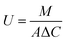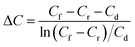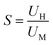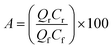Open Access Article
Open Access ArticleRemediation and recycling of inorganic acids and their green alternatives for sustainable industrial chemical processes†
Chhavi
Agarwal
*ab and
Ashok K.
Pandey‡
 *abc
*abc
aRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400085, India. E-mail: cagarwal@barc.gov.in; ashok.pandey@kccollege.edu.in; akpandey.brns@gmail.com
bHomi Bhabha National Institute, Mumbai 400094, India
cK. C. College, HSNC University, Mumbai 400020, India
First published on 7th August 2023
Abstract
The uses of inorganic acids as a solvent, leachant, and lixiviant are widespread in many commercially important industrial processes, including mining, metallurgy, metal-processing, nuclear fuel reprocessing, pickling, cleaning, leaching, etching, electroplating, metal-refining, and many others. Unchecked disposal of acidic waste into the environment poses serious threats to the flora and fauna as well as results in corrosion of metallic structures, undesired changes to the pH of soil and water, and many other problems. Because of their potentially harmful effects on the environment, acid recovery from acidic waste solutions is a crucial problem in addition to the resource preservation. Therefore, greener solvent based chemical processes have been gaining increasing attention in recent years. However, it is unclear whether the greener solvent will replace the inorganic acids in the foreseeable future. At present, developing efficient and sustainable methods for recovering and recycling inorganic acids from industrial effluents offers a co-operative approach to lessen the serious ecological disturbances, over exploitations of resources, and financial effects. The recovery and recycling of acids from acidic waste solutions has been proposed using a variety of methods such as solvent extraction, ion exchange resin, membrane technology, or hyphenated technologies which is a combination of two or more integrated technologies. The membrane technology is one option for recovering and recycling of inorganic acids that is easy to use, affordable, and environmentally benign. This is due to the small and straightforward equipment needed, better throughput, and lack of chemical requirements or secondary waste production during the acid recovery process. It is important to note that hyphenated technologies are very effective for zero waste discharge, do not require any further post-processing or chemical agents, along with other advantages inherent to the selected methods. The many procedures for recovering acids and water from waste solutions documented in the literature will be examined in this review, along with their benefits and drawbacks, as well as any problems that need to be fixed before they can be used on an industrial scale.
Environmental significanceAcidic waste products from the mining, resin regeneration, hydrometallurgy, electroplating, and numerous chemical industries pose risks to the environment's flora, fauna, and ecological stability. A huge amount of aqueous acidic wastes is thought to be produced annually worldwide. Thus, creating sustainable green technology and recycling acid from watery waste streams are both crucial from an environmental perspective. Membrane technology, which produces no secondary waste and is effective in recovering acids with high purity, is a promising approach. One of the most popular membrane technologies is based on diffusion dialysis using anion exchange membranes. For the commercial use of membrane technology, a number of challenges must be handled, probably in combination with other suitable methods for the circular economy leading to zero waste discharges. |
1. Introduction
Numerous industries such as the hydrometallurgy, electroplating, acidic vanadium leaching, and aluminum foil industries, and nuclear fuel fabrication and reprocessing facilities frequently use inorganic and organic acids as a solvent, leachant, or lixiviant.1–4 Therefore, it is not possible to prevent the generation of extremely toxic and corrosive acidic waste water, the discharge of which is strictly forbidden.5–7 However, acidic wastewater is frequently released into the environment untreated, endangering both human health and the ecology. The most often used inorganic acids in various industrial operations are H2SO4, HNO3, HCl, and H3PO4.In metal processing industries such as electroplating, one of the important stages in the metal finishing process is washing metal surfaces using an acidic solution. This is typically done by “pickling” or submerging the metal in an acid bath. Pickling is a crucial stage in the hot-dip galvanizing process because it removes oxidized layers from the surface of fabricated steel parts.8,9 For pickling, various acids such as HCl, HNO3, or H2SO4 are employed. The used pickling solutions are regarded as hazardous wastes because of their corrosive nature, high acid level, and metal contents. The Environment Protection Agency (EPA) hazardous waste list states that spent pickle liquor with 5–10% free acid and 5–15% ferrous ions is categorized as hazardous waste.10 The cost and environmental impact of the hot-dip galvanizing industry are significantly impacted by way of disposal of the pickling solution. In several industries, zinc is often produced via electrowinning by eliminating the contaminants from the used electrolytic solution through a bleed.11,12 A typical bleed composition is 15 to 16% H2SO4, 5 to 10% ZnSO4, 4.5 to 5% MgSO4, 1 to 5% MnSO4, 200 ppm chloride, 300 to 400 ppm Ca, and trace amounts of Fe, Ni, Co, Cu, and Cd.13 Therefore, ZnSO4 and H2SO4 are the two most important components, and these can be recycled into the process if contaminants are eliminated. Also, in the metal plating and finishing industries, aluminum anodizing is a key process in which aluminium parts are anodized and etched using sulfuric acid (H2SO4) and sodium hydroxide (NaOH). Along with this, the oxide scale from the surface of metals is removed using the pickling procedure with acid baths.14 Therefore, these industries also produce waste streams with significant flow rates of alkali and acid.15,16
The manufacturing of semiconductors and liquid crystals involves a number of steps, such as resistor coating, light exposure, etching, resistor removal, and rinsing, all of which produce significant amount of acid waste. The aluminum–molybdenum alloy or silver placed on the glass substrate is dissolved during the etching process using mixed acids that include acetic acid, nitric acid, and phosphoric acid. The amount of waste acid produced during the etching and cleaning procedures is increasing fast due to the rapid growth of the semiconductor and liquid crystal industries in Korea, totaling to over 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes in 2005.17 At present, it would probably continue to rise unless an effective treatment method is implemented. The acid waste (etching waste) generated by the use of the etching solution often contains metals like Al and Mo together with 50–70% phosphoric acid, 2–10% nitric acid, and 1–10% acetic acid.18 These numbers vary based on the etching process's reaction conditions and reaction states.
000 tonnes in 2005.17 At present, it would probably continue to rise unless an effective treatment method is implemented. The acid waste (etching waste) generated by the use of the etching solution often contains metals like Al and Mo together with 50–70% phosphoric acid, 2–10% nitric acid, and 1–10% acetic acid.18 These numbers vary based on the etching process's reaction conditions and reaction states.
In the nuclear, chemical, and metallurgical industries, HNO3 is employed as a dissolving agent.19–22 Due to the usage of nitric acid in dissolving, extraction, fuel manufacturing, and other processes, the nuclear industry's fuel reprocessing is also one of the main sources of high concentrations of nitric acid in aqueous waste. Additionally, a significant amount of radioactive corrosion and fission products, including Mo, Zr, Sr, Cr, Ni, Mn, Y, La, Ce, Pr, Nd, and Sm, are present in this highly acidic waste.23 The major objective of the remediation for such radioactive waste is to produce these radionuclides with a high specific activity in a small amount of acid, and nitrate-free solution that can be safely and readily stored or immobilized in suitable matrices.24
Apart from this, acid mine drainage (AMD), a problem that affects mining companies worldwide, results in one of the major uncontrolled release of acid to the environment.25 AMD is a pervasive and potentially harmful source of contamination from mine sites even after mining has been stopped. When rocks and sediments containing sulfide minerals like pyrite and pyrrhotite are exposed to the atmosphere in an oxidizing environment, AMD waters are created, which are best described as low pH, high metal, and high sulfate-bearing waters.26–29 Both biotic and abiotic, or inorganic chemical oxidation processes, can result in pyrite oxidation. Degradation of biological and abiotic materials can be brought about by oxygen (direct oxidation) or oxygen plus iron (indirect oxidation).30 Apart from sulfuric acid soil, these mining sources also include piles of ore and rock debris, open cuts, tailing pits, and dams.27 AMD also contains iron, aluminum, and zinc as well as other potentially toxic elements. Regular releases of untreated AMD carry the risk of contaminating nearby water sources and sediments, which would be detrimental to biodiversity.31 The United States, Canada, Australia, and South Africa all appear to have sizable mining operations.32–41 The United States (US) Forest Service forecasts that AMD from coal mining in the eastern US will contaminate more than 6000 km impacted by acid discharges from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 50
000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mines in the western half of the country, which exposed forest lands to these discharges.33–35 AMD has been attributed to be due to a significant number of abandoned mine sites and the enormous volume of pyrite-bearing tailings from coal and gold mines in these countries. However, AMD contains acid in only pH ranges in contrast to the acid waste generated during various industrial processes which have high acidity generally in the mol L−1 range.
000 mines in the western half of the country, which exposed forest lands to these discharges.33–35 AMD has been attributed to be due to a significant number of abandoned mine sites and the enormous volume of pyrite-bearing tailings from coal and gold mines in these countries. However, AMD contains acid in only pH ranges in contrast to the acid waste generated during various industrial processes which have high acidity generally in the mol L−1 range.
At present, the inorganic acids cannot be separated from these industrial manufacturing operations. The generation of these inorganic acids raises a number of questions, nevertheless, about their potential effects on the environment and sustainability.
2. Implications of acid discharges to environment
Inorganic acids can significantly damage the environment due to their corrosive nature and are potential for pollution, if not handled properly. The major environmental effects associated with inorganic acids are described below.2.1. Ecological impacts
Inorganic acids, when released into water bodies can cause water pollution by lowering the pH of water. Acidic water can harm aquatic ecosystems, disrupting the balance of aquatic organisms and impairing their growth, reproduction, and overall health.29,30,42,43 Acidification can harm these organisms directly or indirectly by disrupting food chains and altering the composition of aquatic communities.44–48 Aquatic organisms, such as fish, amphibians, and invertebrates, are particularly sensitive to changes in pH. Acid rain, caused by the deposition of acid-forming pollutants, can further impact terrestrial ecosystems, including forests and sensitive plant species.48 Acidic water can also leach metals such as aluminum and mercury, and other contaminants from soils and sediments. These potentially toxic elements are generally hydrolyzed above pH 4 and remain as precipitates/insoluble substances under normal environmental conditions. These metal contaminants, however, turn very soluble at pH 3, and as a result, their concentrations in ground water may rise above the level that is not acceptable for use by humans. This may further exacerbate water pollution.49,502.2. Corrosion and infrastructure damage
Inorganic acids are highly corrosive substances that can damage the infrastructure, including pipelines, storage tanks, and equipment.51–53 Acidic substances can corrode metals, leading to structural failures and leaks.2.3. Air pollution
Inorganic acids can release toxic fumes and gases when exposed to air, particularly at higher temperatures.44 For example, sulfuric acid can release sulfur dioxide (SO2), a major contributor to air pollution and acid rain. Nitric acid can release nitrogen oxides (NOx), which are potent air pollutants that contribute to smog formation and respiratory issues. These emissions can have regional and even global impacts on air quality and ecosystem health.2.4. Water pollution
Wastewater treatment is a pressing problem that affects people worldwide because it poses serious risks to ecological systems and the quality of available drinking water.4,54,55 The issues are consistently made worse by the industry's rapid development and the rise in living standards.56–582.5. Soil and land pollution
Improper disposal or accidental spills of inorganic acids can contaminate soils and land areas. Acidic substances can alter soil pH, making it inhospitable for many plant species. Acidic soil can also cause the leaching of nutrients and essential minerals, reducing soil fertility and impacting agricultural productivity.In general, to mitigate the environmental effects associated with inorganic acids, it is important to implement proper handling, storage, and disposal practices, which includes:
(1) Using appropriate containment systems and protective measures to prevent leaks and spills.
(2) Implementing effective treatment processes to neutralize and remove/recycle inorganic acids from wastewater before discharge.
(3) Employing proper waste management techniques to minimize the release of acids into the environment.
(4) Promoting the use of green alternatives and sustainable practices to reduce reliance on inorganic acids.
(5) Adhering to environmental regulations and standards to minimize the potential environmental impacts.
By adopting responsible practices and considering alternative solutions, industries can minimize the negative environmental effects of inorganic acids and work towards more sustainable and environmentally friendly chemical processes.
3. Sustainability and circular economy
The sustainable use of inorganic acids in industrial applications depends on several factors, including their production, use, disposal, and potential alternatives and require consideration of the following points.3.1. Environmental impact
Inorganic acids can have significant environmental impacts throughout their life cycle. The production of inorganic acids often involves energy-intensive processes and the release of greenhouse gases. Additionally, improper handling and disposal of inorganic acids can lead to water and soil pollution, as well as the emission of toxic fumes. Sustainable inorganic acid use requires minimizing emissions, reducing waste generation, and implementing proper disposal and treatment methods.3.2. Resource efficiency
Inorganic acids are often derived from non-renewable resources, such as sulphur, nitrogen, or minerals. Sustainable practices involve efficient production methods, optimizing resource use, reducing consumption, and exploring alternative sources or processes, thereby reducing reliance on virgin materials.3.3. Health and safety
Inorganic acids can pose risks to human health and safety, especially if not handled properly. Workers involved in their production and use may be exposed to corrosive substances and toxic fumes. Sustainable industrial practices prioritize worker safety through proper training, the use of personal protective equipment (PPE), and the adoption of less hazardous alternatives or processes.3.4. Circular economy approach
Adopting a circular economy approach can enhance the sustainability of inorganic acid uses. This involves minimizing waste generation and exploring opportunities for recycling, reusing, or repurposing inorganic acids. Recycling and recovery technologies can reduce the need for new acid production, conserve resources, and minimize environmental impacts.3.5. Exploration of green alternatives
As mentioned earlier, exploring and adopting green alternatives to inorganic acids can significantly contribute to sustainability. Organic acids, bio-based solvents, enzymatic processes, and supercritical fluids are examples of alternatives that offer reduced environmental impacts and improved safety profiles. Evaluating and implementing these alternatives can lead to more sustainable industrial chemical processes.3.6. Regulatory compliance
Compliance with environmental regulations and standards is essential for sustainable use of inorganic acids. Regulations often aim to control emissions, manage waste disposal, and ensure worker safety. Adhering to these regulations and actively seeking ways to exceed compliance can promote sustainability and minimize environmental risks.In general, the sustainability of inorganic acid use in industrial applications depends on a holistic approach that considers environmental, social, and economic aspects. By implementing sustainable practices, exploring alternatives, optimizing resource use, and prioritizing worker safety, industries can reduce the environmental impact associated with inorganic acid use and contribute to a more sustainable future. The key components of sustainability of inorganic acid waste management are illustrated in Fig. 1.
As discussed earlier, the circular economy of recycling inorganic acids involves the recovery, reuse, and regeneration of these acids to minimize waste generation, conserve resources, and reduce environmental impact. This requires a hybrid multitasking setup. It has been reported in the literature that membrane based technologies and integrated technologies are very effective for the circular economy of recycling inorganic acids and other resources including water with zero waste discharge.59,60 However, there are no analyses indicating cost implications of circular economy by recycling of the resources from the aqueous wastes. When inorganic acids cannot be economically recovered or regenerated, these acidic waste waters should undergo proper treatment (pH adjustment and removal of hazardous components) and neutralization (termed remediation) to minimize their environmental impact. This ensures that the waste acid can be safely discharged or further processed without causing harm to the environment. Therefore, the present review is focussed on three approaches of inorganic acid waste management i.e. (i) remediation of low acidic waste water, (ii) recycling of high acidic waste water, and (iii) green alternative to inorganic acids.
4. Remediation methods
Remediations methods are effective for the treatment of low acidity, typically in pH range, waste waters such as due to AMD. Active and passive remediation strategies can be employed for AMD remediation and comprehensive descriptions of several active and passive remediation techniques have been published earlier.30,43,61–65 In general, passive treatment techniques precipitate metal sulfides by establishing reducing conditions and using organic materials as alkaline agents. One of the primary distinctions between passive and active treatment is the minimal or periodic maintenance and examination.29 In situations with low acid loads and little change in the water flow rate, passive treatment applications may be economically advantageous. However, they are not appropriate for circumstances requiring more than 150 kg of CaCO3 per day.29 Aerobic wetlands, compost reactors, anoxic and open limestone drains and channels, bioreactors, and permeable reactive barriers are examples of passive treatment techniques. According to Wu et al., the use of constructed wetlands (CWs) for the treatment of different industrial effluents, particularly acidic waste, has greatly evolved.66 The CWs, usually referred to as treatment wetlands, are engineered systems that are created and built to enhance water quality while requiring relatively little external energy and being simple to use and maintain. As an alternative to traditional mechanical systems for treating wastewater from small towns, CWs have been installed all over the world.67,68 According to the unique properties of industrial effluents, proper pretreatment, in-flow dilutions through re-circulated effluents, pH correction, plant selection, and intensifications in the wetland bed, such as aeration and bioaugmentation, are suggested. Despite advancements in design and operational strategies as well as the use of intensified systems, CW systems operating as standalone technologies are sometimes unable to meet the requirements of these new guidelines as a result of the deteriorating environment leading to stricter discharge standards, including the emphasis on effluent reuse. The development of treatment systems integrating CWs with other treatment technologies is mainly intended to achieve increased treatment efficiency.69 Also, the wetland uses are limited to AMD with near neutral and low acidic properties (pH surpasses 4.5), and they are unable to cope with and adapt to sudden variations in water quality and flow rates.70 Therefore, prior expert knowledge of the physical and chemical behavior of in situ treatment ponds or pits harboring AMD is required for the effective implementation of passive treatments such as wetlands. Although passive treatment takes little upkeep, refurbishment is usually necessary and adds to the expense.71 Moreover, the toxic metal buildup and its associated long-term effects need to be carefully evaluated.There are numerous primary chemical and physical active treatment strategies for AMD such as chemical neutralization, precipitation (pH control and electrochemical processes), sulphate reduction by biological/microbial mediation, flocculation, adsorption and ion exchange, and filtering, as well as crystallization.72 Chemical neutralization is by far the most popular and widely used active primary treatment and broadly uses neutralizing agents such as caustic soda (sodium hydroxide), lime73 and limestone,74 fly ash,75 natural clinker,76 lignite,77 magnesium oxide, and hydroxides.77–81 Of these neutralizing agents, limestone, being inexpensive is most commonly employed.82–85 The AMD treatment by neutralization and toxic element removal with unmodified and modified limestone has been evaluated by Iakovleva et al.86 They have observed that the modification of limestone with sodium chloride and process water with high content of chlorides, sodium hydroxide and sodium carbonate are efficient and inexpensive methods of modification. However, in general, neutralization and precipitation through pH change alone is insufficient, particularly in the presence of metals like arsenic, molybdenum, mercury, selenium, and chromium. Therefore, a second phase of treatment is also required. Also, the majority of reducing agents have typically unpredictable process parameters, which is not desirable. Although electrochemical reduction provides a highly controlled and secure method of nitrate destruction, maintaining electrochemical reactors over long term poses a significant difficulty.87 Additionally, the formation of watery sludge (greater than 90% water) containing potentially toxic elements is a major problem with the neutralization strategy. The cost of dewatering the sludge is considerable. Due to this, active AMD treatment methods are typically more expensive than passive ones, especially after the mining process has stopped.88
The industrial low acidic waste water discharges containing nitrate could be treated with the artificial denitrification processes.89 Some of these methods are: bio-mediated denitrification,90–95 catalyzed chemical,96–102 electrochemical methods,96,103–107 photocatalytic reduction,108 and hydrothermal decomposition.109,110 Although biological denitrification is a well-developed technology for converting nitrate to nitrogen, it is sluggish and difficult to manage, and the presence of other metal ions in the discharges inhibits bacterial growth. Similar to this, other systems have flaws that prevent them from being improved upon or applied, such as expensive capital expenditure, high energy and alkali consumption, and environmental damage.
The various remediation methods used for the mitigation of adverse effects of acidic waste water discharges are shown in Fig. 2.
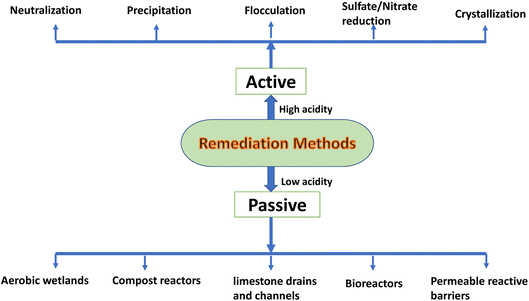 | ||
| Fig. 2 Different practices used commonly for the remediation of acid in waste waters generated by the acidic mine drainage and industrial activities. | ||
5. Recycling and regeneration
Recycling of inorganic acids involves the recovery and reuse of these acids, reducing the need for new acid production and minimizing waste generation. Therefore, recycling and regeneration methods offer better approach than the remediation approach for the management of acidic aqueous wastes, particularly when the concentration of inorganic acids is high enough to make it viable for the circular economy.5.1. Regeneration by treatments
Removing metal ions and other components through chemical or physical processes is one of the main strategies for the regeneration and recycling of the acid from spent acidic solutions generated in the steel, electroplating, and semiconductor industries. Due to its strong acidity and oxidative capacity, piranha solution, a mixture of hydrogen peroxide (1 part) and sulfuric acid (3 parts), is frequently employed in the industrial processing, manufacturing, and laboratories especially to clean surfaces with organic contamination.111–120 The electrochemical oxidation of hydrogen peroxide provides the basis for the regeneration of sulfuric acid from the utilized piranha solution.121In the steel industry, HCl and H2SO4 solutions are typically used to treat steels, while mixtures of HNO3 and HF solutions are used to treat carbon steels. Inhibitors are also included in pickling solutions to lessen the acidity of the pickling solutions' reaction with metals. These pickling solutions are deemed exhausted when the acid concentration falls by 75–85% while the metal content increases to 150–250 kg m−3, resulting in a drop in the pickling rate. The extinguished baths are regarded as special hazardous wastes because of their caustic character and high concentration of acids and metals.122 There are various methodologies for the treatment of spent pickling liquors to regenerate acids and recover metal salts as shown in Fig. 3.
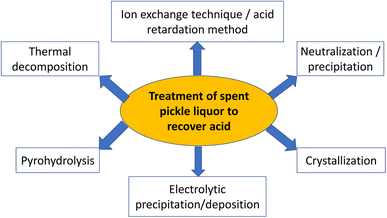 | ||
| Fig. 3 Different techniques and methodologies employed for the regeneration and recovery of acids from the spent pickle liquors. | ||
A brief description of different strategies employed to recover acids from the spent pickle liquors is given below.
| 4FeCl2 + 4H2O + O2 → 8HCl + 2Fe2O3 | (1) |
The fluidized bed reactor's (FBR) separating vessel receives the used pickle liquor, which is subsequently concentrated in a venturi loop using hot reactor gases. From this loop, a portion of the concentrated pickle liquor is continually supplied into the reactor's fluidized bed. According to eqn (1), the fluidized bed is made up of iron oxide granules, acid, and water that are evaporated at 850 °C, turning iron(II) chloride into iron(III) oxide and hydrochloric acid. The spray roasting method is an additional hydrochloric acid regeneration choice. While the equipment utilized varies slightly depending on the roasting procedure, the general operating principle remains the same. In the spray roasting reactor, the pyrohydrolytic separation of iron(II) chloride and water occurs at a temperature of around 450 °C. The heated reactor gases are cooled and the acid is pre-concentrated in a venturi recuperator, which receives the used acid. After that, the concentrate is sprayed from above directly into the fired reactor. The tiny drops evaporate as they fall because of the hot burn gases.124 According to eqn (1), the ferrous chloride is separated into iron oxide and hydrochloric gas using steam and airborne oxygen. The iron oxide that results from this process is collected at the reactor's base and moved pneumatically to an oxide bin.
According to Ozdemir et al., crystallization is an effective way to get rid of ferrous chloride in water after hydrochloric acid pickling.134 To recover metals from used baths and the HNO3/HF acid mixture, Kerstin and Rasmuson built a prototype plant with a continuous crystallizer.135 Another crystallization method developed by Kerstin and Rasmuson is intended to treat pickling water that contains Fe(III) and Cr(III) ions.136 In this method, while Cr(III)F3·3H2O and FeF3·3H2O precipitate, the mixture of regenerated acids (HF/HNO3) is circulated. Galvez et al. investigated the crystallization procedures for recovery of HF and HNO3 from exhaust baths, and they observed that the presence of Cr affects the speed of crystal formation.137 In order to recover HF and HNO3 and separate metals as fluorides from water stripped off, Hermoso et al. developed a crystallization technique.138 This technique also offers nickel recovery with yields more than 72% and purity close to 100%. A method for making iron sulphate heptahydrate from pickling solutions that comprise FeCl2, HCl, and H2O is also patented by Kehrmann.139 The PHAR (pickling hydrochloric acid regeneration) technique is illustrated by Brown and Olsen as having both technical and financial advantages.140 By lowering the crystallization temperature from 270 K to 265 K, it is able to boost iron removal from 72% to 85%. In this procedure, sulfuric acid is introduced to the exhausted baths of hydrochloric acid, and when the temperature falls, iron sulphate heptahydrate crystallizes and hydrochloric acid is released. Energy use, prices, and CO2 emissions are lowered with respect to old systems by 95%, 52%, and 91%, respectively. To regenerate HCl and make iron sulphate heptahydrate, Olsen and Blumenschein developed a technique for the treatment of spent pickling water that contains both HCl and FeCl2.141 In this instance, the regeneration is carried out using sulfuric acid, which, when reacting with FeCl2 at ambient pressure, produces iron sulphate heptahydrate and HCl. While the acid is recycled, the salts are separated. Additionally, wastewaters are not produced, which lowers capital and operating costs. But this approach has the following drawbacks: (i) the crystallization requires considerable energy, (ii) it is challenging to remove strong metallic ions like Fe ions from the waste acids, and (iii) an economically viable way to handle the crystal that has been removed is not there.
(i) Significant energy consumption during the thermal decomposition of waste acid containing low Fe ions, which makes it economically not viable.
(ii) Loss of hydrochloric acid as HCl fumes.
(iii) Difficulty in maintaining the required Fe concentration in real applications.
(iv) Higher maintenance cost due to the corrosion of the equipment caused by wet Cl2 and HCl gases.
5.2. Solvent extraction
The purification and recovery of resources (metals) from waste solutions is accomplished using the well-known treatment technique known as solvent extraction.144–146,158–162 The dispersion of a solute or solutes between two immiscible liquids or phases is known as solvent extraction. It frequently happens with organic and aqueous solutions. The organic phase typically consists of an extractant dissolved in a diluent, but a modifier and a synergistic agent may be needed in some circumstances.158 While the synergistic agent is used to improve and enhance the extractant, the modifier is added to the system to improve its physical features like to cause phase disengagement after equilibrating the two phases. Nowadays, due to technological advancements, rising consumer demand for higher purity products, and call for more environmentally friendly transportation methods, a higher number of stable solvents (extractants) are available for use in hydrometallurgy. These solvents have the potential to exhibit excellent selectivity for a specific metal ion. When the flow rates of waste water and the concentration of the solute are both high, solvent extraction is economically viable. But, when the concentration of the pollutant to be recovered is very low, it is not a feasible method.144–146,158 However, the majority of acidic waste streams typically include low levels of acids, typically 10–20 g L−1.155–157 A major obstacle to the efficient concentration of diluted acidic solution through evaporation is the higher energy requirement. The ability to recover different acids has been attributed to a number of extractants, including TEHA (tris-2-ethylhexylamine), Alamine-336 (a mixture of trioctyl/decyl amines), TBP (tri-butyl phosphate), and Cyanex 923 (a mixture of alkyl phosphine oxides).144–146,158–161![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group in TBP and TOP can behave as a strong Lewis-base, which allows it to form acid–base complexes.
O) group in TBP and TOP can behave as a strong Lewis-base, which allows it to form acid–base complexes.
According to Hesford and Mckey,177 TBP extracted mineral acids in the following order: H2SO4 > HF > HClO4 > HNO3 > H3PO4 > HCl. Lee et al. carried out tests in a related investigation to recover important metals and regenerate nitric acid from the spent nitric acid solutions.178 According to one study, 50% TBP was used in five counter-current stages with a three-volume ratio of organic (O) to aqueous (A) to extract 95% of the nitric acid from a feed solution containing 250 g L−1 of nitric acid. Hoon-shin et al. studied the removal of HNO3 using distilled water at 60 °C. At an O/A ratio of 1.5, a five-stage counter-current test resulted in a stripping efficiency of over 94%.176 After stripping, a high purity product of 99.8% HNO3 was obtained.176 When employing distilled water as a stripping agent, similar results were obtained indicating that nitric acid was removed from the loaded organic phase that contained 80 g L−1 nitric acid. After five interactions, there was a noticeable recovery of nitric acid (>99%) in the aqueous phase.175–182 There are several reports on the recovery of HNO3 using organo-phosphorous based extractants.182
5.3. Membrane based methods
Membrane based methods are most promising for the recycling of acids from aqueous wastes containing higher concentrations of inorganic acids. The important membrane-based methods and their effective combination based integrated technologies for inorganic acid recoveries are described below.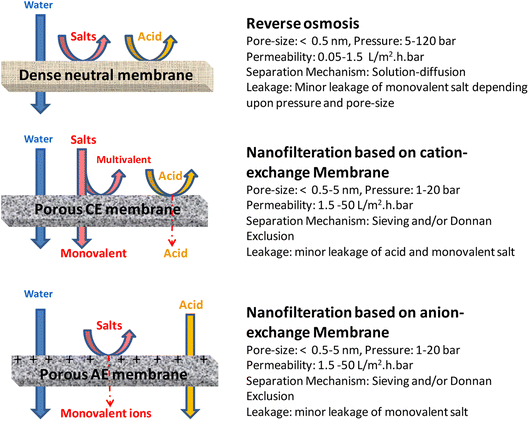 | ||
| Fig. 4 Schematic illustration of separation of acid, salts, water in aqueous waste using reverse osmosis (RO) and nanofiltration (NF) membrane-based processes. | ||
The use of NF to treat hydrometallurgical streams provided a good alternative to established practices. The experimental results demonstrated that a semi-aromatic poly(piperazine amide) membrane may be used to recover strong acids (H2SO4 and HCl) from hydrometallurgical streams. When the pH is below 1.0, this membrane has a positively charged surface that favors the transport of anions while impeding the transport of metallic species that are present as cations.216 For the recovery of phosphoric acid from leached sewage sludge ash, a cutting-edge treatment process has been developed. For the purpose of recovering H3PO4, a hollow ultrafiltration membrane has been modified and transformed into a nanofiltration (NF) LbL membrane using layer-by-layer (LbL) polyelectrolyte deposition.217
As NF exhibits strong rejections for multivalent ions (>90%), while permitting monovalent ions (like H+) to pass through the membrane, it has the necessary qualities to regulate acidic mining waste. It must be emphasized that the most polymeric NF membranes based on polyamide are only stable at pH > 2.218–223 The majority of investigations involving polyamide-based membranes at high sulfuric acid concentrations have demonstrated minimal acid rejection and high metal ion rejection. However, ongoing contact with acidic streams will cause the membrane to deteriorate.219,220 The polyamide active layers have been protected by being coated with or having their chemical composition changed with sulfonated polysulfone groups in an effort to boost the stability of NF membranes in strongly acidic solutions.224
It is well known that positive charge can be introduced into the membrane surface in order to selectively separate out positively charged components, which has a substantial impact on the regulation of charge in NF membranes.225 For the purpose of recovering hydrochloric acid, a water-based coating method was used to form an acid-recovering nanofiltration (NF) membrane that possesses both acid resistance and selective acid permeability. To accomplish this, a loose polyethersulfone NF membrane was dip-coated with branched-polyethyleneimine and an epoxy linker before being heated in a sealed oven with a high-humidity atmosphere. The resulting membrane had a positive surface charge with a zeta potential and showed the positive-charge separation membrane performance order of MgCl2 > MgSO4 > NaCl > Na2SO4. The selective permeation of hydrochloric acid was demonstrated by Mg rejection and Cl permeation studies to be achieved with Mg rejection over 95% and Cl permeation above 70%, allowing the acid to be recovered by getting permeate at the same pH as the input. Moreover, the NF membrane's selective separation capability and flow rate remained constant for a month.
Using a NF membrane, Mendoza-Roca et al. cleaned the acidic wastewaters from the pickling and tanning processes.199 They obtained highly filtered water and attained substantial sulphate retention (>90%). However, the concentrate still contained all the acidic components and metal ions, which needed to be eliminated using customary procedures. Jakobs et al. also treated industrial nitric acid solutions with an NF membrane.197 The nitric acid solution used in the manufacture of image tubes could be converted into a purified version attributed to the NF membrane's cation exclusion and acid permeation capabilities. The NF membrane method had a recycling rate of 80–90%. This outcome demonstrates unequivocally that acid wastewater can be filtered using NF membranes. The quantity of pollutants released is decreased by using NF membrane systems to recover sulfuric acid solutions from sulfuric acid wastewater generated during copper smelting. It appears that no research has been done to ascertain which kind of membrane is ideal for recovering sulfuric acid solution from wastewater used in copper refining. Moreover, the effects of highly acidic environments on membranes have not been extensively studied. Therefore, more investigation is required into the selective ion rejection capabilities and resilience of NF membranes in extremely acidic circumstances for the treatment of sulfuric acid wastewater.
Two polyamide membranes and a polyacrylonitrile-based membrane were successfully used to recover a pure sulfuric acid solution, as demonstrated by a combination of permeate flow, sulphate permeation, and metal ion rejection.226 Because of their poor acid stabilities, polyamide membranes were shown to be inadequate for treating effluents from the copper refining process in studies on both short- and long-term performance. The polyacrylonitrile-based composite membrane, on the other hand, demonstrated outstanding acid stability and provided better than 90% metal ion rejection for 430 days, with the exception of calcium ions. The recovery performance in a 1-ton per day pilot-scale process employing copper-refining wastewater showed that, even at a 95% recovery rate, 90% of the metal ions were rejected.
As specified earlier, in comparison to RO, NF permits greater permeate fluxes, although it is still very effective in retaining organic molecules and multivalent salts.227 Moreover, NF has a membrane isoelectric point (IEP) that is known to be between 3 and 6 and to be charged. As a result, at acidic pH, the membrane surface displays a positive charge,228,229 a characteristic that has a direct impact on the mechanisms regulating NF retention. This allows the positively charged NF membrane to effectively reject multivalent metal cations while allowing acidic anions to pass through the membrane nearly unhindered when treating acidic fluids.230 Because of these features, NF is a very promising method for removing metal species from acid streams. In contrast, RO is a very effective procedure for retaining dissolved salts and organic molecules with low molar masses, achieving 99% retention.227 Its primary use is in the desalination of seawater, where it generates 20% of the world's desalinated water.231 Due to its qualities, RO has grown more and more appealing as a method for treating industrial effluents, especially when water recovery is sought.232,233 According to Ahsan et al.,234 RO can be used to change the concentration of existing solutions because it also generates a highly concentrated stream while creating a high quality permeate. By utilizing RO, those authors were able to produce a concentrate with an acid concentration that was 400% higher than the feed acetic acid solution.
Several studies have reported the integration of NF and RO for the treatment of acidic streams polluted with metals. Purification of phosphoric acid,235,236 nitric acid used in the manufacture of picture tubes,237 recovery of metals and acids from electroplating effluents,238 and purification of sulfuric acid are its few uses.239 In addition to the aforementioned applications, the combination of NF and RO has shown to be a viable treatment for pressure oxidation process effluent, a waste product from the gold mining sector.240 In this work, four flat-sheet NF membranes were used to study the separation of phosphoric acid from washing waste waters.203
The major problem associated with NF and RO are fouling of membranes and degradation of membranes (such as polyamide) in high acidity over a period of time, and high energy consumption. For sustainable application of RO and NF for acid recovery, these problems should be addressed along with possibility of coupling this method with other membrane-based technologies for the management of generated waste with high salt content. Ceramic NF membranes have a great degree of chemical stability, which makes them effective for handling acidic waste.220 Nonetheless, it is necessary to create ceramic NF membranes with smaller pore sizes in order to lessen convective flow's influence on ion transport. This might facilitate greater ion rejections by ceramic NF membranes.
MD is a combination of thermal and membrane desalination. Both the thermal energy, required to heat the feed solution, and the electrical energy, needed to power the circulation pumps, are consumed in MD systems. Therefore, a low-grade heat source and condenser are necessary for this process. Being an energy-intensive process, the technology immediately lost its appeal because it was discovered that MD required more energy than RO.244 However, RO relies on expensive electricity and the cost of electricity has increased, making it appear unsustainable. MD, on the other hand, can more easily utilize waste heat or solar thermal energy. Many benefits of MD-based acid recovery are:245
• A total (theoretical) rejection of non-volatiles such as cells, macromolecules, colloids, and ions.
• Reduced operational temperatures compared to traditional distillation.
• Reduced operating pressures compared to typical membrane separation techniques driven by pressure.
• Less sensitivity to process variable changes (e.g. pH and salts).
• Chemical resistance and good to exceptional mechanical properties.
• Smaller vapor gaps than with traditional distillation methods.
• It can be used to recover more fresh water from brine after RO.
However, there are limitations to MD-based processes, including their high energy intensity (even if heat is often of low-grade energy), sensitivity to surfactants, and the need for separate treatment for volatiles like hydrochloric acid, ammonia, or carbonates that pass through the membrane. The literature has generally identified four different configurations of the MD system, based on how the cold side permeate is handled.246 These are:
(1) Direct contact MD (DCMD), in which liquid phases and the membrane come into direct contact. This is the simplest setup that can provide a respectably high flux. It can be used for applications like desalination and the concentration of aqueous solutions (like acids).247
(2) Air gap MD (AGMD), in which a space called an air gap sits between the condensation surface and membrane. Although this arrangement has the lowest flux, the energy efficiency is maximum. For the majority of MD applications, the air gap structure can be widely used, especially when thermal energy is expensive or scarce.248
(3) Vacuum MD (VMD), where the permeate side is air or vapor at lower pressure, and where the permeate is, if necessary, condensed in a different device. This arrangement is advantageous when volatiles are being eliminated from an aqueous solution.249
(4) Sweep Gas MD (SGMD), in which the created vapor is transported using stripping gas. This arrangement is employed when volatiles are eliminated from an aqueous solution.250
MD has found many potential applications such as production of high-purity water from salty water, removal of potentially toxic elements from waste water,251 recovery of HCl from cleaning solutions in electroplating,252 concentration of sulfuric acid to recover lanthanide compounds in the apatite phosphogypsum extraction process,253 elimination of radioisotopes leading to reduction of nuclear industry waste volume,254 and the removal of volatile organic components from diluted aqueous solutions.255,256 A synthetic solution containing only H2SO4 and HCl was examined by Tomaszewska and Mientka for the separation of H2SO4 and HCl.247 As the concentration of H2SO4 in the feed increases, it has been reported that the presence of sulfuric acid in the feed reduces the solubility of HCl and hence increases HCl flux. Acid recovery from various process acidic solutions employing the MD approach was also described in the studies by Guiqing et al. and Tang and Zhou.257,258 These studies of DCMD for acid recovery focused on treating mostly synthetic and particular real solutions. MD was examined using actual leach solutions that contained either HCl or H2SO4. The salt rejection rate for the H2SO4 actual leach solution was greater than 99.9%, and the concentration of free acid increased from 1.08 mol L−1 to almost 4.60 mol L−1. The membrane rejected >99.9% of the acid.259 Currently, MD has been primarily explored at the laboratory scale such as for treatment of wastewater from a power plant (in Singapore) and for wastewater treatment in a chemical plant (The Netherlands).260–266 Along with this, some of the pilot plants undergoing field trials are for RO concentrate treatment, ground water treatment, and solar heat utilization.267–269 The concept is viable, although it has some practical problems, like scaling on MD membranes.270 In rural Victoria, Australia, a RO-MD trial using a solar-powered direct contact MD system produced results that were comparable.271 Flux decreased as a result of membrane scaling, however it was quickly restored with an acid clean. The possibility of lessening scaling on MD membranes has not yet been investigated extensively. The idea of minimizing the usage of extra chemicals is of importance in remote mining operations. Considering that the scaling salts are most likely to occur in the higher temperature area of the DCMD hot cycle loop, a filter might be used to catch them before they reach the membrane.
At present, MD based processes have not been used for the recovery of acids other than laboratory scale. However, MD has potential when used in combination with other processes for the recovery of acids and aqueous waste treatment.
5.3.3.1. Advantages and disadvantages. Out of all the membrane-based processes, DD is particularly attractive due to its cost-effective nature as no pressure or electric power is needed for driving the separation and hence the energy consumption is low. Only power is required for solution pumping. This leads to lower installation and operating costs.273,275–278 One such interesting account of the economic benefits of a continuous flow diffusion dialyzer plant has been given in ref. 276, where processing 10 tonnes per day of waste solution to recover sulphuric acid has been discussed. It has been estimated that the major economic benefits are achieved from the saving of chemicals and within a short span of time, the high profits could recover the investment costs. The DD process also results in recovering pure acids due to the high selectivity of AEMs for acids. Moreover, the DD process supersedes all the other acid recovery processes owing to its environment friendly nature as it is free of any chemicals or post-processing.
However, in general for a DD process, the processing capacity and efficiency is relatively low (e.g., 11.3 L m−2 d−1 for the commercial DF-120 membrane with an acid recovery of 85–90%), thus requiring large membrane areas for industrial applications.273 This drawback is due to the low acid permeation of AEMs used. Besides, the process consumes huge quantity of water in the receiver compartment.
5.3.3.2. Systems studied. The DD process has been comprehensively applied for the acid recovery of both inorganic as well as organic acids. In inorganic acids, the majority of studies have been focused on recovery of hydrochloric acid279 and only a few studies are there on the recovery of sulphuric acid,280,281 nitric acid282,283 and hydrofluoric acid.284,285 Different types of organic carboxylic acids have also been recovered using the DD process.286,287
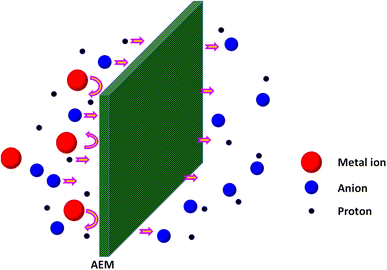
5.3.3.3. Basic principle of a DD process. As stated earlier, the DD process is based on the spontaneous transport of counter-ions due to their concentration gradient, wherein Donnan exclusion and electro-neutrality conditions are maintained.272,273 When an anion-exchange membrane separates a two compartment system with the feed containing the acidic solution (AX) and the receiver containing water, as shown in Fig. 5, the anions i.e. the X− are allowed to transport across the membrane and the cations A+ are repelled as a result of Donnan criteria of co-ion rejection. But since the receiver is electrically neutral, the positively charged H+ ions also drag along with the anion, X−, thereby preserving the electrical neutrality condition.288 The diffusion of H+ ions is preferred relative to other cations, owing to their smaller size, monovalent nature and higher mobility. The proton mobility has been reported to be an order of magnitude higher than those of other cations in aqueous solution owing to its diffusion via some special transport mechanisms, such as the vehicle mechanism and Grotthuss mechanism.289–292 In the vehicle mechanism, the proton moves through the medium as a water cluster by molecular diffusion, while in the Grotthuss mechanism, the protons move from oxygen to oxygen by simultaneously breaking and forming hydrogen bonds. Therefore, in a DD process, a net transport of HX, i.e. acid from the feed to the receiver takes place, leading to its recovery.
5.3.3.4. Models. The species permeation in a DD process is chiefly described by two types of models such as the solution-diffusion model and the three-phase membrane model.273,293 In the solution diffusion model, the transport of species across the concentration gradient is governed solubility/interaction of the species with the membrane matrix. The species may interact with the membrane via electrostatic or non-electrostatic interactions. In a DD process, the separation between the protons and the metal ions is achieved owing to their difference in the solubility and mobilities in the membrane. The second model, the three-phase membrane model, segregates the membrane matrix into three regions: a hydrophobic region, comprising the hydrophobic membrane matrix which is responsible for membrane stability, a functional group containing active region; and an interstitial region. This model attributes the transport of acids to the hopping mechanism of anions and the dragging mechanism of protons and other cations through active and interstitial regions in an anion-exchange membrane. In the literature, different approaches such as the Nernst–Planck equation, Teorell–Meyer–Sievers,294 and a lumped parameter model295 have been used to theoretically model a DD process.296
5.3.3.5. Efficacy of diffusion dialysis. The acid is generally desired to be recovered from a solution containing a mixture of other salts. Therefore, it is important to assess the process in terms of preferential separation of the acid over other salts. The permeation rate of protons will directly govern the acid recovery rate and is designated as the acid diffusion coefficient (U). This can be calculated as:
where M is the amount of the component transported in moles per h, A the effective area in square meters, and ΔC the average concentration of the two chambers in moles per cubic meters and defined as below297,298
Here C is the acid concentration. The subscripts f, r and d represent the feed, recovered acid and depleted solution (dialysate), respectively. Here, it should be noted that there will be volume changes in the cell chambers during the water transport through the membrane and therefore (Cf − Cr − Cd) ≠ 0 during the experiment.298
The separation factor (S) with respect to one species over another is given as the ratio of dialysis coefficients (U) of the two species present in the solution:
The % acid recovery can be represented as:
5.3.3.6. Desirable membrane characteristics. AEMs are the critical component for the DD module. A promising AEM must possess high proton permeability, strong salt rejection, good thermal and chemical stabilities, proper water uptake, poor water permeability and low swelling ratio.299–301 The membrane properties such as ion-exchange capacity, water uptake, and membrane thickness can govern the acid and other salt transport through the membrane. The commercial anion exchange membranes for acid recovery are generally homogeneous, and are prepared from different polymers such as poly(ether sulfone) (PES),302 polysulfone (PSF),303,304 poly vinyl chloride (PVC), polyvinylidene fluoride (PVDF),305 poly ether ketone, polystyrene (PS), poly phenylene oxide (PPO)306–308etc. Some of the commercial anion-exchange membranes explored for recovering acids are DF-120 (Shandong Tianwei Membrane Technology Co., China), Selemion DSV (Asahi Glass, Japan) and Neosepta (Tokuyama Co., Japan). The properties of these membranes have been compiled in detail in ref. 273. Though these membranes have good mechanical strength and stability, the polymer main chains are generally hydrophobic which restricts the ion permeability during the DD process. Some membranes with a hydrophilic matrix such as poly(vinyl) alcohol,309–311 poly(ethylene) oxide etc. are available, but are not preferred for DD owing to their higher swelling leading to low mechanical stability. Also, these membranes have poor thermal and chemical stabilities. Nevertheless, hydrophilic nature of these membranes enhances the mobility of protons through the membranes and also retards fouling properties in the membrane. The unfavorable characteristics of these membranes have been dealt with by introducing cross-links in the polymer matrix using alkoxysilanes and aldehydes. Along with the homogeneous membranes, there have been several attempts to modify interfaces of ion-exchange membranes and synthesize asymmetrical membranes, to improve the membrane properties such as the ion transport and selectivity, thereby improving the efficacy of the DD process.312,313 Lin et al. prepared asymmetric ultrafiltration membranes with a thin active layer (<1 μm thick) on a porous support and achieved significantly higher UH+ and S compared to the commercial DF-120 membrane.303 Kim et al. attempted to improve the properties of the commercially available Neosepta-AFX by interfacial polymerization of pyrrole and could achieve higher dialysis coefficients due to the repelling properties of poly(pyrrole) to cations.313 In one of the studies based on poly(aromatic ether sulfone) (BPAES), series-connected hexacation segments could be synthesized using 1,4-diazabicyclo[2.2.2]octane (DABCO) and dibromomethane and a high separation factor of H+ to Fe2+ (SH+/Fe2+) = 127 and a dialysis coefficient of hydrogen ion (UH+) value of 18.92 × 10−3 m h−1 could be achieved.314
Also, most of the AEMs used in DD have quaternary ammonium groups as ion exchange groups. However, these ion-exchange groups have low thermal and chemical stability and low ion permeability compared with other ion exchange groups.324 Some studies have reported the DD studies using membranes with pyridinium, imidazolium, and methylthiazolium functional groups.311,315–324 Some of the relevant references on acid recovery by diffusion dialysis have been summarized in Table S1 (ESI†).
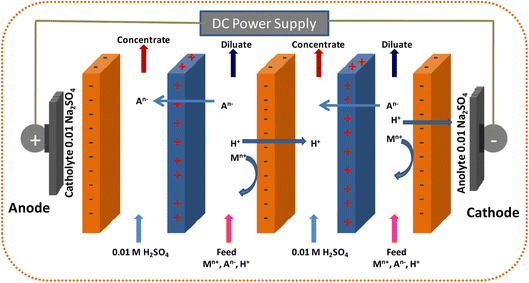 | ||
| Fig. 6 Schematic of the ED cell configuration typically used for the recovery of acids and concentration of salts. | ||
When compared to traditional selective technologies (such as adsorption, filtration, and IE), ED offers many benefits, including: (i) high ionic species separation efficiency, (ii) low operating pressure because there is no liquid movement across the membranes, (iii) the addition of functionalized membranes to selectively remove differently charged ions, (iv) a small operating footprint, (v) decreased sludge formation, and (vi) adoptable and easy to scale up.
Since ED concurrently creates streams that are both ion-rich and ion-deficient, it has been applied to a number of processes, including water desalination,330 salt preconcentration,331 acid/base reuse,332 and precious metal and nutrient recovery.333 The ED approach using cation-exchange membranes (CEM) proved successful in recovering acids from solutions containing salts (NaNO3, NaCl, and Na2SO4).334 Base solutions were utilized as the catholyte in the tests, and various sodium salts served as the anolyte. The outcomes demonstrate that, under the right conditions, electrodialysis appears to be a workable technique for the acid recovery. The recovery of diluted inorganic acids and sodium hydroxide is the subject of several published ED research studies. Bipolar electrodialysis was investigated by Trivedi et al. and Gineste et al. to concentrate diluted acid and caustic liquors utilizing salt solution.335,336 With applied voltages between 10 and 25 V, this method produced concentrated compounds of excellent purity, but operating costs were considerable because of the significant electrical consumption. Inorganic acid recovery from pickling baths used in the stainless-steel industry was explored by Negro et al.,337 while nitric acid recovery from ammonium nitrate-containing effluents was investigated by Ali et al.338 The recovery investigation specifically for the aluminum industry was carried out by Greben et al.,339 who successfully produced 140 g L−1 sulfuric acid with less than 1.2 g L−1 aluminum content by applying ED to a solution derived from electrolytic anodizing of aluminum. Nevertheless, anodizing plants' diluted acid liquors may include up to 20 g L−1 of aluminum in these.
To recover acids and water from diluted acidic wastewater streams from the aluminum anodizing industry,340 the operating conditions in the ED process were adjusted. Studies on an industrial scale showed that thus produced concentrated acid and water solutions were of a high enough standard to be reused for the anodizing of aluminum. This lead to a 90% reduction in the amount of effluents delivered to the wastewater treatment facility and very low amounts of pollutants were released during ED treatment. The aluminum galvanic method was able to accomplish in a closed cycle, which reduces its negative effects on the environment and the economy. Overall, electrodialysis provides the aluminum sector a promising alternative.
Recovery of several commercial acids by ED processes has demonstrated that the AEM has a significant proton leak.341,342 Therefore, research efforts had been concentrated on examining proton transport behavior in the ion-exchange membranes (IEMs) and incorporating the proton blockage mechanism in AEMs.341–344 Due to the tiny hydrated ion radius of protons, some related studies show that the migration rate of protons is substantially higher than that of other cations in ED.345,346 According to the “Grotthuss mechanism” and “vehicle mechanism”,347,348 the protonated water forms clusters for the former, and the protons move through the AEM as water clusters through molecular diffusion. The Donnan theory-based electrostatic repulsive force interaction is typically the best option to stop ion transmission.349–352 The greater ion exchange capacity (IEC) of the membrane would, however, cause it to contain more water, which would facilitate more proton leakage.344,345,353 This demonstrates the significance of water in proton transport. Consequently, utilizing weakly dissociated anion-exchange groups,341,354 adding hydrophobic groups,344,355 and enhancing crosslinking can improve proton blocking capabilities.345 By grafting functional groups containing tertiary amine on the PVDF main chain, for instance, shows that acid-blocking in AEMs is possible.355
According to the description above, a high level of AEM stability and appropriate proton blockage performance are necessary for successful acid recovery. As a result, acid-conditioned PVDF-co-HFP is chemically stable and may be a promising option for acid recovery applications. It is anticipated that the AEM with cross-linking would exhibit the benefit of effective proton blockade. Yu et al. modified poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-co-HFP) with 1-vinylimidazole before crosslinking with 1,4-dibromobutane to create a sequence of proton blockage cross-linking in an AEM.356 The PVDF-co-HFP-based AEM was reported to have an apparent asymmetric structure on its two sides, with a smooth and dense surface and a rough and porous surface. The fluorinated backbone of AEM's asymmetric structure and hydrophobicity result in greater proton blocking performance, higher electrical efficiency, and less energy-intensive acid recovery. Also investigated as a proton blocker in ED are polymer inclusion anion-exchange membranes (PIMs), which exhibit superior proton blocking without significantly impairing performance.357 Jia et al. suggested the model assessments on an ED process for acid recovery for a potential prediction and effective optimization.358 Using a series of thoughtfully planned studies, the detrimental effects of concentration diffusion and electro-migration on the acid recovery process were initially isolated. The undesirable movement of protons was shown to be significantly influenced by the potential gradient and concentration difference, according to the results. A thorough study of the features of the acid recovery system and ED stack configuration helps to accurately forecast the related negative contributions. In particular, the acid leakage is nearly proportional to the relevant driving forces. Proton selective membranes have a challenge in balancing perm-selectivity and ionic flux.359 The production of acid–base pairs between basic 1-vinylimidazole(VI) monomers and acidified sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) was suggested as a straight forward method for creating H+ transfer channels in the membranes to address this problem (SPPO-H). The membranes are compacted and prevented from transferring Zn2+ by the hydrogen bonding networks that are based on the acid–base interaction. On the other hand, electrostatic interactions between sulfonic acid groups and imidazole groups might facilitate the transfer of H+, resulting in a sizable flux of H+ through the membranes of such acid–base pairs. As a result, the finished membranes show a significant H+ penetration but a very low Zn2+ leakage. This study demonstrates that zinc hydrometallurgy effluents can be treated using membranes having the acid–base pairs.
The reverse permeation of acid ions and water, which results from a large concentration difference between the concentrated chamber and the dilute chamber, significantly reduces the concentration effect of the targeted acids that are separated from acidic effluents by the electrodialysis process.360 For the recovery of HCl, HNO3, H2SO4, and H3PO4 in comparison to the TWEDM membrane modules consisting of loose ion exchange membranes, Wang et al. proposed the TWEDMS membrane modules (fabricated by Shandong Tianwei Membrane Technology Co. Ltd.) consisting of compact ion exchange membranes.360 The TWEDMS membrane modules can offer higher concentration differences and current efficiencies for the recovery of HCl, HNO3, and H2SO4 because they suppress the reverse penetration of acid ions and water from the compact membrane structure. The ideal operating conditions must be attained in order to guarantee maximum ED performances. To achieve such ideal operating conditions, a dynamic optimization technique called the orthogonal collocation approach was used.361
The most notable difference between selective electrodialysis (SED) and regular electrodialysis (ED) is the use of a monovalent selective ion-exchange membrane in place of the common cation exchange membrane (CEM) or anion exchange membrane (AEM). Hence, this new electric-driven method has special benefits for monovalent and divalent ion fractionation, such as the separation of lithium and magnesium,362–364 proton and metal ion separation,365–367 NaCl/Na2SO4 separation,368 and others. It is possible to separate the inorganic acids from the metal ion-containing solutions by utilizing the SED process's ion fractionation capability. The concept of SED for acid recovery has been put forward in several published publications.365–367
Contrary to electrodialysis, both electro-electrodialysis and bipolar membrane electrodialysis involve water electrolysis, as seen in Fig. 7. Both H+ and OH− ions are produced when water splits at the electrode or membrane interface (bipolar membrane). Water splitting in bipolar membranes, however, has a number of benefits, one of which is reduced electricity consumption.
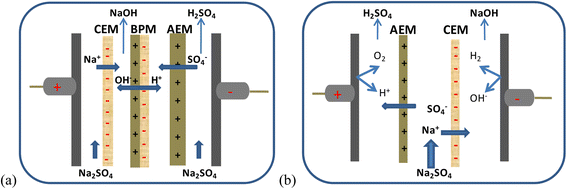 | ||
| Fig. 7 Schematic representations of bipolar membrane electrodialysis (a) and electro-electrodialysis (b). | ||
Bipolar electrodialysis for the purification of bases and acids has also been studied. As an illustration, the acid recovery procedure utilized the waste solutions of acid (HCl or H2SO4) and iron salt obtained during standard electrodialysis.369 In comparison to the feeding solution for traditional electrodialysis, bipolar electrodialysis produced an acid solution that was 51-fold (in the case of hydrochloric acid) and 63-fold (in the case of sulfuric acid) more concentrated. Iron salt was barely present in the recovered acid solution (0.12 and 0.13%). When compared to the feeding solution for the bipolar electrodialysis procedure, the base purification process produced a NaOH solution that was nine times more concentrated. The recovered base was contaminated to varying extents (1.75 to 2.50%). For the purpose of economically recovering chromic acid in plating operations, electro-electrodialysis (EED) is a promising technique.370,371 In a single phase, rinse water treatment, removal of metallic contaminants from the process, and recovery of the plating chemicals from rinse water are all accomplished. In one process, it might be possible to recover chromic acid, get rid of metallic impurities, and clean static rinses. However, there are constraints on the process. The main ones are the applied AEM's weak resistance to the oxidative chromic acid solution and the increase in AEM resistance, particularly at the beginning of the process, as a consequence of the formation of polychromates in the membrane.
6. Hyphenated methods
As shown in Table S2 (ESI†), there are various techniques for treating aqueous acidic wastes. However, it is clearly seen from Table S2 (ESI†) that none of these techniques support the circular economy's goal of zero waste discharge through the recycling of water, acid, and other materials. The majority of membrane processes generate concentrate streams that are highly metal-rich and can be used for: (i) selective precipitation for metal recovery, with the supernatant (i.e., water) being discharged to natural receiving bodies or being recycled internally, (ii) solvent extraction and ion-exchange for the selective recovery of a target metal (e.g., REEs, Cu and Zn), and (iii) electrowinning for the electrodeposition of one particular metal (e.g. Cu). The recovery of a purified acid stream is quite practical with some technologies, such as NF, DD, and MD, and can be used for processes to: (i) dissolve the raw minerals, (ii) remove impurities from the metal surface, (iii) regenerate the solvent extractant, (iv) regenerate the ion-exchange resin, and (v) regenerate the electrolyte in the electrowinning baths. However, other technologies such as ED, FO, RO, and MD obtain a stream that contains water rather than acids. In this situation, water can be either discharged to the natural water receiving bodies or reused internally. The following describes a few of the hyphenated membrane technologies that use conventional techniques or other membrane methods that have been documented in the literature.6.1. Diffusion dialysis-electrodialysis
By utilizing the anion-exchange membrane's permanent proton-metal ion selectivity, diffusion dialysis (DD) is a novel concentration-gradient membrane technique for acid recovery but faces problems such as high water consumption and insufficient acid recovery. It was proposed that an integrated system works better than the typical diffusion-controlled devices for acid recovery and gradually increases productivity and efficiency with “minimum water usage”. One promising approach for recovering acids uses selective electrodialysis (SED), which employs a potential difference as its driving force.372 By combining these two methods, acid reclamation efficiency could be overall improved. In recent years, DD and ED processes have been merged to recover a variety of resources. However, the DD and ED individually use water at different stages of cleaning and acid recovery when operated in non-continuous modes, requiring almost double the water volume as in any other technique. But, it is possible to obtain a single pass of recoverable permeate compartment attributed to the benefit of an integrated system. This approach results in a 50% reduction in consumable water.372,373 Although it takes longer than conventional DD methods, such an integrated system provides minimal treatment costs. Fig. 8 depicts the typical combining methods involving DD-ED.374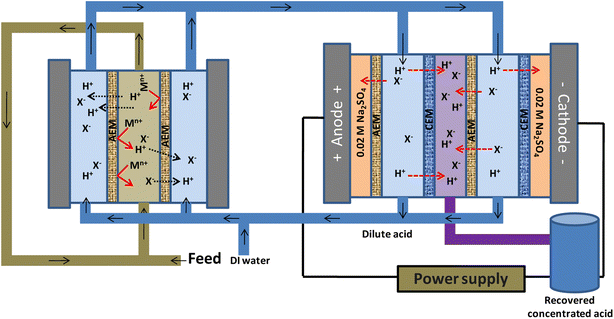 | ||
| Fig. 8 Conceptual illustration of diffusion dialysis integrated selective electrodialysis cell for obtaining concentrated acids. | ||
Zhang et al. published a high recovery ratio (74.9%) for the integrated technique to recover HCl from wastewater of chemosynthetic aluminum foil solutions.375 According to this study, it is important to evaluate integrated systems, reclamation insights, and recovery phenomena for various acids derived from metallurgical wastewater models.375 However, only the recovery of HCl through an integrated process has been documented; there have been no reports of the recovery of HNO3 or H2SO4 using an integrated system. Due to their large quantities in metallurgical effluents,376 the recovery of these acids is necessary. In an effort to enhance acid recovery and preserve water, Chen et al. built an integrated concentration and electro-driven membrane system.377 They also established the nonlinear relationship between mass transfer and water usage in DD. According to the study's findings, selective electrodialysis used a 0.6 V cell potential difference at a 14.9 fold reverse concentration difference to recover 80% of the secondary acid. In this way, the integrated system reduced the water consumption in the DD unit by 50% while still achieving 93% acid recovery, with the concentrated acid reaching a concentration that was 13% higher than the initial concentration of waste acid. Also, the system demonstrated excellent potentially toxic element rejection (90%), exhibited clear benefits by requiring less alkali reagent and releasing lower amount of nitrates into the environment, and highlighted its enormous potential for industrial waste acid reclamation.
Upadhyay et al. explored the electrodialysis integrated diffusion dialysis system using poly(ethylene) based IEMs to recover and enrich acids from metallurgical effluent.374 The pilot scale diffusion dialysis (DD) and electrodialytic (ED) cells were integrated in a series to compare performance, taking advantage of high-permselectivity and counter-ion transport numbers in membrane-driven processes. The poly(ethylene)-based inter-penetrating type cation and anion exchange membranes (In-CEM and In-AEM) were developed, which had ionic conductivities between 8 and 12 mS cm−1 and a counter-ion transport number (tm) in the range of 0.80–0.95. The recoveries of HCl, HNO3, and H2SO4 were assessed for process effectiveness using the proton dialysis coefficient (vH+), separation factor (Sf), and metal-ion leakage (LFen+). The counter-ion diffusivity, thermodynamic variables, dissociation coefficient, and ion's inherent trans-membrane properties for various acids were used to support this acid reclamation research. The vH+ and Sf values for DD as a standalone technique were 0.35 to 0.50 × 10−3 m h−1 and 6–7, respectively. In contrast, the vH+ and Sf were enhanced by 30 and 15 fold, respectively, employing the integrated system. The highest possible vH+ values for HCl, HNO3, and H2SO4 were 14 × 10−3 m h−1, 11 × 10−3 m h−1, and 10.2 × 10−3 m h−1, respectively. The Sf values for HCl, HNO3, and H2SO4 were 88.6, 91.0, and 106.0, respectively.
