Iodine-mediated synthesis of (E)-vinyl sulfones from sodium sulfinates and cinnamic acids in aqueous medium†
Jian Gao,
Junyi Lai and
Gaoqing Yuan*
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640, P. R. China. E-mail: gqyuan@scut.edu.cn
First published on 28th July 2015
Abstract
With water as the reaction medium, a green and efficient method has been developed for the synthesis of (E)-vinyl sulfones via I2-mediated decarboxylative cross-coupling reactions of sodium sulfinates with cinnamic acids. This synthetic route could effectively avoid the use of toxic organic solvents and transition metal catalysts, and the target products could be obtained with moderate to excellent yields under green and mild conditions.
Since 1960s, transition metal catalysts have increasingly attracted one's attention owing to their versatile reactivity and practical applications in organic synthesis,1 especially for the formation of carbon–carbon and carbon–heteroatom bonds.2 Nevertheless, transition-metal-catalyzed coupling reactions have long been plagued by instinctive drawbacks of the catalytic systems, such as expensive, toxic catalysts and ligands, extra additives or co-catalysts, harsh reaction conditions and so on.3 So chemists desire to look for greener and more efficient synthetic routes,4 and recent researches have revealed the feasibility to replace transition metal catalysts with greener and cheaper iodine or iodide reagents.5 For example, Yotphan and co-workers recently reported iodine-catalyzed oxidative amination of sodium sulfonates.5h
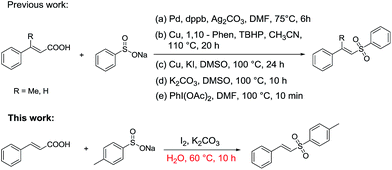
Vinyl sulfones, as key functional units, exhibit a broad range of biological and parmaceutical activities in biological and medicinal chemistry,6 and show great synthetic value as building blocks in organic transformations.7 To date various synthetic methods have been developed,8 mainly including (1) direct coupling reaction of alkenes or alkynes with sulfone resources (sulfinic acid,9 sodium sulfinate,10 sulfonyl hydrazide,11 thiols12 and dimethyl sulfoxide5b,13), and (2) decarboxylative coupling reaction of cinnamic acids with sodium sulfinate (Scheme 1).14 In Scheme 1, there have been some new and efficient Pd- or Cu-catalyzed decarboxylative coupling reactions for the synthesis vinyl sulfones from cinnamic acid and sodium sulfinate. However, all of them need toxic metals, ligands, oxidants and high temperature (Scheme 1a–c).
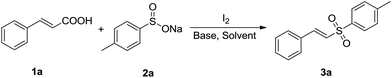
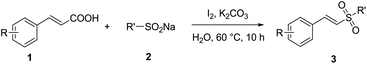
It is noteworthy that Jiang's group has reported a simple and efficient route for the synthesis of vinyl sulfones without any catalysts (Scheme 1d), and very recently Kuhakarn's group has reported a highly efficient method by PhI(OAc)2 mediated decarboxylative sulfonylation in a very short time (Scheme 1e). However, both of them still suffer from toxic organic solvents and high temperature. It is well known that water as green reaction medium has practical advantages over organic solvents and attracted increasing attention in organic synthesis.15 Herein, we reported a green and efficient method for the synthesis of vinyl sulfones via I2-mediated decarboxylative cross-coupling reaction of sodium sulfinates with cinnamic acids, using water as a solvent at lower temperature.
Firstly, we chose trans-cinnamic acid (1a) and sodium 4-methyl benzenesulfinate (2a) as model substrates to examine various reaction conditions, and the results were shown in Table 1. We did not get the desired product when the reaction of 1a with 2a was performed with 1 equiv. of I2 in the absence of a base at room temperature for 5 h (entry 1). In the presence of base K2CO3 (1.0 equiv.), (E)-1-methyl-4-(styrylsulfonyl)benzene (3a) could be obtained with 10% yield (entry 2). We attempted to prolong reaction time to 10 h, but the yield of 3a was not obviously improved (only 23%, entry 3). Luckily, when the reaction temperature was raised to 40 °C, the yield of 3a was drastically increased to 72% (entry 4). Higher temperatures (50 °C, 60 °C and 70 °C, entries 5–7) were further tested, and a best yield was obtained (91%, entry 6). When 0.5 equiv. of I2 or 0.5 equiv. of K2CO3 was added to the reaction system, the yield of 3a declined to approximately half of the best yield (entries 8 and 9). Besides, the reaction efficiency was not improved when excess K2CO3 (2.0 equiv.) was used (entry 10). At last, various bases (Cs2CO3, NaOAc, KOH, CH3ONa and Et3N) and solvents (CH3CN, DMF, EtOAc, CH3OH and DMSO) were screened, respectively. These results showed that K2CO3 was an optimal base and water was the most suitable solvent in this process (entries 11–20).
| Entry | I2 (equiv.) | Base (equiv.) | Solvent | Time (h) | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (0.6 mmol), base (1.0 equiv.), I2 (1.0 equiv.), H2O (2 mL) at 60 °C for 10 h.b Determined by GC-MS using dodecane as the internal standard.c Under N2 atmosphere. | ||||||
| 1 | 1.0 | No | H2O | 5 | rt | Trace |
| 2 | 1.0 | K2CO3 (1.0) | H2O | 5 | rt | 10 |
| 3 | 1.0 | K2CO3 (1.0) | H2O | 10 | rt | 23 |
| 4 | 1.0 | K2CO3 (1.0) | H2O | 10 | 40 | 72 |
| 5 | 1.0 | K2CO3 (1.0) | H2O | 10 | 50 | 80 |
| 6 | 1.0 | K2CO3 (1.0) | H2O | 10 | 60 | 91 |
| 7 | 1.0 | K2CO3 (1.0) | H2O | 10 | 70 | 84 |
| 8 | 0.5 | K2CO3 (1.0) | H2O | 10 | 60 | 40 |
| 9 | 1.0 | K2CO3 (0.5) | H2O | 10 | 60 | 41 |
| 10 | 1.0 | K2CO3 (2.0) | H2O | 10 | 60 | 80 |
| 11 | 1.0 | Cs2CO3 (1.0) | H2O | 10 | 60 | 90 |
| 12 | 1.0 | NaOAc (1.0) | H2O | 10 | 60 | 14 |
| 13 | 1.0 | KOH (1.0) | H2O | 10 | 60 | 30 |
| 14 | 1.0 | CH3ONa (1.0) | H2O | 10 | 60 | 35 |
| 15 | 1.0 | Et3N (1.0) | H2O | 10 | 60 | 22 |
| 16 | 1.0 | K2CO3 (1.0) | CH3CN | 10 | 60 | 7 |
| 17 | 1.0 | K2CO3 (1.0) | DMF | 10 | 60 | 5 |
| 18 | 1.0 | K2CO3 (1.0) | EtOAc | 10 | 60 | 3 |
| 19 | 1.0 | K2CO3 (1.0) | CH3OH | 10 | 60 | 52 |
| 20 | 1.0 | K2CO3 (1.0) | DMSO | 10 | 60 | 5 |
| 21c | 1.0 | K2CO3 (1.0) | H2O | 10 | 60 | 87 |
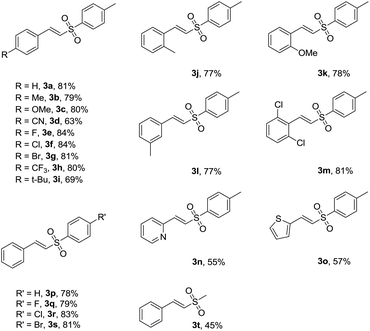
With the optimal reaction conditions in hand, the scope of substrates was investigated and the results are summarized in Table 2. On one hand, we examined the reaction of sodium 4-methyl benzenesulfinate (2a) with cinnamic acid derivatives in the standard reaction conditions. Some cinnamic acids which have electron-withdrawing substituents on the phenyl ring (4-CN, 4-F, 4-Cl, 4-Br, 4-CF3 and 2,6-2Cl) could proceed smoothly to afford the corresponding products in available yields (63–84%) (3d–h, 3m). Also, cinnamic acids with electron-donating substituents on the phenyl ring (4-Me, 4-MeO, 4-t-Bu, 2-Me, 2-MeO and 3-Me) were examined, and the yields of the corresponding products ranged from 69% to 81% (3b–c, 3i–l). These results did not obviously change in comparison with those of substrates having electron-withdrawing substituents. In addition, (E)-2-(2-tosylvinyl)pyridine (3n) and (E)-2-(2-tosylvinyl)thiophene (3o) could be obtained with 55% and 57% yields, respectively. On the other hand, the reactions of trans-cinnamic acid (1a) with various sodium sulfinates were tested. Various sodium sulfinates with 4-H, 4-F, 4-Cl and 4-Br groups substituted on phenyl rings all proceeded smoothly to give good yields (78–83%) (3p–s). Moreover, (E)-(2-(methyl sulfonyl)vinyl)benzene (3t) was obtained with 45% yield.
It is worth noting that the reaction was performed on a 1.0 g scale to afford 3a with 81% yield, indicating that the reaction is scalable and practical (Scheme 2).
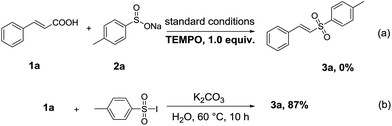
To understand the reaction mechanism better, some control experiments have been explored. The desired product 3a was obtained with a good yield under nitrogen atmosphere, which eliminated the influence of oxygen on this reaction very well (Table 1, entry 21). The reaction of 1a with 2a was proceeded under the standard conditions in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), and no 3a was detected by GC, which implied a radical pathway should be involved (Scheme 3a). When using 4-methylbenzene-1-sulfonyl iodide as the substrate instead of 2a and iodine, the yield of 3a was almost unchanged (Scheme 3b). The result suggested that 4-methylbenzene-1-sulfonyl iodide should be an intermediate in this transformation. The synthetic procedure of 4-methylbenzene-1-sulfonyl iodide was given in the ESI.† In addition, it should be pointed out that I2 is almost converted to iodine anions after the reaction in two steps (i.e., the reaction of ArSO2Na with I2, and the reaction of ArSO2I with cinnamic acid) in the present standard conditions, which is confirmed by iodometric titration.
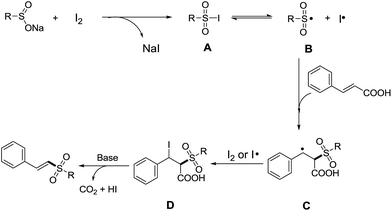
Based on the results of control experiments, a possible reaction mechanism was proposed in Scheme 4. It is easy to generate intermediate A from sodium sulfinate and iodine, and the intermediate A undergoes homolysis to give a sulfonyl radical (B) and an iodine radical.16 Subsequently, the radical B is added to the double bond of cinnamic acid to afford intermediate C,14b–e,17 which is combined with iodine or iodine radical to generate intermediate D.10b,d Finally, the intermediate D undergoes the elimination of carbon dioxide and hydrogen iodide to provide the desired vinyl sulfones with the help of a base.14c,17
In conclusion, we have developed a green and efficient method for the synthesis of vinyl sulfones via I2-mediated decarboxylative coupling reaction using environmentally friendly water as the reaction medium. This reaction shows its fascinating application prospect in organic synthesis. Compared with the reported methods, this route seems to be greener and more efficient.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (21172079) and the Science and Technology Planning Project of Guangdong Province (2011B090400031) for financial support.Notes and references
- For reviews on transition-metal-catalyzed coupling reactions, please see: (a) C. C. C. J. Seechurn, M. O. Kiching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062 CrossRef PubMed; (b) X. F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986 RSC; (c) F. S. Han, Chem. Soc. Rev., 2013, 42, 5270 RSC; (d) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed; (e) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (f) I. Bauer and H. J. Knölker, Chem. Rev., 2015, 115, 3170 CrossRef CAS PubMed.
- For reviews on transition-metal-catalyzed coupling reactions of C–C bond or C–heteroatom formation, please see: (a) W. Shi, C. Liu and A. W. lei, Chem. Soc. Rev., 2011, 40, 2761 RSC; (b) B. J. Li and Z. J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (c) R. Kumar and E. V. V. Eycken, Chem. Soc. Rev., 2013, 42, 1121 RSC; (d) S. G. Modha, V. P. Mehta and E. V. V. Eycken, Chem. Soc. Rev., 2013, 42, 5042 RSC; (e) C. Shen, P. F. Zhang, Q. Sun, S. Q. Bai, T. S. Andy Hor and X. G. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC; (f) G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435 CrossRef CAS PubMed.
- (a) H. Li, L. Wang, Y. Zhang and J. B. Wang, Angew. Chem., Int. Ed., 2012, 51, 2943 CrossRef CAS PubMed; (b) H. Zhang, R. Y. Shi, A. X. Ding, L. J. Lu, B. R. Chen and A. W. Lei, Angew. Chem., Int. Ed., 2012, 51, 12542 CrossRef CAS PubMed; (c) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC; (d) C. L. Sun and Z. J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (e) S. Roscales and A. G. Csákÿ, Chem. Soc. Rev., 2014, 43, 8215 RSC.
- (a) B. A. F. Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. V. Medrano, Green Chem., 2010, 12, 2099 RSC; (b) Y. L. Gu and F. Jérôme, Chem. Soc. Rev., 2013, 42, 9550 RSC; (c) G. L. Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644 RSC; (d) B. H. Lipshutz and S. Ghorai, Green Chem., 2014, 16, 3660 RSC.
- (a) X. J. Pan, J. Gao, J. Liu, J. Y. Lai, H. F. Jiang and G. Q. Yuan, Green Chem., 2015, 17, 1400 RSC; (b) J. Gao, X. J. Pan, J. Liu, J. Y. Lai, L. M. Chang and G. Q. Yuan, RSC Adv., 2015, 5, 27439 RSC; (c) A. K. Banerjee, W. Vera, H. Mora, M. S. Laya, L. Bedoya and E. V. Cabrera, J. Sci. Ind. Res., 2006, 65, 299 CAS; (d) T. Nobuta, N. Tada, A. Fujiya, A. Kariya, T. Miura and A. Itoh, Org. Lett., 2013, 15, 574 CrossRef CAS PubMed; (e) M. A. Kumar, P. Swamy, M. Naresh, M. M. Reddy, C. N. Rohitha, S. Prabhakar, A. V. S. Sarma, J. R. P. Kumar and N. Narender, Chem. Commun., 2013, 49, 1711 RSC; (f) W. Xu, U. Kloeckner and B. J. Nachtsheim, J. Org. Chem., 2013, 78, 6065 CrossRef CAS PubMed; (g) P. R. Adiyala, D. Chandrasekhar, J. S. Kapure, C. N. Reddy and R. A. Maurya, Beilstein J. Org. Chem., 2014, 10, 2065 CrossRef PubMed; (h) C. Buathongjan, D. Beukeaw and S. Yotphan, Eur. J. Org. Chem., 2015, 1575 CrossRef CAS PubMed.
- (a) S. Y. Woo, J. H. Kim, M. K. Moon, S. H. Han, S. K. Yeon, J. W. Choi, B. K. Jang, H. J. Song, Y. G. Kang, J. W. Kim, J. Lee, D. J. Kim, O. Hwang and K. D. Park, J. Med. Chem., 2014, 57, 1473 CrossRef CAS PubMed; (b) E. Dunny, W. Doherty, P. Evans, J. P. G. Malthouse, D. Nolan and A. J. S. Knox, J. Med. Chem., 2013, 56, 6638 CrossRef CAS PubMed; (c) W. Doherty, J. James, P. Evans, L. Martin, N. Adler, D. Nolan and A. Knox, Org. Biomol. Chem., 2014, 12, 7561 RSC; (d) B. A. Frankel, M. Bentley, R. G. Kruger and D. G. McCafferty, J. Am. Chem. Soc., 2004, 126, 3404 CrossRef CAS PubMed.
- (a) J. Aziz, S. Messaoudi, M. Alami and A. Hamze, Org. Biomol. Chem., 2014, 12, 9743 RSC; (b) M. N. Noshi, A. El-awa, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2007, 129, 11242 CrossRef CAS PubMed; (c) H. M. Li, J. Song, X. F. Liu and L. Deng, J. Am. Chem. Soc., 2005, 127, 8948 CrossRef CAS PubMed.
- For reviews on other routes of synthesis of vinyl sulfones, please see: (a) D. C. Reeves, S. Rodriguez, H. Lee, N. Haddad, D. Krishnamurthy and C. H. Senanayake, Tetrahedron Lett., 2009, 50, 2870 CrossRef CAS PubMed; (b) R. Chawla, R. Kapoor, A. K. Singh and D. S. Yadav, Green Chem., 2012, 14, 1308 RSC; (c) J. L. G. Ruano, J. Alemán and C. G. Paredes, Org. Lett., 2006, 8, 2683 CrossRef CAS PubMed; (d) N. Kamigata, H. Sawada and M. Kobayashi, J. Org. Chem., 1983, 48, 3793 CrossRef CAS.
- W. Wei, J. L. Li, D. S. Yang, J. W. Wen, Y. T. Jiao, J. M. You and H. Wang, Org. Biomol. Chem., 2014, 12, 1861 CAS.
- (a) Y. L. Xu, J. W. Zhao, X. D. Tang, W. Q. Wu and H. F. Jiang, Adv. Synth. Catal., 2014, 356, 2029 CrossRef CAS PubMed; (b) V. Nair, A. Augustine, T. G. George and L. G. Nair, Tetrahedron Lett., 2001, 42, 6763 CrossRef CAS; (c) Y. C. Luo, X. J. Pan and G. Q. Yuan, Tetrahedron, 2015, 73, 2119 CrossRef PubMed; (d) N. Zhang, D. S. Yang, W. Wei, L. Yuan, Y. J. Cao and H. Wang, RSC Adv., 2015, 5, 37013 RSC; (e) N. Taniguchi, Tetrahedron, 2014, 70, 1984 CrossRef CAS PubMed; (f) N. Taniguchi, Synlett, 2012, 23, 1245 CrossRef CAS; (g) P. Katurn, S. Chiampanichayakul, K. Korworapan, M. Pohmakotr, V. Reutrakul, T. Jaipetch and C. Kuhakarn, Eur. J. Org. Chem., 2010, 5633 CrossRef PubMed.
- (a) G. W. Rong, J. C. Mao, H. Yan, Y. Zheng and G. Q. Zhang, J. Org. Chem., 2015, 80, 4697 CrossRef CAS PubMed; (b) X. W. Li, Y. L. Xu, C. Jiang, C. R. Qi and H. F. Jiang, Chem.–Eur. J., 2014, 20, 7911 CrossRef CAS PubMed; (c) S. Tang, Y. Wu, W. Q. Liao, R. P. Bai, C. Liu and A. W. Lei, Chem. Commun., 2014, 50, 4496 RSC.
- Q. C. Xue, Z. J. Mao, Y. Shi, H. B. Mao, Y. X. Cheng and C. J. Zhu, Tetrahedron Lett., 2012, 53, 1851 CrossRef CAS PubMed.
- Y. J. Jiang and T. P. Loh, Chem. Sci., 2014, 5, 4939 RSC.
- (a) R. Q. Guo, Q. W. Gui and D. D. Wang, Catal. Lett., 2014, 144, 1377 CrossRef CAS; (b) B. V. Rokade and K. R. Prabhu, J. Org. Chem., 2014, 79, 8110 CrossRef CAS PubMed; (c) Q. Jiang, B. Xu, A. Zhao, Y. R. Zhao, Y. Y. Li, N. N. He and C. C. Guo, J. Org. Chem., 2014, 79, 7372 CrossRef CAS PubMed; (d) Y. L. Xu, X. T. Tang, W. G. Hu, W. Q. Wu and H. F. Jiang, Green Chem., 2014, 16, 3720 RSC; (e) P. Katrun, S. Hlekhlai, J. Meesin, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram and C. Kuhakarn, Org. Biomol. Chem., 2015, 13, 4785 RSC; (f) H. S. Li and G. Liu, J. Org. Chem., 2014, 79, 509 CrossRef CAS PubMed.
- (a) L. Shi, Y. Y. Liu, Q. F. Liu, B. Wei and G. S. Zhang, Green Chem., 2012, 14, 1372 RSC; (b) M. B. Gawande, A. D. B. Bonifácio, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC; (c) N. E. Leadbeater, Chem. Commun., 2005, 2881 RSC; (d) H. D. Velazquez and F. Verpoort, Chem. Soc. Rev., 2012, 41, 7032 RSC; (e) Y. L. Gu, Green Chem., 2012, 14, 2091 RSC; (f) B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744 RSC.
- (a) W. E. Truce and G. C. Wolf, J. Org. Chem., 1971, 36, 1727 CrossRef CAS; (b) P. Katrun, C. Mueangkaew, M. Pohmakotr, V. Reutrakul, T. Jaipetch, D. Soorukram and C. Kuhakarn, J. Org. Chem., 2014, 79, 1778 CrossRef CAS PubMed; (c) E. Truce, D. L. Heuring and G. C. Wolf, J. Org. Chem., 1980, 45, 406 CrossRef; (d) L. M. Harwood, M. Julia and G. L. Thuiller, Tetrahedron, 1980, 36, 2483 CrossRef CAS; (e) D. C. Craig, G. L. Edwards and C. A. Muldoon, Synlett, 1977, 1441 Search PubMed.
- Q. Jiang, J. Jia, A. Zhao and C. C. Guo, J. Org. Chem., 2015, 80, 3586 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization of products, and NMR spectral charts. See DOI: 10.1039/c5ra10896a |
| This journal is © The Royal Society of Chemistry 2015 |