Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent developments in 2D MXene-based materials for next generation room temperature NO2 gas sensors
Sithara
Radhakrishnan
and
Chandra Sekhar
Rout
 *
*
Centre for Nano and Material Sciences, Jain (Deemed-to-be University), Jain Global Campus, Kanakapura, Bangalore 562112, Karnataka, India. E-mail: r.chandrasekhar@jainuniversity.ac.in; csrout@gmail.com
First published on 15th August 2023
Abstract
MXenes with distinctive structures, good electrical conductivity and abundant functional groups have shown great potential in the fabrication of high performance gas sensors. Since the sensing mechanism of MXene-based gas sensors often involves a surface-dominant process, they can work at room temperature. In this regard, a significant amount of research has been carried out on MXene-based room temperature gas sensors and they can be viewed as one of the possible materials for NO2 sensing applications in the future. In this review, we focus on the most recent research and improvements in pure MXenes and their nanocomposites for NO2 gas sensing applications. First, we have explored the mechanisms involved in MXenes for NO2 gas sensing. Following that, other ways to tune the MXene sensing performance are investigated, including nanocomposite formation with metal oxides, polymers, and other 2D materials. A comparative analysis of the RT NO2 sensor performance based on MXenes and their hybrids is provided. We also discuss the major challenges of using MXene-related materials and the areas that can further advance in the future for the development of high-performance room temperature NO2 gas sensors.
1. Introduction
Human health has been severely harmed as a result of air pollution caused by urbanisation. Along with medical and technological advancements, the usage of synthetic fertilisers for greater crop production has resulted in steady population growth while also increasing nitrogen dioxide (NO2) gas emissions into the atmosphere. NO2 constitutes one of the most toxic gases, with a pungent odour, and it deteriorates human health when exposed to ppm levels for an extended period of time.1–3 When compared to CO2, NO2 is one of the main greenhouse gases that contribute to global warming and is responsible for stratospheric ozone depletion.4–6 Hence, the design and fabrication of high performance NO2 sensors operated at room temperature are very important to monitor the presence of low concentration gas molecules effectively. Accurate measurement of NO2 gas under real environmental conditions at room temperature with high selectivity, and reversibility at low cost is a challenging task.7–9In the past few decades, electrical signal-based NO2 gas detection has been reported using sensors made of a variety of materials including metallic nanoparticles/metal-oxide,10–14 polymers13,15–17 and two-dimensional materials (2D) such as MoS2.18–21 Recently, there has been a lot of interest in research on 2D materials, including MXenes, because of their properties like high surface area to volume ratio, layer-dependent tuneable mechanical, electrical, optical, and physicochemical properties arising from quantum confinement, and low dimensionality effects.22–24 Among these 2D materials, graphene and transition metal dichalcogenide (TMDs)-based gas sensors are widely explored due to their excellent mechanical properties, high carrier mobility, and remarkable electrical and optical properties. Despite having an excellent sensor response and response time, the NO2 sensors based on graphene suffered from long-recovery time whereas TMD-based sensors suffer from incomplete recovery due to its high adsorption.25 This limitation motivated researchers to explore other 2D materials including MXenes. The interaction of gas molecules with sensing materials is an indelible feature of any gas-sensing process. Recently, MXene-based gas sensors have received a lot of attention due to their several advantages. Further, they have already shown applications in the fields of electrochemical energy storage devices, flexible electronic devices and so on due to their excellent thermal and chemical stability. This family of 2D transition metal nitrides/carbides that possess intrinsic metallic conductivity demonstrate excellent gas sensing performance due to the properties ascribed above.26 MXenes have the chemical formula of Mn+1XnTx (n = 1–4) where M represents the transition metal, X represents C or N, and Tx corresponds to the functional groups –OH, –O, –F, etc.27,28 MXene-based materials have attracted considerable interest for applications in gas sensing due to a variety of advantages such as high surface area, metallic conductivity, high mechanical flexibility, hydrophilicity, presence of abundant active sites, tuneable surface chemistry, and improved stability, among others.29–32 Various approaches, such as doping, defect and vacancy engineering, heterostructure formation and modification with charge blocking layers and functional groups, and so on, are also used to improve the performance of the sensor in terms of its selectivity, limit of detection (LOD), response and recovery times, etc.
Among the various reported MXenes, Ti3C2Tx is the most explored one for gas-sensing applications. Since the average thickness of the reported Ti3C2Tx layer (∼2 nm) is much less than the depletion layer thickness, the sensing mechanism is expected to be a surface-dominated process. As a result, this kind of MXene-based gas sensor can operate at room temperature.33 However, Ti3C2Tx has drawbacks such as slow response kinetics and irreversibility that limit its use in RT gas sensor technology. Literature studies proved that modification of the constituents is not the only factor affecting the properties of MXene but functional group modification also plays an important role in determining its optical, mechanical and electrical properties. Theoretical studies proved that O-terminated MXenes are the best candidate for NH3 sensing due to their semiconductor electronic characteristics. Given the comparable atomic structures of the MXenes with different terminations, one wonders whether these new forms of MXenes could be used as NO2 gas sensors.34 In this review, we focused on and discussed the recent literature on NO2 sensors operated at room temperature based on pristine and heterostructures of MXenes. We discussed the sensing mechanisms and different approaches to tune the sensing performance. Finally, the challenges and future perspectives of this research field for the development of high performance NO2 sensors are discussed. Fig. 1 summarizes the major contents of the review.
2. NO2 gas sensing mechanisms of 2D materials and MXene-based sensors
In this section, we will discuss the mechanism involved in the MXene-based NO2 gas sensors. NO2 is generally a secondary product primarily generated from NO sources as given by eqn (1)| 2NO + O2 → 2NO2 | (1) |
Because of the unpaired electron character of nitrogen in NO2, it has an electron-accepting nature and acts as a strong oxidizing agent. Hence, the electrons from the sensing materials are taken by NO2 molecules.35
The sensing mechanisms of 2D material-based gas sensors are primarily explained using two well-established models. Specifically, (i) charge transfer mechanisms and (ii) the ionosorption model. In the case of charge transfer mechanisms, electrons or holes act as the charge carriers depending on the type of materials (i.e., p-type or n-type) being used as the active component of the sensor device (Fig. 2). Furthermore, the direction of the flow of charge transfer depends upon whether reducing or oxidising gas molecules are used as analytes. Additionally, the reactivity of the adsorbates and adsorbents, and their adsorption energy all affect how the analytes interact with the sensing materials.9,36,37 The schematic representation of the charge transfer process used in 2D material-based gas sensors is shown in Fig. 2.38
 | ||
| Fig. 2 A schematic representation of the charge transfer mechanism caused by gas adsorption on layered 2D materials. (a) The transport of electrons to and from adsorbent materials while gas molecules interact with the surface, depending on the distance, site of adsorption, gas type, molecule orientation and (b) charge transfer mechanism and density difference plots for O2, H2O, NH3, NO, NO2, and CO interacting with monolayer MoS2, reproduced with permission from 2013 Yue et al.; license Springer.38 | ||
MXenes have been investigated as an active material for gas sensing applications. As previously stated, the high conductivity, huge specific surface area, hydrophilicity and surface terminations of MXenes make them attractive for use in NO2 gas sensors.39 The fundamental sensing mechanism of pure MXenes involved adsorption or desorption of gas analytes onto the sensing layer. The majority of the presented research elucidated the process involved in reducing gas sensing; however, they did not address the interaction of oxidising gases such as NO2.32 In 2017, it was discovered that Ti3C2Tx responds positively to reducing gases (methanol, ethanol, ammonia, and acetone). As a result, it was hypothesised that Ti3C2Tx was a p-type semiconductor and the gas sensing response was caused by the predominant charge carrier transfer by the interaction between the gas analyte and Ti3C2Tx.40 In 2018 and 2019, it was found that MXene-based gas sensors always provide a positive response for all gases, regardless of their kind (oxidising or reducing).40–42 Thus, p-type semiconductor technology is not the appropriate mechanism for MXene gas sensing. Therefore, two additional factors were presented to represent the positive resistance change towards various gas analytes (i) MXene is a metallic compound rather than a semiconductor and this metallic sensing layer always hinders the charge-carrier transport.41 This behaviour is completely distinct and is independent of the electron-donating/accepting properties of analytes and the dominant charge carrier type (i.e. p or n) of the sensing channel. In this context, (ii) interlayer spacing could be another cause for MXene's increased resistance to different gas analytes. Gas sensing, which occurs as a result of interlayer swelling after gas adsorption, impedes out-of-plane electron transport and increases electrical resistance.43 The MXenes' metallic conductivity and interlayer spacing is a one-of-a-kind trait that occasionally makes the gas sensing process somewhat different and more exciting than the sensing mechanism of normal semiconducting materials. The surface functional groups also play a major role in the sensing mechanism as the hydrophilic group (–OH and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) enhances the gaseous interaction with MXenes. Besides functional groups, terminal groups also affect the sensing mechanism. For example, O-terminated MXenes are the best candidates for NH3 sensing due to their semiconductor electronic characteristics.32 In other studies, done by Hu et al. they demonstrated that S-terminated MXene is the best candidate for NOx gas sensors.34
O) enhances the gaseous interaction with MXenes. Besides functional groups, terminal groups also affect the sensing mechanism. For example, O-terminated MXenes are the best candidates for NH3 sensing due to their semiconductor electronic characteristics.32 In other studies, done by Hu et al. they demonstrated that S-terminated MXene is the best candidate for NOx gas sensors.34
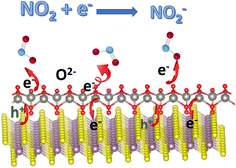
Besides the charge-transfer mechanism, Zhang et al. employed an ionosorption model to better understand the sensing mechanisms of V2CTx MXenes, which could also be applied to other MXenes and hybrids.44 Here O2−(ads) ions interact and contribute to sensing processes at room temperature or low temperatures. Because this review is about MXene-based room temperature sensors, O2−(ads) oxygen ion species play an important role here, and other oxygen species contribute to gas sensors that work at higher temperatures.45–47 O2−(ads) oxygen ions are adsorbed over the surface of active sensor materials such as MXenes at ambient temperature via the following reactions:
| O2(gas) → O2(ads) | (2) |
| O2(ads) + e− → O2−(ads) (room temperature) | (3) |
The width of the junction potential and the electron depletion layer at the grain boundaries increase when the gas sensors come in contact with an oxidising gas like NO2. This is because the gas molecules not only absorb electrons from the active materials but also interact with the adsorbed oxygen ion species (O2−(ads)). Following are several formulas for the chemical reaction caused by the interaction of NO2 gas molecules at room temperature or at low temperatures (Fig. 3):
| NO2(gas) + e− → NO2−(ads) | (4) |
| 2NO2−(ads) + O2−(ads) + e− → 2O−(ads) + 2NO2−(ads) | (5) |
When air is introduced to gas sensors in the reverse process, NO2− interacts with the holes and releases electrons once more, resulting in the formation of NO2 gas molecules.
