Open Access Article
Open Access ArticleCs3VO(O2)2CO3: an exceptionally thermostable carbonatoperoxovanadate with an extremely large second-harmonic generation response†
Guohong
Zou
 *a,
Zhien
Lin
*a,
Zhien
Lin
 a,
Hongmei
Zeng
a,
Hongil
Jo
a,
Hongmei
Zeng
a,
Hongil
Jo
 b,
Seong-Ji
Lim
c,
Tae-Soo
You
b,
Seong-Ji
Lim
c,
Tae-Soo
You
 c and
Kang Min
Ok
c and
Kang Min
Ok
 *b
*b
aCollege of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: zough@scu.edu.cn
bDepartment of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea. E-mail: kmok@cau.ac.kr
cDepartment of Chemistry, Chungbuk National University, Cheongju, Chungbuk 28644, Republic of Korea
First published on 24th September 2018
Abstract
A novel nonlinear optical (NLO) carbonatoperoxovanadate, Cs3VO(O2)2CO3, with an exceptionally high thermostability was successfully synthesized by introducing highly polarizable Cs+ cations and inorganic polydentate carbonate anions into asymmetric peroxovanadates. The structure of Cs3VO(O2)2CO3 is composed of distorted [VO(O2)2CO3]3− units and charge balancing Cs+ cations. The title compound exhibits the largest NLO intensity ever found in the current carbonate NLO materials, i.e., 23.0 times that of KH2PO4 (KDP). The remarkably strong second-harmonic generation (SHG) response originates from the synergistic effect of the exceedingly polarizable Cs+ cations, distortive polyhedra of the V5+ cation, delocalized π orbitals in CO3 groups, and distorted localized π orbitals in O2 groups. First-principles calculations indicated that introducing the polarizable cations into peroxovanadates not only induces the enhancement of the SHG response but also improves the thermal stability of the framework.
The development and availability of nonlinear optical (NLO) crystals,1–15 key materials for solid state lasers to produce continuously tunable coherent light by means of cascaded frequency conversion, have attracted extensive interest owing to their various adaptable applications. A superb NLO material16–19 should meet the following criteria: (1) larger SHG coefficient (dij) than d36 of KDP, (2) wide transparency range, (3) suitable birefringence to achieve the phase-matching condition, (4) large laser damage threshold, (5) good physicochemical and mechanical properties, and (6) easy growth of high quality large crystals. After decades of efforts by chemists and materials scientists to promote the development of novel NLO materials, a number of excellent NLO materials covering a broad wavelength range from ultraviolet (UV) and visible to mid-infrared (IR) have been discovered. Several widely utilized representative NLO materials include KH2PO4 (KDP),20 KTiOPO4 (KTP),21 LiNbO3,22 LiB3O5 (LBO),23 β-BaB2O4 (β-BBO),24 and AgGaS2 (AGS).25 However, each of the NLO materials has some specific drawbacks such as unbefitting birefringence and toxicity of raw materials.26,27 Thus, the demand for superior performing flawless NLO materials that can satisfy the increasing needs of the scientific and technological development still remains high. Since the SHG coefficient is a crucial factor that directly affects the efficiency of the laser, a systematic synthesis of novel NLO materials with strong SHG response is essential.
Employing noncentrosymmetric (NCS) chromophores during the initial syntheses as building blocks has been suggested to be a very effective strategy toward new NLO materials.28–30 Moreover, introducing more than two NCS chromophores into a compound could further induce a strong SHG response through the cooperative effect of the asymmetric chromophores. A few NCS chromophores include polar displacement of a d10 cation center, distorted polyhedra with d0 transition metal cations and/or stereochemically active lone pairs (SCALPs) resulting from the second-order Jahn–Teller (SOJT) effect, and delocalized π-orbital anionic groups.31–40 Recently, Ye and coworkers have proven that O22− anion groups could also make huge contributions to the NLO effect as the symmetric distribution of localized π-orbital electrons is broken when the O22− groups are coordinated to a d0 cation, V5+.41 We have recently demonstrated that employing inorganic polydentate carbonato groups in NCS peroxovanadates induced a sharp enhancement of the SHG response.42 Therefore, carbonatoperoxovanadates can be used as latent capacity NLO materials for practical applications. Thus far, however, the significant influence of charge balancing cations on the overall SHG intensity has not been well recognized compared to that of the anionic group theory when designing new NLO materials.43 Recently, a few novel NLO materials revealing large SHG responses with highly polarizable cations such as Li2CsPO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44–46 and Ba4B11O20F47 have been reported, where the increased polarization of cations with larger radii was beneficial to strong SHG intensity. Guided by these ideas, we have introduced a large alkali metal cation, Cs+, into a carbonatoperoxovanadate and successfully synthesized a novel highly thermostable NLO material, Cs3VO(O2)2CO3, in high yield. Surprisingly, Cs3VO(O2)2CO3 exhibits an SHG intensity of ca. 23.0 times that of KDP, the strongest SHG response ever found in NLO carbonates. In this manuscript, the origin of the extremely strong SHG intensity and the high stability of Cs3VO(O2)2CO3 is elucidated by comparison with other stoichiometrically equivalent alkali metal carbonatoperoxovanadates.
44–46 and Ba4B11O20F47 have been reported, where the increased polarization of cations with larger radii was beneficial to strong SHG intensity. Guided by these ideas, we have introduced a large alkali metal cation, Cs+, into a carbonatoperoxovanadate and successfully synthesized a novel highly thermostable NLO material, Cs3VO(O2)2CO3, in high yield. Surprisingly, Cs3VO(O2)2CO3 exhibits an SHG intensity of ca. 23.0 times that of KDP, the strongest SHG response ever found in NLO carbonates. In this manuscript, the origin of the extremely strong SHG intensity and the high stability of Cs3VO(O2)2CO3 is elucidated by comparison with other stoichiometrically equivalent alkali metal carbonatoperoxovanadates.
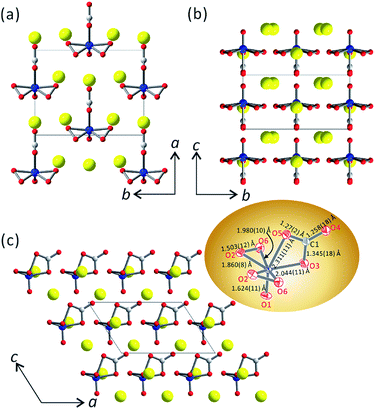
Yellow plate-like crystals of Cs3VO(O2)2CO3 were synthesized via a modified solution-evaporation method (Fig. S1†). The powder X-ray diffraction (PXRD) pattern of the ground crystals of Cs3VO(O2)2CO3 confirms the phase purity (Fig. S2†). Cs3VO(O2)2CO3, isostructural to A3VO(O2)2CO3 (A = K and Rb),41,42 crystallizes in the polar NCS space group of Cm (no. 8). The structure of Cs3VO(O2)2CO3 is composed of isolated [VO(O2)2CO3]3− complex anions and Cs+ ions as charge balancing cations (Fig. 1). V5+ cations reveal a seven-coordinate VO3(O2)2 distorted pentagonal bipyramid (pbp) geometry with oxygen atoms (O3 and O5) in a carbonato group, a double-bonded oxygen atom (O1) at the axial position, and two sets of oxygen atoms (O2 and O6) in peroxo groups. The equatorial plane, defined by four oxygen atoms (two O2 and two O6) from two peroxide anions (O2)2− and the carbonato oxygen atom (O3), is perpendicular to a crystallographic mirror plane that is composed of a (CO3)2− group, a V5+ cation, and the double-bonded oxygen atom (O1). The bonds between V5+ and oxide ligands in the apical position are significantly weaker than those between V5+ and equatorial O ligands due to the trans influence [2.311(11) Å vs. 1.860(8)–2.044(11) Å]. The observed V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oapical bond distance of 1.624(11) Å is similar to the average value of 1.609 Å, found in peroxovanadates. The C–O bond lengths in the (CO3)2− planar triangular group are in the range of 1.258(18)–1.345(18) Å. The different C–O bond distances observed in the (CO3)2− groups are attributed to the presence of further bonds of oxygen with other atoms. The short C–O bond length between C1 and the terminal oxygen O4 [1.258(18) Å] indicates a significant double-bond character. The stronger the C–O bonds [C–O5 (1.27(2) Å) vs. the C–O3 bond (1.345(18) Å)], the weaker the corresponding V–O bonds [V–O5 (2.311(11) Å) vs. V–O3 (2.044(11) Å)]. The VO3(O2)2 distorted pbps and the planar triangular CO3 groups share their edges. The interconnection results in isolated [VO(O2)2CO3] units. Interestingly, the [VO(O2)2CO3] groups align parallel to CO3 in the same direction, which should be extremely beneficial to generating a gigantic macroscopic SHG effect (Fig. 1).48,49
Oapical bond distance of 1.624(11) Å is similar to the average value of 1.609 Å, found in peroxovanadates. The C–O bond lengths in the (CO3)2− planar triangular group are in the range of 1.258(18)–1.345(18) Å. The different C–O bond distances observed in the (CO3)2− groups are attributed to the presence of further bonds of oxygen with other atoms. The short C–O bond length between C1 and the terminal oxygen O4 [1.258(18) Å] indicates a significant double-bond character. The stronger the C–O bonds [C–O5 (1.27(2) Å) vs. the C–O3 bond (1.345(18) Å)], the weaker the corresponding V–O bonds [V–O5 (2.311(11) Å) vs. V–O3 (2.044(11) Å)]. The VO3(O2)2 distorted pbps and the planar triangular CO3 groups share their edges. The interconnection results in isolated [VO(O2)2CO3] units. Interestingly, the [VO(O2)2CO3] groups align parallel to CO3 in the same direction, which should be extremely beneficial to generating a gigantic macroscopic SHG effect (Fig. 1).48,49
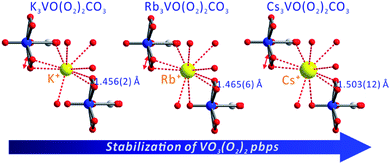
Thermogravimetric analysis (TGA) indicates that Cs3VO(O2)2CO3 is thermally stable up to 300 °C (Fig. S3†). Upon further heating under a nitrogen atmosphere, Cs3VO(O2)2CO3 completely decomposes to Cs4V2O7, Cs2O, and CO2 in two steps in the range of 300–1000 °C. Interestingly, Cs3VO(O2)2CO3 exhibits the highest thermal decomposition temperature (300 °C) among all reported peroxovanadates. In fact, the thermal stability of a series of isostructural compounds, A3VO(O2)2CO3 (A = K, Rb, and Cs), obviously increases as the radii of alkali metal cations increase (see Table 1). A closer structural examination suggests that the thermostability of A3VO(O2)2CO3 seems to be strongly affected by the alkali metal cations' size. Although the VO3(O2)2 pbps in A3VO(O2)2CO3 are stabilized by the coordinated bidentate carbonato ligands, the extent of distortion is varied by the different alkali metal cations. Specifically, two unique K+ cations in K3VO(O2)2CO3 interact with 9 oxide ligands with the K–O contact lengths in the range of 2.6561(18)–3.160(2) Å, and two kinds of Rb+ cations in Rb3VO(O2)2CO3 contact with 9 and 10 oxides with the Rb–O lengths in the range of 2.769(4)–3.550(4).41,42 In Cs3VO(O2)2CO3, the interactions between two unique Cs+ cations and oxide ligands are even longer and reveal Cs–O contact lengths in the range of 2.965(8)–3.687(8) Å.
| Compound | dec. T (°C) | E g (eV) | d(O–O) in (O2)2− (Å) |
|---|---|---|---|
| K3VO(O2)2CO3 | 230 | 2.57 | 1.456 |
| Rb3VO(O2)2CO3 | 250 | 2.68 | 1.465 |
| Cs3VO(O2)2CO3 | 300 | 2.81 | 1.503 |
As seen in Fig. 2, the small ionic size of K+ in K3VO(O2)2CO3 requires a smaller coordination environment. Substantial interaction between K+ cations and oxide ligands results in peroxo ligands with shorter O–O distances (1.456 Å) and more strained VO3(O2)2 pbps. However, the large Cs+ cations in Cs3VO(O2)2CO3 with a larger coordination moiety reveal peroxo ligands with longer O–O distances (1.503 Å) and less strained and more stable VO3(O2)2 pbps. Accordingly, the thermal stability of A3VO(O2)2CO3 (A = K, Rb, and Cs) increases in the K < Rb < Cs order attributable to the stability of the corresponding pbps.
The infrared (IR) spectrum of Cs3VO(O2)2CO3 revealed intense broad bands at 1370 and 1590 cm−1 attributed to the C–O stretching vibrations in the triangular CO3 groups and bands at 850 and 700–640 cm−1 due to the nonplanar bending vibrations of CO3 plane triangles (Fig. S4†). Bands observed at 1020 and 920 cm−1 were assigned to the characteristic absorption of νV–O. The assignments are consistent with other metal carbonatoperoxovanadates.50
The UV-vis diffuse reflectance spectrum of Cs3VO(O2)2CO3 was collected, and the absorption (K/S) data were calculated by the Kubelka–Munk function (Fig. 3).51,52 The optical band gap for Cs3VO(O2)2CO3 is 2.81 eV, indicating that the material is a wide band-gap semiconductor. The transmittance spectrum (Fig. S5†) and the UV absorption spectrum show that there is no absorption from 0.35 to 2.5 μm, suggesting that Cs3VO(O2)2CO3 has wide transparent regions ranging from near-UV to mid-IR. Also in A3VO(O2)2CO3 (A = K, Rb, and Cs), the experimental band gaps increase with increasing the radius of cations (Table 1 and Fig. S7†). The observed band gaps are closely related to the distortion of VO3(O2)2 pbps because the main contribution of the lowest part of the conduction band is d- and p-orbitals of V and O atoms, respectively (Fig. S9†). As we described earlier, the highly distorted VO3(O2)2 pbps in contact with the smaller coordination environment of K+ reveal a relatively smaller band gap, whereas the less strained VO3(O2)2 pbps interacting with the large Cs+ exhibit a larger band gap. In fact, the experimental results are in good agreement with the calculated band gaps (Fig. S10†).
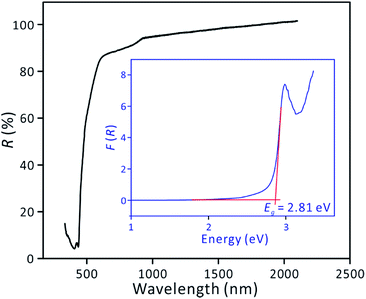 | ||
| Fig. 3 UV-vis-NIR transmittance spectrum of Cs3VO(O2)2CO3. The inset shows an optical diffuse reflectance spectrum of Cs3VO(O2)2CO3. | ||
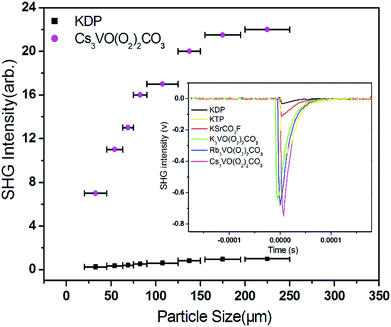
The SHG responses of Cs3VO(O2)2CO3 were measured on sieved ground crystals (Fig. 4). The SHG signals increase gradually with the increasing particle size of samples at the beginning and then tend to remain constant from 155 μm, indicating that Cs3VO(O2)2CO3 is type I phase-matchable. The title compound exhibits an extremely strong SHG response of ca. 23.0 times that of KDP, almost 7 times that of KSrCO3F, which is the largest among those of all the NLO carbonates, including CsPbCO3F (13 × KDP),32 RbCdCO3F (9 × KDP),53 and K4Eu2(CO3)3F4 (8 × KDP),54 reported to date. In addition, as seen in Fig. 4 and Table 2, the SHG efficiencies of the isostructural compounds A3VO(O2)2CO3 (A = K, Rb, and Cs) increase with increasing the size of alkali metal cations. The anionic group theory indicates that the values of intra-atomic dipole transitions in anionic groups are larger than those of the transitions occurring from the cations to the anionic groups which are off-site transitions.43,55 Thus, the NLO coefficients of Cs3VO(O2)2CO3 mainly originate from CO3 plane triangle groups. The major determinants for the NLO coefficients of the title compound are two: the structural criterion (C) and the density of anionic groups (n/V) ([CO3]2− in this case). A high C value (100%) results from the optimal arrangement of CO3 plane triangle groups in A3VO(O2)2CO3 (A = K, Rb, and Cs) as those in KSrCO3F,31 with all CO3 groups arranging in parallel and orienting in the same direction. Thus, the density of CO3 groups determines the macroscopic NLO coefficients of these four carbonate NLO materials.
| Compound | SHG (×KDP) | Structural criterion, C | Density of [CO3]2−, (n/V) (Å) | (n/V) × C (Å) |
|---|---|---|---|---|
| K3VO(O2)2CO3 | 20.0 | 1.00 | 0.0051 | 0.0051 |
| Rb3VO(O2)2CO3 | 21.0 | 1.00 | 0.0046 | 0.0046 |
| Cs3VO(O2)2CO3 | 23.0 | 1.00 | 0.0041 | 0.0041 |
| KSrCO3F | 3.3 | 1.00 | 0.0089 | 0.0089 |
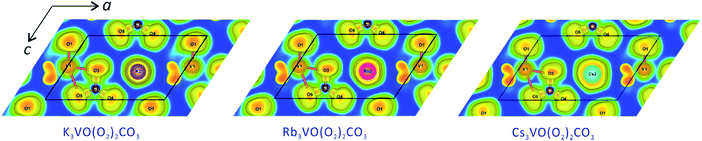
Interestingly, Cs3VO(O2)2CO3 exhibits an unexpectedly strong SHG response (7.0 × KSrCO3F), although the density of aligned CO3 groups in Cs3VO(O2)2CO3 is less than half compared to that of KSrCO3F (Table 2). Therefore, we believe that there are some other factors that affect the striking SHG intensity of Cs3VO(O2)2CO3 in addition to the anionic group theory. The distorted environment of VO3(O2)2 pbps in Cs3VO(O2)2CO3 should be an important contribution factor, which was confirmed by the calculated dipole moment value of 12.4 D (debyes) with a net moment of ca. 24.8 D for a unit cell along the c-direction. In addition, polarizability of Cs+ cations is another crucial factor influencing the SHG response. Comparing the three isostructural compounds, A3VO(O2)2CO3 (A = K, Rb, and Cs), we found that the SHG responses obviously increase with increasing the radii of cations. The electron localization function (ELF) diagrams of A3VO(O2)2CO3 (A = K, Rb, and Cs) are displayed in the crystallographic ac-plane in Fig. 5. It is clear that O1, O3, O4, and O5 atoms forming the VO3(O2)2 pbps reveal strongly distorted ELF values. In particular, the distortion of O3 forming the trigonal (CO3)2− group toward nearby cations increases as the polarizability of cations increases from K to Rb and to Cs. Therefore, the increasing trend of SHG response of these compounds should be attributed to the increasing polarizability of cations as well as the interatomic interactions with neighboring O atoms.
The calculated linear optical results show that the refractive index dispersion curves display strong anisotropy and follow the order of nz > ny ≈ nx with a moderate birefringence (Δn) (0.105@1064 nm), which is in favor of phase-matching during the SHG process (Fig. S11†). On the basis of the space group and Kleinman symmetry,56,57 there exist six non-zero independent SHG coefficient tensors for Cs3VO(O2)2CO3 (d11, d12, d13, d15, d24, and d33). The absolute value of d13, the highest tensor, in the static limit is calculated to be 8.7 pm V−1 at a wavelength of 1064 nm, which agrees well with our experimental value (Fig. S12†).
Conclusions
A novel superb NLO carbonatoperoxovanadate material, Cs3VO(O2)2CO3, has been successfully designed and synthesized by introducing highly polarizable cations and inorganic polydentate carbonato ligands into asymmetric peroxovanadates. Cs3VO(O2)2CO3 exhibits a thermal decomposition temperature of 300 °C, which is the highest among those of all the reported peroxovanadates. Cs3VO(O2)2CO3 also reveals an extremely strong SHG intensity of 23.0 × KDP, which is the largest value ever observed in the current carbonate NLO materials. Detailed structural investigations along with theoretical calculations confirmed that the extremely large SHG intensity originates from the cooperation of the NCS chromophores composed of CO3 planar triangle groups with delocalized π orbitals, O22− groups with distorted localized π orbitals, highly polarizable Cs+ cations, and SOJT distortive V5+ cation polyhedra. We found that careful tuning of the polarizability and the size of alkali metal cations is extremely important to design novel NLO materials with excellent performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 21401178, 21501161 and 21875146) and the National Research Foundation of Korea funded by the Ministry of Science and ICT (No. 2014M3A9B8023478, 2015R1A1A1A05027845, 2016R1A2A2A05005298 and 2018R1A5A1025208).Notes and references
- P. S. Halasyamani and K. R. Poeppelmeier, Chem. Mater., 1998, 10, 2753–2769 CrossRef CAS.
- H. W. Yu, H. P. Wu, S. L. Pan, Z. H. Yang, X. L. Hou, X. Su, Q. Jing, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2014, 136, 1264–1267 CrossRef CAS PubMed.
- K. M. Ok, Acc. Chem. Res., 2016, 49, 2774–2785 CrossRef CAS PubMed.
- T. T. Tran, J. Young, J. M. Rondinelli and P. S. Halasyamani, J. Am. Chem. Soc., 2016, 139, 1285–1295 CrossRef PubMed.
- G. Zou, C. Lin, H. Jo, G. Nam, T. S. You and K. M. Ok, Angew. Chem., Int. Ed., 2016, 55, 12078–12082 CrossRef CAS PubMed.
- L. Huang, Q. Wang, C. Lin, G. Zou, D. Gao, J. Bi and N. Ye, J. Alloys Compd., 2017, 724, 1057–1063 CrossRef CAS.
- T. T. Tran, N. Z. Koocher, J. M. Rondinelli and P. S. Halasyamani, Angew. Chem., Int. Ed., 2017, 56, 2969–2973 CrossRef CAS PubMed.
- G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS PubMed.
- X. Wang, Y. Wang, B. Zhang, F. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS PubMed.
- B. Zhang, G. Shi, Z. Yang, F. Zhang and S. Pan, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS PubMed.
- X. Dong, L. Huang, Q. Liu, H. Zeng, Z. Lin, D. Xu and G. Zou, Chem. Commun., 2018, 54, 5792–5795 RSC.
- M. Mutailipu, M. Zhang, B. Zhang, L. Wang, Z. Yang, X. Zhou and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 6095–6099 CrossRef CAS PubMed.
- Q. Wang, F. He, L. Huang, D. Gao, J. Bi and G. Zou, Cryst. Growth Des., 2018, 18, 3644–3653 CrossRef CAS.
- Q. Wang, C. Lin, G. Zou, M. Liu, D. Gao, J. Bi and L. Huang, J. Alloys Compd., 2018, 735, 677–683 CrossRef CAS.
- Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef CAS PubMed.
- L. Kang, S. Luo, H. Huang, N. Ye, Z. Lin, J. Qin and C. Chen, J. Phys. Chem. C, 2013, 117, 25684–25692 CrossRef CAS.
- S. Zhao, L. Kang, Y. Shen, X. Wang, M. A. Asghar, Z. Lin, Y. Xu, S. Zeng, M. Hong and J. Luo, J. Am. Chem. Soc., 2016, 138, 2961–2964 CrossRef CAS PubMed.
- M. Luo, F. Liang, Y. Song, D. Zhao, F. Xu, N. Ye and Z. Lin, J. Am. Chem. Soc., 2018, 140, 3884–3887 CrossRef CAS PubMed.
- M. Luo, F. Liang, Y. Song, D. Zhao, N. Ye and Z. Lin, J. Am. Chem. Soc., 2018, 140, 6814–6817 CrossRef CAS PubMed.
- G. A. Samara, Ferroelectrics, 1973, 5, 25–37 CrossRef CAS.
- F. C. Zumsteg, J. D. Bierlein and T. E. Gier, J. Appl. Phys., 1976, 47, 4980–4985 CrossRef CAS.
- G. D. Boyd, R. C. Miller, K. Nassau, W. L. Bond and A. Savage, Appl. Phys. Lett., 1964, 5, 234–236 CrossRef CAS.
- C. Chen, Y. Wu, A. Jiang, B. Wu, G. You, R. Li and S. Lin, J. Opt. Soc. Am. B, 1989, 6, 616–621 CrossRef CAS.
- C. T. Chen, B. C. Wu and A. D. Jiang, Sci. Sin., Ser. B, 1985, 28, 235–243 Search PubMed.
- A. Harasaki and K. Kato, Jpn. J. Appl. Phys., 1997, 36, 700–703 CrossRef CAS.
- H. Huang, J. Yao, Z. Lin, X. Wang, R. He, W. Yao, N. Zhai and C. Chen, Chem. Mater., 2011, 23, 5457–5463 CrossRef CAS.
- S. C. Wang and N. Ye, J. Am. Chem. Soc., 2011, 133, 11458–11461 CrossRef CAS PubMed.
- H.-S. Ra, K. M. Ok and P. S. Halasyamani, J. Am. Chem. Soc., 2003, 125, 7764–7765 CrossRef CAS PubMed.
- Y.-Z. Huang, L.-M. Wu, X.-T. Wu, L.-H. Li, L. Chen and Y.-F. Zhang, J. Am. Chem. Soc., 2010, 132, 12788–12789 CrossRef CAS PubMed.
- W.-L. Zhang, W.-D. Cheng, H. Zhang, L. Geng, C.-S. Lin and Z.-Z. He, J. Am. Chem. Soc., 2010, 132, 1508–1509 CrossRef CAS PubMed.
- G. H. Zou, N. Ye, L. Huang and X. S. Lin, J. Am. Chem. Soc., 2011, 133, 20001–20007 CrossRef CAS PubMed.
- G. H. Zou, L. Huang, N. Ye, C. S. Lin, W. D. Cheng and H. Huang, J. Am. Chem. Soc., 2013, 135, 18560–18566 CrossRef CAS PubMed.
- S. Zhao, P. Gong, L. Bai, X. Xu, S. Zhang, Z. Sun, Z. Lin, M. Hong, C. Chen and J. Luo, Nat. Commun., 2014, 5, 1–7 CAS.
- L. Huang, G. H. Zou, H. Q. Cai, S. C. Wang, C. S. Lin and N. Ye, J. Mater. Chem. C, 2015, 3, 5268–5274 RSC.
- S. Zhao, P. Gong, S. Luo, S. Liu, L. Li, M. A. Asghar, T. Khan, M. Hong, Z. Lin and J. Luo, J. Am. Chem. Soc., 2015, 137, 2207–2210 CrossRef CAS PubMed.
- M. Xia, X. Jiang, Z. Lin and R. Li, J. Am. Chem. Soc., 2016, 138, 14190–14193 CrossRef CAS PubMed.
- S. Zhao, Y. Yang, Y. Shen, B. Zhao, L. Li, C. Ji, Z. Wu, D. Yuan, Z. Lin, M. Hong and J. Luo, Angew. Chem., Int. Ed., 2016, 56, 540–544 CrossRef PubMed.
- F. F. Mao, C. L. Hu, X. Xu, D. Yan, B. P. Yang and J. G. Mao, Angew. Chem., Int. Ed., 2017, 56, 2151–2155 CrossRef CAS PubMed.
- Y. Shen, S. Zhao and J. Luo, Coord. Chem. Rev., 2018, 366, 1–28 CrossRef CAS.
- M. L. Liang, Y. X. Ma, C. L. Hu, F. Kong and J.-G. Mao, Dalton Trans., 2018, 47, 1513–1519 RSC.
- Y. Song, M. Luo, F. Liang, N. Ye and Z. Lin, Chem. Commun., 2018, 54, 1445–1448 RSC.
- G. Zou, H. Jo, S. J. Lim, T. S. You and K. M. Ok, Angew. Chem., Int. Ed., 2018, 57, 8619–8622 CrossRef CAS PubMed.
- N. Ye, Q. Chen, B. Wu and C. Chen, J. Appl. Phys., 1998, 84, 555–558 CrossRef CAS.
- L. Li, Y. Wang, B. H. Lei, S. Han, Z. Yang, K. R. Poeppelmeier and S. Pan, J. Am. Chem. Soc., 2016, 138, 9101 CrossRef CAS PubMed.
- Y. Shen, Y. Yang, S. Zhao, B. Zhao, Z. Lin, C. Ji, L. Li, P. Fu, M. Hong and J. Luo, Chem. Mater., 2016, 28, 7110–7116 CrossRef CAS.
- X. Cheng, M. H. Whangbo, G. C. Guo, M. Hong and S. Deng, Angew. Chem., Int. Ed., 2018, 57, 3933–3937 CrossRef CAS PubMed.
- H. P. Wu, H. W. Yu, Z. H. Yang, X. L. Hou, X. Su, S. L. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215–4218 CrossRef CAS PubMed.
- P. A. Maggard, T. S. Nault, C. L. Stern and K. R. Poeppelmeier, J. Solid State Chem., 2003, 175, 27–33 CrossRef CAS.
- P. Yu, L.-J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227–2235 CrossRef CAS PubMed.
- A. Butler, M. J. Clague and G. E. Meister, Chem. Rev., 1994, 94, 625–638 CrossRef CAS.
- P. Kubelka, Z. Tech. Phys., 1931, 12, 593–603 Search PubMed.
- J. Tauc, Mater. Res. Bull., 1970, 5, 721–729 CrossRef CAS.
- G. Zou, G. Nam, H. G. Kim, H. Jo, T.-S. You and K. M. Ok, RSC Adv., 2015, 5, 84754–84761 RSC.
- J. D. Grice, V. Maisonneuve and M. Leblanc, Chem. Rev., 2007, 107, 114–132 CrossRef CAS PubMed.
- C. Chen, Y. Wu and R. Li, Int. Rev. Phys. Chem., 1989, 8, 65–91 Search PubMed.
- J. F. Nye, Physical Properties of Crystals, Oxford University Press, Oxford, UK, 1957 Search PubMed.
- D. A. Kleinman, Phys. Rev., 1962, 126, 1977–1979 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1851047. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc03672a |
| This journal is © The Royal Society of Chemistry 2018 |