Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water recycling public toilets based on onsite electrochemical wastewater treatment†
Pragadeesh
Subramani
 a,
Milan
Basil
a,
Praveen
Rosario
a,
Milan
Basil
a,
Praveen
Rosario
 a,
Dijin Ramachandran
Jalaja
b,
Vaishali
Choudhary
a,
Dijin Ramachandran
Jalaja
b,
Vaishali
Choudhary
 c,
Jayakumar
Renganathan
c,
Ligy
Philip
c,
Jayakumar
Renganathan
c,
Ligy
Philip
 c,
Kangwoo
Cho
e,
Claire
Welling
c,
Kangwoo
Cho
e,
Claire
Welling
 d,
Sonia
Grego
d,
Sonia
Grego
 d and
Clèment
Cid
d and
Clèment
Cid
 *e
*e
aDuke India Services Private Ltd, Bangalore, India
bERAM Scientific, Trivandrum, India
cDepartment of Civil Engineering, IIT Madras, Chennai, India
dCenter for WaSH-AID, Duke University, Durham, NC 27701, USA
eCalifornia Institute of Technology, Pasadena CA 91125, USA. E-mail: ccid@caltech.edu
First published on 13th November 2023
Abstract
The long-term performance of an anaerobic bioreactor combined with electrochemical oxidation and integrated into a self-contained public bathroom under daily use was investigated over 14 months in Coimbatore (Tamil Nadu, India). With varying daily number of users and across all seasons, the electrochemical treatment of the bioreactor effluent consistently generated a highly chlorinated (>400 mg Cl2 per L) and clear effluent that was negative to a fecal coliform assay. During 8 months of testing under water recycling conditions, the treatment maintained its performance for disinfection, as well as solid and nitrogen removal, due to breakpoint chlorination; however, the COD removal capacity of the system was slightly reduced. The strongly oxidizing condition of this electrochemical based disinfection raised the concern of generation of toxic disinfection byproducts (DPBs). This study also examined the formation of chloroform and haloacetic acid DBPs under non-recycling and recycling conditions.
Water impactOnsite treatment of toilet effluents is a potential source of non-potable water for reuse that simultaneously addresses the global need for safely managed sanitation. This study reports the long-term field testing of a small-scale single toilet system re-using its highly chlorinated treated effluent for flushing. Water reuse resulted in considerable water saving; however, it also affected the water quality. |
1. Introduction
Effective treatment of blackwater, human waste in the effluent of water-flush toilets, is essential for public health and environment.1 The 2030 Sustainable Development Goals (SDGs) of the United Nations include SDG 6 aimed to ensure “availability and sustainable management of water and sanitation for all”. In combination with 2.4 billion people who lack improved sanitation services worldwide, water scarcity is a large global risk due to climate change and changing consumption patterns, thus making water reuse a major priority.2The development of SDG 6 has triggered large-scale sanitation programs in Africa,3 the “Toilet Revolution” in China4 and a Clean India Swachh Bharat Mission in India.5
Onsite small-scale treatment to remove pathogens that cause diseases and recover resources from waste where it is generated (household and community toilets) without any need for its transport has recently gained extensive research interest.6 A few onsite treatment systems including onsite water re-use have reached maturation to be tested in a relevant environment, and to leverage electrooxidation,7,8 membrane bioreaction9 and electrochemical treatment combined with constructed wetlands10 with granular activated carbon11 and solid/liquid separation.12
A critical element of urban sanitation are public toilets, and a great emphasis of the Swachh Bharat mission in India has been the construction of public toilets in areas not necessarily covered by sewers.13,14
This work describes the field testing and characterization of onsite treatment and water reuse on the scale of single public toilet booth that could operate without sewer connection. The electrochemical generation of reactive chlorine species (RCS) that include free chlorine (FCl) is at the core of the treatment technology (electrochemical oxidation, EO) and the removal of organic matter primarily by anaerobic bio-digestion.
In collaboration with Eram Scientific Instruments (Trivandrum, Kerala India), the authors integrated an electronic public toilet booth with automated flush (e-Toilet Model Beta with a biological and electrochemical oxidation (EO) system as described in a previous study7). Although a previously published study has shown the effectiveness of combined biological and EO for the treatment of raw and domestic sewage with water reused for toilet flushing,8 that installation size exceeded 500 L per day allowed for the tolerance of variability in wastewater flows and composition.
This study reports the evaluation of a small-scale system (130 L per day) under the condition of re-use of its highly chlorinated effluent for toilet flushing. The system was operated beyond the chloride oxidation breakpoint to remove ammonium and to ensure high levels of free chlorine for robust disinfection. Such conditions generated a high-quality effluent, which under water reuse, we anticipated, would have a negative impact on the microbial community in the biological reactor. The strongly oxidizing condition of this electrochemical based disinfection raises the concern of generation of toxic disinfection byproducts (DPBs) including previously reported high-level trihalomethanes and haloacetic acids (HAAs).15
This field-testing study was conducted in a real-life condition in public toilets in India where users had unrestricted access with the purpose of measuring 1) the properties of the treated effluent under the highly variable real-life use; 2) the effect of water reuse for toilet flushing on the quality of blackwater; and 3) the formation of organic DBPs under water reuse conditions.
