Fluorescent calix[4]arene chemosensor for acidic and basic amino acids in pure aqueous media†
Yao-Wei Xua,
Li-Lin Tana,
Jun-Min Liu*a,
Li-Min Xiaoc,
Shao-Yong Li*b and
Cheng-Yong Su*a
aMOE Laboratory of Bioinorganic and Synthetic Chemistry/KLGHEI of Environment and Energy Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: liujunm@mail.sysu.edu.cn; cesscy@mail.sysu.edu.cn
bTianjin Key Laboratory on Technologies Enabling Development of Clinical Therapeutics and Diagnostics (Theranostics), School of Pharmacy, Basic Medical Research Center, Tianjin Medical University, Tianjin 300070, China. E-mail: lishaoyong@tijmu.edu.cn
cSchool of Computer Science and Engineering, Beihang University, Beijing 100191, China
First published on 16th June 2014
Abstract
A modified calix[4]arene based receptor 2, conjugated with four bithiophene–cyanoacrylic acid groups, not only recognizes acidic amino acids (Asp and Glu) by quenching fluorescence, but also shows highly selective sensing for basic amino acids (Lys and Arg) by turning on fluorescence in sodium phosphate buffer solution without any organic solvent. The UV-Vis spectroscopy binding analysis and ESI-MS studies indicate that the above complexes have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. 1H NMR and molecular modeling studies suggest a supramolecular complex structure like the relationship between a lid and cup, but not a typical host-guest relationship of the cavity. A plausible mechanism involving intramolecular charge transfer (ICT) is proposed.
1 stoichiometry. 1H NMR and molecular modeling studies suggest a supramolecular complex structure like the relationship between a lid and cup, but not a typical host-guest relationship of the cavity. A plausible mechanism involving intramolecular charge transfer (ICT) is proposed.
Introduction
Amino acids and their derivatives are fundamental substrates involved in a wide variety of biological processes, and also play an important role in the area of design and preparation of pharmaceuticals, as they are part of the synthetic process in the production of drug intermediates and protein-based drugs.1 Therefore the selective recognition of these compounds by synthetic receptors is of particular significance for understanding the interactions between biological molecules as well as design of catalysis systems, new pharmaceutical agents, and separation materials. Of particular relevance are the studies performed in water, where most of the biological processes take place.2 Recently, considerable efforts have been made to develop macrocyclic receptors with specific properties and functions revealing their affinity and selectivity towards certain amino acids.3 However, most of these artificial receptors have suffered from low sensitivity and selectivity between various naturally occurring amino acids.4 Some of them, especially fluorescent chemosensors, achieved high selectivity in their recognition but unsatisfactorily worked in organic or organic-aqueous solution.5 Thus, the development of synthetic receptors for the recognition of amino acids in aqueous media is highly desirable.During the last couple of years, we have been interested in preparing and studying new calixarene derivatives, which show interesting properties as molecular receptors, molecular devices, organogelators, and fluorescent chemosensors.6 On the other hand, organic D-π-A dyes that contain oligothiophene as both donor groups and π bridge, and 2-cyanoacrylic acid as acceptor, are potential fluorescent chemosensors because of their excellent photophysical properties, such as large molar extinction coefficients, long absorption and emission wavelengths, and moderate fluorescence quantum yields. Moreover, the carboxylic acid group of the cyanoacrylic acid acceptor is a representative hydrophilic group and excellent donor/acceptor of hydrogen bonds. When grafted with oligothiophene–cyanoacrylic acid groups, calix[4]arene derivatives will become more watersoluble with the potential to recognize amino acids in aqueous media. In a preliminary communication, we reported the ability of a calix[4]arene derivative (1) conjugated with four thiophene–cyanoacrylic acid groups to act as a selective fluorescent ratiometric chemosensor for acidic amino acids.7 This led us to undertake an in-depth study of the recognition process by simple structural modification such as variation of the conjugation pathway. Thus, the receptor 2 conjugated with four bithiophene–cyanoacrylic acid groups has been designed by addition of a thiophene unit into 1. The results turn out interestingly that 2 not only recognizes acidic amino acids (Asp and Glu) by fluorescence quenching, but also shows highly selective sensing for basic amino acids (Lys and Arg) by fluorescence turn-on in sodium phosphate buffer solution without any organic solvent. In this paper, we describe a comprehensive study by a multidisciplinary approach using UV-Vis absorption and fluorescence spectroscopy, mass spectrometry, nuclear magnetic resonance, and molecular modeling, aiming to propose a binding mode and a reasonable supramolecular structure which can explain the selectivity observed by fluorescence measurements.
Results and discussion
Synthesis
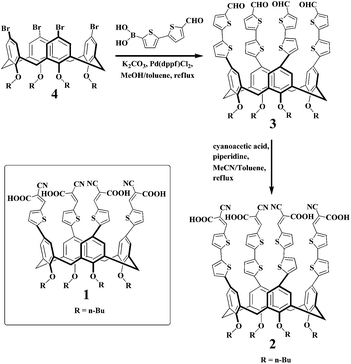
The synthesis of 2 is depicted in Scheme 1. A Suzuki reaction between 45c and 2TFB (5′-formyl-2,2′-bithiophene-5-yl boronic acid) was successfully executed in the presence of anhydrous K2CO3 and PdCl2(dppf) to produce 3. A Knoevenagel reaction between 3 and cyanoacetic acid then took place under piperidine to finally result in 2 with a high yield. The structures of 2 and 3 were confirmed by 1H NMR, 13C NMR, MS spectra, and EA. The cone conformation of every compound is clearly shown in the signal pattern of the 1H NMR.8 Meanwhile, the configuration of the bithiophene–cyanoacrylic acid group of 2 can be assigned a Z-geometry since the chemical shift of its alkenic proton is at δ 8.21 ppm in its 1H NMR spectrum.9The UV spectrophotometric titration of 2 with amino acids
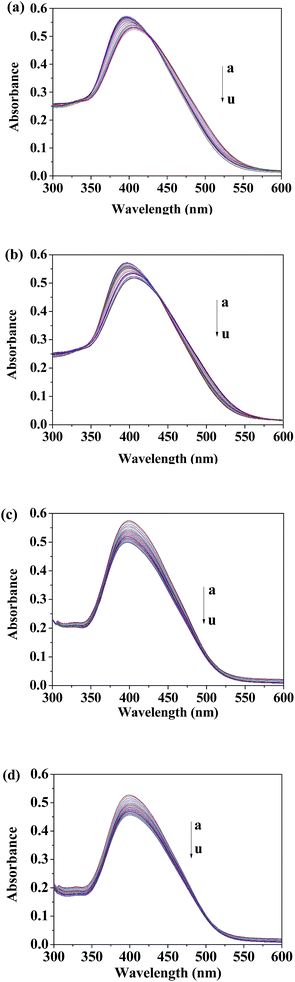
In the absorption spectrum of 2, maximum bands were observed at 400 nm attributable to the π–π* transition with charge-transfer character. Addition of Asp/Glu resulted in a decrease of the absorption intensity at 500 nm and an increase of the intensity at 400 nm with a slight blue-shift and the isosbestic point at 425 nm, which indicates complex formation (Fig. 1a and b). On the other hand, the titration of 2 with Lys/Arg showed a gradual decrease in absorption at 250–600 nm over an amino acid concentrations range of 50–1000 μM, above which a plateau was achieved, suggesting the presence of equilibrium between 2 and its Lys/Arg complex (Fig. 1c and d).The fluorometric titration of 2 with amino acids
In our previous study, calix[4]arene derivative 1 could bind amino acids, which inspired us to test the presence of special amino acids by inspecting the change of fluorescence intensity of 2 in the presence of amino acids. In the fluorescent spectrum of 2, the maximum excitation and emission wavelengths were observed at 400 and 566 nm, respectively. All titrations of 2 with 20 naturally occurring amino acids revealed that acidic amino acids (Asp/Glu) exhibit a decrease while basic amino acids (Lys/Arg) show an increase in fluorescence intensity, respectively. However, no obvious spectral changes occurred to 2 in the presence of 10 equiv. of Gln, Asn, Phe, His, Ala, Tyr, Gly, Trp, Ser, Met, Cys, Leu, Thr, Ile, Pro, and Val (Fig. 2 and S1†). These results suggested that acidic and basic amino acids might bind much stronger with 2 than other tested amino acids. Thus, 2 could act as a selective supramolecular fluorescence probe for Asp/Glu and Lys/Arg.To gain insight into the recognition behavior of 2 and Asp/Glu, the fluorescence emission spectral titration experiments were carried out. It was found that upon the addition of Asp/Glu to 2, the emission band at 566 nm gradually decreased with a slight red-shift within 10 nm. When the amino acid concentrations were 10 equiv. of 2, approximately 87% and 89% fluorescence quenching were observed for Asp and Glu, respectively (Fig. 3a and b). On the basis of fluorescence emission titrations, the association constants for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of 2 and acidic amino acids were calculated to be 7.87 × 104 M−1 and 1.36 × 105 M−1 for Asp and Glu according to the Stern–Volmer equation, respectively.
1 complexes of 2 and acidic amino acids were calculated to be 7.87 × 104 M−1 and 1.36 × 105 M−1 for Asp and Glu according to the Stern–Volmer equation, respectively.
To explore the recognition property of 2 to Lys/Arg, we have also carried out similar fluorescence titration experiments. The titration of 2 with basic amino acids showed gradual enhancement in the fluorescence intensity from 1 to 10 equiv. of Lys/Arg, and achieved a plateau afterward (Fig. 3c and d). The Scatchard-type equation was used to calculate the binding constants between 2 and basic amino acids, which were about 3.48 × 103 M−1 and 8.11 × 103 M−1 for Lys and Arg, respectively.
ESI MS studies on formation of the complexes
The electrospray ionization (ESI) mass spectrometry was also used to characterize the complexes between 2 and amino acids in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The salient peak at m/z 1832.2 for [2·Lys + H]+ was found by using the solution of 2 and Lys in 8
1 molar ratio. The salient peak at m/z 1832.2 for [2·Lys + H]+ was found by using the solution of 2 and Lys in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (v/v/v) dimethylacetamide, H2O, and acetonitrile (Fig. 4), which provided further evidence for formation of a 1
100 (v/v/v) dimethylacetamide, H2O, and acetonitrile (Fig. 4), which provided further evidence for formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stable complex between 2 and Lys. Similarly, formation of the 1
1 stable complex between 2 and Lys. Similarly, formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between 2 and Asp, Glu, and Arg was also evidenced by the ESI mass spectrometry, in which the salient peaks at m/z 1819.1, 1833.2, and 1860.2 for [2·Asp + H]+, [2·Glu + H]+, and [2·Arg + H]+, respectively, were all observed (Fig. S2†).
1 complexes between 2 and Asp, Glu, and Arg was also evidenced by the ESI mass spectrometry, in which the salient peaks at m/z 1819.1, 1833.2, and 1860.2 for [2·Asp + H]+, [2·Glu + H]+, and [2·Arg + H]+, respectively, were all observed (Fig. S2†).
1H NMR studies on Formation of the Complexes
To gain a better understanding for the interaction of these four amino acids with the sensor, we have conducted a 1H NMR spectroscopic investigation. From the 1H NMR spectra of 2, substrates, and their mixtures at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, it is found that one H signal of CβH2 in Lys undergoes an obvious downfield shift from 1.64 ppm to 1.71 ppm (marked by black dot in Fig. 5), contrarily, the CεH2 signal in Lys undergoes an obvious upfield shift from 3.06 ppm to 2.77 ppm (marked by white dot in Fig. 6), respectively. The CH2 group signal of Asp undergoes a slight downfield shift from 2.83, 2.77 ppm to 2.85, 2.79 ppm (marked by white and black dots in Fig. S3†). The CαH group signal of Glu undergoes an obvious downfield shift from 3.31 ppm to 3.40 ppm (marked by white dot in Fig. S4†). One H signal of CδH2 in Arg undergoes an obvious downfield shift from 2.92 ppm to 3.10 ppm (marked by white dot in Fig. S7†). Moreover, partial signal of the other two CH2 groups, CβH2 and CγH2, in Arg also undergoes an obvious downfield shift from 1.43 ppm to 1.74 ppm (marked by black dot in Fig. S5†).
1 molar ratio, it is found that one H signal of CβH2 in Lys undergoes an obvious downfield shift from 1.64 ppm to 1.71 ppm (marked by black dot in Fig. 5), contrarily, the CεH2 signal in Lys undergoes an obvious upfield shift from 3.06 ppm to 2.77 ppm (marked by white dot in Fig. 6), respectively. The CH2 group signal of Asp undergoes a slight downfield shift from 2.83, 2.77 ppm to 2.85, 2.79 ppm (marked by white and black dots in Fig. S3†). The CαH group signal of Glu undergoes an obvious downfield shift from 3.31 ppm to 3.40 ppm (marked by white dot in Fig. S4†). One H signal of CδH2 in Arg undergoes an obvious downfield shift from 2.92 ppm to 3.10 ppm (marked by white dot in Fig. S7†). Moreover, partial signal of the other two CH2 groups, CβH2 and CγH2, in Arg also undergoes an obvious downfield shift from 1.43 ppm to 1.74 ppm (marked by black dot in Fig. S5†).
The downfield shift of CH or CH2 in Asp, Glu, Lys and Arg shows that significant interaction exists between these molecules and 2, but they are not included by the cavity of 2. The upfield shift of CεH2 in Lys may result from two interaction modes: (i) its branched NH2 exists as NH3+ and is included by cavity of 2; (ii) its branched NH2 changes into NH2 from NH3+, then interacts with the carboxylic and amino groups of 2. The second mode embodies the actual existing state of the branched NH2 of Lys under its UV and Fluorescent titration condition. Coleman reported that calix[4]arene sulphonate includes the branched NH3+ of Lys with its cavity and induces an obvious upfield shift (Δδ > 1.5 ppm at pH = 5, Δδ > 1.0 ppm at pH = 1) of CεH2 when host and guest was mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.10 Although the cavity structure of 2 can't be wholly equated to that of the reported calix[4]arene sulphonate, the comparable slight upfield (Δδ = 0.29 ppm) is subjective to result from the second factor. Noticeably, 1H NMR spectrum of 2 has no distinct change on the addition of these four substrates, implying acidic and basic amino acids can be only caught by bithiophene–cyanoacrylic acid groups at the upper rim of 2, such as the relation of lid and cup, but not included by its cavity.11 Based on the NMR analysis, it can be deduced that the selectivity toward amino acid of 2 could be improved when the dimension of its “cup” is changed to fully match that of one “lid”.
1 molar ratio.10 Although the cavity structure of 2 can't be wholly equated to that of the reported calix[4]arene sulphonate, the comparable slight upfield (Δδ = 0.29 ppm) is subjective to result from the second factor. Noticeably, 1H NMR spectrum of 2 has no distinct change on the addition of these four substrates, implying acidic and basic amino acids can be only caught by bithiophene–cyanoacrylic acid groups at the upper rim of 2, such as the relation of lid and cup, but not included by its cavity.11 Based on the NMR analysis, it can be deduced that the selectivity toward amino acid of 2 could be improved when the dimension of its “cup” is changed to fully match that of one “lid”.
Molecular Modeling and Proposal of a Supramolecular Complex Structure
The binding mechanism of 2 toward Asp, Glu, Lys, and Arg was further investigated through molecular modeling with the Gaussian 03 package.12 Because a branched carboxylic group or amino group exists in these four amino acids, it is speculated that these additional groups should play an important role in the high selectivity through H-bond interactions with the cyanoacrylic acid groups on 2, cooperating with the characteristic groups of amino acids. Based on this deduction and the NMR analysis results, the optimization of the complexes was executed by placing a corresponding amino acid in a position maximally close to two opposite cyanoacrylic acid groups pointing inward of 2. Compound 2, 2–Asp, 2–Glu, 2–Lys and 2–Arg complex were optimized at the level of B3LYP/6-31G, the results of which are shown in Fig. 6 and Table S1–7.† 2 has an elongated shape, and its four bithiophene–cyanoacrylic acid groups are almost coplanar with their corresponding benzene rings. In the four complexes, amino acids are bridged on the top of 2 by catching its three bithiophene–cyanoacrylic acid groups with different number of H-bonds: O198–H207⋯O116, O115–H1927⋯O201, O102–H188⋯O200, N194–H202⋯O198 and N194–H203⋯N80 (2–Asp complex); O199–H200⋯O116, O115–H192⋯O210, O102–H188⋯O201, O202–H211⋯O102, and N194–H203⋯N80 (2–Glu complex); N200–H214⋯O116, O102–H188⋯O201, O202–H216⋯O102, and N194–H203⋯N80 (2–Lys complex); N203–H217⋯O116, O102–H188⋯O201, O202–H215⋯O102, O115–H192⋯N199, and N194–H205⋯N80 (2–Arg complex). Under these H-bonding interactions from amino acids, the bithiophene rings on three bridged “arms” of 2 are promoted to rotate far away from their conjoint benzene rings. In all of complexes, the branched carboxylic or amino group expectedly cooperates with the characteristic groups of amino acids in multiple H-bond formations and thereof strengthens the interaction with 2. The difference of H-bond fashion in four complexes results in different stabilization energy (Table S5:† 26.6 kcal mol−1 of 2–Asp complex; 33.5 kcal mol−1 of 2–Glu complex; 20.5 kcal mol−1 of 2–Lys complex; and 31.6 kcal mol−1 of 2–Arg complex), suggesting the interaction between 2 and Glu is slightly stronger than that between 2 and Asp and the interaction between 2 and Arg is slightly stronger than that between 2 and Lys, which is consistent with the results from fluorescence and UV-Vis titrations.Mechanism of amino acid sensing
To account for the observed sensing of acidic and basic amino acid ion by 2, a plausible mechanism involving intramolecular charge transfer (ICT) is proposed. When a fluorophore contains an electron donating group conjugated to an electron-withdrawing group, it undergoes ICT from the donor to the acceptor upon excitation by light. The consequent change of the dipole moment leads to a larger Stokes shift, which is influenced by the microenvironment of the fluorophore. 2, which consists of a n-butyl substituted calix[4]arene donor, a 2-cyanoacrylic acid acceptor, and an oligothiophene spacer, is an ideal fluorescent chemosensor. Intramolecular charge transfer (ICT) occurs from a n-butyl substituted calix[4]arene donor to 2-cyanoacrylic acid acceptor upon photoexcitation of 2. The hydrogen bonding with the electron acceptor could increase or decrease the flow of charge from the donor to the acceptor, thereby affecting the ICT process. When one NH2 groups and two electron-withdrawing COOH groups in an Asp/Glu interact with the acceptor groups, the excited state is more stabilized by the Asp/Glu than the ground state, which leads to a red shift of the absorption and emission spectra. Moreover, the interaction between 2 and Asp/Glu provides a fluorescence quenching pathway. The fluorescence quenching of 2 may occur by the excitation energy transfer or charge transfer from 2 to the Asp/Glu. Conversely, the binding between 2 and Lys/Arg involves simultaneous coordination by two electron-donating NH2 groups and one COOH group. The consequent electron-donating effect by interaction with NH2 groups depolarizes the n-butyloxy group through 2-cyanoacrylic acid of the oligothiophene group leading to a decreased ICT emission band which consequently results in a blue shift of 5 nm (peak shift from 576 to 571 nm). This is also supported by fluorescence titration studies involving Asp/Glu and Lys/Arg. In the case of other amino acids, the absence of significant binding with the 2-cyanoacrylic acid is the main reason for the absence of any significant emission response.Conclusions
In summary, a fluorescent chemosensor based on calix[4]arene derivative 2 conjugated with four bithiophene–cyanoacrylic acid groups at the upper rim can selectively recognize acidic amino acids (Asp and Glu) by fluorescence quenching, but also shows highly selective sensing for basic amino acids (Lys and Arg) by fluorescence turn-on over a wide range of naturally occurring amino acids in sodium phosphate buffer solution, without any organic solvent, which may result from multiple H-bond interactions between them. This outstanding property of 2 may prompt it to be widely applied in the analysis of acidic and basic amino acids in pure aqueous media.Acknowledgements
This work was supported by the NSF of China (21272292, 61232009, 61370059, 9122220121173272, 21121061, and 21272173), Doctoral Fund of Ministry of Education of China (20101102110018, 20120171130006), and Science and Technology Planning Project of Guangdong Province (2011J2200053).Notes and references
- I. S. Krylov, B. A. Kashemirov, J. M. Hilfinger and C. E. McKenna, Mol. Pharmaceutics, 2013, 10, 445–458 CrossRef CAS PubMed.
- (a) X.-P. He, Z. Song, Z.-Z. Wang, X.-X. Shi, K. Chen and G.-R. Chen, Tetrahedron, 2011, 67, 3343–3347 CrossRef CAS PubMed; (b) D.-T. Shi, X.-L. Wei, Y. Sheng, Y. Zhang, X.-P. He, J. Xie, G. Liu, Y. Tang, J. Li and G.-R. Chen, Sci. Rep., 2014, 4, 4252–4257 Search PubMed.
- (a) L. Mutihac, J. H. Lee, J. S. Kim and J. Vicens, Chem. Soc. Rev., 2011, 40, 2777–2796 RSC; (b) Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- (a) F. Perret, N. Morel-Desrosiers and A. W. Coleman, J. Supramol. Chem., 2002, 2, 533–536 CrossRef CAS; (b) E. Da Silva and A. W. Coleman, Tetrahedron, 2003, 59, 7357–7364 CrossRef CAS; (c) A. Buryak and K. Severin, J. Am. Chem. Soc., 2005, 127, 3700–3701 CrossRef CAS PubMed; (d) R. Ahuja, N. K. Singhal, B. Ramanujam, M. Ravikumar and C. P. Rao, J. Org. Chem., 2007, 72, 3430–3442 CrossRef CAS PubMed; (e) R. Joseph, J. P. Chinta and C. P. Rao, J. Org. Chem., 2010, 75, 3387–3395 CrossRef CAS PubMed; (f) M. Torvinen, R. Neitola, F. Sansone, L. Baldini, R. Ungaro, A. Casnati, P. Vainiotalo and E. Kalenius, Org. Biomol. Chem., 2010, 8, 906–915 RSC.
- (a) H. Aït-Haddou, S. L. Wishur, V. M. Lynch and E. V. Anslyn, J. Am. Chem. Soc., 2001, 123, 11296–11297 CrossRef; (b) A. Acharya, B. Ramanujam, J. P. Chinta and C. P. Rao, J. Org. Chem., 2011, 76, 127–137 CrossRef CAS PubMed; (c) R. Zadmard, P. Ataeian and M. Khalili-Foumeshi, Org. Chem. Int., 2011 DOI:10.1155/2011/171374.
- (a) S.-Y. Li, Y.-W. Xu, J.-M. Liu and C.-Y. Su, Int. J. Mol. Sci., 2011, 12, 429–455 CrossRef CAS PubMed; (b) J.-M. Liu, J.-Y. Shi, Y.-W. Xu, C.-Y. Su and S.-Y. Li, Supramol. Chem., 2011, 23, 419–424 CrossRef CAS; (c) J.-M. Liu, M. Tonigold, B. Bredenkötter, T. Schröder, J. Mattay and D. Volkmer, Tetrahedron Lett., 2009, 50, 1303–1306 CrossRef CAS PubMed; (d) J.-H. Mai, J.-M. Liu, S.-Y. Li and H.-F. Jiang, Chin. Chem. Lett., 2009, 20, 1191–1194 CrossRef CAS PubMed; (e) J.-M. Liu, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron, 2007, 63, 9939–9946 CrossRef CAS PubMed; (f) J.-M. Liu, J.-H. Bu, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2006, 47, 1905–1908 CrossRef CAS PubMed; (g) J.-M. Liu, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, J. Inclusion Phenom. Macrocyclic Chem., 2005, 51, 165–171 CrossRef CAS; (h) J.-M. Liu, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2004, 45, 6071–6074 CrossRef CAS PubMed; (i) R.-H. Wang, J.-M. Liu, J.-H. Mai and S.-J. Liao, Chin. J. Org. Chem., 2008, 28, 1213–1217 CAS; (j) G.-B. Pan, J.-M. Liu, H.-M. Zhang, L.-J. Wan, Q.-Y. Zheng and C.-L. Bai, Angew. Chem., Int. Ed, 2003, 42, 2747–2751 CrossRef CAS PubMed; (k) G.-B. Pan, J.-H. Bu, D. Wang, J.-M. Liu, L.-J. Wan, Q.-Y. Zheng and C.-L. Bai, J. Phys. Chem. B, 2003, 107, 13111–13116 CrossRef CAS; (l) J.-M. Liu, Y.-S. Zheng, Q.-Y. Zheng, J. Xie, Me.-X. Wang and Z.-T. Huang, Tetrahedron, 2002, 58, 3729–3736 CrossRef CAS; (m) J.-M. Liu, Q.-Y. Zheng, J.-Y. Yang, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2002, 43, 9202–9212 Search PubMed.
- S.-Y. Li, Y.-W. Xu, S.-Q. Zeng, L.-M. Xiao, H.-Q. Duan, X.-L. Lin, J.-M. Liu and C.-Y. Su, Tetrahedron Lett., 2012, 53, 2918–2921 CrossRef CAS PubMed.
- C. Jaime, J. de Mendoza, P. Prados, P. M. Nieto and C. Sanchez, J. Org. Chem., 1991, 56, 3372–3376 CrossRef CAS.
- L. F. Tietze, Comprehensive Organic Synthesis, ed. B. M. Trost, Pergamon Press, New York, 1991, vol. 2, ch. 1.11, pp. 341–394 Search PubMed.
- N. Douteau-Guevel, A. W. Coleman, J.-P. Morel and N. Morel-Desrosiers, J. Phys. Org. Chem., 1998, 11, 693–696 CrossRef CAS.
- G. Arena, A. Casnati, A. Contino, A. Magrì, F. Sansone, D. Sciotto and R. Ungaro, Org. Biomol. Chem., 2006, 4, 243–249 CAS.
- M. J. Frisch, et al., GAUSSIAN 03, Revision B.04, Gaussian, Inc., Wallingford, CT, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of syntheses of 2 and 3; UV-Vis titrations of 2 with Glu and Arg; fluorescent titrations of 2 with Glu and Arg; ESI-MS spectra of 2 and Asp, 2 and Glu, 2 and Arg; 1H NMR spectra of 2 and Asp, 2 and Glu, 2 and Arg; Cartesian coordinates of 2, 2 and Asp, 2 and Glu, 2 and Lys, 2 and Arg from B3LYP/6-31G optimization; hydrogen bonds and energies in 2 and Asp, 2 and Glu, 2 and Lys, 2 and Arg based on computational study (B3LYP/6-31G). See DOI: 10.1039/c4ra04074k |
| This journal is © The Royal Society of Chemistry 2014 |