 Open Access Article
Open Access ArticleSynthesis and properties of SiO2/SiO2@Ag two-phase STFs†
Caiting Heab,
Qiushi Wang *ab,
Xiaoya Jiaab,
Jie Liuab,
Runjun Sunab and
Meiyu Chen*ab
*ab,
Xiaoya Jiaab,
Jie Liuab,
Runjun Sunab and
Meiyu Chen*ab
aSchool of Textile Science and Engineering, Xi'an Polytechnic University, Xi'an, Shaanxi 710048, China. E-mail: yuanshijidi@163.com; wangqiushi@xpu.edu.cn
bKey Laboratory of Functional Textile Material and Product (Xi'an Polytechnic University), Ministry of Education, Xi'an, Shaanxi 710048, China
First published on 23rd January 2023
Abstract
Soft body armor with a strain-sensing function using conductive shear thickening fluids (STFs) has gradually gained research interest. In this study, conductive SiO2@Ag core–shell microspheres were synthesized and the influence of process parameters on their properties was evaluated. Subsequently, SiO2 and SiO2@Ag were used as dispersed phases to prepare two-phase STFs, the effect of the core–shell microspheres' proportion on the rheological properties of the STFs was investigated, and its mechanism was discussed. The results indicated that SiO2@Ag core–shell microspheres were coated with elemental silver and when the concentration of sodium hydroxide and glucose were 0.07 and 0.09 mol L−1, respectively, the coating surface was the most uniform and compact, and the conductivity reached the minimum value of 0.56 Ω cm. The two-phase STFs exhibited good and reversible shear thickening behaviors and the critical shear rate decreased with increasing core–shell microsphere concentration. Additionally, when the mass fraction of SiO2 and SiO2@Ag core–shell microspheres was 45% and 20%, respectively, the thickening rate was 325%, and the resistance of two-phase STFs decreased simultaneously with the emergence of shear thickening that reached the lowest value of 795.16 kΩ. This study provides a novel strategy for synthesizing conductive STFs for strain-sensing flexible stab-resistant composites.
1 Introduction
Shear Thickening Fluids (STFs) are non-Newtonian fluids whose physical state rapidly changes from liquid to solid state when subjected to shear action exceeding the critical rate,1–3 and this phase transition is reversible and reproducible.4,5 STFs are extensively used in dampers,6 hydraulic couplers,7 shock absorption,8 and other engineering fields. In the 1990s, Professor Wagner from Delaware State University and Dr Wetzel from the US Army Research Laboratory successfully expanded the application of STFs to the field of personal protection.9,10The rheological properties of STFs served as the foundation for their use in personal protection applications. The concentration, type, and size of the dispersed phase influence the microstructure and rheological properties of the STFs.11,12 Grover et al.11 concluded that the optimal process conditions whereby STFs reduced the impact velocity of the projectile were achieved at a concentration of 67% and particle size of 600 nm, and the increased friction and volume occupation between the SiO2 particles with larger sizes was proposed to be the main reason for the enhancement in the STFs energy absorption. Gurgen et al.3,13 and Hasanzadeh et al.14,15 investigated the influence of the addition of silicon carbide (SiC) and multi-walled carbon nanotubes (MWCNT) as the dispersed phase on the rheological properties of the two-phase STFs, which weakened the shear thickening effect by the degradation of the suspensions' network structure. Li et al.16 used oxygen plasma treatment to graft hydroxyl, carboxyl, and other oxygen functional groups onto the MWCNT surface, and the formation of hydrogen bonds between the oxygen functional groups and particles in the STFs was reported to be the main reason for the improved shear thickening effect.
In addition to meeting the basic rheological property requirements, multi-phase STFs can be synthesized by introducing conductive carbon-based additions to provide intelligent functions such as strain or force sensing.17,18 Chen et al.7 prepared conductive STFs using carbon nanotubes (CNTs). The mass fraction of SiO2 and CNTs affected the resistance of C-STFs, which was negatively correlated with STFs thickening. Zhang et al.19 prepared a C-STF/Ecoflex material for sensors by adding CNTs to STFs, and developed a flexible composite for multifunction applications. Wang and Liu et al.20,21 obtained a flexible material with a high sensing sensitivity and excellent protective performance by combining conductive STFs with CNTs and Kevlar fabric, which monitored the different motion states of the human body. Zhou22 et al. used shear-toughened gel (SSG), two-dimensional MXene, and Kevlar to prepare the MXene/SSG/Kevlar composites with good electrical conductivity impact resistance. Zhang et al.23 prepared impregnated conductive Shear Thickening Gel (STG) by adding carbon black (CB) into a polyurethane sponge (PUS). The developed STG-CB-PUS composite material effectively tracked human movements, such as walking, running, and jumping. Zhao et al.24 developed a CB-STG/Kevlar composite that exhibited good mechanical-electrical coupling characteristics, and the resistance of the material was linearly related to the impact force. Fan25 et al. used reduced graphene oxide (RGO) and nano-silica/shear-thickened gel to manufacture the SiO2/STG/RGO@Kevlar composites as a protective layer with good impact resistance, and strain sensing.
From above, carbon-based materials, such as CNTs and CB, were generally selected to add into STFs to prepare conductive STFs, further endowing STFs-based flexible composites with sensing functions. However, no research has been reported on the preparation of conductive STFs using metal materials with lower resistivities, such as Ag and Cu. Therefore, we synthesize SiO2@Ag core–shell microspheres by planting Ag nanoparticles on the SiO2 microsphere surface, aiming to design the STFs with both shear thickening and strain-sensing characteristics.
2 Experimental section
2.1 Materials
Monodispersed silica microspheres (SiO2) with a particle size of 500 nm were purchased from Shanghai Bowei Applied Materials Technology Co. Ltd. Glucose (C6H12O6), sodium hydroxide (NaOH), silver nitrate (AgNO3), ammonia (NH4OH), anhydrous ethanol, (3-mercaptopropyl) trimethoxysilane (MPTMS), and polyethylene glycol 200 (PEG200) were all of the analytical grade and purchased from Sinopharm Group Chemical Reagent Co. Deionized water was used for all the experiments.2.2 Synthesis of SiO2@Ag core–shell microspheres
A certain mass of SiO2 modified by surface sulfhydrylation (see the ESI† for the detailed method) was added into a reducing solution containing a specific concentration of aqueous C6H12O6 and NaOH, and ultrasonically dispersed for 10–15 min. Ammonia was added dropwise to a certain mass of the plating solution (0.12 mol L−1 AgNO3 aqueous solution) until the turbidity of the mixture was clarified. Subsequently, the resultant mixture was added dropwise to the reducing solution under 300 rad min−1 magnetic stirring at 1 mL min−1, and the reaction was complete after 40 min. After the reaction was completed, the resulting mixture was centrifuged, washed with anhydrous ethanol for 3–5 times, and then dried at 60 °C in a vacuum oven without additional protective atmosphere to obtain SiO2@Ag core–shell microspheres. The flow chat is shown in Fig. 1a. | ||
| Fig. 1 Flow chart of (a) SiO2@Ag core–shell microsphere synthesis, (b) SiO2/SiO2@Ag two-phase STFs preparation. | ||
Based on the preliminary experimental results, the appropriate concentration ranges of NaOH and C6H12O6 were 0.03–0.07 mol L−1 and 0.09–0.13 mol L−1, respectively. The specific experimental protocols are listed in Table S1 in the ESI.†
2.3 Synthesis of STFs
STFs were synthesized using the ultrasonic dispersion method. First, a certain amount of SiO2 was weighed and dried at 60 °C. Subsequently, it was added to a beaker containing an appropriate amount of anhydrous ethanol, and ultrasonically dispersed for 30 min. A certain amount of PEG200 was added under continuous ultrasonic dispersion, followed by a certain amount of SiO2@Ag core–shell microspheres, and the mixture was continuously sonicated until a uniform STF suspension formed. Finally, the residual anhydrous ethanol and air bubbles were removed by vacuum drying at 60 °C for 24 h, and the resulting suspension was sealed and stored for later use. A photo of the prepared two-phase STFs is shown in Fig. S1 in the ESI.† The flow chat is shown in Fig. 1b. The specific experimental protocols for synthesizing the two-phase STFs are listed in Table S2 in the ESI.†2.4 Testing and characterization
3 Results and discussion
3.1 Determination of process parameters for SiO2@Ag core–shell microsphere synthesis
 | ||
| Fig. 4 SEM images and illustration (secondary magnification) of SiO2@Ag core–shell microspheres synthesized with different process parameters. | ||
For the N7Cy series of samples, the reducing agent concentration also affects the surface coating morphology. As shown in Fig. 4, for samples N7C3 and N7C5, owing to the low concentration of the reducing agent, silver ions in solution are reduced to silver at a slow rate, which limits the number of particles adhered to the SiO2 surface, and thereby the coating layer is thin. When the C6H12O6 concentration was higher than 0.09 mol L−1, the silver particles agglomerated into large particles that adhered to the SiO2 surface, which formed a non-dense coating. In particular, for the N7C13 sample, the C6H12O6 concentration was too high, resulting in a highly reducible solution. In this case, the reaction rate is too rapid, and the rate of silver ion reduction is greater than the rate of Ag particle adhesion to the SiO2 surface, and therefore the Ag particles in solutions easily formed agglomerates.
Based on the above SEM results, sample N7C9 exhibited the best coating effect, and the silver particles of the coating layer were relatively dense and uniform. In this case, the reaction rate was relatively moderate, the silver ion reduction rate and Ag particle adhesion to the SiO2 surface were balanced, and the reduced silver preferentially nucleated on the SiO2 surface, forming a continuous and dense coating layer. The TEM photograph of the N7C9 specimen clearly shows the SiO2 core and silver-plating shell of the microspheres, and the thickness of the shell is about 13 nm in Fig. 5.
The content and distribution of Ag on the surface of the N7C9 sample were further analyzed. The proportion of the main elemental content on the sample surface and the mapping sweep distribution of elemental Ag determined by EDS are shown in Fig. 6. The magnification of the EDS mapping sweep image is 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times which are twice that of the SEM photo of N7C9 sample. Based on the distribution of green spots, elemental Ag was uniformly distributed on the surface of the SiO2 microspheres. Additionally, the main elements on the surface of the SiO2@Ag core–shell microspheres were O, Si, and Ag, and the proportion of elemental Ag was 0.76%.
000 times which are twice that of the SEM photo of N7C9 sample. Based on the distribution of green spots, elemental Ag was uniformly distributed on the surface of the SiO2 microspheres. Additionally, the main elements on the surface of the SiO2@Ag core–shell microspheres were O, Si, and Ag, and the proportion of elemental Ag was 0.76%.
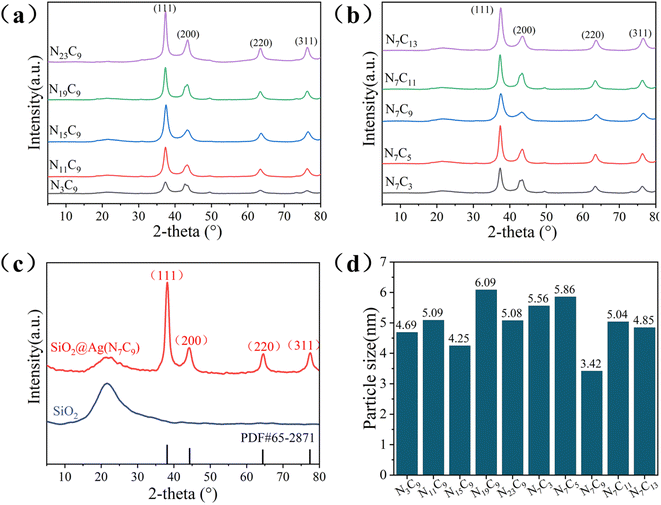 | ||
| Fig. 7 XRD patterns of (a) and (b)NxCy series samples and (c)SiO2 and the (d) average grain size of NxCy series samples. | ||
As shown in Fig. 7d, the grain size of the silver plating layer of NxCy series specimens was calculated by Scherrer equation. And the N7C9 specimen has the smallest average grain size of 3.42 nm, which is consistent with the morphology of microspheres in Fig. 4.
Based on the morphological analysis and resistivity test results, the optimal process parameters for synthesizing SiO2@Ag core–shell microspheres are a C6H12O6 concentration of 0.09 mol L−1 and NaOH concentration of 0.11 mol L−1.
3.2 Rheological properties of the two-phase STFs system
Fig. 9 shows the relationship between the shear rate and (a) viscosity and (b) shear stress, as well as (c) the mechanism of the two-phase STFs system. As shown in Fig. 9a, after SiO2@Ag core–shell microsphere addition, the rheological properties of the two-phase STFs system were consistent with the variation in viscosity of the single-phase sample, which exhibited the behavior of non-Newtonian fluid. Because the density of the core–shell microspheres was larger than that of SiO2, the density of the two-phase STFs system increased with the increase in the mass fraction of the core–shell microspheres. For STFs with a higher density, the adhesion between solid particles is stronger, and therefore the overall viscosity of the fluid increases.3 Additionally, the decrease in the critical shear rate may be attributed to the increased viscosity of the two-phase STFs system. According to eqn (1) for calculating Fhydrodynamic,28 viscosity is proportional to the fluid power, and thus the two-phase dispersed phase in the STF system forms hydroclusters more easily. For all the curves, the viscosity of the STF60:5 system increased from 0.84 to 4.75 Pa s at the critical shear rate, and the thickening rate was 565%. Compared with the single-phase STFs system with 65% SiO2 concentration, the maximum viscosity was increased by 47.37%. The viscosity of STF45:20 increased from 2.77 to 9 Pa s at the critical shear rate, and the thickening rate was 325%. However, the viscosity of the STF40:25 system increased from 7.25 to 17.3 Pa s at the critical shear rate, and the thickening rate was only 239%. Although the SiO2 and SiO2@Ag core–shell microspheres both participate in hydroclusters' formation which determines the shear thickening behavior, the weaken of shear thickening with the higher ratio of SiO2@Ag might be due to the decrease of Si–OH on the SiO2@Ag microspheres, leading the diminish of the hydrogen bond between Si–OH and the –OH groups in PEG200, as shown in Fig. 9(e2 and e3).
 | (1) |
Fig. 9b shows that shear stress increased linearly with increasing shear rate during the initial stage and then increased rapidly when the critical shear rate was reached, indicating nonlinear growth. Moreover, the maximum shear stress increased with increasing mass fraction of the SiO2@Ag core–shell microspheres. Notably, the critical shear stress of STFs with varying ratios of the dispersed phases was not different. However, the critical shear stress of the STFs system with a relatively higher SiO2@Ag core–shell microsphere content was slightly increased.
Non-Newtonian fluids are intermediate between the solid and liquid state, and exhibit viscoelastic behavior. Based on the viscoelastic theory24, the storage modulus (G′) and loss modulus (G′′) are representative of the elastic and viscous strength of the system, respectively. The relationship between strain and the (c) energy storage modulus and (d) loss modulus of the two-phase STFs system is shown in Fig. 9. STF with different ratios of the dispersed phase exhibited the process of thinning and thickening by shear, and the overall trend was consistent. Under lower strain, G′ and G′′ remained unchanged because the network structure damage caused by fluid repulsion and Brownian motion was rapidly restored. Subsequently, with increasing strain, the G′ and G′′ curves both exhibited a downward trend. In this case, intermolecular force and Brownian motion are insufficient to restore the damaged structure. However, G′′ was always greater than G′, which is mainly attributed to energy dissipation and indicates the viscous nature of the system. After the critical strain was reached, the STFs system was mainly dominated by fluid force, leading to the rapid formation of hydroclusters. Both G′ and G′′ curves increased, and G′′ was still larger than G′. In this case, G′′ is dominant, indicating that the system still exhibits viscous behavior, which is consistent with the steady-state rheological behavior of the system, which exhibited a relatively low thickening rate.
3.3 Relationship between the rheological and electrical properties of the two-phase STF system
The relationship between the steady-state rheological and electrical properties of STF45:20 and PEG200 is shown in Fig. 11a and b. Comparing the shear rate-viscosity and resistance curves of PEG200 and the two-phase STFs system, the viscosity and resistance of PEG200 do not change with increasing shear rate, but the resistance and viscosity of STF45:20 decreased and increased, respectively, exhibiting a negative correlation. During the shear thinning stage of the STFs system, the resistance remained stable at 936.77 kΩ. When the system exhibited shear thickening, the resistance rapidly decreased to the lowest value of 795.16 kΩ with the rapid increase in the viscosity. Thus, the variation in the viscosity and resistance occurred simultaneously during the shear process. Because when the STFs system is in the shear thinning stage, there is less contact between the dispersed phase particles owing to the repulsive forces between the molecules, and therefore electron transfer in the polymer solution is difficult, as shown in Fig. 9(e1 and e2). However, when the system is in the shear thickening stage, the rapidly formed SiO2 and SiO2@Ag mixed particle hydroclusters have surface contact and Ag on the core–shell microsphere surface more efficiently performs electron transfer, thus reducing the resistance of the system, as shown in Fig. 9(e3).The relationship between the resistance of the two-phase STFs system and the shear rate for different ratios of the dispersed phases is shown in Fig. 11c. Although no SiO2@Ag core–shell microspheres were added to the STF65:0 system, the variation in resistance also exhibited a similar trend. Because SiO2 is a semiconductor, the SiO2 clusters formed in the shear thickening process will also transfer electrons owing to contact with each other, but the resistance transfer efficiency is relatively low. Therefore, the resistance of the STFs system is considerable. For the STF60:5 system, the stable resistance without shear thickening was 1423.15 kΩ, and the minimum resistance was 1030.39 kΩ, whereas the stable resistance for the STF45:20 system was 936.77 kΩ and the minimum resistance was 795.16 kΩ. Thus, with increasing conducting SiO2@Ag core–shell microspheres, the stable resistance of the STFs system during shear thinning process decreased and the lowest resistance in the shear thickening process decreased significantly. Additionally, a positive correlation was observed between the resistance change rate and thickening rate of the two-phase STFs system, and the thickening rate of the STF40:25 system was 239% with a resistance change rate of 17.17%. Furthermore, the thickening rate of the STF60:5 system was 565% with a resistance change rate of 27.6%. Thus, the higher the shear thickening, the more pronounced the change in resistance. This phenomenon further confirms that the synchronous variation in the resistance and viscosity of the two-phase STFs system in Fig. 11a is caused by the change of the microscopic agglomeration of the dispersed phase during the shear thickening process. However, the resistance of the STF system did not continue to decrease with the further increase in the proportion of the SiO2@Ag core–shell microspheres. In particular, the stability and minimum resistance of STF40:25 were higher than those of STF45:20. Based on the analysis of the shear thickening mechanism of the two-phase STFs system in Section 3.2.1, the surface of the core–shell microspheres possesses limited Si–OH groups, and hydrocluster formation during the shear thickening process depends more on the contact friction and intermolecular force between SiO2 and the core–shell microspheres. In this case, the excessive addition of core–shell microspheres hinders the ability of SiO2@Ag to participate in SiO2 hydrocluster formation during shear thickening completely, and thus the core–shell microspheres are partially dispersed in the polymer solution. Compared with STF45:20, the SiO2 content in STF40:25 was relatively lower, and the number of core–shell microspheres that could participate in agglomeration was also lower, and thus it was more difficult to form a conductive pathway, leading to an increase in the resistance of the two-phase STFs system.
Based on the above studies on the rheological properties and resistance changes of the SiO2/SiO2@Ag two-phase STFs system, the two-phase conductive STF45:20 system with a specific ratio of SiO2 and SiO2@Ag in the dispersed phase exhibited a good shear thickening effect. Moreover, a remarkable electrical phenomenon was observed that the resistance of the system decreases rapidly with shear thickening. Therefore, it is possible to fabricate flexible composites with both anti-stab performance and sensing function.
4 Conclusions
In this study, the relationship between the process parameters of conducting SiO2@Ag core–shell microspheres and their surface morphology and electrical properties was investigated, and the influence of the ratio of the dispersed phases on the rheological properties and resistance of the SiO2/SiO2@Ag two-phase STFs system was evaluated, the main conclusions are as follows:(1) By investigating the effect of the NaOH and C6H12O6 concentrations on the electroless silver plating process, it is demonstrated that the alkaline environment and reducing agent concentration influenced the morphology of the SiO2@Ag core–shell microspheres. The optimal process parameters of the NaOH and C6H12O6 concentrations were 0.07 and 0.09 mol L−1, respectively. Under these conditions, the elemental Ag on the SiO2@Ag core–shell microsphere surface was uniform and compact and exhibited the lowest resistivity (0.56 Ω cm).
(2) SiO2/SiO2@Ag two-phase STFs exhibited a significant shear thickening effect and good reversibility. With increasing SiO2@Ag core–shell microsphere concentration, the critical shear rate of the system decreased, and the initial and maximum viscosities increased.
(3) When the shear thickening phenomenon occurs in the two-phase STFs system, the resistance decreases simultaneously with the viscosity increase. Moreover, the resistance was the lowest when the shear thickening effect was the most significant. The two-phase STF45:20 system exhibited a thickening rate of 325% and minimum resistance of 795.16 kΩ, which was determined to be suitable for fabricating flexible STF-based stab-resistant materials with strain-sensing functions.
(4) During the shear thickening process of the two-phase STFs system, strong surface contact occurs owing to the rapid formation of SiO2 and SiO2@Ag hydroclusters, the Ag on the core–shell microsphere surface is more effective for electron transfer, thereby rapidly reducing the resistance of the STFs system. Thus, the resistance and shear thickening behavior simultaneously induce.
The conductive SiO2@Ag core–shell microspheres investigated in this study provide a novel strategy for the synthesis of multiphase conductive STFs. Moreover, this study provides a new opportunity to explore STFs-based flexible stab-resistant materials for application in the field of strain sensing body armor.
Author contributions
Caiting He: Investigation, data curation, visualization, writing-original draft. Qiushi Wang: conceptualization, methodology, funding acquisition. Xiaoya Jia: data curation, visualization. Jie Liu: validation, visualization, Runjun Sun: methodology, resources. Meiyu Chen: conceptualization, writing-review&editing supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Scientific Research Program Funded by the Shaanxi Provincial Education Department (Program No. 21JY015), the Doctoral Scientific Research Foundation of Xi'an Polytechnic University (Program No. BS2020004) and the Science and Technology Guidance Foundation of China National Textile and Apparel Council (Program No. 2020041).References
- F. J. Galindo-Rosales, S. Martínez-Aranda and L. Campo-Deaño, CorkSTFμfluidics – a novel concept for the development of eco-friendly light-weight energy absorbing composites, Mater. Des., 2015, 82, 326–334 CrossRef.
- A. Majumdar, B. S. Butola and A. Srivastava, Optimal designing of soft body armour materials using shear thickening fluid, Mater. Des., 2013, 46, 191–198 CrossRef CAS.
- S. Gürgen, W. Li and M. C. Kuşhan, The rheology of shear thickening fluids with various ceramic particle additives, Mater. Des., 2016, 104, 312–319 CrossRef.
- L. L. Sun, D. S. Xiong and C. Y. Xu, Application of shear thickening fluid in ultra high molecular weight polyethylene fabric, J. Appl. Polym. Sci., 2013, 129(4), 1922–1928 CrossRef CAS.
- B. J. Maranzano and N. J. Wagner, The effects of particle size on reversible shear thickening of concentrated colloidal dispersions, J. Chem. Phys., 2001, 114(23), 10514–10527 CrossRef CAS.
- H. Zhou, L. Yan, W. Jiang, S. Xuan and X. Gong, Shear thickening fluid–based energy-free damper: Design and dynamic characteristics, J. Intell. Mater. Syst. Struct., 2014, 27(2), 208–220 CrossRef.
- Q. Chen, M. Liu, S. H. Xuan, W. Q. Jiang, S. S. Cao and X. L. Gong, Shear dependent electrical property of conductive shear thickening fluid, Mater. Des., 2017, 121, 92–100 CrossRef CAS.
- B. Liu, C. Du and Y. Fu, The preparation process of shear thickening materials and the application in hydraulic shock absorber, IOP Conf. Ser.: Earth Environ. Sci., 2021, 639(1), 012040 CrossRef.
- Y. S. Lee, E. D. Wetzel and N. J. Wagner, The Ballistic Impact Characteristics of Kevlar Woven Fabrics Impregnated with a Colloidal Shear Thickening Fluid, J. Mater. Sci., 2003, 38(13), 2825–2833 CrossRef CAS.
- D. P. Kalman, R. L. Merrill, N. J. Wagner and E. D. Wetzel, Effect of particle hardness on the penetration behavior of fabrics intercalated with dry particles and concentrated particle-fluid suspensions, ACS Appl. Mater. Interfaces, 2009, 1(11), 2602–2612 CrossRef CAS PubMed.
- G. Grover, S. K. Verma, A. Thakur, I. Biswas and D. Bhatacharjee, The effect of particle size and concentration on the ballistic resistance of different shear thickening fluids, Mater. Today: Proc., 2020, 28, 1472–1476 CAS.
- X. Feng, S. Li, Y. Wang, Y. Wang and J. Liu, Effects of different silica particles on quasi-static stab resistant properties of fabrics impregnated with shear thickening fluids, Mater. Des., 2014, 64, 456–461 CrossRef CAS.
- S. Gurgen and M. C. Kushan, The stab resistance of fabrics impregnated with shear thickening fluids including various particle size of additives, Composites, Part A, 2017, 94, 50–60 CrossRef CAS.
- M. Hasanzadeh and V. Mottaghitalab, Tuning of the rheological properties of concentrated silica suspensions using carbon nanotubes, Rheol. Acta, 2016, 55(9), 759–766 CrossRef CAS.
- M. Hasanzadeh, V. Mottaghitalab, H. Babaei and M. Rezaei, The influence of carbon nanotubes on quasi-static puncture resistance and yarn pull-out behavior of shear-thickening fluids (STFs) impregnated woven fabrics, Composites, Part A, 2016, 88, 263–271 CrossRef CAS.
- D. Li, R. Wang, X. Liu, S. Fang and Y. Sun, Shear-thickening fluid using oxygen-plasma-modified multi-walled carbon nanotubes to improve the quasi-static stab resistance of Kevlar fabrics, Polymers, 2018, 10(12), 1356 CrossRef PubMed.
- J. S. Zhang, Y. Wang, H. X. Deng, J. Y. Zhou, S. Liu and J. P. Wu, et al., A high anti-impact STF/Ecoflex composite structure with a sensing capacity for wearable design, Composites, Part B, 2022, 233, 109656 CrossRef CAS.
- M. Zarei and J. Aalaie, Application of shear thickening fluids in material development, J. Mater. Res. Technol., 2020, 9(5), 10411–10433 CrossRef.
- J. Zhang, Y. Wang, H. Deng, J. Zhou, S. Liu and J. Wu, et al., A high anti-impact STF/Ecoflex composite structure with a sensing capacity for wearable design, Composites, Part B, 2022, 233, 109656 CrossRef CAS.
- S. Wang, S. Xuan, M. Liu, L. Bai, S. Zhang and M. Sang, et al., Smart wearable Kevlar-based safeguarding electronic textile with excellent sensing performance, Soft Matter, 2017, 13(13), 2483–2491 RSC.
- M. Liu, S. S. Zhang, S. Liu, S. S. Cao, S. Wang and L. F. Bai, et al., CNT/STF/Kevlar-based wearable electronic textile with excellent anti-impact and sensing performance, Composites, Part A, 2019, 126, 105612 CrossRef CAS.
- J. Zhou, J. Zhang, M. Sang, S. Liu, F. Yuan and S. Wang, et al., Advanced functional Kevlar composite with excellent mechanical properties for thermal management and intelligent safeguarding, Chem. Eng. J., 2022, 428, 131878 CrossRef CAS.
- S. Zhang, S. Wang, Y. Wang, X. Fan, L. Ding and S. Xuan, et al., Conductive shear thickening gel/polyurethane sponge: A flexible human motion detection sensor with excellent safeguarding performance, Composites, Part A, 2018, 112, 197–206 CrossRef CAS.
- C. Zhao, Y. Wang, S. Cao, S. Xuan, W. Jiang and X. Gong, Conductive shear thickening gel/Kevlar wearable fabrics: A flexible body armor with mechano-electric coupling ballistic performance, Compos. Sci. Technol., 2019, 182, 107782 CrossRef CAS.
- T. Fan, Z. Sun, Y. Y. Zhang, Y. Q. Li, Z. K. Chen and P. Huang, et al., Novel Kevlar fabric composite for multifunctional soft body armor, Composites, Part B, 2022, 242, 110106 CrossRef CAS.
- A. Sakthisabarimoorthi, S. A. M. B. Dhas and M. Jose, Fabrication and nonlinear optical investigations of SiO 2 @Ag core-shell nanoparticles, Mater. Sci. Semicond. Process., 2017, 71, 69–75 CrossRef CAS.
- J. C. Flores, V. Torres, M. Popa, D. Crespo and J. M. Calderón-Moreno, Preparation of core–shell nanospheres of silica–silver: SiO2@Ag, J. Non-Cryst. Solids, 2008, 354(52–54), 5435–5439 CrossRef CAS.
- D. P. Kalman and N. J. Wagner, Microstructure of shear-thickening concentrated suspensions determined by flow-USANS, Rheol. Acta, 2009, 48(8), 897–908 CrossRef CAS.
- M. Yu, X. Qiao, X. Dong and K. Sun, Shear thickening effect of the suspensions of silica nanoparticles in PEG with different particle size, concentration, and shear, Colloid Polym. Sci., 2018, 296(7), 1119–1126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06895h |
| This journal is © The Royal Society of Chemistry 2023 |