6.2. Nanofiltration-electrodialysis
It is anticipated that NF and SED will be cutting-edge technology for the acid recovery. It should be noted that the driving forces for the NF and SED are different, and the ion separation mechanisms for both types of membranes are also different. It is unknown which one is more suitable for treating industrial wastewater in terms of acid recovery, metal ion rejection, current efficiency, and energy consumption. As a result, Hussain et al. investigated selective electrodialysis and nanofiltration for acid recovery from molybdenum metallurgical wastewater.378 A considerable amount of acidic wastewater containing significant levels of copper, molybdenum, and other non-valuable metals is generated by the molybdenum mining industry. Using a combination of selective electrodialysis (SED) and nanofiltration (NF), the separation of metal ions (Mo6+, Fe2+, Ca2+, and Na+) and recovery of sulfuric acid from molybdenum metallurgical effluent were examined in this work. SED was optimized using a constant voltage of 6 V and a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the diluent and concentrate chambers, with the highest acid recovery being 89.6% and the highest Mo6+ rejection rate being 98.1%. In comparison, the NF process achieved the highest acid recovery rate of 59.1% and the highest Mo6+ rejection rate of 96.3% at an optimal operating pressure of 1.0 MPa. In terms of this wastewater treatment, a comparison of SED and NF reveals that SED is more competitive than NF due to its lower energy usage, higher acid recovery rate, and higher acid purity. Moreover, NF and SED together could achieve an acid recovery rate of approximately 94.2%. Also, it was possible to post-process the leftover solution into MoO3 nanoparticles. In view of this, it's reasonable to conclude that SED and NF are both promising technologies for acid recovery from molybdenum metallurgical wastewater. Fig. 9 depicts the standard schematic flow sheet that was used in this work.378
1 between the diluent and concentrate chambers, with the highest acid recovery being 89.6% and the highest Mo6+ rejection rate being 98.1%. In comparison, the NF process achieved the highest acid recovery rate of 59.1% and the highest Mo6+ rejection rate of 96.3% at an optimal operating pressure of 1.0 MPa. In terms of this wastewater treatment, a comparison of SED and NF reveals that SED is more competitive than NF due to its lower energy usage, higher acid recovery rate, and higher acid purity. Moreover, NF and SED together could achieve an acid recovery rate of approximately 94.2%. Also, it was possible to post-process the leftover solution into MoO3 nanoparticles. In view of this, it's reasonable to conclude that SED and NF are both promising technologies for acid recovery from molybdenum metallurgical wastewater. Fig. 9 depicts the standard schematic flow sheet that was used in this work.378
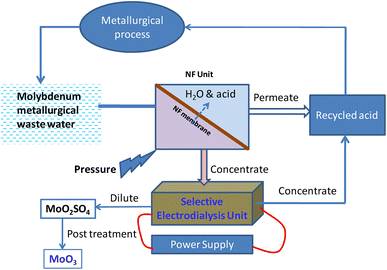 | ||
| Fig. 9 Diagram of the NF-ED integrated molybdenum metallurgical process flowsheet for recovering metal ions and acids. | ||
6.3. Membrane distillation integrated technologies
Kesieme et al. examined the use of membrane distillation and solvent extraction for water and acid recovery from acidic mining waste and process solutions.379 In this work, direct contact membrane distillation (DCMD) and solvent extraction (SX) are combined to recover acid and byproducts from acidic mining waste solution. By applying DCMD to concentrate the waste solution for effective acid and metal recovery using SX, it has been possible to obtain high water recovery and concentrate the acid for the reuse. Fig. 10 illustrates a proposed flow-sheet for recovering water, sulfuric acid, and metal values. H2SO4 concentration rose from 0.85 mol L−1 in the feed solution to 4.44 mol L−1 in the concentrate during the DCMD phase using the synthetic acidic waste solution. The efficiency of the metal and sulphate separation was >99.99%, while the overall water recovery was greater than 80%. The concentrated solution was first treated to recover water using DCMD, followed by sulfuric acid recovery using SX using an organic phase made up of 50% TEHA and 10% ShellSol A150 in octanol. From the waste solution, which contained 245 g L−1 H2SO4 and metals with varying concentrations, more than 80% H2SO4 was recovered in a single contact. With only 2.4 g L−1 H2SO4 remaining in the raffinate after three stages of consecutive extraction, approximately 99% of the acid could be removed. Using water at 60 °C, the extracted acid was easily separated from the loaded organic solution.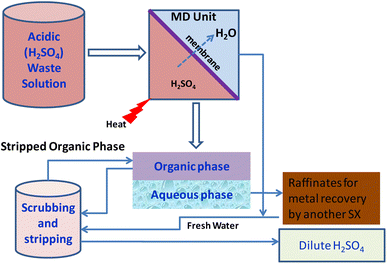 | ||
| Fig. 10 A conceptual flow-sheet to recover water, sulphuric acid and metals (inorganic salts) by the integrated MD-SX process. | ||
A unique membrane-integrated waste acid recovery technique from pickling solution has been developed by Culcasi et al. combining membrane distillation (MD) and diffusion dialysis (DD) technologies with a reactive precipitation unit, where iron ions can be isolated from the zinc-rich solution, and can result in continuous treatment and regeneration of pickling solution.380 To maintain ideal HCl and iron concentrations in the pickling tank and to separate iron and zinc ions in a reactive precipitation unit, the proposed method integrates DD and MD processes with a reactive precipitation unit. Fig. S2 (ESI†) provides a conceptual schematic illustration of MD-DD combined technology for the acid recovery.
A mixture of waste acids with aluminum and/or molybdenum impurities is produced during the etching operations used to make semiconductors. These waste acids include acetic acid (HAc), nitric acid (HNO3), and phosphoric acid (H3PO4). By using vacuum distillation and diffusion dialysis, Kim et al. attempted to recover phosphoric acid from mixed waste acids of the semiconductor industry.381 To recover phosphoric acid from mixed waste acids, diffusion dialysis using a newly developed anion exchange membrane technique was followed by vacuum distillation.
Many combinations of membrane distillation and other techniques are possible, including MD-ion exchange, MD-bioreactor (MDBR), MD-crystallizer, etc.8,382 The simultaneous creation of both fresh water (>98% ion rejection) and solution concentration is made achievable by MD-ion exchange-based technology.383 The benefits of this integration include: (i) the potential for simultaneous recovery of valuable low concentration elements (such as REEs) from AMD using a selective ion exchange adsorbent in a single system, (ii) the potential for improved performance of the ion exchange adsorbent due to increased AMD concentration (while producing high quality water) under MD and thermal conditions; and (iii) the reuse capacity of the ion exchange adsorbent.
The MDBR process combines a membrane bioreactor with MD to recover water while also treating wastewater. Submerged and stepwise are the two basic operational setups that are typically used.384 The main advantages of this integration is that MDBR operates at low operating pressures and moderate temperature, achieving excellent rejection of non-volatile compounds and producing high quality permeate that is independent of biological activity. In comparison to standalone MD, operation of an increased thermophilic biological process enables greater organic biodegradation, which delays membrane fouling and wetting. Furthermore, MD-crystallizer integration has also been studied.385,386 This combination can concentrate feed solution while selectively crystallizing salt (close to zero liquid discharge). The integrated MD-crystallizer process reduces scaling on the MD membrane by continuously crystallizing salt. This small integrated system may produce high-quality water and recover valuable metals as salt crystals for small-scale treatment with low flow rates (like AMD), and hardly any chemicals are needed for the salt precipitation. Yadav et al. recently reviewed the membrane distillation crystallization technology for the zero liquid discharge along with resource recovery.387 The operational difficulties, such as crystal deposition (scaling) on the membrane surface, the pore wetting phenomena, and the economic implications are also examined in this review.387 The appropriate membrane synthesis is highly important for any MD standalone or integrated technology.388
7. Emerging green alternatives
Green alternatives for inorganic acids involve the use of environmentally friendly substitutes that have reduced environmental impacts and are derived from renewable resources as described below.7.1. Organic acids
Organic acids can often serve as greener alternatives to inorganic acids. These acids are derived from natural sources and can be biodegradable, making them more environmentally friendly. For example, citric acid, acetic acid (vinegar), and lactic acid are commonly used as alternatives to inorganic acids in various applications. They can be effective for tasks such as pH adjustment, cleaning, and metal processing.7.2. Bio-based solvents
Bio-based solvents derived from renewable resources offer greener alternatives to traditional inorganic acids. Solvents such as limonene (derived from citrus fruits), ethanol (derived from biomass), or terpenes (derived from plant sources) can be used as substitutes in applications where inorganic acids are traditionally employed. These solvents are often less toxic, have lower volatile organic compound (VOC) emissions, and can be more readily biodegradable.7.3. Enzymatic processes
Enzymes offer an environmentally friendly alternative to inorganic acids in various industrial processes. Enzymes are biodegradable proteins that can act as catalysts for specific reactions. They operate under milder conditions and can provide high specificity, reducing the need for harsh acids. Enzymatic processes can be employed in tasks such as cleaning, surface treatment, and organic synthesis.7.4. Supercritical fluids
Supercritical fluids, such as carbon dioxide (CO2) in its supercritical state, can be utilized as green alternatives to inorganic acids. Supercritical fluids possess unique solvent properties, making them effective in various applications. They are non-toxic and non-flammable, and can be easily separated from the product. Supercritical fluids can be employed in tasks such as extraction, cleaning, and synthesis, reducing the need for traditional acidic solvents.7.5. Green chemistry principles
The principles of green chemistry advocate for the design and use of inherently safer chemicals and processes. By adopting these principles, industries can minimize the use of inorganic acids and prioritize greener alternatives. This includes strategies such as using less hazardous reagents, optimizing reaction conditions to reduce waste and energy consumption, and designing processes that generate less hazardous by-products. It's important to note that the choice of green alternatives depends on the specific application, process requirements, and availability of suitable substitutes. Transitioning to greener alternatives for inorganic acids can contribute to sustainable industrial practices, reduce environmental impact, and promote a more circular and resource-efficient economy. One of the most promising alternatives to inorganic acids without the need to change existing technology is deep eutectic solvents (DESs). After their emergence as a concept some 15 years ago, DESs are today one of the most prominent areas of research within the larger fields of green chemistry and engineering.389–392 The term “DES” typically refers to self-associating mixtures of two or more Bronsted/Lewis acids and bases that exhibit a “eutectic” character upon combination when compared to their native constituents and exhibit a “deep” (i.e. large) melting point depression.390,393,394 The construction of a vast network of intra- and intermolecular interactions, specifically hydrogen bonding between the involved species, is responsible for the formation of such systems.390,393 Numerous applications of DESs have been prompted by their “green” qualities e.g. minimal vapor pressure, lack of toxicity, ease of preparation, biodegradability, etc.390,395–397 In addition to this, DESs have interesting physicochemical properties such as a broad polarity range, electrical conductivity, high surface tension, and good thermal stability.390,394,398 The majority of DES applications at present have been in the metal finishing industry, and their relative toxicity must be compared to that of the aqueous mineral acids they are intended to replace. The environmental effects of the DES based on choline chloride and ethylene glycol in electroplating applications have been studied by Haerens et al.399 Both components are easily biodegradable and have no negative environmental effects, and therefore, the resulting DES is no different.DESs can offer adequate media for the industry's many technological objectives.400,401 In appropriate systems, it may be possible to replace ecologically harmful metal coatings, deposit new alloys and semiconductors, and use new coating techniques to deposit corrosion-resistant metals like Ti, Al, and W.402–404 When it comes to technologically significant plating systems like Ni, Co, and Cr, where many of the aqueous precursors are proven carcinogens, DESs may provide workable alternatives to get around regulatory constraints. DESs have been used to study a variety of metal reduction processes, including those involving Zn,403–406 Sn, Cu,407,408 Ni,409 Ag,410 Cr,411 Al,412 Co, and Sm.413
Stainless steels have generally benefited from electropolishing, but DESs have also made it possible to electropolish aluminum, titanium, Ni/Co alloys, and super alloys. Stainless steel has received the majority of attention when it comes to electropolishing in deep eutectic solvents, but aluminum, titanium, Ni/Co alloys, and super alloys have also been studied.397 Using anodic electrolytic etching with an ethylene glycol:choline chloride DES, a new process has been created for removing surface oxide scale from single crystal aerospace castings of nickel-based super alloys generally used in turbine blades.414 This technique makes it possible to remove scale from cast components, allowing the detection of flawed components prior to the pricey and time-consuming heat treatment procedure.
In a recent review, it has been examined how crucial processes including metal winning, corrosion remediation, and catalyst preparation depend on the dissolving of metals and metal oxides.415 The processing and reprocessing of metals is the source of a huge volume of aqueous waste, with the treatment of acidic and basic byproducts being both energy and chemical expensive.
Many deep eutectic solvents have been the subject of in-depth studies regarding the solubility of metal oxides. A number of metal oxides have been demonstrated to be dissolved by type III DESs,416 and ligands like urea, thiourea, and oxalate are well-known complexants for many metals and can be added to the DES. Electrochemistry can be used to separate metals from complicated mixtures. However, this method has a drawback in that all of the liquids are completely miscible with water and cannot be used for a biphasic extraction. Three DESs based on choline chloride have been reported to dissolve 17 metal oxides in the elemental mass series Ti through to Zn.417 The careful selection of the hydrogen bond donor was resulted in the selectivity for extracting specific metals from complicated matrices.390
In general, DESs have the potential to replace inorganic acids, but this field is still in its infancy, in part because of unknown cost and technological factors.
8. Conclusions
Based on the review of the various technologies used to treat the aqueous acid waste produced by various industries, hyphenated technologies may be very effective for the circular economy and would have zero waste discharges into the environment. By combining membrane technologies with solvent extraction, ion-exchange, crystallization, precipitation, and other processes, numerous hyphenated technologies are made available. Combining two membrane technologies with distinct advantages is also possible, for example, diffusion dialysis with electrodialysis, membrane distillation or nanofiltration to produce pure and concentrated acid, salt remediation, and water reuse. However, the requirements for the acid waste produced by various sectors vary. Thus, it is necessary to integrate various approaches in order to fulfil the demand for a specific acidic waste discharge, to achieve the zero-waste discharge, and circular economy goals. Another interesting development would be the use of deep eutectic solvents as superior eco-friendly alternatives to inorganic acids. There has been a lot of research done on the potential uses of deep eutectic solvents in solvometallurgy, mineral dissolution, electroplating, and electropolishing, among other things. Deep eutectic solvents offer superior qualities to inorganic acids and are non-toxic, biodegradable, and recyclable, but still there are significant technological and financial barriers to overcome. Consequently, it may not be overstated to say that deep eutectic solvents could eventually replace inorganic acids in order to foster circular economy without putting undue strain on the environment or natural resources.Author contributions
C. Agarwal: conceptualization, data curation, resources, visualization, writing – original draft. A. K. Pandey: conceptualization, investigation, methodology, writing – review & editing.Conflicts of interest
The authors declare that they have no known conflict of interest that could have appeared to influence the work reported in this paper.Acknowledgements
No specific grant was given to this research by funding organizations in the public, private, or not-for-profit sectors. Prof. Hemlata K. Bagla, Vice-Chancellor of the HSNC University in Mumbai, is thanked by AKP for her support to this study. For his significant interest in the current work, CA sincerely thanks Dr P. K. Mohapatra, Head, Radiochemistry Division, BARC, Mumbai.References
- A. Agrawal and K. K. Sahu, An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries, J. Hazard. Mater., 2009, 171, 61–75 CrossRef CAS PubMed.
- Y. Nleya, G. S. Simate and S. Ndlovu, Sustainability assessment of the recovery and utilisation of acid from acid mine drainage, J. Cleaner Prod., 2016, 113, 17–27 CrossRef CAS.
- S. Tomiyama and T. Igarashi, The potential threat of mine drainage to groundwater resources, Curr Opin Environ Sci Health, 2022, 27, 100347 CrossRef.
- A. Gallego-Schmid and R. R. Z. Tarpani, Life cycle assessment of wastewater treatment in developing countries: A review, Water Res., 2019, 153, 63–79 CrossRef CAS PubMed.
- S. Tatjana, S. Vrishali, G. Ganapathy, T. David and P. Rohini, Wastewater discharge standards in the evolving context of urban sustainability–The case of India, Front. Environ. Sci., 2020, 8, 30 CrossRef.
- Z. Hana, C. Léa, C. Sandrine, T. Marc, K. Fatma, Z. Hatem, S. Sami and Q. Marianne, Effect of acidic industrial effluent release on microbial diversity and trace metal dynamics during resuspension of coastal sediment, Front Microbiol, 2018, 9, 3103 CrossRef.
- B. P. Degens, Acidic water discharge criteria for saline aquatic ecosystems in the WA Wheatbelt – a technical discussion paper, Salinity and Land Use Impacts Series, Report no. SLUI 65, Department of Water, Government of Western Australia, 2013, p. 30, ISSN 1449–5252 Search PubMed.
- M. Regel-Rosocka, A. Cieszynská and M. Wísniewski, Methods of regeneration of spent pickling solutions from steel treatment plants, Pol. J. Chem. Technol., 2007, 9, 42–45 CrossRef CAS.
- G. Leonzio, Recovery of metal sulphates and hydrochloric acid from spent pickling liquors, J. Cleaner Prod., 2016, 129, 417–426 CrossRef CAS.
- Listed Hazardous Wastes from Specific Sources Appendix C, http://www.cesenvironmental.com/KList.
- V. D. Grebenyuk, G. V. Sorokin, S. V. Verbich, L. H. Zhiginas, V. M. Linkov, N. A. Linkov and J. J. Smit, Combined sorption technology of heavy metal regeneration from electroplating rinse waters, Water SA, 1996, 22, 381–384 CAS.
- P. L. Kempster, W. H. J. Hattingh and H. R. van Vliet, Summarized Water Quality Criteria, No. TR 108, Department of Environment Affairs of South Africa, 1980 Search PubMed.
- A. M. Eyal, A. M. Baniel, K. Hadju and J. Mizrahi, New process for recovery of zinc sulfate and sulfuric acid from zinc electrowinning bleed solutions, Solvent Extr. Ion Exch., 1990, 8, 209–222 CrossRef CAS.
- M. Regel-Rosocka, A review on methods of regeneration of spent pickling solutions from steel processing, J. Hazard. Mater., 2010, 177, 57–69 CrossRef CAS.
- E. Álvarez-Ayuso, Approaches for the treatment of waste streams of the aluminium anodising industry, J. Hazard. Mater., 2009, 164, 409–414 CrossRef.
- X. Tongwen, Electrodialysis processes with bipolar membranes (EDBM) in environmental protection-A review, Resour., Conserv. Recycl., 2002, 37, 1–22 CrossRef.
- H. S. Lee, J. Y. Kim, J. W. Ahn, S. S. So and D. H. Chung, A basic research on the separation of mixed acid containing acetic acid and nitric acid discharged from semiconductor manufacturing process, in Digests of the 2006 Fall Meeting and 28th International Conference, KIRR, South Korea, November, 2006, pp. 137–141 Search PubMed.
- J.-Y. Kim, C.-H. Shin, H. Choi and W. Bae, Recovery of phosphoric acid from mixed waste acids of semiconductor industry by diffusion dialysis and vacuum distillation, Sep. Purif. Technol., 2012, 90, 64–68 CrossRef CAS.
- M. J. Servis, D. T. Wu and C. B. Jenifer, Network analysis and percolation transition in hydrogen bonded clusters: Nitric acid and water extracted by tributylphosphate, Phys. Chem. Chem. Phys., 2017, 19, 11326–11339 RSC.
- S. P. Hlushak, J. P. Simonin, B. Caniffi, P. Moisy, C. Sorel and O. Bernard, Description of partition equilibria for uranyl nitrate, nitric acid and water extracted by tributyl phosphate in dodecane, Hydrometallurgy, 2011, 109, 97–105 CrossRef CAS.
- M. C. Assuncao, G. Cote, M. Andre, H. Halleux and A. Chagnes, Phosphoric acid recovery from concentrated aqueous feeds by a mixture of di-isopropyl ether (DiPE) and tri-n-butylphosphate (TBP): extraction data and modeling, RSC Adv., 2017, 7, 6922 RSC.
- A. G. Baldwin, J. Servis, Y. Yang, Y. N. Bridges, D. T. Wu and J. C. Shafer, The structure of tributyl phosphate solutions: Nitric acid, uranium (VI), and zirconium (IV), J. Mol. Liq., 2017, 246, 225–235 CrossRef CAS.
- V. Chavan, S. Patra, A. K. Pandey, V. Thekkethil, M. Iqbal, J. Huskens, D. Sen, S. Mazumder, A. Goswami and W. Verboom, Understanding nitric acid-induced changes in the arrangement of monomeric and polymeric methacryloyl diglycolamides on their affinity toward f-element ions, J. Phys. Chem. B, 2015, 119, 212–218 CrossRef CAS PubMed.
- O. Prakash, A. M. Mhatre, R. Tripathi, A. K. Pandey, P. K. Yadav, S. A. Khan and P. Maiti, Lithium-irradiated poly(vinylidene fluoride) nanohybrid membrane for radionuclide waste management and tracing, ACS Appl. Polym. Mater., 2021, 3, 2005–2017 CrossRef CAS.
- G. Naidu, S. Ryu, R. Thiruvenkatachari, Y. Choi, S. Jeong and S. Vigneswaran, A critical review on remediation, reuse, and resource recovery from acid mine drainage, Environ. Pollut., 2019, 247, 1110–1124 CrossRef CAS.
- B. Dold, Sustainability in metal mining: from exploration, over processing to mine waste management, Rev. Environ. Sci. Biotechnol., 2008, 7, 275–285 CrossRef CAS.
- DIIS, Australian Energy Update 2016, Department of Industry Innovation and Science (DIIS), 2016, pp. 1–32 Search PubMed.
- M. Kalin, A. Fyson and W. N. Wheeler, The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage, Sci. Total Environ., 2006, 366, 395–408 CrossRef CAS PubMed.
- J. Taylor, S. Pape and N. Murphy, A summary of passive and active treatment technologies for acid and metalliferous drainage (AMD), in 5th Australian Workshop on Acid Mine Drainage, Fremantle, Australia, 2005 Search PubMed.
- G. S. Simate and S. Ndlovu, Acid mine drainage: challenges and opportunities, J. Environ. Chem. Eng., 2014, 2, 1785–1803 CrossRef CAS.
- M. J. Winterbourn, W. F. McDiffett and S. J. Eppley, Aluminium and iron burdens of aquatic biota in New Zealand streams contaminated by acid mine drainage: effects of trophic level, Sci. Total Environ., 2000, 254, 45–54 CrossRef CAS.
- G. Hilson, An overview of land use conflicts in mining communities, Land use policy, 2002, 19, 65–73 CrossRef.
- J. Easton, Acid Mine Drainage: A Reusable Water Resource, Industrial Water World, 2018. http://www.waterworld.com/articles/iww/print/volume-14/issue-6/features/acid-mine-drainage-a-reusable-water-resource.html Search PubMed.
- USDA, Acid Mine Drainage Form Impact of Hard Rock Mining on the National Forests: A Management Challenge, USDA Forest Service, 1993, vol. 1505, p. 12. Progr. Aid (Wash. D.C.) Search PubMed.
- P. Ziemkiewicz, J. Skousen and J. Simmons, Long-term performance of passive acid mine drainage treatment systems, Mine Water Environ., 2003, 22, 118–129 CrossRef CAS.
- MEND, List of Potential Information Requirements in Metal Leaching/Acid Rock Drainage Assessment and Mitigation Work, Mining Environment Neutral Drainage Program, Canada Centre for Mineral and Energy Technology, 2001, http://mend-nedem.org/mend-report/mend-manual-volume-1-summary Search PubMed.
- S. E. Mhlongo and F. Amponsah-Dacosta, A review of problems and solutions of abandoned mines in South Africa, Int. J. Min., Reclam. Environ., 2016, 30, 279–294 CrossRef.
- A. F. Britt, A. Schofield, M. Sexton, R. Simpson, D. C. Champion, A. Hughes, A. P. Hitchman, D. L. Huston, P. Kay, A. Senior and D. Summerfield, Australia's Identified Mineral Resources 2016, Geoscience Australia, Canberra, 2016 Search PubMed.
- A. F. Britt, A. Schofield, M. Sexton, R. Simpson, D. C. Champion, M. Smith, A. P. Hitchman, D. L. Huston, P. Kay, A. Senior, D. Summerfield and D. Roberts, Australia's Identified Mineral Resources 2017, Geoscience Australia, Canberra, 2017 Search PubMed.
- K. Venkateswarlu, R. Nirola, S. Kuppusamy, P. Thavamani, R. Naidu and M. Megharaj, Abandoned metalliferous mines: ecological impacts and potential approaches for reclamation, Rev. Environ. Sci. Biotechnol., 2016, 15, 327–354 CrossRef.
- R. W. Fitzpatrick, G. Grealish, P. Shand, S. L. Simpson, R. H. Merry and M. D. Raven, Acid Sulfate Soil Assessment in Finniss River, Currency Creek, Black Swamp and Goolwa Channel, South Australia, CSIRO Land and Water Science Report, 26/09, CSIRO, Adelaide, 2009 Search PubMed.
- G. Montavon, M. Fuhrmann and B. Hock, Impacts of acid deposition on soil and water systems, Environ. Sci. Pollut. Res., 2008, 15, 455–464 Search PubMed.
- A. Akcil and S. Koldas, Acid Mine Drainage (AMD): causes, treatment and case studies, J. Cleaner Prod., 2006, 14, 1139–1145 CrossRef.
- P. Grennfelt, A. Engleryd, M. Forsius, Ø. Hov, H. Rodhe and E. Cowling, Acid rain and air pollution: 50 years of progress in environmental science and policy, Ambio, 2020, 49, 849–864 CrossRef CAS PubMed.
- J. P. Baker and S. W. Christensen, Effects of Acidification on Biological Communities in Aquatic Ecosystems, in Acidic Deposition and Aquatic Ecosystems-Regional Case Studies, ed. D. F. Charles, Springer-Verlag, New York, 1991, vol. 83–106 Search PubMed.
- J. H. Lee and J. W. Kang, Environmental effects of acid mine drainage on stream ecosystems, Ecotoxicol. Environ. Saf., 2013, 92, 29–36 Search PubMed.
- C. T. Driscoll, G. B. Lawrence, A. J. Bulger, T. J. Butler, C. S. Cronan, C. Eagar, K. F. Lambert, G. E. Likens, J. L. Stoddard and K. C. Weathers, Acidic Deposition in the Northeastern United States: Sources and Inputs, Ecosystem Effects, and Management Strategies: The effects of acidic deposition in the northeastern United States include the acidification of soil and water, which stresses terrestrial and aquatic biota, BioScience, 2001, 51, 180–198 CrossRef.
- D. Mackay and A. Fraser, Ecotoxicity of inorganic acids: A review, Environ. Toxicol. Chem., 2000, 19, 714–722 Search PubMed.
- I. A. Neamtiu, S. R. Al-Abed, J. L. McKernan, C. L. Baciu, E. S. Gurzau, A. O. Pogacean and S. M. Bessler, Metal contamination in environmental media in residential areas around Romanian mining sites, Rev. Environ. Health, 2017, 32, 215–220 CAS.
- E. Bonnail, R. C. Lima, E. B. Chamizo, M. J. Salamanca and P. Cruz-Hernandez, Biomarker responses of the freshwater clam Corbicula fluminea in acid mine drainage polluted systems, Environ. Pollut., 2018, 242, 1659–1668 CrossRef CAS.
- H. Okochi, H. Kameda, S. Hasegawa, N. Saito, K. Kubota and M. Igawa, Deterioration of concrete structures by acid deposition – An assessment of the role of rain water on deterioration by laboratory and field exposure experiments using mortar specimens, Atmos. Environ., 2000, 34, 2937–2945 CrossRef CAS.
- A. Singh and M. Agrawal, Acid rain and its ecological consequences, J. Environ. Biol., 2008, 29, 15–24 CAS.
- R. Sersale, G. Frigione and L. Bonavita, Acid deposition and concrete attack: main influence, Cem. Concr. Res., 1998, 28, 19–24 CrossRef CAS.
- M. Kamali, D. P. Suhas, M. E. Costa, I. Capela and T. M. Aminabhavi, Sustainability considerations in membrane-based technologies for industrial effluents treatment, Chem. Eng. J., 2019, 368, 474–494 CrossRef CAS.
- V. Krstić, T. Urošević and B. Pešovski, A review on adsorbents for treatment of water and wastewaters containing copper ions, Chem. Eng. Sci., 2018, 192, 273–287 CrossRef.
- X. Qu, P. J. J. Alvarez and Q. Li, Applications of nanotechnology in water and wastewater treatment, Water Res., 2013, 47, 3931–3946 CrossRef CAS.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Sustainable technologies for water purification from heavy metals: review and analysis, Chem. Soc. Rev., 2019, 48, 463–487 RSC.
- M. Xie, H. K. Shon, S. R. Gray and M. Elimelech, Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction, Water Res., 2016, 89, 210–221 CrossRef CAS.
- N. Shehata, D. Egirani, A. G. Olabi, A. Inayat, M. A. Abdelkareem, K.-J. Chae and E. T. Sayed, Membrane-based water and wastewater treatment technologies: Issues, current trends, challenges, and role in achieving sustainable development goals, and circular economy, Chemosphere, 2023, 320, 137993 CrossRef CAS PubMed.
- J. López, O. Gibert and J. L. Cortina, Integration of membrane technologies to enhance the sustainability in the treatment of metal-containing acidic liquid wastes. An overview, Sep. Purif. Technol., 2021, 265, 118485 CrossRef.
- K. K. Kefeni, T. A. M. Msagati and B. B. Mamba, Acid mine drainage: prevention, treatment options, and resource recovery: A review, J. Cleaner Prod., 2017, 151, 475–493 CrossRef CAS.
- D. B. Johnson and K. B. Hallberg, Acid mine drainage remediation options: A review, Sci. Total Environ., 2005, 338, 3–14 CrossRef.
- I. Moodley, C. M. Sheridan, U. Kappelmeyer and A. Akcil, Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products, Miner. Eng., 2018, 126, 207–220 CrossRef CAS.
- A. Roy Chowdhury, D. Sarkar and R. Datta, Remediation of acid mine drainage impacted water, Curr. Pollut. Rep., 2015, 1, 131–141 CrossRef CAS.
- O. Gibert, T. S. Rötting, J. L. Cortina, J. de Pablo, C. Ayora, J. Carrera and J. Bolzicco, In-situ remediation of acid mine drainage using a permeable reactive barrier in Aznalcóllar (Sw Spain), J. Hazard. Mater., 2011, 191, 287–295 CrossRef CAS PubMed.
- S. B. Wu, S. Wallace, H. Brix, P. Kuschk, W. K. Kirui, F. Masi and R. J. Dong, Treatment of industrial effluents in constructed wetlands: Challenges, operational strategies and overall performance, Environ. Pollut., 2015, 201, 107–120 CrossRef CAS.
- J. Garcia, D. P. L. Rousseau, J. Morato, E. Lesage, V. Matamoros and J. M. Bayona, Contaminant removal processes in subsurface-flow constructed wetlands: A review, Crit. Rev. Environ. Sci. Technol., 2010, 40, 561–661 CrossRef CAS.
- J. Vymazal, Constructed wetlands for wastewater treatment: five decades of experience, Environ. Sci. Technol., 2011, 45, 61–69 CrossRef CAS PubMed.
- R. Liu, Y. Zhao, L. Doherty, Y. Hu and X. Hao, A review of incorporation of constructed wetland with other treatment processes, Chem. Eng. J., 2015, 279, 220–230 CrossRef CAS.
- M. A. Caraballo, F. MacÍas, T. S. Rötting, J. M. Nieto and C. Ayora, Long term remediation of highly polluted acid mine drainage: A sustainable approach to restore the environmental quality of the Odiel river basin, Environ. Pollut., 2011, 159, 3613–3619 CrossRef CAS.
- J. G. Skousen, A. Sexstone and P. F. Ziemkiewicz, Acid mine drainage control and treatment, Agronomy, 2000, 41, 68–131 Search PubMed.
- M. Brown, B. Barley and H. Wood, Mine Water Treatment: Technology, Application and Policy, IWA Publishing, London, 2002 Search PubMed.
- A. N. Silveira, R. Silva and J. Rubio, Treatment of acid mine drainage (AMD) in South Brazil: comparative active processes and water reuse, Int. J. Miner. Process., 2009, 93, 103–109 CrossRef.
- B. J. Watten, P. L. Sibrell and M. F. Schwartz, Acid neutralization within limestone sand reactors receiving coal mine drainage, Environ. Pollut., 2005, 137, 295–304 CrossRef CAS PubMed.
- W. M. Gitari, L. F. Petrik, O. Etchebers, D. L. Key, E. Iwuoha and C. Okujeni, Passive neutralisation of acid mine drainage by fly ash and its derivatives: a column leaching study, Fuel, 2008, 87, 1637–1650 CrossRef CAS.
- C. A. Ríos, C. D. Williams and C. L. Roberts, Removal of heavy metals from acid mine drainage (AMD) using coal fly ash, natural clinker and synthetic zeolites, J. Hazard. Mater., 2008, 156, 23–35 CrossRef.
- D. Mohan and S. Chander, Removal and recovery of metal ions from acid mine drainage using lignite-A low cost sorbent, J. Hazard. Mater., 2006, 137, 1545–1553 CrossRef CAS PubMed.
- E. Repo, E. R. Petrus, M. Sillanpää and J. K. Warchoł, Equilibrium studies on the adsorption of Co(II) and Ni(II) by modified silica gels: one-component and binary systems, Chem. Eng. J., 2011, 172, 376–385 CrossRef CAS.
- E. Yoĝurtcuoĝlu and M. Uçurum, Surface modification of calcite by wet-stirred ball milling and its properties, Powder Technol., 2011, 214, 47–53 CrossRef.
- Y. Zhao, S. Yang, D. Ding, J. Chen, Y. Yang, Z. Lei, C. Feng and Z. Zhang, Effective adsorption of Cr(VI) from aqueous solution using natural Akadama clay, J. Colloid Interface Sci., 2013, 395, 198–204 CrossRef CAS PubMed.
- M. E. Argun and S. Dursun, A new approach to modification of natural adsorbent for heavy metal adsorption, Bioresour. Technol., 2008, 99, 2516–2527 CrossRef CAS PubMed.
- P. L. Sibrell, B. J. Watten, T. A. Haines and B. W. Spaulding, Limestone fluidized bed treatment of acid-impacted water at the Craig Brook National Fish Hatchery Maine, USA, Aquac Eng, 2006, 34, 61–71 CrossRef.
- H. A. Aziz, M. N. Adlan and K. S. Ariffin, Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) removal from water in Malaysia: post treatment by high quality limestone, Bioresour. Technol., 2008, 99, 1578–1583 CrossRef CAS PubMed.
- W. H. Strosnider and R. W. Nairn, Effective passive treatment of high-strength acid mine drainage and raw municipal wastewater in Potosí, Bolivia using simple mutual incubations and limestone, J. Geochem. Explor., 2010, 105, 34–42 CrossRef CAS.
- A. M. Silva, R. M. F. Lima and V. A. Leão, Mine water treatment with limestone for sulfate removal, J. Hazard. Mater., 2012, 221–222, 45–55 CrossRef CAS PubMed.
- E. Iakovleva, E. Mäkilä, J. Salonen, M. Sitarz, S. Wang and M. Sillanpää, Acid mine drainage (AMD) treatment: Neutralization and toxic elements removal with unmodified and modified limestone, Ecol. Eng., 2015, 81, 30–40 CrossRef.
- M. Duca and M. T. M. Koper, Powering denitrification: the perspectives of electrocatalytic nitrate reduction, Energy Environ. Sci., 2012, 5, 9726–9742 RSC.
- J. E. Santos Jallath, F. M. Romero, R. Iturbe Argüelles, A. Cervantes Macedo and J. Goslinga Arenas, Acid drainage neutralization and trace metals removal by a two-step system with carbonated rocks, Estado de Mexico, Mexico, Environ. Earth Sci., 2018, 77, 86 CrossRef.
- J. L. Steimke and C. M. Boyd, Nitrate Destruction Literature Survey and Evaluation Criteria, Report SRNL-STI-2010-00565, 2011 Search PubMed.
- J. Y. Park and Y. J. Yoo, Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose, Appl. Microbiol. Biotechnol., 2009, 82, 415–429 CrossRef CAS PubMed.
- M. I. M. Soares, Biological denitrification of groundwater, Water Air Soil Pollut., 2000, 123, 183–193 CrossRef CAS.
- S. A. Saad, L. Welles, B. Abbas, C. M. Lopez-Vazquez, M. C. M. van Loosdrecht and D. Brdjanovic, Denitrification of nitrate and nitrite by ‘Candidatus Accumulibacter phosphatis’ clade IC, Water Res., 2016, 105, 97–109 CrossRef CAS.
- V. Mateju, S. Cizinska, J. Krejci and T. Janoch, Biological water denitrification-A review, Enzyme Microb. Technol., 1992, 14, 170–183 CrossRef CAS.
- K. M. Hiscock, J. W. Lloyd and D. N. Lerner, Review of natural and artificial denitrification of groundwater, Water Res., 1991, 25, 1099–1111 CrossRef CAS.
- S. Ghafari, M. Hasan and M. K. Aroua, Bio-electrochemical removal of nitrate from water and wastewater-A review, Bioresour. Technol., 2008, 99, 3965–3974 CrossRef CAS PubMed.
- J. C. Fanning, The chemical reduction of nitrate in aqueous solution, Coord. Chem. Rev., 2000, 199, 159–179 CrossRef CAS.
- X. Song, Z. Chen, X. Wang and S. Zhang, Ligand effects on nitrate reduction by zero-valent iron: role of surface Complexation, Water Res., 2017, 114, 218–227 CrossRef CAS PubMed.
- S. Jung, S. Bae and W. Le, Development of Pd-Cu/Hematite catalyst for selective nitrate reduction, Environ. Sci. Technol., 2014, 48, 9651–9658 CrossRef CAS PubMed.
- Y. Xie, H. Cao, Y. Li, Y. Zhang and J. C. Crittenden, Highly selective PdCu/amorphous Silica_Alumina (ASA) catalysts for groundwater denitration, Environ. Sci. Technol., 2011, 45, 4066–4072 CrossRef CAS PubMed.
- A. Garron and F. Epron, Use of formic acid as reducing agent for application in catalytic reduction of nitrate in water, Water Res., 2005, 39, 3073–3081 CrossRef CAS PubMed.
- F. A. Marchesini, S. Irusta, C. Querini and E. Miro, Spectroscopic and catalytic characterization of Pd-In and Pt-In supported on Al2O3 and SiO2, active catalysts for nitrate hydrogenation, Appl. Catal., A, 2008, 348, 60–70 CrossRef CAS.
- A. P. Murphy, Chemical removal of nitrate from water, Nature, 1991, 350, 223–225 CrossRef CAS.
- V. Rosca, M. Duca, M. T. de Groot and M. T. M. Koper, Nitrogen cycle electrocatalysis, Chem. Rev., 2009, 109, 2209–2244 CrossRef CAS PubMed.
- N. Barrabes and J. Sa, Catalytic nitrate removal from water, past, present and future perspectives, Appl. Catal., B, 2011, 104, 1–5 CrossRef CAS.
- J. Shen, Y. Y. Birdja and M. T. M. Koper, Electrocatalytic nitrate reduction by a cobalt protoporphyrin immobilized on a pyrolytic graphite electrode, Langmuir, 2015, 31, 8495–8850 CrossRef CAS PubMed.
- L. Su, K. Li, H. Zhang, M. Fan, D. Ying, T. Sun, Y. Wang and J. Jia, Electrochemical nitrate reduction by using a novel Co3O4/Ti cathode, Water Res., 2017, 120, 1–11 CrossRef CAS PubMed.
- J. Katsounaros, M. Dortsiou and G. Kyriacou, Electrochemical reduction of nitrate and nitrite in simulated liquid nuclear wastes, J. Hazard. Mater., 2009, 171, 323–327 CrossRef PubMed.
- T. Yang, K. Doudrick and P. Westerhoff, Photocatalytic reduction of nitrate using titanium dioxide for regeneration of ion exchange brine, Water Res., 2013, 47, 1299–1307 CrossRef CAS.
- J. L. Cox, R. T. Hallen and M. A. Liliga, Thermochemical nitrate destruction, Environ. Sci. Technol., 1994, 28, 423–428 CrossRef CAS PubMed.
- A. C. Hutson and A. Sen, Unprecedented homogeneous reduction of ionic nitrogen oxo compounds to ammonia by organics in sulfuric acid, J. Am. Chem. Soc., 1994, 116, 4527–4528 CrossRef CAS.
- K. J. Seu, A. P. Pandey, F. Haque, E. A. Proctor, A. E. Ribbe and J. S. Hovis, Effect of surface treatment on diffusion and domain formation in supported lipid bilayers, Biophys. J., 2007, 92, 2445–2450 CrossRef CAS PubMed.
- K. J. Ziegler, Z. Gu, H. Peng, E. L. Flor, R. H. Hauge and R. E. Smalley, Controlled oxidative cutting of single-walled carbon nanotubes, J. Am. Chem. Soc., 2005, 127, 1541–1547 CrossRef CAS PubMed.
- K. J. Ziegler, Z. Gu, J. Shaver, Z. Chen, E. L. Flor, D. J. Schmidt, C. Chan, R. H. Hauge and R. E. Smalley, Cutting single-walled carbon nanotubes, Nanotechnology, 2005, 16, S539–S544 CrossRef PubMed.
- R. Voelkel, Wafer-scale micro-optics fabrication, Adv. Opt. Technol., 2012, 1, 135–150 CrossRef CAS.
- D. Knudsen, B. Harnish, R. Toth and M. Yan, Creating microstructures on silicon wafers using UV-crosslinked polystyrene thin films, Polym. Eng. Sci., 2009, 49, 945–948 CrossRef CAS.
- P. Schulz, Method for reducing metal contamination of silicon wafers during semiconductor manufacturing, US Pat., US5853491, 1998 Search PubMed.
- L. He, F. Lin, X. Li, Z. Xu and H. Sui, Enhancing heavy oil liberation from solid surfaces using biodegradable demulsifiers, J. Environ. Chem. Eng., 2016, 4, 1753–1758 CrossRef CAS.
- G. Kenanakis and N. Katsarakis, ZnO nanowires on glass via chemical routes: a prospective photocatalyst for indoors applications, J. Environ. Chem. Eng., 2014, 2, 1416–1422 CrossRef CAS.
- G. Kenanakis and N. Katsarakis, Chemically grown TiO2 on glass with superior photocatalytic properties, J. Environ. Chem. Eng., 2014, 2, 1748–1755 CrossRef CAS.
- J. Singh, A. Mehta, M. Rawat and S. Basu, Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction, J. Environ. Chem. Eng., 2018, 215, 121–124 Search PubMed.
- D. S. Carretero, C.-p. Huang, J.-H. Tzeng and C.-p. Huang, The recovery of sulfuric acid from spent piranha solution over a dimensionally stable anode (DSA) Ti-RuO2 electrode, J. Hazard. Mater., 2021, 406, 124658 CrossRef CAS PubMed.
- L. Dahlgren, Treatment of spent pickling acid from stainless steel production: A review of regeneration technologies with focus on the neutralisation process for implementation in chinese industry, Master of Science thesis, Industrial Ecology, Royal Institute of Technology, Stockholm, 2010, ISSN 1402-7615.
- G. Cusano, M. R. Gonzalo, F. Farrell, R. Remus, S. Roudier and L. Delgado Sancho, Best Available Techniques (BAT) Reference Document for the Main Non-ferrous Metals Industries, EUR 28648, Publications Office of the European Union, Luxembourg, 2017, ISSN 1831-9424 Search PubMed.
- V. Baltazar, G. B. Harris and C. W. White, The selective recovery and concentration of sulfuric acid by electrodialysis, Hydrometallurgy, 1992, 30, 463–481 CrossRef CAS.
- K. R. Buban, M. J. Collins and I. M. Masters, Zinc and iron control: overview iron control in zinc pressure leach processes, JOM, 1999, 51, 23–25 CrossRef CAS.
- K. J. Torfs and J. Vliegen, Proceedings of the 2nd International Symposium on Iron Control in Hydrometallugy, Ottawa, Canada, ed. J. E. Dutrizac and G. B. Harris, Metallurgical Society of the CIM, Canada, October 20–23, 1996, pp. 135–146 Search PubMed.
- M. R. C. Ismael and J. M. R. Carvalho, Iron recovery from sulphate leach liquors in zinc hydrometallurgy, Min. Eng., 2003, 16, 31–39 CrossRef CAS.
- U. Kerney, Treatment of spent pickling acid from hot dip galvanising, Resour., Conserv. Recycl., 1994, 10, 145–151 CrossRef.
