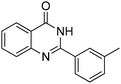
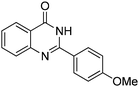
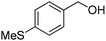
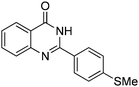
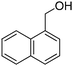
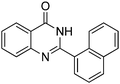
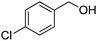
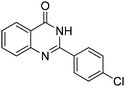
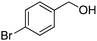
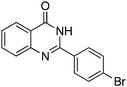
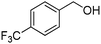
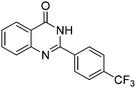
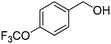
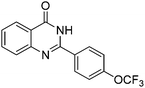
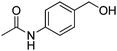
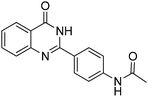
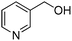
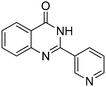
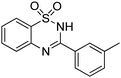
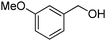
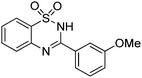
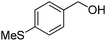
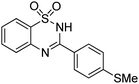
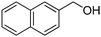
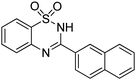
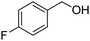
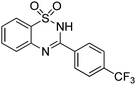
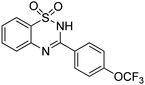
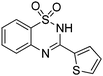
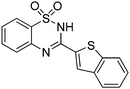
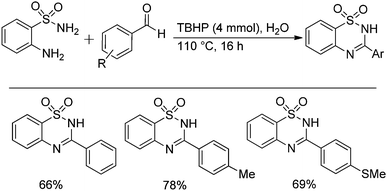
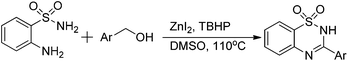Oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols and aldehydes†
Muhammad
Sharif
bd,
Julita
Opalach
bc,
Peter
Langer
bc,
Matthias
Beller
b and
Xiao-Feng
Wu
*ab
aDepartment of Chemistry, Zhejiang Sci-Tech University, Xiasha Campus, Hangzhou, Zhejiang Province, People's Republic of China 310018. E-mail: xiao-feng.wu@catalysis.de
bLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
cUniversität Rostock, Institut für Chemie, Albert-Einstein-Str. 3a, 18059 Rostock, Germany
dDepartment of Chemistry, Comsats Institute of Information Technology, 22060, Abbottabad, Pakistan
First published on 30th October 2013
Abstract
An interesting procedure for the zinc-catalyzed oxidative transformation of ready available 2-aminobenzamide, 2-aminobenzenesulfonamide with benzyl alcohols has been developed. Various quinazolinones and benzothiadiazine 1,1-dioxides were prepared in moderate to good yields under identical conditions. The reactions of both aromatic aldehydes and aliphatic aldehydes with 2-aminobenzamide under catalyst free conditions were described as well. In water media, the products were formed in good yields.
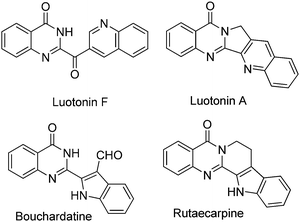
Heterocyclic compounds synthesis is one of the main branches of organic synthesis, due to the recognized importance of heterocycles in natural products, advanced materials, crop protecting agents, and pharmaceuticals.1 Among the numerous known heterocyclic compounds, quinazolinones (Scheme 1) and benzothiadiazine 1,1-dioxides are attractive frameworks because of their reported anticancer, antiviral, anti-inflammatory, as well as anti-microbial activity properties.2 Moreover, quinazolinones are used as ligands for benzodiazepine and AMPA receptors in the CNS system or as DNA binders as well.3 Regarding their prevalence, plentiful methodologies have been developed for heterocycles preparation, cascade reaction, domino reaction, multicomponent reaction are representative examples. For the synthesis of quinazolinones and benzothiadiazine 1,1-dioxides,4 the reaction of carboxylic acid derivatives (such as benzoic acids, benzoyl chlorides and etc.) with 2-aminobenzamide and 2-aminobenzenesulfonamide are the typical procedures.5 The oxidation of 2-amido benzonitriles are also known and some other methodologies were developed as well.6 More recently, the reaction of 2-aminobenzamide or 2-aminobenzenesulfonamide with benzyl alcohols come into the view of synthetic chemists and Pd, Ru or Ir are the usual applied catalysts.7 Remarkably, in 2013, an iodine-catalyzed one-pot two-step oxidative synthesis of quinazolinones from alcohols and 2-aminobenzamide was reported.7d The reaction using DMSO as oxidant, using DMC as solvent at 100 °C, the reaction via the oxidation of alcohols to aldehydes as the key step and then followed by cyclization to give quinazolinones.
We recently reported a zinc-catalyzed oxidative amidation of benzyl alcohols, numbers of amides were prepared by using TBHP as oxidant.8 In view of copious advantages of zinc catalysis9 and our incessant research interests in developing zinc-catalyzed oxidation reactions,10 we became interested in applying our procedure in the direct oxidative synthesis of quinazolinones and benzothiadiazine 1,1-dioxides from 2-aminobenzamide and 2-aminobenzenesulfonamide with benzyl alcohols. As we expected, various desired products were prepared in good to excellent yields under our conditions in one-pot one-step manner.
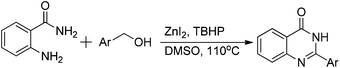
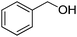
Based on our previous report,8 the initial experiment was carried out in H2O (2 mL) at 110 °C. But no product was produced with 23% conversion of benzyl alcohol in the presence of ZnI2 (10 mol%) and TBHP (70% in H2O; 4 eq.). 10% of quinazolinone was formed in toluene with 41% of benzyl alcohol converted under the same conditions, while only 5% yield was resulted in DMF. To our delight, 95% of our desired product was formed by using DMSO as solvent. With decreased temperature (80 °C) in DMSO, the yield of quinazolinone drops dramatically and lot of N-(2-carbamoylphenyl)benzamide was isolated.
With the best reaction conditions in hand, we carried out the generality of this methodology with different benzyl alcohols (Table 1 and 2). Methyl-, methoxy-, methylthio-substituted benzyl alcohols were successfully converted and gave the corresponding products in 73–85% yields (Table 1, entries 2–4). Naphthyl substituted quinazolinones were produced in 69–71% yield under the same conditions (Table 1, entries 5 and 6). Additionally, halogen-substituted and electron-withdrawing group-substituted benzyl alcohols can be reacted as well and the desired products were isolated in 63–76% yields (Table 1, entries 7–12). Notably, moderate to good yields of heterocycle decorated quinazolinones were prepared under identical conditions as well (Table 1, entries 13–17). 2-Aminobenzylamine was tested as substrate as well, but only traces of desired quinazolinone was detected and together with diverse by-products.
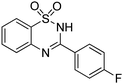
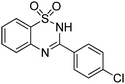
Besides quinazolinones, benzothiadiazine 1,1-dioxides can be produced by simply replacing 2-aminobenzamide with 2-aminobenzenesulfonamide. 13 examples of benzothiadiazine 1,1-dioxides with different substituents were isolated in good yields (Table 2). Both electron-donating and electron-withdrawing functional groups were tolerable, as well as heterocyclic compounds.
Regarding the reaction pathway, we believe N-(2-carbamoylphenyl)benzamide or N-(2-sulfamoylphenyl)benzamide should be the key intermediate which could also be prepared by the reaction of 2-aminobenzamide with aldehydes under even catalyst free conditions. Here, notably, a InCl3-catalyzed condensation of aromatic aldehydes with 2-aminobenzamides to quinazolinones was reported in 2012.11 The reaction works at room temperature, but need MeCN as the organic solvent. As the demands of sustainable development, we think it's interesting and necessary to develop a more general catalyst free system for the quinazolinones synthesis.12,13
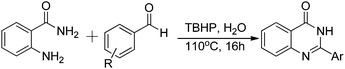
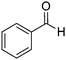
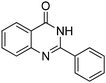
By simply changing the solvent from DMSO to water, excellent yield of quinazolinone was isolated under a catalyst free condition. As shown in Table 3, ortho-, para-, and meta-alkyl substituted benzaldehydes were successfully reacted with 2-aminobenzamide and gave the corresponding products in good yields (Table 3, entries 2–4). Methoxyl and methylthio can be tolerated as well (Table 3, entries 5 and 6). 2-Naphthyl substituted quinazolinones were prepared in good yields (Table 3, entries 7 and 8). Several halogen and electron-withdrawing functional groups are tolerable as well and gave the desired products in good to excellent yields (Table 3, entries 9–14).
| Entry | Aldehyde | Product | Yieldb[%] |
|---|---|---|---|
| a 2-Aminobenzamide (1 mmol), aldehydes (1 mmol), H2O (2 mL), TBHP (70% in water, 4 mmol), 110 °C, 16 h. b Isolated yields. | |||
| 1 |
|
|
90% |
| 2 |
|
|
60% |
| 3 |
|
|
75% |
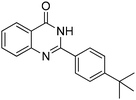
| 4 |
|
|
78% |
| 5 |
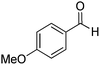
|
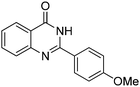
|
83% |
| 6 |
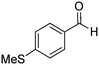
|
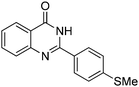
|
70% |
| 7 |
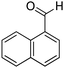
|
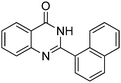
|
74% |
| 8 |
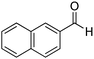
|
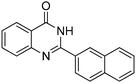
|
76% |
| 9 |
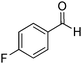
|
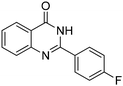
|
88% |
| 10 |
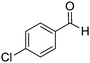
|
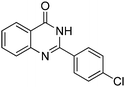
|
73% |
| 11 |
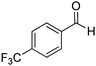
|
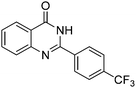
|
77% |
| 12 |
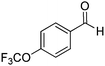
|
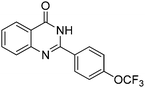
|
71% |
| 13 |
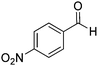
|
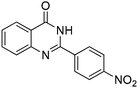
|
55% |
| 14 |
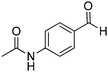
|
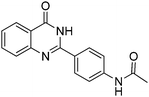
|
68% |
| 15 |
|
|
68% |
| 16 |
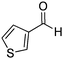
|
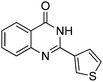
|
80% |
| 17 |
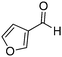
|
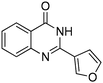
|
70% |
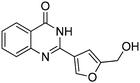
| 18 |
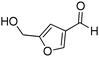
|
|
69% |
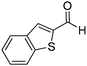
| 19 |
|
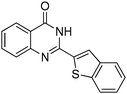
|
69% |
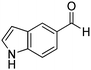
| 20 |
|
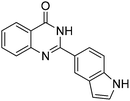
|
65% |
| 21 |
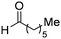
|
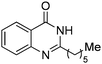
|
88% |
| 22 |
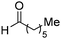
|
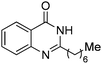
|
83% |
| 23 |
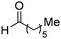
|
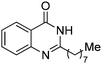
|
80% |
| 24 |
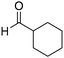
|
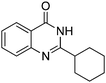
|
62% |
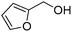
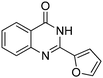
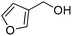
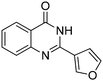
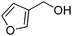
Several heterocyclic aldehydes were applied as substrates because of the interesting biological activities of heterocycles, the corresponding 2-heterocyclic substituted quinazolinones were synthesized straightforward in good yields (Table 3, entries 15–20). Remarkably, even 5-(hydroxymethyl)furan-3-carbaldehyde can be applied as starting material as the hydroxymethyl group is potentially reactive under oxidative conditions (Table 3, entry 18). Additionally, aliphatic aldehydes were reacted with 2-aminobenzamide as well and gave the corresponding alkyl-substituted products in good yields (62–88%; Table 3, entries 21–24) which are difficult in previous methodology.
Besides the preparation of quinazolinone derivatives, this green methodology can also be applied in the synthesis of benzothiadiazine 1,1-dioxides (Scheme 2). Three examples of benzothiadiazine 1,1-dioxides were produced in good yields under the same conditions. Here, the cyclization step may responsible for the need of relative high temperature, which could be decreased by the assistant of Lewis acid.
In conclusion, an interesting methodology for quinazolinones and benzothiadiazine 1,1-dioxides preparation has been developed. Under the assistant of ZnI2/TBHP, various of the desired heterocycles were isolated in good to excellent yields. By using aldehydes instead of benzyl alcohols as substrates, the reactions can be carried out under catalyst free conditions in water. All the products were prepared in good yields.
Experimental section
General comments
All reactions were carried out under air. Reactions were monitored by TLC analysis (pre-coated silica gel plates with fluorescent indicator UV254, 0.2 mm) and visualized with 254 nm UV light or iodine. Chemicals were purchased from Aldrich, Alfa Asar and unless otherwise noted were used without further purification. All compounds were characterized by 1H NMR, 13C NMR, GC-MS and HRMS spectroscopy. 1H spectra were recorded on Bruker AV 300 and AV 400 spectrometers. 13C NMR spectra were recorded at 282 MHz. EI (70 eV) mass spectra were recorded on MAT 95XP (Thermo ELECTRON CORPORATION). GC was performed on Agilent 6890 chromatograph with a 30 m HP5 column. HRMS was performed on MAT 95XP (EI) and Agilent 6210 Time-of-Flight LC/MS (ESI). GC-MS was performed on Agilent 5973 chromatograph Mass Selective Detector. All yields reported refer to isolated yields.General procedure for the oxidative synthesis of quinazolinone
ZnI2 (10 mol%) and a stirring bar were added to a 50 mL pressure tube. Then, DMSO (2 mL), benzyl alcohol (1 mmol), and 2-aminobenzamide (1 mmol) were added. At the end, TBHP (70% in H2O; 4 eq.) was added and the final solution was kept at 110 °C temperature for 16 h. The mixture was cooled to room and solvent was removed under vacuum. The pure product can be isolated by either washed with water, ethyl acetate and finally hexane or recrystallized from MeOH.General procedure for the catalyst free synthesis of quinazolinone
In a 25 mL pressure tube equipped with a stirring bar, aldehyde (1 mmol), 2-aminobenzamide (1 mmol), H2O (2 mL), and tert-butyl peroxide (70% in H2O; 4 mmol) were injected by syringe. Then tube was closed and heated up to 110 °C for 16 hours. When the reaction completed, cool the reaction mixture to room temperature. The pure product can be isolated by either simply filtration or recrystallized from MeOH.2-Phenylquinazolin-4(3H)-one
Yield: (200 mg, 91%); 1H NMR (300 MHz, DMSO-d6): δ = 7.53–7.64 (m, 4H), 7.76–7.90 (m, 2H), 8.17–8.23 (m, 3H), 12.6 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 121.9 (C), 126.8 (CH), 127.5 (CH), 128.4 (C), 128.7 (2CH), 129.5 (2CH), 132.3 (CH), 133.6 (C), 135.5 (CH), 149.6 (C), 153.3 (C), 163.2 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 222 (85), 119 (100), 104 (10), 92 (13), 90 (13), 77 (20). HRMS (ESI): calc. for C14H10N2O1: 222.07876; found: 222.07887.2-(4-Methoxyphenyl)quinazolin-4(3H)-one
Yield: (201 mg, 80%); 1H NMR (300 MHz, CDCl3): δ = 3.76 (s, 3H), 8.26–8.30 (m, 1H), 8.60–8.72 (m, 2H), 8.91–9.02 (m, 1H), 9.07–9.10 (m, 1H), 9.44–9.49 (1H), 12.5 (s, 1H, NH2). 13C NMR (CDCl3): δ = 56.3 (OCH3), 113.4 (CH), 118.5 (CH), 121.6 (CH), 126.7 (CH), 127.5 (CH), 129.1 (CH), 130.6 (C), 130.8 (C), 134.9 (CH), 135.5 (C), 138.1 (C), 149.1 (CH), 160.2 (C), 163.1 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 252 (100), 251 (93), 223 (19), 222 (40), 221 (18), 119 (31), 91 (18), 90 (16). HRMS (EI): calc. for C15H12N2O2: 252.08933; found: 252.08895.2-(4-(Methylthio)phenyl)quinazolin-4(3H)-one
Yield: (195 mg, 73%); 1H NMR (300 MHz, DMSO-d6): δ = 2.56 (s, 3H), 7.35–7.40 (m, 2H), 7.81–7.92 (m, 5H), 8.31–8.42 (m, 1H), 12.8 (s, 1H, NH).13C NMR (CDCl3): δ = 15.4 (SCH3), 124.8 (2CH), 126.0 (CH), 126.8 (CH), 127.9 (CH), 128.6 (CH), 129.0 (2CH), 129.5 (C), 135.7 (C), 149.5 (CH), 150.6 (C), 152.5 (C), 163.1 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 268 (100), 119 (78), 92 (10), 90 (11). HRMS (ESI): calc. for C15H12N2O1S: 268.06649; found: 268.06631.2-(m-Tolyl)quinazolin-4(3H)-one
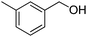
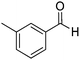
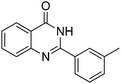
Yield: (200 mg, 85%); 1H NMR (300 MHz, DMSO-d6): δ = 2.45 (s, 3H, CH3), 7.44–7.59 (m, 3H), 7.76–7.79 (m, 1H), 7.81–7.90 (m, 1H), 7.99–8.03 (m, 1H), 8.06 (s, 1H), 8.17–8.21 (m, 1H), 12.6 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 20.9 (CH3), 120.9 (C), 124.8 (CH), 125.8 (CH), 126.5 (CH), 127.4 (CH), 128.2 (CH), 128.5 (CH), 131.9 (CH), 132.6 (C), 134.6 (CH), 137.9 (C), 148.7 (C), 152.3 (C), 162.2 (CO).GC-MS (EI, 70 eV): m/z (%) [M+] 236 (80), 119 (100), 92 (13), 90 (15). HRMS (ESI): calc. for C15H12N2O1: 236.09441; found: 236.09433.2-(4-Fluorophenyl)quinazolin-4(3H)-one
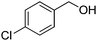
Yield: (190 mg, 79%); 1H NMR (300 MHz, DMSO-d6): δ = 7.38–7.76 (m, 2H), 7.52–7.58 (m, 1H), 7.74–7.78 (m, 1H), 7.84–7.89 (m, 1H), 8.16–8.19 (m, 1H), 8.25–8.32 (m, 1H), 12.6 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 120.3 (2CH), 120.8 (2CH), 125.8 (CH), 130.8 (C), 131.5 (C), 132.2 (CH), 134.2 (CH), 135.4 (C), 139.5 (CH), 156.3 (C), 167.3 (CO), 168 (d, J = 249.8 Hz, CF3). GC-MS (EI, 70 eV): m/z (%) [M+] 240 (100), 122 (13), 120 (12), 119 (93), 95 (18), 92 (15), 90 (14). HRMS (ESI): calc. for C14H9N2O1F1: 240.06934; found: 240.06911.2-(4-Chlorophenyl)quinazolin-4(3H)-one
Yield: (161 mg, 63%); 1H NMR (300 MHz, DMSO-d6): δ = 7.35–7.43 (m, 1H), 7.54–7.60 (m, 1H), 7.64–7.69 (m, 2H), 8.18–8.27 (m, 2H), 12.6 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 115.1 (C), 116.1 (CH), 123.4 (CH), 128.4 (CH), 129.7 (2CH), 131.9 (2CH), 133.6 (C), 135.6 (C), 136.1 (CH), 140.7 (C), 150.9 (C), 163.0 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 256 (73), 119 (100), 111 (11), 92 (14), 90 (14), 75 (12). HRMS (ESI): calc. for C15H9N2O1Cl: 256.03979; found: 256.03921.2-(4-(Trifluoromethyl)phenyl)quinazolin-4(3H)-one
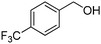
Yield: (203 mg, 70%); 1H NMR (300 MHz, DMSO-d6): δ = 7.57–7.63 (m, 1H), 7.80–7.98 (m, 4H), 8.19–8.23 (m, 1H), 8.39–8.42 (m, 2H), 12.8 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 122.2 (C), 123.0 (C), 126.4 (2CH), 126.9 (CH), 128.1 (CH), 128.6 (CH), 129.7 (2CH), 131.8 (C), 135.7 (CH), 137.5 (C), 149.3 (C), 152.2 (C), 163.1 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 290 (100), 145 (23), 119 (99), 92 (17), 90 (16). HRMS (ESI): calc. for C15H9N2O1F3: 290.06615; found: 290.06587.2-(4-(Trifluoromethoxy)phenyl)quinazolin-4(3H)-one
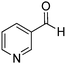
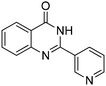
Yield: (232 mg, 70%); 1H NMR (300 MHz, DMSO-d6): δ = 7.55–7.60 (m, 3H), 7.76–7.79 (m, 1H), 7.86–7.91 (m, 1H), 8.18–8.22 (m, 1H), 8.32–8.36 (m, 2H), 12.7 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 121.7 (2CH), 121.9 (C), 122.6 (C), 126.8 (CH), 126.5 (d, J = 259.7 Hz, OCF3), 128.4 (CH), 131.0 (2CH), 132.9 (C), 135.6 (CH), 149.5 (C), 151.3 (C), 152.3 (C), 163.2 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 306 (100), 119 (92), 92 (17), 90 (14). HRMS (ESI): calc. for C15H9N2O2F3: 306.06106; found: 306.06090.2-(Pyridin-3-yl)quinazolin-4(3H)-one
Yield: (150 mg, 68%); 1H NMR (300 MHz, DMSO-d6): δ = 7.56–7.66 (m, 2H), 7.79–7.83 (m, 1H), 7.87–7.93 (m, 1H), 8.19–8.23 (m, 1H), 8.51–8.56 (m, 1H), 8.78 (d, J = 4.95 Hz, 1H), 9.34 (s, 1H), 12.8 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 121.9 (C), 124.2 (CH), 126.6 (CH), 127.6 (CH), 128.3 (C), 129.5 (C), 135.4 (CH), 136.1 (CH), 149.2 (CH), 151.4 (CH), 152.5 (C), 162.9 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 223 (90), 119 (100), 92 (18), 90 (13), 78 (11). HRMS (ESI): calc. for C13H9N3O1: 223.07401; found: 223.07411.2-(Thiophen-2-yl)quinazolin-4(3H)-one
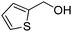
Yield: (164 mg, 72%); 1H NMR (300 MHz, DMSO-d6): δ = 7.49–7.56 (m, 1H), 7.71–7.76 (m, 2H), 7.82–7.93 (m, 2H), 8.15–8.19 (m, 1H), 8.64–8.65 (m, 1H), 12.5 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 120.9 (C), 125.8 (CH), 126.3 (CH), 127.0 (CH), 127.2 (CH), 127.3 (CH), 128.6 (CH), 134.5 (CH), 135.3 (C), 148.2 (C), 148.8 (C), 162.0 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 228 (100), 119 (59), 92 (11), 90 (12). HRMS (ESI): calc. for C12H8N2OS1: 228.03519; found: 228.03515.2-(Furan-2-yl)quinazolin-4(3H)-one
Yield: (127 mg, 60%); 1H NMR (300 MHz, DMSO-d6): δ = 6.67–6.80 (m, 1H), 7.49–7.56 (m, 1H), 7.65–7.68 (m, 1H), 7.70–7.74 (m, 1H), 7.81–7.87 (m, 1H), 8.02–8.04 (m, 1H), 8.14–8.18 (m, 1H), 12.4 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 112.5 (CH), 114.4 (CH), 121.0 (C), 125.9 (CH), 126.4 (CH), 127.1 (CH), 134.6 (CH), 144.0 (C), 146.0 (CH), 146.5 (C), 154.5 (C), 161.6 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 212 (100), 211 (17), 90 (10). HRMS (ESI): calc. for C12H8N2O2: 212.05803; found: 212.05760.2-(Furan-3-yl)quinazolin-4(3H)-one
Yield: (142 mg, 67%); 1H NMR (300 MHz, DMSO-d6): δ = 7.54–7.59 (m, 2H), 7.69–7.73 (m, 1H), 7.83–7.89 (m, 1H), 8.09–8.19 (m, 3H), 12.3 (s, 1H, NH). GC-MS (EI, 70 eV): m/z (%) [M+] 212 (100), 211 (17), 90 (10). HRMS (ESI): calc. for C12H8N2O2: 212.05803; found: 212.05760.2-(Benzo[b]thiophen-2-yl)quinazolin-4(3H)-one
Yield: (195 mg, 70%); 1H NMR (300 MHz, DMSO-d6): δ = 7.17–7.25 (m, 1H), 7.44–7.61 (m, 4H), 7.74–7.78 (1H), 7.86–7.97 (2H), 8.08–8.10 (1H), 8.18–8.23 (1H), 12.9 (s, 1H, NH). 13C NMR (DMSO-d6) δ = 114.9, 115.5, 117.7, 123.9, 124.9, 125.0, 125.6, 127.1, 127.9, 133.7, 135.1, 135.8, 140.8, 148.5, 164.3 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 278 (100), 159 (12), 119 (38), 89 (14). HRMS (ESI): calc. for C16H10N2O1S1: 278.05084; found: 278.05082.2-(Naphthalen-1-yl)quinazolin-4(3H)-one
Yield: (188 mg, 69%); 1H NMR (300 MHz, DMSO-d6): δ = 7.60–7.72 (m, 4H), 7.77–7.95 (m, 3H), 8.07–8.11 (m, 1H), 8.19–8.29 (m, 3H), 12.7 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 121.2 (C), 125.0 (CH), 125.2 (CH), 125.8 (CH), 126.3 (CH), 126.7 (CH), 127.0 (CH), 127.4 (CH), 127.6 (CH), 128.3 (CH), 130.0 (CH), 130.4 (C), 133.1 (C), 134.5 (CH), 135.3 (C), 148.7 (C), 161.8 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 272 (92), 153 (14), 127 (27), 119 (100), 92 (15), 90 (10). HRMS (ESI): calc. for C18H12N2O1: 272.09441; found: 272.09424.2-(Naphthalen-2-yl)quinazolin-4(3H)-one
Yield: (194 mg, 75%); 1H NMR (300 MHz, DMSO-d6): δ = 7.42–7.92 (m, 7H), 7.96–8.51 (m, 4H), 12.7 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 121.2 (C), 125.0 (CH), 125.2 (CH), 125.8 (CH), 125.9 (CH), 126.3 (CH), 126.8 (CH), 127.0 (CH), 127.4 (CH), 127.6 (CH), 128.3 (CH), 130.2 (C), 130.3 (C), 133.7 (C), 133.1 (C), 134.5 (CH), 148.7 (C), 153.6 (C), 161.9 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 272 (53), 272 (100).N-(4-(4-Oxo-3,4-dihydroquinazolin-2-yl)phenyl)acetamide
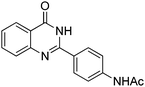
Yield: (209 mg, 75%); 1H NMR (300 MHz, DMSO-d6): δ = 2.13 (s, 3H, CH3), 7.49–7.56 (m, 1H), 7.76–7.89 (m, 4H), 8.14–8.22 (m, 3H), 10.3 (s, 1H, NH), 12.4 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 24.1, 118.3, 120.7, 125.8, 126.2, 126.7, 127.2, 128.5, 134.5, 142.1, 143.9, 151.8, 162.5 (CO), 168.7 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 279 (92), 237 (100), 119 (81), 92 (15), 90 (10), 43 (28). HRMS (ESI): calc. for C16H13N3O2: 279.10023; found: 279.10026.3-Phenyl-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
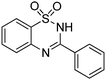
Yield: (222 mg, 86%); 1H NMR (300 MHz, DMSO-d6): δ = 7.35–7.93 (m, 7H), 8.02–8.15 (m, 2H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 119.0 (CH), 122.0 (C), 123.7 (CH), 127.1 (CH), 128.7 (2CH), 129.3 (2CH), 132.3 (C), 133.3 (CH), 135.9 (C), 155.2 (C). GC-MS (EI, 70 eV): m/z (%) [M+] 258 (58), 194 (12), 155 (100), 91 (62), 64 (22). HRMS (ESI): calc. for C13H10N2OS1: 258.04575; found: 258.04581.3-(3-Methoxyphenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (256 mg, 89%); 1H NMR (300 MHz, DMSO-d6): δ = 3.91 (s, 3H, OCH3), 7.29–7.38 (m, 1H), 7.45–7.54 (m, 1H), 7.56–7.61 (m, 2H), 7.74–7.92 (m, 2H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 56.6 (OCH3), 114.3, 118.4, 119.4, 119.5, 121.4, 122.4, 124.3, 124.6, 127.7, 131.0, 134.1, 136.4, 155.5, 160.3. GC-MS (EI, 70 eV): m/z (%) [M+] 288 (74), 287 (17), 155 (100), 91 (58), 63 (17). HRMS (ESI): calc. for C14H12N2O3S1: 288.05631; found: 288.05583.3-(4-(Methylthio)phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (160 mg, 60%); 1H NMR (300 MHz, DMSO-d6): δ = 2.55 (s, 3H, CH3), 7.34–7.38 (m, 1H), 7.47–7.55 (m, 2H), 7.67–7.77 (m, 1H), 7.83–7.91 (m, 2H), 8.02–8.03 (m, 2H), 12.3 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 117.4, 122.4, 123.6, 124.8, 125.0, 126.6, 128.5, 129.6, 133.1, 134.6, 147.6, 154.2.3-(m-Tolyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (240 mg, 88%); 1H NMR (300 MHz, DMSO-d6): δ = 2.44 (s, 3H, CH3), 7.48–7.54 (m, 3H), 7.62–7.66 (m, 1H), 7.71–7.77 (m, 1H), 7.82–7.88 (m, 3H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 21.8 (CH3), 119.3, 122.4, 124.2, 126.3, 127.6, 129.4, 132.7, 134.0, 134.4, 136.4, 139.4, 155.8. GC-MS (EI, 70 eV): m/z (%) [M+] 262 (62), 208 (11), 155 (100), 91 (57), 64 (17). HRMS (ESI): calc. for C14H12N2O2S1: 272.06140; found: 272.06137.3-(4-Fluorophenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (207 mg, 75%); 1H NMR (300 MHz, DMSO-d6): δ = 7.39–7.58 (m, 3H), 7.64–7.68 (m, 1H), 7.74–7.81 (m, 1H), 7.88–7.92 (m, 1H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 116.5 (d, J = 24.5 Hz), 116.8, 122.5, 124.9 (d, J = 33.9 Hz), 128.3, 129.8, 132.9, (d, J = 24.5 Hz), 133.4, 134.4, 165.7, (d, J = 250.3 Hz), 167.3. GC-MS (EI, 70 eV): m/z (%) [M+] 276 (57), 212 (15), 155 (100), 91 (69), 64 (26). HRMS (ESI): calc. for C13H9N2O3S1F1: 276.03633; found: 276.03625.3-(Furan-3-yl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
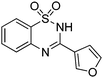
Yield: (188 mg, 76%); 1H NMR (300 MHz, DMSO-d6): δ = 7.13–7.15 (m, 1H), 7.48–7.59 (m, 2H), 7.73–7.79 (m, 1H), 7.84–7.88 (m, 1H), 7.95–7.96 (m, 1H), 11.9 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 109.1, 118.1, 120.6, 121.8, 123.5, 126.7, 133.3, 135.4, 145.4, 147.2, 149.6, 153.1. GC-MS (EI, 70 eV): m/z (%) [M+] 248 (71), 155 (100), 91 (61), 64 (31).N-(4-(1,1-dioxido-2H-benzo[e][1,2,4]thiadiazin-3-yl)phenyl)acetamide
Yield: (211 mg, 67%); 1H NMR (300 MHz, DMSO-d6): δ = 2.06 (s, 3H, CH3), 7.23–7.27 (m, 1H), 7.34–7.38 (m, 1H), 7.47–7.57 (m, 3H), 7.68–7.75 (m, 1H), 7.83–7.88 (m, 1H), 8.01–8.03 (m, 1H), 9.91 (s, 1H, NH), 12.3 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 24.7, 118.3, 119.4, 123.3, 124.4, 127.4, 127.6, 133.9, 135.4, 137.8, 138.7, 148.4, 156.8 (CO), 168.9 (CO).3-(4-Chlorophenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (201 mg, 69%); 1H NMR (300 MHz, DMSO-d6): δ = 7.16-7.59 (m, 3H), 7.70–7.92 (m, 3H), 8.03–8.15 (m, 2H), 12.3 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 118.4, 121.4, 123.3, 123.6, 126.8, 128.9, 130.1, 133.1, 135.4, 137.7, 147.6, 153.6. GC-MS (EI, 70 eV): m/z (%) [M+] 292 (44), 228 (11), 155 (100), 91 (57), 64 (22), 63 (16). HRMS (ESI): calc. for C13H9N2O2S1Cl: 292.00733; found: 292.00741.3-(4-(Trifluoromethyl)phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (270 mg, 79%); 1H NMR (300 MHz, DMSO-d6): δ = 7.13–7.34 (m, 1H), 7.53–7.67 (m, 2H), 7.76–7.82 (m, 1H), 8.05 (d, J = 7.98 Hz, 2H), 8.29 (d, J = 7.98 Hz, 2H), 12.4 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 115.8, (d, J = 12.3 Hz), 118.5, 121.4, 123.4, 123.7, 125.1 (d, J = 270.1 Hz), 127.0, 129.2, 132.7, 133.2, 135.3, 153.4. GC-MS (EI, 70 eV): m/z (%) [M+] 326 (55), 307 (10), 262 (10), 155 (100), 91 (79), 64 (22). HRMS (ESI): calc. for C14H9N2O2S1F3: 326.03313; found: 326.03281.3-(4-(Trifluoromethoxy)phenyl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (284 mg, 83%); 1H NMR (300 MHz, DMSO-d6): δ = 7.43–7.7.69 (m, 4H), 8.08–8.22 (m, 4H), 13.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 119.4, 121.9, 122.4, 124.3, 126.2 (d, J = 263.3 Hz), 127.8 (dd, J = 90.3 Hz), 130.2, 131.7, 132.6, 134.1, 136.3, 153.3 (d, J = 170.4 Hz), 154.5. GC-MS (EI, 70 eV): m/z (%) [M+] 342 (39), 326 (24), 155 (100), 138 (10), 91 (77), 64 (26). HRMS (ESI): calc. for C14H9N2O3S1F3: 342.02805; found: 342.02803.3-(Thiophen-2-yl)-2H-benzo[e][1,2,4]thiadiazine 1,1-dioxide
Yield: (169 mg, 64%); 1H NMR (300 MHz, DMSO-d6): δ = 7.24–7.27 (m, 1H), 7.49–7.55 (m, 1H), 7.60–7.65 (m, 1H), 7.73–7.78 (m, 1H), 7.81–7.82 (m, 1H), 7.86–7.89 (m, 1H), 8.60–8.62 (m, 1H), 12.0 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 119.1, 122.5, 124.3, 127.1, 127.7, 128.5, 129.1, 132.8, 134.1, 136.3, 151.0. GC-MS (EI, 70 eV): m/z (%) [M+] 264 (79), 207 (20), 155 (100), 138 (11), 91 (64), 64 (30). HRMS (ESI): calc. for C11H8N2O2S2: 264.00217; found: 264.00211.2-(4-(tert-Butyl)phenyl)quinazolin-4(3H)-one
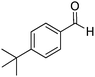
Yield: (216 mg, 78%); 1H NMR (300 MHz, DMSO-d6): δ = 1.35 (s, 9H), 7.50–7.61 (m, 3H), 7.73–7.88 (m, 2H), 8.14–8.21 (m, 2H), 8.46 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 31.7 (3CH3), 35.35 (C), 121.8 (C), 126.3 (2CH), 126.7 (CH), 127.2 (CH), 128.2 (CH), 128.4 (2CH), 130.8 (C), 135.4 (CH), 149.6 (C), 153.1 (C), 155.2 (C), 163.2 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 278 (85), 263 (100).2-(2,6-Dimethylphenyl)quinazolin-4(3H)-one
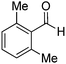
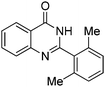
Yield: (125 mg, 60%); 1H NMR (300 MHz, DMSO-d6): δ = 2.19 (s, 6H, 2CH3), 7.63 (d, J = 7.63 Hz, 1H); 7.18–7.24 (m, 1H), 7.34 (t, J = 7.35 Hz, 1H); 7.59 (d, J = 7.63 Hz, 1H); , 7.72 (d, J = 7.91 Hz, 1H); 7.88 (d, J = 7.35 Hz, 1H), 8.21 (d, J = 7.63 Hz, 2H); 12.5 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 19.8 (2CH3), 121.9 (C), 126.6 (CH), 127.5 (CH), 128.2 (2CH), 129.4 (CH), 129.9 (CH), 135.3 (CH), 135.4 (C), 136.2 (C), 141.9 (C), 149.6 (C), 151.8 (C), 162.5 (CO).2-(4-Nitrophenyl)quinazolin-4(3H)-one
Yield: (173 mg, 65%); 1H NMR (300 MHz, DMSO-d6): δ = 6.72 (t, J = 7.63 Hz, 1H), 6.80 (d, J = 8.52 Hz, 1H), 7.27–7.33 (m, 1H), 7.37 (s, 1H), 7.63–7.66 (m, 1H), 7.78 (dt, J = 8.62 Hz, 1H), 8.29 (dt, J = 8.62 Hz, 2H), 8.56 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 115.5, 115.8, 118.4, 124.5, 128.3, 128.9, 134.5, 148.2, 148.3, 150.3, 164.3(CO). GC-MS (EI, 70 eV): m/z (%) [M+] 267 (100), 221 (32), 192 (13), 119 (36), 92 (8), 90 (11).2-(5-(Hydroxymethyl)furan-3-yl)quinazolin-4(3H)-one
Yield: (166 mg, 69%); 1H NMR (300 MHz, DMSO-d6): δ = 4.55 (s, 2H, OCH2), 5.52 (s, 1H, OH), 6.61 (d, J = 3.43 Hz, 1H), 7.49–7.55 (m, 1H), 7.63 (d, J = 3–52 Hz, 1H), 7.71–7.76 (m, 1H), 7.81–7.87 (m, 1H), 8.13.8.17 (m, 1H), 12.5 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 56.7 (OCH2), 110.3 (CH), 115.3 (CH), 122.2 (C), 126.8 (CH), 127.3 (CH), 128.1 (CH), 135.6 (CH), 144.9 (C), 145.9 (C), 149.7 (C), 160.3 (C), 162.5 (CO).2-Hexylquinazolin-4(3H)-one
Yield: (202 mg, 88%); 1H NMR (300 MHz, DMSO-d6): δ = 0.87 (t, J = 7.30 Hz, 3H), 1.23–1.39 (m, 6H), 1.74 (Pent. 2H), 2.61 (t, J = 7.63 Hz, 2H), 7.44–7.49 (m, 1H), 7.60–7.63 (m, 1H), 7.78–7.81 (m, 1H), 8.09–8.13 (m, 1H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 14.8 (CH3), 22.9 (CH2), 27.7 (CH2), 29.1 (CH2), 31.8 (CH2), 35.4 (CH2), 121.7 (C), 126.6 (CH), 126.8 (CH), 127.7 (CH), 135.1 (CH), 149.9 (C), 158.4 (C), 162.8 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 230 (5), 187 (11), 160 (100). HRMS (ESI): calc. for C14H18N2O1: 230.14136; found: 230.14113.2-Heptylquinazolin-4(3H)-one
Yield: (202 mg, 83%); 1H NMR (300 MHz, DMSO-d6): δ = 0.87 (t, J = 7.30 Hz, 3H), 1.23–1.39 (m, 8H), 1.74 (Pent. 2H), 2.61 (t, J = ,7.63 Hz, 2H), 7.44–7.49 (m, 1H), 7.60–7.63 (m, 1H), 7.78–7.81 (m, 1H), 8.09–8.13 (m, 1H), 12.2 (s, 1H, NH). 13C NMR (DMSO-d6): δ = 14.8 (CH3), 23.0 (CH2), 27.8 (CH2), 29.3 (CH2), 29.5 (CH2), 32.1 (CH2), 35.5 (CH2), 121.7 (C), 126.6 (CH), 126.8 (CH), 127.7 (CH), 135.1 (CH), 149.9 (C), 158.8 (C), 163.1 (CO). GC-MS (EI, 70 eV): m/z (%) [M+] 244 (5), 187 (10), 160 (100). HRMS (ESI): calc. for C15H20N2O1: 244.15701; found: 244.15698.2-Cyclohexylquinazolin-4(3H)-one
Yield: (141 mg, 62%); 1H NMR (300 MHz, DMSO-d6): δ = 1.21–1.40 (m, 3H), 1.54–1.73 (3H), 1.79–1.96 (4H), 7.47 (t, J = 7.45 Hz, 1H), 7.63 (d, J = 8.40 Hz, 1H), 7.79 (d, J = 7.45 Hz, 1H), 8.11 (d, J = 8.40 Hz, 1H), 12.1 (s, 1H, NH). 3C NMR (DMSO-d6): δ = 26.3 (CH2), 26.5 (2CH2), 31.2 (2CH2), 43.8 (CH), 121.9 (C), 126.6 (CH), 126.8 (CH), 127.9 (CH), 135.1 (CH), 149.9 (C), 161.7 (C), 162.9 (CO).Notes and references
- For selected reviews on this topic, see: (a) D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 2007, 36, 1095–1108 RSC; (b) B. Willy and T. J. J. Müller, Curr. Org. Chem., 2009, 13, 1777–1790 CrossRef CAS; (c) L. F. Tietze, G. Brasche and K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006 Search PubMed; (d) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS PubMed; (e) X.-F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113, 1–35 CrossRef CAS PubMed; (f) J. Alvarez-Builla, J. J. Vaquero and J. Barluenga, Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011 Search PubMed; (g) B. Heller and M. Hapke, Chem. Soc. Rev., 2007, 36, 1085–1094 RSC; (h) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269–10310 CrossRef CAS PubMed.
- (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787–9826 CrossRef CAS PubMed; (b) S. Sinha and M. Srivastava, Prog. Drug Res., 1994, 43, 143–238 CAS; (c) S. E. de Laszlo, C. S. Quagliato, W. J. Greenlee, A. A. Patchett, R. S. L. Chang, V. J. Lotti, T.-B. Chen, S. A. Scheck, K. A. Faust, S. S. Kivlighn, T. S. Schorn, G. J. Zingaro and P. K. S. Siegl, J. Med. Chem., 1993, 36, 3207–3210 CrossRef CAS; (d) N. J. Liverton, D. J. Armstrong, D. A. Claremon, D. C. Remy, J. J. Baldwin, R. J. Lynch, G. Zhang and R. J. Gould, Bioorg. Med. Chem. Lett., 1998, 8, 483–486 CrossRef CAS; (e) W. Zhang, J. P. Mayer, S. E. Hall and J. A. Weigel, J. Comb. Chem., 2001, 3, 255–256 CrossRef CAS; (f) A. Gopalsamy and H. Yang, J. Comb. Chem., 2000, 2, 378–381 CrossRef CAS PubMed; (g) A. Scozzofava, T. Owa, A. Mastrolorenzo and C. T. Supuran, Curr. Med. Chem., 2003, 10, 925–953 CrossRef; (h) A. Scozzafava, A. Casini and C. T. Supuran, Curr. Med. Chem., 2002, 9, 1167–1185 CrossRef CAS; (i) S. Khelili, N. Kihal, M. Yekhlef, P. de Tullio, P. Lebrun and B. Pirotte, Eur. J. Med. Chem., 2012, 54, 873–878 CrossRef CAS PubMed; (j) P. de Tullio, A.-C. Servais, M. Fillet, F. Gillotin, F. Somers, P. Chiap, P. Lebrun and B. Pirotte, J. Med. Chem., 2011, 54, 8353–8361 CrossRef CAS PubMed.
- (a) D. Arora, H. Kumar, D. Malhotra and M. Malhotra, Pharmacologyonline, 2011, 3, 659–668 Search PubMed; (b) N. Malecki, P. Carato, G. Rigo, J. F. Goossens, R. Houssin, C. Bailly and J. P. Henichart, Bioorg. Med. Chem., 2004, 12, 641–647 CrossRef CAS PubMed.
- For a review on quinazolinones synthesis, see: D. J. Connolly, D. Cusack, T. P. O'Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153–10202 CrossRef CAS PubMed.
