Open Access Article
Open Access ArticleSilica supported lanthanum trifluoroacetate and trichloroacetate as an efficient and reusable water compatible Lewis acid catalyst for synthesis of 2,4,5-triarylimidazoles via a solvent-free green approach†
Dnyaneshwar Purushottam Gholap,
Ramdas Huse,
Sudarshan Dipake and
Machhindra K. Lande *
*
Department of Chemistry, Dr Babasaheb Ambedkar Marathwada University, Aurangabad, Maharashtra, India. E-mail: mkl_chem@yahoo.com
First published on 11th January 2023
Abstract
In the present research article, we have developed solid heterogenous silica supported lanthanum trifluoroacetate and trichloroacetate as green Lewis acid catalysts. These catalysts were synthesized by a novel, simple, cheap, clean, and environment friendly method. The physicochemical properties of the prepared catalysts were well studied and characterized by sophisticated spectroscopic techniques such as FTIR, TGA, XRD, EDX, SEM, TEM and BET analysis. The catalyst was utilized in the synthesis of arylimidazole derivatives via green protocols under solvent-free conditions at 70 °C with a higher yield, mild reaction conditions and a short reaction time. The catalyst works superiorly in water as well as in various organic solvents as a reusable and easily recoverable catalyst.
1. Introduction
Heterogeneous catalysts are considered as a building block, rapidly growing and emerging area in the field of catalysis to promote diverse reactions.1 The development and design of novel heterogeneous catalysts are in high demand due to their wide range of applications in organic transformations in various fields of green chemistry.2–4 The heterogenous catalysts show several advantages such as reusability and recyclability with easy processability compared to homogenous catalysts.5 The Lewis acid catalysts having chemical stability, thermal stability and inherent Lewis acidity are well known for promoting numerous reactions including biomass conversion, biodiesel production and synthesis of both natural and synthetic organic molecules.6 Lewis acid catalysed reactions have a great scope due to their distinctive reactivity, selectivity and mild reaction conditions.7,8In 1991, the first ever water compatible Lewis acid lanthanide triflate was revealed by Kobayashi.9 lanthanide triflates have been literature known compound at that time however their role and use in organic transformations have been restrained. Prior to it, lanthanide triflate was used as homogeneous catalysts for the synthesis of amidine in anhydrous organic solvent.10 Until this synthesis, it was widely assumed that Lewis acid catalysis required to be carried out in a completely anhydrous state. The major distinguishing characteristics of lanthanide triflates are that they are stable and operate as Lewis acids in water. Following this first report, not only homogeneous lanthanide triflates but also rare earth metal triflates that are water-compatible Lewis acids were reported.11 In recent times, silica supported lanthanide triflates, rare earth metal triflates and silica supported trifluoroacetic acid or trichloroacetic acid heterogenous Lewis acid catalysts were developed and utilized in many organic transformations.12,13 These heterogeneous Lewis acid catalysts carry out a variety of useful reactions and have a number of notable advantages, making them environmentally friendly green Lewis acid catalysts.14 This unique properties of green Lewis acid catalysts have also made them ideal alternatives to the conventional Lewis acid catalysts, since they are needed in a stoichiometric amount, stable and function as Lewis acid in both water and organic solvents, found to be water-competent, easily retrieved and reused after a reaction without losing their catalytic activity.15 Moreover, these green Lewis acids are highly efficient chemoselective, regioselective and stereoselective catalysts for different series of organic transformations.16
However, both homogeneous and heterogeneous lanthanide triflates and rare earth metal triflates catalyst have several limitations and restrictions, mainly they are highly sensitive to moisture and hygroscopic in nature, require strict storage under anhydrous, moisture-free, and dry conditions. They are also expensive catalysts in terms of price, and their synthesis required specific inert reaction condition. To overcome these problems, we have developed silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts as a competent alternative for the lanthanide triflates and rare earth metal triflates catalysts. The presently synthesized silica supported catalysts are nonhygroscopic, moisture insensitive and less expensive as they are prepared by commercially available starting materials at normal reaction conditions. Further, they are required in a stoichiometric amount, stable, and function as Lewis acid in both water and organic solvents, and found to be water competent. They can also be easily recovered and reused after a reaction without losing their catalytic activity.
In recent years, imidazole-containing heterocycles have very high demands as they are present in a wide spectrum of bioactive compounds with a variety of medicinal and biological applications. The imidazole nucleus has been found in a variety of pharmacologically active compounds, including histamine and histidine hormone.17 Notably, imidazole nuclei can be found in a variety of pharmacologically active molecules, such as eprosartan,18 losartan,19 and clotrimazole,20 alpidem,21 flumazenil.21 However, a variety of heterocyclic frameworks containing imidazole cores have piqued our interest due to their biological actions and importance in therapeutics and medicine.22,23 In this context, the synthesis of a five-membered nitrogen-containing ring structure is still a hot topic. A literature survey of several methods for the synthesis of highly functionalized tetra- and trisubstituted imidazole scaffolds reveals that24–29 some of these procedures suffer from tedious synthetic paths, harsh reaction conditions, prolonged reaction time, corrosiveness, toxicity, cost, and catalyst reusability. As a result of this simple, environment friendly, and versatile processes are still required. Now-a-days, multicomponent reaction protocol became a tool, which has been approved as an advanced tool for renewable and sustainable synthetic organic chemistry. The efficiency of catalytic techniques used in multicomponent reaction protocol has made it easier to gain access to novel libraries of organic compounds with great biological potential.
In this concern, we have introduced a new and greener route for the one-pot synthesis of 2,4,5-triaryl substituted imidazole derivatives from benzil, aldehyde, and ammonium acetate under solvent-free conditions at 70 °C with a high product yield in a shorter period of time with easy and simple reaction workup. The presently synthesized catalysts showed significant catalytic activity in these derivative syntheses.
2. Experimental section
2.1. Materials and methods
All the chemicals and solvents were purchased by Sigma-Aldrich, Merck and Molychem companies of high purity and used directly in the reaction protocol without further purification. The powder X-ray diffraction technique was utilized with well-calibrated instrument a Bruker D8-advanced diffractometer with Cu Kα radiation ranges from 5° and 60° (2θ values). The FTIR spectra were obtained on a Shimadzu FTIR 8300 spectrophotometer. The elemental composition was carried via objects 8724. The specific surface area and pore volume were determined on Quantachrome instruments version 3.01 with Brunauer–Emmett–Teller method. Morphology of the Lewis acid catalyst samples was analyzed by FE-SEM on instrument Nova Nano SEM NPEP303 and high-resolution transmission electron microscopy images were captured using an instrument JEOL JEM 2100 Plus microscope. All the organic transformations progress were preliminary monitored by thin-layer chromatography (TLC). The 1H NMR spectra (400 MHz) and 13C NMR spectra (100 MHz) were run on a Bruker (400 MHz) spectrometer using DMSO-d6 solvent and tetramethyl silane (TMS) as an internal reference. All reactions were carried out under reflux conditions using a laboratory equipment oil bath and magnetic stirrer. Melting points were recorded on the digital melting point apparatus.2.2. Synthesis of lanthanum trifluoroacetate (La(OCOCF3)3·nH2O) and trichloroacetate (La(OCOCCl3)3·nH2O)
The lanthanum trifluoroacetate and trichloroacetate were prepared by new and slight modification in Fujihara method30 by direct reaction between lanthanum acetate (1 g) with a slightly excess amount of trifluoroacetic acid/trichloroacetic acid (4 g) respectively in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 equivalence ratio in 100 ml round bottom flask. The reaction mixture was refluxed at 60 °C for 10–12 hours. The white product obtained in the reaction was further separated via vacuum filtration to remove unreacted acid and side product acetic acid from the reaction mixture. The product was washed with n-hexane and dried in an oven at 50 °C for 2–3 hours (Scheme 1).
