Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Halogen bonding and chalcogen bonding mediated sensing
Robert
Hein
 and
Paul D.
Beer
and
Paul D.
Beer
 *
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk
First published on 11th May 2022
Abstract
Sigma–hole interactions, in particular halogen bonding (XB) and chalcogen bonding (ChB), have become indispensable tools in supramolecular chemistry, with wide-ranging applications in crystal engineering, catalysis and materials chemistry as well as anion recognition, transport and sensing. The latter has very rapidly developed in recent years and is becoming a mature research area in its own right. This can be attributed to the numerous advantages sigma–hole interactions imbue in sensor design, in particular high degrees of selectivity, sensitivity and the capability for sensing in aqueous media. Herein, we provide the first detailed overview of all developments in the field of XB and ChB mediated sensing, in particular the detection of anions but also neutral (gaseous) Lewis bases. This includes a wide range of optical colorimetric and luminescent sensors as well as an array of electrochemical sensors, most notably redox-active host systems. In addition, we discuss a range of other sensor designs, including capacitive sensors and chemiresistors, and provide a detailed overview and outlook for future fundamental developments in the field. Importantly the sensing concepts and methodologies described herein for the XB and ChB mediated sensing of anions, are generically applicable for the development of supramolecular receptors and sensors in general, including those for cations and neutral molecules employing a wide array of non-covalent interactions. As such we believe this review to be a useful guide to both the supramolecular and general chemistry community with interests in the fields of host–guest recognition and small molecule sensing. Moreover, we also highlight the need for a broader integration of supramolecular chemistry, analytical chemistry, synthetic chemistry and materials science in the development of the next generation of potent sensors.
1. Introduction
Halogen bonding (XB) and chalcogen bonding (ChB), the non-covalent interactions between a positively charged region on a polarised, electron deficient halogen or chalcogen atom (the σ–hole) and a Lewis base, have emerged as powerful and potent additions to the supramolecular chemistry tool-box, being increasingly exploited in catalysis,1–6 crystal engineering7–12 and most notably molecular recognition.13–21 Stimulated by the importance of negatively charged species in a plethora of biological, industrial and environmental spheres, the XB-mediated recognition of anions, in particular, has significantly advanced in recent years.14,16,22–24 Due to their comparatively lower charge-density, stronger hydration as well as pH-dependence, the selective recognition of anions is significantly more challenging than cations, especially in aqueous environments.25–27 XB and ChB are ideally suited to address this challenge, as they typically imbue both enhanced selectivity and binding strength in comparison to hydrogen bonding (HB) analogues. This can be attributed to a variety of factors including a stricter adherence to a 180° binding geometry, lower solvent dependency, larger hydrophobicity and improved electronic tuneability.14,16,28,29These combined advantages have facilitated sigma–hole mediated anion recognition in aqueous media, importantly including pure water.30–32 As a result, increasing attention is being directed at the application of this capability in transmembrane anion transport,33–37 ion extraction and remediation38,39 as well as anion sensing.40–42 The latter is highly relevant in many real-world scenarios, in particular environmental and healthcare monitoring. The reversible nature of non-covalent binding interactions in supramolecular host–guest systems is ideally suited for repeat and long-term sensing applications. To this end, the generation of anion sensors by integration of suitable optical or electrochemical reporter groups into hydrogen bond donor anion receptor structural frameworks has received enormous attention over the past few decades.27,40–45 In contrast, XB and ChB mediated anion sensing is a relatively new phenomenon, often demonstrating enhanced selectivity, binding strength and enhanced signal transducing capabilities in comparison to HB based sensing analogues.
Herein, we provide an overview of this field with a particular focus on the analytical performance of XB and ChB anion sensors. Where possible we contrast these sensors with analogous HB systems, highlighting the origins of enhanced XB and ChB sensing performances in the context of fundamental host–guest recognition principles.
2. Sigma–hole interactions and sensing
2.1 General overview and scope of the review
To date, ion-selective electrodes (ISEs) are the main employed anion sensors. Simple and cheap, they nevertheless often fail certain sensing criteria, as they possess thermodynamically limited sensitivities and require frequent (re)calibration.46–49 In order to satisfy a broad(er) range of application criteria, improved or complementary sensing approaches are thus highly desired. This has spurred on the development of a large range of alternative optical or electrochemical supramolecular anion sensors,40–43,50 wherein the use of σ–hole interactions is increasingly recognised as a particularly potent means to achieve higher degrees of selectivity and sensitivity as well as guest recognition and sensing in aqueous media.This review gives a broad state-of-the art overview of all types of XB and ChB based sensors for anions, and also neutral Lewis bases and gases with a focus on their sensing performance in the context of sigma–hole recognition. Furthermore, we highlight how XB and ChB sensor systems contribute to fundamental aspects of supramolecular interactions as well as how they can aid in future developments in the fields of supramolecular host–guest chemistry and (ion) sensing in general.
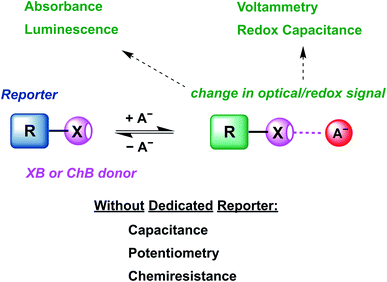
The review begins with an introduction of the intrinsic properties of σ–hole interactions (Section 2.2) and their influence on relevant sensor parameters (Section 2.3). This is followed by a discussion of relevant examples of XB and ChB colorimetric and luminescent sensors (Section 3) as well as redox-active sensors (Section 4.1). The vast majority of these sensors are constructed by covalent appendage of reporter groups to XB or ChB receptors, whose optical or electrochemical properties are reversibly modulated in the presence of the bound guest analyte, as schematically shown in Fig. 1.
Nevertheless, a range of sensors omitting any dedicated reporter groups have been developed, including capacitive or chemiresistive sensors as discussed in Sections 4.2 (other electrochemical sensors) and 5 (other sensors). Finally, we provide an overview of future developments in the rapidly advancing field of sigma–hole mediated sensing and (an)ion sensing more generally (Section 6).
2.2 Brief introduction to sigma–hole interactions
Appending a group 14–17 atom (X) with a substituent of higher electron-withdrawing capability (R) induces an anisotropic distribution of electron density such that a region of positive electrostatic potential is formed along the elongation of the R–X σ-bond (Fig. 2). This so-called σ–hole can then non-covalently interact with Lewis bases (LBs), forming halogen (XB), chalcogen (ChB), pnictogen (PnB) or tetrel bonds (TrB), for X = group 17, 16, 15 and 14, respectively.14 The strength of these σ–hole interactions is, of course, not only dependent on the nature and Lewis basicity of the acceptor, but is also highly tuneable via modification of the σ–hole donor scaffold (for examples see Fig. 2). Specifically, “deeper”, that is more electropositive, σ–holes are formed when the appended substituent is more electron-withdrawing. Similarly, larger, more polarisable and less electronegative donor atoms form more potent σ–holes, such that XB (and ChB) strength generally increases for the analogues descending the respective main group, i.e. I > Br > Cl and Te > Se > S.14† Notably for XB the σ–hole is highly localised along the elongation of the R–X σ-bond, resulting in highly directional, linear non-covalent bond formation with Lewis bases, often exceeding 170° for the R–X⋯LB bond angle. These effects are primarily rationalised within an electrostatic bonding framework, that is based on the concept of the σ–hole. While conceptually useful and straight-forward, this description is not always sufficient to describe all sigma–hole properties. Specifically, a range of other factors, including polarisation, dispersion forces, hydrophobic effects and orbital interactions need to be considered in an accurate description of the nature of σ–hole bonding.14 This notably includes (partial) charge transfer from the Lewis base into the σ* orbital of the donor.51 This interaction between an occupied orbital of the Lewis base guest and an empty donor orbital is not only highly directional, but can also be a substantial driving force for sigma–hole bonding, giving rise to a significant degree of covalent character.29,52–54 This has also been experimentally observed for ChB interactions29 as well as halide-XB complexes.55 Recently, π-covalency was also shown to play a role in XB bond formation with halides.56 These observations may account for specific selectivity preferences, an improved sensor sensitivity as well as a generally lowered solvent dependence of σ–hole interactions in comparison to HB.29,57,58 For more in-depth discussions on the nature of σ–hole interactions the interested reader is referred to recent reviews.14,51,59–61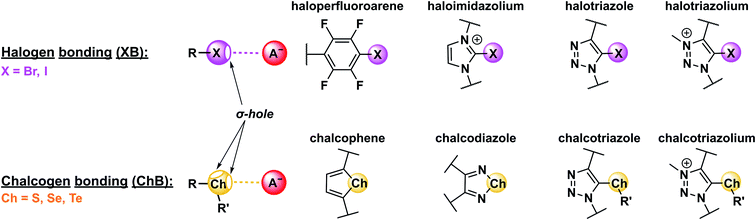 | ||
| Fig. 2 Schematic depiction of halogen (XB) and chalcogen bonding (ChB) interactions with an anion as archetypical Lewis base. Shown are also commonly employed XB and ChB donor motifs. | ||
Of further note is that, depending on their hybridisation, ChB donors can form one (sp2) or two sigma holes (sp3). The “additional” substituent of sp3 hybridised ChB donors (in comparison to only one substituent on typical XB or HB donors), hereby presents a potent means of further tuning the σ–hole strength, or providing additional functionalities, such as optical or electrochemical reporter groups.62
2.3 Relevant sensor parameters
As alluded to above, an important goal in (supramolecular) sensor development is the capability of sensing in aqueous media. Their lower solvent dependence renders σ–hole interactions particularly potent in this regard. Similarly, the inherent characteristics of σ–hole interactions are highly relevant, and often beneficial, in addressing some of the other main goals in sensing and directly impacts most of the relevant sensing parameters as described in the following (Fig. 3).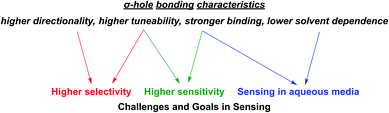 | ||
| Fig. 3 Important σ–hole bonding characteristics (in comparison to commonly employed HB and electrostatic interactions) and their impact on relevant challenges and goals in sensor development. | ||
However, it also should be noted that highly selective recognition is not an absolute requirement for the construction of potent sensors. Firstly, depending on the specific application scenario, as well as the desired sensitivity, the co(existence) of certain levels of competing anions may be tolerated, in particular if their concentrations are low or their binding sufficiently weaker so as to not significantly compete with recognition and sensing of the target analyte.
In addition, the requirement for a high degree of selectivity of any one sensor may be overcome by employing an array of multiple different receptive sensor units such that a broader data set is obtained. From this, the desired sensory parameters, that is the concentration of one or multiple species, can then be obtained by, for example, deep-learning algorithms or more classical approaches such as linear discriminate analysis (LDA) or principal component analysis (PCA).63–66
This is an established approach to sense various analytes, including ions,63–65,67–69 but remains thus far unexplored in the context of sigma–hole mediated sensing. Sigma–hole interactions can offer much in this regard, as their typically contrasting selectivity and sensing patterns can complement that of more established sensors based on HB or electrostatic interactions.
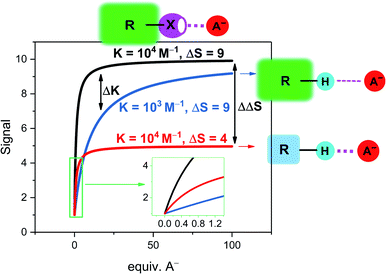 | ||
Fig. 4 Simulated response isotherms of a generic non-covalent host–guest sensor based on the reporter group approach. The binding isotherms are simulated according to a simple 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric binding model with [H] = 100 μM and an initial free host signal of SH = 1.70 Depicted are three exemplary cases: black line: strong binding (K = 104 M−1) and a large signal change of the host–guest complex (SHG = 10, ΔS = 9) as typically encountered in sigma–hole based sensors. The blue and red lines represent typical examples of less potent HB sensors in which either binding is weaker (blue line, K = 103 M−1) or in which the signal magnitude is smaller (red line, SHG = 5, ΔS = 4). This highlights the different mechanisms by which sigma–hole based sensors typically outperform related HB analogues in terms of sensitivity. Inset: magnification at low guest concentrations. 1 host–guest stoichiometric binding model with [H] = 100 μM and an initial free host signal of SH = 1.70 Depicted are three exemplary cases: black line: strong binding (K = 104 M−1) and a large signal change of the host–guest complex (SHG = 10, ΔS = 9) as typically encountered in sigma–hole based sensors. The blue and red lines represent typical examples of less potent HB sensors in which either binding is weaker (blue line, K = 103 M−1) or in which the signal magnitude is smaller (red line, SHG = 5, ΔS = 4). This highlights the different mechanisms by which sigma–hole based sensors typically outperform related HB analogues in terms of sensitivity. Inset: magnification at low guest concentrations. | ||
3. Optical sensors
3.1 Colorimetric sensors
Perhaps the simplest example of a colour dependence in which XB plays a pivotal role is that of dissolved iodine; violet in the gas-phase as well as in non-polar solvents, while brownish in solvents of higher polarity. Over a century ago in 1903, Lachmann attributed this observation to formation of solvent·I2 adducts,74 whose wavelength of absorption is increasingly hypsochromically shifted for solvents with higher Lewis basicity.15,75While this, and many of the following examples, can scarcely be considered useful real-life sensors, the use of UV-vis spectroscopy has, due to its simplicity and ubiquity, received enormous attention in the study of host–guest interactions, including sigma–hole bonding and sensing.76
In fact, many examples of XB-based anion receptors have been shown to undergo changes in absorbance upon anion recognition. This includes, for example, acyclic bimetallic rhenium(I)-containing XB iodotriazole receptors,77 acyclic and macrocyclic zinc(II)-porphyrin iodotriazole XB hosts,78,79 XB iodopyridinium helicates80 and XB/HB iodoperfluoroaryl urea receptors.81
Recently, the groups of Huber and Rosokha specifically employed UV-vis spectroscopy to prove the formation of “anti-electrostatic” XB between halide anions and an anionic iodo-cyclopropenylium XB host in solution (Fig. 5).82 Similarly, UV-vis spectroscopy was employed to investigate guest binding in a range of ChB receptors.36,62,83–86 A few selected examples of such XB and ChB receptors that undergo changes in absorbance upon anion binding are shown in Fig. 5.
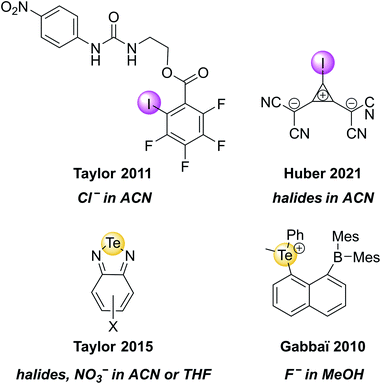 | ||
| Fig. 5 Selected examples of XB and ChB receptors that undergo changes in UV-vis absorbance upon exposure to the highlighted anions. | ||
However, it must be noted that in the majority of these examples no systematic sensing studies were carried out and that the absorbance changes in most systems are only small; very few simple receptors undergo significant (naked-eye visible) changes.78,87,88
A powerful, but comparably rare strategy to induce large scale, naked-eye colorimetric changes upon anion recognition is the use of receptor co-conformational changes in mechanically interlocked molecules (MIMs) such as rotaxanes or catenanes upon guest binding.41 XB-mediated anion sensing via this strategy has been investigated by the Beer group in a range of [2] and [3]rotaxane shuttles.89–91
For instance, the bistable rotaxanes 1.XB/HB were developed as colorimetric anion sensors, undergoing halide binding-induced co-conformational changes and a concomitant colour change (Fig. 6).90 Specifically, in the absence of a coordinating anion guest, the electron-rich hydroquinone-containing macrocycle resides preferentially at the NDI station of the axle, resulting in an orange colour arising from donor–acceptor charge-transfer interactions. Upon addition of Cl− or I−, convergent anion binding from the macrocycle's isophthalamide HB donors and the axle's (iodo)triazolium XB/HB donors induces a shuttling of the macrocycle to the triazolium station, thereby disrupting the donor–acceptor charge-transfer interactions resulting in a loss of colour.
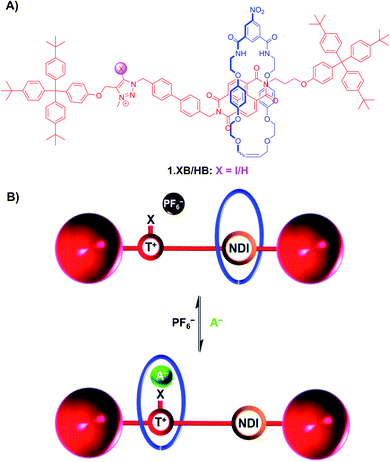 | ||
| Fig. 6 (A) Chemical structure of bistable (iodo)triazolium-NDI [2]rotaxanes 1.XB/HB. (B) Schematic depiction of anion recognition-induced shuttling of the macrocycle from the NDI to the triazolium, resulting in a naked-eye colour change from orange to colourless due to disruption of the donor–acceptor charge-transfer interactions between the hydroquinone-containing macrocycle and the NDI station. Reproduced from ref. 90 with permission from the Royal Society of Chemistry. | ||
Importantly, in the presence of 1 equiv. of halide anions in CDCl3 both 1.XB and 1.HB displayed an (almost) quantitative occupation of the triazolium station of at least 92% and 100%, respectively (Table 1). However, in the absence of coordinating anions, only the XB shuttle displayed a preferential occupation of the NDI station (62%), while in the HB system the macrocycle preferentially resided at the triazolium station (76%). This shows that the XB rotaxane is a superior shuttle, with larger changes in station occupancy upon anion recognition. This enhanced shuttling, and thus sensing, capability of 1.XB was also confirmed in more polar solvent systems. As shown in Table 1, in more competitive CDCl3/MeOD 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and CDCl3/MeOD 1
1 and CDCl3/MeOD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 both rotaxanes displayed reduced shuttling capabilities in the presence of halides, albeit with a generally superior performance of 1.XB. Notably in these competitive solvents the XB system showed a much more significant shuttling performance in the presence of I− with an impressive 30% station occupancy change in CDCl3/MeOD 1
1 both rotaxanes displayed reduced shuttling capabilities in the presence of halides, albeit with a generally superior performance of 1.XB. Notably in these competitive solvents the XB system showed a much more significant shuttling performance in the presence of I− with an impressive 30% station occupancy change in CDCl3/MeOD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while 1.HB displayed a 10-fold worse shuttling behaviour (3% change, Table 1).
1, while 1.HB displayed a 10-fold worse shuttling behaviour (3% change, Table 1).
| Anion | 1.XB | 1.HB | |||||
|---|---|---|---|---|---|---|---|
| Trz | NDI | Δ(A−–PF6−) | Trz | NDI | Δ(A−–PF6−) | ||
| CDCl3 | PF6− | 38% | 62% | — | 76% | 24% | — |
| Cl− | 92% | 8% | 54% | 100% | 0% | 24% | |
| I− | 95% | 5% | 57% | 100% | 0% | 24% | |
CDCl3/MeOD 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PF6− | 52% | 48% | — | 67% | 33% | — |
| Cl− | 100% | 0% | 48% | 87% | 13% | 20% | |
| I− | 92% | 8% | 40% | 70% | 30% | 3% | |
CDCl3/MeOD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
PF6− | 33% | 67% | — | 33% | 67% | — |
| Cl− | 48% | 52% | 15% | 49% | 51% | 16% | |
| I− | 63% | 37% | 30% | 36% | 64% | 3% | |
Building on these results, a more elaborate, structurally related XB/HB four station [3]rotaxane containing two triazolium and two NDI stations, as well as two hydroquinone-isophthalamide macrocycles was developed.89 Upon addition of Cl− or NO3− in CDCl3, the macrocycles undergo a concerted pincer-type shuttling motion from the peripheral NDI stations to the central triazolium stations and, as in the previous example, induce a colour change from orange to colourless. Binding of the smaller halide anion proceeds via 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest stoichiometric binding, while the larger, trigonal planar NO3− binds strongly with a 1
2 host–guest stoichiometric binding, while the larger, trigonal planar NO3− binds strongly with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry, bridging the axle and both macrocycles. Of further note is not only the expectedly enhanced XB recognition performance of the XB [3]rotaxane, but also a rare NO3− selectivity over Cl− and a range of other oxoanions.
1 stoichiometry, bridging the axle and both macrocycles. Of further note is not only the expectedly enhanced XB recognition performance of the XB [3]rotaxane, but also a rare NO3− selectivity over Cl− and a range of other oxoanions.
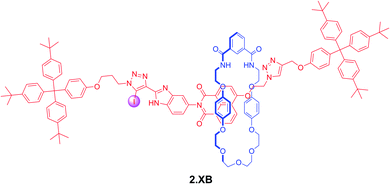
In a more recent example, Klein et al. prepared the bistable [2]rotaxane shuttle 2.XB for anion and pH dependent molecular motion and sensing (Fig. 7).91 In analogy to the previous examples, the hydroquinone-isophthalamide-containing macrocycle of the neutral, unprotonated rotaxanes preferentially resides on the axle electron deficient naphthalimide motif, resulting in a yellow colouration. In CDCl3 neither protonation of the benzimidazole nor presence of Cl− alone induced any shuttling of the macrocycle. Only in the presence of both acid and anion was macrocycle translocation to the benzimidazolium–iodotriazole anion binding station observed, resulting in loss of colour as well as fluorescence emission increase. The rotaxane shuttle thus behaves as a molecular logic AND gate, requiring both a coordinating anion as well as anion binding enhancement via benzimidazole-protonation to function.
3.2 Luminescent sensors
In an effort to provide a more sensitive and useful sensor readout in comparison to the colorimetric sensors discussed above, the development of luminescent sigma–hole-based probes has gained significant attention in the last decade. To this end, a diverse range of XB, and to a much lesser extent ChB, acyclic, macrocyclic and interlocked receptor architectures have been endowed with various organic and transition-metal based luminescent motifs, providing a simple and highly sensitive means of sensing of various anions.41–43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent system, wherein 1H NMR studies revealed strong 1
1 solvent system, wherein 1H NMR studies revealed strong 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
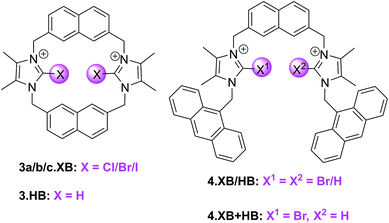
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric binding of Br− and I− to the syn-isomers of the bromo- and iodo-imidazolium hosts 3b.XB and 3c.XB (K > 10
1 host–guest stoichiometric binding of Br− and I− to the syn-isomers of the bromo- and iodo-imidazolium hosts 3b.XB and 3c.XB (K > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 for 3b.XB).
000 M−1 for 3b.XB).
In contrast, a large range of oxoanions (H2PO4−, NO3−, SO42−, AcO−, BzO−) as well as F− and Cl− did not bind to these hosts. Similarly, neither the weaker XB Cl-donor derivative 3a.XB nor the anti conformer of 3c.XB (capable of only forming one XB-anion interaction) bound any of the tested guests, while binding of the three halides Cl−, Br− and I− to the HB host 3.HB was very weak (<85 M−1).
Fluorescence sensing studies mirrored these trends; neither 3.HB nor 3a.XB responded to any anions, while significant enhancement of the naphthalene emission, in particular of the initially weaker low-energy band, was observed for both the bromo- and syn iodo-imidazolium hosts in the presence of Br− and I− (Fig. 9). Interestingly, 3b.XB displayed more significant emission enhancements of up to 5.8× in the presence of I− (K = 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1) than Br− (2×, K = 2880 M−1), while the iodo-imidazolium receptor displayed preferential Br− binding and enhancements (6.4×, K = 95
100 M−1) than Br− (2×, K = 2880 M−1), while the iodo-imidazolium receptor displayed preferential Br− binding and enhancements (6.4×, K = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1), with weaker I− binding (1.6×, K = 3710 M−1). This highlights the enormous potency of XB for tuneable, highly selective halide sensing in aqueous media.
500 M−1), with weaker I− binding (1.6×, K = 3710 M−1). This highlights the enormous potency of XB for tuneable, highly selective halide sensing in aqueous media.
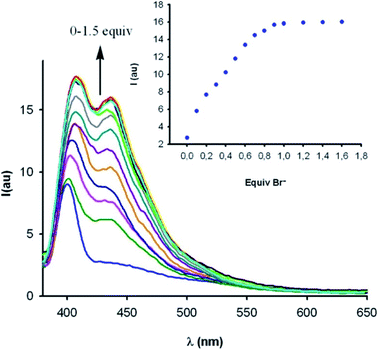 | ||
Fig. 9 Fluorescence emission response of macrocyclic iodo-imidazolium host 3c.XB (10 μM) in CH3OH/H2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of increasing concentrations of bromide. Reproduced with permission from ref. 92 copyright 2012 American Chemical Society. 1 in the presence of increasing concentrations of bromide. Reproduced with permission from ref. 92 copyright 2012 American Chemical Society. | ||
Building on this motif, the groups of Caballero and Molina developed related, anthracene appended acyclic (bromo)imidazolium receptors 4.XB/HB as well as the mixed XB and HB receptor 4.XB+HB.93,94 In ACN 4.XB displayed no fluorescence changes in the presence of Cl−, Br−, I− and various oxoanions, most notably HSO4−, AcO− and BzO−, whereas F− and SO42− induced significant fluorescence quenching.93 In contrast, HP2O73− induced notable emission turn-on, while in the presence of H2PO4− a higher-wavelength emission band appears, arising from formation of anthracene excimers. As a result of this unique response pattern, this XB probe can thus selectively sense the dihydrogen phosphate anion, as further confirmed by competition experiments; only in the presence of 2 equiv. of HP2O73− or SO42− were changes in the H2PO4−-induced excimer band observed. Interestingly, 4.HB displayed only quenching in the presence of F−, SO42−, HP2O73− and H2PO4−, with no appearance of excimer emission. The mixed 4.XB+HB receptor displays response patterns that are generally identical to those of the XB probe, with an additional moderate quenching response towards AcO−.94 In a later study a related tripodal bromoimidazolium anthracene receptor displayed similar selective detection of H2PO4−via the same excimer response mechanism.95 The same group also developed a tetra bromoimidazole-tetraphenylethylene as an ion-pair receptor, capable of selective emission turn-on sensing of HSO4− in the presence of co-bound Zn2+ in ACN.96
In analogy to many of the other sensors described in the other sections of this review, the ubiquitous 5-iodo-1,2,3-triazole motif has been exploited in a large range of fluorescent XB anion sensors. For example, Zapata et al. developed a range of bis(halotriazolium-pyrene) hosts for the sensing of pyrophosphate and dihydrogenphosphate in acetone via pyrene excimer formation (akin to the above mentioned 4.XB system).97 Fluorescent H2PO4− sensing in ACN was also reported by emission enhancements of a BINOL-bis(triazolium) system.98
Aggregation-induced emission (AIE) has over the last two decades rapidly emerged as a new paradigm in a plethora of luminescence applications, but remains notably underexplored in the context of host–guest ion sensing.99 Recently, Docker and Shang et al. conducted a systematic binding and sensing study of a range of iodo-triazole appended tetraphenylethene (TPE) receptors as AIE platforms.100 The tetra-XB receptor 5.XB displayed expectedly strong Cl− recognition in d8-THF and responded to various anions via significant fluorescent enhancements, with a notable halide selectivity, as shown in Fig. 10. The origins of this response were ascribed to anion binding-induced AIE, as supported by DLS and TEM measurements, confirming the presence of ≈100 nm sized particles only in the presence of anions. This fluorescent AIE response was notably not observed for the HB analogue 5.HB nor for a weaker XB donor receptor analogue containing phenyl instead of perfluorophenyl substituents. Similarly, the mono-XB TPE derivative displayed no significant emission response. In spite of strong halide binding by all three possible doubly-substituted XB TPE isomers, only the 1,1-diXB-ethene isomer exhibited chloride-induced AIE, highlighting the importance of the spatial orientation of XB donor sites to effect AIE.
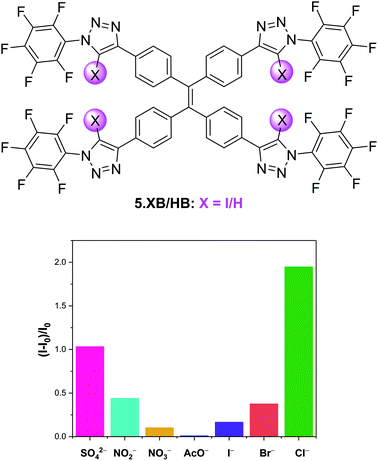 | ||
| Fig. 10 Relative emission intensity increase of 10 μM tetraphenylethene AIE anion sensor 5.XB in the presence of 10 equiv. of various anions in THF. Reproduced from ref. 100 under the terms of the CC BY license. | ||
The authors further demonstrated that the E and Z-derivatives of the other doubly-substituted 1,2-diXB-ethene XB TPE receptor can be interconverted by light, whereby the relative composition in the photostationary state is dependent on anion presence.
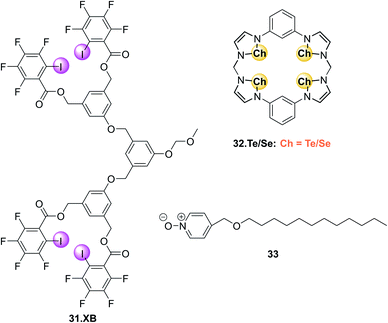
Another recent research focus in the development of XB fluorescent sensors has been their operation in (pure) aqueous environments. To this end Beer and co-workers have developed a range of water-soluble optically responsive XB receptors, including a benzene bis(iodotriazolium) host for emission turn-on sensing of ReO4− in HEPES buffer (pH = 7.4)32 as well as a naphthalimide-appended XB foldamer receptor for sensing of I− in water via fluorescence enhancement.31
In another example, the Beer group recently developed the XB coumarin-appended receptor 6.XB as a hydrosulfide (HS−) selective fluorescent probe.101 Formed upon dissolution of the toxic H2S gas, the sensing of HS− remains a formidable challenge, in spite of its increasing relevance in environmental and medicinal settings.102,103 Thus far, the vast majority of HS− sensors are irreversible optical chemodosimeters,104,105 while host–guest recognition of this anion remains largely underexplored.106–108 Receptor 6.XB not only presents a rare example of a reversible supramolecular HS− host but is capable of selective sensing of this analyte in water.101 As shown in Fig. 11, addition of up to 10 equiv. of HS− to a buffered solution of 6.XB induced notable coumarin emission quenching of up to 60%, while neither Cl−, Br− nor I− induced an appreciable response. While the XB sensor displayed strong HS− binding (K = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1) and a sensitive fluorescence response (LOD = 14 μM), the HB analogue 6.HB did not display any anion detection capability. These findings were further corroborated by DFT and molecular dynamics simulations, highlighting a unique potency of XB for the recognition of HS−.
500 M−1) and a sensitive fluorescence response (LOD = 14 μM), the HB analogue 6.HB did not display any anion detection capability. These findings were further corroborated by DFT and molecular dynamics simulations, highlighting a unique potency of XB for the recognition of HS−.
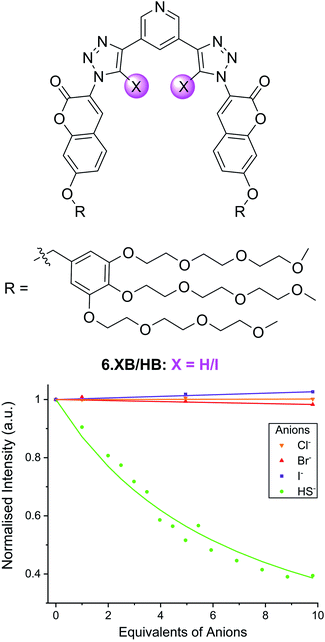 | ||
| Fig. 11 Fluorescence emission intensity response of 10 μM hydrosulfide-selective coumarin-containing 6.XB in the presence of 10 equiv. of various anions in 10 mM HEPES buffer (pH = 7.4). Reproduced from ref. 101 under the terms of the CC BY license. | ||
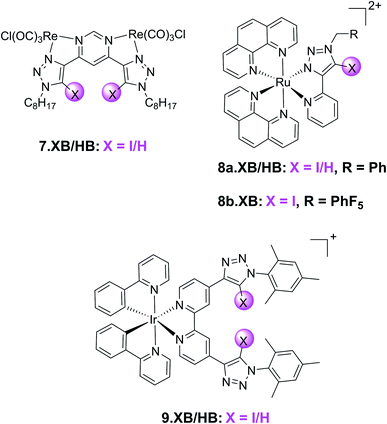
In addition to the organic fluorophores discussed above, various organometallic and transition metal-based emissive XB sensors have been developed. Their typically modular synthesis and bright, highly tuneable emission profiles renders them potent motifs in optical ion sensors.109 This has been exploited in a range of XB fluorescent anion sensors, an early example of which is the neutral, bimetallic pyrimidine-(iodo)triazole 7.XB/HB system containing Re(I) carbonyl reporter groups (Fig. 12).110 Preorganisation and polarization of the triazole binding site by this organometallic motif enabled strong binding of a range of halides and oxoanions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3/MeOD, with strongest binding observed for iodide at 7.XB with K > 10
1 CDCl3/MeOD, with strongest binding observed for iodide at 7.XB with K > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1. Preliminary sensing studies in ACN revealed absorbance changes and luminescence enhancements of both receptors in the presence of the halides, phosphate and sulfate.
000 M−1. Preliminary sensing studies in ACN revealed absorbance changes and luminescence enhancements of both receptors in the presence of the halides, phosphate and sulfate.
A similar design concept was exploited in receptors 8a–b.XB/HB by the group of Ghosh.111,112 In ACN 8a.XB displayed significant emission enhancement of the Ru(phen)2py-triazole MLCT band of 5 and 17-fold in the presence of HP2O73− and H2PO4−, respectively, while a large range of other oxoanions and halides did not induce significant responses.111 This correlated with stronger binding of the latter anion, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric binding constant of K = 194
1 host–guest stoichiometric binding constant of K = 194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, bound 3.5-fold more strongly than pyrophosphate. This was also confirmed by competition experiments; even in the presence of 10 equiv. of various competing anions the sensor response towards 1 equiv. of H2PO4− was largely unaltered. In addition, the authors reported a notable increase in the MLCT luminescence lifetimes of the probe, from ≈6 ns of the free receptor to ≈34 and 109 ns in the presence of HP2O73− and H2PO4−, respectively. For the HB analogue 8a.HB the sensing performance towards these anions was expectedly reduced, with lower binding constants, larger LODs as well as reduced life-time enhancements. This is also reflected in a significant sensing performance for 8a.XB in up to 20% water in ACN (albeit with lower response magnitudes), while 8a.HB was incapable of sensing the phosphate anions in mixtures containing 10% or more water.
000 M−1, bound 3.5-fold more strongly than pyrophosphate. This was also confirmed by competition experiments; even in the presence of 10 equiv. of various competing anions the sensor response towards 1 equiv. of H2PO4− was largely unaltered. In addition, the authors reported a notable increase in the MLCT luminescence lifetimes of the probe, from ≈6 ns of the free receptor to ≈34 and 109 ns in the presence of HP2O73− and H2PO4−, respectively. For the HB analogue 8a.HB the sensing performance towards these anions was expectedly reduced, with lower binding constants, larger LODs as well as reduced life-time enhancements. This is also reflected in a significant sensing performance for 8a.XB in up to 20% water in ACN (albeit with lower response magnitudes), while 8a.HB was incapable of sensing the phosphate anions in mixtures containing 10% or more water.
In a subsequent study, the same group also investigated the more polarized pentafluorophenyl appended receptor analogue 8b.XB.112 Unsurprisingly, the XB receptor displayed enhanced binding of both HP2O73− and H2PO4− (K = 8.9 × 105 and 2.76 × 106 M−1 in ACN, respectively), over 10-fold larger than the benzyl-appended 8a.XB. In addition, 8b.XB also displayed a larger switch-on response of 25-fold in the presence of H2PO4−, while the enhancements in the presence of HP2O73− were comparably somewhat attenuated (3.6-fold increase). The LOD was low towards both anions (≈11 and 91 nM, respectively).
The first example of a XB receptor containing the ubiquitous, luminescent cyclometallated Ir(ppy)2-motif113,114 was reported by Schubert and co-workers in 2020.115 Containing an additional 4,4-bis-iodotriazole bipy ligand, 9.XB displayed significantly enhanced Cl− binding (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1) in comparison to its HB congener in ACN (5000 M−1). Both Br− and OAc− were also bound, albeit weaker. 9.XB exhibited a significant luminescence response towards chloride with a low LOD of 11 nM, while the perturbations induced by the other anions were notably smaller.
000 M−1) in comparison to its HB congener in ACN (5000 M−1). Both Br− and OAc− were also bound, albeit weaker. 9.XB exhibited a significant luminescence response towards chloride with a low LOD of 11 nM, while the perturbations induced by the other anions were notably smaller.
Acyclic fluorescent XB sensors based on other XB donor motifs include, for example, iodo-naphthoquinone receptors for sensing of SO42− in ACN116 and iodo-pyridinium receptors for sensing of various halides and oxoanions in DCM.117
The first example of a fluorescent XB interlocked host, and, to the best of our knowledge, the first example of XB-mediated fluorescent sensing in general, was reported by Caballero et al. in early 2012.122 In ACN the bis-bromo-imidazolium [2]catenane 10.XB, containing naphthalene reporter groups, displayed selective fluorescence switch-on only in the presence of Cl− or Br−, with strong binding of 3.71 × 106 M−1 and 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1, respectively (Fig. 13). In contrast, a large range of other anions, including F−, I−, AcO−, H2PO4−, NO3− and HCO3− did not induce any fluorescence response. Similarly, the monomeric macrocyclic host precursor did not respond to any anions, highlighting the unique selectivity imbued by the preorganized, interlocked catenane XB binding cavity.
000 M−1, respectively (Fig. 13). In contrast, a large range of other anions, including F−, I−, AcO−, H2PO4−, NO3− and HCO3− did not induce any fluorescence response. Similarly, the monomeric macrocyclic host precursor did not respond to any anions, highlighting the unique selectivity imbued by the preorganized, interlocked catenane XB binding cavity.
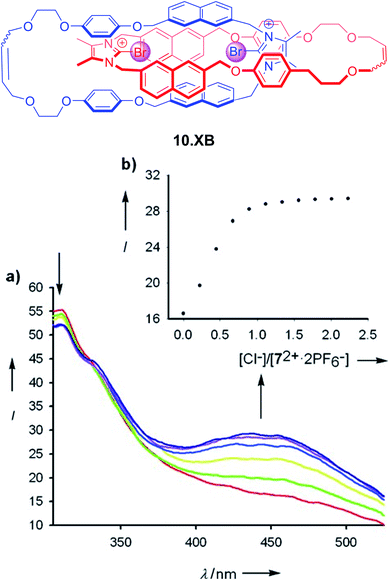 | ||
| Fig. 13 All-XB bromo-imidazolium-napthalene catenane 10.XB for fluorescent halide sensing. (a) Emission spectra of 10 μM 10.XB upon addition of Cl− in ACN. (b) The corresponding response isotherm at 445 nm. Reproduced with permission from ref. 122 copyright 2011 Wiley. | ||
Another structurally related hetero-[2]catenane containing one XB iodo-triazolium macrocycle as well as a HB isophthalamide macrocycle component, was reported for fluorescent sensing of halides and oxoanions in ACN.123 All tested anions induced emission enhancements of the naphthalene emission, which were largest for the oxoanions AcO− and H2PO4− (+73 and +58% intensity increase in the presence of 20 equiv. anion). The response towards the halides was notably smaller with +29, +13 and +4% emission modulation for Cl−, Br− and I−, respectively, thereby displaying a contrasting oxoanion selectivity in comparison to the bromo-imidazolium [2]catenane sensor 10.XB.
Fluorescent reporter motifs have also been incorporated into various rotaxane receptors. This includes, for example, a XB tris(iodo-triazole) rotaxane containing an anthracene reporter appended to the rotaxanes' macrocycle, capable of Cl− sensing in ACN.124
Lim et al. also prepared a chiral XB [3]rotaxane fluorescent sensor 11.XB for biologically relevant dicarboxylates (Fig. 14).1251H NMR titrations in CDCl3/CD3OD/D2O 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 revealed significantly different binding modes between the rotaxane host and chloride and the dicarboxylates S-glutamate, R-glutamate, fumarate and maleate. While Cl− was bound in a 1
1 revealed significantly different binding modes between the rotaxane host and chloride and the dicarboxylates S-glutamate, R-glutamate, fumarate and maleate. While Cl− was bound in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest stoichiometry with the halide binding within each individual interlocked cavity, dicarboxylate binding proceeded in a 1
2 host–guest stoichiometry with the halide binding within each individual interlocked cavity, dicarboxylate binding proceeded in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 fashion via formation of sandwich-type complexes.
1 fashion via formation of sandwich-type complexes.
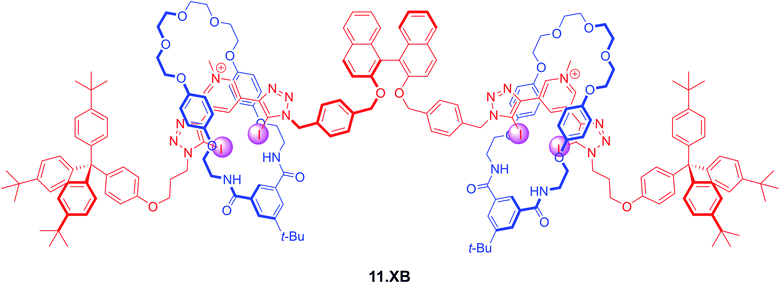 | ||
Fig. 14
S-BINOL-based fluorescent [3]rotaxane 11.XB for fluorescent discrimination of S/R-glutamate enantiomers and fumarate/maleate geometric dicarboxylate isomers in CHCl3/MeOH/H2O 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
From these 1H NMR studies significant Cl− binding (K1:1 = 2610 M−1) was ascertained, while the chemical shift perturbations in the presence of the dicarboxylate guests were too small to be reliably analysed. However, fluorescence sensing studies in the same solvent system revealed strong dicarboxylate recognition with K of up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 for S-glutamate, as reflected in almost complete BINOL fluorescence quenching. Impressively, binding of the R-glutamate enantiomer was 5.7-fold attenuated, attesting to the unique potential of chiral interlocked hosts and sensors; the axle alone not only bound both guests much more weakly (K ≈ 1600 M−1) but also displayed no significant degree of enantiodiscrimination.
200 M−1 for S-glutamate, as reflected in almost complete BINOL fluorescence quenching. Impressively, binding of the R-glutamate enantiomer was 5.7-fold attenuated, attesting to the unique potential of chiral interlocked hosts and sensors; the axle alone not only bound both guests much more weakly (K ≈ 1600 M−1) but also displayed no significant degree of enantiodiscrimination.
Similarly, the [3]rotaxane sensor displayed a significant preference for the more extended fumarate (K = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1), with significantly weaker binding of the maleate geometric isomer (K = 4180 M−1).
400 M−1), with significantly weaker binding of the maleate geometric isomer (K = 4180 M−1).
A range of XB strapped-porphyrin receptors including BODIPY-containing rotaxanes 12a–b.XB were recently reported by Tse et al. (Fig. 15).79 The XB strapped-porphyrin macrocycle alone displayed significant changes (red-shift) in its Soret absorption band upon titration with halides in acetone, revealing strong anion binding which was enhanced up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold in comparison to the unfunctionalized parent Zn-tetraphenylporphyrin. This XB macrocyclic component was then integrated into a range of [2]rotaxanes, which showed significantly enhanced halide binding affinities in comparison to an analogous porphyrin-free rotaxane in d6-acetone and d6-acetone/D2O 98
000-fold in comparison to the unfunctionalized parent Zn-tetraphenylporphyrin. This XB macrocyclic component was then integrated into a range of [2]rotaxanes, which showed significantly enhanced halide binding affinities in comparison to an analogous porphyrin-free rotaxane in d6-acetone and d6-acetone/D2O 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as elucidated by 1H NMR studies. This can be attributed to an enhanced preorganisation and polarization of the interlocked binding cavity by axle-triazole Zn-porphyrin coordination. Unfortunately, this negated the ability of the metallo-porphyrin to act as a chromophoric reporting group; even in the presence of a >1000-fold excess of halides no colorimetric changes were observed. In order to restore the sensing capabilities of the rotaxanes, fluorescent BODIPY reporter groups were incorporated as axle components into 12a–b.XB. In acetone, both rotaxanes responded to Cl−, Br−, I−, OAc− and SO42−via BODIPY fluorescence quenching, whereby binding strength (and response magnitude) were larger for the more polarized perfluorophenyl-containing 12b.XB for all anions, as representatively shown for Cl− in Fig. 15. In the presence of 2% water in acetone, only 12b.XB responded to Cl− and Br− with K = 1090 and 650 M−1, respectively, while 12a.XB did not respond to any anion. By virtue of the redox-activity of the Zn-porphyrin motif, the rotaxanes were also investigated as voltammetric anion sensors. In DCM, all rotaxanes displayed large cathodic voltammetric perturbations in the presence of HSO4−, OAc− and Cl−, of up to −222 and −252 mV for OAc− and Cl− with 12a.XB, respectively. These XB rotaxanes represent rare examples of dual optical and electrochemical sigma–hole-mediated sensing.
2 as elucidated by 1H NMR studies. This can be attributed to an enhanced preorganisation and polarization of the interlocked binding cavity by axle-triazole Zn-porphyrin coordination. Unfortunately, this negated the ability of the metallo-porphyrin to act as a chromophoric reporting group; even in the presence of a >1000-fold excess of halides no colorimetric changes were observed. In order to restore the sensing capabilities of the rotaxanes, fluorescent BODIPY reporter groups were incorporated as axle components into 12a–b.XB. In acetone, both rotaxanes responded to Cl−, Br−, I−, OAc− and SO42−via BODIPY fluorescence quenching, whereby binding strength (and response magnitude) were larger for the more polarized perfluorophenyl-containing 12b.XB for all anions, as representatively shown for Cl− in Fig. 15. In the presence of 2% water in acetone, only 12b.XB responded to Cl− and Br− with K = 1090 and 650 M−1, respectively, while 12a.XB did not respond to any anion. By virtue of the redox-activity of the Zn-porphyrin motif, the rotaxanes were also investigated as voltammetric anion sensors. In DCM, all rotaxanes displayed large cathodic voltammetric perturbations in the presence of HSO4−, OAc− and Cl−, of up to −222 and −252 mV for OAc− and Cl− with 12a.XB, respectively. These XB rotaxanes represent rare examples of dual optical and electrochemical sigma–hole-mediated sensing.
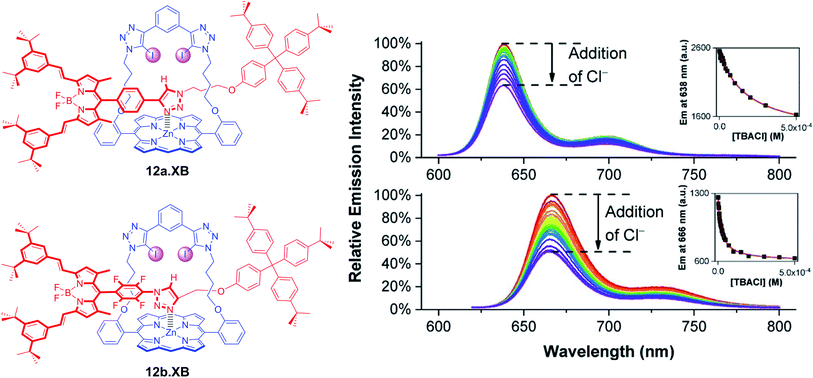 | ||
| Fig. 15 Fluorescence response of 1 μM XB BODIPY-containing strapped porphyrin rotaxanes 12a.XB (top) and 12b.XB (bottom) towards Cl− in acetone. The insets show the corresponding response isotherms, highlighting stronger binding and a larger response for the more polarised, perfluorobenzene-containing 12b.XB. Reproduced from ref. 79 under the terms of the CC BY license. | ||
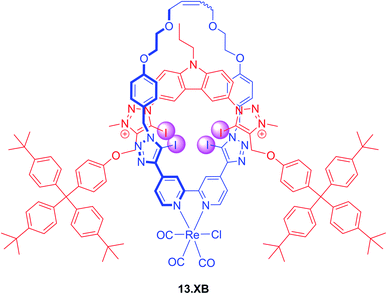
The incorporation of transition metal-based luminescent reporters into interlocked XB hosts has also been investigated by Beer and co-workers, including the all-halogen bonding rotaxane 13.XB (Fig. 16).126 In ACN containing 10 or 20% H2O this Re(I)(bipy)-containing receptor displayed emission quenching in the presence of Cl−, Br− and I− while various oxoanions only induced minor or negligible perturbations. Halide recognition proceeded with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest stoichiometry in both solvents with K1:1 of up to 138
2 host–guest stoichiometry in both solvents with K1:1 of up to 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 for iodide, while Cl− and Br− were bound weaker and F− did not bind at all. Even in ACN/H2O 1
000 M−1 for iodide, while Cl− and Br− were bound weaker and F− did not bind at all. Even in ACN/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selective halide fluorescence quenching was still observed, following the same binding trend (I− > Br− > Cl−) with K1:1 of up to 24
1 selective halide fluorescence quenching was still observed, following the same binding trend (I− > Br− > Cl−) with K1:1 of up to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1.
000 M−1.
A similar Re(I)(CO)3Cl-bistriazole rotaxane was also developed, which was however not luminescent.127 Langton et al. also integrated a Ru(bpy)32+ reporter motif into a water-soluble XB rotaxane and demonstrated Br−, I− and SO42− sensing in pure water, albeit with modest emission enhancements in the presence of excess anion of 3, 6 and 20%, respectively.128 In spite of a larger maximum response for sulfate, the halide anions were bound more strongly as their maximum emission response was reached at a concentration of 1 mM, while a higher concentration of 8 mM SO42− was required to induce signal saturation.
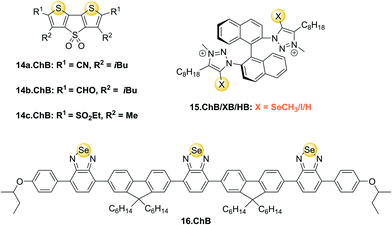
This has been exploited in catalysis,1 anion binding and, in the afore-mentioned seminal work, for anion transport.36 Developed as transmembrane anionophores, DTT receptors 14a–c.ChB were also investigated as anion receptors and optical sensors. All three receptors displayed significant fluorescence emission quenching upon addition of up to 20 mM chloride in THF, with largest quenching and strongest binding of 885 M−1 and 204 M−1 observed for 14a.ChB and 14c.ChB, respectively. Both 14b.ChB (149 M−1) and a mono-cyano derivative of 14a.ChB (69 M−1) displayed weaker binding as well as smaller fluorescent responses. All three receptors also underwent small changes in absorbance in the presence of chloride. In contrast, as expected PF6− did not induce any optical responses, while smaller fluorescence emission quenching of 14a.ChB and 14c.ChB was also reported in the presence of NO3−, in good agreement with weaker binding of this anion of 161 M−1 to 14a.ChB.
Beer and co-workers reported a series of chiral ChB/XB/HB (S)-BINOL based triazolium receptors 15.ChB/XB/HB for the recognition and fluorescent detection of stereo- and geometric dicarboxylate isomers in acetone/H2O 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.130 While 15.XB displayed significant chiral discrimination in the recognition of the enantiomers of tartrate and N-Boc-glutamate, both 15.HB and in particular 15.ChB did not display significant levels of enantioselectivity towards these chiral anion guests as elucidated by 1H NMR binding studies. In contrast, all receptors displayed a considerable degree of binding discrimination of the geometric isomers maleate/fumarate as well as phthalate/isophthalate with a preference for the more extended fumarate or isophthalate in all cases. For the former pair, 15.ChB displayed the largest discrimination with Kfum/Kmal = 5.5, significantly better than 15.HB with Kfum/Kmal = 2.0, while 15.XB decomposed upon exposure to malate.
15.130 While 15.XB displayed significant chiral discrimination in the recognition of the enantiomers of tartrate and N-Boc-glutamate, both 15.HB and in particular 15.ChB did not display significant levels of enantioselectivity towards these chiral anion guests as elucidated by 1H NMR binding studies. In contrast, all receptors displayed a considerable degree of binding discrimination of the geometric isomers maleate/fumarate as well as phthalate/isophthalate with a preference for the more extended fumarate or isophthalate in all cases. For the former pair, 15.ChB displayed the largest discrimination with Kfum/Kmal = 5.5, significantly better than 15.HB with Kfum/Kmal = 2.0, while 15.XB decomposed upon exposure to malate.
Similarly, both sigma–hole hosts displayed enhanced preference for isophthalate over phthalate in comparison to the HB congener. Interestingly, all three hosts displayed significantly different fluorescent anion sensing properties (Fig. 18).
