Advanced research and prospects on polymer ionic liquids: trends, potential and application
Olga
Lebedeva
 *a,
Dmitry
Kultin
*a,
Dmitry
Kultin
 a and
Leonid
Kustov
a and
Leonid
Kustov
 abc
abc
aDepartment of Chemistry, Lomonosov Moscow State University, Moscow, 119991, Russia. E-mail: lebedeva@general.chem.msu.ru
bN.D. Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky Prospect 47, Moscow, 119991, Russia
cInstitute of Ecology and Engineering, National Science and Technology University “MISiS”, Leninsky Prospect 4, Moscow, 119049, Russia
First published on 26th September 2023
Abstract
The purpose of this short perspective review is to summarize the recent literature on the main trends in the use of polymer ionic liquids (PILs) published in the period from 2021 to 2023. Only the most promising areas that will play crucial roles in the very near future are considered. These areas include, in the authors’ opinion, the biochemical and medical applications of PILs; sorption and separation of gases and liquid-phase sorption; solar cells; chemical sensors, supercapacitors and polymer transistors; and catalysts (including electro- and photocatalysts). The advantages of and approaches for improving the efficiency as well as the green approach for the synthesis and use of PILs are discussed.
Introduction
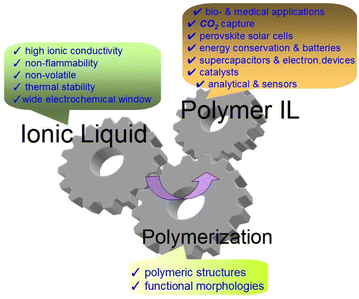
Poly-(ionic liquid)s (PILs) are polymerized ionic liquids (ILs) or polyelectrolytes that contain a polymer backbone and carry ionic liquid (IL) moieties in each of the repeating links.1 According to the strict definition, PILs are ionic polymers with full ionicity (100% ionic).2 However, ionic polymers with lower ionicity levels are also often considered PILs in publications. For example, the work3 define PILs as polymers containing ions in their side chain or backbone. Some advantageous properties of IL monomers, such as high ionic conductivity, thermal stability, very low vapor pressure and nonflammability, are integrated into the polymers. The polymer chains, in turn, endow ILs with unique aggregation structures and morphologies that are not available for monomeric ILs (Fig. 1).Important properties and features of PILs can be noted:
• diversity in macromolecular design;
• chemical stability;
• thermal stability;
• film-forming ability;
• gas permeation properties;
• tunable ion conductivity;
• broad electrochemical stability window;
• universal compatibility;
• one-step synthesis;
• sustainable material;
• low toxicity.
The variety, designability and versatility of ILs and polymer segments significantly enrich the properties and applications of PILs, which have attracted close attention in the fields of polymer chemistry and materials science.1
This brief review does not address the considerable number of papers that cover encapsulated ILs on polymeric carriers (such a recent review, for example, is ref. 4), which are not PILs as defined in the introduction of this article.
Green aspect and Green approach to PILs
In a recent critical review, R. A. Sheldon5 notes that the main cause of global environmental problems is waste. And to solve this problem, preventing pollution at the source through “green” and sustainable chemistry becomes relevant.Factor E emphasizes the need to develop cleaner and waste-free processes. A zero-waste process requires a reduction in the quantity of all materials consumed and a reduction in the cost of waste production and disposal. An important contribution to the E Factor is a solvent and water (so-called complete E Factors (cEF) that include solvents and water with no recycling). To date, no E-factor data have been reported for processes in PILs.
Ionic liquids create a number of environmental problems related to their synthesis, toxicity and complexity of biological decomposition. On the contrary, PILs are characterized by more sustainable and one-step synthesis, they are more stable in water and other solvents, and they can be applied in sustainable reactions in organic and pharmaceutical chemistry.6
Currently, more attention should be paid to the processes of PIL synthesis, including the economical reaction of atoms, the reaction of green raw materials, the reaction of environmentally friendly solvents, the intensification of chemical processes and computer-aided design. It is necessary to take into account such advantages as high synthesis efficiency, good product quality, low waste product generation, low energy consumption, and mild conditions.
PILs are relatively new materials that need comprehensive study; the “dilemma” can be cited as an example. It has been found that a poly ionic liquid may be toxic to one organism and harmless to another.7 Antibacterial hydrogels containing imidazolium salt-based PILs can quickly kill bacteria and also have low toxicity towards human skin fibroblasts (section “Biochemical and medical applications of PILs”).
Biochemical and medical applications of PILs
Antimicrobial drugs
Use in bactericidal surface coatings seems to be one of the most promising applications of antibacterial PILs in medicine. Briefly, but quite fully, research on this topic until 2021 is reported in a review.2 PILs are proposed for application as components of ionogels, films and membranes, which show significant antimicrobial and antifouling activity. The review focuses on the use of quaternary ammonium compounds (QACs) and ionic liquids, in particular PILs, as biocides ranging from simple antiseptics to tunable antimicrobials. A brief review of the antimicrobial activity of various subclasses of ionic liquids, which are often considered as useful antimicrobial agents, is given. The hope was expressed2 that this will serve as a starting point for future research on these classes of biocides. The goal of another publication3 is to engage scientists (pharmacists and medicinal chemists) in the full understanding of the roles played by PILs in order to accelerate their progression from laboratory research to industrialization. Advances in the use of PILs in biomedicine over the past five years until 2022 were reviewed, with an emphasis on applications in proteomics, drug delivery, etc. The need to improve the safety and especially the stability of gels has attracted the attention of researchers to self-repairing materials, such as gels based on PILs,8 in which self-repair is carried out by hydrogen bonds.Polymers can be chemically or physically cross-linked to form 3D gel networks.8 Chemical cross-linking occurs through the formation of covalent bonds, and physical cross-linking is formed through, for example, van der Waals forces.
According to the article8 the use of ionic liquid gels for clinical purposes has not yet been approved. The following problems and directions for the use of PILs were formulated:
• it is crucial to investigate harmless and non-toxic substances for the preparation of ionic liquid gels. Usually initiators and crosslinking agents are required, which can pose a biohazard;
• the characteristics of the gels can be adjusted by developing ILs with multiple structures or adapted to biomedical requirements;
• clarifying the biodegradability of ionic liquid-based gels is necessary to balance their antibacterial efficacy and biocompatibility with mammalian cells;
• secondary damage caused by the gels must be repaired during wound healing. It is most important to distinguish the stages of wound healing in real time (remote monitoring), which can provide accurate information for gel replacement;
• irritant-sensitive gels and gels for noninvasive drug delivery have great application prospects that deserve further in-depth study.
The characteristics of ionic liquid gels, including favorable mechanical properties, self-repair ability, electrical conductivity, and ability to prevent freezing, have ensured their future applications in related fields.
Design of sustainable and functional packaging materials by using natural resources
R. A. Sheldon notes that single-use plastics will be based on renewable raw materials and designed for efficient recycling.5 The findings of one study9 provide important information for the design and sustainable preparation of packaging materials using sustainable resources and green chemistry technologies. The growing concern about the potential contamination of the ecosystem by microplastics has spurred the search for and development of environmentally friendly polymeric packaging membrane materials.In the work9 took advantage of the structural features of chitosan and betaine hydrochloride (BHC) and successfully synthesised fully bioactive and water-soluble poly aprotonic/protonic ionic liquids (PAPILs) through the interaction of the amino groups of CS and the carboxyl groups of BHC (Fig. 2). A water-soluble PAPIL membrane with smooth surface morphology and tensile strength of 62.9 MPa was successfully fabricated. The PAPIL membrane also exhibited satisfactory biocompatibility, excellent antibacterial activity and high oxygen barrier properties. This material has been successfully applied to the packaging of liquid detergents and pesticides, and the potential of the PAPIL membrane for water-based application scenarios has been demonstrated.
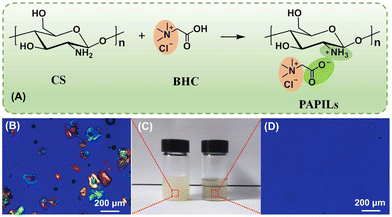 | ||
| Fig. 2 The synthesis of novel material: the protonation of the amino groups in CS by the carboxyl groups in BHC to form PAPILs in water (A). Optical and polarizing images of 3 wt% CS in water (B, left in C) and in 0.3 M BHC aqueous solution (right in C and D) – the PAPILs solution is viscous and transparent. Reproduced from ref. 9 with permission from Elsevier, 2023. | ||
Drug release
In the review10 concluded that nanogels/microgels based on polymer ionic liquids are still poorly understood from the point of view of their biomedical applications. Several papers have been cited here demonstrating the suitability of polymerized IL for drug delivery. The review10 reports a study of chitin nanofibers as fillers for calcium alginate beads for slow release of 5-fluorouracil. It was observed that the inclusion of chitin nanofibers significantly improved the slow release of the drug at pH 7.4.The article11 briefly describe the properties and structure of PIL-microspheres, the classification of PIL-microspheres and prospects for some applications. On the basis of the analysis, the following proposals were made:
• PIL-microspheres have great potential for drug-loading applications. The specific surface area can be increased by adding mesopores or micropores to the microspheres, which can improve the ability of the microspheres to load the drug;
• PIL-microspheres have great potential and a market for purifying water of contaminants, especially heavy metal impurities.
Current problems of PIL-microspheres, such as low drug loading, poor (non-selective) targeting and a relatively small number of species and structures, need to be addressed and still limit their use.11
It has been observed that polymerizable ILs can impart certain exceptional properties to the corresponding polymeric ILs (PILs) compared with other polymers, such as low cost and good biocompatibility. Due to the presence of cations and anions, PILs demonstrate a wide range of selectivity, and thus the properties of PILs can be easily tuned. An amphiphilic block-polymer containing a PIL block with a target ligand, a photosensitive block, and a pH-sensitive block was designed to form self-assembled drug-loaded nanoparticles with a size of ≈80 nm in aqueous solutions (Fig. 3).12
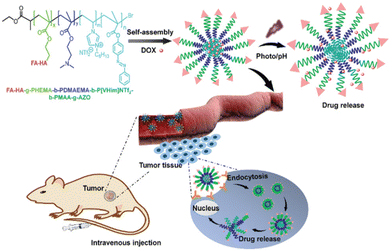 | ||
| Fig. 3 A schematic illustration of the preparation of stimuli-responsive PILs based on polymer nanoparticles for cancer therapy. Reproduced from ref. 12 with permission from The Royal Society of Chemistry, 2020. | ||
Bioanalysis and sensors
One study13 is devoted to increasing the efficiency of solid-phase microextraction according to the “fiber-in-tube” principle. Cross-linked zwitterionic ionic liquid-functionalized nitinol wires for solid-phase fiber-in-tube microextraction and ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) were applied in the analysis of beta-amyloid peptide (Aβ) binding protein in biological fluids. It is expected that future experiments will use the fiber-in-tube method to determine Aβ peptide in real cerebrospinal fluid samples. A bioassay study was designed to examine the correlation of peptide Aβ in patients with Alzheimer's disease and healthy patients.The synthesis of a highly stable electrochemical biosensor for pesticide detection has been described.14 PILs were first introduced to create a biosensor for acetylcholinesterase. Acetylcholinesterase was captured by PIL microspheres by an emulsion polymerization reaction, where negatively charged Au nanoparticles can be immobilized by positively charged PILs, resulting in improved catalytic performance. Positively charged PILs not only provide a biocompatible microenvironment around the enzyme molecule, stabilizing its biological activity and preventing leakage, but also act as a modifiable interface that allows loading of other components with electron transfer properties onto the polymer substrate. This provides an efficient electron transfer channel for trapped molecules. The claimed biosensor has good thermal stability, repeatability, selectivity, and high accuracy in rapid analysis of fruits and vegetables in the field under various climatic conditions, and in the analysis of tap water and other types of samples.14
Photon-crystalline PIL spheres based on imidazolium cation and dicyandiamide anion doped with luminogens that demonstrate aggregation-induced emission (AIE) have been developed.15 The urea group was introduced in the monomer and cross-linker of ionic liquids (ILs) because it can provide hydrogen-bonding sites for effective interaction with target analytes. Since different non-covalent interactions are involved in PIL fragments, the single sphere exhibits different binding affinities to a wide range of psychoactive substances. The development of a sensing platform capable of identifying them remains a great challenge due to limitations related to the complexity of synthesis, the scope of sensor applications and the extensibility of the receptors. The theoretical calculations further reveal the underlying interactions between PIL receptors and the substances to be analyzed and provide guidelines for future sensing element design. Although only a few types of sensing elements were demonstrated in this work, the high degree of flexibility and extensibility of introducing different functional anions into the sensing platform undoubtedly allows for a significant number of sensing spheres with different properties (Fig. 4). This demonstrates great potential for accurate and sensitive identification of complex multi-analyte samples including narcotic substances, which is undoubtedly driven by increasing concern for public health and safety.
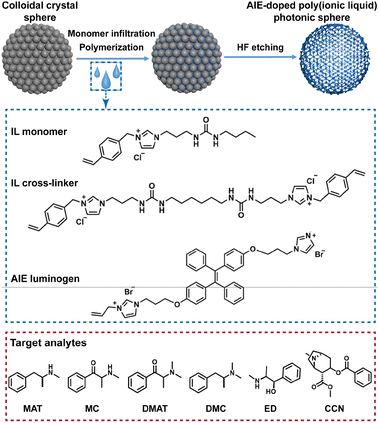 | ||
| Fig. 4 Schematic illustration of the preparation of the AIE-doped PIL photonic spheres and the chemical structures of the IL monomer, IL crosslinker, AIE luminogen, and target analytes, including methylamphetamine (MAT), N,N-dimethylamphetamine (DMAT), methcathinone (MC), N,N-dimethyl cassidone (DMC), ephedrine (ED) and cocaine (CCN). Reproduced from ref. 15 with permission from Wiley, 2023. | ||
Multidimensional sensor devices based on a molecule or particle basis are a very promising concept with numerous advantages for the identification of multiple analytes, e.g., contributing to the development of high-throughput screening in sustainable chemistry and biology.
PILs as electronic skin
Self-healing materials are being intensively researched for mimicking natural systems capable of partial or complete self-repair after damage inflicted on them.16 The copolymer of 1-vinylimidazole paired with bis(trifluoromethylsulfonyl) imide group and ethylacrylate was obtained by a simple synthetic route (Fig. 5). This study achieved the inherent self-reduction function of the materials by taking the electrostatic interaction of ionic pairs in a polyionic liquid as a basis. The synthesised copolymer can completely repair the damage after 3 h at 60 °C, and the recovered samples can bear a certain weight, which does not lead to any wound rupture. In addition, the flexible segment in the copolymer is very useful for improving the ionic conductivity of the PIL as an ion transport channel. At the same time, the ionic conductivity can be as high as 10−6 C cm−1. A solid foundation has been laid to improve its safety and reliability. The material's self-healing ability has good prospects for sustainable development as well as applications in the field of energy equipment as a solid electrolyte or artificial intelligence as a strain sensor.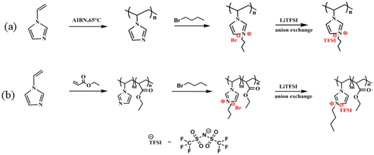 | ||
| Fig. 5 Schematic diagram of the synthesis of polymers in different ways (a and b) discussed in ref. 16. Reproduced from ref. 16 with permission from Elsevier, 2023. | ||
A novel PIL hydrogel with high extensibility, mechanical strength, electrical conductivity, and antibacterial and biocompatible properties was obtained.17 This material (Fig. 6) has high elasticity and sensitivity as a directly wearable ionic skin for human motion detection. Simultaneously achieving the combination of a single hydrogel system and superior integrated characteristics (i.e., mechanical strength, electrical sensitivity, broad-spectrum antibacterial activity, and biocompatibility) is challenging. A novel poly(ionic liquid) hydrogel consisting of poly(acrylamide-co-lauryl methacrylate-co-methyl-uracil-imidazolium chloride-co-2-acryloylamino-2-methyl-1-propane sulfonic acid) (AAm-LMA-MUI-AMPS) was prepared by a micellar copolymerization. Due to the numerous supramolecular cross-links and hydrophobic association in the mesh, the hydrogel exhibited excellent mechanical properties (624 kPa at rupture and 1243 kPa in compression), shell-like elastic modulus (46.2 kPa), stretchability (1803%) and mechanical strength (after 200 cycles at 500% strain, it can be fully recovered). The hydrogel sensor was found to have a certain degree of antibacterial activity and good biocompatibility and, as a consequence, low toxicity. Compared with previously presented hydrogel sensors, the off-the-shelf poly (ionic liquid) hydrogel may well fulfil multiple functions in a single hydrogel system, making it a promising material for direct wearable applications.
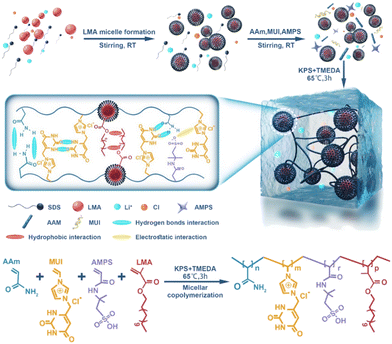 | ||
| Fig. 6 Schematic illustration for the synthesis mechanism of the hydrogel discussed in ref. 17. Reproduced from ref. 17 with permission from ACS, 2023. | ||
One study18 reports the development of a hierarchical response network for the preparation of highly stretchable, self-healing and stable in a wide temperature range composite ionogels based on deep eutectic solvents (DESCI). Polymerisable deep eutectic solvents consisting of choline chloride and acrylic acid monomers dispersed in polyethylene glycol matrix were used in this work. A chemically cross-linked network of the derived DESCIs could serve as a strong cross-linking site to impart high mechanical strength and elasticity, while a network with high-density hydrogen bonds and dynamic reversibility could provide excellent self-healing ability and ductility. This design provides DESCI with high a mechanical strength (≈181 kPa), high extensibility (>730%) and self-healing ability at room temperature. The DESCI-based strain gauge exhibits high sensitivity (GF value is 0.81), fast response time of 0.25 s at 10% strain, and stable resistance values at different applied stress frequencies. This study offers an interesting route to develop self-healing ion-conducting gel materials that are stable over a wide temperature range and have high mechanical performance. The used solvents (choline chloride) as well as the synthesis method make the obtained material low-toxic and useful in corresponding sustainable chemistry.
CO2 capture, sorption, separation, catalysis, and green aspects of PILs
CO2 levels in the atmosphere are increasing exponentially. The current effects of the climate change are dictating the crucial need for new and sustainable materials for CO2 capture.It has been noted19 that there are many opportunities to develop more efficient adsorbents and catalysts that allow CO2 capture and conversion more efficiently at lower temperatures and pressures. There are several promising challenges and areas for research that the work19 suggest for further development of new materials, including:
• development of innovative ionic polymers with a high surface area (SBET), controlled porosity, and high ionic fragment density for optimized CO2 capture and simultaneous conversion;
• improving synthesis strategies to reduce the cost of PILs and ILs;
• expanding the range of substrates, not just epoxides (Fig. 7A) for the practical application by converting CO2 into other attractive chemicals.
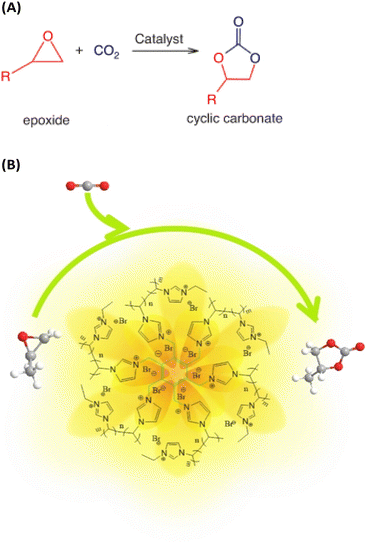 | ||
| Fig. 7 (A) Synthesis of cyclic carbonates from CO2 and epoxides. Reproduced from ref. 19 with permission from CSIRO Publisher, 2021. (B) Schematic representation of CO2 capture with the participation of poly(ionic liquid)s with high ionic density (HDPILs). Reproduced from ref. 20 with permission from Wiley, 2023. | ||
It is worth noting that most of the polymers studied in CO2 conversion can also be used as heterogeneous catalysts in other types of chemical reactions.
Converting CO2 into other attractive chemicals
PILs have proved themselves to be particularly good for the synthesis of cyclic carbonates from CO2 and epoxides as one of the validated routes in line with atomic economy to achieve CO2 conversion.20 Combining the advantages of high activity and easy separation for the heterogeneous catalytic reaction system, a series of heterogeneous catalysts based on poly(ionic liquid)s with high ionic density (HDPILs) was designed and synthesized. Looser structures and high ionic density were obtained by using muti-center ionic liquids with different numbers of substituents as cross-linking agents. Ply[PhSVIM]Br, the copolymerized HDPILs with six substituents on the benzene ring, was optimal (Fig. 7B). The catalyst exhibited excellent catalytic performance for CO2 cycloaddition with 98% conversion and 99% selectivity under optimal conditions.20In one study,21 conjugated acid-alkaline bifunctional PILs with high basicity and high density of ionic centers for heterogeneous catalytic cycloaddition of CO2 with epoxide for the synthesis of a cyclic carbonate were developed. High yields of glycidyl at 90 °C and 1 MPa were obtained. The work claim to have found higher catalytic performance than conventional hydrogen bonding donor (HBD) PILs. Monomers of 2-(1-vinyl-1H-imidazol-3-ium-3-yl) acetate with HBDs exhibit high cycloaddition catalytic activity. The introduction of the monomer into the PILs increases the CO2 concentration available for the cycloaddition reaction, thereby increasing its rate (Fig. 8). According to the characteristics of the polymer, the reaction product is also easily separated from the catalyst. In summary, the researchers believe that their work opens a new direction and mechanism for the binding of CO2 with high-performance chemicals under milder conditions.21
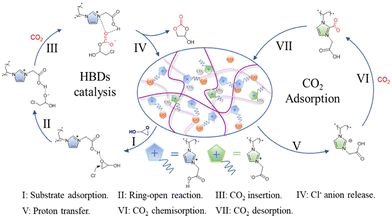 | ||
| Fig. 8 Mechanism diagram of the cycloaddition reaction between glycidyl and CO2 catalyzed by P-DVB-CE/CCImCl. Reproduced from ref. 21 with permission from Elsevier, 2023. | ||
Porous sulfonyl-binuclear carbonate PILs were used for one-pot fixation of dilute CO2 into dimethyl carbonate.22 The one-pot synthesis process not only solves the thermodynamic stability dilemma of CO2 by adding high-energy epoxides, but also eliminates the separation of intermediates due to the bifunctionality of the catalysts, thus realising a win–win situation of carbon footprint utilisation and catalytic process optimisation. The direct use of dilute CO2 avoids the additional enrichment and purification operations required to obtain high-purity CO2, allowing it to be used in a green catalytic process (Fig. 9).
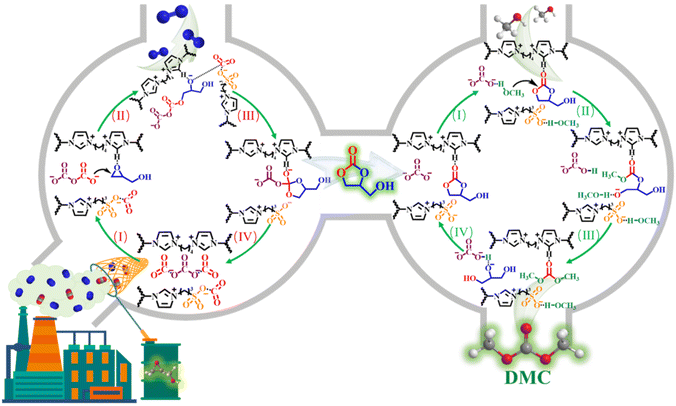 | ||
| Fig. 9 Catalytic mechanism of diluted CO2 coupling reaction. Reproduced from ref. 22 with permission from Elsevier, 2023. | ||
Cycloaddition of dilute CO2 still remains a challenge. Novel porous sulfonyl-dinuclear carbonate poly (ionic liquids) (SC-PILs) have been successfully obtained. Relying on the synergistic effect between the specific surface area increased by etching with basic anions and the basic sites opened by carbonate anion exchange, SC-PILs were able to implement the directed conversion of simulated flue gas (15% CO2 + 85% N2) without any solvent or co-catalyst, and the dimethyl carbonate yield was increased from 17% to 80%. The theoretical DFT calculation was used to deeply understand the difference in activation of the dinuclear CO32−-anion on CO2 compared with the mononuclear CH3COO− anion and the mechanism of [CO32− + CO2] adduct ring opening on epoxides.22
CO2 separation
PILs with dicationic pending moieties as gas separation membranes have been studied.23 The incorporation of ILs into Poly(norbornene)s by post-polymerization resulted in unusual materials with synergistic properties. These membranes showed a moderate permeability for CO2 varying nonlinearly with the variation of the composition, and there was an inverse trend with respect to selectivity for the CO2/N2 mixture, which is the usual trade-off between permeability and selectivity (the Robeson dependence).A series of pyridinium-based PILs containing a hydrophilic ethylene oxide group (PEO-group) has been synthesized.24 They combine a high vapor permeability with high H2O/CO2 and H2O/N2 separation factors. This makes it possible to use them as membranes for removing water from flue gas vapor or dehydrating air. PILs were prepared by the N-quaternization reaction of a precursor copolymer containing 2.5 substituted pyridine links together with PEO side chains, followed by the anion metathesis reaction.
CO2 sorption
Another means of CO2 capture is presented in the review-article.25 This review discussed the CO2 sorption capacities of PILs in comparison with their corresponding ILs. The inclusion of hydroxyl groups in the polycation increased the interaction with CO2 molecules. The COSMO-RS model was used to understand the molecular-level behavior of PILs in terms of their activity coefficients. The biodegradability and toxicity of proposed PILs should be properly evaluated to avoid environmental pollution.A new and simple method for obtaining hierarchical nitrogen-doped porous carbons was demonstrated, and the researchers are confident that this type of carbon material can solve not only the threat to the environment, but also the problem of energy shortage.26 Nitrogen-doped hierarchical porous carbons with a rich pore structure were obtained by direct carbonization of the compound PIL/potassium ferricyanide. The hierarchical porous structure is characterized by the synergistic effect of mesopores and micropores in the CO2 capture process. The mesopores can provide a continuous channel for transporting CO2 molecules; at the same time, the microporous structure provides enough space for mass storage of CO2 molecules (Fig. 10). In practical terms, this carbon material can be easily obtained, regenerated, and reused repeatedly with almost no reduction in the CO2 uptake.
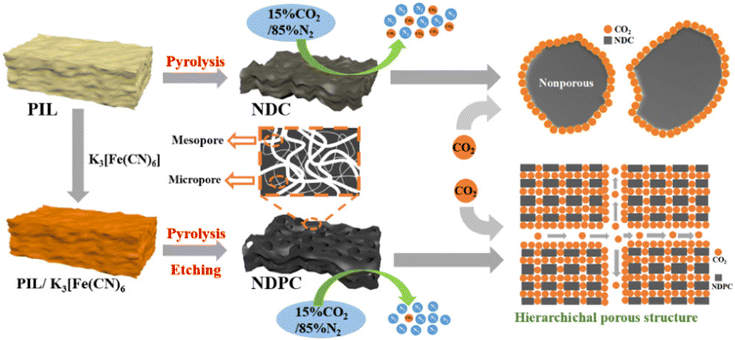 | ||
| Fig. 10 Preparation of N-doped hierarchically porous carbon derived from PIL and potassium ferricyanide and mechanism diagram of CO2 adsorption on NDC and NDPC adsorbents. Reproduced from ref. 26 with permission from ACS, 2021. | ||
The development and synthesis of porous materials as effective CO2 adsorbents have attracted much attention for solving environmental problems related to CO2 emissions. A new amino acid-functionalized PIL poly[1-(p-vinylbenzyl)-3-methylimidazolium glycinate] with good thermal stability and outstanding CO2 adsorption performance was successfully synthesized by polymerization followed by functionalization.27 The work showed that the foamed porous-P([VBMI][Gly]) has a regular cellular structure and uniform pore size distribution. The specific surface area and pore volume are significantly improved compared with the initial sample. The CO2 adsorption capacity of porous P([VBMI][Gly]) can reach 165 mg CO2 per g PIL, which is almost 60% higher than that of the initial sample. Supercritical foaming technology was first developed to produce an ionic liquid homopolymer. The effects of the foaming temperature, pressure, and saturation time on the morphology and CO2 adsorption performance were studied. After 12 cycles of adsorption–desorption, the CO2 adsorption capacity decreased by about 25%.
PIL-chitosan aerogels (AEROPILs) in the form of balls with high porosity (94.6–97.0%) and surface area (270–744 m2 g−1) were successfully obtained.28 AEROPILs were first used as CO2 sorbents. The combination of PILs with chitosan aerogels generally increased the CO2 sorption capacity of these materials, reaching a maximum CO2-capture capacity (0.70 mmol g−1 at 25 °C and 1 bar). Such significant results were obtained by combining PILs and aerogels to create a stable CO2 sorbent in which the porosity can be controlled. The properties of aerogels allow for production strategies that provide porosity control.28
Various polymeric adsorbents for CO2 removal were compared in a review.29 It was highlighted that PILs are the most promising materials for this field. Unique polyelectrolytes obtained by direct polymerization of ionic liquid monomers and structural modification of existing polymers can increase CO2 absorption to a greater extent than other ILs due to the crystalline structure at low pressures, integration of anions and cations with each other in the structure of PILs, and the nature and architecture of the main polymer framework and side groups.
CO2 capture using polymer-based sorbents (even without the use of PILs) is still in its infancy, and there is a long way to go to improve the sorbents and processes that must be developed as soon as possible to mitigate the rapid global climate change.29
Catalysts (including electro- and photocatalysts)
First of all, a recent review30 devoted in particular to the use of Imidazolium-based PILs for green catalytic reactions should be mentioned. It was noted that PILs offer the possibility of creating greener catalytic tools for various transformations, as they facilitate the transition from batch to continuous flow mode. ILs also enable the fabrication of devices using additive manufacturing to create advanced and complex geometries. In this review, the properties of catalysts/substrates/products in pure, poly- and deposited ILs are broadly presented and can be used to help readers select suitable ILs and derivatives for the desired catalytic system.Catalytic green treatment
Excellent stability and versatility make an iron-based PIL/polydopamine composite an effective heterogeneous Fenton catalyst for practical applications in wastewater treatment. The composite can be used in a wide pH range and can be recycled seven times without an obvious decrease in catalytic performance, and it can also be used for the decomposition of organic pollutants. The conclusion was drawn31 that the catalyst exhibits a higher activity than most of the described heterogeneous catalysts.The development and production of catalysts for the destruction of environmental pollutants is of great interest. The development of such hydrogel-based materials with both suitable mechanical and catalytic properties remains a challenge (as for other functional applications, as we have already noted in earlier sections). A polyacrylamide/dimethylaminoethyl methacrylate bromododecane p(AM/DMLB) hydrogel equipped with PIL microspheres as reinforcement points as well as catalytic centres was successfully synthesized.32 The hydrogel has excellent self-adhesive and self-healing properties due to the internal hydrophobic bonding. The efficiency of the material can be dramatically improved by using loaded Pd nanoparticles without the use of other additives and crosslinking agents (to increase the stability of nanoparticle retention), which can obviously significantly improve the catalytic performance. This material has not yet been tested in industrial practice, but excellent antibacterial activity has been noted. According to this work the p(AM/DMLB)/PILs-MPs hydrogels have the potential to be highly efficient catalysts and have high adsorption capacity for sustainable industrial applications.
The problem of color removal of dyeing wastewater has been around for decades. PIL (such as polyvinyl-ethyl-imidazolium bromide [ViEtIm]Br) modified with reduced graphene oxide (rGO), rGO-poly[ViEtIm]Br, has been proposed as a highly efficient open carrier to establish a decomposition platform for industrial azo dyes.33 It was prepared by assembling the integrated catalyst rGO-poly[ViEtIm][heteropolyanions]. The material demonstrated near “perfect” catalytic performance in the decomposition of azo dyes such as methyl orange. Excellent decomposition possibility has been demonstrated for the catalytic removal of methyl orange. In contrast to the previous work, under optimized experimental conditions, the decomposition efficiency of this catalyst for methyl orange reached 98.7% within 3 hours. In addition, after the catalyst was recycled 5 times, the removal efficiency of methyl orange could still reach 95%, which indicates the exceptional suitability of this type of catalyst for recycling.
Fuel and its purification
A potential strategy to synthesize magnetic PILs for tandem reactions has been proposed,34 which could serve as a platform for green and sustainable chemistry applications. Using magnetic polystyrene microspheres as the core and alkali imidazolium ionic liquid brush-type polymers as covalent bonds, robust magnetic ionic liquid brush-type polymers were synthesized. Due to the features of brush-type ionic liquid polymers (BILPs), [Im][OH]/MP-B showed a high catalytic activity. With a wide range of substrates, [Im][OH]/MP-B was applied in tandem Knoevenagel–Michael reactions for the synthesis of 4 H-pyrans (achieving 99.99% conversion and 93.6% yield) and tandem transesterification reactions for sustainable biodiesel production (achieving 99.9% conversion and 95.2% yield) (Fig. 11). Notably, the generated material was effortlessly extracted by magnetic separation and reused many times without any obvious changes in catalytic performance, composition or microstructure, indicating promising opportunities for green chemistry procedures.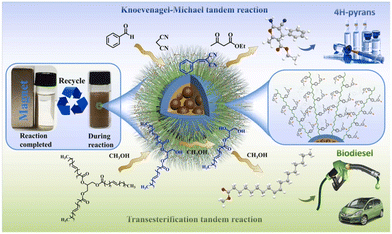 | ||
| Fig. 11 Schematic representation of the tandem Knoevenagel–Michael reactions for the for sustainable biodiesel production in ref. 34. Reproduced from ref. 34 with permission from Elsevier, 2023. | ||
One study35 describes the preparation of a cobalt-containing polyvinylimidazole ionic liquid catalyst and coupling with persulfate for room-temperature ultra-deep desulfurisation. The catalyst has both the advantages of ionic liquid and polymer and overcomes the poor fluidity of ionic liquid, so it is very suitable for catalytic oxidative desulfurisation. The morphology of the catalyst was a microsphere with a diameter of 28 μm, and its interior was a two-dimensional thin-layer nanowire with a width of about 20 nm and a mesoporous carbon framework. The specific surface area was 10.54 m2 g−1 and the average pore diameter was 3.5 nm. The removal efficiency of dibenzothiophene was more than 99% (in the model reaction) and the sulphur removal extent was over 90% even after five cycles. DBTO2 was identified as the main desulfurisation product and the desulfurisation mechanism included the stage of Co(II)-catalysed potassium monopersulfate conversion to form –SO4−, which then oxidised the DBT molecules. The desulfurisation process followed zero-order reaction kinetics. The high degree of purification enables the proposed method to meet the requirements of sustainable chemistry.
Refining of biomass
Sulfonic acid-functionalized PILs were synthesized and applied to catalyze the conversion of cellulose to levulinic acid. PILs were more thermostable than the corresponding low-molecular-weight ILs. As an advantage of PILs, it should be noted that levulinic acid can be easily extracted from the reaction mixture, and the catalysts themselves lose activity insignificantly even after five cycles of use.36 The catalyst also proved to be good for refining other types of biomass.“Green” photocatalysis
The synergistic effect of embedding perovskite halide nanocrystals into PILs has been observed in the production of composites for the photodegradation of organic dyes. Such a visible light photocatalyst has a high absorption coefficient and resistance to defects. The resulting composites show better light resistance, moisture capacity, and stability. The heat resistance compared with the original perovskite halide nanocrystals is about 200 days.37Electrocatalysis
A prospective and one of the most highly promising electrocatalytic reactions today is certainly the nitrogen reduction reaction (NRR). By itself, this reaction is intended to be a replacement for the Haber–Bosch process, but it is devoid of many disadvantages, with the absence of toxic reagents, water as a solvent, etc., and therefore can be called a Green reaction of the future. The use of PILs as an electrolyte due to the following properties has the potential to significantly improve nitrogen conversion:• the absence of a competitive hydrogen reduction reaction (or the suppression of this reaction) in the case of aprotic PILs;
• the solubility of nitrogen in some types of PILs should be higher than in classical solvents;
• electrochemical and increased thermal stability of PILs.
FeS2–SnS2 heterogeneous nanoplates with high defect content were grown in situ on poly(1-vinyl-3-ethylimidazolium bromide)-functionalized polypyrrole/graphene oxide38 using interface and defect engineering. The materials demonstrated the best electrocatalytic performance with respect to NRR among a series of electrocatalysts prepared using different modifiers under the same reaction conditions. However, as the report notes, the stability and durability of such an electrocatalyst with respect to NRR remain unsatisfactory so far.
Porous cobaltous thiocyanate-functionalized PILs were reported.39 These composites have ultrahigh capacity and reversible NH3 absorption. The goal was to obtain highly efficient NH3 sorbents; nevertheless, increasing the efficiency of NRR could be a strategy for designing the use of such sorbents.
Perovskite solar cells
Over the past decade, perovskite photovoltaics have approached other currently available technologies in terms of sophistication and efficiency, and have established themselves as the most promising type of solar cell. PIL engineering was considered in perovskite photovoltaics.40 The use of PILs for their high conductivity, ability to chemically “tune” the structure, and environmental compatibility, appears to provide a potential solution to some of the problems of this type of solar cell. Such problems include poor durability (due to the tendency for the surface to break down) and low mechanical strength, as well as environmental impact. A small but informative review40 briefly describes the use of and role played by PILs (mostly merely ILs) as universal additives, solvents and modifiers, in the charge transport layer, and in interfacial design and stability. Prospects for designing a selection of ILs that meet the requirements for highly efficient and stable next-generation perovskite solar cells are discussed.Lead sequestration in perovskite photovoltaic device
One study41 provides a promising solution to complex lead-binding problems, reducing environmental impact and, in addition, promoting the proliferation of perovskite solar cells (Fig. 12). A transparent bilayer shield made of waterproof and adhesive poly[1-(3-propionic acid)-3-vinylimidazolium] bis(trifluoromethanesulfonyl)imide (PPVI-TFSI) was synthesized and applied to isolate lead in perovskite solar cells. This eventually greatly improved the stability of the solar cell under diverse conditions (including pure water, acidic water, alkaline water, salt water and hot water). At the same time, PPVI-TFSI has an adsorption capacity of 516 mg g−1 towards Pb and excellent adsorption kinetics, which is useful for downstream treatment of damaged devices. In the final part of the work, a demonstrable wheat germination test was performed, which should serve as evidence that the proposed approach helped to prevent lead leakage in discarded devices.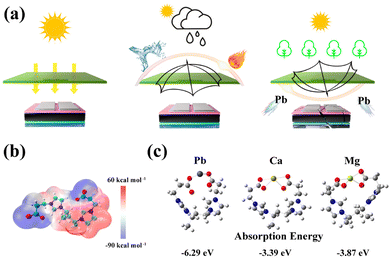 | ||
| Fig. 12 (a) Ambidextrous protective shield manufactured by PPVI-TFSI. (b) Electrostatic potential distribution for PPVI-TFSI. (c) Optimized structures and absorption energy for PIL toward different cations. Reproduced from ref. 41 with permission from ACS, 2023. | ||
Preparation and research of the multifunctional intermediate layer
PIL was applied42 to introduce a multifunctional intermediate layer in order to improve the efficiency and stability of the device with perovskites with small and wide band gaps and different cell architectures (pin and nip). The presence of PIL on the perovskite surface reduces no-radiation losses to 60 MeV in the pure material, indicating effective passivation of the surface trap, thereby increasing the quantum yield of external photoluminescence to 7%. The PIL treatment results in a bifunctional surface where the insulating areas act as a blocking layer, reducing charge recombination at the interface and increasing open circuit voltage (VOC), while at the same time, the passivated neighboring areas provide more efficient charge extraction, increasing the fill factor (FF) (Fig. 13). As a result, these solar cells exhibit excellent VOC and FF values of 1.17 V and 83%, respectively, with conversion efficiencies of up to 21.4% in the best devices.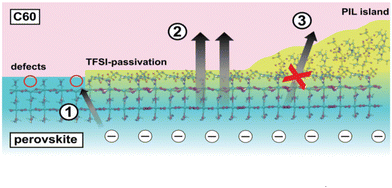 | ||
| Fig. 13 Schematic representation of the perovskite/ETL interface in the presence of PIL based on experimental and theoretical results. (1) Possible non-radiative recombination pathway at the perovskite surface due to trapping at MA vacancies. (2) TFSI-passivated regions with reduced trap density and enhanced charge extraction. Here electrons do not undergo non-radiative recombination due to defects and can be effectively extracted. (3) The thick insulating polymer islands effectively reduce the interface area physically spacing the perovskite and C60. As such, the interface recombination happening across this layer is reduced. Reproduced from ref. 42 with permission from The Royal Society of Chemistry, 2021. | ||
A new multifunctional intermediate layer based on imidazolium polyionic liquid [PeIm] [TFSI] was prepared.42 A detailed mechanistic view of the PIL-perovskite system combining a large variety of experimental methods with theoretical calculations has been presented (Fig. 14). It is suggested how a combination of efficient surface-passivating components, such as TFSI, embedded in an insulating polymer matrix can reduce the emission-free recombination in the perovskite and at the interfaces, improve charge extraction and prolong the stability simultaneously.
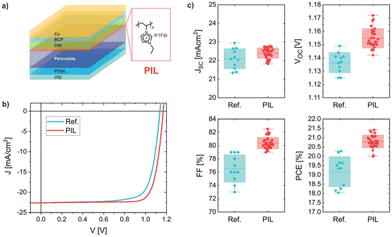 | ||
| Fig. 14 (a) Pin-type device layer architecture with the chemical structure of the poly(ionic liquid) (PIL) inserted between the perovskite and C60. (b) Typical current–voltage characteristic for a reference untreated cell and for a PIL-modified cell in reverse scan at 0.025 V s−1. (c) Device parameter statistics for reference and the PIL-containing cells. Boxes indicate the standard deviation. Reproduced from ref. 42 with permission from The Royal Society of Chemistry, 2021. | ||
Based on the results of this publication, the widespread use of PILs in perovskite research is expected in the future, including also inorganic perovskites and tandem solar cells.42
The topic of this section is now on an upward trend and deserves special attention. We would like to conclude this discussion by noting a few more works.
PILs as perovskite defect-passivating agents
An ionic liquid polymer (poly[Se-PbI][BF4]) was developed to form high-quality perovskite films.43 The novelty of the publication44 is the use of PILs as additives for the absorber layer, which were used as perovskite passivating agents. PILs were chosen because of their high ionic conductivity and hydrophobic nature in the macromolecular structure. A direct comparison of the performance of devices obtained by adding a passivating IL (tBMPy-TFSI) and its polymeric version, PIL, to the perovskite layer was shown. It has been demonstrated45 that degradation caused by ion migration can be eliminated by introducing multifunctional poly(ion-liquid) additives (PILs), resulting in ultra-stable perovskite solar cells. The presence of PILs is sufficient to create an “ionic polymer network” that provides defect passivation and ionic immobilization functionality by simultaneously forming a physical barrier and chemical bonding. It was noted45 that longevity is a key limitation for perovskite-based hybrid photovoltaic systems.Batteries and energy conservation
A review article46 has described the synthetic methodologies for PIL composites, as well as the fascinating properties of the hybrid materials. The new family of redox-active PILs can be applied in several electrochemical energy storage technologies, such as lithium batteries, electrocatalysts in fuel cells and metal–air batteries or electrolytes in organic redox flow batteries. The integration of PILs into different substances has provided unique aspects in composites, especially the synergistic effects that arise from each component. The review in detail considers the synthesis and properties of PIL–metal nanoparticle composites (Pd, Rh, Au, Pt), metal oxide NP composites (Fe3O4, TiO2, VxOy), carbon materials (CNT, graphene composites), silica, metal salts, polyoxometalates, organic polymers, organic molecules, MOF/POF, biosubstances, electrode composites, and IL-composites. The structure–property relationships, physicochemical behaviors and green aspects need better understanding and development.The progress of the emergence and use of new ILs for energy storage and conversion was noted in a brief review.47 The following were considered as new ILs:
• deep eutectic solvents (DESs);
• poly(ionic liquids) (PILs);
• ionic liquid crystals (ILCs);
• redox-active ionic liquids (RAILs);
• polymeric deep eutectic solvents (PDES), which are considered as a new family of PILs (described in the section “Basic and theoretical aspects of the study of PILs”).
Increased reaction rates, selectivity, and productivity in various chemical reactions are their distinct advantages over traditional ILs. The work cites numerous examples and concludes that, given their unique physical and chemical properties, electrochemical behavior, and self-assembled structures, the new ILs have become promising materials for various applications, particularly energy storage and conversion.47
Liquid (at room temperature) PILs were developed and synthesized.48 It is believed that energy applications can be achieved by tying these RTILs to liquid membrane supports and to diffusion membranes, which will allow their use for energy storage applications.
Zn–air batteries
Combining biomaterials with a variety of PILs creates tremendous opportunities for advanced solid-state electrolytes for solid-state batteries. Flexible zinc–air solid-state batteries49 offer a high power density of 135 MW cm−2, a specific capacity of 775 mA h g−1, and ultra-long cyclic stability with 240 hours of continuous operation for 720 cycles, far superior to today's solid-state batteries. Creation of safe and environmentally friendly solid electrolytes with intrinsic hydroxide-ion conductivity for flexible zinc–air batteries is highly desirable, but extremely challenging, as illustrated in Fig. 15.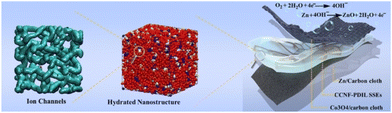 | ||
| Fig. 15 Safe, environmentally benign, hierarchically nanostructured CCNF-PDIL solid-state electrolytes with reinforced concrete architecture are constructed by nanoconfined polymerization of dual-cation ionic liquid (PDIL, concrete) within a robust three-dimensional porous cationic cellulose nanofiber matrix (CCNF, reinforcing steel) for flexible zinc–air batteries. Reproduced from ref. 49 with permission from Wiley, 2022. | ||
Li-solid-state batteries
The concept of using natural biomaterials and multipurpose PILs to prepare solid-state electrolytes offers great opportunities for the development of safe and environmentally friendly flexible solid-state batteries.50A PIL-coated anion-exchange membrane with poly(diallyldimethylammonium bis(trifluoromethanesulfonyl)imide) on a commercial polypropylene separator (PP) was successfully developed as a Cu–Li battery separator at 3.3 V.50 The separator is positively charged and can effectively block the migration of copper ions from the cathode to the anode, thereby greatly increasing the Coulomb efficiency and stability of the Cu–Li battery. The PIL/PP separator also exhibits excellent ionic conductivity with rapid anion transfer due to the special molecular structure of PIL with a positively charged polymer backbone. Such a dual-function separator can improve the overall electrochemical performance of metal-to-metal batteries and makes them potential candidates for large-scale energy storage systems.
PILs combine the properties of a polymer and ionic liquids, so their application has several advantages over ionic liquids. In a paper,51 poly(1-[2-(2-(2-(methacryloyloxy)ethoxy)ethoxy)ethyl]-3-methylimidazolium bis(trifluoromethylsulfonyl)imide)-co-poly(poly(ethylene glycol) methyl ether methacrylate) was synthesized and blended with basic polymer and lithium salt to prepare a PIL gel electrolyte membrane for use in a lithium battery. According to the measured characteristics, another promising material was obtained for use as an electrolyte in high-performance lithium batteries to improve the capacity density and power density.
Na|NaFePO4 batteries
PIL has been used to develop non-solvent solid polymer electrolytes (SPE) for Na|NaFePO4 batteries based on polymer electrolyte membranes with a high salt concentration containing (in addition to PIL) mixed anions, block copolymer polystyrene-b-poly(diallyldimethylammonium)bis(trifluoromethanesulfonyl)imide-b-polystyrene and NaFePO4. SPEs are attracting growing attention in the development of post-lithium battery technologies because of the safety and performance advantages of solid-state batteries. The resulting Na|NaFePO4 cell operating at 70° C and good Coulomb efficiency (98%) demonstrates the promising potential of these solvent-free triblock copolymer electrolytes in Na metal batteries.52Organic solid-state batteries
The aim of the research53 was to create new liquid polymeric materials as polymeric electrolytes for organic solid-state batteries. Polymers with chloride counterions based on acrylic ionic liquid monomers with a stepwise changing chemical structure were synthesized and investigated. PIL meshes were obtained by UV-volume polymerization. The main focus of the study was on the ionic conductivity of the samples. The electrochemical stability was investigated by the linear potential sweep method and revealed the stability of the selected samples in the voltage range from −2 to +2 V. So, this study is another step toward obtaining polymer electrolytes with ion centers and ionic conductivity for solid-state batteries.Supercapacitors and other electronic devices
It is quite possible that supercapacitors will become one of the components of renewable energy. Supercapacitors are suitable temporary energy storage devices for energy collection systems. The widespread use of PILs will reduce the serious environmental problems associated with organic electrolytes.The design of ionic diodes based on supercapacitors with adjustable bias direction using PIL was carried out.54 The supercapacitor-diode is a device that combines the characteristics of a diode in an electrical bilayer capacitor and can be used to extend conventional supercapacitors to new technological applications, and can play a crucial role in network stabilization, signal propagation and logic operations. Typically, the supercapacitor-diode can realize charge accumulation only in the positive bias direction. It is proposed to use the devices with an adjustable bias direction realized using a polycationic IL or a polyanionic IL (Fig. 16) as an electrolyte.
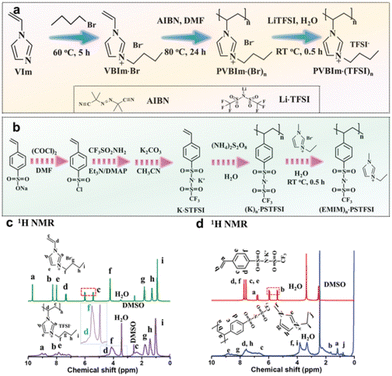 | ||
| Fig. 16 Synthesis and characterization of two poly(ionic liquids). (a) Synthetic scheme of PVBIm·(TFSI)n. (b) Synthetic scheme of (EMIM)n·PSTFSI. (c) 1H NMR for VBIm·Br and PVBIm·(TFSI)n. (d) 1H NMR for K·STFSI and (EMIM)n·PSTFSI. Reproduced from ref. 54 with permission from Wiley, 2021. | ||
It is believed that the new devices containing PILs have an outstanding lifetime (4500 cycles), which represents a breakthrough in the development of high-performance capacitive ion diodes.54
PILs incorporating poly(pyridinium bromides) and poly(pyridinium trifluoroacetates) were synthesized using an end pyridine-containing monomer and a dibromalkyl LC monomer with cholesterol groups.55 The ionic conductivity of these new PILs increased as the temperature increased from 95 to 242 °C. The temperature-dependent conductivity of the PILCs exhibited a transition from Arrhenius-like behavior to Vogel–Fulcher–Tamman-like behavior in nematic phases. The excellent temperature-dependent conductivity characteristics of these PILCs could provide potential applications in temperature-controlled electronic switches.
Stable viologen-based electrochromic devices based on multi-functional PIL membranes have been discussed.56 A polymeric ionic liquid, poly(vinylidene fluoride-co-difluoro-vinylideneamino-oxoethyl-1-butylimidazolium-co-vinylidene-oxoethyl-1-butylimidazolium tetrafluoroborate), has been successfully synthesized to form stable electrofusion membranes as quasi-solid electrolytes for use in electrochromic devices. The device demonstrated a significant change in transmittance up to 73.0%. The synthesized membranes retained 95.9% and 88.3% of their initial change in transmittance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of continuous switching, respectively.
000 cycles of continuous switching, respectively.
Progress in the development of high-performance thin-film electronics based on highly conductive solid polymer materials suitable for high-speed printing has been demonstrated in one study.57 The proliferation of high-performance thin-film electronics depends on the development of highly conductive solid polymer materials. The synthesis and properties of well-defined poly[(ILM-r-PEGM)-b-PhEtM] (decode) cationic and anionic block copolymers for use in printed electronics have been reported. The block copolymers were found to readily self-organize into a lamellar structure, providing a mechanically strong region and a low glassy region for ionic charge transfer. These structures are promising in terms of green and sustainable chemistry. These new polymers were characterized by very high ionic conductivity and enhanced capacitance values when incorporated into thin film parallel plate capacitors.
The role played by various PILs used as electrolyte for optoelectronic device technology was briefly discussed.58 Particular attention was paid to the dye-sensitized solar cells, which are a new generation of solar cells that convert sunlight energy into electricity with simple device manufacture (and at a reasonable price). However, traditional devices of this type have the significant disadvantage of using organic solvents as the electrolyte, which causes serious environmental problems. PILs with superior properties have the potential to solve exactly this problem too.
A simple strategy to fabricate porous carbon materials with high capacitive performance using PIL poly(1-cyanomethyl-3-vinylimidazolium bis(trifuoromethanesulfonyl)imide) (PCMVImTf2N) and biomacromolecular composites was proposed.59 A porous carbon material doped with nitrogen and sulfur was investigated by carbonizing a composite of PIL (PCMVImTf2N) and zein biomacromolecule, which was electrostatically cross-linked after treatment with an ethanol solution with ammonia (Fig. 17).
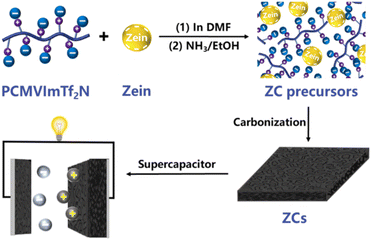 | ||
| Fig. 17 Scheme of fabrication and application of the eco-friendly supercapacitor using PIL. Reproduced from ref. 59 with permission from Elsevier, 2023. | ||
The surface area of the supercapacitor could reach 764 m2 g−1, and the total pore volume was 0.41 cm3 g−1. Remarkably, the specific capacitance of the electrode obtained using the material was 338 Fg−1 in 2.0 M KOH at a current density of 0.1 A g−1 and showed excellent cycling stability. After 5000 cycles, the specific capacitance was still stable.59 Zein, which contains both hydrophobic and hydrophilic amino acids is an amphiphilic, non-toxic, and good biodegradable biopolymer.
Analytical chemistry and PILs
Amperometric gas sensors
PILs can be used as membranes in analytical gas sensors.60 One way to overcome the fluidity of the initial ILs is to create polymeric membranes. To form stable membranes on miniature flat electrode devices, IL 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([C2mim][NTf2]) was mixed with PIL poly(diallyldimethylammonium bis(trifluoromethylsulfonyl)imide) (poly[DADMA][NTf2]) at room temperature. The optimal ratio (60/40 wt%) for IL/PIL was found. The obtained membranes were tested for detection of O2, SO2, and NH3. IL/PIL membranes can be used as new design materials for highly reliable voltammetric/amperometric detection of various gases. It was shown60 that PILs have promise for use in amperometric gas sensors, and future applications can be explored by further tuning the chemical structure to improve analytical performance, including CO2 detection.Extraction and chromatography
PIL-absorbents for the extraction of n-butanol from aqueous solutions have been studied.61 An in-depth study of imidazolium-based polymeric ionic liquids (PILs) as absorbents was carried out in bioreactors with two-phase separation, to remove n-butanol from aqueous solutions. Poly(1-vinyl-3-alkylimidazolium) bromides containing alkyl groups of different lengths, structure and composition, as well as analogues containing various inorganic and carboxylate anions were synthesized for the work. A series of equilibrium experiments produced a complete three-phase P[(VC12-linIm)(Br)]/n-BuOH/water diagram, which can easily be used for a simplified n-butanol extraction process. The same team went on to use PILs to extract succinic acid from aqueous solutions62 using a polyionic liquid based on 1-vinyl-3-dodecylimidazolium containing a hydroxide counterion. The researchers concluded that the obtained material is sufficiently suitable for the extraction of succinic acid during its bioproduction, as well as to facilitate the control of pH of the aqueous solution.Cross-linked PIL coatings for imidazolium-based solid-phase microextraction were developed in a study63 for the selective extraction of pesticides. Solid-phase microextraction is a solvent-free extraction technique and is an alternative to conventional extraction methods. A comparison with a commercial PDMS/DVB fiber was carried out. The main characteristics of the sorbent were optimized using high-performance liquid chromatography with ultraviolet detection. The PIL sorbent coating consisting of aromatic fragments in both monomer and cross-linking agent showed the best selectivity and the highest affinity to pesticides across all the PIL sorbent coatings tested. A four-fold increase in pesticide extraction and a nine-fold decrease in cannabinoid extraction were observed compared with the data previously obtained for the method described. It is suggested that future work in this direction will focus on modifying the structure of the sorbent coatings and extraction platform in order to increase the selectivity of the sorbent coating and to extract the full range of pesticides.63
Two nickel-based PIL coatings-sorbents in HS-SPME combined with GC-MS were successfully developed and applied64 for the detection of volatile and semi-volatile amines, including pyridine, triallylamine, tripropylamine, 3-ethylpyridine, aniline and 2,6-DTBP in water samples.
One review6 concerned the preparation of improved dispersible adsorption materials with desired properties using imidazolium-based PILs. Providing tunable properties suitable for the desired applications can be achieved by changing the combinations of functional groups. Possibilities for the best use of imidazolium-based PILs were shown. Methods to improve the adsorption materials were suggested:
• study of surface functionalization methods using such materials as carbohydrate polymers, metal–organic frameworks (MOFs), nanomaterials, and inorganic compounds;
• development of new, intelligent and environmentally friendly materials derived from imidazolium-based PILs that exhibit universal sensitivity to various complex matrices, including the environment, food, and biological media;
• applying a combination of modern computer modeling techniques, such as molecular docking modeling;
• introduction of hybrid adsorbents based on imidazolium-based PIL by combining imidazolium-based PIL with other environmentally friendly renewable materials, such as clay and polysaccharide biopolymers (cellulose, lignin and chitosan);
• consideration of the properties and advantages of imidazolium-based PILs and the use of good detection methods by chromatography, sensors, or electrophoresis.
In conclusion, imidazolium-based PILs have attracted considerable attention for dispersive solid-phase extraction (DSPE) and for advances in their dynamic characteristics over the past few years. General protocols for the synthesis of imidazolium-based PILs have been developed, the analytical performance of the adsorbents under study in DSPE has been described, and the application of PILs in DSPE and its variations has been summarized (Fig. 18).6 The advantages of various substituents in imidazolium-based PILs were reviewed.
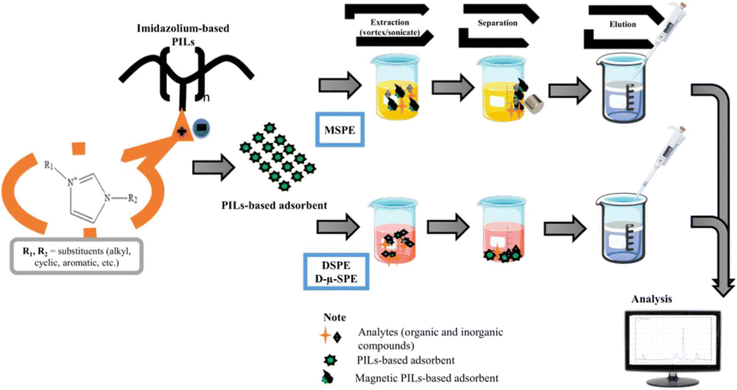 | ||
| Fig. 18 The general scheme of analytical approaches using imidazolium PILs described in ref. 6. Reproduced from ref. 6 with permission from Elsevier, 2023. | ||
Basic and theoretical aspects of the study of PILs
PDES (polymeric deep eutectic solvents) as a new family of PILs
A recent work65 developed novel Brønsted–Lewis acidic polyvinylpyrrolidone [HPVP]Cl/ZnCl2 deep eutectic solvent based on commercially available linear polyvinylpyrrolidone (PVP) that is converted via the common in situ polymerization routes to PDESs (Fig. 19). This protocol offers a highly atom-economical approach for precisely designed PDESs with total control over average molecular weight, Mw, as a fundamental polymeric property. It was demonstrated that [HPVP]Cl/ZnCl2 catalysts can be favorably utilized to synthesize bio-active bis(indolyl)methane compounds in a sustainable, reusable, effective, and scalable manner (76–98%).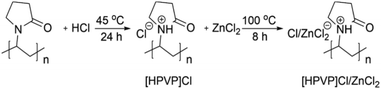 | ||
| Fig. 19 Scheme of synthesis of Brønsted–Lewis [HPVP]Cl/ZnCl2 deep eutectic solvent polymer via quaternization of PVP. Reproduced from ref. 65 with permission from Elsevier, 2023. | ||
Experimental studies of the basic properties of PILs
A series of magnetic PILs (MPILs) based on poly(acrylamide-co-diallyl dimethylammonium chloride) has been synthesized. In the past decade, the MPIL field has expanded to a variety of applications including catalysis, organic templates and metal ion sources for in situ metal nanoparticle synthesis, magnetic separations, antibacterial materials, optical and fluorescent quantum dots for biological labelling, and molecular magnet development. PILs were mixed with Co2+, Fe3+ and Co2+/Fe3+ in different molar ratios to form a series of MPILs. These novel MPILs were used to study the molecular structure and binding between polymers and metals using comprehensive spectroscopic studies in both dry and liquid states.66 Infrared, X-ray photoelectron, UV-visible and Raman spectroscopy showed the presence of metal coordination of both iron and cobalt chlorides with the acrylamide co-monomer. It was demonstrated that MPILs can be tuned using metal ion species and concentration to increase the magnetic susceptibility and modify the metal ion coordination structure and binding to the PIL copolymer. The control of the coordination structure and binding in PILs is important as these materials allow the combination of the advantages of polymers with antibacterial properties and magnetosensitivity, in the case of MPILs. It is noted that understanding the interaction of transition metal ions in MPIL systems will guide the design and selection criteria for other magnetically sensitive functional PIL materials.Researchers synthesized three imidazolium-based PIL analogs with different alkyl spacer lengths between the ion pair and the main chain (P[ECnVim][PF6]).67 The effect of the alkyl chain spacer length on the electrorheological effect (ER) and dielectric properties of P[ECnVim][PF6] microsphere suspensions was studied. It was shown that as the length of the alkyl chain spacer increased, the electrorheological effect increased, but the operating temperature range of the ER effect became narrower. The structure–property relationship was established in the design of PIL-based ER materials.
The aim of the research68 was to obtain polyelectrolytes with increased ionic conductivity and electrochemical stability. The work fills a gap in the fundamental knowledge concerning the influence of the recurring unit charge density on the structure–property ratio of PILs. Within this field, a series of eight cationic TFSI-based PILs was prepared by (1) polyaddition by copper(I)-catalyzed azide–alkyne cycloaddition using cationic ILMS and (2) subsequent N-alkylation of poly(1,2,3-triazole) intermediate products. The synthesized PILs differed in the number of charge carriers per monomer unit (from 1 to 4 ion pairs) and the nature of the cation combinations (1,2,3-triazolium, imidazolium, or ammonium, as well as 1,2,3-triazolium/imidazolium or 1,2,3-triazolium/ammonium). Such PILs could be potential candidates for the next generation of safe and flexible polymer-based electrochemical devices requiring high solid-state conductivity, such as supercapacitors, smart windows, or actuators. When the application requires high electrochemical stability, it is preferable to include more stable cations such as ammonium or imidazolium in the polymer backbone. Consequently, future applications of PILs with mixed type cations may include lithium-ion batteries.
The influence of the PIL's nanostructure on the macromolecular conformation of the polymer chains was first studied by extensive use of small-angle neutron scattering on a series of poly(1-vinyl-3-alkylimidazolium)s with different alkyl and anion side chain lengths.69 In solution, rotational radii were found to increase with increasing side chain length as a consequence of tightening interactions between neighboring monomers. In general, however, the nonmonotonic evolution of the rotation radius reflects changes in chain flexibility and potential screening of electrostatic interactions. In addition, on a smaller scale, the use of small-angle neutron scattering provides experimental estimates of both chain diameter and correlation length between neighboring chains, a comparison of which reveals clear evidence of interpenetration of alkyl side chains. These structural features make valuable contributions to the understanding of the dynamic properties of PILs.
It has been shown that the electrostatic interaction and, consequently, the number of dissociated counterions determine the conformation and dynamics of PIL chains in solutions.70 The researchers demonstrated that condensation of counterions occurs in PIL solutions, indicating the ability to control the properties of PIL solutions simply by changing the dielectric permittivity of the solvent. Since PILs have recently attracted considerable attention due to their wide range of applications (which are described in the course of our review), these results may provide useful information about the rheological properties of PIL solutions, optimize process operations for PILs, and thus develop a PIL-based material with improved system performance.
The effect of the ion diameter of ionic liquids on the shear viscosity of polymerized ionic liquids in ILs has been studied.71 When both PIL and IL contain large PFSI anions (a ≈ 0.57 nm), the specific viscosity first decreases with increasing IL concentration in the low IL concentration range, reaches a minimum, and then increases with increasing IL concentration in the high IL concentration range (Fig. 20). The results demonstrate that the diameter of IL ions plays an important role in controlling the charge shielding mechanism of PILs in IL solutions and thus affecting the viscoelastic properties of PIL solutions, which may be one of the essential properties of PIL as a sustainable material.
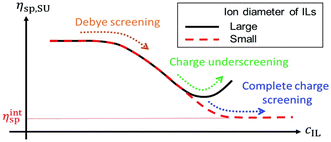 | ||
| Fig. 20 The dependence of the specific viscosity (on the Y axis) against ion concentration of IL or PIL (on the X axis) obtained from the resulting equation in ref. 71. Reproduced from ref. 71 with permission from The Royal Society of Chemistry, 2022. | ||
Theoretical description of PIL-based systems
One work72 correctly notes that the number of electrochemical applications of ionic liquids and their polymerized forms is increasing, while PIL-based systems have almost no theoretical description (as well as IL-based systems, by the way). They offered a brief prospective overview of the current state of the art in this field (as of early 2022).72 The study73 developed a theoretical model that describes the behavior of a polymeric ionic liquid and its solution on a charged electrode. The model includes the conformational entropy of polymerized cations within the framework of Lifshitz theory and electrostatic and volume interactions of counterions in the mean-field approximation. The researchers obtained a new analytical expression for the differential capacitance in the framework of the linear theory, which is a function of microscopic ionic parameters and is converted to well-known expressions in the limit modes. Numerically calculated differential capacitance curves analyzing potential and ion concentration profiles are discussed. It is noted that the model can be considered as a theoretical basis for the description of gel electrolytes based on PILs, which is one of the current applications of PILs.Other prospective applications
There are also new (and prospective) works that were not included in the thematic sections of this review (see above).74–77 In this section, some of these studies are discussed in more detail.Conductive hybrid graphene fiber
A conductive hybrid fiber coated with graphene was designed and produced using an improved wet spinning process. PIL-Cl and PIL-Ac were synthesized to promote the formation of CMC/PIL/graphene fibers. PIL in this work was shown to have absolute advantages whether it was used to facilitate fiber production or to impart conductivity to the fibers. The fabrication of CMC/PIL/graphene fibers not only extends the field of application of conductive hybrid fibers, but also provides a new method of fiber fabrication via electrostatic interaction (Fig. 21).74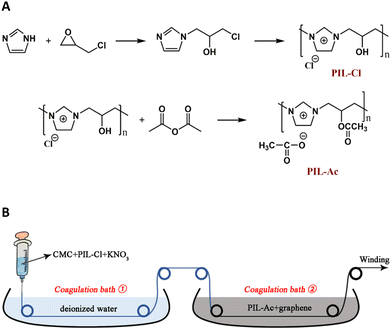 | ||
| Fig. 21 (A) The synthetic routes to PIL-Cl and PIL single bond Ac. (B) Preparation of graphene coated carboxymethyl cellulose (CMC) fibers. Reproduced from ref. 74 with permission from Elsevier, 2022. | ||
Environmentally friendly adhesive at low temperatures
The synthesis of a new class of PILs, which have excellent dynamic adhesion strength on various substrates, is reported.75 These adhesives are highly transparent and have excellent resistance to ultra-low temperatures and organic solvents. They are expected to function as environmentally friendly adhesives in harsh environments where most previously described adhesives exhibit poor performance. The work attributes the outstanding dynamic interfacial adhesion to a combination of electrostatic interactions and hydrogen bonds. During shear stripping, electrostatic interactions determine the movement of charges toward the surface of the template. These discoveries can be applied in the development of strategies for creating adhesive materials with special functions (Fig. 22).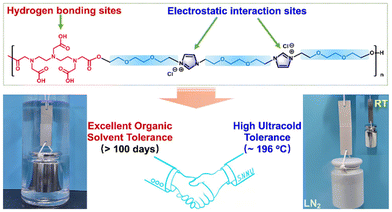 | ||
| Fig. 22 The interfacial adhesion to a combination of electrostatic interactions and hydrogen bonds, obtained from Deng et al.75 Reproduced from ref. 75 with permission from ACS, 2022. | ||
High-efficiency superlubricant
The PILs series with a flexible and electron-rich polymer chain attached to the cation was developed as lubricants for steel-to-steel systems.76 Compared with widely used poly-alpha-olefin and commercial lubricants, PILs-lubricants have markedly lower friction and better surface protection. Macroscale superlubricity for PILs based on 2,5-furandicarboxylic acid is achieved without a run-in period at 75 °C and above. Surface characteristics and molecular dynamics simulations show that the superlubricity is achieved by a strong lubricating film immediately formed on the steel surface due to the rapid adsorption of 2,5-furandicarboxylic acid-IL ions in several locations. This work provides new insights into the design of macroscale superlubricity systems.76Conclusions
Advanced research and prospects on polymer ionic liquids – trends, potential and application – have been briefly outlined in this review, taking into account the point of view of the green approach.Scientific and technological breakthroughs can be expected in biochemical and medical applications; CO2 capture, sorption, and gas separation; perovskite solar cells; in creating and improving electronic devices, energy storage devices, catalysts; analytical chemistry; and in other applications as well.
The following areas of research and benefits of PILs should be highlighted:
• producing PILs is one of the effective strategies for using ion-conducting materials for a wide range of applications;
• extension of the modification of the composition and properties of PILs based on a combination with modern computer modeling techniques;
• properties of PILs (polarity, magnetic properties, catalytic activity) can easily be modified or added by changing the structure of counterions during subsequent processing; after adsorption; PILs can easily be regenerated without any loss of adsorption properties;
• attractive for industrial applications is the cost, comparable to the cost of standard organic solvents;
• and of course, from a green point of view, the described advantages of PILs (for example, sustainable material, low toxicity, etc.) are undoubtedly very important.
Author contributions
We declare the equal contribution of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Russian Science Foundation (grant no. 23-73-30007).References
- W. Wang, in Encyclopedia of Ionic Liquids, ed. S. Zhang, Springer Nature Singapore, Singapore, 2022, pp. 1043–1058 Search PubMed.
- A. N. Vereshchagin, N. A. Frolov, K. S. Egorova, M. M. Seitkalieva and V. P. Ananikov, Int. J. Mol. Sci., 2021, 22, 6793 CrossRef CAS PubMed.
- C. Liu, F. Raza, H. Qian and X. Tian, Biomater. Sci., 2022, 10, 2524–2539 RSC.
- N. Hussain Solangi, F. Hussin, A. Anjum, N. Sabzoi, S. Ali Mazari, N. M. Mubarak, M. Kheireddine Aroua, M. T. H. Siddiqui and S. Saeed Qureshi, J. Mol. Liq., 2023, 374, 121266 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- A. H. Mohamed, N. A. Noorhisham, K. Bakar, N. Yahaya, S. Mohamad, S. Kamaruzaman and H. Osman, Microchem. J., 2022, 178, 107363 CrossRef CAS.
- H. Wang, X. Hu, Y. Su, J. Xu, H. Fang, J. Liu, Y. Kang, X. Li, Z. Chen and K. Song, J. Phys.: Conf. Ser., 2023, 2468, 012057 CrossRef.
- Y. Gao, W. Zhang, L. Li, Z. Wang, Y. Shu and J. Wang, Chem. Eng. J., 2023, 452, 139248 CrossRef CAS.
- L. Zhang, H. Sheng, R. Liu, M. Yang, Y. Guo, Q. Xu, L. Hu, S. Liang and H. Xie, Int. J. Biol. Macromol., 2023, 230, 123182 CrossRef CAS PubMed.
- I. B. Qader and K. Prasad, Pharm. Res., 2022, 39, 2367–2377 CrossRef CAS PubMed.
- R. Zhang, A. Ahmed, B. Yu, H. Cong and Y. Shen, J. Mol. Liq., 2022, 362, 119706 CrossRef CAS.
- B. Lu, G. Zhou, F. Xiao, Q. He and J. Zhang, J. Mater. Chem. B, 2020, 8, 7994–8001 RSC.
- I. D. Souza, J. L. Anderson and M. E. C. Queiroz, Anal. Chim. Acta, 2022, 1193, 339394 CrossRef CAS PubMed.
- Y. Wan, H. Wang, L. Zhang, Y. Chen, S. Li, J. Zhou, Q. Zhang and L. Xia, Microchim. Acta, 2022, 189, 300 CrossRef CAS PubMed.
- C. Liu, W. Li, W. Zhang, H. Zhao, G. He, C. Li, C. Wang and G. Li, Chem. – Eur. J., 2023, 29, e202203616 CrossRef CAS PubMed.
- C. Wang, H. Meng, Y. Wang, W. Ye and C. Wang, J. Mol. Liq., 2023, 383, 121981 CrossRef CAS.
- D. Fu, G. Huang, Y. Xie, M. Zheng, J. Feng, K. Kan and J. Shen, ACS Appl. Mater. Interfaces, 2023, 15, 11062–11075 CrossRef CAS PubMed.
- Q. Zhou, Y. Wang, T. Zhu, M. Lian, D. H. Nguyen and C. Zhang, Compos. Commun., 2023, 41, 101658 CrossRef.
- R. Jamil, L. C. Tomé, D. Mecerreyes and D. S. Silvester, Aust. J. Chem., 2021, 74, 767 CrossRef CAS.
- Q. Zhao, X. Yao, Q. Su, L. Deng, J. Chen, Y. Li, L. Dong, Z. Yang and W. Cheng, ChemCatChem, 2023, 15, e202300754 CrossRef CAS.
- D. Jiang, Y. He, J. Zhang, J. Yin, J. Ding, S. Wang and H. Li, J. Mol. Liq., 2023, 375, 121284 CrossRef CAS.
- J. Ding, P. Wang, Y. He, L. Cheng, X. Li, C. Fang, H. Li, H. Wan and G. Guan, Appl. Catal., B, 2023, 324, 122278 CrossRef CAS.
- S. Ravula, K. E. O'Harra, K. A. Watson and J. E. Bara, Membranes, 2022, 12, 264 CrossRef CAS PubMed.
- A. Ioannidi, C. Anastasopoulos, D. Vroulias, J. Kallitsis, T. Ioannides and V. Deimede, Sep. Purif. Technol., 2022, 280, 119790 CrossRef CAS.
- R. Soni and R. Biswas, Recent Innovations Chem. Eng., 2022, 15, 72–85 CAS.
- Q. Guo, C. Chen, F. Xing, W. Shi, J. Meng, H. Wan and G. Guan, ACS Omega, 2021, 6, 7186–7198 CrossRef CAS PubMed.
- L. Sun, M. Gao and S. Tang, Chem. Eng. J., 2021, 412, 128764 CrossRef CAS.
- R. V. Barrulas, C. López-Iglesias, M. Zanatta, T. Casimiro, G. Mármol, M. R. Carrott, C. A. García-González and M. C. Corvo, Int. J. Mol. Sci., 2021, 23, 200 CrossRef PubMed.
- A. Sattari, A. Ramazani, H. Aghahosseini and M. K. Aroua, J. CO2 Util., 2021, 48, 101526 CrossRef CAS.
- P. Migowski, P. Lozano and J. Dupont, Green Chem., 2023, 25, 1237–1260 RSC.
- R. Li, Y. Guo, G. Ma, D. T. Sun, J. Lin, T. Xue, R. Qiu, S. Yan, S. Yang, Y. Wang, Y. Hong, Y. Su, H. Wang, L. Peng and J. Li, Environ. Sci.: Nano, 2023, 10, 1232–1243 RSC.
- X. Lv, A. Lv, S. Tian, T. Xie and S. Sun, Eur. Polym. J., 2023, 182, 111713 CrossRef CAS.
- H. Zhang, D. Zhang, D. Zhang, X. Shao, T. Zhang, R. Wu and X. Ji, Langmuir, 2023, 39, 739–749 CrossRef CAS PubMed.
- J. Chen, D. Shi, Q. Wu, K. Chen, Y. Zhang, X. Xu and H. Li, Colloids Surf., A, 2023, 665, 131209 CrossRef CAS.
- H. Xu, A. Niu, Z. Yang, F. Wu, X. Guo, X. Wei and J. Zhang, Fuel, 2023, 334, 126762 CrossRef CAS.
- S. Shi, W. Wu, P. Liu and G. Xiao, ChemCatChem, 2023, 15, e202201274 CrossRef CAS.
- S. Miralles-Comins, M. Zanatta, A. F. Gualdrón-Reyes, J. Rodriguez-Pereira, I. Mora-Seró and V. Sans, Nanoscale, 2023, 15, 4962–4971 RSC.
- H. Mao, H. Yang, S. Zhang, J. Liu, D. Ma, S. Wu, D. Liu, H. Li, X.-M. Song and T. Ma, Catal. Today, 2022, 424, 113806 CrossRef.
- L. Luo, J. Li, X. Chen, X. Cao, Y. Liu, Z. Wu, X. Luo and C. Wang, Chem. Eng. J., 2022, 427, 131638 CrossRef CAS.
- F. Wang, D. Duan, M. Singh, C. M. Sutter-Fella, H. Lin, L. Li, P. Naumov and H. Hu, Energy Environ. Mater., 2023, 6, e12435 CrossRef CAS.
- H. Chen, G.-H. Zhang, Q.-H. Zhu, J. Fu, S. Qin, L. He and G.-H. Tao, ACS Appl. Mater. Interfaces, 2023, 15, 13637–13643 CrossRef CAS PubMed.
- P. Caprioglio, D. S. Cruz, S. Caicedo-Dávila, F. Zu, A. A. Sutanto, F. Peña-Camargo, L. Kegelmann, D. Meggiolaro, L. Gregori, C. M. Wolff, B. Stiller, L. Perdigón-Toro, H. Köbler, B. Li, E. Gutierrez-Partida, I. Lauermann, A. Abate, N. Koch, F. De Angelis, B. Rech, G. Grancini, D. Abou-Ras, M. K. Nazeeruddin, M. Stolterfoht, S. Albrecht, M. Antonietti and D. Neher, Energy Environ. Sci., 2021, 14, 4508–4522 RSC.
- J. Yang, W. Sheng, R. Li, L. Gong, Y. Li, L. Tan, Q. Lin and Y. Chen, Adv. Energy Mater., 2022, 12, 2103652 CrossRef CAS.
- S. Mariotti, D. Mantione, S. Almosni, M. Ivanović, T. Bessho, M. Furue, H. Segawa, G. Hadziioannou, E. Cloutet and T. Toupance, J. Mater. Chem. C, 2022, 10, 16583–16591 RSC.
- Y. Shen, G. Xu, J. Li, X. Lin, F. Yang, H. Yang, W. Chen, Y. Wu, X. Wu, Q. Cheng, J. Zhu, Y. Li and Y. Li, Angew. Chem., 2023, 135, e202300690 CrossRef.
- S.-Y. Zhang, Q. Zhuang, M. Zhang, H. Wang, Z. Gao, J.-K. Sun and J. Yuan, Chem. Soc. Rev., 2020, 49, 1726–1755 RSC.
- R. Wang, C. Fang, L. Yang, K. Li, K. Zhu, G. Liu and J. Chen, Small Sci., 2022, 2, 2200048 CrossRef CAS.
- S. Li, H. Lindsay, V. Mannari and J. Texter, J. Mol. Liq., 2023, 376, 121403 CrossRef CAS.
- M. Xu, H. Dou, Z. Zhang, Y. Zheng, B. Ren, Q. Ma, G. Wen, D. Luo, A. Yu, L. Zhang, X. Wang and Z. Chen, Angew. Chem., Int. Ed., 2022, 61, e202117703 CrossRef CAS PubMed.
- K. Xue, Y. Zhao, P. Lee and D. Y. W. Yu, Energy Environ. Mater., 2023, 6, e12395 CrossRef CAS.
- H. Niu, M. Ding, N. Zhang, X. Li, X. Su, X. Han, N. Zhang, P. Guan and X. Hu, Mater. Chem. Phys., 2023, 293, 126971 CrossRef CAS.
- S. Malunavar, A. Gallastegui, X. Wang, F. Makhlooghiazad, D. Mecerreyes, M. Armand, M. Galceran, P. C. Howlett and M. Forsyth, ACS Appl. Polym. Mater., 2022, 4, 8977–8986 CrossRef CAS.
- L. Ehrlich, D. Pospiech, U. L. Muza, A. Lederer, J. Muche, D. Fischer, P. Uhlmann, F. Tzschöckell, S. Muench, M. D. Hager and U. S. Schubert, Macromol. Chem. Phys., 2023, 224, 2200317 CrossRef CAS.
- J. Feng, Y. Wang, Y. Xu, H. Ma, G. Wang, P. Ma, Y. Tang and X. Yan, Adv. Mater., 2021, 33, 2100887 CrossRef CAS PubMed.
- X. Tang, X. Chang, B. Zhu, L. Cui, B. Jiang, F. Meng and G. Yan, Polym. Adv. Technol., 2022, 33, 4317–4329 CrossRef CAS.
- H.-F. Yu, C.-T. Li and K.-C. Ho, Sol. Energy Mater. Sol. Cells, 2023, 250, 112072 CrossRef CAS.
- D. R. Nosov, B. Ronnasi, E. I. Lozinskaya, D. O. Ponkratov, L. Puchot, P. Grysan, D. F. Schmidt, B. H. Lessard and A. S. Shaplov, ACS Appl. Polym. Mater., 2023, 5, 2639–2653 CrossRef CAS PubMed.
- S. Zafar and M. Imran, in Advanced Applications of Ionic Liquids, Elsevier, 2023, pp. 391–413 Search PubMed.
- Y. Zhang, H. Song, Z. Dai and Y. Xiong, J. Nanopart. Res., 2023, 25, 34 CrossRef CAS.
- S. Doblinger, C. E. Hay, L. C. Tomé, D. Mecerreyes and D. S. Silvester, Anal. Chim. Acta, 2022, 1195, 339414 CrossRef CAS PubMed.
- R. Biro, A. J. Daugulis and J. S. Parent, AIChE J., 2022, 68, e17676 CrossRef CAS.
- J. Alexiou, R. Biro, A. Tremblay, A. Daugulis and J. S. Parent, React. Funct. Polym., 2022, 181, 105412 CrossRef CAS.
- V. R. Zeger, D. S. Bell, J. S. Herrington and J. L. Anderson, J. Chromatogr. A, 2022, 1680, 463416 CrossRef CAS PubMed.
- K. Yavir, P. Eor, A. Kloskowski and J. L. Anderson, J. Sep. Sci., 2021, 44, 2620–2630 CrossRef CAS PubMed.
- M. Shahiri Haghayegh, N. Azizi, S. Seyyed Shahabi and Y. Gu, J. Mol. Liq., 2023, 387, 122677 CrossRef CAS.
- K. Foley, L. Condes and K. B. Walters, Mol. Syst. Des. Eng., 2023, Advance Article, 10.1039/D3ME00076A.
- F. He, B. Xue, X. Zhao and J. Yin, Polymer, 2023, 268, 125714 CrossRef CAS.
- M. Cotessat, D. Flachard, D. Nosov, E. I. Lozinskaya, D. O. Ponkratov, D. F. Schmidt, E. Drockenmuller and A. S. Shaplov, New J. Chem., 2021, 45, 53–65 RSC.
- T. Outerelo Corvo, A. Jourdain, S. O'Brien, F. Restagno, E. Drockenmuller and A. Chennevière, Macromolecules, 2022, 55, 4111–4118 CrossRef CAS.
- A. Matsumoto, R. Ukai, H. Osada, S. Sugihara and Y. Maeda, Macromolecules, 2022, 55, 10600–10606 CrossRef CAS.
- A. Matsumoto and A. Q. Shen, Soft Matter, 2022, 18, 4197–4204 RSC.
- N. N. Kalikin, A. L. Kolesnikov and Y. A. Budkov, Curr. Opin. Electrochem., 2022, 36, 101134 CrossRef CAS.
- Y. A. Budkov, N. N. Kalikin and A. L. Kolesnikov, Phys. Chem. Chem. Phys., 2022, 24, 1355–1366 RSC.
- J. Wang, Y. Liang, S. Huang, W. Jin, Z. Li, Z. Zhang, C. Ye, Y. Chen, P. Wei, Y. Wang and Y. Xia, Carbohydr. Polym., 2022, 280, 119009 CrossRef CAS PubMed.
- X. Deng, J. Tang, W. Guan, W. Jiang, M. Zhang, Y. Liu, H.-L. Chen, C.-L. Chen, Y. Li, K. Liu and Y. Fang, ACS Nano, 2022, 16, 5303–5315 CrossRef CAS PubMed.
- H. Chen, T. Cai, H. Li, X. Ruan, C. Jiao, R. Atkin, Y. Wang, P. Gong, X. Zhou, J. Yu and N. Jiang, Tribol. Int., 2023, 182, 108349 CrossRef CAS.
- O. Lebedeva, D. Kultin and L. Kustov, Nanomaterials, 2021, 11, 327 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |