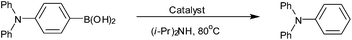
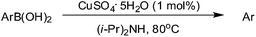Copper-catalyzed protodeboronation of arylboronic acids in aqueous media†
Chun Liu*,
Xinmin Li,
Yonghua Wu and
Jieshan Qiu
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Linggong Road 2, 116024, Dalian, China. E-mail: chunliu70@yahoo.com; Fax: +86 411-84986182; Tel: +86 411-84986182
First published on 17th October 2014
Abstract
A general and efficient protocol for the CuSO4·5H2O-catalyzed protodeboronation of arylboronic acids in aqueous ethanol is described. This catalytic system exhibits high activity towards a wide range of arylboronic acids. The results demonstrate that the protodeboronation reaction is promoted by oxygen.
Organoboronic acids are valuable reagents for organic syntheses due to their unique reactivity, high stability and low toxicity, thus allowing handling without special precautions.1–4 Over the past decades, arylboronic acids and their derivatives have been widely used in various reactions, in particular, in the transition-metal-catalyzed Suzuki–Miyaura coupling reaction which is known as one of most powerful carbon–carbon bond forming reactions.5–8 The protodeboronation is a common, sometimes even dominant side reaction in the Suzuki–Miyaura coupling reaction.9,10 However, little work has been reported in investigating the mechanism of protodeboronation and in applying the protodeboronation to organic syntheses. As early as 1930, Ainley et al.11 reported that the protodeboronation of arylboronic acids could be performed slowly in boiling aqueous solution with stoichiometric amounts of metallic salts (CuSO4, CrBr3, ZnCl2). Subsequently, Kuivila and co-workers12–14 focused on studying the mechanism of protodeboronation in an acidic, alkaline or metal-mediated condition. In 2006, Liu et al.15 reported a nano-palladium-catalyzed protodeboronation of arylboronic acids. In the presence of 1 mol% palladium nano-particles and 1.1 equiv. K2CO3, the protodeboronation of phenylboronic acid provided the corresponding product in 98% yield after 10 h under N2 atmosphere, and a few examples were employed in this approach. Perrin's group16 studied the protodeboronation of electron-deficient aryl and heteroarylboronic acids. Of these, the 2,6-disubstituted-boronic acids underwent rapid and quantitative protodeboronation at pH 12. These findings provide an insight into the stability of ortho-substituted arylboronates for use in the Suzuki–Miyaura cross-couplings. To the best of our knowledge, there are no reports on the cheap metal-catalyzed efficient protodeboronation of arylboronic acids.
The protodeboronation is usually considered as no practical value for organic syntheses. Fortunately, remarkable progress in applying the protodeboronation to organic syntheses has recently been made by several groups. Aggarwal's group17–20 reported a series of investigations on applying the protodeboronation of boronic esters to stereo-selective synthesis of natural and non-natural products. For example, using 1.5 equiv. of CsF, 1.1 equiv. of water in dioxane or using 1.5 equiv. TBAF·3H2O in pentane at 45 °C, various boronic pinacol esters afforded the protodeboronation products in excellent yields and selectivity.17 Furthermore, this method has been successfully applied to a short synthesis of the sesquiterpene, (S)-turmerone. Carreño's group21 reported the reactions of heteroaromatic compounds with quinonyl boronic acids proceeded by 1,4-addition and protodeboronation, leading directly to the Friedel–Crafts alkylation products. The boronic acid group is essential to trigger the Friedel–Crafts process. Very recently, Cheon's group22,23 reported metal-free thermal protodeboronation of electron-rich arylboronic acids. Several reaction parameters were investigated in the paper, and this protocol was successfully applied to the synthesis of ortho- and meta-functionalized phenols using the boronic acid moiety as a blocking group, respectively.
Green chemistry has become a target either in academic institutions or in industry.24–28 Nowadays, chemists have an increasing interest in developing green processes, as the sustainability has become an important issue in every area of human activity.29–31 Chemists have known protodeboronation of arylboronic acids for many years. However, the protodeboronation reactions mostly either took place under relatively harsh conditions or suffered from a limited substrate scope. Therefore, the development of a mild and efficient process of protodeboronation remains a desirable goal in synthetic organic chemistry. Herein, we report a green and general protocol for the copper-catalyzed protodeboronation of arylboronic acids in aqueous ethanol.
We initially investigated the effect of different metal catalyts on the model reaction of protodeboronation of 4-(diphenylamino)phenylboronic acid in air at 80 °C. The results are summarized in Table 1. In the absence of metal catalyst, only a 54% isolated yield was obtained in 1.5 h (Table 1, entry 1). The addition of copper catalysts led to a dramatic increase in activity, and the protodeboronation reactions provided satisfied results when using 10 mol% CuI, CuCl, Cu(OAc)2·H2O and CuSO4·5H2O as catalysts (Table 1, entries 2–5). Interestingly, the protodeboronation reaction could afford a 96% yield even the CuSO4·5H2O was reduced to 1 mol% (Table 1, entry 6). The results demonstrated clearly that copper ions can promote the protodeboronation reaction, which is consistent with Kuivil's report.14 On the other hand, HgSO4, ZnCl2 and Co(OAc)2·4H2O showed rather poor catalytic activity under the same reaction conditions (Table 1, entries 7–9). FeCl2 provided 57% isolated yield, nearly the same as that under metal-free conditions (Table 1, entry 10). Thus, we selected 1 mol% CuSO4·5H2O as the catalyst for next research.
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 4-(diphenylamino)phenylboronic acid (0.2 mmol), catalyst (10 mol%), (i-Pr)2NH (0.2 mmol), EtOH/H2O (0.5 mL/0.5 mL), 80 °C, 1.5 h, under air. The reaction was monitored by TLC.b Isolated yields.c CuSO4·5H2O (1 mol%). | ||
| 1 | — | 54 |
| 2 | CuI | 94 |
| 3 | CuCl | 84 |
| 4 | Cu(OAc)2·H2O | 86 |
| 5 | CuSO4·5H2O | 95 |
| 6 | CuSO4·5H2O | 96c |
| 7 | HgSO4 | 30 |
| 8 | ZnCl2 | 34 |
| 9 | Co(OAc)2·4H2O | 14 |
| 10 | FeCl2 | 57 |
As reported in the literature,14 base plays an important role for improving the reactivity of protodeboronation, thus we next studied the impact of different bases on the same model reaction. As shown in Table 2, only a 14% yield was observed when the protodeboronation reaction proceeded without any bases (Table 2, entry 1). However, a 59% yield was obtained in 1.5 h when 0.1 equiv. K2CO3 was added to the reaction mixture (Table 2, entry 2). Increasing the amounts of K2CO3 could raise the yield evidently, and a 93% yield was obtained with 1.0 equiv. K2CO3 (Table 2, entry 4). It is supposed that arylboronic acid may form arylboronate anion [ArB(OH)3]− under basic condition, which can undergo protodeboronation more easily. A series of bases were subsequently examined, Et3N, (i-Pr)2NH and DBU delivered the desired products in high yields (Table 2, entries 8–10), while only moderate yields were observed with K3PO4·3H2O, NaOH and NH3·H2O (Table 2, entries 5–7). Hence, (i-Pr)2NH is the best base for this catalytic system.
| Entry | Base | Equivalent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-(diphenylamino)phenylboronic acid (0.2 mmol), CuSO4·5H2O (1 mol%), base (0.2 mmol), EtOH/H2O (0.5 mL/0.5 mL), 80 °C, 1.5 h, under air. The reaction was monitored by TLC.b Isolated yields. | |||
| 1 | — | — | 14 |
| 2 | K2CO3 | 0.1 | 59 |
| 3 | K2CO3 | 0.2 | 75 |
| 4 | K2CO3 | 1.0 | 93 |
| 5 | K3PO4·3H2O | 1.0 | 63 |
| 6 | NaOH | 1.0 | 54 |
| 7 | NH3·H2O | 1.0 | 54 |
| 8 | Et3N | 1.0 | 93 |
| 9 | (i-Pr)2NH | 1.0 | 96 |
| 10 | DBU | 1.0 | 93 |
As literatures reported, the Suzuki reactions4,32 and self-homocoupling reactions33 which using arylboronic acids as reagents could be promoted by oxygen. However, the effect of reaction atmosphere on the protodeboronation has never been studied. Thus, the next investigation was to study the effect of atmosphere on the protodeboronation reaction. As shown in Table 3, the protodeboronation of several arylboronic acids were performed in different atmospheres. Indeed, the atmosphere had an effect on the protodeboronation which gave higher yields under oxygen than in nitrogen (Table 3, entries 1c–4c vs. 1b–4b). For example, the protodeboronation of 4-cyanophenylboronic acid provided a 92% yield under air in 1.5 h (Table 3, entry 2a), while the isolated yield was decreased to a 64% in nitrogen (Table 3, entry 2b). The same protodeboronation reaction performed in an oxygen atmosphere could achieve 94% yield in 1.5 h. Using 4-(diphenylamino)-phenylboronic acid as substrate, the protodeboronation provided higher yield in oxygen than in nitrogen (Table 3, entry 4c vs. 4a). The kinetic studies were performed on the protodeboronation of 4-(diphenylamino)-phenylboronic acid under air, nitrogen and oxygen, respectively. The results are illustrated in Fig. 1. It is clear that the reaction proceeded faster in oxygen. For example, a 53% yield was obtained in 5 min under oxygen, higher than a 38% yield in nitrogen in the same reaction time. After 30 min, 85% and 80% yields were reached in oxygen and air, respectively, while a 71% yield was obtained under nitrogen. These results reflected that molecular oxygen plays a crucial role in such a copper-catalyzed protodeboronation reaction. More interestingly, when the reactions were performed without any copper catalyst, similar yields were afforded under the different atmospheres (Table 3, entries 4a–4c in parentheses). When using 1 mol% CuI as catalyst, 92% and 94% yields were reached in air and oxygen, respectively (Table 3, entries 5a and 5c). However, only a 56% yield was obtained under nitrogen, which was similar with the yield without copper catalyst (Table 3, entries 5b vs. 4a in parentheses). These results suggest that Cu(II) was actually the active species in this copper-catalyzed protodeboronation reaction, and the oxygen might play the role of the final oxidant which lead to regenerate Cu(II) species for the next catalytic cycle.
| Entry | Ar–B(OH)2 | Atmosphere | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: arylboronic acid (0.2 mmol), CuSO4·5H2O (1 mol%), (i-Pr)2NH (0.2 mmol), EtOH/H2O (0.5 mL/0.5 mL), 80 °C, 1.5 h.b Isolated yields yields in parentheses are obtained without using copper catalyst.c GC yields.d 1 mol% CuI as catalyst. | |||
| 1a | 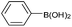 |
Air | 88c |
| 1b | N2 | 70c | |
| 1c | O2 | 90c | |
| 2a | 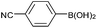 |
Air | 92c |
| 2b | N2 | 64c | |
| 2c | O2 | 94c | |
| 3a | 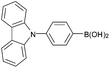 |
Air | 95 |
| 3b | N2 | 71 | |
| 3c | O2 | 94 | |
| 4a | 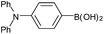 |
Air | 96 (54) |
| 4b | N2 | 76 (50) | |
| 4c | O2 | 97 (52) | |
| 5a | 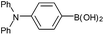 |
Air | 92d |
| 5b | N2 | 56d | |
| 5c | O2 | 94d | |
With the optimized conditions at hand, we further explored the scope and limitations of substrates for this protocol and the results are shown in Table 4. Unlike other protocols,16 the electronic nature of the substituent group had little effect on the protodeboronation reactions in this system. Arylboronic acids bearing electron-deficient or electron-rich groups all exhibited high reactivity and provided the corresponding products in good to excellent yields (Table 4, entries1–7). Moreover, ortho-substituted substrates also afforded good yields (Table 4, entries 8 and 9), while the same substrates could not undergo protodeboronation in a metal-free protocol.23 Various polycyclic aromatic boronic acids were examined in this protocol (Table 4, entries 10–15). For example, 10-phenylanthracen-9-ylboronic acid afforded the desired product in 94% yield (Table 4, entry 12). However, 9-phenyl-9H-carbazol-3-ylboronic acid gave only a 64% yield (Table 4, entry 15). The present protocol was further extended to the synthesis of heterocyclic compound. For example, 3-pyridylboronic acid afforded the product in 88% yield (Table 4, entry 16) and 3-thioenylboronic acid gave the desired product in 92% yield (Table 4, entry 19). We also attempted to extend this protocol to arylboronic esters, however, 4-(diphenyl-amino)phenylboronic pinacol ester provided a 61% yield after 24 h (Table 4, entry 20).
| Entry | Arylboronic acid | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: Arylboronic acid (0.2 mmol), CuSO4·5H2O (1 mol%), (i-Pr)2NH (0.2 mmol), EtOH/H2O (0.5 mL/0.5 mL), 80 °C, 1.5 h, under air. The reaction was monitored by TLC.b GC yields.c Isolated yields.d 24 h, isolated yields. | |||
| 1 | 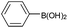 |
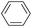 |
88 |
| 2 |  |
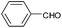 |
95 |
| 3 | 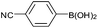 |
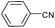 |
92 |
| 4 | 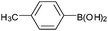 |
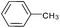 |
91 |
| 5 | 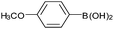 |
 |
92 |
| 6 | 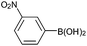 |
 |
91 |
| 7 | 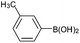 |
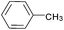 |
89 |
| 8 | 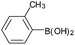 |
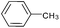 |
88 |
| 9 | 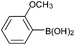 |
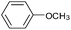 |
94 |
| 10 | 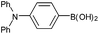 |
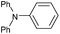 |
96c |
| 11 | 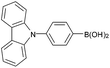 |
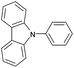 |
95c |
| 12 | 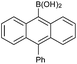 |
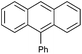 |
94c |
| 13 |  |
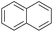 |
90 |
| 14 | 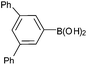 |
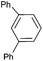 |
92c |
| 15 |  |
 |
64c |
| 16 | 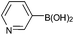 |
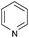 |
88 |
| 17 | 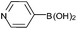 |
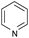 |
87 |
| 18 | 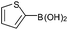 |
 |
86 |
| 19 | 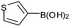 |
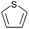 |
92 |
| 20 | 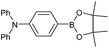 |
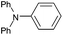 |
61d |
Although the detailed mechanism of the copper-catalyzed protodeboronation reaction of arylboronic acids is unclear, on the basis of the results described above, a tentative mechanism is proposed as shown in Fig. 2. Initially, arylboronic acid as mild organic lewis acids easily reacts with H2O to generate arylboronate anion. Then, Cu(II) ion attacks this tetrahedral adduct to form the intermediate of arylcopper complex 1. Subsequently, arylcopper complex undertakes the protodemetallation process with the (i-Pr)2NH2+, delivering the arenes and Cu(I) ion. The Cu(I) is oxidized by oxygen to Cu(II) species which enters into the next catalytic circle.
Conclusions
In conclusion, we have developed a simple and highly active protocol for the copper-catalyzed protodeboronation of arylboronic acids in aqueous media. A wide range of substrates, including electron-deficient or electron-rich groups, readily underwent the protodeboronation to give excellent yields. It is noteworthy that the reaction could be promoted by oxygen. Further investigations on the precise mechanism and synthetic application of this protocol are currently underway in our laboratory.Experimental
General remarks
Arylboronic acids and metal catalysts were purchased from Alfa Aesar. Other chemicals were obtained commercially and used without any prior purification. 1H NMR spectra were recorded on a Bruker Advance II 400 spectrometer using TMS as the internal standard. The yields were determined by GC using biphenyl as an internal standard for the liquid products, and the solid products were isolated by short chromatography on a silica gel (200–300 mesh) column using petroleumether (60–90 °C), unless otherwise noted. Compounds described in the literature were characterized by 1H NMR spectra compared to reported data.Typical procedure for protodeboronation of arylboronic acids
A mixture of arylboronic acid (0.2 mmol), CuSO4·5H2O (1 mol%), (i-Pr)2NH (0.2 mmol) and EtOH/H2O (0.5 mL/0.5 mL) was stirred at 80 °C in air for 1.5 h. The mixture was added to brine (2 mL) and extracted two times with ethyl acetate (2 mL). The combined organic layers were dried over sodium sulfate and the yield was determined by GC analysis with biphenyl as internal standard or the product was isolated by short chromatography on a silica gel (200–300 mesh) column using petroleumether (60–90 °C).Acknowledgements
The authors thank the financial support from the National Natural Science Foundation of China (21276043) and the Ministry of Education (The Program for New Century Excellent Talents in University, NCET-10-0283).Notes and references
- C. J. Li, Chem. Rev., 1993, 93, 2023–2035 CrossRef CAS.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, John Wiley & Sons, 2012 Search PubMed.
- C. Liu, Q. Ni, P. Hu and J. Qiu, Org. Biomol. Chem., 2011, 9, 1054–1060 CAS.
- T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508–7510 CrossRef CAS.
- J. P. Wolfe, R. A. Singer, B. H. Yang and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 9550–9561 CrossRef CAS.
- N. Miyaura, in Cross-coupling reactions, Springer, 2002, pp. 11–59 Search PubMed.
- W. Han, C. Liu and Z.-L. Jin, Org. Lett., 2007, 9, 4005–4007 CrossRef CAS PubMed.
- D. V. Partyka, Chem. Rev., 2011, 111, 1529–1595 CrossRef CAS PubMed.
- A. J. Lennox and G. C. Lloyd-Jones, Angew. Chem., Int. Ed., 2013, 52, 7362–7370 CrossRef CAS PubMed.
- A. D. Ainley and F. Challenger, J. Chem. Soc., 1930, 2171–2180 RSC.
- H. G. Kuivila and K. Nahabedian, J. Am. Chem. Soc., 1961, 83, 2159–2163 CrossRef CAS.
- H. G. Kuivila, J. F. Reuwer Jr and J. A. Mangravite, Can. J. Chem., 1963, 41, 3081–3090 CrossRef CAS.
- H. G. Kuivila, J. F. Reuwer and J. A. Mangravite, J. Am. Chem. Soc., 1964, 86, 2666–2670 CrossRef CAS.
- R. Y. Lai, C. L. Chen and S. T. Liu, J. Chin. Chem. Soc., 2006, 53, 979–985 CAS.
- J. Lozada, Z. Liu and D. M. Perrin, J. Org. Chem., 2014, 79, 5365–5368 CrossRef CAS PubMed.
- S. Nave, R. P. Sonawane, T. G. Elford and V. K. Aggarwal, J. Am. Chem. Soc., 2010, 132, 17096–17098 CrossRef CAS PubMed.
- T. G. Elford, S. Nave, R. P. Sonawane and V. K. Aggarwal, J. Am. Chem. Soc., 2011, 133, 16798–16801 CrossRef CAS PubMed.
- S. Roesner, J. M. Casatejada, T. G. Elford, R. P. Sonawane and V. K. Aggarwal, Org. Lett., 2011, 13, 5740–5743 CrossRef CAS PubMed.
- C. G. Watson and V. K. Aggarwal, Org. Lett., 2013, 15, 1346–1349 CrossRef CAS PubMed.
- M. Veguillas, M. Ribagorda and M. C. Carreño, Org. Lett., 2011, 13, 656–659 CrossRef CAS PubMed.
- S.-J. Ahn, C.-Y. Lee, N.-K. Kim and C.-H. Cheon, J. Org. Chem., 2014, 79, 7277–7285 CrossRef CAS PubMed.
- C.-Y. Lee, S.-J. Ahn and C.-H. Cheon, J. Org. Chem., 2013, 78, 12154–12160 CrossRef CAS PubMed.
- R. T. Baker and W. Tumas, Science, 1999, 284, 1477–1479 CrossRef CAS.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807–810 CrossRef CAS PubMed.
- C.-J. Li, Chem. Rev., 2005, 105, 3095–3166 CrossRef CAS PubMed.
- C.-J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68–82 RSC.
- C.-J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197–13202 CrossRef CAS PubMed.
- C. Wei and C.-J. Li, J. Am. Chem. Soc., 2002, 124, 5638–5639 CrossRef CAS PubMed.
- N. Uhlig and C.-J. Li, Chem. Sci., 2011, 2, 1241–1249 RSC.
- Z. Jia, F. Zhou, M. Liu, X. Li, A. S. Chan and C. J. Li, Angew. Chem., Int. Ed., 2013, 52, 11871–11874 CrossRef CAS PubMed.
- X. Rao, C. Liu, Y. Xing, Y. Fu, J. Qiu and Z. Jin, Asian J. Org. Chem., 2013, 2, 514–518 CrossRef CAS.
- K. A. Smith, E. M. Campi, W. R. Jackson, S. Marcuccio, C. G. Naeslund and G. B. Deacon, Synlett, 1997, 1, 131–132 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail and characterization of protodeboronation products. See DOI: 10.1039/c4ra11659c |
| This journal is © The Royal Society of Chemistry 2014 |