Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-based coordination cages as dynamic crosslinks in acrylamide hydrogels†
Chaolei
Hu
,
Damien W.
Chen
,
Sylvain
Sudan
and
Kay
Severin
 *
*
Institut des Sciences et Ingénierie Chimiques, École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: kay.severin@epfl.ch
First published on 27th February 2025
Abstract
Soft polymer networks with palladium cages as crosslinks can be obtained by combining polymeric N-donor ligands with PdII salts in organic solvents. Herein, we describe an alternative procedure that enables the preparation of hydrogels with PdnL2n-type junctions. The gels were obtained by photoinitiated copolymerization of palladium cages with acrylamide monomers in water. Cages with varying nuclearities (n = 2, 4, or 12) and different acrylates were employed. The material properties could be tuned by changing the crosslinker density. Thermoresponsive hydrogels were obtained when NIPAm was used as the monomer. The dynamic nature of the Pd-based crosslinks allows the creation of stimuli-responsive hydrogels. In particular, we were able to alter the network topology of a hydrogel by anion-induced conversion of Pd4L8 crosslinks into Pd2L4-type junctions.
Introduction
Palladium-based coordination cages of the general formula [PdnL2n]X2n (X = weakly coordinating anion) can be obtained by thermal equilibration of PdII salts with ditopic nitrogen-donor ligands.1 The Pd–N bonds in these assemblies are strong enough to ensure that the cages are stable in coordinating solvents. Despite their high stability, Pd–N bonds remain sufficiently labile, allowing for stimuli-induced structural rearrangements. Changes in the nuclearity n of PdnL2n complexes have been achieved with light,2 by a change of the solvent,3 or by the addition of chemical templates.4Another interesting feature of PdnL2n-based coordination cages is their ability to serve as hosts for different guests. The versatile host–guest chemistry has enabled applications in fields such as catalysis5 or medicinal chemistry.6
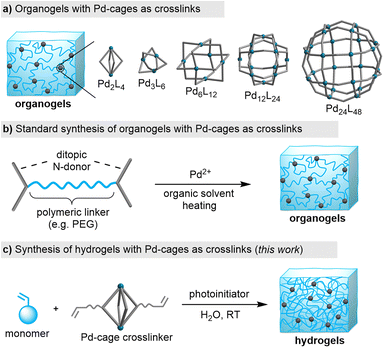
Traditionally, coordination cages (also referred to as ‘metal–organic cages’) have been studied primarily in solution and in the crystalline state. However, these metallosupramolecular structures are increasingly being explored within the context of materials science, with a special focus on polymer networks.7 PdnL2n-type cages with nuclearities ranging from n = 2 to 24 have been incorporated into organogels (Fig. 1a).7–11 The typical synthetic route for producing such gels involves using N-donor ligands, which are connected by a polymeric linker (e.g. a polyethylene glycol chain). The polymer networks are then obtained by combining the ligand with a PdII salt in an organic solvent at slightly elevated temperatures (Fig. 1b).
Organogels with PdnL2n-type cages as crosslinks can display high branch functionalities8 and the material properties are broadly tunable.9 The dynamic nature of the Pd–N bonds enables the formation of stimuli-responsive gels. For example, light-responsive gels were obtained by using photochromic N-donor ligands10 or external photoacids.11 Moreover, the gel properties can be altered by adding suited guest molecules.12
We sought to investigate whether PdnL2n cages could be incorporated into other types of gel matrices, particularly acrylamide hydrogels.13 The thermal stability of PdII salts in water poses a challenge, as heating can lead to their decomposition. This makes the thermal equilibration of polymeric ligands with PdII salts potentially problematic. Indeed, our initial attempts to synthesize hydrogels using polyethylene glycol-linked dipyridyl ligands were compromised by the apparent formation of metallic Pd during the heating of the ligand/PdII mixture in water, as indicated by the coloration of the solution (see the ESI, Fig. S17†).
In view of these difficulties, we decide to explore an alternative approach: the copolymerization of acrylamide monomers with pre-formed PdnL2n cages having polymerizable side chains. We have recently shown that this strategy can be employed for the synthesis of nanogels.14 We now demonstrate that bulk acrylamide hydrogels can be obtained by photoinitiated copolymerization of di-, tetra-, and dodecanuclear Pd cages with different acrylamide monomers (Fig. 1c). A post-synthetic change in the network topology was achieved by guest-induced rearrangement of Pd4L8 crosslinks into Pd2L4 connections.
Results and discussion
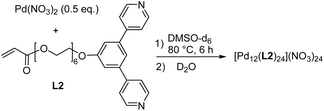
For the synthesis of hydrogels with PdnL2n-based coordination cages, we needed water-soluble cages with polymerizable side chains. Toward this goal, we first prepared the dipyridyl ligand L1 (Scheme 1). The synthesis of L1 was accomplished in four steps with an overall yield of 51% (for details, see the ESI, Section 2.1†). The PEG8 chain of L1 was expected to provide solubility in water, while the terminal acrylate group could be used for copolymerization reactions.Ligand L1 was then combined with 0.5 equivalents of Pd(NO3)2 in CD3CN. After heating the mixture for two hours at 80 °C, the 1H NMR spectrum indicated the formation of a highly symmetric complex (Fig. S18†). Analysis of the product by mass spectrometry confirmed that the expected dinuclear cage [Pd2(L1)4](NO3)4 had formed (Fig. S20†).
Following synthesis and analysis in CD3CN, the solvent was exchanged for D2O. Complex [Pd2(L1)4](NO3)4 could be re-dissolved without problems at 5 mM concentrations, and the analytical data confirmed that the assembly had not undergone any structural changes upon switching the solvent (Fig. S24†).
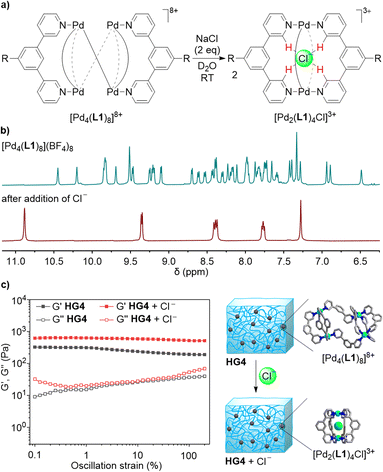
When ligand L1 was combined with [Pd(CH3CN)4](BF4)2 instead of Pd(NO3)2, a different outcome was observed. The 1H NMR spectrum of the CD3CN solution after thermal equilibration was very complex. Moreover, the spectrum was found to be concentration- and temperature-dependent. After solvent switch to D2O, the spectrum revealed the presence of a defined, low-symmetry assembly (Fig. 2a). The mass spectrum showed that this species was a tetranuclear complex (Fig. 2b).
The two most common structural motifs for Pd4L8 complexes are macrocyclic15 and tetrahedral.16 Furthermore, there are examples of interlocked (Pd2L4)2 cages.17 Recently, we described a fourth structural motif: a doubly-bridged (Pd2L3)(μ-L)2(Pd2L3) cage, the formation of which was templated by an ion pair.18 The low apparent symmetry of [Pd4(L1)8](BF4)8, with an eightfold splitting of the ligands signals (Fig. 2a), suggested that [Pd4(L1)8](BF4)8 displays a (Pd2L3)(μ-L)2(Pd2L3)-type of structure. Molecular modeling of [Pd4(L1)8](BF4)8, excluding the PEG side chains, revealed that the ligands adopt two distinct conformations. A convergent orientation of the pyridyl groups is observed for the ligands within the Pd2(L1)3 units, and a divergent orientation of the N-donor groups is found for the ligands bridging the two Pd2(L1)3 units (Fig. 2c).
The striking difference between the reactions with Pd(NO3)2 and [Pd(CH3CN)4](BF4)2 can be attributed to the fact that nitrate is able to template the formation of Pd2L4 cages with bridging 1,3-di(pyridin-3-yl)benzene ligands (one nitrate anion is encapsulated in the cage cavity).19 The BF4 anion is less suited as a template, and the inherent strain of the dinuclear Pd2(L1)4 complex19b cannot be compensated. As a result, the system rearranges to give an entropically disfavored but less strained tetranuclear assembly.
In addition to the di- and tetranuclear complexes [Pd2(L1)4](NO3)4 and [Pd4(L1)8](BF4)8, we synthesized the dodecanuclear cage [Pd12(L2)24](NO3)24 (Scheme 2). The required ligand L2 was obtained in four steps following a related synthetic procedure as used for L1 (for details, see the ESI, Section 2.1†). The 1,3-di(pyridin-4-yl)benzene part in ligand L2 is well known to promote the formation of cuboctahedral PdII cages,20 and the analytical data of [Pd12(L2)24](NO3)24 are in line with the anticipated structure (for details, see the ESI, Section 2.5†).