- U. Kerney, Possible ways for recycling of spent zinc containing pickling solutions from hot dip galvanizing workshops, Metallurgy, 1992, 46, 907–911 CAS.
- J. Dufour, J. O. Marron, C. Negro, R. Latorre, A. Formoso and F. Lopenz-Mateos, Mechanism and kinetic control of the oxyprecipitation of sulphuric liquors from steel pickling, Chem. Eng. J., 1997, 68, 173–187 CrossRef CAS.
- G. Pourcelly, I. Tugas and C. Gavach, Electrotransport of sulphuric acid in special anion exchange membranes for the recovery of acids, J. Membr. Sci., 1994, 97, 99–107 CrossRef CAS.
- A. Lopez-Delgoda, F. J. Alguacil and F. A. Lopez, Recovery of iron from bio-oxidized sulphuric pickling wastewater by precipitation as basic sulphates, Hydrometallurgy, 1997, 45, 97–112 CrossRef.
- H. Lu, J. Wang, T. Wang, N. Wang, Y. Bao and H. Hao, Crystallization techniques in wastewater treatment: An overview of applications, Chemosphere, 2017, 173, 474–484 CrossRef CAS.
- T. Ozdemir, C. Oztin and N. S. Kincal, Treatment of waste pickling liquors: process synthesis and economic analysis, Chem. Eng. Commun., 2006, 193, 548–563 CrossRef.
- M. F. Kerstin and A. C. Rasmuson, Recycling of waste pickle acid by precipitation of metal fluoride hydrates, Miner. Eng., 2007, 20, 950–955 CrossRef.
- M. F. Kerstin and A. C. Rasmuson, Crystallization of metal fluoride hydrates from mixed hydrofluoric and nitric acid solutions, Part I, Iron (III) and Chromium (III), J. Cryst. Growth, 2010, 312, 2351–2357 CrossRef.
- J. L. Galvez, J. Dufour, C. Negro and F. Lopez-Mateos, Kinetics of K2FeF5.H2O (s) and CrF3.2H2O (s) crystallization from stainless steel spent pickling baths, Ind. Eng. Chem. Res., 2007, 46, 5221–5227 CrossRef CAS.
- J. Hermoso, J. Dufour, J. L. Gálvez, C. Negro and F. López-Mateos, Nickel hydroxide recovery from stainless steel pickling liquors by selective precipitation, Ind. Eng. Chem. Res., 2005, 44, 5750–5756 CrossRef CAS.
- A. Kehrmann, Method of producing ferrous sulfate heptahydrate, US Pat., 7097816B2, 2006 Search PubMed.
- C. J. Brown and D. R. Olsen, Regeneration of hydrochloric acid pickle liquors by crystallization, in Third International Symposium on Iron Control in Hydrometallurgy, Montreal, Canada, 2006 Search PubMed.
- D. R. Olsen and C. D. Blumenschein, System and method for converting the spent remnant of a first pickling acid solution into a useable second pickling acid solution, US Pat., 7351391B1, 2008 Search PubMed.
- M. Watanabe and S. Nishimura, Process for Recovery of Waste H2SO4 and HCl, US Pat., 4177119, 1979 Search PubMed.
- K. Jozsef, M. Andor and S. Miklos, Method for the cyclic electrochemical processing of sulfuric acid-containing pickle waste liquors, US. Pat., 3969207, 1976 Search PubMed.
- U. K. Kesieme, Mine Waste Water Treatment and Acid Recovery Using Membrane Distillation and Solvent Extraction, PhD thesis, Victoria University Melbourne, 2015.
- U. K. Kesieme and H. Aral, Novel application of membrane distillation and solvent extraction for acid and water recovery from acidic mining and process solutions, J. Environ. Chem. Eng., 2015, 3, 2050–2056 CrossRef CAS.
- U. K. Kesieme, H. Aral, M. Duke, N. Milne and C. Y. Cheng, Recovery of sulphuric acids from waste and process solutions using solvent extraction, Hydrometallurgy, 2013, 138, 14–20 CrossRef CAS.
- M. Dejak, Acid purification and recovery using resin sorption technology-An update, presented at AESF/EPA Pollution Prevention Conference, 1994 Search PubMed.
- C. J. Brown and P. Eng, Recovery of stainless steel pickle liquors: purification Vs regeneration, CISA International Steel Congress, Beijing, China, Tech Paper, Eco-Tec Inc., Pickering, Ontario, Canada, 2002, vol. 158, pp. 1–14 Search PubMed.
- C. J. Brown, Fluid treatment process and apparatus, US. Pat., 4673507, 1987 Search PubMed.
- V. Nenov, N. Dimitrova and I. Dobrevsky, Recovery of sulphuric acid from waste aqueous solutions containing arsenic by ion exchange, Hydrometallurgy, 1997, 44, 43–52 CrossRef CAS.
- K. A. Kraus, F. Nelson and J. F. Baxter, Anion-exchange studies. Separation of sulfuric acid from metal sulfates by anion exchange, J. Am. Chem. Soc., 1953, 75, 2768–2770 CrossRef CAS.
- I. P. Shamritska, V. P. Meleshko and L. A. Poluhina, Theory and practice of sorption processes, Voronezh, 1971, 5, 76 Search PubMed.
- I. A. Poluhina and I. P. Shamritska, Theory and practice of sorption processes, Voronezh, 1972, 7, 32 Search PubMed.
- C. J. Brown, D. Davey and P. J. Simmons, Purification of sulfuric acid anodizing solutions, Plat. Surf. Finish., 1979, 54–57 CAS.
- C. J. Brown, D. Davey and P. J. Simmons, Recovery of nitric acid from solutions used for treating metal surfaces, Plat. Surf. Finish., 1980, 67, 60–62 CAS.
- M. J. Hatch and J. A. Dillon, Acid retardation-simple physical method for separation of strong acids from their salts, Ind. Eng. Chem. Process Des. Dev., 1963, 2, 253–263 CrossRef CAS.
- E. Petkova, H. Vassilev and V. Shkodrova, Separation of waste plating solution sulphuric acid from metal cations by anion exchange, Hydrometallurgy, 1981, 6, 291–297 CrossRef CAS.
- U. K. Kesieme, A. Chrysanthou, M. Catulli and C. Y. Cheng, Optimal use of tris-2-ethylhexylamine to recover hydrochloric acid and metals from leach solutions and comparison with other extractant, J. Environ. Chem. Eng., 2018, 6, 3177–3184 CrossRef CAS.
- A. Agrawal, S. Kumari, B. C. Ray and K. K. Sahu, Extraction of acid and iron from sulphate waste pickle liquor of a steel industry by solvent extraction, Hydrometallurgy, 2007, 88, 55–66 CrossRef.
- A. Agrawal, S. Kumari and K. K. Sahu, Liquid-liquid extraction of sulphuric acid from zinc bleed stream, Hydrometallurgy, 2008, 92, 42–47 CrossRef CAS.
- A. Agrawal and K. K. Sahu, An overview of the recovery of acid from spent solutions from steel and electroplating industries, J. Hazard. Mater., 2009, 171, 61–75 CrossRef CAS PubMed.
- U. K. Kesieme, N. Milne, C. Y. Cheng, H. Aral and M. Duke, Recovery of water and acid from leach solution using direct contact membrane distillation, Water Sci. Technol., 2014, 69, 868–875 CrossRef CAS PubMed.
- A. Agrawal, S. Kumari and K. K. Sahu, Comparative performance assessment of solvents for the extraction of H2SO4 from spent electrolytic bleed stream of copper industry, J. Mol. Liq., 2016, 220, 82–91 CrossRef CAS.
- D. S. Fleet, Solvent extraction in hydrometallurgy: the role of organophosphorous extractants, J. Organomet. Chem., 2005, 690, 2426–2438 CrossRef.
- G. M. Ritecy, Solvent Extraction Principles and Application to Process Metallurgy, revised ed, Elsevier Science, 2006, vol. 2, pp. 613–638 Search PubMed.
- A. M. Eyal, B. Hazan and R. Bloch, Recovery and concentration of strong acid from dilute solution through LLXII: reversible extraction with branched – chain amines, Solvent Extr. Ion Exch., 1991, 9, 221–222 Search PubMed.
- A. M. Eyal, B. Hazan and R. Bloch, Recovery and concentration of strong acid from dilute solution through LLXIII: Temperature swing based process, Solvent Extr. Ion Exch., 1991, 9, 223–226 CrossRef CAS.
- A. M. Eyal and R. Canari, pH dependence of carboxylic and mineral acid extraction by amine based extractant: Effect of pKa, amine basicity and diluents properties, Ind. Eng. Chem. Res., 1995, 34, 1789–1798 CrossRef CAS.
- W. Liao, Q. Shang and G. Yu, Three phase extraction study of Cyanex 923 – n-heptane/H2SO4 system, Talanta, 2002, 57, 1085–1092 CrossRef CAS.
- W. Liao, G. Yu, S. Yue and D. Li, Kinetics of cerium (IV) extraction from H2SO4-HF medium with Cyanex 923, Talanta, 2002, 56, 613–618 CrossRef CAS.
- F. J. Alguacil and F. A. Lopez, The extraction of mineral acid by phosphine oxide Cyanex 923, Hydrometallurgy, 1996, 42, 245–255 CrossRef CAS.
- W. A. Rickelton, The extraction of mineral acids with liquid phosphine oxide, in Solvent Extraction in the Process Industries, ed. D. H. Logsdail and M. J. Slater, Elsevier Applied Science, London, 1993, vol. 2, p. 731 Search PubMed.
- D. F. Haghshenas, D. Darvishi, H. Rafieipour, E. K. Alamdari and A. A. Salardini, A comparison between TEHA and Cyanex 923 on the separation and recovery of sulphuric from aqueous solutions, Hydrometallurgy, 2009, 97, 173–179 CrossRef CAS.
- K. Sarangi, E. Padhan, P. V. Sarma, K. H. Park and R. P. Das, Removal/recovery of hydrochloric acid using Alamine 336, Aliquat 336, TBP and Cyanex 923, Hydrometallurgy, 2006, 84, 125–129 CrossRef CAS.
- S. K. Jaiswal, D. Mandal and R. V. Visweswara Rao, Recovery and reuse of nitric acid from effluents containing free nitric acid in absence and presence of metal nitrates, Chem. Eng. J., 2015, 266, 271–278 CrossRef CAS.
- C. Hoon-Shin, J. Y. Kim, H. S. Kim, H. S. Lee, D. Mohaptara, J. G. Ahn and W. Bae, Recovery of nitric acid from waste etching using solvent extraction, J. Hazard. Mater., 2009, 163, 729–734 CrossRef.
- E. Hesford and H. A. C. McKay, The extraction of mineral acids by tri-n-butyl phosphate (TBP), J. Inorg. Nucl. Chem., 1960, 13, 156–164 CrossRef CAS.
- M. Lee, G. J. Ahn and J. W. Ahn, Recovery of copper, tin and lead from the spent nitric etching solutions of printed circuit board and regeneration of the etching solution, Hydrometallurgy, 2003, 70, 23–29 CrossRef CAS.
- P. Kulkarni, Recovery of uranium (VI) from acidic wastes using tri-noctylphosphine oxide and sodium carbonate based liquid membranes, Chem. Eng. J., 2003, 92, 209–214 CrossRef CAS.
- Y. Zhang, M. Valiente and M. Muhammed, Extraction of nitric acid and phosphoric acid with tri-butyl phosphate, Solvent Extr. Ion Exch., 1989, 7, 173–200 CrossRef CAS.
- S. Mishra, S. Ganesh, P. Velavendan, N. K. Pandey, C. Mallika, U. K. Mudali and R. Natarajan, Thermodynamics of solubility of tri-n-butyl phosphate in nitric acid solutions, Adv. Chem. Eng. Res., 2013, 2, 55–60 Search PubMed.
- T. Sekine and Y. Hasegawa, Solvent Extraction Chemistry, Publisher Marcel Dekker, New York, 1977 Search PubMed.
- M. C. Assuncao, G. Cote, M. Andre, H. Halleux and A. Chagnes, Phosphoric acid recovery from concentrated aqueous feeds by a mixture of di-isopropyl ether (DiPE) and tri-n-butylphosphate (TBP): extraction data and modeling, RSC Adv., 2017, 7, 6922 RSC.
- A. G. Baldwin, J. Servis, Y. Yang, Y. N. Bridges, D. T. Wu and J. C. Shafer, The structure of tributyl phosphate solutions: Nitric acid, uranium (VI), and zirconium (IV), J. Mol. Liq., 2017, 246, 225–235 CrossRef CAS.
- G. A. Yagodin, V. V. Sergievskii, A. V. Bogdanov, N. S. Smironova and Y. A. Kiryanov, The extraction of phosphoric acid by tri-n-butyl phosphate, Russ. J. Inorg. Chem., 1981, 26, 879 Search PubMed.
- L. L. Krupatkin and T. A. Shcherbakova, Extraction of Phosphoric acid by tributyl phosphate, Radiokhimiya, 1969, 11, 37 Search PubMed.
- H. Ahmed, H. Diamonta, C. Chaker and R. Abdelhamid, Purification of wet process phosphoric acid by solvent extraction with TBP and MIBK mixtures, Sep. Purif. Technol., 2007, 55, 212–216 CrossRef.
- R. Rautenbach and A. Gröschl, Separation potential of nanofiltration membranes, Desalination, 1990, 77, 73–84 CrossRef CAS.
- A. W. Mohammad, Y. H. Teowa, W. L. Ang, Y. T. Chung, D. L. Oatley-Radcliffe and N. Hilal, Nanofiltration membranes review: recent advances and future prospects, Desalination, 2015, 356, 226–254 CrossRef CAS.
- I. Vergili, Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources, J. Environ. Manage., 2013, 127, 177–187 CrossRef CAS PubMed.
- F.-f. Chang, W.-j. Liu and X.-m. Wang, Comparison of polyamide nanofiltration and low-pressure reverse osmosis membranes on As(III) rejection under various operational conditions, Desalination, 2014, 334, 10–16 CrossRef CAS.
- M. Homayoonfal, A. Akbari and M. R. Mehrnia, Preparation of polysulfone nanofiltration membranes by UV-assisted grafting polymerization for water softening, Desalination, 2010, 263, 217–225 CrossRef CAS.
- S. Bunani, E. Yörükoğlu, G. Sert, Ü. Yüksel, M. Yüksel and N. Kabay, Application of nanofiltration for reuse of municipal wastewater and quality analysis of product water, Desalination, 2013, 315, 33–36 CrossRef CAS.
- G. Li, W. Wang and Q. Du, Applicability of nanofiltration for the advanced treatment of landfill leachate, J. Appl. Polym. Sci., 2010, 166, 2343–2347 Search PubMed.
- L. M. Ortega, Ŕ. Lebrunb, I. M. Nöel and R. Hausler, Application of nanofiltration in the recovery of chromium (III) from tannery effluents, Sep. Purif. Technol., 2005, 44, 45–52 CrossRef CAS.
- M. Unlu, H. Yukseler and U. Yetis, Indigo dyeing wastewater reclamation by membrane-based filtration and coagulation processes, Desalination, 2009, 240, 178–185 CrossRef CAS.
- D. Jakobs and G. Baumgarten, Nanofiltration of nitric acidic solutions from picture tube production, Desalination, 2002, 145, 65–68 CrossRef CAS.
- A. M. Mika, A. K. Pandey and R. F. Childs, Ultra-low pressure water softening with pore-filled membranes, Desalination, 2001, 140, 265–275 CrossRef CAS.
- M. V. Galiana-Aleixandre, A. Iborra-Clar, A. Bes-Pifi, J. A. Mendoza-Roca, B. Cuartas-Uribe and M. I. Iborra-Clar, Nanofiltration for sulfate removal and water reuse of the pickling and tanning processes in a tannery, Desalination, 2005, 179, 307–313 CrossRef CAS.
- A. O. Aguiar, L. H. Andrade, B. C. Ricci, W. L. Pires, G. A. Miranda and M. C. S. Amaral, Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions, Sep. Purif. Technol., 2016, 170, 360–369 CrossRef CAS.
- T. Yun, J. W. Chung and S.-Y. Kwak, Recovery of sulfuric acid aqueous solution from copper-refining sulfuric acid wastewater using nanofiltration membrane process, J. Environ. Manage., 2018, 223, 652–657 CrossRef CAS PubMed.
- G. Shang, G. Zhang, C. Gao, W. Fu and L. i. Zeng, A novel nanofiltration process for the recovery of vanadium from acid leach solution, Hydrometallurgy, 2014, 142, 94–97 CrossRef CAS.
- A. R. Guastalli, J. Labanda and J. Llorens, Separation of phosphoric acid from an industrial rinsing water by means of nanofiltration, Desalination, 2009, 243, 218–228 CrossRef CAS.
- M. P. González, R. Navarro, I. Saucedo, M. Avila, J. Revilla and C. h. Bouchard, Purification of phosphoric acid solutions by reverse osmosis and nanofiltration, Desalination, 2002, 147, 315–320 CrossRef.
- T. Yun and S.-Y. Kwak, Recovery of hydrochloric acid using positively-charged nanofiltration membrane with selective acid permeability and acid resistance, J. Environ. Manage., 2020, 260, 110001 CrossRef CAS PubMed.
- W. R. Bowen, A. W. Mohammad and N. Hilal, Characterisation of nanofiltration membranes for predictive purposes - Use of salts, uncharged solutes and atomic force microscopy, J. Membr. Sci., 1997, 126, 91–105 CrossRef CAS.
- W. Cheng, C. Liu, T. Tong, R. Epsztein, M. Sun, R. Verduzco, J. Ma and M. Elimelech, Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength, J. Membr. Sci., 2018, 559, 98–106 CrossRef CAS.
- J. Lopez, M. Reig, O. Gibert and J. L. Cortina, Recovery of sulphuric acid and added value metals (Zn, Cu and rare earths) from acidic mine waters using nanofiltration membranes, Sep. Purif. Technol., 2019, 212, 180–190 CrossRef CAS.
- J. Tanninen and M. Nyström, Separation of ions in acidic conditions using NF, Desalination, 2002, 147, 295–299 CrossRef CAS.
- L. Paugam, S. Taha, G. Dorange, P. Jaouen and F. Quéméneur, Mechanism of nitrate ions transfer in nanofiltration depending on pressure, pH, concentration and medium composition, J. Membr. Sci., 2004, 231, 37–46 CrossRef CAS.
- T. J. K. Visser, S. J. Modise, H. M. Krieg and K. Keizer, The removal of acid sulphate pollution by nanofiltration, Desalination, 2001, 140, 79–86 CrossRef CAS.
- M. Mullett, R. Fornarelli and D. Ralph, Nanofiltration of mine water: impact of feed pH and membrane charge on resource recovery and water discharge, Membranes, 2014, 4, 163–180 CrossRef PubMed.
- E. Cséfalvay, V. Pauer and P. Mizsey, Recovery of copper from process waters by nanofiltration and reverse osmosis, Desalination, 2009, 240, 132–142 CrossRef.
- C. Niewersch, K. Meier, T. Wintgens and T. Melin, Selectivity of polyamide nanofiltration membranes for cations and phosphoric acid, Desalination, 2010, 250, 1021–1024 CrossRef CAS.
- A. L. Ahmad and B. S. Ooi, A study on acid reclamation and copper recovery using low pressure nanofiltration membrane, Chem. Eng. J., 2010, 156, 257–263 CrossRef CAS.
- J. Lopez, M. Reig, O. Gibert and J. L. Cortina, Increasing sustainability on the metallurgical industry by integration of membrane nanofiltration processes: Acid recovery, Sep. Purif. Technol., 2019, 226, 267–277 CrossRef CAS.
- L. Paltrinieri, K. Remmen, B. Müller, L. Chu, J. Köser, T. Wintgens, M. Wessling, L. C. P. M. de Smet and E. J. R. Sudhölter, Improved phosphoric acid recovery from sewage sludge ash using layer-by-layer modified membranes, J. Membr. Sci., 2019, 587, 117162 CrossRef CAS.
- B. C. Ricci, C. D. Ferreira, A. O. Aguiar and M. C. S. Amaral, Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent, Sep. Purif. Technol., 2015, 154, 11–21 CrossRef CAS.
- B. S. Ooi, in Inorganic Acids Recovery by Nanofiltration, Springer, Berlin Heidelberg, 2015, pp. 1–2 Search PubMed.
- J. López, M. Reig, X. Vecino, O. Gibert and J. L. Cortina, Comparison of acid-resistant ceramic and polymeric nanofiltration membranes for acid mine waters treatment, Chem. Eng. J., 2020, 382, 122786 CrossRef.
- R. Fornarelli, M. Mullett and D. Ralph, Factors Influencing Nanofiltration of Acid Mine Drainage, Reliab. Mine Water Technol., 2013, pp. 563–568 Search PubMed.
- H. Al-Zoubi, A. Rieger, P. Steinberger, W. Pelz, R. Haseneder and G. Hartel, Nanofiltration of acid mine drainage, Desalin. Water Treat., 2010, 21, 148–161 CrossRef CAS.
- H. Al-Zoubi, A. Rieger, P. Steinberger, W. Pelz, R. Haseneder and G. Hartel, Optimization study for treatment of acid mine drainage using membrane technology, Sep. Sci. Technol., 2010, 45, 2004–2016 CrossRef CAS.
- K. Meschke, N. Hansen, R. Hofmann, R. Haseneder and J. U. Repke, Characterization and performance evaluation of polymeric nanofiltration membranes for the separation of strategic elements from aqueous solutions, J. Membr. Sci., 2018, 546, 246–257 CrossRef CAS.
- P. S. Zhong, N. Widjojo, T. S. Chung, M. Weber and C. Maletzko, Positively charged nanofiltration (NF) membranes via UV grafting on sulfonated polyphenylene sulfone (SPPSU) for effective removal of textile dyes from wastewater, J. Membr. Sci., 2012, 417, 52–60 CrossRef.
- B. G. Reis, A. L. B. Araújo, C. C. Vieira, M. C. S. Amaral and H. C. Ferraz, Assessing potential of nanofiltration for sulfuric acid plant effluent reclamation: Operational and economic aspects, Sep. Purif. Technol., 2019, 222, 369–380 CrossRef CAS.
- R. W. Baker, Membrane Technology, Wiley Online Library, 2000 Search PubMed.
- G. Hagmeyer and R. Gimbel, Modelling the rejection of nanofiltration membranes using zeta potential measurements, Sep. Purif. Technol., 1999, 15, 19–30 CrossRef CAS.
- J. Schaep and C. Vandecasteele, Evaluating the charge of nanofiltration membranes, J. Membr. Sci., 2001, 188, 129–136 CrossRef CAS.
- A. I. Schäfer, A. G. Fane and T. D. Waite, Nanofiltration: Principles and Applications, Elsevier, 2005 Search PubMed.
- F. Fu and Q. Wang, Removal of heavy metal ions from wastewaters: a review, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- L. Qi, X. Wang and Q. Xu, Coupling of biological methods with membrane filtration using ozone as pre-treatment for water reuse, Desalination, 2011, 270, 264–268 CrossRef CAS.
- E. Kurt, D. Y. Koseoglu-Imer, N. Dizge, S. Chellam and I. Koyuncu, Pilot-scale evaluation of nanofiltration and reverse osmosis for process reuse of segregated textile dyewash wastewater, Desalination, 2012, 302, 24–32 CrossRef CAS.
- L. Ahsan, M. S. Jahan and Y. Ni, Recovering/concentrating of hemicellulosic sugars and acetic acid by nanofiltration and reverse osmosis from prehydrolysis liquor of kraft based hardwood dissolving pulp process, Bioresour. Technol., 2014, 155, 111–115 CrossRef CAS PubMed.
- K. Khaless, H. Chanouri, S. Amal, A. Ouaattou, E. M. Mounir, H. Haddar and R. Benhida, Wet process phosphoric acid purification using functionalized organic nanofiltration membrane, Separations, 2022, 9, 100 CrossRef CAS.
- M. Gonzalez, I. Saucedo, R. Navarro, P. Pradanos, L. Palacio, F. Martinez, A. Martin and A. Hernandez, Effect of phosphoric and hydrofluoric acid on the structure and permeation of a nanofiltration membrane, J. Membr. Sci., 2006, 281, 177–185 CrossRef CAS.
- J. Luo and Y. Wan, Effects of pH and salt on nanofiltration-A critical review, J. Membr. Sci., 2013, 438, 18–28 CrossRef CAS.
- X. Wei, X. Kong, S. Wong, H. Xiang, J. Wang and J. Chen, Removal of heavy metals from electroplating wastewater by thin film composite nanofiltration hollow-fiber membranes, Ind. Eng. Chem. Res., 2013, 52, 17583–17590 CrossRef CAS.
- J. Tanninen, M. Manttari and M. Nystrom, Effect of electrolyte strength on acid separation with NF membranes, J. Membr. Sci., 2007, 294, 207–212 CrossRef CAS.
- B. C. Ricci, C. D. Ferreira, L. S. Marques, S. S. Martins, B. G. Reis and M. C. S. Amaral, Assessment of the chemical stability of nanofiltration and reverse osmosis membranes employed in treatment of acid gold mining effluent, Sep. Purif. Technol., 2017, 174, 301–311 CrossRef CAS.
- A. Yadav, Membrane Distillation Process for High Saline and Waste Water Treatment, PhD thesis, 20EE19A16001, Academy of Scientific and Innovative Research, India, 2022.
- A. Alkhudhiri, N. Darwish and N. Hilal, Membrane distillation: A comprehensive review, Desalination, 2012, 287, 2–18 CrossRef CAS.
- I. Hitsov, T. Maere, K. De Sitter, C. Dotremont and I. Nopens, Modelling approaches in membrane distillation: A critical review, Sep. Purif. Technol., 2015, 142, 48–64 CrossRef CAS.
- M. S. El-Bourawi, Z. Ding, M. Ma and M. Khayet, A framework for better understanding membrane distillation separation process, J. Membr. Sci., 2006, 285, 4–29 CrossRef CAS.
- K. W. Lawson and D. R. Lloyd, Membrane distillation review, J. Membr. Sci., 1997, 124, 1–25 CrossRef CAS.
- A. M. Alklaibi and N. Lior, Membrane-distillation desalination: Status and potential, Desalination, 2005, 171, 111–131 CrossRef CAS.
- M. Tomaszewska and A. Mientka, Separation of HCl from HCl – H2SO4 solution by membrane distillation, J. Membr. Sci., 2009, 240, 244–250 CAS.
- A. S. Josson, R. Wimmerstedt and A. C. Harrysson, Membrane distillation-A theoretical study of evaporation through microporous membranes, Desalination, 1985, 56, 237–249 CrossRef.
- S. Bandini, C. Gostoli and G. C. Sarti, Separation efficiency in vacuum membrane, Distillation, J. Membr. Sci., 1992, 73, 217–229 CrossRef CAS.
- M. Khayet, P. Godino and J. I. Mengual, Nature of flow on sweeping gas membrane distillation, J. Membr. Sci., 2000, 170, 243–255 CrossRef CAS.
- P. P. Zolotarev, V. V. Ugrozov, I. B. Volkina and V. M. Nikulin, Treatment of waste water for removing heavy metals by membrane distillation, J. Hazard. Mater., 1994, 37, 77–82 CrossRef CAS.
- M. Tomaszewska, M. Grayta and A. W. Morawski, Recovery of hydrochloric acid from metal pickling solutions by membrane distillation, Sep. Purif., 2001, 23, 591–600 CrossRef.
- M. Tomaszewska, Concentration of the extraction fluid from sulfuric acid treatment of phosphogypsum by membrane distillation, J. Membr. Sci., 1993, 78, 277–282 CrossRef CAS.
- G. Zakrzewska-Trznadel, M. Harasimowicz and A. G. Chmielewski, Concentration of radioactive components in liquid low-level radioactive waste by membrane distillation, J. Membr. Sci., 1999, 163, 257–264 CrossRef CAS.
- F. A. Banat and J. Simandl, Removal of benzene traces from contaminated water by vacuum membrane distillation, Chem. Eng. J., 1996, 51, 1257–1265 CrossRef CAS.
- M. J. Semmens, R. Qin and A. Zander, Using a microporous hollow-fiber membrane to separate VOCs from water, J. AWWA, 1989, 81, 162–167 CrossRef CAS.
- Z. Guiqing, Z. Qixiu, Z. Kanggen and L. Aiping, Study on concentrating sulphuric acid solution by membrane distillation with metal PTFE composite membrane, J. Cent. South Univ. Technol., 1999, 6, 95–98 CrossRef.
- J. Tang and K. Zhou, Hydrochloric acid recovery from rare earth chloride solution by vacuum membrane distillation, Rare Met., 2006, 25, 287–292 CrossRef CAS.
- U. K. Kesieme, Mine Waste Water Treatment and Acid Recovery Using Membrane Distillation and Solvent Extraction, PhD thesis, Victoria University Melbourne, 2015.
- A. M. Alklaibi and N. Lior, Membrane-distillation desalination: Status and potential, Desalination, 2005, 171, 111–131 CrossRef CAS.
- M. Tomaszewska and A. Mientka, Separation of HCl from HCl – H2SO4 solution by membrane distillation, J. Membr. Sci., 2009, 240, 244–250 CAS.
- A. S. Josson, R. Wimmerstedt and A. C. Harrysson, Membrane distillation-A theoretical study of evaporation through microporous membranes, Desalination, 1985, 56, 237–249 CrossRef.
- S. Bandini, C. Gostoli and G. C. Sarti, Separation efficiency in vacuum membrane, Distillation, J. Membr. Sci., 1992, 73, 217–229 CrossRef CAS.
- M. Khayet, P. Godino and J. I. Mengual, Nature of flow on sweeping gas membrane distillation, J. Membr. Sci., 2000, 170, 243–255 CrossRef CAS.
- E. Curcio and E. Drioli, Membrane distillation and related operations: A review, Sep. Purif., 2005, 34, 35–86 CrossRef CAS.
- Operation and Evaluation of Memstill Pilot Plant, 2008, http://www.pub.gov.sg/research/Key_Projects/Pages/Membrane3.aspx?Print2=yes.
- Z. Yan, Y. Jiang, L. Liu, Z. Li, X. Chen, M. Xia, G. Fan and A. Ding, Membrane Distillation for Wastewater Treatment: A Mini Review, Water, 2021, 13, 3480 CrossRef CAS.
- M. Tomaszewska, M. Grayta and A. W. Morawski, Mass transfer of HCl and H2O across the hydrophobic membrane during membrane distillation, J. Membr. Sci., 2000, 166, 149–157 CrossRef CAS.
- F. Suárez, S. W. Tyler and A. E. Childress, A theoretical study of a direct contact membrane distillation system coupled to a salt-gradient solar pond for terminal lakes reclamation, Water Res., 2010, 44, 4601–4615 CrossRef PubMed.
- C. R. Martinetti, A. E. Childress and T. Y. Cath, High recovery of concentrated RO brines using forward osmosis and membrane distillation, J. Membr. Sci., 2009, 331, 31–39 CrossRef CAS.
- N. Dow, M. Duke, J. Zhang, T. O'Rielly, J.-D. Li, S. Gray, E. Ostarcevic and P. Atherton, Demonstration of solar driven membrane distillation in remote Victoria, in Australian Water Association, Ozwater10, Brisbane, Queensland, Australia, March 8–10, 2010 Search PubMed.
- H. Strathmann, Ion-Exchange Membrane Separation Process, Elsevier, 2004 Search PubMed.
- J. Luo, C. Wu, T. Xu and Y. Wu, Diffusion dialysis-concept, principle and applications, J. Membr. Sci., 2011, 366, 1–16 CrossRef CAS.
- K. A. Stancheva, Application of dialysis, Oxid. Commun., 2008, 31, 758–775 CAS.
- T. W. Xu, Ion exchange membranes: state of their development and perspective, J. Membr. Sci., 2005, 263, 1–29 CrossRef CAS.
- J. K. Jeong, M. S. Kim and B. S. Kim, Recovery of H2SO4 from waste acid solution by diffusion dialysis method, J. Hazard. Mater., 2005, 124, 230–235 CrossRef CAS PubMed.
- S. Jung Oh, S.-H. Moon and T. Davis, Effects of metal ions on diffusion dialysis of inorganic acids, J. Membr. Sci., 2000, 169, 95–105 CrossRef.
- T. Xu and W. Yang, Tuning the diffusion dialysis performance by surface cross-linking of PPO anion exchange membranes—simultaneous recovery of sulfuric acid and nickel from electrolysis spent liquor of relatively low acid concentration, J. Hazard. Mater., 2004, 109, 157–164 CrossRef CAS PubMed.
- Z. Palatý and A. Žáková, Separation of HCl + NiCl2 Mixture by Diffusion Dialysis, Sep. Sci. Technol., 2007, 42, 1965–1983 CrossRef.
- Z. Palatý and A. Žáková, Separation of H2SO4 + ZnSO4 mixture by diffusion dialysis, Desalination, 2004, 169, 277–285 CrossRef.
- J. Jeong, M. Kim, B. Kim, S. Kim, W. Kim and J. Lee, Recovery of H2SO4 from waste acid solution by a diffusion dialysis method, J. Hazard. Mater., 2005, 124, 230–235 CrossRef CAS PubMed.
- S. Lan, X. Wen, Z. Zhu, F. Shao and C. Zhu, Recycling of spent nitric acid solution from electrodialysis by diffusion dialysis, Desalination, 2011, 278, 227–230 CrossRef CAS.
- V. Chavan, C. Agarwal, V. C. Adya and A. K. Pandey, Hybrid organic-inorganic anion-exchange pore-filled membranes for the recovery of nitric acid from highly acidic aqueous waste streams, Water Res., 2018, 133, 87–98 CrossRef CAS PubMed.
- T. Xu and W. Yang, Industrial recovery of mixed acid (HF + HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes, J. Membr. Sci., 2003, 220, 89–95 CrossRef CAS.
- Z. Palatý and H. Bendová, Permeability of a Fumasep-FAD Membrane for Selected Inorganic Acids, Chem. Eng. Technol., 2018, 41, 385–391 CrossRef.
- A. Narębska, M. Staniszewski and A. Narȩbska, Separation of Carboxylic Acids from Carboxylates by Diffusion Dialysis, Sep. Sci. Technol., 2008, 43, 490–501 CrossRef.
- Z. Palatý, P. Stoček, H. Bendová and P. Prchal, Continuous dialysis of carboxylic acids: Solubility and diffusivity in Neosepta-AMH membranes, Desalination, 2009, 243, 65–73 CrossRef.
- T. Xu and W. Yang, Tuning the diffusion dialysis performance by surface cross-linking of PPO anion exchange membranes-simultaneous recovery of sulfuric acid and nickel from electrolysis spent liquor of relatively low acid concentration, J. Hazard. Mater., 2004, 109, 157–164 CrossRef CAS PubMed.
- M. Ekimova, C. Kleine, J. Ludwig, M. Ochmann, T. E. G. Agrenius, E. Kozari, D. Pines, E. Pines, N. Huse, P. Wernet, M. Odelius and E. T. J. Nibbering, From local covalent bonding to extended electric field interactions in proton hydration, Angew. Chem., Int. Ed., 2022, 61, e202211066 CrossRef CAS PubMed.
- Y. Lorrain, G. Pourcelly and C. Gavach, Influence of cations on the proton leakage through anion-exchange membranes, J. Membr. Sci., 1996, 110, 181 CrossRef CAS.
- O. Vendrell and H.-D. Meyer, Proton transfer and hydrated proton in small water systems, in Multidimensional Quantum Dynamics, ed. H.-D. Meyer, F. Gatti and G. A. Worth, Wiley, 2009, DOI:10.1002/9783527627400.ch23.
- I. Tugas, G. Pourcelly and C. Gavach, Electrotransport of protons and chloride ions in anion exchange membranes for the recovery of acids. Part I. Equilibrium properties, J. Membr. Sci., 1993, 85, 183–194 CrossRef CAS.
- J. G. Wijmans and R. W. Baker, The solution-diffusion model: a review, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- A. Elmidaoui, J. Molenat and C. Gavach, Competitive diffusion of hydrochloric acid and sodium chloride through an acid dialysis membrane, J. Membr. Sci., 1991, 55, 79–98 CrossRef CAS.
- M.-S. Kang, K.-S. Yoo, S.-J. Oh and S.-H. Moon, A lumped parameter model to predict hydrochloric acid recovery in diffusion dialysis, J. Membr. Sci., 2001, 188, 61–70 CrossRef CAS.
- D. M. Stachera and R. F. Childs, Tuning the acid recovery performance of poly(4-vinylpyridine)-filled membranes by the introduction of hydrophobic groups, J. Membr. Sci., 2001, 187, 213–225 CrossRef CAS.
- C. Wang, C. Wu, Y. Wu, J. Gu and T. Xu, Polyelectrolyte complex/PVA membranes for diffusion dialysis, J. Hazard. Mater., 2013, 261, 114–122 CrossRef CAS PubMed.
- S. B. Tuwiner, L. P. Miller and W. E. Brown, Diffusion and Membrane Technology, Reinhold, New York, 1962 Search PubMed.
- Y. He, J. Pan and T. Xu, Facile preparation of 1,8-Diazabicyclo[5.4.0]undec-7-ene based high performance anion exchange membranes for diffusion dialysis applications, J. Membr. Sci., 2015, 491, 45–52 CrossRef CAS.
- K. Emmanuel, B. Erigene and T. Xu, Facile synthesis of pyridinium functionalized anion exchange membranes for diffusion dialysis application, Sep. Purif. Technol., 2016, 167, 108–116 CrossRef CAS.
- F. Sun, C. Wu, Y. Wu and T. Xu, Porous BPPO-based membranes modified by multisilicon copolymer for application in diffusion dialysis, J. Membr. Sci., 2014, 450, 103–110 CrossRef CAS.
- M. Ersoz, I. Gugul and A. Şahin, Transport of acids through polyether–sulfone anion-exchange membrane, J. Colloid Interface Sci., 2001, 237, 130–135 CrossRef CAS PubMed.
- X. Lin, S. Kim, D. M. Zhu, E. Shamsaei, T. Xu, X. Fang and H. Wang, Preparation of porous diffusion dialysis membranes by functionalization of polysulfone for acid recovery, J. Membr. Sci., 2017, 524, 557–564 CrossRef CAS.
- P. Mondal, N. S. Samanta, A. Kumar and M. K. Purkait, Recovery of H2SO4 from wastewater in the presence of NaCl and KHCO3 through pH responsive polysulfone membrane: Optimization approach, Polym. Test., 2020, 86, 106463 CrossRef CAS.
- P. P. Sharma, V. Yadav, A. Rajput and V. Kulshrestha, Acid resistant PVDF based copolymer alkaline anion exchange membrane for acid recovery and electrodialytic water desalination, J. Membr. Sci., 2018, 563, 561–570 CrossRef CAS.
- L. Wang, F. Zhang, Z. Li, J. Liao, Y. Huang, Y. Lei and N. Li, Mixed-charge poly(2,6-dimethyl-phenylene oxide)anion exchange membrane for diffusion dialysis in acid recovery, J. Membr. Sci., 2018, 549, 543–549 CrossRef CAS.
- Y. He, J. Pan, L. Wu, L. Ge and T. Xu, Facile preparation of 1,8-Diazabicyclo[5.4.0] undec-7-ene based high performance anion exchange membranes for diffusion dialysis applications, J. Membr. Sci., 2015, 491, 45–52 CrossRef CAS.
- H. S. Park, D. H. Kim, J. S. Park, S. H. Moon, Y. Lee, K. H. Yeon and M. S. Kang, Surface modification and use of polymer complex agents to mitigate metal crossover of anion-exchange membranes, J. Colloid Interface Sci., 2014, 430, 24–30 CrossRef CAS PubMed.
- Y. Wu, C. Wu, Y. Li, T. Xu and Y. Fu, PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent, J. Membr. Sci., 2010, 350, 322–332 CrossRef CAS.
- Y. Wu, J. Luo, L. Yao, C. Wu, F. Mao and T. Xu, PVA/SiO 2 anion exchange hybrid membranes from multisilicon copolymers with two types of molecular weights, J. Membr. Sci., 2012, 399–400, 16–27 CrossRef CAS.
- K. Emmanuel, B. Erigene, C. Cheng, A. N. Mondal, Md. M. Hossain, M. I. Khan, N. U. Afsar, L. Ge, L. Wu and T. Xu, Facile synthesis of pyridinium functionalized anion exchange membranes for diffusion dialysis application, Sep. Purif. Technol., 2016, 167, 108–116 CrossRef CAS.
- X. Lin, E. Shamsaei, B. Kong, J. Z. Liu, T. Xu and H. Wang, Fabrication of asymmetrical diffusion dialysis membranes for rapid acid recovery with high purity, J. Mater. Chem. A, 2015, 3, 24000–24007 RSC.
- D. Kim, J. Park, S. Seo, J. Park, S. Jung, Y. S. Kang, J. Choi and M. Kang, Development of thin anion-exchange pore-filled membranes for high diffusion dialysis performance, J. Membr. Sci., 2013, 447, 80–86 CrossRef CAS.
- J. Feng, J. Chen, B. Wei, S. Liao, Y. Yu and X. Li, Series-connected hexacations cross-linked anion exchange membranes for diffusion dialysis in acid recovery, J. Membr. Sci., 2019, 570–571, 120–129 CrossRef CAS.
- X. Lin, L. Wu, Y. Liu, A. L. Ong, S. D. Poynton, J. R. Varcoe and T. Xu, Alkali resistant and conductive guanidinium-based anion-exchange membranes for alkaline polymer electrolyte fuel cells, J. Power Sources, 2012, 217, 373–380 CrossRef CAS.
- C. Wu, Y. Wu, J. Luo, T. Xu and Y. Fu, Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process, J. Membr. Sci., 2010, 356, 96–104 CrossRef CAS.
- Y. Wu, J. Luo, L. Zhao, G. Zhang, C. Wu and T. Xu, QPPO/PVA anion exchange hybrid membranes from double crosslinking agents for acid recovery, J. Membr. Sci., 2013, 428, 95–103 CrossRef CAS.
- C. Cheng, Z. Yang, J. Pan, B. Tong and T. Xu, Facile and cost effective PVA based hybrid membrane fabrication for acid recovery, Sep. Purif. Technol., 2014, 136, 250–257 CrossRef CAS.
- K. Emmanuel, C. Cheng, B. Erigene, A. N. Mondal, Md. M. Hossain, M. I. Khan, N. UI Afsar, G. Liang, L. Wu and T. Xu, Imidazolium functionalized anion exchange membrane blended with PVA for acid recovery via diffusion dialysis process, J. Membr. Sci., 2016, 497, 209–215 CrossRef CAS.
- H. Hu and W. Song, One-step fabrication of methylthiazole-functionalized anion exchange membranes for diffusion dialysis, Open J. Phys. Chem., 2018, 8, 100–109 CrossRef CAS.
- X. Lin, L. Wu, Y. Liu, A. L. Ong, S. D. Poynton, J. R. Varcoe and T. Xu, Alkali resistant and conductive guanidinium-based anion-exchange membranes for alkaline polymer electrolyte fuel cells, J. Power Sources, 2012, 217, 373–380 CrossRef CAS.
- C. Yang, S. Wang, W. Ma, L. Jiang and G. Sun, Comparison of alkaline stability of quaternary ammonium- and 1,2-methylimidazolium-based alkaline anion exchange membranes, J. Membr. Sci., 2015, 487, 12–18 CrossRef CAS.
- A. Voge, V. Deimede and J. K. Kallitsis, Synthesis and properties of alkaline stable pyridinium containing anion exchange membranes, RSC Adv., 2014, 4, 45040–45049 RSC.
- B. Qiu, B. Lin, L. Qiu and F. Yan, Alkaline imidazolium- and quaternary ammonium- functionalized anion exchange membranes for alkaline fuel cell applications, J. Mater. Chem., 2012, 22, 1040–1045 RSC.
- L. Gurreri, A. Tamburini, A. Cipollina and G. Micale, Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives, Membranes, 2020, 10, 146 CrossRef CAS PubMed.
- T. Xu and C. Huang, Electrodialysis-based separation technologies: A critical review, AIChE J., 2008, 54, 3147–3159 CrossRef CAS.
- Y. Mei and C. Y. Tang, Recent developments and future perspectives of reverse electrodialysis technology: A review, Desalination, 2018, 425, 156–174 CrossRef CAS.
- H. Strathmann, Electrodialysis, a mature technology with a multitude of new applications, Desalination, 2010, 264, 268–288 CrossRef CAS.
- S. Koter and M. Kultys, Modeling the electric transport of sulfuric and phosphoric acids through anion-exchange membranes, Sep. Purif. Technol., 2010, 73, 219–229 CrossRef CAS.
- C. Charcosset, A review of membrane processes and renewable energies for desalination, Desalination, 2009, 245, 214–231 CrossRef CAS.
- M. Turek, Dual-purpose desalination-salt production electrodialysis, Desalination, 2003, 153, 377–381 CrossRef CAS.
- A. M. Lopez, M. Williams, M. Paiva, D. Demydov, T. D. Do, J. L. Fairey, Y. P. J. Lin and J. A. Hestekin, Potential of electrodialytic techniques in brackish desalination and recovery of industrial process water for reuse, Desalination, 2017, 409, 108–114 CrossRef CAS.
- Y. Zhang, B. Van der Bruggen, L. Pinoy and B. Meesschaert, Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis, J. Membr. Sci., 2009, 332, 104–112 CrossRef CAS.
- Y. Oztekin and Z. Yazicigil, Recovery of acids from salt forms of sodium using cation-exchange membranes, Desalination, 2007, 212, 62–69 CrossRef CAS.
- G. S. Trivedi, B. G. Shah, S. K. Adhikary, V. K. Indusekhar and R. Rangarajan, Studies on bipolar membranes. Part II - Conversion of sodium acetateto acetic acid and sodium hydroxide, React. Funct. Polym., 1997, 32, 209–215 CrossRef CAS.
- J. L. Gineste, G. Pourcelly, Y. Lorrain, F. Persin and C. Gavach, Analysis of factors limiting the use of bipolar membranes: A simplified model to determine trends, J. Membr. Sci., 1996, 112, 199–208 CrossRef CAS.
- C. Negro, M. A. Blanco, F. Ĺopez-Mateos, A. M. C. P. DeJong, G. LaCalle, J. Van Erkel and D. Schmal, Free acids and chemicals recovery from stainless steel pickling baths, Sep. Sci. Technol., 2001, 36, 1543–1556 CrossRef CAS.
- M. Ali, M. Rakib, S. Laborie, P. Viers and G. Durand, Coupling of bipolar membrane electrodialysis and ammonia stripping for direct treatment of wastewaters containing ammonium nitrate, J. Membr. Sci., 2004, 244, 89–96 CrossRef.
- N. Y. Greben, V. P. Pivowarov, N. Y. Rodzik and I. G. Kovarskii, Regeneration of sulphuric acid from a sulphuric acid electrolyte used for electrolytic anodizing of aluminium, Russ. J. Appl. Chem., 1992, 65, 619–623 Search PubMed.
- B. Yuze, M. I. Aydin, H. Yildiz, B. Hasançebi, H. Selcuk and Y. Kadmi, Optimal performance of electrodialysis process for the recovery of acid wastes in wastewater: Practicing circular economy in aluminum finishing industry, Chem. Eng. J., 2022, 434, 134755 CrossRef.
- L. Wang, Z. Li, Z. Xu, F. Zhang, J. E. Efome and N. Li, Proton blockage membrane with tertiary amine groups for concentration of sulfonic acid in electrodialysis, J. Membr. Sci., 2018, 555, 78–87 CrossRef CAS.
- A. T. Cherif, C. Gavach, T. Cohen, P. Dagard and L. Albert, Sulfuric acid concentration with an electro-electrodialysis process, Hydrometallurgy, 1988, 21, 191–201 CrossRef CAS.
- L. Ma, L. Gutierrez, R. Verbeke, A. D'Haese, M. Waqas, M. Dickmann, R. Helm, I. Vankelecom, A. Verliefde and E. Cornelissen, Transport of organic solutes in ion-exchange membranes: mechanisms and influence of solvent ionic composition, Water Res., 2021, 190, 116756 CrossRef CAS PubMed.
- R. Xie, P. Ning, G. Qu, J. Li, M. Ren, C. Du, H. Gao and Z. Li, Self-made anion-exchange membrane with polyaniline as an additive for sulfuric acid enrichment, Chem. Eng. J., 2018, 341, 298–307 CrossRef CAS.
- J. Liao, H. Ruan, X. Gao, Q. Chen and J. Shen, Exploring the acid enrichment application of piperidinium-functionalized cross-linked poly(2,6-dimethyl-1,4- phenylene oxide) anion exchange membranes in electrodialysis, J. Membr. Sci., 2021, 621, 118999 CrossRef CAS.
- T. J. Peckham and S. Holdcroft, Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes, Adv. Mater., 2010, 22, 4667–4690 CrossRef CAS PubMed.
- N. Agmon, The Grotthuss mechanism, Chem. Phys. Lett., 1995, 244, 456–462 CrossRef CAS.
- K.-D. Kreuer, R. Albrecht and W. Werner, Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors, Angew. Chem., 1982, 94, 224 CrossRef CAS.
- S. Yang, Y. Liu, J. Liao, H. Liu, Y. Jiang, B. Van der Bruggen, J. Shen and C. Gao, Codeposition modification of cation exchange membranes with dopamine and crown ether to achieve high K+ electrodialysis selectivity, ACS Appl. Mater. Interfaces, 2019, 11, 17730–17741 CrossRef CAS PubMed.
- J. Liao, X. Yu, N. Pan, J. Li, J. Shen and C. Gao, Amphoteric ion-exchange membranes with superior mono-/bi-valent anion separation performance for electrodialysis applications, J. Membr. Sci., 2019, 577, 153–164 CrossRef CAS.
- H. Li, Y. Gao, L. Pan, Y. Zhang, Y. Chen and Z. Sun, Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes, Water Res., 2008, 42, 4923–4928 CrossRef CAS PubMed.
- G. Q. Chen, K. Wei, A. Hassanvand, B. D. Freeman and S. E. Kentish, Single and binary ion sorption equilibria of monovalent and divalent ions in commercial ion exchange membranes, Water Res., 2020, 175, 115681 CrossRef CAS PubMed.
- Y. Qiu, Y. Lv, C. Tang, J. Liao, H. Ruan, A. Sotto and J. Shen, Sustainable recovery of high-saline papermaking wastewater: optimized separation for salts and organics via membrane-hybrid process, Desalination, 2021, 507, 114938 CrossRef CAS.
- R. Simons, Development of an acid impermeable anion exchange membrane, Desalination, 1990, 78, 297 CrossRef CAS.
- R.-q. Guo, B.-b. Wang, Y.-x. Jia and M. Wang, Development of acid block anion exchange membrane by structure design and its possible application in waste acid recovery, Sep. Purif. Technol., 2017, 186, 188–196 CrossRef CAS.
- S. Yu, H. Qian, J. Liao, J. Dong, L. Yu, C. Liu and J. Shen, Proton blockage PVDF-co-HFP-based anion exchange membrane for sulfuric acid recovery in electrodialysis, J. Membr. Sci., 2022, 653, 120510 CrossRef CAS.
- N. Zhang, Y. Liu, R. Liu, Z. She, M. Tan, D. Mao, R. Fu and Y. Zhang, Polymer inclusion membrane (PIM) containing ionic liquid as a proton blocker to improve waste acid recovery efficiency in electrodialysis process, J. Membr. Sci., 2019, 581, 18–27 CrossRef CAS.
- Y.-x. Jia, F.-j. Li, X. Chen and M. Wang, Model analysis on electrodialysis for inorganic acid recovery and its experimental validation, Sep. Purif. Technol., 2018, 190, 261–267 CrossRef CAS.
- L. Ge, X. Liu, G. Wang, B. Wu, L. Wu, E. Bakangura and T. Xu, Preparation of proton selective membranes through constructing H+ transfer channels by acid–base pairs, J. Membr. Sci., 2015, 475, 273–280 CrossRef CAS.
- W. Wang, C. Yang, W. Wang, R. Fu and H. Wang, Novel compact ion exchange membranes through suppressing reverse permeation for high-efficiency recovery of inorganic acids, Sep. Purif. Technol., 2022, 289, 120725 CrossRef CAS.
- F. S. Rohman and N. Aziz, Optimization of batch electrodialysis for hydrochloric acid recovery using orthogonal collocation method, Desalination, 2011, 275, 37–49 CrossRef CAS.
- P. Ji, Z. Ji, Q. Chen, J. Liu, Y. Zhao, S. Wang, F. Li and J. Yuan, Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis, Sep. Purif. Technol., 2018, 207, 1–11 CrossRef CAS.
- Y. Qiu, L. Yao, C. Tang, Y. Zhao, J. Zhu and J. Shen, Integration of selectrodialysis and selectrodialysis with bipolar membrane to salt lake treatment for the production of lithium hydroxide, Desalination, 2019, 465, 1–12 CrossRef CAS.
- A. T. K. Tran, Y. Zhang, D. De Corte, J.-B. Hannes, W. Ye, P. Mondal, N. Jullok, B. Meesschaert, L. Pinoy and B. Van der Bruggen, P-recovery as calcium phosphate from wastewater using an integrated selectrodialysis/crystallization process, J. Cleaner Prod., 2014, 77, 140–151 CrossRef CAS.
- M. Reig, C. Valderrama, O. Gibert and J. L. Cortina, Selectrodialysis and bipolar membrane electrodialysis combination for industrial process brines treatment: monovalent-divalent ions separation and acid and base production, Desalination, 2016, 399, 88–95 CrossRef CAS.
- P. Song, M. Wang, B. Zhang, Y. Jia and Y. Chen, Fabrication of proton permselective composite membrane for electrodialysis-based waste acid reclamation, J. Membr. Sci., 2019, 592, 117366 CrossRef.
- M. Reig, X. Vecino, C. Valderrama, O. Gibert and J. L. Cortina, Application of selectrodialysis for the removal of As from metallurgical process waters: recovery of Cu and Zn, Sep. Purif. Technol., 2018, 195, 404–412 CrossRef CAS.
- Y. Zhang, S. Paepen, L. Pinoy, B. Meesschaert and B. Van der Bruggen, Selectrodialysis: fractionation of divalent ions from monovalent ions in a novel electrodialysis stack, Sep. Purif. Technol., 2012, 88, 191–201 CrossRef CAS.
- J. Wiśniewski, G. Wiśniewska and T. Winnicki, Application of bipolar electrodialysis to the recovery of acids and bases from water solutions, Desalination, 2004, 169, 11–20 CrossRef.
- I. Frenzel, H. Holdik, D. F. Stamatialis, G. Pourcelly and M. Wessling, Chromic acid recovery by electro-electrodialysis: I. Evaluation of anion-exchange membrane, J. Membr. Sci., 2005, 261, 49–57 CrossRef CAS.
- I. Frenzel, H. Holdik, D. F. Stamatialis, G. Pourcelly and M. Wessling, Chromic acid recovery by electro-electrodialysis: II. Pilot scale process, development, and optimization, Sep. Purif. Technol., 2005, 47, 27–35 CrossRef CAS.
- J. Yan, H. Wang, R. Fu, R. Fu, R. Li, B. Chen, C. Jiang, L. Ge, Z. Liu, Y. Wang and T. Xu, Ion exchange membranes for acid recovery: Diffusion Dialysis (DD) or Selective Electrodialysis (SED)?, Desalination, 2022, 531, 115690 CrossRef CAS.
- J. N. Mathur, M. S. Murali, M. V. B. Krishna, V. Ramachandhran, M. S. Hanra and B. M. Misra, Diffusion dialysis aided electrodialysis process for concentration of radionuclides in acid medium, J. Radioanal. Nucl. Chem., 1998, 232, 237–240 CrossRef CAS.
- P. Upadhyay, S. Mishra, J. Sharma, S. Panja and V. Kulshrestha, Recovery and enrichment of acid from metallurgical wastewater model by electrodialysis integrated diffusion dialysis system using poly(ethylene) based IEMs, Sep. Purif. Technol., 2023, 304, 122353 CrossRef CAS.
- X. Zhang, C. Li, X. Wang, Y. Wang and T. Xu, Recovery of hydrochloric acid from simulated chemosynthesis aluminum foils wastewater: An integration of diffusion dialysis and conventional electrodialysis, J. Membr. Sci., 2012, 409–410, 257–263 CrossRef CAS.
- J. P. McKaveney, The determination of inorganic acids in metal or mineral processing solutions, JOM, 1978, 30, 11–13 CrossRef CAS.
- Q. Chen, G. Zhang, R. Liu, Q. Ji and H. Liu, Integration of concentration and electro-driven membrane system for effective water-saved acid recycling performance, Resour., Conserv. Recycl., 2023, 191, 106885 CrossRef CAS.
- A. Hussain, H. Yan, N. U. Afsar, H. Wang, J. Yan, C. Jiang, Y. Wang and T. Xu, Acid recovery from molybdenum metallurgical wastewater via selective electrodialysis and nanofiltration, Sep. Purif. Technol., 2022, 295, 121318 CrossRef CAS.
- U. K. Kesieme, Hal Aral, Application of membrane distillation and solvent extraction for water and acid recovery from acidic mining waste and process solutions, J. Environ. Chem. Eng., 2015, 3, 2050–2056 CrossRef CAS.
- A. Culcasi, R. Gueccia, S. Randazzo, A. Cipollina and G. Micale, Design of a novel membrane-integrated waste acid recovery process from pickling solution, J. Cleaner Prod., 2019, 236, 117623 CrossRef CAS.
- J.-Y. Kim, C.-H. Shin, H. Choi and W. Bae, Recovery of phosphoric acid from mixed waste acids of semiconductor industry by diffusion dialysis and vacuum distillation, Sep. Purif. Technol., 2012, 90, 64–68 CrossRef CAS.
- G. Chen, Y. Ye, N. Yao, N. Hu, J. Zhang and Y. Huang, A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage, J. Cleaner Prod., 2021, 329, 129666 CrossRef CAS.
- G. Naidu, S. Jeong, M. A. H. Johir, A. G. Fane, J. Kandasamy and S. Vigneswaran, Rubidium extraction from seawater brine by an integrated membrane distillation-selective sorption system, Water Res., 2017, 123, 321–331 CrossRef CAS PubMed.
- J. Phattaranawik, A. G. Fane, A. C. S. Pasquier and W. Bing, A novel membrane bioreactor based on membrane distillation, Desalination, 2008, 223, 386–395 CrossRef CAS.
- A. Ali, C. A. Quist-Jensen, E. Drioli and F. Macedonio, Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment, Desalination, 2018, 434, 161–168 CrossRef CAS.
- F. Macedonio, E. Curcio and E. Drioli, Integrated membrane systems for seawater desalination: Energetic and exergetic analysis, economic evaluation, experimental study, Desalination, 2007, 203, 260–276 CrossRef CAS.
- A. Yadav, P. K. Labhasetwar and V. K. Shahi, Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives, Sci. Total Environ., 2022, 806(Pt 2), 150692 CrossRef CAS PubMed.
- L. Eykens, K. De Sitter, C. Dotremont, L. Pinoy and B. Van der Bruggen, Membrane synthesis for membrane distillation: A review, Sep. Purif. Technol., 2017, 182, 36–51 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Novel solvent properties of choline chloride/urea mixtures, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids, J. Am. Chem. Soc., 2004, 126, 9142 CrossRef CAS PubMed.
- C. J. Clarke, W. C. Tu, O. Levers, A. Brohl and J. P. Hallett, Green and Sustainable Solvents in Chemical Processes, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- I. Wazeer, M. Hayyan and M. K. Hadj-Kali, Deep eutectic solvents: designer fluids for chemical processes, J. Chem. Technol. Biotechnol., 2018, 93, 945–958 CrossRef CAS.
- E. Durand, J. Lecomte and P. Villeneuve, From green chemistry to nature: The versatile role of low transition temperature mixtures, Biochimie, 2016, 120, 119–123 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, Natural deep eutectic solvents - Solvents for the 21st century, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- L. I. N. Tome, V. Baiao, W. da Silva and C. M. A. Brett, Deep eutectic solvents for the production and application of new materials, Appl. Mater. Today, 2018, 10, 30–50 CrossRef.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Deep eutectic solvents: syntheses, properties and applications, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep eutectic solvents (DESs) and their applications, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- G. Garcia, S. Aparicio, R. Ullah and M. Atilhan, Deep eutectic solvents: Physicochemical properties and gas separation applications, Energy Fuels, 2015, 29, 2616–2644 CrossRef CAS.
- K. Haerens, E. Matthijs, A. Chmielarz and B. Van der Bruggen, The use of ionic liquids based on choline chloride for metal deposition: A green alternative?, J. Environ. Manage., 2009, 90, 3245–3252 CrossRef CAS PubMed.
- A. P. Abbott, G. Frisch and K. S. Ryder, Electroplating Using Ionic Liquids, Annual Review of Materials Research, ed. D. R. Clarke, Annual Reviews, Palo Alto, CA, 2013, vol. 43, pp. 335–358 Search PubMed.
- E. L. Smith, Deep eutectic solvents (DESs) and the metal finishing industry: where are they now?, Trans. IMF, 2013, 91, 241–248 CrossRef CAS.
- A. P. Abbott, J. C. Barron, M. Elhadi, G. Frisch, S. J. Gurman, A. R. Hillman, E. L. Smith, M. A. Mohamoud and K. S. Ryder, Electrolytic Metal Coatings and Metal Finishing Using Ionic Liquids, ECS Trans., 2009, 16, 47 CrossRef CAS.
- A. P. Abbott and K. J. McKenzie, Application of ionic liquids to the electrodeposition of metals, Phys. Chem. Chem. Phys., 2006, 8, 4265–4279 RSC.
- A. P. Abbott, K. S. Ryder and U. Konig, Electrofinishing of Metals Using Eutectic Based Ionic Liquids, Trans. Inst. Met. Finish., 2008, 86, 196–204 CrossRef CAS.
- A. P. Abbott, J. C. Barron and K. S. Ryder, Electrolytic deposition of Zn coatings from ionic liquids based on choline chloride, Trans. Inst. Met. Finish., 2009, 87, 201–207 CrossRef CAS.
- A. P. Abbott, J. C. Barron, G. Frisch, K. S. Ryder and A. F. Silva, The effect of additives on zinc electrodeposition from deep eutectic solvents, Electrochim. Acta, 2011, 56, 5272–5279 CrossRef CAS.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. J. McKenzie and K. S. Ryder, Electrodeposition of copper composites from deep eutectic solvents based on choline chloride, Phys. Chem. Chem. Phys., 2009, 11, 4269–4277 RSC.
- A. M. Popescu, A. Cojocaru, C. Donath and V. Constantin, Electrochemical study and electrodeposition of copper(I) in ionic liquid-reline, Chem. Res. Chin. Univ., 2013, 29, 991–997 CrossRef CAS.
- A. P. Abbott, K. El Ttaib, K. S. Ryder and E. L. Smith, Electrodeposition of nickel using eutectic based ionic liquids, Trans. Inst. Met. Finish., 2008, 86, 234–240 CrossRef CAS.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. S. Ryder and D. Weston, The electrodeposition of silver composites using deep eutectic solvents, Phys. Chem. Chem. Phys., 2012, 14, 2443–2449 RSC.
- A. P. Abbott, G. Capper, D. L. Davies and R. K. Rasheed, Ionic liquid analogues formed from hydrated metal salts, Chem. –Eur. J., 2004, 10, 3769–3774 CrossRef CAS PubMed.
- H. M. A. Abood, A. P. Abbott, A. D. Ballantyne and K. S. Ryder, Do all ionic liquids need organic cations? Characterisation of [AlCl2·nAmide]+AlCl4− and comparison with imidazolium based systems, Chem. Commun., 2011, 47, 3523–3525 RSC.
- E. Gómez, P. Cojocaru, L. Magagnin and E. Valles, Electrodeposition of Co, Sm and SmCo from a deep eutectic solvent, J. Electroanal. Chem., 2011, 658, 18–24 CrossRef.
- A. P. Abbott, N. Dsouza, P. Withey and K. S. Ryder, Electrolytic processing of superalloy aerospace castings using choline chloride-based ionic liquids, Trans. Inst. Met. Finish., 2012, 90, 9–14 CrossRef CAS.
- A. P. Abbott, G. Frisch, J. Hartley and K. S. Ryder, Processing of metals and metal oxides using ionic liquids, Green Chem., 2011, 13, 471–481 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. Rasheed and P. Shikotra, Selective extraction of metals from mixed oxide matrixes using choline-based ionic liquids, Inorg. Chem., 2005, 44, 6497–6499 CrossRef CAS PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, Solubility of metal oxides in deep eutectic solvents based on choline chloride, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00112a |
| ‡ Superannuated from Radiochemistry Division, BARC, Mumbai-400085, India. |
| This journal is © The Royal Society of Chemistry 2023 |