Open Access Article
Open Access ArticleMechanistic understanding of humin formation in the conversion of glucose and fructose to 5-hydroxymethylfurfural in [BMIM]Cl ionic liquid†
Zhanwei Xua,
Yiwen Yang ab,
Peifang Yana,
Zhi Xiaa,
Xuebin Liu*c and
Z. Conrad Zhang
ab,
Peifang Yana,
Zhi Xiaa,
Xuebin Liu*c and
Z. Conrad Zhang *a
*a
aState Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: zczhang@yahoo.com
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cEnergy Innovation Laboratory, Applied Chemistry and Physics Centre of Expertise, BP Group Technology, Dalian 116023, P. R. China. E-mail: xuebin.liu@se1.bp.com
First published on 18th September 2020
Abstract
Humin formation is one of the key issues that hinders economical 5-HMF production from hexose sugars such as glucose and fructose. In this work, the mechanism of humin formation in glucose/fructose conversion to HMF was studied in an ionic liquid system (1-butyl-3-methylimidazolium chloride, [BMIM]Cl) with CrCl3 as the catalyst. Elemental analysis, XRD, FT-IR, and TEM were applied to study the molecular structure and morphology of the solid humins. The possible intermediates to form solid humins were investigated by HPLC-MS. We synthesized furanic model compounds that mimic the experimentally identified humin intermediates to investigate the mechanism of humin growth at an early stage. The results showed that a furan compound bearing a hydroxymethyl and an electron-donating group was unstable due to three types of reactions: (1) bimolecular ether formation reactions; (2) intermolecular addition reaction; (3) furan ring opening reaction with water. The stability of a furan compound in [BMIM]Cl was increased when the hydroxymethyl group of a furan compound was protected by a methyl group, and the stability was further enhanced with an additional electron-withdrawing group (such as an aldehyde group) on the furan ring. Protecting the hydroxymethyl group of 5-HMF with a methyl group allows easy separation of the products from the [BMIM]Cl solvent through extraction.
Introduction
Lignocellulosic biomass is mainly composed of lignin, cellulose, and hemicellulose. One of the biomass utilization pathways is to transform biomass to platform chemicals, which are further processed to various value-added chemicals.1–7 Among products that can be obtained from cellulose, 5-hydroxymethylfurfural (5-HMF) has been recognized as a potentially versatile platform chemical.8–10 5-HMF and its derivatives offer the potential to replace an array of compounds derived from fossil fuels, enabling biomass to be a viable feedstock for the production of liquid fuels and chemicals.11–13 Especially, 2,5-furandicarboxylic acid, the oxidation product of 5-HMF, is an alternative of purified terephthalic acid (PTA) monomer.14–16Up to now, hexose sugars, such as glucose and fructose have been successfully used to produce 5-HMF with acid catalysts in various solvents, such as aqueous solutions with/without organic solvents, organic solvents, and ionic liquids.17 But economical industrial production of 5-HMF from hexose sugars remains hindered mostly due to separation and purification of 5-HMF from byproducts, in particular humins (Scheme 1). The formation of humins also largely attributes to the reduced 5-HMF yield. Therefore, a mechanistic understanding of humins formation is significantly important for the minimization of humins in economical 5-HMF production.
Sumerskii et al. synthesized humins by acid hydrolysis of saccharides and furan compounds including 5-HMF under conditions similar to an industrial process of acid-catalyzed hydrolysis of wood (0.5% H2SO4, 2 h, 175–180 °C) in water. Ether formation between the hydroxymethyl groups of bimolecular 5-HMF, and intermolecular acetal reaction between the aldehyde group and hydroxymethyl group of 5-HMF were proposed as the possible humins formation pathways.18 Lund et al. proposed that, in acid-catalyzed conversion of 5-HMF, rehydration of 5-HMF generated 2,5-dioxo-6-hydroxyhexanal (DHH), followed by aldol condensation of DHH with the carbonyl groups to form humins.19 van Zandvoort et al. synthesized a series of humins through acid-catalyzed dehydration of pentose or hexose in aqueous solution. It was proposed that humins was formed through condensation reactions at the α-position of furan ring of 5-HMF, and nucleophilic attack on the aldehyde group of 5-HMF by β-position of furan rings.20 Maruani et al. studied the acidic hydrothermal dehydration of glucose to humins. Water-soluble oligomers of glucose were identified to react with 5-HMF through acetal reaction to form humins.21
The effect of organic solvent on humins formation was also studied. For instance, based on study of the 5-HMF derived humins formed in the Brønsted acid catalyzed degradation of 5-HMF, Vlachos et al. proposed that dimethylsulfoxide (DMSO) as a co-solvent was found to interact with aldehyde group of 5-HMF, thus stabilized the aldehyde group against nucleophilic attack, such as the aldol condensation of DHH, and the nucleophilic attack of the α- and β-position of furan ring.22 Fu et al. studied the humins formation mechanism in glucose dehydration to 5-HMF using tetrahydrofuran (THF) and compressed CO2 as the mixed solvent. It was also found that THF and compressed CO2 could be used for suppressing oligomers generated through the cross-condensation of 5-HMF and levulinic acid (LA), self-condensation of 5-HMF, and degradation of glucose/fructose in H2O.23 In general, in aqueous systems with/without organic solvents, reactions between 5-HMF and its derivative oxygenates such as DHH and LA (from the reaction of 5-HMF with water) were considered responsible for the formation of byproduct humins.
Compared with aqueous and organic solutions, certain ionic liquids are effective solvents for one-pot synthesis of 5-HMF from hexoses under mild conditions. Cr(II, III) salts were reported as catalysts for isomerization/dehydration of glucose to 5-HMF in ionic liquids, such as 1-butyl-3-methylimidazolium chloride ([BMIM]Cl).24–28 Ionic liquids are also applied in 5-HMF synthesis from fructose or even cellulose.29 For hexoses conversion in [BMIM]Cl with CrCl2 or CrCl3 as catalysts, typical 5-HMF selectivity is about 70% and 90% with glucose and fructose as feedstock, respectively. Byproducts were dominated by humins.24–28 Significant efforts have been made to reduce the humins formation such as adopting proper ligands for CrCl3, introducing an organic phase for in situ 5-HMF extraction, and reducing sugar feed concentration.24 A biphasic system consisting of [BMIM]Cl and glycol dimethyl ether (GDE) significantly increased the 5-HMF yield by minimizing the residence time of 5-HMF in the IL reaction media.24 However, the humins byproducts remained substantial even under optimized conditions. 5-HMF alone was also found stable in certain IL phases but reactive for humins formation in aqueous media.18,28 In view of the observed difference in byproduct formation, e.g. levulinic acid is a common byproducts in aqueous solvent but it is absent in most IL solvents, it is conceivable that the humins formation mechanism in aqueous phase systems should be different from that in IL solvent systems. Therefore, a better fundamental understanding of humins formation in IL will be valuable for economical 5-HMF production process with minimized humins formation.
In this work, we studied individual model compounds as well as mixtures of model compounds in [BMIM]Cl with and without the presence of CrCl3 catalyst at various conditions. Solid and soluble humins were prepared in [BMIM]Cl containing CrCl3 by using glucose or fructose as a feedstock under intensified conditions to accelerate the formation of humins. The humins samples were characterized in detail to identify possible precursors for humins at an early stage and to determine the structure and morphology of aged solid humins. The main objective of this work was to understand the mechanism of humins formation by identifying the precursors and the reactions at an early stage. The roles of hydroxymethyl and aldehyde groups of 5-HMF in forming humins precursors and possible reactions thereof were investigated using model compounds of furan derivatives. The reactivity of model compounds of furan derivatives was further studied by using substituents with various electronic properties.
Results and discussion
Our approach was to first obtain compositional and structural information of humins formed in ionic liquids. Solid humins was prepared through CrCl3 catalyzed isomerization/dehydration of glucose/fructose in [BMIM]Cl ionic liquid under intensified conditions as shown in Table 1. It should be noted that a high weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for sugar
2 for sugar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [BMIM]Cl solvent was used to intensify humins formation, as high glucose concentration always leads to a poor 5-HMF selectivity due to dominant humins.24 A temperature as high as 130 °C was also used for the same purpose. The effect of reaction temperatures on solid humins formation was explored (entries 1–4, Table 1). The carbon yield of solid humins increased from 29.7% to 78.7% with elevating reaction temperature from 100 °C to 130 °C, with a decrease in the 5-HMF yield. Based on elemental analysis, the total C wt% of the solid humins was slightly increased with increased temperature, probably as the dehydration reaction involved in solid humins formation was enhanced at elevated temperatures. The yield of soluble humins decreased with increasing the temperature from 100 °C to 130 °C, suggesting that the soluble humins may be intermediates for the formation of solid humins. In a [BMIM]Cl system, water, formed from dehydration of sugar, was increased with reaction time. We therefore further studied the effect of water on humins formation (entries 5–8, Table 1). Generally, with increasing water concentration, the yield of soluble humins increased while the yield of solid humins decreased. Water may suppress the dehydration of soluble humins to solid humins, resulting a decrease in the yield of solid humins. Small amount of water can increase 5-HMF yield from 32.5% to 39.9% (entries 5 and 6, Table 1). Adding more water caused a steady decline of 5-HMF yield, as water may participate the formation of soluble humins (entries 6–8, Table 1). The solid humins from fructose was also prepared under similar conditions (entry 9, Table 1). Compared with glucose feedstock (entry 5, Table 1), more fructose feedstock could be converted to 5-HMF. The C wt% of solid humins from fructose or glucose were all around 60%. It should be noticed that no nitrogen was detected in all solid humins, indicating that [BMIM]Cl did not participate in solid humins formation. As a result, solid humins formation under intensified reaction conditions and separation by filtration may be a feasible method for the recycled use of [BMIM]Cl solvent.
[BMIM]Cl solvent was used to intensify humins formation, as high glucose concentration always leads to a poor 5-HMF selectivity due to dominant humins.24 A temperature as high as 130 °C was also used for the same purpose. The effect of reaction temperatures on solid humins formation was explored (entries 1–4, Table 1). The carbon yield of solid humins increased from 29.7% to 78.7% with elevating reaction temperature from 100 °C to 130 °C, with a decrease in the 5-HMF yield. Based on elemental analysis, the total C wt% of the solid humins was slightly increased with increased temperature, probably as the dehydration reaction involved in solid humins formation was enhanced at elevated temperatures. The yield of soluble humins decreased with increasing the temperature from 100 °C to 130 °C, suggesting that the soluble humins may be intermediates for the formation of solid humins. In a [BMIM]Cl system, water, formed from dehydration of sugar, was increased with reaction time. We therefore further studied the effect of water on humins formation (entries 5–8, Table 1). Generally, with increasing water concentration, the yield of soluble humins increased while the yield of solid humins decreased. Water may suppress the dehydration of soluble humins to solid humins, resulting a decrease in the yield of solid humins. Small amount of water can increase 5-HMF yield from 32.5% to 39.9% (entries 5 and 6, Table 1). Adding more water caused a steady decline of 5-HMF yield, as water may participate the formation of soluble humins (entries 6–8, Table 1). The solid humins from fructose was also prepared under similar conditions (entry 9, Table 1). Compared with glucose feedstock (entry 5, Table 1), more fructose feedstock could be converted to 5-HMF. The C wt% of solid humins from fructose or glucose were all around 60%. It should be noticed that no nitrogen was detected in all solid humins, indicating that [BMIM]Cl did not participate in solid humins formation. As a result, solid humins formation under intensified reaction conditions and separation by filtration may be a feasible method for the recycled use of [BMIM]Cl solvent.
| Entry | Water (g) | T (°C) | Solid huminsb (%) | Soluble huminsc (%) | HMFb (%) | Elemental analysis of solid humins | |
|---|---|---|---|---|---|---|---|
| N (wt%) | C (wt%) | ||||||
| a Reaction conditions: [BMIM]Cl (10.0 g), glucose (5.0 g), and CrCl3 (0.74 g) were mixed, and reaction time was 4 h.b Carbon yield was given.c Soluble humins was calculated based on carbon mass balance after accounting the total amount of 5-HMF and solid humins.d Reaction time was 2 h.e Fructose (5.0 g) was the feedstock. | |||||||
| 1 | 0 | 100 | 29.7 | 30.9 | 39.4 | 0 | 59.4 |
| 2 | 0 | 110 | 52.6 | 15.5 | 31.9 | 0 | 60.5 |
| 3 | 0 | 120 | 67.9 | 10.5 | 21.6 | 0 | 61.5 |
| 4 | 0 | 130 | 78.7 | 7.4 | 13.9 | 0 | 62.4 |
| 5d | 0 | 110 | 33.8 | 33.7 | 32.5 | 0 | 59.6 |
| 6d | 0.2 | 110 | 31.9 | 28.2 | 39.9 | 0 | 59.9 |
| 7d | 0.5 | 110 | 22.7 | 45 | 32.3 | 0 | 59.7 |
| 8d | 1.0 | 110 | 17.5 | 56.6 | 25.9 | 0 | 58.6 |
| 9d,e | 0 | 110 | 33.9 | 24 | 42.1 | 0 | 61.1 |
Solid humins appeared brown in its optical microscope image (Fig. 1a) while its TEM image showed large pieces of aggregated amorphous solid (Fig. 1b). XRD of solid humins confirmed it was an amorphous solid (Fig. S1, ESI†). TG/DTG of solid humins (Fig. S2, ESI†) at a N2 atmosphere showed a maximum decomposition temperature around 420 °C with 50 wt% of initial solid humins remained. The residual weight in TG/DTG analyses could be even higher (from 60.5 to 87.1 wt%) while the corresponding H wt% of the residual from 4.2 to 0.7 wt%, indicating a further dehydration.
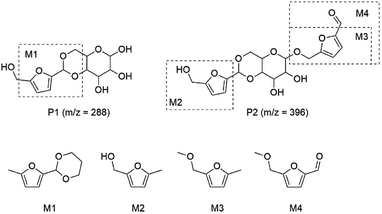
To understand the possible humins intermediates in [BMIM]Cl phase, we applied HPLC-MS to identify the possible precursors in the brown [BMIM]Cl phase. The peak in HPLC-MS spectrum (m/z = 288) may be assigned to humins precursor P1 in Scheme 2, formed through the acetal reaction between aldehyde group of 5-HMF and diol group of glucose. The peak with m/z = 396 may be assigned to humins precursor P2 in Scheme 2, formed through the reaction between hydroxyl group of 5-HMF and glucose moiety of P1. The humins precursors P1 and P2 were also detected in acid-catalyzed hydrolysis of cellulose in ionic liquids by Alexis T. Bell et al.30 However, no direct evidence for the proposed reaction mechanism with compounds P1 and P2 as precursors for solid humins is available because compounds P1 and P2 are too instable to prepare. Therefore, we chose 2-(5-methylfuran-2-yl)-1,3-dioxane (M1), (5-methylfuran-2-yl)methanol (M2), 2-(methoxymethyl)-5-methylfuran (M3), and 5-(methoxymethyl)furan-2-carbaldehyde (M4) as model compounds for humins precursors P1 and P2 to understand possible reactions pathways involved in humins formation at an early stage (Scheme 2).
The reactivity of acetal group of model compounds M1 and M2 was first studied. M1 is chosen as the model compound because M1 has the acetal group but without the reactive hydroxymethyl group, which is replaced as a methyl group in M1 instead. As shown in Table S1, ESI,† M1 reacted with water releasing aldehyde and diol within minutes. Therefore, one of the methods to suppress the formation of acetal-type humins precursors is by adding certain amount of water in [BMIM]Cl to regenerate 5-HMF and glucose.
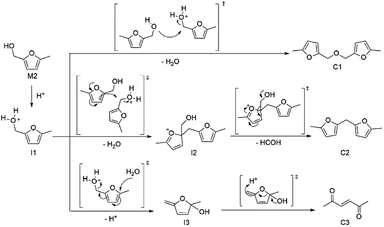
We further studied the reactivity of furfuryl alcohol moiety of humins precursors P1 and P2 with M2 as the model compound, where the reactive acetal group is replaced by a stable methyl group (Scheme 3). As shown in Table 2, M2 became more reactive with increasing reaction temperatures (entries 1 to 4, Table 2). Three compounds, including 5,5′-(oxybis(methylene))bis(2-methylfuran) (C1), bis(5-methylfuran-2-yl)methane (C2), and hex-3-ene-2,5-dione (C3), were detected in reaction media under 90 °C by GC-MS analysis (entry 2, Table 2). The proposed pathways to generate the three compounds C1–C3 from model compound M2 are shown in Scheme 3. The hydroxyl group of M2 first reacts with a proton to generate the intermediate I1. The intermediate I1 may undergo three types of reactions to generate compounds C1–C3: (1) the intermediate I1 reacts with the hydroxyl group of M2 to generate ether compound C1 through intermolecular nucleophilic substitution reaction; (2) the intermolecular electrophilic substitution forms intermediate I2, followed by rearrangement reaction to generate C2; (3) protonated hydroxyl group induces furan ring opening reaction with water through intermediate I3 to form C3. Compounds C1 and C2 may further react with water in the presence of acid to generate C3-type ketone compound. Compound C3 may further react with aldehyde or ketone groups through Aldol condensation. Such complex reactions may contribute to the growth of solid humins from sugar, 5-HMF, humins precursors P1, P2, and so on. To further verify the above proposed three types of reactions in solid humins formation, the structure of solid humins was studied by IR (Fig. S2, ESI†). For the typical solid humins, the peak (1672 cm−1) is assigned to aldehyde group from 5-HMF. The peak (1708 cm−1) is assigned to ketone group, which is assigned to C3-type structure formed through the reaction of furan ring with water. The peaks at 1510 and 1579 cm−1 are ascribed to furan ring stretches, which probably belong to C1 and C2-type furan structures.
| Entry | Water (mg) | Temperature (°C) | Conversion (%) |
|---|---|---|---|
| a Reaction conditions: [BMIM]Cl (0.5 g), CrCl3 (6.6 mg), substrate (56 mg), 90 °C, 0.5 h, 600 rpm, under air.b HCl was used as an acid catalyst instead of CrCl3. | |||
| 1 | 0 | 80 | 12.2 |
| 2 | 0 | 90 | 21.2 |
| 3 | 0 | 100 | 61.6 |
| 4 | 0 | 110 | 84.8 |
| 5 | 20 | 90 | 35.9 |
| 6 | 50 | 90 | 65.3 |
| 7 | 100 | 90 | 86.2 |
| 8b | 20 | 90 | 23.8 |
According to the proposed mechanism shown in Scheme 3, water may suppress the dehydration processes involved in the formation of C1 and C2. However, experimental results show that water actually enhanced model compound M2 conversion in [BMIM]Cl (entries 2, and 5–7, Table 2). Water seems to promote the furan ring opening reaction to generate C3 (Scheme 3). In the presence of Brønsted acid as a catalyst, compounds C2 and C3 were all detected, but C1 was not detected (entry 8, Table 2), suggesting that the ether C1 formation through intermolecular nucleophilic substitution reaction was probably catalyzed by Cr catalyst.
In order to prevent the reactive hydroxymethyl group from participating humins formation, we further studied if the reactivity of hydroxymethyl of M2 can be passivated or not. The hydroxyl group of M2 is then replaced with a methoxy group as the model compound M3. Compared with M2 (entry 1, Table 2), the M3 conversion decreased to 5.7% at the same reaction conditions (entry 1, Table 3), indicating that etherification of hydroxymethyl group passivates its reactivity and inhibits side reactions as shown in Scheme 3. Although passivated, model compound M3 can be converted as shown in Scheme 4. GC-MS analysis results indicate that M3 can be reversibly converted back to M2 or reacted with water to form ring opening compound 1-methoxyhexane-2,5-dione (C4) through intermediate I4. Intermediates similar to compound C4 may react with aldehyde group of 5-HMF or glucose through aldol condensation to form solid humins.19 Scheme 4 also implies water may promote M3 conversion to M2 and C4, which is in line with our experimental results shown in Table 3. As shown in entries 1–4 of Table 3, M3 conversion was increase from 5.7% to 64.4% when the amount of water added was increased from 0 to 100 mg. Therefore, based on the above results and compared with the reactivity of M2 (entries 1–4, Table 2), the hydroxy group on a furan ring can be passivated by ether group but only to certain extent.
| Entry | Water (mg) | Temperature (°C) | Conversion (%) |
|---|---|---|---|
| a Reaction conditions: [BMIM]Cl (0.5 g), CrCl3 (6.6 mg), substrate (0.5 mmol), 90 °C, 0.5 h, 600 rpm, under air. | |||
| 1 | 0 | 90 | 5.7 |
| 2 | 20 | 90 | 8.1 |
| 3 | 50 | 90 | 22.3 |
| 4 | 100 | 90 | 64.4 |
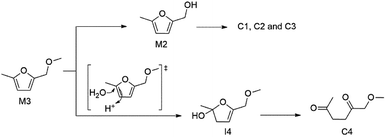 | ||
| Scheme 4 Proposed mechanism on the decomposition of model compound 2-(methoxymethyl)-5-methylfuran (M3). | ||
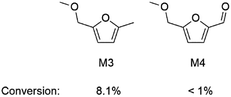
Finally, 5-(methoxymethyl)furan-2-carbaldehyde (M4) was chosen as a model compound for the humins precursor P2 (Scheme 2). Unlike the methyl group (electron-donating group) of M3, the formyl group of M4 is an electron-withdrawing group. Compared to M3 in [BMIM]Cl in the presence of Cr catalyst and water (M3 conversion is 8.1%, Scheme 5), the stability of M4 was further increased as its conversion was less than 1%, suggesting that the stability of a furanic compound in our reaction system may benefit from an electron-withdrawing group. We also found that only 12% of 5-HMF was extracted to a glycol dimethyl ether (GDE) phase (Table S2, ESI†). Our previous work showed that the hydrogen bond of [BMIM]Cl with 5-HMF impeded 5-HMF extraction from [BMIM]Cl phase.24 It was found that model compound M4 was easily extracted from [BMIM]Cl phase, with 82% of M4 extracted to GDE as the hydrogen bond of [BMIM]Cl with M4 was broken by the replacement of hydroxyl group by a methyl group (Table S2, ESI†). Thus, model compound M4 may be an alternative product of 5-HMF from sugar, as M4 may be stable enough to minimize humins formation and easily separated from [BMIM]Cl through extraction.
Conclusions
The mechanism of humins formation in the conversion of glucose and fructose in [BMIM]Cl solvent with CrCl3 catalyst was studied by characterization of solid humins prepared under intensified conditions. The yield of solid humins was increased with increasing temperature and decreasing amount of water. No nitrogen was detected in all solid humins, indicating that [BMIM]Cl did not participate in humins formation. The separation of the solid humins by filtration provides feasible method for recycled use of [BMIM]Cl. From HPLC-MS analysis of separated brown liquid [BMIM]Cl phase, the compounds P1 and P2 identified from the reaction of 5-HMF with glucose are proposed to be the primary soluble humins precursors for the generation of solid humins. 2-(5-Methylfuran-2-yl)-1,3-dioxane (M1), (5-methylfuran-2-yl)methanol (M2), 2-(methoxymethyl)-5-methylfuran (M3), and 5-(methoxymethyl)furan-2-carbaldehyde (M4) were studied as model compounds for humins precursors P1 and P2. M1 regenerates aldehyde and diol in the presence of water. The stability of model compounds M2, M3, and M4 in [BMIM]Cl followed the order: M2 (bearing hydroxymethyl group) < M3 (bearing methoxymethyl group) < M4 (bearing methoxymethyl and electron-withdrawing aldehyde groups). The conversion of M2 bearing free hydroxyl group was increased with increasing temperature and amount of water. The protonated hydroxymethyl group of M2 reacts with oxygen or carbon of other furanic compounds through substitution reaction. M2 also reacts with water to form ketone. M3 reacts with water to form M2, or forms ketone through furan ring opening reaction. Based on the reaction results of model compounds, the precursors of solid humins may undergo furan ring opening reaction with water to form ketones at an early stage. The precursors of solid humins may also grow with other furanic compounds through addition reaction of protonated hydroxymethyl group with oxygen and carbon of furan ring. Model compound M4 may be an alternative of 5-HMF from sugar, as M4 was stable and easily separated from [BMIM]Cl through extraction. The optimization to enhance 5-HMF selectivity based on the above understanding of humins formation mechanism, are underway in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was financially supported by BP company.Notes and references
- Y. Liu, Y. Nie, X. Lu, X. Zhang, H. He, F. Pan, L. Zhou, X. Liu, X. Ji and S. Zhang, Green Chem., 2019, 21, 3499 RSC.
- S. Kang, J. Fu and G. Zhang, Renewable Sustainable Energy Rev., 2018, 94, 340 CrossRef CAS.
- Y. Li, J. Wang, X. Liu and S. Zhang, Chem. Sci., 2018, 9, 4027 RSC.
- A. Shrotri, H. Kobayashi and A. Fukuoka, Acc. Chem. Res., 2018, 51, 761 CrossRef CAS.
- I. Bodachivskyi, U. Kuzhiumparambil and D. B. G. Williams, ChemSusChem, 2018, 11, 642 CrossRef CAS.
- L. T. Mika, E. Cséfalvay and A. Németh, Chem. Rev., 2018, 118, 505 CrossRef CAS.
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC.
- K. I. Galkin and V. P. Ananikov, ChemSusChem, 2019, 12, 2976 CrossRef CAS.
- B. Wozniak, S. Tin and J. G. de Vries, Chem. Sci., 2019, 10, 6024 RSC.
- W. Fan, C. Verrier, Y. Queneau and F. Popowycz, Curr. Org. Synth., 2019, 16, 583 CrossRef CAS.
- H. Wang, C. Zhu, D. Li, Q. Liu, J. Tan, C. Wang, C. Cai and L. Ma, Renewable Sustainable Energy Rev., 2019, 103, 227 CrossRef CAS.
- S. Chen, R. Wojcieszak, F. Dumeignil, E. Marceau and S. Royer, Chem. Rev., 2018, 118, 11023 CrossRef CAS.
- X. Kong, Y. Zhu, Z. Fang, J. A. Kozinski, I. S. Butler, L. Xu, H. Song and X. Wei, Green Chem., 2018, 20, 3657 RSC.
- P. Pal and S. Saravanamurugan, ChemSusChem, 2019, 12, 145 CrossRef CAS.
- M. Sajid, X. Zhao and D. Liu, Green Chem., 2018, 20, 5427 RSC.
- B. R. Caes, R. E. Teixeira, K. G. Knapp and R. T. Raines, ACS Sustainable Chem. Eng., 2015, 3, 2591 CrossRef CAS.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397 CrossRef CAS.
- I. V. Sumerskii, S. M. Krutov and M. Y. Zarubin, Russ. J. Appl. Chem., 2010, 83, 320 CrossRef CAS.
- S. K. R. Patil, J. Heltzel and C. R. F. Lund, Energy Fuels, 2012, 26, 5281 CrossRef CAS; S. K. R. Patil and C. R. F. Lund, Energy Fuels, 2011, 25, 4745 CrossRef.
- I. van Zandvoort, Y. H. Wang, C. B. Rasrendra, E. R. H. van Eck, P. C. A. Bruijnincx, H. J. Heeres and B. M. Weckhuysen, ChemSusChem, 2013, 6, 1745 CrossRef CAS.
- V. Maruani, S. Narayanin-Richenapin, E. Framery and B. Andrioletti, ACS Sustainable Chem. Eng., 2018, 6, 13487 CrossRef CAS.
- G. Tsilomelekis, M. J. Orella, Z. Lin, Z. Cheng, W. Zheng, V. Nikolakis and D. G. Vlachos, Green Chem., 2016, 18, 1983 RSC.
- X. Fu, J. Dai, X. Guo, J. Tang, L. Zhu and C. Hu, Green Chem., 2017, 19, 3334 RSC.
- J. Zhou, Z. Xia, T. Huang, P. Yan, W. Xu, Z. Xu, J. Wang and Z. C. Zhang, Green Chem., 2015, 17, 4206 RSC.
- A. Chinnappan, C. Baskar and H. Kim, RSC Adv., 2016, 6, 6399 RSC.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548 RSC.
- Q. Hou, M. Zhen, W. Li, L. Liu, J. Liu, S. Zhang, Y. Nie, C. Bai, X. Bai and M. Ju, Appl. Catal., B, 2019, 253, 1 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597 CrossRef CAS.
- Y. Su, H. M. Brown, X. Huang, X. Zhou, J. E. Amonette and Z. C. Zhang, Appl. Catal., A, 2009, 361, 117 CrossRef CAS.
- S. J. Dee and A. T. Bell, ChemSusChem, 2011, 4, 1166 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05641c |
| This journal is © The Royal Society of Chemistry 2020 |