2. Material and methods
2.1 Technology description
ERAM e-Toilets are prefabricated single-user booths that are equipped with a self-cleaning and washing (flush and floor wash) mechanism. The ERAM Beta e-Toilet model was connected to an anaerobic baffled reactor (ABR) followed by electrochemical treatment technology that was developed at the California Institute of Technology (Caltech), described in detail in an earlier work.7The ABR was a 4-chamber 2200 L capacity system whose purpose was to reduce the nutrient and organic load before wastewater was electrochemically treated. The electrochemical treatment was conducted in an electrochemical reactor (ECR) 50 L tank (44 L effective treatment volume). The electrodes were a set of 7 pairs of Ir–Ru–Ti anodes and stainless-steel cathodes (Entrustech, China) with an effective anode surface area of 2041 cm2. The electrodes were powered at a constant voltage of 4.1 V with a typical current of 80 Å (range 71–90 Å) and a current density of 27 Å m−2. For a 3 hour batch treatment cycle of 44 L, the total energy requirement was 3.6 MJ.
The electrochemically treated wastewater was pumped through a polishing 10 μm pore filter cartridge and stored in a 100 L treated water tank (TWT) and, when reused, was connected to toilet flushing and periodic floor washing (Fig. 1A).
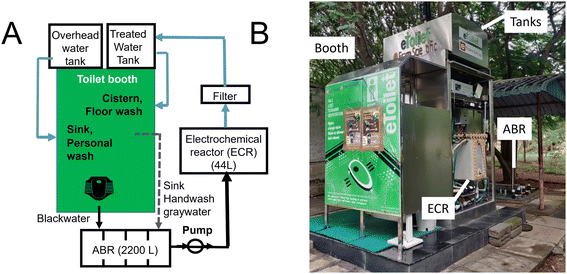 | ||
| Fig. 1 A) Schematic of the apparatus. B) Picture of the system (doors of the ECR cabinet were removed to show the ECR tank). | ||
Freshwater from a bore-well was connected to an overhead water tank (OHT) and was used to supply the personal wash wand (i.e., bidet) and a small handwash sink inside the booth. The e-Toilet effluent was connected via a splitter valve to either the ABR treatment or to a nearby sewer line.
NaCl addition to wastewater enhanced the chlorine production during ECR treatment. NaCl was added by pouring a NaCl solution through the hand wash sink, which was plumbed to the ABR4. The volume of wastewater in the ABR4 was estimated based on liquid level, and the appropriate weight of food-grade NaCl dissolved in 1 liter of tap water was added to result in the desired molarity in ABR4.
Sampling points throughout the treatment pathway allowed for the full characterization of the process.
The top of each of the 4 ABR chambers (ABR1 through ABR4) featured a sampling port and wastewater was sampled using a macerator pump connected to a hose lowered in the chamber at a pre-determined depth (i.e. 50 cm from bottom). Two spigots along the plumbing line allowed sampling by gravity the system effluent, namely, the ECR-out after the polishing filter and the TWT.
2.2 Test site, users, and operation
The e-Toilet booth apparatus was installed next to an existing public toilet in a car parking lot at PSG research hospital in the city of Coimbatore, Tamil Nadu India. The e-Toilet was designated for male users and was visited mostly by car drivers and visitors of the hospital.The e-Toilet software system electronically tracked the number of users entering the booth and water usage such as flushes. Additional electronic controls drove and recorded the ECR cycles and tank level sensors as described in an earlier work.7
The system operated in two configurations “open loop” and “closed loop”. In the open loop, the electro-chemically treated water was not reused but discharged to sewer and the overhead tank supplies bore-well water for all uses (handwash, personal wash, floor wash and flush events). In closed loop, the treated wastewater was recycled and the Treated Water Tank (TWT) was connected to the toilet flush and floor wash pipes while fresh water from OHT continued to feed the sink and personal wash.
The system was installed in October 2018, and after commissioning and baseline data collection, a study that started in late 2019 was interrupted multiple times by shutdowns and people's movement restrictions ordered by the government facing the COVID-19 pandemic. The data reported in this study were collected from August 2021 through September 2022.
2.3 Analytical measurements
Samples from ABR1 and ABR4 chambers as well as the treated ECR effluent were collected in autoclaved glass bottles and transported in a secondary container to a laboratory located on the same research hospital campus. Immediately after transport, the ABR 1 and ABR 4 samples were pasteurized in a water bath at 60 °C for 90 minutes to ensure the safety of personnel from SARS-CoV-2. A separate study found no difference due to pasteurization under these conditions in the physico-chemical parameters of wastewater (Rosario et al., submitted). The ECR effluent samples were not heat-treated.Electrical conductivity and pH were measured using a Myron L Ultrameter II, Model 6P.
Physico-chemical parameters of wastewater were analyzed using commercially available test kits and Multiparameter Portable Colorimeter (DR900, Hach). Free Chlorine (FCl) and Total Chlorine (TCl) were measured with Hach kits Method 8021/8167. A dry thermostat reactor (DRB 200, Hach) was used for the digestion step, and vendor protocols were followed to measure Chemical Oxygen Demand (COD) by Reactor Digestion (Method 800), Total Nitrogen (TN) by Persulfate Digestion (Method 10072) and Total Phosphorus by Acid Persulfate Digestion (Method 8190).
Total suspended solids (TSS) were measured according to the EPA 160.2 method. Turbidity was measured in Nephelometric Turbidity Units (NTU) using a turbidimeter (2100 Q, Hach).
Disinfection was confirmed by measuring the fecal coliform levels according to the most probable number (MPN) assay adapted from the literature and described in an earlier work11 with a limit of detection of 3 MPN per mL. Chloride measurements were performed at third-party NABL-certified laboratory (T. Stanes, Coimbatore) analyzed as per Indian standard IS:3025 part 32.
2.4 Disinfection byproduct analytical methods
The minimum detection limit of 0.06 mg L−1 was estimated by injecting seven replicates of 1 mg L−1 of chloroform standards. The product of the standard deviation of the replicates and t-statistic with n − 1 degree of freedom is defined as MDL. The linearity was determined in ultra-pure water with a blank and various analytical chloroform standards ranging between 0.1 and 500 mg L−1 concentrations. The calibration curves were linear with R2 > 0.998.
The accuracy of the analysis was established by performing recovery studies, whereby 1 mg L−1 of chloroform standards was spiked at the beginning of the extraction process.
3. Results and discussion
3.1 Operation
The toilet system under test was a mature version of earlier prototypes, and for this study, it underwent long-term testing under steady use. Our intent was to assess the consistency of the treatment under the highly variable real-life use and assess the impact of water reuse for toilet flushing (closed loop) on the operation of the system and the quality of the treated water.We recorded data for 52 weeks of up-time over a 14 month period, of which 23 weeks in the open loop were followed by 29 weeks in the closed loop. Table 1 summarizes the operational results of this field testing.
| Open loop | Closed loop | Total | |
|---|---|---|---|
| Dates | 1 Aug 2021–17 Jan 2022 | Jan 18 2022–Sept 07 2022 | Aug 2021–Sep 2022 |
| Duration (weeks) | 23 | 29 | 52 |
| Users (number) | 2566 | 2189 | 4755 |
| Users per day | 16 ± 8 | 12 ± 7 | |
| ECR hours (cycles) | 540 (180) | 1041 (347) | 1581 (527) |
| Volume treated (L) | 7920 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 268 268 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 188 188 |
| Volume reused (L) | 0 | 8404 | 8404 |
| Free chlorine (mg L−1) (av ± st dev) | 609 ± 208 | 417 ± 95 | 451 ± 142 |
| Total chlorine (mg L−1) (av ± st dev) | 645 ± 204 | 445 ± 100 | 480 ± 145 |
The study reflects nearly 5000 users of the toilet (the number of users was recorded automatically with daily resolution, see ESI† Fig. S1). The daily use of the toilet was higher at the beginning of the study due to increased foot-traffic to an adjacent COVID-19 visitor screening site belonging to PSG Hospitals that was later dismantled. The system's treatment capacity of 350 L per day exceeded the treatment requirement dictated by the number of the users (150 L per day at peak use of 30 users per day), and thus, the ECR was not run when TWT was full.
We conducted a detailed evaluation of water use for flush and personal wash from a large dataset, and obtained an estimate of 5.1–5.3 L per use of which 1.0–1.5 L per use are for personal wash (a detailed water use analysis is reported in ESI† material Section S2).
3.2 Chlorination and use of NaCl as an additive
The Ir–Ru–Ti anodes in the ECR oxidize Cl− to produce RCS, incl. FCl. In prior studies, a similar type of electrode achieved breakpoint chlorination after 3 hours of EO at [Cl−] > 20 mM under similar conditions.16 The bore-well freshwater connected to the system has a high mineral content (typical EC = 2400 μS cm−1), including 9 ± 1.5 mM Cl−.The influent ABR1 had an EC = 4065 ± 250 μS cm−1 and 15 mM Cl−, a Cl− concentration in agreement with expected contribution from urine.17
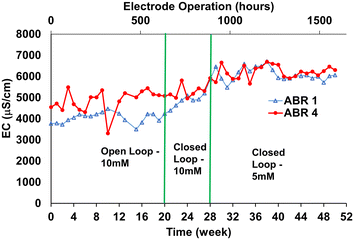
In the first two weeks of field testing, we observed that the chlorination breakpoint could not be consistently achieved with the as-received influent (n = 10 tests). Thus, from week 2 onward, 10 mM of NaCl was added to ABR4 as described in the Methods section of this manuscript. During field testing in the open loop, the average ABR4 EC = 4903 ± 503 μS cm−1 was thus higher than ABR1 EC. The EC for ABR1 (influent) and ABR4 (post-biological treatment) measured over the course of the study is shown in Fig. 2.
When the loop was closed, the EC started to increase in ABR1 as expected by the influx of high chloride concentration effluents instead of freshwater for toilet flushing. ABR1 EC reached 6000 μS cm−1. We observed that addition of NaCl continued to be a requirement to prevent drops in EC due to dilution with freshwater used for personal cleaning (estimated to be 20% of the total volume, see details in ESI† S2). We also observed that the system required EC ∼ 6000 μS cm−1 to consistently achieve breakpoint, which we interpreted as due electrode aging. The electrode hours of operation are reported on the top horizontal axis in Fig. 2. The chlorine evolution reaction rates of the electrode conducted at the beginning and the end of the study are reported in Fig. S3 of the ESI† Section and illustrated a reduction in the FCl production of aged electrode for the same Cl− concentration.
As a result of Cl− addition, the system consistently produced FCl and TCl exceeding 400 mg L−1.
Breakpoint was achieved throughout the duration of the study, even when the electrodes aged beyond 1000 h of electrolysis, as evidenced by the ammonia level below detectable levels at all timepoints mentioned in ESI† Fig. S4.1.
A major design requirement for the system was to produce pathogen-free effluents safe for water re-use for flushing. No pathogens were detected in the effluent in this study. An assay for a fecal contamination indicator, fecal coliform bacteria, was periodically performed in both the ECR effluent and the TWT (when in closed loop). The fecal coliform content of the ECR effluent was found to be consistently below the detection limit of the assay (3 MPN per mL) (n = 16).
Given the long-term operation of the ECR, mineral scaling was observed on the electrodes and it was removed by using a similar reverse polarity scheme as the one described by Varigala et al.8 The reverse polarity cleaning was carried out as preventive maintenance prior to any observed decrease in oxidation efficiency at an average interval of 30 ± 12 cycles during the open loop and at a longer interval of 64 ± 18 cycles in the closed loop.
3.3 Effluent physico-chemical parameters in open and closed loops
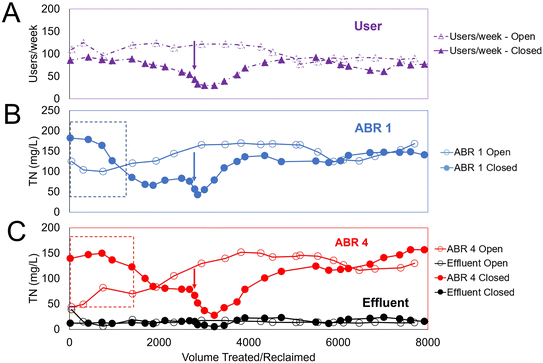
Fig. 3 reports the values of TN across the treatment stages and compares results between the open and closed loop configuration. The figure also includes the use number to support the interpretation of TN decreases associated with a longer retention time.The dashed rectangle insert in Fig. 3B highlights TN decreasing in ABR1 as soon as the loop closed. This sudden decrease was probably due to the effect of highly chlorinated recycled water reacting with nitrogen compounds in ABR1. The same effect can be observed in ABR4 (Fig. 3C), lagging by a week in time and 400 L of treated water, as a result of the fluid transport in the ABR. TN values in ABR1 and ABR4 stabilized at around 6 weeks of closed loop operation and 2000 L reused by the system. A technical incident at week 11 prevented the usage of the system for a period of 4 weeks, leading to a sharp decrease in TN values due to extended treatment and sluggish restart of the system with weekly users below 50 users per week.
After this decline, a ramp up on TN was observed in both chambers with a similar one-week delay as observed beforehand. Steady values similar to those in the open loop were observed at 4000 L until the end of the study.
Overall, the TN reduction obtained by EO was significant with effluent TN = 15 ± 7 mg L−1 in the open loop, and similar TN = 16 ± 9 mg L−1 during the entire closed loop period. The ammonia results (in ESI† Fig. S4) followed closely the TN trends over time, and also exhibited no difference between the close and open loops. An effluent ammonia concentration of 0 mg L−1 was measured, as expected in a system operated beyond the chlorination breakpoint.
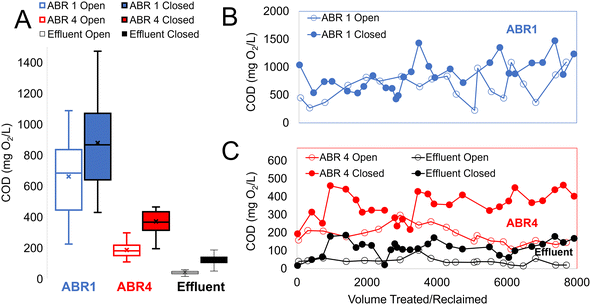
Fig. 4 illustrates the COD values measured in open and closed loops. This treatment system reduces organics in the effluent thanks to both digestion in the ABR and oxidation in the ECR. Fig. 4A shows that the mean COD values increase in the closed loop as compared to the open loop across all sampling points. In ABR1, COD = 662 ± 257 mg O2 per L (open) increased to COD = 879 ± 277 mg O2 per L (closed), in ABR4 COD = 186 ± 48 mg O2 per L (open) nearly doubles to COD = 371 ± 109 mg O2 per L (closed) and importantly, the effluent COD = 41 ± 19 mg O2 per L (open) increased to COD = 118 ± 45 mg O2 per L in the closed loop. Fig. 4B and C show the COD values relative to volume treated and illustrate that, despite the variability of the values due to the fluctuating nature of the influent, the closed loop COD values are consistently higher over the open loop values.
Because the number of users in the closed loop does not increase over the open loop, we interpret the COD increase as a reflection of the highly chlorinated effluent impact on the microbial population of the biodigester and its ability to remove organics.
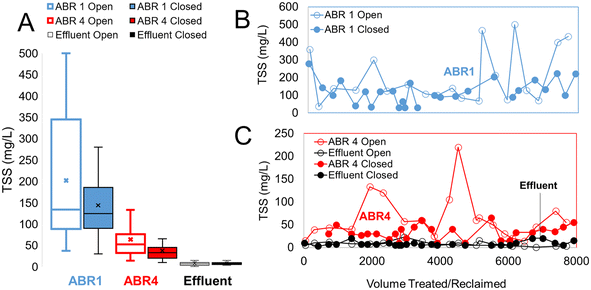
The effect of chlorinated recycled water on TSS is a reduction in the values in ABR (TSS = 202 ± 150 mg L−1 (open loop) to TSS = 144 ± 99 mg L−1 (closed loop) (Fig. 5)). Similarly, in ABR4, TSS = 64 ± 49 mg L−1 in the open loop decreases to TSS = 38 ± 24 mg L−1 in the closed loop. The effluent featured consistently low solids with average TSS = 8 mg mL−1 (range = 1 to 23 mg L−1) and average turbidity = 4.2 NTU (range 1–27 NTU). We interpret the reduction in closed loop of TSS in ABR 1 and ABR 4 as the impact of the chlorinated influent that breaks down organic portion of the suspended solid, thus reducing TSS.
3.4 Benchmarking
In order to evaluate the quality of the wastewater treatment of the tested system, we compared our results to quality targets of available water and wastewater quality standards. We compared the field testing results to an international standard for non-sewered sanitation systems, the ISO 30500 (ref. 18) and specifically the Category A limits for unrestricted urban reuse, which explicitly includes treated wastewater reuse for toilet flushing. The limited applicability of local and national regulatory framework to novel small-scale wastewater technologies particularly with water recycling has been discussed in the literature.19,20 Indeed, the country of this field testing (India) has not issued a national requirement for water reuse for recycling technologies or onsite wastewater treatment systems such as the one tested. Nonetheless, for reference, in Table 2 we compare our results against the current India standard established by Indian National Green Tribunal (NGT), New Delhi in 2019 for sewage treatment plants, thus for systems that are significantly larger scale output measured in million liters per day.21 The 2019 NGT has different thresholds for urban areas of different populations and we reported in Table 2 the limits for Metropolitan Cities (population more than 1 million) since the test site city of Coimbatore population is 3 million people in 2023.| Parameters | Open loop (n = 20) | Closed loop (n = 29) | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | % results meeting | Influent | Effluent | % results meeting | |||||
| Av ± SD | Av ± SD | ISO Cat Aa | NGT 2019a | Av ± SD | Av ± SD | ISO Cat Aa | NGT 2019a | ISO 30500 | NGT 2019b | |
| a Pass % of all measurements relative to the respective reference limits. b For class I cities, with more than 1 million population. c The threshold is not a concentration, but a load reduction percentage. | ||||||||||
| TSS (mg L−1) | 202 ± 150 | 8 ± 5 | 80% | 100% | 144 ± 99 | 8 ± 5 | 86% | 100% | ≤10 | 30 |
| COD (mg L−1) | 662 ± 257 | 41 ± 19 | 80% | 95% | 879 ± 277 | 118 ± 45 | 10% | 24% | ≤50 | 100 |
| pH | 7.8 ± 0.2 | 7.7 ± 0.4 | 100% | 100% | 7.7 ± 0.3 | 8.3 ± 0.8 | 86% | 100% | 6–9 | 5.5–9.0 |
| TN (mg L−1) | 150 ± 33 | 15 ± 7 | 95% | 70% | 113 ± 44 | 16 ± 9 | 90% | 59% | 70%c | 15 |
| TP (mg L−1) | 55 ± 10 | 22 ± 4 | 0% | 0% | 78 ± 20 | 37 ± 13 | 0% | 0% | 80%c | 1 |
Table 2 reports the effluent parameter values averaged over the period of the study and the percentage of measured values that meet the respective reference limit. Additional data are provided in ESI† S5.
The effluent TSS concentration met the NGT standard requirements for 100% of the measurements and the ISO 30500 standard 80% or more. We note that according to the field testing requirements for environmental parameters of the effluent described in ISO 30500, all the required thresholds of at least 4 out of 5 test events (i.e., 80% of the measurements), and results are not to be averaged.
While TSS results were similar in open and closed loops, the COD was significantly higher in the closed loop than in the open loop. The COD values fully met both standard requirements in the open loop but only 10% and 24% of the times in the closed loop for the ISO 30500 and NGT standards. The pH in closed loop was higher on average and exceeded pH = 9 in 14% of the measurements. Variations in the pH in EO processes can be caused by changes in the buffering capacity of the solution as well as the increase in parasitic oxidation reactions such as oxygen evolution reactions, directly in completion with CER.
The effluent TN values met the ISO limit (70% removal), and was on average very close to the NGT limit of 15 mg L−1: it met the limit 70% of the time in the open loop and 59% of the time in the closed loop.
The treatment removed only 50% of the TP and thus effluent TP exceeded the limits of both reference standards.
We did not compare the microbiological results obtained in this study to the ISO 30500 and NGT standards because the procedure followed in this study measured yielded results in MPN per mL that cannot be converted in the units required by standards. Nevertheless, the fecal coliform content was always observed to be below the limit of detection of the assay for all measurements.
3.5 Chloroform disinfection byproducts
This field-testing study evaluated the production of DBP chloroform (CFM) and haloacetic acids (HAAs) from the treatment and compared the results in the open and closed loops.CFM has been reported as the main trihalomethane byproduct when chlorine reacts with organic materials.22 For CFM quantification, the liquid–liquid extraction was conducted onsite to avoid analyte loss due to evaporation during shipment to the laboratory. To ensure the procedure was carried out properly at the field site, the results of the same specimen (14 samples over 10 shipments) were compared for onsite extraction and extracted at a later date at the analytical laboratory. Differences ranged up to 150%, but were on average 10% and typically within a 20% reduction in the laboratory measurement. Thus, carrying out analyte extraction onsite provided the most accurate extraction method. As an additional quality control, shipments also included an additional treated wastewater sample spiked with 1 mg L−1 of CFM and extracted on site to determine the percentage recovery of CFM. We report data from samples with a recovery range between 52% and 200%.
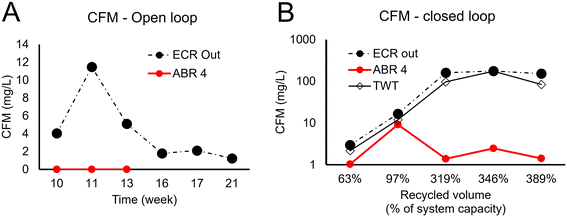
The CFM concentration over six separate measurements in the open loop ranged between 1 and 12 mg L−1. (Fig. 6A). The sample-to-sample variability was attributed to the composition of the wastewater. The incoming wastewater in the ECR from ABR4 contained no CFM in the open loop (n = 3). These CFM values compared well with the CFM concentration reported with similar electrodes setup at breakpoint chlorination with 30 mM chloride wastewater, CFM = 2.1 mg L−1 (17.5 μM).15 In drinking water, 1–12 mg L−1 CFM DBPs generated by the treatment are 3 to 50 times that mentioned in the guidelines for CFM (0.3 mg L−1 according to WHO 2021 (ref. 23) and 0.2 mg L−1 according to the 2012 Bureau of Indian standards (BIS)24). No guidelines are available for the limit of CFM in treated wastewater effluents.
Fig. 6B shows the CFM concentration (log scale) in the closed loop versus the percentage recycled wastewater volume.
As the system reached one complete volume recycled water, the electrolysis produced 12 mg L−1 of CFM and the ABR wastewater contained similar CFM concentrations, as expected. However, when the system achieved 3 and 4 recirculation volumes, the CFM concentration after electrolysis increased dramatically and consistently to over 100 mg L−1. Such concentration was 500 times higher than the BIS guidelines for drinking water.
Surprisingly the values in the ABR where such CFM treated water was recycled remained at a value of 1–10 mg L−1. It has been reported that anaerobic conditions favor biodegradation of chloroform25 and additionally CFM is highly volatile, thus these factors may justify the low ABR values in the closed loop. The high CFM values produced by the ECR are thus not due to an accumulation effect. We observed that in the closed loop, the pH increases relative to the open loop, and it has also been reported that the pH increase results in THM increase.22,26 However, the relatively modest increase in pH (in average from pH = 7.7 to pH 8.3) cannot unambiguously justify the observation. The addition of NaCl in a reaction of organics and chlorine also favors CFM formation,27,28 and in the closed loop, the Cl− concentration in the influent to the ECR nearly doubled from an estimated 22 mM to 49 mM (ESI† S6).
We hypothesize that ABR adaptation to highly chlorinated influent could alter the microbial behavior and fraction of effluent organics resulting in a composition that is highly favorable to CFM formation. The increased concentration of chlorine can create two opposite effects: 1) the solubilization and hydrolysis of the organic matter can be increased due to the strong oxidative nature of the FCl, which could increase the COD load in the effluent (as shown in Fig. 4), 2) the more dissolved organic matter could easily react with residual chlorine to produce CFM.29 Thus, a combination of increased pH, increased NaCl and altered organic fractionation may collectively be the cause of the significant increase of CFM in the closed loop.
3.6 Halo acetic acid disinfection byproducts
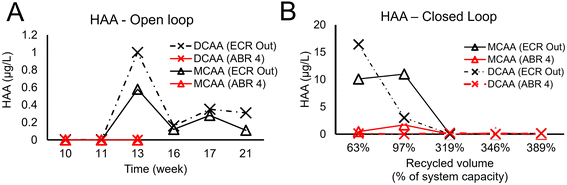
HAA measurements were conducted in the open and closed loops for the same wastewater samples as measured for CFM (Fig. 7). The HAA compounds were generated at much lower concentration than CFM, namely, μg L−1 instead of mg L−1.The observed values for MCAA and DCAA in the open loop exhibited sample-to-sample variability ranging between 1 μg L−1 and the detection limit of 0.002 μg L−1 (Fig. 7A).
Because the HAA compounds are not volatile, we anticipated them to accumulate over time in the closed loop while surprisingly, the opposite was observed. After closing the loop, the MCAA and DCAA values were measured to be as high as 16 μg L−1, and indeed, had to be diluted for the measurement; however, this byproduct generation ceased to occur after the recycled volume increased to 300% of the system capacity (Fig. 7B). TCAA is not reported in Fig. 7 and was negligible for all measurements except for an increase to 12 μg L−1 during the first closed loop point.
Several recovery studies were performed in spiking wastewater with 1 μg L−1 and 50 μg L−1 HAA as quality control of these measurements. The results of this study indicated that the system produces HAA byproducts at concentrations below 10 μg L−1. These levels are quite different from previous work reporting, for similar electrolysis conditions, concentrations 1000 times higher, namely 2, 15 and 6 mg L−1 for MCAA/DCAA/TCAA, respectively.15
The values measured under open- and closed-loop conditions were below the WHO guidelines for drinking water MCAA = 20 μg L−1, DCAA = 50 μg L−1 and TCAA = 200 μg L−1 (there are no HAA regulations in Indian standards for discharge/drinking water).
The same hypothesis of altered microbial community we invoked for the CFM production could justify the HAA reduction observation, as the effects of chlorine disinfectant on microbial community have been reported to delay the production of volatile fatty acids, which are highly plausible precursors of HAA.29 Additionally, it has been reported that the rate of CFM production is faster and it competes with HAA for residual chlorine.30 Similar results were reported by Jasper et al.15 whereby chloroform production was predominant on both TiO2/IrO2 and BDD electrodes. It should be noted that other than chlorine, pH and temperature, the nature of organic matter significantly affects the production of CFM and HAA. For instance, effluent anaerobic digester contains simpler organic matters such as volatile fatty acid alcohols, which are known to favor CFM production over HAAs.31 Thus, the nature of organic matter and microbial community in the anaerobic reactor can significantly affect the formation of DBPs.
4. Conclusions
An onsite blackwater treatment and water-reuse system connected to a single public e-Toilet booth under frequent daily use has been evaluated with weekly measurements over 14 months.With varying daily number of users and across all seasons, the electrochemical treatment of the ABR effluent consistently generated a highly chlorinated (>400 mg L−1) and clear effluent that was negative to a fecal coliform assay.
The wastewater treatment achieved effective removal of solids, organics and nitrogen, and modest removal of phosphorus as benchmarked against Indian and international wastewater standards.
Onsite recycling of treated wastewater for toilet flushing is an appealing approach to address discharge in the environment and achieve water efficiency; however, one can expect negative effects of highly chlorinated water on the biological activity of the ABR.
Over 8 months of test under water recycling conditions, the treatment maintained its solids and nitrogen removal performance due to breakpoint chlorination; however, the COD removal capacity of the system was somewhat reduced.
The strongly oxidizing condition of this electrochemical based disinfection raised the concern of generation of toxic disinfection byproducts (DPBs). This study examined the formation of chloroform and HAA DBPs, and found that the CFM concentration was 10 times above the limit of drinking water, while the HAA values were modest. Under wastewater recycling, the CFM generation increased dramatically and this could be due to an increased and altered organic load.
These findings demonstrate that the evaluated system provides robust ability to carry out onsite treatment of wastewater at the scale of a single toilet. The reuse of highly chlorinated wastewater for toilet flushing demonstrated excellent water savings; however, it likely induced alterations in the biology and chemistry of the digester and resulted in increased byproduct generation. Additional studies are needed to elucidate the role of the microbial community and organic precursors responsible for the formation of DBPs during highly chlorinated wastewater recycling.
The authors recommend including risk assessment for discharge or reuse of treated wastewater containing these levels of DBP and mitigation strategies for DBP generation such as optimization of the electrochemical operation.
Author contributions
PS: investigation, data curation, writing – original draft; MB: investigation, data curation, visualization; PR: investigation, data curation, visualization, writing – original draft; DRJ: resources; VC: investigation, data curation, writing – original draft; JR: methodology; LP: conceptualization, validation, writing – review & editing; KC: formal analysis, validation; CW: validation, visualization; SG: conceptualization, validation, writing – original draft, project administration; CC: conceptualization, validation, writing – review & editing, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests.Acknowledgements
We gratefully acknowledge PSG Institute of Medical Sciences and Research, Coimbatore for supporting the test site installation and operation on their campus. This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation grant INV001513 to Duke University and grants INV003227 and INV047212 to Caltech. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.References
- WHO/UNICEF, Progress on household drinking water, sanitation and hygiene 2000–2020: five years into the SDGs, Geneva, 2021, ISBN (WHO) 978–92–4-003084-8 Search PubMed.
- L. Zhou, H. Wang, Z. Zhang, J. Zhang, H. Chen, X. Bi, X. Dai, S. Xia, L. Alvarez-Cohen and B. E. Rittmann, Novel perspective for urban water resource management: 5R generation, Front. Environ. Sci. Eng., 2021, 15, 1–13 CrossRef.
- G. Nhamo, C. Nhemachena and S. Nhamo, Is 2030 too soon for Africa to achieve the water and sanitation sustainable development goal?, Sci. Total Environ., 2019, 669, 129–139 CrossRef CAS.
- R. Yan, S. Cheng, J. Chen, X. Li, S. Sharma, S. M. N. Uddin, H.-P. Mang, C. Chen, Z. Li and T. Li, Operating status of public toilets in the Hutong neighborhoods of Beijing: An empirical study, J. Environ. Manage., 2021, 287, 112252 CrossRef PubMed.
- B. Pathak and I. Chakravarty, Sanitation and health: a movement visualizing Gandhi's dream, Indian J. Med. Res., 2019, 149(Suppl 1), S73 Search PubMed.
- W. Zhang, H. Chu, L. Yang, X. You, Z. Yu, Y. Zhang and X. Zhou, Technologies for pollutant removal and resource recovery from blackwater: a review, Front. Environ. Sci. Eng., 2023, 17(7), 83 CrossRef CAS PubMed.
- C. A. Cid, Y. Qu and M. R. Hoffmann, Design and preliminary implementation of onsite electrochemical wastewater treatment and recycling toilets for the developing world, Environ. Sci.: Water Res. Technol., 2018, 4(10), 1439–1450 RSC.
- S. K. Varigala, M. Hegarty-Craver, S. Krishnaswamy, P. Madhavan, M. Basil, P. Rosario, A. Raj, V. Barani, C. A. Cid, S. Grego and M. Luettgen, Field testing of an onsite sanitation system on apartment building blackwater using biological treatment and electrochemical disinfection, Environ. Sci.: Water Res. Technol., 2020, 6(5), 1400–1411 RSC.
- C. Castro, H. Shyu, L. Xaba, R. Bair and D. Yeh, Performance and onsite regeneration of natural zeolite for ammonium removal in a field-scale non-sewered sanitation system, Sci. Total Environ., 2021, 776, 145938 CrossRef CAS PubMed.
- G. V. Talekar, P. Sharma, A. Yadav, P. Clauwaert, K. Rabaey and S. Mutnuri, Sanitation of blackwater via sequential wetland and electrochemical treatment, npj Clean Water, 2018, 1(1), 14 CrossRef CAS.
- C. M. Welling, S. Sasidaran, P. Kachoria, S. Hennessy, B. J. Lynch, S. Teleski, H. Chaudhari, K. L. Sellgren, B. R. Stoner and S. Grego, Field testing of a household-scale onsite blackwater treatment system in Coimbatore, India, Sci. Total Environ., 2020, 713, 136706 CrossRef CAS.
- T. Koottatep, S. K. Chapagain, C. Polprasert, A. Panuvatvanich and K.-H. Ahn, Sanitation situations in selected Southeast Asian countries and application of innovative technologies, Environ. Dev. Sustain., 2018, 20, 495–506 CrossRef.
- G. Dandabathula, P. Bhardwaj, M. Burra, P. V. P. Rao and S. S. Rao, Impact assessment of India's Swachh Bharat Mission–Clean India Campaign on acute diarrheal disease outbreaks: Yes, there is a positive change, J. Family Med. Prim. Care, 2019, 8(3), 1202 CrossRef PubMed.
- Y. Brief, Swachh Bharat Mission (Urban): Need vs. Planning, 2018 Search PubMed.
- J. T. Jasper, Y. Yang and M. R. Hoffmann, Toxic byproduct formation during electrochemical treatment of latrine wastewater, Environ. Sci. Technol., 2017, 51(12), 7111–7119 CrossRef CAS PubMed.
- K. Cho, Y. Qu, D. Kwon, H. Zhang, C. A. Cid, A. Aryanfar and M. R. Hoffmann, Effects of anodic potential and chloride ion on overall reactivity in electrochemical reactors designed for solar-powered wastewater treatment, Environ. Sci. Technol., 2014, 48(4), 2377–2384 CrossRef CAS PubMed.
- C. Rose, A. Parker, B. Jefferson and E. Cartmell, The characterization of feces and urine: a review of the literature to inform advanced treatment technology, Crit. Rev. Environ. Sci. Technol., 2015, 45(17), 1827–1879 CrossRef CAS.
- ISO30500, Non-Sewered Sanitation Systems – Prefabricated Integrated Treatment Units – General Safety And Performance Requirements For Design And Testing, in International Organization for Standardization, 2018, ISO 030500:2018.
- E. Reynaert, A. Hess and E. Morgenroth, Making Waves: Why water reuse frameworks need to co-evolve with emerging small-scale technologies, Water Res.: X, 2021, 11, 100094 CAS.
- C. A. Cid, F. Abiola and M. Starkl, Can International Nonsewered Sanitation Standards Help Solve the Global Sanitation Crisis?, Environ. Sci. Technol., 2022, 56(2), 699–706 CrossRef CAS.
- NGT, National Green Tribunal, Principal Bench, New Delhi Order 30.4.2019 Sewage Disposal Norms, 2019 Search PubMed.
- B. W. Waters and Y. C. Hung, The effect of organic loads on stability of various chlorine-based sanitisers, Int. J. Food Sci. Technol., 2014, 49(3), 867–875 CrossRef CAS.
- W.H.O., A global overview of national regulations and standards for drinking-water quality, 2021 Search PubMed.
- IS10500, Indian standard drinking water–specification (second revision), Bureau of Indian Standards (BIS), New Delhi, 2012 Search PubMed.
- H. Wang, R. Yu, J. Webb, P. Dollar and D. L. Freedman, Anaerobic biodegradation of chloroform and dichloromethane with a Dehalobacter enrichment culture, Appl. Environ. Microbiol., 2022, 88(4), e0197021 CrossRef.
- Y.-C. Hung, B. W. Waters, V. K. Yemmireddy and C.-H. Huang, pH effect on the formation of THM and HAA disinfection byproducts and potential control strategies for food processing, J. Integr. Agric., 2017, 16(12), 2914–2923 CrossRef CAS.
- V. M. Gómez-López, A. Marín, M. S. Medina-Martínez, M. I. Gil and A. Allende, Generation of trihalomethanes with chlorine-based sanitizers and impact on microbial, nutritional and sensory quality of baby spinach, Postharvest Biol. Technol., 2013, 85, 210–217 CrossRef.
- S. M. Iskander, T. Zeng, E. Smiley, S. C. Bolyard, J. T. Novak and Z. He, Formation of disinfection byproducts during Fenton's oxidation of chloride-rich landfill leachate, J. Hazard. Mater., 2020, 382, 121213 CrossRef CAS PubMed.
- Z. Shao, X. Guo, Q. Qu, K. Kang, Q. Su, C. Wang and L. Qiu, Effects of chlorine disinfectants on the microbial community structure and the performance of anaerobic digestion of swine manure, Bioresour. Technol., 2021, 339, 125576 CrossRef CAS PubMed.
- X. Wang, H. Zhang, Y. Zhang, Q. Shi, J. Wang, J. Yu and M. Yang, New insights into trihalomethane and haloacetic acid formation potentials: correlation with the molecular composition of natural organic matter in source water, Environ. Sci. Technol., 2017, 51(4), 2015–2021 CrossRef CAS.
- L. Liang and P. C. Singer, Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water, Environ. Sci. Technol., 2003, 37(13), 2920–2928 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00454f |
| This journal is © The Royal Society of Chemistry 2024 |