Equilibrating the key parameters of thermally activated delayed fluorescence emitters towards efficient red/near-infrared OLEDs†
Jinming
Fan
ab,
Yulin
Xu
a,
Nengquan
Li
 a,
Jingsheng
Miao
a,
Changjiang
Zhou
c,
Tengxiao
Liu
d,
Minrong
Zhu
a and
Xiaojun
Yin
a,
Jingsheng
Miao
a,
Changjiang
Zhou
c,
Tengxiao
Liu
d,
Minrong
Zhu
a and
Xiaojun
Yin
 *a
*a
aShenzhen Key Laboratory of New Information Display and Storage Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518060, People's Republic of China. E-mail: xiaojunyin@szu.edu.cn
bCollege of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, People's Republic of China
cCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou, 310014, P. R. China
dCollege of Chemistry and Environmental Science, Wuhan Institute of Bioengineering, Wuhan, 430415, People's Republic of China
First published on 4th November 2022
Abstract
Equilibrating the critical parameters associated with the thermally activated delayed fluorescence process proposes the prerequisite for realizing high performance electroluminescence devices. Herein, precise manipulations relying on the model near-infrared emitter are successfully demonstrated, affording improved maximum external quantum efficiencies of 18.9% (@630 nm) and 12.6% (@680 nm).
Effective utilization of the triplet excitons in organic light-emitting diodes (OLEDs) is one of the fundamental issues to adapt to the commercial requirements of high device performances and durability1,2 since the radiative transition from triplet to ground states (S0) in purely organic emitters is typically spin-forbidden and excess triplet excitons are the prime factor for efficiency roll-offs as well.3–7 Recycling the dark-state triplet excitons into spin-allowed singlet excitons via the thermally activated reverse intersystem crossing (RISC) channel (i.e., thermally activated delayed fluorescence mechanism, TADF) can fully realize the potential of organic emitters in OLEDs.8–10 To pursue a high RISC rate constant (kRISC), a small singlet–triplet energy gap (ΔEST) between the lowest singlet state (S1) and triplet state (T1) is one of the basic principles.11 However, a small ΔEST requires highly separated frontier orbitals between the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs), which will inevitably bring about inefficient spin–orbit coupling (SOC) between the relevant excited states and small S1 → S0 transition dipole moments
 synchronously.11–13 Undoubtedly, an adequate balance among these key parameters (e.g., kRISC, ΔEST, and SOC values) that are associated with the TADF process proposes a reliable hypothesis to obtain desirable TADF emitters with high luminous efficiency, yet tremendous challenges to thoroughly equilibrate these parameters are still remaining.14–16 In particular, the trade-off relationships are more complicated in the long wavelength emission region due to the energy gap law;17–19 that is, the vibrational relaxation of non-radiative transition from electronically excited S1 or T1 to the zeroth vibration level of the S0 state is remarkably accelerated along with the narrowing of emission gaps.20–22
synchronously.11–13 Undoubtedly, an adequate balance among these key parameters (e.g., kRISC, ΔEST, and SOC values) that are associated with the TADF process proposes a reliable hypothesis to obtain desirable TADF emitters with high luminous efficiency, yet tremendous challenges to thoroughly equilibrate these parameters are still remaining.14–16 In particular, the trade-off relationships are more complicated in the long wavelength emission region due to the energy gap law;17–19 that is, the vibrational relaxation of non-radiative transition from electronically excited S1 or T1 to the zeroth vibration level of the S0 state is remarkably accelerated along with the narrowing of emission gaps.20–22
The development of red/near-infrared (NIR) TADF materials is urgently needed, arising from the wide application prospects in OLEDs, night-vision displays, bioimaging, phototherapy, telecommunications, etc.23–27 To date, multiple seminal works and new perspectives have been proposed to overcome the interference of key parameters in red/NIR-TADF emitters,28–32 including the incorporation of the non-adiabatic coupling effect,33,34 multi-resonance skeletons with shallow potential energy surfaces,20 intramolecular hydrogen-bonding,35 regulation of the locally excited and charge-transfer (CT) triplet state, etc.14,36 In view of the decisive role of energy gap law for red/NIR emitters, radiative decay rate (kr,s) of S1 is the primary concern.37,38 Within the typical donor–acceptor (D–A) type red/NIR TADF molecules, the triarylamine derivatives comprising free rotation phenyl groups commonly serve as the D component to endow them with low reorganization energy and high luminous efficiency.39–43 For example, Qiao et al. demonstrated a high photoluminescence quantum yield (ΦPL) of 97.4% (dpTPAAP) with an emission peak at 624 nm,33 Xu and co-workers showed an excellent ΦPL of 90% (pCNQ–TPA) with an emission peak even at 691 nm,44 and Ma et al. revealed a very high oscillator strength (f) of 0.72 (DTPS-PT) for the S0 → S1 excitations,45 while the drawbacks are a relatively large ΔEST and low kRISC resulting in an inferior device efficiency.46–48 With this in mind, precisely regulating the TADF parameters of triarylamine derivative based D–A type red/NIR emitters may offer a reliable means to further improve their electroluminescence (EL) performances.
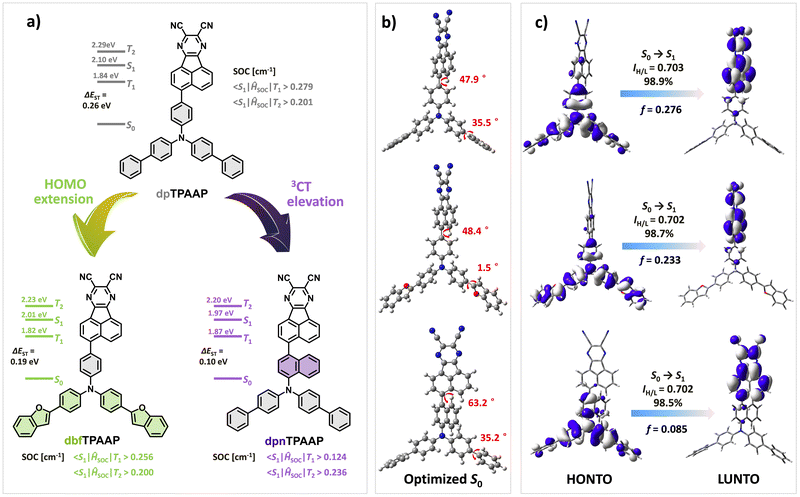
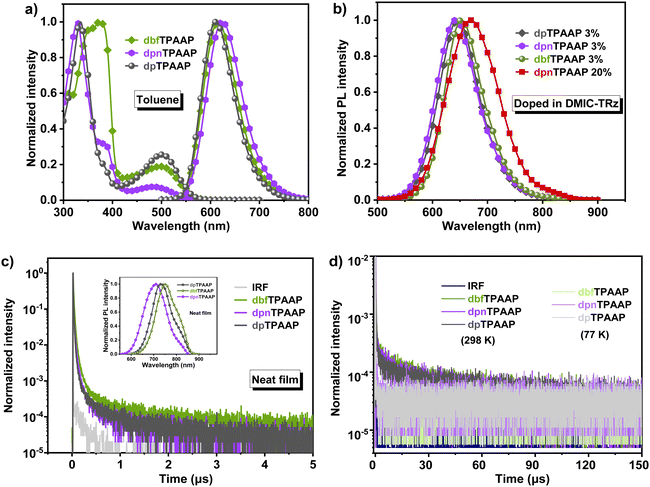
To highlight the fine-tuning strategy, two new triarylamine based D adopted with acenaphtho[1,2-b]pyrazine-8,9-dicarbonitrile A, namely, dbfTPAAP and dpnTPAAP were elaborately designed and synthesized (Fig. 1a). In comparison with the previously reported dpTPAAP,33 the altering of peripheral phenyl to benzofuryl on dbfTPAAP will be expected to extend their HOMO extension and afford enhanced intermolecular CT features, while the introduced naphthyl on dpnTPAAP will appropriately restrict the rotatable of π-bridge and output a smaller ΔEST and a higher kRISC. Theoretical calculations fully confirm that both dbfTPAAP and dpnTPAAP demonstrate more balanced parameters than the reference dpTPAAP, i.e., a small ΔEST, a high f and SOC value, which will facilitate a higher ΦPL and exciton utilization. As expected, both dbfTPAAP and dpnTPAAP show bright pure red emission (∼620 nm) in dilute toluene, along with an improved ΦPL of 81% and 86% for dbfTPAAP and dpnTPAAP (3 wt% in 5-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-7,7-dimethyl-5,7-dihydroindeno[2,1-b]-carbazole, DMIC-TRz matrix),49 respectively. According to the transient photoluminescence (PL) decay curves, compared to the primary dpTPAAP, the kRISC/kISC, and kr,s/nonradiative decay rate (knr,s) values are enhanced 22% and 45% for dbfTPAAP and 78% and 163% for dpnTPAAP, respectively, which implies a reinforced ability to harvest triplet excitons. EL devices employing them as red/NIR emitters were fabricated; compared to the 15.1% (@630 nm) maximum external quantum efficiency (EQEmax) and turn-on voltage (Von) of 2.4 V for the reference dpTPAAP, the EQEmax and Von were remarkably improved to 18.1% (@632 nm)/2.3 V, and 18.9% (@630 nm)/2.3 V for dbfTPAAP and dpnTPAAP, respectively. In addition, even at a high doping concentration of 20 wt%, a high EQEmax of 12.6% (@680 nm) can be achieved by dpnTPAAP due to the balanced TADF parameters.
Initially, density functional theory (DFT) calculations using the Gaussian 16 program package at the B3LYP(D3BJ)/Def2-SVP level were performed.50 As expected, all these three investigated TADF molecules show spatially separated HOMO and LUMO and resulting typical CT attribute of the S1 (Fig. 1c). With the introduction of benzofuryl, the HOMO distribution of dbfTPAAP was obviously extended to the periphery of the D moiety, affording a 0.07 eV rise of the HOMO level and equally narrowing of ΔEST (Fig. 1a and Fig. S26, ESI†), which were beneficial for the RISC process and broadened intermolecular CT interactions. Meanwhile, the calculated SOC values of dbfTPAAP between the excited S1 and T1 〈S1|ĤSOC|T1〉 (0.256 cm−1) or S1 and T2 〈S1|ĤSOC|T2〉 (0.200 cm−1) as well as the dihedral angle (48.4°) between the D and A segments (Fig. 1b), and f values (0.233) for S0 → S1 excitations (Fig. 1c) were comparable to those of the reference dpTPAAP. In stark contrast, the parameters relevant to the TADF path were remarkably changed with the altering of the π-bridge to naphthyl, i.e., a smaller ΔEST (0.10 eV), a higher T1 level (3CT, 1.87 eV), a larger dihedral angle (63.2°) but depressed SOC and f values (Fig. 1). To further investigate the impact on fluorescence efficiency along with the fine-tuning of TADF parameters, the Huang–Rhys factors (HRFs) at different vibration modes (v) were calculated using MOMAP in the DUSHIN module.51–53 As revealed in Fig. 2a, the dominated HRFs of S1 → S0 on dpTPAAP involve both low-frequency twisting vibration modes (v = 11.5 cm−1) and high-frequency stretching vibrations that are associated with the fluctuations of bond length within different segments (e.g., v = 581.4, 687.3 and 789.5 cm−1). Obviously, the introduction of the large steric hindrance naphthyl π-bridge can significantly restrain both the high- and low-frequency vibrations (Fig. 2b), and therefore the nonradiative energy consumptions of S1 can be substantially suppressed,54 while for dbfTPAAP, an additional swing vibration model (v = 51.4 cm−1) on the peripheral benzofuryl can be observed instead (Fig. 2c), indicating a negative effect to restrain the knr,s from this point of view.
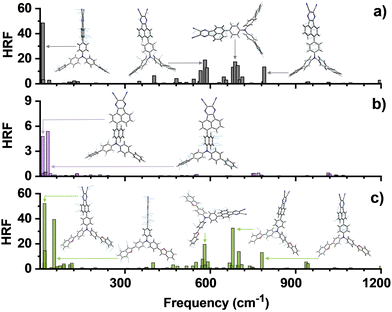 | ||
| Fig. 2 The HRFs versus frequencies, and the relatively characteristic vibration modes for S1 → S0 transition of (a) dpTPAAP, (b) dpnTPAAP and (c) dbfTPAAP. | ||
The synthetic routes to dbfTPAAP and dpnTPAAP are described in Scheme S1 (ESI†), and dpTPAAP was prepared according to a previous document.33 The key intermediates of 5-(4-(bis(4-(benzofuran-2-yl)phenyl)amino)phenyl)acenaphthylene-1,2-dione (A3) and 5-(5-(di([1,1′-biphenyl]-4-yl)amino)naphthalen-1-yl)acenaphthylene-1,2-dione (B3) were synthesized by using a modified Suzuki–Miyaura cross-coupling reaction with a considerable yield of 76% and 56%, respectively. Thereafter, the acid catalyzed cyclization between the A3/B3 and 2,3-diaminomaleonitrile yielded the target compounds of dbfTPAAP and dpnTPAAP with good yields of 67% and 58%, respectively. The chemical structures of dbfTPAAP and dpnTPAAP were thoroughly characterized with 1H NMR, 13C NMR, and high resolution mass spectrometry (HRMS, APCI) (Fig. S1–S25, ESI†). All these compounds display favorable thermal stability with high decomposition temperatures (Td, identified with 5% weight loss) of 489 °C (dbfTPAAP), 425 °C (dpnTPAAP) and 440 °C (dpTPAAP) according to the thermogravimetric analysis (Fig. S27, ESI†). Compared to the dpTPAAP (128 °C), the glass transition temperature (Tg) of dpnTPAAP was elevated to 184 °C, implying a more rigid structure conformation of the latter than the former (Fig. S28, ESI†). The experimental value of HOMO/LUMO levels was estimated from their onset potential of cyclic voltammetry curves (Fig. S29, ESI† and Table 1), i.e., −5.15 eV/−3.47 eV for dbfTPAAP, −5.21 eV/−3.47 eV for dpnTPAAP and −5.19 eV/−3.46 eV for dpTPAAP, which were in line with the general trend of DFT results.
| Emitter | λ abs [nm] | λ PL [nm] | E HOMO/ELUMOb [eV] | S1/T1c [eV] | ΔESTd [eV] | Φ PL [%] | Φ p [%] | Φ d [%] | τ p [ns] | τ d [μs] | k r,s [107 s−1] | k nr,s [106 s−1] | k ISC [107 s−1] | k RISC [104 s−1] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Measured in 1 × 10−4 mol L−1 toluene solutions. b Calculated from the onset potential of cyclic voltammetry curves in the oxidation or reduction process. c Estimated from the onset of the fluorescence and phosphorescence spectra at 77 K, respectively. d ΔEST = S1 − T1. e Total ΦPL (3 wt% doped in DMIC-TRz). f Φ PL contributions of the prompt component (p) and delayed (d) component, respectively. g The PL lifetimes of prompt (τp) and delayed (τd) decay components measured in 3 wt% doped DMIC-TRz film under an argon atmosphere. h Calculated kr,s and knr,s values from S1 to S0. i Calculated ISC (kISC) and RISC (kRISC) rate constants. | ||||||||||||||
| dbfTPAAP | 500 | 616 | −5.19/−3.46 | 2.26/2.16 | 0.10 | 81 | 49 | 32 | 20.6 | 48.7 | 1.9 | 4.5 | 2.5 | 2.6 |
| dpnTPAAP | 486 | 625 | −5.21/−3.47 | 2.27/2.23 | 0.04 | 86 | 64 | 22 | 34.3 | 51.1 | 1.6 | 2.6 | 1.0 | 1.9 |
| dpTPAAP | 501 | 611 | −5.15/−3.47 | 2.26/2.12 | 0.14 | 78 | 47 | 31 | 21.2 | 67.5 | 1.7 | 4.9 | 2.5 | 1.8 |
Fig. 3 demonstrates the single-crystal structure of the dbfTPAAP and corresponding packing patterns. The dihedral angle between the D and A moieties is 41.15°, in accord with the DFT results (Fig. 3a), but distinct results can be observed between the triphenylamine fragment and the peripheral benzofuryls, which can be understood in terms of the differentiated intermolecular interactions. As displayed in Fig. 3b, the measured π–π distance between the A plane and one of the benzofuryl substituted triphenylamine legs is as small as 3.30 Å, implying strong intermolecular CT interactions between the aforementioned two planes. In addition, the measured C–H⋯N distance between the cyano group of A and the benzofuryl of the other triphenylamine legs is as short as 2.64 Å, which is within the coverage of the hydrogen bond. Therefore, such staggered intermolecular interactions among different monomers offer an enhanced CT feature within the P21/n space groups (Fig. 3c).
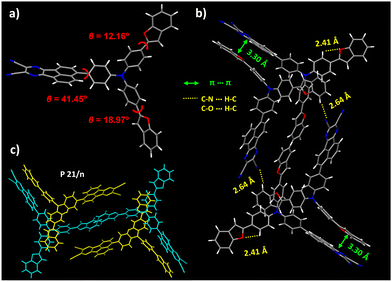 | ||
| Fig. 3 (a) Single-crystal structure of the dbfTPAAP with solvent molecules omitted, (b) packing patterns in one space unit and (c) highlighted with two different colors. | ||
The photophysical properties of these emitters both in dilute toluene and film states were investigated and the results are shown in Fig. 4 and Table 1. As revealed in Fig. 4a, all the three emitters demonstrate two distinct absorption bands, i.e., an intense high energy absorption band ranging from 300 to 400 nm assigned to the composition of π–π* and n–π* transitions of the D and A moieties, while the low energy absorption band at around 500 nm belongs to the typical intramolecular CT transitions from the D to the A segments Accordingly, an obvious redshift can be observed on these fluorescence emission spectra with the increase of solvent polarity (Fig. S30, ESI†), agreeing with the typical intermolecular CT transition feature. Compared to the dpTPAAP, the emission peaks of dbfTPAAP and dpnTPAAP in toluene were slightly red-shifted to 616 and 625 nm, respectively. All the three emitters demonstrate remarkably shifted emission peaks in neat films extending to the NIR region, but the peaks of both dbfTPAAP and dpTPAAP were larger than that of the dpnTPAAP due to the contribution of intermolecular CT transitions (inset of Fig. 4c). Steady state PL spectra of the doped films were collected in the DMIC-TRz matrix, and feedback deep-red emission of ∼650 nm at 3 wt%, NIR emission of ∼671 nm at 20 wt% (Fig. 4b).
To further investigate their TADF behaviors along with the fine-tuning of molecular parameters, transient PL decay curves of these emitters both in neat and doped films were measured. In comparison with the neat samples (Fig. 4c), all the three red/NIR TADF emitters distinctly exhibited a second-order exponential PL decay, consisting of a prompt component and a delayed component (Fig. 4d). The fitted lifetimes (at 298 K) of the prompt (τp) and delayed (τd) components are 20.6 ns and 48.7 μs for dbfTPAAP, 34.3 ns and 51.1 μs for dpnTPAAP, and 21.2 ns and 67.5 μs for dpTPAAP, respectively (Fig. 3d and Fig. S32, ESI†). As expected, the delayed fluorescence components of all the three samples were enhanced distinctly with the temperature increase from 77 K to 298 K (Fig. 4d), conforming to the typical TADF behaviors.40 Obviously, both the extension of HOMO delocalization and narrowing of the ΔEST value of the triphenylamine-based TADF molecules are beneficial for decreasing their τd values and therefore avoiding undesired quenching of triplet excitons in OLEDs. The ΦPL of 3 wt% doped DMIC-TRz was collected as well, and slightly enhanced ΦPL of 81% (dbfTPAAP) and 86% (dpnTPAAP) in contrast to the 78% (dpTPAAP) were obtained. According to the proportion of ΦPL in prompt (Φp) or delayed fluorescence (Φd), the kISC and kRISC of the three samples were estimated. Compared to the dpTPAAP, the kRISC and kRISC/kISC values of both dbfTPAAP and dpnTPAAP were remarkably optimized. In addition, the kr,s and kr,s/knr,s values are improved synchronously (Table 1), indicating a successful strategy via parameter optimization as well as preferable potential to adapt to the application in OLEDs.
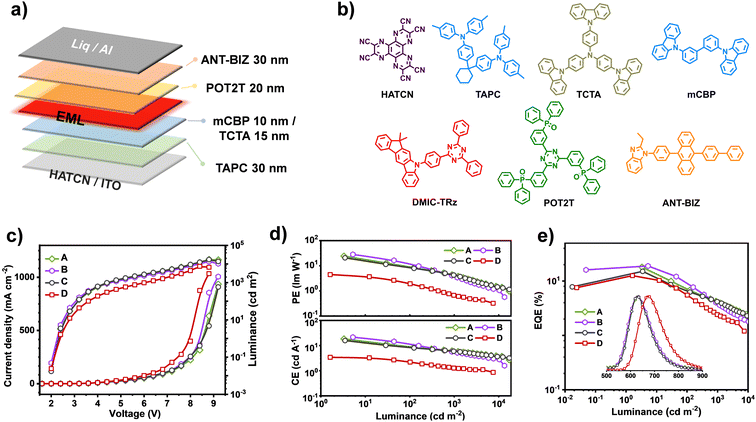
To evaluate the EL properties of these red/NIR TADF emitters, vacuum evaporated multilayer OLEDs with the configuration of “ITO/HATCN (5 nm)/TAPC (30 nm)/TCTA (15 nm)/mCBP (10 nm)/EMLs (30 nm)/POT2T (20 nm)/ANT-BIZ (30 nm)/Liq (2 nm)/Al (100 nm)” were fabricated (Fig. 5a). Herein, the dipyrazino[2,3-f:2′,3′-h]quinoxaline-2,3,6,7,10,11-hexacarbonitrile (HATCN) with a deep LUMO level was incorporated as the hole injection layer, the 4,4′,4′′-tris(carbazol-9-yl)triphenyl-amine (TCTA) and 1,1-bis((di-4-tolylamino)phenyl)-cyclohexane (TAPC) were employed as hole-transporting layers, and 1-(4-(10-([1,1′-biphenyl]-4-yl)anthracen-9-yl)phenyl)-2-ethyl-1H-benzo[d]imidazole (ANT-BIZ) was used as the electron-transporting layer. The 3,3-di(9H-carbazol-9-yl)biphenyl (mCBP) and (1,3,5-triazine-2,4,6-triyl)tris(benzene-3,1-diyl)tris(diphenylphosphineoxide) (POT2T) were introduced as electron/hole blocking layers, respectively (Fig. 5b). The EMLs were composed of 3 wt% dbfTPAAP, 3 wt% dpnTPAAP, 3 wt% dpTPAAP or 20 wt% dpnTPAAP in the DMIC-TRz matrix, denoted as devices A, B, C and D respectively.
As revealed in Table 2, devices A–C with the same low dopant concentrations (3 wt% in DMIC-TRz) all display pure red-emission (EL peaks ∼630 nm). Among the three devices, owing to the more balanced TADF parameters of the dbfTPAAP and dpnTPAAP than the dpTPAAP, the CEmax, PEmax, and EQEmax of devices A and B are obviously improved in contrat to the device C, which comforms to the higher key TADF parameters (kRISC, kRISC/kISC, kr,s, kr,s/knr,s and ΦPL) of the former two than the latter one. The optimal device performance was obtained by device B (dpnTPAAP) with an EQEmax of 18.9%, a CEmax of 21.6 cd A−1 and a PEmax of 28.2 lm W−1, which represented a 25%, 37% and 36% improvement accordingly with reference to the device C (dpTPAAP). Moreover, slightly decreased Von (0.1 V) of the devices A and B compared to the C can be observed as well (Fig. 5c), which may originate from the better matched energy levels or more balanced hole/electron transport of the dbfTPAAP and dpnTPAAP than the primary dpTPAAP. With the increase of loading of dpnTPAAP (20 wt% in DMIC-TRz, device D), the ELpeak significantly shifted to the deep-red region (680 nm), and a considerably high EQEmax of 12.6% can be maintained as well (Fig. 5d). Notably, all these devices demonstrate obvious efficiency roll-off at high brightness (Table 2), which can be ascribed to the typical triplet–triplet annihilation mechanism.46 In addition, non-doped devices by employing the two new emitters were fabricated as well, and the emission peaks were successfully extended to the NIR region (∼780 nm) together with a considerable EQEmax of around 1% (Fig. S33, ESI†).
| Devices | V on [V] | L max [cd m−2] | CEmaxc [cd A−1] | PEmaxd [Im W−1] | EQEe [%] | ELpeakf [nm] |
|---|---|---|---|---|---|---|
| a V on, turn-on voltage at 1 cd m−2. b L max, the maximum luminance of the devices. c CEmax, the maximum current efficiency. d PEmax, the maximum power efficiency. e EQE values, following the sequence of maximum, at 100 cd m−2, and at 1000 cd m−2,respectively. f The EL emission peaks at the same driving voltage of 2.8 V. | ||||||
| A | 2.3 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 355 355 |
18.5 | 24.3 | 18.1/9.1/4.2 | 632 |
| B | 2.3 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557 557 |
21.6 | 28.2 | 18.9/7.2/3.9 | 630 |
| C | 2.4 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 355 355 |
15.8 | 20.7 | 15.1/6.6/4.1 | 630 |
| D | 2.4 | 7523 | 3.4 | 4.5 | 12.6/5.6/2.4 | 680 |
Conclusions
In conclusion, two new triarylamine based D–A type red/NIR TADF emitters with fine-tuning molecular parameters were elaborately designed and synthesized. In comparison with the model molecule of dpTPAAP, the incorporation of planar electron-rich end-capping groups (i.e., benzofuryl) at the periphery of triarylamine legs can efficiently enlarge the HOMO distributions and subsequently strengthen their intermolecular CT interactions, while the suppression of nonradiative energy consumptions and synchronous increase of kRISC can be realized by introducing a block π-bridge (i.e., naphthyl). Notably, the fine-tuning strategy offers a directed way to predictably equilibrate the key TADF parameters and obtain desirable red/NIR TADF emitters with improved device performances. Consequently, EL devices employing these red/NIR emitters were fabricated, and an EQEmax approaching 20% (@ 630 nm, with 25% improvement in contrast to the reference one), or 12.6% (@680 nm) was achieved.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the Natural Science Foundation of China (No. 51803124, 51903158 and 51903252), the Shenzhen Science and Technology Program (No. KQTD20170330110107046 and ZDSYS 20210623091813040), and Natural Science Foundation of Hubei Provincial (2021CFB398). The authors also thank the Instrumental Analysis Center of Shenzhen University for Analytical Support. They thank Kui Gong, Yibin Hu and Yin Wang (all from HZWTECH) for help and discussions regarding this study.References
- Y. Xu, P. Xu, D. Hu and Y. Ma, Chem. Soc. Rev., 2021, 50, 1030–1069 RSC.
- J. Song, H. Lee, E. G. Jeong, K. C. Choi and S. Yoo, Adv. Mater., 2020, 32, 1907539 CrossRef CAS PubMed.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Brase, Adv. Mater., 2021, 33, 2005630 CrossRef CAS PubMed.
- P. Jiang, J. Miao, X. Cao, H. Xia, K. Pan, T. Hua, X. Lv, Z. Huang, Y. Zou and C. Yang, Adv. Mater., 2022, 34, 2106954 CrossRef CAS PubMed.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- X. Yin, G. Xie, Y. Peng, B. Wang, T. Chen, S. Li, W. Zhang, L. Wang and C. Yang, Adv. Funct. Mater., 2017, 27, 1700695 CrossRef.
- X. Yin, T. Zhang, Q. Peng, T. Zhou, W. Zeng, Z. Zhu, G. Xie, F. Li, D. Ma and C. Yang, J. Mater. Chem. C, 2015, 3, 7589–7596 RSC.
- Y. J. Yu, X. Q. Wang, J. F. Liu, Z. Q. Jiang and L. S. Liao, iScience, 2021, 24, 102123 CrossRef CAS PubMed.
- Y. Liu, C. Li, Z. Ren, S. Yan and M. R. Bryce, Nat. Rev. Mater., 2018, 3, 18020 CrossRef CAS.
- X. Cai, J. Xue, C. Li, B. Liang, A. Ying, Y. Tan, S. Gong and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, 202200337 Search PubMed.
- X. K. Chen, D. Kim and J. L. Bredas, Acc. Chem. Res., 2018, 51, 2215–2224 CrossRef CAS PubMed.
- P. K. Samanta, D. Kim, V. Coropceanu and J. L. Bredas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- X.-K. Chen, S.-F. Zhang, J.-X. Fan and A.-M. Ren, J. Phys. Chem. C, 2015, 119, 9728–9733 CrossRef CAS.
- U. Balijapalli, Y. T. Lee, B. S. B. Karunathilaka, G. Tumen-Ulzii, M. Auffray, Y. Tsuchiya, H. Nakanotani and C. Adachi, Angew. Chem., Int. Ed., 2021, 60, 19364–19373 CrossRef CAS PubMed.
- S. Kothavale, W. J. Chung and J. Y. Lee, J. Mater. Chem. C, 2022, 10, 6043–6049 RSC.
- Y. Liu, J. Yang, Z. Mao, X. Chen, Z. Yang, X. Ge, X. Peng, J. Zhao, S. J. Su and Z. Chi, ACS Appl. Mater. Interfaces, 2022, 14, 33606–33613 CrossRef PubMed.
- Y.-C. Wei, S. F. Wang, Y. Hu, L.-S. Liao, D.-G. Chen, K.-H. Chang, C.-W. Wang, S.-H. Liu, W.-H. Chan, J.-L. Liao, W.-Y. Hung, T.-H. Wang, P.-T. Chen, H.-F. Hsu, Y. Chi and P.-T. Chou, Nat. Photonics, 2020, 14, 570–577 CrossRef CAS.
- K. Zhang, X. Zhang, J. Fan, Y. Song, J. Fan, C. K. Wang and L. Lin, J. Phys. Chem. Lett., 2022, 13, 4711–4720 CrossRef CAS PubMed.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS PubMed.
- B. Zhao, H. Wang, C. Han, P. Ma, Z. Li, P. Chang and H. Xu, Angew. Chem., Int. Ed., 2020, 59, 19042–19047 CrossRef CAS PubMed.
- K. Tuong, Ly, R.-W. Chen-Cheng, H.-W. Lin, Y.-J. Shiau, S.-H. Liu, P.-T. Chou, C.-S. Tsao, Y.-C. Huang and Y. Chi, Nat. Photonics, 2016, 11, 63–68 CrossRef.
- A. Zampetti, A. Minotto and F. Cacialli, Adv. Funct. Mater., 2019, 29, 1807623 CrossRef.
- J. H. Kim, J. H. Yun and J. Y. Lee, Adv. Opt. Mater., 2018, 6, 1800255 CrossRef.
- Y. J. Yu, Y. Hu, S. Y. Yang, W. Luo, Y. Yuan, C. C. Peng, J. F. Liu, A. Khan, Z. Q. Jiang and L. S. Liao, Angew. Chem., Int. Ed., 2020, 59, 21578–21584 CrossRef CAS PubMed.
- H. Y. Zhang, H. Y. Yang, M. Zhang, H. Lin, S. L. Tao, C. J. Zheng and X. H. Zhang, Mater. Horiz., 2022, 9, 2425–2432 RSC.
- U. Balijapalli, R. Nagata, N. Yamada, H. Nakanotani, M. Tanaka, A. D'Aléo, V. Placide, M. Mamada, Y. Tsuchiya and C. Adachi, Angew. Chem., Int. Ed., 2021, 60, 8477–8482 CrossRef CAS PubMed.
- J. X. Chen, W. W. Tao, W. C. Chen, Y. F. Xiao, K. Wang, C. Cao, J. Yu, S. Li, F. X. Geng, C. Adachi, C. S. Lee and X. H. Zhang, Angew. Chem., Int. Ed., 2019, 58, 14660–14665 CrossRef CAS PubMed.
- J. X. Chen, H. Wang, Y. F. Xiao, K. Wang, M. H. Zheng, W. C. Chen, L. Zhou, D. Hu, Y. Huo, C. S. Lee and X. H. Zhang, Small, 2022, 18, 2201548 CrossRef CAS PubMed.
- P. Data, P. Pander, M. Okazaki, Y. Takeda, S. Minakata and A. P. Monkman, Angew. Chem., Int. Ed., 2016, 55, 5739–5744 CrossRef CAS PubMed.
- Y. Zhang, Y. Wang, J. Song, J. Qu, B. Li, W. Zhu and W.-Y. Wong, Adv. Opt. Mater., 2018, 6, 1800466 CrossRef.
- J. Fan, J. Miao, N. Li, Y. Zeng, C. Ye, X. Yin and C. Yang, J. Mater. Chem. C, 2022, 10, 10255–10261 RSC.
- J. Xue, J. Xu, J. Ren, Q. Liang, Q. Ou, R. Wang, Z. Shuai and J. Qiao, Sci. China: Chem., 2021, 64, 1786–1795 CrossRef CAS.
- D.-H. Kim, A. D’Aléo, X.-K. Chen, A. D. S. Sandanayaka, D. Yao, L. Zhao, T. Komino, E. Zaborova, G. Canard, Y. Tsuchiya, E. Choi, J. W. Wu, F. Fages, J.-L. Brédas, J.-C. Ribierre and C. Adachi, Nat. Photonics, 2018, 12, 98–104 CrossRef CAS.
- Z. Cai, X. Wu, H. Liu, J. Guo, D. Yang, D. Ma, Z. Zhao and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 23635–23640 CrossRef CAS PubMed.
- J. X. Chen, Y. F. Xiao, K. Wang, D. Sun, X. C. Fan, X. Zhang, M. Zhang, Y. Z. Shi, J. Yu, F. X. Geng, C. S. Lee and X. H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2478–2484 CrossRef CAS PubMed.
- U. Balijapalli, R. Nagata, N. Yamada, H. Nakanotani, M. Tanaka, A. D'Aleo, V. Placide, M. Mamada, Y. Tsuchiya and C. Adachi, Angew. Chem., Int. Ed., 2021, 60, 8477–8482 CrossRef CAS PubMed.
- Y. Xiao, H. Wang, Z. Xie, M. Shen, R. Huang, Y. Miao, G. Liu, T. Yu and W. Huang, Chem. Sci., 2022, 13, 8906–8923 RSC.
- Y. Y. Wang, K. N. Tong, K. Zhang, C. H. Lu, X. Chen, J. X. Liang, C. K. Wang, C. C. Wu, M. K. Fung and J. Fan, Mater. Horiz., 2021, 8, 1297–1303 RSC.
- Y. L. Zhang, Q. Ran, Q. Wang, Y. Liu, C. Hanisch, S. Reineke, J. Fan and L. S. Liao, Adv. Mater., 2019, 31, 1902368 CrossRef CAS PubMed.
- J. Liu, Z. Li, T. Hu, T. Gao, Y. Yi, P. Wang and Y. Wang, Adv. Opt. Mater., 2022, 10, 2102558 CrossRef CAS.
- M. Zhao, M. Li, W. Li, S. Du, Z. Chen, M. Luo, Y. Qiu, X. Lu, S. Yang, Z. Wang, J. Zhang, S. J. Su and Z. Ge, Angew. Chem., Int. Ed., 2022, 202210687 Search PubMed.
- D. G. Congrave, B. H. Drummond, P. J. Conaghan, H. Francis, S. T. E. Jones, C. P. Grey, N. C. Greenham, D. Credgington and H. Bronstein, J. Am. Chem. Soc., 2019, 141, 18390–18394 CrossRef CAS PubMed.
- Z. Li, D. Yang, C. Han, B. Zhao, H. Wang, Y. Man, P. Ma, P. Chang, D. Ma and H. Xu, Angew. Chem., Int. Ed., 2021, 60, 14846–14851 CrossRef CAS PubMed.
- J. Jiang, Z. Xu, J. Zhou, M. Hanif, Q. Jiang, D. Hu, R. Zhao, C. Wang, L. Liu, D. Ma, Y. Ma and Y. Cao, Chem. Mater., 2019, 31, 6499–6505 CrossRef CAS.
- H.-Y. Yang, H.-y Zhang, M. Zhang, X.-c Fan, H. Lin, S.-L. Tao, C.-J. Zheng and X.-H. Zhang, Chem. Eng. J., 2022, 448, 137717 CrossRef CAS.
- J. Xue, Q. Liang, R. Wang, J. Hou, W. Li, Q. Peng, Z. Shuai and J. Qiao, Adv. Mater., 2019, 31, 1808242 CrossRef PubMed.
- C. Li, R. Duan, B. Liang, G. Han, S. Wang, K. Ye, Y. Liu, Y. Yi and Y. Wang, Angew. Chem., Int. Ed., 2017, 56, 11525–11529 CrossRef CAS PubMed.
- X. Lv, R. Huang, S. Sun, Q. Zhang, S. Xiang, S. Ye, P. Leng, F. B. Dias and L. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 10758–10767 CrossRef CAS PubMed.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, J. R. C. M. A. Robb, G. Scalmani, V. Barone, H. N. G. A. Petersson, X. Li, M. Caricato, A. V. Marenich, B. G. J. J. Bloino, R. Gomperts, B. Mennucci, H. P. Hratchian, A. F. I. J. V. Ortiz, J. L. Sonnenberg, D. Williams-Young, F. L. F. Ding, F. Egidi, J. Goings, B. Peng, A. Petrone, D. R. T. Henderson, V. G. Zakrzewski, J. Gao, N. Rega, W. L. G. Zheng, M. Hada, M. Ehara, K. Toyota, R. Fukuda, M. I. J. Hasegawa, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, K. T. T. Vreven, J. A. Montgomery Jr., J. E. Peralta, M. J. B. F. Ogliaro, J. J. Heyd, E. N. Brothers, K. N. Kudin, T. A. K. V. N. Staroverov, R. Kobayashi, J. Normand, A. P. R. K. Raghavachari, J. C. Burant, S. S. Iyengar, M. C. J. Tomasi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, R. L. M. J. W. Ochterski, K. Morokuma, O. Farkas and A. D. J. F. J. B. Foresman, Gaussian 16, Revision A.03, Gaussian, Inc.: Wallingford CT, 2016 Search PubMed.
- Z. Shuai, Chin. J. Chem., 2020, 38, 1223–1232 CrossRef CAS.
- Z. Shuai and Q. Peng, Nat. Sci. Rev., 2017, 4, 224–239 CrossRef CAS.
- Z. Shuai and Q. Peng, Phys. Rep., 2014, 537, 123–156 CrossRef CAS.
- J. Fan, Y. Zhang, Y. Zhou, L. Lin and C.-K. Wang, J. Phys. Chem. C, 2018, 122, 2358–2366 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General information, synthetic procedures, characterization data, NMR and HRMS spectra, theoretical calculations, PL spectra, crystal and device data. CCDC 2184275. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2tc03933h |
| This journal is © The Royal Society of Chemistry 2022 |