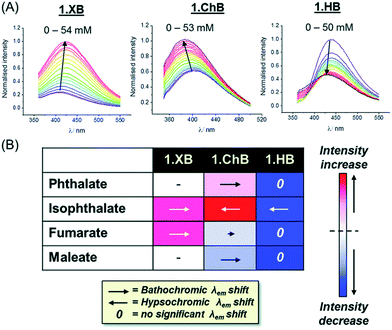 | ||
Fig. 18 (A) Fluorescence emission spectra of 15.ChB/XB/HB (labelled 1.ChB/XB/HB in the figure) in the presence of increasing concentrations of isophthalate in acetone/H2O 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. (B) Summary of fluorescence response patterns of each receptor towards geometric dicarboxylate isomers. The length of the arrows is indicative of the magnitude of the shift in λmax while the shade of red/blue denotes the magnitude of fluorescence enhancement/quenching. Reproduced from ref. 130 with permission from the Royal Society of Chemistry. 15. (B) Summary of fluorescence response patterns of each receptor towards geometric dicarboxylate isomers. The length of the arrows is indicative of the magnitude of the shift in λmax while the shade of red/blue denotes the magnitude of fluorescence enhancement/quenching. Reproduced from ref. 130 with permission from the Royal Society of Chemistry. | ||
While addition of all anions induced large emission enhancement for 15.XB, 15.HB showed quenching in all cases. In contrast, 15.ChB showed more nuanced response patterns with emission enhancements towards (iso)phthalate and quenching in the presence of fumarate/maleate. In addition, the ChB host also displayed unique changes in the emission wavelengths with opposite, hypso- and bathochromic shifts for isophthalate and phthalate, respectively.
Recently, Che and co-workers reported a fluorescent ChB sensor for the detection of the toxic trimethylarsine gas.131 This was achieved by formation of bundled nanofibers constructed from the tris(benzoselenadiazole) receptor 16.ChB which responded to exposure of the analyte vapours by a decrease in emission intensity (Fig. 19). With a LOD of 0.44 ppb and a fast response time of ≈3 s, this sensor performed significantly better than its sulfur-donor benzothiadiazole analogue (LOD = 67 ppb, response time of 54 s). This is indicative of the response arising from ChB-mediation recognition, as further supported by DFT calculations. The sensor displayed an impressive level of selectivity, with no notable response to a large range of solvent vapours, including H2O, methanol, ethanol and acetone at significantly higher levels. In addition, selective trimethylarsine detection was also demonstrated in complex matrices, including car exhaust, smoke and hair spray.
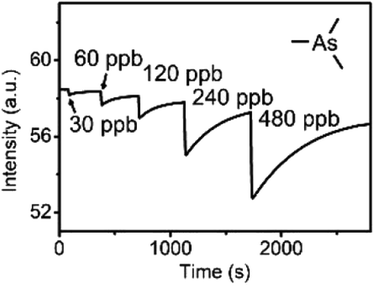 | ||
| Fig. 19 Fluorescence emission intensity of bundled nanoribbons of 16.ChB upon exposure to increasing concentration of trimethyl arsine gas. Reproduced with permission from ref. 131 copyright 2021 American Chemical Society. | ||
The same group also developed a structurally related ChB benzoselenadiazole sensor for assessment of meat freshness by fluorescent sensing of gaseous dimethyl sulfide.132
4. Electrochemical sensors
Owing to their low cost, high sensitivity, flexibility, and scalability, electrochemical sensors are at the forefront of sensor development,133 in particular for the sensing of biomolecules,134 but also for small molecules and ions.40 The latter most notably includes ion-selective electrodes, which, as alluded to in Section 2.1, are thus far the only widely and generically applied ion sensors. This can in part be attributed to a century-long development of potentiometric techniques, their low cost and (operational) simplicity.46–49 Nevertheless, these are not available for various (an)ions and, depending on the specific application scenario, can fail to address certain sensing criteria (e.g. selectivity, or the capability to monitor small changes in concentration), see also Section 4.2.§ This has sparked significant research into alternative electroanalytical supramolecular host–guest ion sensing methodologies, in particular voltammetric sensors based on redox-active receptors as discussed in the following Section.4.1 Redox-active sensors
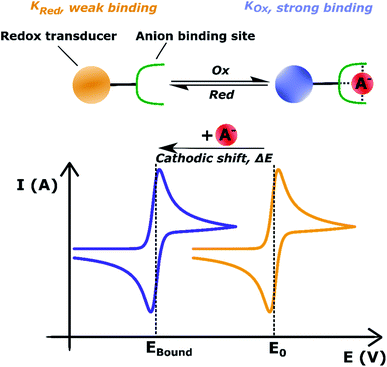
In turn, voltammetric modulation of the receptor's redox state affects the guest binding properties, with stronger anion binding in the higher, more cationic oxidation state (KOx) than in the lower, neutral or anionic redox state (KRed). The specific signalling pathways and fundamentals that underpin these observations are well-established in the literature, but will not be discussed in detail herein.40,135–138 However, note that in the most general case the magnitude of the voltammetric perturbation is given by 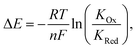 and is thus only dependent on the ratio of KOx/KRed (often denoted the binding enhancement factor, BEF).40 This is to say that the signal magnitude in a voltammetric (an)ion sensor is determined by how strongly guest binding is affected by receptor oxidation/reduction. From this consideration it becomes apparent that a stronger electronic communication between the redox and receptive sites is a key factor for sensor performance. It is thus not surprising that sigma hole interactions, which, as discussed in Section 2.2, display a high degree of electronic tuneability, are particularly potent in voltammetric (anion) sensors.139
and is thus only dependent on the ratio of KOx/KRed (often denoted the binding enhancement factor, BEF).40 This is to say that the signal magnitude in a voltammetric (an)ion sensor is determined by how strongly guest binding is affected by receptor oxidation/reduction. From this consideration it becomes apparent that a stronger electronic communication between the redox and receptive sites is a key factor for sensor performance. It is thus not surprising that sigma hole interactions, which, as discussed in Section 2.2, display a high degree of electronic tuneability, are particularly potent in voltammetric (anion) sensors.139
The first demonstration of a high sensitivity of redox control of XB was reported by Schöllhorn and co-workers in 2014, wherein it was shown that the voltammetric properties of redox active Lewis bases can be modulated in the presence of neutral XB donors (note that this is the “reverse” case as depicted in Fig. 20 and in subsequent examples).140 For instance, in ACN the reduction of tetrachloro-p-quinone (TCQ) is facilitated in the presence of iodo-perfluoro-alkynes/arynes. Specifically, CV experiments showed that single-electron reduction of TCQ to TCQ−˙ is not perturbed by addition of 1-iodo-perfluorohexane, while the second reductive couple TCQ−˙/TCQ2− undergoes significant anodic voltammetric shifts of up to 140 mV in the presence of up to 100 equivalents of XB donor. This is indicative of XB formation, and concomitant decrease in electron-density, which occurs only for the stronger, dianionic Lewis base TCQ2−.
Shortly thereafter the Beer group reported the first examples of redox active XB iodotriazole voltammetric anion sensors 17.XB and 18.XB (Fig. 21A).141 In this case, and all following examples, the XB receptor itself is redox-active, such that the XB donor strength is electrochemically modulated and sensing of redox-inactive Lewis basic analytes, in particular anions, is enabled. In ACN both receptors responded to presence of up to 10 equiv. of Cl− or Br− with moderate cathodic shifts of ≈−30 and ≈−20 mV, respectively (Fig. 21B), which is notably larger than the response of their HB congeners 17.HB and 18.HB. In particular the response of 18.HB was strongly diminished with −6 and 0 mV for Cl− and Br−, respectively, highlighting the crucial role of the XB interaction in sensing the halide anions.
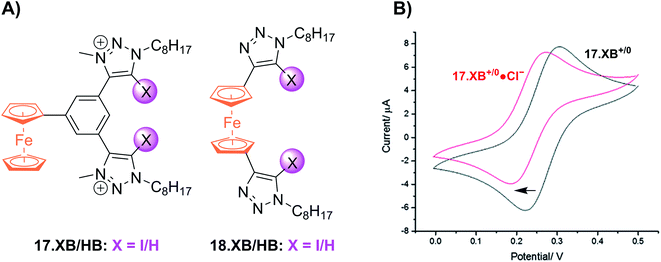 | ||
| Fig. 21 (A) Chemical structures of the first XB ferrocenyl voltammetric anion sensors. (B) CVs of 17.XB in ACN in the absence (black) and presence of 10 equiv. Cl− (red). Adapted from ref. 141 with permission from the Royal Society of Chemistry. | ||
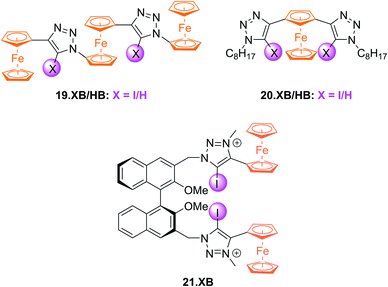
Subsequently, a range of XB Fc-containing acyclic receptors were prepared by different groups. For example, Zapata, Caballero and Molina reported trisferrocene-bis((iodo)triazole) receptors 19.XB/HB (Fig. 22) as oxoanion sensors in DCM/ACN 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.142 Notably, the receptors display two redox waves as a result of strong electronic coupling between the two chemically inequivalent Fc environments, whereby the outer Fc motifs are simultaneously oxidised first, while the inner Fc is subsequently oxidised at a ≈400 mV higher potential. In addition, the sensors display comparably complex voltammetric response patterns, characterised by a two-wave slow exchange behaviour and response magnitudes that differ significantly for both redox waves. For example, addition of OAc− or SO42− to 19.XB did not perturb the first, more cathodic redox wave, but induced moderate perturbations of the second redox couple of −65 and −52 mV, respectively. In contrast, in the presence of H2PO4− or HP2O73− both redox couples were cathodically perturbed, in particular the more anodic wave, displaying shifts of −327 and −252 mV, respectively. Furthermore, and in contrast to all other solution-phase examples discussed herein, 19.XB shows somewhat smaller voltammetric shift perturbations than its HB analogue. These observations may arise as a result of the dominance of strong coulombic electrostatic interactions between the anions and the di/tricationic receptors in the low polarity solvent system.
1.142 Notably, the receptors display two redox waves as a result of strong electronic coupling between the two chemically inequivalent Fc environments, whereby the outer Fc motifs are simultaneously oxidised first, while the inner Fc is subsequently oxidised at a ≈400 mV higher potential. In addition, the sensors display comparably complex voltammetric response patterns, characterised by a two-wave slow exchange behaviour and response magnitudes that differ significantly for both redox waves. For example, addition of OAc− or SO42− to 19.XB did not perturb the first, more cathodic redox wave, but induced moderate perturbations of the second redox couple of −65 and −52 mV, respectively. In contrast, in the presence of H2PO4− or HP2O73− both redox couples were cathodically perturbed, in particular the more anodic wave, displaying shifts of −327 and −252 mV, respectively. Furthermore, and in contrast to all other solution-phase examples discussed herein, 19.XB shows somewhat smaller voltammetric shift perturbations than its HB analogue. These observations may arise as a result of the dominance of strong coulombic electrostatic interactions between the anions and the di/tricationic receptors in the low polarity solvent system.
 | ||
| Fig. 22 Chemical structures of Fc-based XB and HB voltammetric sensors for oxoanions, azide and chiral anions. | ||
Lim et al. further developed the voltammetric Fc-based sensors 20.XB/HB and 21.XB. The former, a constitutional isomer of the afore-discussed 18.XB/HB was developed for sensing of azide in ACN/H2O 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.143 Receptor 20.XB notably displayed larger responses towards this anion of −40 mV over Cl−, Br− or OAc− (max. −22 mV for Br−, all at 10 equiv.) as well as significantly enhanced signal magnitudes in comparison to 20.HB (−18 mV for N3−).
1.143 Receptor 20.XB notably displayed larger responses towards this anion of −40 mV over Cl−, Br− or OAc− (max. −22 mV for Br−, all at 10 equiv.) as well as significantly enhanced signal magnitudes in comparison to 20.HB (−18 mV for N3−).
The chiral 21.XB represents a rare example of voltammetric enantioselective sensing of various chiral anions, achieved in ACN.144 This (S)-BINOL-based XB probe displayed a larger response for the R-enantiomer of both N-Boc-alanine and N-Boc-leucine with ΔER/ΔES of 1.11 and 1.41, respectively and a preferential response towards the S-enantiomer of BINOL-phosphate with ΔER/ΔES = 0.67. These voltammetric enantioselectivities are not only in very good agreement with those obtained by 1H NMR binding titrations of the neutral receptor but are also larger than those observed in a previous Fc-urea HB sensor.145 These observations highlight the potential of XB systems not only as potent voltammetric sensors with typically enhanced response magnitudes in comparison to HB analogues, but also enhanced enantiodiscrimination, presumably arising from the stricter geometric preferences imposed by XB.
In an effort to further enhance the selectivity of such voltammetric anion sensors, Lim and Beer recently integrated a Fc-reporter group into an all-XB rotaxane 22.XB.146 In the competitive solvent mixture of ACN/acetone/H2O 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, this sensor displayed a modest but notably selective response towards excess Br− of −22 mV over Cl− and SCN−. This selectivity is in good agreement with the binding preference of the native rotaxane as elucidated by 1H NMR binding titrations (Fig. 23).
1, this sensor displayed a modest but notably selective response towards excess Br− of −22 mV over Cl− and SCN−. This selectivity is in good agreement with the binding preference of the native rotaxane as elucidated by 1H NMR binding titrations (Fig. 23).
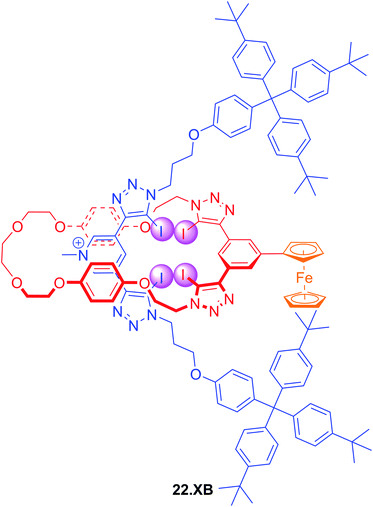 | ||
Fig. 23 Ferrocene-containing all XB [2]rotaxane for selective voltammetric sensing of bromide in ACN/acetone/H2O 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The groups of Beer as well as Schöllhorn and Fave also investigated a range of other redox transducers in XB anion sensors, such as viologen-based systems for detection of various halides, whereby all studies revealed an important contribution of XB in obtaining (enhanced) voltammetric responses.147–149 The latter groups further conducted a range of systematic studies into XB iodo-tetrathiafulvalene (TTF) voltammetric sensors.138,150 As shown in Fig. 24, iodo-TTF 23.XB displays two reversible oxidative couples in DMF, corresponding to step-wise one-electron oxidation to 23.XB+˙ and 23.XB2+, which both respond to the presence of increasing chloride concentrations by well-defined, continuous cathodic shifts. As expected, various control experiments proved that XB formation was the crucial driving force in halide recognition and sensing.138,150
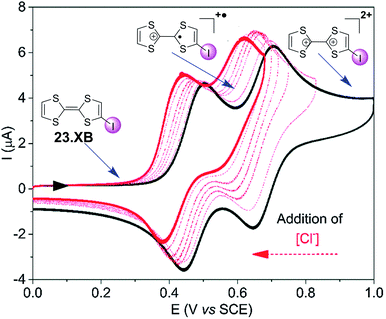 | ||
| Fig. 24 Chemical structure of iodo-TTF 23.XB and its corresponding CV in DMF in the absence (black) and presence (red) of increasing concentrations of Cl−. Depicted are also the different redox states at different potentials (structures and blue arrows). Adapted from ref. 138 with permission from the PCCP Owner Societies. | ||
The sensor further displayed somewhat smaller cathodic perturbations in the presence of Br−, while OTf−, NO3− and H2O did not induce any response (Fig. 25). By fitting of the voltammetric binding isotherms to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric Nernst binding model the authors further extracted absolute halide binding constants to all receptor oxidation states.40,138
1 host–guest stoichiometric Nernst binding model the authors further extracted absolute halide binding constants to all receptor oxidation states.40,138
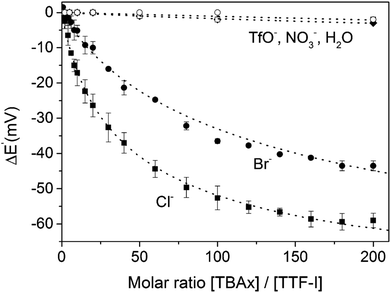 | ||
| Fig. 25 Cathodic voltammetric perturbations of the first oxidative couple of iodo-TTF 23.XB in DMF upon addition of various anions. Reproduced from ref. 138 with permission from the PCCP Owner Societies. | ||
Unsurprisingly, the neutral, native 23XB displayed only very weak halide anion binding constants (≤20 M−1), while binding to the monocationic 23XB+˙ was significantly switched on with K = 425 and 131 M−1 for Cl− and Br−, respectively. A further increase in chloride binding to 23XB2+ of K = 6730 M−1 was extracted, while the analogous binding constant for Br− could not be obtained as Br− oxidation overlapped with the second oxidative TTF couple in CV. These observations saliently highlight the unique advantages of voltammetric anion sensors; the transient generation of a more cationic, higher oxidation state increases anion binding to such an extent that sensing in competitive solvent media, in which the native receptor often displays negligible anion binding, is possible. This concept was also recently exploited for the sensing of anions in competitive aqueous/organic solvent systems at a range of interfacial XB anion sensors, as discussed in more detail in Section 4.1.2.57,71,72,137
The same groups later reported a systematic investigation of rarely studied electrolyte effects on the anion sensing performance of a methylated iodo-TTF derivative of 23.XB.150 The authors showed that different electrolyte anions BF4−, MsO−, TfO−, NO3−, ClO4−, PF6− or BArF4− ([tetrakis[3,5-bis(trifluoromethyl)phenyl]borate]) can significantly influence the sensing properties of (XB) voltammetric anion sensors. For instance, the cathodic shift perturbation of XB sensor trimethyl-iodo-TTF towards 100 equiv. Cl− ranged between −36 and −49 mV, corresponding to an up to 2.6-fold difference in KOx, depending on the electrolyte. These observations highlight that even “non-coordinating” electrolyte anions may significantly compete with anion binding and associated signal transduction in redox-active sensors. This was recently corroborated by a systematic comparison of NMR, UV-vis and voltammetrically determined anion binding constants in XB viologen derivatives in the absence and presence of electrolytes.149
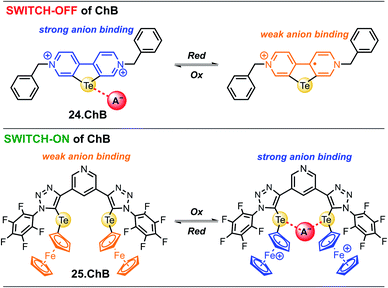
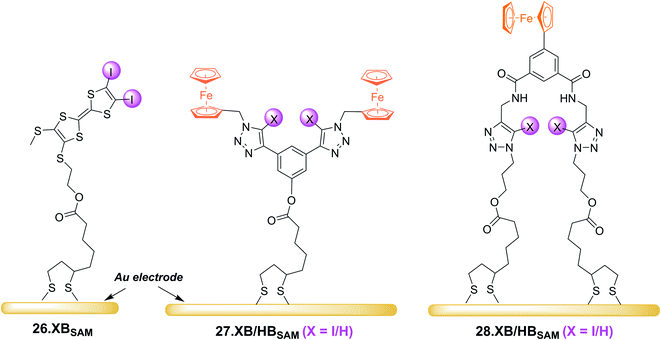
In 2022, Hein et al. reported the first examples of ChB voltammetric anion sensors including the dicationic telluro-viologen derivative 24.ChB as well as the neutral pyridine bis(ferrocenyltellurotriazole) 25.ChB (Fig. 26).62
The telluro-viologen 24.ChB displayed moderately strong halide binding in competitive CD3CN/D2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a modest preference for bromide (K = 1036 M−1) while its lighter Se congener bound all halides much more weakly (K = 182 M−1 for Br−), yet still stronger than the HB viologen analogue (K = 139 M−1), confirming a significant ChB participation in anion recognition. Voltammetric anion sensing studies in the same solvent system confirmed significant cathodic responses of the first reductive viologen couple of the telluoroviologen 24.ChB, which were again largest for bromide (ΔEmax = −61 mV), and slightly smaller for chloride (−57 mV) and iodide (−49 mV). In contrast, the oxoanions HSO4− and NO3− induced smaller responses of ≤−36 mV. These perturbations were only observed for the first reductive couple; the second reduction couple was not perturbed in the presence of any anion, see Fig. 27. This indicates that upon first mono-electron reduction, the receptor's potent ChB ability is switched-OFF i.e. the bound anion is expelled. A further reduction has thus no additional effect on anion binding and no shifts of the second couple are observed. Importantly, both the lighter ChB seleno-congener as well as the unfunctionalized HB viologen responded to anions much more weakly, with largest responses towards Cl− of −22 and −17 mV, respectively. Of further note is that 24.ChB also responded to the halides via naked eye-visible changes in absorbance (red-shift), thereby acting as a dual-output anion sensor.
1 with a modest preference for bromide (K = 1036 M−1) while its lighter Se congener bound all halides much more weakly (K = 182 M−1 for Br−), yet still stronger than the HB viologen analogue (K = 139 M−1), confirming a significant ChB participation in anion recognition. Voltammetric anion sensing studies in the same solvent system confirmed significant cathodic responses of the first reductive viologen couple of the telluoroviologen 24.ChB, which were again largest for bromide (ΔEmax = −61 mV), and slightly smaller for chloride (−57 mV) and iodide (−49 mV). In contrast, the oxoanions HSO4− and NO3− induced smaller responses of ≤−36 mV. These perturbations were only observed for the first reductive couple; the second reduction couple was not perturbed in the presence of any anion, see Fig. 27. This indicates that upon first mono-electron reduction, the receptor's potent ChB ability is switched-OFF i.e. the bound anion is expelled. A further reduction has thus no additional effect on anion binding and no shifts of the second couple are observed. Importantly, both the lighter ChB seleno-congener as well as the unfunctionalized HB viologen responded to anions much more weakly, with largest responses towards Cl− of −22 and −17 mV, respectively. Of further note is that 24.ChB also responded to the halides via naked eye-visible changes in absorbance (red-shift), thereby acting as a dual-output anion sensor.
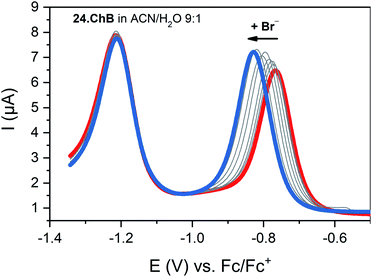 | ||
Fig. 27 SWVs of telluroviologen ChB sensor 24.ChB in ACN/H2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 upon addition of up to 50 mM Br−. Only the first reductive couple is responsive, indicative of complete ChB deactivation upon mono-reduction. Reproduced with permission from ref. 62 copyright 2022 American Chemical Society. 1 upon addition of up to 50 mM Br−. Only the first reductive couple is responsive, indicative of complete ChB deactivation upon mono-reduction. Reproduced with permission from ref. 62 copyright 2022 American Chemical Society. | ||
In contrast to the reductive switch-OFF telluro-viologen system, the neutral ferrocenyltellurotriazole receptor 25.ChB was investigated as a ChB switch-ON sensor via ferrocene oxidation.
In its natively neutral state 25.ChB displayed very low ChB potency as elucidated by 1H NMR anion binding studies; even in the less competitive acetone only H2PO4− displayed modest binding of 111 M−1.
Nevertheless, the sensor displayed large cathodic perturbations of the ferrocene/ferrocenium redox couple upon addition of various halides (Cl−, Br−) as well as oxoanions (H2PO4−, HSO4−, NO3−), in a range of competitive solvent systems, including ACN, ACN/H2O 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ACN/H2O 9
1 and ACN/H2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, attesting to the strong ChB switch-ON upon receptor oxidation. In ACN, 25.ChB responded with the following selectivity trend: H2PO4− > Cl− > Br− > HSO4− > NO3−, with perturbations of up to −217 mV for H2PO4−. This corresponds to an impressively large binding-enhancement factor BEF = 4800. Even in the presence of 10% water in ACN the sensor still displayed cathodic voltametric shifts of up to −42 mV towards bromide and a notably altered selectivity trend: Br− > H2PO4− ≈ Cl− > HSO4− ≈ NO3−. In comparison to other similar XB/HB voltammetric sensors, 25.ChB displays in general significantly enhanced Br− responses, which are up to 2.4-fold larger than those observed for structurally similar 27.XB in both ACN and ACN/H2O 19
1, attesting to the strong ChB switch-ON upon receptor oxidation. In ACN, 25.ChB responded with the following selectivity trend: H2PO4− > Cl− > Br− > HSO4− > NO3−, with perturbations of up to −217 mV for H2PO4−. This corresponds to an impressively large binding-enhancement factor BEF = 4800. Even in the presence of 10% water in ACN the sensor still displayed cathodic voltametric shifts of up to −42 mV towards bromide and a notably altered selectivity trend: Br− > H2PO4− ≈ Cl− > HSO4− ≈ NO3−. In comparison to other similar XB/HB voltammetric sensors, 25.ChB displays in general significantly enhanced Br− responses, which are up to 2.4-fold larger than those observed for structurally similar 27.XB in both ACN and ACN/H2O 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.57 This confirms a particularly potent redox-dependent binding-modulation of ChB (large BEF and large ΔE), enabled by a high sensitivity of ChB on its electronic environment28 as well as the uniquely close spatial coupling of the redox and Te-donor binding sites, a design principle with significant future potential. Importantly, these findings establish redox-control of ChB as a powerful, reversible approach for high fidelity switch-OFF or switch-ON modulation of ChB anion recognition and sensing.
1.57 This confirms a particularly potent redox-dependent binding-modulation of ChB (large BEF and large ΔE), enabled by a high sensitivity of ChB on its electronic environment28 as well as the uniquely close spatial coupling of the redox and Te-donor binding sites, a design principle with significant future potential. Importantly, these findings establish redox-control of ChB as a powerful, reversible approach for high fidelity switch-OFF or switch-ON modulation of ChB anion recognition and sensing.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. In contrast, the response of 26.XBSAM follows a more complex slow-exchange two-wave pattern with emergence of a new peak at lower potentials, a result of altered kinetic binding profiles. These results nicely illustrate the afore-mentioned advantages of interfacial sensing, that is circumvented solubility constraints and improved response magnitudes. The latter was justified by elucidation of the Cl− binding constants to 26.XBSAM and 26.XBSAM+˙, which were with KRed ≈ 1000 M−1 and KOx ≈ 570.000 M−1, not only individually larger than those in solution, but whose ratio (i.e. the BEF) was also significantly enhanced, corresponding to a larger response magnitude (ΔE ∝ KOx/KRed).
7. In contrast, the response of 26.XBSAM follows a more complex slow-exchange two-wave pattern with emergence of a new peak at lower potentials, a result of altered kinetic binding profiles. These results nicely illustrate the afore-mentioned advantages of interfacial sensing, that is circumvented solubility constraints and improved response magnitudes. The latter was justified by elucidation of the Cl− binding constants to 26.XBSAM and 26.XBSAM+˙, which were with KRed ≈ 1000 M−1 and KOx ≈ 570.000 M−1, not only individually larger than those in solution, but whose ratio (i.e. the BEF) was also significantly enhanced, corresponding to a larger response magnitude (ΔE ∝ KOx/KRed).
An enhanced interfacial response of the bis(ferrocene-(iodo)-triazole) sensors 27.XB/HBSAM towards the oxoanions HSO4−, H2PO4− and NO3− in various ACN/H2O mixtures of up to 30% water was also reported by Patrick et al. in 2021.57
Interestingly, the HB congener 27.HBSAM displayed a slightly enhanced response towards these oxoanions in comparison to 27.XBSAM, while under diffusive conditions 27.XBdif outperformed 27.HBdif in response to all anions, including Cl− and Br−, in a range of ACN/H2O mixtures of up to 20% water. This unexpected observation potentially arises from differing interfacial receptor organizational or hydration differences as elucidated by various surface analyses. Particularly noteworthy in this study is a rare demonstration of XB/HB (interfacial) voltammetric sensing in highly competitive aqueous media of up to 30% water (in which the diffusive receptors are not soluble), again highlighting the utility of surface-immobilisation in generating potent, potentially real-life relevant electrochemical anion sensors. This work also presents the first comprehensive study into solvent effects in voltammetric anion sensors. Unsurprisingly, the voltammetric shift magnitude of both sensors, in solution and at the surface, generally decreased upon increasing water content, particularly strongly for H2PO4−, a reflection of its large hydration enthalpy. A noteworthy exception to this trend is the solution-phase performance of 27.XBdif, whose response to the halides was largely independent of water content (note that this effect could not be studied at the interfacial receptors due to poor voltammetric reversibility of 27.XB/HBSAM in the presence of halides). Importantly, this trend was not observed for 27.HBdif; with increasing water content the anion sensing performance of 27.XBdifrelatively increased in comparison to the HB sensor, attesting to the potency of XB anion sensing in aqueous media.
A detailed investigation into the transduction mechanisms that govern the response mechanisms and enhanced signal magnitudes of interfacial voltammetric sensors was recently reported by Hein et al.137 As shown in Fig. 29, the XB/HB ferrocene-isophthalamide-(iodo)triazole interface 28.XB/HBSAM displayed significantly enhanced sensory responses towards a range of oxoanions as well as halides in ACN/H2O 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
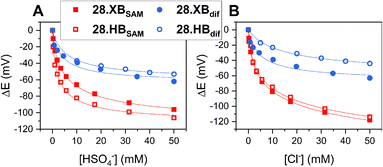 | ||
Fig. 29 Comparison of cathodic voltammetric shifts under diffusive conditions of 28.XB/HBdif (blue circles) and on the surface (28.XB/HBSAM (red squares)) in ACN/H2O 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 upon titration with (A) HSO4− and (B) Cl−. Filled symbol represent the XB receptors while empty symbols represent the HB receptors. A pronounced surface enhancement was also observed for all other tested anions (Br−, H2PO4− and NO3−). Adapted from ref. 137 with permission from the Royal Society of Chemistry. 1 upon titration with (A) HSO4− and (B) Cl−. Filled symbol represent the XB receptors while empty symbols represent the HB receptors. A pronounced surface enhancement was also observed for all other tested anions (Br−, H2PO4− and NO3−). Adapted from ref. 137 with permission from the Royal Society of Chemistry. | ||
In analogy to the 27.XB/HB sensor system, the interfacial response towards the oxoanions HSO4−, H2PO4− and NO3− was slightly augmented for 28.HBSAM, while 28.XBSAM displayed a modest preference towards the halides Cl− and Br−, in agreement with previous observations of a typical XB recognition preference towards (softer) halides.14,23
In good agreement with the above-described voltammetric studies is also the consistently augmented solution-phase performance of 28.XBdif towards all anions. All of these observations were rationalised in the context of a novel dielectric model, highlighting the importance of through-space and through-bond charge interactions and their screening in environments of different dielectric.
As a result of its well-defined anion sensing performance and high voltammetric stability the groups of Beer and Davis further developed 28.XB/HBSAM as anion sensors for real-time continuous flow sensing. The detection of anions in aqueous media under flow conditions is of high relevance across various applications, including long-term health and water monitoring, but remains underdeveloped. Interfacial supramolecular sensors are ideally suited to address this challenge as they can be easily re-used by simple washing. To demonstrate this capability, Patrick and Hein et al. developed a 3D-printed electrochemical flow cell (Fig. 30A) through which electrolyte was continuously pumped over a 28.XB/HBSAM-modified gold electrode.72
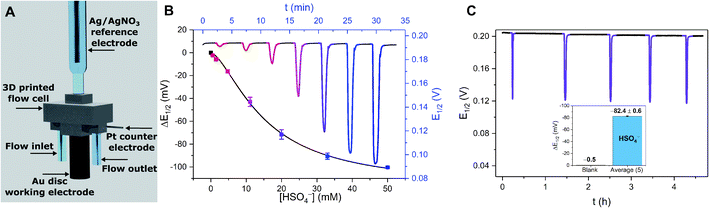 | ||
Fig. 30 (A) Schematic depiction of the 3D-printed electrochemical flow cell. (B) Sensogram (blue axes) and corresponding isotherm (black axes) of 28.XBSAM under continuous flow to increasing concentrations of HSO4− in ACN/H2O 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as measured by continuous SWV. Each spike corresponds to injections of increasing [HSO4−] up to 50 mM. (C) Voltammetric response of 28.XBSAM towards five additions of 20 mM HSO4− under continuous electrolyte flow over 4.5 h. The inset shows the voltammetric shift in response to the blank electrolyte and the average of all five HSO4− additions. Reproduced from ref. 72 under the terms of the CC BY license. 1 as measured by continuous SWV. Each spike corresponds to injections of increasing [HSO4−] up to 50 mM. (C) Voltammetric response of 28.XBSAM towards five additions of 20 mM HSO4− under continuous electrolyte flow over 4.5 h. The inset shows the voltammetric shift in response to the blank electrolyte and the average of all five HSO4− additions. Reproduced from ref. 72 under the terms of the CC BY license. | ||
A continuous sensor signal readout (that is the E1/2) of the sensor was obtained by repeat SWV voltammetry and analysis of the voltammograms with a custom MATLAB script, affording a highly stable signal baseline with a temporal resolution of ≈4 s. Injection of anion aliquots (HSO4−, H2PO4− or Cl−) into the flow then induced response spikes in the sensograms (Fig. 30B), the magnitudes of which were pleasingly identical to that obtained under standard, “static” conditions. Importantly, upon washing with fresh electrolyte, the sensor's response quickly returned to its baseline, confirming complete anion removal. Impressively, the sensor could be continuously operated over a 4.5 h period (corresponding to 3700 voltammetric scans), with a highly reproducible response to repeat HSO4− injections and minimal baseline drift of ≤5 mV (Fig. 30C).
Surface-immobilisation of ion receptors is associated with a further unique advantage over solution-phase sensing; an ability to utilise other electroanalytical techniques. This most notably includes electrochemical impedance/capacitance spectroscopy (EIS/ECS). The former is well-established for the sensing of ions at redox-inactive receptive electrode surfaces, whereby a signal-generating solution-phase redox probe (typically ferri/ferrocyanide) is employed.153–155
Upon interfacial ion recognition, electrostatic interactions between the charged redox probe and the receptive surface are altered such that a change in the charge-transfer resistance is observed (Faradaic impedance).
In contrast, faradaic capacitance spectroscopy relies on changes in the capacitive, that is charge-storing, properties of a surface-bound redox transducer.156 This redox capacitance Cr is highly sensitive to changes in local (dielectric) properties and is well-established for biosensing.157–159
In 2021, Patrick and Hein et al. demonstrated for the first time the utility of this approach for ion sensing.71 This study was carried out on the same XB receptive interface 28.XBSAM, enabling a direct comparison with the afore-discussed voltammetric sensing format. Redox capacitance spectroscopy at a fixed AC frequency was employed to resolve the interfacial redox capacitance Cr which reports on the redox density of states (DOS) of the electro-active interface. Resolving this DOS (green triangles, Fig. 31A) affords, in the first instance, analogous information as standard SWV (black trace), i.e. it reports on anion binding-induced cathodic shifts (Fig. 31B).
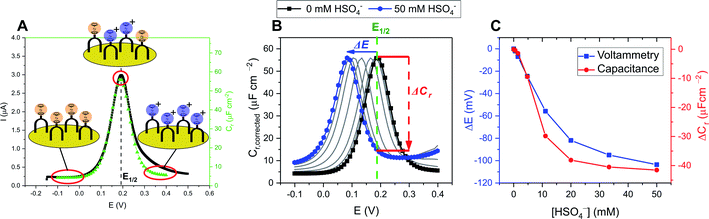 | ||
Fig. 31 (A) Comparison of SWV (black) and redox capacitance Cr (green triangles) as a function of electrode potential (i.e. redox density of states (DOS)) of 28.XBSAM in ACN/H2O 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Schematically depicted is the ratio of oxidised and reduced anion receptors at different potential regimes whereby ferrocene/ferrocenium units are shown in orange and blue, respectively. (B) Normalised redox capacitance Cr as function of potential in response to increasing concentrations of HSO4−. The blue arrow indicates the “standard” potential shift as typically resolved voltammetrically. The dashed red arrow indicates the drop in Cr at E1/2 (dotted black line). (C) Comparison of voltammetric (blue) and redox capacitive response isotherms at E1/2 (red). Reproduced with permission from ref. 71 copyright 2021 American Chemical Society. 1. Schematically depicted is the ratio of oxidised and reduced anion receptors at different potential regimes whereby ferrocene/ferrocenium units are shown in orange and blue, respectively. (B) Normalised redox capacitance Cr as function of potential in response to increasing concentrations of HSO4−. The blue arrow indicates the “standard” potential shift as typically resolved voltammetrically. The dashed red arrow indicates the drop in Cr at E1/2 (dotted black line). (C) Comparison of voltammetric (blue) and redox capacitive response isotherms at E1/2 (red). Reproduced with permission from ref. 71 copyright 2021 American Chemical Society. | ||
However, in contrast to voltammetry, each point within this Cr DOS distribution is recorded at equilibrium, i.e. does not require a potential sweeping. Instead, Cr can be continually measured at a constant, freely chosen electrode potential and provides a simple, constant, direct sensor readout. For example, if Cr is continually monitored at the initial E1/2 of the interface then anion binding induces a drop in signal, as Cr now lies in the anodic tail of the redox distribution. The sensing isotherm obtained in this manner is similar to that obtained by standard voltammetry (Fig. 31C), however the binding and response are somewhat enhanced due to a self-amplification effect in the redox capacitive format.
As a result of its direct readout, necessitating no further data analysis, and its high temporal resolution (≈2.5 s) this novel methodology is ideally suited for real-time, continuous flow ion sensing, as investigated in the same 3D-printed flow cell as used for previous voltammetric studies. As shown in Fig. 32A, injection of HSO4− aliquots of increasing concentration induced noticeable response spikes, whose magnitude and “direction” are dependent on the applied electrode potential. In addition to this signal switch-on/off control the apparent binding constant (i.e. “steepness”) of the response isotherm can be modulated by judicious choice of potential (Fig. 32B) by up to 1 order of magnitude (Kapp at +100 mV = 320 M−1vs. 36 M−1 at −200 mV). Concomitantly, the sensor's LOD can be tuned in this manner, and is with ≈45 μM (for measurements at E1/2), lower than in an optimised voltammetric format,72 which cannot be additionally tuned. With a higher temporal resolution, direct sensor readout as well as improved and tuneable analytical performance, this redox capacitive sensing format is thus vastly superior to standard voltammetry. In addition to supporting improved ion sensing capabilities, preliminary investigations suggest that the redox capacitive readout maybe used to elucidate interfacial host–guest binding kinetics and thus also presents a novel tool in the fundamental study of interfacial electro-active supramolecular host–guest systems.
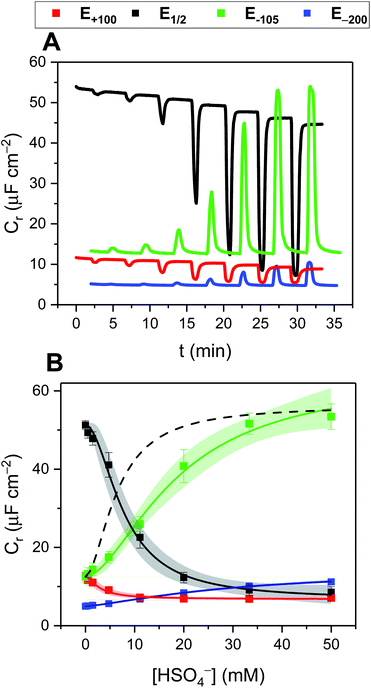 | ||
| Fig. 32 (A) Redox capacitance response of 28.XBSAM towards HSO4− at E1/2 (black), E−105 (green), E−200 (blue) and E+100 (red) under continuous electrolyte flow in a custom 3D-printed flow cell (see Fig. 31A). Each spike in (A) represents the response towards aliquots of HSO4− of increasing concentrations with absolute signal increasing or decreasing depending on the initial surface polarisation. (B) The corresponding baseline-corrected response isotherms. The dashed black line represents, for simpler comparison, the mirrored capacitance response at E1/2, highlighting the steeper response slope and enhanced anion binding magnitude at E1/2vs. at E−105. Reproduced with permission from ref. 71 copyright 2021 American Chemical Society. | ||
4.2 Other electrochemical sensors
Exploiting the uniquely potent performance of XB for anion sensing in water, Hein et al. recently demonstrated, for the first time non-faradaic capacitive anion sensing at XB and HB foldamer molecular films 29.XB/HBSAM (Fig. 33).162
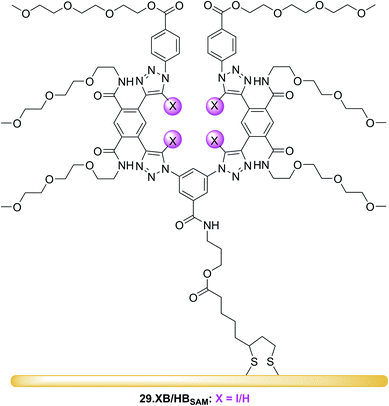 | ||
| Fig. 33 XB and HB foldamer SAMs for anion sensing in pure water via non-faradaic capacitance spectroscopy. | ||
In pure water this sensor responded selectively to the environmentally and biologically relevant charge-diffuse anions ReO4−, I− and SCN− by an increase in the interfacial capacitance (Fig. 34A) while neither Cl−, Br− nor ClO4− induced any response. This is notably different to the recognition behaviour of the parent foldamer receptor in solution-phase, where 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric binding with ReO4−, I−, SCN−, Br− and ClO4− was ascertained via isothermal titration calorimetry (ITC), with β = K1K2 of up to 1.45 × 1010 M−2 for I−. In contrast, binding was significantly attenuated at the surface for 29.XB/HBSAM (K ≤ 360 M−1), and proceeds via formation of 1
1 host–guest stoichiometric binding with ReO4−, I−, SCN−, Br− and ClO4− was ascertained via isothermal titration calorimetry (ITC), with β = K1K2 of up to 1.45 × 1010 M−2 for I−. In contrast, binding was significantly attenuated at the surface for 29.XB/HBSAM (K ≤ 360 M−1), and proceeds via formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complexes, as suggested by interfacial binding isotherm analysis according to the Langmuir adsorption model. As representatively shown in Fig. 34B, the XB sensor 29.XBSAM outperformed its HB congener in all cases, with not only higher maximum signal magnitudes but also increased binding strength.
1 host–guest complexes, as suggested by interfacial binding isotherm analysis according to the Langmuir adsorption model. As representatively shown in Fig. 34B, the XB sensor 29.XBSAM outperformed its HB congener in all cases, with not only higher maximum signal magnitudes but also increased binding strength.
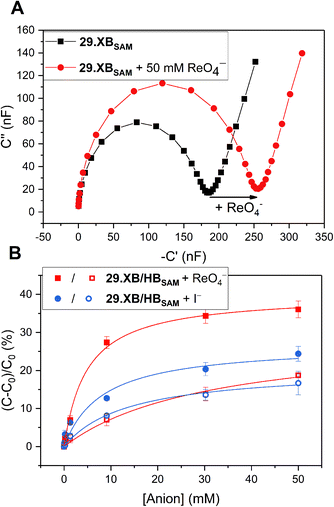 | ||
| Fig. 34 (A) Capacitive Nyquist plots of 29.XBSAM in the absence and presence of 50 mM ReO4− showing an increased capacitance in the presence of bound anion. (B) Relative capacitive sensor response of 29.XBSAM (filled symbols) and 29.HBSAM (empty symbols) in response to ReO4− (red) and I− (blue). Reproduced from ref. 162 with permission of the Royal Society of Chemistry. | ||
The binding of ReO4− in particular was enhanced significantly for 29.XBSAM (K = 231 M−1) in comparison to 29.HBSAM (K = 11 M−1).
Relatedly, the LOD of 29.XBSAM was ≈3-fold improved in all cases and was lowest for I− (14 μM). The physicochemical origins of these capacitive sensor response patterns were also later analysed and justified within a mesoscopic model.163 Of note is that, in principle, this sensor can, akin to the redox capacitive sensor 28.XBSAM, be operated at a fixed frequency and freely chosen electrode potential and may thus be used for real-time flow anion sensing in pure water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) αi, where E0 is a constant, αi the activity of the ion and
αi, where E0 is a constant, αi the activity of the ion and 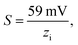 where zi is the charge of the ion. For a monovalent ion an ideal “Nernstian” response of 59 mV per decade change of ion concentration is thus expected. In order to render the ISE selective toward the target analyte, ion receptors (ionophores) are typically incorporated into the hydrophobic membrane component of the ISE. To date, the vast majority of anion ISEs rely on Lewis acid metal complexes or traditional HB receptors as ionophores.40 Surprisingly, σ–hole receptors have, despite their typically contrasting selectivity patterns and larger hydrophobicity, only very recently been explored in ISEs. Specifically, the first, and thus far only, example of a XB ionophore 30.XB was reported by Lim, Goh and co-workers for the potentiometric sensing of iodide in pure water.166
where zi is the charge of the ion. For a monovalent ion an ideal “Nernstian” response of 59 mV per decade change of ion concentration is thus expected. In order to render the ISE selective toward the target analyte, ion receptors (ionophores) are typically incorporated into the hydrophobic membrane component of the ISE. To date, the vast majority of anion ISEs rely on Lewis acid metal complexes or traditional HB receptors as ionophores.40 Surprisingly, σ–hole receptors have, despite their typically contrasting selectivity patterns and larger hydrophobicity, only very recently been explored in ISEs. Specifically, the first, and thus far only, example of a XB ionophore 30.XB was reported by Lim, Goh and co-workers for the potentiometric sensing of iodide in pure water.166
Initial 1H NMR studies in d6-acetone indicated convergent XB mediated I− recognition with moderate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric binding of K = 260 M−1. In contrast, I− binding of the HB analogue 30.HB was significantly attenuated with K = 2.75 M−1. Incorporation of the ionophores into polymeric membranes of varying composition produced a series of ISEs, all of which responded with a near-Nernstian response of ≈50 mV/decade and a LOD of ≈1.25 μM to iodide. The authors then conducted a range of selectivity studies in the presence of the potentially interfering anions Cl−, Br−, NO3−, SCN− and ClO4−. Based on the Hofmeister series, anions of low hydrophilicity, i.e. more hydrophobic ones, can more easily ingress into the membrane to induce the largest interference, as observed for a control membrane without ionophore, which displayed the expected selectivity pattern of: ClO4− > SCN− > I− > NO3− > Br− > Cl−.
1 host–guest stoichiometric binding of K = 260 M−1. In contrast, I− binding of the HB analogue 30.HB was significantly attenuated with K = 2.75 M−1. Incorporation of the ionophores into polymeric membranes of varying composition produced a series of ISEs, all of which responded with a near-Nernstian response of ≈50 mV/decade and a LOD of ≈1.25 μM to iodide. The authors then conducted a range of selectivity studies in the presence of the potentially interfering anions Cl−, Br−, NO3−, SCN− and ClO4−. Based on the Hofmeister series, anions of low hydrophilicity, i.e. more hydrophobic ones, can more easily ingress into the membrane to induce the largest interference, as observed for a control membrane without ionophore, which displayed the expected selectivity pattern of: ClO4− > SCN− > I− > NO3− > Br− > Cl−.
While incorporation of the 30.HB ionophore into the membrane did not appreciably alter this selectivity trend, the XB ionophore induced moderate enhancements in I− selectivity over all other tested anions with a notable, modest, preference for I− over SCN−, indicating that specific XB mediated I− recognition takes place within the membrane.
As shown in Fig. 35, the I− selectivity over the more hydrophilic halides was sufficiently large such that 10 mM Cl− or Br− did not interfere. In contrast, the more hydrophobic SCN− and in particular ClO4− significantly interfered with I− determination at much lower levels of 0.1 mM. Nevertheless, this study provides an important first foray into the exploitation of σ–hole interactions in ISEs which will undoubtedly receive more attention in the future, in particular for the sensing of softer anions.
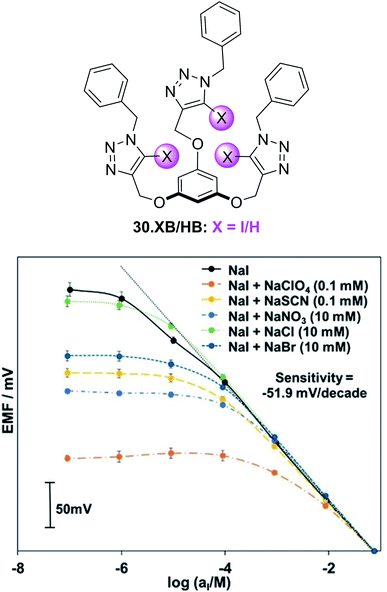 | ||
| Fig. 35 Response of an ISE containing ionophore 30.XB toward iodide in the absence of presence of competing anions. Reproduced with permission from ref. 166 copyright 2021 American Chemical Society. | ||
5. Other sensors
Akin to electrochemical sensors, chemiresistive sensors have emerged as simple, cheap and scalable sensing devices.¶ They respond to analyte presence by changes in the conductance of a sensing material immobilised between two electrodes, a concept that has been exploited in particular for sensing of gases,167 but also ions.168–170In 2016, the group of Swager demonstrated for the first time the utility of XB “selectors” as host motifs to enable selective chemiresistive sensing of pyridine gas.171 To this end they modified single-walled carbon nanotubes (SWCNTs) with haloaryl XB hosts by solvent-free ball-milling. The resulting selector-modified SWCNTs were then immobilized between gold electrodes and their conductance G measured in the absence and presence of pyridine (Fig. 36A).
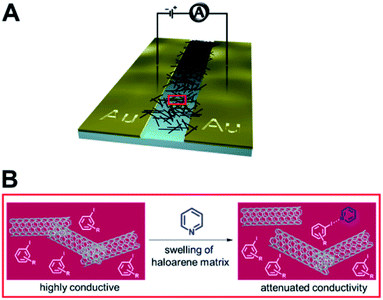 | ||
| Fig. 36 (A) Schematic depiction of a chemiresistive sensor based on a carbon nanotube matrix between two gold electrodes. (B) Incorporation of haloaryl XB selectors into the sensor matrix enables sensing of pyridine vapours by recognition-induced swelling of the matrix and an associated decrease in conductance. Reproduced with permission from ref. 171 copyright 2016 American Chemical Society. | ||
As shown in Fig. 37, p-dihalobenzene selectors enabled sensing of low concentrations of pyridine gas (<25 ppm) by a decrease in electrical conductance, induced by swelling of the sensing matrix (Fig. 36B). Importantly, the p-diiodobenzene host enabled more sensitive sensing than the bromo or chloro-congeners, consistent with a sensor response arising from XB-mediated recognition.
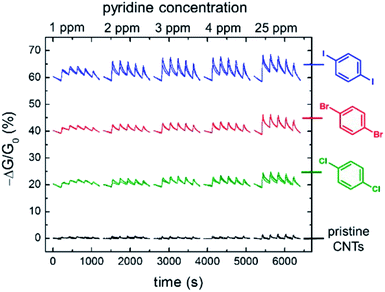 | ||
| Fig. 37 Conductance response of chemiresistive gas sensors containing different dihaloaryl XB selectors in the repeating presence of increasing concentrations of pyridine in N2 carrier gas. For clarity the relative conductance responses are offset by 20%. Reproduced with permission from ref. 171 copyright 2016 American Chemical Society. | ||
Specifically, the p-diiodobenzene-containing sensor displayed the largest response of −ΔG/G0 = 5.1 ± 0.9% in response to only 3 ppm pyridine, while much higher pyridine concentrations were required to induce significant responses when the other selectors were employed. Further evidence for the crucial role of XB in this sensor was also obtained by experiments using iodo- or bromodurene as mono-haloaryl selectors as well as studies with 4-methylpyridine as analyte, which due to its enhanced Lewis basicity induced larger responses. Of further note is the response of these XB sensors displaying a relatively high level of selectivity; depending on the selector, even very high concentrations (>1000 ppm) of the potential interferents acetonitrile, benzene, isopropanol or hexanes induced only minor changes in conductance.
Similar chemiresistive gas sensors based on various p-dihalobenzene XB selectors and a SWCNT matrix were also developed for the detection of cyclohexanone and dimethyl-dinitro-butane (DMNB).172 These analytes were chosen as model compounds for detection of nitro-containing explosives, the latter also serving as a tagant or marker compound in certain plastic explosives. Unsurprisingly, the diiodoaryl based selectors outperformed their bromo-counterparts for the sensing of cyclohexanone, with linear conductance decreases of up to 12%. Initial experiments indicate that the sensor can also detect the less volatile DMNB. A variety of other analytes also induced significant responses (EtOH, ACN, EtOAc, cyclohexane and acetone), however this was attributed to their much higher vapour pressures and thus higher concentrations under the experimental conditions.
Notably in both of these studies, the sensor was easily regenerated by exposure to pure N2 gas, confirming the reversibility of the analyte–sensor interaction and enabling facile sensor re-use.171,172
In 2016 Liu et al. reported a XB organogelator 31.XB containing multiple peripheral iodoperfluoroarene moieties as a visual and rheological Cl− sensor (Fig. 38).173
In acetone/hexane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 the free gelator formed an organogel, which collapsed into a solution within 10 min upon addition of 0.8 equiv. Cl−. In contrast, the same amount of Br− only induced a small degree of gel–sol transition while HSO4−, NO3−, CN− or I− had no effect on the gel. Only at higher concentrations (2 equiv. for Br− and 5 equiv. for I−) was dissolution of the gel achieved. These observations are in good agreement with the anion association constants of the gelator, determined by 1H NMR titrations in acetone, which were largest for Cl− (650 M−1) and smaller for Br− (390 M−1) and I− (140 M−1), while the other anions did not bind significantly.
8 the free gelator formed an organogel, which collapsed into a solution within 10 min upon addition of 0.8 equiv. Cl−. In contrast, the same amount of Br− only induced a small degree of gel–sol transition while HSO4−, NO3−, CN− or I− had no effect on the gel. Only at higher concentrations (2 equiv. for Br− and 5 equiv. for I−) was dissolution of the gel achieved. These observations are in good agreement with the anion association constants of the gelator, determined by 1H NMR titrations in acetone, which were largest for Cl− (650 M−1) and smaller for Br− (390 M−1) and I− (140 M−1), while the other anions did not bind significantly.
A similar anion binding-induced transformation of a supramolecular assembly was also reported based on ChB quasi-calix[4]-chalcogenadiazole hosts 32.Te and 32.Se, which upon interaction with a pyridine N-oxide surfactant 33 in water self-assembled into vesicle or nanofibers, respectively (Fig. 38).174 Upon exposure to halide anions, or lowering the pH, these formations disassembled. In the case of the vesicles formed by 32.Te and 33, this was exploited as a proof-of-principle for release of the chemotherapeutic doxorubicin (DOX). Specifically, DOX-loaded vesicles were ruptured by addition of Cl− or Br−, resulting in DOX release and increase of DOX fluorescence, thereby presenting an indirect halide sensor.
6. Conclusions and outlook
The considered employment of sigma–hole interactions in the development of sensors, in particular for anions, but also neutral (gaseous) Lewis basic analytes, has significantly matured in the last decade. This includes a large range of both optical and electrochemical sensing approaches, in particular those based on fluorescent as well as voltammetric readouts. In addition, various other sensor formats, most notably chemiresistors as well as (redox)capacitive sigma–hole sensors have been developed recently. In all these formats an improved performance of the sigma–hole sensor (in particular XB) in comparison to a structurally analogous HB sensor is typically observed, including enhanced response magnitudes and sensitivities, lower LODs and/or enhanced/altered selectivity patterns. This arises as a result of enhanced binding magnitudes and/or enhanced signal transduction, which in turn can be attributed to the inherent characteristics of sigma–hole interactions, most notably lower solvent dependencies, higher hydrophobicities, stricter geometric binding preferences and altered thermodynamic binding contributions. As a result of these combined advantages, increasingly sophisticated and potent XB sensors have been developed, in particular in the last ≈5 years. This includes, for example, systems capable of anion sensing in increasingly competitive media, including pure water,101,162,166 as well as continuous, real-time sensing systems.71,72,131,171,172These significant advances in a comparably short amount of time attest to the enormous future potential of sigma–hole based sensors for real-life relevant applications and provide an excellent foundation for a broad range of research activities in sensor development and related applications as well as fundamental host–guest studies. We believe further efforts in this field will/should focus on the following:
6.1 Sensing in aqueous media
In spite of the aforementioned examples, anion sensing in predominantly aqueous media remains a highly important but formidable challenge, which can, in part, be attributed to the significant synthetic complexity of receptive, water-soluble probes. This can partially be circumvented by use of non-diffusive sensing formats (e.g. surface immobilisation) thereby negating the need for solubilising groups. Similarly, the omission of reporter groups can reduce synthetic complexity, but this can only be done if a sensing approach without a transducer is used, e.g. in potentiometric, chemiresistive or impedimetric/capacitive formats.6.2 Fundamental studies into and exploitation of (other) sigma–hole interactions
While XB and, to a lesser extent ChB based sensors, are established, the application of pnictogen bonding (PnB) and tetrel bonding (TrB) as non-covalent supramolecular interactions for sensing has not been reported to date. Nevertheless, there is an increasing interest in the design and application of such receptors,14,16,175–177 and it is expected that they would display potent sensory performance. In light of the recently developed electroactive ChB systems62 it appears that redox-control of PnB or TrB may be a particularly promising approach to not only generate novel sensors but to also gain fundamental insights into their intrinsic bonding properties.177 Such fundamental investigations have already been carried out on a range of XB systems, in particular via voltammetric methodologies,54,125–128 which allow for the transient reversible generation of a differently charged (potentially not otherwise accessible) species. This enables simultaneous investigation of the sigma–hole properties of a receptor in multiple redox states, an approach that will undoubtedly prove useful in a continued investigation of sigma–hole properties.6.3 Development of novel sensing approaches and mechanisms
We envision a further exploration of recently developed or (in the context of XB/ChB-mediated recognition) underexplored methodologies such as impedimetric or (redox)capacitive methodologies,71,162 chemiresistive171,172 or potentiometric approaches.166 In addition, other sensing principles are ripe for exploration in concert with sigma–hole interactions, such as indicator displacement assays (IDAs)178 or the recently developed transporter–liposome–fluorophore (TLF) approach which relies on the quenching of a vesicle-encapsulated fluorophore by the analyte ion.179 In the latter, a selectivity enhancement is achieved by use of a transmembrane ion transporter, such that only ions which can cross the vesicle membrane and quench the encapsulated fluorophore induce a response. Due to their typically improved or contrasting anion transport selectivities and efficacies, sigma–hole anionophores have much to offer as potent anionophores in TLF assays.33–36,175,1806.4 Device and materials integration
As highlighted in Section 2.3, sensor integration into condensed matter, in particular surfaces, membranes and polymeric architectures will be an indispensable avenue towards enabling many real-life relevant sensing applications such as flow sensors and microfluidic devices, as only in these formats the main advantage of the non-covalent sensing approach, its reversibility, can be exploited.68,71,72,181 In addition to sensor re-usability and more facile device integration, this is associated with various other benefits, including surface enhancement effects, circumventing solubility constraints, and the use of otherwise inaccessible sensing formats/readouts (e.g. impedance/capacitance/chemiresistance).40,137,151In spite of the enormous potential, surface immobilisation or material-integration sigma–hole mediated sensing remains comparably underdeveloped, and is only established in electrochemical57,71,72,137,152,162 and chemiresistive formats.171,172 Interfacial XB or ChB optical sensors remain even more embryonic; the only example being the benzoselenadiazole fibres developed by Che for fluorescent gas sensing.131 Clearly there is significant untapped potential in further exploration of XB and ChB (sensing) materials,182,183 particularly in thin films and (interfacial) polymers184–186 in electrochemical, optical and other sensing formats.
Author contributions
RH and PDB co-wrote/edited the review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from Nuclear Waste Services. RH would like to thank Somerville College, University of Oxford for support through a Fulford Junior Research Fellowship. We thank Sophie C. Patrick and Hui Min Tay, University of Oxford, for helpful comments. We also acknowledge Prof. Jason J. Davis, University of Oxford, for useful discussions.Notes and references
- S. Benz, J. López-Andarias, J. Mareda, N. Sakai and S. Matile, Angew. Chem., 2017, 129, 830 CrossRef.
- R. L. Sutar and S. M. Huber, ACS Catal., 2019, 9, 9622 CrossRef CAS.
- K. T. Mahmudov, M. N. Kopylovich, M. F. C. G. da Silva and A. J. Pombeiro, Dalton Trans., 2017, 46, 10121 RSC.
- W. Wang, H. Zhu, S. Liu, Z. Zhao, L. Zhang, J. Hao and Y. Wang, J. Am. Chem. Soc., 2019, 141, 9175 CrossRef CAS PubMed.
- D. Bulfield and S. M. Huber, Chem. - Eur. J., 2016, 22, 14434 CrossRef CAS PubMed.
- R. Weiss, E. Aubert, P. Pale and V. Mamane, Angew. Chem., Int. Ed., 2021, 60, 19281 CrossRef CAS PubMed.
- J. Teyssandier, K. S. Mali and S. De Feyter, ChemistryOpen, 2020, 9, 225 CrossRef CAS PubMed.
- P. Metrangolo, G. Resnati, T. Pilati and S. Biella, in Halogen Bonding: Fundamentals and Applications, ed. P. Metrangolo and G. Resnati, Springer, Heidelberg, 2008, vol. 4, pp. 105–136 Search PubMed.
- A. Dhaka, O. Jeannin, I.-R. Jeon, E. Aubert, E. Espinosa and M. Fourmigué, Angew. Chem., Int. Ed., 2020, 59, 23583 CrossRef CAS PubMed.
- M. Fourmigué and A. Dhaka, Coord. Chem. Rev., 2020, 403, 213084 CrossRef.
- N. Biot and D. Bonifazi, Chem. - Eur. J., 2020, 26, 2904 CrossRef CAS PubMed.
- M. Saccone and L. Catalano, J. Phys. Chem. B, 2019, 123, 9281 CrossRef CAS PubMed.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118 CrossRef CAS PubMed.
- J. Y. C. Lim and P. D. Beer, Chem, 2018, 4, 731 CAS.
- M. Erdelyi, Chem. Soc. Rev., 2012, 41, 3547 RSC.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- P. C. Ho, P. Szydlowski, J. Sinclair, P. J. W. Elder, J. Kübel, C. Gendy, L. M. Lee, H. Jenkins, J. F. Britten, D. R. Morim and I. Vargas-Baca, Nat. Commun., 2016, 7, 11299 CrossRef CAS PubMed.
- N. Biot and D. Bonifazi, Coord. Chem. Rev., 2020, 413, 213243 CrossRef CAS.
- Y.-J. Zhu, Y. Gao, M.-M. Tang, J. Rebek and Y. Yu, Chem. Commun., 2021, 57, 1543 RSC.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386 CrossRef CAS PubMed.
- M. R. Scholfield, C. M. V. Zanden, M. Carter and P. S. Ho, Protein Sci., 2013, 22, 139 CrossRef CAS PubMed.
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645 RSC.
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS.
- G. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, M. Sansotera and G. Terraneo, Chem. Soc. Rev., 2010, 39, 3772 RSC.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648 RSC.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974 CrossRef CAS PubMed.
- P. D. Beer and P. A. Gale, Angew. Chem., Int. Ed., 2001, 40, 486 CrossRef CAS PubMed.
- A. Docker, C. H. Guthrie, H. Kuhn and P. D. Beer, Angew. Chem., Int. Ed. Engl., 2021, 60, 21973 CrossRef CAS PubMed.
- D. J. Pascoe, K. B. Ling and S. L. Cockroft, J. Am. Chem. Soc., 2017, 139, 15160 CrossRef CAS PubMed.
- M. J. Langton, S. W. Robinson, I. Marques, V. Félix and P. D. Beer, Nat. Chem., 2014, 6, 1039 CrossRef CAS PubMed.
- A. Borissov, I. Marques, J. Y. C. Lim, V. Félix, M. D. Smith and P. D. Beer, J. Am. Chem. Soc., 2019, 141, 4119 CrossRef CAS PubMed.
- J. Y. C. Lim and P. D. Beer, Chem. Commun., 2015, 51, 3686 RSC.
- L. E. Bickerton, A. J. Sterling, P. D. Beer, F. Duarte and M. J. Langton, Chem. Sci., 2020, 11, 4722 RSC.
- L. E. Bickerton, A. Docker, A. J. Sterling, H. Kuhn, F. Duarte, P. D. Beer and M. J. Langton, Chem. - Eur. J., 2021, 27, 11738 CrossRef CAS PubMed.
- A. Vargas Jentzsch and S. Matile, J. Am. Chem. Soc., 2013, 135, 5302 CrossRef CAS PubMed.
- S. Benz, M. Macchione, Q. Verolet, J. Mareda, N. Sakai and S. Matile, J. Am. Chem. Soc., 2016, 138, 9093 CrossRef CAS PubMed.
- A. V. Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 1 Search PubMed.
- A. Docker, J. G. Stevens and P. D. Beer, Chem. - Eur. J., 2021, 27, 14600 CrossRef CAS PubMed.
- Y. C. Tse, A. Docker, Z. Zhang and P. D. Beer, Chem. Commun., 2021, 57, 4950 RSC.
- R. Hein, P. D. Beer and J. J. Davis, Chem. Rev., 2020, 120, 1888 CrossRef CAS PubMed.
- H. M. Tay and P. Beer, Org. Biomol. Chem., 2021, 19, 4652 RSC.
- D. A. McNaughton, M. Fares, G. Picci, P. A. Gale and C. Caltagirone, Coord. Chem. Rev., 2021, 427, 213573 CrossRef CAS.
- P. A. Gale and C. Caltagirone, Chem. Soc. Rev., 2015, 44, 4212 RSC.
- R. Hein and P. D. Beer, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2021, vol. DOI:10.1016/B978-0-12-820206-7.00132-3 DOI:10.1016/B978.
- T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS.
- P. Bühlmann and L. D. Chen, in Supramolecular Chemistry: From Molecules to Nanomaterials, Wiley, 2012, vol. 5, p. 2539 Search PubMed.
- E. Bakker, P. Bühlmann and E. Pretsch, Chem. Rev., 1997, 97, 3083 CrossRef CAS PubMed.
- K. N. Mikhelson, Ion-selective electrodes, Springer, 2013 Search PubMed.
- E. Zdrachek and E. Bakker, Anal. Chem., 2019, 91, 2 CrossRef CAS PubMed.
- A. Caballero, F. Zapata and P. D. Beer, Coord. Chem. Rev., 2013, 257, 2434 CrossRef CAS.
- N. Tarannam, R. Shukla and S. Kozuch, Phys. Chem. Chem. Phys., 2021, 23, 19948 RSC.
- B. Inscoe, H. Rathnayake and Y. Mo, J. Phys. Chem. A, 2021, 125, 2944 CrossRef CAS PubMed.
- S. M. Huber, E. Jimenez-Izal, J. M. Ugalde and I. Infante, Chem. Commun., 2012, 48, 7708 RSC.
- V. Angarov and S. Kozuch, New J. Chem., 2018, 42, 1413 RSC.
- S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. L. Thompson, P. Kennepohl and P. D. Beer, J. Am. Chem. Soc., 2015, 137, 499 CrossRef CAS PubMed.
- C. W. Kellett, P. Kennepohl and C. P. Berlinguette, Nat. Commun., 2020, 11, 3310 CrossRef CAS PubMed.
- S. C. Patrick, R. Hein, A. Docker, P. D. Beer and J. J. Davis, Chem. - Eur. J., 2021, 27, 10201 CrossRef CAS PubMed.
- C. C. Robertson, R. N. Perutz, L. Brammer and C. A. Hunter, Chem. Sci., 2014, 5, 4179 RSC.
- P. Politzer and J. S. Murray, ChemPhysChem, 2013, 14, 278 CrossRef CAS PubMed.
- I. Alkorta, J. Elguero and A. Frontera, Crystals, 2020, 10, 180 CrossRef CAS.
- L. Vogel, P. Wonner and S. M. Huber, Angew. Chem., Int. Ed., 2019, 58, 1880 CrossRef CAS PubMed.
- R. Hein, A. Docker, J. J. Davis and P. D. Beer, J. Am. Chem. Soc., 2022 DOI:10.1021/jacs.2c02924.
- Z. Wang, M. A. Palacios and P. Anzenbacher, Anal. Chem., 2008, 80, 7451 CrossRef CAS PubMed.
- Y. Liu, T. Minami, R. Nishiyabu, Z. Wang and P. Anzenbacher, J. Am. Chem. Soc., 2013, 135, 7705 CrossRef CAS PubMed.
- J. Han, B. Wang, M. Bender, K. Seehafer and U. H. F. Bunz, ACS Appl. Mater. Interfaces, 2016, 8, 20415 CrossRef CAS PubMed.
- B. Debus, H. Parastar, P. Harrington and D. Kirsanov, TrAC, Trends Anal. Chem., 2021, 145, 116459 CrossRef CAS.
- C. Zhong, C. Hu, R. Kumar, V. Trouillet, F. Biedermann and M. Hirtz, ACS Appl. Nano Mater., 2021, 4, 4676 CrossRef CAS.
- L. Basabe-Desmonts, F. Benito-López, H. J. G. E. Gardeniers, R. Duwel, A. van den Berg, D. N. Reinhoudt and M. Crego-Calama, Anal. Bioanal. Chem., 2008, 390, 307 CrossRef CAS PubMed.
- R. Zimmerman, L. Basabe-Desmonts, F. van der Baan, D. N. Reinhoudt and M. Crego-Calama, J. Mater. Chem., 2005, 15, 2772 RSC.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305 RSC.
- S. C. Patrick, R. Hein, P. D. Beer and J. J. Davis, J. Am. Chem. Soc., 2021, 143, 19199 CrossRef CAS PubMed.
- S. C. Patrick, R. Hein, M. Sharafeldin, X. Li, P. D. Beer and J. J. Davis, Chem. - Eur. J., 2021, 27, 17700 CrossRef CAS PubMed.
- T. Bunchuay, A. Docker, U. Eiamprasert, P. Surawatanawong, A. Brown and P. D. Beer, Angew. Chem., Int. Ed., 2020, 59, 12007 CrossRef CAS PubMed.
- A. Lachman, J. Am. Chem. Soc., 1903, 25, 50 CrossRef CAS.
- J. Kleinberg and A. W. Davidson, Chem. Rev., 1948, 42, 601 CrossRef CAS PubMed.
- P. L. Wash, S. Ma, U. Obst and J. Rebek, J. Am. Chem. Soc., 1999, 121, 7973 CrossRef CAS.
- T. K. Mole, W. E. Arter, I. Marques, V. Félix and P. D. Beer, J. Organomet. Chem., 2015, 792, 206 CrossRef CAS.
- L. C. Gilday, N. G. White and P. D. Beer, Dalton Trans., 2013, 42, 15766 RSC.
- Y. Cheong Tse, R. Hein, E. J. Mitchell, Z. Zhang and P. D. Beer, Chem. - Eur. J., 2021, 27, 14550 CrossRef CAS PubMed.
- C. J. Massena, N. B. Wageling, D. A. Decato, E. Martin Rodriguez, A. M. Rose and O. B. Berryman, Angew. Chem., Int. Ed., 2016, 55, 12398 CrossRef CAS PubMed.
- M. G. Chudzinski, C. A. McClary and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 10559 CrossRef CAS PubMed.
- C. Loy, J. M. Holthoff, R. Weiss, S. M. Huber and S. V. Rosokha, Chem. Sci., 2021, 12, 8246 RSC.
- G. E. Garrett, G. L. Gibson, R. N. Straus, D. S. Seferos and M. S. Taylor, J. Am. Chem. Soc., 2015, 137, 4126 CrossRef CAS PubMed.
- R. Zeng, Z. Gong, L. Chen and Q. Yan, ACS Macro Lett., 2020, 9, 1102 CrossRef CAS.
- G. E. Garrett, E. I. Carrera, D. S. Seferos and M. S. Taylor, Chem. Commun., 2016, 52, 9881 RSC.
- H. Zhao and F. P. Gabbaï, Nat. Chem., 2010, 2, 984 CrossRef CAS PubMed.
- L. Maugeri, E. M. G. Jamieson, D. B. Cordes, A. M. Z. Slawin and D. Philp, Chem. Sci., 2017, 8, 938 RSC.
- Y. Cheong Tse, R. Hein, E. J. Mitchell, Z. Zhang and P. D. Beer, Chemistry, 2021, 27, 14550 CrossRef CAS PubMed.
- T. A. Barendt, A. Docker, I. Marques, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 11069 CrossRef CAS PubMed.
- T. A. Barendt, S. W. Robinson and P. D. Beer, Chem. Sci., 2016, 7, 5171 RSC.
- H. A. Klein, H. Kuhn and P. D. Beer, Chem. Commun., 2019, 55, 9975 RSC.
- F. Zapata, A. Caballero, N. G. White, T. D. W. Claridge, P. J. Costa, V. t. Félix and P. D. Beer, J. Am. Chem. Soc., 2012, 134, 11533 CrossRef CAS PubMed.
- P. Sabater, F. Zapata, A. Caballero, N. de la Visitación, I. Alkorta, J. Elguero and P. Molina, J. Org. Chem., 2016, 81, 7448 CrossRef CAS PubMed.
- F. Zapata, S. J. Benítez-Benítez, P. Sabater, A. Caballero and P. Molina, Molecules, 2017, 22, 2273 CrossRef PubMed.
- P. Sabater, F. Zapata, A. Bastida and A. Caballero, Org. Biomol. Chem., 2020, 18, 3858 RSC.
- P. Sabater, F. Zapata, B. López, I. Fernández, A. Caballero and P. Molina, Dalton Trans., 2018, 47, 15941 RSC.
- F. Zapata, A. Caballero, P. Molina, I. Alkorta and J. Elguero, J. Org. Chem., 2014, 79, 6959 CrossRef CAS PubMed.
- L. González, F. Zapata, A. Caballero, P. Molina, C. Ramírez de Arellano, I. Alkorta and J. Elguero, Chem. - Eur. J., 2016, 22, 7533 CrossRef PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- A. Docker, X. Shang, D. Yuan, H. Kuhn, Z. Zhang, J. J. Davis, P. D. Beer and M. J. Langton, Angew. Chem., Int. Ed., 2021, 60, 19442 CrossRef CAS PubMed.
- E. J. Mitchell, A. J. Beecroft, J. Martin, S. Thompson, I. Marques, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2021, 60, 24048 CrossRef CAS PubMed.
- X. Wang, L. An, Q. Tian and K. Cui, RSC Adv., 2019, 9, 33578 RSC.
- S. L. Malone Rubright, L. L. Pearce and J. Peterson, Nitric Oxide, 2017, 71, 1 CrossRef CAS PubMed.
- V. S. Lin and C. J. Chang, Curr. Opin. Chem. Biol., 2012, 16, 595 CrossRef CAS PubMed.
- Y. Luo, Y. Zuo, G. Shi, H. Xiang and H. Gu, Anal. Bioanal. Chem., 2022, 414, 2809–2839 CrossRef CAS PubMed.
- M. D. Hartle, R. J. Hansen, B. W. Tresca, S. S. Prakel, L. N. Zakharov, M. M. Haley, M. D. Pluth and D. W. Johnson, Angew. Chem., Int. Ed., 2016, 55, 11480 CrossRef CAS PubMed.
- N. Lau, L. N. Zakharov and M. D. Pluth, Chem. Commun., 2018, 54, 2337 RSC.
- J. Vázquez and V. Sindelar, Chem. Commun., 2018, 54, 5859 RSC.
- R. Hein and P. D. Beer, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2021, DOI:10.1016/B978-0-12-820206-7.00132-3.
- T. K. Mole, W. E. Arter, I. Marques, V. Félix and P. D. Beer, J. Organomet. Chem., 2015, 792, 206 CrossRef CAS.
- B. Chowdhury, S. Sinha and P. Ghosh, Chem. - Eur. J., 2016, 22, 18051 CrossRef CAS PubMed.
- S. Mondal, A. Rashid and P. Ghosh, J. Organomet. Chem., 2021, 952, 122027 CrossRef CAS.
- K. K.-W. Lo, S. P.-Y. Li and K. Y. Zhang, New J. Chem., 2011, 35, 265 RSC.
- I. Omae, J. Organomet. Chem., 2016, 823, 50 CrossRef CAS.
- R. Kampes, R. Tepper, H. Görls, P. Bellstedt, M. Jäger and U. S. Schubert, Chem. - Eur. J., 2020, 26, 14679 CrossRef CAS PubMed.
- E. Navarro-García, M. D. Velasco, F. Zapata, A. Bauzá, A. Frontera, C. Ramírez de Arellano and A. Caballero, Dalton Trans., 2019, 48, 11813 RSC.
- J. Sun, A. M. S. Riel and O. B. Berryman, New J. Chem., 2018, 42, 10489 RSC.
- K. M. Bąk, K. Porfyrakis, J. J. Davis and P. D. Beer, Mater. Chem. Front., 2020, 4, 1052 RSC.
- M. J. Langton and P. D. Beer, Acc. Chem. Res., 2014, 47, 1935 CrossRef CAS PubMed.
- A. Docker and P. D. Beer, in Halogen Bonding in Solution, 2021, p. 83, DOI:10.1002/9783527825738.ch3.
- J. Y. Lim, I. Marques, A. L. Thompson, K. E. Christensen, V. Félix and P. D. Beer, J. Am. Chem. Soc., 2017, 139, 3122 CrossRef CAS PubMed.
- A. Caballero, F. Zapata, N. G. White, P. J. Costa, V. Félix and P. D. Beer, Angew. Chem., 2012, 124, 1912 CrossRef.
- J. M. Mercurio, A. Caballero, J. Cookson and P. D. Beer, RSC Adv., 2015, 5, 9298 RSC.
- X. Li, J. Y. Lim and P. D. Beer, Chem. - Eur. J., 2018, 24, 17788 CrossRef CAS PubMed.
- J. Y. Lim, I. Marques, V. Félix and P. D. Beer, Angew. Chem., 2018, 130, 593 CrossRef.
- B. R. Mullaney, A. L. Thompson and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11458 CrossRef CAS PubMed.
- M. J. Langton, Y. Xiong and P. D. Beer, Chem. - Eur. J., 2015, 21, 18910 CrossRef CAS PubMed.
- M. J. Langton, I. Marques, S. W. Robinson, V. Félix and P. D. Beer, Chem. - Eur. J., 2016, 22, 185 CrossRef CAS PubMed.
- K. Strakova, L. Assies, A. Goujon, F. Piazzolla, H. V. Humeniuk and S. Matile, Chem. Rev., 2019, 119, 10977 CrossRef PubMed.
- J. Y. Lim, I. Marques, V. Félix and P. D. Beer, Chem. Commun., 2018, 54, 10851 RSC.
- L. Cui, Y. Gong, X. Yu, C. Lv, X. Du, J. Zhao and Y. Che, ACS Sens, 2021, 6, 2851 CrossRef CAS PubMed.
- X. Yu, Y. Gong, H. Ji, C. Cheng, C. Lv, Y. Zhang, L. Zang, J. Zhao and Y. Che, ACS Sens, 2022 DOI:10.1021/acssensors.2c00079.
- E. Bakker and M. Telting-Diaz, Anal. Chem., 2002, 74, 2781 CrossRef CAS PubMed.
- C. Jiang, G. Wang, R. Hein, N. Liu, X. Luo and J. J. Davis, Chem. Rev., 2020, 120, 3852 CrossRef CAS PubMed.
- S. R. Miller, D. A. Gustowski, Z. H. Chen, G. W. Gokel, L. Echegoyen and A. E. Kaifer, Anal. Chem., 1988, 60, 2021 CrossRef CAS.
- P. D. Beer, P. A. Gale and G. Z. Chen, Coord. Chem. Rev., 1999, 185, 3 CrossRef.
- R. Hein, X. Li, P. D. Beer and J. J. Davis, Chem. Sci., 2021, 12, 2433 RSC.
- R. Oliveira, S. Groni, C. Fave, M. Branca, F. Mavre, D. Lorcy, M. Fourmigue and B. Schollhorn, Phys. Chem. Chem. Phys., 2016, 18, 15867 RSC.
- C. Fave and B. Schöllhorn, Curr. Opin. Electrochem., 2019, 15, 89 CrossRef CAS.
- S. Groni, T. Maby-Raud, C. Fave, M. Branca and B. Schöllhorn, Chem. Commun., 2014, 50, 14616 RSC.
- J. Y. C. Lim, M. J. Cunningham, J. J. Davis and P. D. Beer, Chem. Commun., 2015, 51, 14640 RSC.
- F. Zapata, A. Caballero and P. Molina, Eur. J. Inorg. Chem., 2017, 2017, 237 CrossRef CAS.
- J. Y. C. Lim and P. D. Beer, Eur. J. Inorg. Chem., 2017, 2017, 220 CrossRef CAS.
- J. Y. C. Lim, I. Marques, L. Ferreira, V. Félix and P. D. Beer, Chem. Commun., 2016, 52, 5527 RSC.
- Y. Willener, K. M. Joly, C. J. Moody and J. H. R. Tucker, J. Org. Chem., 2008, 73, 1225 CrossRef CAS PubMed.
- J. Y. C. Lim and P. D. Beer, Eur. J. Org. Chem., 2019, 2019, 3433 CrossRef CAS.
- B. R. Mullaney, M. J. Cunningham, J. J. Davis and P. D. Beer, Polyhedron, 2016, 116, 20 CrossRef CAS.
- G. Creste, S. Groni, C. Fave, M. Branca and B. Schöllhorn, Faraday Discuss., 2017, 203, 301 RSC.
- E. Engelage, H. Hijazi, M. Gartmann, L. M. Chamoreau, B. Schöllhorn, S. M. Huber and C. Fave, Phys. Chem. Chem. Phys., 2021, 23, 4344 RSC.
- R. Oliveira, S. Groni, A. Vacher, F. Barrière, D. Lorcy, M. Fourmigué, E. Maisonhaute, B. Schöllhorn and C. Fave, ChemistrySelect, 2018, 3, 8874 CrossRef CAS.
- N. H. Evans, H. Rahman, J. J. Davis and P. D. Beer, Anal. Bioanal. Chem., 2012, 402, 1739 CrossRef CAS PubMed.
- H. Hijazi, A. Vacher, S. Groni, D. Lorcy, E. Levillain, C. Fave and B. Schollhorn, Chem. Commun., 2019, 55, 1983 RSC.
- S. Zhang and L. Echegoyen, J. Am. Chem. Soc., 2005, 127, 2006 CrossRef CAS PubMed.
- J. Wei, Z. Guo, X. Chen, D.-D. Han, X.-K. Wang and X.-J. Huang, Anal. Chem., 2015, 87, 1991 CrossRef CAS PubMed.
- S. Zhang, A. Palkar and L. Echegoyen, Langmuir, 2006, 22, 10732 CrossRef CAS PubMed.
- P. R. Bueno and J. J. Davis, Anal. Chem., 2014, 86, 1337 CrossRef CAS PubMed.
- J. Piccoli, R. Hein, A. H. El-Sagheer, T. Brown, E. M. Cilli, P. R. Bueno and J. J. Davis, Anal. Chem., 2018, 90, 3005 CrossRef CAS PubMed.
- A. Baradoke, R. Hein, X. Li and J. J. Davis, Anal. Chem., 2020, 92, 3508 CrossRef CAS PubMed.
- J. Lehr, F. C. B. Fernandes, P. R. Bueno and J. J. Davis, Anal. Chem., 2014, 86, 2559 CrossRef CAS PubMed.
- N. Wanichacheva, E. R. Soto, C. R. Lambert and W. G. McGimpsey, Anal. Chem., 2006, 78, 7132 CrossRef CAS PubMed.
- S. Flink, F. C. Van Veggel and D. N. Reinhoudt, J. Phys. Chem. B, 1999, 103, 6515 CrossRef CAS.
- R. Hein, A. Borissov, M. D. Smith, P. D. Beer and J. J. Davis, Chem. Commun., 2019, 55, 4849 RSC.
- P. R. Bueno, R. Hein, A. Santos and J. J. Davis, Phys. Chem. Chem. Phys., 2020, 22, 3770 RSC.
- J. Bobacka, A. Ivaska and A. Lewenstam, Chem. Rev., 2008, 108, 329 CrossRef CAS PubMed.
- P. Bühlmann and L. D. Chen, Ion-Selective Electrodes With Ionophore-Doped Sensing Membranes, Wiley, 2012 Search PubMed.
- G. E. K. K. Seah, A. Y. X. Tan, Z. H. Neo, J. Y. C. Lim and S. S. Goh, Anal. Chem., 2021, 93, 15543 CrossRef CAS PubMed.
- Y. C. Wong, B. C. Ang, A. Haseeb, A. A. Baharuddin and Y. H. Wong, J. Electrochem. Soc., 2019, 167, 037503 CrossRef.
- S. J. Choi, B. Yoon, J. D. Ray, A. Netchaev, L. C. Moores and T. M. Swager, Adv. Funct. Mater., 2020, 30, 1907087 CrossRef CAS.
- V. Montes-García, R. F. de Oliveira, Y. Wang, A. Berezin, P. Fanjul-Bolado, M. B. González García, T. M. Hermans, D. Bonifazi, S. Casalini and P. Samorì, Adv. Funct. Mater., 2021, 31, 2008554 CrossRef.
- B. Yoon and S.-J. Choi, Sens. Actuators, B, 2021, 346, 130461 CrossRef CAS.
- J. G. Weis, J. B. Ravnsbæk, K. A. Mirica and T. M. Swager, ACS Sens, 2016, 1, 115 CrossRef CAS.
- A. K. A. Jaini, L. B. Hughes, M. M. Kitimet, K. J. Ulep, M. C. Leopold and C. A. Parish, ACS Sens, 2019, 4, 389 CrossRef CAS PubMed.
- Z.-X. Liu, Y. Sun, Y. Feng, H. Chen, Y.-M. He and Q.-H. Fan, Chem. Commun., 2016, 52, 2269 RSC.
- L. Chen, J. Xiang, Y. Zhao and Q. Yan, J. Am. Chem. Soc., 2018, 140, 7079 CrossRef CAS PubMed.
- L. M. Lee, M. Tsemperouli, A. I. Poblador-Bahamonde, S. Benz, N. Sakai, K. Sugihara and S. Matile, J. Am. Chem. Soc., 2019, 141, 810 CrossRef CAS PubMed.
- G. Park, D. J. Brock, J.-P. Pellois and F. P. Gabbaï, Chem, 2019, 5, 2215 CAS.
- G. Park and F. P. Gabbaï, Chem. Sci., 2020, 11, 10107 RSC.
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118 CrossRef CAS.
- S. R. Marshall, A. Singh, J. N. Wagner and N. Busschaert, Chem. Commun., 2020, 56, 14455 RSC.
- A. Vargas Jentzsch, D. Emery, J. Mareda, P. Metrangolo, G. Resnati and S. Matile, Angew. Chem., Int. Ed., 2011, 50, 11675 CrossRef CAS PubMed.
- F. Benito-Lopez, S. Scarmagnani, Z. Walsh, B. Paull, M. Macka and D. Diamond, Sens. Actuators, B, 2009, 140, 295 CrossRef CAS.
- R. Furlan de Oliveira, V. Montes-García, A. Ciesielski and P. Samorì, Mater. Horiz., 2021, 8, 2685 RSC.
- J. Zheng, A. Suwardi, C. J. E. Wong, X. J. Loh and Z. Li, Nanoscale Adv., 2021, 3, 6342 RSC.
- S. Byrne and K. M. Mullen, RSC Adv., 2016, 6, 33880 RSC.
- M. Schmittel and H. Lin, J. Mater. Chem., 2008, 18, 333 RSC.
- R. T. Bronson, D. J. Michaelis, R. D. Lamb, G. A. Husseini, P. B. Farnsworth, M. R. Linford, R. M. Izatt, J. S. Bradshaw and P. B. Savage, Org. Lett., 2005, 7, 1105 CrossRef CAS PubMed.
Footnotes |
| † This trend is not strictly true for other σ–hole interactions such as PnB or TrB, for further information see ref. 14. |
| ‡ The term “sensitivity” is ill-defined in the literature and can refer to both the LOD or the slope of the dose–response curve. Herein, we use the latter definition. |
| § Note that the selectivity of ISEs is in principle governed by the supramolecular design of the ionophore hosts. However, in comparison to other sensing approaches the selectivity is further affected by the inherent partitioning of ions into the ISE membrane. Hence, ions of lower hydration enthalpy often interfere with membrane based ISEs. |
| ¶ While very similar, chemiresistors are technically not electrochemical devices, as no chemical change is occurring as a result of current/voltage perturbations. However, this differentiation is generally not relevant and chemiresistors usually possess the advantages that are associated with typical electrochemical techniques. |
| This journal is © The Royal Society of Chemistry 2022 |