Conjugated polymers for high-efficiency organic photovoltaics
Xiaowei
Zhan
* and
Daoben
Zhu
Beijing National Laboratory for Molecular Sciences and CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: xwzhan@iccas.ac.cn
First published on 7th January 2010
Abstract
Organic photovoltaics (OPVs) are a promising cost-effective alternative to silicon-based solar cells, and possess low-cost, light-weight, and flexibility advantages. Recently, great advances have been achieved in the development of novel photovoltaic materials and device structures, and the power conversion efficiencies can now reach 7.7%. In this review we summarize the most recent developments in conjugated polymers for high-efficiency OPV devices. We focus on correlations of polymer chemical structures with properties, such as absorption, bandgap, energy levels, mobilities, and photovoltaic performances. This structure-property relationship analysis may guide rational structural design and evaluation of photovoltaic materials.
 Xiaowei Zhan | Xiaowei Zhan obtained a Ph.D. degree in chemistry from Zhejiang University in 1998. He was then a postdoctoral researcher at Institute of Chemistry, Chinese Academy of Sciences (ICCAS) from 1998 to 2000, and in 2000 he was promoted to Associate Professor at ICCAS. Dr Zhan worked in the University of Arizona and Georgia Institute of Technology from 2002 to 2006 as Research Associate and Research Scientist. He has been a professor at ICCAS since 2006. His research interests are the development of organic and polymeric materials for organic electronics and photonics. |
 Daoben Zhu | Daoben Zhu is a professor and Director of the Organic Solids Laboratory of the Institute of Chemistry, Chinese Academy of Sciences. He was selected as an academician of the Chinese Academy of Sciences in 1997. He finished his graduate courses at the East China University of Science and Technology in 1968. From 1977 to 1979 and from 1985 to 1986, as a visiting-scholar and visiting-scientist he performed research in the Max-Planck Institute for Medical Research in Heidelberg, Germany, respectively. His research interests include molecular materials and devices. |
Introduction
Fossil fuels such as coal, oil, and gas are the most used energy sources. Fossil fuel production and use causes a number of environmental problems, and also their availability is diminishing. The need to develop renewable energy sources has become urgent. The development of photovoltaic (PV) cells, which transform inexhaustible solar energy into electricity, is therefore one of the most promising long-term solutions for clean, renewable energy. Currently, the main barrier that prevents PV technology from providing a large fraction of energy is the high cost of silicon-based solar cells.Organic photovoltaics (OPVs) are a promising cost-effective alternative to silicon-based solar cells, and possess low-cost, light-weight, and flexibility advantages. Since the discovery of photoinduced charge transfer in composites of conjugated polymers and C60,1polymer solar cells (PSCs) have attracted considerable attention. Recently, polymer photovoltaic materials and devices are a rapidly developing research field. So far, the highest power conversion efficiency (PCE) for small-area PSCs is 7.7%,2 still far below the 10% that is often regarded as being a prerequisite for large-scale commercial applications.
Yu et al.3 and Halls et al.4 pioneered the “bulk heterojunction” (BHJ) concept, which is the best OPV device architecture so far. BHJ is a blend of bicontinuous and interpenetrating donor and acceptor components in a bulk volume. Such a nanoscale network exhibits a donor–acceptor phase separation in a 10–20 nm length scale, which is within a distance less than the exciton diffusion length. BHJ significantly increases the donor–acceptor interfacial area, leading to enhanced efficiency. Moreover, BHJ devices can be fabricated by high-throughput processing methodologies, such as spin coating, inkjet printing, gravure and flexographic printing.
In a typical BHJPSC, the photoactive blend layer, sandwiched between two electrodes, is composed of a conjugated polymer donor and an acceptor (small molecule or polymer). The synthesis of new conjugated polymers with small bandgaps, strong and broad absorption, appropriate energy levels, and high carrier mobilities will be the key to allowing plastic solar cells to become a viable energy source for this century. Several reviews have summarized the synthesis and use of conjugated polymers in OPVs5–12 as well as device physics.13–20 In the present review, we focus on the representative conjugated polymer donors, polymer acceptors and double-cable polymers for high-efficiency PSCs. Progress in the past decade has been substantial, but continued development of PV materials will require a better understanding of the relationships between molecular structure, electronic structure, materials microstructure, charge transport and photovoltaic properties than is currently available. For these reasons, we will survey and analyze what is currently known concerning structure/property relationships of photovoltaic polymers.
Conjugated polymer donors
Polythiophene derivatives
Polythiophenes, particularly regioregular poly(3-alkylthiophene)s (P3ATs), are a widely used class of polymer donors in PSCs due to their excellent thermal and chemical stability as well as good light-harvesting and charge-transporting properties. Table 1 provides a summary of electronic properties as well as PSC data for representative polythiophene derivatives (Fig. 1).21–25 The length of alkyl group in P3ATs plays an important role in determining the solubility, crystallinity and morphology. Nguyen et al. investigated effect of alkyl groups (butyl, hexyl, octyl, decyl, and dodecyl) on performance of P3AT:fullerene BHJ PSCs.26 Longer alkyl lengths (≥ C8) facilitate diffusion rates of the fullerene soluble derivative PCBM in the polymer matrix, leading to larger scale of phase separation, reduced interfacial area, and finally lower efficiency. PSCs based on regioregular poly(3-butylthiophene) (P3BT) exhibited one order of magnitude lower PCEs compared to its counterpart poly(3-hexylthiophene) (P3HT), due to poor solubility, poor crystallinity, smaller conjugation length, and lower carrier mobility.27| λ max abs/nm | E g/eV | μ h/cm2V−1 s−1 | HOMO/LUMO/eV | D/PC61BM (w/w) | J sc/mA cm−2 | V oc/V | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| a λ max abs = absorption peak in film; Eg = optical bandgap; μh = hole mobility; D = donor; Jsc = short-circuit current density; Voc = open-circuit voltage; FF = fill factor; PCE = power conversion efficiency. b 50 mW cm−2. | ||||||||||
| 1a | 552 | 1.8 | — | −4.8/−2.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.9 | 0.60 | 0.41 | 2.41 | 21 |
| 1b | — | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.77 | 0.61 | 0.62 | 3.73 | 22 |
| 1c | 630 | 1.6 | 0.02 | −4.8/−3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | 0.52 | — | 2.7b | 23 |
| 1d | 540 | 1.8 | — | −4.9/−3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10.3 | 0.72 | 0.43 | 3.18 | 21 |
| 1e | 550 | 1.8 | — | −4.7/−3.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13.7 | 0.68 | 0.37 | 3.45 | 24 |
| 1f | 660 | 1.4 | — | −5.1/−3.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.4 | 0.63 | 0.54 | 3.2 | 25 |
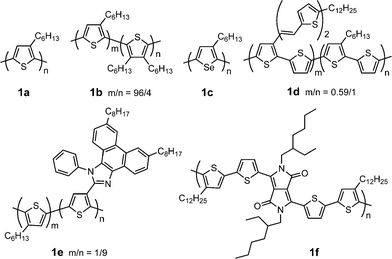 | ||
| Fig. 1 Chemical structure of some polythiophene derivatives. | ||
P3HT (1a, Fig. 1) gave the best device performance in the P3AT family. So far, the P3HT/PCBM system is one of the most efficient BHJ PSCs with PCEs of ca. 5%. The device performance is susceptible to the molecular weight and regioregularity of P3HT and device fabrication methods. The molecular weight and polydispersity of P3HT exhibit significant impacts on PSC performance;28 only P3HT with a number-average molecular weight greater than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 can achieve a PCE over 2.5%. Fréchet et al. investigated the influence of the regioregularity of P3HT on P3HT/PC61BM PSC performance.29 All three polymers (86%, 90%, and 96% regioregularities) exhibit similar mobilities (10−3 cm2V−1 s−1); their blends with PC61BM gave similar PCEs (ca. 4%). They introduced a small amount of 3,4-dihexylthiophene into P3HT to reduce the regioregularity and to weaken the crytallization formation.221b (Fig. 1) exhibited similar device efficiency but better thermal stability compared to 1a. This is ascribed to the suppression of the crystallization-driven phase separation by introduction of a controlled amount of disorder into the polymer backbone. The success of the P3HT/PCBM system is largely associated with careful control and optimization of the active layer morphology, such as using different casting solvents and film-forming speed, solvent and thermal annealing, additives to the active layer, optical spacer, anode and cathode interfacial layer, and tandem structure.13–19
000 can achieve a PCE over 2.5%. Fréchet et al. investigated the influence of the regioregularity of P3HT on P3HT/PC61BM PSC performance.29 All three polymers (86%, 90%, and 96% regioregularities) exhibit similar mobilities (10−3 cm2V−1 s−1); their blends with PC61BM gave similar PCEs (ca. 4%). They introduced a small amount of 3,4-dihexylthiophene into P3HT to reduce the regioregularity and to weaken the crytallization formation.221b (Fig. 1) exhibited similar device efficiency but better thermal stability compared to 1a. This is ascribed to the suppression of the crystallization-driven phase separation by introduction of a controlled amount of disorder into the polymer backbone. The success of the P3HT/PCBM system is largely associated with careful control and optimization of the active layer morphology, such as using different casting solvents and film-forming speed, solvent and thermal annealing, additives to the active layer, optical spacer, anode and cathode interfacial layer, and tandem structure.13–19
Poly(3-hexylselenophene) (P3HS, 1c) is an analogue of P3HT with selenium replacing the sulfur atom. P3HS exhibits lower LUMO level (similar HOMO), smaller bandgap, and significantly red-shifted absorption than P3HT.23 Moreover, P3HS displays crystalline morphology and FET mobility (0.02–0.04 cm2V−1 s−1) similar to that of P3HT. The solar cell device based on P3HS/PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) produced a PCE of 2.7% after optimal thermal annealing, slightly lower than that of P3HT/PC61BM (3.0%).
1, w/w) produced a PCE of 2.7% after optimal thermal annealing, slightly lower than that of P3HT/PC61BM (3.0%).
To enhance absorbance in the short-wavelength region (< 500 nm) of polythiophene, Liet al. attached bi(thienylenevinylene) conjugated side chain to the polythiophene backbone (1d).21 This two-dimensional conjugated polythiophene shows a broad absorption plateau from 350 to 650 nm and much stronger absorbance than that of P3HT in the wavelength 350–480 nm. The maximum PCE of the PSCs based on 1d/PC61BM reached 3.18%, which is 38% enhancement in comparison with that (2.41%) of P3HT under the same experimental conditions. Wei and co-workers reported a new regioregular polythiophene with (octylphenanthrenyl)imidazole conjugated side chain (1e).24 The electron-withdrawing ability of this side chain induces efficient intramolecular charge transfer from the donor thiophene main chain to the acceptor side chain. Thus, charge separation is facilitated through sequential electron transfer from the main chain to the side chain and then to PC61BM. The faster electron transfer leads to a higher PCE (3.45%) compared to P3HT.
To red shift absorption spectrum and to lower HOMO level of polythiophene, Janssen and co-workers copolymerized electron-rich quaterthiophene with electron-deficient diketopyrrolopyrrole unit to afford a D–A copolymer (1f).251f exhibits lower HOMO and LUMO levels, smaller bandgap (1.4 eV), and significantly red-shifted absorption than P3HT. The best device using 1f/PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) gave a PCE of 3.2%. By utilizing PC71BM as the acceptor, the PCE was further improved to 4.0%.
2, w/w) gave a PCE of 3.2%. By utilizing PC71BM as the acceptor, the PCE was further improved to 4.0%.
Poly(arylenevinylene)s and poly(aryleneethynylene)s
Table 2 provides a summary of electronic properties as well as PSC data for representative poly(arylenevinylene)s and poly(aryleneethynylene)s (Fig. 2).30–34Poly[(2-methoxy-5-(3′,7′-dimethyloctyloxy))-1,4-phenylenevinylene] (MDMO-PPV, 2a) is the best donor among the poly(p-phenylenevinylene)s (PPVs) in BHJ PSCs. Shaheen et al. reported devices based on 2a:PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, w/w) with a PCE of 2.5%.35 Tajima et al. synthesized fully regioregular 2a and utilized for PSCs.30 Significant improvements in short-circuit current (JSC), fill factor (FF) and PCE were achieved for the devices with regioregular 2a compared to those for its regiorandom counterpart, which benefited from both higher hole mobility (resulting from the higher crystallinity) of the regioregular polymer and better mixing morphology of the blend. A PCE of 3.1% was achieved with regioregular MDMO-PPV, which is the highest reported for the PPV:PC61BM system so far.
4, w/w) with a PCE of 2.5%.35 Tajima et al. synthesized fully regioregular 2a and utilized for PSCs.30 Significant improvements in short-circuit current (JSC), fill factor (FF) and PCE were achieved for the devices with regioregular 2a compared to those for its regiorandom counterpart, which benefited from both higher hole mobility (resulting from the higher crystallinity) of the regioregular polymer and better mixing morphology of the blend. A PCE of 3.1% was achieved with regioregular MDMO-PPV, which is the highest reported for the PPV:PC61BM system so far.
| λ max abs/nm | E g/eV | μ h/cm2V−1 s−1 | HOMO/LUMO/eV | D/PC61BM (w/w) | J sc/mA cm−2 | V oc/V | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| a λ max abs = absorption peak in film; Eg = optical bandgap; μh = hole mobility; D = donor; Jsc = short-circuit current density; Voc = open-circuit voltage; FF = fill factor; PCE = power conversion efficiency. | ||||||||||
| 2a | 540 | — | 3 × 10−10 | −5.4/— | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6.2 | 0.71 | 0.7 | 3.1 | 30 |
| 2b | 556 | 1.7 | 3 × 10−3 | −5.3/−3.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
5.47 | 0.86 | 0.43 | 2.01 | 31 |
| 2c | 650 | 1.6 | — | −5.2/−3.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10.72 | 0.67 | 0.33 | 2.37 | 32 |
| 2d | 554 | 1.9 | — | −5.4/−3.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
15.43 | 0.82 | 0.39 | 4.93 | 33 |
| 2e | 611 | 1.8 | 1 × 10−2 | −5.1/−3.3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
5.67 | 0.79 | 0.49 | 2.22 | 34 |
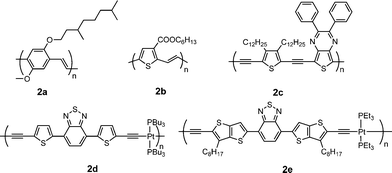 | ||
| Fig. 2 Chemical structure of some poly(arylenevinylene)s and poly(aryleneethynylene)s. | ||
Poly(thienylenevinylene)s (PTVs) exhibit low bandgap (1.5–1.8 eV) and high hole mobility (ca. 0.22 cm2V−1 s−1),36 which makes them attractive candidates for photovoltaic applications. However, PTVs are inherently nonemissive due to their extremely short singlet excited-state lifetime,37 and thus far the PCEs of the PSCs based on PTV donors are rather low (< 0.5%),38 probably because their photoexcitation decay primarily occurs via intrachain relaxation rather than photoinduced electron transfer from the PTVs to the acceptor.39 Li and co-workers synthesized a new PTV derivative with an electron-withdrawing carboxylate side chain (2b).312b shows unexpected emission, narrower bandgap, lower HOMO level, and higher hole mobility in comparison with poly(3-hexylthienylenevinylene) (P3HTV). The PCE of the PSC based on 2b:PC61BM reached 2.01%, which is ca. 10× enhancement compared to that for P3HTV. The PCE of 2.01% is the highest reported for the PSCs based on PTVs so far.
Poly(aryleneethynylene)s (PAEs) are a class of conjugated polymers with extended conjugation and rigid backbone for strong interchain interactions due to the internal triple bonds. Ashraf et al. synthesized a D–A PAE derivative containing thieno[3,4-b]pyrazine as the acceptor and thiophene as the donor (2c).32 This polymer exhibits broad absorption from 300 to 800 nm and low bandgap of ca. 1.6 eV. The PSC based on 2c:PC61BM gave a PCE of 2.37%.
Introduction of platinum into PAE main chain enhances π-conjugation and electron delocalization along the polymer chain due to overlap of the d-orbital of the Pt with the p-orbital of the alkyne unit.40 On the other hand, efficient intersystem crossing facilitates the formation of triplet excited states due to strong spin-orbital coupling in this system. It is well known that triplet excited states have longer lifetimes and thus allow longer exciton diffusion lengths compared to the singlet excited states. Wong et al. reported a platinum-containing D–A PAEpolymer (2d).33 The PSCs based on 2d/PC61BM gave a Jsc of 15.43 mA cm−2, a Voc of 0.82 V and a PCE of 4.93%.41 Jen and co-workers synthesized a similar polymer (2e) using thieno[3,2-b]thiophene instead of thiophene in 2d.34 Despite this polymer being amorphous, a high FET mobility of 1 × 10−2 cm2V−1 s−1 was achieved. The average PCEs of PSCs based on 2e:PC61BM or PC71BM are 2.22 and 3.73%, respectively, without thermal annealing. Jenekhe and co-workers synthesized eleven Pt-bridged organometallic donor–acceptor conjugated PAEpolymers, incorporating various electron acceptors. By varying the electron-accepting strength in the donor–acceptor architecture, they demonstrate molecular engineering of the absorption bands, HOMO/LUMO levels, charge transport, and photovoltaic properties. The highest PCE was observed in blends of the polymers with PC71BM to be 2.41%.41
Conjugated polymers based on fused ring blocks
Molecules containing fused ring systems intend to maximize the π-orbital overlap and possibly to induce face-to-face π-stacking, facilitating charge transport through intermolecular hopping. On the other hand, the fused aromatic rings can make the polymer backbone more rigid and coplanar, therefore enhancing effective π-conjugation, lowering bandgap and extending absorption. Thus, fluorene, silafluorene, carbazole, indolocarbazole, benzodithiophene, dithienocyclopentadiene, dithienosilole, dithienopyrrole, and other fused-ring blocks are introduced into π-conjugated polymeric systems to obtain good performance in solar cells. Table 3 provides a summary of electronic properties as well as PSC data for representative conjugated polymers based on fused ring building blocks (Fig. 3).2,42–57| λ max abs/nm | E g/eV | μ h /cm2V−1 s−1 | HOMO/LUMO/eV | D/PC61BM (w/w) | J sc/mA cm−2 | V oc/V | FF | PCE (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| a λ max abs = absorption peak in film; Eg = optical bandgap; μh = hole mobility; D = donor; Jsc = short-circuit current density; Voc = open-circuit voltage; FF = fill factor; PCE = power conversion efficiency. b 90 mW cm−2. c 80 mW cm−2. d PC71BM. | ||||||||||
| 3a | — | — | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
7.70 | 1.00 | 0.54 | 4.2 | 42 |
| 3b | 576 | 1.9 | — | −5.5/−3.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
6.92 | 0.89 | 0.63 | 3.6b | 43 |
| 3c | 579 | 2.0 | 1 × 10−4 | −5.2/−3.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5d 2.5d |
9.8 | 0.81 | 0.69 | 5.4 | 44 |
| 3d | 565 | 1.8 | 1 × 10−3 | −5.4/-- | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.5 | 0.90 | 0.51 | 5.4c | 45 |
| 3e | 542 | 1.9 | 1 × 10−5 | −6.3/−3.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
6.0 | 1.0 | 0.63 | 3.70 | 46 |
| 3f | 525 | 2.0 | 6 × 10−5 | −5.6/−3.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4.33 | 0.94 | 0.51 | 2.06 | 47 |
| 3g | — | 1.9 | 1 × 10−4 | −5.3/−3.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
9.62 | 0.99 | 0.50 | 4.74 | 48 |
| 3h | — | 1.9 | — | −5.2/−3.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.17 | 0.69 | 0.57 | 3.6 | 49 |
| 3i | 510 | 2.1 | 8 × 10−4 | −5.1/−3.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
5.3 | 0.77 | 0.53 | 2.2 | 50 |
| 3j | 590 | 1.7 | 3 × 10−3 | −5.4/−3.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
6.2 | 0.8 | 0.51 | 2.5 | 51 |
| 3k | 690 | 1.6 | 4 × 10−4 | −4.9/−3.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.5 | 0.58 | 0.65 | 4.76 | 52 |
| 3l | 682 | 1.6 | 8 × 10−4 | −5.1/−3.3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13.0 | 0.74 | 0.61 | 5.90 | 53 |
| 3m | 690 | 1.6 | 2 × 10−4 | −5.1/−3.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5d 1.5d |
14.7 | 0.7 | 0.64 | 6.58 | 54 |
| 3n | — | 1.6 | 7 × 10−4 | −5.2/−3.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5d 1.5d |
15.2 | 0.76 | 0.67 | 7.73 | 2 |
| 3o | 775 | 1.4 | 2 × 10−2 | −5.3/−3.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.0 | 0.63 | 0.47 | 2.67 | 55 |
| 3p | 740 | 1.5 | 3 × 10−3 | −5.1/−3.3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d 1d |
12.7 | 0.68 | 0.55 | 5.10 | 56 |
| 3q | 771 | 1.4 | — | −4.8/−3.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
11.9 | 0.54 | 0.44 | 2.8 | 57 |
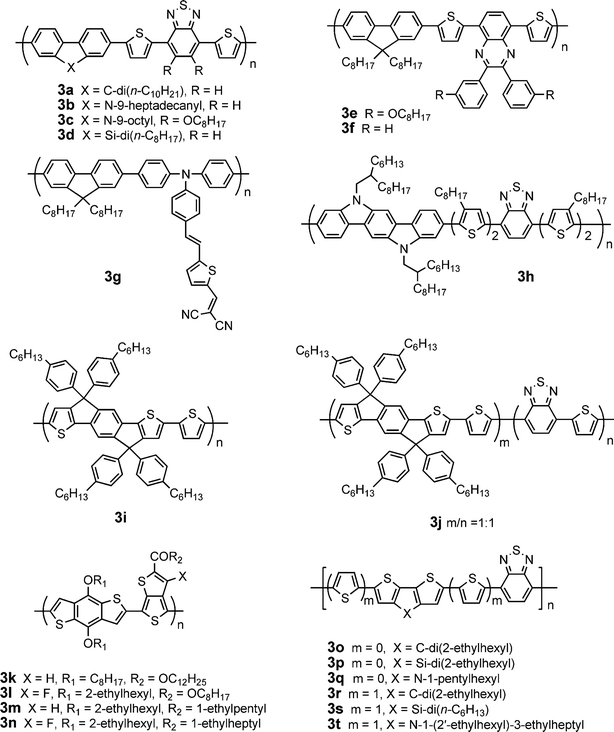 | ||
| Fig. 3 Chemical structure of some conjugated polymers based on fused ring blocks. | ||
Polyfluorenes possess good thermal and photochemical stability, low-lying HOMO level, high mobility, and excellent blue-emitting properties.58 However, their optical bandgap is ca. 3.3 eV, which is too large to efficiently harvest sunlight. Incorporating an electron donating and/or accepting units into the main chain can lower bandgap and extend absorption of the polymers. Andersson and co-workers synthesized a fluorene-dithienylbenzothiadiazolecopolymer with a bandgap of ca. 1.9 eV.59 The PSC based on this polymer in combination with PC61BM gave a PCE of 2.2%. A similar polymer with dioctyl groups at 9 position of fluorene gave a higher PCE of 2.84%.60 Replacing dioctyl with didecyl (3a, Fig. 3) leads to further enhancement of PCE to 4.2%, which is the highest reported for fluorene-based polymers/PC61BM devices.42 Thus, the substituents at 9 position of fluorene exhibit significant impacts on device performance although the fluorene-dithienylbenzothiadiazolecopolymers have similar electronic properties.
Inganäs and co-workers reported an alternating copolymer (3e) derived from fluorene and 5,8-dithienylquinoxaline with a very low-lying HOMO of −6.3 eV.46 The PSCs based on 3e:PC61BM displayed a PCE of 3.7%. 3f without alkoxygroups on the phenyl rings, a close analogue of 3e, was utilized in an active layer of 3f:PC61BM;47 the device showed a much lower PCE of 2.06%. Andersson et al.47 and Kitazawa et al.61 reported PCEs of 2.9 and 5.5% based on 3f:PC71BM, respectively. Jen et al. have synthesized a series of new fluorene-based conjugated polymers with an electron-rich conjugated main chain and a D-π-bridge-A conjugated side chain.48 The polymer3g exhibited a Eg of 1.9 eV, a low HOMO (−5.3 eV) and a FET mobility of 1 × 10−4 cm2V−1 s−1. The PSCs based on this polymer in conjunction with PC71BM exhibited high VOC (0.99 V), high JSC (9.62 mA cm−2), FF of 0.50, and high PCE (4.74%).
Carbazole is an electron-rich and widely used building block for synthesis of conjugated polymers. 2,7-Linked carbazolepolymers have better π-conjugation and charge migration along the main chain compared to their 3,6-linked counterparts. Leclerc and co-workers synthesized a copolymer composed of alternating 2,7-carbazole and dithienyl-benzothiadiazole (3b).43 The PSC device based on 3b:PC61BM showed a PCE of 3.6% at AM1.5, 90 mW cm−2. The PCE of solar cells based on 3b was increased from 3.6% to 6.1% by replacing PC61BM with PC71BM together with titanium oxide as the optical spacer and hole blocking layer.62 Leclerc et al. further reported a series of poly(2,7-carbazole) derivatives containing different electron-deficient moieties (quinoxaline, benzothiadiazole, benzooxadiazoleetc).63 The polymers with symmetric benzene-based acceptors have better structural organization in the solid state than those with asymmetric pyridine-based acceptors. The PCEs of PSCs based on the polymers containing symmetric benzene-based acceptors are higher than those for their asymmetric pyridine-based counterparts. Bo et al. reported an analogue of 3b with dioctoxygroups on benzothiadiazole (3c) giving a PCE of 5.4% in combination with PC71BM.44 The PSC based on 3c/PC71BM exhibited a very high FF (0.69), indicating a balanced charge transport in the device. Indeed, FET measurements on the blend used in optimized solar cells confirmed a quite balanced mobility of ∼1 × 10−4 cm2V−1 s−1 for holes and 3 × 10−4 cm2V−1 s−1 for electrons under vacuum.
Siloles are attractive electron-accepting building blocks for the design and synthesis of organic semiconductors due to relatively large electron affinities and relatively high electron mobilities.64 Cao and co-workers pioneered synthesis of silole-based polymers for PSC applications. An alternating copolymer of 9,9-dihexylfluorene with 1,1-dimethyl-3,4-diphenyl-2,5-bis(2′-thienyl)-silole exhibited low HOMO level (−5.71 eV) and hole mobility of 4.5 × 10−5 cm2V−1 s−1.65 In combination with PC61BM the OPV device gave a PCE of 2.01%. By condensing a silole ring with two phenyl rings, the silafluorene combines the advantages of both silole and fluorene intrinsic properties. Leclerc et al. synthesized a copolymer of 2,7-silafluorene and dithienylbenzothiadiazole (3d).66 The PCE of PSCs based on 3d:PC61BM is 1.6%. Cao et al. reported a much higher PCE of 5.4% under illumination of AM1.5, 80 mW cm−2 from 3d:PC61BM blend.45 The high Voc (0.9 V) is attributed to the low-lying HOMO (−5.39 eV); the high Jsc is attributed to higher mobility (1 × 10−3 cm2V−1 s−1) and broader absorption compared to its fluorene counterpart. It should be noted that these fluorene, carbazole, and silafluorene-based polymers generally give a higher Voc of ca. 0.9–1.0 V due to their lower HOMOs (< −5.5 eV).
Indolo[3,2-b]carbazole can be regarded as two carbazoles condensed together. The large coplanar π-conjugated indolo[3,2-b]carbazole is a promising electron-donating building block for synthesis of conjugated polymers due to high mobility.67 Lu et al. synthesized a copolymer consisting of benzothiadiazole, oligothiophene and indolocarbazole units (3h).49 The absorption edge in the solid state significantly red-shifted 100 nm compared to that in solution, suggesting strong intermolecular interaction induced by crystallinity. The PSC based on a blend of 3h/PC61BM exhibited a high PCE of 3.6%.
Ko et al. synthesized a copolymer of pentacyclic fused thiophene–phenylene–thiophene (TPT) unit with thiophene (3i).50 This rigid planar structure facilitates effective π-conjugation and strong intermolecular interactions. The FET hole mobility for 3i is 8.3 × 10−4 cm2V−1 s−1. 3i in combination with PC61BM and PC71BM displayed PCEs of 2.2 and 3.3%, respectively. Introduction of benzothiadiazole into the main chain of 3i leads to a lower bandgap (1.7 eV) and a higher hole mobility of 3.4 × 10−3 cm2V−1 s−1 for 3j.51 Blending 3j with PC61BM and PC71BM gave PCEs of 2.5 and 4.3%, respectively. Later, they synthesized a series of fused TPT-based low-bandgap conjugated polymers containing various electron-withdrawing comonomers.68 These copolymers exhibited a wide range of optical bandgaps (1–1.8 eV); Eg can be reduced upon increasing the electron-withdrawing strength of the acceptor moieties.
The large planar benzo[1,2-b:4,5-b′]dithiophene (BDT) unit has emerged as an attractive building block for conjugated polymers. Copolymers of BDT and bithiophene exhibited a high FET mobility of 0.25 cm2V−1 s−1 and enhanced stability.69 Yu and co-workers synthesized a copolymer of BDT with thienothiophene (3k).52Thienothiophene promotes stabilization of the quinoid structure and planarity along the polymer backbone. The ester-substituent on thienothiophene enhances solubility and oxidation stability of the polymer. The PSCs based on 3k/PC61BM afforded a high PCE of 4.76%; the PCE was improved to 5.3% using PC71BM instead of PC61BM. The high PCEs are related to the rigidity and planarity of the fused-ring backbone. Later, they introduced an electron-withdrawing fluorine atom onto thienothiophene (3l) to lower HOMO.53 The Voc and PCE of PSCs based on 3l/PC61BM increased to 0.74 V and 5.9%, respectively. To further lower the HOMO, Hou et al. replaced the estergroup in 3k and 3l with a ketonegroup. The HOMO levels of 3k and 3l were reduced by 0.2 (3m)54 and 0.1 eV (3n)2, respectively. The average PCE of 3m:PC71BM reached 6.3% with a champion PCE of 6.58%.54 In particular, the PSCs based on 3n:PC71BM gave a very high Jsc (15.2 mA cm−2), a high Voc (0.76 V), a very high FF (0.67), and a world record PCE (7.73%).2 Yang and co-workers reported a series of BDT-based polymers with different counits and systematically investigated their structure-property relationships.70
Dithienocyclopentadiene (DTC) derivatives have attracted considerable attention due to their fully coplanar structure, low bandgap, and strong intermolecular interactions.71 Müllen and co-workers reported a copolymer of 4,4-dihexyldecyl-substituted DTC and benzothiadiazole with a high FET mobility of 0.17 cm2V−1 s−1.72 Mühlbacher et al. synthesized a similar polymer with two ethylhexylgroups on DTC (3o).553o exhibited narrow bandgap (1.4 eV), low HOMO (−5.3 eV), and high mobility (2 × 10−2 cm2V−1 s−1). Unlike the homopolymer of DTC, 3o showed a significant red shift of the absorption spectrum in film form compared to that in solution, indicating stronger intermolecular interactions when benzothiadiazole units are incorporated. When blended with PC61BM and PC71BM, the PSC devices gave PCEs of 2.67 and 3.5%, respectively. By adding a small amount of 1,8-octanedithiols into the 3o:PC71BM solution prior to spin coating, the device efficiency was further improved to 5.5%, due to formation of an better bulk morphology which enhances both photoconductivity and charge carrier lifetime.73 Heeger and co-workers reported an efficient tandem cell (PCE = 6.5%) with two active layers composed of the wider bandgap P3HT and the smaller bandgap 3o.74
A series of random copolymers consisting of DTC, benzothiadiazole, bithiophene with different D:A ratio were also synthesized;75 PCEs up to 3% was obtained. A copolymer of DTC and dithienylbenzothiadiazole (3r) exhibited significantly blue-shifted (70 nm) absorption, two orders of magnitude lower mobility, and lower PCE (2.1% with PC61BM) compared to its analogue 3o.76
Replacing the carbon atom with silicon in DTC yields a dithienosilole (DTS) having a silole ring condensed with dithiophene. Yang and co-workers reported a DTS-benzothiadiazolecopolymer (3p), structurally similar to 3o.563p exhibited a low optical bandgap (1.45 eV) and high hole mobility (3 × 10−3 cm2V−1 s−1). The PSC based on 3p:PC71BM exhibited a PCE of 5.1%. Replacing the 2-ethylhexylgroup with n-dodecyl in 3p also gave a high PCE (5.0% with PC71BM).77 A copolymer of DTS and dithienylbenzothiadiazole (3s) exhibited significantly blue-shifted (ca. 100 nm) absorption and much lower PCE (0.18% with PC61BM) compared to its analogue 3p.78 Yang and co-workers reported an analogue of 3s with didodecylgroups on DTS giving a PCE of 3.43% in combination with PC71BM.79
In addition to the tricyclic DTC and DTS blocks used as excellent donor components in low-bandgap polymers, dithieno[3,2-b:2′,3′-d]pyrrole (DTP) attracted considerable attention as another fused bithiopene member having a pyrrole ring condensed with dithiophene due to strong electron-donating and charge-transporting properties.80 Geng et al. synthesized a copolymer consisting of alternating DTP and benzothiadiazole units (3q).57 This polymer shows strong absorption in the wavelength range of 600–900 nm. The PSC based on 3q:PC61BM gave a PCE of 2.80%. Hashimoto and co-workers reported another D–A conjugated copolymer (3t) constituted of alternating DTP and dithienylbenzothiadiazole units.813t exhibited significantly blue-shifted (ca. 74 nm) absorption and lower PCE (2.18% with PC61BM) compared to its analogue 3q. In general, copolymers of DTC, DTS, or DTP with dithienylbenzothiadiazole exhibited significantly blue-shifted absorption and lower PCE compared to their benzothiadiazole-containing analogues.
Conjugated polymer acceptors
Although fullerenes, particularly the solution-processible derivative known as PCBM, are the most commonly used acceptors in BHJ OPVs, perylene diimide (PDI) small molecules and polymers have attracted interest as alternative acceptor materials since they exhibit large absorptivities, high electron mobilities, and electron affinities similar to those of fullerenes. Zhan et al. reported the synthesis of the first soluble rylene-based fully conjugated polymer. This copolymer of PDI and dithienothiophene, 4a (Fig. 4), exhibits broad absorption (300-850 nm), low bandgap (1.7 eV) and high electron mobility (1.3 × 10−2 cm2V−1 s−1).82 All-polymer solar cells based on 4a acceptor in conjunction with a bis(thienylenevinylene)-substituted polythiophene (2TV-PT) donor21a gave a PCE of 1.03%. More recently, all-polymer solar cells based on a related donor tris(thienylenevinylene)-substituted polythiophene (3TV-PT) and a related acceptor PDI-bis(dithienothiophene)polymer (4b) exhibits a PCE as high as 1.48% by optimizing the donor/acceptor ratio.83 Increasing dithienothiophene from 1 (4a) to 3 (4c) in the main chain of the PDI copolymer leads to lower PCE for PDI polymer:3TV-PT device (Table 4).84 Using an alternating PDI-phenylenevinylenecopolymer (4d) acceptor and poly(3-phenyl hydrazone thiophene) donor in PSCs, Mikroyannidis et al. obtained a PCE of 2.3% under white-light illumination calibrated to an AM1.5 intensity of 30 mW cm−2 after annealing at 80 °C for 10 min.85 Babel and Jenekhe reported a high electron mobility (0.1 cm2V−1 s−1) ladder polymer (4e),93 closely related to PDI polymers. The ladder polymer has been used to fabricate bilayer PSCs in conjunction with a PPV donor with PCE of 1.5% at AM1.5, 80 mW cm−2.86| λ max abs/nm | E g/eV | μ e/cm2V−1 s−1 | HOMO/LUMO/eV | Donor | J sc/mA cm−2 | V oc/V | FF | PCE (%) | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| a λ max abs = absorption peak in film; Eg = optical bandgap; μe = electron mobility; Jsc = short-circuit current density; Voc = open-circuit voltage; FF = fill factor; PCE = power conversion efficiency; 2TV-PT = bis(thienylenevinylene)-substituted polythiophene; 3TV-PT = tris(thienylenevinylene)-substituted polythiophene; PPHT = poly(3-phenyl hydrazone thiophene); PPV = poly(p-phenylenevinylene); POPT = poly[3-(4-n-octyl)-phenylthiophene]; PPV2 = poly[2,5-dimethoxy-1,4-phenylene-1,2-ethenylene-2-methoxy-5-(2-ethylhexyloxy)-(1,4-phenylene-1,2-ethenylene)] (M3EH-PPV); PV-PT = poly[3-(10-n-octyl-3-phenothiazinevinylene)thiophene-co-2,5-thiophene]; P3HT = poly(3-hexylthiophene); PPV3 = poly[2-methoxy-5-(3,7-dimethyloctyloxy)-1,4-phenylenevinylene] (MDMO-PPV). b 30 mW cm−2. c 80 mW cm−2. | ||||||||||
| 4a | 630 | 1.7 | 1 × 10−2 | −5.8/−3.9 | 2TV-PT | 4.2 | 0.63 | 0.39 | 1.03 | 82 |
| 4b | 647 | 1.5 | — | −5.5/−3.8 | 3TV-PT | 5.02 | 0.69 | 0.43 | 1.48 | 83 |
| 4c | 678 | 1.5 | — | −5.4/−4.0 | 3TV-PT | 2.80 | 0.69 | 0.40 | 0.77 | 84 |
| 4d | 504 | 1.7 | 8 × 10−3 | −5.8/−4.0 | PPHT | 2.98 | 0.60 | 0.39 | 2.32b | 85 |
| 4e | 590 | 1.7 | 0.1 | −5.9/−4.0 | PPV | 2.15 | 1.10 | 0.50 | 1.5c | 86 |
| 4f | — | — | — | —/−3.7 | POPT | — | — | — | 2.0 | 87 |
| 4g | — | — | — | −6.1/−3.4 | PPV2 | 3.57 | 1.36 | 0.35 | 1.70 | 88 |
| 4h | — | 2.0 | — | −5.8/−3.7 | PV-PT | 3.14 | 0.85 | 0.29 | 0.8 | 89 |
| 4i | — | — | 8 × 10−5 | −5.4/−3.2 | P3HT | — | — | — | 1.80 | 90 |
| 4j | 473 | 2.2 | — | −5.4/−3.1 | PPV3 | 3.0 | 1.40 | 0.37 | 1.5 | 91 |
| 4k | 450 | — | — | —/−4.0 | P3HT | — | — | — | 1.6 | 92 |
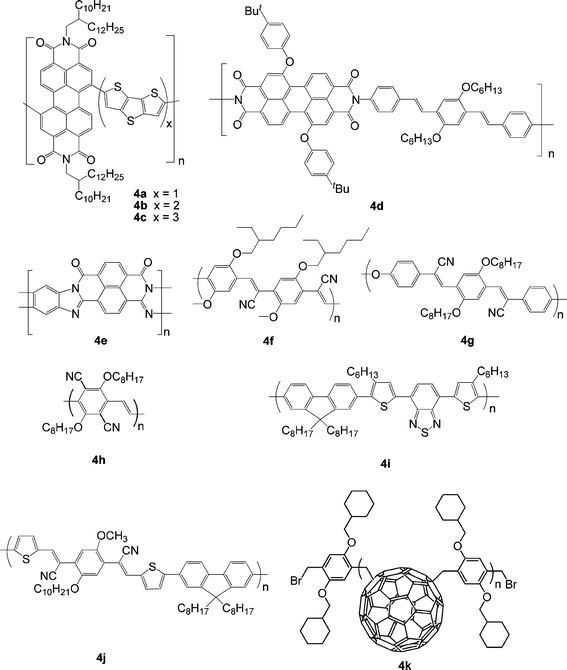 | ||
| Fig. 4 Chemical structure of some polymer acceptors. | ||
Replacing vinylene with cyanovinylene in poly[2-methoxy-5-(2′-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV) lowers both HOMO and LUMO levels by ∼0.5 eV, both increasing the ease of reduction and decreasing the ease of oxidation, and having only minor effects upon absorption and fluorescence spectra and bandgap. Cyano-substituted PPVs (CN-PPVs) display relatively high electron affinities and considerable electron-transport properties as a result of the electron-withdrawing effect of the cyano group. CN-PPVs can function as a suitable electron acceptor in all-polymer solar cells in either bilayer or BHJ configuration.4 For example, Friend et al. fabricated a bilayer device using 4f as acceptor and poly[3-(4-n-octyl)-phenylthiophene] (POPT) as donor through a lamination technique, achieving a PCE of 1.9% at AM1.5, 77 mW cm−2.94 Very recently, Fréchet and co-workers reported a bilayer device using 4f as an acceptor and regioregular POPT as a donor through spin coating; a PCE of 2% was achieved, which is the highest reported to date for an all-polymer OPV device.87
Carter and co-workers fabricated a polymer blend device using poly[2,5-dimethoxy-1,4-phenylene-1,2-ethenylene-2-methoxy-5-(2-ethylhexyloxy)- (1,4-phenylenevinylene-1,2-ethenylene)] (M3EH-PPV) as donor and poly[oxa-1,4-phenylene-1,2-(1-cyano)ethenylene-2,5-dioctyloxy-1,4-phenylene -1,2-(2-cyano)ethenylene-1,4-phenylene] (4g) as acceptor; a PCE of 1% was obtained at AM1.5, 80 mW cm−2.95 Later, Kietzke et al. reported all-polymer solar cells based on M3EH-PPV:4g with a much higher PCE (1.7%).88 They proposed that due to the much lower solubility of M3EH-PPV in chlorobenzene compared to 4g, a vertically composition graded layer other than a homogeneous blend is formed during spin coating, facilitating both exciton separation and efficient transport of the separated charges to the electrodes. Kietzke et al. reported studies on bilayer devices using CN-PPV acceptors with varying LUMO levels and M3EH-PPV.96 It was found that the open-circuit voltage decreases and the quantum efficiency increases with lowering the LUMO level of the acceptor. Li and co-workers synthesized a soluble n-type conjugated polymer poly(1,4-dioctyloxyl- 2,5-dicyano-p-phenylenevinylene) (4h)97 and fabricated all-polymer solar cells using polymer blend of poly[3-(10-n-octyl-3-phenothiazinevinylene)thiophene-co-2,5-thiophene] (PV-PT) as donor and 4h as acceptor;89 a PCE of 0.8% was achieved after thermal annealing.
McNeill et al. reported that the copolymer of fluorene and 4,7-bis(3-hexylthienyl)-2,1,3-benzothiadiazole (4i) shows an ambipolar nature and is capable of functioning as an efficient electron acceptor in blends with donor P3HT.90 A PCE of 1.8% was achieved under simulated sunlight for optimized 4i/P3HT devices. They also studied the effect of thermal annealing on performance of device based on P3HT:4i;98 an enhanced PCE from 0.14% to 1.2% was observed after annealing. Thermal annealing was found to increase charge generation efficiency through increasing the efficiency of charge separation, not only due to an increase in the degree of phase separation but also due to an order of magnitude increase in the hole mobility of the P3HT phase.
Koetse et al. reported a polymer/polymerBHJ solar cell based on a mixture of MDMO-PPV as the donor and an alternating copolymer poly{9,9-dioctylfluorene-2,7-diyl-alt-1,4-bis[2-(5-thienyl)-1-cyanovinyl]-2-methoxy-5-(3,7-dimethyl-octyloxy)benzene} (4j) as the acceptor; a maximum external quantum efficiency of 52% at 530 nm and a PCE of 1.5% was achieved.91
To simultaneously exploit the expected benefits of polymers and the electron acceptor properties of fullerene, Hiorns et al. synthesized a main-chain C60polymer, poly{(1,4-fullerene)-alt-[1,4-dimethylene-2,5-bis(cyclohexylmethyl ether)phenylene]} (4k), using atom transfer radical addition.92 A blend of P3HT:4k was demonstrated by atomic force microscopy (AFM) to yield nanoclusters (ca. 20 nm) favorable to exciton capture. Even without optimization of the chemical structure or the device, this prototype device gave a promising PCE of 1.6%.
It is well known that mixtures of polymers tend to phase segregate due to the low entropy mixing. The feature size of phase separation in polymer/polymer blend generally is hundreds nm, while in the case of polymer/PCBM system, the phase separation is only tens nm. Thus the donor/acceptor interfacial area for charge separation in the polymer/PCBM systems is much larger than that in the polymer/polymer system. Given the fact that typical exciton diffusion length in disordered blend layer is about 10 nm, the large scale phase separation in polymer/polymer system is not favorable for efficient exciton dissociation, leading to lower efficiencies.89 Very recently, Shuai et al. carried out the dynamic Monte Carlo simulation for the all-polymer solar cells based on 4a/2TV-PT blend. The simulations indicate that a 5% PCE could be achieved with an optimum combination of charge mobility and morphology (the feature size is around 10 nm).99
Double-cable polymers
One of the main problems for BHJ solar cells is fine tuning the complicated physical interactions between donor–donor, acceptor–acceptor, and donor–acceptor to obtain an ideal morphology with a well-defined nanostructure. To solve this problem fullerene acceptor was attached to a donor polymer as a pendant side chain to form a so-called double-cable polymer, which can be regarded as a molecular heterojunction.7,9,100–103 Such a structure facilitates exciton dissociation and homogeneous distribution to prevent severe phase separation. After photoinduced electron transfer, the double cable creates two ideal channels for both hole transporting in the conjugated main chain and electron hopping between neighboring pendant fullerenes.Janssen and co-workers used a double-cable PPV-type polymer with a pendent fullerene as a single component (5a, Fig. 5) in PSCs.104 Photoluminescence quenching was observed in thin film of this polymer, indicating efficient photoinduced electron transfer from the main chain to the side chain. The PSC exhibited a Jsc of 0.42 mA cm−2, a Voc of 0.83 V, and an FF of 0.29. A possible origin for the low efficiency is that the content of fullerene is below the percolation threshold for the formation of acceptor network, thus limiting electron transport. Li and co-workers synthesized a double-cable polythiophene with pendent C60 (5b).105 The phenylenevinylene side chain is used to increase the conjugation of the polymer and to enhance absorption in the short-wavelength region. The role of the long aliphatic spacer linking the backbone and C60 is to improve solubility and to prevent donor–acceptor interaction in the ground state. The maximum PCE of the PSC based on this polymer reached 0.52%, which is the best value reported for a double-cable polymer so far.
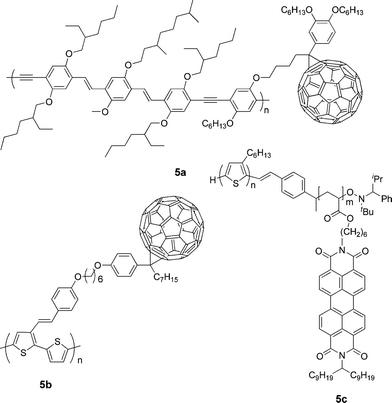 | ||
| Fig. 5 Chemical structure of some double-cable polymers. | ||
Several studies have employed donor–acceptor diblock copolymers in which a polyacrylate with pendant PDI serves as the acceptor block; they undergo microscale phase segregation, as single-component active layers in OPVs. Devices based on block copolymers with a 4-(diphenylamino)styrene donor block exhibited PCEs of 0.11%,106 while annealed devices based on 5c, in which the donor block is regioregular P3HT, gave a PCE of 0.49%.107
The fast charge recombination and inefficient electron hopping and transport is probably responsible for the lower PCEs of double-cable polymers compared to donor/acceptor blend BHJ solar cells.105
Conclusion and outlook
Due to limited scope we have just reviewed some representative conjugated polymer donors, polymer acceptors and double-cable polymers for high-efficiency solar cells. So far thousands of OPV materials have been discovered, most of these materials give low PCEs (< 2% for polymer/fullerene and < 1% for all-polymer system). The basic requirements for an ideal donor or acceptor polymer material include: (a) good solubility and film-forming properties; (b) strong and broad bandwidth absorption; (c) high hole or electron mobility; (d) suitable HOMO/LUMO energy levels; and (e) high purity and molecular weight. The reasons for low PCEs of OPV devices are: (a) the OPV materials do not meet one or some of the above criteria; (b) the donor and acceptor mismatch in terms of energy levels and morphology; and (c) the device configuration and fabrication have not well optimized.High-efficiency polymer solar cells have been achieved thanks to innovations of photovoltaic materials and device fabrication technology, indicating a bright future for this research field. A key challenge related to organic photovoltaics is to control electronic structures, film morphology and device properties of organic semiconductors by modifying their chemical structures. Therefore, we have surveyed what is known about the relationships between chemical structures and optical, electronic and device properties for most important donor, acceptor, and double-cable polymers for high-performance PSCs, since such an understanding is a basic requirement for rational design of the photovoltaic materials. To summarise:
(1) Extending the absorption and decreasing the bandgap of a polymer donor to match solar radiation is one of the main ways to improve Jsc and efficiencies. A polymer donor with bandgap < 2 eV is necessary but not sufficient for a high PCE.11 D–A polymers based on fused dithiophene tricyclic blocks such as dithienocyclopentadiene, dithienosilole, dithienopyrrole and benzodithiophene (Table 3) can exhibit relatively low Eg (< 1.6 eV), broad absorption, high Jsc (> 10 mA cm−2), and high PCEs (> 5% with PC71BM).
(2) Given that the PCE is linearly proportional to Voc which is related directly to the energy difference between the HOMO level of the donor and the LUMO level of the acceptor, a lower HOMO of a donor would help to achieve a higher Voc and higher PCE. In general, a polymer with a HOMO below −5.4 eV tends to give Voc > 0.8 V.11Polymers based on fused biphenyl tricyclic blocks such as fluorene, carbazole, and silafluorene (Table 3) can exhibit relatively large Eg (∼ 1.9 eV), low HOMOs (< −5.5 eV), high Voc (> 0.9 V), and high PCEs (> 3.5% with PC61BM).
(3) Another basic requirement for a polymer donor is high hole mobility. The high mobility (> 1 × 10−3 cm2V−1 s−1) of a donor helps to achieve a high Jsc and high PCE. Polymers based on fused ring blocks (Table 3) tend to exhibit relatively high mobilities and high PCEs.
(4) Among the acceptor polymers the polymers based on PDI, CN-PPV, fluorene, and fullerenes are promising for all-polymer OPVs. However, the large scale phase separation and smaller donor/acceptor interfacial area in the polymer/polymer system lead to inefficient exciton dissociation and lower efficiencies compared to the polymer/fullerene system.
(5) The PSCs based on double-cable polymers give much lower PCEs due to fast charge recombination and inefficient electron transport compared to donor/acceptor blend BHJ solar cells.
(6) The PCE is more a device parameter than an intrinsic photovoltaic material parameter. High efficiency achievement is a systematic engineering of the excellent properties of materials with careful optimization of the various device fabrication conditions. So far the best performances (> 6%) of PSCs were achieved from blends of fused-ring-based polymer donors with PC71BM. An interdisciplinary approach such as novel photovoltaic materials and new advanced device concepts will probably bring high-efficiency (> 10%) and low-cost plastic solar cells to final commercialization.
Acknowledgements
This material is based upon work supported in part by the NSFC (Grants 50873107, 20774104, 20721061), the MOST (Grants 2006AA03Z220, 2006CB932100), and the Chinese Academy of Sciences.References and notes
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 CrossRef CAS.
- H.-Y. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 Search PubMed.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CrossRef CAS.
- J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti and A. B. Holmes, Nature, 1995, 376, 498 CrossRef CAS.
- K. M. Coakley and M. D. McGehee, Chem. Mater., 2004, 16, 4533 CrossRef CAS.
- C. Winder and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 1077 RSC.
- J. Roncali, Chem. Soc. Rev., 2005, 34, 483 RSC.
- S. Gunes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef.
- A. Cravino, Polym. Int., 2007, 56, 943 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2007, 91, 954 CrossRef CAS.
- J. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709 CrossRef CAS.
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551 CrossRef CAS.
- X. Yang and J. Loos, Macromolecules, 2007, 40, 1353 CrossRef CAS.
- A. Pivrikas, N. S. Sariciftci, G. Juska and R. Osterbacka, Progr. Photovolt.: Res. Appl., 2007, 15, 677 Search PubMed.
- M. Jorgensen, K. Norrman and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2008, 92, 686 CrossRef.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58 CrossRef.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323 CrossRef CAS.
- L.-M. Chen, Z. Hong, G. Li and Y. Yang, Adv. Mater., 2009, 21, 1434 CrossRef CAS.
- B. Kippelen and J.-L. Brédas, Energy Environ. Sci., 2009, 2, 251 RSC.
- (a) J. Hou, Z. Tan, Y. Yan, Y. He, C. Yang and Y. Li, J. Am. Chem. Soc., 2006, 128, 4911 CrossRef CAS; (b) Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952 CrossRef CAS.
- K. Sivula, C. K. Luscombe, B. C. Thompson and J. M. J. Fréchet, J. Am. Chem. Soc., 2006, 128, 13988 CrossRef CAS.
- (a) M. Heeney, W. Zhang, D. J. Crouch, M. L. Chabinyc, S. Gordeyev, R. Hamilton, S. J. Higgins, I. McCulloch, P. J. Skabara, D. Sparrowe and S. Tierney, Chem. Commun., 2007, 5061 RSC; (b) A. M. Ballantyne, L. Chen, J. Nelson, D. D. C. Bradley, Y. Astuti, A. Maurano, C. G. Shuttle, J. R. Durrant, M. Heeney, W. Duffy and I. McCulloch, Adv. Mater., 2007, 19, 4544 CrossRef CAS.
- Y.-T. Chang, S.-L. Hsu, G.-Y. Chen, M.-H. Su, T. A. Singh, E. W.-G. Diau and K.-H. Wei, Adv. Funct. Mater., 2008, 18, 2356 CrossRef CAS.
- M. M. Wienk, M. Turbiez, J. Gilot and R. A. J. Janssen, Adv. Mater., 2008, 20, 2556 CrossRef CAS.
- L. H. Nguyen, H. Hoppe, T. Erb, S. Gunes, G. Gobsch and N. S. Sariciftci, Adv. Funct. Mater., 2007, 17, 1071 CrossRef CAS.
- PSCs based on blend of P3BT nanowire/PC61BM or PC71BM gave PCEs of 2.2 and 3.0%, respectively. H. Xin, F. S. Kim and S. A. Jenekhe, J. Am. Chem. Soc., 2008, 130, 5424 Search PubMed.
- (a) P. Schilinsky, U. Asawapirom, U. Scherf, M. Biele and C. J. Brabec, Chem. Mater., 2005, 17, 2175 CrossRef CAS; (b) R. C. Hiorns, R. de Bettignies, J. Leroy, S. Bailly, M. Firon, C. Sentein, A. Khoukh, H. Preud'homme and C. Dagron-Lartigau, Adv. Funct. Mater., 2006, 16, 2263 CrossRef CAS; (c) W. Ma, J. Y. Kim, K. Lee and A. J. Heeger, Macromol. Rapid Commun., 2007, 28, 1776 CrossRef CAS.
- C. H. Woo, B. C. Thompson, B. J. Kim, M. F. Toney and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 16324 CrossRef CAS.
- K. Tajima, Y. Suzuki and K. Hashimoto, J. Phys. Chem. C, 2008, 112, 8507 CrossRef CAS.
- L. Huo, T. L. Chen, Y. Zhou, J. Hou, H.-Y. Chen, Y. Yang and Y. Li, Macromolecules, 2009, 42, 4377 CrossRef CAS.
- R. S. Ashraf, M. Shahid, E. Klemm, M. Al-Ibrahim and S. Sensfuss, Macromol. Rapid Commun., 2006, 27, 1454 CrossRef CAS.
- W.-Y. Wong, X.-Z. Wang, Z. He, A. B. Djurisic, C.-T. Yip, K.-Y. Cheung, H. Wang, C. S. K. Mak and W.-K. Chan, Nat. Mater., 2007, 6, 521 CrossRef CAS.
- N. S. Baek, S. K. Hau, H.-L. Yip, O. Acton, K.-S. Chen and A. K.-Y. Jen, Chem. Mater., 2008, 20, 5734 CrossRef CAS.
- S. E. Shaheen, C. J. Brabec, N. S. Sariciftci, F. Padinger, T. Fromherz and J. C. Hunnelen, Appl. Phys. Lett., 2001, 78, 841 CrossRef CAS.
- H. Fuchigami, A. Tsumura and H. Koezuka, Appl. Phys. Lett., 1993, 63, 1372 CrossRef CAS.
- A. J. Brassett, N. F. Colaneri, D. D. C. Bradley, R. A. Lawrence, R. H. Friend, H. Murata, S. Tokito, T. Tsutsui and S. Saito, Phys. Rev. B: Condens. Matter, 1990, 41, 10586 CrossRef CAS.
- (a) A. P. Smith, R. R. Smith, B. E. Taylor and M. F. Durstock, Chem. Mater., 2004, 16, 4687 CrossRef CAS; (b) J. H. Hou, Z. A. Tan, Y. J. He, C. H. Yang and Y. F. Li, Macromolecules, 2006, 39, 4657 CrossRef CAS; (c) S. Zhang, H. Fan, Y. Liu, G. Zhao, Q. Li, Y. Li and X. Zhan, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2843 CrossRef CAS.
- I. W. Hwang, Q. H. Xu, C. Soci, B. Q. Chen, A. K. Y. Jen, D. Moses and A. J. Heeger, Adv. Funct. Mater., 2007, 17, 563 CrossRef CAS.
- H. Masai, K. Sonogashira and N. Hagihara, Bull. Chem. Soc. Jpn., 1971, 44, 2226 CAS.
- Janssen and co-workers commented on such a high efficiency for 2d: based on the optical data and the 70 nm film thickness of the active layer, the highest PCE should not exceed 2.2%. J. Gilot, M. M. Wienk and R. A. J. Janssen, Nat. Mater., 2007, 6, 704 Search PubMed Later, Jenekhe and co-workers reported a PCE of 1.4% obtained from the device based on 2d. P.-T. Wu, T. Bull, F. S. Kim, C. K. Luscombe and S. A. Jenekhe, Macromolecules, 2009, 42, 671 CrossRef CAS.
- L. H. Slooff, S. C. Veenstra, J. M. Kroon, D. J. D. Moet, J. Sweelssen and M. M. Koetse, Appl. Phys. Lett., 2007, 90, 143506 CrossRef.
- N. Blouin, A. Michaud and M. Leclerc, Adv. Mater., 2007, 19, 2295 CrossRef CAS.
- R. Qin, W. Li, C. Li, C. Du, C. Veit, H.-F. Schleiermacher, M. Andersson, Z. Bo, Z. Liu, O. Inganas, U. Wuerfel and F. Zhang, J. Am. Chem. Soc., 2009, 131, 14612 CrossRef CAS.
- E. Wang, L. Wang, L. Lan, C. Luo, W. Zhuang, J. Peng and Y. Cao, Appl. Phys. Lett., 2008, 92, 033307 CrossRef.
- A. Gadisa, W. Mammo, L. M. Andersson, S. Admassie, F. Zhang, M. R. Andersson and O. A. Inganäs, Adv. Funct. Mater., 2007, 17, 3836 CrossRef CAS.
- L. J. Lindgren, F. Zhang, M. Andersson, S. Barrau, S. Hellstrom, W. Mammo, E. Perzon, O. A. Inganäs and M. R. Andersson, Chem. Mater., 2009, 21, 3491 CrossRef CAS.
- F. Huang, K.-S. Chen, H.-L. Yip, S. K. Hau, O. Acton, Y. Zhang, J. Luo and A. K.-Y. Jen, J. Am. Chem. Soc., 2009, 131, 13886 CrossRef CAS.
- J. Lu, F. Liang, N. Drolet, J. Ding, Y. Tao and R. Movileanu, Chem. Commun., 2008, 5315 RSC.
- S. H. Chan, C. P. Chen, T. C. Chao, C. Ting, C. S. Lin and B. T. Ko, Macromolecules, 2008, 41, 5519 CrossRef CAS.
- C. P. Chen, S. H. Chan, T. C. Chao, C. Ting and B. T. Ko, J. Am. Chem. Soc., 2008, 130, 12828 CrossRef CAS.
- Y. Liang, Y. Wu, D. Feng, S. T. Tsai, H. J. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS.
- Y. Liang, D. Feng, Y. Wu, S. T. Tsai, G. Li, C. Ray and L. Yu, J. Am. Chem. Soc., 2009, 131, 7792 CrossRef CAS.
- J. Hou, H.-Y. Chen, S. Zhang, R. I. Chen, Y. Yang, Y. Wu and G. Li, J. Am. Chem. Soc., 2009, 131, 15586 CrossRef CAS.
- D. Mühlbacher, M. Scharber, M. Morana, Z. Zhu, D. Waller, R. Gaudiana and C. Brabec, Adv. Mater., 2006, 18, 2884 CrossRef.
- J. H. Hou, H. Y. Chen, S. Q. Zhang, G. Li and Y. Yang, J. Am. Chem. Soc., 2008, 130, 16144 CrossRef CAS.
- W. Yue, Y. Zhao, S. Shao, H. Tian, Z. Xie, Y. Geng and F. Wang, J. Mater. Chem., 2009, 19, 2199 RSC.
- (a) L. L. Chua, J. Zaumseil, J. F. Chang, E. C. W. Ou, P. K. H. Ho, H. Sirringhaus and R. H. Friend, Nature, 2005, 434, 194 CrossRef CAS; (b) X. Zhan, Y. Liu, X. Wu, S. Wang and D. Zhu, Macromolecules, 2002, 35, 2529 CrossRef CAS; (c) U. Scherf and E. J. W. List, Adv. Mater., 2002, 14, 477 CrossRef CAS; (d) M. T. Bernius, M. Inbasekaran, J. O'Brien and W. Wu, Adv. Mater., 2000, 12, 1737 CrossRef CAS.
- M. Svensson, F. Zhang, S. C. Veenstra, W. J. H. Verhees, J. C. Hummelen, J. M. Kroon, O. Inganäs and M. R. Andersson, Adv. Mater., 2003, 15, 988 CrossRef CAS.
- F. Zhang, K. G. Jespersen, C. Bjorstrom, M. Svensson, M. R. Andersson, V. Sundstrom, K. Magnusson, E. Moons, A. Yartsev and O. Inganäs, Adv. Funct. Mater., 2006, 16, 667 CrossRef CAS.
- D. Kitazawa, N. Watanabe, S. Yamamoto and J. Tsukamoto, Appl. Phys. Lett., 2009, 95, 053701 CrossRef.
- S. H. Park, A. Roy, S. Beaupre, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 Search PubMed.
- (a) N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. Neagu-Plesu, M. Belletete, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS; (b) N. Blouin and M. Leclerc, Acc. Chem. Res., 2008, 41, 1110 CrossRef CAS.
- (a) X. Zhan, S. Barlow and S. R. Marder, Chem. Commun., 2009, 1948 RSC; (b) X. Zhan, C. Risco, F. Amy, C. Chan, W. Zhao, S. Barlow, A. Kahn, J. L. Bredas and S. R. Marder, J. Am. Chem. Soc., 2005, 127, 9021 CrossRef CAS; (c) H. Murata, G. G. Malliaras, M. Uchida, Y. Shen and Z. H. Kafafi, Chem. Phys. Lett., 2001, 339, 161 CrossRef CAS; (d) J. W. Chen and Y. Cao, Macromol. Rapid Commun., 2007, 28, 1714 CrossRef CAS; (e) S. Yamaguchi and K. Tamao, J. Chem. Soc., Dalton Trans., 1998, 3693 RSC.
- F. Wang, J. Luo, K. X. Yang, J. W. Chen, F. Huang and Y. Cao, Macromolecules, 2005, 38, 2253 CrossRef CAS.
- P.-L. T. Boudreault, A. Michaud and M. Leclerc, Macromol. Rapid Commun., 2007, 28, 2176 CrossRef CAS.
- (a) Y. Li, Y. Wu and B. S. Ong, Macromolecules, 2006, 39, 6521 CrossRef CAS; (b) P.-L. T. Boudreault, S. Wakim, N. Blouin, M. Simard, C. Tessier, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2007, 129, 9125 CrossRef CAS.
- C.-Y. Yu, C.-P. Chen, S.-H. Chan, G.-W. Hwang and C. Ting, Chem. Mater., 2009, 21, 3262 CrossRef CAS.
- H. Pan, Y. Li, Y. Wu, P. Liu, B. S. Ong, S. Zhu and G. Xu, J. Am. Chem. Soc., 2007, 129, 4112 CrossRef CAS.
- J. Hou, M. H. Park, S. Zhang, Y. Yao, L. M. Chen, J. H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS.
- P. Coppo and M. L. Turner, J. Mater. Chem., 2005, 15, 1123 RSC.
- M. Zhang, H. N. Tsao, W. Pisula, C. Yang, A. K. Mishra and K. Müllen, J. Am. Chem. Soc., 2007, 129, 3472 CrossRef CAS.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222 CrossRef CAS.
- Z. Zhu, D. Waller, R. Gaudiana, M. Morana, D. Muhlbacher, M. Scharber and C. Brabec, Macromolecules, 2007, 40, 1981 CrossRef CAS.
- A. J. Moule, A. Tsami, T. W. Bunnagel, M. Forster, N. M. Kronenberg, M. Scharber, M. Koppe, M. Morana, C. J. Brabec, K. Meerholz and U. Scherf, Chem. Mater., 2008, 20, 4045 CrossRef CAS.
- R. C. Coffin, J. Peet, J. Rogers and G. C. Bazan, Nat. Chem., 2009, 1, 657 Search PubMed.
- L. Liao, L. M. Dai, A. Smith, M. Durstock, J. P. Lu, J. F. Ding and Y. Tao, Macromolecules, 2007, 40, 9406 CrossRef CAS.
- L. Huo, H.-Y. Chen, J. Hou, T. L. Chen and Y. Yang, Chem. Commun., 2009, 5570 RSC.
- (a) T. T. Steckler, X. Zhang, J. Hwang, R. Honeyager, S. Ohira, X. H. Zhang, A. Grant, S. Ellinger, S. A. Odom, D. Sweat, D. B. Tanner, A. G. Rinzler, S. Barlow, J. L. Brédas, B. Kippelen, S. R. Marder and J. R. Reynolds, J. Am. Chem. Soc., 2009, 131, 2824 CrossRef CAS; (b) J. Liu, R. Zhang, G. Sauvé, T. Kowalewski and R. D. McCullough, J. Am. Chem. Soc., 2008, 130, 13167 CrossRef CAS; (c) S. Zhang, Y. Guo, H. Fan, Y. Liu, H. Chen, G. Yang, X. Zhan, Y. Liu, Y. Li and Y. Yang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5498 CrossRef CAS.
- E. Zhou, M. Nakamura, T. Nishizawa, Y. Zhang, Q. Wei, K. Tajima, C. Yang and K. Hashimoto, Macromolecules, 2008, 41, 8302 CrossRef CAS.
- X. Zhan, Z. Tan, B. Domercq, Z. An, X. Zhang, S. Barlow, Y. Li, D. Zhu, B. Kippelen and S. R. Marder, J. Am. Chem. Soc., 2007, 129, 7246 CrossRef CAS.
- Z. A. Tan, E. J. Zhou, X. W. Zhan, X. Wang, Y. F. Li, S. Barlow and S. R. Marder, Appl. Phys. Lett., 2008, 93, 073309 CrossRef.
- X. Zhan, Z. Tan, E. Zhou, Y. Li, R. Misra, A. Grant, B. Domercq, X. Zhang, Z. An, X. Zhang, S. Barlow, B. Kippelen and S. R. Marder, J. Mater. Chem., 2009, 19, 5794 RSC.
- J. A. Mikroyannidis, M. M. Stylianakis, G. D. Sharma, P. Balraju and M. S. Roy, J. Phys. Chem. C, 2009, 113, 7904 CrossRef CAS.
- M. M. Alam and S. A. Jenekhe, Chem. Mater., 2004, 16, 4647 CrossRef CAS.
- T. W. Holcombe, C. H. Woo, D. F. J. Kavulak, B. C. Thompson and J. M. J. Fréchet, J. Am. Chem. Soc., 2009, 131, 14160 CrossRef CAS.
- T. Kietzke, H.-H. Horhold and D. Neher, Chem. Mater., 2005, 17, 6532 CrossRef CAS.
- G. Sang, Y. Zou, Y. Huang, G. Zhao, Y. Yang and Y. Li, Appl. Phys. Lett., 2009, 94, 193302 CrossRef.
- C. R. McNeill, A. Abrusci, J. Zaumseil, R. Wilson, M. J. McKiernan, J. J. M. Halls, N. C. Greenham and R. H. Friend, Appl. Phys. Lett., 2007, 90, 193506 CrossRef.
- M. M. Koetse, J. Sweelssen, K. T. Hoekerd, H. F. M. Schoo, S. C. Veenstra, J. M. Kroon, X. Yang and J. Loos, Appl. Phys. Lett., 2006, 88, 083504 CrossRef.
- R. C. Hiorns, E. Cloutet, E. Ibarboure, L. Vignau, N. Lemaitre, S. Guillerez, C. Absalon and H. Cramail, Macromolecules, 2009, 42, 3549 CrossRef CAS.
- A. Babel and S. A. Jenekhe, J. Am. Chem. Soc., 2003, 125, 13656 CrossRef CAS.
- M. Granstrom, K. Petritsch, A. C. Arias, A. Lux, M. R. Andersson and R. H. Friend, Nature, 1998, 395, 257 CrossRef CAS.
- A. J. Breeze, Z. Schlesinger, S. A. Carter, H. Tillmann and H.-H. Horhold, Sol. Energy Mater. Sol. Cells, 2004, 83, 263 CrossRef CAS.
- T. Kietzke, D. A. M. Egbe, H.-H. Horhold and D. Neher, Macromolecules, 2006, 39, 4018 CrossRef CAS.
- Y. P. Zou, J. H. Hou, C. H. Yang and Y. F. Li, Macromolecules, 2006, 39, 8889 CrossRef CAS.
- C. R. McNeill, J. J. M. Halls, R. Wilson, G. L. Whiting, S. Berkebile, M. G. Ramsey, R. H. Friend and N. C. Greenham, Adv. Funct. Mater., 2008, 18, 2309 CrossRef CAS.
- L. Meng, Y. Shang, Q. Li, Y. Li, X. Zhan, Z. Shuai, R. G. E. Kimber and A. B. Walker, J. Phys. Chem. B DOI:10.1021/jp907167u.
- A. Cravino, G. Zerza, M. Maggini, S. Bucella, M. Svensson, M. R. Andersson, H. Neugebauer and N. S. Sariciftci, Chem. Commun., 2000, 2487 RSC.
- A. Cravino and N. S. Sariciftci, J. Mater. Chem., 2002, 12, 1931 RSC.
- A. Cravino and N. S. Sariciftci, Nat. Mater., 2003, 2, 360 CrossRef CAS.
- F. Zhang, M. Svensson, M. R. Anderson, M. Maggini, S. Bucella, E. Menna and O. Inganäs, Adv. Mater., 2001, 13, 1871 CrossRef CAS.
- A. M. Ramos, M. T. Rispens, J. K. J. van Duren, J. C. Hummelen and R. A. J. Janssen, J. Am. Chem. Soc., 2001, 123, 6714 CrossRef CAS.
- Z. Tan, J. Hou, Y. He, E. Zhou, C. Yang and Y. Li, Macromolecules, 2007, 40, 1868 CrossRef CAS.
- S. King, M. Sommer, S. Huettner, M. Thelakkat and S. A. Haque, J. Mater. Chem., 2009, 19, 5436 RSC.
- Q. Zhang, A. Cirpan, T. P. Russell and T. Emrick, Macromolecules, 2009, 42, 1079 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
