usually, catalysts of smaller particle size give higher mass activity, because smaller sized particles possess a larger ratio of surface atoms to the total atoms per mass and give a larger number of electrocatalytic active sites. As the particle size is decreased, most of the catalyst surface becomes active and the normalization by mass becomes more accurate. In addition, normalizing the catalytic activity per unit mass of catalyst could provide the end-users with a cost of operation. In this regard, the normalization can also be done per weight of the metal centre e.g., Cu, Ni or Zn.
Many claims have been made regarding the performance of MOF electrocatalysts for the CO2RR which have been subject to criticism, especially by more experienced electrochemists. It is argued that comparing the activity metric of one MOF catalyst to another without recourse to a method of normalization is not only misleading but can result in over-rating catalyst performance. A survey of MOF electrocatalysts for the CO2RR has been carried out in an effort to identify the method of current normalization and expose errors in comparison of the electrocatalytic activities of different MOFs (Tables 3–5). First, some authors failed to clearly present the method they used to normalize their current, as they compared the activity metric of their catalyst with other catalysts from different laboratories, for example,112,155,156 presenting the performance of their MOF electrocatalyst for CO2 reduction with no clear idea of the type of surface area used in normalizing the catalyst current. Misinformation of this sort has made it impossible to compare the performance of these catalysts objectively with other MOF electrocatalysts for CO2 reduction.
| Electrocatalyst | Synthetic method | Electrode potential | FE (%) | Peak jtotal (mA cm−2) | Current normalization | Main product | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|---|
| CR-MOF | Solution self-assembly | −0.78 | 98 | ∼7.1 | GSA | HCOOH | 0.5 M KHCO3 | 102 |
| HKUST-1 | Room temperature synthesis | −2.5 vs. Ag/Ag+ | 51 | 19 | N/A | Oxalic acid | 0.01 M TBATFB in DMF | 112 |
| Al2(OH)2TCPP-Co | Microwave | −0.7 V vs. RHE | 76 | ∼1 | N/A | CO | 0.5 M KHCO3 | 103 |
| Fe_MOF-525 | Electrophoretic deposition | −1.3 vs. NHE | 50 | 4 | GSA | CO | 1 M TBATF6 in DMF | 125 |
| Cu2(CuTCPP) | Vigorous stirring at temperature of 85 °C | −1.55 V vs. Ag/Ag+ | 68.4 | 1.0 | GSA | Acetate | CH3CN with 1 M H2O and 0.5 M EMIMBF4 | 100 |
| Cu2(CuTCPP) | Vigorous stirring at temperature of 85 °C | −1.40 V to −1.65 V | 38.8% to 85.2% | 3.5 | GSA | Formate | CH3CN with 1 M H2O and 0.5 M EMIMBF4 | 100 |
| HKUST-1 | Electrophoretic deposition | −2.2 vs. Ag/Ag+ | 80 | 3 | ECSA | CH4 | BMIMBF4 | 157 |
| HKUST-1 | Solvent-free synthesis by oven heating | −0.9 vs. Ag/Ag+ | 15.9 | 10 | GSA | CH3OH and C2H5OH | 0.5 M KHCO3 | 158 |
| ZIF-8 | Room temperature synthesis under vigorous stirring | −1.14 vs. SCE | 65 | ∼3 | N/A | CO | 0.5 M NaCl | 155 |
| Electrocatalyst | Synthetic method | Electrode potential | FE (%) | Peak jtotal (mA cm−2) | Current normalization | Main product | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ligand doped ZIF-8 | Room temperature synthesis | −1.2 V vs. RHE | 90 | 10.1 | N/A | CO | 0.1 M KHCO3 | 156 |
| CuII/ade-MOFs | Room temperature synthesis | −1.4 V vs. RHE | 45 | 8.5 | ECSA | C2H4 | 0.1 M KHCO3 | 159 |
| CuII/ade-MOFs | Room temperature synthesis | −1.4 V vs. RHE | 50 | 15 | ECSA | CH4 | 0.1 M KHCO3 | 159 |
| 2D c-MOF | Solvothermal synthesis | −0.7 V vs. RHE | 88 | 8 | N/A | CO | 0.1 M KHCO3 | 160 |
| Re-SURMOF/FTO | Liquid-phase epitaxy | −1.6 V vs. NHE | 93 ± 5 | >2 | N/A | CO | 0.1 M TBAH in CH3CN + 5% CF3CH2OH | 124 |
| M-PMOF | Hydrothermal | −0.8 V vs. RHE | 98.7 | 38.9 | GSA | CO | 0.5 M KHCO3 | 104 |
| FeTCPP-UiO-66 | Thermal treatment | 0.45 | 100 | 1.3 | ECSA | CO | 0.1 M KHCO3 | 161 |
| Cu2O@Cu-MOF | Hydrothermal | −1.71 | 63.2 | −14 | ECSA | CH4 | 0.1 M KHCO3 | 162 |
| Cu-SIM NU-1000 | Heat treatment | −0.82 | 28 | −1.2 | GSA | HCOO− | 0.1 M NaClO4 | 163 |
| Ag2O/layered ZIF composite | Refluxed at high temperature | −1.2 V vs. RHE | 80.5 | 26 | ECSA | CO | 0.25 M K2SO4 | 164 |
| TCPP(Co)/Zr-BTB | Thermal treatment | −0.769 vs. RHE | 85.1 | 6 | GSA | CO | 0.5 M KHCO3 | 165 |
| PCN-222(Fe) | Thermal treatment | 1.2 | 91 | −0.6 | GSA | CO | 0.5 M KHCO3 | 107 |
| HKUST-1 mediate Cu | Thermal treatment | −1.07 V vs. RHE | 45 | 262 | GSA | C2H4 | 1 M KOH | 166 |
| Electrocatalyst | Electrode potential | FE (%) | Peak jtotal (mA cm−2) | Current normalization | Main product | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| OD Cu/C-1000(Cu-based HKUST-1) | −0.1 V vs. RHE | 45.2–71.2 | ∼8.9 | GSA | CH3OH and C2H5OH | 0.1 M KHCO3 | 167 |
| ZIF/MWCNT | –0.86 V vs. RHE | 100 | 7.7 | ECSA | CO | 0.1 M NaHCO3 | 168 |
| ZIF-8 to Ni SAs/N-C | −1.0 V vs. RHE | 71.9 | 10.48 | GSA | CO | 0.5 M KHCO3 | 169 |
| Co/Zn ZIFs derived Co-N2 | −0.8 V vs. RHE | 94.7 | 18.1 | N/A | CO | 0.5 M KHCO3 | 170 |
| C-AFC©ZIF-8 | −0.43 V vs. RHE | 93 | 2.8 | N/A | CO | 0.1 M KHCO3 | 171 |
| Ni SAs/NCNTs | −1.0 V vs. RHE | 95 | 57.1 | GSA | CO | 0.5 M KHCO3 | 172 |
| ZIF-8 derived NC | −0.5 V vs. RHE | 95.4 | 1 | N/A | CO | 0.5 M KHCO3 | 173 |
| ZIF-8 derived NC | −0.93 vs. RHE | 78 | 1.1 | N/A | CO | 0.1 M KHCO3 | 174 |
| ZIF-8 derived Fe–N–C | −0.6 V vs. RHE | 91 | 17.8 | N/A | CO | 0.5 M KHCO3 | 175 |
| ZIF-8 derived Fe-N | −0.4 vs. RHE | 90 | 6.8 | GSA | CO | 0.5 M NaHCO3 | 176 |
In another scenario, some reports have wrongly compared the catalytic activity of MOF electrocatalysts normalized by geometrical area with other reports whose catalytic activity was normalized by ECSA, and vice versa.102,124,125,161,169,177–179 In addition to reports on MOFs catalyst normalized per geometrical area, information on the number of active sites per catalyst loading were not stated in most cases, making it difficult to make a comparative judgement of the activity of the catalysts. For instance, it has been claimed that polyoxometalate-based MOFs (PMOFs) show excellent catalytic activity towards the CO2RR, however, the catalyst was normalized by geometrical area and quantitative information on the active site loading per surface area was not provided as the entire catalyst cannot be assumed to be active toward CO2 reduction.104
5. Recent progress in utilizing MOFs for electrocatalysts for CO2 reduction
5.1. Pristine MOFs as electrocatalysts for CO2 reduction
The use of pristine MOFs in catalysing CO2 reduction was pioneered by Hinogami et al.,102 who reported the use of a Cu rubeanate framework (CR-MOF) containing coordinatively unsaturated metal sites as catalyst for the CO2RR to formic acid at 98% selectivity and 30% faradaic efficiency. Kumar et al.112 reported the electrochemical conversion of CO2 to oxalic acid (H2C2O4) at 90% selectivity, 51% faradaic efficiency on a Cu3(BTC)2 (1,3,5-benzenetricarboxylic acid [BTC]) thin film catalyst deposited on a glassy carbon electrode (Fig. 12). In Fig. 12(a), the cyclic voltammogram shows distinct reversible oxidation and reduction of Cu(II) to Cu(I) at 0.14 and 0.02 V vs. SCE, respectively. A reduction peak at 0.5 V vs. SCE and a sharp oxidation peak at 0.102 V vs. SCE indicate the presence of the Cu(0) to Cu(I) redox couple. Cu3(BTC)2 exhibited a cyclic voltammetric response indicative of copper being in the Cu2+ ionic state, whereas Cu electrodes in 0.1 M KCl solution did not exhibit such behaviour (Fig. 12(a) inset). Fig. 12(b) shows the redox activities of a GCE and Cu3(BTC)2 coated GCE in electrolyte without CO2 bubbling (1 and 3) and in CO2-saturated 0.01 M TBATFB/DMF (2 and 4), indicating that the direct reduction potential of CO2 starts at a potential more positive on a Cu3(BTC)2 coated GC electrode (−1.12 V vs. Ag/Ag+) than a bare GC electrode (−1.75 V vs. Ag/Ag+), and also showing that cathodic evolution current density is increased from 2.27 mA cm−2 to 19.22 mA cm−2. These two pioneering studies have proven the importance of nature and density of active site (metal centre) and the electronic environment (ligand) in deciding the product selectivity and efficiency. | ||
| Fig. 12 (a) CV readings of Cu3(BTC) + GCE (b) CV scan showing redox activities of (1) GCE, (2) GCE in presence of CO2, (3) Cu3(BTC)2 + GCE and (4) Cu3(BTC)2 + GCE in CO2-saturated 0.01 M TBATFB/DMF. Reproduced from Kumar et al.112 Copyright 2012, Elsevier. | ||
In situ growth of MOFs into a thin layer has been recently explored to control MOF thickness which can enhance mass and charge transfer, thereby promoting higher catalytic activity towards CO2 reduction. Kornienko et al.103 demonstrated this idea by synthesizing a thin-film of cobalt–porphyrin MOF (Al2(OH)2TCPP-Co) via atomic layer deposition (ALD) on a transparent conducting fluorine-doped tin oxide (FTO) support. An analysis of this MOF's voltammogram trace showed that it displayed an enhanced current density under CO2-saturated solutions relative to argon-saturated solutions and an irreversible catalyst peak, producing CO and H2, with selectivity for CO reaching up to 76% at −0.7 V vs. RHE (Fig. 13(a) and (c)). The stability of the MOF catalyst was tested over an extended period of 7 h at −0.7 V vs. RHE in CO2-saturated aqueous bicarbonate buffer, reaching a stable current density after several minutes, generating 16 mL of CO with estimated turnover number of 1400 (Fig. 13(d)). According to the author, the thin-layer configuration of Al2(OH)2TCPP-Co MOF was observed to enhance the reduction of CO2 to CO. In another finding by Wu et al.,100 layering of copper porphyrin MOF (Cu2(CuTCPP) into nanosheets was reported to enhance the catalytic conversion of CO2 into formate (FE = 68.4% at −1.55 V) and acetate (Fig. 14). Furthermore, spectroscopic studies showed that Cu2(CuTCPP) was converted into copper clusters (CuO, Cu2O and Cu4O3) which in turn catalysed the transformation of CO2 to formate and acetate.
 | ||
| Fig. 13 (a) CV scan of the Al2(OH)2TCPP-Co MOF catalyst showing a current increase in a CO2 environment relative to an argon-saturated environment. (b) Varying scan rate with corresponding redox potentials (c) plot of the faradaic efficiency at the potential range of −0.5 to 0.9 V vs. RHE; (d) MOF stability test with corresponding FE measurement. Reproduced from Kornienko et al.103 Copyright 2015, American Chemical Society. | ||
 | ||
| Fig. 14 (a) Crystal structure of Cu2(CuTCPP) MOF nanosheets; (b) schematic of electrochemical system showing electrocatalytic reduction of CO2 on Cu2(CuTCPP) nanosheets. Reproduced with permission from Wu et al.100 Copyright 2019, Royal Society of Chemistry. | ||
The choice of electrolyte has also been shown to enhance performance and product selectivity of catalytic MOFs toward the CO2RR. For instance, Kang et al.157 observed that ionic liquids (ILs) used as the electrolyte were able to influence the selective conversion of CO2 to CH4 using Zn-MOF catalyst. Imidazolium based ILs can interact with CO2 by physical absorption,180 thereby serving as both robust electrolytes and CO2 activation promoters.181 The cyclic voltammetry profile shows that the CO2 reduction activities of Zn-MOF in CO2-saturated saturated IL 1-butyl-3-methylimidazolium tetrafluoroborate (BMIMBF4) gives a current density of 3 mA cm−2 at reduction peak of about −2.2 V vs. Ag/Ag+ while the current density of the N2-saturated system was negligible (Fig. 15(a)). After 2 h of electrolysis, CH4 was the dominant product (FE = 80% at −2.2 V vs. Ag/Ag+), showing no decrease in current density with time, suggesting that the Zn-MOF electrode and the IL were stable (Fig. 15(b) and (c)).
 | ||
| Fig. 15 (a) CV scans in CO2-saturated and (b) stability test of different Zn-MOF/CP cathodes. (c) Amount of CH4 (ACH4, volume at standard temperature and pressure) generated in 2 h under different potentials. (d) Tafel plot for CH4 of the Zn-MOF/CP cathode using Zn-MOF synthesized at x = 0.38. Reproduced from Kang et al.157 Copyright 2016, Wiley. | ||
Downsizing the dimensionality of MOFs into 2D nanosheets destined for catalytic application can increase their surface area thereby exposing more metal active sites and improve electrical conductivity by decreasing the electron transfer distance from the ultrathin nanosheets to the current collector of the electrodes.182 The use of 2D MOFs as electrocatalysts for the CO2RR was demonstrated by Yang et al.159 where they attributed the significant catalytic activity and product affinity of their Cu-MOF nanosheets to the cathodized restructuring of the framework. Similarly, Zhang et al.165 showed that using 2D porphyrin-based MOF (TCPP(Co)/Zr-BTB) to catalyse the CO2RR resulted in the production of CO with satisfactory faradaic efficiency and selectivity.
5.2. MOF composites as electrocatalysts for CO2 reduction
Another strategy to obtain catalytically active MOFs is by incorporating catalytic/electrically conducting molecules which can be achieved either via in situ synthesis or post-synthetic modification.141 Since an in situ synthesis strategy is very difficult to achieve owing to kinetic and steric challenges, and the chemical effect that guest species may have on the reaction mixture, post synthetic modification has been more explored to incorporate molecules such as metal ions, metal nanoparticles or organic linker-based functional groups into the framework.183 Findings reveal that doping of MOF ligands could improve their electron-donating ability thus affecting their electrocatalytic activity towards the CO2RR.6 For example, Dou et al.156 found that doping ZIF-8 (zeolitic imidazolate framework) with 1,10-phenanthroline molecules enhanced the charge transfer ability of the MOF, thereby promoting the catalytic conversion of CO2 into CO (FECO = 90.47% and jCO = 10 mA cm−2 at the −1.1 V vs. RHE) compared to pristine ZIF-8 (FECO = 50%) (Fig. 16).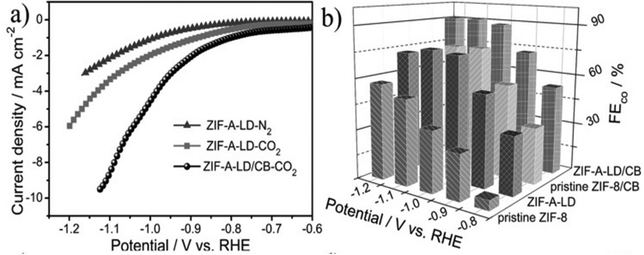 | ||
| Fig. 16 (a) LSV curves of ZIF-8 activated and 1,10-phenanthroline ligand doped product (ZIF-A-LD) in N2- and CO2-saturated 0.1 M KHCO3 solution in comparison with ZIF-A-LD/CB (b) FECO. Reproduced from Dou et al.156 Copyright 2019, Wiley. | ||
Functionalizing the metal component of MOFs (active sites) with catalytic metals/NPs can create more coordinatively unsaturated sites (CUSs) within MOFs thereby boosting catalytic activities for the CO2RR.141 Feng et al.160 demonstrated this strategy by using a MOF constructed from zinc-bis(dihydroxy) complex as node and copper–phthalocyanine as ligand to drive the CO2RR producing syngas. In situ XAS was able to confirm that ZnO4 being the active sites was responsible for the CO2RR while CuN4 act as supports to promote proton and electron transport. In this system, the authors claim that the CuN4 might be responsible for drawing electrons and H2O to produce protons which are reduced to molecular H2. On the other hand, the adsorbed CO2 on ZnO4 complexes is reduced to *COOH by coupling with protons/electrons from the CuN4 sites and electrode/electrolyte, releasing CO as the final product (Fig. 17). The possibility of having dual metallic sites in a catalyst is highly desirable, considering the overall effect it will have on electrocatalytic performance towards the CO2RR. However, this strategy has not been widely explored and therefore presents exciting opportunities for further research.
 | ||
| Fig. 17 (a) Zn K-edge Fourier transform EXAFS spectra of Zn foil, ZnO and PcCu-O8-Zn samples. (b) Cu K-edge Fourier transform EXAFS spectra of Cu foil, CuO and PcCu-O8-Zn samples (c) schematic HER and CO2RR reaction processes for PcCu-O8-Zn (d) faradaic efficiency of CO and H2 for PcCu-O8-Zn/CNT, PcCu-O8-Cu/CNT, PcZn-O8-Zn/CNT and PcZn-O8-Cu/CNT at −0.7 V vs. RHE. Reproduced from Feng et al.160 Copyright 2020, SpringerNature. | ||
It has been suggested that incorporating conductive materials (e.g. carbon black or graphene oxides) or catalytic species into MOFs via guest–host interaction is a plausible way to improve the performance in MOFs.141 Ye et al.124 adopted this strategy to enhance the catalytic performance of HKUST-1 MOF by grafting into its framework a catalytically active carboxylate-based complex-ReL(CO)3Cl (L = 2,2′-bipyridine-5,5′-dicarboxylic acid) (Fig. 18). The author reported that a thin film of this catalyst on FTO achieved selectivity towards CO formation of up 93 ± 5%, in comparison to just Re-linkers as catalysts. According to the authors, the higher % FECO of this Re-SURMOF was attributed to its highly oriented structure on the electrode surface.
 | ||
| Fig. 18 (a) Schematic description of the preparation of a Re-SURMOF on fluorine-doped titanium oxide (FTO) electrode (b) CV scan and (c) FE of the Re-SURMOF and Re-linker at different applied potentials. Reproduced from Ye et al.124 Copyright 2016, Royal Society of Chemistry. | ||
Wang et al.104 proposed the implementation of a polyoxometalate MOF (PMOF) catalyst prepared from reduced polyoxometalates (POMs) and metalloporphyrin (M-TCPP). Among the metal polyoxometalate MOFs tested, Co-PMOF exhibited superior performance (99% FECO at 38.9 mA cm−2) over metal polyoxometalate MOFs. The performance of this Co-PMOF was attributed to synergistic cooperation between Co-porphyrin and POMs.
It is important to note again the lack of standards in the normalization process of electrochemical activities as described in Section 1.4. While comparisons in catalytic activity are made across different experiments and materials, those comparisons, on occasion “benchmarking” using geometrical areas, surface areas measured from BET curves or electrochemical surface areas from capacitive curves, are often not only unreliable but also misleading.
5.3. MOF-derived materials as electrocatalysts for CO2 reduction
Despite the popularity of research on the use of pristine MOFs as electrocatalysts for the CO2RR, lack of electrical conductivity and structural stability are still major issues for most MOFs,8 and have cast doubt in the minds of many among the electrochemical community on the use of MOFs as electrocatalysts. While significant amount of reports on MOFs electrocatalysts claim to exhibit long term stability, few, if any, of these works, have performed an appropriate post-reaction analysis of the catalyst to justify these claims.184 A few reports have suggested that upon exposure of the MOF or MOF-composite to electrochemical conditions, the integrity of the MOF may be compromised resulting in the “segregation” of the metal centre and the formation of active metal clusters supported on organic matrix.58 With this in mind, some groups have proposed the use of MOFs as sacrificial “templates” to generate more stable carbon-supported catalysts for efficient CO2 reduction. MOFs, upon calcination/pyrolysis at various temperatures, can generate carbon-derived metal catalysts with inherited porosity and surface area. These MOF-derived materials are claimed to possess improved physicochemical and electrocatalytic properties including large surface area, better electronic conductivity (in comparison to MOFs), evenly dispersed single-atom active sites, clusters or NPs.185 One example of this strategy is given by Zhao et al.167 who pyrolysed HKUST-1 to produce an oxide-derived Cu/carbon (OD Cu/C) catalyst. During the CO2RR, the OD Cu/C showed formation of alcohols at potentials as low as −0.1 V vs. RHE. The improvement in selectivity and activity of this catalyst was linked to the mutual interaction between the matrix of porous carbon and the highly dispersed copper. Nitrogen-doped carbon materials (N-Cs) obtained from pyrolysis of MOFs have been described as electrocatalyst materials with good electrical conductivity and sufficient mesoporous surface for effective transport.183 The work of Guo et al.168 demonstrated the effectiveness of using hybrid material obtained from pyrolysis of zeolitic imidazolate frameworks (ZIFs) and multi-walled carbon nanotubes (MWCNTs) to catalyse the CO2RR, producing CO at almost 100% efficiency, overpotential of 740 mV and a current density of 7.7 mA cm−2 (Fig. 19). Upon adding Fe into the composite, higher FECO of 97% was observed at low overpotentials. The excellent CO2RR activities of this material were attributed to synergistic cooperation between the inherent active sites in the hybrid material and the carbon nanotube (CNT) support which promotes electron and mass transport. | ||
| Fig. 19 (a) Schematic showing the pyrolysis of ZIFs on multi-walled carbon nanotubes (MWCNT) which aid interparticle conductivity and enhance the mass transport in CO2RR. (b) FE(CO) and (d) partial CO current for different catalysts at different applied potentials. Reproduced with permission from Guo et al.168 Copyright 2017, Royal Society of Chemistry. | ||
Promising electrocatalytic performance exhibited by MOF-derived catalysts has frequently been ascribed to “single atom catalysts” (SACs),186 through direct evidence that the active sites are in fact single atoms is generally lacking. Zhao et al.169 reported Ni-single atoms dispersed in nitrogen-doped porous carbon (Ni-SAs/N–C) prepared from ZIF-8 as precursor. Ni-SAs/N–C successfully catalysed CO2 to CO at 71.9% faradaic efficiency, current density of 10.48 mA cm−2, overpotential of 0.89 V and high TOF of 5273 h−1, performing better than Ni foam and Ni NPs (Fig. 20). The clearly improved CO2 reduction performance of Ni SAs/N–C may be attributed to the increased number of surface-active sites, lower adsorption energy of CO over active sites, and improved electronic conductivity.187 In a similar report, Wang et al.170 used ZIF-8 as sacrificial template to obtained a cobalt metal catalyst anchored on nitrogen-doped carbon (Co-SAs/Nx–C) which performed excellently catalysing CO2 to CO, achieving FE of 94%, high current density of 18.1 mA cm−2 and TOF of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 h−1. In comparison to the work of Zhao et al., it was reported that regulating the coordination number of the dispersed metal species obtained from ZIF-8 can enhance its CO2RR activity. Some relevant works171–176 have also demonstrated the use of the pyrolysis approach to obtain metal–N–C catalyst materials which have shown promising performance towards CO2 reduction in terms of selectivity and activity.
200 h−1. In comparison to the work of Zhao et al., it was reported that regulating the coordination number of the dispersed metal species obtained from ZIF-8 can enhance its CO2RR activity. Some relevant works171–176 have also demonstrated the use of the pyrolysis approach to obtain metal–N–C catalyst materials which have shown promising performance towards CO2 reduction in terms of selectivity and activity.
 | ||
| Fig. 20 (a) LSV curves in the N2-saturated (dotted line) or CO2-saturated (solid line) 0.5 M KHCO3 electrolyte at a scan rate of 10 mV s−1. (b) FEs of CO and (c) partial CO current density plots and TOFs of Ni SAs/N–C and Ni NPs/N–C at different applied potentials. (d) Stability of Ni SAs/N–C at a potential of −1.0 V vs. RHE for Ni SAs/N–C during 60 h. Reproduced from Zhao et al.169 Copyright 2017, American Chemistry Society. | ||
6. Summary and future outlook
Achieving sustainable energy and a clean environment is the driving force behind a rapid surge of interest in the electrocatalytic potential of MOFs for CO2 conversion fuelled by their excellent properties. In the last decade, the drive to obtain a highly competitive, viable and cost-effective alternative catalyst for the CO2RR has created unprecedented efforts to harness electrocatalytic potential of MOFs. Despite this development, there are still some challenges preventing the industrial application of MOFs as electrocatalysts for CO2 reduction.Fig. 21 provides a comparative analysis of the CO2RR performance across pristine MOFs, MOF composites, and MOF-derived materials, highlighting key parameters such as faradaic efficiency, overpotential, current density, stability and conductivity. This visualization underscores the fundamental trade-offs between these material classes, demonstrating how MOF modifications and derivatization significantly enhance catalytic activity and durability. While pristine MOFs benefit from high surface areas and tunable active sites, their poor conductivity and limited stability hinder their practical application. MOF composites offer improved charge transport and catalytic synergy, while MOF-derived materials, particularly carbonized or metal-based derivatives, exhibit the highest conductivity and long-term stability. This comparison reinforces the need for continued material innovation, particularly in optimizing conductivity and structural integrity, to unlock the full potential of MOF-based catalysts for CO2 electroreduction.
 | ||
| Fig. 21 Summary of the overall performance metrics of the three types of MOF discussed in the review. | ||
One key issue underlying the poor performance of MOFs compared to conventional inorganic catalysts is electrical conductivity. Therefore, building electrical conductivity into MOFs can push their limit, in terms of electrocatalytic performance, beyond the current catalysts. The general requirement for constructing MOFs with high conductivity is to select building blocks with loosely bound charge carriers that are capable of creating conducting pathways for charge transport via redox hopping and/or band transport. Carefully selecting building components which include mixed-valence metal ions, ligands with conjugated double bonds or nitrogen- or sulfur-containing ligands, can lead to the fabrication of MOFs with higher intrinsic conductivity. Taking advantage of the porosity, a guest-based conductive MOF can be built by way of incorporating conducting materials such as metallic species, redox-active molecules, or conductive polymers into the MOF framework.
Another major issue hindering the electrocatalytic performance of MOFs is stability, as many MOFs collapse into metal oxides/clusters during electrochemical reaction.58 Because the metal–carbon bond in most MOFs is rather weak, they easily become degraded, losing their structural integrity, thereby limiting their operability in electrocatalysis. A few measures have been suggested to address MOF stability problems, such as de novo synthesis of MOFs by linking carboxylate-based ligands (hard bases) with high-valent metal ions (hard acids) or linking azolate ligands (soft bases) with low-valent metal ions (soft acids). The introduction of hydrophobic/rigid ligands, and phosphonate/carboxyphosphonate ligands into MOF synthesis have also been suggested as a way to improve MOF stability.
The stability of MOFs can also be affected by the strength of their attachment to the electrode surface. Finding an effective method for preparing MOF-modified electrodes could play an important role in creating a strong attachment of the MOF catalyst to the electrode and by extension enhance the MOF electrochemical response. Drop casting is one facile method for immobilizing MOFs on conductive electrodes for electrochemical studies, however, the ease of delamination of the catalyst from the substrate during electrochemical studies is a major drawback. Additionally, controlling the resulting MOF film's thickness and morphology is challenging. As a result, it is highly desirable to use alternative preparation methods such as direct solvothermal synthesis, electrochemical synthesis etc., which result in strong chemical bonds between the MOF and the substrate.
Having strong MOF attachment to the electrode surface is not sufficient to assure long-term stability as the supporting electrolyte and current flow can also affect the MOF structure. Considerable attention should be paid to the stability of the MOF in aqueous electrolytes and in the presence of bicarbonate ions, which is the preferred medium to carry out CO2 electrolysis. Therefore, the development of stable MOFs which will be tolerant to electrochemical environments is vital for future CO2RR electrocatalysis applications.
To complement the stability of MOFs, studies of catalytic performance should be accompanied by extensive material characterization, either during or after reaction, to ensure the MOF structure is maintained under reaction conditions. The method of catalyst normalization is crucial in rating and benchmarking the performance of catalysts, as comparing the activities without due consideration of the method of normalization is not only misleading but can lead to overrating or underrating the catalyst performance. While some authors failed to clearly present the method used in normalizing their current, others erroneously compared the activity metric of MOF electrocatalysts normalized geometrically to those normalized electrochemically. It is therefore necessary to be cautious in comparing the performance of catalysts without taking into account the method of catalyst normalization.
In addition, to confirm the stability of the materials, further post mortem analysis should be performed. Post-reaction analysis techniques play a crucial role in understanding the active sites and reaction mechanisms of electrocatalysts, particularly for CO2RR. For instance, in situ X-ray absorption spectroscopy (XAS) has been widely used to monitor the oxidation state and coordination environment of metal centres during CO2RR, providing insights into the dynamic transformations of catalysts, such as the reduction of Cu oxides into metallic Cu species, which directly influences product selectivity.188 High-resolution transmission electron microscopy (HRTEM) has been instrumental in visualizing structural changes at the atomic level, as seen in the formation of defect-rich structures in carbon-supported single-atom catalysts, which enhance CO2 adsorption and conversion efficiency.188,189 Similarly, operando Raman spectroscopy has been used to track reaction intermediates in metal–nitrogen–carbon (M–N–C) catalysts, revealing the role of nitrogen coordination in stabilizing CO2RR intermediates.190 These techniques have been successfully applied to transition metal oxides, single-atom catalysts and bimetallic systems, but remain underutilized in MOF-based electrocatalysis. Implementing these methods in MOF-based CO2RR research would provide real-time insights into MOF stability, electronic structure evolution, and potential catalyst degradation, paving the way for the rational design of highly selective and stable MOF-based CO2RR electrocatalysts.
An overview of research on MOFs as electrocatalysts reveals that they have been deployed in pristine form as catalysts, as porous supports for the incorporation of catalytically active material or as precursors for dispersed metal catalysts, showing that they can reduce CO2 into CO, formic acid and even hydrocarbons and alcohols. Several strategies have been attempted to improve the catalytic performance of MOFs for the CO2RR.
In situ MOF growth into a thin layer has been reported to control MOF thickness which can enhance mass and charge transfer. Downsizing the dimensionality of MOFs into 2D nanosheets can increase the exposure of metal active sites and improve electrical conductivity by decreasing the electron transfer distance from the ultrathin nanosheets to the current collector of the electrodes. Compositing MOFs with conductive materials (such as carbon black or graphene oxides) or catalytic species via guest–host interaction has been demonstrated as a plausible means of inducing catalytic activity in MOFs.
Research involving the use of different MOF morphologies such as ultrathin 2D nanosheets, nanocages and nanowires for CO2 reduction remains a tiny subset of the total and therefore channelling effort in this direction could yield results in producing efficient electrocatalysts that can promote mass transport and electronic transfer during electrocatalysis.
For the development of optimal catalysts, future studies should explore the tunability of MOFs to understand the role of the active metal centres, pore size or substituents on the linkers. Furthermore, theoretical studies can help understand the effect of metal centre and linker on the strength of interaction between the catalysts and reaction intermediates. This information is essential to elucidate and understand the reaction mechanisms, identify the rate-limiting step and to predict optimal active sites.
Additionally, using MOFs with multi-metal sites present an opportunity for further tuning their catalytic properties. Although, MOF-derived dispersed metal catalysts have recently emerged as a new frontier in catalysis science and have attracted extensive research attention due to their excellent activities toward CO2 reduction, there remains a need to devise more efficient routes for the synthesis of catalysts having flexible structures and active sites, and for much better characterization of the latter.
Author contributions
All authors have contributed substantially and given approval to the final version of the manuscript.Data availability
We would like to confirm that no primary research results, software or code have been included in this work, and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no competing financial interests or personal relationships that could have influenced, or appeared to influence, the contents of this paper.Acknowledgements
The authors acknowledge the Petroleum Technology Development fund (PTDF), Nigeria for funding this research work by awarding a scholarship to Oriyomi Ogunbanjo (PTDF/ED/PHD/OOO/1553/19).References
- K. Obileke, H. Onyeaka, O. Omoregbe, G. Makaka, N. Nwokolo and P. Mukumba, Bioenergy from bio-waste: a bibliometric analysis of the trend in scientific research from 1998–2018, Biomass Convers. Biorefin., 2020, 12, 1077–1092 Search PubMed.
- A. K. Singh, L. Gu, A. Dutta Chowdhury and A. Indra, Exploring Ligand-Controlled C2 Product Selectivity in Carbon Dioxide Reduction with Copper Metal–Organic Framework Nanosheets, Inorg. Chem., 2023, 62, 8803–8811 Search PubMed.
- C. J. Liu, U. Burghaus, F. Besenbacher and Z. L. Wang, Preparation and Characterization of Nanomaterials for Sustainable Energy Production, ACS Nano, 2010, 4, 5517–5526 Search PubMed.
- O. Omoregbe, A. N. Mustapha, R. Steinberger-Wilckens, A. El-Kharouf and H. Onyeaka, Carbon capture technologies for climate change mitigation: A bibliometric analysis of the scientific discourse during 1998–2018, Energy Rep., 2020, 6, 1200–1212 Search PubMed.
- M. Scibioh and B. Viswanathan, Carbon dioxide to Chemical and fuel, in CO2 Conversion—Relevance and Importance, ed. K. I. Marinakis, and M. W. Fishe, John Fedor, Oxford, 2018, vol. 1, pp. 1–22 Search PubMed.
- A. A. Al-Omari, Z. H. Yamani and H. L. Nguyen, Electrocatalytic CO2 Reduction: From Homogeneous Catalysts to Heterogeneous-Based Reticular Chemistry, Molecules, 2018, 23, 2835 Search PubMed.
- P. Shao, L. Yi, S. Chen, T. Zhou and J. Zhang, Metal–organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers, J. Energy Chem., 2020, 40, 156–170 Search PubMed.
- D. Narváez-Celada and A. S. Varela, CO2 electrochemical reduction on metal–organic framework catalysts: current status and future directions, J. Mater. Chem. A, 2022, 10, 5899–5917 Search PubMed.
- R. Kortlever, J. Shen, K. J. Schouten, F. Calle-Vallejo and M. T. Koper, Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide, J. Phys. Chem. Lett., 2015, 6, 4073–4082 Search PubMed.
- J. T. Feaster, C. Shi, E. R. Cave, T. Hatsukade, D. N. Abram, K. P. Kuhl, C. Hahn, J. K. Nørskov and T. F. Jaramillo, Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes, ACS Catal., 2017, 7, 4822–4827 Search PubMed.
- Z. Chen, X. Duan, W. Wei, S. Wang and B. J. Ni, Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution, J. Mater. Chem. A, 2019, 7, 14971–15005 Search PubMed.
- Q. Shi, C. Zhu, D. Du and Y. Lin, Robust noble metal-based electrocatalysts for oxygen evolution reaction, Chem. Soc. Rev., 2019, 48, 3181–3192 Search PubMed.
- M. Wang, S. Wang, H. Yang, W. Ku, S. Yang, Z. Liu and G. Lu, Carbon-Based Electrocatalysts Derived From Biomass for Oxygen Reduction Reaction: A Mini review, Front. Chem., 2020, 8, 116 Search PubMed.
- J. Liu, D. Zhu, C. Guo, A. Vasileff and S. Z. Qiao, Design Strategies toward Advanced MOF-Derived Electrocatalysts for Energy-Conversion Reactions, Adv. Energy Mater., 2017, 7, 1700518 Search PubMed.
- U. Kayal, B. Mohanty, P. Bhanja, S. Chatterjee, D. Chandra, M. Hara, B. Kumar Jena and A. Bhaumik, Ag nanoparticle-decorated, ordered mesoporous silica as an efficient electrocatalyst for alkaline water oxidation reaction, Dalton Trans., 2019, 48, 2220–2227 Search PubMed.
- H. Zhang, X. Liu, Y. Wu, C. Guan, A. K. Cheetham and J. Wang, MOF-derived nanohybrids for electrocatalysis and energy storage: current status and perspectives, Chem. Commun., 2018, 54, 5268–5288 Search PubMed.
- K. Naka, Metal Organic Framework (MOF), in Encyclopedia of Polymeric Nanomaterials, ed. S. Kobayashi and K. Müllen, Springer, Berlin, Heidelberg, 2015, vol. 148, pp. 1233–1238 Search PubMed.
- M. S. Alhumaimess, MOF-derived nanohybrids for electrocatalysis and energy storage: current status and perspectives, J. Saudi Chem. Soc., 2020, 24, 461–473 Search PubMed.
- V. F. Samanidou and E. A. Deliyanni, Metal Organic Frameworks: Synthesis and Application, Molecules, 2020, 25, 960 Search PubMed.
- O. M. Yaghi, G. Li and H. Li, Selective binding and removal of guests in a microporous metal–organic framework, Nature, 1995, 378, 703–706 Search PubMed.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal–organic framework, Nature, 1999, 402, 276–279 Search PubMed.
- J. Li, L. Wang, Y. Liu, Y. Song, P. Zeng and Y. Zhang, The research trends of metal–organic frameworks in environmental science: a review based on bibliometric analysis, Environ. Sci. Pollut. Res., 2020, 27, 19265–19284 Search PubMed.
- Y. Yang, Y. Yang, Y. Liu, S. Zhao and Z. Tang, Metal–Organic Frameworks for Electrocatalysis: Beyond Their Derivatives, Small Sci., 2021, 1, 2100015 Search PubMed.
- Z. Di, Y. Qi, X. Yu and F. Hu, The Progress of Metal–Organic Framework for Boosting CO2 Conversion, Catalysts, 2022, 12, 1582 Search PubMed.
- C. Li, H. Zhang, M. Liu, F. F. Lang, J. Pang and X. H. Bu, Recent progress in metal–organic frameworks (MOFs) for electrocatalysis, Ind. Chem. Mater., 2023, 1, 9–38 Search PubMed.
- J. Li, H. Luo, B. Li, J. G. Ma and P. Cheng, Application of MOF-derived materials as electrocatalysts for CO2 conversion, Mater. Chem. Front, 2023, 7, 6107–6129 Search PubMed.
- B. Y. Guan, X. Y. Yu, H. B. Wu and X. W. Lou, Complex Nanostructures from Materials based on Metal–Organic Frameworks for Electrochemical Energy Storage and Conversion, Adv. Mater., 2017, 29, 1703614 Search PubMed.
- D. Li, H. Q. Xu, L. Jiao and H. L. Jiang, Metal–organic frameworks for catalysis: State of the art, challenges, and opportunities, Energy Chem., 2019, 1, 100005 Search PubMed.
- R. Li, F. Liu, Y. Zhang, M. Guo and D. Liu, Nitrogen, Sulfur Co-Doped Hierarchically Porous Carbon as a Metal-Free Electrocatalyst for Oxygen Reduction and Carbon Dioxide Reduction Reaction, ACS Appl. Mater. Interfaces, 2020, 12, 44578–44587 Search PubMed.
- H. Yang, Y. Wu, Q. Lin, L. Fan, X. Chai, Q. Zhang, J. Liu, C. He and Z. Lin, Composition Tailoring via N and S Co-doping and Structure Tuning by Constructing Hierarchical Pores: Metal-Free Catalysts for High-Performance Electrochemical Reduction of CO2, Angew. Chem., Int. Ed., 2018, 57, 15476–15480 Search PubMed.
- S. Bhattacharyya, C. Das and T. K. Maji, MOF derived carbon based nanocomposite materials as efficient electrocatalysts for oxygen reduction and oxygen and hydrogen evolution reactions, RSC Adv., 2018, 8, 26728–26754 Search PubMed.
- M. X. Yang, L. J. Chen, Y. Z. Ye, X. Y. Lin and S. Lin, Four 3D Co(II) MOFs based on 2,4,6-tris(4-pyridyl)-1,3,5-triazine and polycarboxylic acid ligands and their derivatives as efficient electrocatalysts for oxygen reduction reaction, Dalton Trans., 2021, 50, 4904–4913 Search PubMed.
- Y. Li, M. Cui, Z. Yin, S. Chen and T. Ma, Metal–organic framework based bifunctional oxygen electrocatalysts for rechargeable zinc–air batteries: current progress and prospects, Chem. Sci., 2020, 11, 11646–11671 Search PubMed.
- Y. Zhu, K. Yue, C. Xia, S. Zaman, H. Yang, X. Wang, Y. Yan and B. Y. Xia, Recent Advances on MOF Derivatives for Non-Noble Metal Oxygen Electrocatalysts in Zinc–Air Batteries, Nano-Micro Lett., 2021, 13, 137 Search PubMed.
- G. Dey Shadab and A. Aijaz, Metal–Organic Framework Derived Nanostructured Bifunctional Electrocatalysts for Water Splitting, ChemElectroChem, 2021, 8, 3782–3803 Search PubMed.
- X. Huang, P. Sheng, Z. Tu, F. Zhang, J. Wang, H. Geng, Y. Zou, C. A. Di, Y. Yi, Y. Sun, W. Xu and D. Zhu, A two-dimensional π–d conjugated coordination polymer with extremely high electrical conductivity and ambipolar transport behaviour, Nat. Commun., 2015, 6, 7408 Search PubMed.
- L. Sun, M. G. Campbell and M. Dincă, Electrically Conductive Porous Metal–Organic Frameworks, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 Search PubMed.
- E. M. Johnson, S. Ilic and A. J. Morris, Design Strategies for Enhanced Conductivity in Metal–Organic Frameworks, ACS Cent. Sci., 2021, 7, 445–453 Search PubMed.
- J. H. Li, Y. S. Wang, Y. C. Chen and C. W. Kung, Metal–Organic Frameworks Toward Electrocatalytic Applications, Appl. Sci., 2019, 9, 2427 Search PubMed.
- B. A. Johnson, A. M. Beiler, B. D. McCarthy and S. Ott, Transport Phenomena: Challenges and Opportunities for Molecular Catalysis in Metal–Organic Frameworks, J. Am. Chem. Soc., 2020, 142, 11941–11956 Search PubMed.
- T. Yue, C. Xia, X. Liu, Z. Wang, K. Qi and B. Y. Xia, Design and Synthesis of Conductive Metal–Organic Frameworks and Their Composites for Supercapacitors, ChemElectroChem, 2021, 8, 1021–1034 Search PubMed.
- P. Li and B. Wang, Recent Development and Application of Conductive MOFs, Isr. J. Chem., 2018, 58, 1010–1018 Search PubMed.
- Y. Kobayashi, B. Jacobs, M. D. Allendorf and J. R. Long, Conductivity, Doping, and Redox Chemistry of a Microporous Dithiolene-Based Metal−Organic Framework, Chem. Mater., 2010, 22, 4120–4122 Search PubMed.
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Stavila, P. Haney, R. A. Kinney, V. Szalai, F. El Gabaly, H. P. Yoon, F. Léonard and M. D. Allendorf, Tunable Electrical Conductivity in Metal–Organic Framework Thin-Film Devices, Sciences, 2014, 343, 66–69 Search PubMed.
- C. W. Kung, K. Otake, C. T. Buru, S. Goswami, Y. Cui, J. T. Hupp, A. M. Spokoyny and O. K. Farha, Increased Electrical Conductivity in a Mesoporous Metal–Organic Framework Featuring Metallacarboranes Guests, J. Am. Chem. Soc., 2018, 140, 3871–3875 Search PubMed.
- Z. Xin, Y. R. Wang, Y. Chen, W. L. Li, L. Z. Dong and Y. Q. Lan, Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction, Nano Energy, 2020, 67, 104233 Search PubMed.
- J. J. Calvo, S. M. Angel and M. C. So, Charge transport in metal–organic frameworks for electronics applications, APL Mater., 2020, 8, 050901 Search PubMed.
- S. Lin, P. M. Usov and A. J. Morris, The role of redox hopping in metal–organic framework electrocatalysis, Chem. Commun., 2018, 54, 6965–6974 Search PubMed.
- L. Sun, T. Miyakai, S. Seki and M. Dincă, Mn2(2,5-disulfhydrylbenzene-1,4-dicarboxylate): A Microporous Metal–Organic Framework with Infinite (–Mn–S–)∞ Chains and High Intrinsic Charge Mobility, J. Am. Chem. Soc., 2013, 135, 8185–8188 Search PubMed.
- S. Lin, P. M. Usov and A. J. Morris, The role of redox hopping in metal–organic framework electrocatalysis, Chem. Commun., 2018, 54, 6965–6974 Search PubMed.
- T. C. Narayan, T. Miyakai, S. Seki and M. Dincă, High Charge Mobility in a Tetrathiafulvalene-Based Microporous Metal–Organic Framework, J. Am. Chem. Soc., 2012, 134, 12932–12935 Search PubMed.
- S. S. Park, E. R. Hontz, L. Sun, C. H. Hendon, A. Walsh, T. Van Voorhis and M. Dincă, Cation-Dependent Intrinsic Electrical Conductivity in Isostructural Tetrathiafulvalene-Based Microporous Metal–Organic Frameworks, J. Am. Chem. Soc., 2015, 137, 1774–1777 Search PubMed.
- L. Sun, S. S. Park, D. Sheberla and M. Dincă, Measuring and Reporting Electrical Conductivity in Metal–Organic Frameworks: Cd2(TTFTB) as a Case Study, J. Am. Chem. Soc., 2016, 138, 14772–14782 Search PubMed.
- D. Sheberla, L. Sun, M. A. Blood-Forsythe, S. Er, C. R. Wade, C. K. Brozek, A. Aspuru-Guzik and M. Dincă, High Electrical Conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a Semiconducting Metal–Organic Graphene Analogue, J. Am. Chem. Soc., 2014, 136, 8859–8862 Search PubMed.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincă, Cu3(hexaiminotriphenylene)2: An Electrically Conductive 2D Metal–Organic Framework for Chemiresistive Sensing, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 Search PubMed.
- A. J. Clough, J. M. Skelton, C. A. Downes, A. A. de la Rosa, J. W. Yoo, A. Walsh, B. C. Melot and S. C. Marinescu, Metallic Conductivity in a Two-Dimensional Cobalt Dithiolene Metal–Organic Framework, J. Am. Chem. Soc., 2017, 139, 10863–10867 Search PubMed.
- C. F. Leong, P. M. Usov and D. M. D’Alessandro, ntrinsically conducting metal–organic frameworks, MRS Bull., 2016, 41, 858–864 Search PubMed.
- W. Zheng, M. Liu and L. Y. S. Lee, Electrochemical Instability of Metal–Organic Frameworks: In Situ Spectroelectrochemical Investigation of the Real Active Sites, ACS Catal., 2020, 10, 81–92 Search PubMed.
- D. Bohra, J. H. Chaudhry, T. Burdyny, E. A. Pidko and W. A. Smith, Modeling the Electrical Double Layer to Understand the Reaction Environment in a CO2 Electrocatalytic System, Energy Environ. Sci., 2019, 12, 3380 Search PubMed.
- P. M. Usov, C. McDonnell Worth, F. Zhou, D. R. MacFarlane and D. M. D’Alessandro, The Electrochemical Transformation of the Zeolitic Imidazolate Framework ZIF-67 in Aqueous Electrolytes, Electrochim. Acta, 2015, 153, 433–438 Search PubMed.
- D. O. Miles, D. Jiang, A. D. Burrows, J. E. Halls and F. Marken, Conformal transformation of [Co(bdc)(DMF)] (Co-MOF-71, bdc = 1,4-benzenedicarboxylate, Electrochem. Commun., 2013, 27, 9–13 Search PubMed.
- W. Zheng and L. Y. S. Lee, Metal–Organic Frameworks for Electrocatalysis: Catalyst or Precatalyst?, ACS Energy Lett., 2021, 6, 2838–2843 Search PubMed.
- M. Ding, X. Cai and H. L. Jiang, Improving MOF Stability: Approaches and Applications, Chem. Sci., 2019, 10, 10209 Search PubMed.
- B. Cui, C. Wang, S. Huang, L. He, S. Zhang, Z. Zhang and M. Du, Efficient multifunctional electrocatalyst based on 2D semiconductive bimetallic metal–organic framework toward non-Pt methanol oxidation and overall water splitting, J. Colloid Interface Sci., 2020, 578, 10–23 Search PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Water Stability and Adsorption in Metal–Organic Frameworks, Chem. Rev., 2014, 114, 10575–10612 Search PubMed.
- X. Zhao, C. Y. Zhu, J. S. Qin, H. Rao, D. Y. Du, M. Zhang, P. She, L. Li and Z.-M. Su, Local protons enhance photocatalytic CO2 reduction by porphyrinic zirconium-organic frameworks, Mater. Chem. Front., 2024, 8, 2439–2446 Search PubMed.
- D. Bůžek, S. Adamec, K. Lang and J. Demel, Metal–organic frameworks vs. buffers: case study of UiO-66 stability, Inorg. Chem. Front., 2021, 8, 720–734 Search PubMed.
- W. Wang, H. Yan, U. Anand and U. Mirsaidov, Visualizing the Conversion of Metal–Organic Framework Nanoparticles into Hollow Layered Double Hydroxide Nanocages, J. Am. Chem. Soc., 2021, 143, 1854–1862 Search PubMed.
- B. Han, K. A. Stoerzinger, V. Tileli, A. D. Gamalski, E. A. Stach and Y. Shao-Horn, Nanoscale Structural Oscillations in Perovskite Oxides Induced by Oxygen Evolution, Nat. Mater., 2017, 16, 121 Search PubMed.
- X. Zhao, H. Ren and L. Luo, Gas Bubbles in Electrochemical Gas Evolution Reactions, Langmuir, 2019, 35, 5392 Search PubMed.
- S. Dou, X. Li and X. Wang, Rational Design of Metal–Organic Frameworks towards Efficient Electrocatalysis, ACS Mater. Lett., 2020, 2, 1251 Search PubMed.
- H. Kim, D. Shin, W. Yang, D. H. Won, H.-S. Oh, M. W. Chung, D. Jeong, S. H. Kim, K. H. Chae, J. Y. Ryu, J. Lee, S. J. Cho, J. Seo, H. Kim and C. H. Choi, Identification of Single-Atom Ni Site Active toward Electrochemical CO2 Conversion to CO, J. Am. Chem. Soc., 2021, 143, 925–933 Search PubMed.
- A. Moysiadou, S. Lee, C.-S. Hsu, H. M. Chen and X. Hu, Mechanism of Oxygen Evolution Catalyzed by Cobalt Oxyhydroxide: Cobalt Superoxide Species as a Key Intermediate and Dioxygen Release as a Rate-Determining Step, J. Am. Chem. Soc., 2020, 142, 11901–11914 Search PubMed.
- S. Yuan, J. S. Qin, C. T. Lollar and H. C. Zhou, Stable Metal–Organic Frameworks with Group 4 Metals: Current Status and Trends, ACS Cent. Sci., 2018, 4, 440–450 Search PubMed.
- W. Lu, Z. Wei, Z. Y. Gu, T. F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle, M. Bosch and H. C. Zhou, uning the structure and function of metal–organic frameworks via linker design, Chem. Soc. Rev., 2014, 43, 5561–5593 Search PubMed.
- G. Ferey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surble and I. Margiolaki, A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area, Science, 2005, 309, 2040–2042 Search PubMed.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 Search PubMed.
- D. Feng, Z. Y. Gu, Y. P. Chen, J. Park, Z. Wei, Y. Sun, M. Bosch, S. Yuan and H. C. Zhou, A Highly Stable Porphyrinic Zirconium Metal–Organic Framework with shp-a Topology, J. Am. Chem. Soc., 2014, 136, 17714–17717 Search PubMed.
- D. Feng, W. C. Chung, Z. Wei, Z. Y. Gu, H. L. Jiang, Y. P. Chen, D. J. Darensbourg and H. C. Zhou, Construction of Ultrastable Porphyrin Zr Metal–Organic Frameworks through Linker Elimination, J. Am. Chem. Soc., 2013, 135, 17105–17110 Search PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 Search PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O’Keeffe and O. M. Yaghi, High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture, Science, 2008, 319, 939–943 Search PubMed.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, High thermal and chemical stability in pyrazolate-bridged metal–organic frameworks with exposed metal sites, Chem. Sci., 2011, 2, 1311–1319 Search PubMed.
- B. D. McCarthy, A. M. Beiler, B. A. Johnson, T. Liseev, A. T. Castner and S. Ott, Analysis of Electrocatalytic Metal–Organic Frameworks, Coord. Chem. Rev., 2020, 406, 213137 Search PubMed.
- N. Zaman, N. Iqbal and T. Noor, Advances and challenges of MOF derived carbon-based electrocatalysts and photocatalyst for water splitting: A review, Arabian J. Chem., 2022, 15, 103906 Search PubMed.
- P. Salcedo-Abraira, S. M. F. Vilela, A. A. Babaryk, M. Cabrero-Antonino, P. Gregorio, F. Salles, S. Navalón, H. García and P. Horcajada, Nickel phosphonate MOF as efficient water splitting photocatalyst, Nano Res., 2021, 14, 450–457 Search PubMed.
- G. K. H. Shimizu, R. Vaidhyanathan and J. M. Taylor, Phosphonate and sulfonate metal organic frameworks, Chem. Soc. Rev., 2009, 38, 1430–1449 Search PubMed.
- M. Moura de Salles Pupo and R. Kortlever, Electrolyte Effects on the Electrochemical Reduction of CO2, ChemPhysChem, 2019, 20, 2926–2935 Search PubMed.
- X. H. Yang, M. Papasizza, A. Cuesta and J. Cheng, Water-In-Salt Environment Reduces the Overpotential for Reduction of CO2 to CO2− in Ionic Liquid/Water Mixtures, ACS Catal., 2022, 12, 6770–6780 Search PubMed.
- P. Anastas and N. Eghbali, Green Chemistry: Principles and Practice, Chem. Soc. Rev., 2010, 39, 301–312 Search PubMed.
- S. Dou, J. Song, S. Xi, Y. Du, J. Wang, Z. F. Huang, Z. J. Xu and X. Wang, Boosting Electrochemical CO2 Reduction on Metal–Organic Frameworks via Ligand Doping, Angew. Chem., Int. Ed., 2019, 58, 4041–4045 Search PubMed.
- Y. Wang, P. Hou, Z. Wang and P. Kang, Zinc Imidazolate Metal–Organic Frameworks (ZIF-8) for Electrochemical Reduction of CO2 to CO, ChemPhysChem, 2017, 18, 3142–3147 Search PubMed.
- K. AlKaabi, Casey R. Wade and M. Dincă, Transparent-to-Dark Electrochromic Behavior in Naphthalene-Diimide-Based Mesoporous MOF-74 Analogs, Chem., 2016, 1, 264–272 Search PubMed.
- C. R. Wade, M. Li and M. Dincă, Facile Deposition of Multicolored Electrochromic Metal–Organic Framework Thin Films, Angew. Chem., Int. Ed., 2013, 52, 13377–13381 Search PubMed.
- B. A. Johnson, A. Bhunia, H. Fei, S. M. Cohen and S. Ott, Development of a UiO-Type Thin Film Electrocatalysis Platform with Redox-Active Linkers, J. Am. Chem. Soc., 2018, 140, 2985–2994 Search PubMed.
- W. Liu and X. B. Yin, Metal–organic frameworks for electrochemical applications, Trends Anal. Chem., 2016, 75, 86–96 Search PubMed.
- Y. Zhou, K. Wang, S. Zheng, X. Cheng, Y. He, W. Qin, X. Zhang, H. Chang, N. Zhong and X. He, Advancements in electrochemical CO2 reduction reaction: A review on CO2 mass transport enhancement strategies, Chem. Eng. J., 2024, 486, 150169 Search PubMed.
- T. Möller, T. Ngo Thanh, X. Wang, W. Ju, Z. Jovanov and P. Strasser, The product selectivity zones in gas diffusion electrodes during the electrocatalytic reduction of CO2, Energy Environ. Sci., 2021, 14, 5995–6006 Search PubMed.
- D. L. T. Nguyen, C. W. Lee, J. Na, M. C. Kim, N. D. K. Tu, S. Y. Lee, Y. J. Sa, D. H. Won, H. S. Oh, H. Kim, B. K. Min, S. S. Han, U. Lee and Y. J. Hwang, Mass Transport Control by Surface Graphene Oxide for Selective CO Production from Electrochemical CO2 Reduction, ACS Catal., 2020, 10, 3222–3231 Search PubMed.
- S. Chen, D. Wu and C. Zhao, Two-dimensional metal–organic frameworks for energy-related electrocatalytic applications, JPhys Energy, 2020, 2, 021002 Search PubMed.
- J.-X. Wu, S. Z. Hou, X. D. Zhang, M. Xu, H. F. Yang, P. S. Cao and Z. Y. Gu, Cathodized copper porphyrin metal–organic framework nanosheets for selective formate and acetate production from CO2 electroreduction, Chem. Sci., 2019, 10, 2199–2205 Search PubMed.
- X. Kang, Q. Zhu, X. Sun, J. Hu, J. Zhang, Z. Liu and B. Han, Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal–organic framework cathode, Chem. Sci., 2016, 7, 266–273 Search PubMed.
- R. Hinogami, S. Yotsuhashi, M. Deguchi, Y. Zenitani, H. Hashiba and Y. Yamada, Electrochemical Reduction of Carbon Dioxide Using a Copper Rubeanate Metal Organic Framework, ECS Electrochem. Lett., 2012, 1, H17–H19 Search PubMed.
- N. Kornienko, Y. Zhao, C. S. Kley, C. Zhu, D. Kim, S. Lin, C. J. Chang, O. M. Yaghi and P. Yang, Metal–organic frameworks for electrocatalytic reduction of carbon dioxide, J. Am. Chem. Soc., 2015, 137, 14129–14135 Search PubMed.
- Y. R. Wang, Q. Huang, C. T. He, Y. Chen, J. Liu, F. C. Shen and Y. Q. Lan, Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2, Nat. Commun., 2018, 9, 4466 Search PubMed.
- O. Shekhah, J. Liu, R. A. Fischer and C. Wöll, MOF thin films: existing and future applications, Chem. Soc. Rev., 2011, 40, 1081–1106 Search PubMed.
- L. Wang, Y. Wu, R. Cao, L. Ren, M. Chen, X. Feng, J. Zhou and B. Wang, Fe/Ni Metal–Organic Frameworks and Their Binder-Free Thin Films for Efficient Oxygen Evolution with Low Overpotential, ACS Appl. Mater. Interfaces, 2016, 8, 16736–16743 Search PubMed.
- B. X. Dong, S. L. Qian, F. Y. Bu, Y. C. Wu, L. G. Feng, Y. L. Teng, W. L. Liu and Z. W. Li, Electrochemical Reduction of CO2 to CO by a Heterogeneous Catalyst of Fe–Porphyrin-Based Metal–Organic Framework, ACS Appl. Energy Mater., 2018, 1, 4662–4669 Search PubMed.
- W. Ju, A. Bagger, G. P. Hao, A. S. Varela, I. Sinev, V. Bon, B. Roldan Cuenya, S. Kaskel, J. Rossmeisl and P. Strasser, Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2, Nat. Commun., 2017, 8, 944 Search PubMed.
- J. Duan, S. Chen and C. Zhao, Ultrathin metal–organic framework array for efficient electrocatalytic water splitting, Nat. Commun., 2017, 8, 15341 Search PubMed.
- J. Li, P. Pršlja, T. Shinagawa, A. J. Martín Fernández, F. Krumeich, K. Artyushkova, P. Atanassov, A. Zitolo, Y. Zhou, R. García-Muelas, N. López, J. Pérez-Ramírez and F. Jaouen, Volcano Trend in Electrocatalytic CO2 Reduction Activity over Atomically Dispersed Metal Sites on Nitrogen-Doped Carbon, ACS Catal., 2019, 9, 10426–10439 Search PubMed.
- H. Sun, Z. Yan, F. Liu, W. Xu, F. Cheng and J. Chen, Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution, Adv. Mater., 2020, 32, 1806326 Search PubMed.
- R. Senthil Kumar, S. Senthil Kumar and M. Anbu Kulandainathan, Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst, Electrochem. Commun., 2012, 25, 70–73 Search PubMed.
- A. M. Szczepkowska, M. Janeta, M. Siczek, W. Tylus, A. M. Trzeciak and W. Bury, Immobilization of Rh(I) precursor in a porphyrin metal–organic framework – turning on the catalytic activity, Dalton Trans., 2021, 50, 9051–9058 Search PubMed.
- R. Matheu, E. Gutierrez-Puebla, M. Á. Monge, C. S. Diercks, J. Kang, M. S. Prévot, X. Pei, N. Hanikel, B. Zhang, P. Yang and O. M. Yaghi, Three-Dimensional Phthalocyanine Metal-Catecholates for High Electrochemical Carbon Dioxide Reduction, J. Am. Chem. Soc., 2019, 141, 17081–17085 Search PubMed.
- N. Sikdar, J. R. C. Junqueira, S. Dieckhöfer, T. Quast, M. Braun, Y. Song, H. B. Aiyappa, S. Seisel, J. Weidner, D. Öhl, C. Andronescu and W. Schuhmann, Metal–Organic Framework derived CuxOyCz Catalyst for Electrochemical CO2 Reduction and Impact of Local pH Change, Angew. Chem., Int. Ed., 2021, 60, 23427–23434 Search PubMed.
- D. Frederichi, M. H. N. O. Scaliante and R. Bergamasco, Structured photocatalytic systems: photocatalytic coatings on low-cost structures for treatment of water contaminated with micropollutants—a short review, Environ. Sci. Pollut. Res., 2021, 28, 23610–23633 Search PubMed.
- J. Albo, D. Vallejo, G. Beobide, O. Castillo, P. Castaño and A. Irabien, Copper-Based Metal–Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols, ChemSusChem, 2017, 10, 1100–1109 Search PubMed.
- G. Genesio, J. Maynadié, M. Carboni and D. Meyer, Recent status on MOF thin films on transparent conductive oxides substrates (ITO or FTO), New J. Chem., 2018, 42, 2351–2363 Search PubMed.
- B. A. Johnson, A. Bhunia and S. Ott, Electrocatalytic water oxidation by a molecular catalyst incorporated into a metal–organic framework thin film, Dalton Trans., 2017, 46, 1382–1388 Search PubMed.
- Z. Dou, J. Yu, H. Xu, Y. Cui, Y. Yang and G. Qian, Facile preparation of continuous indium metal–organic framework thin films on indium tin oxide glass, Thin Solid Films, 2013, 544, 296–300 Search PubMed.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, Step-by-Step Route for the Synthesis of Metal−Organic Frameworks, J. Am. Chem. Soc., 2007, 129, 15118–15119 Search PubMed.
- B. Liu and R. A. Fischer, Liquid-phase epitaxy of metal organic framework thin films, Sci. China: Chem., 2011, 54, 1851–1866 Search PubMed.
- D. J. Li, Q. H. Li, Z. G. Gu and J. Zhang, A surface-mounted MOF thin film with oriented nanosheet arrays for enhancing the oxygen evolution reaction, J. Mater. Chem. A, 2019, 7, 18519–18528 Search PubMed.
- L. Ye, J. Liu, Y. Gao, C. Gong, M. Addicoat, T. Heine, C. Wöll and L. Sun, Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency, J. Mater. Chem. A, 2016, 4, 15320–15326 Search PubMed.
- I. Hod, M. D. Sampson, P. Deria, C. P. Kubiak, O. K. Farha and J. T. Hupp, Fe-Porphyrin-Based Metal–Organic Framework Films as High-Surface Concentration, Heterogeneous Catalysts for Electrochemical Reduction of CO2, ACS Catal., 2015, 5, 6302–6309 Search PubMed.
- A. Bétard and R. A. Fischer, Metal–Organic Framework Thin Films: From Fundamentals to Applications, Chem. Rev., 2012, 112, 1055–1083 Search PubMed.
- J.-L. Zhuang, A. Terfort and C. Wöll, Formation of oriented and patterned films of metal–organic frameworks by liquid phase epitaxy: A review, Coord. Chem. Rev., 2016, 307, 391–424 Search PubMed.
- Y. Liu, Y. Wei, M. Liu, Y. Bai, X. Wang, S. Shang, J. Chen and Y. Liu, Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes, Angew. Chem., Int. Ed., 2021, 60, 2887–2891 Search PubMed.
- M. V. Varsha and G. Nageswaran, Review—Direct Electrochemical Synthesis of Metal Organic Frameworks, J. Electrochem. Soc., 2020, 167, 155527 Search PubMed.
- I. Hod, W. Bury, D. M. Karlin, P. Deria, C. W. Kung, M. J. Katz, M. So, B. Klahr, D. Jin, Y. W. Chung, T. W. Odom, O. K. Farha and J. T. Hupp, Directed Growth of Electroactive Metal–Organic Framework Thin Films Using Electrophoretic Deposition, Adv. Mater., 2014, 26, 6295–6300 Search PubMed.
- H. Al-Kutubi, J. Gascon, E. J. R. Sudhölter and L. Rassaei, Electrosynthesis of Metal–Organic Frameworks: Challenges and Opportunities, ChemElectroChem, 2015, 2, 462–474 Search PubMed.
- M. Hartmann, S. Kunz, D. Himsl, O. Tangermann, S. Ernst and A. Wagener, Adsorptive Separation of Isobutene and Isobutane on Cu3(BTC)2, Langmuir, 2008, 24, 8634–8642 Search PubMed.
- W. J. Li, J. Lü, S. Y. Gao, Q. H. Li and R. Cao, Electrochemical preparation of metal–organic framework films for fast detection of nitro explosives, J. Mater. Chem. A, 2014, 2, 19473–19478 Search PubMed.
- S. Khazalpour, V. Safarifard, A. Morsali and D. Nematollahi, Electrochemical synthesis of pillared layer mixed ligand metal–organic framework: DMOF-1–Zn, RSC Adv., 2015, 5, 36547–36551 Search PubMed.
- N. Campagnol, T. R. C. Van Assche, M. Li, L. Stappers, M. Dincă, J. F. M. Denayer, K. Binnemans, D. E. De Vos and J. Fransaer, On the electrochemical deposition of metal–organic frameworks, J. Mater. Chem. A, 2016, 4, 3914–3925 Search PubMed.
- L. L. Jiang, X. Zeng, M. Li, M. Q. Wang, T. Y. Su, X. C. Tian and J. Tang, Rapid electrochemical synthesis of HKUST-1 on indium tin oxide, RSC Adv., 2017, 7, 9316–9320 Search PubMed.
- M. Li and M. Dincă, Reductive Electrosynthesis of Crystalline Metal–Organic Frameworks, J. Am. Chem. Soc., 2011, 133, 12926–12929 Search PubMed.
- M. Li and M. Dincă, Selective formation of biphasic thin films of metal–organic frameworks by potential-controlled cathodic electrodeposition, Chem. Sci., 2014, 5, 107–111 Search PubMed.
- X. Kang, B. Wang, K. Hu, K. Lyu, X. Han, B. F. Spencer, M. D. Frogley, F. Tuna, E. J. L. McInnes, R. A. W. Dryfe, B. Han, S. Yang and M. Schröder, Quantitative Electro-Reduction of CO2 to Liquid Fuel over Electro-Synthesized Metal–Organic Framework, J. Am. Chem. Soc., 2020, 142, 17384–17392 Search PubMed.
- M. Mohd and A. Musheer, Historical Developments in Synthesis Approaches and Photocatalytic Perspectives of Metal–Organic Frameworks, in Photocatalysts – New Perspectives, ed. S. A. Nasser, S. A. Saleh and A. Ahmed, IntechOpen, Rijeka, 2022, vol. 14 Search PubMed.
- S. S. A. Shah, T. Najam, M. Wen, S. Q. Zang, A. Waseem and H. L. Jiang, Metal–Organic Framework-Based Electrocatalysts for CO2 Reduction, Small Struct, 2022, 3, 2100090 Search PubMed.
- I. M. Ferrer, C. C. L. McCrory, J. C. Peters and T. F. Jaramillo, Benchmarking of Heterogeneous CO2 Reduction Reaction Electrocatalysts, ECS Meeting Abstracts, 2015, MA2015-01, 1755.
- P. Connor, J. Schuch, B. Kaiser and W. Jaegermann, The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst, Z. Phys. Chem., 2020, 234, 979–994 Search PubMed.
- E. L. Clark, J. Resasco, A. Landers, J. Lin, L. T. Chung, A. Walton, C. Hahn, T. F. Jaramillo and A. T. Bell, Standards and Protocols for Data Acquisition and Reporting for Studies of the Electrochemical Reduction of Carbon Dioxide, ACS Catal., 2018, 8, 6560–6570 Search PubMed.
- J. Resasco, L. D. Chen, E. Clark, C. Tsai, C. Hahn, T. F. Jaramillo, K. Chan and A. T. Bell, Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide, J. Am. Chem. Soc., 2017, 139, 11277–11287 Search PubMed.
- D. Voiry, M. Chhowalla, Y. Gogotsi, N. A. Kotov, Y. Li, R. M. Penner, R. E. Schaak and P. S. Weiss, Best Practices for Reporting Electrocatalytic Performance of Nanomaterials, ACS Nano, 2018, 12, 9635–9638 Search PubMed.
- C. Wei, S. Sun, D. Mandler, X. Wang, S. Z. Qiao and Z. J. Xu, Approaches for measuring the surface areas of metal oxide electrocatalysts for determining their intrinsic electrocatalytic activity, Chem. Soc. Rev., 2019, 48, 2518–2534 Search PubMed.
- J.-I. Jung, M. Risch, S. Park, M. G. Kim, G. Nam, H. Y. Jeong, Y. Shao-Horn and J. Cho, Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis, Energy Environ. Sci., 2016, 9, 176–183 Search PubMed.
- C. Wei and Z. J. Xu, The Comprehensive Understanding of as an Evaluation Parameter for Electrochemical Water Splitting, Small Methods, 2018, 2, 1800168 Search PubMed.
- S. Anantharaj and S. Kundu, Do the Evaluation Parameters Reflect Intrinsic Activity of Electrocatalysts in Electrochemical Water Splitting?, ACS Energy Lett., 2019, 4, 1260–1264 Search PubMed.
- K. A. Stoerzinger, M. Risch, B. Han and Y. Shao-Horn, Recent Insights into Manganese Oxides in Catalyzing Oxygen Reduction Kinetics, ACS Catal., 2015, 5, 6021–6031 Search PubMed.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices, J. Am. Chem. Soc., 2015, 137, 4347–4357 Search PubMed.
- T. Bligaard, R. M. Bullock, C. T. Campbell, J. G. Chen, B. C. Gates, R. J. Gorte, C. W. Jones, W. D. Jones, J. R. Kitchin and S. L. Scott, Toward Benchmarking in Catalysis Science: Best Practices, Challenges, and Opportunities, ACS Catal., 2016, 6, 2590–2602 Search PubMed.
- J. B. Goodenough and K. S. Park, The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc., 2013, 135, 1167–1176 Search PubMed.
- Y. Wang, P. Hou, Z. Wang and P. Kang, Zinc Imidazolate Metal–Organic Frameworks (ZIF-8) for Electrochemical Reduction of CO2 to CO, ChemPhysChem, 2017, 18, 3142–3147 Search PubMed.
- S. Dou, J. Song, S. Xi, Y. Du, J. Wang, Z. F. Huang, Z. J. Xu and X. Wang, Boosting Electrochemical CO2 Reduction on Metal–Organic Frameworks via Ligand Doping, Angew. Chem., Int. Ed., 2019, 58, 4041–4045 Search PubMed.
- X. Kang, Q. Zhu, X. Sun, J. Hu, J. Zhang, Z. Liu and B. Han, Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal–organic framework cathode, Chem. Sci., 2016, 7, 266–273 Search PubMed.
- J. Albo, D. Vallejo, G. Beobide, O. Castillo, P. Castano and A. Irabien, Copper-Based Metal–Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols, ChemSusChem, 2017, 10, 1100–1109 Search PubMed.
- F. Yang, A. Chen, P. L. Deng, Y. Zhou, Z. Shahid, H. Liu and B. Y. Xia, Highly efficient electroconversion of carbon dioxide into hydrocarbons by cathodized copper–organic frameworks, Chem. Sci., 2019, 10, 7975–7981 Search PubMed.
- H. Zhong, M. Ghorbani-Asl, K. H. Ly, J. Zhang, J. Ge, M. Wang, Z. Liao, D. Makarov, E. Zschech, E. Brunner, I. M. Weidinger, J. Zhang, A. V. Krasheninnikov, S. Kaskel, R. Dong and X. Feng, Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal–organic frameworks, Nat. Commun., 2020, 11, 1409 Search PubMed.
- F. Mao, Y. H. Jin, P. F. Liu, P. Yang, L. Zhang, L. Chen, X. M. Cao, J. Gu and H. G. Yang, Accelerated proton transmission in metal–organic frameworks for the efficient reduction of CO2 in aqueous solutions, J. Mater. Chem. A, 2019, 7, 23055–23063 Search PubMed.
- X. Tan, C. Yu, C. Zhao, H. Huang, X. Yao, X. Han, W. Guo, S. Cui, H. Huang and J. Qiu, Restructuring of Cu2O to Cu2O@Cu–Metal–Organic Frameworks for Selective Electrochemical Reduction of CO2, ACS Appl. Mater. Interfaces, 2019, 11, 9904–9910 Search PubMed.
- C. W. Kung, C. O. Audu, A. W. Peters, H. Noh, O. K. Farha and J. T. Hupp, Copper Nanoparticles Installed in Metal–Organic Framework Thin Films are Electrocatalytically Competent for CO2 Reduction, ACS Energy Lett., 2017, 2, 2394–2401 Search PubMed.
- X. L. Jiang, H. H. Wu, S. J. Chang, R. Si, S. Miao, W. X. Huang, Y. H. Li, G. X. Wang and X. H. Bao, Boosting CO2 electroreduction over layered zeolitic imidazolate frameworks decorated with Ag2O nanoparticles, J. Mater. Chem. A, 2017, 5, 19371–19377 Search PubMed.
- X. D. Zhang, S. Z. Hou, J. X. Wu and Z. Y. Gu, Two-Dimensional Metal–Organic Framework Nanosheets with Cobalt-Porphyrins for High-Performance CO2 Electroreduction, Chem. – Eur. J., 2020, 26, 1604 Search PubMed.
- D. H. Nam, O. S. Bushuyev, J. Li, P. De Luna, A. Seifitokaldani, C. T. Dinh, F. P. Garcia de Arquer, Y. Wang, Z. Liang, A. H. Proppe, C. S. Tan, P. Todorovic, O. Shekhah, C. M. Gabardo, J. W. Jo, J. Choi, M. J. Choi, S. W. Baek, J. Kim, D. Sinton, S. O. Kelley, M. Eddaoudi and E. H. Sargent, Metal–Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction, J. Am. Chem. Soc., 2018, 140, 11378–11386 Search PubMed.
- K. Zhao, Y. Liu, X. Quan, S. Chen and H. Yu, CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework, ACS Appl. Mater. Interfaces, 2017, 9, 5302–5311 Search PubMed.
- Y. Guo, H. Yang, X. Zhou, K. Liu, C. Zhang, Z. Zhou, C. Wang and W. Lin, Electrocatalytic reduction of CO2 to CO with 100% faradaic efficiency by using pyrolyzed zeolitic imidazolate frameworks supported on carbon nanotube networks, J. Mater. Chem. A, 2017, 5, 24867–24873 Search PubMed.
- C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang, J. Wang, J. Yang, S. Wei, Y. Wu and Y. Li, Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2, J. Am. Chem. Soc., 2017, 139, 8078–8081 Search PubMed.
- X. Q. Wang, Z. Chen, X. Y. Zhao, T. Yao, W. X. Chen, R. You, C. M. Zhao, G. Wu, J. Wang, W. X. Huang, J. L. Yang, X. Hong, S. Q. Wei, Y. Wu and Y. D. Li, Regulation of Coordination Number over Single Co Sites: Triggering the Efficient Electroreduction of CO2, Angew. Chem., Int. Ed., 2018, 57, 1944–1948 Search PubMed.
- Y. F. Ye, F. Cai, H. B. Li, H. H. Wu, G. X. Wang, Y. S. Li, S. Miao, S. H. Xie, R. Si, J. Wang and X. H. Bao, Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe–N active sites for efficient oxygen and carbon dioxide electroreduction, Nano Energy, 2017, 38, 281–289 Search PubMed.
- P. L. Lu, Y. J. Yang, J. N. Yao, M. Wang, S. Dipazir, M. L. Yuan, J. X. Zhang, X. Wang, Z. J. Xie and G. J. Zhang, Facile synthesis of single-nickel-atomic dispersed N-doped carbon framework for efficient electrochemical CO2 reduction, Appl. Catal., B, 2019, 241, 113–119 Search PubMed.
- Y. Zheng, P. Cheng, J. Xu, J. Han, D. Wang, C. Hao, H. R. Alanagh, C. Long, X. Shi and Z. Tang, MOF-derived nitrogen-doped nanoporous carbon for electroreduction of CO2 to CO: the calcining temperature effect and the mechanism, Nanoscale, 2019, 11, 4911–4917 Search PubMed.
- S. An, G. Zhang, T. Wang, W. Zhang, K. Li, C. Song, J. T. Miller, S. Miao, J. Wang and X. Guo, High-Density Ultra-Small Clusters and Single-Atom Fe Sites Embedded in Graphitic Carbon Nitride (G-C3N4) for Highly Efficient Catalytic Advanced Oxidation Processes, ACS Nano, 2018, 12, 9441 Search PubMed.
- C. C. Yan, Y. F. Ye, L. Lin, H. H. Wu, Q. K. Jiang, G. X. Wang and X. H. Bao, Improving CO2 electroreduction over ZIF-derived carbon doped with Fe–N sites by an additional ammonia treatment, Catal. Today, 2019, 330, 252–258 Search PubMed.
- T. N. Huan, N. Ranjbar, G. Rousse, M. Sougrati, A. Zitolo, V. Mougel, F. Jaouen and M. Fontecave, Electrochemical Reduction of CO2 Catalyzed by Fe–N–C Materials: A Structure-Selectivity Study, ACS Catal., 2017, 7, 1520–1525 Search PubMed.
- W. Zhu, Y. J. Zhang, H. Zhang, H. Lv, Q. Li, R. Michalsky, A. A. Peterson and S. Sun, Active and Selective Conversion of CO2 to CO on Ultrathin Au Nanowires, J. Am. Chem. Soc., 2014, 136, 16132–16135 Search PubMed.
- Q. Lu, J. Rosen, Y. Zhou, G. S. Hutchings, Y. C. Kimmel, J. G. Chen and F. Jiao, A selective and efficient electrocatalyst for carbon dioxide reduction, Nat. Commun., 2014, 5, 3242 Search PubMed.
- S. Lin, C. S. Diercks, Y.-B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science, 2015, 349, 1208–1213 Search PubMed.
- C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke and E. J. Maginn, Why Is CO2 So Soluble in Imidazolium-Based Ionic Liquids?, J. Am. Chem. Soc., 2004, 126, 5300–5308 Search PubMed.
- D. R. MacFarlane, M. Forsyth, P. C. Howlett, J. M. Pringle, J. Sun, G. Annat, W. Neil and E. I. Izgorodina, Ionic Liquids in Electrochemical Devices and Processes: Managing Interfacial Electrochemistry, Acc. Chem. Res., 2007, 40, 1165–1173 Search PubMed.
- Q. Cui, G. Qin, W. Wang, K. R. Geethalakshmi, A. Du and Q. Sun, Novel two-dimensional MOF as a promising single-atom electrocatalyst for CO2 reduction: A theoretical study, Appl. Surf. Sci., 2020, 500, 143993 Search PubMed.
- L. Chen and Q. Xu, Metal–Organic Framework Composites for Catalysis, Matter, 2019, 1, 57–89 Search PubMed.
- F. Schüth, M. D. Ward and J. M. Buriak, Common Pitfalls of Catalysis Manuscripts Submitted to Chemistry of Materials, Chem. Mater., 2018, 30, 3599–3600 Search PubMed.
- W. Chaikittisilp, K. Ariga and Y. Yamauchi, A new family of carbon materials: synthesis of MOF-derived nanoporous carbons and their promising applications, J. Mater. Chem. A, 2013, 1, 14–19 Search PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements, Nat. Chem., 2011, 3, 634 Search PubMed.
- S. Back, J. Lim, N. Y. Kim, Y.-H. Kim and Y. Jung, Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements, Chem. Sci., 2017, 8, 1090–1096 Search PubMed.
- J. Vavra, T.-H. Shen, D. Stoian, V. Tileli and R. Buonsanti, Real-time Monitoring Reveals Dissolution/Redeposition Mechanism in Copper Nanocatalysts during the Initial Stages of the CO2 Reduction Reaction, Angew. Chem., Int. Ed., 2021, 60, 1347–1354 Search PubMed.
- L. Xiao, G. Wang, X. Huang, S. Zhou, R. Zhou, Y. Jiang, S. Liu, G. Li, H. Zheng, S. G. Sun and H. G. Liao, Efficient CO2 reduction MOFs derivatives transformation mechanism revealed by in-situ liquid phase TEM, Appl. Catal., B, 2022, 307, 121164 Search PubMed.
- D. Zhang, X. Liu, Y. Zhao, H. Zhang, A. V. Rudnev and J. F. Li, In situ Raman spectroscopic studies of CO2 reduction reactions: from catalyst surface structures to reaction mechanisms, Chem. Sci., 2025, 16, 4916 Search PubMed.
| This journal is © the Partner Organisations 2025 |






