Accurate determination of enantiomeric excess of an amino acid using an extended-gate-type organic transistor†
Yijing
Zhang
 a,
Yui
Sasaki
a,
Yui
Sasaki
 abc,
Xiaojun
Lyu
abc,
Xiaojun
Lyu
 a,
Jun-ichi
Ogawa
d,
Hidenosuke
Itoh
d and
Tsuyoshi
Minami
a,
Jun-ichi
Ogawa
d,
Hidenosuke
Itoh
d and
Tsuyoshi
Minami
 *a
*a
aInstitute of Industrial Science, The University of Tokyo, 4-6-1 Komaba, Meguro-ku, Tokyo, 153-8505, Japan. E-mail: tminami@g.ecc.u-tokyo.ac.jp
bResearch Center for Advanced Science and Technology, The University of Tokyo, 4-6-1, Komaba, Meguro-ku, Tokyo, 153-8904, Japan
cJST PRESTO, 4-1-8 Honcho, Kawaguchi, Saitama, 332-0012, Japan
dYokogawa Electric Corporation, 2-9-32 Nakacho, Musashino, Tokyo 180-0006, Japan
First published on 29th May 2025
Abstract
An extended-gate-type organic field effect transistor functionalized with molecularly imprinted polymer has shown chiral selectivity to L-histidine and discriminated it from amino acid families. Indeed, the chiral sensor device has simultaneously determined four points of the enantiomeric excesses of the chiral amino acid.
The homochirality of amino acids is a significant feature in biochemical processes.1 In addition, chiral amino acids are essential components in various industrial fields, including pharmaceuticals, foods and drinks, cosmetics, etc.2 Thus, the determination of enantiomeric excess (ee) of chiral amino acids is required in many fields. Enantiomeric purities of amino acids have been conventionally determined using high-performance liquid chromatography (HPLC) equipped with a chiral column.3 Although the conventional method enables reliable chiral analysis, the requirements of large-sized apparatuses, trained personnel, and time-consuming measurements pose facile chiral detection. Thus, we propose a chemical sensor device for enantioselective detection and its ee determination with high accuracy. Among amino acids, L-histidine (His) was selected as the main target from the viewpoint of the detection significance in diagnosis.4
To date, various chiral receptors have been vigorously developed based on molecular recognition chemistry.5 The fundamental strategy to design chiral receptors is the introduction of rigidity into a recognition scaffold, which is required for the discrimination of a slight structural difference in enantiomers.5d,e For example, rigid receptor skeletons such as atropisomers (e.g., binaphthyl5f and benzophenone5g) and macrocycles (e.g., cucurbit[n]uril5h and cyclodextrin5i) have been vigorously used for the discrimination of structural differences in amino acid families and their % ee determination. However, high synthetic efforts to obtain such rigid receptors that satisfy complementarity with chiral amino acids remain challenging tasks in sensing applications. In addition, the high rigidity of receptors causes low water solubility, which poses chiral sensing in aqueous media.5f,g To overcome the above issues, we here employed a molecular imprinting method to easily obtain chiral receptors without a synthetic burden and to perform chiral sensing in aqueous media.
Molecularly imprinted polymers (MIPs) are artificial recognition materials created by polymerization of functional monomers in the presence of templates (i.e., analytes).6 The specificity of MIPs is derived from three-dimensional recognition scaffolds provided by multiple interactions between functional monomers and templates. By selecting appropriate monomers, a MIP layer can be easily fabricated on an electrode through electrochemical polymerization.6c,d Therefore, a solid-state MIP layer on an electrode enables chiral sensing at an interface between the MIP electrode and aqueous media containing a chiral analyte.6e The chiral recognition information is visualized by connecting the electrode to an appropriate transducer unit.
A compact switching device based on a field-effect transistor (FET) has emerged as a promising platform for various applications.7 The applicability of solution processes in device fabrication has accelerated the manufacturing of organic FET (OFET)-based chemical sensors.8 By integrating appropriate molecular recognition materials, the OFET can demonstrate quantitative changes in transistor characteristics such as threshold voltages (VTHs) and drain currents (IDSs) upon analyte capture.9 Notably, the amplification ability of OFETs contributes to sensitive detection over conventional electrochemical methods.6d,e Meanwhile, the instability of organic semiconductive layers of OFETs under ambient conditions is a bottleneck for chemical sensing in aqueous media.10 Considering this, an extended-gate structure was selected as a configuration of a MIP-attached OFET-based chiral sensor (MIP-OFET) in this study.11 The OFET device is isolated from a sensing gate in this structure, which qualitatively and quantitatively detects changes in transistor characteristics upon analyte capture at an electrode functionalized with a MIP layer for L-His (Fig. 1). To the best of our knowledge, the combination of organic transistors and MIPs have been applied to the detection of chiral amino acids, whereas the % ee determination has never been reported.12 To this end, we strategically employed two crucial techniques using density functional theory (DFT) calculations for an optimal MIP design and a powerful machine learning method (i.e., support vector machine, SVM) for accurate data analysis (vide infra).
In this study, 1,2-diaminobenzene was selected as a functional monomer for MIP against L-His (Fig. 1). The monomer can be polymerized on an Au electrode by an electrochemical method.13 In addition, the polymerized structure is favorable for selective recognition based on multiple hydrogen bonds with a specific analyte.6d Herein, two hydrogen bonds between two monomers and one carboxy group of the template were expected. One of the monomers interacting with the carbonyl group could also contribute to a hydrogen bond with the primary amino group of L-His. The appropriate molar ratio between the monomer and the template was determined by both DFT calculations and experimental investigations. For example, the pre-organized structure at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (= 1,2-diaminobenzene: L-His) indicates insufficient interactions between the monomers and the template (Fig. S1(a), ESI†). Meanwhile, although a slightly more stable complex was observed at a 5
1 molar ratio (= 1,2-diaminobenzene: L-His) indicates insufficient interactions between the monomers and the template (Fig. S1(a), ESI†). Meanwhile, although a slightly more stable complex was observed at a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, the surplus monomer did not contribute to interactions with the template (see the ESI†). In contrast, four monomers efficiently contributed to hydrogen bonds with all binding sites of the template at a 4
1 molar ratio, the surplus monomer did not contribute to interactions with the template (see the ESI†). In contrast, four monomers efficiently contributed to hydrogen bonds with all binding sites of the template at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Fig. 2(a) and Fig. S2(a), ESI†). The isosurface figures of the Independent Gradient Model (IGM)14 indicate pre-organized structures of 1,2-diaminobenzene and a template (i.e., L- or D-His) at a 4
1 molar ratio (Fig. 2(a) and Fig. S2(a), ESI†). The isosurface figures of the Independent Gradient Model (IGM)14 indicate pre-organized structures of 1,2-diaminobenzene and a template (i.e., L- or D-His) at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Fig. 2). The same conditions of fragments (i.e., 1,2-diaminobenzene) that optimized for L-His were also applied to D-His in DFT calculations. The DFT calculations revealed a difference in the binding energy of the pre-organized structures between L-(−155.6 kJ mol−1) and D-His (−122.3 kJ mol−1) owing to the following reasons. The bluish-green ellipsoid areas suggest noncovalent interactions between the monomers and the chiral template (i.e., L- or D-His). Among them, hydrogen bonds provided