Aerogel templating on functionalized fibers of nanocellulose networks†
Thanh-Dinh
Nguyen
 a,
Wadood Y.
Hamad
a,
Wadood Y.
Hamad
 b and
Mark J.
MacLachlan
b and
Mark J.
MacLachlan
 *a
*a
aDepartment of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia V6T 1Z1, Canada. E-mail: mmaclach@chem.ubc.ca
bFPInnovations, 2665 East Mall, Vancouver, British Columbia V6T 1Z4, Canada
First published on 10th July 2018
Abstract
We present novel spaghetti-like gel fibers of gelatin-functionalized cellulose nanocrystals (gCNCs) and aerogel templating of semiconductors on the gCNC scaffolds. An injection method is first used to make CNC aerogel fibers by crosslinking with gelatin (GE). The gelation of the injectable fibers can be conducted by manipulating interactions between CNCs and GE to preserve the chiral nematic order. The gCNC fibers are then used as templates to make porous TiO2/gCNC aerogel composites with structural organization imparted by the chiral arrangement and hierarchical organization of the gCNC fibers. The uniqueness of this templating concept lies in its ability to truly replicate spindle-shaped features of CNCs in freestanding TiO2 nanofibrillar aerogels. The affinity of functional groups of gelatin on the CNC aerogels with metal-based precursors causes the attachment of TiO2 nanoparticles around cellulose nanorods to form core–shell-like aerogel composites. The uniform integration of these components leads to the retention of TiO2 aerogel fibers after thermal removal of the gCNC template. To further demonstrate the use of gCNC fibers for templating, they are used to fabricate polypyrrole composites that retain the chiral structure of the aerogel. These new templated fibers are promising materials for supercapacitor electrodes, sensors, catalysts, and dye-sensitized solar cells. The results described here demonstrate a new and generalizable method to construct mesoporous materials with an open-network structure through helicoidal aerogel templating at the nanoscale.
Introduction
Porous semiconducting aerogels have been explored for a variety of technologies such as optical switching, catalysis, energy storage, sorption, filtration, and insulation.1 Distinctive optoelectronic properties of such aerogel materials are a function of their open network, high surface area, nanosized features, and bandgap.2 Recent efforts have shown the successful synthesis of well-defined porous conducting networks of titania,3 quantum dots,4 molybdenum sulfide,5 and polypyrrole.6 Control of the morphology and hierarchical structure of conducting nanostructured aerogels is of great interest to study device fabrication. Aerogel templating is a straightforward strategy to construct highly porous materials with organized structures determined by templates.7 Finding new templating methods to fabricate conducting aerogels with tunable structures is thus still a major goal to enable these materials for diverse applications.Carbohydrates and proteins have hierarchical fibrous structures that arise from the complexity of their primary units at the molecular and nanoscale levels.8 Colloidal fibrils of these biopolymers and their derivatives can self-organize into secondary structures such as hydrogels, liquid crystals, and thin films with anisotropic mechanical properties.9 Besides novel applications emerging from their inherent structures, the assembled biopolymer networks can serve as intriguing templates for other materials, a process known as biotemplating.10 Templating of semiconductors with biopolymer aerogels is of great interest to create new materials with sophisticated structures. Fabricating new structural forms of hierarchically nanostructured networks of biopolymers is thus an interesting way to extend biotemplating for developing new aerogel materials.
Cellulose nanocrystals (CNCs) are an exciting class of colloidal template for the fabrication of diverse materials with unique photonic and mechanical properties.11 Negatively charged CNCs with surface sulfate groups are obtained by sulfuric acid hydrolysis of native cellulose microfibers.12 CNCs can self-assemble into lyotropic liquid crystals (LCs) with a chiral nematic structure.13 In the chiral nematic LC, CNCs are arranged into aligned layers, where the orientation of CNCs in adjacent layers rotates.14 When the pitch of the rotation in the chiral nematic liquid crystals matches the wavelengths of incident light, they show the diffraction of visible light and appear iridescent.15 Thin films of CNCs that retain this chiral nematic structure are of interest for optical coatings16 and as templates for new photonic materials.11
Lyotropic cellulose LCs can form optically active hydrogels that can convert to hierarchically nanostructured aerogels.17–19 The gelation of CNCs generally occurs by interaction of CNCs with crosslinkers,20 electrostatic attraction,21,22 hydrothermal-induced desulfation,23 cationic functionality,24 or ice-assisted assembly.25 CNC hydrogels can be solidified to recover aerogels by freeze drying or critical point drying. These efforts have resulted in elegant examples of composite supercapacitors,26 flexible aerogels,27 and superabsorbent aerogels.28 The enlarged porosity of amphiphilic networks leads these functional cellulose aerogels to have unique capacitative, mechanical, and absorbent properties, but most of them disrupt the chiral nematic order of CNCs.25,29–32 We recently solidified the LC phase of phase-separated CNCs into chiral nematic aerogels for soft-templating of periodic silica replicas.33 There have been some studies of using CNC gels as templates for functional porous materials;11,33,34 however, freestanding semiconductor aerogels (e.g., TiO2 monoliths) templated on chiral nematic CNC networks are underdeveloped.
Here we present a reliable method to produce aerogel fibers of CNCs crosslinked by gelatin. Controlling the surface diffusion-induced crosslinking of CNCs with gelatin enabled the ability to preserve the chiral nematic arrangement of CNCs in the gel fibers. We used these fibers as novel biotemplates to form titania and polypyrrole composite aerogels with chiral nematic structure. The use of gelatin is crucial as it both crosslinks CNCs and simultaneously presents surface functional groups to support fully deposited semiconductors (e.g., titania and polypyrrole) on the CNC nanofibrillar aerogels. Upon calcination of the composites, freestanding aerogel fibers of titania are obtained with structural integrity at the nanoscale level.
Experimental
Hierarchical gCNC aerogel fibers (HCF)
Gelatin (1.4 g) was mixed with 20 mL CNC aqueous suspension (2.5 wt%, pH ∼ 6.5) with sequential stirring for 10 min and sonicating for 20 min using an Ultrasonic Cleaner (120 W, 60 Hz) to dissolve it at room temperature to yield a homogeneous gel dispersion. Portions of this mixture (2 mL) were transferred into a 3 mL syringe equipped with a 1 mm-diameter needle and immediately injected into fresh water to obtain hydrogel fibers that resemble cooked spaghetti. The hydrogels were collected and then immersed in ethanol for 1 h to obtain gCNC alcogels. The resulting alcogels (∼3 g) were dried with supercritical CO2 to obtain ∼200 mg of gCNC aerogel fibers.Twisted gCNC aerogel fibers (TCF)
The dilute CNC aqueous suspension (2.5 wt%, pH ∼ 6.5) was evaporated on Petri dishes in a fumehood at room temperature to concentrate the CNCs to ∼10 wt%. Portions of this suspension (2 mL) were transferred into a 3 mL syringe equipped with a 1 mm-diameter needle and immediately injected into a fresh ∼6 wt% GE aqueous solution. The injection of the concentrated lyotropic cellulose LCs afforded spaghetti-like fibers stable in the GE aqueous solution. Note that to accelerate the surface adsorption of GE crosslinkers onto CNC fibers, it is important to use a high concentration of GE (≥3 wt%) since lower GE concentrations led to the dissolution of CNC fibers. Because the 6 wt% GE aqueous solution itself gelled at room temperature, the mixture was warmed at 50 °C for 48 h to maintain homogeneity of the GE solution and to allow the GE crosslinkers to adsorb onto the hydrogel, fixing the fibers in place. This method afforded multidomain, chiral nematic gCNC hydrogel fibers. The resulting hydrogels were collected, washed with copious water, and then solidified into twisted gCNC aerogel fibers (TCF) by supercritical CO2 drying.TiO2/gCNC aerogel composites and TiO2 aerogel fibers
The prepared gCNC alcogel fibers (∼3.5 g) were dispersed in 75 mL isopropanol and then 0.5 mL titanium diisopropoxide bis(acetylacetonate) (TDAA) was added. Distilled water (20 mL) was added, then the sample was heated to 60 °C with gentle stirring for 20 h to slowly hydrolyze TDAA to TiO2. The resulting mixtures remained transparent in the early stages of the reaction and gradually turned cloudy after ∼30 min. After 20 h, the products were washed thorough with ethanol to remove residual species, yielding light yellow fibrous composites. The TiO2/gCNC composites were dried with supercritical CO2 to obtain TiO2/gCNC aerogel fibers. The TiO2/gCNC composites were calcined in air at 540 °C for 6 h to generate TiO2 aerogels. The typical reaction synthesis yielded ∼6 mg of the calcined TiO2 from ∼180 mg of TiO2/HCF aerogels recovered from ∼3.4 g of hydrogel composite fibers.PPy/gCNC aerogel composite fibers
The prepared gCNC alcogel fibers (∼3.5 g) were redispersed in 50 mL distilled water. Pyrrole (0.2 mL) was added to the above mixtures under gentle stirring for 10 min. This gave an initial concentration of pyrrole of about 3.8 mg L−1. 10 mg (NH4)2S2O8 was then added to the mixtures to facilitate the oxidative polymerization of pyrrole into polypyrrole on gCNC aerogel fibers. The mixtures visibly changed color to violet in the early stages of reaction, then to black. After 20 h, black PPy/gCNC hydrogel fibers were isolated, were immersed in ethanol, then were dried from supercritical CO2 to obtain black PPy/gCNC aerogel fibers.Results and discussion
Gelatin (GE) is a protein with important characteristics of reversible thermal gelation.35 The structure of gelatin is complex and contains many amino acids; a typical segment of gelatin is shown in Scheme 1.36 Gelatin has been combined with cellulose (e.g., microfibers and spindle-shaped nanocrystals) to prepare composite gels.37–40 To the best of our knowledge, unique structured features of spaghetti-like fibers and chiral nematic structures of gelatin-crosslinked CNC aerogels and as well their helicoidal aerogel templating are virtually unexplored. In this work, the homogeneous combination of CNCs and GE into GE-crosslinked CNC (gCNC) fibers was performed to control the structural organization using two different experimental protocols. Typically, mixed GE/CNC gels were injected into water to obtain hierarchical (non-twisted) gCNC fibers denoted as HCF. Alternatively, the injection of lyotropic liquid crystalline CNCs into a GE aqueous solution yielded twisted gCNC fibers denoted as TCF. These gCNC fibers were subsequently used as aerogel templates for materials design.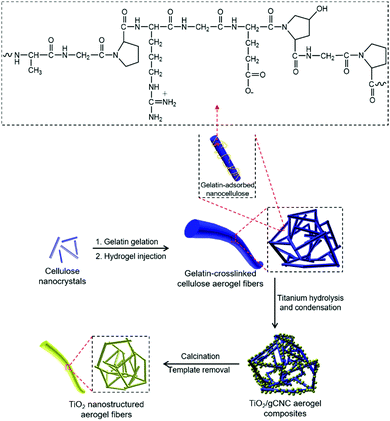 | ||
| Scheme 1 Schematic of aerogel templating of TiO2 fibers on hierarchical gCNC nanofibrillar networks. | ||
We found the addition of GE to lyotropic CNCs (2.5 wt%, pH ∼ 6.5) yielded homogeneous aqueous gels at ambient conditions. Qualitatively, the extent of the gelation of the resulting mixture scales with GE loading in the CNC suspension. It was interesting to find that the injection of the GE/CNC aqueous mixture by syringe into water led to the immediate precipitation of gel fibers from the aqueous phase. In control experiments, no fibers can be obtained from the injection of the aqueous dispersion of either CNCs or GE alone, even using high concentrations and at various pH levels. This suggests that intermolecular interactions between cellulose and gelatin are critical for the formation of hydrogel networks.
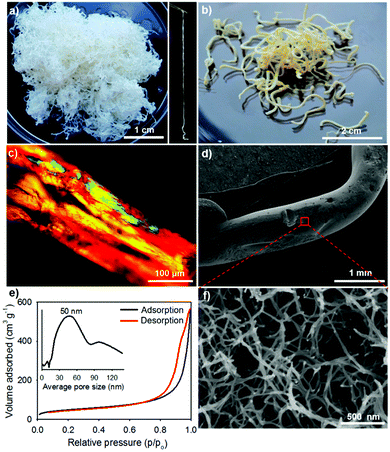
The GE-crosslinked CNC (gCNC) fibers are millimeters in diameter and tens of centimeters in length, and have relatively smooth surfaces. They are stable in water for months without any apparent change to their shape. The gCNC fibers are semi-transparent, flexible, and swollen, characteristic of soft hydrogels, which overall look like cooked spaghetti strands (Fig. 1a). The hydrogels were immersed in ethanol to obtain gCNC alcogels that were subsequently dried using supercritical CO2 to form opaque gCNC aerogels with shape and size retention (Fig. 1b).
Powder X-ray diffraction (PXRD) patterns (Fig. S1, ESI†) show that the solidified fibers contain cellulose I crystallites, resembling pristine CNCs.41 No diffraction peaks of GE could be observed, even with high GE loading, indicating that the GE is amorphous.42 Elemental analyses confirmed ∼7.3 wt% nitrogen (N), indicating the presence of GE (theoretical ∼18 wt% N) in the CNC fibers. IR spectra (Fig. S2, ESI†) of the fibers show distinct stretching bands associated with vibrational modes of cellulose (e.g., C–O–C pyranose ring stretching vibration at 1050 cm−1 and CH2 scissoring motion at 1430 cm−1)43 along with vibrational bands at 1628 cm−1 for amide I (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch), at 1525 cm−1 for amide II (C–N stretch/N–H in-plane bend), and 1230 cm−1 for amide III (C–N stretch/N–H in-plane bend) characteristic of gelatin.44 Thermal analyses (Fig. S3, ESI†) show that the aerogel fiber first dehydrated below 250 °C and then started to decompose at about 320 °C, which is higher thermal stability than each component. These analyses suggest that the hydrogels form by physical crosslinking of CNCs with GE through hydrogen-bonding interactions of the peptide with hydroxyl and sulfate groups of CNCs.
O stretch), at 1525 cm−1 for amide II (C–N stretch/N–H in-plane bend), and 1230 cm−1 for amide III (C–N stretch/N–H in-plane bend) characteristic of gelatin.44 Thermal analyses (Fig. S3, ESI†) show that the aerogel fiber first dehydrated below 250 °C and then started to decompose at about 320 °C, which is higher thermal stability than each component. These analyses suggest that the hydrogels form by physical crosslinking of CNCs with GE through hydrogen-bonding interactions of the peptide with hydroxyl and sulfate groups of CNCs.
Electron microscopy studies revealed that the gCNC fibers with diameters of millimeters are actually constructed from smaller nanofibers that form a hierarchical aerogel network. Polarized optical microscope images (POM, Fig. 1c) of the large fibers show that they are highly anisotropic (strong birefringence), but there is no evidence of a fingerprint texture often seen in CNC films and characteristic of chiral nematic order. The small, interconnected fibers are ∼20 nm in diameter compared with ∼5–10 nm-diameter CNCs, suggesting bundles of several CNCs are present (Fig. 1d, f and Fig. S6, ESI†). The hierarchical gCNC aerogel fibers (HCF) have a surface area of ∼170 m2 g−1 and pore size distribution centered around 50 nm (N2 sorption, Fig. 1e), consistent with the enlarged porous networks viewed by SEM. It is noteworthy that the injection of CNCs into the gelatin solution gives hydrogel fibers of gCNCs that form highly porous structures on solidification as evidenced by gas sorption isotherms and SEM in Fig. 1e and f. In contrast, traditional casting of CNC suspensions yields films with low porosity.45
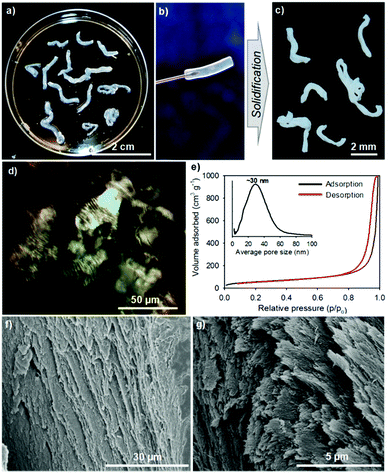
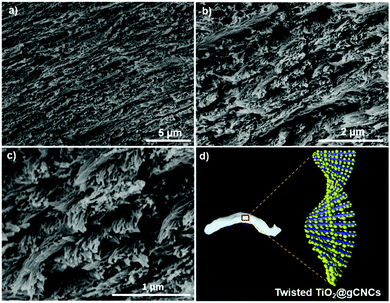
Unfortunately, the direct mixing of lyotropic cellulose nanocrystal LCs with GE leads to the disruption of the chiral nematic organization of CNCs in the gel fibers (as observed in HCF). To address this obstacle and produce aerogel fibers with chiral nematic order, we adsorbed GE through lyotropic LCs to crosslink nanocellulose into twisted gCNC hydrogel fibers (TCF). In a typical procedure, the CNC aqueous suspension (2.5 wt%, pH ∼ 6.5) was concentrated up to ∼10 wt% by water evaporation at room temperature. The concentrated cellulose LCs were injected by syringe into a 6 wt% GE aqueous solution. The mixture was warmed to 50 °C and held at this temperature for 48 h to accelerate the adsorption of GE within lyotropic cellulose LCs for hydrogel crosslinking. Similarly to the GE/CNC mixtures, the hydrogel injection of CNCs in the GE aqueous medium also yielded spaghetti-like gel fibers that maintain during aging (Fig. 2a and b). These CNC hydrogel fibers are more transparent and have larger diameters than the cloudy hydrogels derived from the GE/CNC aqueous mixtures. POM images (Fig. 2d) of these CNC hydrogel fibers show the fingerprint texture characteristic of chiral nematic order that resembles that of lyotropic cellulose LCs.13
After supercritical CO2 drying of the CNC hydrogels, aerogels with the shape integrity of fibers were obtained (Fig. 2c). SEM images (Fig. 2f and g) of the CNC aerogel fibers show left-handed twisting of CNC nanorods in transverse plane to form a chiral nematic structure with pitch of ∼1 μm at the macroscopic scale. Nitrogen sorption isotherms (Fig. 2e) of the helicoidal CNC aerogel fibers show a surface area of ∼210 m2 g−1 and uniform pore size distribution with a maximum peak at ∼30 nm. This indicates the preservation of the chiral nematic order of CNCs in the aerogel fibers led to lower pore size but higher surface area compared with the HCF samples. IR spectra (Fig. S7d, ESI†) also show distinct stretching bands of gelatin among the CNC fibers. These results assume the surface adsorption of GE to CNC fibers without disrupting the LC phase, in which GE may intercalate between crosslinked CNC assemblies, thus forming the twisted gCNC gel fibers (TCF).
Owing to the gelation properties of proteins, many gelatin/cellulose composite gels have been prepared by self-assembly or injection.37–40 For example, nanocellulose was used as a crosslinker for gelatin to improve mechanical and thermal properties of composite hydrogels.38,39 Injectable hydrogels of nanocellulose were also made by crosslinking with alginate/gelatin or carboxymethyl cellulose/dextran.37,46 None of these reports demonstrates a chiral form of the cellulose, and the injectable hydrogels do not usually show fiber-shaped objects. Our experiment provides the first evidence that injecting CNCs in the presence of GE can yield spaghetti-like aerogel fibers that can retain the chiral nematic structure of CNCs.
Considering the chirality and high porosity of these novel gCNC aerogels, they are promising for applications in biomedical engineering, fibril processing, absorption, scaffolding, and chiral separations.18,47,48 These gCNC materials may also be novel precursors to prepare hydrophobic nanofibrillar aerogels by surface modification for oil separations.49,50 We sought to take advantage of the chemical functionality of GE on CNCs in order to template other functional aerogel materials. Because titanium dioxide (TiO2) is a well-known semiconductor that has been extensively used for photocatalysis and electro-optic devices,51 we selected this as a representative example to demonstrate the concept of aerogel templating on gCNC scaffolds. Both forms of the gCNC fibers with hierarchical and twisted structures (HCF and TCF) were used as aerogel templates for titania.
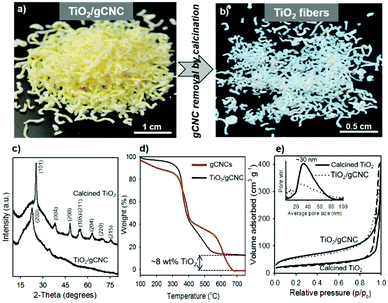
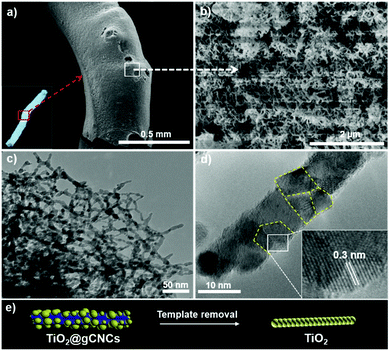
Titanium diisopropoxide bis(acetylacetonate) (TDAA) was slowly hydrolyzed and condensed around the injectable gCNC fibers to form TiO2/gCNC composites. In both cases (HCF and TCF), the products collected from the mixtures are slightly yellow, solidified fibers with a fiber-like shape analogous to that of the pristine gCNC aerogels, suggesting the uniform deposition of titania (Fig. 3a and Fig. S15a, ESI†). Next, the calcination of these composites removed the gCNC template, yielding TiO2 aerogels. It was surprising to obtain freestanding TiO2 fibers with the shape integrity of the TiO2/HCF composites, albeit with some shrinkage (Fig. 3b). Scheme 1 shows the synthetic route to freestanding TiO2 aerogel fibers templated on the gelatin-crosslinked CNC scaffolds in the fibers.
We examined the structures of the TiO2/HCF composites before and after calcination. Prior to calcination, the cellulose remains crystalline, but the titania is amorphous in the TiO2/HCF composites (PXRD, Fig. 3c). Thermogravimetric analysis (TGA) results (Fig. 3d) show that the mass retention of the composites at 550 °C is ∼8 wt% for TiO2 after decomposition of CNCs and gelatin below this temperature. This corresponds to only ∼8 wt% TiO2 in the original aerogel composites, which is also consistent with the result (∼4.2 wt% Ti) of energy-dispersive X-ray (EDX) analysis (Fig. S8, ESI†). Conversely, the gCNC aerogel sample shows a total mass loss from the complete decomposition of CNCs and gelatin above 680 °C and no ceramic solids left behind after the analysis. After calcination of the TiO2/HCF composites under air at 540 °C, PXRD of the product shows only anatase TiO2, and the signals of CNCs are absent. Elemental analyses show no C or N in the calcined product, and EDX spectra show only TiO2. Thus, the calcination of the TiO2/HCF composites affords an aerogel made of anatase TiO2 crystals.
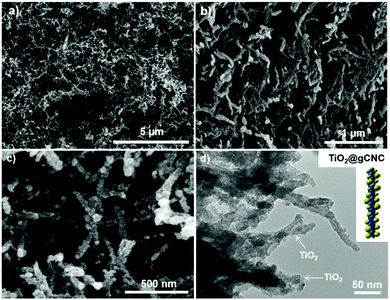
The TiO2/HCF composites were assessed by SEM (Fig. 4a–c and Fig. S10, ESI†). The TiO2/gCNC composite fibers appear structurally similar to the pristine gCNC aerogels, but fibrous components within larger fibers (∼0.7 mm) appear rougher and have larger diameters (∼30 nm). At high magnification, the composites show TiO2 nanoparticles densely distributed over the entire surface of the individual nanobundles. The uniform decoration seems to create a continuous TiO2 nanocluster layer on the gCNC nanofibers. Transmission electron microscopy (TEM) images (Fig. 4d and Fig. S11, ESI†) of these samples further reveal a hybrid open network that consists of ∼12 nm-sized TiO2 particles distributed on individual bundles of the gCNC assemblies. These results confirm the surface deposition of amorphous TiO2 nanoparticles on individual gCNC bundles to form an interconnected fibrillar hybrid structure within aerogels. We also carried out a control experiment of using mesoporous pure CNC films52 to support hydrolyzed TiO2 particles, but the composite films were destroyed after removal of the cellulose template. This suggests that the amino, carboxyl, and/or carbonyl groups of gelatin have strong affinity to metal ions to attach titanium-based precursors on gCNCs.
Remarkably, freestanding aerogel fibers of TiO2 nanoparticles were conserved during calcination of the TiO2/HCF composites. This TiO2 fiber has an aerogel network of nanorods (SEM, Fig. 5a, b and Fig. S12, ESI†) with hierarchical organization analogous to that of the TiO2/gCNC composites. Notably, the TiO2 nanoparticles in the aerogels remain separated and distributed rather than agglomerated after calcination. TEM images (Fig. 5c and Fig. S13, ESI†) of the TiO2 aerogel fibers also confirm a porous open network of interconnected nanorods with ∼10–15 nm diameter, a little larger than individual CNCs (∼5–10 nm). High-resolution TEM (HRTEM) images (Fig. 5d and Fig. S14, ESI†) of the aerogel fibers show anatase TiO2 nanorods that are made up of ∼5 nm-sized tiny particles with a lattice spacing of ∼0.3 nm, confirming that they were constructed from oriented attachment. This indicates that the TiO2 nanoparticles on the gCNC bundles underwent thermal shrinkage and consequently fused with neighboring ones to form a pearl-necklace nanostructure after template removal. These results provide evidence for the successful transfer of the spaghetti-like aerogel of gCNCs into hierarchical aerogels of interconnected TiO2 nanorods.
Nitrogen sorption studies show that the aerogel fibers of the as-prepared and calcined TiO2/HCF composites both have macro-mesoporous structures characteristic of an aerogel and in agreement with the electron microscopy observations (Fig. 3e). The specific surface area and pore size are ∼200 m2 g−1 and ∼25 nm and ∼90 m2 g−1 and ∼30 nm for TiO2/gCNC and for TiO2, respectively. Removal of the template from the TiO2/HCF composites clearly decreases the surface area, but still results in TiO2 fibers with considerable porosity.
Structural and compositional analyses were performed for the TiO2 aerogels templated by twisted gCNC fibers (TCF). TGA analysis shows the TiO2/TCF composite contains ∼5 wt% TiO2 that is consistent with the result (∼2.5 wt% Ti) of EDX analysis (Fig. S16 and S17a, ESI†). This titania loading is a little lower than that in the TiO2/HCF composites, possibly due to the limited adsorption of functional GE molecules on CNCs compared with the direct mixing of GE with CNCs. PXRD patterns (Fig. S17b, ESI†) also reveal a mixture of crystalline nanocellulose and amorphous titania in the TiO2/TCF aerogel composites that transformed to anatase titania crystals after calcination. It is apparent from SEM images that the chiral nematic structure of CNC aerogels was retained in the TiO2/TCF composites (Fig. 6). Unlike the TiO2/HCF composites, the TiO2 components deposited on CNCs in TiO2/TCF could not be observed by SEM, possibly due to the tiny nanoparticle sizes. This structural feature is likely related to the low concentration of GE adsorbed on CNCs. We found that the calcination of the helicoidal TiO2/gCNC composites under air recovers aerogels of anatase TiO2 pearl-necklace nanofibrils, but the twisted chiral order present in the TiO2/gCNC composites is severely diminished by major shrinkage of the fibers (Fig. S18, ESI†). We also attempted to prevent the shrinkage of the TiO2 aerogel fibers to preserve the order by first carbonizing the composite and then calcining under air. Regrettably, the resulting TiO2 aerogels show exclusively a layered nanofibrillar network rather than the chiral nematic structure (Fig. S20, ESI†).
A few studies of using cellulose as an anisotropic template for constructing metal oxide nanofibrils have been reported. For example, Korhonen et al.53 templated inorganic hollow nanotubes (TiO2, ZnO, Al2O3) with native cellulose nanofilaments by atomic layer deposition. Liu et al.54 prepared macroporous Fe2O3 nanostructured fibrils templated from cellulose microfibrils. These approaches use native forms of cellulose such as filaments and microfibers rather than CNCs. These natural cellulose templates have much larger size (diameter and length) and poorer organization than CNCs. As a result, these templated oxide materials are generally large, irregular fibers and do not form aerogel networks.
Because the diameters of CNCs (∼10 nm) are much smaller than those of native cellulose fibers (TEM image in Fig. S6a, ESI†), it is challenging to accomplish the full attachment of oxide nanoparticles on the backbone of individual CNCs. As a result, there is no previous demonstration of truly transforming nanorod-like structures of single CNCs or assemblies of several CNCs into freestanding oxide replicas by means of templating. Some recent works have used CNCs to template oxide (e.g., TiO2) materials, but they were mesoporous films55 and nanosized powders with low surface areas and pores56 rather than aerogel nanofibrillar networks. Another work used CNCs with considerably larger size to prepare TiO2/CNC tube-like nanofibrils by a hydrothermal reaction, but the authors did not investigate the removal of template in these composites.57 To the best of our knowledge, our method presents the first evidence of the aerogel templating of freestanding metal oxide (e.g., TiO2) nanofibers using CNCs. The high surface area and enlarged porosity of our CNC-templated hierarchical TiO2 aerogels lend them to applications in photocatalysis and photovoltaics.58
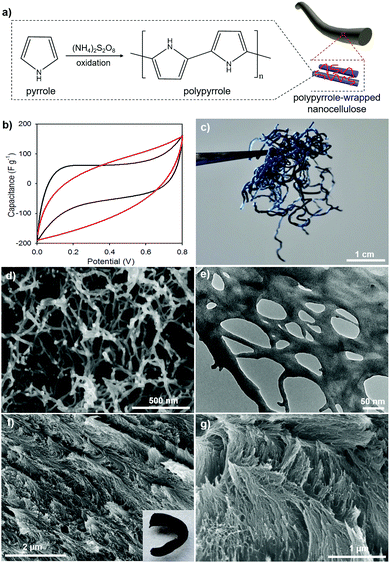
To generalize our new method, we developed polypyrrole (PPy) composites templated by gCNC aerogel fibers; both HCF and TCF were used as scaffolds. PPy/gCNC composites were prepared by oxidative polymerization of pyrrole within the gCNC template. The gCNC fibers remain intact, but turn to a homogeneous black color as the uniform polymerization proceeds (Fig. 7a, inset of part 7f). After supercritical CO2 drying, the PPy/gCNC composites show negligible shrinkage but are more rigid than the gCNC aerogels. Elemental analyses show that the PPy/HCF fibers contain 8.5 wt% N compared to 7.3 wt% N in the gCNC fibers. The presence of polypyrrole in the composites was also confirmed by IR spectroscopy (Fig. S23, ESI†). N 1s X-ray photoelectron spectroscopy (XPS) analysis (Fig. S24, ESI†) of the PPy/HCF fibers show that nitrogen components are mainly found in an amide bonding environment (399.8 eV) (from gelatin) with additional contributions of pyrrolic-like nitrogen (400.9 eV) and quaternary-like nitrogen (402.5 eV) of pyrrole. PXRD patterns (Fig. S25, ESI†) of the composites show diffraction peaks only for CNCs, verifying that the PPy is amorphous. The composites show an increased thermal stability relative to the pristine gCNC samples (TGA, Fig. S26, ESI†). The structural phase and chemical composition of the PPy/TCF composites were also found to be similar to those in the PPy/HCF materials (Fig. S30, ESI†).
Electron microscopy images (Fig. 7d–g) reveal the structural integrity of the composites during pyrrole polymerization. PPy is uniformly distributed on gCNCs interconnected within the fibers. The PPy/HCF composites have a hierarchical aerogel network that looks like a web under TEM (Fig. 7e and Fig. S29, ESI†), whereas the PPy/TCF composites retain the chiral nematic organization of the CNC scaffolds (Fig. 7f and g). N2 sorption measurements (Fig. S27, ESI†) confirm that the PPy/HCF composites maintain the macro–mesoporous structure with the surface area (∼150 m2 g−1) and pore size (∼20 nm) lower than for the pristine gCNC aerogels, consistent with the PPy occupying some of the pore space.
These conducting PPy composite aerogel fibers were evaluated as supercapacitor electrodes using cyclic voltammetry (CV). CV curves of these PPy composites show a leaf-like shape at a slow scan rate of 5 mV s−1 (Fig. 7b). The PPy/HCF and PPy/TCF both show similar performance as their specific capacitance was estimated to be ∼60 F g−1. This value is consistent with our recent results of PPy/cellulose thin films,59 but is much lower than for some previously reported PPy/cellulose materials, due to the nature of both non-conductive cellulose nanocrystals and gelatin.26,60–62 However, the nitrogen enrichment of PPy and gelatin in these cellulose composites makes them interesting as novel precursors to nitrogen-doped carbon fibers for improved supercapacitance behavior.63 The conducting characteristics of PPy or carbonized substances suggest that the porous conducting composite fibers may be useful as supercapacitors for portable electronic devices.64
Conclusions
In summary, we have shown for the first time the aerogel templating of freestanding TiO2 nanofibrils on a scaffold of CNCs interconnected within gelatin-crosslinked fibers. Hydrogel injection was used to fabricate gCNC spaghetti-like fibers. By controlling the crosslinking process, it was possible to produce gCNC fibers with multidomain, chiral nematic order. The role of gelatin is crucial for the templating on CNCs in the gel networks as its functional groups have affinity to metal-based precursors to achieve the uniform deposition of guests. We used these gCNC fibers as aerogel templates for the structural replication of functional materials. The homogeneous incorporation of secondary components on the gel networks afforded intact fibers of TiO2/gCNC and polypyrrole/gCNC composites. These novel composites and their carbonized forms are attractive for energy storage applications, such as batteries, supercapacitors, and electrocatalysts. We investigated the thermal removal of the gCNC template in the composites and consequently obtained the first example of freestanding titania aerogel fibers with nanostructural integrity. Our templating method may be extended to other components to construct new aerogel materials for diverse applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the NSERC (Canada) for funding through a Discovery Grant.Notes and references
- A. Eychmüller, Angew. Chem., Int. Ed., 2005, 44, 4839–4841 CrossRef PubMed.
- C. Ziegler, A. Wolf, W. Liu, K. A. Herrmann, N. Gaponik and A. Eychmüller, Angew. Chem., Int. Ed., 2017, 56, 13200–13221 CrossRef PubMed.
- Y. Liu, R. Che, G. Chen, J. Fan, Z. Sun, Z. Wu, M. Wang, B. Li, J. Wei, Y. Wei, G. Wang, G. Guan, A. A. Elzatahry, A. A. Bagabas, A. M. A. Enizi, Y. Deng, H. Peng and D. Zhao, Sci. Adv., 2015, 1, e1500166 Search PubMed.
- J. L. Mohanan, I. U. Arachchige and S. L. Brock, Science, 2005, 307, 397–400 Search PubMed.
- X. Gao, X. Wang, X. Ouyang and C. Wen, Sci. Rep., 2016, 6, 27207 CrossRef PubMed.
- S. Liu, F. Wang, R. Dong, T. Zhang, J. Zhang, Z. Zheng, Y. Mai and X. Feng, Small, 2017, 13, 1604099 CrossRef PubMed.
- I. Smirnova and P. Gurikov, Annu. Rev. Chem. Biomol. Eng., 2017, 8, 307–334 CrossRef PubMed.
- J. F. Lutz, J. M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 16024 CrossRef.
- I. W. Hamley, Soft Matter, 2010, 6, 1863–1871 RSC.
- S. Sotiropoulou, Y. Sierra-Sastre, S. S. Mark and C. A. Batt, Chem. Mater., 2008, 20, 821–834 CrossRef.
- M. Giese, L. K. Blusch, M. K. Khan and M. J. MacLachlan, Angew. Chem., Int. Ed., 2015, 54, 2888–2910 CrossRef PubMed.
- L. Zhong, S. Fu, X. Peng, H. Zhan and R. Sun, Carbohydr. Polym., 2012, 90, 644–649 CrossRef PubMed.
- P. X. Wang, W. Y. Hamad and M. J. MacLachlan, Nat. Commun., 2016, 7, 11515 CrossRef PubMed.
- J. Majoinen, E. Kontturi, O. Ikkala and D. G. Gray, Cellulose, 2012, 19, 1599–1605 CrossRef.
- X. Mu and D. G. Gray, Langmuir, 2014, 30, 9256–9260 CrossRef PubMed.
- Y. Zhao, G. Gao, D. Liu, D. Tian, Y. Zhu and Y. Chang, Carbohydr. Polym., 2017, 174, 39–47 CrossRef PubMed.
- J. Ma, X. Li and Y. Bao, RSC Adv., 2015, 5, 59745–59757 RSC.
- K. J. De France, T. Hoare and E. D. Cranston, Chem. Mater., 2017, 29, 4609–4631 CrossRef.
- N. Lavoine and L. Bergstrom, J. Mater. Chem. A, 2017, 5, 16105–16117 RSC.
- F. Jiang and Y. L. Hsieh, ACS Appl. Mater. Interfaces, 2017, 9, 2825–2834 CrossRef PubMed.
- M. Chau, S. E. Sriskandha, D. Pichugin, H. Thérien-Aubin, D. Nykypanchuck, G. Chauve, M. Méthot, J. Bouchard, O. Gang and E. Kumacheva, Biomacromolecules, 2015, 16, 2455–2462 CrossRef PubMed.
- A. L. Oechsle, L. Lewis, W. Y. Hamad, S. G. Hatzikiriakos and M. J. MacLachlan, Chem. Mater., 2018, 30, 376–385 CrossRef.
- L. Lewis, M. Derakhushandeh, S. G. Hatzikiriakos, W. Y. Hamad and M. J. MacLachlan, Biomacromolecules, 2016, 17, 2747–2754 CrossRef PubMed.
- M. Hasani, E. D. Cranston, G. Westman and D. G. Gray, Soft Matter, 2008, 4, 2238–2244 RSC.
- G. Chu, D. Qu, E. Zussman and Y. Xu, Chem. Mater., 2017, 29, 3980–3988 CrossRef.
- X. Yang, K. Shi, I. Zhitomirsky and E. D. Cranston, Adv. Mater., 2015, 27, 6104–6109 CrossRef PubMed.
- Q. Y. Mi, S. R. Ma, J. Yu, J. S. He and J. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 656–660 CrossRef.
- F. Jiang and Y. L. Hsieh, J. Mater. Chem. A, 2014, 2, 6337–6342 RSC.
- Y. Lu, H. Liu., R. Gao, S. Xiao, M. Zhang, Y. Yin, S. Wang, J. Li and D. Yan, ACS Appl. Mater. Interfaces, 2016, 8, 29179–29185 CrossRef PubMed.
- R. T. Olsson, M. A. S. Azizi Samir, G. Salazar-Alvarez, L. Belova, V. Ström, L. A. Berglund, O. Ikkala, J. Nogués and U. W. Gedde, Nat. Nanotechnol., 2010, 5, 584–588 CrossRef PubMed.
- Y. Kobayashi, T. Saito and A. Isogai, Angew. Chem., Int. Ed., 2014, 53, 10394–10397 CrossRef PubMed.
- M. Chau, K. J. De France, B. Kopera, V. R. Machado, S. Rosenfeldt, L. Reyes, K. J. W. Chan, S. Förster, E. D. Cranston, T. Hoare and E. Kumacheva, Chem. Mater., 2016, 28, 3406–3415 CrossRef.
- Y. T. Xu, Y. Dai, T. D. Nguyen, W. Y. Hamad and M. J. MacLachlan, Nanoscale, 2018, 10, 3805–3812 RSC.
- J. P. F. Lagerwall, C. Schütz, M. Salajkova, J. Noh, J. H. Park, G. Scalia and L. Bergström, NPG Asia Mater., 2014, 6, e80 CrossRef.
- M. Tamura, F. Yanagawa, S. Sugiura, T. Takagi, K. Sumaru and T. Kanamori, Sci. Rep., 2015, 5, 15060 CrossRef PubMed.
- A. O. Elzoghby, J. Controlled Release, 2013, 172, 1075–1091 CrossRef PubMed.
- K. Wang, K. C. Nune and R. D. K. Misra, Acta Biomater., 2016, 36, 143–151 CrossRef PubMed.
- R. Dash, M. Foston and A. J. Ragauskas, Carbohydr. Polym., 2013, 91, 638–645 CrossRef PubMed.
- S. Y. Ooi, I. Ahmad and M. C. I. Mohd Amin, Ind. Crops Prod., 2016, 93, 227–234 CrossRef.
- W. Treesuppharat, P. Rojanapanthu, C. Siangsanoh, H. Manuspiya and S. Ummartyotin, Biotechnol. Rep., 2017, 15, 84–91 CrossRef PubMed.
- W. Y. Hamad and T. Q. Hu, Can. J. Chem. Eng., 2010, 88, 392–402 Search PubMed.
- C. Zhuang, F. Tao and Y. Cui, RSC Adv., 2015, 5, 52183–52193 RSC.
- P. Garside and P. Wyeth, Stud. Conserv., 2003, 48, 269–275 CrossRef.
- V. Y. Birshtein and V. M. Tul'chinskii, Chem. Nat. Compd., 1982, 18, 697–700 CrossRef.
- T. D. Nguyen, W. Y. Hamad and M. J. MacLachlan, Chem. Commun., 2013, 49, 11296–11298 RSC.
- X. Yang, E. Bakaic, T. Hoare and E. D. Cranston, Biomacromolecules, 2013, 14, 4447–4455 CrossRef PubMed.
- V. A. Dowling, J. A. M. Charles, E. Nwakpuda and L. B. McGown, Anal. Chem., 2004, 76, 4558–4563 CrossRef PubMed.
- H. Liu, B. Geng, Y. Chen and H. Wang, ACS Sustainable Chem. Eng., 2017, 5, 49–66 CrossRef.
- H. Peng, H. Wang, J. Wu, G. Meng, Y. Wang, Y. Shi, Z. Liu and X. Guo, Ind. Eng. Chem. Res., 2016, 55, 832–838 CrossRef.
- A. Mulyadi, Z. Zhang and Y. Deng, ACS Appl. Mater. Interfaces, 2016, 8, 2732–2740 CrossRef PubMed.
- D. Fattakhova-Rohlfing, A. Zaleska and T. Bein, Chem. Rev., 2014, 114, 9487–9558 CrossRef PubMed.
- M. Schlesinger, M. Giese, L. K. Blusch, W. Y. Hamad and M. J. MacLachlan, Chem. Commun., 2015, 51, 530–533 RSC.
- J. T. Korhonen, P. Hiekkataipale, J. Malm, M. Karppinen, O. Ikkala and R. H. A. Ras, ACS Nano, 2011, 5, 1967–1974 CrossRef PubMed.
- S. Liu, L. Zhang, J. Zhou, J. Xiang, J. Sun and J. Guan, Chem. Mater., 2008, 20, 3623–3628 CrossRef.
- A. Ivanova, D. Fattakhova-Rohlfing, B. E. Kayaalp, J. Rathousky and T. Bein, J. Am. Chem. Soc., 2014, 136, 5930–5937 CrossRef PubMed.
- J. Xue, F. Song, X. W. Yin, Z. L. Zhang, Y. Liu, X. L. Wang and Y. Z. Wang, ACS Sustainable Chem. Eng., 2017, 5, 3721–3725 CrossRef.
- M. M. Chamakh, D. Ponnamma and M. A. A. Al-Maadeed, Results Phys., 2017, 7, 590–592 CrossRef.
- M. Dahl, Y. Liu and Y. Yin, Chem. Rev., 2014, 114, 9853–9889 CrossRef PubMed.
- E. Lizundia, T. D. Nguyen, J. L. Vilas, W. Y. Hamad and M. J. MacLachlan, J. Mater. Chem. A, 2017, 5, 19184–19194 RSC.
- Q. Niu, Y. Guo, K. Gao and Z. Shao, RSC Adv., 2016, 6, 109143–109149 RSC.
- Z. Shi, H. Gao, J. Feng, B. Ding, X. Cao, S. Kuga, Y. Wang, L. Zhang and J. Cai, Angew. Chem., Int. Ed., 2014, 126, 5484–5488 CrossRef.
- K. Shi, X. Yang, E. D. Cranston and I. Zhitomirsky, Adv. Funct. Mater., 2016, 26, 6437–6445 CrossRef.
- T. D. Nguyen, K. E. Shopsowitz and M. J. MacLachlan, J. Mater. Chem. A, 2014, 2, 5915–5921 RSC.
- I. Shown, A. Ganguly, L. C. Chen and K. H. Chen, Energy Sci. Eng., 2015, 3, 2–26 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Details of materials synthesis, structural analyses, photographs, and extended electron microscopy images. See DOI: 10.1039/c8qm00200b |
| This journal is © the Partner Organisations 2018 |