Bimetallic Ni–Co selenide heterostructure aerogel for highly efficient overall water splitting†
Hongchen
Liu
,
Fan
Yang
*,
Fengjiang
Chen
,
Sai
Che
 ,
Neng
Chen
,
Chong
Xu
,
Ni
Wu
,
Wenkai
Wei
and
Yongfeng
Li
,
Neng
Chen
,
Chong
Xu
,
Ni
Wu
,
Wenkai
Wei
and
Yongfeng
Li
 *
*
State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Changping, Beijing 102249, China. E-mail: yangfan@cup.edu.cn; yfli@cup.edu.cn
First published on 25th January 2023
Abstract
Exploring transition metal-based electrocatalysts with excellent performance toward alkaline overall water splitting is of significant importance for the hydrogen economy but remains challenging. Herein, we designed and prepared a bimetallic Ni–Co selenide heterostructure aerogel (NiSe2–CoSe2) for highly efficient overall water splitting via facile spontaneous gelation and selenium vapor deposition. The optimized sample exhibited extremely low overpotentials of 65/220 mV for the HER/OER at a geometric current density of 10 mA cm−2 in 1 M KOH electrolyte. Assembled as an electrolyzer for overall water splitting, NiSe2–CoSe2 only required a low cell voltage of 1.56 V to achieve 10 mA cm−2 with decent stability, which was comparable to those of commercial noble-metal catalysts (Pt/C + RuO2). The exceptional performance was attributed to the unique porous morphology of the aerogel with abundant active sites and the bimetallic selenide heterostructure with excellent intrinsic activity. Density functional theory (DFT) calculations further revealed the ideal adsorption performance for reactant intermediates at the heterogeneous phase boundaries. This work provides an anticipated perspective of transition metal selenide bifunctional electrocatalysts for overall water splitting.
Introduction
Nowadays, the ever-worsening global energy crisis and environmental pollution push the development of renewable energy, especially toward ‘green hydrogen’ produced by water splitting.1,2 Currently, noble metal-based catalysts show the best performance for electrocatalytic water splitting with a low operating voltage. However, high cost and low reserves dramatically prevent their large-scale application.3,4 In this case, the development of highly active non-noble metal electrocatalysts has been a hot research topic in recent years, such as transition metal (Ni, Co, or Fe, etc.) alloys5–7 or compounds (phosphides, sulfides, selenides, nitrides or oxides, etc.).8–12 Although some of the materials demonstrate electrocatalytic hydrogen or oxygen evolution reaction (HER or OER) activities comparable to those of precious metal catalysts, the research and applications of those materials in overall water splitting are insufficient. To this end, it is particularly crucial to design and develop bifunctional non-noble metal electrocatalysts with low overpotential and good stability for highly efficient overall water splitting.Basically, high performance electrocatalysts for the HER require moderate adsorption/desorption properties with H*, while high performance electrocatalysts for the OER need balanced binding energy of O* between OH* and OOH*.3,13 In this regard, transition metal-based selenides (TMSes) have been widely studied as a newly discovered family for water electrolysis owing to their unique features.10 As sulfide analogs, TMSes have balanced hydrogen bonding energy, leading to favorable catalytic performance toward the HER.14 Meanwhile, some TMSes with a cubic pyrite-type crystal structure (e.g. NiSe2, CoSe2, etc.) can efficiently coordinate with water/hydroxide and undergo oxidation and reduction on an anode to construct a oxide/hydroxide shell with advanced OER catalytic activity and stability.15,16 Besides, the unique metal-like properties of selenium allow excellent electrical conductivity of TMSes, which leads to desirable charge transfer, reaction kinetics, and energy conversion efficiency.17,18 In order to further improve the intrinsic electrocatalytic capability of TMSes, there are some recent reports on construction of heterostructures to adjust the interface electronic structures, such as NiSe2–NiFe2Se4, Ni2P–NiSe2, NiSe2–CoSe2, etc.19–21 By constructing heterostructures, the charge transfer efficiency within the catalyst can be significantly enhanced due to the electricity differences of the two phases, while new active sites with a moderately regulated electronic structure that adapts for the adsorption of reaction intermediates generate at the heterogeneous phase boundaries.22–24 To maximally expose those highly active sites in TMSe-based heterostructures, different morphological regulation strategies have been proposed, such as designing ultra-thin nanosheets or nickel foam-based nanoarrays.25,26 However, the electrochemical active surface area and the amount of mass transfer paths of these structures still remain to be improved. Recently, metal aerogels have been widely researched due to their unique properties between powders and self-supporting materials. Compared with other powder materials, metal aerogels have three-dimensional interconnected network structures without carriers, which not only present a high specific area with plenty of active sites, but also provide abundant pathways for mass transfer.27,28 Benefiting from the advantages of the aerogel structure, some transition-metal-based structures have been reported as high-performance water electrolysis catalysts.29,30 For example, we have designed and prepared a P, Mo-codoped Ni aerogel (Ni–Mo–P aerogel) for electrocatalytic overall water splitting in our previous work. In contrast to the nanowires with the same composition, the Ni–Mo–P aerogel exhibits a larger electrochemical surface area and a better HER performance.31 Nevertheless, there are few reports dedicated to combining the TMSes or TMSe-based heterostructures and the unique aerogel morphology for efficient overall water splitting.
In this work, a NiSe2–CoSe2 aerogel was prepared by spontaneous gelation and selenium vapor deposition. The bifunctional NiSe2–CoSe2 catalyst exhibited an impressive performance for the HER (65 mV@10 mA cm−2) and the OER (220 mV@10 mA cm−2), and the assembled symmetrical overall water splitting system required a low cell voltage of 1.56 V to achieve a current density of 10 mA cm−2. The eminent performance of the optimized sample was attributed to the following two aspects: (1) the heterogeneous structure constructed by NiSe2 and CoSe2 led to the generation of active sites with ideal adsorption capability for reactant intermediates at the interface. Meanwhile, the charge transfer efficiency of the material was significantly improved due to the electrical property contrast of the two phases. (2) The NiSe2–CoSe2 heterostructure with higher intrinsic activity was successfully prepared with an aerogel morphology, providing a high specific surface area with lots of exposed active sites. This work provides a new perspective for the design of TMSe-based electrocatalysts for overall water splitting.
Materials and methods
Materials and reagents
Nickle chloride hexahydrate (NiCl2·6H2O, AR) and cobalt chloride hexahydrate (CoCl2·6H2O, AR) were purchased from Sinopharm Chemical Reagent Co. Sodium borohydride (NaBH4, 98%) was purchased from TianJin Fuchen Chemical Reagents Factory. Selenium (Se, 99.99%, powder, 100) was purchased from Adamas-beta. Pt/C (20 wt%) and RuO2 were purchased from Alfa Aesar.Ni–Co aerogel synthesis
In a typical preparation of the Ni–Co aerogel, 15 mL of NaBH4 (0.1 M) solution was quickly dropped into the mixed solution of 10 mL Ni2+ (0.05 M) and Co2+ (0.05 M). After 8 hours of gelation, the Ni–Co hydrogel was formed at the bottom of the flask. After 20 h of freeze drying, moisture was removed and the Ni–Co aerogel (Ni–Co-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was obtained. As comparison samples, the molar ratio of Ni2+ and Co2+ was changed to 2
1) was obtained. As comparison samples, the molar ratio of Ni2+ and Co2+ was changed to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 0
0 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to prepare the Ni–Co-2
1 to prepare the Ni–Co-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Ni–Co-1
1, Ni–Co-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, pure Ni and Co aerogels, respectively.
2, pure Ni and Co aerogels, respectively.
NiSe2–CoSe2 aerogel synthesis
To fabricate the NiSe2–CoSe2 aerogel, 20 mg of the Ni–Co-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aerogel and excess Se powder were placed at two separate positions in porcelain boats, respectively, of which Se powder was placed on the upstream side of the furnace. Then, the samples were heated up to 400 °C at 5 °C min−1 and maintained at this temperature for 120 min with Ar gas flowing at 50 sccm to obtain the NiSe2–CoSe2 aerogel. The comparison samples prepared in the previous step were selenized in the same way to get NiSe2–CoSe2-2
1 aerogel and excess Se powder were placed at two separate positions in porcelain boats, respectively, of which Se powder was placed on the upstream side of the furnace. Then, the samples were heated up to 400 °C at 5 °C min−1 and maintained at this temperature for 120 min with Ar gas flowing at 50 sccm to obtain the NiSe2–CoSe2 aerogel. The comparison samples prepared in the previous step were selenized in the same way to get NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NiSe2–CoSe2-1
1, NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, NiSe2 and CoSe2 aerogels, respectively. To investigate the influence of selenide temperature and determine the best reaction conditions, NiSe2–CoSe2-300 and NiSe2–CoSe2-500 aerogels were prepared by changing the reaction temperature to 300 °C and 500 °C, respectively.
2, NiSe2 and CoSe2 aerogels, respectively. To investigate the influence of selenide temperature and determine the best reaction conditions, NiSe2–CoSe2-300 and NiSe2–CoSe2-500 aerogels were prepared by changing the reaction temperature to 300 °C and 500 °C, respectively.
Materials characterization
Scanning electron microscopy (SEM) measurements were performed on a SU8010 microscope at an accelerating voltage of 5 kV. Transmission electron microscopy (TEM) and energy disperse spectroscopy (EDS) images were recorded using a Tecnai G2 F20 field emission transmission electron microscope. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) results were obtained from an Agilent ICPOES 730. Powder X-ray diffraction (XRD) was studied using a Bruker D8 Advance diffractometer. X-ray photoelectron spectroscopy (XPS) data of the samples were recorded on a Thermo Fisher K-Alpha American with an Al K X-ray source.Electrochemical measurements
Samples on carbon-cloth-based electrodes were prepared for all the electrochemical measurements. In this context, 5.0 mg of the sample was dispersed in the mixed solution with 0.25 mL ethanol and 0.25 mL deionized water, containing 20 μL of Nafion (5%) under sonication for 30 min to form a homogeneous catalyst ink. Then, the obtained ink was drop-cast onto the surface of carbon cloth (1 cm × 1 cm) followed by drying at 60 °C for 5 h to achieve the loading amount of 1.0 mg cm−2 for the sample on carbon cloth. For a comparison, Pt/C and RuO2 on carbon cloth were also prepared for evaluating HER, OER and overall water splitting by the same method.All the electrochemical properties of the samples were measured on a CHI 760 electrochemical workstation (CH Instruments, Inc., Shanghai) integrated with a three-electrode system at room temperature. Sample modified carbon cloth, graphite rod, and Hg/HgO acted as working, counter, and reference electrodes, respectively. The conversion potential of E (RHE) was obtained according to the following equation:
| E(RHE) = E(Hg/HgO) + 0.059 × pH + 0.098 |
Electrocatalytic performance of the HER and OER were tested by linear-sweep voltammetry in 1 M KOH solution at a scan rate of 5 mV s−1. The potential range of the HER and OER was −0.8 to −1.4 V and 0 to 0.8 V vs. the standard Hg/HgO electrode, respectively. All the polarization curves of the HER and OER were adjusted with 90% IR-correction according to the ohmic resistance of the solution (Rs), and the values of Rs was obtained from the electrochemical impedance spectroscopy (EIS) results under the open circuit potential. Besides, electrocatalytic performance of overall water splitting were tested by LSV on an electrolytic cell consisting of two carbon cloth electrodes with the optimized sample under the operating voltage from 1.0 to 2.0 V (without IR-correction). EIS measurements were applied to evaluate the charge transfer ability of the catalysts at a frequency range of 0.01 to 105 Hz under the voltage of −1.1 V. Double-layer capacitance (Cdl) values were assessed by cyclic voltammetry (CV) in the non-faradaic potential region ranging from −0.3 to −0.5 V vs. standard Hg/HgO electrode at 10 mV s−1, 50 mV s−1, 100 mV s−1, 150 mV s−1, and 200 mV s−1. Stability of samples was tested by chronoamperometric measurement under the voltages to achieve a current density of 10 mA cm−2.
Theoretical calculations
Density functional theory (DFT) calculation was performed using the DMol3 code as implemented in Materials Studio. The generalized gradient approximation of the Perdew–Burke–Ernzerhof (GGA-PBE) function was used to treat all the energy changes. The core treatment was effective core potentials (ECP) and the basis set was DNP v4.4. The k-point was set as 3 × 3 × 1 and the size of vacuum region was 15 Å. The Ni–Co model was formed by Ni(111) and Co(111) layers, and NiSe2, CoSe2, NiSe2–CoSe2 models were formed by NiSe2(210) and CoSe2(210) layers. The typical Ni–Co surface was modified by the U × 2 and V × 2 super cell of the Ni(111)–Co(111) layer, while all the selenide surfaces were modified by the U × 2 and V × 1 super cell of corresponding NiSe2(210), CoSe2(210) or NiSe2(210)–CoSe2(210) layers. The adsorption energies of hydrogen atoms and reaction intermediates of the OER on the structures were calculated by the following equation:| ΔG = ΔE + ΔZPE − TΔS |
In the equation, the ΔZPE and ΔS in the formula are the zero-point energy change and the entropy change between the surface and the adsorbed reaction intermediate molecule, which were obtained from the frequency calculation function of the DMol3 code. T was the temperature of the system, which was set to 298.15 K in all the calculations. Besides the ΔE was the binding energy of reaction intermediates and could be calculated by the following equation:
| ΔE = E(surf+i) − E(surf) − Ei |
Results and discussion
Catalyst synthesis and characterizations
The synthetic procedure of the NiSe2–CoSe2 aerogel involved three facile steps (Fig. 1). First of all, the Ni–Co hydrogel was synthesized via the spontaneous NaBH4 reduction and gelation process. The corresponding reactions of this step was described by the following equations:| Ni2+ + NaBH4 + 2H2O → Ni + NaBO2 + 2H+ + 2H2 |
| Co2+ + NaBH4 + 2H2O → Co + NaBO2 + 2H+ + 2H2 |
| NaBH4 + 2H2O → NaBO2 + 4H2 |
During this step, the in situ generated H2 gas from the reduction of Ni, Co elements and the hydrolysis of NaBH4 served as the gas template, directing the formation of porous interconnected networks (Fig. S1, ESI†).31,32 Then, the corresponding aerogel was obtained by freeze-drying without damaging the morphology of the gel. Finally, the Ni–Co aerogel was annealed under a selenium vapor atmosphere to obtain the NiSe2–CoSe2 aerogel.
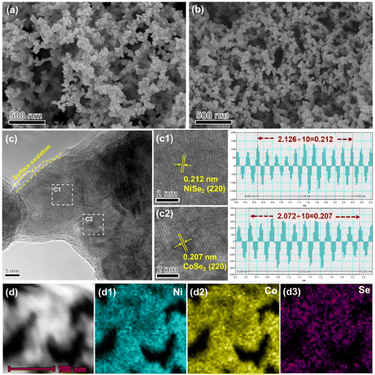
The morphologies and structures of the NiSe2–CoSe2 aerogel were characterized by SEM. To be specific, the Ni–Co precursor and NiSe2–CoSe2 aerogel exhibited the fluffy overall morphology in the large-scale images (Fig. S2(a) and (b), ESI†). Moreover, the Ni–Co aerogel presented a three-dimensional interconnected network formed by bimetallic alloy nanoparticles (Fig. 2(a)). After selenization, the NiSe2–CoSe2 aerogel still maintained the unique porous morphology (Fig. 2(b)), which could be further confirmed by the high-resolution SEM images (Fig. S2(c) and (d), ESI†) and low-resolution TEM images (Fig. S3, ESI†). Such a shaggy and porous structure provided abundant active sites and mass transfer pathways.32,33 According to reported literatures and our previous works,28,31,34 the type and mole ratio of metals are the key factors affecting the morphology of the aerogel. Notably, the NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and NiSe2 aerogels with a higher Ni content tended to form the network structure composed of comparatively small nanoparticles (Fig. S4(a) and (c), ESI†), while the NiSe2–CoSe2-1
1 and NiSe2 aerogels with a higher Ni content tended to form the network structure composed of comparatively small nanoparticles (Fig. S4(a) and (c), ESI†), while the NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and CoSe2 were entirely comprised of larger nanoparticles without an appreciable network skeleton (Fig. S4(b) and (d), ESI†).35 In addition, the selenization temperature also played an important role in modulating the morphology. NiSe2–CoSe2-300 was identified to retain the porous network structure, while NiSe2–CoSe2-500 agglomerated obviously and gradually lost the aerogel morphology (Fig. S5, ESI†). The HRTEM of the NiSe2–CoSe2 aerogel is shown in Fig. 2(c). Because of contact with air, a thick oxide layer was formed on the surface of aerogel.34,36 Based on the results of Fast Fourier Transform (FFT), the (220) crystal planes of NiSe2 (0.212 nm, Fig. 2(c1)) and CoSe2 (0.207 nm, Fig. 2(c2)) were observed, which preliminarily proved the formation of the NiSe2–CoSe2 heterostructure.21,26 Besides, the STEM-mapping of the NiSe2–CoSe2 aerogel showed that Ni, Co, and Se were evenly distributed in the skeleton (Fig. 2(d1)–(d3)), which revealed the construction of the selenide aerogel. Meanwhile, the Ni, Co, and Se contents of NiSe2–CoSe2, NiSe2–CoSe2-2
2 and CoSe2 were entirely comprised of larger nanoparticles without an appreciable network skeleton (Fig. S4(b) and (d), ESI†).35 In addition, the selenization temperature also played an important role in modulating the morphology. NiSe2–CoSe2-300 was identified to retain the porous network structure, while NiSe2–CoSe2-500 agglomerated obviously and gradually lost the aerogel morphology (Fig. S5, ESI†). The HRTEM of the NiSe2–CoSe2 aerogel is shown in Fig. 2(c). Because of contact with air, a thick oxide layer was formed on the surface of aerogel.34,36 Based on the results of Fast Fourier Transform (FFT), the (220) crystal planes of NiSe2 (0.212 nm, Fig. 2(c1)) and CoSe2 (0.207 nm, Fig. 2(c2)) were observed, which preliminarily proved the formation of the NiSe2–CoSe2 heterostructure.21,26 Besides, the STEM-mapping of the NiSe2–CoSe2 aerogel showed that Ni, Co, and Se were evenly distributed in the skeleton (Fig. 2(d1)–(d3)), which revealed the construction of the selenide aerogel. Meanwhile, the Ni, Co, and Se contents of NiSe2–CoSe2, NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and NiSe2–CoSe2-1
1 and NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 were analyzed by SEM-EDS (Fig. S6, ESI†) and further confirmed by the results of ICP-OES (Table S1, ESI†). The proportion of Ni and Co in each sample conformed well to our design, while the Se contents were 30–40 wt%.
2 were analyzed by SEM-EDS (Fig. S6, ESI†) and further confirmed by the results of ICP-OES (Table S1, ESI†). The proportion of Ni and Co in each sample conformed well to our design, while the Se contents were 30–40 wt%.
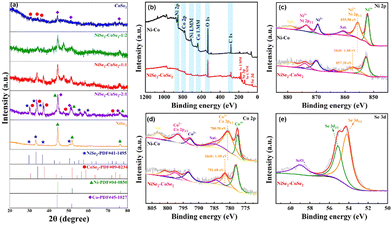
The crystalline structure of NiSe2–CoSe2 and comparison samples were investigated by XRD (Fig. 3(a)). The patterns of NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NiSe2–CoSe2-2
1, NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and NiSe2–CoSe2-1
1 and NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aerogels exhibited the characteristic diffraction peaks of NiSe2 (PDF#41-1495) and CoSe2 (PDF#09-0234). Meanwhile, the selenides on the surface of the aerogel prevented selenium vapor from contacting the Ni and Co inside the skeleton, so that the corresponding peaks of Ni (PDF#04-0850) and Co (PDF#45-1027) could be observed in the patterns.19 Compared with the standard cards, the shift and superposition of NiSe2 and CoSe2 characteristic peaks at 30°–40° could be observed, which was attributed to the lattice distortion caused by the interaction between the two selenide crystals, thus proving the formation of a bimetallic selenide heterostructure.37,38 In addition, the NiSe2 aerogel showed good crystallinity with distinct characteristic peaks of Ni and NiSe2, but the crystallinity of the CoSe2 aerogel was comparatively poor due to the easy formation of an amorphous oxide layer on the surface.39,40 Therefore, the crystallization degree of the aerogels could be regulated by constructing a bimetallic selenide heterostructure to expose highly-active crystal planes.41
2 aerogels exhibited the characteristic diffraction peaks of NiSe2 (PDF#41-1495) and CoSe2 (PDF#09-0234). Meanwhile, the selenides on the surface of the aerogel prevented selenium vapor from contacting the Ni and Co inside the skeleton, so that the corresponding peaks of Ni (PDF#04-0850) and Co (PDF#45-1027) could be observed in the patterns.19 Compared with the standard cards, the shift and superposition of NiSe2 and CoSe2 characteristic peaks at 30°–40° could be observed, which was attributed to the lattice distortion caused by the interaction between the two selenide crystals, thus proving the formation of a bimetallic selenide heterostructure.37,38 In addition, the NiSe2 aerogel showed good crystallinity with distinct characteristic peaks of Ni and NiSe2, but the crystallinity of the CoSe2 aerogel was comparatively poor due to the easy formation of an amorphous oxide layer on the surface.39,40 Therefore, the crystallization degree of the aerogels could be regulated by constructing a bimetallic selenide heterostructure to expose highly-active crystal planes.41
XPS analysis was used to analyze the chemical states of Ni–Co and NiSe2–CoSe2 aerogels. As demonstrated in Fig. 3(b), the survey spectrum of Ni–Co and NiSe2–CoSe2 aerogels demonstrated the co-presence of Ni, Co, C, and O fundamental elements. The characteristic peak of Se 3d was observed for the NiSe2–CoSe2 aerogel, which indicated the generation of selenides. The high-resolution Ni 2p and Co 2p spectra were respectively manifested in Fig. 3(c) and (d), and four major peaks of Ni and Co with two satellite peaks were identified. For the Ni–Co aerogel, the peaks located at 852.1 and 869.5 eV were assigned to Ni3+, and the peaks at 855.5 and 873.4 eV were attributed to Ni2+ of Ni 2p3/2 and Ni2+ of Ni 2p1/2, respectively. Similarly, the peaks located at 777.5 and 792.8 eV were fitted to Co3+, and the peaks at 780.5 and 796.6 eV were assigned to the Co2+ 2p3/2 and Co 2+ of Co 2p1/2, respectively.21 For NiSe2–CoSe2, the peaks of Ni2+ and Co2+ showed obvious positive shifts of 1.80 eV and 1.10 eV, respectively, indicating the electronic structure regulation due to selenization. Such alteration of electron densities around the bimetallic atoms could benefit the adsorption of OH− ions and the desorption of H* during alkaline electrocatalytic water splitting.42Fig. 3(e) showed the Se 3d spectra of the NiSe2–CoSe2 aerogel. The peaks at 54.2 and 55.1 eV were assigned to Se2− of Se 3d5/2 and Se2− of Se 3d3/2, while the peak at 59.0 eV was attributed to SeOx due to air oxidation.43
Electrocatalytic activity toward the HER
The electrocatalytic activity of NiSe2–CoSe2 toward the HER was investigated through LSV in 1.0 M KOH under a scan rate of 5 mV s−1. For comparison, NiSe2, CoSe2, Ni–Co aerogels, and commercial Pt/C on carbon cloth with similar mass loading were also tested (Fig. 4(a), and the curves before IR-correction are shown in Fig. S7, ESI†). The Pt/C showed the lowest overpotential of 34 mV@10 mA cm−2, which was consistent with the reported literature.44 The NiSe2–CoSe2 also afforded remarkable HER activity with an overpotential of only 65 mV@10 mA cm−2. It obviously outperformed the counterparts of the NiSe2 aerogel (131 mV@10 mA cm−2), CoSe2 aerogel (173 mV@10 mA cm−2), and Ni–Co aerogel (94 mV@10 mA cm−2), indicating the synergic enhancement of the bimetallic selenide heterostructure. In order to determine the best ratio of Ni and Co, we also tested the HER performance of NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (84 mV@10 mA cm−2) and NiSe2–CoSe2-1
1 (84 mV@10 mA cm−2) and NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aerogels (92 mV@10 mA cm−2) (Fig. S8(a) (ESI†), and the curves before IR-correction are shown in Fig. S8(b), ESI†). It was found that the sample with the balanced ratio of Ni and Co (1
2 aerogels (92 mV@10 mA cm−2) (Fig. S8(a) (ESI†), and the curves before IR-correction are shown in Fig. S8(b), ESI†). It was found that the sample with the balanced ratio of Ni and Co (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed the best performance, indicating the optimized construction of a heterogeneous interface under this ratio. To optimize the selenization temperature, we tested the HER performance of NiSe2–CoSe2-300 (95 mV@10 mA cm−2) and NiSe2–CoSe2-500 (133 mV@10 mA cm−2) as well (Fig. S8(c), ESI,† and the curves before IR-correction are shown in Fig. S8(d), ESI†). NiSe2–CoSe2-300 showed similar HER performance with the Ni–Co aerogel, indicating that NiSe2–CoSe2-300 was barely selenized at 300 °C. Meanwhile, the performance of NiSe2–CoSe2-500 decreased significantly due to structural agglomeration under the elevated temperature of 500 °C. Thus, we determined that 400 °C was the optimized selenization temperature. Tafel plots were further conducted to analyze the electrocatalytic performance. In Fig. 4(b), the NiSe2–CoSe2 aerogel delivered a Tafel slope of 57.54 mV dec−1, lower than those of NiSe2 (95.36 mV dec−1), CoSe2 (115.68 mV dec−1), and Ni–Co (75.68 mV dec−1) aerogels. The low Tafel slope on the NiSe2–CoSe2 aerogel indicated the accelerated H2 generation with the applied overpotential, in accordance with its high activity.3 Tafel slopes of aerogels with different metal proportions and different selenization temperatures were also calculated in Fig. S9 (ESI†), which were also consistent with their HER performances. The cyclic stability of the NiSe2–CoSe2 aerogel with the best performance was tested by cyclic voltammetry and chronoamperometric measurement at an initial current density of 10 mA cm−2 (Fig. 4(c) and (d)). A tiny deflection of LSV results was observed before and after 2000 CV cycles, verifying the impressive stability of the NiSe2–CoSe2 aerogel. After a 40 h test, the current density retained 97% of the initial value, which further indicated the favorable stability of the NiSe2–CoSe2 aerogel.
1) showed the best performance, indicating the optimized construction of a heterogeneous interface under this ratio. To optimize the selenization temperature, we tested the HER performance of NiSe2–CoSe2-300 (95 mV@10 mA cm−2) and NiSe2–CoSe2-500 (133 mV@10 mA cm−2) as well (Fig. S8(c), ESI,† and the curves before IR-correction are shown in Fig. S8(d), ESI†). NiSe2–CoSe2-300 showed similar HER performance with the Ni–Co aerogel, indicating that NiSe2–CoSe2-300 was barely selenized at 300 °C. Meanwhile, the performance of NiSe2–CoSe2-500 decreased significantly due to structural agglomeration under the elevated temperature of 500 °C. Thus, we determined that 400 °C was the optimized selenization temperature. Tafel plots were further conducted to analyze the electrocatalytic performance. In Fig. 4(b), the NiSe2–CoSe2 aerogel delivered a Tafel slope of 57.54 mV dec−1, lower than those of NiSe2 (95.36 mV dec−1), CoSe2 (115.68 mV dec−1), and Ni–Co (75.68 mV dec−1) aerogels. The low Tafel slope on the NiSe2–CoSe2 aerogel indicated the accelerated H2 generation with the applied overpotential, in accordance with its high activity.3 Tafel slopes of aerogels with different metal proportions and different selenization temperatures were also calculated in Fig. S9 (ESI†), which were also consistent with their HER performances. The cyclic stability of the NiSe2–CoSe2 aerogel with the best performance was tested by cyclic voltammetry and chronoamperometric measurement at an initial current density of 10 mA cm−2 (Fig. 4(c) and (d)). A tiny deflection of LSV results was observed before and after 2000 CV cycles, verifying the impressive stability of the NiSe2–CoSe2 aerogel. After a 40 h test, the current density retained 97% of the initial value, which further indicated the favorable stability of the NiSe2–CoSe2 aerogel.
Electrocatalytic activity towards OER and overall water splitting
The OER polarization curve of the NiSe2–CoSe2 aerogel and contrast samples were also determined through LSV in 1.0 M KOH under a scan rate of 5 mV s−1 (Fig. 5(a), and the curves before IR-correction are shown in Fig. S10, ESI†). The oxidation peaks could be observed at around 1.33 V in the curves of NiSe2 and CoSe2 aerogels, which represented the reconstruction process and the generation of OOH*.42 In contrast with the single metallic selenides, the Ni–Co aerogel exhibited a lower onset voltage of oxidation at 1.25 V, indicating that the facilitated charge transfer efficiency enhanced the reconstruction process. As Ni–Co was further selenized to NiSe2–CoSe2, the onset voltage of oxidation decreased to 1.23 V, demonstrating that the bimetallic selenide heterostructure had the surface that favored to reconstruct and generate active sites with excellent OER performance.10 Benefiting from the rapid reconstruction, NiSe2–CoSe2 exhibited the lowest overpotential of 220 mV@10 mA cm−2, significantly better than that of NiSe2 (304 mV), CoSe2 (289 mV) and Ni–Co (265 mV). Notably, the performance of the NiSe2–CoSe2 aerogel was superior to the commercial RuO2 (251 mV@10 mA cm−2), demonstrating that the selenide heterostructure possessed great application potential in the electrocatalytic OER process. Besides, NiSe2–CoSe2 exhibited a Tafel slope of 99.57 mV dec−1, lower than that of NiSe2 (157.71 mV dec−1), CoSe2 (131.52 mV dec−1) and Ni–Co (109.47 mV dec−1), which indicated the favorable reaction kinetics of the Ni–Co selenide heterostructure (Fig. 5(b)). To further illustrate the optimal metal ratio and reaction temperature, the OER properties of NiSe2–CoSe2-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NiSe2–CoSe2-1
1, NiSe2–CoSe2-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, NiSe2–CoSe2-300, and NiSe2–CoSe2-500 aerogels were also tested (Fig. S11 and S12, ESI†). Similar to the HER, the sample with a metal ratio of 1
2, NiSe2–CoSe2-300, and NiSe2–CoSe2-500 aerogels were also tested (Fig. S11 and S12, ESI†). Similar to the HER, the sample with a metal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and selenization temperature of 400 °C had the best OER performance. All of the above results indicated that the optimized NiSe2–CoSe2 had the best OER performance due to its balanced ratio of NiSe2/CoSe2 and favorable morphology. Stability of NiSe2–CoSe2 toward the OER was also evaluated by cyclic voltammetry and chronoamperometric measurements at an initial current density of 10 mA cm−2 (Fig. 5(c) and (d)). A deflection of LSV results was observed before and after 2000 CV cycles. Such a change in performance mainly came from the sample falling out caused by oxygen bubble overflow. After a 40 h test, 93% of the initial value of the current density was retained, indicating the favorable stability of the NiSe2–CoSe2 aerogel toward the OER.
1 and selenization temperature of 400 °C had the best OER performance. All of the above results indicated that the optimized NiSe2–CoSe2 had the best OER performance due to its balanced ratio of NiSe2/CoSe2 and favorable morphology. Stability of NiSe2–CoSe2 toward the OER was also evaluated by cyclic voltammetry and chronoamperometric measurements at an initial current density of 10 mA cm−2 (Fig. 5(c) and (d)). A deflection of LSV results was observed before and after 2000 CV cycles. Such a change in performance mainly came from the sample falling out caused by oxygen bubble overflow. After a 40 h test, 93% of the initial value of the current density was retained, indicating the favorable stability of the NiSe2–CoSe2 aerogel toward the OER.
In another regard, the excellent performance of NiSe2–CoSe2 toward both the HER and OER in an alkaline medium could also be accounted for the high charge transfer efficiency and large electrochemically active surface area (ECSA)-introduced efficient charge transfer and abundant active sites. The charge transfer resistances (Rct) of NiSe2–CoSe2 and comparison samples were tested by EIS analysis (Fig. 6(a)). Compared with NiSe2 (39.70 Ω), CoSe2 (59.51 Ω), and Ni–Co (25.87 Ω) aerogels, the NiSe2–CoSe2 showed the lowest Rct of 12.16 Ω, indicating the enhanced charge transfer ability offered by the heterostructure. Besides, we also tested the conductivities of other prepared samples (Fig. S13, ESI†). NiSe2–CoSe2 showed the best electron conductive performance, which further supported the optimal preparation conditions. After that, electrochemical double-layer capacitance (Cdl) was applied to estimate the electrochemical surface area of different samples (Fig. 6(b)) based on CV curves (Fig. S14, ESI†). The Cdl value of the NiSe2–CoSe2 aerogel was calculated to be 18.50 mF cm−2, which was higher than that of NiSe2 (3.64 mF cm−2), CoSe2 (3.25 mF cm−2), and Ni–Co (1.16 mF cm−2) aerogels, indicating that the heterostructure activated additional exposed active sites. The same electrochemical tests were performed on other comparison samples (Fig. S15, ESI†), and the NiSe2–CoSe2 aerogel exhibited a higher Cdl value than other aerogels, suggesting that the sufficient construction of the heterostructure based on the optimal preparation conditions could fully activate catalytic sites and provide a large electrochemical surface area. Besides, the optimized NiSe2–CoSe2 aerogel exhibited a Cdl value superior to some reported selenides or heterostructure electrocatalysts with other nanostructures (Table S2, ESI†), proving plenty of active sites and abundant mass transfer pathways provided by the aerogel skeleton.
On the basis of good activities of HER and OER, an overall water splitting cell was prepared with two NiSe2–CoSe2 electrodes under alkaline conditions (Fig. 6(c)). The NiSe2–CoSe2 aerogel device exhibited a low potential of 1.56 V@10 mA cm−2, which was equal to the performance of Pt/C + RuO2 (1.56 V). Note that the ratio of H2 and O2 was close to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S16, ESI†), indicating that the faradaic efficiency (FE) was nearly 100% for each gas evolution reaction.21 Besides, the activity of NiSe2–CoSe2 was also comparable with other reported selenides or heterostructure electrocatalysts (Table S3, ESI†). In addition to catalytic activity, the stability of the overall water splitting cell was also investigated. Fig. 6(d) shows that the NiSe2–CoSe2 device had decent durability, with a current retention of 91% after 40 h operation. Furthermore, the XRD patterns of NiSe2–CoSe2 after the stability test of overall water splitting are shown in Fig. S17 (ESI†). The characteristic peaks of selenides (NiSe2 and CoSe2) and metal elements (Ni and Co) that are identical to the unreacted sample could be observed in the XRD pattern of the cathodic NiSe2–CoSe2 aerogel, suggesting that the overall structure and composition of the tested hybrid was unaltered. This result could indicate the superior electrochemical stability of the selenides heterostructure in the HER process.21 Moreover, there were no obvious peaks in the XRD pattern of the anodic NiSe2–CoSe2 aerogel, which was attributed to the transformation of crystal structures from selenides and metal elements to reconstructed hydroxides (Ni(OH)2 and Co(OH)2) and hydroxyl oxides (NiOOH and CoOOH) in the OER process.42
1 (Fig. S16, ESI†), indicating that the faradaic efficiency (FE) was nearly 100% for each gas evolution reaction.21 Besides, the activity of NiSe2–CoSe2 was also comparable with other reported selenides or heterostructure electrocatalysts (Table S3, ESI†). In addition to catalytic activity, the stability of the overall water splitting cell was also investigated. Fig. 6(d) shows that the NiSe2–CoSe2 device had decent durability, with a current retention of 91% after 40 h operation. Furthermore, the XRD patterns of NiSe2–CoSe2 after the stability test of overall water splitting are shown in Fig. S17 (ESI†). The characteristic peaks of selenides (NiSe2 and CoSe2) and metal elements (Ni and Co) that are identical to the unreacted sample could be observed in the XRD pattern of the cathodic NiSe2–CoSe2 aerogel, suggesting that the overall structure and composition of the tested hybrid was unaltered. This result could indicate the superior electrochemical stability of the selenides heterostructure in the HER process.21 Moreover, there were no obvious peaks in the XRD pattern of the anodic NiSe2–CoSe2 aerogel, which was attributed to the transformation of crystal structures from selenides and metal elements to reconstructed hydroxides (Ni(OH)2 and Co(OH)2) and hydroxyl oxides (NiOOH and CoOOH) in the OER process.42
Theorical calculation
To further understand the origin of the observed excellent performance of both the HER and OER on the NiSe2–CoSe2 aerogel, theoretical calculations were conducted using density functional theory (DFT) with the Materials Studio-DMol.3 Considering the complexity of the aerogel with surface oxidation and metals without selenide, we simplified the model and focused on the interactions within the bimetallic selenide heterostructure. Based on the XRD results, we formed the models of Ni–Co, NiSe2, CoSe2, and NiSe2–CoSe2, and all structures are shown in Fig. 7(a). For the HER process, we mainly focused on the adsorption properties of metal sites (Ni or Co site) for H* (Fig. 7(b)). In the NiSe2–CoSe2 structure, the Ni site exhibited stronger free energy of hydrogen adsorption ( , −0.26 eV) than that of the Co site (−0.18 eV), indicating that electrons transferred from CoSe2 to NiSe2 at the heterointerface. In contrast, the Co site had the
, −0.26 eV) than that of the Co site (−0.18 eV), indicating that electrons transferred from CoSe2 to NiSe2 at the heterointerface. In contrast, the Co site had the  closer to zero (ideal state), demonstrating the favorable adsorption properties and high activity toward the HER. Meanwhile, the Co site in NiSe2–CoSe2 had markedly better adsorption properties toward H* than that of Ni–Co (−0.32 eV), NiSe2 (−0.40 eV) and CoSe2 (−0.49 eV), indicating the superior activity toward the HER on the heterointerface between NiSe2 and CoSe2.21 Furthermore, we proposed the detailed OER mechanism of NiSe2–CoSe2 by DFT calculations. The OER reaction could be divided into the following 4 steps:
closer to zero (ideal state), demonstrating the favorable adsorption properties and high activity toward the HER. Meanwhile, the Co site in NiSe2–CoSe2 had markedly better adsorption properties toward H* than that of Ni–Co (−0.32 eV), NiSe2 (−0.40 eV) and CoSe2 (−0.49 eV), indicating the superior activity toward the HER on the heterointerface between NiSe2 and CoSe2.21 Furthermore, we proposed the detailed OER mechanism of NiSe2–CoSe2 by DFT calculations. The OER reaction could be divided into the following 4 steps:| M + OH− = M·OH* + e− |
| M·OH* + OH− = M·O* + H2O + e− |
| M·O* + OH− = M·OOH* + e− |
| M·OOH* + OH− = M + O2 + H2O + e− |
The calculated results (Fig. 7(c)) showed that the third electron transfer step to form *OOH exhibited the largest ladder span with an energy barrier of 1.86 eV, which was the rate-limiting step of the OER.45 Besides, the free energy difference between the intermediates HO* and OOH* was a definite value (about 3.2 eV). When the difference of the energy barriers between HO* to O* and O* to OOH* was smaller, the OER process would be easier.46 For the NiSe2–CoSe2, the difference value between the two steps was only 0.43 eV, which further validated the excellent reaction kinetics. All the theoretical calculations were in good agreement with the experimental results.
Conclusions
In summary, we designed and synthesized a NiSe2–CoSe2 aerogel via spontaneous gelation followed by a facile chemical vapor deposition selenide process. The bifunctional NiSe2–CoSe2 aerogel showed splendid HER, OER and overall water splitting capabilities with low potentials and favorable stabilities. Systematic characterization, electrochemical testing and DFT calculations illustrated that the superior performances were attributed to the large effective surface area of the aerogel with a three-dimensional interconnected network structure, as well as the high intrinsic activity of the heterostructure formed by NiSe2 and CoSe2. Our work afforded an uncomplicated approach to construct potentially scalable electrocatalysts using TMSe-based aerogels.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (No. 22278431 and No. 21776302) and the Science Foundation of China University of Petroleum, Beijing (No. 2462020YXZZ033) for financial support of this work.Notes and references
- I. Staffell, D. Scamman, A. Velazquez Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, The role of hydrogen and fuel cells in the global energy system, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- J. Schmidt, K. Gruber, M. Klingler, C. Klöckl, L. Ramirez Camargo, P. Regner, O. Turkovska, S. Wehrle and E. Wetterlund, A new perspective on global renewable energy systems: Why trade in energy carriers matters, Energy Environ. Sci., 2019, 12, 2022–2029 RSC.
- J. Zhu, L. Hu, P. Zhao, L. Y. S. Lee and K. Y. Wong, Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles, Chem. Rev., 2020, 120, 851–918 CrossRef CAS PubMed.
- E. Fabbri, A. Habereder, K. Waltar, R. Kötz and T. J. Schmidt, Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction, Catal. Sci. Technol., 2014, 4, 3800–3821 RSC.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Nørskov, Computational high-throughput screening of electrocatalytic materials for hydrogen evolution, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- M. Chen, D. Liu, B. Zi, Y. Chen, D. Liu, X. Du, F. Li, P. Zhou, Y. Ke, J. Li, K. H. Lo, C. T. Kwok, W. F. Ip, S. Chen, S. Wang, Q. Liu and H. Pan, Remarkable synergistic effect in cobalt-iron nitride/alloy nanosheets for robust electrochemical water splitting, J. Energy Chem., 2021, 65, 405–414 CrossRef.
- Y. Zhou, B. Tang, S. Wang and J. Long, Cu-MOF@Co-MOF derived Co–Cu alloy nanoparticles and N atoms co-doped carbon matrix as efficient catalyst for enhanced oxygen reduction, Int. J. Hydrogen Energy, 2020, 45, 15785–15795 CrossRef CAS.
- A. Parra-Puerto, K. L. Ng, K. Fahy, A. E. Goode, M. P. Ryan and A. Kucernak, Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes, ACS Catal., 2019, 9, 11515–11529 CrossRef CAS.
- Y. Zhao, S. Wei, K. Pan, Z. Dong, B. Zhang, H. H. Wu, Q. Zhang, J. Lin and H. Pang, Self-supporting transition metal chalcogenides on metal substrates for catalytic water splitting, Chem. Eng. J., 2021, 421, 129645 CrossRef CAS.
- X. Peng, Y. Yan, X. Jin, C. Huang, W. Jin, B. Gao and P. K. Chu, Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting, Nano Energy, 2020, 78, 105234 CrossRef CAS.
- M. S. Balogun, Y. Huang, W. Qiu, H. Yang, H. Ji and Y. Tong, Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting, Mater. Today, 2017, 20, 425–451 CrossRef CAS.
- I. Concina, Z. H. Ibupoto and A. Vomiero, Semiconducting metal oxide nanostructures for water splitting and photovoltaics, Adv. Energy Mater., 2017, 7, 1–29 Search PubMed.
- J. Mohammed-ibrahim and H. Moussab, Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting – A review, J. Energy Chem., 2021, 56, 299–342 CrossRef.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- S. Anantharaj and S. Noda, Nickel selenides as pre-catalysts for electrochemical oxygen evolution reaction: A review, Int. J. Hydrogen Energy, 2020, 45, 15763–15784 CrossRef CAS.
- X. Xia, L. Wang, N. Sui, V. L. Colvin and W. W. Yu, Recent progress in transition metal selenide electrocatalysts for water splitting, Nanoscale, 2020, 12, 12249–12262 RSC.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Strong Electronic Interaction in Dual-Cation-Incorporated NiSe2 Nanosheets with Lattice Distortion for Highly Efficient Overall Water Splitting, Adv. Mater., 2018, 30, 1–7 Search PubMed.
- Y. Chen, J. Zhang, P. Guo, H. Liu, Z. Wang, M. Liu, T. Zhang, S. Wang, Y. Zhou, X. Lu and J. Zhang, Coupled Heterostructure of Mo-Fe Selenide Nanosheets Supported on Carbon Paper as an Integrated Electrocatalyst for Efficient Hydrogen Evolution, ACS Appl. Mater. Interfaces, 2018, 10, 27787–27794 CrossRef CAS PubMed.
- J. Yuan, X. Cheng, H. Wang, C. Lei, S. Pardiwala, B. Yang, Z. Li, Q. Zhang, L. Lei, S. Wang and Y. Hou, A Superaerophobic Bimetallic Selenides Heterostructure for Efficient Industrial-Level Oxygen Evolution at Ultra-High Current Densities, Nano-Micro Lett., 2020, 12, 1–12 Search PubMed.
- C. Liu, T. Gong, J. Zhang, X. Zheng, J. Mao, H. Liu, Y. Li and Q. Hao, Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution, Appl. Catal., B, 2020, 262, 1–8 Search PubMed.
- D. Chen, Z. Xu, W. Chen, G. Chen, J. Huang, J. Huang, C. Song, C. Li and K. (Ken) Ostrikov, Just add water to split water: ultrahigh-performance bifunctional electrocatalysts fabricated using eco-friendly heterointerfacing of NiCo diselenides, J. Mater. Chem. A, 2020, 8, 12035–12044 RSC.
- B. Wang, C. Tang, H. F. Wang, X. Chen, R. Cao and Q. Zhang, A Nanosized CoNi Hydroxide@Hydroxysulfide Core–Shell Heterostructure for Enhanced Oxygen Evolution, Adv. Mater., 2019, 31, 1–7 Search PubMed.
- Y. Li, W. Wang, B. Huang, Z. Mao, R. Wang, B. He, Y. Gong and H. Wang, Abundant heterointerfaces in MOF-derived hollow CoS2–MoS2 nanosheet array electrocatalysts for overall water splitting, J. Energy Chem., 2021, 57, 99–108 CrossRef CAS.
- C. Zhang, Y. Xue, L. Hui, Y. Fang, Y. Liu and Y. Li, Graphdiyne@NiOx(OH)y heterostructure for efficient overall water splitting, Mater. Chem. Front., 2021, 5, 5305–5311 RSC.
- K. Xiao, L. Zhou, M. Shao and M. Wei, Fabrication of (Ni,Co)0.85Se nanosheet arrays derived from layered double hydroxides toward largely enhanced overall water splitting, J. Mater. Chem. A, 2018, 6, 7585–7591 RSC.
- X. Zhang, Y. Ding, G. Wu and X. Du, CoSe2@NiSe2 nanoarray as better and efficient electrocatalyst for overall water splitting, Int. J. Hydrogen Energy, 2020, 45, 30611–30621 CrossRef CAS.
- Y. Li, S. Guo, T. Jin, Y. Wang, F. Cheng and L. Jiao, Promoted synergy in core-branch CoP@NiFe–OH nanohybrids for efficient electrochemical-/photovoltage-driven overall water splitting, Nano Energy, 2019, 63, 103821 CrossRef CAS.
- H. Wang, Q. Fang, W. Gu, D. Du, Y. Lin and C. Zhu, Noble Metal Aerogels, ACS Appl. Mater. Interfaces, 2020, 12, 52234–52250 CrossRef CAS PubMed.
- O. Lori, N. Zion, H. C. Honig and L. Elbaz, 3D metal carbide aerogel network as a stable catalyst for the hydrogen evolution reaction, ACS Catal., 2021, 11, 13707–13713 CrossRef CAS.
- Z. Lin, S. Liu, Y. Liu, Z. Liu, S. Zhang, X. Zhang, Y. Tian and Z. Tang, Rational design of Ru aerogel and RuCo aerogels with abundant oxygen vacancies for hydrogen evolution reaction, oxygen evolution reaction, and overall water splitting, J. Power Sources, 2021, 514, 230600 CrossRef CAS.
- B. Zhang, F. Yang, X. Liu, N. Wu, S. Che and Y. Li, Phosphorus doped nickel-molybdenum aerogel for efficient overall water splitting, Appl. Catal., B, 2021, 298, 120494 CrossRef CAS.
- L. Liu, L. X. Chen, A. J. Wang, J. Yuan, L. Shen and J. J. Feng, Hydrogen bubbles template-directed synthesis of self-supported AuPt nanowire networks for improved ethanol oxidation and oxygen reduction reactions, Int. J. Hydrogen Energy, 2016, 41, 8871–8880 CrossRef CAS.
- A. A. Dubale, Y. Zheng, H. Wang, R. Hübner, Y. Li, J. Yang, J. Zhang, N. K. Sethi, L. He, Z. Zheng and W. Liu, High-Performance Bismuth-Doped Nickel Aerogel Electrocatalyst for the Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2020, 59, 13891–13899 CrossRef CAS PubMed.
- B. Cai and A. Eychmüller, Promoting Electrocatalysis upon Aerogels, Adv. Mater., 2019, 31, 1–16 Search PubMed.
- K. Deng, T. Ren, Y. Xu, S. Liu, Z. Dai, Z. Wang, X. Li, L. Wang and H. Wang, Transition metal M (M = Co, Ni, and Fe) and boron co-modulation in Rh-based aerogels for highly efficient and pH-universal hydrogen evolution electrocatalysis, J. Mater. Chem. A, 2020, 8, 5595–5600 RSC.
- F. Chen, F. Yang, H. Liu, S. Che, G. Zhang, C. Xu and Y. Li, One-pot preparation of surface vulcanization Co-Fe bimetallic aerogel for efficient sulfadiazine degradation, Chem. Eng. J., 2022, 430, 132904 CrossRef CAS.
- B. Jansi Rani, G. Ravi, R. Yuvakkumar, B. Saravanakumar, M. Thambidurai, C. Dang and D. Velauthapillai, CoNiSe2Nanostructures for Clean Energy Production, ACS Omega, 2020, 5, 14702–14710 CrossRef CAS.
- C. Liu, T. Gong, J. Zhang, X. Zheng, J. Mao, H. Liu, Y. Li and Q. Hao, Engineering Ni2P-NiSe2 heterostructure interface for highly efficient alkaline hydrogen evolution, Appl. Catal., B, 2020, 262, 1–8 Search PubMed.
- A. Badruzzaman, A. Yuda, A. Ashok and A. Kumar, Recent advances in cobalt based heterogeneous catalysts for oxygen evolution reaction, Inorg. Chim. Acta, 2020, 511, 119854 CrossRef CAS.
- V. D. Nithya, Recent advances in CoSe2 electrocatalysts for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2021, 46, 36080–36102 CrossRef CAS.
- Y. Yang, W. Zhang, Y. Xiao, Z. Shi, X. Cao and Y. Tang, CoNiSe 2 heteronanorods decorated with layered-double-hydroxides for efficient hydrogen evolution, Appl. Catal., B, 2019, 242, 132–139 CrossRef CAS.
- X. Yun, T. Lu, R. Zhou, Z. Lu, J. Li and Y. Zhu, Heterostructured NiSe 2/CoSe 2 hollow microspheres as battery-type cathode for hybrid supercapacitors: Electrochemical kinetics and energy storage mechanism, Chem. Eng. J., 2021, 426, 131328 CrossRef CAS.
- L. Wei, J. Luo, L. Jiang, L. Qiu, J. Zhang, D. Zhang, P. Xu and D. Yuan, CoSe 2 nanoparticles grown on carbon nanofibers derived from bacterial cellulose as an efficient electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2018, 43, 20704–20711 CrossRef CAS.
- V. H. Hoa, D. T. Tran, D. C. Nguyen, D. H. Kim, N. H. Kim and J. H. Lee, Molybdenum and Phosphorous Dual Doping in Cobalt Monolayer Interfacial Assembled Cobalt Nanowires for Efficient Overall Water Splitting, Adv. Funct. Mater., 2020, 30, 1–12 CrossRef.
- H. Dau, C. Limberg, T. Reier, M. Risch and S. Roggan, The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis, ChemCatChem, 2010, 2, 724–761 CrossRef CAS.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01082h |
| This journal is © the Partner Organisations 2023 |