by the monomers contributed to interactions with the primary amino group and the carboxy group of L-His (Fig. 2(a) and Fig. S2(a), ESI†). The abovementioned interactions were also observed in the pre-organized structure of D-His, likewise L-His (Fig. 2(b) and Fig. S2(b), ESI†). On the other hand, a noncovalent interaction between the monomer and the secondary amino moiety of the imidazole ring of the template was only observed in the pre-organized structure with L-His, which implies a key interaction to distinguish L-His from D-His. According to the determined molar ratio, 1,2-diaminobenzene was electrochemically polymerized on an Au electrode in the presence of L-His. The surface of a thermally deposited Au electrode was modified by an Au nanostructure with a needle-like shape to enhance adhesion of the MIP layer to the extended-gate electrode.6d,e The conductivity of the Au extended-gate electrode decreased upon polymerization of 1,2-diaminobenzene by cyclic voltammetry (CV), which suggested the accumulation of an insulating polymer layer (Fig. S5, ESI†).13 The extraction of the template from the polymerized electrode was performed by CV in a basic solution. The details of electrode fabrication are summarized in the ESI.†
1 molar ratio (Fig. 2). The same conditions of fragments (i.e., 1,2-diaminobenzene) that optimized for L-His were also applied to D-His in DFT calculations. The DFT calculations revealed a difference in the binding energy of the pre-organized structures between L-(−155.6 kJ mol−1) and D-His (−122.3 kJ mol−1) owing to the following reasons. The bluish-green ellipsoid areas suggest noncovalent interactions between the monomers and the chiral template (i.e., L- or D-His). Among them, hydrogen bonds provided by the monomers contributed to interactions with the primary amino group and the carboxy group of L-His (Fig. 2(a) and Fig. S2(a), ESI†). The abovementioned interactions were also observed in the pre-organized structure of D-His, likewise L-His (Fig. 2(b) and Fig. S2(b), ESI†). On the other hand, a noncovalent interaction between the monomer and the secondary amino moiety of the imidazole ring of the template was only observed in the pre-organized structure with L-His, which implies a key interaction to distinguish L-His from D-His. According to the determined molar ratio, 1,2-diaminobenzene was electrochemically polymerized on an Au electrode in the presence of L-His. The surface of a thermally deposited Au electrode was modified by an Au nanostructure with a needle-like shape to enhance adhesion of the MIP layer to the extended-gate electrode.6d,e The conductivity of the Au extended-gate electrode decreased upon polymerization of 1,2-diaminobenzene by cyclic voltammetry (CV), which suggested the accumulation of an insulating polymer layer (Fig. S5, ESI†).13 The extraction of the template from the polymerized electrode was performed by CV in a basic solution. The details of electrode fabrication are summarized in the ESI.†
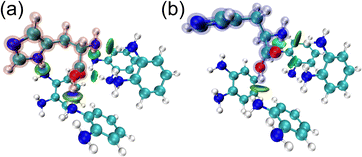 | ||
Fig. 2 The isosurface IGM figure of the optimized complexes of 1,2-diaminobenzene and (a) L- or (b) D-His at a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The blueish-green ellipsoid areas indicate non-covalent interactions. 1 molar ratio. The blueish-green ellipsoid areas indicate non-covalent interactions. | ||
Next, the detectability of the MIP electrode for L-His was evaluated by differential pulse voltammetry (DPV). The detection mechanism of the MIP made of 1,2-diaminobenzene for L-His relies on hydrogen bonds.15 Thus, pH conditions for all chiral sensing were set to 6.0, considering the pKa of His.16 Fig. S6 (ESI†) displays a concentration-dependent decrease in currents of the MIP electrode upon adding L-His (0.0–10 mM) in a K3Fe(CN)6 solution. The phenomenon can be explained by the behavior of electroactive ions (i.e., [Fe(CN)6]3−) inside the cavity of MIP on the Au electrode.6c,17 In the absence of L-His, the electroactive ions inside the cavity of MIP can approach the Au electrode surface, whereas their approach was prevented by the capturing of L-His inside the cavity of MIP. Therefore, the current decrease upon adding L-His in the DPV measurement indicated the analyte capture by the MIP electrode.
Subsequently, the OFET was employed for chiral sensing. In the OFET fabrication, a combination of aluminum oxide and tetradecylphosphonic acid was used as a dielectric layer to operate at low voltage (<|3| V).18 In addition, the semiconductive layer made of poly{2,5-bis(3-tetradecylthiophen-2-yl)thieno[3,2-b]thiophene}19 was formed by a drop-casting process. The surface of the device was entirely covered with a hydrophobic material (i.e., CYTOP™) for passivation. The basic transistor characteristics were evaluated under ambient conditions (Fig. S9, ESI†). The details of the device fabrication process and measurements are summarized in the ESI.† The manufactured OFET device and the MIP-attached extended-gate electrode were connected using a conductive cable to perform chiral sensing. The MIP-OFET showed concentration-dependent VTH shifts upon adding L-His (0.0–1.0 mM) in a phosphate buffer solution (100 mM) at pH 6.0 at 25 °C (Fig. 3). Moreover, the limit of detection was estimated to be 73 μM based on the 3σ method.20 In contrast to L-His, the MIP-OFET did not respond to D-His (Fig. 3). Although a slight fluctuation of the response to D-His due to the enantiomeric purity of commercially available L-His (94.9% ee determined by HPLC, Fig. S14, ESI†) used in MIP fabrication was observed, this demonstration revealed the high enantioselectivity of the MIP-OFET.
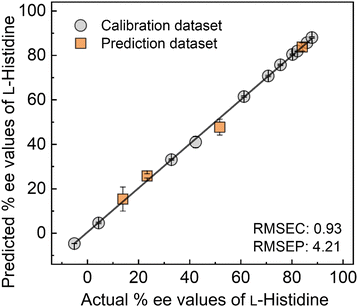
Furthermore, we investigated the discriminatory ability of the MIP-OFET to L-His from L-type aromatic amino acids (i.e., tyrosine (Tyr), phenylalanine (Phe), and tryptophan (Trp)) and L-lysine (Lys). Fig. 4 displayed the highest response to L-His over other L-amino acids.21 The selectivity test clarified the significance of the optimization of the MIP design considering interactions with the chiral template. Indeed, the DFT calculation results revealed the unstable complexation with L-type aromatic amino acids (i.e., Tyr, Phe, or Trp) than L-His (Table S2, ESI†). As the next attempt, the MIP-OFET was applied to determine the % ee of L-His. In general, chemical sensors show non-linear responses in complicated sensing situations, which cause difficulty in the precise determination of analyte information. Thus, we decided to apply SVM for data processing of slight differences in transistor characteristics depending on the % ee changes (Fig. S15, ESI†). The SVM is one of the machine learning methods that is capable of building linear calibration lines even in non-linear responses.22 In addition, the SVM enables the prediction of unknown chemical information based on the supervised dataset. In this assay, the transfer characteristics (VGS–IDS) of the MIP-OFET upon changing in % ee of L-His were collected to obtain an inset data for regression analysis using SVM (Fig. S15(a), ESI†). Fig. 5 shows the distribution of four predicted plots (i.e., 13.9% ee, 23.4% ee, 51.9% ee, and 84.1% ee as orange square plots) on the calibration line (i.e., gray circle plots). The values of the root-mean-square errors of calibration (RMSEC) and prediction (RMSEP) indicate the accuracy of the built model and predictions. The % ee determination against four test plots revealed the usability of the chiral sensor system combined with the SVM.
In summary, we demonstrated chiral analysis, especially % ee determination, using the extended-gate-type OFET functionalized with the MIP layer. The pre-organized structure of 1,2-diaminobenzene as the functional monomer and L-His as the template was optimized by using DFT calculations. In addition, the visualized structure clarified the significant noncovalent interaction of the monomer and the template to distinguish L-His from D-His. Indeed, the MIP-OFET showed chiral selective response to L-His and discriminated from L-amino acid families. Finally, the % ee determination was conducted by applying SVM. The estimated % ee values based on the SVM analysis of the changes in the transistor characteristics indicated the feasibility of accurate chiral analysis without using a stationary apparatus. Through this demonstration, we revealed that the employment of calculation methods such as DFT and machine learning will not only expand the potential of conventional sensor technologies but also contribute to accurate analysis against challenging analytes.
Y. Sasaki gratefully acknowledges the financial support from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant No. JP24K17667) and JST PRESTO (Grant No. JPMJPR23H2). T. Minami thanks JSPS KAKENHI (Grant No. JP23H03864 and JP24K01315) and JST CREST (Grant No. JPMJCR2011). All authors thank Miyuki Kato for performing HPLC analysis (Fig. S14, ESI†).
Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the ESI.†Notes and references
- A. Banreti, S. Bhattacharya, F. Wien, K. Matsuo, M. Réfrégiers, C. Meinert, U. Meierhenrich, B. Hudry, D. Thompson and S. Noselli, Nat. Commun., 2022, 13, 7059 CrossRef CAS PubMed.
- Y.-P. Xue, C.-H. Cao and Y.-G. Zheng, Chem. Soc. Rev., 2018, 47, 1516–1561 RSC.
- (a) I. Ilisz, R. Berkecz and A. Péter, J. Sep. Sci., 2006, 29, 1305–1321 CrossRef CAS PubMed; (b) G. Carenzi, S. Sacchi, M. Abbondi and L. Pollegioni, Amino Acids, 2020, 52, 849–862 CrossRef CAS PubMed.
- (a) J. Y. Oh, Y. S. Lee, K. H. Min, G. Y. Hur, S. Y. Lee, K. H. Kang, C. K. Rhee, S. J. Park, A. Khan, J. Na, Y. H. Park and J. J. Shim, Int. J. Chronic Obstruct. Pulm. Dis., 2018, 13, 1809–1818 CrossRef CAS PubMed; (b) Y. Okusha, Y. Hirai, H. Maezawa, K. Hisadome, N. Inoue, Y. Yamazaki and M. Funahashi, J. Physiol. Sci., 2017, 67, 467–474 CrossRef CAS.
- For reviews, see: (a) M. Hu, H.-T. Feng, Y.-X. Yuan, Y.-S. Zheng and B. Z. Tang, Coord. Chem. Rev., 2020, 416, 213329 CrossRef CAS; (b) M. Quan, X.-Y. Pang and W. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202201258 CrossRef CAS PubMed; (c) J. Guo, J. Hou, J. Hu, Y. Geng, M. Li, H. Wang, J. Wang and Q. Luo, Chem. Commun., 2023, 59, 9157–9166 RSC; (d) J. S. S. K. Formen, J. R. Howard, E. V. Anslyn and C. Wolf, Angew. Chem., Int. Ed., 2024, 63, e202400767 CrossRef CAS . For examples, see: ; (e) Y. Sasaki, S. Kojima, V. Hamedpour, R. Kubota, S. Takizawa, I. Yoshikawa, H. Houjou, Y. Kubo and T. Minami, Chem. Sci., 2020, 11, 3790–3796 RSC; (f) Y.-Y. Zhu, X.-D. Wu, S.-X. Gu and L. Pu, J. Am. Chem. Soc., 2019, 141, 175–181 CrossRef CAS PubMed; (g) H. Kim, S. M. So, C. P.-H. Yen, E. Vinhato, A. J. Lough, J.-I. Hong, H.-J. Kim and J. Chin, Angew. Chem., Int. Ed., 2008, 47, 8657–8660 CrossRef CAS PubMed; (h) T. Minami, N. A. Esipenko, B. Zhang, L. Isaacs and P. Anzenbacher, Jr., Chem. Commun., 2014, 50, 61–63 RSC; (i) Y. Sun, Y. Wang, Y. Wu, X. Wang, X. Li, S. Wang and Y. Xiao, Anal. Chem., 2018, 90, 9264–9271 CrossRef CAS.
- For reviews, see: (a) K. Haupt, P. X. Medina Rangel and B. T. S. Bui, Chem. Rev., 2020, 120, 9554–9582 CrossRef CAS; (b) T. Takeuchi and H. Sunayama, Chem. Commun., 2018, 54, 6243–6251 RSC For examples, see: ; (c) Q. Zhou, M. Wang, S. Yagi and T. Minami, Nanoscale, 2021, 13, 100–107 RSC; (d) Y. Sasaki, Y. Zhang, H. Fan, K. Ohshiro, Q. Zhou, W. Tang, X. Lyu and T. Minami, Sens. Actuators, B, 2023, 382, 133458 CrossRef CAS; (e) Q. Zhou, Y. Sasaki, K. Ohshiro, H. Fan, V. Montagna, C. Gonzato, K. Haupt and T. Minami, J. Mater. Chem. B, 2022, 10, 6808–6815 RSC.
- G. Horowitz, Adv. Mater., 1998, 10, 365–377 CrossRef CAS.
- T. Minamiki, T. Minami, Y.-P. Chen, T. Mano, Y. Takeda, K. Fukuda and S. Tokito, Commun. Mater., 2021, 2, 8 CrossRef CAS.
- Y. Sasaki and T. Minami, Phys. Status Solidi A, 2023, 220, 2300469 CrossRef CAS.
- M. E. Roberts, S. C. B. Mannsfeld, N. Queraltó, C. Reese, J. Locklin, W. Knoll and Z. Bao, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12134–12139 CrossRef CAS.
- (a) J. van der spiegel, I. Lauks, P. Chan and D. Babic, Sens. Actuators, 1983, 4, 291–298 CrossRef CAS; (b) Y. Sasaki, K. Ohshiro, X. Lyu, T. Kawashima, M. Kamiko, H. Tanaka, A. Yamagami, Y. Ueno and T. Minami, Chem. Commun., 2024, 60, 9930–9933 RSC.
- (a) L. Zhang, Z. Liu, C. Xiong, L. Zheng, Y. Ding, H. Lu, G. Zhang and L. Qiu, Org. Electron., 2018, 61, 254–260 CrossRef CAS; (b) Z. Iskierko, A. Checinska, P. S. Sharma, K. Golebiewska, K. Noworyta, P. Borowicz, K. Fronc, V. Bandi, F. D'Souza and W. Kutner, J. Mater. Chem. C, 2017, 5, 969–977 RSC.
- S. Bilal, A.-U.-H. A. Shah and R. Holze, Electrochim. Acta, 2012, 85, 358–368 CrossRef CAS.
- C. Lefebvre, G. Rubez, H. Khartabil, J.-C. Boisson, J. Contreras-García and E. Hénon, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 RSC.
- R. N. V. Krishna Deepak and R. Sankararamakrishnan, Biochemistry, 2016, 55, 3774–3783 CrossRef CAS PubMed.
- M. Hennig and B. H. Geierstanger, J. Am. Chem. Soc., 1999, 121, 5123–5126 CrossRef CAS.
- S. Motia, B. Bouchikhi, E. Llobet and N. El Bari, Talanta, 2020, 216, 120953 Search PubMed.
- H. Klauk, U. Zschieschang, J. Pflaum and M. Halik, Nature, 2007, 445, 745–748 CrossRef CAS PubMed.
- I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. MacDonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M. L. Chabinyc, R. J. Kline, M. D. McGehee and M. F. Toney, Nat. Mater., 2006, 5, 328–333 CrossRef CAS PubMed.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education Canada, 5th edn, 2005 Search PubMed.
- Weak responses to other amino acids were observed in the selectivity test. In this regard, a polymerized layer without using the template exhibited a weak DPV current response toward L-His (Fig. S7, ESI†). Thus, the observed responses to other amino acids were presumably derived from physical adsorption to the MIP electrode.
- (a) T. Minami, N. A. Esipenko, B. Zhang, M. E. Kozelkova, L. Isaacs, R. Nishiyabu, Y. Kubo and P. Anzenbacher Jr., J. Am. Chem. Soc., 2012, 134, 20021–20024 CrossRef CAS PubMed; (b) L. Hamel, Knowledge Discovery with Support Vector Machines, John Wiley & Sons, Inc., 2009 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Regents and materials, apparatuses and methods, basic transistor characteristics, characterization of the MIP electrode, evaluation of the detectability of the MIP electrode, determination of enantiomeric purity of target histidine. See DOI: https://doi.org/10.1039/d5cc02191j |
| This journal is © The Royal Society of Chemistry 2025 |