- For selected examples, see: (a) T. M. Potewar, R. N. Nadaf, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Synth. Commun., 2005, 35, 231–241 CrossRef CAS PubMed; (b) Y. Imai, S. Sato, R. Takasawa and M. Ueda, Synthesis, 1981, 35–36 CrossRef CAS; (c) Y.-P. Zhu, Z. Fei, M.-C. Liu, F.-C. Jia and A.-X. Wu, Org. Lett., 2013, 15, 378–381 CrossRef CAS PubMed; (d) K. C. Majumdar and S. Ganai, Beilstein J. Org. Chem., 2013, 9, 503–509 CrossRef CAS PubMed; (e) S. Hirota, R. Kato, M. Suzuki, Y. Soneta, T. Otani and T. Saito, Eur. J. Org. Chem., 2008, 2075–2083 CrossRef CAS; (f) A. Rolfe and P. R. Hanson, Tetrahedron Lett., 2009, 50, 6935–6937 CrossRef CAS PubMed; (g) S. Hirota, T. Sakai, N. Kitamura, K. Kubokawa, N. Kutsumura, T. Otani and T. Saito, Tetrahedron, 2010, 66, 653–662 CrossRef CAS PubMed; (h) C. Blackburn, A. Achab, A. Elder, S. Ghosh, J. Guo, G. Harriman and M. Jones, J. Org. Chem., 2005, 70, 10206–10209 CrossRef CAS PubMed; (i) O. B. Pawar, F. R. Chavan, S. S. Sakate and B. D. Shinde, Chin. J. Chem., 2010, 28, 69–71 CrossRef CAS; (j) M. M. Heravi, N. Tavakoli-Hoseini and F. F. Bamoharram, Synth. Commun., 2011, 41, 707–714 CrossRef CAS; (k) X.-S. Wang, K. Yang, M.-M. Zhang and C.-S. Yao, Synth. Commun., 2010, 40, 2633–2646 CrossRef CAS; (l) N. Tavakoli-Hoseini and A. Davoodnia, Synth. React. Inorg. Met.-Org. Chem., 2012, 42, 76–81 CrossRef CAS; (m) J. Hanusek, M. Sedlak, P. Simunek and V. Sterba, Eur. J. Org. Chem., 2002, 1855–1863 CrossRef CAS.
- For selected examples, see: (a) B. Baudoin, Y. Ribeill and N. Vicker, Synth. Commun., 1993, 23, 2833–2837 CrossRef CAS; (b) B. P. Bandgar, Synth. Commun., 1997, 27, 2065–2068 CrossRef CAS; (c) M. Dabiri, M. Baghbanzadeh and A. S. Delbari, J. Comb. Chem., 2008, 10, 700–703 CrossRef CAS PubMed; (d) H. B. Jalani, A. N. Pandya, D. H. Panday, J. A. Sharma, V. Sudarsanam and K. K. Vasu, Tetrahedron Lett., 2010, 53, 4062–4064 CrossRef PubMed; (e) L. Xu, Y. Jiang and D. Ma, Org. Lett., 2012, 14, 1150–1153 CrossRef CAS PubMed; (f) A. V. Lygin and A. de Meijere, Org. Lett., 2009, 11, 389–392 CrossRef CAS PubMed; (g) B. Ma, Y. Wang, J. Peng and Q. Zhu, J. Org. Chem., 2011, 76, 6362–6366 CrossRef CAS PubMed; (h) J. E. R. Sadig, R. Foster, F. Wakenhut and M. C. Willis, J. Org. Chem., 2012, 77, 9473–9486 CrossRef CAS PubMed.
- (a) A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, Org. Biomol. Chem., 2012, 12, 240–243 RSC; (b) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730–7736 CrossRef CAS PubMed; (c) H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046–7051 CrossRef CAS PubMed; (d) W. Ge, X. Zhu and Y. Wei, RSC Adv., 2013, 3, 10817–10822 RSC.
- X.-F. Wu, M. Sharif, A. Pews-Davtyan, P. Langer, K. Ayub and M. Beller, Eur. J. Org. Chem., 2013, 2783–2787 CrossRef CAS.
- For reviews on zinc-catalyzed organic reactions, see: (a) X.-F. Wu, Chem. Asian J., 2012, 7, 2502–2509 CrossRef CAS PubMed; (b) X.-F. Wu and H. Neumann, Adv. Synth. Catal., 2012, 354, 3141–3160 CrossRef CAS; (c) S. Enthaler, ACS Catal., 2013, 3, 150–158 CrossRef CAS.
- (a) X.-F. Wu, Chem.–Eur. J, 2012, 18, 8912–8915 CrossRef CAS PubMed; (b) X.-F. Wu, Tetrahedron Lett., 2012, 53, 3397–3399 CrossRef CAS PubMed; (c) X.-F. Wu, Tetrahedron Lett., 2012, 53, 4328–4331 CrossRef CAS PubMed; (d) Z.-Z. Song, J.-L. Gong, M. Zhang and X.-F. Wu, Asian J. Org. Chem., 2012, 1, 214–217 CrossRef CAS; (e) X.-F. Wu, Tetrahedron Lett., 2012, 53, 6123–6126 CrossRef CAS PubMed; (f) X.-F. Wu, C. B. Bheeter, H. Neumann, P. H. Dixneuf and M. Beller, Chem. Commun., 2012, 48, 12237–12239 RSC; (g) M. Zhang and X.-F. Wu, Tetrahedron Lett., 2013, 54, 1059–1062 CrossRef CAS PubMed.
- N. Mulakayala, B. Kandagatla, Ismail, R. K. Rapolu, P. Rao, C. Mulakayala, C. S. Kumar, J. Iqbal and S. Oruganti, Bioorg. Med. Chem. Lett., 2012, 22, 5063–5066 CrossRef CAS PubMed.
- For most recent reviews on topic, see: (a) Metal-Catalyzed reactions in water, ed. P. H. Dixneuf and V. Cadierno, Wiley-VCH, 2013 Search PubMed; (b) R. Noyori, Chem. Commun., 2005, 1807–1811 RSC; (c) M.-O. Simon and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC; (d) X. Han and M. Poliakoff, Chem. Soc. Rev., 2012, 41, 1428–1436 RSC; (e) R. B. N. Baig and R. S. Varma, Chem. Soc. Rev., 2012, 41, 1559–1584 RSC; (f) B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744–5767 RSC.
- (a) H. Adolfsson, in Modern Oxidation Methods, ed, J.-E. Bäckvall, Wiley, New York, 2nd edn 2011 Search PubMed; (b) I. W. C. E. Arends, Angew. Chem., 2006, 118, 6398–6400 ( Angew. Chem., Int. Ed. , 2006 , 45 , 6250–6252 ) CrossRef; (c) J. Wang, C. Liu, J. Yuan and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 2256–2259 CrossRef CAS PubMed; (d) X.-F. Wu, M. Sharif, J.-B. Feng, H. Neumann, A. Pews-Davtyan, P. Langer and M. Beller, Green Chem., 2013, 15, 1956–1961 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra45765f |
| This journal is © The Royal Society of Chemistry 2014 |