Water turbidity sensing using a smartphone†
I. Hussaina,
K. Ahamadb and
P. Nath*a
aApplied Photonics and Nanophotnics Laboratory, Department of Physics, Tezpur University, Napaam 784028, India. E-mail: pnath@tezu.ernet.in
bDepartment of Civil Engineering, Tezpur University, Napaam 784028, India
First published on 15th February 2016
Abstract
This paper demonstrates a rapid, cost-effective and field-portable smartphone based turbidimeter that measures turbidity of water samples collected from different natural water resources and in drinking water. The working of the designed sensor is based on a Mie-scattering principle where suspended micro (μ-) particles in water medium scatter a strong light signal along the normal direction of the incoming light signal, which can be detected by an infra-red (IR) proximity sensor embedded in the smartphone. Two freely available android applications were used to measure the irradiance of the scattered flux and analyse the turbidity of the medium. With the designed sensor, water turbidity variation as low as 0.1 NTU can be measured accurately in the turbidity value ranging from 0 to 400 NTU. The sensor responses for these ranges of turbid media are found to be linear. A high repeatability in the sensor characteristics is also been observed. The optics design involved for the development of the proposed smartphone turbidimeter is simple and is robust in operation. The designed sensing technique could emerge as a truly portable, user-friendly and inexpensive turbidity sensing tool that would be useful for different in-field applications.
Introduction
The presence of organic and inorganic μ-particles in water bodies affects the lives of aquatic living organisms.1 Also, the presence of such μ-particles in drinking water above the threshold level may cause serious health hazards to our society.2 Thus, accurate monitoring of such μ-particles in water medium bears a great relevance as far as water quality monitoring of natural water resources and drinking water is concerned. Turbidity is a measure of water clarity which means how much the suspended μ-particle in water medium affect the passage of light through it. Highly turbid water causes strong scattering of the incident light signal which in turn, may lead to reduction in photosynthesis process of aquatic flora. This may affect the production of dissolved oxygen in aquatic medium.3 Further, a highly turbid water or water having high levels of suspended solids absorbs more sunlight and may cause rise in temperature of the water bodies. This may subsequently affects the aquatic lives of some living organisms.4 Highly turbid water medium is a favorable condition for growth of waterborne pathogens.5 All suspended inorganic, organic and μ-organisms in water causes light scattering which can be estimated by commercially available turbidimeter such as 2100Q portable turbidimeter from Hach Inc.6 Two of the common approaches for monitoring of water turbidity are Jackson candle method and Secchi disk.7 Both of these techniques depend on the observers' perception thus, turbidity readings may vary from observer to observer. In recent years several groups have demonstrated the usability of optical fibers for monitoring of turbidity in different liquid medium.8,9 Very recently, single photon counting based technique10 has been proposed to monitor turbidity of liquid medium. Using this technique water turbidity as low as 0.1 NTU could be measured accurately. Another optical approach that measures turbidity in liquid medium is based on light scattering principle.11 Nephelometric 90° light scattering measurement is considered to be the standard method for turbidity measurement in nephelometric turbidity unit (NTU).12 This approach is based on intensity comparison of the scattered light beam from a given water sample under investigation to the laboratory prepared standard reference turbid medium such as formazin standard suspension.13 The technique is primarily based on Mie-scattering principle where quasi-collimated light beam from an IR source is allowed to incident on a turbid medium and the scattered flux from this medium is monitored at right angle to the direction of the incident beam. For overall estimation of suspended particle concentration where size of the particles may vary from less than 2 μm to 2000 μm, Mie-scattering principle is valid. Hence, for monitoring of overall quality of water, nephelometric technique is being widely used.With the improved hardware and software, smart built-in sensors and freely downloadable applications that enable easy data analysis and interpretation make smartphones a primary choice for many researchers across the globe to develop it as alternative sensing tools for different physical, chemical and biological sensing applications.14–19 High megapixel imaging sensor of the smartphone finds its applications in imaging and detection of different biological samples.20,21 Using the rear camera of the smartphone and simple optical components, the smartphone based optical set-up can measure optical phase difference as small as π/256 in an optical interference process.22 Dutta et al.23 have successfully demonstrated the measurement of optical absorption bands of different colored dyes and pH level of different water bodies. Koydemir et al.24 have demonstrated that using the imaging sensor of the smartphone, presence of waterborne parasites such as Giardia lamblia cysts in drinking water can be detected.
The integrated proximity and ambient light sensor embedded in the front panel of the smartphone is meant for optimizing battery power consumption by the phone. This sensor assembly is composed of one low power IR light emitting diode (LED) and two photodiodes. One photodiode is sensitive to both visible and IR spectrum used for ambient light sensing and the second photodiode is sensitive primarily to IR light for proximity detection. For all smartphones, the peak emission wavelength range of the IR-LED matches the peak wavelength response of the IR detector which is found to be in the wavelength range of 700–900 nm. In the present work by exploring the IR-detector of the smartphone and by using simple optical components and freely available applications, an attempt has been made to design a sensitive, cost-efficient and truly field portable turbidimeter. To analyze the IR sensor data, freely available android platform based applications namely ‘light meter’25 and ‘stanXY’26 have been used. The designed smartphone turbidimeter is based on Mie-scattering principle where light signal from an IR-LED is allowed to incident on a turbid medium and scattered light from the medium is captured at right angle by the smartphone IR detector. For scattered flux receiving at right angle to the direction of the incoming signal, the irradiance of the scattered beam depends on the concentration of the μ-particles present in the medium and least sensitive to the dimension of the particle size.27 The sensor responses for both laboratory grade formazin standard solutions and water samples collected from different locations of Sonitpur district of Assam have been observed and evaluated.
Materials and methods
To prepare formazin standard turbid medium, analytical grade reagent hexamethylenetetramine ((CH2)6N4) (product no. 398160) and hydrazine sulfate (H6N2O4S) (product no. 489735) were procured from Sigma-Aldrich Inc. All chemicals have been used as received without further processing. Following the standard procedure described by the U.S Environmental Protection Agency,28 a 400 NTU standard turbid medium has been prepared initially in the laboratory. The synthesis procedure is as follows – 1.000 g of hydrazine sulfate is dissolved in 100 mL of distilled water in a volumetric flask and labeled as solution 1. 10.000 g of hexamethylenetetramine is dissolved in 100 mL of distilled water in a volumetric flask and labeled it as solution 2. Now in a 200 mL volumetric flask 5 mL each of the solution 1 and 2 are mixed and total volume of the solution is made to 100 mL by adding 90 mL of distilled water. The resultant solution would be ready for use after keeping it for 24 hours in room temperature. The turbidity of the resultant solution is estimated to be 400 NTU. From this mother solution other low turbid medium can be prepared by using the following equation:29
 | (1) |
Using the above guiding equation, different formazin standard turbid media have been prepared in the laboratory with turbidity value ranging from 0.1 NTU to 400 NTU.
Working principle
The proposed sensing system is based on standard ISO-7027, International Organization for Standardization for water quality in which the scattered radiation flux from a turbid medium is measured at right angle to the direction of the incoming signal.30 According to Mie-scattering principle, the radiation flux scattered by a suspended particles is given by the following equation:31,32
 | (2) |
Again, the relationship between turbidity in NTU to the total suspended solid (TSS) in mg L−1 is given by:33
| NTU = a(TSS)b | (3) |
Experimental setup
Fig. 1(a) shows the schematic of the experimental set-up of our designed smartphone turbidimeter. To develop the turbidimeter, the proximity sensor of Sony Experia E3 smartphone was utilized. Sony Xperia series smartphones contain Avago APDS-9930 or ams AG (TAOS) TMD2771 integrated proximity and ambient light sensor chip.34–36 The detail specifications of the phone used and spectral response of the IR detector is usually found in the wavelength range of 830–890 nm. In general, all smartphones irrespective of its model and variant, the peak spectral response of the embedded IR photo-detector is found to be in this wavelength range. An IR LED with peak emission wavelength 870 nm (product no. L12756 Hamamatsu) is powered from the smartphone battery by using a mini USG-OTG (on-the-go) cable is used to illuminate the turbid medium. The USB port in smartphones is based on defined recognized protocols which make this port universal for all smartphones.37,38 Any device compatible with the USB port provides a current rating of 500 mA at 5 V. In the present work the IR LED is connected to the USB-OTG through a 220 Ω resistor. The emitted IR beam from the LED is collimated using a plano-convex lens (7 mm diameter, focal length 11 mm, Edmund Optics 32-404) and allowed to fall on the turbid medium. The light signal scattered from this turbid medium is received at right angle to the direction of the incoming beam by the IR detector of the smartphone. In order to ensure only ∼90° scattered light signal is being received by the detector, a 1 mm diameter pinhole (Edmund Optics 56-291) is placed in front of the detector. For the present set-up, the separation between the pin hole and the sample holder is 25 mm. Considering that the light scattering is taking place from the center of the sample holder, then the scattered flux arriving at the IR detector will vary within the range 90° ± 1.14° which is well below the limit set by Environmental Protection Agency (EPA) U.S. guideline.30 Detail calculation for measurement of scattering angle in the present optical set-up is provided in the ESI.† Fig. 1(b) shows the photo image of the designed smartphone turbidimeter while Fig. 1(c) shows the photograph of the optical set-up which has been attached to the smartphone. All optical components including the smart phone have been mounted in a custom developed plastic holder made of Nylon. The inner wall of the Nylon block is blackened so that affect of the ambient light on the IR detector is minimum. Low cost, high mechanical strength and superior resistance to wear from chemicals make Nylon material a primary choice for fabricating the optical holder in the present investigation. The overall dimension of the setup was measured to be 140 mm in length, 80 mm in both breadth and 40 mm in width; the weight of the proposed device along with the phone was approximately 250 g. | ||
| Fig. 1 (a) A schematic diagram of the proposed setup with different components (b) photo image of the designed smartphone turbidimeter (c) inside view of the turbidimeter compartment. | ||
Workflow of the smartphone application
Fig. 2 shows the process flow of the proposed smartphone turbidimeter. The freely available ‘light meter’ app for android smartphone measures the irradiance of the scattered flux in LUX unit. This specific app measures the average value through recording of minimum and the maximum variation of the scattered flux irradiance for a specific period of time. In order to plot the variation of the scattered flux intensity with turbidity another free app ‘stanXY’ was used in the present work. Using the app characteristic plot of the scattered flux irradiance versus turbidity for standard turbid medium was first obtained. To measure the turbidity of an unknown medium, the reading of the scattered flux irradiance is plotted in the standard calibration curve. Thus, by using these two smartphone applications, the turbidity of any unknown samples can be estimated easily with the designed sensing set-up.Experimental result
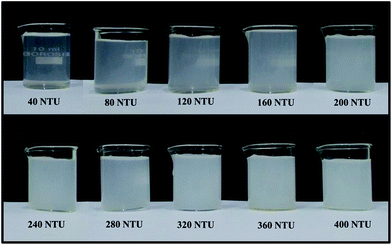
The designed smartphone turbidimeter was first calibrated with the formazin standard turbid medium. Formazin standard medium of different turbidity value ranging from 40 NTU to 400 NTU in step of 40 NTU have been prepared from the 400 NTU stock solution. To obtain low value turbid medium from the stock solution, the dilution procedure was followed as explained in the material and method section. Fig. 3 shows the photo images of 10 different turbid medium of formazin standard solutions.Prior to study the sensor characteristics, turbidity value of all the formazin standard samples have been measured using laboratory grade standard turbidimeter (Systronics India, μC turbidity meter model no. 135). Turbidity of the prepared samples were measured by the designed smartphone turbidimeter. The average value of scattered flux irradiance of all the considered samples have been measured for a period of 5 seconds. Fig. 4(a) shows the characteristic plot of the normalized scattered flux intensity with the variation in turbidity value for different Formazin standard media. The linear fitted line with the value of co-efficient of regression R2 = 0.99727 implies that turbidity of any sample can be measured almost precisely and accurately with our designed sensor. Fig. 4(b) shows the residual versus fitted graph that ensures the validity of our regression model. From this regression analysis, following equation can be used to measure turbidity of unknown water samples:
 | (4) |
 | ||
| Fig. 4 (a) Smartphone sensor response curve for high turbidity value of formazin standards solutions and (b) residual plot of sensor response curve. | ||
The relation between the scattered flux irradiance versus turbidity of the standard medium is plotted using ‘stanXY’ application software and the screenshot image of the smartphone reading is shown in Fig. 2.
Again, according to EPA U.S., the turbidity limit in drinking water, should be below 1.0 NTU. Water turbidity of 1.0 NTU or less is protective against cryptosporidium break-through in the medium. According to Indian standards (IS), the permissible value of turbidity in drinking water is ∼1.0 NTU and in the absence of alternate source, this value can be as high as 5.0 NTU.39,40 The performance of the designed sensor is therefore further evaluated for low turbid medium. 10 more samples of formazin standard medium with turbidity value ranging from 0.1 NTU to 1 NTU in step incremental value of 0.1 NTU and another 10 more samples with turbidity value ranging from 1 NTU to 10 NTU in step incremental value of 1 NTU have been prepared in the laboratory. The turbidity value of the prepared samples have been measured 10 times with our designed sensor and the average values were recorded. Fig. 5(a) and b illustrate the sensor response of the designed sensor for all the considered samples. A fairly linear sensor response has been observed even at the low turbidity value of the medium. Here, 0.0 NTU is considered for distilled water when it was placed in the optical path of the set-up. An offset has been noticed in the designed set-up which was attributed to the thermal fluctuations of the detector and output power fluctuations of the IR-source. The sensor characteristic curve in Fig. 5(b) implies that even for low turbid media the sensor response found to be linear with R2 = 0.9914. Fig. 5(c) shows the uncertainty in measurements produced for 10 sets of readings for each sample in the turbidity range 0.0 NTU to 1 NTU. The maximum uncertainty in measurement is found to be approximately 0.065 NTU. This indicate that the proposed senor can reliably measure the turbidity variation as low as 0.1 NTU with good accuracy.
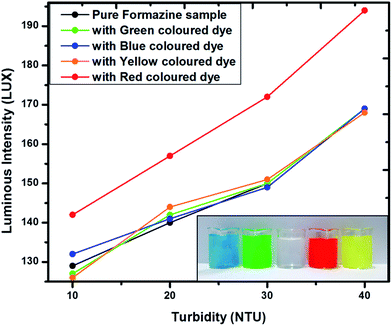
Due to the presence of algae, phytoplankton or other different organic substances in natural water bodies, there could be a colour variation in water medium which may affect the sensor response. Therefore, the sensor characteristics have further been evaluated for different coloured turbid media. In order to investigate this specific characteristic of the sensor we consider four different Formazin standard samples with turbidity values 10 NTU, 20 NTU, 30 NTU and 40 NTU. Turbidity value of most of the environmental water bodies are found in these range (10–40 NTU). The prepared samples were then mixed with four coloured dyes namely red, green, yellow and blue prepared in distilled water. Fig. 6 shows the sensor response curves for these different coloured samples with reference to the clean formazin samples. Inset figure shows photograph of the prepared coloured Formazin samples along with the clear Formazin sample. It has been observed that except the red coloured turbid medium, the response of the designed sensor for all other coloured media and clear Formazin sample were almost same. This is attributed to the spectral range of the IR-detector response (given in ESI†) covers the red region (700 nm) of the visible spectrum and this has perturbed the sensor characteristics. To measure the turbidity of red coloured medium, an IR filter (850–860 nm) should be placed in the path between the IR detector and the sample holder so that it could eliminate the affect due to color of the medium.
The usability of the designed sensor for in-field applications has also been evaluated. Water samples from different sources in Sonitpur district of Assam has been collected during June–July 2015. Fig. 7 shows the geographical map of Sonitpur district adapted from Google maps with red spots indicating the locations from where water samples have been collected. Also, 3 more clay mixed water samples have been prepared in the laboratory. Turbidity value of the field-collected and clay mixed water media have been measured initially with the standard turbidimeter. We then measure the turbidity value of all these samples by our designed turbidimeter. Fig. 8 illustrates the histogram representation of the turbidity measurement provided by the designed smartphone sensor and the standard turbidimeter. The error bar of the experimental data yielded by the smartphone turbidimeter indicates that the sensor has very low experimental error and this implies high reliability of the designed sensor for in-field turbidity sensing applications.
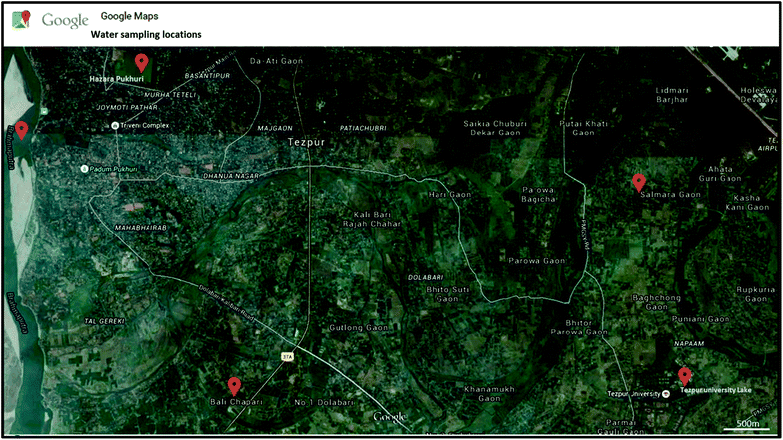 | ||
| Fig. 7 Satellite image shows a part of Sonitpur district of Assam India. Water samples collected locations have been marked in red in this image. | ||
Owing to the involvement of low cost components, we believe that the proposed sensing scheme could emerge as a potentially inexpensive tool for sensing of water quality with high accuracy and repeatability. The net cost involved for development of the smartphone turbidimeter excluding the smartphone was ∼$86. The response of the proximity sensor for three different smartphones namely Sony Xperia E3, Asus Zenfone2 and Moto G xt1033 have been evaluated for same incident IR-radiation. It has been found that for same version light meter application almost similar responses have been observed for all the three different smartphones. The photo images of the detector response of these phones have been provided in the ESI.† By exploiting μ-internet facility it is possible to share the in-field turbidity data with the central water quality monitoring laboratory anywhere in the world in almost in real time environment. With the user friendly apps for data recording and analysis, the proposed sensing system can even be used by an inexperienced person to monitor turbidity of different water medium.
In the present work the proximity sensor of the smartphone has been explicitly exploited along with the freely available smartphone applications for reliable and accurate estimation of turbidity of water medium. Although smartphone based platform turbidimeter are commercially available such as from Aguaclara41 and Arbor Scientific,42 the working principle of the present smartphone turbidimeter is different from those sensors. In the commercially available turbidimeters, the smartphone has been used primarily for data acquisition which has been transferred from an external turbidity sensor. The smartphone proximity sensor is not utilized as a detector in those sensors. The use of separate photo-detector, battery unit are required for interfacing with the smartphones which significantly increases its cost. In the present sensor, we have utilized the smartphone both for detection and data analysis purpose. Further the use of smartphone battery reduces its overall cost significantly. Table 1 compares the performance of our designed sensor with respect to its commercially available counterparts.
| Device specifications | AguaClara's smartphone turbidimeter | Arbor scientific's smartphone turbidity sensor module | Proposed smartphone turbidimeter | Remarks |
|---|---|---|---|---|
| Hardware used | (1) External turbidity sensor which includes: (a) LED, (b) external battery, (c) photo-detectors, (d) analog to digital converter, (e) sample cell, (f) USB/bluetooth interface | (1) External turbidity sensor which includes: (a) LED, (b) external battery, (c) photo-detectors, (d) analog to digital converter, (e) sample cell, (f) wi-fi module | (1) Infra-red LED | The smartphone battery is used to power the IR LED. No external photo detector is used to measure scattered light flux from the turbid medium |
| (2) USB-OTG cable | ||||
| (3) Sample cell | ||||
| (4) Lens and pinhole | ||||
| (5) Plastic holder | ||||
| (6) Smartphone | ||||
| (2) Smartphone | (2) Smartphone | |||
| Software used | (1) Turbidimeter app | (1) NeuLog software | (1) Light meter app (free) | Here two freely available apps have been used for detection and analysis of scattered flux radiation. These apps are compatible with any android smartphone |
| (2) Communication software package | (2) Phone web browser | (2) ‘stanXY’ app (free) | ||
| Operational range | Unknown | 0–200 NTU | 0–400 NTU | The proposed device has a higher dynamic range |
| Sensitivity | Unknown | 0.08 NTU | 0.1 NTU | The sensitivity is nearly comparable to its commercially available counterparts |
| Cost (excluding smartphone) | $100 | $111.99 | ∼$86.00 | The cost is less as compared to the others |
Conclusion
A cost-effective, robust and field portable turbidimeter using the proximity sensor of the smartphone has been demonstrated. The IR detector of the proximity sensor of the smartphone has been utilized to measure scattered radiation flux form turbid water samples. Using simple optical set-up and freely available applications, turbidity of different Formazin standard medium have been measured successfully by the designed sensor. The device performance was found to be reliable while monitoring the turbidity value of drinking and environmental water bodies. The designed sensor can be used for water turbidity monitoring in flood prone areas. The performance of the sensor has been compared with the laboratory grade standard turbidimeter and good correlation was found. The proposed sensing device may also find applications in clinical and biological investigations which will be performed in the future course of work.Acknowledgements
P. Nath gratefully acknowledges the valuable guidance received from B. Cunningham on similar line of work during visit to University of Illinois at Urbane Champaign, USA. Authors acknowledge A. J. Thakur, and A. Mohanta, Department of Chemical Sciences, Tezpur University (TU) for providing chemicals for preparation of Formazin standard solutions. Authors thank P. Kalita, Department of Civil Engineering (TU) for assistance with the standard turbidity meter measurements. Authors also acknowledge the support received from Workshop, Mechanical Engineering Department (TU) for developing the plastic holder in the present work. I. Hussain gratefully acknowledges the financial support received from UGC, New Delhi for awarding Maulana Azad National fellowship for Minority students 2014–15.References
- http://water.epa.gov/type/rsl/monitoring/vms55.cfm, (accessed September 2015).
- http://www.hc-sc.gc.ca/ewh-semt/pubs/water-eau/turbidity/index-eng.php, (accessed September 2015).
- B. M. Wilen and P. Balmer, Water Sci. Technol., 1998, 38, 25–33 CAS
.
- J. T. Kirk, in Perspectives in Southern Hemisphere Limnology, Springer, 1985, pp. 195–208 Search PubMed
.
- J. E. Ongerth, J. - Am. Water Works Assoc., 1990, 82, 85–96 CrossRef CAS
.
- http://www.hach.com/2100qportableturbidimeter/product%20?id=%207640450963, (accessed September 2015).
- R. W. Preisendorfer, Limnol. Oceanogr., 1986, 31, 909–926 CrossRef CAS
.
- L. Bilro, S. A. Prats, J. L. Pinto, J. J. Keizer and R. N. Nogueira, Meas. Sci. Technol., 2010, 21, 10701 Search PubMed
.
- A. F. Bin Omar and M. Z. Bin MatJafri, Sensors, 2009, 9, 8311–8335 CrossRef CAS PubMed
.
- Y. X. Yang, H. Q. Wang, Y. Y. Cao, H. Q. Gui, J. G. Liu, L. Lu, H. B. Cao, T. Z. Yu and H. You, Opt. Laser Technol., 2015, 73, 44–49 CrossRef
.
- W. McCluney, J. - Water Pollut. Control Fed., 1975, 252–266 Search PubMed
.
- F. W. Gilcreas, Am. J. Public Health Nation's Health, 1966, 56, 387–388 CrossRef CAS
.
- http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/007_07_10_methods_method_180_1.pdf, (accessed September 2015).
- S. Cho, T. S. Park, T. G. Nahapetian and J. Y. Yoon, Biosens. Bioelectron., 2015, 74, 601–611 CrossRef CAS PubMed
.
- A. Roda, L. Cevenini, D. Calabria, M. M. Calabretta and E. Michelini, Luminescence, 2014, 29, 90 Search PubMed
.
- A. Sun, T. Wambach, A. G. Venkatesh and D. A. Hall, IEEE Biomedical Circuits and Systems Conference : Healthcare Technology, 2014, 2014, 312–315 Search PubMed
.
- L. Zhang, W. Yang, Y. Yang, H. Liu and Z. Gu, Analyst, 2015, 140, 7399–7406 RSC
.
- S. Lee, G. Kim and J. Moon, J. Nanosci. Nanotechnol., 2014, 14, 8453–8457 CrossRef CAS PubMed
.
- D. Gallegos, K. D. Long, H. J. Yu, P. P. Clark, Y. X. Lin, S. George, P. Nath and B. T. Cunningham, Lab Chip, 2013, 13, 2124–2132 RSC
.
- A. Ozcan, Lab Chip, 2014, 14, 3187–3194 RSC
.
- S. C. B. Gopinath, T. H. Tang, Y. Chen, M. Citartan and T. Lakshmipriya, Biosens. Bioelectron., 2014, 60, 332–342 CrossRef CAS PubMed
.
- I. Hussain and P. Nath, Appl. Opt., 2015, 54, 5739–5742 CrossRef CAS PubMed
.
- S. Dutta, D. Sarma and P. Nath, AIP Adv., 2015, 5, 057151 CrossRef
.
- H. C. Koydemir, Z. Gorocs, D. Tseng, B. Cortazar, S. Feng, R. Y. L. Chan, J. Burbano, E. McLeod and A. Ozcan, Lab Chip, 2015, 15, 1284–1293 RSC
.
- https://play.google.com/store/apps/details?id=net.mannoun.lightmeter%26hl=en, (accessed September 2015).
- https://play.google.com/store/apps/details?id=com.apdla.stanxy%26hl=en, (accessed September 2015).
- Inorganic species part 1, Water analysis, ed. R. A. Minear and L. H. Keith, vol. 1, Academic press, 1982, pp. 182–233 Search PubMed
.
- U.S. Environmental Protection Agency, Methods for Determination of Inorganic Substances in Environmental Samples.EPA-600/R/93/100-Draft, Environmental Monitoring Systems Lab., Cincinnati, Ohio, 1993 Search PubMed
.
- www.hach.com/asset-get.download%20en.jsa?code=61799, (accessed September 2015).
- ISO 7027:1999, Water quality – Determination of turbidity, https://www.iso.org/obp/ui/#iso:std:iso:7027:ed-3:v1:en, (accessed October 2015).
- H. C. Hulst and H. van de Hulst, Light scattering by small particles, Dover publication, New York, 1957 Search PubMed
.
- T. F. Sutherland, P. M. Lane, C. L. Amos and J. Downing, Mar. Geol., 2000, 162, 587–597 CrossRef
.
- C. Holliday, T. C. Rasmussen and W. P. Miller, Proceedings of the 2003 Georgia Water Resources Conference, Institute of Ecology, The University of Georgia, Athens, Georgia, 2003 Search PubMed
.
- http://specdevice.com/showspec.php?id=6fca-4f5b-ffff-fffff133d5e9, (accessed October 2015).
- http://www.avagotech.com/products/optical-sensors/integrated-ambient-light-proximity-sensors/apds-9930, (accessed October 2015).
- http://ams.com/eng/Products/Light-Sensors/Ambient-Light-Sensor-Proximity-Detection, (accessed October 2015).
- E. H. Doeven, G. J. Barbante, A. J. Harsant, P. S. Donnelly, T. U. Connell, C. F. Hogan and P. S. Francis, Sens. Actuators, B, 2015, 216, 608–613 CrossRef CAS
.
- http://www.usb.org/developers/onthego, (Accessed October 2015).
- www.epa.ie/pubs/advice/drinkingwater/2015_04_21_ParametersStandaloneDoc.pdf, (accessed October 2015).
- Drinking water- specifications, IS 10500: 2012, http://cgwb.gov.in/NEW/Documents/WQ-standards.pdf. (accessed October 2015).
- https://confluence.cornell.edu/pages/viewpageattachments.action?pageId=190483240%26metadataLink=true, (accessed january 2016).
- http://www.arborsci.com/media/datasheet/NL-2310_DS.pdf. (accessed january 2016).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02483a |
| This journal is © The Royal Society of Chemistry 2016 |