3 equivalence ratio in 100 ml round bottom flask. The reaction mixture was refluxed at 60 °C for 10–12 hours. The white product obtained in the reaction was further separated via vacuum filtration to remove unreacted acid and side product acetic acid from the reaction mixture. The product was washed with n-hexane and dried in an oven at 50 °C for 2–3 hours (Scheme 1).
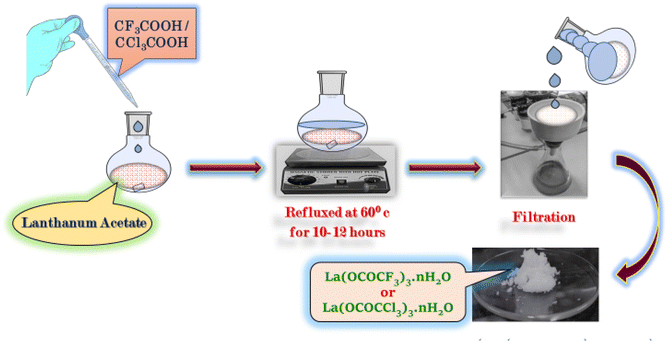 | ||
| Scheme 1 Synthesis of lanthanum trifluoroacetate (La(OCOCF3)3·nH2O) and trichloroacetate (La(OCOCCl3)3·nH2O). | ||
2.3. Synthesis of silica supported lanthanum trifluoroacetate (La(OCOCF3)3·nH2O/SiO2) and trichloroacetate (La(OCOCF3)3·nH2O/SiO2) Lewis acid catalyst
The silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalyst was prepared according to literature known procedures31 with slight modifications. The lanthanum trifluoroacetate/trichloroacetate (1 g) was added to methanol solvent (50 ml) in 100 ml round bottom flask connected with a reflux condenser and a magnetic stirrer bar. The supporting material Kieselgel K100 or silica gel (10 g), was adding up and the resultant slurry of the reaction mixture was well stirred at room temperature for 7–8 hours. The solvent was then evaporated in a sonicator at 70 °C for 1 hour. Finally solid porous form of catalyst was kept in a vacuum desiccator for 2–3 hours to obtain its anhydrous form (Scheme 2).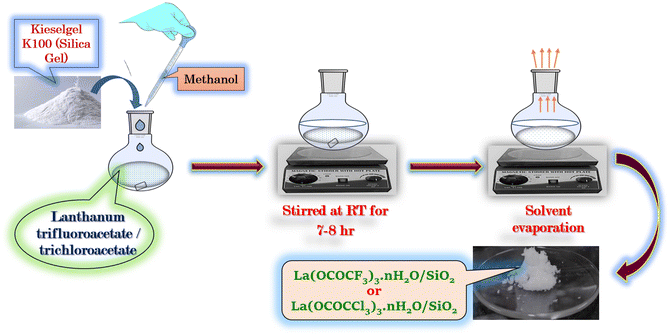 | ||
| Scheme 2 Silica supported lanthanum trifluoroacetate (La(OCOCF3)3·nH2O/SiO2) and trichloroacetate (La(OCOCCl3)3·nH2O/SiO2) Lewis acid catalyst. | ||
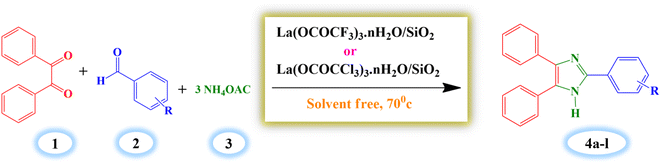
2.4. General procedure for synthesis of 2,4,5-triarylimidazole derivatives (4a–l)
A mixture of benzil (1 mmol), benzaldehyde (1 mmol), ammonium acetate (3 mmol) and silica supported lanthanum trifluoroacetate catalyst (0.04 g) were stirred in oil bath at 70 °C for 14 minutes under solvent-free condition. The progress of reaction was supervised by thin layer chromatography technique (TLC). The reaction mixture was diluted with hot ethanol (15 ml) once the reaction was completed, and it was then filtered to separate the catalyst. Further, the filtrate of reaction mixture was poured into crushed ice to obtain crude solid product which was filtered and recrystallized by using hot ethanol to get a pure crystal of product. The recovered catalyst was washed with ethanol and dried in an vacuum desiccator for 2–3 hours for further reuse.3. Result and discussion
3.1. Catalyst characterization
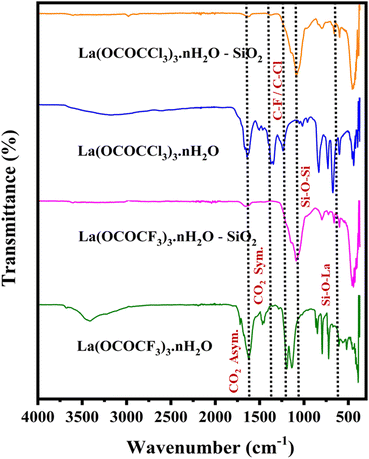 | ||
| Fig. 1 FT-IR analysis of lanthanum trifluoroacetate, silica supported lanthanum trifluoroacetate, lanthanum trichloroacetate and silica supported lanthanum trichloroacetate composites. | ||
The FTIR spectra of synthesized catalysts exhibited five characteristic frequencies. The unsupported lanthanum trifluoroacetate and trichloroacetate have FTIR spectrum at 1633 and 1622 cm−1 is associated with a CO2 asymmetric vibration and band appears at 1462, 1452 cm−1 represent, CO2 symmetric vibration. While, silica supported lanthanum trifluoroacetate and trichloroacetate shows CO2 asymmetric vibration band at 1658, 1645 cm−1. This modification in higher vibrational value in silica supported catalyst is the result of interaction of silica and lanthanum trifluoroacetate and trichloroacetate. Another characteristics doublet spectrum at 1165, 1138 and 1198 cm−1 assigned to C–F, C–Cl and C–O bond vibrations of the trifluoroacetate or trichloroacetate groups, while bands in the 850 and 835 cm−1 indicate δasCF3 and δasCCl3.32 Moreover, FTIR spectrum of silica-supported catalyst showed lower shift and observed new sharp additional bands appears at 1082 cm−1 and 663 cm−1 assigned to Si–O–Si & La–O–Si bond vibrations, attribute more clear confirmation of the successful functionalization and strong electrostatic interaction of lanthanum trifluoroacetate and trichloroacetate with the mesoporous silica support material. The above FTIR results reflect the successful functionalization of silica-supported catalysts having Lewis acidic sites which are actively involved in the synthesis of 2,4,5-triarylimidazoles.
