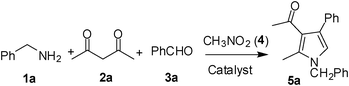
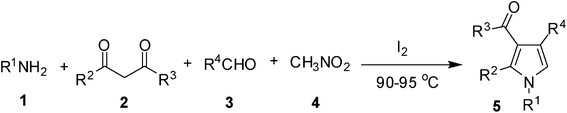
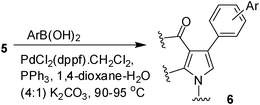
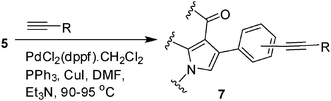
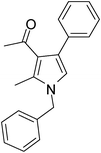
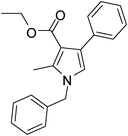
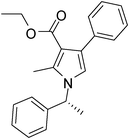
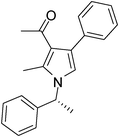
Iodine catalyzed four-component reaction: a straightforward one-pot synthesis of functionalized pyrroles under metal-free conditions†
G. Rajeshwar
Reddy
ab,
T. Ram
Reddy
a,
Suju C.
Joseph
a,
K. Sateesh
Reddy
a and
Manojit
Pal
*c
aCustom Pharmaceutical Services, Dr Reddy's Laboratories Limited, Bollaram Road Miyapur, Hyderabad, 500 049, India
bChemistry Division, Institute of Science and Technology, JNT University, Kukatpally, Hyderabad, 500072, Andhra Pradesh, India
cInstitute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad, 500 046, India. E-mail: manojitpal@rediffmail.com
First published on 28th February 2012
Abstract
An iodine-catalyzed four-component reaction of 1,3-dicarbonyl compounds, amines, aldehydes and nitroalkanes afforded polysubstituted pyrroles under a metal free condition. Simplicity, low cost and good yields are the key features of this methodology. The mechanism of this four component process is discussed and structural elaboration of one of the compounds synthesized using Suzuki and Sonogashira coupling is presented.
Introduction
The pyrrole framework in addition to its occurrence in many natural products has found wide applications in the area of drug discovery.1 A range of pharmacological properties e.g. antitumor, anti-inflammatory, antibacterial, antioxidant and antifungal activities of this important class of heterocycle are known in the literature. Thus, a number of synthetic methods have been developed for the construction of a pyrrole ring. The classical methods used frequently for the synthesis of pyrroles include Hantzsch,2 Knorr,3 and Paal–Knorr4 reactions. While these methods are very useful and effective their uses however suffer from several drawbacks such as functional group compatibility, regiospecificity, harsh reaction conditions and multistep synthetic operation. Among the various strategies1 developed to overcome these problems, multi-component reactions (MCRs) have found wide applications in the synthesis of pyrroles.5 MCRs allow the union of three or more starting materials in a single synthetic operation leading to increased molecular diversity and complexity in a fast and experimentally simple fashion. MCRs have become an important tool in organic synthesis especially for diversity-oriented synthesis. The advantages of MCRs over classical stepwise methods include (i) high atom economy and bond-forming efficiency, (ii) avoids isolation and purifications of any intermediates thereby (iii) minimizing waste, labor, and cost.6 Thus, synthesis of functionalized pyrroles has been reported via a Fe(III)-catalyzed four-component coupling reaction of 1,3-dicarbonyl compounds, amines, aldehydes, and nitroalkanes.7 More recently, we have reported a Pd-mediated MCR for the synthesis of functionalized pyrroles of potential medicinal value.8 All these methods however, involve the use of a metal catalyst the removal of which often requires cumbersome workup and purifications. As an inexpensive and commercially available reagent iodine has attracted considerable interest due to its non-hazardous nature and efficiency in various organic transformations.9 Herein we report a metal free synthesis of polysubstituted pyrroles via a four component reaction catalyzed by molecular iodine.Results and discussion
It is known that generation of (i) β-enaminocarbonyl derivatives from β-dicarbonyl and amines10a and (ii) nitroalkenes from aldehyde and nitroalkane can be catalyzed by molecular iodine.10b Moreover, the iodine catalyzed Michael reaction is well known.10c It was therefore hypothesized that construction of a pyrrole ring via Michael reaction of β-enamino ketones or esters and nitroalkenes followed by cyclization could be facilitated by iodine (Fig. 1).10d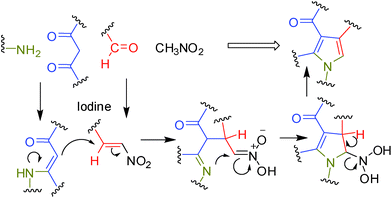 | ||
| Fig. 1 Probable route to construct a pyrrole ring catalyzed by I2. | ||
Accordingly, we examined the reaction of benzylamine (1a), acetyl acetone (2a), benzaldehyde (3a) and nitromethane (4) in the presence of a catalyst under nitrogen. Since our initial goal was to identify an alternative catalyst—preferably non-metal—a range of relevent agents were examined (Table 1). All the reactions were generally carried out at 90–95 °C without using an additional solvent. The use of p-toluenesulfonic acid (p-TSA) (entry 1, Table 1), CF3SO3H (entry 2, Table 1) and solid supported catalyst such as NaHSO3-SiO2 (entries 3 and 4, Table 1) was examined but found to be less effective. The desired product 5 was isolated only in 25–30% yield. The use of iodine however accelerated the MCR and increased the product yield significantly (entry 5, Table 1) affording compound 5 in 85% yield. The reaction was carried out using 0.1 mmol of iodine. The yield of the product was decreased when 0.05 mmol of iodine was used (entry 6, Table 1) or suppressed when the reaction was performed at room temperature (entry 7, Table 1). The effect of using a solvent e.g. DMF (entry 8, Table 1) or THF, was also examined and found to be counter productive. The MCR did not proceed well in the absence of iodine (entry 9, Table 1) or when Amberlyst-15 was used as a catalyst (entry 10, Table 1).
| Entry | Catalyst (mmol) | T/°C | Time (h) | %Yieldb |
|---|---|---|---|---|
| a All the reactions were carried out using compound 1 (1.5 mmol), 2 (1.0 mmol), 3a (1.0 mmol) and 4 (3.0 mL) in presence of a catalyst under nitrogen. b Isolated yield. c The reaction was carried out in DMF. | ||||
| 1 | p-TSA (0.1) | 90–95 | 14 | 30 |
| 2 | CF3SO3H (0.1) | 90–95 | 14 | 30 |
| 3 | NaHSO3-SiO2 (0.1) | 90–95 | 24 | 25 |
| 4 | NaHSO3-SiO2 (0.15) | 90–95 | 24 | 30 |
| 5 | I2 (0.1) | 90–95 | 6.0 | 85 |
| 6 | I2 (0.05) | 90–95 | 6.0 | 65 |
| 7 | I2 (0.1) | Room temp | 24 | 15 |
| 8 | I2 (0.1) | 90–95 | 6.0 | 60c |
| 9 | No catalyst | 90–95 | 24 | 11 |
| 10 | Amberlyst-15 (10%w/w) | 90–95 | 24 | 10 |
To demonstrate the utility of the present MCR a variety of polyfunctionalized pyrroles (6) was synthesized (Table 2) in good yields. Both aryl and alkyl amines e.g. benzyl, (R)- and (S)-phenylethyl, 1-naphthyl, 2-hydroxyethyl and cyclohexyl amine were employed in the present MCR. Both acetyl acetone (2a) and ethyl acetoacetate (2b) participated well in this reaction. The MCR proceeded smoothly with a number of aryl aldehydes containing various substitution patterns.
| Entry | 1; R1 | 2; R2; R3 | 3; R4 | Time (h) | Products | %Yieldb |
|---|---|---|---|---|---|---|
| a All the reactions were carried out using amine 1 (1.5 mmol), 1,3-dicarbonyl compound 2 (1.0 mmol), aryl aldehyde 3 (1.0 mmol) and nitromethane 4 (3.0 mL) in presence of iodine (0.10 mmol) at 90–95 °C under nitrogen. b Isolated yield. | ||||||
| 1 | 1a; PhCH2 | 2a; CH3; CH3 | 3a; Ph | 6 | 5a | 85 |
| 2 | 1a | 2b; CH3; OEt | 3a | 8 | 5b | 76 |
| 3 | 1b; (R)-Ph(CH3)CH | 2b | 3a | 8 | 5c | 75 |
| 4 | 1b | 2a | 3a | 7 | 5d | 85 |
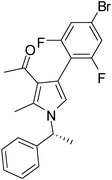
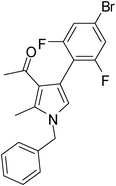
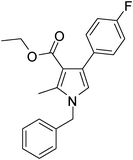
| 5 | 1b | 2a | 3b; 2,6-diF-4-BrC6H2 | 7.5 | 5e | 72 |
| 6 | 1a | 2a | 3b | 6.5 | 5f | 81 |
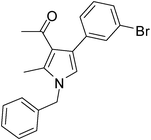
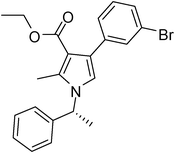
| 7 | 1a | 2a | 3c; 3-BrC6H4 | 6.0 | 5g | 85 |
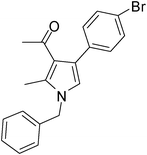
| 8 | 1a | 2a | 3d; 4-BrC6H4 | 7.5 | 5h | 80 |
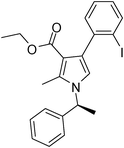
| 9 | 1c; (S)-Ph(CH3)CH | 2b | 3e; 2-IC6H4 | 8.0 | 5i | 70 |
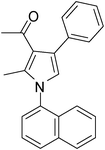
| 10 | 1d; 1-Naphthyl | 2a | 3a | 6.5 | 5j | 60 |
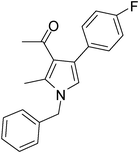
| 11 | 1a | 2a | 3f; 4-FC6H4 | 6.5 | 5k | 80 |
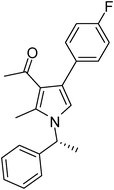
| 12 | 1b | 2a | 3f | 7.0 | 5l | 75 |
| 13 | 1a | 2b | 3f | 8.0 | 5m | 77 |
| 14 | 1b | 2b | 3c | 7.5 | 5n | 69 |
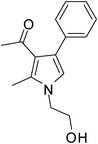
| 15 | 1e; HOCH2CH2– | 2b | 3a | 8.0 | 5o | 70 |
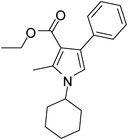
| 16 | 1f; c-Hexyl | 2b | 3a | 7.0 | 5p | 65 |
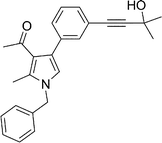
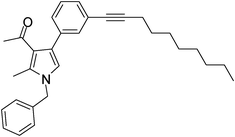
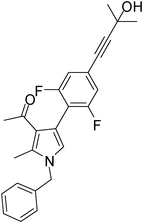
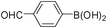
To expand the scope of the present solvent free MCR, structural elaboration of one of the compounds synthesized e.g.5g was carried out using Suzuki (Table 3) and Sonogashira coupling (Table 4). A number of boronic acids were employed to couple with compound 5 in the presence of PdCl2(dppf)·CH2Cl2, PPh3 and K2CO3 in 1,4-dioxane-H2O at 90–95 °C. The desired product 6 was isolated in 75–83% yield. Similarly, a number of terminal alkynes were coupled with 5 in the presence of PdCl2(dppf)·CH2Cl2, PPh3, CuI and Et3N in DMF at 90–95 °C to give desired alkynes 7 in 78–83% yield.
| Entry | Halide 5 | ArB(OH)2 or ester | Products (6) | Timeb(h) | %Yieldb |
|---|---|---|---|---|---|
a All the reactions were carried out using compound 5 (1.0 mmol), aryl boronic acid (1.5 mmol), PdCl2(dppf)·CH2Cl2 (0.10 mmol), PPh3 (0.10 mmol) and K2CO3 (2.0 mmol) in 1,4-dioxane-H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (5.0 mL) at 90–95 °C under nitrogen.
b Isolated yield. 1) (5.0 mL) at 90–95 °C under nitrogen.
b Isolated yield.
|
|||||
| 1 | 5e |
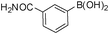
|
6a | 6.5 | 75 |
| 2 | 5e |
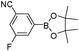
|
6b | 7.0 | 79 |
| 3 | 5g |
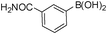
|
6c | 6.0 | 77 |
| 4 | 5g |

|
6d | 6.5 | 75 |
| 5 | 5i |
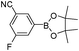
|
6e | 7.0 | 76 |
| 6 | 5h |
|
6f | 6.0 | 84 |
| Entry | Halide 5 | Alkynes | Products (7) | Timeb(h) | %Yieldb |
|---|---|---|---|---|---|
| a All the reactions were carried out using compound 5 (1.0 mmol), a terminal alkyne (1.5 mmol), PdCl2(dppf)·CH2Cl2 (0.10 mmol), PPh3 (0.10 mmol), CuI (0.1 mmol) and Et3N (3.0 mmol) in DMF (6 mL) at 90–95 °C under nitrogen. b Isolated yield. | |||||
| 1 | 5h | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –CH2CH2CH2Cl –CH2CH2CH2Cl |
7a | 8.0 | 80 |
| 2 | 5h | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –CH2(CH2)6CH3 –CH2(CH2)6CH3 |
7b | 7.0 | 81 |
| 3 | 5g | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –C(CH3)2OH –C(CH3)2OH |
7c | 7.5 | 83 |
| 4 | 5g | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –CH2(CH2)6CH3 –CH2(CH2)6CH3 |
7d | 8.5 | 79 |
| 5 | 5f | ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –C(CH3)2OH –C(CH3)2OH |
7e | 9.0 | 78 |
Mechanistically, the reaction seems to proceed via the path shown in Fig. 1. While the precise role of iodine in the present MCR was not clearly understood possibly the Michael reaction11 of β-enamino carbonyl compounds with nitroalkene (both generated in situ) was accelerated in presence of iodine. To gain further evidences the β-enamino ketone (8a) prepared from 1a and 2a was reacted with nitroalkene (9a) prepared separately from the benzaldehyde (3a) and the nitromethane (4) in the presence and absence of iodine. While the reaction proceeded well in the first case (< 80% conversion after 6 h) but it was sluggish in the second case (> 40% conversion after 6 h) indicating the key role played by iodine in the Michael addition step. The formation of β-enamino carbonyl compound via the reaction of amine and the 1,3-dicarbonyl compound could also be accelerated by iodine.12 The interaction of molecular iodine with carbonyl oxygen perhaps played a vital role here. This type of interaction has been described in the literature earlier.13a For example, nucleophilic addition of indole to a carbonyl compound was catalyzed efficiently by molecular iodine.13b Additionally, the Michael reaction of indole and imidazole with unsaturated carbonyl compound has also been carried out successfully in the presence of a catalytic amount of iodine.10c It was therefore suggested that a halogen bond between the carbonyl oxygen and iodine molecule plays a key role in the remarkable catalytic effect of iodine observed in these reactions.13a A halogen bond is defined as intermolecular uncovalent interaction between a halogen atom and electron-donor atom such as O or N (similar to hydrogen bond).14 Nevertheless, once again to gain further evidence the nitroalkene 9a was reacted with 1a and 2a in the presence and absence of iodine. The reaction was completed within 6 h in the first case affording the desired product 5a whereas very little product was formed in the second case. Overall, all these observations not only suggested that the reaction proceed via formation of a nitroalkene but the central role played by iodine in facilitating the MCR.
Conclusions
In conclusion, a facile and efficient synthesis of multisubstituted pyrroles derivatives has been developed via a I2-mediated MCR. The methodology employs readily available and inexpensive starting materials under metal and solvent free conditions. To the best of our knowledge this is the first example of Grob and Camenisch's pyrrole synthesis11a catalyzed by iodine via one-pot four-component reactions. The methodology should find wide usage both in academia and pharma industries.Experimantal
General methods
Unless stated otherwise, reactions were performed under nitrogen atmosphere using oven dried glassware. Reactions were monitored by thin layer chromatography (TLC) on silica gel plates (60 F254), visualizing with ultraviolet light or iodine spray. Flash chromatography was performed on silica gel (230–400 mesh) using distilled hexane, ethyl acetate, dichloromethane. 1H NMR and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 solution by using 400 or 200 and 100 MHz spectrometers, respectively. Proton chemical shifts (δ) are relative to tetramethylsilane (TMS, δ = 0.00) as internal standard and expressed in ppm. Spin multiplicities are given as s (singlet), d (doublet), t (triplet) and m (multiplet) as well as b (broad). Coupling constants (J) are given in hertz. Infrared spectra were recorded on a FT-IR spectrometer. Melting points were determined using Buchi B-540 melting point apparatus and are uncorrected. MS spectra were obtained on a Agilent 6430 series Triple Quard LC-MS/MS spectrometer. High-resolution mass spectra (HRMS) were recorded using a Waters LCT Premier XE instrument.General method for the synthesis of functionalized pyrrole derivatives (5)
To a solution of amine 1 (1.5 mmol), 1,3-dicarbonyl compound 2 (1.0 mmol), aryl aldehyde 3 (1.0 mmol) and nitromethane 4 (3.0 mL) was added I2 (0.10 mmol) with stirring under nitrogen at room temp. The mixture was then stirred at 90–95 °C and the progress was monitored by TLC. After completion of the reaction the mixture was cooled to room temp, diluted with water (5 mL) and extracted with ethyl acetate (3 × 5 mL). The organic layers were collected, combined, washed with brine (5 mL), dried over Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography on silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–4
9–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ethyl acetate–petroleum ether to afford the desired compound.
6 ethyl acetate–petroleum ether to afford the desired compound.
Brown colour solid (245 mg, 0.85 mmol, 85%); mp 52–54 °C; IR (neat) 3585, 3019, 2929, 1887, 1647, 1560, 1453, 1354, 1215, 1073 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.03 (s, 3H), 2.43 (s, 3H), 5.05 (s, 2H), 6.53 (s, 1H), 7.09 (d, J = 6.8 Hz, 2H), 7.25–7.55 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 31.0, 120.0, 121.9, 125.8, 126.6, 127.3, 128.1, 129.0, 135.0, 136.2, 197.5; HRMS: m/z calcd for C20H19NO (M + 1) 290.1467; found 290.1541.
Dark brown sticky liquid (242 mg, 0.76 mmol, 76%); IR (neat) 3405, 3017, 2982, 1949, 1687, 1527, 1454, 1284, 1216 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.13 (t, J = 6.8 Hz, 3H), 2.47 (s, 3H), 4.17 (q, J = 6.8 Hz, 2H), 5.06 (s, 2H), 6.58 (s, 1H), 7.08 (d, J = 6.8 Hz, 2H), 7.23–7.38 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.4, 14.0, 50.5, 59.3, 111.0, 120.3, 126.0, 127.4, 128.8, 129.2, 135.8, 136.3, 165.8; HRMS: m/z calcd for C21H21NO2 (M + 1) 320.1572; found 320.1646.
Off white solid (250 mg, 0.75 mmol, 75%); mp 47–49 °C; IR (neat) 3384, 3060, 2981, 1949, 1802, 1693, 1527, 1412, 1276 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.12 (t, J = 6.8 Hz, 3H), 1.83 (d, J = 6.8 Hz, 3H), 2.44 (s, 3H), 4.16 (q, J = 6.8 Hz, 2H), 5.39 (q, J = 6.8 Hz, 1H), 6.72 (s, 1H), 7.08 (d, J = 7.2 Hz, 2H), 7.23–7.40 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.3, 13.9, 22.1, 55.0, 59.3, 110.8, 116.7, 125.7, 126.0, 127.4, 128.8, 129.2, 136.1, 142.0, 165.9; HRMS: m/z calcd for C22H23NO2 (M + 1) 334.1729; found 334.1809.
Off-white solid (257 mg, 0.85 mmol, 85%); mp 126–129 °C; IR (neat) 3401, 3013, 2401, 1950, 1647, 1511, 1406, 1272, 1215, 1029 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.83 (d, J = 7.2 Hz, 3H), 2.03 (s, 3H), 2.41 (s, 3H), 5.40 (q, J = 7.2 Hz, 1H), 6.67 (s, 1H), 7.10 (d, J = 7.2 Hz, 2H), 7.24–7.38 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.4, 22.0, 54.8, 116.5, 121.9, 125.6, 126.5, 127.6, 128.1, 129.2, 134.9, 136.5, 141.9, 197.8; HRMS: m/z calcd for C21H21NO (M + 1) 304.1623; found 304.1694.
Brown sticky liquid (300 mg, 0.72 mmol, 72%); IR (neat) 3400, 3089, 2927, 1651, 1557, 1417, 1269, 1216, 1026, 851, 687 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.83 (d, J = 7.6 Hz, 3H), 2.10 (s, 3H), 2.41 (s, 3H), 5.41 (q, J = 7.6 Hz, 1H), 6.77 (s, 1H), 7.07 (d, J = 7.6 Hz, 2H), 7.12–7.18 (m, 2H), 7.26–7.35 (m, 3H); 13C NMR (CDCl3, 100 MHz) δ 11.7, 22.2, 29.6, 55.2, 108.7, 113.3, 115.1, 116.3, 118.6, 120.1, 122.2, 125.4, 127.6, 128.9, 135.6, 141.7, 159.0, 161.1, 195.7; HRMS: m/z calcd for C21H18F2NO (M + 1) 418.0540; found 418.0633.
Brown colour solid (326 mg, 0.81 mmol, 81%); mp 96–99 °C; IR (neat) 3390, 3031, 2925, 1807, 1651, 1557, 1417, 1353, 1181, 1025, 852, 752 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.10 (s, 3H), 2.45 (s, 3H), 5.08 (s, 2H), 6.62 (s, 1H), 7.07 (d, J = 7.2 Hz, 2H), 7.09–7.17 (m, 2H), 7.25–7.37 (m, 3H); 13C NMR (CDCl3, 100 MHz) δ 11.7, 29.4, 50.4, 108.9, 113.0, 115.8, 116.4, 120.2, 122.1, 126.5, 127.7, 128.8, 129.2, 135.7, 136.1, 161.4, 195.4; HRMS: m/z calcd for C20H16BrF2NO (M + 1) 404.0383; found 404.0461.
Off-white solid (312 mg, 0.85 mmol, 85%); mp 105–107 °C; IR (neat) 3397, 2941, 2400, 1949, 1806, 1653, 1559, 1420, 1215, 1180, 1071, 755 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.06 (s, 3H), 2.42 (s, 3H), 5.05 (s, 2H), 6.54 (s, 1H), 7.08 (d, J = 6.8 Hz, 2H), 7.21–7.42 (m, 6H), 7.49 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 31.0, 50.2, 120.3, 121.8, 122.1, 124.2, 126.5, 127.8, 128.1, 129.2, 131.9, 135.3, 136.2, 138.3, 196.9; HRMS: m/z calcd for C20H18BrNO (M + 1) 368.0572; found 368.0638.
Dark brown sticky liquid (293 mg, 0.80 mmol, 80%); mp 115–117 °C; IR (neat) 3399, 3031, 2923, 1646, 1555, 1454, 1377, 1279, 1010, 701 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.05 (s, 3H), 2.42 (s, 3H), 5.05 (s, 2H), 6.52 (s, 1H), 7.08 (d, J = 6.8 Hz, 2H), 7.20–7.28 (m, 2H), 7.29–7.41 (m, 4H), 7.48 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 31.0, 50.3, 120.0, 121.9, 124.5, 126.6, 127.3, 128.8, 129.0, 130.8, 131.2, 132.2, 134.1, 135.2, 136.3, 197.1; HRMS: m/z calcd for C20H18BrNO (M + 1) 368.0572; found 368.0626.
Pale yellow colour solid (321 mg, 0.70 mmol, 70%); mp 77–80 °C; IR (neat) 3436, 3045, 2977, 1686, 1531, 1409, 1283, 1155, 1090, 1016, 637 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.94 (t, J = 7.2 Hz, 3H), 1.83 (d, J = 7.2 Hz, 3H), 2.46 (s, 3H), 4.03 (q, J = 7.2 Hz, 2H), 5.41 (q, J = 7.2 Hz, 1H), 6.67 (s, 1H), 6.93–6.97 (m, 1H), 7.09 (d, J = 6.8 Hz, 2H), 7.23–7.34 (m, 5H), 7.87 (d, J = 7.6 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.2, 13.6, 22.2, 55.0, 59.0, 102.5, 111.7, 116.8, 125.6, 127.1, 128.7, 130.5, 135.8, 138.1, 142.2, 165.4; HRMS: m/z calcd for C22H22INO2 (M + 1) 460.0695; found 460.0794.
Dark brown sticky liquid (195 mg, 0.60 mmol, 60%); IR (neat) 3584, 3352, 3051, 2933, 1922, 1601, 1573, 1514, 1434, 1313, 1281 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.87 (s, 3H), 2.17 (s, 3H), 6.76 (s, 1H), 7.24–7.32 (m, 5H), 7.42–7.55 (m, 3H), 7.75–7.88 (m, 3H), 8.02–8.03 (m, 1H); 13C NMR (CDCl3, 100 MHz) δ 19.4, 29.0, 97.4, 109.5, 118.8, 120.7, 122.6, 123.3, 124.7, 126.2, 128.1, 129.8, 134.1, 142.0, 196.3; HRMS: m/z calcd for C23H19NO (M + 1) 326.1467; found 326.1547.
Dark brown sticky liquid (245 mg, 0.80 mmol, 80%); IR (neat) 3684, 3019, 2927, 2400, 1645, 1557, 1511, 1281, 1215, 1016, 835 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.02 (s, 3H), 2.43 (s, 3H), 5.05 (s, 2H), 6.50 (s, 1H), 7.02–7.09 (m, 3H), 7.21–7.38 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 30.9, 50.2, 114.8, 115.0, 120.0, 121.9, 124.6, 126.5, 127.2, 128.1, 129.4, 130.7, 132.2, 135.0, 136.4, 163.0, 197.1; HRMS: m/z calcd for C20H18FNO (M + 1) 308.1372; found 308.1443.
Brown colour solid (240 mg, 0.75 mmol, 75%); mp 131-133 °C; IR (neat) 3430, 3019, 2400, 1645, 1510, 1420, 1215, 1016, 756, 669 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.83 (d, J = 6.8 Hz, 3H), 2.01 (s, 3H), 2.40 (s, 3H), 5.40 (q, J = 6.8 Hz, 1H), 6.64 (s, 1H), 7.02–7.10 (m, 4H), 7.22–7.37 (m, 5H); 13C NMR (CDCl3, 100 MHz) δ 11.4, 22.0, 31.0, 54.8, 114.9, 115.1, 116.5, 121.9, 124.5, 125.4, 127.1, 128.8, 130.7, 132.5, 135.1, 141.7, 162.6, 197.4; HRMS: m/z calcd for C21H20FNO (M + 1) 322.1529; found 322.1594.
Brown sticky liquid (259 mg, 0.77 mmol, 77%); IR (neat) 3681, 3018, 2937, 1951, 1887, 1686, 1605, 1528, 1424, 1284, 1065, 756, 668 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.13 (t, J = 6.8 Hz, 3H), 2.46 (s, 3H), 4.17 (q, J = 6.8 Hz, 2H), 5.05 (s, 2H), 6.54 (s, 1H), 6.99–7.07 (m, 3H), 7.25–7.35 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ 11.1, 14.0, 50.4, 59.3, 110.9, 114.0, 120.2, 125.1, 126.0, 127.4, 128.8, 129.2, 130.6, 131.7, 136.4, 162.0, 165.6; HRMS: m/z calcd for C21H20FNO2 (M + 1) 338.1478; found 338.1541.
Off-White solid (283 mg, 0.69 mmol, 69%); mp 103–105 °C; IR (neat) 3677, 3019, 2984, 1686, 1605, 1527, 1425, 1302, 1260, 1215, 1027, 668 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.15 (t, J = 6.8 Hz, 3H), 1.83 (d, J = 7.2 Hz, 3H), 2.45 (s, 3H), 4.17 (q, J = 7.2 Hz, 2H), 5.37 (q, J = 6.8 Hz, 1H), 6.71 (s, 1H), 6.96 (d, J = 7.2 Hz, 2H), 7.07 (d, J = 7.2 Hz, 2H), 7.17–7.37 (m, 4H), 7.60 (d, J = 8.8 Hz, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.3, 13.9, 22.0, 55.0, 59.4, 110.7, 114.6, 117.0, 121.3, 124.3, 125.3, 127.0, 128.8, 132.1, 133.9, 136.8, 138.2, 141.8, 165.6; HRMS: m/z calcd for C22H22BrNO2 (M + 1) 412.0834; found 412.0915.
Dark brown sticky liquid (170 mg, 0.70 mmol, 70%); IR (neat) 3390, 2924, 1702, 1632, 1551, 1413, 1282, 1070 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.01 (s, 3H), 2.49 (s, 3H), 3.90 (t, J = 5.2 Hz, 2H), 4.01 (t, J = 5.2 Hz, 2H), 6.57 (s, 1H), 7.26–7.48 (m, 5H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 30.8, 48.4, 61.6, 120.6, 121.5, 126.5, 128.2, 129.1, 133.0, 135.2, 136.0, 198.0; HRMS: m/z calcd for C15H17NO2 (M + 1) 244.1259; found 244.1333.
Dark brown sticky liquid (202 mg, 0.65 mmol, 65%); IR (neat) 3018, 2983, 2858, 1889, 1687, 1603, 1557, 1449, 1379, 1278, 1215, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.08 (t, J = 7.2 Hz, 3H), 1.19–1.48 (m, 4H), 1.56–1.70 (m, 2H), 1.74–1.77 (m, 2H), 1.89–2.01 (m, 2H), 2.54 (s, 3H), 3.91 (m, 1H), 4.16 (q, J = 7.2 Hz, 2H), 6.62 (s, 1H), 7.20–7.39 (m, 5H); 13C NMR (CDCl3, 100 MHz) δ 11.0, 13.9, 25.2, 33.8, 55.1, 59.1, 109.9, 115.8, 125.7, 127.3, 128.9, 135.2, 136.2, 166.0; HRMS: m/z calcd for C20H25NO2 (M + 1) 312.1885; found 312.1951.
General method for the synthesis of 4-biaryl substituted pyrroles (6)
To a stirring solution of compound 5 (1.0 mmol) in 1,4-dioxane (4.0 mL) and water (1.0 mL) was added arylboronic acid (1.5 mmol), PdCl2(dppf)·CH2Cl2 (0.10 mmol), PPh3 (0.10 mmol) and K2CO3 (2.0 mmol) with stirring. The mixture was then stirred at 90–95 °C under nitrogen for the time indicated in Table 3. After completion of the reaction the mixture was cooled to room temp, diluted with water (5 mL) and extracted with ethyl acetate (3 × 5 mL). The organic layers were collected, combined, washed with brine (5 mL), dried over Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography on silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–3
9–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ethyl acetate–petroleum ether to afford the desired compound.
7 ethyl acetate–petroleum ether to afford the desired compound.
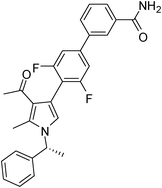
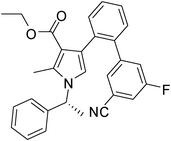
Brown sticky liquid (383 mg, 0.75 mmol, 75%); IR (neat) 3467, 3177, 3017, 2929, 1670, 1604, 1517, 1483, 1267, 1215, 1071, 855, 667 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.85 (d, J = 6.8 Hz, 3H), 2.14 (s, 3H), 2.44 (s, 3H), 5.41 (q, J = 6.8 Hz, 1H), 6.84 (s, 1H), 7.10 (d, J = 7.6 Hz, 2H), 7.22–7.32 (m, 4H), 7.34–7.36 (m, 1H), 7.44–7.51 (m, 1H), 7.52–7.57 (m, 1H), 7.64–7.75 (m, 2H), 7.82 (d, J = 4.8 Hz, 1H), 8.10 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.7, 22.1, 29.6, 55.1, 109.7, 112.8, 114.4, 118.7, 119.3, 122.3, 125.6, 126.1, 128.8, 129.1, 131.6, 132.0, 134.2, 135.4, 138.8, 140.8, 141.7, 161.7, 169.1, 196.2; HRMS: m/z calcd for C28H24F2N2O2 (M + 1) 459.1806; found 459.1877.
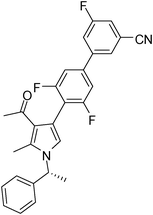
Light brown solid (362 mg, 0.79 mmol, 79%); mp 66–68 °C; IR (neat) 3683, 3019, 2926, 2229, 1951, 1737, 1652, 1569, 1409, 1354, 1271, 1029, 865, 668 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.86 (d, J = 6.8 Hz, 3H), 2.16 (s, 3H), 2.44 (s, 3H), 5.43 (q, J = 6.8 Hz, 1H), 6.85 (s, 1H), 7.09 (d, J = 7.6 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 7.26–7.39 (m, 4H), 7.54 (d, J = 6.0 Hz, 1H), 7.68 (s, 1H) ; 13C NMR (CDCl3, 100 MHz) δ 11.7, 22.2, 29.7, 55.2, 108.9, 109.8, 110.1, 114.5, 117.2, 118.6, 122.4, 125.7, 126.4, 127.7, 128.9, 135.4, 137.9, 141.7, 142.5, 159.6, 161.3, 162.0, 163.8, 195.7; HRMS: m/z calcd for C28H21F3N2O (M + 1) 459.1606; found 459.1684.
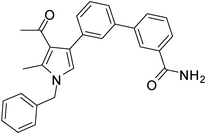
Dark brown sticky liquid (314 mg, 0.77 mmol, 77%); IR (neat) 3686, 3019, 2400, 1625, 1523, 1423, 1215, 1016, 669 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.09 (s, 3H), 2.45 (s, 3H), 5.08 (s, 2H), 6.60 (s, 1H), 7.11 (d, J = 7.6 Hz, 2H), 7.24–7.39 (m, 6H), 7.44–7.62 (m, 5H), 7.83 (d, J = 7.6 Hz, 1H), 7.88 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.6, 31.9, 50.3, 112.9, 118.7, 120.3, 122.0, 125.2, 126.6, 127.8, 128.9, 129.3, 130.6, 131.4, 135.4, 136.4, 137.2, 138.8, 142.2, 163.8, 197.1; HRMS: m/z calcd for C27H24N2O2 (M + 1) 409.1838; found 409.1920.
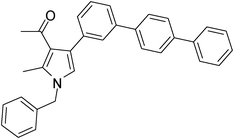
Off-White solid (331 mg, 0.75 mmol, 75%); mp 135–136 °C; IR (neat) 3018, 2938, 2401, 1688, 1605, 1527, 1424, 1384, 1284, 1216, 1029 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.11 (s, 3H), 2.45 (s, 3H), 5.07 (s, 2H), 6.60 (s, 1H), 7.11 (d, J = 6.8 Hz, 2H), 7.25–7.37 (m, 5H), 7.42–7.56 (m, 3H), 7.58–7.70 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 31.1, 50.3, 120.2, 122.0, 125.3, 126.6, 127.0, 128.3, 135.2, 136.4, 139.7, 140.1, 197.4; HRMS: m/z calcd for C32H27NO (M + 1) 442.2093; found 442.2185.
Pale yellow viscous liquid (343 mg, 0.76 mmol, 76%); IR (neat) 3310, 3070, 2937, 2233, 1694, 1606, 1591, 1417, 1329, 1259, 1152, 860, 691 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.02 (t, J = 7.2 Hz, 3H), 1.73 (d, J = 6.8 Hz, 3H), 2.36 (s, 3H), 3.99 (q, J = 7.2 Hz, 2H), 5.32 (q, J = 6.8 Hz, 1H), 6.40 (s, 1H), 6.91 (d, J = 7.6 Hz, 2H), 7.17–7.20 (m, 2H), 7.26–7.37 (m, 8H); 13C NMR (CDCl3, 100 MHz) δ 11.3, 12.9, 14.0, 29.6, 54.8, 59.4, 110.8, 115.2, 116.4, 117.6, 121.4, 123.8, 125.4, 127.2, 128.0, 129.2, 131.5, 136.1, 141.7, 163.7, 165.6; HRMS: m/z calcd for C29H25FN2O2 (M + 1) 453.1900; found 453.1981.
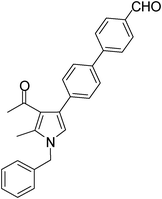
White solid (330 mg, 0.84 mmol, 84%), mp 119–120 °C; IR (neat) 3584, 3368, 2710, 2400, 1603, 1424, 1217, 1021 cm−1; 1H NMR (CDCl3, 400 MHz) δ 2.12 (s, 3H), 2.45 (s, 3H), 5.08 (s, 2H), 6.60 (s, 1H), 7.11 (d, J = 7.2 Hz, 2H), 7.26–7.45 (m, 5H), 7.66 (d, J = 8.0 Hz, 2H), 7.80 (d, J = 7.6 Hz, 2H), 7.97 (d, J = 8.4 Hz, 2H), 10.06 (s, 1H); 13C NMR (CDCl3, 100 MHz) δ 11.5, 31.1, 50.3, 55.3, 120.3, 122.0, 125.0, 126.6, 127.1, 128.9, 130.2, 136.3, 137.7, 146.6, 191.8, 197.3 ; HRMS: m/z calcd for C27H23NO2 (M + 1) 394.1729; found 394.1803.
General method for the synthesis of alkynyliaryl substituted pyrroles (7)
To a stirring solution of aryl halide (1.0 mmol), DMF (6.0 mL), was added a terminal alkyne (1.5 mmol), CuI (0.1 mmol), PdCl2 (dppf)·CH2Cl2 (0.1 mmol), PPh3 (0.1 mmol) and Et3N (3.0 mmol) with stirring. The mixture was then stirred at 90–95 °C under nitrogen for the time indicated in Table 4. After completion of the reaction the mixture was cooled to room temp, diluted with water (5 mL) and extracted with ethyl acetate (3 × 5 mL). The organic layers were collected, combined, washed with brine (5 mL), dried over Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography on silica gel using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–2
9–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ethyl acetate–petroleum ether to afford the desired compound.
8 ethyl acetate–petroleum ether to afford the desired compound.
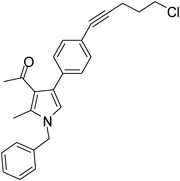
Pale yellow colour solid (311 mg, 0.80 mmol, 80%); mp 152–153 °C; IR (neat) 3443, 2962, 2236, 1915, 1648, 1513, 1421, 1261, 1095, 802 cm−1; 1H NMR (CDCl3, 500 MHz) δ 2.04 (s, 3H), 2.08 (t, J = 7.0 Hz, 2H), 2.42 (s, 3H), 2.63 (t, J = 7.0 Hz, 2H), 3.74 (t, J = 6.5 Hz, 2H), 5.05 (s, 2H), 6.54 (s, 1H), 7.09 (d, J = 7.0 Hz, 2H), 7.24–7.39 (m, 7H) ; 13C NMR (CDCl3, 100 MHz) δ 11.5, 16.9, 20.8, 29.7, 31.1, 43.7, 50.3, 80.3, 88.3, 120.1, 122.0, 125.2, 126.6, 127.8, 128.9, 129.0, 131.4, 135.8, 136.4, 197.4; HRMS: m/z calcd for C25H24ClNO (M + 1) 390.1546; found 390.1629.
Brown colour liquid (344 mg, 0.81 mmol, 81%); IR (neat) 3283, 3011, 2928, 2232, 1949, 1648, 1510, 1419, 1378, 1216, 1029 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.89 (t, J = 6.8 Hz, 3H), 1.21–1.39 (m, 4H), 1.43–1.47 (m, 2H), 1.57–1.64 (m, 6H), 2.04 (s, 3H), 2.39–2.43 (m, 5H), 5.05 (s, 2H), 6.53 (s, 1H), 7.09 (d, J = 6.8 Hz, 2H), 7.22–7.39 (m, 7H) ; 13C NMR (CDCl3, 100 MHz) δ 11.3, 13.9, 19.3, 22.5, 28.6, 29.0, 30.9, 31.7, 50.1, 80.3, 90.7, 120.0, 121.8, 122.2, 125.2, 126.5, 127.7, 128.8, 131.2, 135.1, 136.3, 197.4; HRMS: m/z calcd for C30H35NO (M + 1) 426.2719; found 426.2790.
Brown colour liquid (308 mg, 0.83 mmol, 83%); IR (neat) 3670, 3394, 3011, 2228, 1950, 1647, 1509, 1420, 1216, 1133, 1029, cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.61 (s, 6H), 2.04 (s, 3H), 2.43 (s, 3H), 5.05 (s, 2H), 6.53 (s, 1H), 7.08 (d, J = 6.8 Hz, 2H), 7.24–7.40 (m, 7H); 13C NMR (CDCl3, 50 MHz) δ 11.5, 31.0, 50.2, 65.5, 81.9, 93.9, 120.2, 121.9, 122.6, 124.9, 126.5, 127.8, 128.0, 129.3, 132.2, 135.2, 136.4, 197.3; HRMS: m/z calcd for C25H25NO2 (M + 1) 372.1885; found 372.1955.
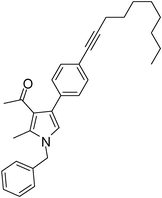
Brown colour liquid (337 mg, 0.79 mmol, 79%); IR (neat) 3678, 3015, 2928, 2226, 1949, 1648, 1599, 1420, 1216, 1078 cm−1; 1H NMR (CDCl3, 400 MHz) δ 0.89 (t, J = 6.8 Hz, 3H), 1.25–1.31 (m, 8H), 1.44–1.58 (m, 2H), 1.59–1.61 (m, 2H), 2.04 (s, 3H), 2.41 (t, J = 7.2 Hz, 2H), 2.42 (s, 3H), 5.05 (s, 2H), 6.53 (s, 1H), 7.07 (d, J = 6.8 Hz, 2H), 7.20–7.38 (m, 7H); 13C NMR (CDCl3, 50 MHz) δ 11.5, 14.1, 19.4, 22.6, 28.7, 29.1, 31.1, 50.2, 80.3, 90.6, 120.1, 121.9, 124.0, 125.1, 127.8, 129.7, 132.2, 135.1, 136.3, 197.3; HRMS: m/z calcd for C30H35NO (M + 1) 426.2719; found 426.2817.
Brown colour solid (317 mg, 0.78 mmol, 78%); mp 79–81 °C; IR (neat) 3672, 3364, 3014, 2926, 2227, 1912, 1643, 1561, 1455, 1377, 1216, 860 cm−1; 1H NMR (CDCl3, 400 MHz) δ 1.53 (s, 3H), 1.61 (s, 3H), 2.08 (s, 3H), 2.45 (s, 3H), 5.08 (s, 2H), 6.65 (s, 1H), 7.01 (d, J = 7.6 Hz, 2H), 7.08 (d, J = 6.8 Hz, 2H), 7.26–7.37 (m, 3H); 13C NMR (CDCl3, 100 MHz) δ 11.7, 29.6, 30.9, 31.2, 50.4, 65.4, 83.9, 95.8, 109.3, 114.1, 122.2, 123.2, 126.5, 127.8, 128.9, 135.7, 136.2, 161.0, 195.8; HRMS: m/z calcd for C25H23F2NO2 (M + 1) 408.1697; found 408.1774.
Acknowledgements
GRR thanks Dr V. Dahanukar and Dr U. K. Syam Kumar for encouragement and the analytical group of DRL for spectral support. MP thanks Prof J. Iqbal for support.References
- For an excellent review, see V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402 RSC.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474 CrossRef.
- L. Knorr, Ber. Dtsch. Chem. Ges., 1884, 17, 1635 CrossRef.
- C. Pall, Ber. Dtsch. Chem. Ges., 1885, 18, 367 CrossRef.
- (a) For an in-depth review, see: G. Balme, Angew. Chem., Int. Ed., 2004, 43, 6238 CrossRef CAS and references therein; (b) For recent examples, see: L. Nagarapu, R. Mallepalli, L. Yeramanchi and R. Bantu, Tetrahedron Lett., 2011, 52, 3401 CrossRef CAS; (c) X. Lin, Z. Mao, X. Dai, P. Lu and Y. Wang, Chem. Commun., 2011, 47, 6620 RSC.
- For recent reviews, see: (a) B. B. Touré and D. G. Hall, Chem. Rev., 2009, 109, 4439 CrossRef; (b) G. Balme, E. Bossharth and N. Monteiro, Eur. J. Org. Chem., 2003, 4101 CrossRef CAS; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS; (d) R. V. A. Orru and M. de Greef, Synthesis, 2003, 1471 CrossRef CAS; (e) J. Zhu, Eur. J. Org. Chem., 2003, 1133 CrossRef CAS; (f) H. Bienaymé, C. Hulme, G. Oddon and P. Schmitt, Chem.–Eur. J., 2000, 6, 3321 CrossRef.
- S. Maiti, S. Biswas and U. Jana, J. Org. Chem., 2010, 75, 1674 CrossRef CAS.
- G. R. Reddy, T. R. Reddy, S. C. Joseph, K. S. Reddy, L. S. Reddy, P. M. Kumar, G. R. Krishna, C. M. Reddy, D. Rambabu, R. Kapavarapu, C. Lakshmi, T. Meda, K. K. Priya, K. V. L. Parsa and M. Pal, Chem. Commun., 2011, 47, 7779 RSC.
- (a) J. S. Yadav, B. V. S. Reddy and S. R. Hashim, J. Chem. Soc., Perkin Trans. 1, 2000, 3025 Search PubMed; (b) J. S. Yadav, B.V. S. Reddy, K. Premalatha and T. Swamy, Tetrahedron Lett., 2005, 46, 2687 CrossRef CAS; (c) J. S. Yadav, B. V. S. Reddy, C. V. Rao, P. K. Chand and A. R. Prasad, Synlett, 2001, 1638 CrossRef CAS; (d) D. Bandyopadhyay, S. Mukherjee and B. K. Banik, Molecules, 2010, 15, 2520 CrossRef CAS; (e) For a review, see: M. J. Mphahlele, Molecules, 2009, 14, 5308 CrossRef CAS.
- (a) S. Gogoi, R. Bhuyan and N. C. Barua, Synth. Commun., 2005, 35, 2811 CrossRef CAS; (b) Y. Ren and C. Cai, Catal. Lett., 2007, 118, 134 CrossRef CAS; (c) B. K. Banik, M. Fernandez and C. Alvarez, Tetrahedron Lett., 2005, 46, 2479 CrossRef CAS.
- (a) C. A. Grob and K. Camenisch, Helv. Chim. Acta, 1953, 36, 49 CrossRef CAS; (b) Z.-H. Guan, L. Li, Z.-H. Ren, J. Li and M.-N. Zhao, Green Chem., 2011, 13, 1664 RSC.
- While the formation of imine from aldehyde 3a and the amine 1a should be faster than other reactions it however appeared that due to the reversible nature of the imine formation both aldehyde and amine was available in the reaction mixture to participate in the process shown in Fig. 1.
- (a) See for example: Y.-h. Wang, L. Li and X.-s. Chen, Chem. Res. Chin. Univ., 2008, 24, 520, DOI:10.1016/S1005-9040(08)60109-9; (b) B. P. Bandgar and K. A. Shaikh, Tetrahedron Lett., 2003, 44, 1959 CrossRef CAS.
- (a) A. C. Legon, Angew. Chem., 1999, 111, 2850 CrossRef; (b) G. P. Desiraju and R. L. Harlow, J. Am. Chem. Soc., 1998, 111, 6757 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Copies of spectra for all new compounds. See DOI: 10.1039/c2ra00982j/ |
| This journal is © The Royal Society of Chemistry 2012 |