Benzo[1,2-b:4,5-b′]dithiophene-based conjugated polymers: band gap and energy level control and their application in polymer solar cells
Lijun
Huo
and
Jianhui
Hou
*
State Key Laboratory of Polymer Physics and Chemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: hjhzlz@iccas.ac.cn
First published on 10th August 2011
Abstract
To pursue high power conversion efficiency (PCE) of polymer solar cells (PSCs), more and more new active layer materials, especially polymeric photovoltaic materials, have been developed in the past several years, and benzo[1,2-b:4,5-b′]dithiophene (BDT) plays a very important role in these polymer materials. The applications of these new polymer materials boost the PCE of polymer solar cells greatly, and recent results indicate that 7–8% PCEs have been achieved by using BDT-based polymers. In this review, we summarize the recent works related to BDT-based polymers for the applications in PSCs and try to reveal the correlations of molecular structures of BDT-based polymers with their band gaps and molecular energy levels.
 Lijun Huo | Lijun Huo received his BE degree in 2001 in fine chemistry from China University of Mining and Technology and obtained his PhD degree in chemistry in 2008 from China University of Mining and Technology, Beijing. From 2008 to 2010, he moved to the University of California, Los Angeles (UCLA), USA, for his postdoctoral research with Prof. Yang Yang. Now he works in the Institute of Chemistry, Chinese Academy of Sciences (ICCAS) as associate professor since 2011. His research interests are the design and synthesis of organic/polymer photovoltaic materials. |
 Jianhui Hou | Jianhui Hou obtained his PhD degree in chemistry from ICCAS in 2006. Then he did postdoctoral research in Prof. Yang Yang's group UCLA as a postdoctoral researcher from 2006 to 2008, and in Oct. 2008, he joined Solarmer Energy Inc. in El Monte, US as a senior researcher and then was promoted to the Director of Research. He has been a professor at the Institute of Chemistry, Chinese Academy of Sciences since Oct. 2010. His research interests are the development of organic and polymeric optoelectronic materials, especially in polymeric photovoltaic materials. |
Introduction
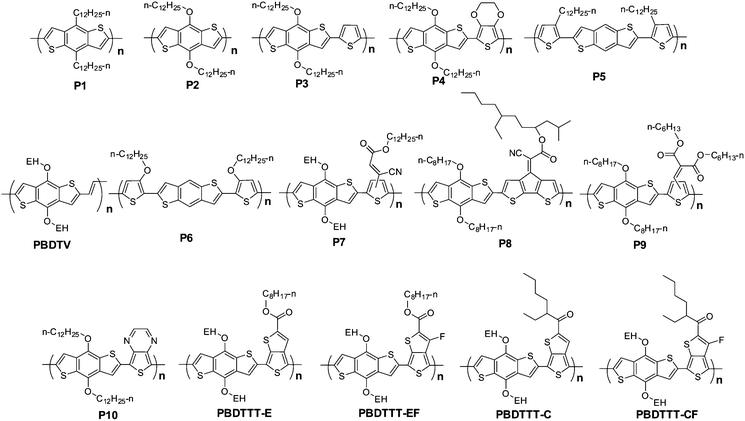
Polymer solar cells (PSCs) are an attractive technology to utilize solar energy due to its potential application in making large area, lightweight and flexible photovoltaic panels. The bulk heterojunction type PSC is the most successful device structure so far, in which the active layer consists of an interpenetrating network of electron donor material and electron acceptor material.1 The bulk heterojunction can be made by coating a solution of electron donor and electron acceptor in an organic solvent. The properties of the electron donor and acceptor, like their absorption spectra, band gaps, molecular energy levels, and so on, are all important issues for achieving high power conversion efficiency (PCE).2 Therefore, to pursue high PCE, more and more new active layer materials, including donors and acceptors, have been designed and developed, and great success has been achieved in the past several years. For example, Hou et al. reported a PCE of 7.74% in 2010, which is the first result over 7%;3 after that several impressive results were reported by different groups. In these works, new materials are the keys to achieve high PCE values. The molecular structures of conjugated polymers with >6.5% PCEs are shown in Scheme 1.3–9 It is very clear that benzo[1,2-b:4,5-b′]dithiophene (BDT) plays a very important role in these highly efficient photovoltaic polymers.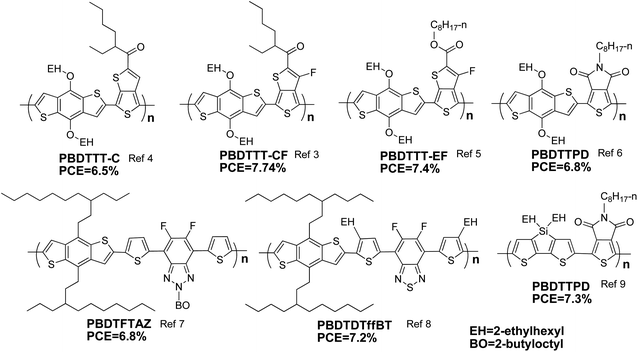 | ||
| Scheme 1 Molecular structures of the polymer photovoltaic materials with PCEs over 6.5%. | ||
BDT has a symmetric and planar conjugated structure, and hence tight and regular stacking can be expected for the BDT-based conjugated polymers. Therefore, BDT-based polymers were firstly used in organic field effect transistors (OFETs). In 2007, a BDT-thiophene-based polymer was reported and exhibited a hole mobility of 0.25 cm2 V−1s−1, which was one of the highest values for polymer-based OFET.10 In 2008, Hou et al. first introduced BDT-based conjugated polymer to polymer solar cells and eight BDT-based photovoltaic polymers were designed, synthesized; band gaps and molecular energy levels of BDT-based polymers were successfully tuned by molecular structure design.11 In the meantime, other groups also exhibited strong interest in this useful conjugated component, and more and more photovoltaic materials with BDT units were developed and reported.
PCE value of a PSC device is directly proportional to three key parameters, short-circuit current (Jsc), open-circuit voltage (Voc) and fill factor (FF).2 From the point of view of the electron donor materials, small band gap, deep HOMO level and high mobility are the keys to realize big Jsc, high Voc and good FF, respectively. In comparison with the polymers with other widely used conjugated building blocks, the polymers based on BDT have a relatively better balance between band gap and HOMO level. As shown in Scheme 2, several conjugated polymers with common units, 4,7-dithiophene-2-yl-2,1,3-benzothiadiazole (DTBT), were selected to make clear comparison.12–17 Based on the results listed in Table 1, it can be seen that the absorption bands of PSiDTBT,17PCPDTDTBT,14 and PDTPDTBT15 match the solar irradiation spectrum better, but the Voc of the PSCs based on them is limited by their high-lying HOMO levels; PFDTBT13 and PCDTBT16 have deep HOMO levels, but their band gaps are too broad to match the solar spectrum well. Additionally, for BDT-based polymers, good mobilities can also be expected due to the symmetric and planar structure of BDT. Therefore, BDT-based polymers made great success in PSCs. This review will focus on BDT-based polymers, and the relationships between molecular structure and molecular energy levels of BDT-based polymers will be concluded and discussed in detail.
 | ||
| Scheme 2 Several photovoltaic polymers with DTBT units. | ||
| E g opt/eV | HOMO/eV | LUMO/eV | V oc/V | J sc/mA cm−2 | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| PBDTDTBT | 1.75 | −5.31 | −3.44 | 0.92 | 10.7 | 0.57 | 5.66 | 12 |
| PFDTBT | 1.90 | −5.52 | −3.50 | 0.97 | 9.1 | 0.51 | 4.5 | 13 |
| PCPDTDTBT | 1.55 | — | — | 0.60 | 8.75 | 0.4 | 2.1 | 14 |
| PDTPDTBT | 1.46 | −5.00 | −3.43 | 0.52 | 9.47 | 0.44 | 2.18 | 15 |
| PCDTBT | 1.88 | −5.45 | −3.60 | 0.86 | 6.8 | 0.56 | 3.6 | 16 |
| PSiDTBT | 1.53 | −4.99 | −3.17 | 0.62 | 10.67 | 0.52 | 3.4 | 17 |
Synthesis of BDT monomers
Starting with thiophene-3-carboxylic acid, the BDT monomers can be synthesized readily. As shown in Scheme 3, benzo[1,2-b:4,5-b′]dithiophene-4,8-dione (M3) is an important intermediate for the synthesis of BDT monomers, which can be synthesized through a 3-step method.11Thiophene-3-carboxylic acid was used as starting material. After dealing with thionyl chloride or oxalyl chloride, thiophene-3-carbonyl chloride (M1) can be obtained, and then it can be aminolyzed by diethyl amine using dichloromethane as solvent to yield N,N-diethylthiophene-3-carboxamide (M2). The total yield of these two steps is over 90%, and the amide can be easily purified by distillation. Then, the amide was reacted with n-butyllithium in THF at 0 °C to produce the benzo[1,2-b:4,5-b′]dithiophene-4,8-dione (M3) with a yield of 75%. The quinoid product can be purified by recrystallization from acetic acid or by sublimation under vacuum.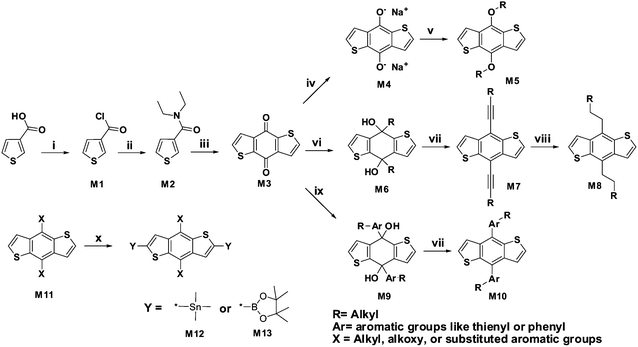 | ||
| Scheme 3 Synthetic procedures of three kinds of BDT monomers. (i) Oxalyl chloride, methylene chloride, ambient temperature, overnight; (ii) diethylamine, methylene chloride, ambient temperature, 30 min; (iii) n-butyllithium, ambient temperature, then water, several hours; (iv) Zn, NaOH, H2O, reflux for 1 h; (v) n-C12H25Br, TBAB, reflux for 6 h; (vi) alkyne lithium; (vii) SnCl2, HCl; (viii) Pd/C, H2; (ix) aromatic lithium; (x) n-butyllithium, ambient temperature, then chlorotrimethylstannane or 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane. | ||
In the reported works, three kinds of functional groups, including alkoxy, alkyl and alkylthiophene, were used as side groups on the 4 and 8 positions of the BDT unit to make solution processable polymers. The alkoxy-substituted BDT can be synthesized easily through a one-pot two-step reaction.11
The quinoid compound was reduced to diol by zinc in a sodium hydroxide solution, and then an excessive amount of alkyl bromide, alkyl iodide or alkyl p-toluenesulfonate was added with catalytic amount of KI and tetrabutylammonium bromide (TBAB). Typically, the yield of the alkoxy substituted BDT (M5) was between 70 and 90%. The alkyl-substituted BDT (M8) compounds can be synthesized by the three step reaction as shown in Scheme 3.18 Firstly, the dione compound was reacted with an alkynyl lithium or alkynyl magnesium halide to make the diol compound (M6), which can be reduced in situ by SnCl2/HCl/H2O easily to produce the alkynyl-substituted BDT compound (M7). Successively, the catalytic hydrogenation was conducted to produce alkyl-substituted BDT (M8). The yield of the three step reaction for alkyl-substituted BDT is over 50%. The alkylthiophene-substituted BDT compounds (M10) were synthesized by the similar reaction for alkynyl-substituted BDTs, but 2-lithium-5-alkyl-thiophene was used instead of alkynyl lithium to form the aromatic diol compound (M9).12
A 4,8-subsituted BDT (M11) has two pairs of protons, and the chemical behaviors of these protons are similar as the protons on a thiophene unit. The protons on 2 and 6 positions exhibit lower pKa value than the protons on 3 and 7 positions, so the 2,6-dilithium salt of BDT can be readily formed by treating the BDT compound with n-butyllithium under room temperature using tetrahydrofuran as solvent. A deprotonation reaction produces 2,6-bis(trialkyltin)-BDT compound (M12) with a yield of >80%. Interestingly, BDTs with branched alkyls are liquid at room temperature, but some of their 2,6-bis(trimethyltin)-substituted derivatives can be purified easily through recrystallization. Therefore, although trimethylstannyl chloride is highly toxic, it was still widely used in making BDT monomers for Stille coupling reactions. It is worthy to mention that a Suzuki coupling reaction can also be employed for the synthesis of some BDT-based polymers (M13).12
Band gap and molecular energy level control of BDT based polymers
The band gaps of active layer materials are of crucial importance to the photovoltaic performance of PSCs. Although great success has been achieved, the mismatch between the absorption band of PSCs and the solar irradiance spectrum is still one of the main obstacles in achieving higher PCE. The application of small band gap materials is an effective way to utilize more sunlight, and consequently, profiting from better harvest of the sunlight, higher Jsc values have already been achieved in the PSCs based on small band gap polymers. Besides band gap, molecular energy level is another crucial issue for achieving high PCE. As reported, the Voc of bulk heterojunction PSCs is directly proportional to the offset between the HOMO level of the electron donor and the LUMO level of the electron acceptor.2,11 For instance, by using an electron acceptor with higher LUMO level than PCBM, the Voc of the poly(3-hexylthiophene) (P3HT)-based PSC can be improved effectively, from 0.6 V to 0.84 V;19 by using an electron donor with deeper HOMO level, the Voc of the PSCs can also be enhanced easily.20How to tune the band gaps and the molecular energy levels of BDT-polymers attracted much attention. By co-polymerizing with different conjugated components or by introducing different functional groups, the band gaps and the molecular energy levels of BDT-based polymers can be tuned in a broad range. In the following sections, the strategies of molecular design will be discussed in detail.
BDT-based polymers with thiophene or its derivatives
As listed in Table 2, the band gap of the alternating copolymer of 4,8-bis-alkyoxy-BDT and thiophene, P3, is ca. 2.06 eV, which is not very suitable for the applications in PSCs.11 In order to minimize the mismatch between the solar irradiation spectrum and absorbance of PSC device, the band gap of the polymer should be reduced. Introducing electron donating functional groups is a feasible method to reduce the band gap of BDT-based polymer. Band gap and HOMO level of the P5 are 2.59 eV and −5.71 eV respectively;21 after replacing its alkyl groups by alkoxy groups, its band gap can be reduced to 1.83 V, and since the alkoxy has strong electron donating effect, HOMO level of P6 reached −4.8 eV, which is 0.91 eV higher than that of P5.22 As a result, after introducing alkoxy groups, the Voc of the PSCs is lowered. For example, the Voc of P4-based PSC is much lower than P3-based PSC.11 To build an alternating copolymer of electron deficient units and electron rich units is another generally used method to make small band gap polymers, and based on this strategy, cyano group and carboxylic ester groups were introduced into BDT-thiophene-based polymers as substituents. The derivatives, P7, P8 and P9 were designed and a 1.13 eV band gap was reported in the polymer P8 (Scheme 4).23,24| HOMO/eV | LUMO/eV | λ max/nm | E g opt/eV | V oc/V | J sc/mA cm−2g | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| P1 | — | — | 450 | 2.22 | — | — | — | — | 18 |
| P2 | −5.16 | −2.67 | 495 | 2.13 | — | — | — | — | 11 |
| P3 | −5.05 | −2.69 | 511 | 2.06 | 0.75 | 3.78 | 0.56 | 1.60 | 11 |
| P4 | −4.56 | −2.66 | 532 | 1.97 | 0.37 | 2.46 | 0.40 | 0.36 | 11 |
| P5 | −5.71 | — | 406 | 2.59 | — | — | — | — | 21 |
| P6 | −4.80 | — | 549 | 1.83 | — | — | — | — | 22 |
| P7 | −5.51 | −3.58 | 551 | 1.93 | 0.85 | 2.79 | 0.34 | 0.79 | 23 |
| P8 | −5.11 | −3.98 | 761 | 1.13 | 0.62 | 0.56 | 0.46 | 0.16 | 24 |
| P9 | −5.03 | −3.0 | 524 | 2.03 | 0.76 | 5.79 | 0.43 | 1.90 | 23 |
| P10 | −4.65 | −3.46 | 780 | 1.05 | 0.22 | 1.41 | 0.35 | 0.11 | 11 |
| PBDTV | −5.07 | −2.86 | 510 | 2.03 | 0.71 | 6.46 | 0.57 | 2.63 | 11,25 |
| PBDTTT-E | −5.01 | −3.24 | 680 | 1.62 | 0.62 | 13.2 | 0.63 | 5.15 | 3 |
| PBDTTT-EF | −5.12 | −3.13 | 682 | 1.63 | 0.74 | 14.5 | 0.69 | 7.4 | 5 |
| PBDTTT-C | −5.12 | −3.35 | 686 | 1.61 | 0.70 | 14.7 | 0.64 | 6.58 | 4 |
| PBDTTT-CF | −5.22 | −3.45 | 674 | 1.60 | 0.76 | 15.2 | 0.67 | 7.73 | 3 |
Besides introducing electron-rich or electron-deficient side groups, designing conjugated polymer with quinoid structures is another effective way to reduce band gap, and this strategy has been already proved to be very effective in other conjugated systems. For BDT-based polymers, P10 was designed based on this strategy.11 In this polymer, 4,8-bis-alkoxyl-BDT was copolymerized with thieno[3,4-b]pyrazine (TPZ), and the band gap of this polymer reached 1.05 eV, which is the lowest one in BDT-based polymers. The design of PBDTTTs, the copolymers based on BDT and thieno[3,4-b]thiophene (TT), is one excellent application of the quinoid structure.3–5 In these polymers, BDT units were alternatively copolymerized with TT units, band gaps of these polymers are ∼1.6 eV. As shown in Scheme 5, in PBDTTT and P10, the quinoid forms can be stabilized by the fused aromatic rings, and hence these polymers exhibit narrower band gaps in comparison with others. More importantly, the molecular energy levels of PBDTTTs can be tuned readily by adding different functional side groups. Liang et al. reported the first PBDTTT polymer, PBDTTT-E in Scheme 4, and the polymer exhibited promising photovoltaic properties, but the Voc of the device was ∼0.6 V only.3,4 In order to reduce the HOMO level of PBDTTT-E, a fluorine atom was introduced to the 5th position of the TT unit and the polymer PBDTTT-EF was designed and prepared.4 Since the fluorine atom has a very strong electron withdrawing effect, a HOMO level of −5.12 eV was obtained, which is 0.1 V lower than that of PBDTTT-E. Ketone exhibited stronger electron withdrawing effect than alkyl ester, and therefore, instead of using alkyl ester as the substituent on TT unit, ketone groups can also be employed to modulate the molecular energy levels of the PBDTTTs. For instance, PBDTTT-C has a very similar molecular structure as PBDTTT-E, but the HOMO level of PBDTTT-C is ∼5.12 eV, which is 0.1 eV higher than that of PBDTTT-E. As a result, Voc of PBDTTT-C based PSCs reached 0.7 V and a PCE of 6.5% was obtained.4 In another work reported by Hou et al., a polymer named as PBDTTT-CF was designed and its HOMO level reached −5.22 eV due to the synergistic electron-withdrawing effect of the fluorine and the ketone group. Based on PBDTTT-CF, a PCE result of 7.74% with a Voc of 0.76 V was achieved.3
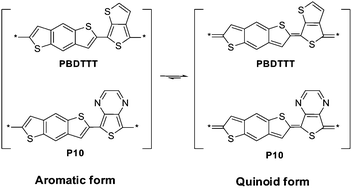 | ||
| Scheme 5 Aromatic and quinoid forms of two BDT-based polymers. | ||
BDT-based polymers with 2,1,3-benzothiadiazole and the like
2,1,3-Benzothiadiazole (BT) has been broadly used as an electron-deficient conjugated component in conjugated polymers. Many conjugated polymers with BT unit have been developed for the applications in optoelectronic or electronic devices.32–34 As an electron deficient unit, when BT units were copolymerized with other conjugated components, like fluorenes or thiophenes, the π-electrons can be delocalized more efficiently and hence band gap of the conjugated polymers can be lowered. Therefore, one of the successful applications of BT in conjugated polymers is to make red emitting materials for polymer light emitting devices (PLEDs).35−39 BT also plays an important role in making small band gap photovoltaic polymers. Many highly efficient photovoltaic polymers with BT units, like PCPDTBT40–42 and PSBTBT,43,44 were developed in the past several years. Over 5% efficiencies were obtained based on these materials.Hou et al. firstly reported a small band gap copolymer of BDT and BT, P11 in Scheme 6.11The band gap and HOMO level of this polymer are ∼1.7 eV and −5.10 eV, respectively. When BDT was copolymerized with 2,1,3-benzoselenadiazole (BSe), the band gap can be reduced to 1.52 eV.11 The quinoxaline units can also be employed, and P1311 and P1426 provide good examples. The 2,3-diphenylpyrido[4,3-b]pyrazine (DPPP) unit in P14 is more deficient in π-electrons than the 2,3-diphenylquinoxaline (DPQ) unit in P13, as the former is hybridized by more nitrogen atoms than the latter. HOMO level of P14 is −5.22 eV, which is ∼0.4 eV lower than that of P13.
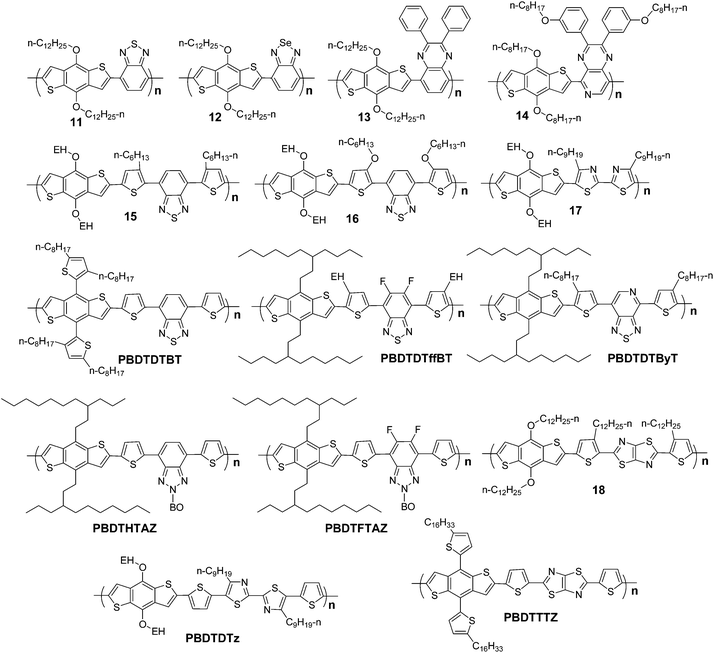 | ||
| Scheme 6 Molecular structures of BDT-based polymers with 2,1,3-benzothiadiazole and the like. | ||
4,7-Di-2-thienyl-2,1,3-benzothiadiazole (DTBT) and its derivatives are broadly used to make photovoltaic polymers and great progress has been achieved by using this unit. Several important materials, like PFDTBT, PFSiDBT, PCDTBT, and PSiDTBT, have been developed and PCEs between 4 and 6% have been reported by different research groups. Hou et al. reported two polymers based on DTBT and BDT, P15 and P16 in Scheme 6.27 There is a little difference between these two polymers. On the thiophene unit of P15, hexyl was used as a substituent; for P16, hexyloxy was used. The HOMO level of P15 is 0.4 eV lower than that of P16, as alkoxy has stronger electron donating effect than alkyl. As a result, the Voc of P15-based PSC is 0.4 V higher than that of P16. Since alkyl still has weak electron donating effect, it can be predicted that removing all alkyl groups on the thiophene units of P16 will reduce the HOMO level further. PBDTDTBT give a good example for this idea. In PBDTDTBT, no alkyl or alkoxy groups are directly linked with the conjugated main chain. The HOMO level of PBDTDTBT is ∼5.3 eV, and thus a Voc of 0.92 V was obtained with a PCE of 5.6% (Table 3).12
| HOMO/eV | LUMO/eV | λ max/nm | E g opt/eV | V oc/V | J sc/mA cm−2 | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 11 | −5.10 | −3.19 | 591 | 1.7 | 0.68 | 2.97 | 0.44 | 0.9 | 11 |
| 12 | −4.88 | −3.33 | 641 | 1.52 | 0.55 | 1.05 | 0.32 | 0.18 | 11 |
| 13 | −4.78 | −3.28 | 601 | 1.63 | 0.6 | 1.54 | 0.26 | 0.3 | 11 |
| 14 | −5.22 | −3.32 | 680 | 1.58 | 0.74 | 4.69 | 0.34 | 1.2 | 26 |
| 15 | −4.8 | — | 531 | 1.85 | 0.84 | 6.28 | 0.37 | 1.95 | 27 |
| 16 | −5.2 | — | 625 | 1.55 | 0.4 | 5.27 | 0.60 | 1.28 | 27 |
| 17 | −5.04 | −3.04 | 530 | 2.0 | 0.73 | 6.63 | 0.42 | 2.03 | 28 |
| 18 | −5.05 | −3.11 | 532 | 1.94 | 0.77 | 6.68 | 0.51 | 2.06 | 28 |
| PBDTDTBT | −5.31 | −3.44 | 596 | 1.75 | 0.92 | 10.7 | 0.57 | 5.66 | 12 |
| PBDTDTffBT | −5.54 | −3.33 | 620 | 1.70 | 0.91 | 12.91 | 0.61 | 7.2 | 8 |
| PBDTDTByT | −5.47 | −3.44 | 685 | 1.51 | 0.85 | 12.78 | 0.58 | 6.32 | 29 |
| PBDTDTz | −5.15 | −2.95 | 530 | 2.0 | 0.86 | 7.84 | 0.57 | 3.82 | 30 |
| PBDTHTAZ | −5.29 | −2.87 | 580 | 1.98 | 0.70 | 11.4 | 0.55 | 4.3 | 7 |
| PBDTFTAZ | −5.36 | −3.05 | 580 | 2.0 | 0.79 | 11.8 | 0.73 | 6.81 | 7 |
| PBDTTTZ | −5.3 | −3.2 | 582 | 2.0 | 0.85 | 10.4 | 0.59 | 5.22 | 31 |
Although the alkyl groups on DTBT unit can cause slight elevation of the HOMO level, they are very helpful to keep good solubility of the polymer. You et al. reported an effective method to circumvent the negative effect of alkyls. They introduced two fluorine atoms on the 5 and 6 positions of BT unit. The target polymer, PDBTDTffBT, shows a band gap of 1.7 eV with a HOMO level of −5.54 eV. This polymer also exhibits excellent photovoltaic performance and a PCE of 7.2% with a high Voc of 0.91 V was recorded.8 They also reported another effective way to modulate the molecular energy level of this kind of polymers. A conjugated component with stronger electron deficient effect than BT was used to replace the BT unit in PBDTDTBT, and thus a new polymer with smaller band gap and lower HOMO level can be obtained, namely PBDTDTByT in Scheme 6. This polymer also exhibits promising photovoltaic properties, and a PCE of 6.32% was recorded.29
The electron withdrawing effect of fluorine can also be utilized in other BDT-contained polymers. You et al. reported two 2-alkyl-benzo[d][1,2,3]triazole (TAZ)-based polymers, PBDTHTAZ and PBDTFTAZ as shown in Scheme 6. After adding two fluorine atoms on the TAZ component, the HOMO level of the polymer can be lowered to 0.07 eV without affecting its band gap.7
Thiazole and thiazolo[5,4-d]thiazole also can be used in BDT-based polymers. PBDTDTz30 and PBDTTTZ31 are two representatives of this kind of materials. Although the band gaps of these two polymers are ∼2.0 eV and their absorption bands are not very broad, they also exhibit good photovoltaic properties. Especially for the PBDTTTZ-based PSCs, a PCE of 5.2% was recorded. Since the absorption band of PBDTTTZ is mainly at the range from 400 to 600 nm, it can be used as blue absorber in PSCs with tandem structure.
BDT polymers with amide, imide and diimide-containing conjugated components
Conjugated organic compounds containing amide, imide or diimide or the like have strong extinction coefficient and also strong photoluminescence and are broadly used as dyes in different applications.45–47 This kind of dye is also introduced in BDT-based polymers as building blocks. For example, phthalimide (PI), naphthalene diimide (NDI), and perylene diimide (PDI) were copolymerized with BDT, and correspondingly, three polymers, P19, PBDTNDI and PBDTPDI, were prepared (Scheme 7). Based on the data listed in Table 4, it can be seen that their band gaps and molecular energy levels are also tunable in a broad range.48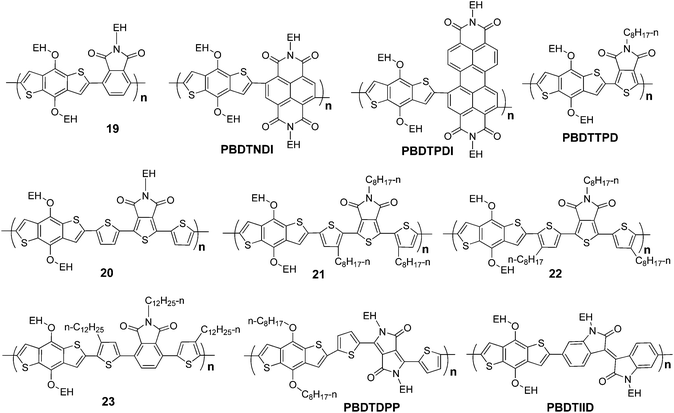 | ||
| Scheme 7 Molecular structures of BDT-based polymers with amide, imide and diimide-containing conjugated components. | ||
| HOMO/eV | LUMO/eV | λ max/nm | E g opt/eV | V oc/V | J sc/mA cm−2 | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 19 | −5.51 | −3.36 | 508 | 2.15 | — | — | — | — | 48 |
| 20 | −5.49 | −3.70 | 516 | 1.84 | 0.76 | 2.9 | 0.43 | 0.95 | 49 |
| 21 | −5.56 | −3.70 | 539 | 1.88 | 0.89 | 7.6 | 0.57 | 3.9 | 49 |
| 22 | −5.66 | −3.83 | 539 | 1.86 | 0.66 | 1.2 | 0.26 | 0.2 | 49 |
| 23 | −5.32 | −3.34 | 500 | 1.98 | 2.96 | 0.93 | 0.56 | 1.54 | 50 |
| PBDTNDI | −5.62 | −3.99 | 604 | 1.63 | — | — | — | — | 48 |
| PBDTPDI | −5.91 | −4.18 | 549 | 1.74 | — | — | — | — | 48 |
| PBDTTPD | −5.56 | −3.75 | 627 | 1.73 | 0.85 | 11.8 | 0.68 | 6.8 | 6 |
| PBDTDPP | −5.16 | −3.51 | 750 | 1.31 | 0.68 | 8.4 | 0.44 | 2.53 | 51 |
| PBDTIID | −5.20 | −3.66 | 688 | 1.54 | 0.71 | 7.93 | 0.34 | 1.91 | 52 |
Thiophene-based imide, N-alkylthieno[3,4-c]pyrrole-4,6-dione (TPD), exhibits promising properties as building block in BDT-based polymers. In 2010, four different research groups reported a series of results of PBDTTPDs-based PSCs.6,53–55 In these works, the same conjugated backbone, BDT-alt-TPD, was selected, and different alkyl side groups were employed. These polymers have very similar band gaps and molecular energy levels, and the best photovoltaic result, ∼6.8%, was reported by Fréchet et al.6 In this work, 2-ethylhexyloxy was used as a substituent on the BDT unit, and three different alkyls, octyl, 3,7-dimethyl-octyl and 2-ethylhexyl, were selected to optimize the photovoltaic properties of PBDTTPDs. They found that the choice of the alkyls impacts structural order and orientation in polymer backbones, which critically affects photovoltaic performance of PSCs.
A more detailed research of BDT-TPD-based polymers was reported by Leclerc et al.49 In this work, the relationship among the length of the alkyl on the TPD units, the morphology of the copolymers, and the photovoltaic properties of the copolymer-based PSCs were fully investigated, providing a good example for the molecular structural optimization of photovoltaic polymers.
3,6-Diaryl-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (DPP) and 1,1′-dialkyl-6,6′-isoindigo (IID) have been applied in paints, plastic ink, electroluminescent devices, and transistors.51,52,56,57 The lactam unit, which can be seen as a derivative of amide, has a strong electron withdrawing effect, and therefore, they can be used as electron withdrawing blocks in conjugated polymers. Recently, several interesting photovoltaic polymers have been developed based on DPP and IID, PBDTDPP51 and PBDTIID.52 Although the photovoltaic performance of these two kinds of materials is not high, their low-lying HOMO levels and small band gaps indicate that these two kinds of polymers have great potential to make high performance photovoltaic devices.
Summary and outlook
As summarized in the monomer synthesis section, three kinds of BDT monomers, alkyl, alkoxy and aryl-substituted BDT, can be prepared readily with high yields. Usually, Suzuki or Stille coupling reactions are used to make BDT-based polymers, since these two kinds of Pd catalyzed coupling reaction have good tolerance to different functional groups; the copolymerization between BDT and various conjugated building blocks gives many kinds of polymers with different properties. The band gaps, the molecular energy levels, the absorption bands and the other properties of BDT-based polymers can be effectively tuned by copolymerizing with different conjugated units or selecting different functional side groups. Many BDT-based conjugated polymers, including several very promising photovoltaic polymer families, like PBDTTTs, PBDTDTBTs, PBDTTPDs, etc., have been already developed by different research groups, and 7–8% PCEs have been already realized. These promising results demonstrate that the BDT unit plays a very important role in photovoltaic materials.In the past several years, people paid much attention to band gap and molecular control of BDT-based polymers. As known, photovoltaic properties of conjugated polymers are also closely related to their morphology or stacking behavior. Recently, several research groups reported the results related to BDT-based polymers and tried to reveal the relationship between their photovoltaic properties and the morphology/stacking property of the polymer/PCBM blends. Although how to control the morphology through molecular structure design is still a great challenge, its importance has been noticed by the researchers in this field and would yield much more promising results in the near future.
On the other hand, from the point of view of chemical structures, although a lot of BDT-based polymers have been reported, fewer efforts have been employed to modify the BDT part. It is reasonable to believe that better results should be achievable if detailed modification of the BDT units can be done. Based on the great success of BDT-based polymers, this kind of polymer will play a very important role in the field of PSC.
Acknowledgements
This work was supported by the Chinese Academy of Sciences.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 CrossRef CAS.
- J. Hou, H.-Y. Chen, S. Zhang, R. I. Chen, Y. Yang, Y. Wu and G. Li, J. Am. Chem. Soc., 2009, 131, 15586 CrossRef CAS.
- Y. Liang and L. Yu, Acc. Chem. Res., 2010, 43, 1227 CrossRef CAS.
- C. Piliego, T. W. Holcombe, J. D. Douglas, C. H. Woo, P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2010, 132, 7595 CrossRef CAS.
- S. C. Price, A. C. Stuart, L. Yang, H. Zhou and W. You, J. Am. Chem. Soc., 2011, 133, 4625 CrossRef CAS.
- H. Zhou, L. Yang, A. C. Stuart, S. C. Price, S. Liu and W. You, Angew. Chem., Int. Ed., 2011, 50, 2995 CrossRef CAS.
- T.-Y. Chu, J. Lu, S. Beaupré, Y. Zhang, J.-R. Pouliot, S. Wakim, J. Zhou, M. Leclerc, Z. Li, J. Ding and Y. Tao, J. Am. Chem. Soc., 2011, 133, 4250 CrossRef CAS.
- H. Pan, Y. Li, Y. Wu, P. Liu, B. S. Ong, S. Zhu and G. Xu, J. Am. Chem. Soc., 2007, 129, 4112 CrossRef CAS.
- J. Hou, M.-H. Park, S. Zhang, Y. Yao, L.-M. Chen, J.-H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS.
- L. Huo, J. Hou, S. Zhang, H.-Y. Chen and Y. Yang, Angew. Chem., Int. Ed., 2010, 49, 1500 CrossRef CAS.
- M.-H. Chen, J. Hou, Z. Hong, G. Yang, S. Sista, L.-M. Chen and Y. Yang, Adv. Mater., 2009, 21, 4238 CrossRef CAS.
- J. Moule, A. Tsami, T. W. Bünnagel, M. Forster, N. M. Kronenberg, M. Scharber, M. Koppe, M. Morana, C. J. Brabec, K. Meerholz and U. Scherf, Chem. Mater., 2008, 20, 4045 CrossRef.
- E. Zhou, M. Nakamura, T. Nishizawa, Y. Zhang, Q. Wei, K. Tajima, C. Yang and K. Hashimoto, Macromolecules, 2008, 41, 8302 CrossRef CAS.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS.
- L. Huo, H.-Y. Chen, J. Hou, T. L. Chen and Y. Yang, Chem. Commun., 2009, 5570 RSC.
- H. Pan, Y. Li, Y. Wu, P. Liu, B. S. Ong, S. Zhu and G. Xu, Chem. Mater., 2006, 18, 3237 CrossRef CAS.
- Y. He, H.-Y. Chen, J. Hou and Y. Li, J. Am. Chem. Soc., 2010, 132, 1377 CrossRef CAS.
- (a) J. Hou, T. L. Chen, S. Zhang, L. Huo, S. Sista and Y. Yang, Macromolecules, 2009, 42, 9217 CrossRef CAS; (b) L. Huo, T. Chen, Y. Zhou, J. Hou, H. Chen, Y. Yang and Y. Li, Macromolecules, 2009, 42, 4377 CrossRef CAS.
- T. T. M. Dang, S.-J. Park, J.-W. Park, D.-S. Chung, C. E. Park, Y.-H. Kim and S.-K. Kwon, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5277 CrossRef CAS.
- M. A. M. Leenen, T. Meyer, F. Cucinotta, H. Thiem, R. Anselmann and L. De Cola, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1973 CrossRef CAS.
- K.-H. Lee, H.-J. Lee, K. Morino, A. Sudo and T. Endo, Macromol. Chem. Phys., 2010, 211, 2490 CrossRef CAS.
- K.-H. Lee, H.-J. Lee, K. Morino, A. Sudo and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1427 CrossRef CAS.
- Y. He, Y. Zhou, G. Zhao, J. Min, X. Guo, B. Zhang, M. Zhang, J. Zhang, Y. Li, F. Zhang and O. Inganäs, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1822 CrossRef CAS.
- M.-C. Yuan, M.-Y. Chiu, C.-M. Chiang and K.-H. Wei, Macromolecules, 2010, 43, 6270 CrossRef CAS.
- J. Hou, H.-Y. Chen, S. Zhang and Y. Yang, J. Phys. Chem. C, 2009, 113, 21202 CAS.
- M. Yang, B. Peng, B. Liu, Y. Zou, K. Zhou, Y. He, C. Pan and Y. Li, J. Phys. Chem. C, 2010, 114, 17989 CAS.
- H. Zhou, L. Yang, S. C. Price, K. J. Knight and W. You, Angew. Chem., Int. Ed., 2010, 49, 7992 CrossRef CAS.
- M. Zhang, H. Fan, X. Guo, Y. He, Z.-G. Zhang, J. Min, J. Zhang, G. Zhao, X. Zhan and Y. Li, Macromolecules, 2010, 43, 8714 CrossRef CAS.
- L. Huo, X. Guo, S. Zhang, Y. Li and J. Hou, Macromolecules, 2011, 44, 4035 CrossRef CAS.
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS.
- X. Zhan and D. Zhu, Polym. Chem., 2010, 1, 409 RSC.
- P.-L. T. Boudreault, A. Najari and M. Leclerc, Chem. Mater., 2010, 23, 456 CrossRef.
- C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897 CrossRef.
- C. Zhong, C. Duan, F. Huang, H. Wu and Y. Cao, Chem. Mater., 2011, 23, 326 CrossRef CAS.
- S. Beaupré, P.-L. T. Boudreault and M. Leclerc, Adv. Mater., 2010, 22, E6 CrossRef.
- P. Herguth, X. Jiang, M. S. Liu and A. K.-Y. Jen, Macromolecules, 2002, 35, 6094 CrossRef CAS.
- F. Perepichka, D. F. Perepichka, H. Meng and F. Wudl, Adv. Mater., 2005, 17, 2281 CrossRef.
- D. Muhlbacher, M. Scharber, M. Morana, Z. Zhu, D. Waller, R. Gaudiana and C. Brabec, Adv. Mater., 2006, 18, 2884 CrossRef.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS.
- J. Hou, H.-Y. Chen, S. Zhang, G. Li and Y. Yang, J. Am. Chem. Soc., 2008, 130, 16144 CrossRef CAS.
- H.-Y. Chen, J. Hou, A. E. Hayden, H. Yang, K. N. Houk and Y. Yang, Adv. Mater., 2009, 22, 371 CrossRef.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef CAS.
- R. P. Ortiz, A. Facchetti and T. J. Marks, Chem. Rev., 2010, 110, 205 CrossRef CAS.
- H. Quante and K. Müllen, Angew. Chem., Int. Ed. Engl., 1995, 34, 1323 CrossRef CAS.
- J. Chen, M.-M. Shi, X.-L. Hu, M. Wang and H.-Z. Chen, Polymer, 2010, 51, 2897 CrossRef CAS.
- A. Najari, S. Beaupré, P. Berrouard, Y. Zou, J.-R. Pouliot, C. Lepage-Pérusse and M. Leclerc, Adv. Funct. Mater., 2011, 21, 718 CrossRef CAS.
- G. Zhang, Y. Fu, Q. Zhang and Z. Xie, Macromol. Chem. Phys., 2010, 211, 2596 CrossRef CAS.
- L. Huo, J. Hou, H.-Y. Chen, S. Zhang, Y. Jiang, T. L. Chen and Y. Yang, Macromolecules, 2009, 42, 6564 CrossRef CAS.
- G. Zhang, Y. Fu, Z. Xie and Q. Zhang, Macromolecules, 2011, 44, 1414 CAS.
- Y. Zou, A. Najari, P. Berrouard, S. Beaupré, B. R. Aïch, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330 CrossRef CAS.
- G. Zhang, Y. Fu, Q. Zhang and Z. Xie, Chem. Commun., 2010, 46, 4997 RSC.
- Y. Zhang, S. K. Hau, H.-L. Yip, Y. Sun, O. Acton and A. K.-Y. Jen, Chem. Mater., 2010, 22, 2696 CrossRef CAS.
- L. Bürgi, M. Turbiez, R. Pfeiffer, F. Bienewald, H.-J. Kirner and C. Winnewisser, Adv. Mater., 2008, 20, 2217 CrossRef.
- R. Stalder, J. Mei and J. R. Reynolds, Macromolecules, 2010, 43, 8348 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |