Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct observation and characterisation of 3-azido-2H-azirines: postulated, but highly elusive intermediates†‡
Kevin
Weigand§
,
Neeraj
Singh§
,
Manfred
Hagedorn
and
Klaus
Banert
*
Chemnitz University of Technology, Organic Chemistry, Strasse der Nationen 62, 09111 Chemnitz, Germany. E-mail: klaus.banert@chemie.tu-chemnitz.de
First published on 3rd November 2016
Abstract
For the first time, successful synthesis of an unknown class of compounds, 3-azido-2H-azirines, which are implicated as highly reactive intermediates in the thermolysis of the corresponding 1,1-diazidoethenes, has been performed. These elusive heterocycles have been detected and characterised by low-temperature NMR and in situ IR spectroscopy. Even the parent compound, 3-azido-2H-azirine, has been observed via low-temperature photolysis of 1,1-diazidoethene, as a highly reactive species with a half-life period of only 12 min at −40 °C.
The azido group, although absent in natural products, is a highly versatile functionality which has found immense applications in various branches of chemistry,1 as well as biology2 and materials science.3 In particular, vinyl azides4 are useful starting materials for the synthesis of strained compounds, for example azirines,5via photolysis or thermolysis and also for ring-enlarged heterocycles of synthetic and biological significance.
Although the parent compound 4 has not yet been reported and only four substituted representatives of 1,1-diazidoethenes, 1, 2a, 2b and 3, were mentioned in the literature,6 a rich chemistry, particularly with reactions of the push–pull olefin 1, was reported by Saalfrank7 and others8 (Scheme 1a). The treatment of 1 with different types of amines, for example, led to nitrogen heterocycles, such as 2H-azirines, 1,2,3-triazoles and tetrazoles, as depicted in Scheme 1b.7,9,10 Unimolecular reactions of 1, 2a and 2b induced by thermolysis or photolysis were also investigated intensively.7,8,10,11 In the case of substrate 1, extrusion of two or three molecules of dinitrogen and formation of the products 7 or 8, respectively, were claimed (Scheme 1c).7,10,11 The thermal reaction of 1 was explained with the help of short-lived intermediate 5 and the subsequent cleavage of the C–C bond in the three-membered ring, combined with the loss of a second molecule of dinitrogen. Finally, a two-step substitution of the cyano group by nucleophilic methanol should result in the generation of 7. Irradiation of a solution of 1 in methanol at −30 °C gave mainly “the thermal product” 7 (70%) and the acrylic ester 8 as a side product (30%).11 The diazo compound 6 and the derived carbene (vinylidene species) were discussed as short-lived intermediates in the surprising formation of 8. It is well known in the chemistry of vinyl azides that these substrates generally lead to high yields of the corresponding 2H-azirines under low-temperature irradiation, whereas thermolysis of the same azides can give varying results: in some cases, heating a solution of a vinyl azide also afforded a high yield of the 2H-azirine, but other such substrates avoided the thermal generation of these heterocycles and furnished quite different products. Thus, the reported unimolecular reactions of 1 are in contrast to the general rules of vinyl azide reactivity.4,5
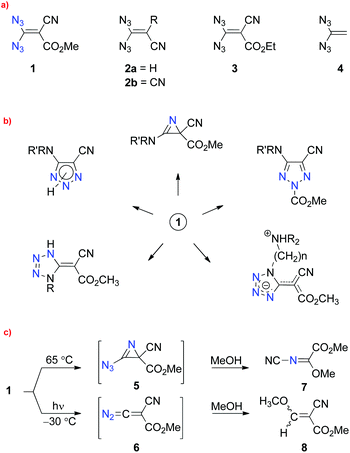 | ||
| Scheme 1 (a) Reported (1, 2a, 2b and 3) and unknown (4) 1,1-diazidoethenes; (b) products prepared from compound 1; (c) proposed intermediates and products in the thermolysis and photolysis of 1. | ||
Because 3-azido-2H-azirines are only postulated,7,10,11 however, never detected species, and intermediates of types 5 and 6 are obviously not only elusive, but also highly interesting, we judged low-temperature photolyses of diazide 1 and the parent compound 4 to be promising. We became further interested in such experiments, since we previously investigated the low-temperature irradiation of open-chain 1,2-diazidoalkenes,12a which led to the first proof of long-sought 2-azido-2H-azirines.12b Such short-lived species turned out to be intermediates in the known transformation of vicinal vinyl diazides into two nitrile fragments, that is accompanied by the cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and loss of two molecules of dinitrogen. In the case of 3-azido-2H-azirines, the electron-donating properties of the azido group should be able to stabilize the azirine ring by reducing the electron deficiency at the sp2 carbon atom. This effect is well documented especially for 3-amino-2H-azirines, which bear, however, a stronger donor.13 On the other hand, it is well known that the direct combination of strain resulting from small rings decreases the stability of azides.
C bond and loss of two molecules of dinitrogen. In the case of 3-azido-2H-azirines, the electron-donating properties of the azido group should be able to stabilize the azirine ring by reducing the electron deficiency at the sp2 carbon atom. This effect is well documented especially for 3-amino-2H-azirines, which bear, however, a stronger donor.13 On the other hand, it is well known that the direct combination of strain resulting from small rings decreases the stability of azides.
Since light energy circumvents the problem of direct thermal agitation, we planned to irradiate 1 at low temperatures and to detect short-lived intermediate 5 by low-temperature NMR spectroscopy. Surprisingly, substrate 1 was not soluble in methanol at −30 °C, and the same is true even for a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
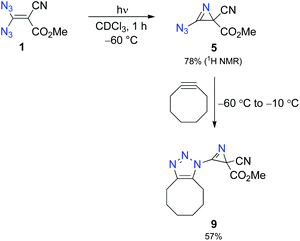
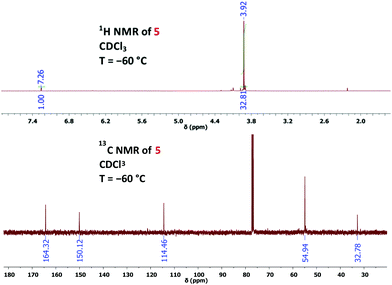
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of chloroform and methanol, although the solubility of 1 in neat chloroform is excellent. Thus, we photolysed a solution of 1 (30 mg) in deuterated chloroform (0.75 mL) with the help of a mercury high-pressure lamp (filter with λ > 320 nm), maintaining the temperature at −60 °C through a cryostat.14 After 1 h, low-temperature NMR analysis showed complete consumption of 1 and the formation of 2H-azirine 5 with 78% yield (Scheme 2 and Fig. 1). Under our conditions, prolonged irradiation (3–4 h) did not lead to a significant decay of azido compound 5, whereas decomposition of this compound occurred when the temperature of its solution was raised above −10 °C. The structural proof of 5 is mainly based on the corresponding 13C NMR data and especially on the characteristic chemical shifts of the 2H-azirine unit with δ (C-2) = 32.8 and δ (C-3) = 150.1. The assignment of these signals was also supported by CIGAR experiments.
1 mixture of chloroform and methanol, although the solubility of 1 in neat chloroform is excellent. Thus, we photolysed a solution of 1 (30 mg) in deuterated chloroform (0.75 mL) with the help of a mercury high-pressure lamp (filter with λ > 320 nm), maintaining the temperature at −60 °C through a cryostat.14 After 1 h, low-temperature NMR analysis showed complete consumption of 1 and the formation of 2H-azirine 5 with 78% yield (Scheme 2 and Fig. 1). Under our conditions, prolonged irradiation (3–4 h) did not lead to a significant decay of azido compound 5, whereas decomposition of this compound occurred when the temperature of its solution was raised above −10 °C. The structural proof of 5 is mainly based on the corresponding 13C NMR data and especially on the characteristic chemical shifts of the 2H-azirine unit with δ (C-2) = 32.8 and δ (C-3) = 150.1. The assignment of these signals was also supported by CIGAR experiments.
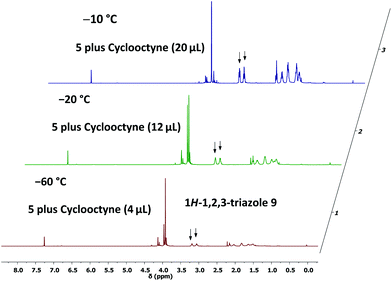
To provide further evidence of the structure of 5, we utilised strain-promoted alkyne–azide cycloaddition with the help of cyclooctyne to obtain the 1H-1,2,3-triazole 9.15,16 Although the product 9 showed limited stability at room temperature, most probably because of the electron-withdrawing properties of the triazolo unit, which increases the electron deficiency at C-3 of the 2H-azirine, we were able to isolate 9 as a straw-coloured oil in 57% yield, and this allowed complete characterisation of the trapping product.14 We also monitored the reaction of 5 with cyclooctyne in deuterated chloroform by using 1H NMR spectroscopy to get an impression of the rate of this cycloaddition (Fig. 2). It turned out that the consumption of 5 was very slow at −60 °C, whereas a rapid formation of 9 was observed at −20 °C.
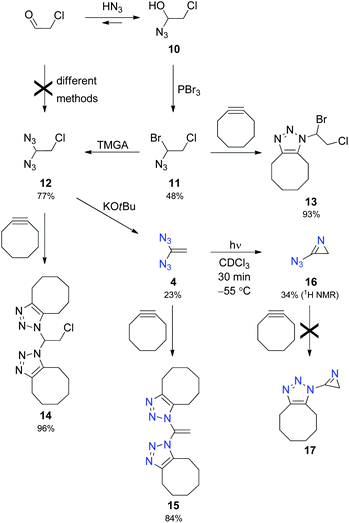
Encouraged by successful characterisation of azirine 5, we were highly motivated to generate the parent compound, 3-azido-2H-azirine (16), which requires the synthesis of unknown 1,1-diazidoethene (4). Obviously, the latter compound cannot be prepared from 1,1-dichloroethene by nucleophilic substitution owing to the absence of electron-withdrawing groups that facilitate the access to diazides 1–3. Therefore, it seemed logical to utilise the chloroethane derivative 12 as a precursor for the desired diazide 4 (Scheme 3). However, all our attempts failed when we tried to directly transform chloroacetaldehyde into 12 by using well-established methods17 for the synthesis of geminal diazides from aldehydes. Subsequently, we employed the multifarious chemistry of α-azido alcohols6a,18 to prepare 12 in a three-step sequence. Thus, we treated chloroacetaldehyde with hydrazoic acid and reacted the resulting α-azido alcohol 10 with phosphorus tribromide to obtain the intermediate product 11 with 48% yield based on the aldehyde. Selective nucleophilic substitution of 11 with the help of N,N,N′,N′-tetramethylguanidinium azide (TMGA) led to the desired diazide 12, which was transformed into 1,1-diazidoethene (4) in the presence of potassium tert-butoxide in diethyl ether.14
The azides 11, 12 and particularly 4 are dangerously explosive and should be handled and characterised in solution only, for example, by IR and NMR spectroscopy. Because of its substitution pattern, 4 is an electron-rich olefin as shown by an upfield shift of the NMR data with δ (13C) = 85.3 and δ (1H) = 4.40 measured for the CH2 group (CDCl3). 1,1-Diazidoethene (4) has a limited stability at room temperature and a half-life period of 220 min at 40 °C in deuterated chloroform. With the help of cyclooctyne, explosive azides 4, 11 and 12 were transformed into the corresponding 1,2,3-triazoles 15, 13 and 14, respectively, and these heterocyclic derivatives were easily isolated and characterised.14,16
A solution of 4 in deuterated chloroform with diethyl ether as an internal standard was irradiated at −55 °C using a mercury high-pressure lamp. After 30 min, NMR low-temperature analysis showed the formation of 3-azido-2H-azirine (16) in 34% yield (Scheme 3). 1H NMR spectroscopy at −40 °C indicated a short half-life period of approximately 12 min. Thus, 16 is very short-lived, and attempts to intercept this species in the presence of cyclooctyne did not lead to the desired cycloadduct 17. This unsuccessful experiment exhibits the limit of trapping unstable azides with the help of cyclooctyne.
The photolysis of 4 was also monitored with solutions of the diazide in toluene-d8 at −55 °C by using NMR spectroscopy (Fig. 3). The product 16 was identified by its high-field 1H NMR signal at δ = 1.49 (singlet) and the characteristic 13C NMR signals at δ = 27.14 (C-2) and δ = 161.25 (C-3), and the coupling constant 1J (13C, 1H) = 182 Hz is also typical of 2H-azirines.5
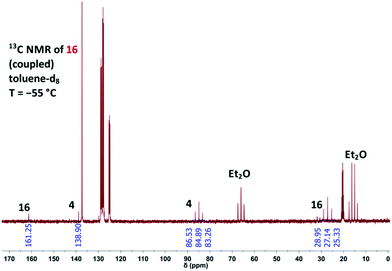 | ||
| Fig. 3 Proton-coupled 13C NMR spectrum measured at −55 °C during the photolysis of 4 in toluene-d8 to generate 3-azido-2H-azirine (16). | ||
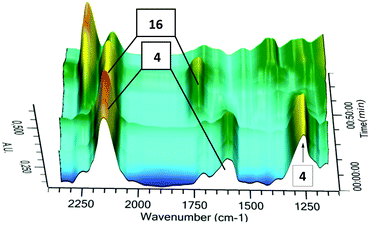
Finally, we utilized in situ IR spectroscopy to detect the progress of the photochemical step 4 → 16 in deuterated chloroform at −45 °C (Fig. 4). Signals at 2120 cm−1 (asym. N3), 1641 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 1311 cm−1 (sym. N3) were assigned to the starting compound 4, whereas signals at 2148 cm−1 (asym. N3) and 1756 cm−1 (C
C) and 1311 cm−1 (sym. N3) were assigned to the starting compound 4, whereas signals at 2148 cm−1 (asym. N3) and 1756 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) were attributed to the product 16. Our IR analysis confirmed the NMR results indicating that 16 is unstable even in solution at low temperatures. Furthermore, it is shown that the parent 3-azido-2H-azirine 16 is significantly less stable than the substituted heterocycle 5. Such an effect of substituents at the 2-position is well known for other 2H-azirines.5,19
N) were attributed to the product 16. Our IR analysis confirmed the NMR results indicating that 16 is unstable even in solution at low temperatures. Furthermore, it is shown that the parent 3-azido-2H-azirine 16 is significantly less stable than the substituted heterocycle 5. Such an effect of substituents at the 2-position is well known for other 2H-azirines.5,19
Conclusions
In summary, we have demonstrated that the irradiation of 1,1-diazidoethenes 1 and 4 leads to 3-azido-2H-azirines 5 and 16, respectively. Although these photoproducts are highly unstable, their structures are unequivocally confirmed by low-temperature NMR and IR spectroscopy as well as trapping reactions. This outcome confirms the general rule that photolysis of vinyl azides results in the formation of 2H-azirines as primary products.4,5 Thus, we were not able to verify previous reports11 from 1982 on the irradiation of 1 since we obtained different results.Compounds 1, 4, 11 and 12 should be considered highly explosive, and effective safety measures should be taken when handling these substances in the pure state. We highly recommend the use of only dilute solutions in appropriate solvents.20
Acknowledgements
This work is part 37 in the series “Reactions of Unsaturated Azides”, for part 36, see ref. 6a. We thank Deutsche Forschungsgemeinschaft for financial support (BA 903/12/1-3).Notes and references
- (a) H. Tanimoto and K. Kakiuchi, Nat. Prod. Commun., 2013, 8, 1021–1034 CAS; (b) Organic Azides, Syntheses and Applications, ed. S. Bräse and K. Banert, Wiley, Chichester, 2010 Search PubMed; (c) S. Bräse, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188–5240 CrossRef PubMed; (d) E. F. V. Scriven and K. Turnbull, Chem. Rev., 1988, 88, 297–368 CrossRef CAS.
- X. Zhang and Y. Zhang, Molecules, 2013, 18, 7145–7159 CrossRef CAS PubMed.
- J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- Reviews on vinyl azides: (a) B. Hu and S. G. DiMagno, Org. Biomol. Chem., 2015, 13, 3844–3855 RSC; (b) K. Banert, in Organic Azides, Syntheses and Applications, ed. S. Bräse and K. Banert, Wiley, Chichester, 2010, pp. 115–166 Search PubMed; (c) S. J. Collier, in Science of Synthesis, ed. G. A. Molander, Thieme, Stuttgart, 2006, vol. 33, pp. 541–563 Search PubMed; (d) K. Banert, in Houben–Weyl, ed. H. Kropf and E. Schaumann, Thieme, Stuttgart, 4th edn, 1993, vol. E15, pp. 818–875 Search PubMed.
- Reviews on 2H-azirines: (a) A. F. Khlebnikov and M. S. Novikov, Tetrahedron, 2013, 69, 3363–3401 CrossRef CAS; (b) J. Backes, in Houben–Weyl, ed. D. Klamann, Thieme, Stuttgart, 4th edn, 1992, vol. E16c, pp. 321–369 Search PubMed; (c) V. Nair, in The Chemistry of Heterocyclic Compounds, Small-Ring Heterocycles, ed. A. Hassner, John Wiley & Sons, Inc., New York, 1983, vol. 42, part 1, pp. 215–332 Search PubMed; (d) W. H. Pearson, B. W. Lian and S. C. Bergmeier, in Comprehensive Heterocyclic Chemistry II, ed. A. Padwa, Pergamon, New York, 1996, vol. 1A, pp. 1–60 Search PubMed; (e) F. Palacios, A. M. Ochoa de Retana, E. Martinez de Marigorta and J. M. de los Santos, Eur. J. Org. Chem., 2001, 2401–2414 CrossRef CAS; (f) T. L. Gilchrist, Aldrichimica Acta, 2001, 34, 51–55 CAS; (g) K. M. L. Rai and A. Hassner, in Advances in Strained and Interesting Organic Molecules, ed. B. Halton, Jai, Greenwich, 2000, vol. 8, pp. 187–257 Search PubMed.
- Reviews including 1,1-diazidoethenes: (a) K. Banert, Synthesis, 2016, 2361–2375 CrossRef CAS; (b) A. P. Häring and S. F. Kirsch, Molecules, 2015, 20, 20042–20062 CrossRef PubMed; (c) K. Banert, in Science of Synthesis, ed. A. de Meijere, Thieme, Stuttgart, 2006, vol. 24, pp. 747–765 Search PubMed; (d) K. Banert, in Houben–Weyl, ed. H. Kropf and E. Schaumann, Thieme, Stuttgart, 4th edn, 1993, vol. E15, pp. 2348–2349 Search PubMed.
- R. W. Saalfrank and H. Maid, Chem. Commun., 2005, 5953–5967 RSC.
- (a) H. K. Hall Jr., M. Ramezanian and F. D. Saeva, Tetrahedron Lett., 1988, 29, 1235–1238 CrossRef; (b) M. Ramezanian, A. B. Padias, F. D. Saeva and H. K. Hall Jr., J. Org. Chem., 1990, 55, 1768–1771 CrossRef CAS; (c) B. Roekens, A. B. Padias and H. K. Hall Jr., J. Chem. Res., Synop., 1993, 162 CAS.
- (a) R. W. Saalfrank, E. Ackermann, M. Fischer and U. Wirth, Chem. Ber., 1987, 120, 2003–2006 CrossRef CAS; (b) R. Carrié, D. Danion, E. Ackermann and R. W. Saalfrank, Angew. Chem., Int. Ed. Engl., 1982, 21, 288 CrossRef; (c) R. Carrié, D. Danion, E. Ackermann and R. W. Saalfrank, Angew. Chem., Int. Ed. Engl., 1982, 668–674 CrossRef; (d) R. W. Saalfrank, M. Fischer, U. Wirth and H. Zimmermann, Angew. Chem., Int. Ed. Engl., 1987, 26, 1160–1161 CrossRef; (e) R. W. Saalfrank, U. Wirth and C.-J. Lurz, J. Org. Chem., 1989, 54, 4356–4359 CrossRef CAS.
- (a) R. W. Saalfrank and U. Wirth, Chem. Ber., 1989, 122, 519–522 CrossRef CAS; (b) R. W. Saalfrank and U. Wirth, Chem. Ber., 1989, 122, 969–973 CrossRef CAS; (c) R. W. Saalfrank, E. Ackermann, M. Fischer, U. Wirth and H. Zimmermann, Chem. Ber., 1990, 123, 115–120 CrossRef CAS; (d) R. W. Saalfrank, C.-J. Lurz, J. Hassa, D. Danion and L. Toupet, Chem. Ber., 1991, 124, 595–608 CrossRef CAS.
- (a) R. Carrié, D. Danion, E. Ackermann and R. W. Saalfrank, Angew. Chem., Int. Ed. Engl., 1982, 21, 287 CrossRef; (b) R. Carrié, D. Danion, E. Ackermann and R. W. Saalfrank, Angew. Chem., Int. Ed. Engl., 1982, 660–667 CrossRef.
- (a) J. R. Fotsing, M. Hagedorn and K. Banert, Tetrahedron, 2005, 61, 8904–8909 CrossRef CAS; (b) K. Banert, J. R. Fotsing, M. Hagedorn, H. P. Reisenauer and G. Maier, Tetrahedron, 2008, 64, 5645–5648 CrossRef CAS.
- H. Heimgartner, Angew. Chem., Int. Ed. Engl., 1991, 30, 238–264 CrossRef.
- See the ESI‡ for further details.
- (a) G. Wittig and A. Krebs, Chem. Ber., 1961, 94, 3260–3275 CrossRef CAS; (b) N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- For other examples of trapping highly unstable or explosive azides with the help of cyclooctyne, see: (a) K. Banert, M. Hagedorn, C. Liedtke, A. Melzer and C. Schöffler, Eur. J. Org. Chem., 2000, 257–267 CrossRef CAS; (b) K. Banert and F. Köhler, Angew. Chem., Int. Ed., 2001, 40, 174–177 CrossRef CAS; (c) K. Banert, Y.-H. Joo, T. Rüffer, B. Walfort and H. Lang, Angew. Chem., Int. Ed., 2007, 46, 1168–1171 CrossRef CAS PubMed; (d) K. Banert, Y.-H. Joo, T. Rüffer, B. Walfort and H. Lang, Tetrahedron Lett., 2010, 51, 2880–2882 CrossRef CAS; (e) K. Banert, M. Hagedorn, J. Wutke, P. Ecorchard, D. Schaarschmidt and H. Lang, Chem. Commun., 2010, 46, 4058–4060 RSC; (f) K. Banert, R. Arnold, M. Hagedorn, P. Thoss and A. A. Auer, Angew. Chem., Int. Ed., 2012, 51, 7515–7518 CrossRef CAS PubMed; (g) K. Banert, S. Richter, D. Schaarschmidt and H. Lang, Angew. Chem., Int. Ed., 2013, 52, 3499–3502 CrossRef CAS PubMed; (h) K. Banert, M. Hagedorn, Z. Wu and X. Zeng, Molecules, 2015, 20, 21328–21335 CrossRef CAS PubMed.
- (a) H. Suzuki and C. Nakaya, Synthesis, 1992, 641–642 CrossRef CAS; (b) K. Nishiyama, M. Oba and A. Watanabe, Tetrahedron, 1987, 43, 693–700 CrossRef CAS; (c) H. Yanai and T. Taguchi, Tetrahedron Lett., 2005, 46, 8639–8643 CrossRef CAS.
- K. Banert, C. Berndt, S. Firdous, M. Hagedorn, Y.-H. Joo, T. Rüffer and H. Lang, Angew. Chem., Int. Ed., 2010, 49, 10206–10209 CrossRef CAS PubMed.
- For an example of such an effect, see: K. Banert, Tetrahedron Lett., 1985, 26, 5261–5264 CrossRef CAS.
- For potential hazards in handling hydrazoic acid and organic azides, see: T. Keicher and S. Löbbecke, in Organic Azides, Syntheses and Applications, ed. S. Bräse and K. Banert, Wiley, Chichester, 2010, pp. 3–27 Search PubMed.
Footnotes |
| † Dedicated to Prof. Adalbert Maercker on the occasion of his 85th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, and copies of NMR as well as IR spectra. See DOI: 10.1039/c6qo00625f |
| § These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |