Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: a review
Abdanne Weyesa
and
Endale Mulugeta
 *
*
Department of Applied Chemistry, School of Applied Natural Science, Adama Science and Technology University, P. O. Box: 1888, Adama, Ethiopia. E-mail: endale.mulugeta@astu.edu.et
First published on 2nd June 2020
Abstract
Recently, quinoline has become an essential heterocyclic compound due to its versatile applications in the fields of industrial and synthetic organic chemistry. It is a vital scaffold for leads in drug discovery and plays a major role in the field of medicinal chemistry. Nowadays there are plenty of articles reporting syntheses of the main scaffold and its functionalization for biological and pharmaceutical activities. So far, a wide range of synthesis protocols have been reported in the literature for the construction of this scaffold. For example, Gould–Jacob, Friedländer, Pfitzinger, Skraup, Doebner–von Miller and Conrad–Limpach are well-known classical synthesis protocols used up to now for the construction of the principal quinoline scaffold. Transition metal catalysed reactions, metal-free ionic liquid mediated reactions, ultrasound irradiation reactions and green reaction protocols are also useful for the construction and functionalization of this compound. The main part of this review focuses on and highlights the above-mentioned synthesis procedures and findings to tackle the drawbacks of the syntheses and side effects on the environment. Furthermore, various selected quinolines and derivatives with potential biological and pharmaceutical activities will be presented.
1. Introduction
Quinoline is the most ubiquitous heterocyclic aromatic compound with a potential for industrial and medicinal applications. 1-Azanapthalene and benzo[b]pyridine are used as alternative names for quinoline (Fig. 1).It has a characteristic double-ring structure containing a benzene ring fused with a pyridine moiety, with the molecular formula C9H7N.1 Quinoline is an essential segment of both natural and synthetic compounds. In particular, the pyranoquinoline ring system has gained considerable attention as it is a core structure, constituting the basic skeleton of a number of alkaloids.2 Generally quinoline is present in pharmacologically active natural products and in synthetic products. This compound is used mainly as a central template for the synthesis of various drugs. Quinoline is a weak tertiary base and can form salts with acids. It exhibits similar reactions to pyridine and benzene and can also participate in both electrophilic and nucleophilic substitution reactions. It is nontoxic to humans.3 For the construction and functionalization of this noble compound and its derivatives, an enormous number of synthesis techniques have been reported, among which conventional or classical methods, transition metal free catalysed methods, ultrasound irradiation reactions and greener chemical processes have been well explored. Most of the time scholars explore classical reaction methodologies, such as Gould–Jacobs,4 Friedländer,5 Pfitzinger,6 Skraup,7 Doebner–von Miller,8 and Conrad–Limpach,9 and modify them with eco-friendly transition metal mediated, ultrasound irradiation reactions or greener protocols.10,11
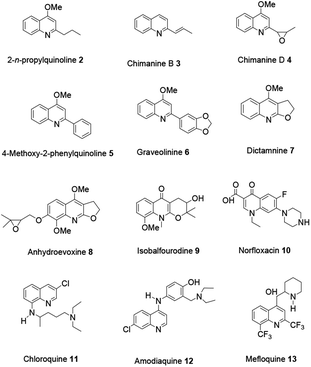
Currently, studies revealing numerous natural products and synthetic derivatives incorporating a quinoline scaffold have attracted scholars' attention because they exhibit a broad range of biological and pharmaceutical activities.
For example antibacterial, antioxidant, anticancer, anti-inflammatory, antimalarial, antifungal and antileishmanial activities have been well studied.12 Shang and co-workers reported a comprehensive review on alkaloids with a quinoline moiety as a core scaffold, isolated from compounds from natural sources and showing bioactivity potential.13 The review comprehensively organized into two focal sections. First various synthesis strategies will be addressed to highlight the original reaction procedures and modifications in the recent literature related to all the synthetic strategies accordingly. Then novel pharmaceutically and biologically active quinolines will be explored (Fig. 2).
2. Synthesis of quinoline and its derivatives
So far scholars have explored copious synthesis protocols to construct and functionalize the quinoline scaffold. Here, we focus on a review of the classical synthesis procedures named Gould–Jacob, Friedländer, Pfitzinger, Skraup, Doebner–von Miller, and Conrad–Limpach reactions, transition metal catalysed reactions, transition metal free ionic liquid mediated reactions, ultrasound irradiation reactions, and greener chemical processes for the synthesis of quinoline and its analogues will be discussed briefly.2.1. Gould–Jacob quinoline synthesis
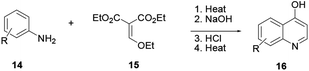
Various quinoline scaffolds with substituents at carbon-4 can be prepared through cascade reactions known as the Gould–Jacob cyclization reaction.4 In this procedure 4-hydroxyquinoline 16 is prepared from aniline (14) and diethyl ethoxymethylenemalonate (15) involving series of reactions to provide quinoline 16 (Scheme 1).This protocol helps in the preparation of various commercially available drugs based on a quinoline scaffold as the core skeleton. For instance, the preparation of the well-known nonsteroidal anti-inflammatory drugs floctafenine and glafenine relies on this synthesis methodology.4
2.2. Friedländer quinoline synthesis
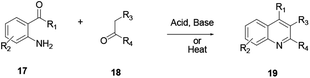
In this procedure ortho-substitution of aniline 17 and aldehyde or ketone 18 with a reactive α-methylene group via condensation followed by cyclodehydration reactions affords compound 19 (Scheme 2). In this reaction procedure, regioselectivity is a challenging issue when unsymmetrical ketones are used.5 The reaction is well catalysed using a base or acid, such as a Brønsted acid or a Lewis acid, and ionic liquids can also activate the reaction well. Furthermore, it can proceed smoothly without a catalyst by heating the mixture. The merit of this reaction procedure is the scope of substrates of various functional groups that are well tolerated on both arylamine and ketone moieties.2.3. Modified Friedländer quinoline synthesis
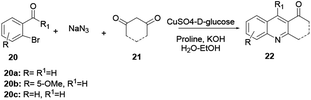
Anand and co-workers operationally developed a simple, highly efficient, practical and convenient one-pot method for the synthesis of a broad scope of quinoline derivatives.6 Here, by using 2-bromobenzaldehyde (20), acyclic or cyclic 1,3-diketone (21) and sodium azide in a three-component reaction protocol in the presence of an air-stable, eco-efficient and inexpensive catalyst, quinoline 22 is prepared in good yields. In this reaction copper salt-D-glucose helps to generate Cu(I) species in situ through reduction in aqueous ethanol as a green solvent, and proline is used as a ligand and proton source to synthesize the target compound (Scheme 3). This protocol follows an Ullmann-type coupling reaction where nucleophilic substitution of Br from 2-bromobenzaldehyde (20) with sodium azide gives an azido complex intermediate. The azido–Cu complex is subjected to reductive elimination followed by dehydrative cyclocondensation, providing the desired quinoline 22.The authors claim that this method worked nicely with both electron-donating and electron-withdrawing substituent groups at the ortho-, meta-, and para-positions of the phenyl ring.6
2.4. Pfitzinger quinoline synthesis
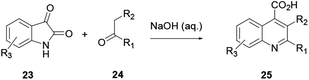
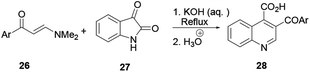
This procedure is also known as the Pfitzinger–Borsche reaction. Here isatin 23 reacting with α-methylene carbonyl compound 24 in the presence of a base in ethanol provides substituted quinoline derivative 25 (Scheme 4).6 In this reaction protocol, isatic acid is formed from isatin 23 and condensed with α-methylene carbonyl compound 24 in the presence of a strong base. Subsequent decarboxylation affords quinoline 25. This procedure is an extension of the Friedländer quinoline synthesis protocol. The reaction basically depends on the more stable isatin varieties instead of the ortho-aminoaryl moieties that are the basic starting materials for the preparation of quinoline via the former reaction protocol.7 Elghamry and co-workers used a similar reaction protocol with minor modifications in a one-step and one-pot method to synthesize quinoline-4-carboxylic acids 28 in water (Scheme 5). Isatin (27) was refluxed with enaminone 26 in the presence of an aqueous solution of KOH or NaOH, and subsequent acidification using dilute HCl to prepare quinoline-4-carboxylic acid (28) in good to excellent yields.7 The authors claimed that using enaminone as a replacement for 1,3-dicarbinols improves the yield and practicality of the reaction.2.5. Skraup/Doebner–von Miller quinoline synthesis
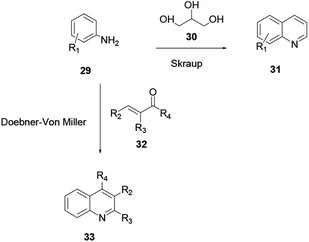
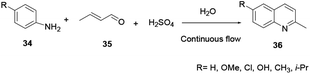
A synthesis of quinoline via aniline and glycerine in the presence of a strong acid and an oxidant under reflux was revealed by Skraup and co-workers.8 Here, a crotonaldehyde intermediate is generated in situ from glycerol 30. Subsequently aniline 29 is added to the reaction under heating to provide quinoline 31. The reaction of substituted acrolein 32 with aniline 29 in the presence of an oxidant to provide quinoline 33 is known as the Doebner–von Miller protocol (Scheme 6).The fundamental drawbacks of the Skraup and Doebner–von Miller syntheses are that both turn out to be violently exothermic during the progress of the reaction, and the variety of oxidants and the highly acidic medium required make isolation of the desired product tedious. Regioselectivity is also a concern when meta or 3,4-disubstituted anilines are used.9 2-methylquinoline and its derivatives have shown substantial biological activities. However, there are different techniques for the synthesis of 2-methylquinoline, and Doebner–von Miller is the best. Yalgin and co-workers report the synthesis of 2-methylquinoline 36 using a modified Doebner–von Miller reaction protocol in the presence of a strong acid in a flow reactor with aniline and acrolein.15 2-Methylquinoline derivative 36 synthesized using a continuous flow in water through Doebner–Miller reaction procedure. This method is a rapid and green route for the synthesis of quinoline derivatives to provide a good to excellent yields (Scheme 7).14
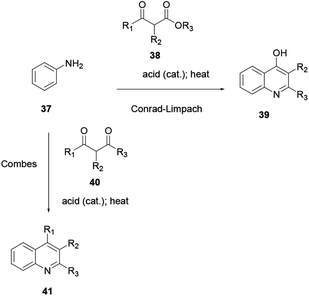
2.6. Combes/Conrad–Limpach quinoline synthesis
The Combes/Conrad–Limpach reaction is the condensation of a primary aryl amine with a 1,3-diketone or β-keto-aldehyde or 1,3-dialdehyde, giving an enamine intermediate which is further heated in a strong acid, and later cyclodehydration to afford quinoline. Here, the condensation of primary aryl amine 37 with β-diketone 40 in acid catalysis, followed by ring closure of a Schiff base, affords quinoline 41 (Scheme 8).10Using the same reaction procedure the Conrad–Limpach scheme can be used to prepare various quinoline derivatives using β-ketoester 38 in place of β-diketone 40. Both procedures follow the condensation of arylamine with β-ketoester or β-diketone by a further cyclodehydration reaction and heating in strong acid to prepare the target compounds.
2.7. Ultrasound irradiation reactions
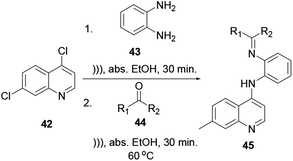
The reaction time, product yields and qualities of this reaction procedure are better than those from the above-mentioned quinoline synthesis protocols. It is one of the greener quinoline synthesis protocols. Using an ultrasound irradiation reaction procedure via two sequential reactions, SN2 followed by a condensation reaction, quinoline 45 can be synthesized in good yield (Scheme 9).12This procedure has the advantages of a short reaction time and easy isolation of the product and provides good-to-excellent yields.16 Quinoline 45 can be easily functionalized at the 4-chloro position with o-phenylenediamine 43, and further reacted with unsymmetrical ketone 44 via a green chemistry protocol to provide quinoline derivative 45.
Xie and co-workers report C2-sulfonation of N-oxide quinoline (46) using sulfonyl chloride 47 in the presence of Zn-dust, with water as a solvent via an ultrasound-assisted protocol, providing quinoline 48 in moderate yield.17 Here, sulfonyl chloride 47 is first reduced using Zn-dust. The generated intermediate is coordinated with N-oxide quinoline 46. Finally, the target compound 48 is synthesized through an intramolecular nucleophilic addition reaction (Scheme 10).
2.8. Transition metal free quinoline synthesis
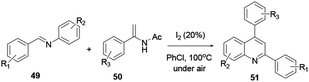
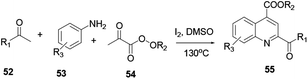
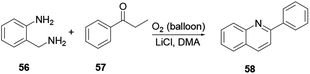
Various scholars report the synthesis of quinoline and its derivatives via metal-free mediated reaction protocols, such as ionic liquid, simple acid or base catalyst, using molecular iodine and catalyst-free reactions. The above-mentioned procedures are considered to be green chemical processes. Conducting reactions using the above strategies can achieve a high level of atom efficiency. The reaction media are recyclable, they provide high yields, have a short reaction time, are practical to operate under mild reaction conditions, conduct the reduction in a number of steps, decrease waste and are eco-friendly.18 Here, two amine derivatives, enamide 49 and imine 50, react in the presence of iodine in air to afford quinoline 51 (Scheme 11). In order to improve the reaction efficiency, various types of catalysts were explored. Among all these catalysts, iodine exhibited the highest yields. Moreover, the reaction has attracted a great deal of attention with its benefits of low toxicity, ability to operate under mild reaction conditions, low-cost starting materials and broad scope of substrates.The three-component reaction of methyl ketone 52, arylamine 53, and α-ketoester 54 in the presence of iodine and a catalytic amount of hydroiodic acid provides quinoline 55. In this reaction the HI co-product acts as a promoter with good functional compatibility (Scheme 12).19 Under transition metal free reaction conditions oxygen acts as an oxidant, where 2-(aminomethyl)-aniline 56 reacts with aromatic ketone 57 to provide the corresponding quinoline 58 with excellent yield (Scheme 13). Wu and co-workers reported that the reaction procedure for the synthesis of quinoline can be achieved under aerobic reaction conditions without the presence of a catalyst, with lithium chloride salt additive in dimethylacetamide solvent.20 Following this reaction procedure ketones bearing strong electron-withdrawing or electron-donating groups could react smoothly and in an eco-friendly way to provide the desired product.
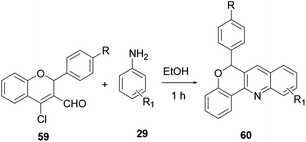
Carbaldehyde 59 reacted with substituted aniline 29 without a catalyst to provide quinoline 60 in excellent yield (Scheme 14).21 The reaction is performed at room temperature, with a short reaction time, providing excellent yield with no catalyst required (Scheme 14).
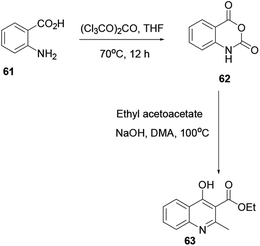
Through a convenient two consecutive steps ethyl-4-hydroxy-2-methylquinoline-3-carboxylate (63) was synthesized. Using solid triphosgene in THF, commercially available 2-aminobenzoic acid 61 converted to isatoic anhydride 62, reacted with the sodium enolate of ethyl acetoacetate in warm DMA, to afford substituted quinoline 63 (Scheme 15).22
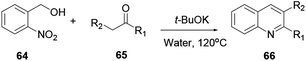
Zhu and co-workers reported an environmentally friendly reaction methodology to prepare quinoline 66.23 In this reaction procedure the cheap and commercially available material 2-nitrobenzyl alcohol (64) reacted with ketone 65 via an intramolecular redox process to afford synthetically important quinoline 66 in moderate yields (Scheme 16).
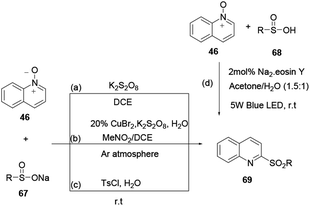
2-Sulfonylquinoline 69 was prepared smoothly without a base or organic solvent from quinoline N-oxide 46 and sodium sulfinate 67 using a metal-free and dual radical coupling reaction protocol devised by Xie and co-workers.24 Sodium sulfinate is well-known for use as a sulfonating reagent in the following reactions, because it is stable to moisture and is safe and easy to handle. Additionally, Han and co-workers report the synthesis of 2-sulfonylquinoline 69 using quinoline N-oxide with sodium sulfinate as starting materials in the presence of K2S2O8 as an oxidant under an inert atmosphere in a mixed solvent system.25 Peng and co-workers amended previously reported protocols and reported the synthesis of 2-sulfonylquinoline 69 from quinoline N-oxide and sodium sulfinate via an easy, metal-free, oxidant-free and solvent-free method under mild reaction conditions using TsCl in water.26 Furthermore, 2-sulfonylquinoline 69 was synthesized from quinoline N-oxide and sulfonic acid 68 using organic dye as a catalyst (Scheme 17).27
The reaction was performed with a greener process protocol, and in ambient air to provide good to excellent yields.
2.9. Transition metal mediated protocols
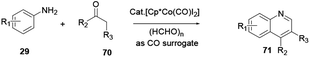
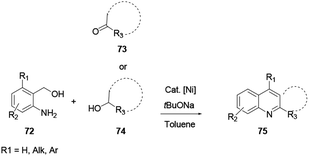
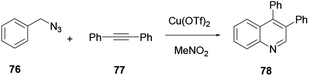
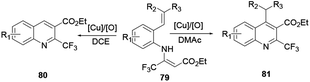
Xu and co-workers reported the one-pot synthesis of substituted quinoline 71 with a broad scope of substrates using a transition Co(III) metal mediated reaction protocol.28 Co(III) catalysis via a cascade of reactions, C–H activation, carbonylation and cyclization of aniline 29 and ketone 70 with paraformaldehyde provide various useful quinoline derivatives (Scheme 18). Benefits of the procedure are the broad scope of substrates with tolerance to various functional groups and the affordance of good to excellent yields. Moreover, it releases water and hydrogen gas as by-products.Das and co-workers reported the synthesis of various polysubstituted quinolines 75 from commercially available α-aminoaryl alcohol 72 and acyclic/cyclic ketones 73 or secondary alcohol 74 via a sequential dehydrogenation and condensation reaction process (Scheme 19).29 The advantage of conducting reactions via this method is that it uses a simple-to-handle and inexpensive nickel(II) catalyst. Luo and co-workers reported 6-chloro-2-dimethyl-4-phenylquinoline, an antiparasitic agent, and 3,4-diphenylquinoline-2(1H)-one, a p38αMAP kinase inhibitor, as biologically active quinoline derivatives. The reaction proceeds through a single-step process and under neutral reaction conditions using a copper triflate catalyst in nitromethane.30 In contrast to an acid-catalysed two-step [4 + 2] cycloaddition and oxidation reaction, it allows a single-step reaction to provide quinoline in high yields and excellent regioselectivity (Scheme 20). N-(2-alkenylaryl)enamine 79 is a strategic precursor for the synthesis of quinoline 80 or 81 using a one-pot copper-catalysed aerobic oxidative cyclization reaction (Scheme 21). Chen and co-workers reported a novel and efficient procedure for the synthesis of 2-trifluoromethylquinolines 80 and 81 using copper-chloride salt under aerobic reaction conditions with various functional group tolerances.31
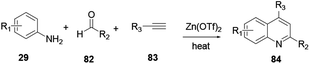
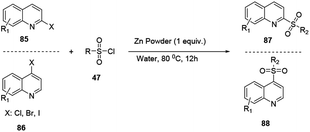
2,3-Disubstituted quinoline 84 was synthesized through multicomponent coupling reactions of alkyne 83, amine 29 and aldehyde 82 using a zinc(II) triflate salt catalyst (Scheme 22).32 The merits of this procedure are that reactions can proceed without the presence of ligands or co-catalysts, under solvent-free and inert reaction conditions. Bao and co-workers recently reported a simple and efficient synthetic protocol for the construction of sulfonylated quinoline in water via a zinc powder mediated coupling reaction.33
Haloquinolines 85 and 86 reacted with sulfonyl chloride in water in the presence of cheap metal zinc powder to afford sulfonylated quinolines 87 and 88, respectively (Scheme 23).
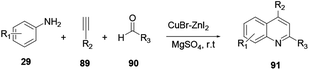
Mondal and co-workers reported a neat ZnII/CuI catalyzed reaction procedure to synthesise quinoline 91 through the uncommon sp2 C–H activation of three-component protocols.34 Aniline 29, alkynes 89 and aldehydes 90 react via a cascade cyclization reaction to provide quinoline 91 (Scheme 24).
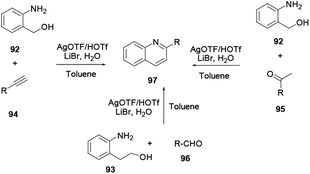
2-Substituted quinoline 97 is prepared from either 2-aminobenzyl alcohol and alkyne/ketone or 2-aminophenethyl alcohol and aldehyde using an AgOTf catalyst.35 Synthetically important heterocyclic anchored quinolines, such as furan, pyrrole and thiophene, can be synthesized via a facile and economic procedure using a silver triflate catalyst in toluene solvent with commercially available precursors and additives, as reported by Xu and co-workers (Scheme 25). Afterwards, the silver triflate catalysed one-step synthesis of quinoline with a broad scope of substrates was reported by Xu and co-workers.36
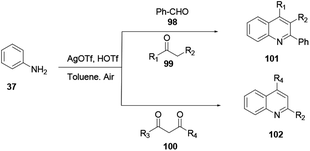
Multicomponent reactions proceeding using aniline 37, aldehyde 98, and ketones 99 or 100 reacting in the presence of a silver triflate catalyst provide quinolines 101 or 102 (Scheme 26).
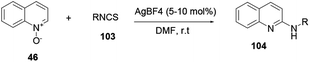
Xie and co-workers reported an effective and suitable protocol for the synthesis of 2-aminoquinoline 104 using an AgBF4 catalyst in DMF solvent.37 The reaction proceeds using isothiocyanate 103 under basic and oxidant-free mild reaction conditions to provide good to excellent yields (Scheme 27).
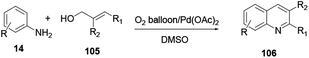
The reaction of commercially available starting materials aniline 14 and aryl allyl alcohol 105 using a palladium acetate catalyst in DMSO solvent via an oxidative cyclization reaction provides quinoline 106 (Scheme 28).38 It does not need to employ any acid, base or additive to promote the reaction. The procedure works with a broad scope of substrates and strong electron-withdrawing substituted materials to furnish moderate to satisfactory yields.
3. Bioactivities of quinolines
Both natural products and synthetic compounds which are anchored with a quinoline scaffold have certainly exhibited a broad range of biological or pharmaceutical activities.39,40 Among them are antibacterial,41,42 antioxidant,43 anticancer,44 anti-inflammatory,45,46 antimalarial,47,48 and antifungal activities.493.1. Antibacterial activity
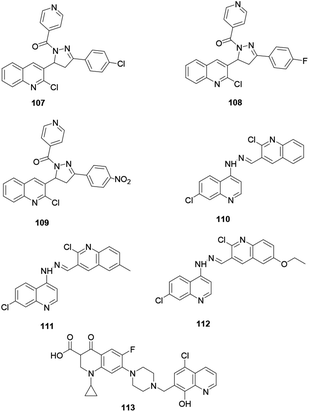
Desai and co-workers reported the synthesis of quinoline derivatives 107, 108 and 109 exhibiting the most powerful antimicrobial activities (Fig. 3).50 The synthesized compounds were screened for their potential antibacterial activity on Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa using ampicillin as a standard drug. The recorded results revealed that these compounds have good bacterial activity with minimum inhibition concentrations of 12.5 μg ml−1 and 50 μg ml−1. Furthermore, the authors claim that the potential activity of these compounds is directly associated with the substituent effect on the ring. These three bioactive quinoline derivatives are linked using hydrazone, as reported by Le and co-workers.51 Quinoline derivatives with a hydrazone linker 110, 111 and 112 showed good growth inhibition of targeted bacteria.Furthermore, Fu and co-workers synthesized hybridized quinoline derivative 113 with a piperazine moiety linker and reported that it showed broad-spectrum antibacterial activities on selected bacteria with MIC values of 0.125–8 μg ml−1.52
3.2. Antioxidant activity
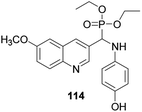
Bazine and co-workers report that quinoline derivative 114 anchored with an α-aminophosphate revealed effective antioxidant activity when compared with standard DPPH (Fig. 4).53 The authors confirmed that the bioactivity was further modified through the introduction of a phenol ring as a substituent to the quinoline scaffold.3.3. Anticancer activity
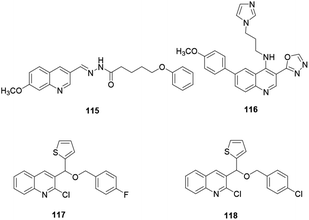
Bingul and co-workers synthesized and reported quinoline derivative 115 for anticancer activity against neuroblastoma cells (Fig. 5). The authors revealed that compound 115 showed reasonable anticancer activities against SH-SY5Y and Kelly neuroblastoma cell lines and decreased the viability of neurocancer cells with substantial selectivity over normal cells.54 Compounds 117 and 118 are quinoline derivatives anchored with a thiophene moiety, reported by Othman and co-workers.55 The above quinolines exhibited powerful anticancer activity against MCF-7 human cancer cells with IC50 values of 38.41 and 28.36 μM, respectively. Additionally, Kundu and co-workers reported that quinoline 116 with an imidazole and 1,3,4-oxadiazole exhibited the highest potency for human topoisomerase 1.563.4. Anti-inflammatory activity
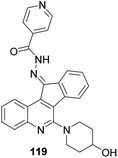
Tseng and co-workers synthesized and reported indeno[1,2-c] quinoline derivatives 119 as a potent anti-TB agent, besides being a potent anti-inflammatory agent with low cytotoxicity (Fig. 6).573.5. Antileishmanial activity
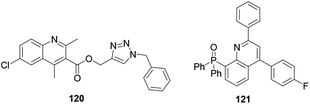
Upadhyay and co-workers synthesized and reported that quinoline derivative 120 anchored with a triazole exhibited antileishmanial activity (Fig. 7).58 The authors revealed that having a chloro-substituent enhanced the activity of the synthesized compound.Phosphorated quinoline 121 is a hybrid and potent antileishmanial agent.59 Phosphorus hybridized with quinoline is a promising basis for strong antileishmanial activity.
3.6. Antimalarial activity
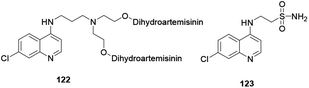
Currently, scholars are exploring how to improve and enhance the antimalarial activity of compounds with a quinoline scaffold. These are mainly synthesized quinoline derivatives hybridized with commercially available and potentially recognized drugs.60Investigators claim that hybridization will result in benefits of cost-effectiveness and minimize the risk of drug–drug interaction. Lombard and co-workers reported quinoline hybridized with artemisinin drug and provided compound 122 (Fig. 8). The hybrid compound exhibited antimalarial activity, although not as much as dihydroartemisinin, but it showed excellent antiplasmodial activity.61 The antimalarially active quinoline–sulfonamide hybrid derivative 123 was synthesized and reported by Verma and co-workers.62 The authors revealed that the hybrid compound exhibited inhibition of the formation of hemozoin.
3.7. Antifungal activity
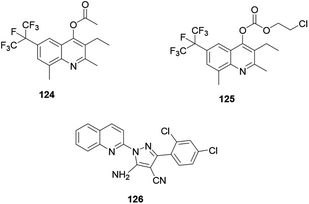
Antifungal active compounds 6-perfluoropropanyl quinoline 124 and 125 were synthesized and reported by Fang and co-workers (Fig. 9).63 The synthesized quinoline derivatives exhibited excellent antifungal activity against Pyricularia oryzae. El Shehry and co-workers synthesized and reported an antifungally active pyrazole–quinoline hybrid 126 (Fig. 9).64 The synthesized compound exhibited good antifungal activity against the target fungal species.4. Conclusions
In conclusion, numerous quinoline derivatives play a big role in the progress of organic synthesis and applications to medicinal chemistry. Recently, researchers have synthesized hybrid quinoline scaffolds with compounds containing other heterocyclic compounds using different procedures. For example, through transitional metal catalysed or metal-free reactions, ultrasound irradiation, or multicomponent one-pot synthesis, modified named reactions can be instigated to synthesize various noble and effective quinoline derivatives. Generally, from the above-mentioned procedures metal-free, ionic liquid and ultrasound irradiation synthesis methodologies meet the requirements of ‘green chemistry’.Until now the synthesis and modification of a quinoline scaffold and investigations to improve its biological and pharmaceutical activities have been continually developing.65,66 Nowadays researchers are focused not only on the synthesis of quinoline and its derivatives but also on developing and designing eco-friendly reaction procedures. Typical metal-free, solvent-free, aqueous media and ionic liquid catalysed reactions are green protocols.67 Quinoline and its derivatives have exhibited a potentially wide range of applications to treat various kinds of human infections, such as bacterial infections, cancer, malaria and fungal infections. The above-mentioned synthesis protocols based on green synthesis procedures are usually suggested for preparing this noble organic compound and its analogues.
Conflicts of interest
Article content has no conflict of interest.References
- S. Jain, V. Chandra, P. K. Jain, K. Pathak, D. Pathak and A. Vaidya, Arabian J. Chem., 2019, 12, 4920–4946 CrossRef CAS.
- P. G. Shobhashana, P. Prasad and M. P. Patel, Heterocycl. Lett., 2017, 7, 819–828 CAS.
- A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman and M. M. Alam, Saudi Pharm. J., 2013, 21, 1–12 CrossRef PubMed.
- T. Jennifer, B. Andrew, K. Stanislaw, W. Ying, C. Manwika and D. Stevan, J. Org. Chem., 2017, 82, 1073–1084 CrossRef PubMed.
- M. F. Mehrjardi, Mini-Rev. Org. Chem., 2017, 14, 187–196 Search PubMed.
- N. Anand, T. Chanda, S. Koley, S. Chowdhury and M. S. Sing, RSC Adv., 2015, 5, 7654–7660 RSC.
- I. Elghamry and Y. Al-Faiyz, Tetrahedron Lett., 2016, 57, 110–112 CrossRef CAS.
- Z. H. Skraup, Monatsh. Chem., 1881, 2, 139–170 CrossRef.
- G. A. Ramann and B. J. Cowen, Tetrahedron Lett., 2015, 56, 6436–6439 CrossRef CAS.
- G. A. Ramann and B. J. Cowen, Molecules, 2016, 21, 986–1009 CrossRef PubMed.
- R. Sharma, P. Kour and A. Kumar, J. Chem. Sci., 2018, 73, 130–155 Search PubMed.
- A. Aboelnaga and T. H. El-Sayed, Green Chem. Lett. Rev., 2018, 11, 254–263 CrossRef CAS.
- X. F. Shang, S. L. Morris-Natschke, Y. Q. Liu, X. Guo, X. S. Xu, M. Goto, J. C. Li, J. Y. Zhang and K. H. Lee, Med Res Rev., 2018, 38, 1614–1660 CrossRef PubMed.
- S. Xiao-Fei, L. M. Susan, L. Ying-Qian, G. Xiao, X. Xiao-Shan, G. Masuo, L. Jun-Cai, Y. Guan-Zhou and L. Kuo-Hsiung, Med. Res. Rev., 2018, 38, 775–828 CrossRef PubMed.
- H. Yalgin, D. Luart and C. Len, J. Flow Chem., 2016, 6, 80–85 CrossRef CAS.
- M. K. Kumawat, P. Parida and D. Chetia, Med. Chem. Res., 2016, 25, 1993–2004 CrossRef CAS.
- (a) L. Y. Xie, Y. J. Li, J. Qu, Y. Duan, J. Hu, K. J. Liu, Z. Cao and W. M. He, Green Chem., 2017, 19, 5642–5646 RSC; (b) Z. Cao, Q. Zhu, Y. W. Lin and W. M. He, Chin. Chem. Lett., 2019, 30, 2132–2138 CrossRef CAS.
- L. Yamin, Z. Xiaoqiang, W. Zhaoyang, C. Jinhui, M. Chaowei, H. Yongqin and H. Guosheng, RSC Adv., 2015, 5, 88214–88217 RSC.
- Q. Gao, S. Liu, X. Wu, J. Zhang and A. Wu, J. Org. Chem., 2015, 80(11), 5984–5991 CrossRef CAS PubMed.
- W. Kun, H. Zhiliang, L. Chao, Z. Heng and L. Aiwen, Chem. Commun., 2015, 51, 2286–2289 RSC.
- A. S. Kumar, R. A. Kumar, V. Satyanarayana, E. P. Reddy, B. J. M. Reddy, D. N. Kumar, A. Khurana, G. Chandraiah and J. S. Yadav, Nat. Prod. Commun., 2017, 12, 1129–1132 CrossRef.
- G. J. Nicholas, D. H. Jared, B. C. Emily, M. B. Samer, H. P. Amy, A. P. Julie, J. K. Jacques and G. D. Matthew, Beilstein J. Org. Chem., 2018, 14, 2529–2536 CrossRef PubMed.
- M. Zhu, C. Wang, W. Tang and J. Xiao, Tetrahedron Lett., 2015, 56, 6758–6761 CrossRef CAS.
- L. Y. Xie, S. Peng, F. Liu, G. R. Chen, W. Xia, X. Yu, W. F. Li, Z. Cao and W. M. He, Org. Chem. Front., 2018, 5, 2604–2609 RSC.
- B. Du, P. Qian, Y. Wang, H. Mei, J. Han and Y. Pan, Org. Lett., 2016, 18, 4144–4147 CrossRef CAS PubMed.
- S. Peng, Y. X. Song, J. Y. He, S. S. Tang, J. X. Tan, Z. Cao, Y. W. Lin and W. M. He, Chin. Chem. Lett., 2019, 30, 2287–2290 CrossRef CAS.
- L. Y. Xie, T. G. Fang, J. X. Tan, B. Zhang, Z. Cao, L. H. Yanga and W. M. He, Green Chem., 2019, 21, 3858–3863 RSC.
- X. Xu, Y. Yang, X. Chen, X. Zhang and W. Yi, Org. Biomol. Chem., 2017, 15, 9061–9065 RSC.
- S. Das, D. Maiti and S. D. Sarkar, J. Org. Chem., 2018, 83, 2309–2316 CrossRef CAS PubMed.
- C.-Z. Luo, P. Gandeepan, Y.-C. Wu, W.-C. Chen and C.-H. Cheng, RSC Adv., 2015, 5, 106012–106018 RSC.
- W. Chen, Q. Ding, Z. Nie and Y. Peng, RSC Adv., 2016, 6, 48767–48773 RSC.
- B. S. Prashant, P. B. Sandeep and S. C. Hemantm, Tetrahedron Lett., 2016, 57, 5753–5756 CrossRef.
- P. Bao, L. Wang, Q. Liu, D. Yang, H. Wang, X. Zhao, H. Yue and W. We, Tetrahedron Lett., 2019, 60, 214–218 CrossRef CAS.
- R. R. Mondal, S. Khamarui and D. K. Maiti, ACS Omega, 2016, 1, 251–263 CrossRef CAS PubMed.
- X. Xu, X. Zhang, W. Liu, Q. Zhao, Z. Wang, L. Yu and F. Shi, Tetrahedron Lett., 2015, 56, 3790–3792 CrossRef CAS.
- X. Xu, W. Liu, Z. Wang, Y. Feng, Y. Yan and X. Zhang, Tetrahedron Lett., 2016, 57, 226–229 CrossRef CAS.
- L. Y. Xie, S. Peng, L. L. Jiang, X. Peng, W. Xia, X. Yu, X. X. Wang, Z. Cao and W. M. He, Org. Chem. Front., 2019, 6, 167–171 RSC.
- J. Xu, J. Sun, J. Zhao, B. Huang, X. Li and Y. Sun, RSC Adv., 2017, 7, 36242–36245 RSC.
- N. J. P. Subhashini, J. Amanaganti, L. Boddu and P. A. Nagarjuna, J. Chem. Pharm. Res., 2013, 5, 140–147 CAS.
- W. Gao, J. Liu, Y. Jiang and Y. Li, Beilstein J. Org. Chem., 2011, 7, 210–217 CrossRef CAS PubMed.
- N. C. Desai, G. M. Kotadiya and A. R. Trivedi, Bioorg. Med. Chem. Lett., 2014, 24, 3126–3130 CrossRef CAS PubMed.
- R. Vlahov, J. Parushev, P. Nickel and G. Snatzke, J. Pure Appl. Chem. Res., 1990, 7, 1303–1306 Search PubMed.
- B. Vivekanand, K. M. Raj and B. H. M. Mruthyunjayaswamy, J. Mol. Struct., 2015, 1079, 214–224 CrossRef CAS.
- V. Spanò, B. Parrino, A. Carbone, A. Montalbano, A. Salvador, P. Brun, D. Vedaldi, P. Diana, G. Cirrincione and P. Barraja, Eur. J. Med. Chem., 2015, 102, 334–351 CrossRef PubMed.
- S. A. El-Feky, Z. K. Abd El-Samii, N. A. Osman, J. Lashine, M. A. Kamel and H. K. Thabet, Bioorg. Chem., 2015, 58, 104–116 CrossRef CAS PubMed.
- M. A. Kerry, G. W. Boyd, S. P. Mackay, O. Meth-cohn and L. Platt, J. Chem. Soc., Perkin Trans. 1, 1999, 2315–2321 RSC.
- S. Vandekerckhove and M. D'hooghe, Bioorg. Med. Chem., 2015, 23, 5098–5119 CrossRef CAS PubMed.
- M. A. Lyon, S. Lawrence, D. J. William and Y. A. Jackson, J. Chem. Soc., Perkin Trans. 1, 1999, 437–442 RSC.
- S. Pramilla, S. P. Garg and S. R. Nautiyal, Indian J. Heterocycl. Chem., 1998, 7, 201–204 Search PubMed.
- N. C. Desai, B. Y. Patel and B. P. Dave, Med. Chem. Res., 2017, 26, 109–119 CrossRef CAS.
- T. D. Le, N. N. Pham and T. C. Nguyen, J. Chem., 2018, 2018, 1–8 CrossRef.
- H.-G. Fu, Z.-W. Li, X.-X. Hu, S.-Y. Si, X.-F. You, S. Tang, Y.-X. Wang and D.-Q. Song, Molecules, 2019, 24, 548–558 CrossRef PubMed.
- I. Bazine, Z. Cheraiet, R. Bensegueni, C. Bensouici and A. Boukhari, J. Heterocycl. Chem., 2020, 1–11 Search PubMed.
- M. Bingul, O. Tan, C. R. Gardner, S. K. Sutton, G. M. Arndt, G. M. Marshall, B. B. Cheung, N. Kumar and D. S. Black, Molecules, 2016, 21, 916–935 CrossRef PubMed.
- D. I. A. Othman, K. B. Selim, M. A.-A. El-Sayed, A. S. Tantawy, Y. Amen, K. Shimizu, T. Okauchi and M. Kitamura, Bioorg. Med. Chem., 2019, 27, 115026–115038 CrossRef PubMed.
- B. Kundu, S. K. Das, S. P. Chowdhuri, S. Pal, D. Sarkar, A. Ghosh, A. Mukherjee, D. Bhattacharya, B. B. Das and A. Talukdar, J. Med. Chem., 2019, 62, 3428–3446 CrossRef CAS PubMed.
- C.-H. Tseng, C.-W. Tung, C.-H. Wu, C.-C. Tzeng, Y.-H. Chen, T.-L. Hwang and Y.-L. Chen, Molecules, 2017, 22, 1001–1016 CrossRef PubMed.
- A. Upadhyay, P. Kushwaha, S. Gupta, R. P. Dodda, K. Ramalingam, R. Kant, N. Goyal and K. V. Sashidhara, Eur. J. Med. Chem., 2018, 154, 172–181 CrossRef CAS PubMed.
- A. Tejería, Y. P. Pertejo, R. M. Reguera, R. C. Andres, R. B. Fouce, C. Alonso, E. M. Encinas, A. Selas, G. Rubiales and F. Palacios, Eur. J. Med. Chem., 2019, 162, 18–31 CrossRef PubMed.
- X. Nqoro, N. Tobeka and B. A. Aderibigbe, Molecules, 2017, 22, 2268–2290 CrossRef PubMed.
- M. C. Lombard, D. D. N'Da, J. C. Breytenbach, N. I. Kolesnikova, C. T. Van Ba, S. Wein, J. Norman, P. Denti, H. Vial and L. Wiesner, Eur. J. Pharm. Sci., 2012, 47, 834–841 CrossRef CAS PubMed.
- S. Verma, S. Pandey, P. Agarwal, P. Verma, S. Deshpande, J. K. Saxena, K. Srivastava, P. M. S. Chauhan and Y. S. Prabhakar, RSC Adv., 2016, 6, 25584–25593 RSC.
- Y.-M. Fang, R.-R. Zhang, Z.-H. Shen, H.-K. Wu, C.-X. Tan, J.-Q. Weng, T.-M. Xu and X. H. Liua, J. Heterocycl. Chem., 2017, 55, 240–245 CrossRef.
- M. F. El Shehry, M. M. Ghorab, S. Y. Abbas, E. A. Fayed, S. A. Shedid and Y. A. Ammar, Eur. J. Med. Chem., 2018, 143, 1463–1473 CrossRef CAS PubMed.
- P. Gisbert, M. Albert-Soriano and I. M. Pastor, Eur. J. Org. Chem., 2019, 4928–4940 CrossRef CAS.
- P. da Silveira, S. Ligia and T. R. A. Vasconcelos, Mini-Rev. Org. Chem., 2019, 16, 602–608 CrossRef.
- V. F. Batista, D. C. G. A. Pinto and A. M. S. Silva, ACS Sustainable Chem. Eng., 2016, 4, 4064–4078 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |