Copper(0)-mediated living radical polymerization of styrene
Jessica
Tom
a,
Ben
Hornby
a,
Andrew
West
a,
Simon
Harrisson
*b and
Sébastien
Perrier
*a
aKey Centre for Polymers and Colloids, School of Chemistry, Building F11, The University of Sydney, NSW 2006, Australia. E-mail: s.perrier@chem.usyd.edu.au
bCSIRO Molecular and Health Technologies, Bayview Ave, Clayton, VIC 3168, Australia. E-mail: simon.harrisson@csiro.au
First published on 19th March 2010
Abstract
We report the controlled polymerization of styrene using zerovalent copper and conventional ATRP ligands as catalysts at 90 °C in toluene. Commercially available PMDETA was found to provide the best control, with good agreement between measured and theoretical molecular weight and a polydispersity of 1.24. This could be reduced to 1.17 by addition of a small amount of Cu(II)Br2.
First reported in 1995,1,2 metal-mediated living radical polymerization is the most widely used living radical polymerization technique. One of the most utilised forms is atom transfer radical polymerization (ATRP), which is highly versatile and capable of controlling the polymerization of many vinyl monomers including styrenes, acrylates, methacrylates, acrylamides, acrylonitrile,3–5 and vinyl acetate.6 A key advantage of ATRP is that it can be performed using commercially available reagents. While ATRP provides a facile approach to the synthesis of novel architectures, its industrial implementation has been limited.7,8 High concentrations of catalyst (usually a copper halide) are required; the catalyst contaminates the polymer, causing discoloration and potentially catalysing degradation. Removal, for example by selective polymer precipitation or using ion-exchange resins,8 is difficult and costly. Several modified ATRP techniques have been developed that require significantly reduced concentrations of copper catalyst:3,9 as little as 50 ppm8 compared to 10,000 ppm in conventional ATRP. At such low concentrations, removal of residual copper is unnecessary. These techniques include initiators for continuous activator regeneration (ICAR) ATRP, activator regenerated by electron transfer (ARGET) ATRP, activators generated by electron transfer (AGET) ATRP and single electron transfer living radical polymerization (SET-LRP).10,11 SET-LRP is distinct in that it uses metallic copper as the catalyst,11 which is cheaper and easier to handle than copper(I) salts. Cu(0) reacts with an alkyl halide to generate Cu(I) halide and an alkyl radical,12 which is capable of initiating polymerization with high efficiency.13 SET-LRP requires the use of dipolar protic and aprotic solvents, such as DMSO, although it has been demonstrated in relatively non-polar solvents, in some cases with various additives.13,14 In addition, SET-LRP has been shown to polymerise electron-withdrawing monomers such as acrylates and vinyl chloride at room temperature or below, with first order kinetics and near-quantitative chain end functionality.11,15
Zerovalent metals are known to accelerate the rate of polymerization in ATRP and have also been used as reducing agents in ARGET ATRP.5,10 However, polymerizations carried out in non-polar solvents with copper metal as the sole catalyst were poorly controlled. The mechanism of polymerization with copper metal is not well understood, and conflicting ideas are presented in the literature.3,5,11
Here we report the first controlled synthesis of polystyrene mediated by copper metal.† The reaction can be carried out in a non-polar solvent, toluene, using the commercially available ligand N,N,N′,N′′,N′′-pentamethyl diethylene triamine (PMDETA). Adequate control is obtained using only copper metal, PMDETA and initiator (ethyl 2-bromopropionate, EBP). As in SET-LRP, improved control is obtained by addition of a small amount of CuBr2 deactivator.
Three ligands with different activities in ATRP were trialled in the copper-mediated polymerization of styrene. The ligand 4,4′-dinonyl-2,2′-bipyridine (diNbpy) has been used extensively in ATRP to control the polymerization of styrene at 110 °C.4PMDETA is a commercially available ligand of intermediate activity. Hexamethylene tris(2-aminoethyl)amine (Me6TREN) is a highly active ligand which is effective in low-copper techniques such as ARGET. Using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of copper
1 ratio of copper![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator and stoichiometric quantities of ligand (2 eq. for diNbpy, 1 eq. for PMDETA and Me6TREN) resulted in higher molecular weight than theory and broad polydispersity (Table 1, entries 1–3). This is thought to be due to uncontrolled free radical polymerization during the early stages of the reaction as a result of insufficient concentration of Cu(II) deactivator.
initiator and stoichiometric quantities of ligand (2 eq. for diNbpy, 1 eq. for PMDETA and Me6TREN) resulted in higher molecular weight than theory and broad polydispersity (Table 1, entries 1–3). This is thought to be due to uncontrolled free radical polymerization during the early stages of the reaction as a result of insufficient concentration of Cu(II) deactivator.
| Entry | Ligand | [Sty]0/[EBP]0/L0/[Cu(0)]0/[CuBr2]0a | Time/h | Conv (%) | M n (theo) | M n (GPC) | PDIc |
|---|---|---|---|---|---|---|---|
| a Polymerizations carried out at 90 °C in toluene (styrene/toluene = 2/1 v/v). b M n(theo) = ([styrene]0/[EBP]0) × conversion. c Determined by GPC in tetrahydrofuran based on polystyrene standards. | |||||||
| 1 | diNbpy | 240/1/2/1/0 | 9.33 | 37 | 9300 | 18800 | 2.79 |
| 2 | PMDETA | 240/1/1/1/0 | 5.43 | 74 | 18600 | 21400 | 1.74 |
| 3 | Me6TREN | 240/1/1/1/0 | 6.58 | 30 | 7800 | 11500 | 4.98 |
| 4 | PMDETA | 240/1/0.5/0.5/0 | 21.83 | 95 | 23900 | 24600 | 1.38 |
| 5 | PMDETA | 240/1/0.35/0.25/0.1 | 21.17 | 85 | 21400 | 21000 | 1.21 |
| 6 | PMDETA | 49/1/0.35/0.25/0.1 | 17.00 | 77 | 4100 | 3500 | 1.20 |
| 7 | PMDETA | 144/1/0.35/0.25/0.1 | 17.00 | 78 | 11800 | 11900 | 1.17 |
| 8 | PMDETA | 480/1/0.35/0.25/0.1 | 17.00 | 48 | 24100 | 23500 | 1.18 |
| 9 | PMDETA | 49/1/0.25/0.25/0 | 17.00 | 82 | 4300 | 3800 | 1.23 |
| 10 | PMDETA | 144/1/0.25/0.25/0 | 17.00 | 64 | 9800 | 9600 | 1.24 |
| 11 | PMDETA | 480/1/0.25/0.25/0 | 17.00 | 39 | 19500 | 19100 | 1.32 |
Of the three ligands trialled, PMDETA gave the most promising results, with high conversion, moderate polydispersity and reasonable agreement with theoretical molecular weight.
Reduction of the initial copper![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator ratio (Table 1, entry 4) to 0.5
initiator ratio (Table 1, entry 4) to 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in a slower reaction, with greatly improved control (Fig. 1). Good agreement between molecular weight and theory was obtained at all stages of the reaction, and the final polydispersity was 1.38. Pseudo-first-order kinetics were observed, indicating an approximately constant concentration of active species.
1 resulted in a slower reaction, with greatly improved control (Fig. 1). Good agreement between molecular weight and theory was obtained at all stages of the reaction, and the final polydispersity was 1.38. Pseudo-first-order kinetics were observed, indicating an approximately constant concentration of active species.
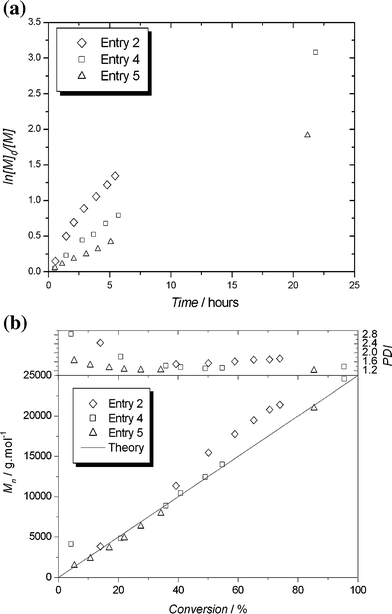 | ||
| Fig. 1 Kinetic plots (a) and evolution of molecular weights and polydispersities against monomer conversion (b) for Cu(0)-LRP of styrene with PMDETA mediated by varying amounts of Cu(0)/CuBr2 (Table 1, entries 2, 4 and 5). | ||
It has been observed that the addition of a small amount of Cu(II)Br2 to SET-LRP polymerizations results in a marked improvement in control of molecular weight and polydispersity.11 Theoretical work by Monteiro et al. shows that the addition of Cu(II)Br2 to the SET-LRP system is necessary if low levels of bimolecular termination, and hence low polydispersities, are required.16 Addition of 0.1 eq. Cu(II)Br2 to the PMDETA/Cu(0) system (Table 1, entry 5), resulted in polymer with a final polydispersity of 1.21. Kinetics were pseudo-first order, and good agreement between measured and theoretical molecular weight was observed throughout the reaction. This reaction was carried out with only 0.25 eq. of Cu(0) relative to initiator, resulting in a very pale green solution that contained dark green particles of unreacted copper. This catalytic system was used to target several different molecular weights both with and without added Cu(II)Br2. In all cases good agreement between measured and expected molecular weights was obtained, with low polydispersities (< 1.2) in the presence of added Cu(II)Br2 (Table 1, entries 6–8) and slightly broader polydispersities in the absence of added Cu(II)Br2 (1.23–1.32, Table 1, entries 9–11).
In order to demonstrate the retention of the halogen chain-end group after polymerisation, a sample of polystyrene (Table 1, entry 5) was chain extended with styrene using ATRP (Fig. 2). Integration of the deconvoluted peaks as shown by GPC analysis indicate that 90% of the initial chains were chain extended, as illustrated by a clear shift of the molecular weight distribution to high molecular weights, with a slight tailing towards low molecular weight, which corresponds to inactive chains, and is reflected by the slight broadening of the polydispersity from 1.21 to 1.33. This chain extension by ATRP shows that the Cu(0)-mediated system provides a good retention of the polymer's active chain ends.
![GPC traces for the block extension of polystyrene synthesised using Cu(0)-LRP (Table 1, entry 5), with styrene by ATRP. [PS]0 : [St]0 : [diNbpy]0 : [CuBr]0 = 1 : 985 : 2 : 1, solvent = toluene (2 : 1 v/v monomer : solvent). 1st block: monomer conversion = 85%, Mn-theo = 21200 g mol−1, Mn-GPC = 21000 g mol−1. 2nd block: monomer conversion = 47.46%, Mn-theo = 69700 g mol−1, Mn-GPC = 48100 g mol−1.](/image/article/2010/PY/b9py00382g/b9py00382g-f2.gif) | ||
Fig. 2
GPC traces for the block extension of polystyrene synthesised using Cu(0)-LRP (Table 1, entry 5), with styrene by ATRP. [PS]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [St]0 [St]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [diNbpy]0 [diNbpy]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr]0 = 1 [CuBr]0 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 985 985![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, solvent = toluene (2 1, solvent = toluene (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v monomer 1 v/v monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent). 1st block: monomer conversion = 85%, Mn-theo = 21200 g mol−1, Mn-GPC = 21000 g mol−1. 2nd block: monomer conversion = 47.46%, Mn-theo = 69700 g mol−1, Mn-GPC = 48100 g mol−1. solvent). 1st block: monomer conversion = 85%, Mn-theo = 21200 g mol−1, Mn-GPC = 21000 g mol−1. 2nd block: monomer conversion = 47.46%, Mn-theo = 69700 g mol−1, Mn-GPC = 48100 g mol−1. | ||
In the copper-mediated polymerization of styrene, the rate of the initiation reaction between Cu(0) and initiator must be balanced with the rate of the deactivation reaction between growing polymer chains and Cu(II)Br2. We found that PMDETA, a ligand forming copper complexes with intermediate activity, is ideal for this Cu(0)-mediated LRP. It is active enough to maintain an adequate rate of deactivation, while not reacting too rapidly with Cu(0), and causing significant build up of radicals.
The Cu(0)/styrene system is advantageous in regards to its simplicity, ease of catalyst removal (Cu(0) can be removed by filtration) and overall lower catalyst concentrations than conventional ATRP. The ligand, PMDETA, is commercially available and inexpensive compared to diNbpy and Me6TREN.
Acknowledgements
The authors would like to thank The University of Sydney for financial assistance. SH acknowledges support from the Cooperative Research Centre for Polymers and CSIRO Molecular and Health Technologies. The authors would also like to acknowledge the editorial office for its helpful suggestions.Notes and references
- M. Kato, M. Kamigaito, M. Sawamoto and T. Higashimura, Macromolecules, 1995, 28(5), 1721–1723 CrossRef CAS.
- J.-S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 1995, 117(20), 5614–5615 CrossRef CAS.
- K. Matyjaszewski, N. V. Tsarevsky, W. A. Braunecker, H. Dong, J. Huang, W. Jakubowski, Y. Kwak, R. Nicolay, W. Tang and J. A. Yoon, Macromolecules, 2007, 40(22), 7795–7806 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101(9), 2921–2990 CrossRef CAS.
- K. Matyjaszewski, S. Coca, S. G. Gaynor, M. Wei and B. E. Woodworth, Macromolecules, 1997, 30(23), 7348–7350 CrossRef CAS.
- H. D. Tang, M. Radosz and Y. Q. Shen, AIChE J., 2009, 55(3), 737–746 CrossRef CAS.
- W. Jakubowski, N. V. Tsarevsky, P. McCarthy and K. Matyjaszewski, Polym. Prepr., 2008, 49(2), 369–370 CAS.
- K. Matyjaszewski, W. Jakubowski, K. Min, W. Tang, J. Huang, W. A. Braunecker and N. V. Tsarevsky, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(42), 15309–15314 CrossRef CAS.
- K. Matyjaszewski, M. Wei, J. Xia and N. E. McDermott, Macromolecules, 1997, 30(26), 8161–8164 CrossRef CAS.
- W. A. Braunecker and K. Matyjaszewski, Prog. Polym. Sci., 2007, 32(1), 93–146 CrossRef CAS.
- V. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro and S. Sahoo, J. Am. Chem. Soc., 2006, 128(43), 14156–14165 CrossRef CAS.
- M. van der Sluis, B. Barboiu, N. Pesa and V. Percec, Macromolecules, 1998, 31(26), 9409–9412 CrossRef CAS.
- V. Percec, B. Barboiu and M. van der Sluis, Macromolecules, 1998, 31(12), 4053–4056 CrossRef CAS.
- P. M. Wright, G. Mantovani and D. M. Haddleton, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(22), 7376–7385 CrossRef CAS.
- G. Lligadas, B. M. Rosen, M. J. Monteiro and V. Percec, Macromolecules, 2008, 41(22), 8360–8364 CrossRef CAS.
- M. J. Monteiro, T. Guliashvili and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2007, 45(10), 1835–1847 CrossRef CAS.
Footnote |
| † Polymerizations were undertaken as follows: the monomer, solvent, ligand and copper source were weighed into a Schlenk tube. The sealed Schlenk tube was subjected to 5 cycles of freeze–pump–thaw degassing before backfilling with nitrogen. The degassed Schlenk tube was placed into a thermostatted oil bath after addition of a known volume of previously degassed initiator. Samples were taken at various intervals and subjected to analysis by GPC and NMR to determine molecular weight and conversion respectively. In the case of chain extensions, the first block was isolated by precipitation into cold methanol. |
| This journal is © The Royal Society of Chemistry 2010 |