| NO2−(ads) + h+ → NO2(g) | (6) |
The above-given reactions are the essence of MXene-based RT NO2 sensors. Fig. 3 describes the schematic illustration of the NO2 sensing mechanisms based on the ionosorption model.48
Also, the synergistic and reverse enhancement effect in MXene composites directly influences the NO2 sensing mechanism depending on the type and properties of the foreign materials. Generally, MXenes are reported as a channel layer and metal oxides are used as a supporting layer during composite formation. In this case, the basic mechanism is very similar to that of the conventional metal oxide semiconductor-based sensors in which effective absorption/desorption of the gas analyte on the surface of sensing materials shows an effective change in device resistance. The gas sensing mechanism of MXene-metal oxide composites is connected to the interfacial interactions and heterojunction development of both involved materials. The type of response (p- or n-type) following composite formation is entirely dependent on the composition of both materials as well as the material that plays the predominant role in carrier conduction.35 Gasso et al. synthesized a WO3/Ti3C2Tx hybrid for NO2 sensing where this composite shows electron transfer from MXene to WO3 leading to the formation of heterojunctions. The oxygen adsorption on the surface leads to an electron depletion layer (EDL) and hole accumulation layer (HAL). When the sensor is exposed to NO2 it captures electrons from the conduction band of WO3 that lead to an increase in resistance.49
The gas sensing mechanism of oxidised MXenes is generally considered as a Schottky barrier (SB) modulation. The oxidizing tendency of Ti3C2 flakes to form a heterojunction consisting of metallic Ti3C2 and semiconducting TiO2 is utilized to form the SB sites. Here Ti3C2 MXene with intrinsic metallic conductivity offers the possibility of SB modulation within the sensing channel itself. Choi et al. demonstrated this in situ formation of multiple SBs in a single NO2 gas sensing channel. In the presence of oxidising gas such as NO2 SB can be shifted upwards which surpasses the transport of electrons and lead to a high gas-sensing response. The sensing mechanism for MXene-polymers also depends upon a number of factors including redox reactions between the gas analytes and hybrids, and charge carrier concentration changes happening in sensing layers.50 Zhao et al. reported the NO2 sensing mechanism of a poly(L-glutamic acid) (γ-PGA)-MXene sensor which is different from the conventional metal oxide semiconductor-based sensors. Here, the gas molecules are adsorbed on the γ-PGA using a non-covalent bond. In the presence of NO2, the resistance of the sensor changes from a negative response to a positive response. Water molecules in the presence of air adsorbed onto the γ-PGA film and hydrolysed. At high concentrations of NO2 excess molecules are adsorbed onto the surface via hydrogen bonding and electrostatic interaction and it may compete with H2O molecules for adsorption by the equation given below; this hinders ion conduction, thereby increasing the resistance. Here, the blocking behaviour of MXene was enhanced by γ-PGA and the acceleration rate was also increased.51
| NO2 + H3O+ + e− ↔ NO + 2H2O | (7) |
Aside from the synthesis method and etching conditions, the MXene surface termination has a considerable impact on NO2 gas sensing. For example, it was recently found that NaOH alkalization can shift the response of the Ti3C2Tx type to negative.52 According to XPS measurements, alkalization increased the O/F ratio of the termination on this Ti3C2Tx MXene from 2.6 to 7.6. As a result, it was claimed that a high O/F termination ratio can change the response type from positive to negative. However, the underlying reasons behind this conversion remain unknown, and it is not clear whether this conversion occurs in other MXenes.52 According to Zhang et al.'s studies, this alkalization treatment with DMSO improves the NO2 gas sensing performance of V2CTx MXene, indicating that this conversion also occurs with other MXenes.44 They demonstrated that the adsorbed H2O and O results in p-type sensing behaviour in V2CTx. With the exposure of V2CTx MXene-based sensors to humid air, the oxygen molecules absorbed on the surface of V2CTx ionized to O2− with the consumption of electrons. As a result, the concentration of the V2CTx MXene major charge carrier increases, increasing conductivity and decreasing resistance. When NO2 molecules are exposed to the V2CTx MXene-based sensors, they are adsorbed on the active sites by surface terminations such as –OH and –O. Electrons can be transferred from the V2CTx to NO2 gas molecules. This charge transfer results in increasing the hole concentration of MXene, which further lowers the resistance and the conductivity of V2CTx MXene-based sensors increase. The studies carried out by Choi et al. also prove that the response of Mo2CTx MXene using tetramethylammonium hydroxide (TMAOH) as the intercalant can change the response type which can be attributed to the high density of MXene surface functional groups and its intrinsic metallic conductivity.53 The DFT studies demonstrated that TMA intercalated MXenes show high adsorption energy towards NO2
3. Recent advancements in NO2 gas sensors using MXene-based materials
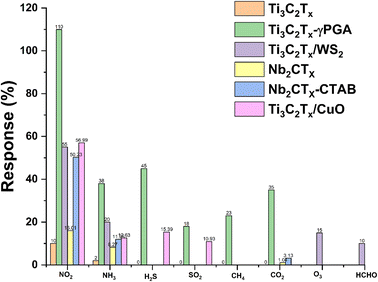
MXenes are an excellent choice for gas-sensing applications due to their abundant active sites, large aspect ratio, availability of abundant functional groups, hydrophilicity, metallic conductivity and tunable surface chemistry. However, MXenes show disadvantages such as irreversibility and low response kinetics, which limit their gas sensing applications.51 The development of MXene heterostructures to modify the physiochemical properties of MXenes is a possible route for the development of high-performance low-temperature sensors. Surface modification, functionalization with noble metals, additive doping, inorganic heterojunction sensitization, light activation, etc. are some of the other approaches which can further tune the properties of MXenes and make them better candidates for RT NO2 gas sensing. MXenes have a massive gas adsorption area, variable layer numbers, and an interlayer swelling effect, making them an amazing gas-sensitive material with a good signal-to-noise ratio.44,54–56Table 1 summarizes the reported NO2 sensing performance of different MXenes and their hybrid-based sensors.| Materials | Dynamic range | Sensor performance, S = Ra/Rg, tres = response time, trec = recovering time, LOD = limit of definition | Sensing environment RT = room temperature (temp., dry air, RT, etc.) | Reference |
|---|---|---|---|---|
| Mo2CTx | 0.125–5 ppm | S = 18.2 | RT, N2 | 53 |
| Nb2CTx | 5–25 ppm | LOD = 67 ppb | RT, air | 56 |
| t res = 39 s | ||||
| t rec = 66 s | ||||
| S = 16.01 for 25 ppm | ||||
| Nb2CTx-CTAB | 5–25 ppm | LOD = 21 ppb | RT, air | 56 |
| t res = 39 s | ||||
| t rec = 78.9 s | ||||
| S = 50.23 for 25 ppm | ||||
| V2CTx | 5–50 ppm | t res = 76 s | 25 °C/RT, air | 60 |
| t rec = 20 s | ||||
| S = 70 for 50 ppm | ||||
| SnO2-MXene | 1–960 ppb | S = 23% for 30 ppb | RT, air | 68 |
| t res = 146 s | ||||
| t rec = 102 s | ||||
| Microwave irradiated SnO2-MXene | 0.1–10 ppm | S = 24.8 for 10 ppm | 150 °C, air | 67 |
| LOD = 0.0153 ppm | ||||
| Facet controlled SnO2/Ti3C2 | 0.05–10 ppm | Response = (ΔR/Ra) = 0.02–1.57 | 25 °C/RT, air | 66 |
| Self-powered SnO2 fibre/sodium L-ascorbate-treated MXene | — | t res = 265 ms | RT, air | 69 |
| t rec = 75.5 ms | ||||
| LOD = 0.03 ppb NO2 | ||||
| Ti3C2/TiO2 | 0.125–5 ppm | S = 16% for 5 ppm | RT, air | 53 |
| LOD = 125 ppb | ||||
| ZnO-Ti3C2Tx UV excited | 5–200 ppb | S = 81% for 50 ppm | RT, air | 70 |
| LOD = 0.2 ppb | ||||
| t res = 17 s | ||||
| t rec = 24 s | ||||
| Ti3C2Tx-ZnO sphere | 5–100 ppm | S = 41.93% for 100 ppm | RT, air | 71 |
| t res = 53 s | ||||
| t rec = 103 s | ||||
| Ti3C2Tx-ZnO nanosheets | — | S = 367.63% to 20 ppm | RT, air | 73 |
| t res = 22 s | ||||
| t rec = 10 s | ||||
| Ti3C2Tx-CuO | 1–50 ppm | S = 56.9% for 50 ppm | RT, air | 74 |
| t res = 16.6 s | ||||
| t rec = 31.3 s | ||||
| Ti3C2Tx/TiO2/rGO | 10–500 ppb | 19.85% for 5 ppm | RT, air | 26 |
| WO3/Ti3C2Tx | 15–500 ppb | t res = 182 s | RT, air | 49 |
| t rec = 75 s | ||||
| (Self-powered) Ti3C2Tx/WO3 | 0.5–50 ppm | S = 510% for 50 ppm | RT, air | 75 |
| Ti3C2-WO3 | 30–1000 ppb | S = 19% for 30 ppb | RT, air | 76 |
| t res = 25 s | ||||
| t rec = 40 s | ||||
| BiOCl-MXene | 100 ppm | S = 34.58 | RT | 77 |
| t res = 3.15 s | ||||
| t rec = 31.05 s | ||||
| MXene-PEODT/PSS | 1 ppm | — | 65 °C | 91 |
| CO3O4@PEI/Ti3C2Tx | 0.3–100 ppm | S = 27.9% @ 100 | RT, air | 78 |
| α-MOC1−x | 0.125–5 ppm | S = 15 | RT, air | 85 |
| LOD = ppb–ppt level | ||||
| Ti3C2Tx/WS2 | 0.1–20 ppm | S = 15.2% @ 1 ppm | RT, air | 86 |
| LOD = 11 ppb | ||||
| t rec = 60 s | ||||
| 2H MoS2/TiC3T2Tx | 30–70 ppm | 65.6% @ 100 ppm | RT, air | 18 |
| MoS2/Ti3C2Tx | 1–50 ppm | 40% @ 20 ppm | RT, air | 19 |
| t res = 525 s | ||||
| t rec = 155 s | ||||
| Mo2TiC2Tx/MoS2 | 0.2–50 ppm | S = 415.8% @ 50 ppm | RT, air | 21 |
| LOD = 2.5 ppb | ||||
| t res = 33.5 s | ||||
| t rec = 140.1 s | ||||
| Black phosphorous QDS/Ti3C2Tx | 50 ppb–10 ppm | LOD = 13 ppb | RT, air | 90 |
| t res = 72 s | ||||
| t rec = 85 s | ||||
| Nb2CTx-APTES | 5–25 ppm | S = 31.52% @ 25 ppm | RT, air | 65 |
| LOD = 15 ppb | ||||
| LOQ = 52 ppb | ||||
| t res = 36 s | ||||
| t rec = 96.8 s | ||||
| Ti3C2Tx/γ-PGA | 2–50 ppm | S = 1127.3% | RT, air | 96 |
| t res = 43.4 s | ||||
| t rec = 3 s | ||||
| Ti3C2/TiO2 | 1–100 ppm | S = 19.76 for 100 ppm | RT, air | 98 |
| Ti3C2Tx@TiO2@MoS2 | 0.02–50 ppm | S = 55.16 (Ra/Rg) @ 50 ppm | RT, air | 98 |
| t res = 1.8 s | ||||
| t rec = 50 s | ||||
| LOD = 23 ppb | ||||
| MXene-derived TiO2-rGO | 0.05–20 ppm | S = 400% for 20 ppm | RT, air | 99 |
| LOD = 50 ppb | ||||
| t res = 130 s | ||||
| t rec = 230 s | ||||
| MXene-derived TiO2-SnS2 | — | 115 for 1000 ppm NO2 | RT, air | 100 |
3.1. Pristine and intercalated MXenes
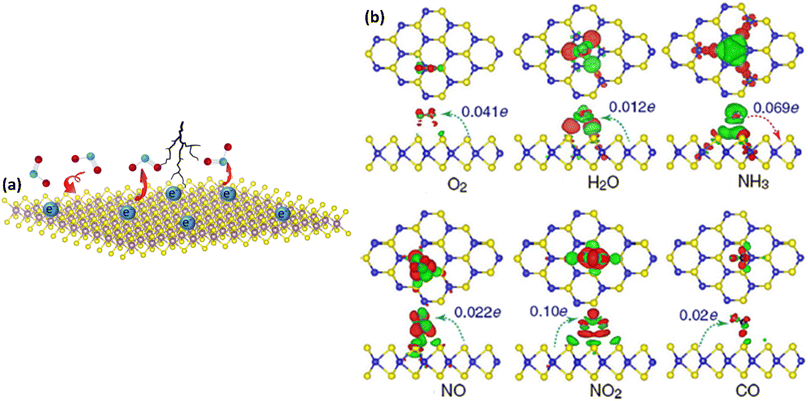
The theoretical studies conducted by Yu et al. first revealed the possibilities of MXenes for gas sensing applications. Their DFT analyses demonstrate that other gas molecules, notably NO2, display distinct adsorption behaviours compared to NH3. Lower adsorption energies of NO2 gas molecules on the Ti2CO2 monolayer reflect weaker interactions between MXene and NO2, implying that this Ti2CO2 MXene is unsuitable for NO2 gas sensing applications.57 Furthermore, Jian et al. revealed that the Ti3C2Tx MXene-based gas sensor is not suited for strong and moderate oxidising gases such as NO2 due to its high proclivity to oxidise to TiOx.33The other pristine MXenes which are reported to show promising NO2 sensing performance are Mo2CTx, Nb2CTx, and V2CTx.44,53,56,58,59 For example, Molybdenum carbide-based NO2 sensors demonstrated a high signal-to-noise ratio with the ability to detect NO2 concentration even at the ppb level and high ambient stability due to their high electrical conductivity, rich density of states near the Fermi level, superior catalytic properties, good resistance to corrosion and low chemical reactivity.59 According to reports, Mo2CTx MXene exhibits a three-phase transition to gas sensing behaviour. Here, the film thickness and the presence of organic intercalants play a crucial role in tuning the performance.53 It is demonstrated that the thin film sensor with 5 nm thick Mo2CTx intercalated by tetramethylammonium hydroxide (TMAOH) shows a p-type gas sensing response whereas it displayed an n-type response without intercalants and films with thickness above 700 nm exhibit conductor type response. Because of the stronger gas molecule binding in the presence of the intercalants, the intercalation technique allows the gas molecules to penetrate, resulting in increased gas sensing performance with sensitivity 30 times greater than that of the deintercalated films. The effect of the thickness of the film is also studied and it demonstrates that the thin film of Mo2CTx shows a better response towards NO2. This is because as the thickness of the film reduces, gas molecules can more easily penetrate into the interlayer space, adsorbing on the surface of MXene flakes faster and resulting in a quick response and short recovery time.53
Rathi et al. employed Nb2CTx to demonstrate that the strong metallic conductivity of MXenes is beneficial for noise reduction and that the abundance of functional groups is favourable for achieving higher sensitivity.56 It has been found that the sensor response of Nb2CTx MXenes treated with cetyltrimethylammonium bromide (CTAB) increases thrice when compared to pure MXenes. The activation of the exposed surface, as well as the interlayer swelling with the hydroxyl groups, play an important role in the adsorption of target molecules, resulting in increased sensing characteristics for delaminated MXene by CTAB.
V2CTx MXenes intercalated by Na+ ions also show 80 times higher response than the as-prepared samples for NO2 gas (5–50 ppm).60 Intercalation by Na atoms swelled the layers of the MXenes which allowed the analyte gases to enter inside for better interaction. Further, the existence of the surface functional groups (–OH) allowed the adsorption of water molecules on MXenes leading to more reactions with NO2 to form NO (3NO2 + H2O → 2HNO3 + NO). Hence, the swelling effect and surface adsorption promoted by the functional groups present in MXenes contributed significantly to achieve enhanced NO2 sensing performance.60
Compared to other MXenes Ti3C2Tx is not much preferred for oxidising gases such as NO2, because it gets easily oxidised to TiO2. The studies done by Jian et al. showed that Ti3C2Tx-based gas sensors give stable response–recovery curves for both reducing and oxidizing gases where this sensor shows a major baseline resistance drift in the case of NO2 gas sensing. This irreversible performance towards NO2 gas could be attributed to Ti3C2Tx oxidation in an oxidising environment. Hence, compared to other MXenes Ti3C2Tx is not much preferred for oxidising gases such as NO2, because it gets easily oxidised to TiO2.33 In this scenario, Chae et al. stored Ti3C2Tx in an aqueous solution at different temperatures and aged for different time intervals. Their studies concluded that MXene which is stored at −80 °C for 5 weeks and as-synthesized samples show the same response towards NO2 at 5 ppm demonstrating that the oxidation stability can be controlled by maintaining the storage conditions.64 To address these issues of oxidation, several techniques including modification with a hydrophobic flurorosilane layer, and polymers were done. This surface modification helps to decrease MXene oxidation and also allows the simultaneous introduction of additional reactive groups. For example, Naveen Kumar et al. demonstrated that the introduction of amine groups over the Nb2CTx MXene helps in the detection of acidic gases such as NO2 by acting as an electron acceptor.65 Rathi et al. also modified Nb2CTx MXene with CTAB and here the stability enhancement was ascribed to the (i) non-covalent bonding between CTAB and Nb2CTx MXene (ii) the long hydrophobic chains in CTAB hinders the interaction of humidity and oxygen with Nb2CTx MXene. CTAB functionalization can aid in the bulk manufacture of stable MXenes under ambient conditions for future device manufacturing in NO2 gas sensors.56
3.2. MXene-based hybrid materials
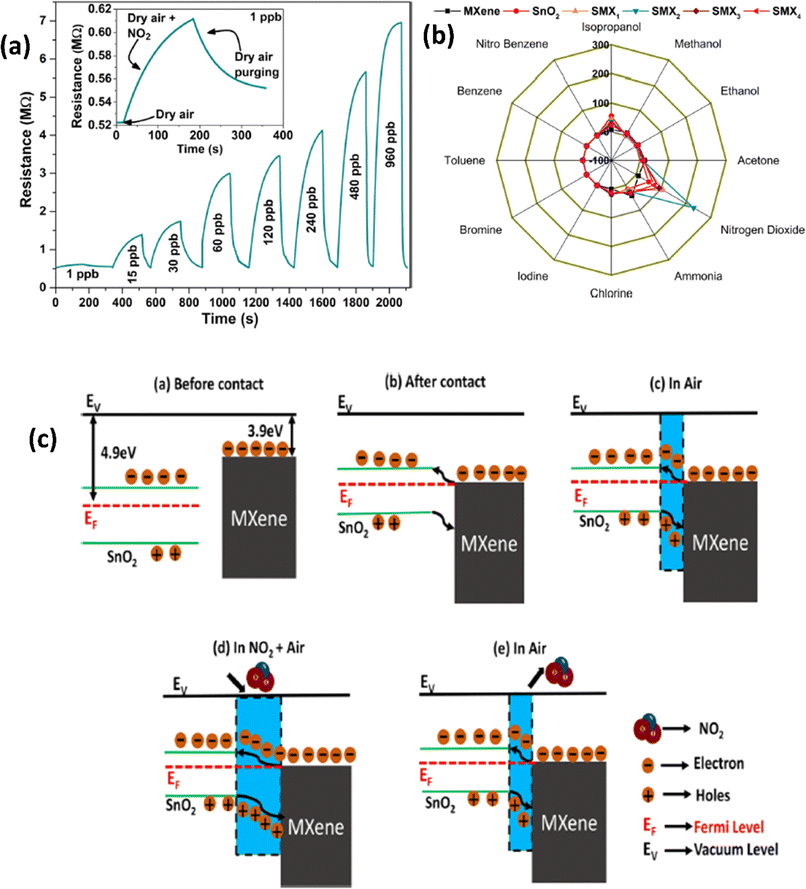 | ||
| Fig. 4 (a) Resistance–time curves for the SnO2/MXene sensor with exposure to different concentrations of NO2. (b) Polar plots for various gases at 30 ppb concentrations for pristine SnO2, MXenes and their heterostructures at room temperature. (c) Schematic representation of the NO2 sensing mechanism of the SnO2/MXene heterostructure, reprinted in part permission from ref. 60 Copyright (2023) Elsevier. | ||
Due to their high surface area, sizable active sites, and modification of the carrier density, MXene-ZnO composites are another type of MXene-metal oxide composite that have been reported to exhibit promising NO2 sensing capabilities.14,70–72 ZnO/Ti3C2Tx MXene nanocomposites displayed an improved response of 3.4 for NO2 (8 ppm) with a recovery time of 254 s and response times of 191 s respectively.14 The crumpled spheres of the heterostructures demonstrated improved NO2 sensing performance due to their increased surface area, an abundance of edges and flaws generated by folding, and the development of the MXene/ZnO p–n junction.71 This heterostructure showed enhanced response from 27.3% to 41.9% for 100 ppm NO2, as well as a significant increase in the recovery rate from 30% to 100%. ZnO1−x/Ti3C2Tx MXene composites with abundant oxygen vacancies showed 2.3 and 14.2-times higher response as compared to the ZnO/Ti3C2Tx and pristine Ti3C2Tx respectively with good linear response (R2 = 0.99509), long-term stability and reproducibility14 (Fig. 5). The sensing mechanisms for the ZnO-MXene heterostructure are predicted to follow a similar mechanism to that of the SnO2-MXene, in which energy bands bend and a Schottky barrier forms at the interface of the two materials.
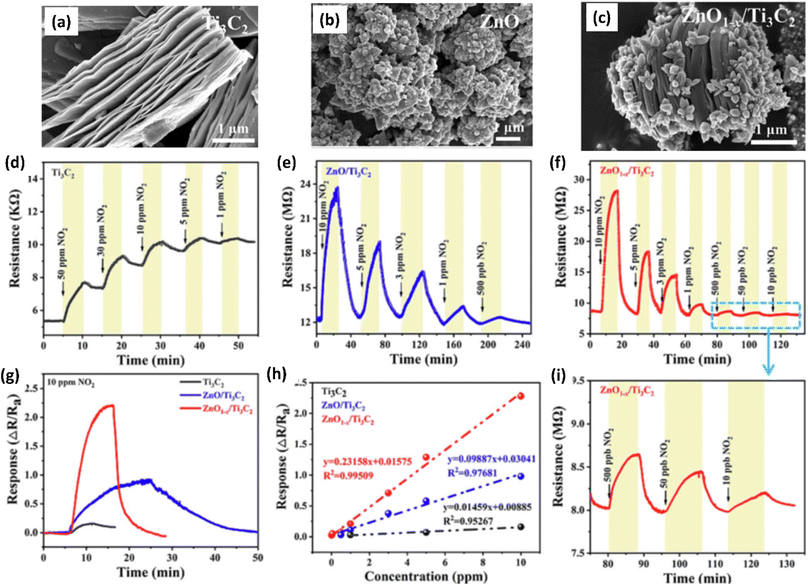 | ||
| Fig. 5 SEM images showing morphology of (a) pristine Ti3C2 (MXene), (b) ZnO and (c) ZnO/Ti3C2 (MXene), (d–f) Response/recovery curves of ZnO/Ti3C2 (MXene) (g) response curves and (h) linear fitting curves of Ti3C2 (MXene) and ZnO/Ti3C2 (MXene) heterostructures at room temperature to gas NO2. (i) Response/recovery curves of ZnO/Ti3C2 (MXene) heterostructures at different concentrations of NO2 (10, 50 and 500 ppb), reprinted with reprinted in part permission from ref. 63 from Copyright (2023) Elsevier. | ||
Room temperature recovery is a big challenge for MXene-based NO2 gas sensors. Thus, pristine MXene-based gas sensors experience incomplete recovery at room temperature which demands sensor operation at a higher temperature. However, thermal treatment for obtaining full recovery is not suitable. Recently, light-assisted recovery of gas sensors has opened up a new prospective avenue for developing RT gas sensors. Light illumination not only aids in sensor recovery but also improves 3S performance (recovery time, low response and sensor response).25 Based on these, Wang et al. showed that under UV illumination, the NO2 sensing efficiency of MXene/ZnO nanorods can be considerably improved due to photo-generated electrons in ZnO reducing the depletion layers and enhancing the conduction path. The sensing response ranged from 21% to 346% for 5–200 ppb NO2 at room temperature, with response and recovery times of 17 s and 24 s for 50 ppb NO2 respectively.70 In the regime of portable, flexible and wearable gas sensors self-powered sensors have garnered a lot of attention. Similarly, Fan et al. demonstrated that MXene/ZnO nanaosheet-based sensors show enhanced performance in the presence of UV illumination. They found out that the main adsorption site for NO2 was present on the surface of ZnO nanosheets, while the Ti3C2Tx MXene plays a major role as a conductive path which helps to accelerate the charge carrier transformation.73
Because of the p-type conductivity (∼1.5 eV) and narrow band gap, CuO is employed to fabricate p–n heterojunctions of CuO/MXene hybrids for NO2 sensing applications.74 (Fig. 6). For 50 ppm NO2, the response of mesoporous MXene-CuO nanocomposites was 5 times higher (56.99%) than pure MXene (11.7%), with ultra-fast response (16.6 s) and recovery time (31.3 s) to 20 ppm NO2 and notable reversibility (over 40 days).
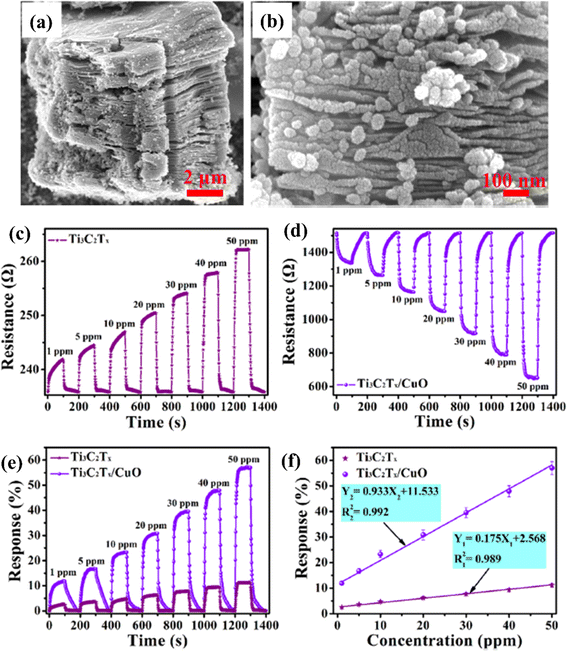 | ||
| Fig. 6 Ti3C2Tx (MXene)/CuO heterostructure for NO2 gas-sensing applications: (a and b) low- and high-resolution FESEM images of the Ti3C2Tx (MXene)/CuO heterostructure, (c and d) dynamic resistance curves of Ti3C2Tx (MXene) and Ti3C2Tx (MXene)/CuO heterostructure-based sensors when exposed to NO2 with different concentrations varying from 1 to 50 ppm at 23 °C, (e) normalized response curves of Ti3C2Tx (MXene) and Ti3C2Tx (MXene)/CuO heterostructure-based sensors, (f) normalised response curves of Ti3C2Tx (MXene) and Ti3C2Tx (MXene)/CuO heterostructure-based gas sensors at 23 °C as a function of NO2 gas concentration, reprinted with reprinted in part permission from ref. 67 from Copyright (2023) Elsevier. | ||
Since, theoretical calculations predicted that both Ti3C2Tx MXene and WO3 have significant NO2 reactivity with adsorption energies (Eads) of −1.12 and 0.54 eV respectively, researchers have investigated NO2 sensing performance of WO3/Ti3C2Tx MXene.49,75,76 Further, MXene sheets treated with sodium L-ascorbate in WO3/Ti3C2Tx MXene hybrids are reported to show enhanced reversibility and stability under varying humid conditions (0–99% RH).49 The sensing mechanisms of MXene-WO3 hybrids are reported to be similar to the proposed mechanisms for other metal oxide-MXene composites. For the MXene-WO3 heterostructures, the MXene platform for WO3 nanorods significantly restricted the aggregation of WO3 and helped to achieve increased interfacial contacts, enhanced surface area for the adsorption of gas and quicker charge transit. Wang et al. reported a high performing self-powered Ti3C2Tx MXene/WO3 sensor powered by triboelectric nanogenerators (TENGs) with a response of 510% for 50 ppm NO2, which was 15 times greater than that of a resistive MXene/WO3 sensor75 (Fig. 7). Heterostructures based on the highly conductive Ti3C2Tx MXene and p-type semiconductor BiOCl offered electronic transmission channels with excellent response and quick response/recovery periods, as well as a lower detection limit (50 ppb) for NO2 gas.77 Sun et al. constructed a NO2 sensor based on Co3O4 nanocrystals decorated on the surface of polyethylenimine (PEI) sheet functionalized MXene.78 MXene provided the electron transport channels whereas high surface area and active sites of the p-type Co3O4 nanocrystals contributed to achieving high NO2 sensing performance with good response and recovery times of 27.9 s and 2 s for NOx gas at RT and at 26% RH, excellent selectivity, reproducibility and an LOD of 30 ppb. Ti3C2Tx/TiO2/rGO heterostructure-based sensors have also been reported to have improved gas sensing performance with superior linearity, great limit of detection as low as 10 ppb, high selectivity, and response of 19.85% for 5 ppm NO2 at ambient temperature.26
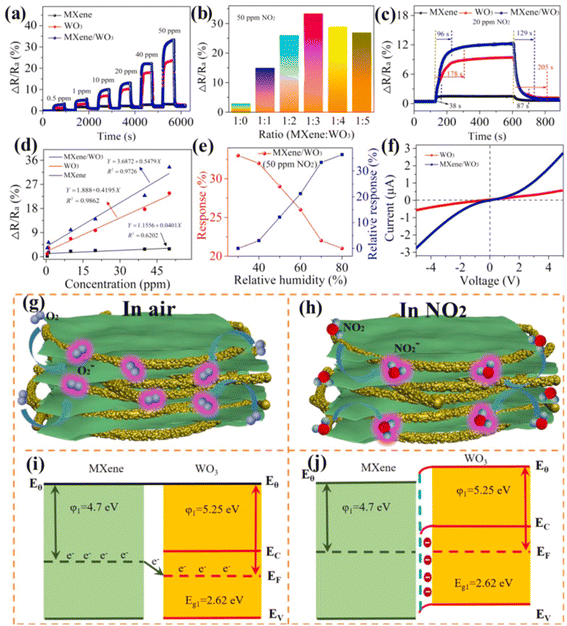 | ||
| Fig. 7 MXene/WO3 heterostructure for NO2 gas sensing: (a) the dynamic response variation of the pristine MXene, WO3 and MXene/WO3 heterostructure-based sensors at different NO2 concentrations. (b) The sensor response of composite materials with different mass ratios. (c) The response and recovery time of the MXene/WO3 heterostructure-based sensor. (d) Response–concentration fitting curves of the three developed sensors. (e) The humidity effect on the MXene/WO3 sensor. (f) The I–V curves of WO3 and the MXene/WO3 sensor. Schematic of the gas-sensing mechanism and energy band structure of the MXene/WO3 heterostructure (g and i) in the presence of air and (h and j) in the presence of NO2 gas, reprinted with reprinted in part permission from ref. 69 from Copyright (2023) Elsevier. | ||
| 2NO2(g) + O2− + e− → 2NO3− | (8) |
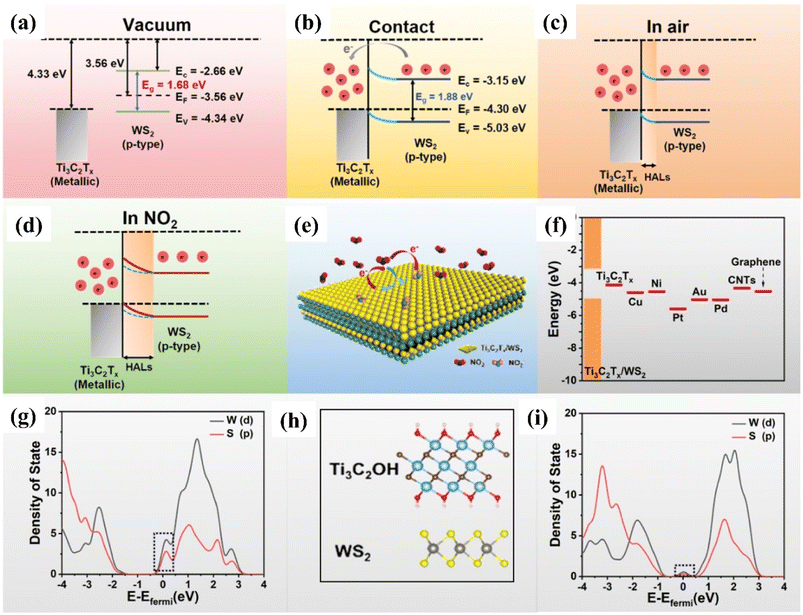 | ||
| Fig. 8 Schematic representation showing the Fermi level position and work function for theTi3C2Tx (MXene)/and WS2 (p-type) semiconductor (a) before contact (b) after contact (c) with air, and (d) NO2 at room temperature. (e) Schematic diagram of the charge transfer process of Ti3C2Tx (MXene)/WS2 heterostructures in the presence of NO2 gas at room temperature. (f) Different material work function. PDOS of the WS2 in contact with the (g) Au and (h) Ti3C2OH, respectively, reprinted with reprinted in part permission from ref. 80 from Copyright (2023) American Chemical Society. | ||
DFT calculations confirmed that the improved NO2 sensing performance is credited to the work function matching, heterojunction regulation effect and suppression of the metal-induced gas states. The enhanced visible light photo-activation effects, optoelectronic properties along with the efficient separation of photo carriers by the 2D/2D heterointerface of Ti3C2Tx/WS2 helped to achieve enhanced NO2 sensing performance with full reversibility, good selectivity, fast response/recovery rate, long stability and low limit of detection (10 ppb).86
MoS2/MXene heterostructures with interconnected networks exhibited highly sensitive and selective NO2 sensing properties due to the presence of abundant Mo active sites, excellent heterointerface contacts and accelerated electrons from the conductive MXene.18,19 The excellent response (65.6%) of the 2H MoS2/Ti3C2Tx MXene heterostructure to 100 ppm NO2 at ambient temperature is ascribed to the quick channels for carrier transportation and a large number of active sites between 2H MoS2 and few layered MXenes.19 For a 2D/2D/2D composite made up of Ti3C2Tx MXene@TiO2@MoS2, the strong interfacial contact between the different components facilitated the charge carrier transfer and spatial separation, resulting in improved sensing performance, with Ti3C2Tx and MoS2 acting as the electron reservoir and main sensitive materials, respectively.20 This NO2 sensor based on Ti3C2Tx MXene@TiO2@MoS2 exhibited good response (Ra/Rg = 55.6 for 50 ppm NO2) which was 3.8, 7.3 and 2.1 times higher than that of TiO2@MoS2, pristine MoS2 and MXene@MoS2 composites respectively. The highly active double transition metal titanium molybdenum carbide (Mo2TiC2Tx) and its hybrids with MoS2 displayed exceptional responsiveness due to their extremely strong surface adsorption (−3.12 eV) for the gas NO2.21 The fabricated Mo2TiC2Tx/MoS2 sensor exhibited a sensitivity of around 7.36% ppm−1, detection limit of 2.5 ppm and room temperature reversibility (Fig. 9). The edge-enriched Mo2TiC2Tx/MoS2 heterostructure is thought to play a crucial role in improving the sensor performance, as both the pristine Mo2TiC2Tx and its MoS2 composite displayed p-type behaviour.
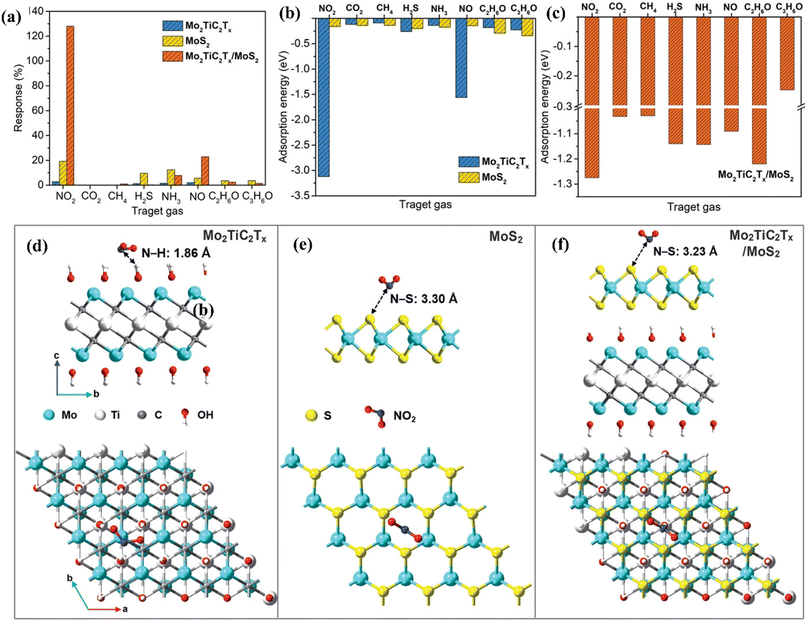 | ||
| Fig. 9 (a) Selective responses for the Mo2TiC2Tx (MXene), pristine MoS2, and Mo2TiC2Tx/MoS2-based sensors in the presence of different gases. (b) Adsorption energies of pristine Mo2TiC2Tx and pristine MoS2 for different gases. (c) Adsorption energies of the Mo2TiC2Tx/MoS2 heterostructure for different gases. Top and side views of the configurations for (d) Mo2TiC2Tx (e) MoS2, and (f) Mo2TiC2Tx/MoS2 heterostructure after adsorption of NO2 molecules, reprinted in part permission from ref. 21 from Copyright (2023) Wiley materials. | ||
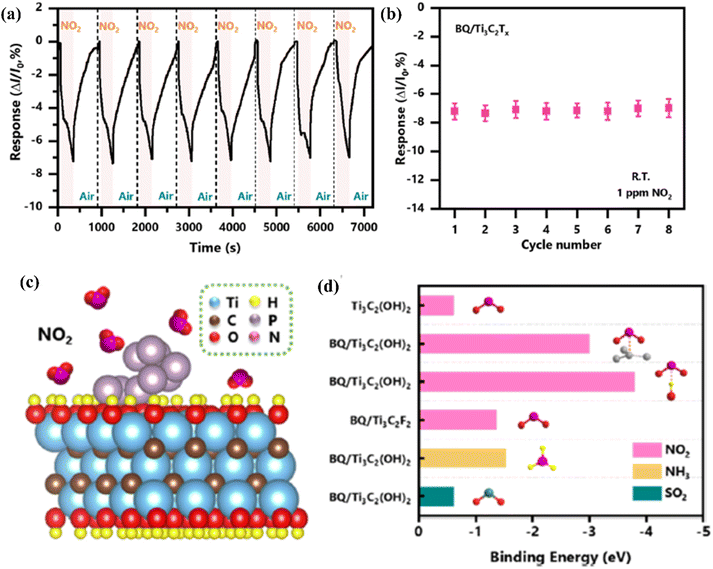 | ||
| Fig. 10 Sensing properties of Ti3C2Tx (MXene) modified with black phosphorus quantum dots in the presence of NO2 (a) cycling test and (b) the summarized responses of the BQ/Ti3C2Tx (MXene) sensor to NO2 (1 ppm). (c) Schematic representation of adsorption of NO2 molecules on the BQ/Ti3C2Tx (MXene) heterostructure surface. (d) Binding energies calculated of gas molecules on the pristine Ti3C2Tx (MXene) and BQ/Ti3C2Tx (MXene) heterostructure with different binding modes and functional groups, reprinted with reprinted in part permission from ref. 85 from Copyright (2023) Elsevier. | ||
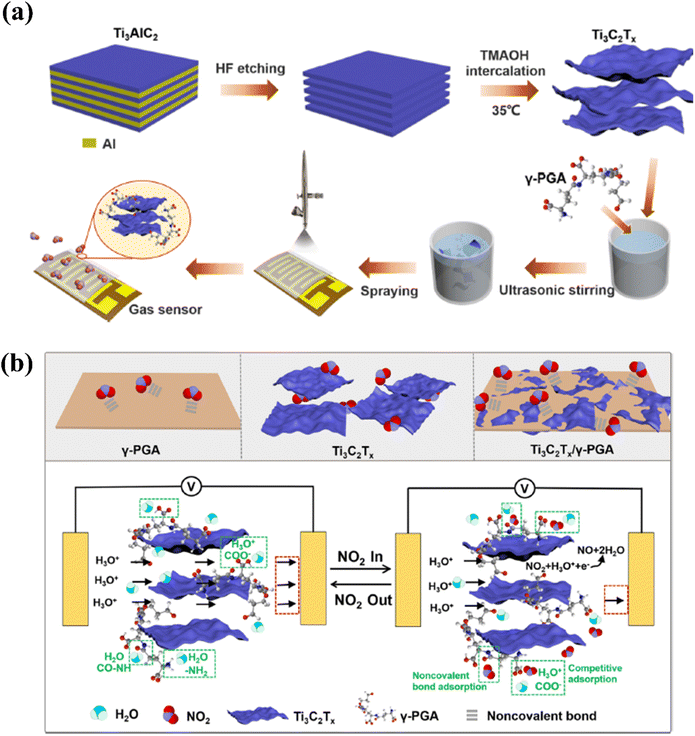 | ||
| Fig. 11 Techniques used to improve the NO2 sensing performance of Ti3C2Tx by modifying with γ-poly(L-glutamic acid): (a) schematic representation of the fabrication of oligo-layer Ti3C2Tx and deposition of the Ti3C2Tx/γ-PGA nanocomposite film. (b) Gas sensor's NO2 gas-sensing mechanism, reprinted with reprinted in part permission from ref. 51 from Copyright (2023) American Chemical Society. | ||
3.3. MXene-derived materials
Ti3C2 MXene-derived semiconducting–metallic Ti3C2–TiO2 hybrid materials are reported to show selective NO2 sensing properties due to the analyte surface charge transfer and the modulation of Schottky barrier (SB) at the interface between the semiconducting and metallic surfaces.97 The TiO2/Ti3C2 composite-based sensors showed selective NO2 sensing performance with excellent sensitivity around 13.7 times greater than pristine Ti3C2 MXene and a limit of detection of 125 ppb (Fig. 12). Similarly, in another study, Liu et al. reported that the composite with an optimal ratio of Ti3C2–TiO2 showed higher response values (86 times) and faster recovery and response times (3.8 and 2 times) to 100 ppm NO2 as compared to the pristine Ti3C2 MXene.98 Theoretical calculations proved that the two Ti–O bonds and the development of a Ti–N bond between the N and O atoms from the NO2 and the nearby Ti atoms from the Ti3C2 in the composites result in a substantial rise in the adsorption energy. Song et al. reported a high-performance NO2 sensor based on MXene-derived TiO2 nanoparticle intercalated between reduced graphene oxide (rGO) assembly in which uniform distribution of the TiO2 nanoparticles and highly wrinkled rGO interconnected porous structure contributed to the sensing.99 The MXene-derived TiO2 spaced rGO gas sensor exhibited a 400% enhancement in NO2 sensitivity with a limit of detection of around 50 ppb, excellent workability under humid conditions and good selectivity. Further, SnS2-MXene-derived TiO2 hybrid materials exhibited a large response of 115 against 1000 ppm NO2 gas with an ultrafast recovery time of 10 s at room temperature.100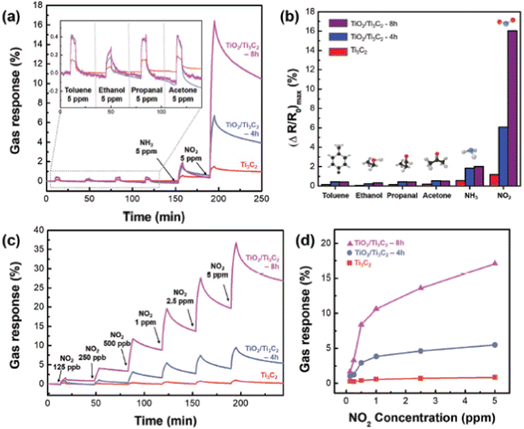 | ||
| Fig. 12 (a) Selective response to gas and (b) maximum change in resistance when exposed to 5 ppm of toluene, ethanol, propanol, acetone, NH3+, and NO2. (c) The TiO2/Ti3C2 real-time gas response curve as a function of NO2 concentration and (d) graph showing maximal resistance change dot at room temperature (NO2, at concentrations ranging from 0.125 to 5 ppm), reprinted in part permission from ref. 93 from Copyright (2023) Wiley materials. | ||
4. Conclusions and future directions
In this review article, we highlighted the state-of-the-art use of MXene-based materials for room temperature NO2 sensing applications. The advantages of MXenes such as their high surface area to volume ratio, tuneable physicochemical properties, high metallic characteristics, hydrophilicity, mechanical flexibility and availability of abundant surface functional groups make them ideal candidates for the fabrication of high performance NO2 sensor applications. Subsequently, we discussed the NO2 sensing mechanisms of pristine and heterostructures of MXenes in detail. Among the various reported MXenes, Ti3C2Tx is the most explored one for NH3 and VOC sensing. However, it possesses small adsorption energy (>−0.8 eV) for NO2 gas suggesting its limited selectivity. The strong interlayer van der Waals force of attraction lead to the self-stacking of MXene nanosheets leading to the obstruction in the diffusion pathways and insufficient utilization of surface active sites, thereby reducing gas-sensing response. NO2 molecules capture electrons from the Ti3C2Tx to produce NO2− and oxidize Ti3C2Tx that leads to poor reversibility. Due to these demerits, the sensing performance of other MXenes such as Mo2CTx and V2CTx are also investigated, and it was discovered that these MXenes have great potential in NO2 gas sensing applications, which opens up the door for unexplored MXenes for NO2 sensing applications. The literature studies prove that the metallic conductivity of MXenes along with interlayer spacing is responsible for positive change in resistance.The strategies such as introducing interlayer spacers (e.g., TiO2), constructing self-supporting architectures and hybrid formation with other semiconducting materials are adopted to overcome the self-restacking problem of MXenes and thereby enhancing the adsorption site. From the literature, we can see that MXene hybrid formation with different metal oxides, TMDs, black phosphorus, polymers, etc. among others helps in the fabrication of MXene-based high-performance NO2 sensors with high response, selectivity, low response and recovery time, etc. The sensor's response value and response speed are improved here due to the sufficient and compact interface contact, which can promote interfacial charge transfer. Furthermore, the in situ formed heterogeneous composite shows potential in gas sensor applications. This in situ heterogeneous composite production incorporates the structural benefits of multi-component materials while preventing the self-stacking of MXene nanosheets.
However, although there are reports on these materials for NO2 sensing still there is plenty of room in this topic which need to be explored for the design of high performance NO2 sensors. At this point, research on monolayer MXenes in gas sensors is in its early stage, despite the fact that Choi et al. revealed that monolayer Mo2CTx performs better against NO2 but performs poorly without intercalants. Thus, the sensing capability of monolayer MXenes falls short of the criteria for practical applications, specifically in terms of its sensitivity and low applicability under ambient conditions.53 This can be clearly seen from Fig. 13, where the pristine MXene shows less response towards NO2 and its modifications with polymers help to enhance its sensitivity towards NO2. Several newly reported MXenes are yet to be explored for the fabrication of high performance NO2 sensor devices.25 Similarly, heterostructures of these newly emerging MXenes with other 2D materials such as TMDs, BP, MBenes, etc. are less explored. Hence, choosing the appropriate combination of materials with MXenes for the fabrication of heterostructures and tuning their properties by defect and vacancy engineering, alloying, doping, intercalation, layer tuning, etc. can allow for high performance selective NO2 gas sensing performance.
Aside from sensor recovery and response time, the immediate response of the gas sensor is an important aspect. The response time of each sensor is determined by how quickly the gas molecules react to the sensing film and change their corresponding parameter. So far, the observed response time of NO2 molecule detection by MXene's has been in the few seconds' range. As a result, developing NO2 sensors capable of responding in milliseconds or microseconds remains difficult, and only one study is now available.69 The technique for improving ultrafast sensors is based mostly on the interaction of gas molecules and MXenes as well as charge transfer in MXenes. By developing MXene-based heterostructures as sensing devices, the rapid charge carrier separation can be increased. Different methods such as photoexcitation, doping, gating, defect and vacancy engineering, surface modification, piezotronic/piezophotonic effects, etc. for the MXene-based NO2 sensor devices are yet to be explored.
Recently, it is revealed that 2D material-based gas sensors with Schottky contact can create highly selective and sensitive sensors by adjusting the Schottky barrier height (SBH), which works as the gate controller to regulate the current flow. As a result of the metallic character of MXenes, SBH-controlled high-performance NO2 selective sensors can be produced by selecting appropriate semiconducting materials for Schottky contact creation.101–104 It is also necessary to strengthen theoretical and experimental efforts with thorough insight and understanding, which will lead to the advancement of high-performance NO2 sensors. Several pathways for developing high-performing electrical contacts also should be identified.
Environmental factors include contaminants of different chemicals, humidity, moisture, corrosion caused by toxic vapours, and residual charges all have a substantial impact on a film's conductivity. These factors significantly lower the gas sensors' stability, reliability and repeatability. The response of MXene-based RT NO2 sensors has been found to decrease with an increase in humidity. This is due to the interaction between adsorbed oxygen O2− and water molecules that led to decreasing the site required for the adsorption of NO2 molecules.68 So, various approaches for reducing humidity interference such as (i) pre-treating the target gas such as NO2 using dehumidifier (ii) modifying materials using hydrophobic materials and (iii) establishing a humidity compensation model are frequently utilized. Zhao et al. demonstrated the humidity compensation model can be established for NO2 gas sensing at a concentration of 2–10 ppm. They also applied statistical regression to find the relationship between gas concentration, humidity and gas sensing response (R). The sensor based on MXene/γ-PGA was able to recognize NO2 with 2 ppm concentration after humidity compensations. Thus, it is necessary to make efforts to improve the sensing devices' stability and response.92
According to WHO, the recommended levels of NO2 exposure for an hour are 82 ppb and for a year are 410 ppb. Long-term exposure to NO2 above that threshold has negative health effects. The MXene-based NO2 sensor's lower detection limit has been measured in ppb. Consequently, it takes a lot of work to produce ultrasensitive NO2 sensors, which is an important task. Finding NO2-sensitive materials that can quickly and easily integrate with MXenes and quickly detect NO2 at lower concentrations is crucial. Additionally, for quick sensor response, such materials need to speed up the transfer of charges.
Also, considering the advantages of 2D MXenes, flexible and wearable sensor devices based on these materials need to be explored. Till now there are only two reports on self-powered NO2 sensors based on MXenes which displayed promising features over conventional gas sensor devices. Hence, this research topic is believed to be an emerging research area in recent years.
Spectroscopic techniques that use electrical shields and laser sources have recently caught the attention of scientists for NO2 detection at trace quantity. The visible range of the absorption spectrum of NO2 molecules provides a significant opportunity for electronic exciton in NO2 molecules. Using spectroscopic methods for NO2 trace detection with MXene sensors could be a novel strategy. Over the past two years, the scientific community has become interested in light-assisted sensing of NO2 gas molecules. The MXene's ability to combine with metal layers has gained a lot of attention in surface plasma resonance (SPR) sensors.105 Thus, the SPR properties of MXenes may be an unconventional approach to construct NO2 gas sensors based on MXenes. New experimental attempts should be directed towards realising the potential of plasmonic in the field of gas sensing. SPR can activate the interface between MXene and metal, changing the refractive index. Thus, a good selection of metal NPs and appropriate wavelengths will aid in the development of high-performance NO2 gas sensors.
By considering these aspects, it can be concluded that MXenes are still in the initial phase of gas sensor research and further exploration into these topics is needed to achieve high performance NO2 sensor devices (Fig. 14). Understanding the NO2 gas sensing mechanisms of MXene-based materials by different in situ/operando spectroscopic studies and by detailed theoretical investigations is of significant importance since it is helpful to design high performance gas sensors.37,106–109 These studies can provide detailed information on the physical and chemical properties of the MXenes upon interaction with the analyte gas molecules. But there is no report on these topics to date. As a result, these study domains must be investigated in the next few years in order to develop superior gas sensor devices.
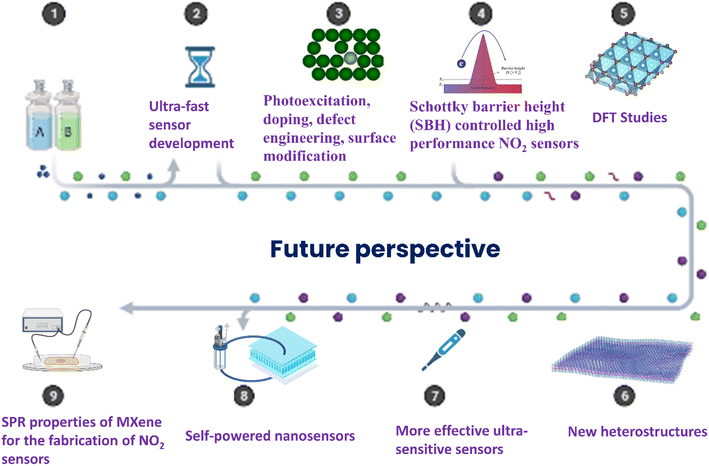 | ||
| Fig. 14 Future perspectives of MXene-based NO2 gas sensors, reprinted with permission from, Sci. Rep., 9, https://doi.org/10.1038/s41598-019-45162-7 and http://biorender.com/ | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial assistance from the SERB Core Research Grant (Grant no. CRG/2022/000897), Department of Science and Technology (DST/NM/NT/2019/205(G)), and Minor Research Project Grant, Jain University (JU/MRP/CNMS/29/2023).References
- L. Calderón-Garcidueñas, B. Azzarelli, H. Acuna, R. Garcia, T. M. Gambling, N. Osnaya, S. Monroy, M. Del Rosario Tizapantzi, J. L. Carson, A. Villarreal-Calderon and B. Rewcastle, Toxicol. Pathol., 2002, 30, 373–389 CrossRef PubMed.
- N. M. Elsayed, Toxicology, 1994, 89, 161–174 CrossRef CAS PubMed.
- J. A. Bernstein, N. Alexis, H. Bacchus, I. L. Bernstein, P. Fritz, E. Horner, N. Li, S. Mason, A. Nel, J. Oullette, K. Reijula, T. Reponen, J. Seltzer, A. Smith and S. M. Tarlo, J. Allergy Clin. Immunol., 2008, 121, 585–591 CrossRef CAS PubMed.
- D. S. Reay, E. A. Davidson, K. A. Smith, P. Smith, J. M. Melillo, F. Dentener and P. J. Crutzen, Nat. Clim. Change, 2012, 2, 410–416 CrossRef CAS.
- A. R. Ravishankara, J. S. Daniel and R. W. Portmann, Science, 2009, 326, 123–125 CrossRef CAS PubMed.
- J. N. Galloway, A. R. Townsend, J. W. Erisman, M. Bekunda, Z. Cai, J. R. Freney, L. A. Martinelli, S. P. Seitzinger and M. A. Sutton, Science, 2008, 320, 889–892 CrossRef CAS PubMed.
- J. Ryu, S. Shim, J. Song, J. Park, H. S. Kim, S.-K. Lee, J. C. Shin, J. Mun and S.-W. Kang, Nanomaterials, 2023, 13, 573 CrossRef CAS PubMed.
- G. Lei, C. Lou, X. Liu, J. Xie, Z. Li, W. Zheng and J. Zhang, Sens. Actuators, B, 2021, 341, 129996 CrossRef CAS.
- M. Mathew and C. S. Rout, J. Mater. Chem. C, 2021, 9, 395–416 RSC.
- C. Jiang, G. Zhang, Y. Wu, L. Li and K. Shi, CrystEngComm, 2012, 14, 2739–2747 RSC.
- S. Liu, Z. Wang, Y. Zhang, Z. Dong and T. Zhang, RSC Adv., 2015, 5, 91760–91765 RSC.
- S. Srivastava, K. Jain, V. N. Singh, S. Singh, N. Vijayan, N. Dilawar, G. Gupta and T. D. Senguttuvan, Nanotechnology, 2012, 23, 205501 CrossRef PubMed.
- T. Hyodo, K. Sasahara, Y. Shimizu and M. Egashira, Sens. Actuators, B, 2005, 106, 580–590 CrossRef CAS.
- S. Liu, M. Wang, C. Ge, X. Zhang, S. Lei, S. Hussain, M. Wang, G. Qiao and G. Liu, Appl. Surf. Sci., 2023, 610, 155440 CrossRef CAS.
- T. Xie, G. Xie, H. Du, Y. Zhou, F. Xie, Y. Jiang and H. Tai, IEEE Sens. J., 2016, 16, 1865–1871 CAS.
- A. Lv, Y. Pan and L. Chi, Sensors, 2017, 17, 213 CrossRef PubMed.
- W. Yuan, L. Huang, Q. Zhou and G. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 17003–17008 CrossRef CAS PubMed.
- V. T. Le, Y. Vasseghian, V. D. Doan, T. T. T. Nguyen, T.-T. Thi Vo, H. H. Do, K. B. Vu, Q. H. Vu, T. Dai Lam and V. A. Tran, Chemosphere, 2022, 291, 133025 CrossRef CAS PubMed.
- Q. Thanh Hoai Ta, N. Ngoc Tri and J.-S. Noh, Appl. Surf. Sci., 2022, 604, 154624 CrossRef CAS.
- Z. Liu, H. Lv, Y. Xie, J. Wang, J. Fan, B. Sun, L. Jiang, Y. Zhang, R. Wang and K. Shi, J. Mater. Chem. A, 2022, 10, 11980–11989 RSC.
- Q. Zhao, W. Zhou, M. Zhang, Y. Wang, Z. Duan, C. Tan, B. Liu, F. Ouyang, Z. Yuan, H. Tai and Y. Jiang, Adv. Funct. Mater., 2022, 32, 2203528 CrossRef CAS.
- R. Frisenda, A. J. Molina-Mendoza, T. Mueller, A. Castellanos-Gomez and H. S. J. van der Zant, Chem. Soc. Rev., 2018, 47, 3339–3358 RSC.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S.-Z. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed.
- X. Zhou, X. Hu, J. Yu, S. Liu, Z. Shu, Q. Zhang, H. Li, Y. Ma, H. Xu and T. Zhai, Adv. Funct. Mater., 2018, 28, 1706587 CrossRef.
- A. V. Agrawal, N. Kumar and M. Kumar, Nano-Micro Lett., 2021, 13, 38 CrossRef PubMed.
- Z. Yang, H. Zou, Y. Zhang, F. Liu, J. Wang, S. Lv, L. Jiang, C. Wang, X. Yan, P. Sun, L. Zhang, Y. Duan and G. Lu, Adv. Funct. Mater., 2022, 32, 2108959 CrossRef CAS.
- K. A. Sree Raj, N. Barman, S. Radhakrishnan, R. Thapa and C. S. Rout, J. Mater. Chem. A, 2022, 10, 23590–23602 RSC.
- S. A. Thomas, A. Patra, B. M. Al-Shehri, M. Selvaraj, A. Aravind and C. S. Rout, J. Energy Storage, 2022, 55, 105765 CrossRef.
- V. Chaudhary, N. Ashraf, M. Khalid, R. Walvekar, Y. Yang, A. Kaushik and Y. K. Mishra, Adv. Funct. Mater., 2022, 32, 2112913 CrossRef CAS.
- M. S. Bhargava Reddy, S. Kailasa, B. C. G. Marupalli, K. K. Sadasivuni and S. Aich, ACS Sens., 2022, 7, 2132–2163 CrossRef CAS PubMed.
- S. Mehdi Aghaei, A. Aasi and B. Panchapakesan, ACS Omega, 2021, 6, 2450–2461 CrossRef CAS PubMed.
- R. Bhardwaj and A. Hazra, J. Mater. Chem. C, 2021, 9, 15735–15754 RSC.
- Y. Jian, D. Qu, L. Guo, Y. Zhu, C. Su, H. Feng, G. Zhang, J. Zhang, W. Wu and M.-S. Yao, Front. Chem. Sci. Eng., 2021, 15, 505–517 CrossRef CAS.
- C. Hu, X. Yu, Y. Li, J. Cheng, Q. Li and B. Xiao, Appl. Surf. Sci., 2022, 592, 153296 CrossRef CAS.
- A. V. Agrawal, N. Kumar and M. Kumar, Nano-Micro Lett., 2021, 13, 38 CrossRef PubMed.
- M. Mathew, P. V. Shinde, R. Samal and C. S. Rout, J. Mater. Sci., 2021, 56, 9575–9604 CrossRef CAS.
- A. Sharma and C. S. Rout, J. Mater. Chem. A, 2021, 9, 18175–18207 RSC.
- Q. Yue, Z. Shao, S. Chang and J. Li, Nanoscale Res. Lett., 2013, 8, 425 CrossRef PubMed.
- S. Nahirniak and B. Saruhan, Sensors, 2022, 22, 972 CrossRef CAS PubMed.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D.-J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CrossRef CAS PubMed.
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi, J. Kim, Y. Gogotsi and H.-T. Jung, ACS Nano, 2018, 12, 986–993 CrossRef CAS PubMed.
- M. Wu, M. He, Q. Hu, Q. Wu, G. Sun, L. Xie, Z. Zhang, Z. Zhu and A. Zhou, ACS Sens., 2019, 4, 2763–2770 CrossRef CAS PubMed.
- H.-J. Koh, S. J. Kim, K. Maleski, S.-Y. Cho, Y.-J. Kim, C. W. Ahn, Y. Gogotsi and H.-T. Jung, ACS Sens., 2019, 4, 1365–1372 CrossRef CAS PubMed.
- Y. Zhang, Y. Jiang, Z. Duan, Q. Huang, Y. Wu, B. Liu, Q. Zhao, S. Wang, Z. Yuan and H. Tai, Sens. Actuators, B, 2021, 344, 130150 CrossRef CAS.
- W.-T. Koo, H.-J. Cho, D.-H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I.-D. Kim, ACS Nano, 2020, 14, 14284–14322 CrossRef CAS PubMed.
- H. Hashtroudi, I. D. R. Mackinnon and M. Shafiei, J. Mater. Chem. C, 2020, 8, 13108–13126 RSC.
- R. Malik, V. K. Tomer, Y. K. Mishra and L. Lin, Applied Physics Reviews, 2020, 7, 021301 CrossRef CAS.
- Y. Han, D. Huang, Y. Ma, G. He, J. Hu, J. Zhang, N. Hu, Y. Su, Z. Zhou, Y. Zhang and Z. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 22640–22649 CrossRef CAS PubMed.
- S. Gasso and A. Mahajan, Mater. Sci. Semicond. Process., 2022, 152, 107048 CrossRef CAS.
- J. Choi, Adv. Funct. Mater., 2020, 2003998 CrossRef CAS.
- Q. Zhao, D. Sun, S. Wang, Z. Duan, Z. Yuan, G. Wei, J.-L. Xu, H. Tai and Y. Jiang, ACS Sens., 2021, 6, 2858–2867 CrossRef CAS PubMed.
- Z. Yang, A. Liu, C. Wang, F. Liu, J. He, S. Li, J. Wang, R. You, X. Yan, P. Sun, Y. Duan and G. Lu, ACS Sens., 2019, 4, 1261–1269 CrossRef CAS PubMed.
- J. Choi, B. Chacon, H. Park, K. Hantanasirisakul, T. Kim, K. Shevchuk, J. Lee, H. Kang, S.-Y. Cho, J. Kim, Y. Gogotsi, S. J. Kim and H.-T. Jung, ACS Sens., 2022, 7, 2225–2234 CrossRef CAS PubMed.
- Y. Wang, J. Fu, J. Xu, H. Hu and D. Ho, ACS Appl. Mater. Interfaces, 2023, 15, 12232–12239 CrossRef CAS PubMed.
- M. Hu, C. Cui, C. Shi, Z.-S. Wu, J. Yang, R. Cheng, T. Guang, H. Wang, H. Lu and X. Wang, ACS Nano, 2019, 13, 6899–6905 CrossRef CAS PubMed.
- K. Rathi, N. K. Arkoti and K. Pal, Adv. Mater. Interfaces, 2022, 9, 2200415 CrossRef CAS.
- X. Yu, Y. Li, J. Cheng, Z. Liu, Q. Li, W. Li, X. Yang and B. Xiao, ACS Appl. Mater. Interfaces, 2015, 7, 13707–13713 CrossRef CAS PubMed.
- Q. Guo, W. Pang, X. Xie, Y. Xu and W. Yuan, J. Mater. Chem. A, 2022, 10, 15634–15646 RSC.
- S.-Y. Cho, J. Y. Kim, O. Kwon, J. Kim and H.-T. Jung, J. Mater. Chem. A, 2018, 6, 23408–23416 RSC.
- Y. Zhang, Y. Jiang, Z. Duan, Q. Huang, Y. Wu, B. Liu, Q. Zhao, S. Wang, Z. Yuan and H. Tai, Sens. Actuators, B, 2021, 344, 130150 CrossRef CAS.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- K. Hantanasirisakul and Y. Gogotsi, Adv. Mater., 2018, 30, 1804779 CrossRef PubMed.
- T. Schultz, N. C. Frey, K. Hantanasirisakul, S. Park, S. J. May, V. B. Shenoy, Y. Gogotsi and N. Koch, Chem. Mater., 2019, 31, 6590 CrossRef CAS.
- Y. Chae, S. J. Kim, S. Y. Cho, J. Choi, K. Maleski, B. J. Lee, H. T. Jung, Y. Gogotsi, Y. Lee and C. W. Ahn, Nanoscale, 2019, 11(17), 8387–8393 RSC.
- A. Naveen Kumar and K. Pal, Mater. Adv., 2022, 3, 5151–5162 RSC.
- S. Liu, M. Wang, C. Ge, S. Lei, S. Hussain, M. Wang, G. Qiao and G. Liu, Sens. Actuators, B, 2022, 365, 131919 CrossRef CAS.
- S. Kang, A. Mirzaei, K. Y. Shin, W. Oum, D. J. Yu, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2023, 375, 132882 CrossRef CAS.
- S. Gasso, M. K. Sohal and A. Mahajan, Sens. Actuators, B, 2022, 357, 131427 CrossRef CAS.
- S. Gasso and A. Mahajan, ACS Appl. Nano Mater., 2023, 6(8), 6678–6692 CrossRef CAS.
- J. Wang, Y. Yang and Y. Xia, Sens. Actuators, B, 2022, 353, 131087 CrossRef CAS.
- Z. Yang, L. Jiang, J. Wang, F. Liu, J. He, A. Liu, S. Lv, R. You, X. Yan, P. Sun, C. Wang, Y. Duan and G. Lu, Sens. Actuators, B, 2021, 326, 128828 CrossRef CAS.
- X. Liu, H. Zhang, Y. Song, T. Shen and J. Sun, Sens. Actuators, B, 2022, 367, 132025 CrossRef CAS.
- C. Fan, J. Shi, Y. Zhang, W. Quan, X. Chen, J. Yang, M. Zeng, Z. Zhou, Y. Su, H. Wei and Z. Yang, Nanoscale, 2022, 14, 3441–3451 RSC.
- F. Guo, C. Feng, Z. Zhang, L. Zhang, C. Xu, C. Zhang, S. Lin, H. Wu, B. Zhang, A. Tabusi and Y. Huang, Sens. Actuators, B, 2023, 375, 132885 CrossRef CAS.
- D. Wang, D. Zhang, J. Guo, Y. Hu, Y. Yang, T. Sun, H. Zhang and X. Liu, Nano Energy, 2021, 89, 106410 CrossRef CAS.
- S. Gasso and A. Mahajan, ACS Sens., 2022, 7, 2454–2464 CrossRef CAS PubMed.
- J. Fan, J. Gao, H. Lv, L. Jiang, F. Qin, Y. Fan, B. Sun, J. Wang, M. Ikram and K. Shi, J. Mater. Chem. A, 2022, 10, 25714–25724 RSC.
- B. Sun, H. Lv, Z. Liu, J. Wang, X. Bai, Y. Zhang, J. Chen, K. Kan and K. Shi, J. Mater. Chem. A, 2021, 9, 6335–6344 RSC.
- E. Lee, Y. S. Yoon and D.-J. Kim, ACS Sens., 2018, 3, 2045–2060 CrossRef CAS PubMed.
- Y. Kim, S. Lee, J.-G. Song, K. Y. Ko, W. J. Woo, S. W. Lee, M. Park, H. Lee, Z. Lee, H. Choi, W.-H. Kim, J. Park and H. Kim, Adv. Funct. Mater., 2020, 30, 2003360 CrossRef CAS.
- W.-T. Koo, H.-J. Cho, D.-H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I.-D. Kim, ACS Nano, 2020, 14, 14284–14322 CrossRef CAS PubMed.
- S. Kumar, V. Pavelyev, P. Mishra, N. Tripathi, P. Sharma and F. Calle, Mater. Sci. Semicond. Process., 2020, 107, 104865 CrossRef CAS.
- G. Sanyal, A. Vaidyanathan, C. S. Rout and B. Chakraborty, Mater. Today Commun., 2021, 28, 102717 CrossRef CAS.
- D. Simon Patrick, P. Bharathi, M. Krishna Mohan, C. Muthamizchelvan, S. Harish and M. Navaneethan, J. Mater. Sci.: Mater. Electron., 2022, 33, 9235–9245 CrossRef CAS.
- W. Quan, J. Shi, H. Luo, C. Fan, W. Lv, X. Chen, M. Zeng, J. Yang, N. Hu, Y. Su, H. Wei and Z. Yang, ACS Sens., 2023, 8, 103–113 CrossRef CAS PubMed.
- Y. Xia, S. He, J. Wang, L. Zhou, J. Wang and S. Komarneni, Chem. Commun., 2021, 57, 9136–9139 RSC.
- N. Mao, J. Tang, L. Xie, J. Wu, B. Han, J. Lin, S. Deng, W. Ji, H. Xu, K. Liu, L. Tong and J. Zhang, J. Am. Chem. Soc., 2016, 138, 300–305 CrossRef CAS PubMed.
- P. Yasaei, B. Kumar, T. Foroozan, C. Wang, M. Asadi, D. Tuschel, J. E. Indacochea, R. F. Klie and A. Salehi-Khojin, Adv. Mater., 2015, 27, 1887–1892 CrossRef CAS PubMed.
- Aaryashree, P. V. Shinde, A. Kumar, D. J. Late and C. S. Rout, J. Mater. Chem. C, 2021, 9, 3773–3794 RSC.
- Q. Xu, B. Zong, Y. Yang, Q. Li and S. Mao, Sens. Actuators, B, 2022, 373, 132696 CrossRef CAS.
- K. Hassan, N. Stanley, T. T. Tung, P. L. Yap, H. Rastin, L. Yu and D. Losic, Adv. Mater. Interfaces, 2021, 8, 2101175 CrossRef CAS.
- Q. Zhao, D. Sun, S. Wang, Z. Duan, Z. Yuan, G. Wei, J.-L. Xu, H. Tai and Y. Jiang, ACS Sens., 2021, 6, 2858–2867 CrossRef CAS PubMed.
- Y. Zhou, J. Qiu, H. Zhao, Y. Wang, J. Li and C. Zou, J. Phys. Chem. Lett., 2022, 13, 9599–9606 CrossRef CAS PubMed.
- H. Zhi, J. Gao and L. Feng, ACS Sens., 2020, 5, 772–780 CrossRef CAS PubMed.
- J. Wu, Z. Wu, W. Huang, X. Yang, Y. Liang, K. Tao, B.-R. Yang, W. Shi and X. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 52070–52081 CrossRef CAS PubMed.
- Q. Zhao, D. Sun, S. Wang, Z. Duan, Z. Yuan, G. Wei, J.-L. Xu, H. Tai and Y. Jiang, ACS Sens., 2021, 6, 2858–2867 CrossRef CAS PubMed.
- Q. T. H. Ta, N. M. Tran, N. N. Tri, A. Sreedhar and J.-S. Noh, Chem. Eng. J., 2021, 425, 131437 CrossRef CAS.
- S. Liu, M. Wang, G. Liu, N. Wan, C. Ge, S. Hussain, H. Meng, M. Wang and G. Qiao, Appl. Surf. Sci., 2021, 567, 150747 CrossRef CAS.
- Y. Song, Y. Xu, Q. Guo, Z. Hua, F. Yin and W. Yuan, ACS Appl. Mater. Interfaces, 2021, 13, 39772–39780 CrossRef CAS PubMed.
- T. Chen, W. Yan, Y. Wang, J. Li, H. Hu and D. Ho, J. Mater. Chem. C, 2021, 9, 7407–7416 RSC.
- Ó. L. Camargo Moreira, W.-Y. Cheng, H.-R. Fuh, W.-C. Chien, W. Yan, H. Fei, H. Xu, D. Zhang, Y. Chen, Y. Zhao, Y. Lv, G. Wu, C. Lv, S. K. Arora, C. Ó Coileáin, C. Heng, C.-R. Chang and H.-C. Wu, ACS Sens., 2019, 4, 2546–2552 CrossRef PubMed.
- A. Singh, Md. A. Uddin, T. Sudarshan and G. Koley, Small, 2014, 10, 1555–1565 CrossRef CAS PubMed.
- Z. Yang, X. Dou, S. Zhang, L. Guo, B. Zu, Z. Wu and H. Zeng, Adv. Funct. Mater., 2015, 25, 4039–4048 CrossRef CAS.
- M. Mathew and C. S. Rout, J. Mater. Chem. C, 2021, 9, 395–416 RSC.
- V. K. Verma, R. Kumar, S. Pal and Y. K. Prajapati, Opt. Mater., 2022, 133, 112977 CrossRef CAS.
- T. Hartman, R. G. Geitenbeek, C. S. Wondergem, W. van der Stam and B. M. Weckhuysen, ACS Nano, 2020, 14, 3725–3735 CrossRef CAS PubMed.
- S. Sänze, A. Gurlo and C. Hess, Angew. Chem., Int. Ed., 2013, 52, 3607–3610 CrossRef PubMed.
- D. Degler, U. Weimar and N. Barsan, ACS Sens., 2019, 4, 2228–2249 CrossRef CAS PubMed.
- A. Bag and N.-E. Lee, J. Mater. Chem. C, 2019, 7, 13367–13383 RSC.
| This journal is © The Royal Society of Chemistry 2023 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. He was ranked top 2% scientists by the Stanford study in 2020–2022.
000. He was ranked top 2% scientists by the Stanford study in 2020–2022.