 | ||
| Fig. 2 XRD analysis of lanthanum trifluoroacetate, silica supported lanthanum trifluoroacetate, lanthanum trichloroacetate and silica supported lanthanum trichloroacetate composites. | ||
Moreover, silica supported lanthanum trifluoroacetate and trichloroacetate shows modified and prominent intense characteristics diffraction peaks at 2θ = 14°, 25°, 29°, 32°, and 49°, 54°. These results provide clear evidence that lanthanum trifluoroacetate and trichloroacetate were finely dispersed on the silica supported material in the La(OCOCF3)3·nH2O/silica and La(OCOCCl3)3·nH2O/silica catalysts. The broad humped diffraction peaks observed in silica supported lanthanum trifluoroacetate and trichloroacetate catalysts compared to the sharp diffraction peaks in unsupported lanthanum trifluoroacetate and trichloroacetate clearly indicate the disturbance in original crystallinity of catalysts. This change in crystallinity of silica supported catalyst is caused by the strong electrostatic interaction of lanthanum trifluoroacetate and trichloroacetate with the silica support material, which converts the supported catalyst to amorphous nature.34
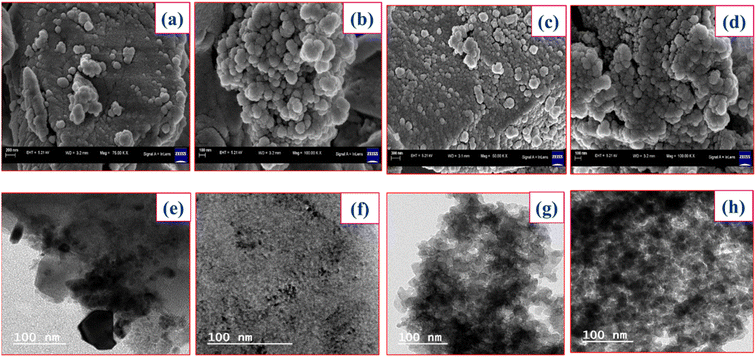
The TEM images of unsupported lanthanum trifluoroacetate and trichloroacetate (Fig. 3e and f) shows that most of the specifically arranged fine particles covered with dark colour. While, the TEM images of silica supported lanthanum trifluoroacetate and trichloroacetate (Fig. 3g and h) indicates proper layered dark colour fine particles on another surface layer of supporting material. This provides strong evidence for uniform dispersion of lanthanum trifluoroacetate and trichloroacetate inside the mesoporous pores of silica support material.
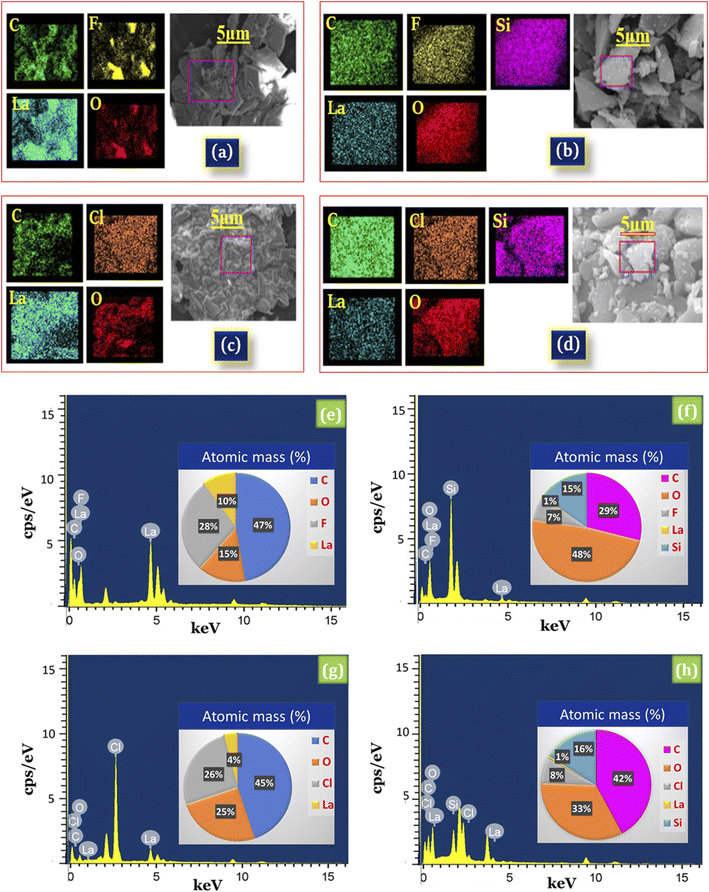
Moreover, the EDX results of silica supported lanthanum trifluoroacetate (La(OCOCF3)3·nH2O/SiO2) showed the presence of La, O, C, and F elements of La(OCOCF3)3·nH2O and Si, and O of silica (Fig. 4b), while silica supported lanthanum trichloroacetate (La(OCOCCl3)3·nH2O/SiO2) showed the presence of La, O, C, and Cl elements of La(OCOCCl3)3·nH2O and Si, and O of silica (Fig. 4d), which strongly indicates the successful formation of silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts. The results of EDX elemental image mapping shown in (Fig. 4e and f), revealed the atomic percentage of La, O, C, F, Cl, Si, and O in the synthesized catalysts.
Further, EDX mapping images provides additional validation or confirmation for a uniform distribution of La, O, C, and F in the desired lanthanum trifluoroacetate (La(OCOCF3)3·nH2O) catalyst system (Fig. 4a), while La, O, C, and Cl in the lanthanum trichloroacetate (La(OCOCCl3)3·nH2O) catalyst system (Fig. 4c). The elemental mapping of Fig. 4b and d represents the construction of a finely dispersed blended material of La, O, C, F, Cl, Si, and O in the synthesized silica supported lanthanum trifluoroacetate (La(OCOCF3)3·nH2O/SiO2) and lanthanum trichloroacetate (La(OCOCCl3)3·nH2O/SiO2) Lewis acid catalyst that is in moral agreement with FT-IR, XRD and SEM-TEM spectral results.
| Entry | Catalyst sample | SBET (m2 g−1) | Dpore (nm) | Vpore (cm3 g−1) |
|---|---|---|---|---|
| 1 | La(OCOCF3)3·nH2O | 21.278 | 9.0766 | 0.0436 |
| 2 | La(OCOCCl3)3·nH2O | 19.198 | 7.5292 | 0.0361 |
| 3 | La(OCOCF3)3·nH2O/SiO2 | 113.545 | 11.102 | 0.3151 |
| 4 | La(OCOCCl3)3·nH2O/SiO2 | 45.001 | 11.958 | 0.1345 |
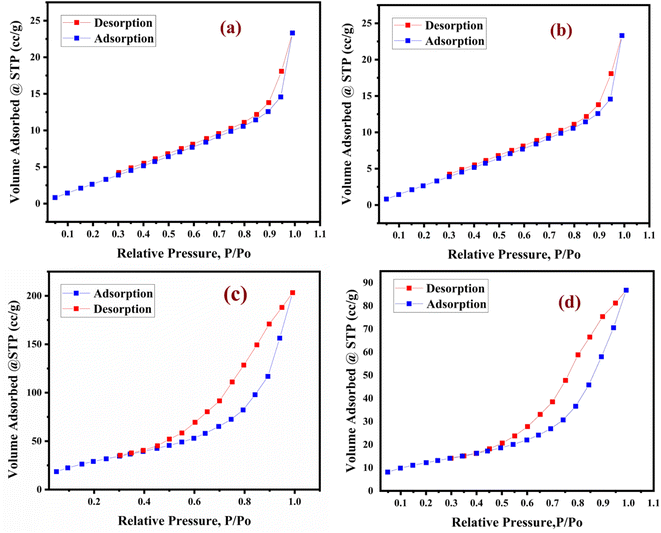
The nitrogen adsorption–desorption isotherm is of type V in nature according to the IUPAC classification. The bulk unsupported lanthanum trifluoroacetate, lanthanum trichloroacetate, silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts exhibit a well expressed H3 hysteresis loop (Fig. 5a–d), in the order of 0.4–1.0P/P0 at high relative pressure, which is most common for mesoporous materials.35 As represented in Table 1, the average pore diameter of the lanthanum trifluoroacetate, lanthanum trichloroacetate, silica supported lanthanum trifluoroacetate and lanthanum trichloroacetate catalyst were 9.0766, 7.5292, 11.102 and 11.958 nm respectively. Also, total pore volume (0.0436–0.3151 cm3 g−1) of the catalysts was calculated using the BJH method (see Fig. 5a–d).
The BET study proved that the surface area of lanthanum trifluoroacetate and trichloroacetate catalysts supported by silica was marginally greater than that of the same catalysts without support. The main cause of this might be the deposition and incorporation of lanthanum trifluoroacetate or trichloroacetate into the pores of the mesoporous silica support material. The increased surface area, average pore diameter and total pore volume of silica supported Lewis acid catalysts may become one of the key features in enhancing catalytic performance.
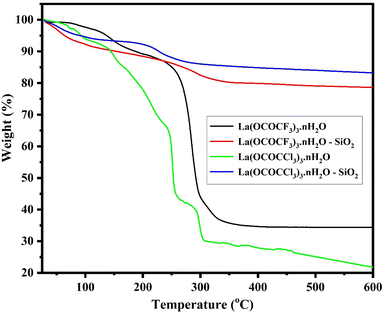 | ||
| Fig. 6 TGA analysis of lanthanum trifluoroacetate, silica supported lanthanum trifluoroacetate, lanthanum trichloroacetate and silica supported lanthanum trichloroacetate composites. | ||
In the TGA of silica supported lanthanum trifluoroacetate and trichloroacetate catalysts, the second characteristic curves (from temperature range 198 °C to 271 °C) with minimum mass loss of 14 and 12% respectively, were assumed to be due to a partial loss of trifluoroacetate and trichloroacetate group of the anhydrous Lewis acid. While third curve (from temperature range 276 °C to 326 °C) with maximum mass loss of 19 and 15% respectively, were associated due to a complete loss of trifluoroacetate and trichloroacetate group of the anhydrous Lewis acid.
Herein, TGA study of these Lewis acid catalyst provides strong confirmation that the thermal stability of supported Lewis acid catalysts were increased than that of the catalyst alone. This must be due to the interaction between silica and bulk lanthanum trifluoroacetate or trichloroacetate catalyst. These advancements in thermal stability of the supported catalyst leads to moderate a range of temperatures over which it can be used without a significant loss in catalytic activity, achieving reactions at higher temperatures as well as preventing sintering of metal in order to prolong life of the catalyst.
3.2. Catalytic activity of silica supported Lewis acid catalysts for the synthesis of 2,4,5-trisubstituted arylimidazole derivatives
The first focus of our studies was to evaluate the efficiency of synthesized Lewis acid catalysts and optimization of the best reaction conditions for the one-pot synthesis of 2,4,5-triarylimidazole derivatives. Benzaldehyde (1 mmol), benzil (1 mmol) and ammonium acetate (3 mmol) were chosen as substrates for the model reaction (Scheme 3).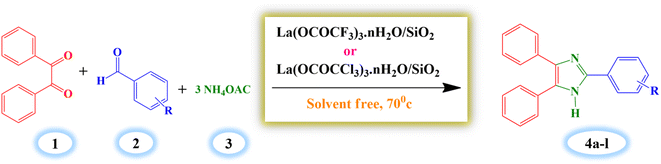 | ||
| Scheme 3 Silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysed synthesis of 2,4,5 triaryl imidazole. | ||
In order to evaluate the efficiency of synthesized Lewis acid catalysts, the model reaction was carried via unsupported Lewis acid catalysts La(OCOCF3)3·nH2O & La(OCOCCl3)3·nH2O and silica supported Lewis acid catalysts La(OCOCF3)3·nH2O/SiO2 & La(OCOCCl3)3·nH2O/SiO2 under solvent free condition (Table 2). The unsupported Lewis acid catalysts show lesser catalytic efficiency in respect of reaction time period and product yields as compared to similar catalysts with silica support. It was found that product yield and reaction time period was improved a lot by the silica supported Lewis acid catalysts. While, pure silica supporting material shows incredibly poor catalytic activity in terms of the expected product yield and reaction time period (Table 2, entry 5).
| Entry | Catalyst sample | Timeb (min) | Yieldc (%) |
|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and synthesized Lewis acid catalysts.b Reaction progress monitored by TLC.c Isolated yields. | |||
| 1 | La(OCOCF3)3·nH2O | 45 | 71 |
| 2 | La(OCOCCl3)3·nH2O | 48 | 68 |
| 3 | La(OCOCF3)3·nH2O/SiO2 | 14 | 96 |
| 4 | La(OCOCCl3)3·nH2O/SiO2 | 17 | 94 |
| 5 | Kieselgel K100 (silica gel) | 220 | 41 |
This indicates that silica supported Lewis acid catalysts La(OCOCF3)3·nH2O/SiO2 & La(OCOCCl3)3·nH2O/SiO2 shows very high catalytic activity/efficiency. The increase in activity of the silica supported Lewis acid catalysts were due to high dispersion of lanthanum trifluoroacetate or trichloroacetate Lewis acid catalyst on silica support. As a result, it would have more surface area and active Lewis and Brønsted sites than the unsupported Lewis acid catalysts La(OCOCF3)3·nH2O & La(OCOCCl3)3·nH2O. Moreover, the comparative study of catalytic efficiency of silica supported lanthanum trifluoroacetate (La(OCOCF3)3·nH2O/SiO2) and lanthanum trichloroacetate (La(OCOCCl3)3·nH2O/SiO2) Lewis acid catalysts were also performed, which clearly indicates that silica supported lanthanum trifluoroacetate shows higher catalytic activity/efficiency than silica supported lanthanum trichloroacetate.
Further, we have monitored the model reaction under many conditions, such as the various solvents, temperature range and catalyst amount, in order to determine the ideal reaction parameters for this multicomponent reaction in presence of synthesized silica supported Lewis acid catalysts. The different range of solvents (polar and non-polar solvent) were utilized to examine the catalytic activity of the synthesized silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts. It was observed that the solvent-free environment produced the best results in terms of reaction time and product yield (Table 3). This must be because in solvent-free conditions, the larger availability of the reactant molecules allows for easy access to the active sites through the pores of the catalyst, and this porous structure produces the best results compared to in a solvent. In a solvent, the presence of solvent molecules reduces the availability of reactant molecules, which lowers the yield of the product in comparison to a solvent-free reaction. Since solvent free reaction condition provided the best results in terms of reaction time and product yield, temperature optimization was also carried out at the solvent free condition, in order to examine the best reaction temperature for the overall synthesis of the 2,4,5-trisubstituted arylimidazole derivatives listed in Table 3. The reaction time and product yield improved gradually and steadily as the temperature range increased from 50 to 70 °C. The results show that 70 °C is the ideal reaction temperature for this one-pot, solvent-free multicomponent synthesis. Furthermore, the reaction progress time and product yield were not significantly affected by the temperature increase above 70 °C.
| Entry | Solvents | Reaction condition (temp.) | Timeb (min) | Yieldc (%) | ||
|---|---|---|---|---|---|---|
| A Catalyst | B Catalyst | A Catalyst | B Catalyst | |||
| a Reaction conditions: benzaldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and (A Catalyst) – silica supported lanthanum trifluoroacetate or (B Catalyst) – silica supported lanthanum trichloroacetate.b Reaction progress monitored by TLC.c Isolated yields. | ||||||
| 1 | Water | Reflux | 64 | 72 | 70 | 68 |
| 2 | Ethanol | Reflux | 38 | 40 | 90 | 89 |
| 3 | DMF | Reflux | 46 | 48 | 76 | 72 |
| 4 | THF | Reflux | 43 | 47 | 81 | 78 |
| 5 | Acetonitrile | Reflux | 68 | 74 | 67 | 62 |
| 6 | Chloroform | Reflux | 65 | 70 | 69 | 67 |
| 7 | Toluene | Reflux | 84 | 90 | 65 | 62 |
| 8 | Solvent free | Reflux | 17 | 22 | 85 | 83 |
| 9 | Solvent free | 50 °C | 20 | 24 | 78 | 74 |
| 10 | Solvent free | 60 °C | 14 | 17 | 85 | 81 |
| 11 | Solvent free | 70 °C | 14 | 17 | 96 | 94 |
| 12 | Solvent free | 80 °C | 14 | 17 | 96 | 94 |
| 13 | Solvent free | 90 °C | 14 | 17 | 96 | 94 |
The determination of appropriate and stoichiometric amount of the catalyst for the reaction is another crucial factor for reaction effectiveness. To find appropriate amount of catalyst required, the model reaction was carried out under solvent free condition in the presence of different amounts (0.01, 0.02, 0.03, 0.04, 0.05, and 0.06 g) of both silica supported lanthanum trifluoroacetate and trichloroacetate lewis acid catalysts and obtained results are summarized in Table 4.
| Entry | Amount of catalyst (g) | Reaction condition (temp.) | Timeb (min) | Yieldc (%) | ||
|---|---|---|---|---|---|---|
| A Catalyst | B Catalyst | A Catalyst | B Catalyst | |||
| a Reaction conditions: benzaldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and (A Catalyst) – silica supported lanthanum trifluoroacetate or (B Catalyst) – silica supported lanthanum trichloroacetate.b Reaction progress monitored by TLC.c Isolated yields. | ||||||
| 1 | 0.01 | 70 °C | 14 | 17 | 67 | 66 |
| 2 | 0.02 | 70 °C | 14 | 17 | 74 | 71 |
| 3 | 0.03 | 70 °C | 14 | 17 | 85 | 81 |
| 4 | 0.04 | 70 °C | 14 | 17 | 96 | 94 |
| 5 | 0.05 | 70 °C | 14 | 17 | 96 | 94 |
| 6 | 0.06 | 70 °C | 14 | 17 | 96 | 94 |
When the amount of these Lewis acid catalysts increases progressively, product yield also increases (Table 4, entries 1–6). The obtained results demonstrate that 0.04 g amount of both silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts gave 96% and 94% yield of product respectively at 70 °C temperature (Table 4, entry 4). An additional increase in the amount (0.05, and 0.06 g) of these Lewis acid catalysts does not increase yield of the product (Table 4, entry 5, 6). This could be as a result of the catalyst reaching its maximum conversion efficiency.
Finally, the 2,4,5-trisubstituted arylimidazole derivatives (4a–l) were synthesized employing the catalytic system under the optimum reaction conditions, and excellent results were achieved, which are well described in Table 5. However, out of this silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts, silica supported lanthanum trifluoroacetate shows more catalytic activity compared to silica supported lanthanum trichloroacetate in respect of all optimization parameters and arylimidazole derivatives (4a–l) synthesis. Actually, this is due to the fact that high electronegative fluorine atoms increase the acidity of Lewis acid catalyst by its more withdrawing nature as compared to chlorine atoms.
| Entry | Substituent ‘R’ | Timeb (min) | Yieldc (%) | M.P. (°C) observed | M.P. (°C) reported | |||
|---|---|---|---|---|---|---|---|---|
| A Catalyst | B Catalyst | A Catalyst | B Catalyst | |||||
| a Reaction conditions: benzaldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and (A Catalyst) – silica supported lanthanum trifluoroacetate or (B Catalyst) – silica supported lanthanum trichloroacetate.b Reaction progress monitored by TLC.c Isolated yields. | ||||||||
| 4a | H | 14 | 17 | 96 | 94 | 269–270 | 270–272 [37] | |
| 4b | 4-OMe | 15 | 18 | 96 | 93 | 229–231 | 231–232 [38] | |
| 4c | 4-Me | 16 | 18 | 95 | 93 | 228–230 | 231–232 [39] | |
| 4d | 4-Br | 14 | 17 | 95 | 92 | 257–260 | 254–256 [40] | |
| 4e | 4-F | 14 | 19 | 96 | 94 | 188–190 | 190 [41] | |
| 4f | 4-Cl | 14 | 17 | 96 | 92 | 261–263 | 257–260 [42] | |
| 4g | 4-NO2 | 16 | 20 | 92 | 90 | 242–245 | 241–242 [43] | |
| 4h | 2-Me | 16 | 19 | 95 | 92 | 253–255 | 255–258 [44] | |
| 4i | 2-Cl | 15 | 18 | 95 | 93 | 189–192 | 188 [45] | |
| 4j | 3-Br | 16 | 17 | 93 | 91 | 293–295 | 298–300 [45] | |
| 4k | 4-OH | 14 | 18 | 96 | 93 | 255–258 | 254–256 [46] | |
| 4l | 3-OH | 15 | 19 | 93 | 91 | 259–261 | 260–261 [47] | |
A comparative study, which includes silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid as a catalyst was performed for the synthesis of 2,4,5-trisubstituted arylimidazole derivatives with various catalysts reported in the literature (see Table 6). In comparison to silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts in solvent-free systems, reactions with other different catalysts required a higher amount of catalyst and longer reaction time period which produces a low yield of the desired product also. As a result, the present Lewis acid catalyst promoted the reactions more successfully than the other catalysts and should become one of the best options for choosing an economically convenient, and environmentally eco-friendly catalyst.
| Entry | Catalyst | Conditions | Timeb (min) | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), benzil (1 mmol), ammonium acetate (3 mmol) and silica supported lanthanum trifluoroacetate or silica supported lanthanum trichloroacetate Lewis acid catalysts.b Reaction progress monitored by TLC.c Isolated yields. | ||||
| 1 | Without catalyst | Reflux | 720 | NR |
| 2 | CH3COOH | Reflux | 120 | 40 [48] |
| 3 | Montmorillonite K10 | EtOH, reflux | 90 | 70 [40] |
| 4 | Polymer–ZnCl2 | EtOH, reflux | 240 | 96 [49] |
| 5 | Nano-SnCl4.SiO2 | Solvent-free/130 °C | 120 | 96 [50] |
| 6 | Ceric ammonium nitrate | EtOH, reflux | 360 | 75 [51] |
| 7 | Yb(OTf)3 | Acetic acid, reflux | 160 | 92 [52] |
| 8 | Y(TFA)3 | Reflux/100 °C | 180 | 95 [53] |
| 9 | ZSM-11 | Solvent-free/110 °C | 30 | 90 [54] |
| 10 | La(OCOCCl3)3·nH2O/SiO2 | Solvent-free, 70 °C | 17 | 94 (this work) |
| 11 | La(OCOCF3)3·nH2O/SiO2 | Solvent-free, 70 °C | 14 | 96 (this work) |
3.3. Proposed plausible structure for active site of silica supported Lewis acid catalyst
The proposed model of active site of silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts is well depicted in Fig. 7. These catalysts mainly indicate Lewis acid sites on lanthanum metal which is attached to the mesoporous surface of silica support material. The trifluoroacetate or trichloroacetate group withdraws the electron density from the metal atom (La) which leads to making it electron deficient and developing Lewis acidity. The interaction of trifluoroacetate or trichloroacetate with surface –OH groups of silica bind them on the silica surface without moderating its Lewis acidity. It is well reported that the silica supported lanthanum trifluoroacetate and trichloroacetate solid heterogeneous catalysts mainly includes Lewis acidity, which is actually originated from the loaded lanthanum trifluoroacetate or trichloroacetate.36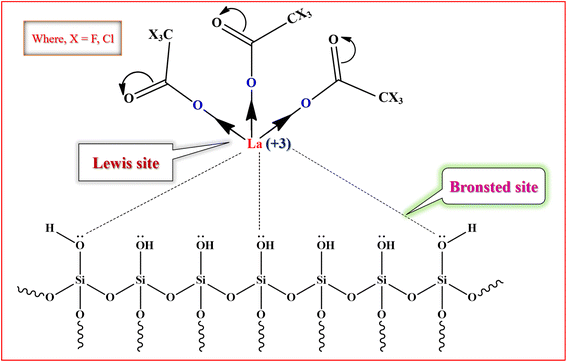 | ||
| Fig. 7 The proposed model of active site of silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalyst. | ||
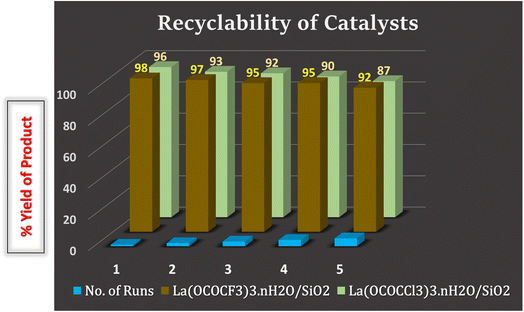
3.4. Recycling of catalyst
The recyclability of the catalysts is one of the key features of the current methodology. The catalysts were recovered from the reaction mixture by means of simple filtration technique, in such a way that after completion of the reaction, reaction mixture was diluted with hot ethanol and filtered for the separation catalyst. Filtered, solid catalysts were washed with the ethanol/water system (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The recovered catalysts were kept under vacuum-oven for 20 min at 80 °C for drying and activation, after which they were weighed and reused in the next run. They were used for five runs without any further treatment in the subsequent reaction. It was found that recovered catalysts almost have consistent activity after several time of use. The present silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts even after the fifth recycling, shows 92% and 87% catalytic efficiency respectively (Fig. 9). These studies proved that the current silica supported green Lewis acid catalysts are highly active, efficient and reusable without a considerable loss in catalytic activity. Furthermore, after the fifth cycle, the recycled catalyst was recovered, washed, dried and weighed. This recovered catalyst was then characterized by using powder XRD analysis for its comparative morphological study with fresh catalyst. The diffraction peaks at 2θ = 14°, 25°, 29°, 32°, 49°, and 54° of recovered catalysts were exactly identical to the fresh silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts (Fig. 8).
1). The recovered catalysts were kept under vacuum-oven for 20 min at 80 °C for drying and activation, after which they were weighed and reused in the next run. They were used for five runs without any further treatment in the subsequent reaction. It was found that recovered catalysts almost have consistent activity after several time of use. The present silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts even after the fifth recycling, shows 92% and 87% catalytic efficiency respectively (Fig. 9). These studies proved that the current silica supported green Lewis acid catalysts are highly active, efficient and reusable without a considerable loss in catalytic activity. Furthermore, after the fifth cycle, the recycled catalyst was recovered, washed, dried and weighed. This recovered catalyst was then characterized by using powder XRD analysis for its comparative morphological study with fresh catalyst. The diffraction peaks at 2θ = 14°, 25°, 29°, 32°, 49°, and 54° of recovered catalysts were exactly identical to the fresh silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalysts (Fig. 8).
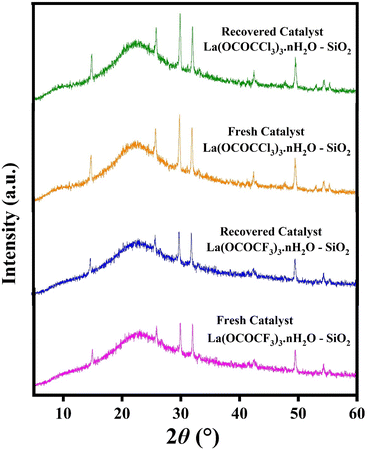 | ||
| Fig. 8 XRD spectrum of fresh and recovered silica supported lanthanum trifluoroacetate and lanthanum trichloroacetate Lewis acid catalysts. | ||
 | ||
| Fig. 9 Recyclability of silica supported lanthanum trifluoroacetate and trichloroacetate Lewis acid catalyst in the synthesis of 2,4,5-triarylimidazole derivatives. | ||
3.5. Plausible reaction mechanism
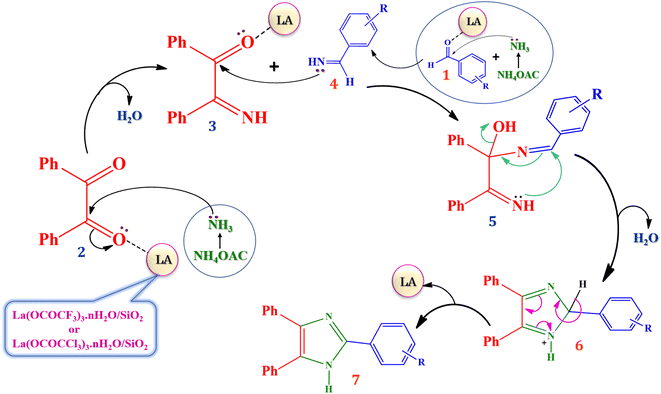
The proposed plausible mechanism for the synthesis of 2,4,5-trisubstituted arylimidazole in presence of currently synthesized green Lewis acid catalyst is well described in Scheme 4, based on literature reports.13,54 The carbonyl groups of benzil (2) and aromatic aldehydes (1) are activated by silica supported lanthanum trifluoroacetate or trichloroacetate Lewis acid catalyst which leads to an increase in electrophilicity of carbonyl carbon. Then nucleophilic attack of the nitrogen atom of ammonia molecule takes place due to the presence of its free lone pair of electron. The ammonia molecules are generated in situ from ammonium acetate which attack on the activated carbonyl carbon of benzil and aromatic aldehydes resulting in the formation of 2-imino-1,2-diphenylethanone intermediate (3) and aryl methanimine intermediate (4). Further, nucleophilic attack of aryl methanimine intermediate (4) to another carbonyl carbon of 2-imino-1,2-diphenylethanone (3) gives 1-(benzylidene amino)-2-imino-1,2-diphenylethanol intermediate (5), which afterward undergoes the cyclization leads to the formation of 2,4,5-triphenyl-2H-(1-imidazolium) intermediate (6) further converted into 2,4,5- trisubstituted arylimidazole derivatives by dehydration. However, the catalyst which regenerated after end of each reaction are available for the next reaction cycle.4. Conclusion
In an overview, we have developed silica supported lanthanum trifluoroacetate and trichloroacetate as water-compatible green Lewis acid catalysts via novel, simple, and eco-friendly method. The morphology and properties of synthesized Lewis acid catalysts were studied by FTIR, TGA, XRD, EDX, SEM, TEM and BET analysis techniques. The synthesized catalysts were utilized in the synthesis of 2,4,5-trisubstituted arylimidazole derivatives under solvent-free green reaction protocols. The silica supported lanthanum trifluoroacetate and trichloroacetate catalysts work superiorly in presence of water as well as in organic solvents without loss in their catalytic activity compared to conventional Lewis acid catalysts. However, the best reaction results were found in solvent-free conditions at 70 °C optimum temperature. This reaction protocol has many advantages as being highly efficient with excellent product yield in shorter reaction time, work-up simplicity and no side reactions. The comparative study of catalytic efficiency of these two catalysts was also performed in terms of reaction time, reaction temperature, and influence of different solvents on yield of the product. This clearly indicates that silica supported lanthanum trifluoroacetate shows higher catalytic activity and efficiency than silica supported lanthanum trichloroacetate.These catalysts offer several remarkable features. They are non-hazardous, water compatible, work superiorly in the different range of solvents under mild reaction conditions with best product yields, easily recovered and reused in reactions. These catalysts were economically valuable as they were synthesized from commercially available cheap starting materials. Moreover, the synthesized silica supported Lewis acid catalysts are non-hygroscopic, moisture insensitive, environment friendly and inexpensive compared to metal triflate Lewis acid catalysts. This will make them one of the best competent alternatives for metal triflate Lewis acid catalysts as well as to the heterogeneous acid catalysts.
Conflicts of interest
The authors declared that there is no conflict of interest in the publication of this research paper.Acknowledgements
The authors Dnyaneshwar Purushottam Gholap and Prof. M. K. Lande gratefully acknowledge UGC-DST SAP for financial assistance. We are also thankful to the Department of Chemistry, Dr Babasaheb Ambedkar Marathwada University, Aurangabad (MS), India for providing laboratory facilities.References
- A. Dhakshinamoorthy, A. Santiago-Portillo, A. M. Asiri and H. Garcia, ChemCatChem, 2019, 11, 899 CrossRef CAS.
- T. Zhang, Chem. Sci., 2021, 12, 12529–12545 RSC.
- A. H. Chughtai and others, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- (a) C. M. Friend and B. Xu, Acc. Chem. Res., 2017, 50, 517–521 CrossRef CAS PubMed; (b) D. S. Aher, K. R. Khillare, L. D. Chavan and S. G. Shankarwar, RSC Adv., 2021, 11, 2783–2792 RSC; (c) D. S. Aher, K. R. Khillare, L. D. Chavan and S. G. Shankarwar, RSC Adv., 2021, 11, 33980–33989 RSC; (d) R. G. Jadhav, D. Singh and A. K. Das, Sustainable Energy Fuels, 2020, 4, 1320–1331 RSC.
- W. Chao, Y. Anyuan and D. Wei-Lin, Appl. Catal., B, 2014, 160–161, 730–741 Search PubMed.
- (a) L. Lin X. Han, B. Han and S. Yang, Chem. Soc. Rev., 2021, 50, 11270–11292 RSC; (b) Q. Yangjian, L. Guangxu, F. Yingjie, S. Wenjie, Y. Eric and L. Wenbin, J. Am. Chem. Soc., 2020, 142, 1746–1751 CrossRef PubMed; (c) O. Awogbemi, D. V. Von Kallon and V. S. Aigbodion, J. Energy Inst., 2021, 98, 244–258 CrossRef CAS.
- S. Kobayashi, M. Sugira, H. kitagawa, W. William and L. Lam, Chem. Rev., 2002, 120, 2227–2302 CrossRef PubMed.
- (a) M. Hiroyuki, F. Risa, S. Yuhei, M. Kazuhiro and O. Takashi, Org. Lett., 2014, 16, 2018–2021 CrossRef PubMed; (b) T. Beisel and G. Manolikakes, Org. Lett., 2013, 15, 6046–6049 CrossRef CAS PubMed; (c) G. A. Meshram, S. S. Deshpande, P. A. Wagh and V. A. Vala, Tetrahedron Lett., 2014, 55, 3557–3560 CrossRef CAS.
- (a) S. Kobayashi, Synlett, 1994, 9, 689–701 CrossRef; (b) R. W. Marshman, Aldrichimica Acta, 1995, 28, 77–84 CAS; (c) S. Kobayashi, Chem. Lett., 1991, 20, 2187–2190 CrossRef.
- J. H. Forsberg, V. T. Spaziano, T. M. Balasubramanian, G. K. Liu, S. A. Kinsley, C. A. Duckworth, J. J. Poteruca, P. S. Brown and J. L. Miller, J. Org. Chem., 1987, 52, 1017–1021 CrossRef CAS.
- S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209–217 CrossRef CAS PubMed.
- B. Kumar, K. Smita, B. Kumar and L. Cumbal, J. Chem. Sci., 2014, 126, 1831–1840 CrossRef CAS.
- (a) D. Song, C. Liu, S. Zhang and G. Luo, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2010, 40, 145–147 CrossRef CAS; (b) K. H. Asressu, C. K. Chan and C. C. Wang, RSC Adv., 2021, 11, 28061–28071 RSC.
- K. N. Tayade, M. Mishra, K. Munusamy and R. S. Somani, Catal. Sci. Technol., 2015, 5, 2427–2440 RSC.
- (a) H. Firouzabadi, N. Iranpoor, A. A. Jafari and M. R. Jafari, J. Organomet. Chem., 2008, 693, 2711–2714 CrossRef CAS; (b) H. Adibi, H. A. Samimi and M. Beygzadeh, Catal. Commun., 2007, 8, 2119–2124 CrossRef CAS.
- (a) A. Corma and H. Garcıa, Chem. Rev., 2003, 103, 4307–4366 CrossRef CAS PubMed; (b) E. B. Mubofu and J. B. F. N. Engberts, J. Phys. Org. Chem., 2004, 17, 180–186 CrossRef CAS.
- (a) L. D. Luca, Curr. Med. Chem., 2006, 13, 1–23 Search PubMed; (b) C. K. Jadhav, A. S. Nipate, A. V. Chate, P. M. Kamble, G. A. Kadam, V. S. Dofe, V. M. Khedkar and C. H. Gill, J. Chin. Chem. Soc., 2021, 68, 1067–1081 CrossRef CAS.
- M. Gaba and C. Mohan, Med. Chem. Res., 2016, 25, 173–210 CrossRef CAS.
- R. Kreutz, Vasc. Health Risk Manage., 2011, 7, 183–192 CrossRef CAS PubMed.
- J. G. Hoogerheide and B. E. Wyka, Clotrimazole, Analytical Profiles of Drug Substances, 1982, vol. 11, pp. 225–255 Search PubMed.
- M. H. Fisher and A. Lusi, J. Med. Chem., 1972, 15, 982–985 CrossRef CAS PubMed.
- (a) J. Li, T. S. Kaoud, C. Laroche, K. N. Dalby and S. M. Kerwin, Bioorg. Med. Chem. Lett., 2009, 19, 6293–6297 CrossRef CAS PubMed; (b) S. Tahlan, S. Kumar and B. Narasimhan, BMC Chem., 2019, 13, 1–27 CrossRef PubMed; (c) M. Sánchez-Moreno, F. Gómez-Contreras, P. Navarro, C. Marín, F. Olmo, M. J. R. Yunta, A. M. Sanz, M. J. Rosales, C. Cano and L. Campayo, J. Med. Chem., 2012, 55, 9900–9913 CrossRef PubMed; (d) N. Zhao, Y. L. Wang and J. Y. Wang, J. Chin. Chem. Soc., 2005, 52, 535–538 CrossRef CAS.
- (a) A. V. Chate, S. P. Kamdi, A. N. Bhagat, C. K. Jadhav, A. Nipte, A. P. Sarkate, S. V. Tiwari and C. H. Gill, J. Heterocycl. Chem., 2018, 55, 2297–2302 CrossRef CAS; (b) V. S. Dofe, A. P. Sarkate, Z. M. Shaikh, C. K. Jadhav, A. S. Nipte and C. H. Gill, J. Heterocycl. Chem., 2018, 55, 756–762 CrossRef CAS; (c) S. Samanta, A. K. Ghosh, S. Ghosh, A. A. Ilina, Y. A. Volkova, I. V. Zavarzin, A. M. Scherbakov, D. I. Salnikova, Y. U. Dzichenka and A. B. Sachenko, Org. Biomol. Chem., 2020, 18, 5571–5576 RSC.
- A. Shaabani, R. Afshari, S. E. Hooshmand and M. Keramati Nejad, ACS Sustainable Chem. Eng., 2017, 5, 9506–9516 CrossRef CAS.
- S. Das Sharma, P. Hazarika and D. Konwar, Tetrahedron Lett., 2008, 49, 2216–2220 CrossRef CAS.
- A. Teimouri and A. N. Chermahini, J. Mol. Catal. A: Chem., 2011, 346, 39–45 CrossRef CAS.
- E. Kanaani and M. Nasr-Esfahani, J. Chin. Chem. Soc., 2019, 66, 119–125 CrossRef CAS.
- Y. Gu, Green Chem., 2012, 14, 2091–2128 RSC.
- Y. L. Wang, J. Luo and Z. L. Liu, J. Chin. Chem. Soc., 2013, 60, 1007–1013 CrossRef CAS.
- (a) S. Fujihara, T. Kato and T. Kimura, J. Sol-Gel Sci. Technol., 2003, 26, 953–956 CrossRef CAS; (b) E. Kemnitz and J. Noack, Dalton Trans., 2015, 44, 19411–19431 RSC.
- V. Sage, J. H. Clark and D. J. Macquarrie, J. Catal., 2004, 227, 502–511 CrossRef CAS.
- Y. A. Opata and J. C. Grivel, J. Anal. Appl. Pyrolysis, 2018, 132, 40–46 CrossRef CAS.
- S. I. Gutnikov, E. V. Karpova, M. A. Zakharov and A. I. Boltalin, Russ. J. Inorg. Chem., 2006, 51, 541–548 CrossRef.
- K. Mantri, K. Komura, Y. Kubota and Y. Sugi, J. Mol. Catal. A: Chem., 2005, 236, 168–175 CrossRef CAS.
- L. Xu, Y. Zhang, J. Zhang, J. Liu, Y. Shi, Y. Li, G. Dang, Y. Cheng, N. Chen and A. Guo, Minerals, 2020, 72, 1–25 Search PubMed.
- (a) C. Khatri, D. Jain and A. Rani, Fuel, 2010, 89, 3853–3859 CrossRef CAS; (b) A. Rani, C. Khatri and R. Hada, Fuel Process. Technol., 2013, 116, 366–373 CrossRef CAS.
- M. Kidwai, P. Mothsra, V. Bansal and R. Goyal, Monatsh. Chem., 2006, 137, 1189–1194 CrossRef CAS.
- H. Naeimi and D. Aghaseyedkarimi, New J. Chem., 2015, 39, 9415–9421 RSC.
- S. Balalaie, A. Arabanian and M. S. Hashtroudi, Monatsh. Chem., 2000, 131, 945–948 CrossRef CAS.
- A. Teimouri and A. N. Chermahini, J. Mol. Catal. A: Chem., 2011, 346, 39–45 CrossRef CAS.
- G. V. M. Sharma, Y. Jyothi and P. S. Lakshmi, Synth. Commun., 2006, 36, 2991–3000 CrossRef CAS.
- H. D. Hanoon, S. M. Radhi and S. K. Abbas, AIP Conf. Proc., 2019, 2144, 020005 CrossRef.
- A. Marzouk, V. M. Abbasov, A. H. Talybov and S. K. Mohamed, World. J. Org. Chem., 2013, 1, 6–10 Search PubMed.
- H. Zheng, Q. Y. Shi, K. Du, Y. J. Mei and P. F. Zhang, Catal. Lett., 2013, 143, 118–121 CrossRef CAS.
- S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539–3546 CrossRef CAS.
- M. Esmaeilpour, J. Javidi and M. Zandi, New J. Chem., 2015, 39, 3388–3398 RSC.
- J. Safari and Z. Zarnegar, Ultrason. Sonochem., 2013, 20, 740–746 CrossRef CAS PubMed.
- H. D. Hanoon, E. Kowsari, M. Abdouss, M. H. Ghasemi and H. Zandi, Res. Chem. Intermed., 2017, 43, 4023–4041 CrossRef CAS.
- L. Wang and C. Cai, Monatsh. Chem., 2009, 140, 541–546 CrossRef CAS.
- B. F. Mirjalili, A. Bamoniri and M. A. Mirhoseini, Sci. Iran., 2013, 20, 587–591 CAS.
- A. Shaabani, A. Maleki and M. Behnam, Synth. Commun., 2009, 39, 102–110 CrossRef CAS.
- H. Weinmann, M. Harre, K. Koenig, E. Merten and U. Tilstam, Tetrahedron Lett., 2002, 43, 593–595 CrossRef CAS.
- R. Wang, C. Liu and G. Luo, Green Chem. Lett. Rev., 2010, 3, 101–104 CrossRef CAS.
- S. S. Dipake, M. K. Lande, A. S. Rajbhoj and S. T. Gaikwad, Res. Chem. Intermed., 2021, 47, 2245–2261 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07021a |
| This journal is © The Royal Society of Chemistry 2023 |