Open Access Article
Open Access ArticleNarrowband blue emission with insensitivity to the doping concentration from an oxygen-bridged triarylboron-based TADF emitter: nondoped OLEDs with a high external quantum efficiency up to 21.4%†
Jianmei
Han‡
ab,
Zhongyan
Huang‡
 a,
Jingsheng
Miao
a,
Yuntao
Qiu
a,
Ziyang
Xie
a and
Chuluo
Yang
a,
Jingsheng
Miao
a,
Yuntao
Qiu
a,
Ziyang
Xie
a and
Chuluo
Yang
 *a
*a
aShenzhen Key Laboratory of Polymer Science and Technology, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, P. R. China. E-mail: clyang@szu.edu.cn
bCollege of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen 518060, P. R. China
First published on 21st February 2022
Abstract
Blue thermally activated delayed fluorescence (TADF) emitters that can simultaneously achieve narrowband emission and high efficiency in nondoped organic light-emitting diodes (OLEDs) remain a big challenge. Herein, we successfully design and synthesize two blue TADF emitters by directly incorporating carbazole fragments into an oxygen-bridged triarylboron acceptor. Depending on the linking mode, the two emitters show significantly different photophysical properties. Benefitting from the bulky steric hindrance between the acceptor and terminal pendants, the blue emitter TDBA-Cz exhibited a high photoluminescence quantum yield (PLQY) of 88% in neat films and narrowband emission. The corresponding non-doped blue device exhibited a maximum external quantum efficiency (EQE) of 21.4%, with a full width at half maximum (FWHM) of only 45 nm. This compound is the first blue TADF emitter that can concurrently achieve narrow bandwidth and high electroluminescence (EL) efficiency in nondoped blue TADF-OLEDs.
Introduction
Organic light-emitting diodes (OLEDs) are currently one of the competitive candidates for future full-color displays due to their recommendable advantages such as high efficiency, light weight, wide viewing angle and superior mechanical flexibility.1 Since the pioneering work of Adachi and co-workers reported in 2011, thermally activated delayed fluorescence (TADF) materials without precious heavy metals have been greeted with an avalanche of publicity as the third-generation OLED emitters.2 The use of metal-free TADF emitters in OLEDs could theoretically achieve 100% internal quantum efficiency by converting electro-generated triplet excitons into singlet excitons via an efficient reverse intersystem crossing (RISC) process.3 Until now, a considerable number of highly efficient TADF-based OLEDs have been successfully reported.4 Generally, the commonly adopted design strategy for blue TADF molecules is to construct a donor–acceptor-type (D–A-type) molecular skeleton with strong intramolecular charge-transfer (ICT) character, which inevitably causes broad electroluminescence (EL) spectra with a full width at half maximum (FWHM) of over 80 nm, thereby resulting in inferior color purity and gigantic energy consumption in OLED displays with the use of color filters.5 To meet the requirements of perfect color purity for practical applications, Lee and coworkers employed the steric hindrance effect to obtain a deep-blue-emitting TADF emitter with a narrow FWHM of 48 nm but with a fairly low external quantum efficiency (EQE) of only 14.0% in 2016.6 Recently, Hatakeyama and coworkers demonstrated that multi-resonance type TADF molecules composed of a rigid skeleton with an orderly arrangement of boron and nitrogen atoms enabled narrow emission and can be employed to construct highly efficient TADF-OLEDs with high color purity. However, this strategy is only suitable for specially designated molecular frameworks and inapplicable to conventional D–A-type TADF emitters.7 More importantly, the majority of TADF emitters suffer from serious concentration-caused emission quenching.4c,8 Consequently, most of the highly efficient TADF OLEDs reported so far can only function well in doped films at low doping concentrations, thereby resulting in complicated manufacturing processes, expensive fabrication, and poor repeatability. To address this drawback, TADF emitters must be dispersed in a suitable host matrix to alleviate the detrimental quenching. However, the selection of desirable host materials for blue emitters is imperative but is technically difficult because of the limited availability of suitable building blocks. To circumvent this issue, nondoped devices could be adopted to construct highly-efficient TADF OLEDs.Compared to doped devices, nondoped devices could not only expand the exciton recombination zone but also simplify device fabrication and improve device performance reproducibility due to the unnecessity of precise control of the dopant concentration. Despite these merits, the realization of highly efficient non-doped blue OLEDs with narrowband emission is ever more challenging and needs to be exploited long-term. To the best of our knowledge, up to now, the most efficient non-doped blue-emitting device was reported to have an EQE up to 22.8% but with a slightly broad FWHM of 63 nm.4b Therefore, there is a desperate need for the development of new blue TADF emitters which could simultaneously achieve high color purity, high efficiency and low driving voltage for practical applications.
Based on the above considerations, we reported a simple but effective design strategy for constructing highly-efficient TADF emitters with narrowband emission. By directly incorporating two carbazole donors into a rigid oxygen-bridged triarylboron acceptor, a novel TADF emitter was successfully synthesized. Such a structural design strategy not only makes the compound capable of realizing highly-efficient doped blue OLEDs with an EQE up to 31.1% at a doping concentration of 10 wt% but also endows the most efficient narrowband and non-doped blue OLED (EQE up to 21.4%) with an extremely small FWHM of only 45 nm so far.
Results and discussion
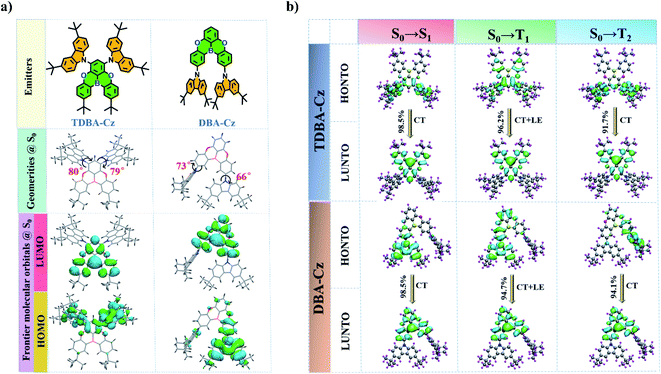
The synthetic routes of the designed compound TDBA-Cz are depicted in Scheme S1 in the ESI,† which involved the aromatic nucleophilic substitution and Ullmann coupling reaction, followed by the tert-butyllithium mediated borylation reactions. For comparison, a reference molecule DBA-Cz was rationally designed and synthesized using precursors DBA-2Br and 3,6-di-tert-butylcarbazole by the classical coupling method according to the reported literature.9 Different from TDBA-Cz, two carbazole units were introduced into the meta position of the boron atom on the other two phenyl rings of the acceptor moiety to elaborate the effect of the substitution site of carbazole units on photophysical characteristics. The chemical structures of all the intermediates and final products were characterized by NMR and high-resolution mass spectroscopy (Fig. S1–S8, ESI†). In addition, as proved by thermogravimetric analysis (TGA, Fig. S9 and S10, ESI†), both TDBA-Cz and DBA-Cz showed excellent thermal stability with a decomposition temperature (Td) of 421 and 390 °C at 5% weight loss, respectively.The design conception is supported by the density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations, as shown in Fig. 1. The detailed computation method is summarized in the ESI.† In the S0 state, benefitting from the bulky steric hindrance between the donor and acceptor moieties, the two compounds exhibited a distorted molecular configuration with large dihedral angles of over 60°, thereby leading to better spatial separation of the HOMO and LUMO distribution. The LUMO was fully located on the oxygen-bridged triarylboron acceptor unit, while the HOMO was predominantly distributed on the carbazole donor and adjacent benzene ring, and even extended to the acceptor moiety. Such an orbital overlap of the HOMO and LUMO resulted in a slightly enlarged ΔEST value of 0.23 eV for the two compounds (Fig. 1a and S11†). To further comprehensively understand the excited state properties of TDBA-Cz and DBA-Cz, the natural transition orbitals (NTOs) for the transition to singlet and triplet excited states were examined (Fig. 1b). For S0 → S1 transition, the HONTO and LUNTO of both compounds are similar to their respective HOMO and LUMO of S0, indicating that singlet emission can be interpreted as charge transfer (CT) emission. While the HONTO of the transition to T1 is mainly distributed on the carbazole donor and adjacent acceptor moiety, the LUNTO is still fully located on the oxygen-bridged triarylboron unit, suggesting the existence of a mixed transition featuring CT character and locally excited (LE) state character in the triplet state. Such LE characteristics in the triplet state are of vital importance in boosting the RISC process by increasing the spin–orbit coupling (SOC) between the S1 state with CT characteristics and triplet states. In addition, the transition to T2 exhibited dominant CT characteristics for both TDBA-Cz and DBA-Cz, which gave rise to a smaller ΔEST than that of the T1 state, so as to facilitate the upconversion of the triplet excitons to the singlet excited state through the RISC process.
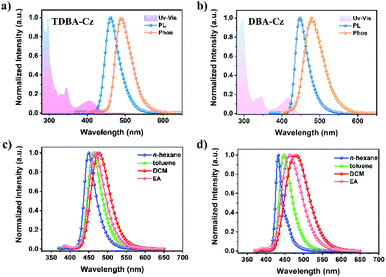
The electrochemical properties of both compounds were measured by cyclic voltammetry (CV). As shown in Fig. S12 and S13 in the ESI,†TDBA-Cz and DBA-Cz exhibited onset oxidation potential values (Eox) of 1.00 and 1.03 eV, respectively. The calculated HOMO energy level (−5.39 eV) of TDBA-Cz is about 30 meV lower than that of DBA-Cz, which is consistent with the aforementioned DFT calculation results. The preliminary photophysical properties of these two compounds were characterized in dilute toluene solution as illustrated in Fig. 2 and Table 1. Both molecules displayed intense and sharp absorption bands ranging from 400 to 450 nm, originating from the intramolecular charge transfer (ICT) transitions. The ICT character could be further evidenced by the solvatochromism experiment upon altering the solvent from low polar n-hexene to high polar dichloromethane (DCM). Compared with DBA-Cz, TDBA-Cz displayed less solvatochromic effect with relatively narrow FWHM as the polarity of the solvent increased, indicating that TDBA-Cz possess a bigger contribution from the LE state in higher polar solvent whereas the reverse is true for DBA-Cz (Tables S1 and S2, ESI†). In toluene solution, TDBA-Cz showed blue emission with a fluorescence spectrum peaking at 461 nm, while DBA-Cz showed deep-blue emission with an emission maximum of 447 nm. Importantly, both compounds exhibited narrowband emission with a small FWHM of about 40 nm, which is significantly smaller than those of typical D–A type TADF emitters and even comparable to those of multi-resonance type emitters.7,10 Furthermore, ΔEST values were calculated to be 0.14 and 0.03 eV for TDBA-Cz and DBA-Cz from their respective fluorescence and phosphorescence spectra. Notably, the photoluminescence (PL) spectrum of DBA-Cz in neat films exhibited a significant bathochromic-shift and broadened FWHM, while the emission of TDBA-Cz closely resembled that of toluene solution. The narrow FWHM for TDBA-Cz in different states was mainly attributed to the trivial conformational changes of the molecular structure between the ground state and singlet excited states. As shown in Fig. 3a and b, the molecular geometrical structures of S0 and S1 states for these two molecules were simulated by root mean square deviation (RMSD) to judge the configurational changes of the excited state relative to the ground state. Generally, the structural variations between S0 and S1 are closely related to the FWHMs of the emission spectra. As for TDBA-Cz, the major discrepancy between S0 and S1 states is the slightly twisted vibrations of the noncoplanar tertiary butyl moieties, with a small average alteration in configuration of 0.329 Å. However DBA-Cz exhibited significant changes not only in the rigid oxygen-bridged triarylboron acceptor but also in carbazole donors between S0 and S1 states of molecular conformation, thereby resulting in an immense average RMSD value of up to 1.35 Å. The above results indicated that the introduction of two carbazole units to the same phenyl ring of the boron-based core could effectively suppress the molecular configuration relaxation of the radiative transition process, thus obtaining narrowband emissions benefiting from small structural changes.
| Compound | λ abs [nm] | λ em [nm] | FWHMa [nm] | E S1 [eV] | E T1 [eV] | ΔESTa [eV] | τ prompt [ns] | τ delay [μs] | k r [107 s−1] | k ISC [108 s−1] | k RISC [106 s−1] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Measured in the toluene solution with a concentration of 10−5 mol L−1. b Measured in neat films of TDBA-Cz and DBA-Cz. | |||||||||||
| TDBA-Cz | 406 | 461 | 43 | 2.89 | 2.75 | 0.14 | 7.6 | 6.1 | 1.38 | 1.17 | 1.40 |
| DBA-Cz | 418 | 447 | 38 | 3.00 | 2.97 | 0.03 | 23.7 | 8.2 | 0.59 | 0.31 | 0.46 |
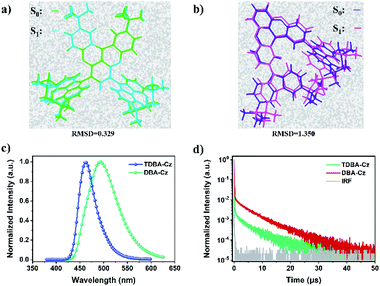 | ||
| Fig. 3 Calculated RMSD constant between S0 and S1 states for (a) TDBA-Cz and (b) DBA-Cz. PL spectra of neat films (c) and transient photoluminescence decay curves (d) of TDBA-Cz and DBA-Cz. | ||
To factually assess the performance of TDBA-Cz and DBA-Cz as TADF emitters, photophysical properties of doped thin films were recorded using bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) as the host material. As depicted in Fig. S14 and S15,† both doped films (dopant concentration of 10 wt%) gave blue emissions peaking at 456 nm and 450 nm with corresponding FWHMs of 46 nm and 47 nm for TDBA-Cz and DBA-Cz, respectively. In addition, on increasing the doping concentration of TDBA-Cz in the DPEPO host, a slight change of the spectral redshift with FWHM narrowing was observed, with an emission peak wavelength ranging from 456 (FWHM: 46 nm) to 462 nm (FWHM: 41 nm) when going from 10 to 100 wt% (Table S3†). Notably, the films possess high PLQYs, which gradually reduce from 100% at a 10 wt% concentration to 88% in the neat film. The high PLQYs confirmed the good suppression of aggregation-caused quenching achieved by rational molecular design, further elucidating potentiality for the fabrication of non-doped OLEDs. In contrast, DBA-Cz displayed a substantial spectral redshift of the emission (450–494 nm) and broadened FWHM (47–82 nm) in DPEPO blends as the doping concentration increased (Fig. S15†), accompanied by a significant PLQY drop from 90% in the 10 wt% DPEPO blends to 52% in the neat film (Fig. S16 and Table S4, ESI†). To further elucidate the TADF nature of these compounds, temperature-dependent transient PL decay curves in thin films were measured. The detailed rate constants including the radiative decay rate constant (kr), intersystem crossing rate constant (kISC) and RISC rate constant (kRISC) were also calculated according to the references, and summarized in Table 1. As shown in Fig. 3d, both TDBA-Cz and DBA-Cz in neat films exhibited typical TADF features with nanosecond-order prompt decays and microsecond-order delayed decays. Moreover, the fluorescence decay was sensitive to temperature, and the intensity of the delayed fluorescence component increased with the temperature increase from 77 to 300 K (Fig. S17, ESI†), thus proving the TADF characteristics of both emitters. Remarkably, the delayed fluorescence lifetime (τd) of TDBA-Cz was 1.3 times shorter than that of DBA-Cz (8.2 μs), which mostly benefits from the fast ISC and RISC process of TDBA-Cz in films. In addition, combined with large rate constants of fluorescence (kF: 1.37 × 107 s−1), concentration quenching of triplet excitons in electroluminescent devices could be efficiently alleviated thereby suppressing efficiency roll-off with increasing luminance. However, DBA-Cz showed relatively small kISC, kF and kRISC values, which were one order of magnitude less than those of TDBA-Cz. According to Fermi's golden rule, kRISC is proportional to the SOC constant between the singlet and triplet states and inversely proportional to the energy difference ΔEST.3c,4b,11 The computed SOC value 〈S1|HSOC|T1〉 (0.336 cm−1) of DBA-Cz is much greater than 〈S1|HSOC|T2〉 (0.018 cm−1), indicating that the RISC process was dominated by the transition from the T1 state to the S1 state. In comparison, TDBA-Cz with a larger ΔEST value and shorter decay lifetime was found to afford a slightly smaller 〈S1|HSOC|T1〉 of 0.218 cm−1 than DBA-Cz, unambiguously indicating that other electronic transition states are involved in the triplet-singlet spin conversion process to accelerate RISC. The further analysis of the NTO proclaimed that the T2 triplet state below the S1 energy level was found to afford a fairly large SOC with its S1 state (〈S1|HSOC|T2〉 of 0.917 cm−1) compared to its T1 counterpart. Therefore, TDBA-Cz is expected to undergo a more efficient RISC process through the interstate coupling with the T2 state.
To better understand the configuration of TDBA-Cz, its single crystal was cultivated by slow evaporation of ethyl acetate solvent at room temperature. As indicated in Fig. S18 in the ESI,† the crystal was monoclinic with space group P21/c. Due to the steric hindrance between the two bulky carbazole groups, the acceptor moiety was slightly distorted out of plane, as shown by its side view in Fig. S18,† with a dihedral angle of 14.39° between the two wing phenyl rings (B/B′). In addition, the dihedral angles between the two carbazole units and the adjacent phenyl ring are 88.76° (A/D) and 58.71° (A/D′), respectively. Such highly twisted configuration implied that the concentration-caused emission quenching could be effectively suppressed. Notably, the twisted conformation prevents the molecules from forming detrimental π⋯π intermolecular interactions. Only weak intermolecular interactions, including CH⋯π (2.768 Å, 2.813 Å, and 2.852 Å) and CH3⋯CH (2.275 Å and 2.833 Å), were observed, which makes TDBA-Cz a suitable emitter for the non-doped OLEDs.
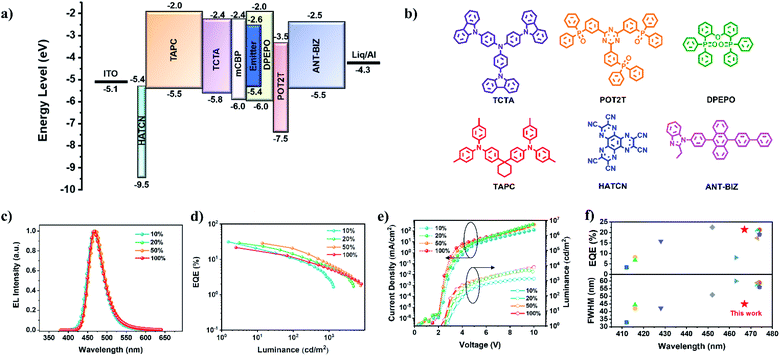
Considering the excellent photophysical properties of TDBA-Cz, multilayer OLEDs with an optimized device configuration of [ITO/HATCN (5 nm)/TAPC (30 nm)/TCTA (15 nm)/mCBP (10 nm)/DPEPO: x wt% TDBA-Cz (15 nm)/POT2T (10 nm)/ANT-BIZ (30 nm)/Liq (2 nm)/Al (100 nm)] were fabricated (x = 10, 20, 50), where HATCN (1,4,5,8,9,11-hexaazatriphenylenehexacarbonitrile) served as the hole-injection layer, TAPC (1,1-bis[(di-4-tolylamino)phenyl]cyclohexane) and ANT-BIZ (1-(4-(10-([1,1′-biphenyl]-4-yl)anthracen-9-yl)phenyl)-2-ethyl-1H-benzo[d]-imidazole) were employed as the hole- and electron-transport layers, respectively, while TCTA (tris(4-carbazolyl-9-ylphenyl)amine), mCBP 3,3′-di(9H-carbazol-9-yl)-1,1′-biphenyl and POT2T ((1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl)(tris(diphenylphosphine oxide)) acted as exciton-blocking layers (EBL). The corresponding energy level diagram and chemical structures of the adopted materials are shown in Fig. 4. The detailed device parameters are summarized in Table 2.
| Emitter | Dopant ratio | V on [V] | λ EL [nm] | FWHMc [nm] | EQEd [%] | CEe [cd A−1] | CIEf (x, y) |
|---|---|---|---|---|---|---|---|
| a Turn-on voltage when brightness is 1 cd m−2. b Maximum EL wavelength. c FWHM of EL spectra. d Maximum EQE. e Maximum CE. f Recorded at a luminance of 1000 cd m−2. | |||||||
| TDBA-Cz | 10% | 2.8 | 466 | 47 | 31.1 | 41.0 | 0.134, 0.129 |
| 20% | 2.7 | 468 | 46 | 28.7 | 39.4 | 0.134, 0.151 | |
| 50% | 2.6 | 470 | 45 | 28.4 | 38.3 | 0.131, 0.165 | |
| 100% | 2.4 | 467 | 45 | 21.4 | 31.5 | 0.138, 0.155 | |
All the devices displayed a low turn-on voltage, which gradually decreased from 2.8 V to 2.6 V with the increase of the doping concentration from 10% to 50 wt%, indicating that excitons generated in the emitting layer are mainly from direct charge trapping on the guest molecules.12 All the devices exhibited blue EL spectra and the increasing doping concentration only resulted in a slight red-shift of the emission maxima from 466 to 470 nm and an unobtrusive FWHM narrowing from 48 nm to 45 nm. The device with a 10 wt% doping concentration achieved the maximum EQE and current efficiency (CE) of up to 31.1% and 41.0 cd A−1 with the CIE coordinates of (0.134, 0.129), which are comparable with the reported efficiencies for the high-performance blue OLEDs.3h,8c,13 When the doping concentration exceeded 10 wt%, the maximum EQE reduced marginally, but still maintained a maximum EQE of up to 28.4% at a doping concentration of 50%, which indicated that the highly twisted structure of TDBA-Cz can efficiently alleviate the concentration quenching at high doping concentrations. However for DBA-Cz, EL spectra showed gradual red-shifts and broadened FWHMs with the increase of doping concentration from 10 to 50 wt%, accompanied by great variations of CIE coordinates from (0.136, 0.147) to (0.147, 0.259). In addition, high EQEs of 30.3% and 29.3% were achieved in devices with doping ratios of 10 and 20 wt%, respectively, which were comparable to those of TDBA-Cz, while the value dramatically dropped to 16.2% when the doping concentration increased to 50 wt% (Fig. S19 and Table S5, ESI†). In addition, compared with DBA-Cz, TDBA-Cz-based devices revealed excellent chromatic stability with minor variations of CIE coordinates within 0.03 with the increase of TDBA-Cz doping concentration (Fig. S20, ESI†). These results demonstrate the superiority of TDBA-Cz as an emitter in blue devices.
Inspired by the excellent device performances of the OLEDs at high doping concentrations, non-doped OLEDs were further fabricated with the aforementioned device configuration. As shown in Fig. 4, the turn-on voltage of the device was further dropped to 2.4 V. A slight red-shift (5 nm) of the EL spectrum could be observed compared with its PL spectrum of the neat film, accompanied by the minor FWHM broadening of only 4 nm. Remarkably, the device displayed a maximum EQE of 21.4% and CE of 31.5 cd A−1, which are among the best performance values reported in blue nondoped OLEDs with the FWHM below 50 nm.4b,8h,14 In contrast, the DBA-Cz-based nondoped device exhibited a relatively low efficiency with a maximum EQE of only 11.3%, which was almost half of the value for TDBA-Cz (Fig. S19 and Table S5, ESI†). Moreover, in comparison with its doped device, the nondoped device of DBA-Cz has a slightly red-shifted emission with a maximum at 478 nm and a broadened FWHM of 63 nm, thereby resulting in poor color purity with the CIE coordinates of (0.170, 0.312). The much better EQE and CIE coordinates of TDBA-Cz further indicate the validity of the molecular design strategy for the nondoped device. Furthermore, the operational lifetime (LT50) of these devices was evaluated at an initial luminance of 100 nit (Fig. S21, ESI†). TDBA-Cz-based devices exhibited lifetimes of 82.7 and 226.7 min for the doped devices with 10 wt% and 50 wt% doping concentrations, respectively, and 200.7 min for the nondoped device, all of which were longer than those of the corresponding DBA-Cz based devices. To date, the reported nondoped blue OLEDs with a narrow FWHM of less than 50 nm possessed an EQEmax less than 15.8% no matter what kinds of emitters are adopted (Table S6†). The EQEmax of this device is about 1.4 times as high as that of the best reported value and represents the highest EQE achieved so far (Fig. 4f). Additionally, the FWHMs of whether doped or nondoped devices are even comparable to those of the multi-resonance TADF-OLEDs. Such superior device performances can be mainly attributed to the efficient RISC process and very large SOC constants, combined with the fast kr, kISC and kRISC values.
Conclusions
In conclusion, a rational molecular design strategy for constructing highly-efficient and narrowband emission TADF emitters was demonstrated by precise peripheral decoration on the oxygen-bridged triarylboron acceptor unit. The bulky steric hindrance between the acceptor and terminal pendant endowed the compound with a highly twisted configuration, and the rigid skeleton structure resulted in an intrinsically narrow emission spectrum and extremely high PLQY whether in doped films or neat films, which is conductive to the effective suppression of concentration quenching. The nondoped blue device based on TDBA-Cz was achieved with a maximum EQE of 21.4%, as well as a narrow FWHM. The fine molecular modulation and the revealed structure–property relationship provide guidance for developing narrowband and high-efficiency donor–acceptor type TADF emitters.Data availability
The datasets supporting this article have been uploaded as part of this manuscript and its corresponding ESI.† Crystallographic data can be obtained from the CCDC.Author contributions
J. Han, J. Miao and C. Yang wrote the manuscript. J. Han and Z. Huang synthesized the compounds. J. Han measured the photophysical, thermal and electrochemical properties of the compounds. J. Miao fabricated and characterized the devices. Y. Qiu performed the temperature-dependent transient PL decay measurements. Z. Xie performed a part of the quantum chemical calculations. C. Yang supervised the project. All authors contributed to the discussion of the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 52130308 and 21805195), Foundation for Basic and Applied Research of Guangdong Province (No. 2020A1515110854), the Shenzhen Science and Technology Program (KQTD20170330110107046), and the Science and Technology Innovation Commission of Shenzhen (JCYJ20180507182244027). We thank the Instrumental Analysis Center of Shenzhen University for the analytical support.Notes and references
- (a) Y. L. Chang, Y. Song, Z. Wang, M. G. Helander, J. Qiu, L. Chai, Z. Liu, G. D. Scholes and Z. Lu, Adv. Funct. Mater., 2013, 23, 705–712 CrossRef CAS; (b) S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lüssem and K. Leo, Nature, 2009, 459, 234–238 CrossRef CAS PubMed; (c) B. Zhang, G. Tan, C. S. Lam, B. Yao, C. L. Ho, L. Liu, Z. Xie, W. Y. Wong, J. Ding and L. Wang, Adv. Mater., 2012, 24, 1873–1877 CrossRef CAS PubMed; (d) Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS; (e) X. Li, G. Xie, M. Liu, D. Chen, X. Cai, J. Peng, Y. Cao and S. Su, Adv. Mater., 2016, 28, 4614–4619 CrossRef CAS PubMed; (f) M.-C. Tang, C.-H. Lee, M. Ng, Y.-C. Wong, M.-Y. Chan and V. W.-W. Yam, Angew. Chem., Int. Ed., 2018, 57, 5463–5466 CrossRef CAS PubMed; (g) J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1332–1334 CrossRef CAS PubMed; (h) B. Li, Z. Li, T. Hu, Y. Zhang, Y. Wang, Y. Yi, F. Guo and L. Zhao, J. Mater. Chem. C, 2018, 6, 2351–2359 RSC; (i) R. Ishimatsu, S. Matsunami, T. Kasahara, J. Mizuno, T. Edura, C. Adachi, K. Nakano and T. Imato, Angew. Chem., Int. Ed., 2014, 53, 6993–6996 CrossRef CAS PubMed; (j) X. Wang, S. Yang, Q. Tian, C. Zhong, Y. Qu, Y. Yu, Z. Jiang and L. Liao, Angew. Chem., Int. Ed., 2021, 60, 5213–5219 CrossRef CAS PubMed; (k) Q. Xue and G. Xie, Adv. Opt. Mater., 2021, 9, 2002204 CrossRef CAS.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Appl. Phys. Lett., 2011, 98, 083302 CrossRef.
- (a) Y. Zhang, D. Zhang, J. Wei, X. Hong, Y. Lu, D. Hu, G. Li, Z. Liu, Y. Chen and L. Duan, Angew. Chem., Int. Ed., 2020, 59, 17499–17503 CrossRef CAS PubMed; (b) Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Angew. Chem., Int. Ed., 2019, 58, 16912–16917 CrossRef CAS PubMed; (c) W. Li, B. Li, X. Cai, L. Gan, Z. Xu, W. Li, K. Liu, D. Chen and S. Su, Angew. Chem., Int. Ed., 2019, 58, 11301–11305 CrossRef CAS PubMed; (d) C. Wu, W. Liu, K. Li, G. Cheng, J. Xiong, T. Teng, C.-M. Che and C. Yang, Angew. Chem., Int. Ed., 2021, 60, 3994–3998 CrossRef CAS PubMed; (e) Y. Qi, W. Ning, Y. Zou, X. Cao, S. Gong and C. Yang, Adv. Funct. Mater., 2021, 31, 2102017 CrossRef CAS; (f) F. Ni, C.-W. Huang, Y. Tang, Z. Chen, Y. Wu, S. Xia, X. Cao, J.-H. Hsu, W.-K. Lee, K. Zheng, Z. Huang, C.-C. Wu and C. Yang, Mater. Horiz., 2021, 8, 547–555 RSC; (g) N. Li, D. Chai, Z. Chen, C. Zhou, F. Ni, Z. Huang, X. Cao, G. Xie, K. Li and C. Yang, Chem. Eng. J., 2020, 396, 125276 CrossRef CAS; (h) H. Lim, H. J. Cheon, S.-J. Woo, S.-K. Kwon, Y.-H. Kim and J.-J. Kim, Adv. Mater., 2020, 32, 2004083 CrossRef CAS PubMed; (i) H. Liu, Z. Liu, G. Li, H. Huang, C. Zhou, Z. Wang and C. Yang, Angew. Chem., Int. Ed., 2021, 60, 12376–12380 CrossRef CAS PubMed.
- (a) J. U. Kim, I. S. Park, C.-Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS PubMed; (b) I. S. Park, K. Matsuo, N. Aizawa and T. Yasuda, Adv. Funct. Mater., 2018, 28, 1802031 CrossRef; (c) M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed; (d) D. Karthik, Y. H. Jung, H. Lee, S. Hwang, B.-M. Seo, J.-Y. Kim, C. W. Han and J. H. Kwon, Adv. Mater., 2021, 33, 2007724 CrossRef CAS PubMed; (e) H. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta and T. Hatakeyama, Angew. Chem., Int. Ed., 2015, 54, 13581–13585 CrossRef CAS PubMed; (f) D. Song, Y. Yu, L. Yue, D. Zhong, Y. Zhang, X. Yang, Y. Sun, G. Zhou and Z. Wu, J. Mater. Chem. C, 2019, 7, 11953–11963 RSC; (g) X. Zheng, R. Huang, C. Zhong, G. Xie, W. Ning, M. Huang, F. Ni, F. B. Dias and C. Yang, Adv. Sci., 2020, 7, 1902087 CrossRef CAS PubMed.
- (a) M. Godumala, S. Choi, M. J. Cho and D. H. Choi, J. Mater. Chem. C, 2019, 7, 2172–2198 RSC; (b) K. Wu, T. Zhang, Z. Wang, L. Wang, L. Zhan, S. Gong, C. Zhong, Z.-H. Lu, S. Zhang and C. Yang, J. Am. Chem. Soc., 2018, 140, 8877–8886 CrossRef CAS PubMed; (c) S. J. Yoon, J. H. Kim, W. J. Chung and J. Y. Lee, Chem.–Eur. J., 2021, 27, 3065–3073 CrossRef CAS PubMed; (d) Y. Gao, W. Guan, L. Yan and Z. Su, J. Mater. Chem. C, 2020, 8, 219–227 RSC; (e) B. Du, X. Wang, F. Chen, Q. Yang, S. Shao, L. Wang, X. Jing and F. Wang, Chem. Commun., 2021, 57, 7144–7147 RSC.
- Y. J. Cho, S. K. Jeon, S.-S. Lee, E. Yu and J. Y. Lee, Chem. Mater., 2016, 28, 5400–5405 CrossRef CAS.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- (a) Y. Im, S. H. Han and J. Y. Lee, J. Mater. Chem. C, 2018, 6, 5012–5017 RSC; (b) Y. J. Cho, S. K. Jeon and J. Y. Lee, Adv. Opt. Mater., 2016, 4, 688–693 CrossRef CAS; (c) Y. H. Lee, S. Park, J. Oh, S.-J. Woo, A. Kumar, J.-J. Kim, J. Jung, S. Yoo and M. H. Lee, Adv. Opt. Mater., 2018, 6, 1800385 CrossRef; (d) Y. J. Cho, B. D. Chin, S. K. Jeon and J. Y. Lee, Adv. Funct. Mater., 2015, 25, 6786–6792 CrossRef CAS; (e) D. R. Lee, M. Kim, S. K. Jeon, S.-H. Hwang, C. W. Lee and J. Y. Lee, Adv. Mater., 2015, 27, 5861–5867 CrossRef CAS PubMed; (f) M. Kim, S. K. Jeon, S.-H. Hwang and J. Y. Lee, Adv. Mater., 2016, 28, 603 CrossRef CAS PubMed; (g) H. L. Lee, K. H. Lee, J. Y. Lee and W. P. Hong, J. Mater. Chem. C, 2019, 7, 6465–6474 RSC; (h) X. Chen, J. Jia, R. Yu, J. Liao, M. Yang and C. Lu, Angew. Chem., Int. Ed., 2017, 56, 15006–15009 CrossRef CAS PubMed; (i) Z. Zhang, E. Crovini, P. L. dos Santos, B. A. Naqvi, D. B. Cordes, A. M. Z. Slawin, P. Sahay, W. Brutting, I. D. W. Samuel, S. Brase and E. Zysman-Colman, Adv. Opt. Mater., 2020, 8, 2001354 CrossRef CAS; (j) X. Liang, Z. Yan, H. Han, Z. Wu, Y. Zheng, H. Meng, J. Zuo and W. Huang, Angew. Chem., Int. Ed., 2018, 57, 11316–11320 CrossRef CAS PubMed.
- G. Meng, X. Chen, X. Wang, N. Wang, T. Peng and S. Wang, Adv. Opt. Mater., 2019, 7, 1900130 CrossRef.
- (a) F. Liu, H. Liu, X. Tang, S. Ren, X. He, J. Li, C. Du, Z. Feng and P. Lu, Nano Energy, 2020, 68, 104325 CrossRef CAS; (b) D. H. Ahn, H. Lee, S. W. Kim, D. Karthik, J. Lee, H. Jeong, J. Y. Lee and J. H. Kwon, ACS Appl. Mater. Interfaces, 2019, 11, 14909–14916 CrossRef CAS PubMed; (c) W. Song, I. Lee and J. Y. Lee, Adv. Mater., 2015, 27, 4358–4363 CrossRef CAS PubMed.
- Y. K. Chen, J. Jayakumar, C. M. Hsieh, T. L. Wu, C. C. Liao, J. Pandidurai, C. L. Ko, W. Y. Hung and C. H. Cheng, Adv. Mater., 2021, 33, 2008032 CrossRef CAS PubMed.
- H. Kaji, H. Suzuki, T. Fukushima, K. Shizu, K. Suzuki, S. Kubo, T. Komino, H. Oiwa, F. Suzuki, A. Wakamiya, Y. Murata and C. Adachi, Nat. Commun., 2015, 6, 8476 CrossRef CAS PubMed.
- (a) K. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi and H. Kaji, Angew. Chem., Int. Ed., 2015, 54, 15231–15235 CrossRef CAS PubMed; (b) P. Ganesan, D.-G. Chen, W.-C. Chen, P. Gnanasekaran, J.-A. Lin, C.-Y. Huang, M.-C. Chen, C.-S. Lee, P.-T. Chou and Y. Chi, J. Mater. Chem. C, 2020, 8, 4780–4788 RSC; (c) Y. H. Lee, S. Park, J. Oh, J. W. Shin, J. Jung, S. Yoo and M. H. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 24035–24042 CrossRef CAS PubMed; (d) I. S. Park, M. Numata, C. Adachi and T. Yasuda, Bull. Chem. Soc. Jpn., 2016, 89, 375–377 CrossRef CAS.
- (a) I. S. Park, K. Matsuo, N. Aizawa, T. Yasuda, S. H. Park, C. W. Koh, C. W. Kim, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Adv. Funct. Mater., 2018, 28, 1802031 CrossRef; (b) J. Hwang, H. Kang, J.-E. Jeong, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Chem. Eng. J., 2021, 416, 129185 CrossRef CAS; (c) S. Zou, F. Xie, M. Xie, Y. Li, T. Cheng, X. Zhang, C.-S. Lee and J. Tang, Adv. Sci., 2020, 7, 1902508 CrossRef CAS PubMed; (d) K. Matsuo and T. Yasuda, Chem. Sci., 2019, 10, 10687–10697 RSC; (e) C. Li, Z. Ren, X. Sun, H. Li and S. Yan, Macromolecules, 2019, 52, 2296–2303 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis and photophysical properties of compounds. CCDC 2130844. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2sc00329e |
| ‡ J. Han and Z. Huang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |