From green to blue economy: Marine biorefineries for a sustainable ocean-based economy
Nathalia Vieira
Veríssimo
 a,
Cassamo Ussemane
Mussagy
a,
Cassamo Ussemane
Mussagy
 a,
Ariane Alves
Oshiro
b,
Carlos Miguel Nóbrega
Mendonça
a,
Valéria de Carvalho
Santos-Ebinuma
b,
Adalberto
Pessoa
Júnior
a,
Ricardo Pinheiro de Souza
Oliveira
a,
Ariane Alves
Oshiro
b,
Carlos Miguel Nóbrega
Mendonça
a,
Valéria de Carvalho
Santos-Ebinuma
b,
Adalberto
Pessoa
Júnior
a,
Ricardo Pinheiro de Souza
Oliveira
 a and
Jorge Fernando Brandão
Pereira
a and
Jorge Fernando Brandão
Pereira
 *bc
*bc
aSchool of Pharmaceutical Sciences, São Paulo University (USP), Av. Prof. Lineu Prestes, no. 580, B16, 05508-000, Cidade de Universitária, São Paulo, SP, Brazil
bDepartment of Engineering of Bioprocesses and Biotechnology, School of Pharmaceutical Sciences, São Paulo State University (UNESP), Rodovia Araraquara-Jaú/Km 01, 14800-903, Araraquara, SP, Brazil
cUniv Coimbra, CIEPQPF, Department of Chemical Engineering, Rua Sílvio Lima, Pólo II – Pinhal de Marrocos, 3030-790 Coimbra, Portugal. E-mail: jfbpereira@eq.uc.pt; Tel: +351 239 798 726
First published on 9th November 2021
Abstract
Despite being a vital asset for global sustenance and economy, ocean aquatic ecosystems are in danger due to the effects of incorrect management of their resources, pollution, and climate change. Considering the seafood industry discards half of its fish-product mass in the ocean, a proper valorization of its residues would decrease not only the ocean contamination but also improve the management of marine resources and increase the sector competitiveness. With these goals in mind, ocean-based industries are adopting new sustainable production models, similar to biorefineries, which are effective for waste valorization, namely, converting low-value biomass into commercially relevant by-products. Based on a deeper knowledge of aquatic feedstocks, the development and implementation of a marine biorefinery can be fundamental to consolidate a “greener” socioeconomic development, similar to that observed in green chemistry. However, biorefineries are sophisticated multi-step systems with numerous feedstocks and commodities. Therefore, their implementation requires expertise in all stages of manufacturing, in addition to a clear vision of all raw materials, residues, and products. In this sense, with this perspective, we provide an initial overview of the current state-of-the-art on marine biorefineries and the sources and applications of their by-products. Afterward, we suggest how to integrate green chemistry and blue economy principles into ocean-based industries, aiming to support a more sustainable, profitable, and conscious ocean economy.
Introduction
The ocean is essential for our survival: it covers 70% of our planet, is the source of half of our oxygen, and is responsible for 17% of animal protein production.1 However, the degradation of aquatic ecosystems is happening at an alarming rate, and there are not enough actions to change this grim outlook. For example, about 35 million tons of fish caught (35% of the total) are discarded into the ocean every year.2 This is not only an irrational wastage of marine resources, but it also pollutes and endangers our aquatic environments. Hence, it is crucial to design smart and sustainable production systems to protect our oceans while preserving the capacity of marine industries to generate jobs and supply food, especially by protecting these vital assets for future generations. In this sense, green and blue economies can provide the tools required for this paradigm shift in ocean-based industrial operations. One model that follows green chemistry precepts and could reduce seafood wastage and pollution is the “biorefinery”, considering it can convert low-value biomass into beneficial and valuable by-(bio)products.3By-products are any secondary substances in a manufacturing process that are not its main commodity, which can be considered marketable compounds or simply waste.4 An industrial residue with the same composition can be discarded as waste or used as a source of commercially relevant by-products by different sectors. Hence, no waste or residue is truly “worthless”, and it is an ecological and financial loss to discard by-products without harnessing their complete potential as a secondary source of raw material.
Nowadays, each industry's processing capacities, values, and sector regulations dictate how their residues are managed. For example, the energy field already has many successful examples of biorefineries for producing biofuels from residues.5 Ideally, more companies should apply a biorefinery approach to harness the most out of their raw materials, reducing wastage and environmental degradation.
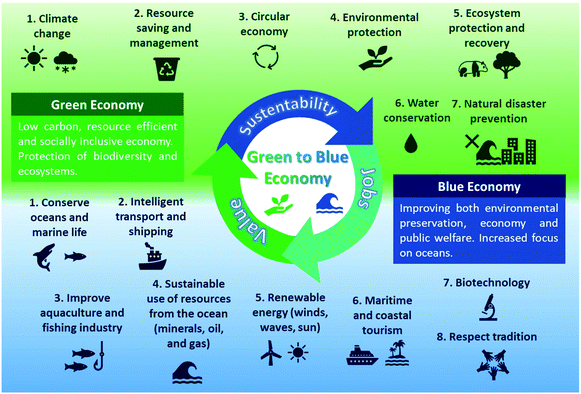
The development of cost-effective, environmentally friendly, and circular technologies for marine-based biomass processing into value-added products are decisive for successfully implementing sustainable ocean-based industries, focusing on strategies that simultaneously contribute to decreasing waste and energetic demand.6,7 By employing a biorefinery concept for marine industries, it is possible to save natural resources and avoid the production of seafood waste while creating jobs and increasing profits. This model embodies the blue economy principles of preserving marine environments while improving the economy and public welfare. Having these principles in mind, in this perspective, we will introduce concepts of the ocean economy along with those of the blue and green economies, while presenting the potential of marine biorefineries for seafood waste valorization. We will also pinpoint sources and applications of seafood waste by-products and discuss the state-of-the-art on marine biorefineries. Finally, we will examine the potential and trends in this field and offer our perspective on the next steps required to spread blue economy practices.
The ocean economy
The ocean economy includes all commercial ocean-based activities, with a mix of well-established and emerging industries.1,8 Established ocean industries operate on different sectors, such as food (e.g., industrial capture of fish and seafood processing), transport (e.g., maritime transport and shipping, port activities), construction (e.g., shipbuilding and marine manufacture and construction), marine education and research, business, tourism (maritime and coastal), energy (shallow-water offshore oil and gas drilling) and dredging.1 As for emerging ocean industries, they apply new technologies to solve current problems and reduce environmental risks. For example, to boost seafood supply and face an increase in demand due to exponential population growth, the fishing industry designed an intensive production model known as aquaculture (controlled cultivation of aquatic organisms).9 The development of modern technologies to extract deep and ultra-deep oil and gas as well as for marine and seabed mining are also helping to prevent shortages of these non-renewable compounds. For example, offshore crude oil and natural gas already represent 30% of this market.10 In addition, exploiting the potential of the sea for the production of renewable energy from waves and wind could help with the growing demand for sustainable energy sources.11 There is also great interest in exploring marine biomolecules using biotechnology for medical and industrial applications due to the vast biodiversity present in the ocean. Furthermore, considering that 80% of the volume of products is transported by sea, maritime safety and surveillance and maritime technology are gaining importance in our globalized commerce.12 To elucidate key areas of the ocean economy, Fig. 1 includes the established and emerging sectors of ocean-based industries defined by the Organization for Economic Cooperation and Development (OECD).12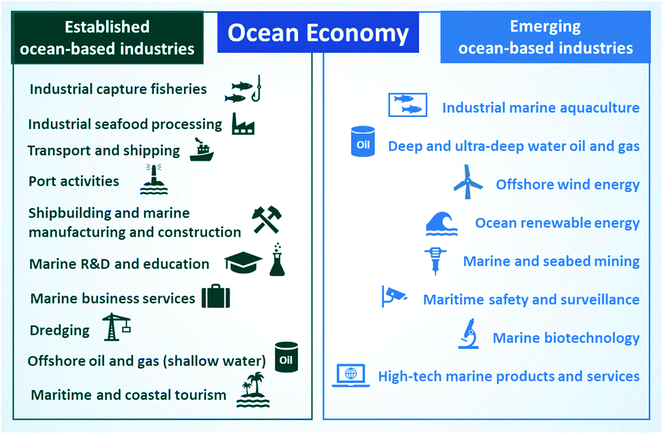 | ||
| Fig. 1 Established and emerging ocean-based industries. Image produced by the authors with Information from OECD.13 | ||
As illustrated in Fig. 1, the ocean economy encompasses and impacts a wide range of industrial and commercial areas. In addition to these traditional fields recognized by OECD, the sea is also used for certain irregular activities, such as piracy and illicit trafficking of people and illegal products. These irregular actions mainly stem from the difficulty of prosecuting and defining jurisdictions for offshore crimes, which facilitate their expansion and turn them into a global concern.14 Interestingly, along with the relevance of traditional sectors, emerging ocean-based industries already have an extensive impact on the economy, supply of food, and extraction of natural resources. The best example is aquaculture, which already accounts for half of seafood production and is the fastest-growing food sector today.9
The ocean economy is so relevant that, if it were a country, it would be the 8th largest economy in 2021, with an annual gross domestic product (GDP) of USD 2.5 trillion.1,15 Moreover, the World-Wide Fund for Nature (WWF) has estimated the value of major ocean assets to be at least USD 24 trillion.1 The impressive value of the oceans is not only economic, but also social, as the fishing industry employs around 200 million people in the capture, harvesting, and processing of fish products, and provides more than 17% of animal protein worldwide.16 From 1990 to 2018, there was a 122% rise in total fish products consumption, propelling a 14% and 527% increase in fish capture and aquaculture, respectively, producing 200 million tons of seafood per year.2 Most of the seafood production is still employed for direct human consumption. In 2018, from 178.6 million tons (live weight) of seafood, 156.4 million tons were used for food products (87.6%), while 22.2 million tons (12.4%) were applied for non-food purposes (i.e., not used for human consumption).2,17 Of the food segment, 44% comprised fresh or chilled fish.2 About non-food products, 80% was reduced to fish meal and oil, while the rest was applied in different sectors, from pharmaceutical and nutraceutical formulations to ornamental fish.2,18 Since FAO members adopted the Code of Conduct for Responsible Fisheries in 1995, the seafood industry is progressing towards more sustainable practices.2,19 For example, 25 to 35% of fish meal and oil are already produced from fish by-products.2 However, the seafood industry still discards countless residues rich in high-value biomolecules that could be valorized and commercialized with efficient processing.
Above all, it is crucial to emphasize the environmental relevance of the seas, which play a leading role in our survival. For example, aquatic ecosystems generate 50% of the oxygen and absorb 30% of the carbon dioxide (CO2) emissions on Earth.1 Therefore, the ocean is a vital asset for global livelihoods and economy, providing reliable sources of food, energy, chemicals, medicines, transport, tourism, and jobs. However, while the ocean's vastness makes it seem unwavering, growing and uncontrolled human activity at sea, ocean waste disposal, and climate change are increasingly putting pressure on “our oceans”, quickly showing the true fragility of “our aquatic ecosystems”.
The excessive extraction of ocean resources and sea pollution is rapidly degrading marine environments, especially destroying these natural biodiversity hotspots. For example, in 1990, 90% of fish stocks were within sustainable biological levels.2 However, these levels declined to an alarming 65.8% in 2017.2 This figure is even more worrying because, according to the Food and Agriculture Organization (FAO), 35% of global fish catches are discarded dead or dying in the ocean.2 There is also the problem of disposal of fish waste in marine environments, which can reduce oxygen levels in seawater, bury or smother sea life, and introduce diseases and invasive or non-native species to the aquatic ecosystems.20 Considering that nearly 70% of the production of marine products depends on a healthy ocean, if no action is taken soon, ocean-based industries and related activities will face a severe crisis in the recent future.1
To counter both of these issues, we believe that creating marine biorefineries for the sustainable valorization of seafood waste/residues can save ocean resources and maximize revenues, as well as, at least partially, reduce marine pollution and minimize the negative environmental impact of human activity. Considering that ocean-based industries discard more than half of seafood mass during processing, there is a wide variety of waste materials that can be used to generate value-added products, namely: algae biomass residues, fish frames, heads, fins, scales, viscera and bones, and marine invertebrates shells, viscera, and skin.21 Therefore, by applying green economy principles and strategies to reuse fishery waste, it would be possible to reduce the environmental problems associated with fishing and aquaculture while increasing revenue and creating jobs.
The green and blue economies
The term “green chemistry” was effectively proposed by Paul Anastas in 1990, during his position as a staff chemist at the US Environmental Protection Agency's (EPA).22 However, concerns about the impacts of industries (mainly the chemical, agricultures, and arms sectors) on the environment emerged around 1950.23 At that time, the main ecological issues were the eutrophication and pollution of potable water sources by synthetic detergents and industrial residues. The environmental impact of detergents was first demonstrated after the cleaning products manufacturer company Henkel began monitoring its surfactant concentrations in the Rhine River (Germany) to assess the biodegradability of its products.24 The discovery of the harmful environmental impacts of synthetic (crude oil-based) tetrapropylenebenzene sulphonate, together with an increased environmental awareness of consumers, initiated the chemical industry's “green shift” to the development and implementation of environmentally-friendly products and processes.23 These efforts also evolved and led to the creation of principles and guidelines to prevent pollution and environmental risks, such as: “Twelve Principles of Green Chemistry” published in 1998 by Paul Anastas and John C. Warner; “The Responsible Care® Global Charter” from the International Council of Chemical Associations (ICCA).23,25,26 These green chemistry principles began to be actively applied in the chemical industry, leading to the effective establishment of the concept of “green economy”.The United Nations (UN) Environment Program defines the green economy as a “low carbon, resource-efficient, and socially inclusive” economy.27 It aims to reduce pollution and carbon emissions, improve resource and energy efficiency, as well as preserve ecosystems and biodiversity.27 The pillars of green economy include:28
1. Climate change – It is crucial to reduce carbon emissions and preserve our forests and oceans to slow down climate change and related effects;
2. Resource-saving and management – The efficient and resourceful use of natural resources and the reduction of wastage are imperative to prevent products and energy shortages and a global environmental crisis;
3. Circular economy – It aims to preserve natural systems by reusing and repurposing waste and residues, thus generating value while maintaining the sustainability and economic viability of the productive chain;
4. Environmental protection – Environmental degradation has negative impacts on all social and economic sectors of society, therefore, preserving the environment is essential to maintain our sustenance and health;
5. Ecosystem protection and recovery – The preservation and recovery of ecosystems and their native biodiversity will have positive social and economic impacts on the world;
6. Water conservation – Water is one of our most vital natural resources, which is primordial to protecting our drinking water sources and ensuring access to clean water for disadvantaged communities around the world;
7. Natural disaster prevention – Large-scale natural disasters are becoming more frequent due to climate change, deforestation, and rising sea levels, making it essential to avoid them as a way for saving human and animal lives, as well as natural ecosystems, urban and rural infrastructures.
Notably, these seven pillars of the green economy presented above are directly aligned with fourteen of the seventeen UN Sustainable Development Goals (SDGs), as schematized in Fig. 2.29
 | ||
| Fig. 2 United Nations (UN) Sustainable Development Goals (SDGs) that are directly related to green economy pillars and aims (in color). Image produced by the authors with pictograms from http://www.un.org.29 | ||
As Fig. 2 shows, the green economy is addressing most of the pressing issues we face today. Therefore, as a direct consequence, the green economy has efforts to accomplish environmental goals, such as clean water and sanitation (SDG 6), affordable and clean energy, responsible consumption and production, climate action, life below water, and life on land (SDGs 7, 12, 13, 14, and 15, respectively), as well as socio-economic aims, like no poverty and zero hunger (SDGs 1 and 2, respectively), allowing decent work and economy growth and reduced inequalities (SDGs 8 and 10, respectively), good health and well-being (SDG 3), industry, innovation and infrastructure and sustainable cities and communities (SDGs 9 and 11, respectively) and partnership for the goals (SDG 17). As schematized in Fig. 2, there are only three SDGs, i.e., quality education (SDG 4), gender equality (SDG 5), and peace, justice, and strong institutions (SDG 16), which are not directly aligned with the green economy precepts. In any case, we must stress out that if society will become “greener”, the world will be more inclusive, fair, and socially sustainable.
Although the use of green economy principles has been a step in the right direction towards more sustainable production systems and economies, there are still not enough efforts to protect our aquatic ecosystems, i.e., “the blue of the oceans is not yet the new green”. While 15% of terrestrial lands are conservation areas, only 1% of the ocean surface is legally protected.30 This difference is quite surprising, especially considering that the initial efforts to define guidelines for protecting the environment aimed to preserve our water resources.23 Additionally, climate change disproportionately affects the oceans, which absorb 90% of heat caused by global warming and 30% of CO2 emissions.31 This increase in heat and CO2 absorption can change sea temperature, cause deoxygenation and ocean acidification.32,33 As a result, these alterations in the marine environment can modify the chemistry and circulation of the oceans, raise sea levels, and reduce marine biodiversity.32,33 With these issues in mind, the term “blue economy” was introduced in 2012 during the Rio + 20 UN Conference on Sustainable Development to expand the blue aspect of the green economy, as a sub-field focused on marine activities and oceans environments.34 In addition to a focus on ocean preservation, the blue economy consolidated the paradigm that sustainability also requires a balance between environmental protection, economic growth, and social justice and inclusion.32 There is still no unified definition of blue economy, but it revolves mainly around a sustainable ocean economy. Table 1 presents blue economy definitions established by leading representative organizations around the world.
| Organization | Definition | Ref. |
|---|---|---|
| Conservation International | “'Blue economy’ refers to the range of economic uses of the ocean and coastal resources—such as energy, shipping, fisheries, aquaculture, mining, and tourism. It also includes economic benefits that may not be marketed, such as carbon storage, coastal protection, cultural values, and biodiversity”. | 35 |
| European Commission | “Blue economy encompasses all industries and sectors related to oceans, seas, and coasts, whether they are based directly in the marine environment (e.g., shipping, seafood, energy generation) or on land (e.g., ports, shipyards, coastal infrastructures)”. | 36 |
| The Center for the Blue Economy | “The ‘blue economy’ comprises the economic activities that create sustainable wealth from the world's oceans and coasts”. | 37 |
| The Commonwealth of Nations | “The ‘blue economy’ is an emerging concept which encourages better stewardship of our ocean or ‘blue’ resources”. | 38 |
| The Ocean Foundation | “The (new) ‘blue economy’ refers to economic activities that are both based in, and which are actively good for the ocean, though definitions vary”. | 39 |
| United Nations | “A blue economy is a long-term strategy aimed at supporting sustainable economic growth through ocean-related sectors and activities while improving human well-being and social equity and preserving the environment”. | 40 |
| World Bank | “Blue economy is the sustainable use of ocean resources for economic growth, improved livelihoods and jobs, and ocean ecosystem health”. | 41 |
| World Wildlife Fund | “A sustainable blue economy is a marine-based economy that: (1) provides social and economic benefits for current and future generations, by contributing to food, security, poverty eradication, livelihoods, income, employment, health, safety, equity, and political stability; (2) restores, protects and maintains the diversity, productivity, resilience, core functions, and intrinsic value of marine ecosystems – the natural capital upon which its prosperity depends; (3) is based on clean technologies, renewable energy, and circular material flows to secure economic and social stability over time while keeping within the limits of one planet”. | 42 |
The definitions shown in Table 1 overall agree that the blue economy is a concept related to the sustainable exploitation of the ocean and its resources. Nonetheless, while the preservation of marine environments is a priority, economic and social variables are also considered for its design. Like the green economy, the blue economy can also be organized around (eight) main pillars, each with challenging principles and goals, as listed below.43
1. Conserve oceans and marine life: The oceans absorb the excess heat and greenhouse gases and provide half the oxygen on earth.1,31 It also provides food, energy and allows the transport at a low (or at least fair) cost of a plethora of products and commodities. Therefore, the maintenance of a healthy ocean and the preservation of marine life is not only a matter of economy, but it is also vital for our survival.
2. Intelligent transport and shipping: Most of the shipping in the world is already by sea, but there is an increasing demand for more economic and green maritime shipping technology and effective sea routes.12
3. Improve aquaculture and fishing industry: With our current population growth and damage to the oceans due to climate change and pollution, it will be necessary to simultaneously maximize the efficiency and sustainability of fisheries and aquaculture's productive chains. These systems must be more efficient and generate less waste, also allowing marine species to regain sustainable biological levels.34
4. Sustainable use of oceans resources: The industries discard more than half of the mass during processing. It is necessary to reduce seafood wastage to decrease the pressure on marine ecosystems and avoid pollution. The valorization of waste can, thus, be a source of revenue and job creation.43
5. Renewable energy: The oceans can provide a reliable source of renewable energy in the form of waves and winds, and can help enable coastal communities to become energy self-sufficient.
6. Maritime and coastal tourism: This sector provides income for many disadvantaged communities, and is a critical source of income for several coastal countries. While irresponsible tourism can damage ecosystems, sustainable maritime and coastal tourism can help preserve and recover natural landmarks. This will be beneficial not only for the restoration of aquatic ecosystems but also for increasing touristic attraction and maximizing revenues.
7. Biotechnology: The biodiversity of sea life is a source for bioprospection and novel bioactive biomolecules, which can be produced biotechnologically through more sustainable systems (e.g., recombinant technology, industrial enzymes, bioactive biomolecules for medical and industrial applications). There is a remarkable potential of application for these marine-based compounds for different medical and industrial applications.
8. Respect tradition: Although the preservation of marine life is a priority, it is also necessary to respect the cultures of coastal communities, discussing and elaborating strategies that allow for environmental protection while preserving traditions.
At this point, we must highlight that the blue economy is not a substitute for the green economy. Rather, it will be an additional and complementary guideline for action in a vulnerable and previously neglected ecosystem – the ocean. To elucidate the pillars of the green and blue economy and how they integrate, Fig. 3 shows a schematic summary of their main goals and principles.
In Fig. 3, the synergy between green and blue economies is evident, both in their principles and goals. The blue economy relies on perpetuating the actions of the green economy, while the green economy requires blue economy principles to advance towards more sustainable development goals. This synergy was very recently considered by the European Commission (EC), when it proposed new guidelines for a sustainable blue economy, as part of the European Green Deal and the Recovery Plan for Europe. The commission emphasized the importance of the blue economy as crucial for achieving economic and environmental sustainability, arguing that “there can't be green without blue”.44 Therefore, it is necessary to keep these fundamentals in mind to address seafood wastage and protect marine ecosystems. They should be used as guidelines for establishing actions to save and manage resources and improve the efficiency and safety of the mining, drilling, shipping, fishing, and aquaculture industries.
While this perspective focuses on discussing circular economy models in the seafood industries, we must emphasize the need for policies to increase the safety and sustainability of maritime shipping, mining, and drilling, which are also crucial to fulfilling the precepts of the blue economy. For example, cargo ships can release toxic substances in the water and air, contribute to the introduction and migration of invasive species, and even physically damage coral reefs.45–47 Onshore and offshore drilling can generate hazardous or problematic discharges,48,49 in addition to the risk of total or partial destruction of natural ecosystems due to oil and gas spills.47,50–52
Despite the relevance of the abovementioned activities, in our opinion, the complete synergy of green and blue economies will be achieved if implemented through a “biorefinery perspective”. Having this in mind, in the next section, we will discuss how biorefineries can integrate green chemistry into the seafood industry. Biorefineries are processing facilities that convert biomass (such as waste) into beneficial by-products.3 Considering the high volume of waste in fishery and aquaculture, a biorefinery model, i.e., the creation of “marine (bio)refinery”, could benefit these industries while protecting the environment and maximizing revenues for society.
Biorefineries and sustainable marine industries
The integration of a biorefinery concept in traditional industries enables a transition to a bio-based economy model (bioeconomy), where renewable sources and the circular economy are the foundation.53 The development of cost-effective, environmentally friendly, and circular technologies for processing biomass and waste, and converting it into value-added compounds are decisive factors to successfully implementing sustainable biorefineries, particularly those that contribute simultaneously to reducing industrial waste and energetic demand.7Initially, the biorefinery model was conceived as a way to promote the switch from a petroleum-based to a bio-based economy, where renewable biological sources are central.7,53,54 Biorefineries are conversion facilities for biomass processing, adapted to their feedstocks, and generally decentralized and located close to its material sources.55,56 Today, this model is much broader, since biorefineries are designed to be highly efficient and accommodate even unrefined and hazardous raw materials (e.g., industrial, agricultural and domestic waste) while generating innocuous and valuable by-products.54,57 Particularly for marine biorefineries, they aim to optimize the exploitation of ocean resources by recovering relevant substances from seafood residues, thus reducing waste creation and adding value to ocean-based processes.58 Marine biorefineries, especially for algae, are already well-described and consolidated.58,59 For example, by applying a biorefinery approach, Baghel et al. had a rate of brown seaweed utilization of 93% in their processing.58 They were able to develop an integrated strategy to recover 3.8 ± 0.2 g of protein concentrate, 10 ± 0.5 g of cellulose 32 ± 1.5 g of alginic acid, and 540 ± 5.5 mL of sap from 1 kg of wet brown seaweed biomass. This is a good example of an integrated process that allowed for recovering more by-products from the algae biomass, such as soluble algae products, protein concentrate, cellulose, and salts, in addition to having their effluents properly treated. Compared with conventional methods, the biorefinery model proposed by Baghel et al. allowed a reduction of the water footprint, and chemical and energy inputs, while providing extra revenue from the production of other commercially relevant substances.58 Thus, considering the excessive amount of waste produced in fisheries and aquacultures, including biorefinery principles in these marine industries can turn a problem (seafood waste) into a source of energy and valuable (bio)products and, of course, an extra income.60
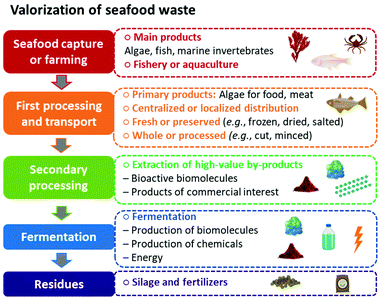
In previous sections, we have highlighted that by applying the blue economy principles and a biorefinery approach, seafood waste can be reduced, and the use of natural resources optimized while increasing revenue and creating jobs. In traditional ocean-based industries, seafood (mainly algae, fish, and marine invertebrates) is obtained through capture by the fishery or farming with aquaculture. The products are usually transported fresh in localized distributions or undergo the first stage of preservation (i.e., frozen, dried, or salted) and pre-preparation (e.g., bleeding, eviscerating, beheading, filleting, and minced) for centralized distribution.60 In conventional seafood industry models, after removing the primary products (seaweed for food applications and meat from fish and marine invertebrates), the residual biomass, wastewater, and lower-value animal parts (e.g., skin, scales, fins, head, viscera, bones, shells) are discarded as seafood waste in the ocean or landfills.2
Seafood waste not only causes pollution and environmental risks but also generates costs for its disposal (if properly managed). However, seafood waste has a complex and highly nutritional composition, with elevated amounts of proteins, oils, pigments, natural polymers, and other bioactive biomolecules of high commercial value. Therefore, with proper and efficient secondary processing, it is possible to extract these valuable by-products from the rich marine residues and increase the economic and environmental sustainability seafood industry. Furthermore, after isolating all higher-value products, the remaining residues can also become feedstock for microbial conversion through the cultivation process to produce more biomolecules and chemicals (e.g., alcohol, acids, biosurfactants, nutraceuticals, and biopharmaceuticals). The cultivation stage can also generate energy to supply the productive chain and ensure the system's energy self-sufficiency. Finally, depending on previous stages of processing, there may be solid residues that the industry can still use in the formulation of fertilizers and silage.
This thoughtful and intelligent process design, which applies green engineering principles, is critical for a successful waste management in a biorefinery approach. Having it in mind, in Fig. 4, we schematized a processing chain to elucidate what should be considered for the effective valorization of resources from seafood waste.
In Fig. 4, we schematized how a biorefinery approach could work for seafood waste valorization. Nonetheless, the feasibility of each stage of the biorefinery strongly relies on the composition and amount of the raw materials, as well as the type of industry they aim to supply. Hence, the first and crucial step in successfully designing a marine biorefinery is an in-depth knowledge of each resource, productive chain, operational limitations, and field of application. It is essential to know not only the composition of the feedstock (in this case, seafood waste from different sources), but especially the potential market value and application of the biomolecules, chemicals, and other by-products that can be isolated or converted from each type of waste. The following section will explore the different sources of seafood waste (i.e., from fishing and aquaculture industries), the products they can generate, and their commercial applications.
Fishing and aquaculture industries waste and products
According to the United Nations Educational, Scientific, and Cultural Organization (UNESCO), researchers estimate that the ocean is home to 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 marine species.61 However, a few of them comprises the majority of the volume of commercial seafood, including as the most prevalent: marine invertebrates – shrimps, oysters, crabs, lobsters, scallops, and squids; and fish – different species of tuna, cod, herring, salmon, anchovy, flounder, and mullet.2 Each type of aquatic species has a distinct composition and, therefore, different (bio)technological potential and applications.
000 marine species.61 However, a few of them comprises the majority of the volume of commercial seafood, including as the most prevalent: marine invertebrates – shrimps, oysters, crabs, lobsters, scallops, and squids; and fish – different species of tuna, cod, herring, salmon, anchovy, flounder, and mullet.2 Each type of aquatic species has a distinct composition and, therefore, different (bio)technological potential and applications.
The type of seafood, final product and processing parameters will directly impact the amount of waste that each fishery and aquaculture industry produces.60 For example, only 45% of catfish mass is used for human consumption. For marine invertebrates, this number is even lower, cf., prawns (40%), crustaceans (39%), shrimp (35%), crabs (32%), and mussel (14%).62 As for algae, certain species are entirely used as food (edible seaweed), generating almost no residues, while other species have no food applications, being cultivated or harvested only for the production of bio-compounds or fuel.63,64 However, it is important to highlight that the composition and concentration of bioproducts of interest in algae will be highly dependent of each algae species, as well as seasonal variations and types of biomasses (i.e., parts of the algae). For example, the alginic acid content in brown seaweeds can vary from 17 to 47% of their dry weight depending on their species, seasonal variations, and parts of the algae.65Ascophyllum nodosum has 22 to 30% of alginic acid in their dry weight, while Laminaria digitata has 25 to 44% in their fronds and 35 to 47% in their stipes, and Laminaria hyperborea present 17 to 33% in their fronds and 25 to 30% in their stipes.65
Overall, about 50% of the seafood mass is discarded.62 Still, these residues can be used by industries as a source of valuable by-products. If processed and preserved correctly, they can conform to the low-risk category 3 by-products of European Union (EU) regulation, viz., “parts of animals that have been passed fit for human consumption in a slaughterhouse but which are not intended for consumption”.62
Seafood waste is a remarkable source of by-products, mainly because it is a matrix rich in nutrients and bioactive molecules. Lipids comprise up to 60% of seafood residues (fish oil),66 where the predominant oil is triacylglycerol, like most other natural oils.66 Particularly, fish waste consists mainly of heads, frames, belly flaps, and parts of the viscera, such as liver and roe (containing valuable proteins and lipids).60 A sizable drawback of fish waste is its fast deterioration, so industries must preserve these residues as soon as possible after capture or harvest.60 Marine invertebrates waste also contains proteins (∼40%), chitin (∼25%), calcium carbonate matrix (∼30%), lipids (∼10%), and highly valuable carotenoids (i.e., pigments) such as astaxanthin (which has an outstanding antioxidant potential).60 As can be seen, there are many commercially relevant substances in seafood waste. In Table 2, we summarized the main classes of these by-products that can be obtained from fishery and aquaculture waste, listing their primary sources and industrial applications.
| Sources | Products | Applications | Ref. |
|---|---|---|---|
| Algae waste | Agar | Food, chemicals, medicines | 63 and 64 |
| Alginate | Food, chemicals, medicines | 63, 67 and 68 | |
| Biodiesel | Alternative fuel | 63 and 69 | |
| Carotenoids (β-carotene, astaxanthin, lutein, and zeaxanthin) | Food, nutraceuticals, medicines | 70–73 | |
| Carrageenan | Food, chemicals, medicines | 64 and 74 | |
| Protein extracts | Food, nutraceuticals, chemicals, medicines, animal feed, fertilizers | 63 and 75 | |
| Silage | Animal feed, fertilizer | 76 | |
| - Fermentation of algae waste | Alcohol and acids | Food, chemicals, fuel | 77 and 78 |
| Marine invertebrates waste | Lipids (oils and fatty acids) | Food, nutraceuticals, medicines | 79–83 |
| Peptones | Animal feed, chemicals, nutraceuticals | 84 | |
| Protein extracts | Food, nutraceuticals, chemicals, medicines, animal feed, fertilizers | 75 | |
| - Fermentation of marine invertebrates waste | Alcohol and acids | Food, chemicals, fuel | 85 |
| - Seashells | Biodiesel | Alternative fuel | 86 |
| Carotenoids (β-carotene, astaxanthin, lutein) | Food, nutraceuticals, medicines | 82 and 87–89 | |
| Chitin | Development of materials | 75 and 90–92 | |
| Inorganic minerals (calcium, iron, selenium, and zinc salts) | Nutraceuticals, medicines, chemicals, fertilizers | 75, 93 and 94 | |
| Hydroxyapatite | Development of materials, medical and dental applications | 93, 95 and 96 | |
| - Squid skin and by-products | Antioxidant peptides | Medicines | 97 |
| Fish sauce | Food | 98 | |
| - Viscera | Enzymes (e.g., carbohydrases, lipases and phospholipases, proteases) | Food, medical, and industrial applications | 99 and 100 |
| Fish waste | Biodiesel | Alternative fuel | 101–103 |
| Fatty acids (monounsaturated fatty acids, omega-3 fatty acids, polyunsaturated fatty acids, saturated fatty acids, saponification of fish oil) | Food, nutraceuticals, medicines | 18, 75, 102, 104 and 105 | |
| Fish silage | Animal feed, fertilizers | 97 and 106–108 | |
| Liposoluble vitamins, oils, phospholipids, squalene | Food, nutraceuticals, medicines | 75, 104 and 109–111 | |
| Peptones | Animal feed, chemicals, nutraceuticals | 84 | |
| Protein extracts | Food, nutraceuticals, chemicals, medicines, animal feed, fertilizers | 75 | |
| - Bones, skin, scales, and fins | Carotenoids (astaxanthin) | Food, nutraceuticals, medicines | |
| Collagen and gelatin | Nutraceuticals and medicines | 112–116 | |
| Hydroxyapatite | Development of materials, medical and dental applications | 93, 117 and 118 | |
| Inorganic minerals (calcium, iron, selenium, and zinc salts) | Nutraceuticals, pharmaceuticals, chemicals, fertilizers | 75 and 93 | |
| - Fermentation of fish waste | Alcohol and acids | Food, chemicals, fuel | 85 |
| - Fish protein hydrolysates | Fish sauce | Food | 97 and 106 |
| - Viscera and liver | Bioactive peptides | Medicines | 97 |
| Enzymes (e.g., carbohydrases, lipases and phospholipases, proteases) | Food, medical and industrial applications | 97, 99, 100 and 119–121 | |
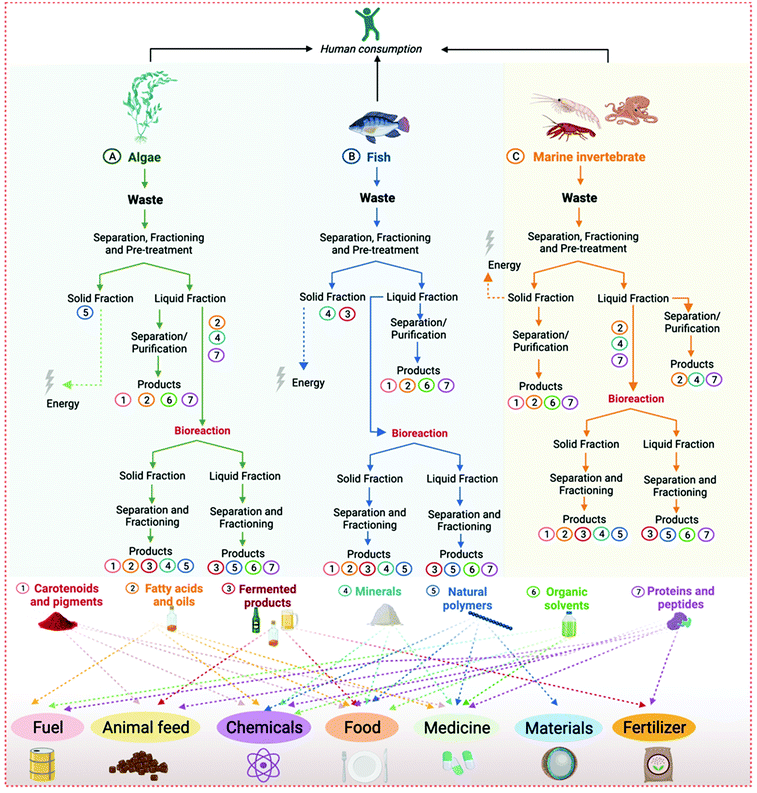
As detailed in Table 2, depending on the type of seafood waste, various by-products can be obtained and used in many industrial sectors, such as food, medicine, chemicals, materials, fertilizers, fuel, and animal feed. These can be extracted and isolated from algae biomass, marine invertebrate shells and viscera, the skin of octopus, fish residues (e.g., bones, skin, scales and fins, viscera, head, and frame), or even from wastewater from the fishery and aquaculture industries. Moreover, the residues can be used as a carbon and nitrogen source to produce other high-value products through microbial bioconversion. Considering the great diversity of by-products that can be obtained from the different classes of seafood waste, in the following paragraphs we discuss the most representative examples according to their chemical nature.
1. Carotenoids: The most prevalent liposoluble pigments in seafood waste are carotenoids.70–72,75,82,87–89,122 Astaxanthin alone comprises 74 to 98% of pigments mass in marine animal shells, with applications in the food and medical industries as a colorant, antioxidant, and vitamin A precursor.75 This carotenoid also has promising properties against certain types of cancer, can enhance the immune systems, and protect cells against radiation and muscle degeneration caused by aging.75 Other commercially relevant carotenoids with antioxidative properties present in seafood are β-carotene (vitamin A precursor),70,87 lutein,71,87 and zeaxanthin. β-Carotene is a natural antioxidant and an alternative source of vitamin A. This carotenoid is associated with aging prevention, benefits to night vision, improved immunity, skin, hair, and nails health, and fat metabolism. In addition to some food and nutraceutical applications, β-carotene has been used in cosmetics, such as skin tanning lotions and anti-aging creams.123 The food industry applies carotenoids as natural colorants in substitution of their synthetic counterparts.124,125 In addition to being healthier, they can also present supplementary nutritional and pharmacological benefits (e.g., vitamin precursors and antioxidants) and are often preferred by consumers.124,125
2. Fatty acids and lipids: The lipids extracted from algae, fish, and marine invertebrate waste can be used for biodiesel production63,69,86,101–103 or in food, nutraceutical, and pharmaceutical industries.75 Seafood oil and fat are valuable sources of liposoluble vitamins (e.g., E, D, and A),75,109 monounsaturated fatty acids,110 omega-3 fatty acids,82,83,102 phospholipids,80,81 polyunsaturated fatty acids,79,80 and saturated fatty acids.75,126 Fish oil is a by-product with many health benefits, for example, anti-inflammatory properties (including control of allergies, arthritis, and auto-immune diseases), protection against coronary heart diseases, low-density lipoprotein (LDL cholesterol) levels, obesity, and hypertension control.75,93 However, there are still drawbacks to fish oil as a food additive and nutraceutical, mainly related to its intense flavors and the low stability of its components.75,93 Besides nutraceutical and food uses, fish oil has other relevant applications. They include feedstock for biodiesel production,127 a consolidated use as drying oil in paints, coatings, and printing inks,128 and for fat liquors production for application in the leather industry.129 In addition to fish oil, shark liver oil is rich in squalene, a high-value marine product. Squalene is an organic molecule of the isoprenoids family (linear triterpene), with several nutraceuticals and therapeutical applications. They include antioxidant, detoxifier, hypocholesterolemia, cardioprotective, and anti-cancer properties.130 Squalene is extensively employed as a principal component of parenteral emulsions for drug and vaccine delivery.131
3. Fermented products: The chemical or biological hydrolysis of seafood can generate seafood protein hydrolysates (SPH) that are formed by small fragments of peptides containing amino acid residues.75 The SPH are used in food, pharmaceutical, and cosmetic formulations due to their bioactivities (e.g., antioxidant, antidiabetic, and immunomodulatory properties) and umami flavor. They also present chelating, hygroscopic, and surfactant properties. For example, the fermentation of seafood can generate different commercial products, such as the traditional fish sauce from Asian culinary made from SPH,97,106,126,132 and also transform seafood waste residues on silage for the production of animal feed and fertilizers.97,106–108
4. Minerals: Marine invertebrates shells and fish bones, fins, and scales are a rich source of minerals and other micronutrients.75,93,94 For example, inorganic minerals such as hydroxyapatite, calcium, phosphate, zinc, selenium, and iron represent almost two-thirds of fish bones. The fish bone powder is a rich source of calcium (around 234 g calcium per kg of dry bone) and other minerals with potential health benefits in growth and metabolism.75 Hydroxyapatite can be used for rapid bone repair after surgery or trauma and as a bone graft in dental applications.93,95,96,117,118 Calcium, phosphate, zinc, and iron are applied as food supplements, chemicals, or fertilizers.75,93,94
5. Natural polymers: Chitin is the most prevalent natural polymer from marine invertebrate shells, while agar, alginate, and carrageenan are the most common polymers from algae biomass.63,75,90–92 It is also possible to process chitin into chitosan, a biodegradable and biocompatible polymer. Chitosan has antimicrobial, antioxidant, anticoagulant, anticancer, and film-forming properties.75,90–92 Because of these characteristics, the medical and dental fields use chitosan for material development. For example, the biomedical field uses chitosan for artificial tissue reconstitution (e.g., skin, bones, and cartilage). In the food industry, natural polymers are applied as biodegradable films and as a flavoring agent, while they are used in the development of drug delivery systems by the pharmaceutical industry.75 The food, chemical, and pharmaceutical industries use agar and alginate as thickeners, plasticizers, and gelling agents.63 Agars are also widely employed in microbiology for the cultivation of microorganisms on solid medium.133 Recently, agar-based films present an alternative to plastic as biodegradable and edible food packaging.134 Analogous to agar, Funori is a polysaccharide mucilage made from the seaweed Gloiopeltis sp., common in Japan.135 It is used as a weak water-soluble adhesive, and it can form a gel at concentrations as low as 1%. Funori has been also added to hygiene and cosmetic formulations and used as a conservation material.136 Carrageenan, also called Irish Moss, is a natural polymer of the sulfated linear polysaccharide family obtained from red seaweed extracts. This biopolymer is currently used as a natural thickening agent in foods, beverages, gels, and lotions.137
6. Organic solvents: Seafood waste can be used as feedstock to produce a series of organic compounds by fermentation, such as alcohols and acids. Most times, the microbial cultivation of seafood waste will aim to produce ethanol and energy,85 but other organic (bio)solvents can be also obtained through an adequate choice of the microbial species.
7. Protein and peptides: This is the most diversified and highly valued group of seafood by-products, with applications in most industrial areas. It includes bioactive peptides (antioxidant and antihypertensive), collagen and gelatin, enzymes, peptones, and protein extracts. In addition to traditional uses in enzymatic reactions or as medicines, there are also novel and disruptive applications of these ocean biomolecules. For example, phycobiliproteins from red algae can be used to produce photovoltaic cells to capture low light underwater.138 Regarding the most common protein and peptide-based products, they include:
(a) Bioactive peptides (antioxidant and antihypertensive) – Usually extracted from the liver and viscera of fish and are mainly applied as medicines.97
(b) Collagen and gelatin – Obtained by thermal denaturation of collagen, they are proteins with applications in pharmaceutical, biomedical, leather, cosmetics, and tissue-engineering industries due to their unique structural and functional features. The fish skins, scales, and fins are rich in collagen, but it is also present in the meat and viscera of fish.75,112–116
(c) Enzymes – Different classes of enzymes can be found on the viscera of fish and marine invertebrates, particularly digestive enzymes such as carbohydrases,97 proteases (e.g., chymotrypsin, pepsin, and trypsin),99,100,119–121 lipases, and phospholipases.97,100 Other enzymes include lipoxygenase, myosin ATPases, polyphenol oxidases, transglutaminases.99,100,119–121 Most enzymes extracted from seafood are used for medical and industrial applications, but lipases and phospholipases are also employed by the food, chemical, and biofuel industries.97,100
(d) Peptones – Peptones are semi-digested proteins that are used as a carbon and nitrogen source. They can be extracted from fish and marine invertebrates waste and used to produce animal feed, chemicals, and nutraceuticals.84
(e) Protein extracts – Seafood is a rich source of animal protein. Protein extracts can be obtained from algae, fish, and invertebrate animal waste. They can be used as food additives, nutraceuticals, chemicals, pharmaceuticals, animal feed, and fertilizers.63,75
To better elucidate the sources and applications of different seafood waste products, a schematic representation associating each product and its respective source(s) and application(s) is presented in Fig. 5.
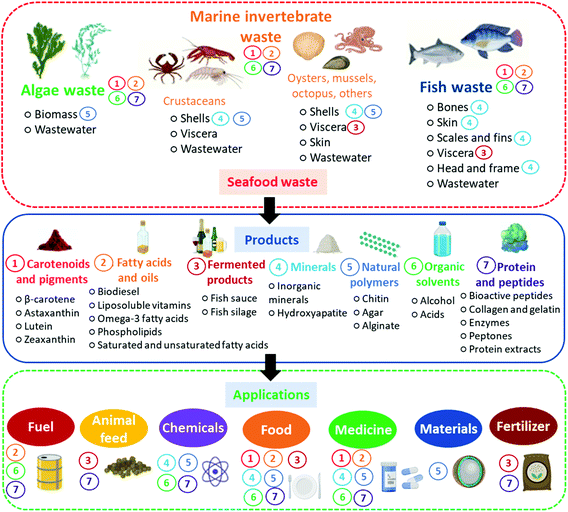 | ||
| Fig. 5 Products from seafood waste and their sources and applications. Products are correlated with their sources and applications by the numbers from 1 to 7 next to them. | ||
As shown in Fig. 5, it is possible to use seafood waste to formulate many products, with direct or indirect application in most industrial areas. Consequently, there are many opportunities for implementing marine biorefineries as an ideal solution for seafood waste valorization. However, biorefineries implementation can be exceptionally complex and requires a study and validation of all processing stages. These stages usually include marine life capture, pre-processing of seafood, its conservation and transport, downstream processing of high-value by-products, fermentation, and formulation of products. In the next section, we present concepts and examples of marine biorefineries, elucidating how they work and their potential applications.
Marine biorefineries for seafood waste valorization
Traditionally, residues and side/by-products are considered low-value substances, which are regularly employed as feed, fertilizers, or simply discarded as waste.2,139 Nevertheless, if properly extracted, isolated, and processed, they can become a valuable source of commercially relevant bioproducts, such as lipids, proteins, pigments, chitin, minerals, among others.60 These substances have a wide range of applications, such as colorants, food supplements, nutraceuticals, pharmaceuticals, cosmetics, and others, as previously listed in Table 2.140,141 It is clear that the valorization of seafood waste (e.g., fish and crustacean industries) is crucial for ecological reasons as well as a source of additional revenue for the productive sector. However, the conversion of residues into commercial goods relies on the successful implementation of large-scale biorefineries that create products with substantial market demand. Their manufacture should also be economically viable and environmentally friendly, ideally respecting the principles of a circular economy. To clarify these aspects, in this section, we propose a conceptual perspective panorama on a potential marine biorefinery (Fig. 6), including a careful analysis of the main steps required for seafood waste valorization, namely: extraction of primary high-value by-products; microbial cultivation for the production of other valuable biomolecules production; process design and integration.The perspective panorama in Fig. 6 represents potential marine biorefineries within a zero-waste model, exemplifying how a biorefinery can work towards an efficient valorization of seafood waste. The processing of seaweed will vary depending on its type, considering that some are edible and can go entirely/partially for food applications, while others are cultivated for the extraction of bioproducts such as alginate, agar, and carrageenan.63 As for fish and marine invertebrates, after removing their “meat” (i.e., the flesh of an animal used for food purposes) for human consumption, the residues can go through the separation of liquid and solid fractions.60 Usually, the seafood solid residues are employed directly in the production of fertilizers or silage for animal feed. Nonetheless, this is not ideal at the beginning of the process, considering they are commercialized as low-value products. In a biorefinery approach, solid residues must undergo separation, fraction, and pre-treatment stages, where promising substances are recovered and the solid fractions pre-processed for further extraction of these high-value biomolecules.60 Particularly for shell-waste, it is necessary to design a pre-treatment for their deproteination and demineralization.142,143
The liquid fraction of marine invertebrates and fish waste, particularly from their viscera, is a rich carbon and nitrogen source (e.g., proteins, carbohydrates, lipids, and minerals).144–146 Therefore, it is possible to directly extract some valuable products (e.g., bioactive molecules, proteins, colorants, lipids), while the rest can become feedstock to produce biomolecules through fermentation (i.e., microbial cultivation).60 Like the solid seafood fractions, the microbial biomass from fermentation can be processed for the recovery of commercially relevant substances. In a similar way, the liquid fraction of the fermented broth can also be processed to obtaining high-value biomolecules (e.g., proteins, pigments, polymers) or organic compounds (e.g., fuel and chemicals). In addition to generating high-value substances, fermentation processes can be included to produce energy for the industrial complex. The solid fractions (biomass) of different stages can be also processed for energy production.
After the pre-treatment of the solid fractions, they will go to the extraction stages. The recovery steps will vary according to the nature of target biomolecules and their final applications, as well as the source and composition of the crude fractions. The separation methods include liquid–liquid extraction, ultrafiltration, evaporation, precipitation, and potentially chromatography according to the degree of purity required. To maintain the sustainability of the biorefinery, it is also essential to include adequate solvent recycling procedures, ideally using solvents produced in the fermentation stage, or biosolvents obtained from renewable sources with minimal ecological impact. After extracting all high-value products, the remaining solid residues can finally be used to produce silage and fertilizers. It is important to emphasize that is also possible to recover chitin and inorganic minerals after deproteination and demineralization of shells (especially for crustacean-waste).
Chitin can be converted into chitosan by its partial deacetylation (above 50%).147 During deacetylation, the N-acetyl-D-glucosamine monomers of chitin are converted into randomly chained N-acetyl-D-glucosamine and D-glucosamine monomers. This process can occur in highly alkaline environments (e.g., 40 to 50% NaOH) or in the presence of chitin deacetylase enzymes (the biocatalytic reaction converts chitin + H2O into chitosan + acetate). The quality and size of chitosan will be strictly dependent on the purity and molecular weight of the starting chitin, as the methods and conditions employed for deacetylation. These marine-based polymers (particularly chitosan) can be used for the manufacture of (bio)materials or the production of glucosamine. These applications have made chitosan a highly valued compound, with an annual market of USD 2.49 billion in 2020, with an expected Compound Annual Growth Rate (CAGR) of around 12% during 2021–2026.148
Although Fig. 6 represents the potential “ideal scenario” for a marine biorefinery (i.e., with circular processing and zero waste), a closed and waste-free system is not always economically or even environmentally viable. For example, by optimizing the processing routes for a marine biorefinery (using fish, crustaceans, and cartilaginous species), Antelo et al. found that it was better to use specific parts of the fish than the whole animal, from both environmental and economic perspectives.149 These researchers also demonstrated that some products (e.g., biopeptides, chondroitin sulfate, and fish enzymes) have a higher potential than others due to their elevated commercial prices and low environmental impacts.149 Nevertheless, they concluded that there is still a need to improve the recovery of chitin, gelatin, and fishmeal from seafood waste as, while profitable, it had a severe environmental cost due to its high energy and large water consumption.149 In addition, Antelo et al. observed that many of the optimal valorization pathways still generated waste, even if in small quantities.149
In our opinion, although a circular process and zero waste are preferred, the pursuit of a “perfect” marine biorefinery should not prevent industries from implementing systems capable of reducing waste or aggregating value to their products. With this information in mind, in the following subsections, we will give examples of seafood waste valorization strategies for marine biorefineries designs, namely, for the extraction, microbial cultivation, and process integration stages.
Extraction of by-products from seafood waste
As previously demonstrated, seafood waste is a complex matrix with a mix of natural polymers, macromolecules, and minerals. Therefore, the extraction of by-products from these residues is usually a multi-step process that involves different separation techniques. The design of the extraction platform will vary depending on the characteristics and properties of the raw substrate and target compounds, as well as desired purity degree of commercial goods. For example, it is possible to use simple physical methods to recover fish oil from seafood waste. Nonetheless, the recovery of chitin and proteins from seashells is more complex, requiring additional demineralization and deproteinization steps to break the strong chemical bonds between the components of the biological matrix.142,143 Furthermore, it is possible to add a cultivation step to improve the recovery of chitin, proteins, lipids, and pigments from seafood.145 Besides helping in the extraction of by-products, microbial cultivation can also allow the production of other relevant biomolecules (e.g., adding carotenogenic microorganisms to produce carotenoids). With so many possibilities and variables, it is necessary to carefully evaluate each processing stage before implementing a new biorefinery. In this subsection, we will explore different strategies for the recovery of by-products from seafood waste. Moreover, the differences between working with algae, fish, or seashell residues will also be discussed.Saturated and unsaturated lipids are the most prevalent by-products of fish waste.66 For their recovery, it is possible to apply even physical separation methods (e.g., heat, filtration, and centrifugation).150 However, the high water content of fish residues can create emulsions, reducing the value of the oil recovered, while complicating subsequent downstream processing.150 For this reason, chemical methods are also often employed, such as liquid–liquid extraction. For example, Khoddami et al. extracted oils and fatty acids from sardine waste (mainly head, liver, and intestine) using liquid–liquid extraction with chloroform and methanol.105 Furthermore, the degree of purity will also influence the extent and complexity of the separation of lipids from fish waste. Although we can separate aqueous and hydrophobic phases employing simple physical methods or liquid–liquid extractions, further processing is needed to isolate lipids with similar physical–chemical properties. For example, saturated and longer alkyl chain lipids generally have a higher fusion point than shorter and unsaturated lipids. Therefore, these distinct physicochemical properties can be exploited for their separation and isolation. Although this processing approach would be more than enough to obtain fish oil for the food and cosmetic industry, it may not be enough for a pharmaceutical formulation, which requires specific lipids with a high purity degree. Lipid refining processes include active filtration using adsorbents, distillation, chemical purification, and membrane separation.150 Each of these purification methods has its advantages and drawbacks, and your selection will depend on the substrate and intended application of the target lipids.
From seashell waste, it is possible to extract carotenoids,151 proteins,152 chitin,153 and inorganic minerals.75 However, it is usually necessary to carry out initial demineralization and deproteinization procedures to extract them, considering the strong linkage between chitin and proteins, calcium carbonate, lipids, and pigments in the shell.142,143 It is possible to recover these compounds by a conventional chemical route using acids for demineralization and alkali treatment for deproteinization. For example, Charoenvuttitham et al. extracted chitin from black tiger shrimp shells using NaOH and different mixtures of acids. With the conventional method using 0.25 M hydrogen chloride (HCl), these researchers obtained 86.5% of chitin from protein and minerals, while achieving 88.1% of purity with a new approach using a solution of formic acid and citric acid at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v).154 Alternatively, some authors have been associating chemical and physical methods to improve chitin deproteinization. For example, Kjartansson and Zivanovic used sonication after adding the acid (HCl) and base (sodium hydroxide, NaOH) to increase the deproteinization of chitin from shrimp (Pandalus borealis) shells.155 However, this approach also resulted in lower yields, which the authors attributed to higher concentrations of depolymerized materials in the wash water.
2 (v/v).154 Alternatively, some authors have been associating chemical and physical methods to improve chitin deproteinization. For example, Kjartansson and Zivanovic used sonication after adding the acid (HCl) and base (sodium hydroxide, NaOH) to increase the deproteinization of chitin from shrimp (Pandalus borealis) shells.155 However, this approach also resulted in lower yields, which the authors attributed to higher concentrations of depolymerized materials in the wash water.
As can be seen with the previous examples, acidic and alkaline chemical treatments are the routine protocols for the recovery of products from shells, mainly due to their low cost, simplicity, high yields, and the possibility to reuse or recycle its components.156 However, the problem with using these traditional chemical extraction methods is that they still rely primarily on large amounts of toxic solvents that pollute and persist in the environment,157,158 as well as with concerns about handling them. Of course, these traditional methods are not sustainable solutions for a transition to a blue economy. Therefore, academics and industries seek substitute practices with lower risks and environmental impacts.
Non-conventional biotechnological protocols (e.g., microbial-based or biocatalytic) could be alternatively applied.142,159 Researchers have been proposing the use of biological methods to recover marine products as an alternative to conventional chemical extraction,160 for example, using enzymes (e.g., proteases) for the deproteinization step during the chitin extraction. Ghorbel-Bellaaj et al. used a crude metalloprotease from Pseudomonas aeruginosa A2 for the deproteinization of shrimp waste. After two hours of hydrolysis at 40 °C, there was up to 85% of chitin deproteinization. The purity of the sample was also high and comparable to a commercial α-chitin.161 In a similar strategy, Manni et al. used proteases produced by Bacillus cereus SV1 to remove 88% of the proteins from chitin from shrimp waste.162 These are good examples of how enzymatic reactions can be employed to extract chitin from seashell waste.
Instead of using crude or isolated proteases, it is also possible to cultivate the seashell residues with microorganisms capable of producing proteases. Following this approach, Ghorbel-Bellaaj et al. evaluated the aptitude of six protease-producing Bacillus species (i.e., B. pumilus A1, B. mojavencis A21, B. licheniformis RP1, B. cereus SV1, B. amyloliquefaciens An6, and B. subtilis A26) to extract chitin from shrimp shell waste during microbial cultivation.160 The researchers used shrimp waste as an alternative carbon and nitrogen source in the liquid culture medium, inducing the bacteria to produce proteases to break the strong bonds between the components of the shells and access its nutrients.160 The proteases of all strains were able to generate at least 80% of deproteinization of chitin, but only a maximum of 67% of demineralization. However, this study found that supplementing the medium with 5% (w/v) glucose improved demineralization rates without decreasing deproteinization.160 With a similar method, Doan et al. analyzed the deproteinization of chitin from shrimp waste with submerged cultivation using the alkaline protease-producing strain Brevibacillus parabrevis, achieving 95% of protein removal after four days of cultivation.163 Ghorbel-Bellaaj et al. also employed a protease-producing microorganism (specifically, Pseudomonas aeruginosa A2) to enhance chitin extraction from shrimp. Using this method, they reached 96% of demineralization and 89% of deproteinization.164 These successful examples make us believe that biological processes (using isolated microbial-produced proteases or protease-producing microorganisms) can be successfully applied to extract chitin from shell waste.
Microbial cultivation processes that generate acids can also be used to replace or enhance chemical deproteination and demineralization of chitin from seashells. For example, Castro et al. improved chitin extraction from crab (Allopetrolisthes punctatus) by applying preliminary a lactic ensilation using Lactobacillus plantarum sp. 47 followed by chemical deproteination and demineralization.165 For that, bacteria were inoculated into the biomass and cultivated in a semi-solid medium until reaching the lactic acid peak production (i.e., 17 mg lactic acid per gram of silage with 10% inoculum, 15% sucrose, 85% biomass). After 60 h of processing, the chitin was recovered from the biomass using 0.4 M NaOH and 0.5 M HCl, allowing removal of 99.6% of minerals and 95.3% of proteins from the biopolymer.165 The additional cultivation step for chitin extraction increased both quality and yield when compared to conventional chemical methods.165 In another study, Bhaskar et al. evaluated the effect of Pediococcus acidolactici cultivation for the recovery of chitin and carotenoids from shrimp waste.166 Using this bioprocess, chitin demineralization and deproteination yields reached 72% and 98%, respectively, while 4.3 g of carotenoids were also recovered, after 72 h of submerged cultivation.166 Lactic fermentation appears as another suitable bio-strategy for recovering biomolecules from seashells.
Despite the benefits of microbial cultivation procedures in the recovery of seashells bioproducts, namely, lower risks and environmental impacts, and high efficiency and specificity, these bioprocesses have not been applied on an industrial scale yet.156 As with other industrial residues, one of the main difficulties in using shellfish waste as a substrate for microbial cultivation is the great variability of properties and quality of residues, which will have a strong impact on the outcome of the process.156 For example, Kjartansson and Zivanovic observed that chitin obtained from North Atlantic shrimp (Pandalus borealis) had more impurities than the same compound from freshwater prawns (Macrobrachium rosenbergii), even using the same extraction method.155 These researchers demonstrated that the composition and structural arrangements of chitin in shells have a major impact on extraction process efficiency. The Pandalus borealis have low lipid content (0.3 to 0.5%), high protein (33 to 40%), and high ash (32 to 38%) dry weight,160 while other species, such as the Metapenaeus monoceros shrimp, have more lipids (6%), lower protein (25%), and higher ash (46%) content. These are considerable differences between species that will directly impact chitin recovery. In addition, there are also seasonal variations that will also change the composition of seafood. These changes will influence purification outcomes and difficult the scaling of microbiological extractions of chitin.160
To summarize seashell waste deproteination and demineralization strategies, in Table 3 we compiled the different methods, detailing the major reactants or systems, as well as deproteination and demineralization yields.
| Source | Type of method | Reactants or systems | Deproteination (%) | Demineralization (%) | Ref. |
|---|---|---|---|---|---|
| a N/A – not available. | |||||
| Shrimp | Chemical | NaOH, formic acid, citric acid | 88 (total purity) | 154 | |
| NaOH, HCl | 87 (total purity) | ||||
| Chemical and physical | HCl, NaOH, sonication | 98 | 97 | 155 | |
| Enzymatic | Metalloprotease from P. aeruginosa A2 | 85 | Not significant | 161 | |
| Protease from B. cereus SV1 | 88 | N/Aa | 162 | ||
| Microbiological | Protease-producing B. spp. | 80 | 67 | 160 | |
| Protease-producing B. parabrevis | 95 | N/Aa | 163 | ||
| Protease-producing P. aeruginosa A2 | 89 | 96 | 164 | ||
| Lactic acid bacteria P. acidolactici | 98 | 72 | 166 | ||
| Crab | Lactic acid bacteria L. plantarum sp. 47 | 95 | 97 | 165 | |
As clearly shown in Table 3, it is possible to achieve high deproteination and demineralization yields using the most distinct methods. This picture is transversal to the other biomolecules, especially if the extraction is applied to the valorization of different seafood residues. For this reason, there is no standard and universal procedure for the recovery of marine substances, and the efficiency of each process is always dependent on the type of seafood waste (e.g., type of organism, concentration, and nature of target biomolecule) and the operational extractions procedures (e.g., extraction time, temperature, biomass, and solvent ratio). Hence, we believe that using biorefinery and circular economy approaches, viz., introducing efficient bio-based pre-treatments, integrating downstream operation units, and including adequate solvent recycling protocols, can overcome some of the current ecological and economical concerns.
Chemical extraction will appear to be the simplest and most convenient compared to biological alternatives. Unfortunately, if the chemical operations are still carried out following traditional methods (e.g., using acids or alkalis) the sustainable environmental appeal of the marine biorefinery will not be achieved. Therefore, we suggest applying strategies that allow the efficient recovery of by-products and simultaneous recycling of the solvents, as well as the use of “green” solvents [e.g., biosolvents, ionic liquids (ILs), and deep eutectic solvents (DES), supercritical fluids], which can be fully, or at least partially, obtained (using sustainable technologies) from renewable raw materials.
In the last two decades, some research groups have proposed the use of ILs and DES as an alternative for the extraction of biomolecules from seafood waste.156,167 ILs are a diverse group of salts with low melting points that are easily tailorable to obtain distinct structures with useful properties for industrial applications (e.g., low vapor pressure, high thermal stability, low toxicity, and solvation capability).168 As ILs, DES are also solvents with outstanding properties suitable for industrial use. However, instead of chemical reactions, they are produced with eutectic mixtures of Brønsted or Lewis acids and bases.169 Additionally, ILs and DES have previously demonstrated great aptitude to act as dissolution agents for biopolymers such as cellulose, lignin, and starch.170,171
Besides the environmental advantages of using ILs and DES, it is possible to exploit their unique properties to integrate different downstream stages, such as demineralization, deproteination, and extraction. For example, Qin et al. used imidazolium-based ILs to completely dissolve crustacean shells and recover chitin powder of high molecular weight and purity.172 This is an interesting approach since it allows not only to obtain pure chitin powder but also other chitin-based products, namely, chitin fibers and films directly from the IL solutions of dissolved shrimp shells. Tolesa et al. also employed ammonium-based ILs for the extraction of chitin and conversion to chitosan from shrimp shells.173 After treating shrimp shells with ILs, 14% of chitin was extracted from the original biomass and successfully converted to chitosan under ILs alkaline conditions. As for DES, Zhongji et al. proposed the direct extraction of chitin from shrimp shells using DES, namely, mixtures of choline chloride as hydrogen bond acceptor and thiourea, urea, glycerol, and malonic acid as hydrogen bond donors. Their results demonstrated that a mixture of choline chloride–malonic acid improves the chitin isolation by almost 20%.167 In a similar study, Saravana et al.174 used different DES to extract chitin from shrimp shells (Marsupenaeus japonicas). Curiously, in this work, the high purity of chitin was also obtained using a mixture of choline chloride–malonic acid with a yield of 19.41%.
Although most studies focus on the recovery of natural polymers, it is possible to recover other classes of biomolecules from seafood waste. For example, some researchers have applied alternative IL-based extraction methods for the recovery of marine pigments from seashells waste, such as astaxanthin.89 Using a combination of ultrasound and a solution of imidazolium-based ILs with biosolvent (ethanol) as extractant agents, Bi et al. reported that astaxanthin extraction yields from shrimp wastes increased 98%.175
Nunes et al. evaluated another non-conventional extraction strategy to recover astaxanthin-rich extract from crab shell wastes using microwaves for pretreatment and supercritical fluid for extraction (ethanol as co-biosolvent).89 This new methodology improved 12-fold the extraction efficiency when compared to the traditional soxhlet protocol. This result demonstrates that the strategy of integrating non-conventional technologies was effectively capable of intensifying astaxanthin extraction from crab wastes.
As presented above, alternative extraction methods and novel solvents, namely, ILs, DES, biosolvents, and supercritical fluids, have great potential to improve the recovery of seafood waste by-products while also reducing hazards and environmental pollution, integrating different processing steps, and enhancing yields. However, our opinion is that the most efficient route to enhance the valorization of seafood waste is to associate the chemical processing of biomass and recovery of by-products with additional bioprocessing stages, in which the microbial cultivation is applied both to digest the raw materials and to produce other high-value biomolecules. With this in mind, in the next subsection, the use of seafood residues as feedstock for the production of bioproducts is discussed.
Microbial production of biomolecules in the seafood industry
Different industries have exploited the application of marine waste to produce value-added bioproducts.176 Seafood waste, viz., heads, tails, fins, chitinous materials, or viscera, represents a rich source of pigments, proteins, lipids, and chitin, which can be recovered or used as low-cost substrates to produce several bioproducts. In most cases, these substrates are subjected to an initial pre-treatment to transform them into suitable feedstocks for cultivation.176 For example, viscera or heads from fish waste are cooked, pressed to remove water, and dried. This pre-treated solid fraction (rich in high contents nitrogen, phosphorus, and calcium)176 have been successfully used as a fertilizer for the production of ice lettuce (Lactuca sativa L.),177 tomato crop,176 and biopharming178 (Fig. 6). On the other hand, the nutrient-rich aqueous supernatant is often used as a basal medium in cultivation procedures to produce several biomolecules.144,145Microbial cultivation using fish waste as a source of carbon and nitrogen is considered a low-cost, safe, and sustainable technique to obtain a wide range of valuable compounds.145 For example, fish waste (from Sardinella anchovia, Lepophidium profundorum, Trachurus lathami) was used as a substrate for the extracellular production of L-lysine by Corynebacterium glutamicum ATCC 21543, achieving 30 g L−1 of this amino acid after 72 h of submerged cultivation.144 Horn et al. studied the bacterial growth of Lactobacillus plantarum in a medium containing cod viscera hydrolysates, observing a 10% increase in biomass yield.179 Fish waste hydrolysate (rich in protein and amino acids) can also be used as a low-cost nitrogen source for the culture medium. Martone et al. observed that soluble fish protein hydrolysates obtained from hake (Merluccius hubssi) filleting waste allowed the growth of different bacteria similarly to the conventional Luria–Bertani (LB) medium. The microorganisms included Halobacterium salinarum, Escherichia coli, Bacillus subtilis, and Staphylococcus epidermidis.
Seafood waste can also be used for the production of ethanol via yeast fermentation.180 Alfonsín et al. used Saccharomyces cerevisiae to metabolize the seaweed Euchema spinosun industrial waste, converting its carbohydrates into ethanol with a 75% efficiency.77 Using the same microorganism, Korzen et al. produced bioethanol from Ulva rigida biomass,78 obtaining 196 mg of glucose per gram of dry weight of algae biomass, while every gram of glucose produced 333 mg of bioethanol. It is important to note that, to facilitate the conversion of carbohydrates from seaweed, these researchers assisted the cultivation process with a sonication unit. In our opinion, this is not only a good example of how algae biomass can be used as a suitable feedstock for the production of alcohol via fermentation but also to demonstrate the importance of process integration (viz. ultrasonic-assisted fermentation).
From the above examples, we can conclude that the complex but nutritional-rich composition of seafood waste makes them promising nutrient additives (particularly as a nitrogen and carbon source) for microbial culture and cultivation.
Beyond the use of seafood waste for microbial cultivation, the enormous potential of exploiting marine ecosystems as a “biotechnology source” for a wide range of medically and industrially relevant biomolecules and microorganisms should be remarked. For example, fluorescent proteins (widely applied as biosensors and biomarkers in research and medicine) were originally isolated from jellyfish and coral reefs.181,182 As presented in Table 2, there are also many medically and industrially relevant enzymes isolated from sea life, such as carbohydrases, lipases, phospholipases, and proteases. Furthermore, microalgae isolated from the sea have numerous applications for the biological production of added-value chemicals and products. For example, Fagundes et al. developed a biotechnological approach for squalene production using agro-industrial wastewater and the microalgae Phormidium autumnale.183 The resulting biomass presented 0.18 g kg−1 of squalene and a high content of unsaturated fatty acids (52%). Interestingly, the research group estimated that at an industrial level, and depending on processing capacities, production rates from 727 to 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 kg per year of squalene can be obtained using this method.183 In fact, we must highlight the importance of these marine-based biotechnological processes in restoring fish habitat and population. For example, to illustrate the impact of replacing shark hunting with the biotechnological production of squalene, between 635 to 4446 sharks would be needed to supply the same amount of squalene that a small industry could produce annually (727 kg).183 Aiming at the implementation of sustainable marine biorefineries within the principles of blue economy, this is a perfect representation of the path we must follow. The use of microalgae or other biotechnological approaches to produce biomolecules is probably one of the most promising alternatives to improve productivity and reduce the environmental and ecological impacts of obtaining substances of marine origin.
750 kg per year of squalene can be obtained using this method.183 In fact, we must highlight the importance of these marine-based biotechnological processes in restoring fish habitat and population. For example, to illustrate the impact of replacing shark hunting with the biotechnological production of squalene, between 635 to 4446 sharks would be needed to supply the same amount of squalene that a small industry could produce annually (727 kg).183 Aiming at the implementation of sustainable marine biorefineries within the principles of blue economy, this is a perfect representation of the path we must follow. The use of microalgae or other biotechnological approaches to produce biomolecules is probably one of the most promising alternatives to improve productivity and reduce the environmental and ecological impacts of obtaining substances of marine origin.
In addition to advances in R&D at the academic level, there are already industries investing in the production of valuable biomolecules using marine microorganisms such as microalgae. Veramaris® (a joint venture of DSM and Evonik) implemented in 2019 an industrial plant for the production of omega-3 fatty acids EPA and DHA from natural marine algae (strain Schizochytrium sp.) for sustainable animal nutrition in salmon farming.184,185 The high nutritional composition of this oil allows a better and healthier fish growth in aquaculture without depleting fish oil and burdening marine resources. This joint venture was established as the largest fermentative manufacturing using algae, following a responsibility purpose to help marine life, as claimed by Veramaris®: “using a pioneering omega-3 from natural algae”, Veramaris® “enables partners along the value chain to become independent of marine resources, making them leading stewards of the ocean”.
The use of biotechnology to obtain bioproducts more efficiently and sustainably has been called “white biotechnology”.59,185 Although this is not a recent phenomenon (the food and detergent industries have been using enzymes to improve their processing and products for years), recent advances in molecular biology, genetic and metabolic engineering have allowed the development of disruptive industrial and medical applications using microorganisms, biomolecules, and other bio-based products, including those obtained from marine ecosystems.186 White biotechnology is closely associated with modern and greener biorefineries, appearing as the key to consolidating more sustainable and profitable practices in the seafood industry.187
The sustainable extraction of by-products directly from seafood waste and the production of valuable biomolecules using seafood residues or microalgae are significant advances towards a blue ocean-based economy. However, it is only possible to create a complete marine biorefinery when integrating these different stages one to another into a cohesive and sustainable production system.
Integration of processes in a marine biorefinery
Establishing integrative platforms for the efficient sequential or simultaneous recovery of by-products from complex seafood waste is fundamental in making these processes more economical and sustainable. The choice of target substances and techniques employed should be rational and based on measurable economic and environmental parameters. To elucidate how biorefineries can be delineated and managed, we will discuss representative examples of laboratory and industrial-scale marine biorefineries for the valorization of seafood waste from various sources (e.g., algae, marine invertebrates, and fish).There are already several studies on the integration of different stages for seafood processing. For example, Deng et al.152 proposed an integrative and low-cost biorefinery platform to separate crustacean shells into several fractions of high-added value bioproducts. This integrative platform includes two biocatalytic steps using recombinant aspartic proteases (for the hydrolysis of proteins) and recombinant chitinase (for chitin hydrolysis), and a liquid extraction using biosolvents (ethyl acetate) for the recovery of astaxanthin. The integrated process resulted in 0.45 g protein hydrolysate, 0.17 g chitin oligomers, 101.3 μg astaxanthin, and 0.33 g mineral residues for 1 g shrimp shell waste biomass, demonstrating its efficiency for chitinous waste processing.152 In another approach, Prabhu et al. developed a biorefinery to extract different products from the green algae Ulva ohno, aiming to reduce waste creation and to maximize the recovery of the product from their biomass.188 These researchers were able to convert 90% of the algae biomass in the form of six by-products, namely, salts, starch, lipids, ulvan (cell wall polysaccharide), proteins, and cellulose. As for fish, Abdollahi and Undeland used a biorefinery approach to extract fish oil and recover gel-forming proteins from salmon and herring residues. To this end, the pH-shift process, heat-induced isolation, and emulsion breaking techniques were properly integrated to extract, in parallel, high-quality gel-forming proteins, and fish oil.189 These are remarkable results at laboratory scale, but it is necessary to evaluate the viability of scaling up these “marine biorefineries” to realize their potential as practicable industrial operations.
In addition to several academic works, some industries have already applied biorefinery concepts for seafood waste valorization. For example, 525 Solutions, a research & development company, proposed a technology that employs ILs in a biorefinery approach to recover chitin from seashell waste.190 Because it can extract natural polymers under mild conditions, this technology produces high molecular weight and pure chitin that cannot be obtained with traditional methods. This company demonstrates that it is possible to develop and implement, at an industrial scale, disruptive technologies following the marine biorefineries precepts and accomplishing green and sustainable practices while maintaining economic viability. Still, even at already established biorefineries, there is space for improvement.
Although the biorefinery precept envisages improving economic value and environmental preservation, if their design and implementation are not careful, it can generate a system that leans towards one or the other. For example, García-Santiago et al. assessed the environmental impacts and gross benefit of a cartilaginous fish biorefinery operating in Spain.191 They found that the extraction of high-value by-products generated a more eco-efficient process (i.e., produced more with fewer resources), but it had a worse environmental performance. García-Santiago et al. concluded that the poor ecological results were because the proposed biorefining processes were still not optimized, mainly in terms of equipment and energy consumption.191 Therefore, this study shows the importance of evaluating different economic and environmental parameters to design the best strategies for waste valorization. Even though we normally infer that more eco-efficient systems are automatically more environmentally friendly, this is not always the case. This controversial outcome may occur due to the complexity and energetic demand for the recovery of certain products outweigh the environmental cost of just treating the waste. For some substances (e.g., bioactive proteins and peptides), their high commercial value and unique potential for medical and industrial applications can justify their recovery.149 However, for low-value compounds that can also be obtained from other sources (e.g., gelatin), the cost of processing, energy, and materials can surpass the selling point of the bioproducts.149 Therefore, a sustainable biorefinery requires much more than just thoughtless processing of waste. It requires an intelligent strategy oriented towards continuous evaluation and optimization. Furthermore, the examination must include adjustment parameters that effectively represent its economic and environmental performance. Thus, our opinion is that the development and assessment of a marine biorefinery must take into account the multiple processing steps, raw materials, products, and distinct evaluation parameters.
Fortunately, there are several well-established methods and tools for assessing the environmental impacts of an industrial process. Some ecological parameters are straightforward and estimate the effect of the systems on single environment issues, such as energy, carbon, and water footprints.192 These can provide an overall idea of the burden of the production chain on energy consumption, climate change, and freshwater scarcity, respectively. However, to determine all the environmental impacts on micro and macro scales, an association of different footprints and more in-depth assessment methods are needed. For example, the ecological footprint combines several parameters to determine the productive space and resources required for a given process to produce its commodities and absorb its generated waste.192,193 There is also the life cycle assessment (LCA), a comprehensive method to determine all direct and indirect environmental impacts of the complete cycle of a production system.194,195 This approach is also referred to as “cradle-to-grave” because it covers all manufacturing phases (i.e., from raw material obtention to manufacturing, distribution, use, and disposal).194 Both the footprints and LCA can be applied depending on the process complexity or implementation phase. For example, a carbon and water footprint can be useful tools for quick feedback during the outline of a new process. However, an LCA is ideal at the latest stages of system development for a reliable assessment of its environmental impacts.
As for the techno-economic analysis, there is also a range of models to estimate the commercial viability of a new industrial complex. Some of the most popular parameters include capital expenditure (i.e., required initial investment), net present value (i.e., how much monetary value the project will add to the company), internal rate of return (i.e., the liquid profit per year), and the payback time (i.e., the time required to recover the investment).53,196 Although it can be overlooked, the market viability analysis must also take into account the public's response to healthy and ecological products. For example, Altintzoglou et al. showed that consumers had an increased interest in products with information regarding their health and environmental benefits.197
Many models and parameters can determine the economic viability and environmental impact of an industrial process. However, it is essential to choose an appropriate combination of methods to accurately predict the outcomes of a production chain. With these examples and issues in mind, it is clear to us that ocean-based industries must integrate and optimize all pre-processing, extraction, cultivation, and formulation stages to obtain an integrated, efficient, sustainable, and profitable marine biorefinery. It is also mandatory to assess different economic and environmental parameters when outlining and implementing each phase of their marine (bio)processing. These steps are crucial for the transition of seafood industries from a linear extractivist model to a circular and sustainable blue economy model, whose fundamental principles are to improve environmental protection, the economy, and public welfare. Nonetheless, although changing seafood production systems is fundamental, it should not be an isolated measure to preserve our marine resources. We should also enforce green and blue economy principles in different industrial, commercial, and social sectors to maintain a healthy and bountiful ocean.
Beyond biorefineries towards a blue economy
Establishing sustainable marine biorefineries is just a fraction of what is necessary to protect our aquatic ecosystems. Although biorefineries can help reduce seafood wastage and marine pollution, they cannot contain overconsumption and global warming damages to the ocean. As presented in the latest report from The Intergovernmental Panel on Climate Change (IPCC) from the UN on August 9th, 2021, global warming has reached unprecedented levels.198 This report warns us that “this is code red for humanity”.199 For the oceans, the IPCC informs us that we can expect more frequent marine heatwaves, ocean acidification, and reduced oxygen levels in the water.198 Furthermore, sea-level rise is likely to be inevitable for at least a hundred years, even if we take drastic measures to halt CO2 emissions in this decade.198 However, the report is also hopeful that it is not too late for action. UN Secretary-General António Guterres was direct in his suggestion to solve the issue: “The solutions are clear. Inclusive and green economies, prosperity, cleaner air, and better health are possible for all if we respond to this crisis with solidarity and courage”.200 Thus, our future relies on the effective implementation of green and blue economy models not only as an alternative but as the only path to preserve our ecosystems and the quality of life.It should also be considered that an estimated 90% of marine species remain undiscovered,201 and UNESCO indicates that half of ocean life will be at the brink of extinction by 2100.202 By degrading the oceans and decreasing marine biodiversity, we are not only impacting current ocean-based production systems. Altogether, we are also losing uncountable future and disruptive biotechnological applications of bioprospecting marine life.
In 2022, it will be ten years since the concept of “blue economy” was first introduced during Rio + 20.34 We suggest policymakers, academics, and activists continue to push forward green and blue economies practices and discuss the issues presented in this perspective during the Rio + 30 in 2022. We also believe that marine biorefineries can play a decisive role in improving the sustainability of ocean-based industries towards a blue economy. However, an integrated and joint effort from all economic and social sectors is necessary to maintain a healthy ocean fit for commercial activities. After all, an ocean economy cannot exist without an ocean.
Conclusions
In this perspective, we assessed the importance of the ocean economy and the urgency of preserving our aquatic environments and resources. We intended to propose how marine biorefineries can benefit the seafood industry and help the transition towards a sustainable and circular production system. This shift could launch the ocean economy into a new cycle, where sustainability, social justice, and revenue are not antagonistic but essential segments of the same structure. This perspective also evaluated the most promising by-products that can be obtained from distinct sources of seafood waste and how and where they can be applied. The design and integration of processing stages of a marine biorefinery were carefully examined in the final sections, where we demonstrated how these aspects are crucial to ensuring the implementation of efficient, profitable, and greener marine biorefineries.As a final message, we believe that despite their complexity, marine biorefineries will be effective systems for seafood waste valorization. They can be additional sources of revenue to ocean-based industries while helping to prevent seafood wastage and sea pollution. Biorefineries embody the most meaningful precepts of the green and blue economies and already have solid industrial applications. Their dissemination, as in the case of the marine sector, should be encouraged and supported by governmental and economic sectors, in particular, considering the current concerns with the shortage of strategic natural resources and the negative consequences of climate change.
Author contributions
JFBP, NVV, and CUM: conceptualization, investigation & visualization; NVV, CUM, and AAO: data curation and writing – original draft; JFBP, RPSO, CMNM, APJ, and VCSE: writing – review & editing; JFBP: supervision and project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
N. V. Veríssimo (2020/14144-8), C. U. Mussagy (2020/08655-0), and C. N. M. Mendonça (2020/13271-6) acknowledge scholarships financial support from FAPESP. The researchers also acknowledge the funding from FAPESP (project 2019/15493-9, 2018/06908-8, 2018/25511-1), CNPq, CAPES (001), and CAPES-PROEX. CIEPQPF is supported by the Fundação para a Ciência e Tecnologia (FCT) through the projects UIDB/EQU/00102/2020 and UIDP/EQU/00102/2020. Some of the figures in this work were created with Bio Render.com (Fig. 4–6). We are also grateful to the blind Reviewers of Green Chemistry for their valuable suggestions to improve the quality of this work.Notes and references
- O. Hoegh-Guldberg, Reviving the ocean economy: The case for action, World Wide Fund, Queensland, Australia, 2015 Search PubMed.
- FAO – Food and Agriculture Organization of the United Nations, The state of world fisheries and aquaculture 2020: Sustainability in action, FAO, Rome, Italy, 2020 Search PubMed.
- D. K. Ng, K. S. Ng and R. T. Ng, in Encyclopedia of Sustainable Technologies, Elsevier, 2017, pp. 299–314 Search PubMed.
- T. Rustad, Electron. J. Environ., Agric. Food Chem., 2003, 2, 458–463 Search PubMed.
- J. H. Clark, R. Luque and A. S. Matharu, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 183–207 CrossRef CAS PubMed.
- A. Cristobal-Sarramian and D. Atzmüller, Agron. Res., 2018, 16, 337–338 Search PubMed.
- S. V. Mohan, G. N. Nikhil, P. Chiranjeevi, C. N. Reddy, M. V. Rohit, A. N. Kumar and O. Sarkar, Bioresour. Technol., 2016, 215, 2–12 CrossRef PubMed.
- J.-G. Winther, M. Dai, T. Rist, A. H. Hoel, Y. Li, A. Trice, K. Morrissey, M. A. Juinio-Meñez, L. Fernandes and S. Unger, Nat. Ecol. Evol., 2020, 4, 1451–1458 CrossRef PubMed.
- J. A. Hargreaves, R. Brummett and C. S. Tucker, in Aquaculture: Farming Aquatic Animals and Plants, John Wiley & Sons, West Sussex, UK, 2019, pp. 617–636 Search PubMed.
- International Energy Agency, Offshore Energy Outlook 2018, International Energy Agency, Online, 2018 Search PubMed.
- P. Q. P. Nguyen, Eur. J. Eng. Technol. Res., 2019, 4, 5–11 CrossRef.
- S. N. Sirimanne, J. Hoffman, W. Juan, R. Asariotis, M. Assaf, G. Ayala, H. Benamara, D. Chantrel, J. Hoffmann and A. Premti, Review of maritime transport 2019, United Nations Conference on Trade and Development, Geneva, Switzerland, 2019 Search PubMed.
- OECD – Organisation for Economic Co-operation and Development, The ocean economy in 2030, IWA Publishing, 2017 Search PubMed.
- C. Bueger and T. Edmunds, Mar. Policy, 2020, 119, 104067 CrossRef.
- World Population Review, GDP Ranked by Country 2021, https://worldpopulationreview.com/countries/countries-by-gdp, (accessed July 31, 2021).
- FAO – Food and Agriculture Organization of the United Nations, The state of world fisheries and aquaculture 2016, FAO, Rome, Italy, 2016 Search PubMed.
- A. G. J. Tacon and M. Metian, Rev. Fish. Sci., 2009, 17, 305–317 CrossRef.
- K. Hill and R. Höfer, in Sustainable solutions for modern economies, RSC Publishing, Cambridge, UK, 2009, Green Chem. Ser. 4, pp. 167–237 Search PubMed.
- FAO – Food and Agriculture Organization of the United Nations, Code of conduct for responsible fisheries, FAO, Rome, Italy, 1995 Search PubMed.
- United States Environmental Protection Agency, Ocean Disposal of Fish Wastes, https://www.epa.gov/ocean-dumping/ocean-disposal-fish-wastes, (accessed July 31, 2021).
- G. Caruso, J. Fish. Sci., 2015, 9, 80–83 Search PubMed.
- P. T. Anastas and E. S. Beach, in Green Chemistry Education, ACS Publications, Washington, USA, 2009 Search PubMed.
- R. Höfer, in Sustainable Solutions for Modern Economies, RSC Publishing, Cambridge, UK, 2009, Green Chem. Ser. 4, pp. 1–11 Search PubMed.
- U. Zoller, Handbook of detergents, Part B: Environmental impact, Marcel Dekker, New York, USA, 2004, vol. 121 Search PubMed.
- J. V. D. Molino, V. Marques, D. de Araújo, A. P. Júnior, P. G. Mazzola and M. S. V. Gatti, Biotechnol. Prog., 2013, 29, 1343–1353 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, UK, 1998 Search PubMed.
- UNEP – United Nations Environment Programme, Green Economy, http://www.unep.org/regions/asia-and-pacific/regional-initiatives/supporting-resource-efficiency/green-economy, (accessed July 31, 2021).
- OECD – Organisation for Economic Co-operation and Development , in Meeting of the Council at Ministerial Level, Organization for Economic Cooperation and Development, 2011.
- UN – United Nations, UN Sustainable Development Goals, https://sustainabledevelopment.un.org/?menu=1300, (accessed March 8, 2020).
- Convention on Biological Diversity, Oceans contain a wealth of biodiversity, https://www.cbd.int/article/biodiversityforwater-1, (accessed July 31, 2021).
- L. Zanna, S. Khatiwala, J. M. Gregory, J. Ison and P. Heimbach, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 1126–1131 CrossRef CAS PubMed.
- O. Hoegh-Guldberg and J. F. Bruno, Science, 2010, 328, 1523–1528 CrossRef CAS PubMed.
- IUCN – International Union for Conservation of Nature, The ocean and climate change, https://www.iucn.org/resources/issues-briefs/ocean-and-climate-change, (accessed July 31, 2021).
- M. Voyer, G. Quirk, A. McIlgorm and K. Azmi, J. Environ. Policy Plann., 2018, 20, 595–616 CrossRef.
- Conservation International, What on Earth is the ‘blue economy’?, https://www.conservation.org/blog/what-on-earth-is-the-blue-economy, (accessed July 31, 2021).
- EC – European Commission, Developing a sustainable blue economy in the European Union, https://ec.europa.eu/commission/presscorner/detail/en/ip_21_2341, (accessed July 31, 2021).
- Center for the Blue Economy, What is the “Blue Economy”?, https://www.middlebury.edu/institute/academics/centers-initiatives/center-blue-economy, (accessed July 31, 2021).
- The Commonwealth, Blue economy, https://thecommonwealth.org/blue-economy, (accessed July 31, 2021).
- The Ocean Foundation, Blue Economy, https://oceanfdn.org/blue-economy/, (accessed July 31, 2021).
- UN – United Nations, Exploring the potential of the blue economy, https://www.un.org/en/desa/exploring-potential-blue-economy, (accessed July 31, 2021).
- World Bank, What is the Blue Economy?, https://www.worldbank.org/en/news/infographic/2017/06/06/blue-economy, (accessed July 31, 2021).
- World Wide Fund for Nature, Principles for a sustainable blue economy, WWF, Online, 2018 Search PubMed.
- The European Commission , The EU blue economy report 2021, The European Commission, Luxembourg, Belgium, 2021.
- EC – European Commission, A European Green Deal, https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en, (accessed August 31, 2021).
- A. K. Jägerbrand, A. Brutemark, J. B. Svedén and M. Gren, Sci. Total Environ., 2019, 695, 133637 CrossRef PubMed.
- R. P. Keller, J. M. Drake, M. B. Drew and D. M. Lodge, Divers. Distrib., 2011, 17, 93–102 CrossRef.
- K. Andersson, F. Baldi, S. Brynolf, J. F. Lindgren, L. Granhag and E. Svensson, in Shipping and the Environment, Springer, Berlin, Germany, 2016, pp. 3–27 Search PubMed.
- T. Bakke, J. Klungsøyr and S. Sanni, Mar. Environ. Res., 2013, 92, 154–169 CrossRef CAS PubMed.
- A. Gbadebo, A. Taiwo and U. Eghele, Indian J. Sci. Technol., 2010, 3, 504–510 CrossRef CAS.
- I. M. Saadoun, in Emerging pollutants in the environment-current and further implications, InTech Open Access Publisher, Online, 2015, pp. 75–104 Search PubMed.
- L. Konkel, Environ. Health Perspect., 2016, 124(12), A230–A235 CrossRef CAS PubMed.
- J. M. Shultz, L. Walsh, D. R. Garfin, F. E. Wilson and Y. Neria, J. Behav. Health Serv. Res., 2015, 42, 58–76 CrossRef PubMed.
- A. Vlysidis, M. Binns, C. Webb and C. Theodoropoulos, Energy, 2011, 36, 4671–4683 CrossRef CAS.
- B. Erickson and P. Winters, Biotechnol. J., 2011, 7, 176–185 CrossRef PubMed.
- B. Kamm, P. R. Gruber and M. Kamm, Biorefineries-industrial processes and products, Wiley-VCH Weinheim, Germany, 2006 Search PubMed.
- E. de Jong and G. Jungmeier, in Industrial Biorefineries & White Biotechnology, Elsevier, Amsterdam, Netherlands, 2015, pp. 3–33 Search PubMed.
- S. Takkellapati, T. Li and M. A. Gonzalez, Clean Technol. Environ. Policy, 2018, 20, 1615–1630 CrossRef CAS PubMed.
- R. S. Baghel, P. Suthar, T. K. Gajaria, S. Bhattacharya, A. Anil and C. R. K. Reddy, J. Cleaner Prod., 2020, 263, 121359 CrossRef CAS.
- A. Pandey, R. Höfer, M. Taherzadeh, M. Nampoothiri and C. Larroche, Industrial biorefineries and white biotechnology, Elsevier, Amsterdam, Netherlands, 2015 Search PubMed.
- F. M. Kerton, Y. Liu, K. W. Omari and K. Hawboldt, Green Chem., 2013, 15, 860–871 RSC.
- UNESCO – United Nations Educational, Scientific and Cultural Organization, Ocean life, https://en.unesco.org/news/ocean-life-marine-age-discovery-0, (accessed August 1, 2021).
- R. L. Olsen, J. Toppe and I. Karunasagar, Trends Food Sci. Technol., 2014, 36, 144–151 CrossRef CAS.
- S. V. Vassilev and C. G. Vassileva, Fuel, 2016, 181, 1–33 CrossRef CAS.
- M. Glicksman, in Food hydrocolloids, Crc Press, 2019, pp. 73–113 Search PubMed.
- FAO – Food and Agriculture Organization of the United Nations, in Training Manual on Gracilaria Culture and Seaweed Processing in China, FAO, Rome, Italy, 1990 Search PubMed.
- S. Ahmadkelayeh and K. Hawboldt, Trends Food Sci. Technol., 2020, 103, 94–108 CrossRef CAS.
- T. A. Fenoradosoa, G. Ali, C. Delattre, C. Laroche, E. Petit, A. Wadouachi and P. Michaud, J. Appl. Phycol., 2010, 22, 131–137 CrossRef CAS.
- M. B. Łabowska, I. Michalak and J. Detyna, Open Chem., 2019, 17, 738–762 Search PubMed.
- P. Nautiyal, K. A. Subramanian and M. G. Dastidar, Fuel Process. Technol., 2014, 120, 79–88 CrossRef CAS.
- M. N. Rammuni, T. U. Ariyadasa, P. H. V. Nimarshana and R. A. Attalage, Food Chem., 2019, 277, 128–134 CrossRef CAS PubMed.
- G. Sanzo, S. Mehariya, M. Martino, V. Larocca, P. Casella, S. Chianese, D. Musmarra, R. Balducchi and A. Molino, Mar. Drugs, 2018, 16, 334 CrossRef PubMed.
- Q. Lu, H. Li, Y. Zou, H. Liu and L. Yang, Algal Res., 2021, 54, 102178 CrossRef.
- S. Y. Koo, K. H. Cha, D.-G. Song, D. Chung and C.-H. Pan, J. Appl. Phycol., 2011, 24, 725–730 CrossRef.
- V. Muhardina, D. Ermaya, Y. Aisyah and S. Haryani, IOP Conference Series: Earth and Environmental Science, 2018, 122, 012074.
- S. F. Bruno, F. J. A. A. Ekorong, S. S. Karkal, M. S. B. Cathrine and T. G. Kudre, Trends Food Sci. Technol., 2019, 85, 10–22 CrossRef CAS.
- W. Cahyaningtyas, R. Kusumawati and J. Basmal, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2021, vol. 733, p. 012110.
- V. Alfonsín, R. Maceiras and C. Gutiérrez, Waste Manage., 2019, 87, 791–797 CrossRef PubMed.
- L. Korzen, I. N. Pulidindi, A. Israel, A. Abelson and A. Gedanken, RSC Adv., 2015, 5, 16223–16229 RSC.
- A. S. Naik and M. Hayes, Trends Food Sci. Technol., 2019, 92, 111–121 CrossRef CAS.
- Q. Shen, G. Song, H. Wang, Y. Zhang, Y. Cui, H. Xie, J. Xue and H. Wang, J. Food Compos. Anal., 2021, 95, 103668 CrossRef CAS.
- P. Subra-Paternault, H. ThongDeng, A. Grélard and M. Cansell, LWT – Food Sci. Technol., 2015, 60, 990–998 CrossRef CAS.
- S. Gulzar, N. Raju, R. C. Nagarajarao and S. Benjakul, Trends Food Sci. Technol., 2020, 100, 307–319 CrossRef CAS.
- A. P. Sánchez-Camargo, M. Â. A. Meireles, A. L. K. Ferreira, E. Saito and F. A. Cabral, J. Supercrit. Fluids, 2012, 61, 71–77 CrossRef.
- G. H. Vieira, R. H. Vieira, A. Macrae and O. V. Sousa, J. Sci. Food Agric., 2005, 85, 1235–1237 CrossRef CAS.
- S. Samant, M. M. Naik, D. C. Vaingankar, S. Y. Mujawar, P. Parab and S. N. Meena, in Advances in Biological Science Research: A Practical Approach, Academic Press, London, UK, 2019, pp. 149–159 Search PubMed.
- A. Perea, T. Kelly and Y. Hangun-Balkir, Green Chem. Lett. Rev., 2016, 9, 27–32 CrossRef CAS.
- S. A. Radzali, B. S. Baharin, R. Othman, M. Markom and R. A. Rahman, J. Oleo Sci., 2014, ess13184 Search PubMed.
- S. Ahmadkelayeh and K. Hawboldt, Trends Food Sci. Technol., 2020, 103, 94–108 CrossRef CAS.
- A. N. Nunes, A. Roda, L. F. Gouveia, N. Fernández, M. R. Bronze and A. A. Matias, ACS Sustainable Chem. Eng., 2021, 9, 3050–3059 CrossRef CAS.
- A. S. Yadav, G. Kolluri, M. Gopi, K. Karthik and Y. Singh, J. Exp. Biol., 2016, 4, 3S Search PubMed.
- S. Kaur and G. S. Dhillon, Crit. Rev. Biotechnol., 2015, 35, 44–61 CrossRef CAS PubMed.
- M. C. Gortari and R. A. Hours, Electron. J. Biotechnol., 2013, 16, 14–14 Search PubMed.
- A. Nawaz, E. Li, S. Irshad, Z. Xiong, H. Xiong, H. M. Shahbaz and F. Siddique, Trends Food Sci. Technol., 2020, 99, 34–43 CrossRef CAS.
- S. Jung, N. S. Heo, E. J. Kim, S. Y. Oh, H. U. Lee, I. T. Kim, J. Hur, G.-W. Lee, Y.-C. Lee and Y. S. Huh, Process Saf. Environ. Prot., 2016, 102, 129–139 CrossRef CAS.
- A. Shavandi, A. E.-D. A. Bekhit, A. Ali and Z. Sun, Mater. Chem. Phys., 2015, 149, 607–616 CrossRef.
- S. Bramhe, T. N. Kim, A. Balakrishnan and M. C. Chu, Mater. Lett., 2014, 135, 195–198 CrossRef CAS.
- N. H. Herpandi, A. Rosma and W. A. Wan Nadiah, Compr. Rev. Food Sci. Food Saf., 2011, 10, 195–207 CrossRef CAS.
- W. Xu, G. Yu, C. Xue, Y. Xue and Y. Ren, Food Chem., 2008, 107, 1597–1604 CrossRef CAS.
- Z. C. Petricorena, Handbook of Food Chemistry, 2015, pp. 403–435 Search PubMed.
- M. T. Morrissey and T. Okada, in Maximising the value of marine by-products, CRC Press, Boca Raton, USA, 2007, pp. 374–396 Search PubMed.
- Y. P. Wu, H. M. Huang, Y. F. Lin, W. D. Huang and Y. J. Huang, Energy, 2014, 70, 43–48 CrossRef CAS.
- T. Lopes da Silva, A. R. Santos, R. Gomes and A. Reis, Environ. Technol. Innovation, 2018, 9, 74–81 CrossRef.
- P. J. García-Moreno, M. Khanum, A. Guadix and E. M. Guadix, Renewable Energy, 2014, 68, 618–624 CrossRef.
- J. Dumay, C. Donnay-Moreno, G. Barnathan, P. Jaouen and J.-P. Bergé, Process Biochem., 2006, 41, 2327–2332 CrossRef CAS.
- A. Khoddami, A. A. Ariffin, J. Bakar and H. M. Ghazali, World Appl. Sci. J., 2009, 7, 127–131 CAS.
- A. Gildberg, J. Aquat. Food Prod. Technol., 2004, 13, 3–11 CrossRef CAS.
- L. F. de Arruda, R. Borghesi and M. Oetterer, Braz. Arch. Biol. Technol., 2007, 50, 879–886 CrossRef.
- U. Hossain and A. Alam, SAARC J. Agric., 2016, 13, 13–25 CrossRef.
- A. Scurria, C. Lino, R. Pitonzo, M. Pagliaro, G. Avellone and R. Ciriminna, Chem. Data Collect., 2020, 25, 100311 CrossRef CAS.
- V. L. V. do Nascimento, V. M. S. Bermúdez, A. L. L. de Oliveira, M. N. Kleinberg, R. T. M. Ribeiro, R. F. A. de Abreu and J. O. B. Carioca, Food Sci. Technol., 2015, 35, 321–325 CrossRef.
- A. Patel, U. Rova, P. Christakopoulos and L. Matsakas, Sci. Total Environ., 2020, 736, 139691 CrossRef CAS PubMed.
- D. Dave, Y. Liu, L. Clark, N. Dave, S. Trenholm and J. Westcott, Bioresour. Technol. Rep., 2019, 7, 100271 CrossRef.
- T. Nagai and N. Suzuki, Food Chem., 2000, 68, 277–281 CrossRef CAS.
- T. Nagai, E. Yamashita, K. Taniguchi, N. Kanamori and N. Suzuki, Food Chem., 2001, 72, 425–429 CrossRef CAS.
- S. Mahboob, J. Food Sci. Technol., 2015, 52, 4296–4305 CrossRef CAS PubMed.
- F. S. Hamdan and N. M. Sarbon, Int. Food Res. J., 2019, 26, 133–140 CAS.
- S. Kongsri, K. Janpradit, K. Buapa, S. Techawongstien and S. Chanthai, Chem. Eng. J., 2013, 215, 522–532 CrossRef.
- M. Ozawa and S. Suzuki, J. Am. Ceram. Soc., 2002, 85, 1315–1317 CrossRef CAS.
- T. S. Espósito, I. P. Amaral, D. S. Buarque, G. B. Oliveira, L. B. Carvalho Jr. and R. S. Bezerra, Food Chem., 2009, 112, 125–130 CrossRef.
- I. Younes, R. Nasri, I. Bkhairia, K. Jellouli and M. Nasri, Food Bioprod. Process., 2015, 94, 453–462 CrossRef CAS.
- R. Saranya and J. Jayapriya, Int. J. Biol. Macromol., 2018, 118, 569–583 CrossRef PubMed.
- P. Stepnowski, G. Ólafsson, H. Helgason and B. Jastorff, Chemosphere, 2004, 54, 413–417 CrossRef CAS PubMed.
- K. Gul, A. Tak, A. K. Singh, P. Singh, B. Yousuf and A. A. Wani, Cogent Food Agric., 2015, 1, 1018696 CrossRef.
- A. Mortensen, Pure Appl. Chem., 2006, 78, 1477–1491 CAS.
- M. Yusuf, M. Shabbir and F. Mohammad, Nat. Prod. Bioprospect., 2017, 7, 123–145 CrossRef CAS PubMed.
- S. Setyahadi, in Seafood Processing By-Products, Springer, New York, USA, 2013, pp. 171–181 Search PubMed.
- C.-Y. Lin and R.-J. Li, Fuel Process. Technol., 2009, 90, 130–136 CrossRef CAS.
- J. La Nasa, J. Mazurek, I. Degano and C. E. Rogge, J. Cult. Herit., 2021, 50, 49–60 CrossRef.
- A. Nasr, Egypt. J. Chem., 2017, 60, 919–928 Search PubMed.
- N. I. Ibrahim, S. Fairus, M. S. Zulfarina and I. N. Mohamed, Nutrients, 2020, 12, 414 CrossRef CAS PubMed.
- C. Fox, Molecules, 2009, 14, 3286–3312 CrossRef CAS PubMed.
- I.-L. Shih, L.-G. Chen, T.-S. Yu, W.-T. Chang and S.-L. Wang, Enzyme Microb. Technol., 2003, 33, 154–162 CrossRef CAS.
- R. Armisén, Agar and agarose biotechnological applications, International Workshop on Gelidium, Springer, Dordrecht, 1991 Search PubMed.
- F. S. Mostafavi and D. Zaeim, Int. J. Biol. Macromol., 2020, 159, 1165–1176 CrossRef CAS PubMed.
- N. T. Ha, C. H. Ha, N. Hayakawa, R. Chujo and S. Kawahara, J. Cult. Herit., 2021, 51, 14–20 CrossRef.
- N. Hayakawa, K. Kida, T. Ohmura, N. Yamamoto, K. Kusunoki and W. Kawanobe, Stud. Conserv., 2014, 59, S230–S231 CrossRef.
- J. Necas and L. Bartosikova, Vet. Med., 2013, 58, 187–205 CrossRef CAS.
- W. Li, Y. Pu, B. Ge, Y. Wang, D. Yu and S. Qin, Int. J. Hydrogen Energy, 2019, 44, 1182–1191 CrossRef CAS.
- D. Pauly and D. Zeller, Mar. Policy, 2017, 77, 176–181 CrossRef.
- C. U. Mussagy, J. Winterburn, V. C. Santos-Ebinuma and J. F. B. Pereira, Appl. Microbiol. Biotechnol., 2019, 103, 1095–1114 CrossRef CAS PubMed.
- C. U. Mussagy, S. Khan and A. M. Kot, Crit. Rev. Food Sci. Nutr., 2021, 1–15 Search PubMed.
- M. B. Kaczmarek, K. Struszczyk-Swita, X. Li, M. Szczęsna-Antczak and M. Daroch, Front. Bioeng. Biotechnol., 2019, 7, 243 CrossRef PubMed.
- Y. Kim and R.-D. Park, J. Korean Soc. Appl. Biol. Chem., 2015, 58, 545–554 CrossRef CAS.
- N. Coello, Bioresour. Technol., 2000, 73, 221–225 CrossRef CAS.
- P. V. Suresh, T. G. Kudre and L. C. Johny, in Waste to Wealth, Springer, Singapore, 2018, pp. 111–139 Search PubMed.
- P. Anacleto, A. L. Maulvault, V. Barbosa, M. L. Nunes and A. Marques, in Encyclopedia of Food and Health, Elsevier, Amsterdam, Netherlands, 2016, pp. 764–771 Search PubMed.
- N. Qavami, B. Naghdi and M. Mehregan, Trakia J. Sci., 2017, 15, 83 CrossRef.
- Imarc, Chitosan Market Size, Price Trends, Analysis & Forecast 2021–2026, https://www.imarcgroup.com/chitosan-market, (accessed October 20, 2021).
- L. T. Antelo, G. M. de Hijas-Liste, A. Franco-Uría, A. A. Alonso and R. I. Pérez-Martín, J. Cleaner Prod., 2015, 104, 489–501 CrossRef CAS.
- P. Jayasinghe and K. Hawboldt, Renewable Sustainable Energy Rev., 2012, 16, 798–821 CrossRef CAS.
- S. S. Pattanaik, P. B. Sawant, K. A. M. Xavier, K. Dube, P. P. Srivastava, V. Dhanabalan and N. K. Chadha, Aquaculture, 2020, 515, 734594 CrossRef CAS.
- J.-J. Deng, H.-H. Mao, W. Fang, Z.-Q. Li, D. Shi, Z.-W. Li, T. Zhou and X.-C. Luo, J. Cleaner Prod., 2020, 271, 122655 CrossRef CAS.
- R. Devi and R. Dhamodharan, ACS Sustainable Chem. Eng., 2018, 6, 846–853 CrossRef CAS.
- P. Charoenvuttitham, J. Shi and G. S. Mittal, Sep. Sci. Technol., 2006, 41, 1135–1153 CrossRef CAS.
- G. T. Kjartansson, S. Zivanovic, K. Kristbergsson and J. Weiss, J. Agric. Food Chem., 2006, 54, 5894–5902 CrossRef CAS PubMed.
- B. Bradić, U. Novak and B. Likozar, Green Process. Synth., 2019, 9, 13–25 Search PubMed.
- N. V. dos Santos, M. Martins, V. C. Santos-Ebinuma, S. P. M. Ventura, J. A. P. Coutinho, S. R. Valentini and J. F. B. Pereira, ACS Sustainable Chem. Eng., 2018, 6, 9383–9393 CrossRef CAS.
- M. G. Freire, A. F. M. Claudio, J. M. M. Araujo, J. A. P. Coutinho, I. M. Marrucho, J. N. C. Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 4966–4995 RSC.
- D. Zhao, W.-C. Huang, N. Guo, S. Zhang, C. Xue and X. Mao, Polymers, 2019, 11, 409 CrossRef PubMed.
- O. Ghorbel-Bellaaj, I. Younes, H. Maâlej, S. Hajji and M. Nasri, Int. J. Biol. Macromol., 2012, 51, 1196–1201 CrossRef CAS PubMed.
- O. Ghorbel-Bellaaj, K. Jellouli, I. Younes, L. Manni, M. O. Salem and M. Nasri, Appl. Biochem. Biotechnol., 2011, 164, 410–425 CrossRef CAS PubMed.
- L. Manni, K. Jellouli, O. Ghorbel-Bellaaj, R. Agrebi, A. Haddar, A. Sellami-Kamoun and M. Nasri, Appl. Biochem. Biotechnol., 2010, 160, 2308–2321 CrossRef CAS PubMed.
- C. T. Doan, T. N. Tran, V. B. Nguyen, T. P. K. Vo, A. D. Nguyen and S.-L. Wang, Int. J. Biol. Macromol., 2019, 131, 706–715 CrossRef CAS PubMed.
- O. Ghorbel-Bellaaj, N. Hmidet, K. Jellouli, I. Younes, H. Maâlej, R. Hachicha and M. Nasri, Int. J. Biol. Macromol., 2011, 48, 596–602 CrossRef CAS PubMed.
- R. Castro, I. Guerrero-Legarreta and R. Bórquez, Biotechnol. Rep., 2018, 20, e00287 CrossRef PubMed.
- N. Bhaskar, P. V. Suresh, P. Z. Sakhare and N. M. Sachindra, Enzyme Microb. Technol., 2007, 40, 1427–1434 CrossRef CAS.
- P. Zhu, Z. Gu, S. Hong and H. Lian, Carbohydr. Polym., 2017, 177, 217–223 CrossRef CAS PubMed.
- N. V. Dos Santos, V. de Carvalho Santos-Ebinuma, A. Pessoa Junior and J. F. B. Pereira, J. Chem. Technol. Biotechnol., 2018, 93, 1845–1863 CrossRef CAS.
- E. L. Smith, J. Thomas, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- J. L. K. Mamilla, U. Novak, M. Grilc and B. Likozar, Biomass Bioenergy, 2019, 120, 417–425 CrossRef CAS.
- M. Jablonský, A. Škulcová, A. Malvis and J. Šima, J. Biotechnol., 2018, 282, 46–66 CrossRef PubMed.
- Y. Qin, X. Lu, N. Sun and R. D. Rogers, Green Chem., 2010, 12, 968–971 RSC.
- L. D. Tolesa, B. S. Gupta and M.-J. Lee, Int. J. Biol. Macromol., 2019, 130, 818–826 CrossRef CAS PubMed.
- P. S. Saravana, T. C. Ho, S.-J. Chae, Y.-J. Cho, J.-S. Park, H.-J. Lee and B.-S. Chun, Carbohydr. Polym., 2018, 195, 622–630 CrossRef CAS PubMed.
- W. Bi, M. Tian, J. Zhou and K. H. Row, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 2243–2248 CrossRef CAS PubMed.
- M. Illera-Vives, S. S. Labandeira, L. M. Brito, A. López-Fabal and M. E. López-Mosquera, Sci. Hortic., 2015, 186, 101–107 CrossRef CAS.
- M. Radziemska, M. D. Vaverková, D. Adamcová, M. Brtnický and Z. Mazur, Waste Biomass Valorization, 2019, 10, 2537–2545 CrossRef CAS.
- W. Y. Mo, Y. B. Man and M. H. Wong, Sci. Total Environ., 2018, 613–614, 635–643 CrossRef CAS PubMed.
- S. J. Horn, S. I. Aspmo and V. G. H. Eijsink, J. Appl. Microbiol., 2005, 99, 1082–1089 CrossRef CAS PubMed.
- S. Maneein, J. J. Milledge, B. V. Nielsen and P. J. Harvey, Fermentation, 2018, 4, 100 CrossRef CAS.
- N. V. dos Santos, C. F. Saponi, T. L. Greaves and J. F. B. Pereira, RSC Adv., 2019, 9, 22853–22858 RSC.
- N. V. Dos Santos, C. F. Saponi, T. M. Ryan, F. L. Primo, T. L. Greaves and J. F. B. Pereira, Int. J. Biol. Macromol., 2020, 164, 3474–3484 CrossRef CAS PubMed.
- M. B. Fagundes, R. G. Vendruscolo, M. M. Maroneze, J. S. Barin, C. R. de Menezes, L. Q. Zepka, E. Jacob-Lopes and R. Wagner, Waste Biomass Valorization, 2018, 10, 1295–1302 CrossRef.
- Evonik, Unique facility begins production of omega-3 fatty acids for sustainable salmon farming - Evonik Industries, https://corporate.evonik.com/en/media/press-releases/corporate/unique-facility-begins-production-of-omega-3-fatty-acids-for-sustainable-salmon-farming-114610.html, (accessed October 20, 2021).
- R. Hagen, in Sustainable Solutions for Modern Economies, RSC Publishing, Cambridge, UK, 2009, Green Chem. Ser. 4, pp. 436–478 Search PubMed.
- G. Frazzetto, EMBO Rep., 2003, 4, 835–837 CrossRef CAS PubMed.
- S. Heux, I. Meynial-Salles, M. J. O'Donohue and C. Dumon, Biotechnol. Adv., 2015, 33, 1653–1670 CrossRef CAS PubMed.
- M. S. Prabhu, A. Israel, R. R. Palatnik, D. Zilberman and A. Golberg, J. Appl. Phycol., 2020, 1–12 Search PubMed.
- M. Abdollahi and I. Undeland, Food Chem., 2020, 332, 127294 CrossRef CAS PubMed.
- 525 Solutions, About the company, http://www.525solutions.com/, (accessed August 8, 2021).
- X. García-Santiago, A. Franco-Uría, L. T. Antelo, J. A. Vázquez, R. Pérez-Martín, M. T. Moreira and G. Feijoo, J. Ind. Ecol., 2021, 25, 789–801 CrossRef.
- K. Fang, R. Heijungs and G. R. de Snoo, Ecol. Indic., 2014, 36, 508–518 CrossRef.
- K. Fang, R. Heijungs and G. De Snoo, Metall. Res. Technol, 2013, 110, 77–86 Search PubMed.
- M. L. Brusseau, in Environmental and Pollution Science, Elsevier, 2019, pp. 585–603 Search PubMed.
- I. Ruiz-Salmón, J. Laso, M. Margallo, P. Villanueva-Rey, E. Rodríguez, P. Quinteiro, A. C. Dias, C. Almeida, M. L. Nunes and A. Marques, Sci. Total Environ., 2020, 144094 Search PubMed.
- M. Torres-ACosta, N. V. Dos Santos, S. P. M. Ventura, J. A. P. Coutinho, M. Rito-Palomares and J. F. B. Pereira, Sep. Purif. Technol., 2021, 254, 117595 CrossRef CAS.
- T. Altintzoglou, P. Honkanen and R. D. Whitaker, J. Cleaner Prod., 2021, 285, 125487 CrossRef.
- IPCC – Intergovernmental Panel on Climate Change, Climate change widespread, rapid, and intensifying, https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/, (accessed August 10, 2021).
- IPCC – Intergovernmental Panel on Climate Change, Climate Change 2021: the Physical Science Basis, IPCC, Geneva, Switzerland, 2021 Search PubMed.
- A. Guterres, Secretary-General Calls Latest IPCC Climate Report ‘Code Red for Humanity’, Stressing ‘Irrefutable’ Evidence of Human Influence, https://www.un.org/press/en/2021/sgsm20847.doc.htm, (accessed August 10, 2021).
- C. Mora, D. P. Tittensor, S. Adl, A. G. Simpson and B. Worm, PLoS Biol., 2011, 9, e1001127 CrossRef CAS PubMed.
- UNESCO – United Nations Educational, Scientific and Cultural Organization, Facts and figures on marine biodiversity | United Nations Educational, Scientific and Cultural Organization, http://www.unesco.org/new/en/natural-sciences/ioc-oceans/focus-areas/rio-20-ocean/blueprint-for-the-future-we-want/marine-biodiversity/facts-and-figures-on-marine-biodiversity/, (accessed August 29, 2021).
| This journal is © The Royal Society of Chemistry 2021 |