Next, we examined if the three cages could be employed as crosslinks for the synthesis of hydrogels. Initial experiments were carried out with the dinuclear cage [Pd2(L1)4](NO3)4 and N,N-dimethylacrylamide (DMA) as the monomer. Mixtures of DMA and the cage were copolymerized in D2O by irradiation at 365 nm using 2,2′-azobis-[2-methyl-N-(2-hydroxyethyl)-propionamide] (VA-86) as photoinitiator. Three different cage concentrations were employed: 0.31, 0.63, or 1.25 mol% with respect to the monomer concentration (400 mM). All three reactions resulted in the formation of hydrogels (HG1–HG3, Fig. 3 and S36†).
The 1H NMR spectra of the gels showed broad peaks in the aromatic region, which matched the signals observed for [Pd2(L1)4](NO3)4 (Fig. S37–39†). The data suggest that the polymerization had not compromised the cage structures.
The mechanical properties of the gels HG1–HG3 were evaluated by oscillatory rheometry. Amplitude sweep tests at 1 rad s−1 were performed with the swollen hydrogels. Over the entire range of oscillation strain, the storage moduli (G′) were higher than the loss moduli (G′′), indicating a strong elastic response from the gels (Fig. 3b). The stiffness of the hydrogel was found to depend on the crosslinker density. The G′ (at 0.1% strain) of the hydrogel increased from a value of 130 Pa for HG3 (0.31 mol% cage) to a value of 743 Pa for HG1 (1.25 mol% cage) (Fig. 3b). Moreover, a higher cage concentration was found to reduce the swelling capacity of the hydrogels (Fig. 3b).
Subsequently, we investigated the influence of the cage nuclearity n on the gel properties. The tetranuclear cage [Pd4(L1)8](BF4)8 and the dodecanuclear cage [Pd12(L2)24](NO3)24 were copolymerized with DMA to give the hydrogels HG4 and HG5, respectively (Fig. 3a). The cage concentrations were chosen so that the gels HG1, HG4, and HG5 all contain the same amount of Pd and ligand L1/L2. As a result, the branch functionality of the gels was expected to increase in the order HG1 < HG4 < HG5, while the concentration of the cages was expected to decrease in the same order.
Rheology measurements revealed an indirect correlation between the nuclearity n of the cage crosslinker and the storage modulus: the G′ values of the hydrogels dropped from 743 Pa for HG1 (n = 2), to 326 Pa for HG4 (n = 4), and to 136 Pa for HG5 (n = 12) (Fig. 3c). The reduced branch concentrations for gels based on cages with a higher nuclearity n are likely responsible for the reduced gel stiffness. As before, we found that the softer gels displayed a higher swelling capacity (Fig. 3c).
In addition to DMA, we have used N-hydroxyethyl acrylamide (HEAA) and N-isopropylacrylamide (NIPAm) as monomers in copolymerization reactions with the dinuclear complex [Pd2(L1)4](NO3)4. In order to allow a comparison with HG1, we have employed a uniform cage concentration of 1.25 mol%.
The polymerizations with HEAA and NIPAm gave stable gels (HG6 and HG7, Fig. 4a and S47†). The highest storage modulus at 0.1% strain was observed for HG6 (1022 Pa), followed by HG1 (743 Pa), and then HG7 (358 Pa). The swelling capacity of HG6 and HG7 was found to be lower than that of HG1 (Fig. 4b).
Polymers based on NIPAm tend to be thermoresponsive.21 To examine if HG7 would display a temperature-dependent behavior, we performed frequency sweep tests at 1% strain at different temperatures. The storage and loss moduli were found to increase with temperature (Fig. 4c and d). The lower critical solution temperature (LCST) of ∼30 °C is in the typical range for NIPAm-based hydrogels.21
As discussed above, Pd2L4 cages with bridging 1,3-di(pyridin-3-yl)benzene ligands can bind nitrate. Cages of this type display an even higher affinity for chloride.19a Since the tetranuclear complex [Pd4(L1)8](BF4)8 contains bridging 1,3-di(pyridin-3-yl)benzene ligands, we hypothesized that chloride could induce a rearrangement into a dinuclear complex. Indeed, when two equivalents of NaCl were added to a solution of [Pd4(L1)8](BF4)8 in D2O (2.5 mM), a quantitative rearrangement into a highly symmetric complex was observed by 1H NMR spectroscopy (Fig. 5b). An analysis by mass spectrometry confirmed that the chloride adduct [Pd2(L1)4Cl]3+ had formed (Fig. 5a).
The possibility to cleanly convert the tetranuclear complex [Pd4(L1)8](BF4)8 into the dinuclear complex [Pd2(L1)4Cl]3+ in homogenous solution suggested that a similar rearrangement could be achieved in the gel state. Two equivalents of NaCl (with respect to cage crosslinks) were added to HG4 and the mixture was incubated for two days at room temperature. Analysis of the gel by 1H NMR spectroscopy confirmed that a rearrangement had occurred (see the ESI, Fig. S54 and S55†). The mechanical properties of the new gel were examined by oscillatory rheometry. The result showed that the chloride-induced rearrangement had increased the stiffness of the gel (Fig. 5c). This finding can be explained by the fact that the concentration of the crosslinks in the gels has increased. The mechanical properties of HG4 after incubation with chloride were similar to what was observed for HG1: the storage modulus at 0.1% strain for HG4 + Cl− was 650 Pa, whereas a value of 743 Pa was found for HG1.
Conclusions
Thus far, soft polymer networks with palladium-based cages as crosslinks were primarily obtained by combining polymeric N-donor ligands with palladium(II) salts in organic solvents. We have introduced an alternative method for creating gels with PdnL2n-type junctions. This approach involves the photo-induced copolymerization of palladium cages with acrylamide monomers in water, providing an efficient pathway to synthesize bulk hydrogels. The method is versatile, allowing for the use of palladium cages with varying nuclearities (n = 2, 4, or 12), as well as different types of acrylamides.By controlling the density of the palladium cages in the network, we were able to change the material properties of the resulting hydrogels. When N-isopropylacrylamide was used as the monomer, a thermoresponsive hydrogel was obtained. The dynamic nature of the Pd-based crosslinks enables the development of stimuli-responsive hydrogels. Specifically, we showed that the material properties of a hydrogel could be altered by the addition of chloride. The observed changes are likely due to a conversion of Pd4L8 crosslinks into Pd2L4-type junctions. The possibility of modifying the gel properties via dynamic metallosupramolecular chemistry represents an interesting feature of hydrogels based on Pd cages.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
C. H. and K. S. initiated the study, C. H. performed the experiments and analyzed the data, D. W. C. carried out the molecular modelling, S. S. performed initial studies with a Pd4L8 complex, and C. H. and K. S. co-wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the École Polytechnique Fédérale de Lausanne (EPFL) and by the Swiss National Science Foundation. We thank Alexandra Thoma (EPFL) and Brian David Ridenour (EPFL) for the help with rheology measurements, and Fabienne Bobard (EPFL) for the TEM measurements and analyses.References
- (a) Z. T. Avery, J. L. Algar and D. Preston, Trends Chem., 2024, 6, 352–364 CrossRef; (b) S. Sharma, M. Sarkar and D. K. Chand, Chem. Commun., 2023, 59, 535–554 RSC; (c) J. E. M. Lewis, Chem. Commun., 2022, 58, 13873–13886 RSC; (d) T. Tateishi, M. Yoshimura, S. Tokuda, F. Matsuda, D. Fujita and S. Furukawa, Coord. Chem. Rev., 2022, 467, 214612 CrossRef CAS; (e) N. B. Debata, D. Tripathy and H. S. Sahoo, Coord. Chem. Rev., 2019, 387, 273–298 CrossRef CAS; (f) S. Saha, I. Regeni and G. H. Clever, Coord. Chem. Rev., 2018, 374, 1–14 CrossRef CAS; (g) M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848–1860 RSC; (h) K. Harris, D. Fujita and M. Fujita, Chem. Commun., 2013, 49, 6703–6712 RSC.
- E. Benchimol, J. Tessarolo and G. H. Clever, Nat. Chem., 2024, 16, 13–21 CrossRef CAS PubMed.
- (a) K. E. Ebbert, F. Sendzik, L. Neukirch, L. Eberlein, A. Platzek, P. Kibies, S. M. Kast and G. H. Clever, Angew. Chem., Int. Ed., 2025, 64, e202416076 CrossRef CAS PubMed; (b) A. Kumar, R. Banerjee, E. Zangrando and P. S. Mukherjee, Inorg. Chem., 2022, 61, 2368–2377 CrossRef CAS PubMed; (c) K. Wu, B. Zhang, C. Drechsler, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2021, 60, 6403–6407 CrossRef CAS PubMed; (d) Y.-H. Li, J.-J. Jiang, Y.-Z. Fan, Z.-W. Wei, C.-X. Chen, H.-J. Yu, S.-P. Zheng, D. Fenske, C.-Y. Su and M. Marboiu, Chem. Commun., 2016, 52, 8745–8748 RSC; (e) T. R. Schulte, M. Krick, C. I. Asche, S. Freye and G. H. Clever, RSC Adv., 2014, 4, 29724–29728 RSC; (f) K. Suzuki, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 2819–2822 CrossRef CAS PubMed.
- E. G. Percástegui, Eur. J. Inorg. Chem., 2021, 4425–4438 CrossRef.
- (a) Z. Ashbridge and J. N. H. Reek, Nat. Synth., 2024, 3, 1197–1207 CrossRef CAS; (b) M. Otte, Eur. J. Org Chem., 2023, 26, e202300012 CrossRef CAS; (c) A. C. Pearcy and J. D. Crowley, Chem.–Eur. J., 2023, 29, e202203752 CrossRef CAS PubMed; (d) R. Saha, B. Mondal and P. S. Mukherjee, Chem. Rev., 2022, 122, 12244–12307 CrossRef CAS PubMed; (e) Y. Xue, X. Hang, J. Ding, B. Li, R. Zhu, H. Pang and Q. Xu, Coord. Chem. Rev., 2021, 430, 213656 CrossRef CAS; (f) A. B. Grommet, M. Feller and R. Klajn, Nat. Nanotechnol., 2020, 15, 256–271 CrossRef CAS PubMed.
- (a) G. Moreno-Alcántar, M. Drexler and A. Casini, Nat. Rev. Chem., 2024, 8, 893–914 CrossRef PubMed; (b) G. Moreno-Alcántar and A. Casini, FEBS Lett., 2022, 597, 191–202 CrossRef PubMed.
- (a) Z. Wang and S. Furukawa, Acc. Chem. Res., 2024, 57, 327–337 CrossRef CAS PubMed; (b) W. Drożdż, A. Ciesielski and A. R. Stefankiewicz, Angew. Chem., Int. Ed., 2023, 62, e202307552 CrossRef PubMed; (c) P. Liu, F. Fang, H. Wang and N. M. Khashab, Angew. Chem., Int. Ed., 2023, 62, e202218706 CrossRef CAS PubMed; (d) E. Sánchez-González, M. Y. Tsang, J. Troyano, G. A. Craig and S. Furukawa, Chem. Soc. Rev., 2022, 51, 4876–4889 RSC; (e) I. Jahović, Y.-Q. Zou, S. Adorinni, J. R. Nitschke and S. Marchesan, Matter, 2021, 4, 2123–2140 CrossRef; (f) Y. Zhu, W. Zheng, W. Wang and H.-B. Yang, Chem. Soc. Rev., 2021, 50, 7395–7417 RSC; (g) P. Sutar and T. K. Maji, Dalton Trans., 2020, 49, 7658–7672 RSC; (h) V. J. Pastore and T. R. Cook, Chem. Mater., 2020, 32, 3680–3700 CrossRef CAS; (i) Y. Gu, J. Zhao and J. A. Johnson, Angew. Chem., Int. Ed., 2020, 59, 5022–5049 CrossRef CAS PubMed; (j) K. C. Bentz and S. M. Cohen, Angew. Chem., Int. Ed., 2018, 57, 14992–15001 CrossRef CAS PubMed; (k) N. Hosono and S. Kitagawa, Acc. Chem. Res., 2018, 51, 2437–2446 CrossRef CAS PubMed.
- (a) A. V. Zhukhovitskiy, M. Zhong, E. G. Keeler, V. K. Michaelis, J. E. P. Sun, M. J. A. Hore, D. J. Pochan, R. G. Griffin, A. P. Willard and J. A. Johnson, Nat. Chem., 2016, 8, 33–41 CrossRef CAS PubMed; (b) A. V. Zhukhovitskiy, J. Zhao, M. Zhong, E. G. Keeler, E. A. Alt, P. Teichen, R. G. Griffin, M. J. A. Hore, A. P. Willard and J. A. Johnson, Macromolecules, 2016, 49, 6896–6902 CrossRef CAS.
- (a) J. Zhao, E. O. Bobylev, D. J. Lundberg, N. J. Oldenhuis, H. Wang, I. Kevlishvili, S. L. Craig, H. J. Kulik, X. Li and J. A. Johnson, J. Am. Chem. Soc., 2023, 145, 21879–21885 CrossRef CAS PubMed; (b) Y. Wang, Y. Gu, E. G. Keeler, J. V. Park, R. G. Griffin and J. A. Johnson, Angew. Chem., Int. Ed., 2017, 56, 188–192 CrossRef CAS PubMed.
- Y. Gu, E. A. Alt, H. Wang, X. Li, A. P. Willard and J. A. Johnson, Nature, 2018, 560, 65–69 CrossRef CAS PubMed.
- R.-J. Li, C. Pezzato, C. Berton and K. Severin, Chem. Sci., 2021, 12, 4981–4984 RSC.
- D. J. Lundberg, C. M. Brown, E. O. Bobylev, N. J. Oldenhuis, Y. S. Alfaraj, J. Zhao, I. Kevlishvili, H. J. Kulik and J. A. Johnson, Nat. Commun., 2024, 15, 3951 CrossRef CAS PubMed.
- For examples of hydrogels with metal–organic cage crosslinks other than PdnL2n-type complexes, see: (a) R. Küng, A. Germann, M. Krüsmann, L. P. Niggemann, J. Meisner, M. Karg, R. Göstl and B. M. Schmidt, Chem.–Eur. J., 2023, 29, e202300079 CrossRef PubMed; (b) Z. Wang, G. A. Craig, A. Legrand, F. Haase, S. Minami, K. Urayama and S. Furukawa, Chem.–Asian J., 2021, 16, 1092–1100 CrossRef CAS PubMed; (c) J. A. Foster, R. M. Parker, A. M. Belenguer, N. Kishi, C. Abell and J. R. Nitschke, J. Am. Chem. Soc., 2015, 137, 9722–9729 CrossRef CAS PubMed; (d) A. Lavalette, J. Hamblin, A. Marsh, D. M. Haddleton and M. J. Hannon, Chem. Commun., 2002, 3040–3041 RSC.
- C. Hu and K. Severin, Angew. Chem., Int. Ed., 2024, 63, e202403834 CrossRef CAS PubMed.
- (a) H. Yu, Z. Guo, N. Han, J. Shi, X. Jiang, Q. Bai, Z. Zhang, P. Wang and M. Wang, Cell Rep. Phys. Sci., 2023, 4, 101631 CrossRef CAS; (b) K. E. Ebbert, E. Benchimol, A. Platzek, C. Drechsler, J. Openy, S. Hasegawa, J. J. Holstein and G. H. Clever, Angew. Chem., Int. Ed., 2024, 63, e202413323 CrossRef CAS PubMed; (c) C.-L. Liu, E. O. Bobylev, S. Dauriac, B. Kauffmann, K. Robeyns, Y. Garcia, J. N. H. Reek and M. L. Singleton, CCS Chem., 2023, 5, 2506–2518 CrossRef CAS; (d) S. Park, D. Kim, D. Kim, D. Kim and O.-S. Jung, Chem. Commun., 2021, 57, 2919–2922 RSC; (e) K. Suzuki, M. Kawano and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 2819–2822 CrossRef CAS PubMed.
- (a) M. R. Black, S. Bhattacharyya, S. P. Argent and B. S. Pilgrim, J. Am. Chem. Soc., 2024, 146, 28233–28241 CAS; (b) A. Walther, I. Regeni, J. J. Holstein and G. H. Clever, J. Am. Chem. Soc., 2023, 145, 25365–25371 CrossRef CAS PubMed; (c) J. A. Findlay, K. M. Patil, M. G. Gardiner, H. I. MacDermott-Opeskin, M. L. O'Mara, P. E. Kruger and D. Preston, Chem.–Asian J., 2022, 17, e202200093 CrossRef CAS PubMed; (d) R.-J. Li, A. Marcus, F. Fadaei-Tirani and K. Severin, Chem. Commun., 2021, 57, 10023–10026 RSC; (e) J. Tessarolo, H. Lee, E. Sakuda, K. Umakoshi and G. H. Clever, J. Am. Chem. Soc., 2021, 143, 6339–6344 CrossRef CAS PubMed; (f) T. Tateishi, T. Kojima and S. Hiraoka, Inorg. Chem., 2018, 57, 2686–2694 CrossRef CAS PubMed; (g) S. M. Jansze, G. Cecot, M. D. Wise, K. O. Zhurov, T. K. Ronson, A. M. Castilla, A. Finelli, P. Pattison, E. Solari, R. Scopelliti, G. E. Zelinskii, A. V. Volgzhanina, Y. Z. Voloshin, J. R. Nitschke and K. Severin, J. Am. Chem. Soc., 2016, 138, 2046–2054 CrossRef CAS PubMed; (h) C. Klein, C. Gütz, M. Bogner, F. Topić, K. Rissanen and A. Lützen, Angew. Chem., Int. Ed., 2014, 53, 3739–3742 CrossRef CAS PubMed; (i) D. K. Chand, K. Biradha, M. Kawano, S. Sakamoto, K. Yamaguchi and M. Fujita, Chem.–Asian J., 2006, 1, 82–90 CrossRef CAS PubMed.
- M. Frank, M. D. Johnstone and G. H. Clever, Chem.–Eur. J., 2016, 22, 14104–14125 CrossRef CAS PubMed.
- S. Sudan, F. Fadaei-Tirani, R. Scopelliti, K. E. Ebbert, G. H. Clever and K. Severin, Angew. Chem., Int. Ed., 2022, 61, e202201823 CrossRef CAS PubMed.
- (a) S. Sudan, D. W. Chen, C. Berton, F. Fadaei-Tirani and K. Severin, Angew. Chem., Int. Ed., 2023, 62, e202218072 CrossRef CAS PubMed; (b) R.-J. Li, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Chem.–Eur. J., 2021, 27, 9439–9445 CrossRef CAS PubMed.
- (a) D. Luo, Z.-J. Yuan, L.-J. Ping, X.-W. Zhu, J. Zheng, C.-W. Zhou, X.-C. Zhou, X.-P. Zhou and D. Li, Angew. Chem., Int. Ed., 2023, 62, e202216977 CrossRef CAS PubMed; (b) D. A. Poole III, E. O. Bobylev, S. Mathew and J. N. H. Reek, Chem. Sci., 2022, 13, 10141–10148 RSC; (c) Y. Gao, S.-Q. Deng, X. Jin, S.-L. Cai, S.-R. Zheng and W.-G. Zhang, Chem. Eng. J., 2019, 357, 129–139 CrossRef CAS; (d) J. Uchida, M. Yoshio, S. Sato, H. Yokoyama, M. Fujita and T. Kato, Angew. Chem., Int. Ed., 2017, 56, 14085–14089 CrossRef CAS PubMed; (e) H. Li, J. Luo and T. Liu, Chem.–Eur. J., 2016, 22, 17949–17952 CrossRef CAS PubMed; (f) S. Sato, M. Ikemi, T. Kikuchi, S. Matsumura, K. Shiba and M. Fujita, J. Am. Chem. Soc., 2015, 137, 12890–12896 CrossRef CAS PubMed; (g) S. Sato, Y. Yoshimasa, D. Fujita, M. Yagi-Utsumi, T. Yamaguchi, K. Kato and M. Fujita, Angew. Chem., Int. Ed., 2015, 54, 8435–8439 CrossRef CAS PubMed; (h) K. Takao, K. Suzuki, T. Ichijo, S. Sato, H. Asakura, K. Teramura, K. Kato, T. Ohba, T. Morita and M. Fujita, Angew. Chem., Int. Ed., 2012, 51, 5893–5896 CrossRef CAS PubMed; (i) M. Tominaga, K. Suzuki, M. Kawano, T. Kusukawa, T. Ozeki, S. Sakamoto, K. Yamaguchi and M. Fujita, Angew. Chem., Int. Ed., 2004, 43, 5621–5625 CrossRef CAS PubMed.
- (a) L. Tang, L. Wang, X. Yang, Y. Feng, Y. Li and W. Feng, Prog. Mater. Sci., 2021, 115, 100702 CrossRef CAS; (b) H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Containing synthetic procedures and experimental details. See DOI: https://doi.org/10.1039/d5sc00335k |
| This journal is © The Royal Society of Chemistry 2025 |