 Open Access Article
Open Access ArticleReplacement of feed by fresh microalgae as a novel technology to alleviate water deterioration in aquaculture†
Fufeng Chen,
Yan Xiao,
Xiongwei Wu,
Yuqing Zhong,
Qian Lu * and
Wenguang Zhou*
* and
Wenguang Zhou*
School of Resources, Environmental & Chemical Engineering, Key Laboratory of Poyang Lake Environment and Resource Utilization, Ministry of Education, Nanchang University, Nanchang 330031, China. E-mail: qianlu1960@foxmail.com; wgzhou@ncu.edu.cn
First published on 2nd June 2020
Abstract
The main aim of this work was to evaluate the feasibility of microalgae-assisted aquaculture and explore the relevant mechanisms. In this regard, our work explored the pollution problems in traditional aquaculture and studied the contribution of microalgae to eutrophication control, oxygen gas production and feed replacement. Besides, potential protection mechanisms of microalgae-assisted aquaculture were studied by bacterial community profile analysis and microscope observation. The results showed that microalgae performed well in nutrient assimilation and oxygen production, thus slowing down the eutrophication and preventing oxygen depletion in aquaculture. Study of the mechanisms revealed that microalgae-assisted aquaculture contained much fewer pathogens and a microalgal biofilm was formed to prevent the eutrophication caused by sludge degradation. It is expected that the findings in this work can support the further development of microalgae-assisted aquaculture and promote the industry upgrade.
1 Introduction
Intensive aquaculture, in recent years, has developed all over the world, bringing with it a whole new set of environmental problems, particularly an increasing production of nutrient-rich wastewater.1,2 Boopathy et al. (2007) reported that concentrations of total nitrogen (TN), total ammonia nitrogen (TAN) and chemical oxygen demand (COD) in aquaculture effluent could reach 395 mg L−1, 101 mg L−1, and 1201 mg L−1, respectively, exceeding the limits of wastewater discharge standard.3 According to the statistics of FAO, from 2000 to 2017, aquaculture production increased by 160.26% and aquaculture value increased by 373.48%. In the foreseeable future, aquaculture will undergo a tremendous growth, further increasing the risks of water pollution and threatening the ecological balance. Aquaculture with high stocking density is regarded as a main way to increase profitability,4 but a large amount of wastewater is produced. Hence, the pollution caused by aquaculture with high stocking density merits more attention from researchers.Most of previous studies focused on the post-treatment of effluent from aquaculture system by traditional wastewater remediation technologies, such as aerobic digestion, anaerobic fermentation, and flocculation.5–7 The wastewater, however, is treated at the expense of high energy consumption and generation of secondary pollutants. For instance, according to the study of McCarty et al. (2011), the energy needs for wastewater treatment by aerobic digestion and anaerobic fermentation is about 0.6 kW h m−3.7 In addition, by traditional technologies, most nutrients in aquaculture wastewater are converted to greenhouse gas (CO2 and CH4) or sludge, which accelerate greenhouse effect or cause secondary pollution.3,8 Hence, it is important to find out an eco-friendly way to control the pollution caused by aquaculture with high stocking density.
Microalgae, which perform well in nutrients assimilation and biomass accumulation, have been intensively studied in nutrients recovery from wastewater. Previous studies successfully employed microalgae in the treatment of various sources of wastewater in both lab-scale and pilot-scale experiments.9,10 In a real-world application, it might be an eco-friendly way to culture microalgae for nutrients recovery from aquaculture wastewater and produce value-added biomass as fish feed. The value-added components, such as polyunsaturated fatty acids, astaxanthin, and carotene, in microalgae can improve the fish immunity. For example, immune response could be enhanced by using marine microalgae as alternative ingredients to dietary fish meal, suggesting an effective role of microalgal components as immunostimulant ingredients.11 Recently, a novel concept of adding fresh microalgae into aquaculture system as an in situ method for wastewater treatment, oxygen gas generation, and aquaculture feed production was proposed.2 Theoretically, this new concept has four main advantages: (1) in situ treatment of wastewater by microalgae-based nutrients removal to delay the water deterioration; (2) generation of oxygen gas to reduce the energy consumption of aeration; (3) yield of biomass to partly replace traditional aquaculture feed and lower the total cost of aquaculture; (4) exploitation of value-added components to improve fish immunity and prohibit the antibiotics abuse.12,13 However, to our knowledge, few studies have provided experimental data to evaluate the feasibility of this novel concept.
This work focusing on the contribution of microalgae in aquaculture system to water quality control will provide reasonable answers to three questions: (1) What are the specific water pollution problems with the increase of stocking density in aquaculture? (2) What are the contributions of microalgae to the management of aquaculture with high stocking density? (3) In this novel aquaculture system, what are the protection mechanisms of microalgae in water quality control? The results of this work will not only prove the feasibility of this novel concept, but also solve the technical problems of aquaculture with high stocking density, promoting the sustainable development of microalgae-based eco-friendly aquaculture.
2 Materials and methods
2.1. Materials
Chlorella sp. used in this work for biomass production and water quality control was preserve in TAP medium, of which the components have been documented in our previous work.14 Microalgae biomass was produced in a 250 mL flask filled with 100 mL medium, under continuous light (120 μmol photons per m2 per s) at room temperature (25 ± 1 °C). The inoculum size of microalgae in flask was set as 0.25 g L−1.The larvae of bighead carp (length: 2.60 cm; weight: 0.49 g), a widely commercialized Asian carp, were cultured in glass containers at room temperature for experiment and aquaculture feed used in this work was obtained from fish farm (Nanchang, China). To support the microalgal photosynthesis in aquaculture system, 12 h light (80 μmol photons per m2 per s) was provided for fish rearing each day.
2.2. Experimental design
This work, aiming at employing microalgae for water quality control in aquaculture with high stocking density and explaining relevant mechanisms, was carried out in three steps: (1) based on the changes of biochemical properties in conventional aquaculture, problems to fish rearing were studied; (2) effects of microalgae addition on water quality of aquaculture system were assessed and nutrients profile of algae grown in aquaculture system was analyzed; (3) bacterial community analysis and microscopic observation were conducted to reveal the potential mechanisms in micro-environment and explain the contribution of microalgae to water quality control.All the experiments and tests in this work were performed in triplicate. The results were expressed as average value ± standard deviation.
2.3. Parameters measurement
Dissolved oxygen (DO) content and pH value were measured by using DO analyzer (Hach, USA) and pH analyzer (Sartorius, Germany).
2.4. Comparison of aquaculture systems
In microalgae-assisted aquaculture with same stocking densities, fresh microalgae were added at the same amount to totally replace traditional aquaculture feed. The system was operated under continuous light at 120 μmol photons per m2 per s. Water deterioration in microalgae-assisted aquaculture was measured to study the contribution of microalgae to the alleviation of acute and chronic toxicities.
Contribution of microalgae to oxygen production and pH change in aquaculture with 8 fish/100 mL was studied by adjusting the daily addition amounts (0.0010, 0.0025, and 0.0050 g per fish) of microalgae. DO contents and pH values in water body were measured daily.
3 Results
3.1. Problems in conventional aquaculture
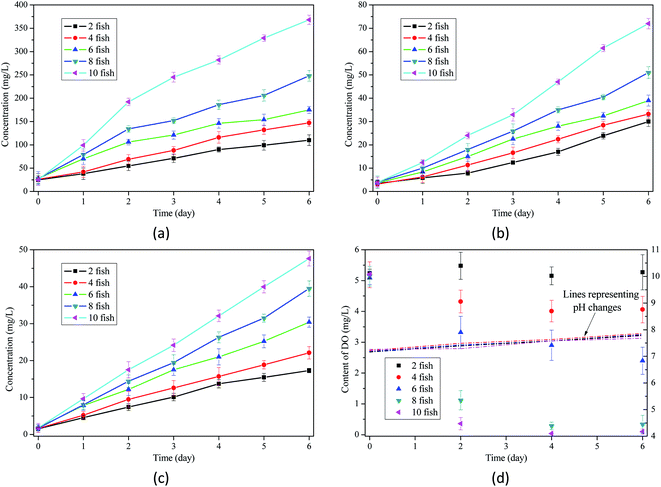 | ||
| Fig. 1 Changes of water quality in conventional aquaculture: (a) concentration of COD; (b) concentration of TN; (c) concentration of NH3–N; (d) values of pH and DO. | ||
Among the water quality parameters, TAN is one of the detrimental factors threatening fish survival. Bhakta (2006) reported that under certain conditions, the semi-lethal concentration of TAN to carp in 96 h was 20 mg L−1, which can be regarded as a threshold for fish culture.18 In this work, with the accumulation of organics in water body over 4 days, concentration of TAN exceeded this threshold, threatening the survival of fish and disturbing the ecological balance in aquaculture. Since the high concentration of TAN was mainly observed after 4 day culture, ammonia toxicity can be considered as a toxicity in aquaculture.
In aquaculture, unionized ammonia (NH3) is highly toxic, whereas the ionized form (NH4+) is nontoxic or less toxic and the percentage of NH3/NH4+ in TAN is closely related with the pH value of aqueous phase.19 Thus, to reduce the toxicity of aquaculture eutrophication to fish, higher percentage of NH4+ in TAN is expected. Weakly acidic environment favors the hydrolysis of ammonia and increases the percentage of ammonium in TAN, creating a better condition for aquatic animals. In aquaculture with different stocking densities, the fluctuation of pH value, which stably ranged between 7.16 and 7.86, would not dramatically change the ratio of NH3/NH4+ in TAN. Therefore, pH fluctuation is not a big concern in the management of aquaculture.
3.2. Alleviation of water deterioration by microalgae
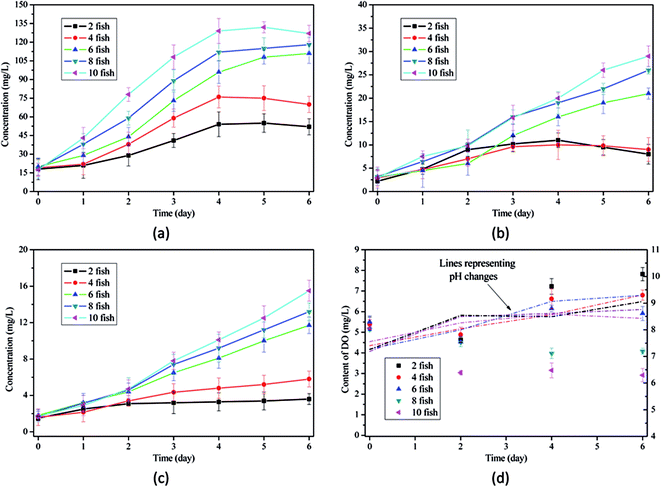 | ||
| Fig. 2 Changes of water quality in microalgae-assisted aquaculture: (a) concentration of COD; (b) concentration of TN; (c) concentration of NH3–N; (d) values of pH and DO. | ||
Fig. 2(d) indicated that the microalgae-based aquaculture had higher pH values (8.42–9.31) than traditional aquaculture by the end of 6 day culture. The main reason is that photosynthetic metabolism of microalgae uptake HCO3− from aqueous phase, at the same time, H+ is continuously consumed with the conversion of HCO3− to CO2.20 With the consumption of H+, the increase of pH value was observed. As a result, the equilibrium between NH3 and NH4+ may be disturbed and higher percentage of NH3 in TAN would threaten the survival of aquatic animals. Hence, given the toxicity of NH3, it is hypothesized that microalgae addition amount might be an important concern in aquaculture.
Microalgae also favored the maintenance of DO content in aquaculture system. As discussed above, oxygen depletion is an acute toxicity to fish survival in aquaculture with high stocking density. In this work, the increase of DO content was observed as long as the stocking densities did exceed 8 fish/100 mL. In aquaculture with high stocking density (8 and 10 fish/100 mL), the reduction of DO content decreased to 1.06 and 2.28 mg L−1 in 6 day culture with the addition of microalgae (Table S1†). Hence, microalgae addition can be a practically-feasible method to replace or partly replace traditional aeration in aquaculture.
It was observed that DO contents reached 10.39 and 5.43 mg L−1 by the end of 6 day culture when the addition amounts of microalgae were 0.0050 and 0.0010 g per fish, respectively, confirming that microalgae addition can greatly increase DO content in aquaculture system and reduce the risks of oxygen depletion.
| Item | Traditional aquaculture feed | Microalgae biomass |
|---|---|---|
| Crude protein (g/100 g DW) | 42.0 ± 2.8 | 50.6 ± 3.2 |
| Crude lipid (g/100 g DW) | 3.1 ± 1.4 | 24.5 ± 4.5 |
| PUFAs (% of fatty acids profile) | — | 42.3 |
| Carotene | — | 2.52 ± 0.28 |
| Chlorophyll (mg g−1 DW) | — | 21.3 ± 1.9 |
3.3. Potential mechanisms in micro-environment
At the level of class, percentages of Gammaproteobacteria in Sample 1 and Sample 2 were 82.50% and 18.41%, respectively (Fig. 3(a)). This result is in accordance with previous study which discovered that Gammaproteobacteria accounted for 84.6% of total bacterial community in traditional aquaculture. Gammaproteobacteria contain a variety of harmful pathogens, not only threatening the fish survival, but also bringing potential safety problems in food chain, creating a detrimental environment for fish rearing in traditional aquaculture.24 On the contrary, microalgae-assisted aquaculture with much less Gammaproteobacteria should be more favourable for fish growth.
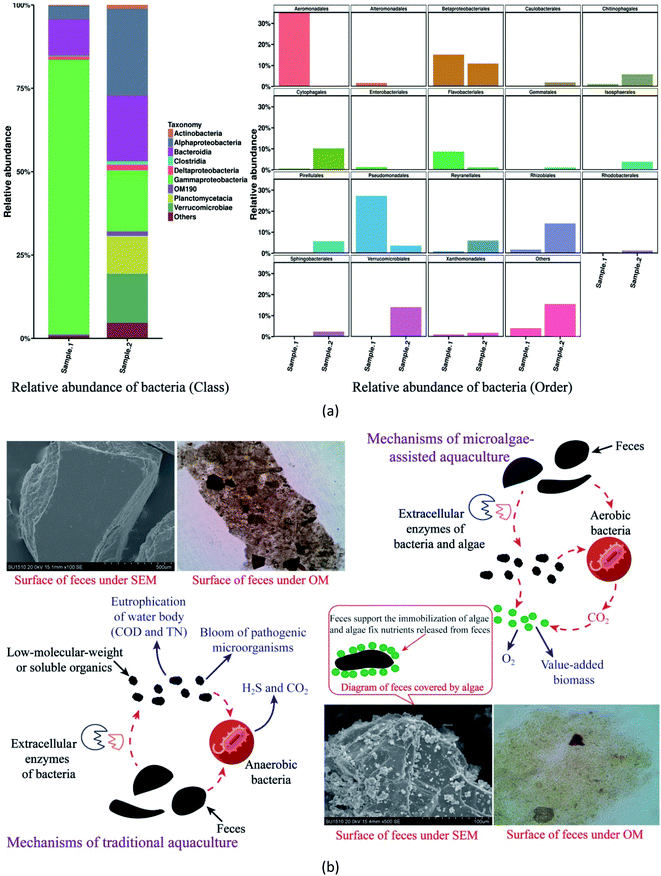 | ||
| Fig. 3 Potential mechanisms in micro-environment: (a) bacterial community profile in aquaculture; (b) sludge in aquaculture. | ||
Fig. 3(a) showed that Alphaproteobacteria, at the level of class, were the dominant bacteria (26.01%) in Sample 2. To our knowledge, Alphaproteobacteria contain both pathogens and beneficial microbes. To specify the roles of Alphaproteobacteria in microalgae-assisted aquaculture, bacterial community profile has to be studied at the level of order. As shown in Fig. 3(a), Rhizobiales, an order of Alphaproteobacteria, were the dominant bacteria (14.18%) in microalgae-assisted aquaculture while traditional aquaculture contained few Rhizobiales (1.76%). The synergistic relationship between Rhizobiales and microalgae in nutrients assimilation has been reported by previous study.25 In this work, since Rhizobiales accounted for 54.52% of Alphaproteobacteria in Sample 2, the high percentage of Alphaproteobacteria in Sample 2 would not be a problem threatening fish rearing in microalgae-assisted aquaculture. In addition to Rhizobiales, other dominant bacteria, Cytophagales and Verrucomicrobiales, at the level of order, are not typical pathogens in aquaculture.
Based on the analysis results of bacterial count and bacterial community profile, it is considered that microalgae in aquaculture system can improve the water quality and create a favorable environment for fish growth. With the inhibition of pathogenic bacteria, the morbidity of aquatic animals can be reduced to a lower level.24 As a result, the abuse of antibiotics in aquaculture can be effectively avoided. On the contrary, in the traditional aquaculture, the diseases of aquatic animals caused by the bloom of pathogenic bacteria are usually treated by a variety of antibiotics. The abuse of antibiotics could not only cause antibiotics resistance in aquatic animals and microorganisms, but also result in ecological disasters in water body. Therefore, microalgae-assisted aquaculture is more favorable to fish rearing than traditional aquaculture.
With the formation of a well-performing protection mechanism, the serious deterioration of water body in microalgae-assisted aquaculture can be avoided, providing a reasonable explanation for the data in Fig. 2. Fig. 3(b) revealed that soluble or low-molecular-weight organics formed with the degradation of sludge were assimilated by microalgae cells in the biofilm for value-added biomass synthesis. In addition, with the continuous production of oxygen gas by photosynthesis, the activity of anaerobic bacteria can be prohibited and the CO2 released by aerobic bacteria can be fixed by algae. Beneficial microalgae can reduce the occurrence possibility of pathogens bloom in aquaculture (Fig. 3(a)). Therefore, the formation of microalgal biofilm played an important role in the well-performing protection mechanism of microalgae-assisted aquaculture.
4 Conclusions
It is concluded that: (1) oxygen depletion and eutrophication are main problems in conventional aquaculture with high stocking density; (2) microalgae can solve the problem of oxygen depletion through photosynthesis and slow down the eutrophication by assimilating nutrients; (3) microalgae in aquaculture prevent the bacterial bloom and change the bacterial community profile, creating a favorable environment for fish rearing; (4) microalgal film covered on the sludge plays an important role in the well-performing protection mechanism of microalgae-assisted aquaculture; (5) application of microalgae biotechnology is expected to solve some problems in aquaculture and realize the industry upgrade.Conflicts of interest
There is no conflict to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (51668044).References
- R. Gothwal and T. Shashidhar, Clean: Soil, Air, Water, 2015, 43(4), 479–489 CAS.
- Q. Lu, P. Han, Y. Xiao, T. Liu, F. Chen, L. Leng, H. Liu and J. Zhou, J. Cleaner Prod., 2019, 217, 573–575 CrossRef.
- R. Boopathy, C. Bonvillain, Q. Fontenot and M. Kilgen, Int. Biodeterior. Biodegrad., 2007, 59(1), 16–19 CrossRef CAS.
- K. Sahin, H. Yazlak, C. Orhan, M. Tuzcu, F. Akdemir and N. Sahin, Aquaculture, 2014, 418, 132–138 CrossRef.
- C. S. Lee, J. Robinson and M. F. Chong, Process Saf. Environ. Prot., 2014, 92(6), 489–508 CrossRef CAS.
- H. Liu, Q. Wang, Y. Sun, K. Zhou, W. Liu, Q. Lu, C. Ming, X. Feng, J. Du and X. Jia, Bioresour. Technol., 2016, 211, 6–15 CrossRef CAS PubMed.
- P. L. McCarty, J. Bae and J. Kim, Domestic wastewater treatment as a net energy producer–can this be achieved?, ACS Publications, 2011 Search PubMed.
- I. Irvan, B. Trisakti, V. Wongistani and Y. Tomiuchi, Int. J. Sci. Eng., 2012, 3(1), 32–35 Search PubMed.
- Q. Lu, J. Li, J. Wang, K. Li, J. Li, P. Han, P. Chen and W. Zhou, Bioresour. Technol., 2017, 244, 542–551 CrossRef CAS PubMed.
- S. A. Razzak, M. M. Hossain, R. A. Lucky, A. S. Bassi and H. de Lasa, Renewable Sustainable Energy Rev., 2013, 27, 622–653 CrossRef CAS.
- M. Messina, C. Bulfon, P. Beraldo, E. Tibaldi and G. Cardinaletti, Aquaculture, 2019, 500, 660–669 CrossRef CAS.
- S. Hemaiswarya, R. Raja, R. R. Kumar, V. Ganesan and C. Anbazhagan, World J. Microbiol. Biotechnol., 2011, 27(8), 1737–1746 CrossRef.
- A. Muller-Feuga Handbook of Microalgal Cultures: Applied Phycology and Biotechnology, Wiley Blackwell, West Sussex, 2nd edn, 2013, pp. 615–627 Search PubMed.
- Q. Lu, W. Zhou, M. Min, X. Ma, C. Chandra, Y. T. Doan, Y. Ma, H. Zheng, S. Cheng and R. Griffith, Bioresour. Technol., 2015, 198, 189–197 CrossRef CAS PubMed.
- Q. Lu, W. Zhou, M. Min, X. Ma, Y. Ma, P. Chen, H. Zheng, Y. T. Doan, H. Liu and C. Chen, Bioresour. Technol., 2016, 201, 33–40 CrossRef CAS PubMed.
- A. Hosikian, S. Lim, R. Halim and M. K. Danquah, Int. J. Chem. Eng., 2010, 1–11 Search PubMed.
- J.-P. Yuan, F. Chen, X. Liu and X.-Z. Li, Food Chem., 2002, 76(3), 319–325 CrossRef CAS.
- J. N. Bhakta, Electronic Journal of Biology, 2006, 2(3), 39–41 Search PubMed.
- Y. Collos and P. J. Harrison, Mar. Pollut. Bull., 2014, 80(1–2), 8–23 CrossRef CAS PubMed.
- Z. Chi, J. V. O'Fallon and S. Chen, Trends Biotechnol., 2011, 29(11), 537–541 CrossRef CAS PubMed.
- R. Cerezuela, F. A. Guardiola, J. Meseguer and M. A. Esteban, Fish Physiol. Biochem., 2012, 38(6), 1729–1739 CrossRef CAS PubMed.
- J. Sargent, L. McEvoy and J. Bell, Aquaculture, 1997, 155(1–4), 117–127 CrossRef CAS.
- A. Mustafa, L. Randolph and S. Dhawale, J. Appl. Aquacult., 2011, 23(2), 136–146 CrossRef.
- M. Broszat, H. Nacke, R. Blasi, C. Siebe, J. Huebner, R. Daniel and E. Grohmann, Mexico, Appl. Environ. Microbiol., 2014, 80(17), 5282–5291 CrossRef PubMed.
- B. P. Hodkinson and F. Lutzoni, Symbiosis, 2009, 49(3), 163–180 CrossRef CAS.
- H. Liu, Q. Lu, Q. Wang, W. Liu, Q. Wei, H. Ren, C. Ming, M. Min, P. Chen and R. Ruan, Bioresour. Technol., 2017, 235, 59–69 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03090b |
| This journal is © The Royal Society of Chemistry 2020 |
