Open Access Article
Open Access ArticleCircumventing challenges in mitochondrial targeting for cancer treatment: leveraging nanoplatforms for effective solutions
Shivani R.
Pandya
 *ab,
Harjeet
Singh
*ab,
Martin F.
Desimone
c,
Jagpreet
Singh
*ab,
Harjeet
Singh
*ab,
Martin F.
Desimone
c,
Jagpreet
Singh
 d,
Noble
George
ab and
Srushti
Jasani
a
d,
Noble
George
ab and
Srushti
Jasani
a
aResearch and Development Cell, Parul university, Vadodara, Gujarat, India. E-mail: shivpan02@gmail.com; mailgsbtm@gmail.com
bParul Institute of Applied Sciences, Parul University, Vadodara, Gujarat, India
cUniversidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Instituto de Química y Metabolismo del Fármaco (IQUIMEFA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Buenos Aires, Argentina
dUniversity Centre for Research and Development (UCRD), Chandigarh University, Mohali, Punjab, India
First published on 21st December 2023
Abstract
Cancer is a highly complex disease that affects lives and causes millions of deaths worldwide. Several approaches are currently employed as cancer treatment options. Among them, mitochondrial targeting offers a highly promising approach to precise cancer treatment. Indeed, various engineered nanomaterials loaded with chemotherapeutic drugs were developed to precisely target mitochondria. This review investigates the challenges of cancer treatment, particularly in photodynamic, photothermal, and chemotherapies. Drawing on insights from mitochondrial biology and targeting materials, it unravels the complex connection between cancer, mitochondria, and nanoplatform-based targeted therapy. The attention is specifically on nanoparticles and their emerging role in precisely targeting mitochondria, offering solutions for effective treatment of cancer.
1. Introduction
Cancer, a complex and concerning issue, continues to pose a worldwide challenge, affecting lives and driving continuous scientific inquiry.1 In 2020, cancer led to nearly 10 million deaths worldwide, accounting for about one in six deaths. These numbers reveal the widespread impact, with around 19.3 million new cancer cases and approximately 10.0 million cancer-related deaths in 2020 alone across 185 countries.2 The intricate characteristics of cancer, marked by cells growing and spreading uncontrollably, have triggered a surge of research aimed at finding effective ways to treat this condition.3 The urgent need for innovative solutions is highlighted by the World Health Organization, which emphasizes cancer as a major global cause of death. This pressing situation underlines the necessity for novel approaches to undertake this challenge.2In the world of cancer, there is a fascinating balance between what we know and what is still unknown. As we study how cancer works at a molecular level and how genes play a role, there are parts that we are still figuring out. For example, some people inherit genes that make them more likely to get certain cancers, but the actual disease often needs additional mutations in their genes. Think of it like a puzzle: a gene called HPC1 can lead to early growth in prostate cancer, but for the disease to become worse, other genes have to be altered too.4,5 Some of these changes can happen because of things around us, and even after cancer starts, changes keep happening that can make the treatment stop working.6 It is like a mystery we are trying to solve, where genes, changes, and treatments all connect in a complicated way.
Several approaches are employed in tackling cancer, spanning from time-tested methods like surgery,7 chemotherapy,8 and radiotherapy9 to more recent innovations such as hormone therapy,10 anti-angiogenic treatment,11 stem cell therapies,12 immunotherapy,13 and dendritic cell-based immunotherapy.14 These strategies collectively constitute the contemporary spectrum of cancer treatment options.12,15 While these methods have shown progress, their drawbacks and potential side effects underscore the necessity for alternative approaches.
Chemotherapy, radiotherapy, and immunotherapy, when employed individually, can harm healthy tissues.16 The effect of the combination of immunotherapy with radio (chemo) therapy on normal tissue complications remains uncertain due to the intricate nature of treatment strategies.17 Additionally, it is crucial to acknowledge that these therapeutic interventions can affect non-cancerous cells alongside malignant ones, given their potential lack of specificity in exclusively targeting cancerous cells. It is important to note that the development of resistance to radiotherapy and chemotherapy can lead to treatment inadequacies or even trigger a recurrence of the malignancy.18–20 Therefore, challenges such as limited solubility, inadequate bioavailability, and indiscriminate biodistribution decrease the efficacy of existing medications and treatment approaches.
As cancer's global impact persists, demanding novel approaches, the intricate balance between known and unknown facets of the disease compels us to explore uncharted territory. In recent times, nanoparticles have emerged as a powerful tool for targeting malignant tumor cells for precise and efficient therapy of cancer.21 Medical nanotechnology involves utilizing materials with sizes in the nanometer range (typically 1–100 nm) for designing and creating therapeutic drugs and devices. These nano-sized materials exhibit distinct physicochemical properties due to their size, setting them apart from larger “bulk materials”. In biotechnology, the nanoparticle definition can extend up to 500 nanometers.22–25 While conventional treatments have limitations, nanotechnology, specifically nanoparticles targeting mitochondria, offers innovative solutions.
Mitochondria offer a highly promising approach to precise cancer treatment. These cellular powerhouses are central to energy regulation and cancer progression, making them a key focus in cancer research. The metabolic shift seen in cancer, notably the Warburg effect, highlights the pivotal role of mitochondria in cancer cells.26,27 Recent advances in understanding mitochondrial pathways and potent inhibitors highlight the potential for targeted interventions to prevent tumor growth. The energy-regulating role of mitochondria and their involvement in cancer progression make them an enticing focal point for therapeutic intervention.28,29 This approach employing different nanoparticles enables controlled drug release, enhancing efficiency with reduced side effects. Their diverse applications have the potential to revolutionize cancer therapy, elevating precision and effectiveness.30
In a recent development, researchers have synergistically integrated nanoparticles (NPs) with conventional chemotherapeutic drugs to fabricate biocompatible and versatile nanoplatforms that precisely target mitochondria.31 The utilization of this technique involves the development of targeted drug delivery, light-triggered hybrid nanostructures (photodynamic/photothermal therapy), and precise nanoparticle delivery to mitochondria, providing a selective, safe, and effective approach for disease treatment that holds promise for overcoming drug resistance while minimizing side-effects.32
This review investigates the challenges of cancer treatment, particularly in photodynamic, photothermal, and chemotherapies. Drawing on insights from mitochondrial biology and targeting materials, it unravels the complex connection between cancer, mitochondria, and nanoplatform-based targeted therapy. The attention is specifically on nanoparticles and their emerging role in precisely targeting mitochondria, offering solutions for effective treatment of cancer.
2. Targeting mitochondria: an important approach in cancer treatment
Mitochondria play an important role in cancer cell proliferation and survival, making them an appealing target for cancer therapy.33,34 Several studies have shown that mitochondrial building blocks play an important role in cancer cell development.34 Mitochondria are the primary producers of ATP, metabolites for macromolecule production, and ROS. Many cancer cells appear to have altered mitochondria (detailed in Section 3), resulting in an increased generation of reactive oxygen species (ROS) that drive cancer cell growth and proliferation.33 As a result, targeting mitochondria has been proven to be an effective strategy in cancer therapy.Classical approaches targeting the mitochondria of cancer cells usually aim at inducing mitochondrial damage or inhibiting mitochondrial function.35 Some of these approaches include mitochondria-targeted photodynamic therapy,36 mitochondria-targeted chemotherapy37 and mitochondria-targeted radiotherapy.38 These approaches have shown promising results in both preclinical and clinical trials, making them an important approach in cancer treatment.
3. Mitochondrial targeting moieties for precise cancer therapy
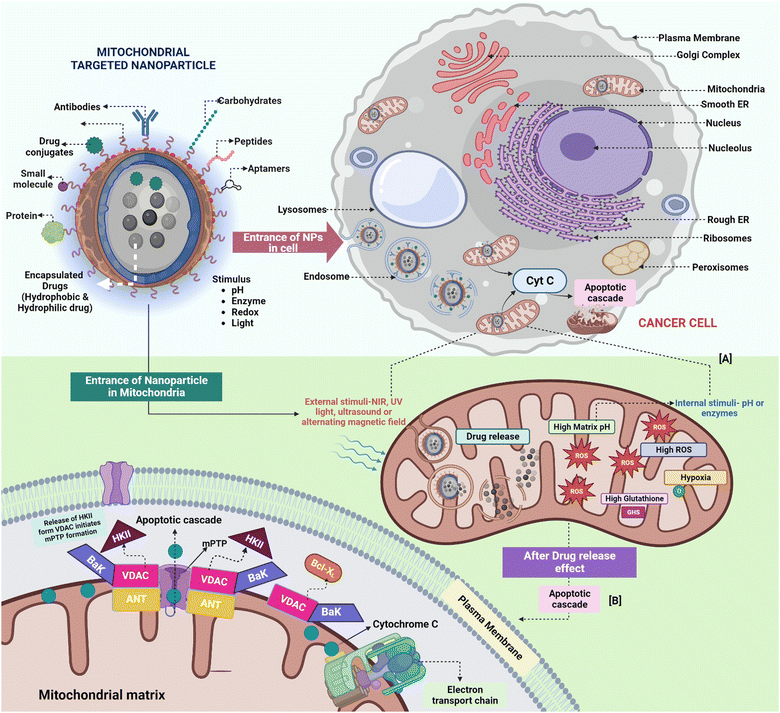
Mitochondrial targeting holds significant promise in addressing existing challenges in the clinical application of chemotherapy and the diagnosis of various disorders. Triphenylphosphonium (TPP) has gained significant recognition as a mitochondrial targeting moiety with extensive research spanning over decades. It is characterized by a positively charged phosphorus atom surrounded by three hydrophobic phenyl groups, which contribute to an effective interaction with the hydrophobic mitochondrial membrane.39,40 TPP exhibits voltage-dependent accumulation within negatively charged membrane compartments, with higher concentrations preferentially observed in mitochondria owing to their more negative membrane potential41,42 (Fig. 1). This distinctive characteristic has paved the way for TPP's utilization as a ligand for targeted mitochondrial delivery in diverse applications, including its conjugation with anticancer agents and vitamin E analogues.43 Consequently, this approach enhances the accumulation of therapeutic compounds within mitochondria, leading to improved therapeutic outcomes.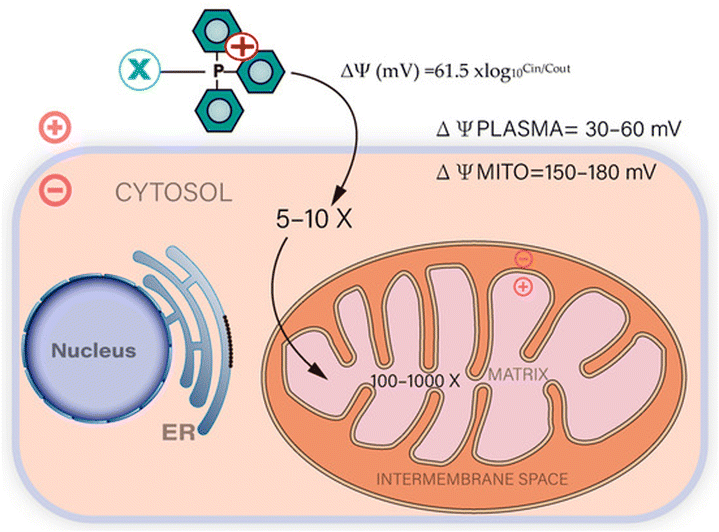 | ||
| Fig. 1 The absorption of compounds conjugated with TPP is facilitated by the plasma and mitochondrial membrane potentials. Mitochondria with a high membrane potential enable the rapid uptake of TPP-conjugated compounds. Reproduced from ref. 44 with permission from MDPI, Copyright 2023 (https://creativecommons.org/licenses/by/4.0/). | ||
In the context of mitochondrial targeting, peptides can be designed by incorporating positively charged and hydrophobic building units. Peptides are promising candidates for mitochondria-targeting ligands due to their advantages, including ease of synthesis, small size, low toxicity, and biocompatibility.45 Mitochondria-penetrating peptides (MPPs) typically contain a highly hydrophobic residue and a positively charged moiety, such as arginine or lysine, positioned alternately. The presence of delocalized positive charge and hydrophobicity allows these peptides to efficiently traverse the plasma membrane and localize within mitochondria.46 In the construction of mitochondria-targeting nanosystems, three commonly utilized peptide types are “mitochondria-targeting signal peptides (MTSs), mitochondria-penetrating peptides (MPPs), and Szeto-Schiller (SS) peptides”.47
Also, some small cationic molecules, such as rhodamine, pyridinium, and cyanine derivatives, can naturally get into mitochondria. These compounds are widely used as staining agents, fluorescent probes, imaging agents, and in photodynamic therapy (PDT) either alone or in conjugation with biologically relevant compounds.48
4. Distinctive mitochondrial attributes in malignant cells
Eukaryotic mitochondria drive energy production through the oxidative metabolism of nutrients, involving NADH/FADH2 oxidation from glycolysis, the TCA cycle, or fatty acid β-oxidation, followed by oxidative phosphorylation, leading to ATP synthesis. This process, termed oxidative phosphorylation (OXPHOS), is fundamental for cellular energy generation.49 However, cancer development triggered by factors like radiation, carcinogens, oncogenes, and oncoproteins induces a shift in energy production. Cancer cells transition from OXPHOS to glycolysis, where glucose is converted to pyruvate and, subsequently, to lactate in the cytosol.50 This glycolytic process allows cancer cells to maintain cytosolic pH, with lactate being transported out of cells via monocarboxylate transporters (MCTs), causing extracellular acidification. This acidic microenvironment impairs oxygen availability, leading to compromised oxygen transfer and the collapse of the electron transport chain (ETC) within mitochondria, thus reducing ATP generation.51 Notably, cancer mitochondria exhibit distinct characteristics: altered membrane potential, elevated ROS, and heightened glutathione (GSH) levels. Cancer cells display a more hyperpolarized mitochondrial membrane potential (ΨIM) of ∼−220 mV compared to normal cells at ∼−140 mV. Mitochondrial ROS in cancer cells intensify tumorigenic features and accelerate mutational accumulation for metastasis. Glutathione (GSH) holds a vital position within the cellular antioxidative system. In cancer cells, its elevated levels are crucial for mitigating excessive reactive oxygen species (ROS) and detoxifying xenobiotics, rendering it an attractive candidate for targeted cancer therapy.52–54 In addition, cancer mitochondria display altered oxygen and pH levels. In an in-depth study by Jiang et al. (2021), the impact of hypoxic conditions on mitochondrial function and glucose metabolism in gastric cancer cells was investigated. The findings uncover that hypoxia affects mitochondrial membrane potential, ROS levels, and crucial gene expressions, thereby promoting glycolysis while inhibiting mitochondrial aerobic respiration. These results illuminate the substantial role of hypoxia in shaping the metabolic attributes of gastric cancer cells.555. Challenges in targeting mitochondria for cancer treatment
Addressing the complexities of utilizing mitochondria in cancer treatment presents a frightening task. This investigation reveals barriers stemming from mitochondrial membrane potential, intricate structure, and cellular heterogeneity. Additionally, it emphasizes the involvement of lipophilic molecules, nanoparticles, and the dynamic tumor microenvironment. Conquering drug resistance and bioavailability hurdles, and refining photodynamic therapy techniques further enhance the intricacy. Despite the substantial potential, effectively overcoming these multidimensional challenges remains vital for the conquest of mitochondrial-centered cancer therapies.5.1. Mitochondrial membrane potential and cancer
Mitochondrial targeting relies on the distinct mitochondrial membrane potential, which is far higher than the plasma membrane potential.42,56 Cancer cells and transformed cells generally show higher mitochondrial potentials. Epithelial cancer cells often have elevated mitochondrial membrane potentials (ΔΨm), linked to increased invasiveness and metastatic potential. Some tumors have elevated ΔΨm, tied to higher glycolysis and resistance to regulated cell death, though not all tumors share this trait.57,585.2. Mitochondrial structure and molecule entry
The mitochondria's intricate four-layer structure (outer membrane, intermembrane space, inner membrane, and matrix) presents a barrier for the entry of diverse molecules into the mitochondria. However, due to a significant electrical potential difference, positively charged molecules can accumulate easily within mitochondria. Also, the passage through the mitochondrial outer membrane (MOM) heavily relies on concentration-dependent passive diffusion.59 To overcome this, lipophilic molecules are preferred due to their membrane permeability, facilitated by hydrophobic interactions.605.3. Lipophilic molecules and cancer heterogeneity
The existence of intratumoral heterogeneity (ITH) makes it difficult to treat cancer effectively, where genetic mutations lead to the development of diverse subpopulations within the tumor. These forms of variability may result in varying responses to the treatments, potentially leading to inadequate elimination of specific subclonal populations.61,625.4. Challenges in hyperthermia and intercellular trafficking
Another problem in cancer treatment is the limited integration of hyperthermia due to its inability to achieve selective cytotoxicity, target tumors effectively, and a lack of comprehensive understanding of its cytotoxic mechanism.63 Intracellular trafficking is another major challenge, and nanoparticles play a crucial role in addressing this problem by endosomal entrapment, as demonstrated through the development of lipid-protamine DNA/hyaluronic acid (LPD) and subsequent advancements like LCP nanoparticles. The incorporation of cationic liposomes, the presence of calcium phosphate cores, and modifications such as PEGylation and ligand conjugation illustrate the potential of nanocarriers in enhancing endosomal escape and facilitating successful mitochondrial targeting for improved drug delivery.645.5. Rapid clearance and circulation issues
Anticancer agents that rely on small molecules face the problem of rapid clearance from the body. This rapid clearance reduces their capacity for precisely targeting cancer cells, ultimately curbing their potential for achieving optimal outcomes in cancer therapy.48 In cancer treatment, nanoparticles' clearance from circulation depends on their properties and interactions with the mononuclear phagocytic system. This system involves macrophages, monocytes, and dendritic cells, impacting NP accumulation in the liver and spleen. Rapid clearance occurs with stiffer, cationic NPs. Surface modifications of NPs with polymers such as PEG would reduce such molecular interactions, extending NP circulation for improved therapeutic outcomes.65–695.6. Hypoxia and pH imbalance
A recent investigation has proposed that hypoxia has an impact on the immune microenvironment, enabling tumor cells to evade immune surveillance and elimination.70 In addition, cancer cells exhibit unique metabolism, favoring enhanced glycolysis, which leads to intracellular alkalinity (pH 7.2 to 7.4) and extracellular acidity (pH 6.2 to 6.8). The acidic tumor microenvironment (TME) promotes resistance, proliferation, and metastasis, unaddressed by current treatments. Current cancer therapies often overlook targeting this pH imbalance resulting from cancer-specific metabolism, contributing to suboptimal treatment outcomes.71–745.7. Drug bioavailability and mitochondrial role
There are significant challenges related to low drug bioavailability, high degradation, and metabolism of drugs in the intestines and liver. These issues can particularly hinder the effective treatment of cancer through oral chemotherapy, affecting the delivery of therapeutic doses to the target site and potentially reducing treatment efficacy.755.8. Mitochondrial adaptation and drug resistance
Mitochondria, as pivotal organelles in cellular energy, exhibit remarkable dynamism and complex integration with signaling cascades. This confers upon cancer cells the capacity to swiftly modulate their bioenergetic and biosynthetic profiles, contributing significantly to multifaceted tumor attributes, including heightened drug resistance. Consequently, the exploration of mitochondrial targeting in cancer treatment to counter drug resistance has gained substantial traction across diverse cancer types.76 As tumors progress, mitochondria play a dual role in maintaining cellular balance and coping with drug-induced stress. For example, in ovarian cancer, mitochondrial fission offers a hypoxia-related advantage to cisplatin-resistant cells over their non-resistant counterparts. These adaptive processes impact mitochondrial metabolism, culminating in drug resistance.77–80 The ever-changing nature and complex attributes of mitochondria underscore the need to investigate innovative agents and approaches targeting these organelles.5.9. Photodynamic therapy: challenges and opportunities
Photodynamic therapy (PDT) is one of the methods used for the treatment of cancer. It employs ROS to induce tumor cell death and trigger immune responses in cancer treatment, yet faces limitations in its scope. Enhancing immune responses holds promise in expanding PDT's clinical impact. Challenges encompass PS (photosensitizer) variability, precise tumor targeting, potential toxicity, immunogenicity, biodistribution, and the search for an ideal PS profile for optimal antitumor efficacy.716. Transforming mitochondrial targeting: nanoparticles as key allies in overcoming cancer therapy challenges
In the pursuit of tackling the complex challenges involved in targeting mitochondria for cancer therapy, nanoparticles have emerged as valuable allies. These tiny particles possess special qualities that make them particularly promising, including their ability to pass through cell membranes and release drugs in a controlled manner.81 These features offer hope for overcoming issues like drug resistance, making drugs more available to cells, and dealing with the intricate structures within cells. In this section, we will explore creative strategies involving nanoparticles, highlighting how they are bringing about significant changes in the complicated field of cancer treatments solely focusing on mitochondria.Nanocarriers alter a drug's pharmacokinetic characteristics, increasing its efficiency and minimizing negative effects. Organic, inorganic, and hybrid nanocarriers are the types of nanocarriers that have been utilized by different research groups for the administration of chemotherapeutic drugs.82–84 Furthermore, their unique attributes enable inorganic nanoparticles to surpass organic nanoparticles as superior drug carriers. Inorganic nanoparticles are better drug carriers than organic nanoparticles because of their unique properties such as high quantum yield, easy surface modification, controlled drug release, low toxicity, improved bioavailability, high drug loading capacity, a longer lifetime, high photostability, and a large surface area.85–87 On the other hand, hybrid nanoparticles, which combine the advantages of both organic and inorganic nanoparticles, may offer improved efficacy and safety compared to their individual counterparts.88,89 Taking into consideration the diverse factors influencing the selection of nanoparticles for cancer treatment, it becomes crucial to weigh the benefits of hybrid nanoparticles, which amalgamate the strengths of both organic and inorganic nanoparticles, against the unique attributes of each type.
6.1. Harnessing nanoparticles for targeting mitochondria as a viable strategy against multi-drug resistance in cancer therapy
Multidrug resistance (MDR) presents a substantial challenge in cancer treatment, contributing to 90% of cancer-related fatalities.90,91 MDR develops either via intrinsic or acquired mechanisms,92 and tumor heterogeneity and cellular invasion exacerbate its impact.93,94 MDR usually results from increased drug efflux pumps, disrupted cell death processes, an altered cancer microenvironment, genetic mutations, and the activation of alternative survival pathways.95Mitochondria, the central organelles for cellular energy supply, exhibit dynamic changes and integrate cellular signaling pathways to provide bioenergetic and biosynthetic flexibility for cancer cells, contributing to multiple aspects of tumor characteristics, including drug resistance.76 As a result, targeting mitochondria for cancer therapy and overcoming drug resistance has become an increasingly prominent focus of research in various forms of cancer.76 A potential strategy for overcoming MDR in cancer therapy involves utilizing mitochondria-targeted nanoparticle therapy.96 Since drug molecules may be effective at the cell's core, directing therapeutic agents to mitochondria provides a dependable and powerful approach to eliminating cancer cells. Nanoparticles can enhance the effectiveness of anticancer treatments and overcome MDR by precisely targeting the mitochondria and subsequently overcoming the challenge of MDR.
Targeting mitochondria is a promising strategy to overcome drug resistance in cancer cells.32 Mitochondrial dysfunction, altered energy metabolism, and altered apoptotic signaling pathways contribute to drug resistance. By specifically delivering drugs to mitochondria or designing drugs that target mitochondrial function, it is possible to disrupt altered energy metabolism, induce mitochondrial dysfunction, and exploit vulnerability in cancer cells.97 This approach holds great potential for enhancing the effectiveness of anticancer therapies and improving patient outcomes.
Customization of these nanocarriers is possible with mitochondrial-targeting compounds like mitochondrial-penetrating peptides or mitochondrial-targeting ligands.98 Prolonging the nanocarrier's in vivo circulation could enhance the utilization of the EPR effect, leading to improved drug uptake by tumor cells and overcoming MDR.96 Most acquired MDR in solid tumors commonly involves overexpression of the P-glycoprotein membrane pump molecule.99 Therefore, it becomes essential to overcome and evade ATP-binding cassette (ABC) transporter molecules.100 Multifaceted nanoplatforms have emerged that typically avoid the interference of ABC transporter molecules, thereby permitting the binding of nanoparticles into the mitochondria and triggering apoptosis by releasing chemotherapeutic medicines into the mitochondria (Fig. 2B).
Jue Tuo et al. (2016) devised a novel approach involving mitochondria-targeting delivery of doxorubicin (DOX) through folic acid-conjugated PEGylated liposomes coated with a berberine derivative. This system was designed to target resistant MCF-7/ADR cells. The utilization of folate as a tumor-targeting ligand facilitated efficient cellular uptake of the nanocarrier, while the berberine derivative facilitated the selective transport of DOX into the mitochondria. This strategy effectively bypassed drug efflux mediated by ABC membrane transporters, a key factor in drug resistance. Consequently, this approach amplified the cytotoxicity and apoptosis-inducing effects of DOX within resistant MCF-7/ADR cells.101 Chen et al. (2017) developed a nanoplatform using “dendrigraft poly L-lysine with specific binding aptamers for nucleolin and cytochrome c”. This dual modified system has been demonstrated to preferentially concentrate in the mitochondria of HeLa and HaCaT cancer cells and rapidly deliver the loaded Dox, which is triggered via higher ATP concentrations in mitochondria. It also modulated mitochondrial membrane potential and evaded P-glycoprotein-mediated drug efflux, a mechanism limiting chemotherapeutic uptake in multidrug-resistant (MDR) cancer cells.102
Yongyan et al. (2020) developed an acid-triggered nanocarrier for targeted nitric oxide delivery to cancer mitochondria, enhancing therapy against MDR and metastasis. This nanocarrier, loaded with nitric oxide and doxorubicin, effectively suppressed ATP production, impaired mitochondrial function, and overcame MDR by inhibiting P-glycoprotein activity. Nitric oxide also hindered tumor-derived microvesicle formation, subsequently reducing metastasis. They found that subcellular targeting of mitochondria in the acidic cancer microenvironment led to better therapeutic outcomes.103
As briefly discussed in the introduction, combinational therapy has considerable potential for extremely effective cancer treatment owing to its unparalleled effectiveness in creating synergistic effects and conquering MDR.104 Chen et al. (2017) developed a multi-organelle-targeting sequential drug delivery system called “DGLipo NPs”. This system effectively treated MDR in cancer as well as monitored Cyt-C release during apoptosis. “DGLipo NPs” achieved precise subcellular drug delivery via c(RGDfK) on the liposomal shell and mitochondria penetrating peptide (MPP) on the DGL core. The pH/CytC responsiveness triggers coordinated release of RA-V and Dox, enhancing subcellular combination treatment efficiency and overcoming MDR in tumor.105 The use of mitochondria-targeted nanoparticles as a prospective technique for overcoming MDR in cancer therapy is depicted in Table 1.
| Sr. no. | Nanoplatform | Cancer cell | Mode of action | Ref. |
|---|---|---|---|---|
| 1. | ROS responsive TPP-DOX@HA-PBPE | MCF7/ADR (resistant breast cancer cell) | Evading P-glycoprotein facilitated drug efflux | 106 |
| 2. | PTX-ss-BBR | A549 (lung cancer cell) | Dissipating the mitochondrial membrane potential and upregulating ROS production and thereby eliciting apoptosis | 107 |
| 3. | BIBR1532-loaded peptide dendrimeric prodrug nanoassembly (B-PDPN) | MCF-7R (resistant breast cancer cell) | B-PDPN broadly inhibits telomerase, diminishing hTERT protein's mitochondrial defence. This boosts ROS, causing DNA harm and apoptosis | 108 |
| 4. | Dual targeting nanocarriers DT-NP to load ROS-responsive pro drug B-DOX. | MCF-7/ADR | DT-NP effectively eradicates MCF-7/ADR cells via targeted DOX release in mitochondria, ROS generation, and GSH reduction | 109 |
| 5. | TPH/PTX nanomicelles | A549/ADR | TPH/PTX induces mitochondrial outer membrane permeabilization by reducing antiapoptotic Bcl-2, leading to cytochrome c release and activating caspase-3 and caspase-9 | 110 |
To summarize, harnessing mitochondria-targeting nanoparticles emerges as a promising strategy to overcome MDR in cancer therapy. Precise mitochondrial delivery via nanoparticles effectively evades drug efflux mechanisms, thereby augmenting therapeutic efficacy. This mitochondrial targeting approach holds potential for synergistic combination therapies, providing innovative solutions to MDR challenges. The evolving convergence of precision medicine and nanomedicine stands poised to revolutionize cancer treatment, transcending MDR and advancing patient outcomes.
6.2. Enhancing drug delivery: nanoparticles targeting mitochondria to overcome lysosomal barriers in chemotherapy
During treatments, the unintended or non-targeted distribution of chemotherapeutic agents reduces their efficacy and potentially increases toxicity. Such drugs harm both cancerous and healthy cells, resulting in side effects such as nausea, vomiting, hair loss, and immunosuppression. Non-targeted administration and premature delivery of drugs predominantly lead to drug resistance, as cancer cells develop resistance to treatment after repeated exposure.91 Consequently, developing precise drug delivery vehicles that specifically target the mitochondria of malignant cells is imperative for effective cancer therapy.111Achieving precise drug delivery within subcellular organelles is a fundamental requirement for the effectiveness of nanocarriers. Mitochondria, having a crucial role in regulating apoptosis, represent a significant target for inducing tumor cell death by disturbing mitochondrial ROS balance during cancer treatment.112 Nevertheless, a significant hurdle in the utilization of nanoparticles for drug delivery lies in the barrier posed by lysosomes. When taken up by cells, nanoparticles are transported to lysosomes, where they may undergo degradation by lysosomal enzymes. This process can lead to a decline in the effectiveness of the delivered drugs.113 Drug leakage into the cytoplasm and lysosomes remains a challenge with mitochondria-targeted nanoparticles.114 Therefore, achieving responsive drug release within mitochondria is of great importance. The rationale for fabricating pH-responsive nanocargos is that the mitochondrial matrix maintains a slightly basic pH range of 7.5–8.2115 due to the expulsion of protons into the intermembrane space during ATP synthesis. In cancer cells, heightened negative polarization of the mitochondrial membrane further elevates the matrix pH, rendering it more alkaline compared to normal cells.116 Both the mitochondrial microenvironment and external stimuli serve as crucial triggers for designing responsive drug delivery systems tailored for cancer treatment.117 Researchers are actively directing their efforts toward exploiting endogenous tumor matrix stimuli to amplify drug release at specific sites (Fig. 2A).118 In 2019, Yanan et al. developed Dox-loaded IR780-CSOSA micelles to target the mitochondria and avoid the lysosomes of MCF-7 and HepG2 tumors. This led to photothermal conversion and controlled drug release upon NIR-laser exposure. The combined effect of photothermal-triggered drug release and heat stress within tumor mitochondria synergistically enhanced ROS generation, notably expediting apoptosis in the surrounding sublethal area.119
Yanan et al. (2017) utilized the alkaline pH environment within cancer cell mitochondria as a stimulus for drug release. They developed pH-responsive CTPP-CSOSA-Cela micelles, demonstrating an impressive 80.17% tumor inhibition rate compared to the standard Cela drug. When tested against MCF-7 and A549 cancer cells, these micelles exhibited faster drug release at pH 8 than at pH 5 and 7. This pH-sensitive behavior minimized drug leakage in the cytoplasm and lysosomes, facilitating accurate drug delivery to the mitochondria and thereby escaping the lysosomal degradation of drugs. Simultaneously, the drug-loaded micelles enhanced ROS production, inducing oxidative stress. This heightened ROS generation triggered the release of cytochrome c, a crucial factor promoting apoptosis. The orchestrated mechanism of drug release and ROS-mediated apoptosis culminated in significantly enhanced therapeutic efficacy against cancer cells.120
Nanoparticles facilitate the meticulous delivery of cancer drugs to mitochondria, mitigating toxicity and augmenting effectiveness. Responsive systems use the endogenous environment of the tumor for controlled drug release. These approaches, involving targeted localization, controlled release, and therapies like photothermal and apoptosis induction, show promise for enhanced cancer treatment.
6.3. Mitochondria-targeting nanoparticles: a paradigm shift in reducing side effects and toxicity in chemotherapy-based cancer treatment
As discussed in the earlier sections, conventional chemotherapy's indiscriminate cytotoxicity affects both cancerous and rapidly dividing non-cancerous cells, leading to side effects and reduced efficacy. Drug resistance in cancer cells worsens targeted precision and treatment effectiveness.91 Stimulating mitochondria-triggered programmed cell death holds significant potential in treating cancer, and there has been a growing focus on utilizing mitochondria as targets for anti-cancer medications as they possess precise anticancer action and have less toxicity.121Nanoparticles are designed to transport therapeutic agents like chemotherapy drugs along with targeting components such as peptides, antibodies, or small molecules.122 These targeting components bind to mitochondrial receptors overexpressed in cancer cells. The nanoparticles encapsulate the therapeutic agents and are coated to improve stability, circulation, and cellular uptake.
This enables specific delivery to cancer cell mitochondria, reducing side effects and enhancing treatment efficacy (Fig. 3).123 Cancer cells have altered mitochondrial physiology compared to normal cells (discussed in Section 3), which creates opportunities for nanoparticles to be preferentially taken up by cancer cell mitochondria.124 Cancer cells are characterized by higher mitochondrial mass, increased membrane potential, and altered redox states. Targeting moieties on the nanoparticles recognize and bind to specific receptors or transporters on the cancer cell surface, facilitating their internalization into the cell.125
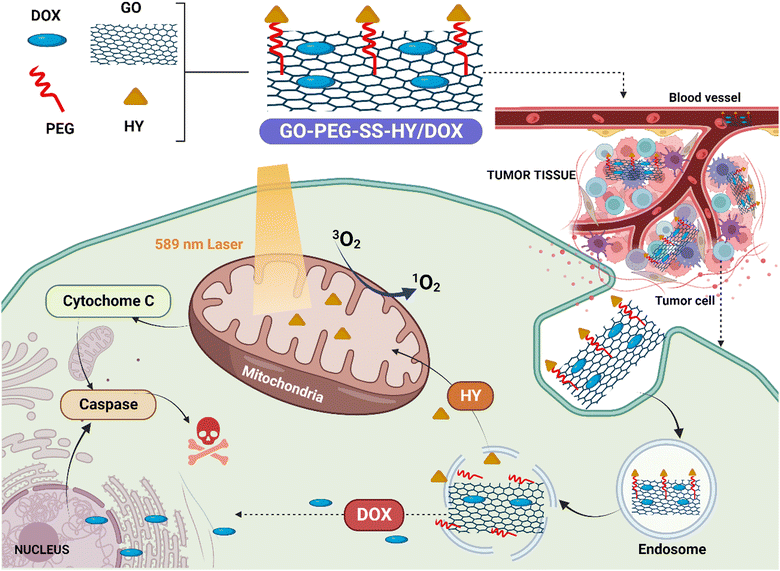 | ||
| Fig. 3 Mitochondria-targeting hypericin-functionalized graphene oxide enhances synergistic anticancer therapy. The illustration depicts HY-functionalized graphene oxide loaded with doxorubicin (GO-PEG-SS-HY/DOX) selectively targeting breast carcinoma cells' mitochondria. The combination of phototherapy and chemotherapy induces mitochondria-mediated apoptosis, enhanced by upregulated key proteins. Normal cell safety is confirmed, highlighting the potential of this platform for improved cancer treatment. [Reproduced with permission from,126 with slight modifications; created with https://BioRender.com.] | ||
The negative membrane potential and the dense double membrane of mitochondria make it challenging to develop ligands that can target them, despite the fact that targeting mitochondria has become a beneficial approach in cancer therapy. To improve the efficacy of these mitochondrial ligands in fighting tumors, they can be linked to nanoparticles.127
Chao et al. (2018) developed hypericin-functionalized graphene oxide loaded with Dox, which resulted in enhanced combined anticancer effects of phototherapy and chemotherapy without any observed side effects.128 This was observed in MD-MB-231 and MCF-7 cells. In addition to that, the functionalized nanoplatform showed low toxicity toward normal cells.126 The study by Bajpai et al. demonstrated that TPP-coated nanoparticles loaded with tigecycline (Mito-TPP-Tig-NPs) exhibited specific targeting to the mitochondria of A549 lung cancer cells, surpassing other cationic nanoparticles. Mito-TPP-Tig-NPs induced damage to mitochondrial morphology and the generation of reactive oxygen species (ROS). All mitochondria-targeted nanoparticles loaded with tigecycline showed enhanced cancer cell killing efficacy in A549 lung cancer cells and HeLa cervical cancer cells compared to free tigecycline. Importantly, Mito-TPP-Tig-NPs displayed significantly lower toxicity towards noncancerous human embryonic kidney cells (HEK293) in comparison to free tigecycline. These findings suggest that the use of antibiotic-loaded mitochondria-targeted nanoparticles holds promise for advancing anticancer therapy.129
In the study by Banik et al., they made a platinum(IV)-based prodrug called Platin-C that has curcumin as an active ligand. This prodrug demonstrates enhanced efficacy in cancer cell lines that are resistant to cisplatin. To deliver Platin-C, a targeted drug delivery system is utilized, employing biodegradable polymer nanoparticles (NPs) functionalized with TPP. The NPs exhibit efficient loading and controlled release of the prodrug, maintaining stability over a week. By using confocal microscopy, it is confirmed that Platin-C targets the mitochondria with the help of the curcumin pendant. The study highlights the mitochondria-directed activity of Platin-C and its NPs in both cisplatin-sensitive and -resistant cell lines, as well as their potential to reduce cellular inflammation markers. This research contributes to the understanding of combining the effects of chemotherapy and inflammation through the use of the cisplatin prodrug approach.130 The dataset presented in Table 2 provides a detailed overview of the different nanoplatforms that are currently being utilized for the targeting of mitochondria, with a focus on the specific moieties that are involved in this process.
| Nanoplatform | Mitochondrial ligand | Cancer cells | Role/effects | Ref. |
|---|---|---|---|---|
| NaGdF4:Yb,Er nanocrystals were modified with a tumor-targeting agent and a mitochondria-targeting moiety | Triphenylphosphonium bromide (TPP) | Glioblastoma-multiforme (GBM) | The effectiveness of sensitization in both in vitro and in vivo scenarios was dependent on the degree of mitochondrial targeting | 131 |
| Hypericin- functionalized graphene oxide loaded with Dox | Hypericin | MD-MB-231, MCF-7 | The effectiveness of both phototherapy and chemotherapy against cancer was improved without any accompanying side effects | 126 |
| Dox-loaded TPCL NPs | TPP | HeLa, HepG2 | The majority of TPCL NPs that were loaded with drugs demonstrated higher efficacy in killing tumors when compared to the free drugs. Additionally, these drug-loaded NPs tended to accumulate more in the mitochondria than the nucleus | 132 |
| DTOS nanoemulsions | Dequalinium (DQA) and α-tocopherol succinate (α-TOS) | HeLa | The DTOS emulsion remained stable at room temperature for three years and inhibited 71.5% of HeLa cells within 24 hours through effective mitochondrial targeting | 133 |
| Targeted-dendrimeric curcumin (TDC) | TPP | Hepa1-6 | In Hepa1-6 tumor-bearing mice, TDC construct treatment led to noteworthy tumor suppression and the longest median survival compared to free curcumin and untargeted constructs | 134 |
| Photosensitizers (IR780) and metformin packed in PEG-PCL liposomes | IR780 | MKN-45P | By using IR780, a combination of PDT and PTT that targets the mitochondria can potentially lead to a more effective combined therapeutic effect | 135 |
| Mitochondria-targeted bovine serum albumin@copper sulfide | Rhodamine-110 | MCF-7 | When exposed to the same near-infrared radiation conditions, the mitochondria-targeted R-BSA@CuS nanocomposites fight cancer much more effectively than the non-targeted BSA@CuS nanocomposites | 136 |
| Mitochondria-targeting magnetothermogenic nanozyme (Ir@MnFe2O4 NPs) | Cyclometalated Ir complex | HeLa | When exposed to an alternating magnetic field (AMF), Ir@MnFe2O4 NPs create localized heat, damaging mitochondria (MHT effect). Simultaneously, CDT disrupts cellular redox balance, increasing cell susceptibility to MHT | 137 |
6.4. Mitochondria-targeted nanoparticles combating tumor hypoxia in cancer therapy
Hypoxia is a major issue for cancer treatment. Hypoxia develops in cancer sites with limited oxygen delivery and is a common characteristic of solid tumors.138 It has been linked to a poor prognosis in cancer patients and is a significant impediment to successful cancer treatment with radiation, chemotherapy, and immunotherapy.139,140 While hypoxia is lethal to most cells, cancer cells can adapt to survive and even thrive under hypoxic conditions, making it difficult to effectively treat the tumor.139 Recent research, however, has demonstrated that nanoparticles may be utilized to specifically target the mitochondria in cancer cells and transport drugs or other therapeutic agents directly to these organelles.91 Additionally, NPs have the potential to avert hypoxia and, in turn, increase the efficacy of cancer therapy.141The most prevalent approach for mitigating tumor hypoxia involves the utilization of nanomaterials to deliver oxygen to a hypoxic microenvironment. Natural red blood cells (RBCs) play a vital role in transporting oxygen from oxygen-rich tissues to oxygen-deprived tissues. Hemoglobin (Hb) within RBCs binds to four oxygen molecules and readily releases them under hypoxic conditions to alleviate hypoxia.142 Through chemical modification or encapsulation using biodegradable materials, Hb-based oxygen carriers can overcome the limitations of cell-free Hb systems while maintaining oxygen-carrying capacity comparable to natural RBCs.143,144 Hb-based oxygen carriers have the ability to penetrate tumor tissues via narrow vascular structures, effectively delivering sufficient oxygen to hypoxic tumors.145
Another strategy is to use nanoparticles that can generate oxygen within tumor mitochondria, thereby overcoming the lack of oxygen and making the therapy more effective. Zhengyang et al. (2019) talk about a photodynamic Mn3O4-MSNs-IR780 nanoparticle that can stop MKN-45P cancer cells from making oxygen by using H2O2 to break down tumors and specifically kill mitochondria. These Mn3O4NPs build up in tumors and react to the H2O2-rich environment inside the tumor by turning H2O2 into O2, which speeds up PDT. The nanoparticles release IR780, which targets the mitochondria and, when hit by a laser, destroys the mitochondria and stops cells from breathing. This stops tumors from getting hypoxic for good and improves the outcome of the treatment. In vitro experiments show that Mn3O4-MSNs-IR780 can sustainably inhibit tumor hypoxia and target mitochondria (Fig. 4).146
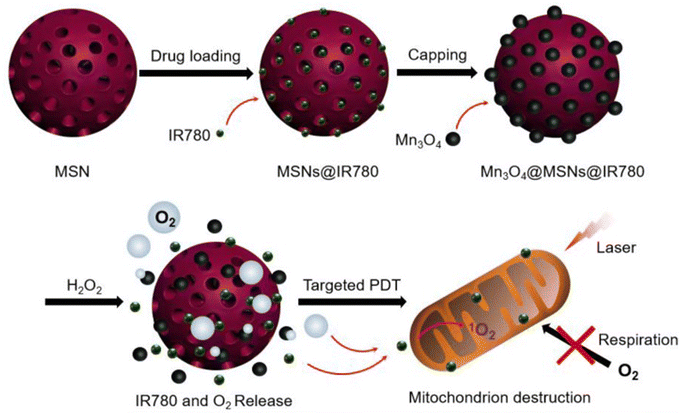 | ||
| Fig. 4 Step-by-step process involved in synthesizing Mn3O4@MSNs@IR780, the mechanism of H2O2-induced release of IR780 and O2, and the procedure for targeted photodynamic therapy (PDT) on mitochondria. Reproduced from ref. 146 with permission from Ivyspring International Publisher, Copyright 2019 (https://creativecommons.org/licenses/by/4.0/). | ||
Ping et al. (2021) came up with a promising way to fight hypoxia-related tumors. They created a TA-MSN-(α-TOS/ICG)-TPP mitochondrial-targeted ROS amplifier to improve photodynamic therapy (Fig. 5). It targets mitochondria, blocking the mitochondrial respiration chain and reducing innate oxygen consumption while inducing endogenous mtROS accumulation. This improves photodynamic therapy by sparing oxygen and overcoming the short lifespan and limited action range of ROS.147
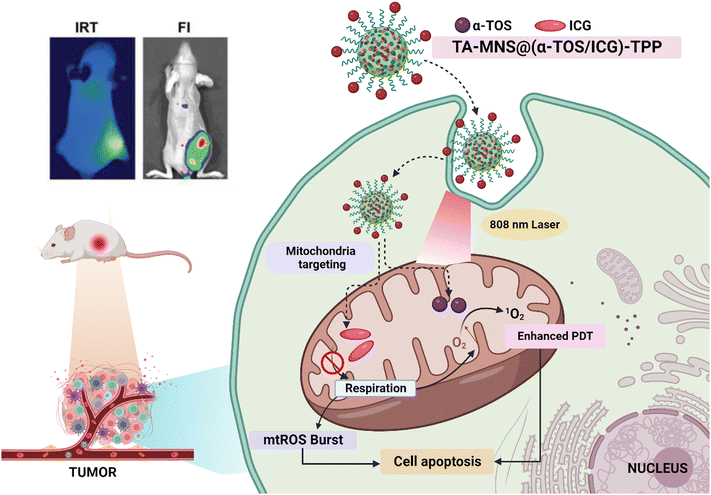 | ||
| Fig. 5 Illustration of targeted ROS amplification for improved PDT in tumor removal. After administration, TA-MSN@(α-TOS/ICG)-TPP nanoparticles gather in tumors and specifically target mitochondria. The released α-TOS curbs mitochondrial respiration, creating excess oxygen and boosting ROS production. This surplus oxygen enhances PDT efficacy within oxygen-rich mitochondria. [Reproduced with permission from ref. 147 with slight modifications from John Wiley and Sons, Copyright 2021; created with https://BioRender.com.] | ||
Nanoparticles targeting the mitochondria in cancer cells have shown potential for overcoming hypoxia, a major issue in cancer treatment. Novel strategies such as hypoxia-activated prodrugs and oxygen-generating nanoparticles have provided opportunities for more effective and targeted cancer therapies with fewer side effects. Further research in this area could lead to improved cancer treatments.
6.5. Unleashing the full potential of cancer therapy with mitochondria targeting NPs: boosting drug bioavailability and reducing rapid clearance
Conventional chemotherapy is a commonly utilized cancer treatment approach, although it has significant disadvantages, including low chemotherapeutic bioavailability.148,149 Bioavailability refers to the extent a chemical or medicine becomes entirely accessible to its designated biological destination.150 Chemotherapeutic drugs have low bioavailability due to their insufficient solubility, instability, and quick removal from the body, limiting their efficacy.151To address these limitations of conventional chemotherapy and enhance its efficacy, a promising strategy involves the utilization of nanoparticles for targeted drug delivery to cancer cells, thereby increasing drug concentration within the tumor while minimizing its impact on healthy cells.111
Another critical aspect affecting nanoparticle efficacy is their size. Smaller particles can damage normal cells and can be filtered by the kidneys, while larger particles may be cleared from the circulation by phagocytes.105 The effectiveness of NPs in drug delivery and therapeutic efficacy is significantly influenced by their surface characteristics, such as their hydrophilicity. NPs coated with hydrophilic substances, like PEG, can avoid clearance by the immune system and thus increase their half-life and bioavailability in the bloodstream.101,152 Therefore, NPs are commonly modified to become hydrophilic, which enhances their penetration and accumulation in tumors, making them more effective in delivering drugs.
Mitochondrial-targeted drug delivery has a lot of potential for resolving the problem of rapid drug clearance from the circulation, which is a prevalent issue in cancer therapy. In this strategy, nanoparticles are created to contain and transport medications selectively to cancer cells' mitochondria, taking advantage of the high mitochondrial density found in these cells.101,153 When drugs are delivered through conventional methods, they are often rapidly cleared from the bloodstream, leading to poor bioavailability and reduced efficacy.154 Unlike traditional drug delivery approaches, which frequently result in rapid drug clearance, mitochondria-targeted nanoparticles provide enhanced drug retention and tailored administration.123 By targeting the mitochondria, these nanoparticles can improve the delivery of drugs to cancer cells while minimizing the exposure of healthy cells to the drugs.123 When compared to traditional drug delivery methods, this focused strategy may result in better therapeutic effectiveness and lower adverse effects.
Positively charged nanoparticles can evade phagocytic clearance, but they may interact with blood components, leading to hemolysis and toxic effects on normal cells.155 Charge-reversal materials have been employed to address these issues. In physiological tissues, negatively charged nanoparticles evade opsonin interactions and MPS clearance. However, in the mildly acidic tumor microenvironment, these particles undergo charge reversal to become positively charged, facilitating interaction with the negatively charged cell membrane156 (Fig. 6).
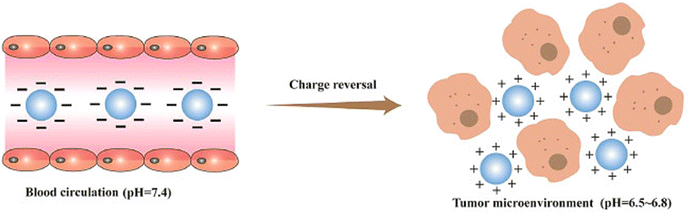 | ||
| Fig. 6 Schematic illustration of how charge reversal particles with a negative charge can effectively evade interaction with opsonin and clearance by the mononuclear phagocyte system (MPS). However, in the mildly acidic environment of a tumor, these particles undergo a charge reversal, becoming positively charged. This positive charge enables them to interact with the negatively charged cell membranes. Reproduced from ref. 155 with permission from Science Direct, Copyright 2021. | ||
In notable research, Hui et al. (2019) developed a DOX-PLGA, CPT, and PD nanoparticle coated with an acidity-triggered cleavable polyanion. This innovative design addressed rapid clearance in the bloodstream by utilizing the surface negative charge of DOX-PLGA/CPT/PD, thereby enhancing accumulation within tumor tissue. In the slightly acidic environment of tumor tissue, DOX-PLGA/CPT/PD underwent conversion to DOX-PLGA/CPT through hydrolysis of amide bonds in PD. The resulting nanomedicine effectively targeted mtDNA in tumor cells, inducing apoptosis and overcoming DOX resistance in MCF-7/ADR breast cancer cells.157
Zhou et al. (2017) developed a redox-triggered intracellular activation of mitochondria targeting nanocarriers, which increased the anticancer efficacy of paclitaxel against MCF-7. LPNPs consisted of PLGA, DLPE-S-S-mPEG4000, and C18-PEG2000-TPP. PEG4000 surface coating masked TPP's positive charge for tumor accumulation. Detachment of PEG4000 under reductive conditions inside cancer cells exposed LPNP's surface charge, leading to quick and precise localization in mitochondria.158
6.6. Targeting mitochondria with nanoparticles: a solution for tumor heterogeneity in cancer treatment
Intratumoral heterogeneity (ITH) refers to genetic variations within tumors, causing disparities between cell populations and posing challenges in cancer therapy, potentially leading to intrinsic and adaptive drug resistance and poor patient outcomes.159 Almost all cancer forms have cell-to-cell differences in “genetic signature, expression of genes, and post-translational alterations”.160The cancer stem cell model is one of two models used to explain the heterogeneity of tumor cells.161 According to this concept, only a few cancer cells can self-renew indefinitely and hence commence and sustain tumor development.162 As a result, tumor-initiating stem cells, also known as cancer stem cells (CSCs), evolve and develop over time, contributing to cancer heterogeneity.163 CSCs are typical cells seen in cancer tissues that have an infinite proliferation capacity and can cause carcinogenesis. Abnormalities in metabolism, proliferation, and apoptosis are observed in CSCs, leading to pathological reprogramming, including remodeling of mitochondrial functions.164 Mitochondria-targeted anti-CSC therapies are being developed to combat cancer by targeting the central role of mitochondria in CSCs.164,165 Ma et al. (2013) designed specialized liposomes containing berberine, utilizing a mitochondria-targeting compound called dequlinium and carboxyl polyethylene glycol-distearoylphosphatidylethanolamine (DQA-PEG2000-DSPE). These liposomes effectively entered cancer stem cells and specifically accumulated within mitochondria. This led to the enhanced release of cytochrome c, triggering apoptosis in breast CSCs.
Choi et al. (2019) investigated the use of reduced graphene oxide-silver nanoparticle nanocomposites (rGO-Ag) to target and eliminate cancer stem cells (CSCs), offering an alternative to conventional chemotherapy. The nanocomposites were synthesized using R-phycoerythrin (RPE) as a biomolecule mediator and evaluated in human ovarian cancer cells and ovarian cancer stem cells (OvCSCs). The results demonstrated that rGO-Ag displayed significant toxicity towards both ovarian cancer cells and OvCSCs. Incubation of OvCSCs with rGO-Ag for three weeks resulted in a notable reduction in the number of A2780 and ALDH+CD133+ colonies. The toxicity of rGO-Ag was attributed to its ability to generate reactive oxygen species, induce lactate dehydrogenase leakage, reduce mitochondrial membrane potential, and enhance the expression of apoptotic genes, leading to mitochondrial dysfunction and potential apoptosis induction. Additionally, rGO-Ag exhibited substantial cytotoxicity against highly tumorigenic ALDH+CD133+ cells. Combining rGO-Ag with salinomycin resulted in significantly higher levels of apoptosis compared to individual treatments, suggesting a potential strategy for selectively eliminating OvCSCs and sensitizing tumor cells. Overall, rGO-Ag shows promise as a novel nanotherapeutic agent for specifically targeting highly tumorigenic ALDH+CD133+ cells and eradicating CSCs, thus highlighting its potential for targeted therapy of tumor-initiating cells (Fig. 7).166
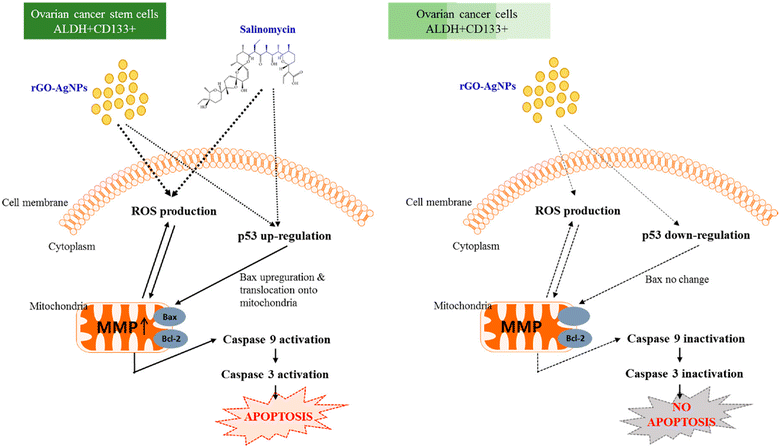 | ||
| Fig. 7 The mechanism by which rGO-Ag and salinomycin elicit toxicity and death in ovarian cancer cells and OvCSCs. Reproduced from ref. 166 with permission from MDPI, Copyright 2018 (https://creativecommons.org/licenses/by/4.0/). | ||
Zhang et al. (2012) created specialized liposomes containing quinacrine, incorporating a targeting element, dequalinium, on the surface. Dequalinium's positive charge facilitated mitochondrial accumulation due to its membrane potential. These modified liposomes notably suppressed MCF-7 cancer stem cells by activating pro-apoptotic BAX, lowering mitochondrial potential, releasing cytochrome c via translocation, and initiating caspase cascade reactions through drug aggregation within mitochondria.167
Another study by Zhao et al. (2019) analyzed the mechanism of action of Sal-AuNPs and indicated ferroptosis, an iron-dependent cell death of breast cancer stem cells, which was achieved as a result of iron accumulation and inhibition of antioxidant properties. This also led to the induction of oxidative stress, mitochondrial dysfunction, and lipid oxidation.168
In conclusion, the development of mitochondria-targeting nanoparticles holds great promise for addressing intratumoral heterogeneity in cancer therapy, particularly in targeting cancer stem cells, and may pave the way for more effective and personalized cancer treatments.
6.7. Revolutionizing cancer treatment monitoring through mitochondria targeting nanoparticles
Conventional cancer treatments such as PTT, PDT, chemotherapy, and radiation therapy have several limitations when it comes to real-time in situ monitoring of their effectiveness.169 One of the major issues is that these therapies may impact both healthy and malignant cells, making it difficult to assess whether the treatment is effective or creating detrimental side effects.91 Furthermore, cancer therapies may not be advantageous for all forms of cancer since cancer cells become resistant to therapy and individuals respond differently to treatment. As a consequence, personalized medicine is an important approach to consider in cancer treatment. Therefore, there is a need for more innovative and personalized approaches for real-time monitoring of cancer treatment that can overcome these limitations and provide more accurate and effective ways to track treatment response.Considering this scenario, Wang et al. (2020) developed mitochondria targeting single-component organic nanoparticles against HeLa cancer cells. After being irradiated with a single 808 nm laser, nanoparticles were able to produce outstanding second near-infrared fluorescence signals for imaging and real-time monitoring of tumor therapy, as well as high photothermal conversion efficiency and singlet oxygen yield. This led to hyperthermia and abundant singlet oxygen, resulting in mitochondrial dysfunction and cell apoptosis.170
Tong et al. (2019), in a different study, developed a novel nanoparticle for targeted activation of fluorescence signals and photodynamic efficacy in cancer cells. The nanoparticle was pH-responsive and composed of a fluorescent copolymer and a mitochondria-targeted photosensitizer. The nanoparticle exhibited enhanced fluorescence signal and singlet oxygen generation in an acidic environment and was quickly endocytosed by cancer cells. The activated photosensitizer induced intrinsic apoptosis in cancer cells, leading to remarkable inhibition of tumor progression without toxicity.171
Juan et al. (2016) have created a new single-component black titanium (B–TiO2–x) nanoplatform for imaging-guided cancer therapy. It showed high stability and compatibility and absorbed NIR to UV light. The nanoplatform achieved simultaneous and synergistic PTT/PDT with high therapeutic efficacy under infrared thermal/photoacoustic dual-modal imaging guidance, triggered by a single NIR laser. This research overcomes the limitations of multi-component nanocomposites, UV light, and high laser power density.172,173 Recent research has yielded encouraging results in the production of targeted nanoparticles for real-time cancer monitoring and therapy, with mitochondria-targeted and pH-responsive nanoparticles being especially successful. These novel techniques have the potential to revolutionize cancer therapy monitoring and result in better outcomes for patients.
6.8. Navigating cellular barriers: nanoparticles for precise mitochondrial treatment
Mitochondria, the cellular organelles responsible for energy production, consist of two bilayers and possess the largest membrane potentials within the cell. This electrochemical gradient allows for the selective accumulation of cationic molecules, which serve as critical monitors of mitochondrial polarization.57 Interestingly, in cancer cells, there is an abnormal accumulation of cytotoxic cationic molecules, which can be attributed to a more negative membrane potential in their mitochondria.42 This phenomenon has important implications for the development of targeted cancer therapies.Functionalized nanoparticles can be designed with a positively charged surface, which allows them to interact with the negatively charged mitochondrial membrane.174 This interaction occurs via electrostatic forces, which can facilitate the delivery of therapeutic agents into the mitochondria. The positively charged nanoparticles can electrostatically adhere to the negatively charged mitochondrial membrane and then be internalized into the mitochondrial matrix.174 This allows for targeted drug delivery to the mitochondria, where the drug can exert its therapeutic effect. The use of positively charged functionalized nanoparticles has been shown to be an effective approach to deliver therapeutics to the mitochondria and induce cancer cell death.25,59
Jia et al. (2019) created mitochondria-targeting nanodrugs against A549 cancer cells that were modified with DSPE-PEG2000 to enhance stability. The nanodrugs were then coated with negatively charged hyaluronic acid to accomplish cancer targeting. The HA coating was engineered to breakdown in cancer tissue by HAase, exposing the positively charged PEG/BD NDs to cells for absorption and subsequent lysosomal escape and mitochondrial targeting. The nanodrugs were discovered to cause apoptosis in A549 cancer cells by dissipating mitochondrial membrane potential and releasing cytochrome c, causing programmed cell death.175
A study conducted by Lei et al. (2020) aimed to improve the effectiveness of mitochondrial targeting drug delivery systems against the PANC-1 tumor by developing novel polysaccharide-based nanoparticles with tumor microenvironment-responsive charge-reversal and mitochondrial targeting abilities. The nanoparticles were loaded with curcumin and had a positively charged core with a pH-sensitive borate ester bond and a negatively charged shell. In vitro experiments showed that the nanoparticles achieved charge-reversal and released more curcumin in the acidic tumor microenvironment, effectively delivering the drug to the mitochondria and enhancing cytotoxicity.176
Nanoparticles that have been modified and have a positively charged surface could be very useful for delivering drugs specifically to mitochondria, especially in cancer cells that have a negative membrane potential. Negatively charged coatings or charge-reversal nanoparticles that can respond to the microenvironment of the tumor can make these drug delivery systems more specific and effective, which could lead to promising results in killing cancer cells.
Table 3 presents a comprehensive dataset of the diverse range of nanoplatforms currently being utilized to target mitochondria, addressing the current limitations of cancer therapy. The information provided covers a wide spectrum of applications, making it a valuable resource for researchers and clinicians seeking to optimize cancer treatment strategies through nanotechnology.
| Nanoplatform | Issue addressed | Rectification | Ref. |
|---|---|---|---|
| Nitric oxide-releasing nanosystem activated by photothermal energy (Cu2−xSe@SiO2) | MDR | Cu2−xSe can generate heat through photothermal conversion in the acidic surroundings of tumor cells, which enhances the release of DOX and triggers NO gas generation. The liberated 'NO' may trigger mitochondrial malfunction, inhibiting ATP production and drug efflux and therefore circumventing MDR | 177 |
| Nanoparticles composed of chondroitin sulfate that are redox-responsive | MDR, unintended delivery and lysosomal escape | When subjected to NIR laser irradiation, P-gp activity is down-regulated and cellular ROS are produced. This causes a reduction in mitochondrial membrane potential and enables lysosomal escape of the drug | 178 |
| Light-activated ROS-responsive polymer micelle nanoplatform | MDR, short-term circulation | Because chondroitin sulfate has a negative polysaccharide component, nanocarriers may extend the time that blood flows, target MCF-7/ADR cells specifically, and produce ROS when exposed to 635 nm red light. This causes apatinib and DOX to be released from micelles. Apatinib inhibits Pgp to recover chemosensitivity to DOX, while excessive ROS induces the PDT effect, leading to cell apoptosis | 179 |
| GQD with ruthenium nitrosyl functionalization | Unintended delivery, side effects, and reduced bioavailability | A fluorescently trackable nanoplatform targets mitochondria in cancer cells. It releases ‘NO’ and creates a photothermal reaction when exposed to 808 nm NIR light, resulting in anti-tumor effectiveness in vitro and in vivo | 180 |
| ROS-triggered TPP-TK-CPI613 nanoplatform | Unintended delivery, reduced bioavailability | The TTCI nanoparticles are very good at targeting mitochondria, so they build up much more in mitochondria. This makes it possible to deliver therapeutic agents very precisely | 181 |
| Dendritic polyglycerol-conjugated gold nanostars (GNSs-dPG-3BP, TPP, and HA) | Reduced bioavailability, tumor heterogeneity | Particles can bind to mitochondria more strongly through 3BP, which stops metabolism and kills cells by releasing cytochrome c. It improves the therapeutic efficiency of targeted PTT while also having a synergistic impact on the elimination of breast CSCs | 182 |
| Carbon-silica nanocapsules with a gold core | Inability to monitor treatment response, off-target toxicity | The nanocapsule is multifunctional and can release drugs in response to NIR light and pH changes. It can deliver drugs precisely to specific areas while protecting healthy tissues. The nanocapsule kills HepG2 cells by increasing ROS levels and lowering the potential of the mitochondrial membrane. Furthermore, it is a multimodal imaging agent for CT and PAT imaging, which can be used to guide therapy | 183 |
| Supramolecular nanoplatform for peroxynitrite-potentiated oxidative therapy | Unintended delivery, side effects, and off-target toxicity | A combined NO-based oxidative therapy uses a drug nanocarrier that targets mitochondria along with a GSH-sensitive NO donor and a pH-sensitive CA prodrug. CA causes ROS to be made in mitochondria, and NO only lets it out in mitochondria to lower GSH levels and make ONOO−, which helps oxidative therapy work and causes mitochondria to die | 184 |
| IR780 and 3BP in PLGA nanocarriers | Inability to monitor treatment response, hypoxic tumor environment | Because of the inherent properties of IR780, nanoplatforms can penetrate deep into the internal areas of tumors and stay in mitochondria. The addition of 3BP suppresses the utilization of oxygen in tumor cells by blocking the mitochondrial respiratory chain, leading to an increase in ROS production. Furthermore, 3BP inhibits tumor cells' high glycolytic ability, causing ATP synthesis to collapse and boosting tumor cell sensitivity to PDT. The nanoplatforms also function as a dual-modal imaging guidance and monitoring agent, capable of PAT and fluorescence imaging | 185 |
| H2O2-activatable BDPP NPs | Hypoxic tumor environment | The lipophilic shells of BDPP NPs may not stop intracellular H2O2 from getting through. This could make O2 and break up the NPs so that the photosensitizer can get into tumor cells. This is because of the mitochondrial-specific feature and H2O2-controllable O2 production, which make the medicine work better both in the lab and in living organisms. The constant creation of O2 by BDPP NPs throughout the PDT process addresses the issue of oxygen overconsumption in PDT, thereby improving the PDT efficiency of cancer treatment | 186 |
| TPP-TK-PPa/DEM NPs | Hypoxic tumor environment | NPs can produce ROS in situ during PDT, which lowers ROS utilization by lowering intracellular GSH levels after being taken inside cells through the slow release of DEM. ROS production during PDT causes significant alterations in mitochondrial membrane potential and shape, eventually leading to apoptosis | 187 |
| The fabrication of biomimetic NPs (oxygen tank NPs) by AIP and RBCm | Hypoxic tumor environment | PFC provides high-capacity external oxygen, whereas ATO slows mitochondrial respiration and mitigates endogenous oxygen consumption. These oxygen regulators may fix hypoxia and show stronger anti-tumor activity through mitochondria-targeted PDT with IR780 | 188 |
| Copper-based chalcogenide nanoplatform (CuS-NiS2) | Inability to monitor treatment response, ROS issues | Under NIR irradiation, CuS-NiS2 causes ROS production, which leads to death via the “Bcl-2/Bax” route in human gastric cancer cells. Furthermore, CuS-NiS2 coupled with NIR laser therapy induces necroptosis in tumor cells by modulating the MLKL/CAPG pathway. According to MRI, CuS-NiS2 demonstrates good contrast enhancement | 189 |
| GNPs-P-Dox-GA | Unintended delivery, MDR | GNPs-P-Dox-GA NPs are converted to tiny particles to release tiny P-Dox-GA particles for effective tumor permeation of tissue. After internalization, Dox-GA is effectively supplied to mitochondria via GA mediation. In drug-resistant HepG2/ADR cells, GNPs-P-Dox-GA had higher cellular uptake, mitochondrial distribution, and ROS generation levels, as well as a lower efflux rate, compared with non-GA-modified carriers | 190 |
| Nanoparticles with pH-responsive charge inversion and mitochondrial targeting (B6-oHA-SS-Ber) | Lysosomal escape, unintended delivery, and reduced bioavailability | The carrier material had a pyridine structure that was sensitive to pH and a disulfide bond that was sensitive to reduction. It also had a surface charge that could change from negative to positive as pH dropped, which helped cells take it in. The positively charged B6 facilitated lysosomal escape | 191 |
| Mitochondria-targeting camptothecin polyprodrug system (MCPS) | Mitochondria membrane barrier and toxicity | MCPS might be able to make water-soluble micelles made of a single molecule that are very stable. This could help drugs stay in tumor cells longer. TPP also promotes the transport of CPT into mitochondria. An intracellular reductant can rupture the disulfide link in MCPS, resulting in increased degradation of mitochondrial DNA and cell death caused by a high amount of ROS | 192 |
| WSSe/MnO2-INH-TPP@CM | Inability to monitor treatment response, ROS issues | MnO2 consumes GSH to yield Mn2+, which functions as a catalyst for INH to produce hydroxyl radicals, which eventually cause cell death. WSSe also offers high PTT performance and CT imaging capacity, as well as cancer/mitochondria dual-targeting potential. Being able to make hydroxyl radicals efficiently and accumulate in tumors efficiently leads to an effective effect that stops tumor growth | 193 |
| PF127/me-IR825 NPs | Inability to monitor treatment response | The NPs have two fluorescence emissions, making them appropriate for both in vitro and in vivo imaging. They have high NIR absorption and are useful for cancer detection and treatment. They are also biocompatible and safe, which bodes well for mitochondrial imaging, the initial stages of cancer detection, and targeted cancer therapy | 194 |
| Cetyltrimethylammonium chloride-loaded mesoporous silica nanoparticles | Mitochondria membrane barrier, unintended delivery | These nanoparticles are easy to take up by cells and build up in the cytoplasm because they are of the right size and can move through cells with the aid of HSA receptors. The positively charged CTAC may be able to target the mitochondria well by interacting with their negatively charged membrane, which hinders mitochondrial function and lowers the levels of both intracellular ATP and mitochondrial potential. MCF-7 cells could die as a result of this | 195 |
| X-ray activated gold nanorod-encapsulated liposome | Mitochondria membrane barrier, unintended delivery | Biodegradable liposome with a size of about 150 nm that contained a photosensitizer (verteporfin) and gold nanorods to enhance radiation. The liposome also had triphenylphosphonium to target mitochondria. The nanoconjugates were able to produce a high amount of harmful singlet oxygen specifically within the mitochondria when exposed to X-ray radiation, leading to the disruption of the membrane potential and eventually causing cancer cell death through apoptosis | 196 |
| Mitochondria-targeted triphenylphosphine (TPP) and AS1411 aptamer-conjugated Au–TiO2 NSs nanoplatform (Au–TiO2-A-TPP) | Lack of precision in SDT, short-term circulation | The nanoplatform displayed excellent biocompatibility, extended circulation duration, and CT imaging capability. It completely inhibited tumor development in both in vitro and in vivo studies, owing to its high ROS yield and dual-targeting capacity | 197 |
| NIR light-regulated PDT nanoplatform (TPP-UCNPs@MOF-Pt) | Hypoxic tumor environment, unintended delivery | The nanoplatform efficiently treats tumor hypoxia by transforming H2O2 into oxygen and boosting ROS levels, which improves PDT efficacy when exposed to NIR light. The mitochondria-targeting characteristic causes significant depolarization of the mitochondrial membrane and activation of the apoptotic pathway, which increases the therapeutic effectiveness even more | 198 |
| Diketopyrrolopyrrole-based photosensitizer, mitochondria-targeting organic nanoparticles (DPP2+ NPs) | Off-target toxicity, unintended delivery | DPP2+ NPs have high cytocompatibility and can generate thermal energy and singlet oxygen when exposed to irradiation. Furthermore, these NPs have a greater probability of entering cells and preferentially target mitochondria, implying that they might be used in mitochondrial photodynamic treatment | 199 |
| Mitochondria targeting nanoprobe | Off-target toxicity, unintended delivery | The designed nanoprobe has a size of less than 10 nm and is terminated with arginine or guanidinium, which enables it to penetrate the cell membrane directly and target mitochondria. This offers a promising approach for mitochondrial drug delivery and therapy | 200 |
| Fluorescent small-molecule (cy-r)-decorated iron oxide nanoprobes (Cy@Fe3O4) | Inability to monitor treatment response, unintended delivery | Mitochondria-specific “fluorescent cyanine dye-based nanosystem” penetrates cancer cells via the organic anion transporting polypeptide channel and attaches to mitochondria as a result of its intense contact with the negatively charged membrane of mitochondria. This dye functions as a small-molecule ligand with tumor-targeting and self-reporting features, allowing for a traceable method for organelle-targeted drug administration in vivo | 201 |
| Biogenic zinc oxide nanoparticles (ZnO NPs) with the aqueous leaf extract of Annona muricata | Unintended delivery, off-target toxicity | Biocompatible and hemocompatible NPs cause depolarization of the mitochondrial membrane potential of A549 and MOLT4 cancer cells leading to apoptosis induction in cancer cells | 202 |
7. Enhancing functional efficiency in cancer treatment through mitochondrial-targeted multifunctional nanoplatforms
Multifunctional nanoparticles are a new and advanced way to treat cancer. They combine different functions into a single carrier to improve tumor management by blocking, tracking, and changing the microenvironment. Rooted in organic, inorganic, or biomimetic backbones, these nanoparticles provide platforms for diverse functional attachments. Challenges encompass streamlining formulation, ensuring cooperation among functions, and yielding simplicity in preparation.203–205 Despite these limitations, recent research has yielded promising multifunctional nanoparticles with substantial potential for various aspects of cancer treatment.206,207 Additionally, nanoparticle-based anti-cancer platforms exhibit novel and efficacious therapeutic approaches characterized by reduced invasiveness and toxicity. Their elevated water solubility eliminates the need for toxic organic solvents, while controlled release, guided by pH or external stimuli, mitigates premature drug detachment as well as lessens systemic toxicity.208–211 Building upon the multifunctional potential of nanoparticles in cancer therapy outlined in the afore-mentioned paragraph, recent advancements have led to specific breakthroughs in targeted drug delivery. Notably, Fang et al. (2019) achieved significant progress by developing pH-responsive charge-reversal nanoparticles, referred to as B6-oHA-SS-Ber, which offer a refined approach to enhancing the efficacy of anticancer drug delivery. They created these nanoparticles for targeted anticancer drug delivery to mitochondria. Through conjugation with vitamin B6 and berberine, these nanoparticles self-assembled into micelles with a hydrodynamic diameter of 173 ± 13 nm. In vitro and in vivo assessments confirmed their superior cytotoxicity, uptake, lysosomal escape, mitochondrial distribution, and tumor growth inhibition compared to alternative formulations.191Docetaxel (DTX) is a potent anticancer agent used broadly but hindered by solubility and toxicity issues. Recent advancements in drug delivery systems (DDS) and nanotechnology have tackled these challenges. Innovative DTX DDS aim to enhance solubility, minimize dose-dependent effects, and improve targeted tumor delivery, resulting in better biodistribution and retention. Advancements in drug delivery have transformed cancer therapy, addressing challenges associated with potent agents like docetaxel (DTX). These innovations improve solubility, minimize toxicity, and enhance targeted treatment, as exemplified by synergistic approaches in specific cancers.212 Chen et al. (2020) found that standard docetaxel-based chemotherapy for prostate cancer faces challenges such as nonspecific targeting, drug resistance, and adverse effects. To address this, synergistic combinations with natural compounds like epigallocatechin-3-gallate (EGCG) have been explored. Yet, poor bioavailability and distribution limit success. A solution emerges through TPGS-conjugated hyaluronic acid and fucoidan-based nanoparticles, encapsulating EGCG and docetaxel. These nanoparticles stop the G2/M cell cycle and stop tumor growth in living things by following pH-sensitive release and cancer cell recognition. This multifunctional nanoparticle system enhances drug synergy, revealing promising potential for prostate cancer treatment.213
Targeting mitochondria offers a promising strategy to mitigate tumor hypoxia, as mitochondrial inhibitors have been shown to reduce the oxygen requirements of tumor cells.214 Lv et al. (2022) introduced a novel strategy to enhance cancer treatment by overcoming the limitations of existing hypoxia-based therapies. A specialized nanoplatform was developed, combining photodynamic therapy, photothermal therapy, and hypoxia-activated chemotherapy for more effective cancer treatment. The nanoplatform utilizes polydopamine-coated hollow copper sulfide nanoparticles to deliver a hypoxia-activated prodrug (TH302). Through targeted accumulation in mitochondria, the nanoplatform maximizes drug efficacy and avoids resistance issues. By using specific lasers, the nanoplatform generates reactive oxygen species and increases hypoxia, amplifying the anti-tumor effects of TH302. Both in vitro and in vivo experiments demonstrate significantly improved anti-cancer activity compared to traditional hypoxia-related treatments. This innovative approach highlights the potential of combining hypoxia-activated drugs with phototherapy for synergistic cancer therapy.215 In conclusion, these studies provide compelling evidence for the efficacy of multifunctional nanoparticles in revolutionizing cancer treatment, offering improved drug delivery, reduced toxicity, and enhanced therapeutic outcomes.
8. Conclusions
In the sphere of cancer therapy, the utilization of nanoparticles as targeted delivery systems has unlocked remarkable potential for precise and effective treatments. Being able to get through cell barriers like the plasma membrane and lysosomal compartments while also taking advantage of the unique traits of cancer cells, like how their mitochondrial functions have changed, could revolutionize the way we treat cancer. The development of mitochondria-targeting nanoparticles has led to a new era of therapeutic strategies, one that addresses challenges across different dimensions of cancer treatment.Adding nanoparticles to cancer treatment not only makes drugs more bioavailable, but it also makes it possible for drugs to be released in a controlled and responsive way inside cells. These nanoparticles deliver drugs precisely while avoiding breaking down too quickly by using pH and ROS levels that are already present in the tumor. By carefully releasing drugs into mitochondria, which is where apoptosis and energy regulation happen in cells, cancer treatments can work much better while being less harmful to healthy tissues. Additionally, nanoparticles' ability to combat tumor hypoxia, a formidable obstacle in cancer treatment, is evidence of their versatility. Therapies like PDT that would otherwise be ineffective can now target mitochondria and produce oxygen inside these organelles. Nanoparticles have demonstrated their potential not only in inducing apoptosis but also in addressing the complexity of cancer heterogeneity, targeting cancer stem cells, and overcoming intrinsic and adaptive drug resistance.
The fusion of multifunctionality and nanoparticle technology heralds a new frontier in cancer treatment. Nanoparticles equipped with multiple functionalities offer a holistic approach to therapy by suppressing tumors, tracking treatment responses, and modulating the tumor microenvironment. The integration of pH-responsive charge-reversal systems, synergistic drug combinations, and hypoxia-activated therapies showcases the power of nanoparticles to revolutionize cancer treatment strategies, resulting in improved outcomes and reduced side effects.
Future prospects
Looking ahead, the landscape of cancer therapy holds immense promise with the continued advancement of nanoparticle-based approaches. As the field evolves, there are several key avenues for further exploration and development. First and foremost, it is imperative to translate these promising strategies from laboratory research to clinical applications through extensive preclinical studies and subsequent clinical trials that validate their safety, efficacy, and long-term impact on human patients. Additionally, the concept of personalized medicine emerges as a significant frontier, with the potential to optimize treatment outcomes by tailoring nanoparticle therapies to individual variations in cancer types and patient responses. Combination therapies, harnessing the synergistic potential of nanoparticle-based modalities, warrant thorough exploration as they could provide enhanced therapeutic effects while circumventing resistance mechanisms. Understanding the biodistribution, clearance, and fate of nanoparticles in the body is crucial for their safe and effective utilization. Furthermore, investigating the immunomodulatory effects of nanoparticles offers an exciting avenue to enhance the body's immune response against cancer, potentially leading to innovative immunotherapeutic strategies. The integration of multimodal imaging capabilities for real-time monitoring and diagnosis can provide comprehensive insights into treatment progress. Lastly, ensuring the biocompatibility and long-term toxicity profiles of nanoparticles is paramount for patient safety and successful clinical translation. In conclusion, the convergence of nanoparticle technology and cancer therapy promises to reshape the landscape of treatment by leveraging precision, versatility, and adaptability to the tumor microenvironment, offering a hopeful trajectory towards improved outcomes and a brighter future for patients globally.Author contributions
Shivani R. Pandya: conceptualization, supervision, review & editing; Harjeet Singh: conceptualization, writing – original draft, writing – review & editing; Martin F. Desimone: review & editing; Jagpreet Singh: review & editing; Noble George: writing – original draft, writing – review & editing; and Srushti Jasani: visualization.Conflicts of interest
There are no conflicts to declare.Notes and references
- Nat. Cancer, 2020, 1, 1–2 Search PubMed.
- J. Ferlay, M. Colombet, I. Soerjomataram, D. M. Parkin, M. Piñeros, A. Znaor and F. Bray, Int. J. Cancer, 2021, 149, 778–789 CrossRef CAS PubMed.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef CAS PubMed.
- P. Rawla, World J. Oncol., 2019, 10, 63–89 CrossRef CAS PubMed.
- H. Erkko, B. Xia, J. Nikkilä, J. Schleutker, K. Syrjäkoski, A. Mannermaa, A. Kallioniemi, K. Pylkäs, S. M. Karppinen, K. Rapakko, A. Miron, Q. Sheng, G. Li, H. Mattila, D. W. Bell, D. A. Haber, M. Grip, M. Reiman, A. Jukkola-Vuorinen, A. Mustonen, J. Kere, L. A. Aaltonen, V. M. Kosma, V. Kataja, Y. Soini, R. I. Drapkin, D. M. Livingston and R. Winqvist, Nature, 2007, 446, 316–319 CrossRef CAS PubMed.
- K. Zhaurova, Nat. Educ., 2008, 1, 49 Search PubMed.
- K. Powell, J. Marquart, T. Olivier and V. Prasad, Future Oncol., 2022, 18, 3955–3959 CrossRef CAS PubMed.
- J. He, H. J. Lee, S. Saha, D. Ruan, H. Guo and C. H. Chan, Cell Death Dis., 2019, 10, 1–16 Search PubMed.
- E. Huynh, A. Hosny, C. Guthier, D. S. Bitterman, S. F. Petit, D. A. Haas-Kogan, B. Kann, H. J. W. L. Aerts and R. H. Mak, Nat. Rev. Clin. Oncol., 2020, 17, 771–781 CrossRef PubMed.
- K. I. Takayama, T. Honma, T. Suzuki, Y. Kondoh, H. Osada, Y. Suzuki, M. Yoshida and S. Inoue, Cancer Res., 2021, 81, 3495–3508 CrossRef CAS PubMed.
- M. Huang, Y. Lin, C. Wang, L. Deng, M. Chen, Y. G. Assaraf, Z. S. Chen, W. Ye and D. Zhang, Drug Resistance Updates, 2022, 64, 100849 CrossRef CAS PubMed.
- D. T. Debela, S. G. Y. Muzazu, K. D. Heraro, M. T. Ndalama, B. W. Mesele, D. C. Haile, S. K. Kitui and T. Manyazewal, SAGE Open Med., 2021, 9 DOI:10.1177/20503121211034366.
- L. Kraehenbuehl, C. H. Weng, S. Eghbali, J. D. Wolchok and T. Merghoub, Nat. Rev. Clin. Oncol., 2022, 19, 37–50 CrossRef CAS PubMed.
- M. Mohsenzadegan, R. W. Peng and R. Roudi, J. Cell. Physiol., 2020, 235, 74–86 CrossRef CAS PubMed.
- S. Charmsaz, D. M. Collins, A. S. Perry and M. Prencipe, Cancers, 2019 DOI:10.3390/cancers11081125.
- S. Gavas, S. Quazi and T. M. Karpiński, Nanoscale Res. Lett., 2021, 16, 1–21 CrossRef PubMed.
- F. Wirsdörfer, S. De Leve and V. Jendrossek, Int. J. Mol. Sci., 2019, 20, 24 CrossRef PubMed.
- Y. P. Liu, C. C. Zheng, Y. N. Huang, M. L. He, W. W. Xu and B. Li, MedComm, 2021, 2, 315–340 CrossRef CAS PubMed.
- C. Rébé and F. Ghiringhelli, Future Oncol., 2015, 11, 2645–2654 CrossRef PubMed.
- A. C. Begg, F. A. Stewart and C. Vens, Nat. Rev. Cancer, 2011, 11, 239–253 CrossRef CAS PubMed.
- J. Hrkach and R. Langer, Drug Delivery Transl. Res., 2020, 10, 567–570 CrossRef PubMed.
- E. S. Ali, S. M. Sharker, M. T. Islam, I. N. Khan, S. Shaw, M. A. Rahman, S. J. Uddin, M. C. Shill, S. Rehman, N. Das, S. Ahmad, J. A. Shilpi, S. Tripathi, S. K. Mishra and M. S. Mubarak, Semin. Cancer Biol., 2021, 69, 52–68 CrossRef CAS PubMed.
- I. Khan, K. Saeed and I. Khan, Arab. J. Chem., 2019, 12, 908–931 CrossRef CAS.
- T. Yokoyama, H. Masuda, M. Suzuki, K. Ehara, K. Nogi, M. Fuji, T. Fukui, H. Suzuki, J. Tatami, K. Hayashi and K. Toda, Nanopart. Technol. Handb., 2008, 3–48 Search PubMed.
- H. Singh, M. F. Desimone, S. Pandya, S. Jasani, N. George, M. Adnan, A. Aldarhami, A. S. Bazaid and S. A. Alderhami, Int. J. Nanomed., 2023, 18, 4727–4750 CrossRef CAS PubMed.
- M. V. Liberti and J. W. Locasale, Trends Biochem. Sci., 2016, 41, 211–218 CrossRef CAS PubMed.
- M. G. V. Heiden, L. C. Cantley and C. B. Thompson, Science, 2009, 324, 1029–1033 CrossRef PubMed.
- L. Frattaruolo, M. Brindisi, R. Curcio, F. Marra, V. Dolce and A. R. Cappello, Int. J. Mol. Sci., 2020, 21, 1–21 Search PubMed.
- Q. Li and Y. Huang, J. Pharm. Invest., 2020, 50, 271–293 CrossRef.
- N. Alrushaid, F. A. Khan, E. A. Al-Suhaimi and A. Elaissari, Pharmaceutics, 2023, 15, 1025 CrossRef CAS PubMed.
- S. Buchke, M. Sharma, A. Bora, M. Relekar, P. Bhanu and J. Kumar, Life, 2022, 12, 657 CrossRef CAS PubMed.
- T. A. Tabish and M. R. Hamblin, Biomater. Biosyst., 2021, 3, 100023 CAS.
- S. Wen, D. Zhu and P. Huang, Future Med. Chem., 2013, 5, 53–67 CrossRef CAS PubMed.
- S. Pustylnikov, F. Costabile, S. Beghi and A. Facciabene, Transl. Res., 2018, 202, 35–51 CrossRef CAS PubMed.
- J. Lopez and S. W. G. Tait, Br. J. Cancer, 2015, 112, 957–962 CrossRef CAS PubMed.
- E. Ortega-Forte, A. Rovira, A. Gandioso, J. Bonelli, M. Bosch, J. Ruiz and V. Marchán, J. Med. Chem., 2021, 64, 17209–17220 CrossRef CAS PubMed.
- Q. T. Hoang, K. A. Huynh, T. G. N. Cao, J. H. Kang, X. N. Dang, V. Ravichandran, H. C. Kang, M. Lee, J. E. Kim, Y. T. Ko, T. Il Lee and M. S. Shim, Adv. Mater., 2023, 35, 2300437 CrossRef PubMed.
- N. Li, L. Yu, J. Wang, X. Gao, Y. Chen, W. Pan and B. Tang, Chem. Sci., 2018, 9, 3159–3164 RSC.
- G. Battogtokh, Y. S. Choi, D. S. Kang, S. J. Park, M. S. Shim, K. M. Huh, Y. Y. Cho, J. Y. Lee, H. S. Lee and H. C. Kang, Acta Pharm. Sin. B, 2018, 8, 862–880 CrossRef PubMed.
- Y. H. Lee, H. I. Park, W. S. Chang and J. S. Choi, Int. J. Biol. Macromol., 2021, 167, 35–45 CrossRef CAS PubMed.
- L. F. Yousif, K. M. Stewart and S. O. Kelley, ChemBioChem, 2009, 10, 1939–1950 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- L. F. Dong, V. J. A. Jameson, D. Tilly, L. Prochazka, J. Rohlena, K. Valis, J. Truksa, R. Zobalova, E. Mahdavian, K. Kluckova, M. Stantic, J. Stursa, R. Freeman, P. K. Witting, E. Norberg, J. Goodwin, B. A. Salvatore, J. Novotna, J. Turanek, M. Ledvina, P. Hozak, B. Zhivotovsky, M. J. Coster, S. J. Ralph, R. A. J. Smith and J. Neuzil, Free Radical Biol. Med., 2011, 50, 1546–1555 CrossRef CAS PubMed.
- X. Cheng, D. Feng, J. Lv, X. Cui, Y. Wang, Q. Wang and L. Zhang, Cancers, 2023, 15, 666 CrossRef CAS PubMed.
- J. Wu, J. Li, H. Wang and C. B. Liu, Expert Opin. Drug Delivery, 2018, 15, 951–964 CrossRef CAS PubMed.
- Z. Zeng, C. Fang, Y. Zhang, C. X. Chen, Y. F. Zhang and K. Zhang, Front. Bioeng. Biotechnol., 2021, 9, 784602 CrossRef PubMed.
- J. Qin, N. Gong, Z. Liao, S. Zhang, P. Timashev, S. Huo and X. J. Liang, Nanoscale, 2021, 13, 7108–7118 RSC.
- M. T. Jeena, S. Kim, S. Jin and J. H. Ryu, Cancers, 2020, 12 Search PubMed.
- J. C. Xin and R. A. Butow, Nat. Rev. Genet., 2005, 6, 815–825 CrossRef PubMed.
- C. Schiliro and B. L. Firestein, Cells, 2021, 10, 1056 CrossRef CAS PubMed.
- D. J. Hanson, S. Nakamura, R. Amachi, M. Hiasa, A. Oda, D. Tsuji, K. Itoh, T. Harada, K. Horikawa, J. Teramachi, H. Miki, T. Matsumoto and M. Abe, Oncotarget, 2015, 6, 33568–33586 CrossRef PubMed.
- M. D. Forrest, bioRxiv, 2015, preprint DOI:10.1101/025197, pp. 1–42.
- S. S. Sabharwal and P. T. Schumacker, Nat. Rev. Cancer, 2014, 14, 709–721 CrossRef CAS PubMed.
- B. Niu, K. Liao, Y. Zhou, T. Wen, G. Quan, X. Pan and C. Wu, Biomaterials, 2021, 277, 121110 CrossRef CAS PubMed.
- J. Jiang, Y. Jiang, Y. G. Zhang, T. Zhang, J. H. Li, D. L. Huang, J. Hou, M. Y. Tian, L. Sun, X. M. Su, Y. Dong and Y. Y. Ma, Transl. Cancer Res., 2021, 10, 817–826 CrossRef CAS PubMed.
- Y. Zhang, C. Zhang, J. Chen, L. Liu, M. Hu, J. Li and H. Bi, ACS Appl. Mater. Interfaces, 2017, 9, 25152–25163 CrossRef CAS PubMed.
- H. M. Begum, C. Mariano, H. Zhou and K. Shen, Cancers, 2021, 13, 5054 CrossRef CAS PubMed.
- P. E. Porporato, N. Filigheddu, J. M. B. S. Pedro, G. Kroemer and L. Galluzzi, Cell Res., 2018, 28, 265–280 CrossRef CAS PubMed.
- Z. Wang, W. Guo, X. Kuang, S. Hou and H. Liu, Asian J. Pharm. Sci., 2017, 12, 498–508 CrossRef PubMed.
- A. S. N. Murthy, S. Das, T. Singh, T. W. Kim, N. Sepay, S. Jeon and J. Im, Drug Delivery, 2022, 29, 270–283 CrossRef CAS PubMed.
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin. Oncol., 2018, 15, 81–94 CrossRef CAS PubMed.
- M. Janiszewska, Oncogene, 2020, 39, 2031–2039 CrossRef CAS PubMed.
- L. Roizin-Towle and J. P. Pirro, Int. J. Radiat. Oncol., Biol., Phys., 1991, 20, 751–756 CrossRef CAS PubMed.
- J. L. Huang, H. Z. Chen and X. L. Gao, J. Drug Targeting, 2018, 26, 398–406 CrossRef CAS PubMed.
- E. Blanco, H. Shen and M. Ferrari, Nat. Biotechnol., 2015, 33, 941–951 CrossRef CAS PubMed.
- N. Hoshyar, S. Gray, H. Han and G. Bao, Nanomedicine, 2016, 11, 673–692 CrossRef CAS PubMed.
- L. Kou, Y. D. Bhutia, Q. Yao, Z. He, J. Sun and V. Ganapathy, Front. Pharmacol., 2018, 9, 27 CrossRef PubMed.
- Y. Hui, X. Yi, F. Hou, D. Wibowo, F. Zhang, D. Zhao, H. Gao and C. X. Zhao, ACS Nano, 2019, 13, 7410–7424 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1 Search PubMed.
- M. Z. Noman, M. Hasmim, A. Lequeux, M. Xiao, C. Duhem, S. Chouaib, G. Berchem and B. Janji, Cells, 2019, 8, 1083 CrossRef CAS PubMed.
- R. Hamaguchi, S. Uemoto and H. Wada, Front. Oncol., 2023, 13, 1223025 CrossRef PubMed.
- E. P. Spugnini, P. Sonveaux, C. Stock, M. Perez-Sayans, A. De Milito, S. Avnet, A. G. Garcìa, S. Harguindey and S. Fais, Biochim. Biophys. Acta, Biomembr., 2015, 1848, 2715–2726 CrossRef CAS PubMed.
- R. A. Cardone, V. Casavola and S. J. Reshkin, Nat. Rev. Cancer, 2005, 5, 786–795 CrossRef CAS PubMed.
- R. J. Gillies, Clin. Exp. Metastasis, 2022, 39, 15–19 CrossRef CAS PubMed.
- A. Parodi, P. Buzaeva, D. Nigovora, A. Baldin, D. Kostyushev, V. Chulanov, L. V. Savvateeva and A. A. Zamyatnin, J. Nanobiotechnol., 2021, 19, 1–19 CrossRef PubMed.
- P. Jin, J. Jiang, L. Zhou, Z. Huang, E. C. Nice, C. Huang and L. Fu, J. Hematol. Oncol., 2022, 15, 1–42 CrossRef PubMed.
- M. Kim, J. Gwak, S. Hwang, S. Yang and S. M. Jeong, Oncogene, 2019, 38, 4729–4738 CrossRef CAS PubMed.
- V. Eisner, M. Picard and G. Hajnóczky, Nat. Cell Biol., 2018, 20, 755–765 CrossRef CAS PubMed.
- Y. Han, B. Kim, U. Cho, I. S. Park, S. I. Kim, D. N. Dhanasekaran, B. K. Tsang and Y. S. Song, Oncogene, 2019, 38, 7089–7105 CrossRef CAS PubMed.
- L. Xie, F. Shi, Y. Li, W. Li, X. Yu, L. Zhao, M. Zhou, J. Hu, X. Luo, M. Tang, J. Fan, J. Zhou, Q. Gao, W. Wu, X. Zhang, W. Liao, A. M. Bode and Y. Cao, Signal Transduction Targeted Ther., 2020, 5, 56, DOI:10.1038/s41392-020-0151-9.
- C. H. Xu, P. J. Ye, Y. C. Zhou, D. X. He, H. Wei and C. Y. Yu, Acta Biomater., 2020, 105, 1–14 CrossRef CAS PubMed.
- T. Kim and T. Hyeon, Nanotechnology, 2014, 25, 012001 CrossRef CAS PubMed.
- H. Sabit, M. Abdel-Hakeem, T. Shoala, S. Abdel-Ghany, M. M. Abdel-Latif, J. Almulhim and M. Mansy, Pharmaceutics, 2022, 14, 1566 CrossRef CAS PubMed.
- G. Chen, Y. Qian, H. Zhang, A. Ullah, X. He, Z. Zhou, H. Fenniri and J. Shen, Appl. Mater. Today, 2021, 23, 101003 CrossRef.
- R. Mitarotonda, E. Giorgi, T. Eufrasio-da-Silva, A. Dolatshahi-Pirouz, Y. K. Mishra, A. Khademhosseini, M. F. Desimone, M. De Marzi and G. Orive, Biomater. Adv., 2022, 135, 212726 CrossRef CAS PubMed.
- R. Mitarotonda, M. Saraceno, M. Todone, E. Giorgi, E. L. Malchiodi, M. F. Desimone and M. C. De Marzi, Ther. Delivery, 2021, 12, 443–459 CrossRef CAS PubMed.
- M. C. De Marzi, M. Saraceno, R. Mitarotonda, M. Todone, M. Fernandez, E. L. Malchiodi and M. F. Desimone, Ther. Delivery, 2017, 8, 1035–1049 CrossRef CAS PubMed.
- M. A. Khan, D. Singh, A. Ahmad and H. R. Siddique, Eur. J. Pharm. Sci., 2021, 164, 105892 CrossRef CAS PubMed.
- D. Chenthamara, S. Subramaniam, S. G. Ramakrishnan, S. Krishnaswamy, M. M. Essa, F. H. Lin and M. W. Qoronfleh, Biomater. Res., 2019, 23, 1–29 CrossRef PubMed.
- Y. Huang and Y. Li, Mol. Pharmaceutics, 2014, 11, 2493–2494 CrossRef CAS PubMed.
- W. H. Talib, A. R. Alsayed, M. Barakat, M. I. Abu-Taha and A. I. Mahmod, Biomedicines, 2021, 9, 1353 CrossRef CAS PubMed.
- M. Kartal-Yandim, A. Adan-Gokbulut and Y. Baran, Crit. Rev. Biotechnol., 2016, 36, 716–726 CrossRef CAS PubMed.
- S. Kapse-Mistry, T. Govender, R. Srivastava and M. Yergeri, Front. Pharmacol., 2014, 5 JUL, 73435 Search PubMed.
- M. Labib and S. O. Kelley, Mol. Oncol., 2021, 15, 1622–1646 CrossRef CAS PubMed.
- K. Bukowski, M. Kciuk and R. Kontek, Int. J. Mol. Sci., 2020, 21, 3233 CrossRef CAS PubMed.
- H. Wang, H. Yin, F. Yan, M. Sun, L. Du, W. Peng, Q. Li, Y. Feng and Y. Zhou, Oncotarget, 2015, 6, 2827–2842 CrossRef PubMed.
- K. S. Allemailem, A. Almatroudi, M. A. Alsahli, A. Aljaghwani, A. M. El-Kady, A. H. Rahmani and A. A. Khan, Int. J. Nanomed., 2021, 16, 3907–3936 CrossRef PubMed.
- R. J. Morris and M. Massi, Advances in Inorganic Chemistry, Academic Press, 2022, vol. 80, pp. 411–509 Search PubMed.
- J. Halder, D. Pradhan, B. Kar, G. Ghosh and G. Rath, Nanomedicine, 2022, 40, 102494 CrossRef CAS PubMed.
- E. L. Giddings, D. P. Champagne, M. H. Wu, J. M. Laffin, T. M. Thornton, F. Valenca-Pereira, R. Culp-Hill, K. A. Fortner, N. Romero, J. East, P. Cao, H. Arias-Pulido, K. S. Sidhu, B. Silverstrim, Y. Kam, S. Kelley, M. Pereira, S. E. Bates, J. Y. Bunn, S. N. Fiering, D. E. Matthews, R. W. Robey, D. Stich, A. D’Alessandro and M. Rincon, Nat. Commun., 2021, 12, 1–19 CrossRef PubMed.
- J. Tuo, Y. Xie, J. Song, Y. Chen, Q. Guo, X. Liu, X. Ni, D. Xu, H. Huang, S. Yin, W. Zhu, J. Wu and H. Hu, J. Mater. Chem. B, 2016, 4, 6856–6864 RSC.
- H. Chen, J. Tian, D. Liu, W. He and Z. Guo, J. Mater. Chem. B, 2017, 5, 972–979 RSC.
- Y. Deng, F. Jia, X. Chen, Q. Jin and J. Ji, Small, 2020, 16, 2001747, DOI:10.1002/smll.202001747.
- P. Gupta, Y. R. Neupane, M. Aqil, K. Kohli and Y. Sultana, Drug Delivery Transl. Res., 2023, 1–28 Search PubMed.
- H. Chen, Y. Wang, Y. Yao, S. Qiao, H. Wang and N. Tan, Theranostics, 2017, 7, 3781–3793 CrossRef CAS PubMed.
- X. C. Zhong, M. H. Shi, H. N. Liu, J. J. Chen, T. T. Wang, M. T. Lin, Z. T. Zhang, Y. Zhou, Y. Y. Lu, W. H. Xu, J. Q. Gao, D. H. Xu, M. Han and Y. D. Chen, Pharm. Dev. Technol., 2021, 26, 21–29 CrossRef CAS PubMed.
- Y. Cheng and Y. Ji, J. Controlled Release, 2020, 318, 38–49 CrossRef CAS PubMed.
- Y. Wu, D. Zhong, Y. Li, H. Wu, X. Xu, J. Yang and Z. Gu, Adv. Healthcare Mater., 2020, 9, 1901739, DOI:10.1002/adhm.201901739.
- L. Xi, J. Wang, Y. Wang and Z. Ge, Macromol. Biosci., 2021, 21, 2100091, DOI:10.1002/mabi.202100091.
- H. Wang, F. Zhang, H. Wen, W. Shi, Q. Huang, Y. Huang, J. Xie, P. Li, J. Chen, L. Qin and Y. Zhou, J. Nanobiotechnol., 2020, 18, 1–21, DOI:10.1186/s12951-019-0562-3.
- S. Senapati, A. K. Mahanta, S. Kumar and P. Maiti, Signal Transduction Targeted Ther., 2018, 3, 7 CrossRef PubMed.
- D. Zhang, L. Wen, R. Huang, H. Wang, X. Hu and D. Xing, Biomaterials, 2018, 153, 14–26 CrossRef CAS PubMed.
- M. Ashrafizadeh, M. Delfi, A. Zarrabi, A. Bigham, E. Sharifi, N. Rabiee, A. C. Paiva-Santos, A. P. Kumar, S. C. Tan, K. Hushmandi, J. Ren, E. N. Zare and P. Makvandi, J. Controlled Release, 2022, 351, 50–80 CrossRef CAS PubMed.
- H. Xiong, S. Du, J. Ni, J. Zhou and J. Yao, Biomaterials, 2016, 94, 70–83 CrossRef CAS PubMed.
- Y. Qin, Z. Wang, X. Wang, T. Zhang, Y. Hu, D. Wang, H. Sun, L. Zhang and Y. Zhu, Mater. Today Adv., 2023, 17, 100328 CrossRef CAS.
- H. Cho, Y. Y. Cho, M. S. Shim, J. Y. Lee, H. S. Lee and H. C. Kang, Biochim. Biophys. Acta, Mol. Basis Dis., 2020, 1866, 165808 CrossRef CAS PubMed.
- X. Jing, H. Hu, Y. Sun, B. Yu, H. Cong and Y. Shen, Small Methods, 2022, 6, 2101437 CrossRef CAS PubMed.
- P. Decuzzi and A. B. Cook, ACS Nano, 2021, 15, 2068–2098 CrossRef PubMed.
- Y. Tan, Y. Zhu, L. Wen, X. Yang, X. Liu, T. Meng, S. Dai, Y. Ping, H. Yuan and F. Hu, Theranostics, 2019, 9, 691–707 CrossRef CAS PubMed.
- Y. Tan, Y. Zhu, Y. Zhao, L. Wen, T. Meng, X. Liu, X. Yang, S. Dai, H. Yuan and F. Hu, Biomaterials, 2018, 154, 169–181 CrossRef CAS PubMed.
- X. Li, Y. Zhao, T. Zhang and D. Xing, Adv. Healthcare Mater., 2021, 10, 2001240 CrossRef CAS PubMed.
- N. R. Stillman, M. Kovacevic, I. Balaz and S. Hauert, npj Comput. Mater., 2020, 6, 92, DOI:10.1038/S41524-020-00366-8.
- B. Dutta, K. C. Barick and P. A. Hassan, Adv. Colloid Interface Sci., 2021, 296, 102509 CrossRef CAS PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- J. W. Rasmussen, E. Martinez, P. Louka and D. G. Wingett, Expert Opin. Drug Delivery, 2010, 7, 1063–1077 CrossRef CAS PubMed.
- C. Han, C. Zhang, T. Ma, C. Zhang, J. Luo, X. Xu, H. Zhao, Y. Chen and L. Kong, Acta Biomater., 2018, 77, 268–281 CrossRef CAS PubMed.
- Z. Xu, X. Chen, Z. Sun, C. Li and B. Jiang, Mater. Today Chem., 2019, 12, 240–260 CrossRef CAS.
- J. J. Wu, J. Zhang, C. Y. Xia, K. Ding, X. X. Li, X. G. Pan, J. K. Xu, J. He and W. K. Zhang, Phytomedicine, 2023, 111 Search PubMed.
- A. Bajpai, N. N. Desai, S. Pandey, C. Shukla, B. Datta and S. Basu, ACS Appl. Bio Mater., 2021, 4, 6799–6806 CrossRef CAS PubMed.
- B. Banik, A. Ashokan, J. H. Choi, B. Surnar and S. Dhar, Dalton Trans., 2023, 52, 3575–3585 RSC.
- J. Xue, D. Duosiken, S. Zhong, J. J. Cao, L. Y. Hu, K. Sun, K. Tao and S. J. Pan, Acta Biomater., 2021, 131, 508–518 CrossRef CAS PubMed.
- D. Y. Cho, H. Cho, K. Kwon, M. Yu, E. Lee, K. M. Huh, D. H. Lee and H. C. Kang, Adv. Funct. Mater., 2015, 25, 5479–5491 CrossRef CAS.
- L. T. Thuy, S. Lee, V. Dongquoc and J. S. Choi, Antioxidants, 2023, 12, 437 CrossRef CAS PubMed.
- S. Kianamiri, A. Dinari, M. Sadeghizadeh, M. Rezaei, B. Daraei, N. E. H. Bahsoun, A. Nomani, M. Sadeghizadeh and A. Nomani, Mol. Pharmaceutics, 2020, 17, 4483–4498 CrossRef CAS PubMed.
- Z. Yang, J. Wang, S. Liu, X. Li, L. Miao, B. Yang, C. Zhang, J. He, S. Ai and W. Guan, Biomaterials, 2020, 229, 119580 CrossRef CAS PubMed.
- H. Tong, Y. Gao, J. Li, J. Li, D. Huang, J. Shi, H. A. Santos and B. Xia, Part. Part. Syst. Charact., 2021, 38, 2100013 CrossRef CAS.
- J. Shen, T. W. Rees, Z. Zhou, S. Yang, L. Ji and H. Chao, Biomaterials, 2020, 251, 120079 CrossRef CAS PubMed.
- T. Hompland, C. S. Fjeldbo and H. Lyng, Cancers, 2021, 13, 1–23 CrossRef PubMed.
- B. Muz, P. de la Puente, F. Azab and A. K. Azab, Hypoxia, 2015, 3, 83 CrossRef PubMed.
- K. Graham and E. Unger, Int. J. Nanomed., 2018, 13, 6049–6058 CrossRef CAS PubMed.
- H. Wang, J. Li, Y. Wang, X. Gong, X. Xu, J. Wang, Y. Li, X. Sha and Z. Zhang, J. Controlled Release, 2020, 319, 25–45 CrossRef CAS PubMed.
- C. Ruan, K. Su, D. Zhao, A. Lu and C. Zhong, Front. Chem., 2021, 9, 649158 CrossRef CAS PubMed.
- M. M. T. Jansman and L. Hosta-Rigau, Adv. Colloid Interface Sci., 2018, 260, 65–84 CrossRef CAS PubMed.
- J. Hu, Q. Wang, Y. Wang, G. You, P. Li, L. Zhao and H. Zhou, J. Colloid Interface Sci., 2020, 571, 326–336 CrossRef CAS PubMed.
- Y. Jia, L. Duan and J. Li, Adv. Mater., 2016, 28, 1312–1318 CrossRef CAS PubMed.
- Z. Yang, J. Wang, S. Ai, J. Sun, X. Mai and W. Guan, Theranostics, 2019, 9, 6809–6823 CrossRef CAS PubMed.
- P. Dong, J. Hu, S. Yu, Y. Zhou, T. Shi, Y. Zhao, X. Wang and X. Liu, Small Methods, 2021, 5, 2100581 CrossRef CAS PubMed.
- F. Fouladi, K. J. Steffen and S. Mallik, Bioconjugate Chem., 2017, 28, 857–868 CrossRef CAS PubMed.
- A. Salvatore, C. Montis, D. Berti and P. Baglioni, ACS Nano, 2016, 10, 7749–7760 CrossRef CAS PubMed.
- Y. Feng, N. X. Li, H. L. Yin, T. Y. Chen, Q. Yang and M. Wu, Mol. Pharmaceutics, 2019, 16, 422–436 CrossRef CAS PubMed.
- C. Li, J. Wang, Y. Wang, H. Gao, G. Wei, Y. Huang, H. Yu, Y. Gan, Y. Wang, L. Mei, H. Chen, H. Hu, Z. Zhang and Y. Jin, Acta Pharm. Sin. B, 2019, 9, 1145–1162 CrossRef PubMed.
- W. Xue, Y. Liu, N. Zhang, Y. Yao, P. Ma, H. Wen, S. Huang, Y. Luo and H. Fan, Int. J. Nanomed., 2018, 13, 5719–5731 CrossRef CAS PubMed.
- P. Gupta, K. A. Jani, D. H. Yang, M. Sadoqi, E. Squillante and Z. S. Chen, Expert Opin. Drug Metab. Toxicol., 2016, 12, 281–289 CrossRef CAS PubMed.
- S. Kumar, S. Singh, S. Senapati, A. P. Singh, B. Ray and P. Maiti, Int. J. Biol. Macromol., 2017, 104, 487–497 CrossRef CAS PubMed.
- J. Di, X. Gao, Y. Du, H. Zhang, J. Gao and A. Zheng, Asian J. Pharm. Sci., 2021, 16, 444–458 CrossRef PubMed.
- M. Merhi, C. Y. Dombu, A. Brient, J. Chang, A. Platel, F. Le Curieux, D. Marzin, F. Nesslany and D. Betbeder, International Journal of Pharmaceutics, Elsevier, 2012, vol. 423, pp. 37–44 Search PubMed.
- H. Yu, J. M. Li, K. Deng, W. Zhou, C. X. Wang, Q. Wang, K. H. Li, H. Y. Zhao and S. W. Huang, Theranostics, 2019, 9, 7033–7050 CrossRef CAS PubMed.
- W. Zhou, H. Yu, L. J. Zhang, B. Wu, C. X. Wang, Q. Wang, K. Deng, R. X. Zhuo and S. W. Huang, Nanoscale, 2017, 9, 17044–17053 RSC.
- X. X. Sun and Q. Yu, Acta Pharmacol. Sin., 2015, 36, 1219–1227 CrossRef CAS PubMed.
- D. Eisenbarth and Y. A. Wang, Oncogene, 2023, 42, 2155–2165 CrossRef CAS PubMed.
- P. R. Prasetyanti and J. P. Medema, Mol. Cancer, 2017, 16, 1–9, DOI:10.1186/S12943-017-0600-4.
- J. N. Rich, Medicine, 2016, 95, S2–S7 CrossRef CAS PubMed.
- A. K. Singh, R. K. Arya, S. Maheshwari, A. Singh, S. Meena, P. Pandey, O. Dormond and D. Datta, Int. J. Cancer, 2015, 136, 1991–2000 CrossRef CAS PubMed.
- H. Duan, Y. Liu, Z. Gao and W. Huang, Acta Pharm. Sin. B, 2021, 11, 55–70 CrossRef CAS PubMed.
- R. Loureiro, K. A. Mesquita, S. Magalhães-Novais, P. J. Oliveira and I. Vega-Naredo, Semin. Cancer Biol., 2017, 47, 18–28 CrossRef CAS PubMed.
- Y. J. Choi, S. Gurunathan and J. H. Kim, Int. J. Mol. Sci., 2018, 19, 710 CrossRef PubMed.
- L. Zhang, H. J. Yao, Y. Yu, Y. Zhang, R. J. Li, R. J. Ju, X. X. Wang, M. G. Sun, J. F. Shi and W. L. Lu, Biomaterials, 2012, 33, 565–582 CrossRef CAS PubMed.
- Y. Zhao, W. Zhao, Y. C. Lim and T. Liu, Mol. Pharmaceutics, 2019, 16, 2532–2539 CrossRef CAS PubMed.
- J. Miao, Y. Huo, G. Yao, Y. Feng, J. Weng, W. Zhao and W. Guo, Angew. Chem., Int. Ed., 2022, 61, e202201815 CrossRef CAS PubMed.
- Q. Wang, J. Xu, R. Geng, J. Cai, J. Li, C. Xie, W. Tang, Q. Shen, W. Huang and Q. Fan, Biomaterials, 2020, 231, 119671 CrossRef CAS PubMed.
- T. Qi, B. Chen, Z. Wang, H. Du, D. Liu, Q. Yin, B. Liu, Q. Zhang and Y. Wang, Biomaterials, 2019, 213, 119219 CrossRef CAS PubMed.
- C. Zarzzeka, J. Goldoni, F. Marafon, W. G. Sganzerla, T. Forster-Carneiro, M. D. Bagatini and L. M. S. Colpini, Biocatal. Agric. Biotechnol., 2023, 50 CAS.
- J. Mou, T. Lin, F. Huang, H. Chen and J. Shi, Biomaterials, 2016, 84, 13–24 CrossRef CAS PubMed.
- P. Zhang, D. Chen, L. Li and K. Sun, J. Nanobiotechnol., 2022, 20, 1–27 CrossRef PubMed.
- J. Song, C. Lin, X. Yang, Y. Xie, P. Hu, H. Li, W. Zhu and H. Hu, J. Controlled Release, 2019, 294, 27–42 CrossRef CAS PubMed.
- L. Fang, H. Lin, Z. Wu, Z. Wang, X. Fan, Z. Cheng, X. Hou and D. Chen, Carbohydr. Polym., 2020, 234, 115930 CrossRef CAS PubMed.
- Y. Huang, J. Zhang, Y. Zhang, L. Shi, X. Qin, B. Lu, Y. Ding, Y. Wang, T. Chen and Y. Yao, Mater. Des., 2021, 211, 110160 CrossRef CAS.
- X. Shi, X. Yang, M. Liu, R. Wang, N. Qiu, Y. Liu, H. Yang, J. Ji and G. Zhai, Carbohydr. Polym., 2021, 254, 117459 CrossRef CAS PubMed.
- X. Wei, L. Liu, X. Guo, Y. Wang, J. Zhao and S. Zhou, ACS Appl. Mater. Interfaces, 2018, 10, 17672–17684 CrossRef CAS PubMed.
- M. Guo, H. J. Xiang, Y. Wang, Q. L. Zhang, L. An, S. P. Yang, Y. Ma, Y. Wang and J. G. Liu, Chem. Commun., 2017, 53, 3253–3256 RSC.
- Y. M. Zhang, M. Xia, R. Ao, L. X. Gao, Y. Tang, J. H. Huang, Y. F. Luo, Z. Z. Chen, B. C. Wang and Z. Huang, Nanomaterials, 2021, 11, 2875 CrossRef CAS PubMed.
- Y. Pan, S. Zhou, C. Liu, X. Ma, J. Xing, B. Parshad, W. Li, A. Wu and R. Haag, Adv. Healthcare Mater., 2022, 11, 2102272 CrossRef CAS PubMed.
- L. Li, C. Chen, H. Liu, C. Fu, L. Tan, S. Wang, S. Fu, X. Liu, X. Meng and H. Liu, Adv. Funct. Mater., 2016, 26, 4252–4261 CrossRef CAS.
- W. Dai, Y. Deng, X. Chen, Y. Huang, H. Hu, Q. Jin, Z. Tang and J. Ji, Biomaterials, 2022, 290, 121854 CrossRef CAS PubMed.
- J. Wen, Y. Luo, H. Gao, L. Zhang, X. Wang, J. Huang, T. Shang, D. Zhou, D. Wang, Z. Wang, P. Li and Z. Wang, J. Nanobiotechnol., 2021, 19, 1–22 CrossRef PubMed.
- H. Chen, C. He, T. Chen and X. Xue, Biomater. Sci., 2020, 8, 3994–4002 RSC.
- Z. Huang, D. Li, F. Guo, T. Xian, H. S. Hu, J. Xu, Y. F. Luo, Z. Z. Chen, B. C. Wang and Y. M. Zhang, Colloids Surf., B, 2022, 220, 112956 CrossRef CAS.
- X. Li, H. Wang, Z. Li, D. Li, X. Lu, S. Ai, Y. Dong, S. Liu, J. Wu and W. Guan, Biomater. Res., 2022, 26, 1–17 CrossRef CAS PubMed.
- J. Chen, R. Zhang, C. Tao, X. Huang, Z. Chen, X. Li, J. Zhou, Q. Zeng, B. Zhao, M. Yuan, M. Ma and Z. Wu, Nanotoxicology, 2020, 14, 774–787 CrossRef CAS PubMed.
- Y. Liu, Z. Zhou, X. Lin, X. Xiong, R. Zhou, M. Zhou and Y. Huang, Biomacromolecules, 2019, 20, 3755–3766 CrossRef CAS PubMed.
- L. Fang, H. Fan, C. Guo, L. Cui, P. Zhang, H. Mu, H. Xu, F. Zhao and D. Chen, J. Biomed. Nanotechnol., 2019, 15, 2151–2163 CrossRef PubMed.
- Y. Wang, T. Zhang, C. Hou, M. Zu, Y. Lu, X. Ma, D. Jia, P. Xue, Y. Kang and Z. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 29330–29340 CrossRef CAS PubMed.
- Y. Cheng, F. Yang, K. Zhang, Y. Zhang, Y. Cao, C. Liu, H. Lu, H. Dong and X. Zhang, Adv. Funct. Mater., 2019, 29, 1903850 CrossRef CAS.
- G. Y. Pan, H. R. Jia, Y. X. Zhu, W. Sun, X. T. Cheng and F. G. Wu, ACS Appl. Nano Mater., 2018, 1, 2885–2897 CrossRef CAS.
- M. Tang, P. Zhang, J. Liu, Y. Long, Y. Cheng and H. Zheng, RSC Adv., 2020, 10, 17050–17057 RSC.
- X. Gu, T. Shu, W. Deng, C. Shen and Y. Wu, J. Mater. Chem. B, 2023, 11, 4539–4547 RSC.
- Y. Cao, T. Wu, W. Dai, H. Dong and X. Zhang, Chem. Mater., 2019, 31, 9105–9114, DOI:10.1021/acs.chemmater.9b03430.
- Y. Chen, Y. Yang, S. Du, J. Ren, H. Jiang, L. Zhang and J. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 35884–35894 CrossRef CAS PubMed.
- C. Li, W. Zhang, S. Liu, X. Hu and Z. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 30077–30084 CrossRef CAS PubMed.
- R. Ray, S. Ghosh, P. Panja and N. R. Jana, ACS Appl. Bio Mater., 2023, 6, 2338–2344, DOI:10.1021/acsabm.3c00187.
- L. Zhou, Y. Wu, Y. Luo, H. Li, X. Meng, C. Liu, J. Xiang, P. Zhang, P. Gong and L. Cai, ACS Appl. Bio Mater., 2019, 2, 5164–5173 CrossRef CAS PubMed.
- S. C. Chabattula, P. K. Gupta, S. K. Tripathi, R. Gahtori, P. Padhi, S. Mahapatra, B. K. Biswal, S. K. Singh, K. Dua, J. Ruokolainen, Y. K. Mishra, N. K. Jha, D. K. Bishi and K. K. Kesari, Mater. Today Chem., 2021, 22, 100618 CrossRef CAS.
- Q. Chen, J. Chen, Z. Yang, J. Xu, L. Xu, C. Liang, X. Han and Z. Liu, Adv. Mater., 2019, 31, 1802228, DOI:10.1002/adma.201802228.
- Q. Zhang, F. Zhang, S. Li, R. Liu, T. Jin, Y. Dou, Z. Zhou and J. Zhang, Theranostics, 2019, 9, 3732–3753 CrossRef CAS PubMed.
- S. Zhou, H. Wang, R. Li, Y. Wang, Z. Wang and L. Feng, ACS Appl. Mater. Interfaces, 2022, 14, 14087–14096 CrossRef CAS PubMed.
- B. Ortiz-Casas, A. Galdámez-Martínez, J. Gutiérrez-Flores, A. Baca Ibañez, P. Kumar Panda, G. Santana, H. A. de la Vega, M. Suar, C. Gutiérrez Rodelo, A. Kaushik, Y. Kumar Mishra and A. Dutt, Mater. Today, 2021, 50, 533–569 CrossRef CAS.
- S. Panda, S. Hajra, A. Kaushik, H. G. Rubahn, Y. K. Mishra and H. J. Kim, Mater. Today Chem., 2022, 26, 101182 CrossRef CAS.
- D. J. Bharali and S. A. Mousa, Pharmacol. Ther., 2010, 128, 324–335 CrossRef CAS PubMed.
- M. Srinivasan, M. Rajabi and S. A. Mousa, Nanomaterials, 2015, 5, 1690–1703 CrossRef CAS PubMed.
- S. R. Pandya and M. Singh, RSC Adv., 2016, 6, 37391–37402 RSC.
- S. R. Pandya, S. Patel, S. Bakshi and M. Singh, Appl. Surf. Sci., 2018, 451, 1–19 CrossRef CAS.
- N. Chaurawal and K. Raza, Heal. Sci. Rev., 2023, 7, 100101 Search PubMed.
- M. L. Chen, C. J. Lai, Y. N. Lin, C. M. Huang and Y. H. Lin, J. Mater. Chem. B, 2020, 8, 10416–10427 RSC.
- M. Benej, X. Hong, S. Vibhute, S. Scott, J. Wu, E. Graves, Q. T. Le, A. C. Koong, A. J. Giaccia, B. Yu, S. C. Chen, I. Papandreou and N. C. Denko, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 10756–10761 CrossRef CAS PubMed.
- J. Lv, S. Wang, D. Qiao, Y. Lin, S. Hu and M. Li, J. Nanobiotechnol., 2022, 20, 1–16 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |