Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An efficient iodide ion chemosensor and a rewritable dual-channel security display material based on an ion responsive supramolecular gel†
Qi
Lin
 *a,
Lu
Liu
a,
Juan
Liu
*b,
Feng
Zheng
a,
You-Ming
Zhang
*a,
Lu
Liu
a,
Juan
Liu
*b,
Feng
Zheng
a,
You-Ming
Zhang
 a,
Hong
Yao
a and
Tai-Bao
Wei
a,
Hong
Yao
a and
Tai-Bao
Wei
 *a
*a
aKey Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: linqi2004@126.com; weitaibao@126.com
bCollege of Chemical Engineering, Northwest University for Nationalities, Lanzhou, 730000, P. R. China. E-mail: liujuan656@126.com
First published on 3rd August 2017
Abstract
By introducing multi-self-assembly driving forces, coordination binding sites and signal groups into the same molecule, a well designed functional gelator G1 was synthesized. The gelator G1 could form a stable Pb2+-coordinated supramolecular metallogel (PbG) accompanied with aggregation-induced fluorescence emission (AIE). PbG shows the reversible selective fluorescent response for I− under a gel–gel state. The detection limit of PbG for I− is 1.0 × 10−7 M. The AIE fluorescence of PbG could be reversibly switched “on–off–on” under gel–gel states via alternatively adding I− and Pb2+ water solution into PbG. Other anions could not induce similar stimuli-response for PbG. Interestingly, when a writing brush dipped in I− water solution was used to write on the xerogel film of PbG, the film did not show any color changes. However under UV at 365 nm, a clear dark writing image appeared. This dark writing could be erased by brushing Pb2+ on the film. More interestingly, when the PbG film containing the invisible I− writing was exposed to iodine vapor, a clear brown writing appeared on the film. However, when this film was placed under the room atmosphere for one minute, the brown writing gradually disappeared. Therefore, the PbG film could act as not only a convenient reversible I− detection test kit, but also an erasable dual-channel security display material.
1. Introduction
In recent years, stimuli-responsive gels,1–8 as a type of smart supramolecular materials, have become one of the most significant realms due to their wide application prospects in chemosensors,9–12 biomaterials,13–16 displays,17 physical materials,18–22 chemical engineering23–26 and other relevant areas.27–32 Taking advantage of the dynamic and reversible nature of noncovalent interactions, such as strong van der Waal's forces, hydrogen bonds and π–π stacking,33 the stimuli-responsive supramolecular gels can sense, process and actuate a response to an external change without assistance.34–36 Moreover, metal ions-coordinated supramolecular gels37 have become a focus due to their tunable coordination binding strength and fascinating redox, optical, electronic, as well as magnetic properties of the metal ions.38,39 These excellent properties would bring benefit to the applications of smart materials.However, although a significant amount of effort has been devoted to the development of metal ions coordinated supramolecular gels,40 it is still a big challenge to design and synthesize supramolecular gels that can optically sense a given chemical stimulus with a specific selectivity. In view of this, as a part of our research interest in supramolecular chemistry,41–45 we attempted to control the stimuli-response properties of supramolecular gels through the competitive coordination between supramolecular gelators, metal ions and guest compounds.
Herein, we designed and synthesized a gelator G1 based on multi-assembly driving forces,46–49 fluorescent signal groups50 and coordination binding sites.51,52 The gelator G1 could form a stable supramolecular organogel in various solvents at very low critical gelation concentrations (CGCs). After the addition of Pb2+ to the G1 ethanol organogel (OG), OG could form a stable Pb2+-coordinated supramolecular metallogel PbG accompanied by the pale blue aggregation-induced fluorescence emission (AIE).53–56 The AIE of PbG could be reversibly controlled by iodide anions with a specific selectivity in gel–gel states. PbG could act as not only a convenient reversible I− detection test kit, but also an erasable dual-channel security display material. It is worth mentioning that the security display materials have become of increasing importance.57,58
2. Experimental
As show in Scheme 1, the compound 3,4,5-tris(hexadecyloxy) benzohydrazide was synthesized according to the literature-reported methods.59G1 was synthesized as follow: p-nitrobenzaldehyde (1 mmol), 3,4,5-tris(hexadecyloxy)-benzohydrazide (1 mmol) and acetic acid (0.1 mL, as a catalyst) were added to ethanol (20 mL). Then, the reaction mixture was stirred under refluxed conditions for 24 hours. After the solvent was removed, the precipitated G1 was yielded and recrystallized with CHCl3–EtOH to get the solid G1. Yield: 60%, m.p.: 106–110 °C, 1H NMR (400 MHz, CDCl3, Fig. S1†) δ 9.30 (s, –NH, 1H), 8.26 (d, J = 8.7 Hz, –ArH, –CH, 3H), 7.90 (s, –ArH, 2H), 7.07 (s, ArH, 2H), 4.01 (d, J = 3.8 Hz, –CH2, 6H), 1.95–1.71 (m, –CH2, 6H), 1.36 (m, –CH2, 78H), 0.88 (t, J = 6.5 Hz, –CH3, 9H). 13C NMR (150 MHz, CDCl3, Fig. S2†) δ 152.76, 123.99, 107.96, 77.19, 76.98, 76.77, 73.59, 73.46, 69.44, 69.16, 60.93, 31.91, 30.30, 29.70, 29.64, 29.62, 29.55, 29.40, 29.38, 29.35, 29.30, 26.06, 22.67, 14.38, 14.08; IR (KBr, cm−1) v: 3455 (–NH), 1715 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1650 (CH
O), 1650 (CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). Anal. calcd. for C62H107N3O6: C 75.03, H 10.40, N 3.98; found: C 75.12, H 10.56, N 3.85. MS: ESI [M + H]+m/z (Fig. S3†) found: 990.8284, calcd: 990.8233.
N). Anal. calcd. for C62H107N3O6: C 75.03, H 10.40, N 3.98; found: C 75.12, H 10.56, N 3.85. MS: ESI [M + H]+m/z (Fig. S3†) found: 990.8284, calcd: 990.8233.
3. Results and discussion
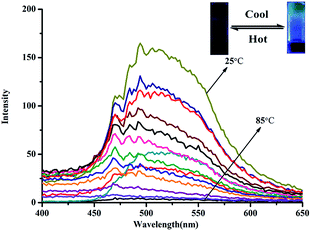
First, we carefully investigated the gelation properties of G1. As shown in Table S1,† the gelator G1 could form a stable supramolecular organogel in various solvents at very low critical gelation concentrations. Among these solvents, the gelator G1 showed the lowest CGC (0.40%, wt/v%, 10 mg mL−1 = 1%) and the highest gel–sol transition temperature (78 °C) in ethanol. The G1-based supramolecular organogel OG in ethanol is more stable than the gel in other solutions. Therefore, we investigated the influence of metal ions on the G1 organogel in ethanol.Interestingly, after the addition of Pb2+ to the G1 ethanol organogel (OG), OG could form a stable Pb2+-coordinated supramolecular metallogel PbG. As shown in Fig. 1, PbG has no fluorescence emission in hot ethanol solution (T > Tgel). However, when the temperature of this hot ethanol solution dropped below the Tgel of PbG, the emission intensity at 340 nm showed an evident increase and reached a steady state, which indicated that the fluorescence emission of metallogel PbG was aggregation-induced emission (AIE).
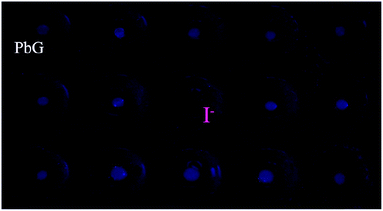
The anion response capability of the supramolecular metallogel PbG was primarily investigated by adding various anions in water solutions (AcO−, HSO4−, H2PO4−, F−, Cl−, Br−, I−, N3−, SCN−, ClO4−, CN−, CO32−, S2− and SO42−, 1 mol L−1) to PbG. As shown in Fig. 2, when adding water solutions of various anions to the small amount of metallogel PbG on a spot plate, only I− could quench the fluorescence of PbG, while other anions could not. These results indicated that PbG could selectively sense I−, which was attributed to I− competitively binding with Pb2+.
Moreover, the I− response properties of PbG were investigated by fluorescence titrations. As shown in Fig. 3, with the addition of I− into PbG, the spectra showed evident red shifts, which was attributed to the coordination of I− with Pb2+. In PbG, Pb2+ coordinated with the gelator through the acylhydrazone moiety. When I− was added into PbG, Pb2+ coordinated with I− and the acylhydrazone moiety was released, which induced the fluorescence spectra of the gel to undergo the red shifts. In addition, the emission intensity at 474 nm decreased with increasing the concentration of I−. The detection limit of the fluorescence spectra changes, which was calculated on the basis of 3δ/S,60 was 2.037 × 10−6 M (Fig. S4†) for I− anion.
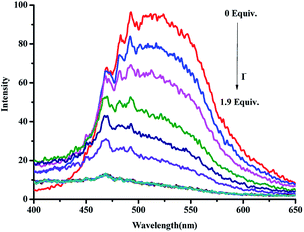 | ||
Fig. 3 Fluorescence spectra of PbG (1%, in ethanol, G1–Pb2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with increasing concentration of I− (using 1 mol L−1 TBA in water solution as the I− sources), λex = 340 nm. 1) with increasing concentration of I− (using 1 mol L−1 TBA in water solution as the I− sources), λex = 340 nm. | ||
Interestingly, after the addition of Pb2+ into the I− containing PbG, the fluorescence of PbG recovered, which was attributed to the Pb2+ coordination with G1 again. These properties make PbG act as an I− and Pb2+ controlled “on–off–on” fluorescent switch. By alternate addition of I− and Pb2+, the switching could be performed reversibly at least for three cycles with a small fluorescent efficiency loss (Fig. 4).
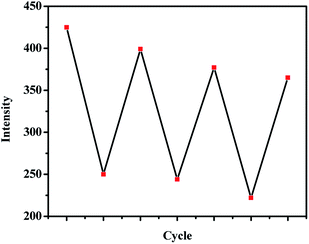 | ||
| Fig. 4 Fluorescent “on–off–on” cycles of PbG, controlled by the alternative addition of I− and Pb2+, λex = 340 nm. | ||
In order to facilitate the use of the metallogel PbG, the I− detection film based on PbG was prepared by pouring the heated ethanol solution of PbG onto a clean glass surface and then drying in air. The PbG film was white under natural light and showed a blue fluorescence emission under UV at 365 nm. When a writing brush dipped in I− water solution was used to write on the film, the film did not show any color changes. However, under UV at 365 nm, a clear dark writing image appeared (Fig. 5). This dark writing image could be erased by brushing Pb2+ on the film. More interestingly, when the PbG film containing the invisible I− writing was exposed to iodine vapor, a clear brown writing appeared on the film. However, when the film was put under the room atmosphere for one minute, the brown writing disappeared gradually. Therefore, the PbG film could act as not only a convenient reversible I− detection test kit, but also an erasable dual-channel security display material.
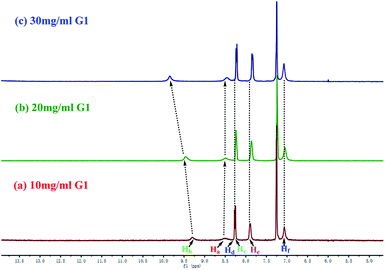
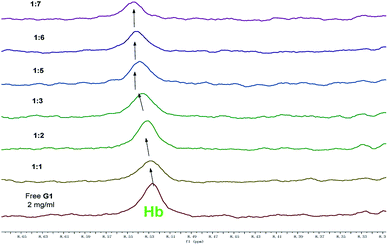
In order to investigate the self-assembly and stimuli-response mechanism of PbG, a series of experiments was carried out. First, in the concentration dependent 1H NMR of G1 (Fig. 6), the –NH– (Hb) and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– (Ha) resonance signals showed significant downfield shifts as the concentration of G1 rose. These results revealed that in the gelation process, the –NH– (Hb) and –N
CH– (Ha) resonance signals showed significant downfield shifts as the concentration of G1 rose. These results revealed that in the gelation process, the –NH– (Hb) and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– (Ha) groups formed hydrogen bonds with the –C
CH– (Ha) groups formed hydrogen bonds with the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups on the adjacent gelators. On the other hand, with a gradual increase in concentration, the 1H NMR signal of phenyl protons (Hc, Hd, He, and Hf) showed an evident upfield shift, indicating that the π–π stacking interactions between the phenyl groups involved in the gelation process.61 Therefore, the gelator G1 self-assembled to supramolecular organogel OG by the hydrogen bonds, π–π stacking as well as the vdW existing in the long alkyl chains.
O groups on the adjacent gelators. On the other hand, with a gradual increase in concentration, the 1H NMR signal of phenyl protons (Hc, Hd, He, and Hf) showed an evident upfield shift, indicating that the π–π stacking interactions between the phenyl groups involved in the gelation process.61 Therefore, the gelator G1 self-assembled to supramolecular organogel OG by the hydrogen bonds, π–π stacking as well as the vdW existing in the long alkyl chains.
The formation mechanism of supramolecular metallogel was also investigated by 1H NMR titrations, as shown in Fig. 7. With the addition of Pb2+, the –NH– (Hb) group on the gelator showed significant downfield shifts, which indicated that the gelator coordinated with Pb2+via the acylhydrazone moiety. In addition, in the IR spectra (Fig. S5†) the stretching vibrations of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C
O and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– of G1 showed obviously shifts from 3455 and 1650 cm−1 to 3583 and 1588 cm−1, respectively. These phenomena indicated that in PbG, Pb2+ coordinated with the nitrogen and oxygen atoms on the acylhydrazone group.
N– of G1 showed obviously shifts from 3455 and 1650 cm−1 to 3583 and 1588 cm−1, respectively. These phenomena indicated that in PbG, Pb2+ coordinated with the nitrogen and oxygen atoms on the acylhydrazone group.
This presumed self-assembly and coordination mechanism was also supported by the Tgel of OG and PbG. For instance, as shown in Fig. 8, under the same condition, the Tgel of OG was significantly higher than that of PbG. The large differences of Tgel between OG and PbG were ascribed to the breakage of intermolecular hydrogen bonds between –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H on one gelator and –C
C–H on one gelator and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O on the other one in OG, which was caused by the coordination of Pb2+ with the gelator G1. Moreover, the Pb2+ coordination process reduced the distance of π–π stacking between the phenyls, which enhanced the aggregation induced emission of PbG.
O on the other one in OG, which was caused by the coordination of Pb2+ with the gelator G1. Moreover, the Pb2+ coordination process reduced the distance of π–π stacking between the phenyls, which enhanced the aggregation induced emission of PbG.
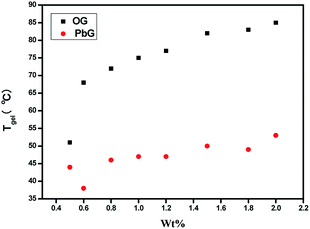 | ||
Fig. 8 Plots of Tgel against the concentrations of organogel OG and metallogels PbG (G1–Pb2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in ethanol. 1) in ethanol. | ||
This proposed mechanism was also supported by the XRD patterns (Fig. S6†). The XRD patterns of OG, PbG and the PbG treated with I− showed the peaks at 2θ = 18.62–27.70°, corresponding to the d spacing 3.5 Å, 3.45 Å, and 3.8 Å, respectively. As shown in Scheme 2 and Fig. S6,† in OG, the peaks at 21.28° and the d spacing 3.5 Å were attributed to the π–π stacking, which existed in the phenyl groups of OG. While, in PbG, the d spacing changed to 3.45 Å, which was attributed to the Pb2+ coordination process reducing the distance of π–π stacking between the phenyls. Meanwhile, the d spacing 3.8 Å was attributed to the interlamellar spacing between the supramolecular chains. In addition, after the formation of PbG, the peaks of OG (at 2θ = 21.28, 23.22, 23.90, 25.30°) disappeared and these peaks reappeared after PbG was treated with I−. These phenomena confirmed that Pb2+ coordinated with OG and induced a change in the XRD pattern of OG, while the addition of I− into PbG induced the competitive coordination of I− with Pb2+ and led to the recovery of XRD peaks. According to 1H NMR, IR and XRD, the ion response mechanism of OG and PbG could be presumed as in Scheme 2.
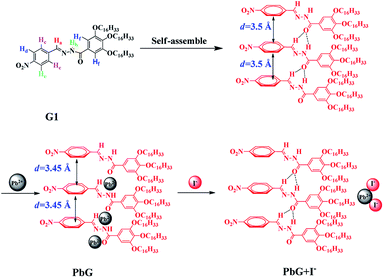 | ||
| Scheme 2 Chemical structure of the G1 and the presumed self assembly and reversible stimuli-response mechanism. | ||
To get further insight into the morphological features of the supramolecular organogel OG, metallogel PbG and PbG treated with I−, SEM studies were carried out with their xerogels, respectively. As shown in Fig. S7,† the SEM images of OG showed an overlapped rugate layer structure. The metallogel PbG also showed overlapped rugate layer structures and the aggregation structure of layer was compacted. However, after I− was added into the PbG xerogel, the micro states experienced obvious changes. There were a large number of micro cavities formed in the xerogel of PbG. These micro cavities provided the PbG xerogel with the properties for the adsorption of iodine vapor. Therefore, the mechanism of iodine vapor-caused color change could be attributed to the iodine vapor adsorption into these micro cavities.
4. Conclusions
In summary, a novel supramolecular gelator G1 has been designed and synthesized. The gelator G1 could form a stable supramolecular metallogel PbG. Through the competitive coordination of Pb2+ and I− with the gelator G1, the aggregation-induced emission of the supramolecular metallogel PbG was controlled as “on–off–on”. More interestingly, after Pb2+ competitive coordinated with I−, there were a large number of micro cavities formed in the PbG xerogel, which enabled the PbG xerogel to absorb the iodine vapor and show a brown color. PbG could act as not only a convenient high selective and sensitive I− detection test kit, but also an erasable dual-channel secret documentation medium.Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 21662031; 21661028; 21574104; 21262032) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC.
- H. Tan, X. Ma and M. Fu, Bull. Mater. Sci., 2013, 36, 153 CrossRef CAS.
- L. Predoana, I. Stanciu, M. Anastasescu, J. M. Calderon-Moreno, M. Stoica, S. Preda, M. Gartner and M. Zaharescu, J. Sol-Gel Sci. Technol., 2016, 78, 589 CrossRef CAS.
- Z. X. Liu, Y. Feng, Z. C. Yan, Y. M. He, C. Y. Liu and Q. H. Fan, Chem. Mater., 2012, 24, 3751 CrossRef CAS.
- Q. Xia, Y. Mao, J. Wu, T. Shu and T. Yi, J. Mater. Chem. C, 2014, 2, 1854 RSC.
- S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 2356 RSC.
- A. S. Lee, S. S. Choi, K. Y. Baek and S. S. Hwang, Inorg. Chem. Commun., 2016, 73, 7 CrossRef CAS.
- S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- V. Lozano, R. Hernández, A. Ardá, J. Jiménez-Barbero, C. Mijangos and M. J. Pérez-Pérez, J. Mater. Chem., 2011, 21, 8862 RSC.
- Y. He, Z. Guo, P. Jin, C. Jiao, H. Tian and W. Zhu, Ind. Eng. Chem. Res., 2015, 54, 2886 CrossRef CAS.
- C. D. Jones and J. W. Steed, Chem. Soc. Rev., 2016, 45, 6546 RSC.
- P. Xue, J. Ding, M. Jin and R. Lu, J. Mater. Chem. C, 2017, 5, 5299 RSC.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS.
- G. Visioli, A. Comastri, D. Imperiale, G. Paredi, A. Faccini and N. Marmiroli, Food Anal. Methods, 2016, 9, 469 CrossRef.
- J. Jielile, A. Jialili, G. Sabirhazi, N. Shawutali, D. Redati, J. Chen, J. Bai and K. Aldyarhan, Appl. Biochem. Biotechnol., 2012, 166, 521 CrossRef CAS.
- Y. Cao, Y. Li, X. Y. Hu, X. Zou, S. Xiong, C. Lin and L. Wang, Chem. Mater., 2015, 27, 1110 CrossRef CAS.
- J. Zhou, X. Du, Y. Gao, J. Shi and B. Xu, J. Am. Chem. Soc., 2014, 136, 2970 CrossRef CAS.
- G. Sun, J. Xiao, M. Lu, H. Wang, X. Chen, Y. Yu, Y. Pan and Y. Wang, Appl. Biochem. Biotechnol., 2015, 175, 400 CrossRef CAS.
- T. Kitahara, M. Shirakawa, S. Kawano, U. Beginn, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 14980 CrossRef CAS.
- X. Y. Hu, T. Xiao, C. Lin, F. Huang and L. Wang, Acc. Chem. Res., 2014, 47, 2041 CrossRef CAS.
- G. Mun, H. Choi, N. Im, J. Ahn, J. Park, H. Seo, Y. Choi, J. H. Lee and J. H. Jung, RSC Adv., 2017, 7, 26827 RSC.
- C. Kim, K. Y. Kim, J. H. Lee, J. Ahn, K. Sakurai, S. S. Lee and J. H. Jung, ACS Appl. Mater. Interfaces, 2017, 9, 3799 CrossRef CAS.
- E. P. Simonenko, A. V. Derbenev, N. P. Simonenko, V. G. Sevastyanov and N. T. Kuznetsov, Russ. J. Inorg. Chem., 2015, 60, 1444 CrossRef CAS.
- Z. Cheng, J. Liao, B. He, F. Zhang, F. Zhang, X. Huang and L. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 1677 CrossRef CAS.
- M. Jiang, J. Zhu, C. Chen, Y. Lu, Y. Ge and X. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 3473 CrossRef CAS.
- Y. Cao, X. Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y. Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762 CrossRef CAS.
- N. V. V. Satya Bhushan and R. N. Nayak, J. Oral Maxillofac. Surg., 2010, 9, 119 CrossRef CAS.
- J. A. Foster, K. K. Damodaran, A. Maurin, G. M. Day, H. P. G. Thompson, G. J. Cameron, J. C. Bernal and J. W. Steed, Chem. Sci., 2017, 8, 78 RSC.
- H. Wang, X. Ji, Z. Li, C. N. Zhu, X. Yang, T. Li, Z. L. Wu and F. Huang, Materials Chemistry Frontiers, 2017, 1, 167 RSC.
- P. Hallegot, G. Hussler, V. Jeanne-Rose, F. Leroy, F. Pineau and H. Samain, J. Sol-Gel Sci. Technol., 2016, 79, 365 CrossRef CAS.
- S. Lee and S. T. Nguyen, Macromolecules, 2013, 46, 9169 CrossRef CAS.
- P. Xue, Q. Xu, P. Gong, C. Qian, A. Ren, Y. Zhang and R. Lu, Chem. Commun., 2013, 49, 5838 RSC.
- T. Ghane, D. Nozaki, A. Dianat, A. Vladyka, R. Gutierrez, J. P. Chinta, S. Yitzchaik, M. Calame and G. Cuniberti, J. Phys. Chem. C, 2015, 119, 6344 CrossRef CAS.
- N. Song, D. X. Chen, Y. C. Qiu, X. Y. Yang, B. Xu, W. Tian and Y. W. Yang, Chem. Commun., 2014, 50, 8231 RSC.
- X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS.
- Y. M. Zhang, B. B. Shi, H. Li, W. J. Qu, G. Y. Gao, Q. Lin, H. Yao and T. B. Wei, Polym. Chem., 2014, 5, 4722 RSC.
- Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Chem.–Eur. J., 2014, 20, 11457 CrossRef CAS.
- K. Lalitha, V. Sridharan, C. U. Maheswari, P. K. Vemula and S. Nagarajan, Chem. Commun., 2017, 53, 1538 RSC.
- Y. Zhang, B. Zhang, Y. Kuang, Y. Gao, J. Shi, X. X. Zhang and B. Xu, J. Am. Chem. Soc., 2013, 135, 5008 CrossRef CAS.
- S. S. Abolmaali, A. Tamaddon, H. Najafi and R. Dinarvand, J. Inorg. Organomet. Polym., 2014, 4, 977 CrossRef.
- Q. Lin, T. T. Lu, X. Zhu, B. Sun, Q. P. Yang, T. B. Wei and Y. M. Zhang, Chem. Commun., 2015, 51, 1635 RSC.
- Q. Lin, T. T. Lu, J. C. Lou, G. Y. Wu, T. B. Wei and Y. M. Zhang, Chem. Commun., 2015, 51, 12224 RSC.
- Q. Lin, Q. P. Yang, B. Sun, J. C. Lou, T. B. Wei and Y. M. Zhang, RSC Adv., 2015, 5, 11786 RSC.
- Q. Lin, F. Zheng, L. Liu, P. P. Mao, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2016, 6, 11928 Search PubMed.
- Q. Lin, P. P. Mao, L. Liu, J. Liu, Y. M. Zhang, H. Yao and T. B. Wei, RSC Adv., 2017, 7, 11206 RSC.
- M. H. Liu and Y. Fang, Chin. Sci. Bull., 2012, 57, 4245 CrossRef.
- C. Zhao, X. Zhang, K. Li, S. Zhu, Z. Guo, L. Zhang, F. Wang, Q. Fei, S. Luo, P. Shi, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2015, 137, 8490 CrossRef CAS PubMed.
- Q. Lin, Q. P. Yang, B. Sun, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Soft Matter, 2014, 10, 8427 RSC.
- Y. Gong, T. Jiao, Q. Hu, N. Cheng, W. Xu, Y. Bi and L. Yu, J. Phys. Chem. C, 2015, 119, 16349 CrossRef CAS.
- X. Ji, Y. Yao, J. Li, X. Yan and F. Huang, J. Am. Chem. Soc., 2013, 135, 74 CrossRef CAS.
- Q. Lin, B. Sun, Q. P. Yang, Y. P. Fu, X. Zhu, Y. M. Zhang and T. B. Wei, Chem. Commun., 2014, 50, 10669 RSC.
- D. H. Qu, Q. C. Wang, Q. W. Zhang, X. Ma and H. Tian, Chem. Rev., 2015, 115, 7543 CrossRef CAS.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- W. Lei, Y. Liu, J. Bai, Y. Dai, Y. Kan, T. Chen and B. Yin, Tetrahedron, 2015, 71, 5465 CrossRef CAS.
- K. Duraimurugan, J. Sivamani, M. Sathiyaraj, V. Thiagarajan and V. Siva, J. Fluoresc., 2016, 26, 1211 CrossRef CAS.
- X. Wu, H. Li, Y. Kan and B. Yin, Dalton Trans., 2013, 42, 16302 RSC.
- A. Kishimura, T. Yamashita, K. Yamaguchi and T. Aida, Nat. Mater., 2005, 4, 546 CrossRef CAS.
- N. Tamaoki, A. V. Parfenov, A. Masaki and H. Matsuda, Adv. Mater., 1997, 9, 1102 CrossRef CAS.
- Y. P. Fu, Q. Lin, T. B. Wei, P. Chen, X. Zhu, X. Liu and Y. M. Zhang, Chem. Reagents, 2013, 35, 367 CAS.
- Analytical Methods Committee, Analyst, 1987, 112, 199 RSC.
- C. Po, Z. Ke, A. Y. Y. Tam, H. F. Chow and V. W. W. Yam, Chem.–Eur. J., 2013, 19, 15735 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06238a |
| This journal is © The Royal Society of Chemistry 2017 |