Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A comparison of non-covalent interactions in the crystal structures of two σ-alkane complexes of Rh exhibiting contrasting stabilities in the solid state†
M. Arif
Sajjad
 a,
Stuart A.
Macgregor
a,
Stuart A.
Macgregor
 *a and
Andrew S.
Weller
*a and
Andrew S.
Weller
 b
b
aInstitute of Chemical Sciences, Heriot-Watt University, Edinburgh, EH14 4AS, UK. E-mail: s.a.macgregor@hw.ac.uk
bDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK
First published on 27th January 2023
Abstract
Non-covalent interactions surrounding the cationic Rh σ-alkane complexes within the crystal structures of [(Cy2PCH2CH2PCy2)Rh(NBA)][BArF4], [1-NBA][BArF4] (NBA = norbornane, C7H12; ArF = 3,5-(CF3)2C6H3), and [1-propane][BArF4] are analysed using Quantum Theory of Atoms in Molecules (QTAIM) and Independent Gradient Model approaches, the latter under a Hirshfeld partitioning scheme (IGMH). In both structures the cations reside in an octahedral array of [BArF4]− anions within which the [1-NBA]+ cation system exhibits a greater number of C–H⋯F contacts to the anions. QTAIM and IGMH analyses indicate these include the strongest individual atom–atom non-covalent interactions between the cation and the anion in these systems. The IGMH approach highlights the directionality of these C–H⋯F contacts that contrasts with the more diffuse C–H⋯π interactions. The accumulative effects of the latter lead to a more significant stabilizing contribution. IGMH %δGatom plots provide a particularly useful visual tool to identify key interactions and highlight the importance of a –{C3H6}– propylene moiety that is present within both the propane and NBA ligands (the latter as a truncated –{C3H4}– unit) and the cyclohexyl rings of the phosphine substituents. The potential for this to act as a privileged motif that confers stability on the crystal structures of σ-alkane complexes in the solid-state is discussed. The greater number of C–H⋯F inter-ion interactions in the [1-NBA][BArF4] system, coupled with more significant C–H⋯π interactions are all consistent with greater non-covalent stabilisation around the [1-NBA]+ cation. This is also supported by larger computed δGatom indices as a measure of cation–anion non-covalent interaction energy.
1. Introduction
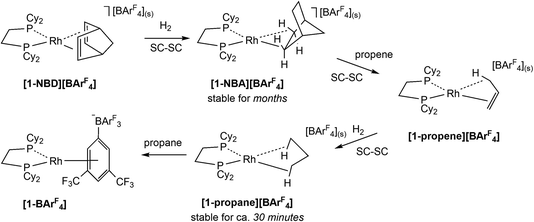
The development of Solid-state Molecular OrganoMetallic (SMOM) chemistry1–3 has enabled the study of transition metal σ-alkane complexes by single crystal X-ray diffraction. Such complexes are key intermediates in C–H activation processes,4–6 but are short-lived in solution even at low temperature.7 SMOM chemistry exploits gas/solid reactions to effect the single crystal-to-single crystal (SC–SC) transformation of alkene precursor complexes, [(R2P(CH2)nPR2)M(alkene)][BArF4], to the corresponding σ-alkane complexes, [(R2P(CH2)nPR2)M(alkane)][BArF4].8 Of these [(Cy2PCH2CH2PCy2)Rh(NBA)][BArF4], [1-NBA][BArF4] (NBA = norbornane, C7H12; ArF = 3,5-(CF3)2C6H3, Scheme 1), is found to be stable for (at least) months under an inert atmosphere and can be synthesized on a gram scale.9,10 Variation of the bidentate phosphine,9,11–13 alkene11,14–16 and counter-anion12,17 has produced a range of crystallographically characterised Rh and Co18 σ-alkane complexes that, in conjunction with computational studies,19 have been shown to exhibit a range of alkane modes that sit along the η1C–H (end-on) to η2C–H (side-on) continuum.11These σ-alkane complexes also exhibit reactivity in the solid state, including fluxional processes,10,14,15 H/D exchange reactions10,15,20 and room temperature alkane dehydrogenation,15 where these last two categories link σ-alkane complexes directly to a C–H bond activation event. Alkane substitution is also possible; for example, exposure of [1-NBA][BArF4], to propene gas (Scheme 1) gives [1-propene][BArF4], in which the Rh–alkene bond is supported by a γ-agostic interaction.1 Hydrogenation of [1-propene][BArF4] then forms [1-propane][BArF4], featuring the lightest to date alkane to be characterised crystallographically as a σ-complex.16 The propane ligand in [1-propane][BArF4] exhibits a 1,3-binding mode with two η2C–H→Rh interactions that are supported by additional contributions from the geminal C–H bonds.
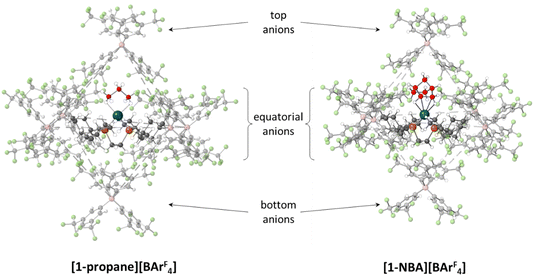
The microenvironments of the Rh cations within the extended crystal structures of [1-NBA][BArF4] and [1-propane][BArF4] are shown in Fig. 1. In both cases the cation resides in a pseudo-octahedral arrangement of [BArF4]− anions with the σ-alkane ligand sitting within a V-shaped pocket defined by two aryl substituents of the adjacent (top) [BArF4]− anion. Despite these similarities, [1-NBA][BArF4] and [1-propane][BArF4] show very different behaviours in the solid state: [1-NBA][BArF4] is stable for months under an inert atmosphere, whereas [1-propane][BArF4] decomposes within 30 minutes via displacement of the alkane by an aryl group of a neighbouring [BArF4]− anion to give zwitterionic [1-BArF4] (Scheme 1). Indeed, crystallographic characterisation of [1-propane][BArF4] required the in situ hydrogenation of [1-propene][BArF4] on a synchrotron beamline (Diamond I19) at 150 K.
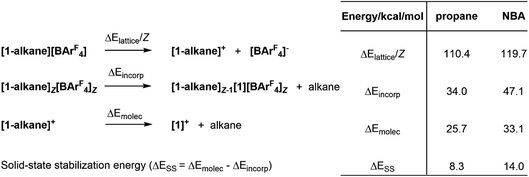
Understanding the stability of these σ-alkane complexes in the solid-state is a central goal for our research in this area, that aims to exploit SMOM chemistry in catalysis.1,17 In an early study we showed that computed NBA binding energies across a range of [(R2P(CH2)nPR2)M(alkane)]+ molecular cations (i.e. neglecting the effect of the solid-state environment, n = 2; R = Cy, iBu, iPr, OiPr; n = 3, R = iPr) showed no correlation with the stability of [(R2P(CH2)nPR2)M(alkane)][BArF4] salts in the solid state.9 Bistoni and co-workers subsequently discussed the role of intramolecular dispersion in the alkane molecular binding energies.21 However, our subsequent work has shown the importance of including the solid-state environment via periodic DFT calculations to achieve reasonable structures and energetics for these σ-alkane complexes.19 In a recent study, normalised lattice energies, alkane incorporation energies and molecular alkane binding energies were computed for a range of [(Cy2P(CH2)2PCy2)M(alkane)][BArF4] complexes (see Fig. 2 for definitions).16 All three measures were consistent with the greater stability of [1-NBA][BArF4] over [1-propane][BArF4]; moreover, a significant, additional stabilization provided by the solid-state environment, ΔESS, was identified and was larger in [1-NBA][BArF4] (14.0 kcal mol−1cf. 8.3 kcal mol−1).
Previously, non-covalent interactions (NCI) plots22 have highlighted broad areas of dispersive stabilisation between the alkane ligand and the neighbouring [BArF4]− anions in these σ-alkane complexes.11,13,14,17,18 In addition, more localised disk-like features have suggested the presence of C–H⋯F contacts and we have commented on the potential role of such interactions in conferring stability in the solid state.17 Here we employ Quantum Theory of Atoms in Molecules (QTAIM) and Independent Gradient Model calculations under the recently proposed Hirshfeld partitioning scheme (IGMH23) to explore further the role of these non-covalent interactions on the stability of σ-alkane complexes. Our study will focus on the [1-NBA][BArF4] and [1-propane][BArF4] systems as these exhibit each end of the range of stabilities seen to date in crystallographically characterised σ-alkane complexes in the solid state.
2. Results and discussion
2.1. Methodology
Geometries for [1-NBA][BArF4]9 and [1-propane][BArF4]16 were taken from the reported crystal structures and partially optimised with periodic-DFT calculations at the PBE-D3 level using the CP2K program (see ESI† for full details). B, C, P and Rh atoms were fixed at their experimentally determined positions while H and F atoms were fully relaxed in order to provide reliable H atom positions and to resolve any rotational disorder associated with the CF3 groups. Unit cell parameters were fixed at the experimental values. Intermolecular interactions were analysed between one central Rh cation and the surrounding pseudo-octahedron of [BArF4]− anions. Short contacts within crystal structures were analysed with CrystalExplorer24,25 with van der Waals radii taken from Alvarez (H: 1.20 Å; C: 1.77 Å; F: 1.46 Å).26 In the following a ‘short contact’ will refer to a distance at or below the sum of the relevant van der Waals radii. Quantum theory of atoms in molecules (QTAIM27) analyses employed the AIMAll program28 and used the extended wavefunction format. Independent gradient model calculations used Multiwfn29 and employed the Hirshfeld partitioning scheme (IGMH method).23 Surfaces were visualised with VMD.302.2. Geometric analysis of the cation microenvironment
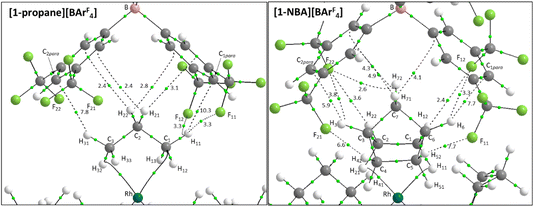
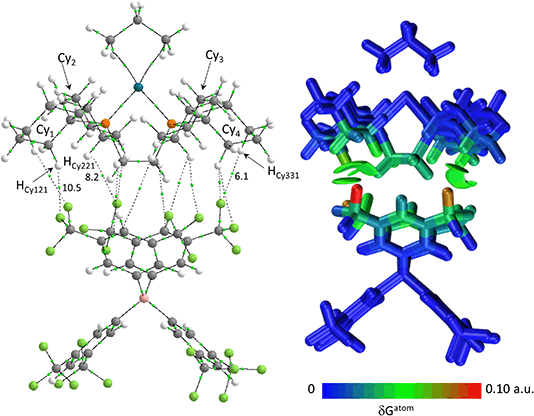
Structures will be analysed in terms of the interaction of the [1-NBA]+ and [1-propane]+ cations with the octahedral array of the six surrounding [BArF4]− anions. Fig. 3 displays Hirshfeld surfaces for the [1-NBA]+ and [1-propane]+ cations within this environment and also defines the anion naming system. The axial direction contains the anions sitting above the alkane (the ‘top’ anion) and adjacent to the phosphine backbone (the ‘bottom’ anion) (here obscured by the Hirshfeld surface of the cation as the view is straight down the axial direction). The equatorial anions behave as two distinct pairs, Eq-1 and Eq-3, and Eq-2 and Eq-4, that are distinguished by their alignment with the adjacent phosphine cyclohexyl substituents. Each pair shows a comparable set of non-covalent interactions, as discussed in more detail below. The anion octahedra are slightly compressed with the axial B⋯B distances somewhat shorter than the trans-equatorial B⋯B distances. Within this the Rh centre sits lower along the axial direction in the NBA complex (Rh⋯Btop = 9.83 Å for [1-NBA][BArF4] and 8.02 Å for [1-propane][BArF4]), possibly reflecting the larger size of this alkane ligand.The Hirshfeld surfaces around the alkanes of the [1-NBA]+ and [1-propane]+ cations are predominantly white and blue indicating that most inter-ion contacts are at or beyond the sum of the van der Waals radii. The red area under the top anion in the [1-propane][BArF4] system corresponds to a short H⋯C contact between a Me hydrogen on the propane and a para-carbon of the top [BArF4]−. An equivalent but weaker feature in [1-NBA]+ involves a bridgehead hydrogen on the NBA. Fingerprint plots indicate that H⋯F cation–anion contacts are most prevalent in both structures ([1-NBA][BArF4]: 61.6%; [1-propane][BArF4]: 63.3%) with H⋯H and H⋯C contacts at around 25% and 12% respectively (see Fig. S1–S4† for individual H⋯X fingerprint plots). A number of H⋯F short contacts (i.e. at or below the sum of the van der Waals radii) are seen and these generally involve cyclohexyl hydrogens rather than the alkane ligands. The intense red spot between the cation and Eq-1 in the Hirshfeld surface of [1-propane]+ is an example of this and corresponds to a short H⋯F contact of 2.22 Å.
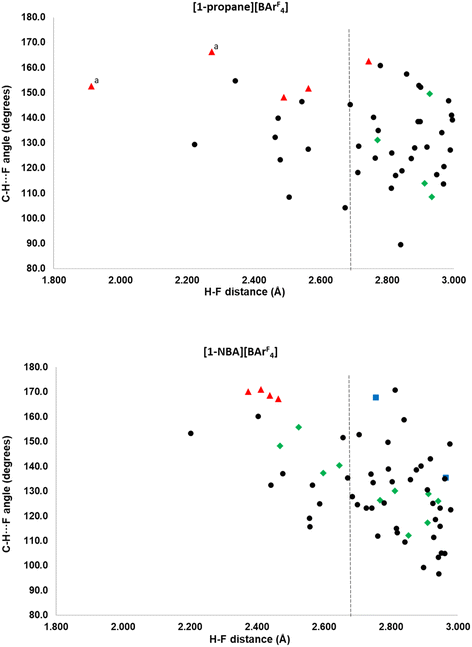
More detail on the distribution of C–H⋯F contacts around the [1-NBA]+ and [1-propane]+ cations that are below 3.0 Å is provided in the scatterplots in Fig. 4. Three classes of cation–anion contacts are seen: (i) alkane C–H⋯F (in green); (ii) phosphine backbone C–H⋯F (blue) and (iii) phosphine cyclohexyl C–H⋯F contacts (black). In addition a small number of anion–anion C–H⋯F contacts are shown in red. The cyclohexyl C–H⋯F contacts are most common and are more numerous for [1-NBA]+ than [1-propane]+ (45 compared to 36). There are no cyclohexyl C–H⋯F contacts to the top anion in either structure. No correlation between H⋯F distance and C–H⋯F angle is seen, beyond the absence of short H⋯F contacts with low C–H⋯F angles. [1-NBA]+ features two C–H⋯F contacts from the phosphine backbone while no such contacts are seen around [1-propane]+. Of the inter-anion C–H⋯F contacts in red (four in [(1-NBA)][BArF4] and seven in [1-propane][BArF4]) all but one is a short contact, and these are also among the closest to linear (150–170°). These features have been taken as evidence for C–H⋯F H-bonding31–33 and this could be promoted by the relative acidity of the C–H H-bond donors that are located between two CF3 substituents on the aryl rings. A more detailed discussion of the role of these inter-anion contacts will be the subject of a separate study.
Comparing the alkane ligands, in [1-propane]+ the propane displays four C–H⋯F contacts below 3.0 Å, although none is below the sum of the van der Waals radii. The NBA in [1-NBA]+ has 10 C–H⋯F contacts, four of which are short contacts. A general trend towards wider C–H⋯F angles with shorter H⋯F distances can be seen in the alkane C–H⋯F data for [1-NBA][BArF4] and this is similar to the data in Boese, Nangia and Desiraju’s early study on fluorobenzenes, where C–H⋯F H-bonding was proposed especially when more acidic C–H bonds are present.32 This is discussed in the context of the present systems after these C–H⋯F contacts have been considered in more detail via electronic structure analyses.
2.3. Electronic structure analyses
QTAIM molecular graphs were computed for all six cation–anion ion-pairs that can be formed within the [1-NBA][BArF4] and [1-propane][BArF4] ‘octahedra’ – i.e. where the central cation is common to all six ion-pairs and is treated in turn with each adjacent anion. The range of C–H⋯F, C–H⋯Caryl and C–H⋯Haryl bond paths characterised are summarised in Table 1. Individual bond critical point (BCP) metrics are listed in the ESI (see Fig. S5–S16†). The C–H⋯F bond paths are most numerous, there being almost twice as many of these as the total of C–H⋯Caryl and C–H⋯Haryl bond paths. Overall the [1-NBA]+ cation exhibits significantly more bond paths to the surrounding anions (88 cf. 72 around [1-propane]+) and this is primarily due to a greater number of C–H⋯F bond paths (61 cf. 46).| Anion | Bond path | [1-Propane][BArF4] | [1-NBA][BArF4] | ||
|---|---|---|---|---|---|
| Numbera | ρ(r)/a.u. × 10−3 | Number | ρ(r)/a.u. × 10−3 | ||
| a Values in parentheses indicate the number of C–H⋯F bond paths involving the cyclohexyl substituents within the conical pocket of anion aryl groups (Cyinner), the cyclohexyl outside that pocket (Cyouter) and, where appropriate, the alkane ligand, respectively (see text for details). b One C⋯F bond path is also present; see ESI for details. c Includes C–H⋯F bond paths from the –C2H4– backbone of the diphosphine ligand. | |||||
| Top | C–H⋯F | 2 | 3.3, 3.3 | 7 | 2.6–7.7 |
| C–H⋯C | 6 | 2.8–10.3 | 5 | 4.1–7.7 | |
| Eq-1 | C–H⋯F | 8 (5/2/1) | 2.2–14.1 | 12 (7/4/1) | 2.0–8.8 |
| C–H⋯C | 4b | 2.0–4.4 | 3 | 2.7–5.4 | |
| C–H⋯H | 1 | 3.5 | 3 | 3.9–5.8 | |
| Eq-2 | C–H⋯F | 10 (5/4/1) | 1.2–8.2 | 12 (7/4/1b) | 1.3–6.9 |
| C–H⋯C | 3 | 1.9–4.4 | 2 | 4.0, 4.7 | |
| C–H⋯H | 2 | 4.5, 5.0 | 2 | 6.6, 7.9 | |
| Eq-3 | C–H⋯F | 7 (4/3) | 1.2–4.5 | 10 (5/4/1) | 2.4–9.1 |
| C–H⋯C | 2 | 3.6, 5.6 | 2b | 3.7, 4.4 | |
| C–H⋯H | 2 | 3.2, 5.6 | 2 | 5.2, 5.4 | |
| Eq-4 | C–H⋯F | 9 (5/4) | 1.5–8.2 | 9 (3/4/2) | 2.6–7.9 |
| C–H⋯C | 2 | 4.6, 7.0 | 2 | 3.1, 3.4 | |
| C–H⋯H | 2 | 4.2, 6.4 | 2 | 3.8, 10.2 | |
| Bottom | C–H⋯F | 10 | 1.5–10.5 | 11 | 1.8–13.0 |
| C–H⋯C | 2 | 1.3, 1.4 | 3 | 2.0–3.5 | |
| C–H⋯H | — | 1 | 2.1 | ||
| Total | C–H⋯F | 46 (29c/13/4) | 1.2–14.1 | 61 (33c/16/12) | 1.3–13.0 |
| C–H⋯C | 19 | 1.9–10.3 | 17 | 2.0–5.4 | |
| C–H⋯H | 7 | 3.2–6.4 | 10 | 2.1–10.2 | |
Within the [1-NBA][BArF4] ion-pair the –{C3H4}– moiety comprising the bridgehead (C3–H3 and C6–H6) and central bridging methylene (C7–H71/C7–H72) bonds behaves as a “propane” fragment and forms two C–H⋯F and five C–H⋯Caryl bond paths that are equivalent to similar bond paths in [1-propane][BArF4]. Consistent with this, the highest ρ(r) values within this set are for the C3–H3⋯C2para and C6–H6⋯C1para bond paths (ρ(r) = 5.9 × 10−3 and 7.7 × 10−3 a.u.). The NBA system has additional bond paths from both pairs of exo-C–H bonds. Of these the C4–H42⋯F21 and C5–H52⋯F11 bond paths correspond to the two shortest C–H⋯F contacts (2.47 Å and 2.53 Å) and have higher ρ(r) values (7.7 × 10−3 a.u. and 6.7 × 10−3 a.u.). The C1–H12⋯F12 and C2–H22⋯F22 bond paths are weaker, with longer contacts (2.77 Å and 2.94 Å) and lower ρ(r) (3.6 × 10−3 a.u. and 2.4 × 10−3 a.u.). The final additional feature of the NBA system is a weak C7–H72⋯F22 bond path (3.02 Å; ρ(r) = 2.6 × 10−3 a.u.).
In general, the C–H⋯F BCP metrics computed here are similar to those reported in previous studies of C–H⋯F contacts and show an exponential relationship between the H⋯F distance and ρ(r) (see Fig. S26†).34–36 Such interactions have been ascribed weak H-bonding character that is enhanced by a more acidic C–H bond.32,33 An electron deficient cationic Rh(I) centre could enhance such interactions via polarization of both the endo-C–H bonds directly involved in the σ-interaction and, through induction effects, the geminal exo-C–H bonds and beyond.37 To assess this, the molecular graphs in Fig. 5 were recomputed with the {(Cy2P(CH2)2PCy2)Rh}+ fragments removed. Computed QTAIM charges confirmed the alkane hydrogen atoms do indeed become more positively charged when the alkane are bound to Rh, however, the same networks of bond paths with virtually unchanged BCP metrics were seen (see Fig. S25†). This lack of influence of the Rh fragment implies at best weak H-bonding character in these C–H⋯F interactions.
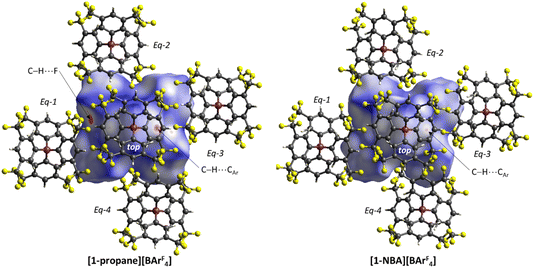
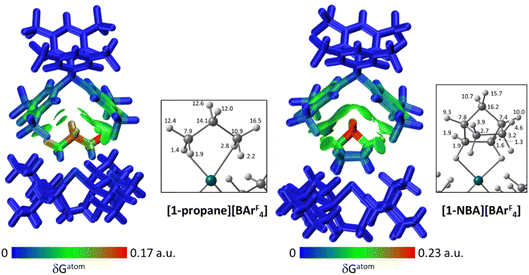
The C–H⋯Caryl bond paths (six around [1-propane]+, five around [1-NBA]+) suggest the presence of C–H⋯π type interactions in both ion-pairs.38–41 Previously, NCI plots have highlighted broad areas of dispersive stabilisation between σ-bound alkanes and the adjacent anions.11,13,14,17,18 Here, we have used the Independent Gradient Model (IGM) approach42,43 to isolate non-covalent interactions between the cation and anion. This method is based on atomic electron densities and uses the descriptor, δG, the difference in the upper limit of the electron density gradient of the non-interacting system vs. the electron density gradient of the interacting system. Here the Hirshfeld partitioning scheme of the molecular electron density proposed by Lu and Chen (and implemented within Multiwfn) is employed to define the atomic electron densities (IGMH approach).23 The results of these IGMH analyses for the top ion-pairs are displayed in Fig. 6, both as isosurfaces of δGinter and as colour-coded %δGatom scores that highlight the atoms that contribute most significantly to these inter-ion interactions. These %δGatom scores are the sum of all the interactions of a given atom on the cation with all the atoms of the anion; individual atom–atom interactions can also be quantified as δGatom indices.
The IGMH isosurfaces amplify the features seen in the QTAIM molecular plots. For [1-NBA][BArF4] distinct disk-like features consistent with exo-C–H⋯F contacts are seen and these are much more pronounced than in [1-propane][BArF4]. The peripheral alkane C–H⋯Cpara (anion) contacts are also common to both structures, but these are more extensive in [1-NBA][BArF4]. This is confirmed by the %δGatom values, where the relative contribution of each atom is represented on a blue–green–red scale (blue: zero; red: the maximum contribution, see also inset for % atomic contributions in the cations). The strongest contributions in [1-NBA][BArF4] are associated with the bridging methylene (C7, H71 and H72) whereas H31 is most prominent in [1-propane][BArF4]. The atoms involved in the exo-C–H⋯F contacts show little lightening of the blue colour, indicating a minimal contribution. This is confirmed by the %δGatom values: the bridgehead and bridging methylene atoms that make up the –{C3H4}– “propane” fragment within the NBA ligand account for 77.0% of the total interaction, whereas the exo-hydrogen atoms, H42 and H52, that are involved in H⋯F short contacts contribute only 4.6% and 3.9% respectively. The δGatom indices, that quantify individual atom–atom contributions, show that the H42⋯F21 and H52⋯F11 pairs are ranked 2nd and 4th strongest (1.8% and 1.7%) and are comparable in strength to the H3⋯C2para and H6⋯C1para pairs (1.9% and 1.8%, see Fig. S11†). These data are also consistent with the similar BCP ρ(r) values computed for the equivalent bond paths. However, H42 and H52 engage in relatively few additional atom–atom contacts whereas all atoms in the –{C3H4}– “propane” moiety have numerous atom–atom contributions that accumulate to make them more significant in the inter-fragment interaction. This difference between the directional C–H⋯F contacts and the more diffuse C–H⋯Caryl contacts is a recurring theme in this study. For [1-propane][BArF4] the equivalent –{C3H4}– moiety accounts for 87.0% of the total interaction, but of this only 3.4% is due to H11⋯F11 and H31⋯F22 atom–atom contributions.
Overall, the alkane ligands dominate the cation component to the non-covalent interactions in these ion-pairs: summing %δGatom values from all atoms of the propane ligand accounts for 94.6% of the interaction while for the NBA ligand the equivalent sum gives 99.5%. These values are summarised in Table 2, where comparison with the other ion-pairs studied below is also made. Also included in Table 2 are the sum of the individual δGatom indices. These give an indication of the accumulative strength of the non-covalent interactions between the cation and anion and show higher values for [1-NBA][BArF4] over [1-propane][BArF4] (for the top anion 1.39 vs. 0.95).
| Ion-pair | [1-Propane][BArF4] | [1-NBA][BArF4] | ||
|---|---|---|---|---|
| % Contribution | ΣδGatom index | % Contribution | ΣδGatom index | |
| Top | Propane | NBA | ||
| 94.6 | 0.95 | 99.5 | 1.39 | |
| Equatorial | Cyinner/Cyouter/propane | Cyinner/Cyouter/NBA | ||
| Eq-1 | 76.0/21.0/2.4 | 1.14 | 75.3/20.2/3.8 | 1.50 |
| Eq-2 | 83.6/14.9/1.1 | 0.82 | 69.3/18.5/11.7 | 1.24 |
| Eq-3 | 80.0/16.0/3.3 | 1.19 | 82.8/13.2/3.2 | 1.38 |
| Eq-4 | 86.4/12.1/1.1 | 1.05 | 72.9/15.4/10.5 | 1.10 |
| Bottom | Cy1–4/C2 backbone | Cy1–4/C2 backbone | ||
| 74.9/24.2 | 0.87 | 69.0/29.1 | 1.13 | |
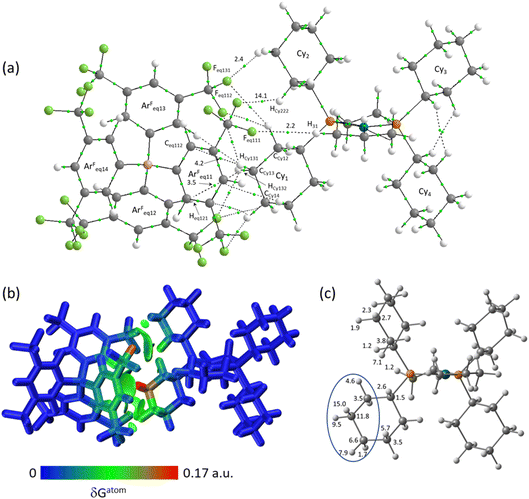
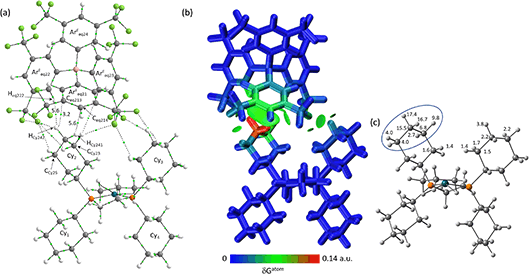
The molecular graph for the ion-pair with Eq-1 in [1-propane][BArF4] is shown in Fig. 7(a). This displays a pattern, common to all these ion-pairs, in which one cyclohexyl substituent (Cyinner, here Cy1) sits within a conical pocket defined by three of the anion aryl groups (ArFeq11, ArFeq12 and ArFeq13) while a second cyclohexyl substituent (Cyouter, here Cy2) sits outside this pocket. The former displays a range of C–H⋯F, C–H⋯Caryl and C–H⋯Haryl bond paths while the latter only exhibits C–H⋯F bond paths. In the Eq-1 ion-pair Cy1 lies approximately parallel to the aromatic ring of ArFeq11 and shows five C–H⋯F bond paths that are distributed across all three ArF groups of the pocket. In contrast the C–H⋯Caryl bond paths are all directed to carbons on ArFeq11 and the shortest of these involves the C3-axial hydrogen and an ortho ring-carbon (HCy131⋯Ceq112 = 3.06 Å; ρ(r) = 4.2 × 10−3 a.u.). All these bond paths have low ρ(r) values below 4.2 × 10−3 a.u. In contrast, the C3-equatorial hydrogen, HCy132, is directed away from ArFeq11 and engages in two C–H⋯F bond paths (ρ(r) = 2.0 × 10−3 a.u. and 3.4 × 10−3 a.u.) and one C–H⋯Haryl bond path to Heq121 (ρ(r) = 3.5 × 10−3 a.u.). Cy2 exhibits two C–H⋯F bond paths, one of which has a very short contact (HCy222⋯Feq112 = 2.22 Å) and the highest ρ(r) value of 14.1 × 10−3 a.u. within this ion-pair and indeed across both systems. This corresponds to the intense red C–H⋯F short contact noted on the Hirshfeld surface in Fig. 3 beside Eq-1.
These different interactions are reflected in the IGMH isosurfaces in Fig. 7(b). The C–H⋯F interactions from Cy2 can be clearly seen with the darker green region equating to the short HCy222⋯Feq112 contact noted above. The orange colour of Feq112 in the %δGatom colouring confirms a significant contribution from that atom: the HCy222⋯Feq112 pair also has the single largest atom–atom δGatom index at 4.1%. More significant, however, is the green isosurface between Cy1 and ArFeq11. In this case the %δGatom colouring highlights the C3-axial hydrogen, HCy131, in red, indicating the largest single atomic contribution (15.0%). The C3-equatorial hydrogen contributes 9.5% – somewhat lower as it is engaged in more directional C–H⋯F interactions. More generally, the cation interaction is dominated by the –{C3H6}– propylene motif centred on the CCy12, CCy13 and CCy14 carbons and ringed in Fig. 7(c). Together these atoms contribute 62.9% of the interaction from the cation.
Within the other equatorial ion-pairs a similar pattern of bond paths is seen, with BCP ρ(r) values generally between 1.0 and 6.0 × 10−3 a.u. Any bond paths with higher BCP ρ(r) values all correspond to short contacts below the sum of the relevant van der Waals radii: there are four such C–H⋯F, two C–H⋯Caryl and one C–H⋯Caryl bond paths, within which the highest ρ(r) value is 8.2 × 10−3 a.u. Of the three remaining equatorial ion-pairs Eq-3 behaves like Eq-1 with one Cy group lying side-on within the conical pocket and the equivalent –{C3H6}– propylene motif centred on the C3 methylene group and contributing 62.0% of the cation interaction. With Eq-2 and Eq-4 the inner cyclohexyl groups (Cy2 and Cy4, respectively) show a more end-on geometry within the pocket (see Fig. 8 for the ion-pair with Eq-2). As a result, both the equatorial and axial hydrogens of the C4 atom (HCy241 and HCy242) engage in C–H⋯Caryl bond paths and this is most clearly seen when visualised as %δGatom in the IGMH plot. This methylene group alone accounts for 49.6% of the cation interaction with Eq-2 and this increases to 76.9% when expanded to include the neighbouring CH2 groups (circled in Fig. 8(c)).
The four equatorial ion-pairs in the [1-NBA][BArF4] system exhibit the same general geometric pattern as in [1-propane][BArF4] with each ion-pair having one inner cyclohexyl group, Cyinner, within the [BArF4]− conical pocket and one outer Cy group, Cyouter, outside that pocket. Geometrically the Eq-1/Eq-3 and Eq-2/Eq-4 ion-pairs again form distinct pairs, with the Cyinner groups being more side-on in the former and interacting primarily through a propylene motif centred on the C3 methylene, while in the latter the Cyinner groups are more end-on and interact most strongly via the propylene motif centred on the C4 methylene. QTAIM molecular graphs, IGMH isosurfaces and %δGatom plots for all ion-pairs are provided in the ESI (see Fig. S18–S25†).
Table 2 compares the data for the equatorial ion-pairs in [1-propane][BArF4] and [1-NBA][BArF4]. Here the %δGatom data have been summed over all atoms of three fragments within the cations: Cyinner, Cyouter and the alkane ligand. In each case Cyinner dominates and a very similar distribution is seen for the Eq-1 and Eq-3 ion-pairs in both systems. The contribution from the alkane ligand is very small (<4%). In contrast, the Eq-2/Eq-4 ion-pairs differ significantly between the two systems: in [1-propane][BArF4] Cyinner contributes 83.6% and 86.4% respectively, whereas this drops to 69.3% and 72.9% in [1-NBA][BArF4]. The major reason for this is a relatively more significant contribution from the NBA ligand (ca. 11% cf. 1% in [1-propane][BArF4]). In all cases the strength of the non-covalent interactions between the cation and the anions, as gauged by the sum of δGatom indices, is significantly greater for [1-NBA][BArF4].
2.4. Discussion
A combination of geometric and electronic structure analyses have revealed significant differences in the non-covalent interactions around the Rh cations in the σ-alkane complexes [1-propane][BArF4] and [1-NBA][BArF4]. The NBA system exhibits more C–H⋯F short contacts and bond paths and the NBA ligand contributes more significantly to inter-ion interactions, not only in the top anion where the alkanes dominate, but also in two of the equatorial anions (Eq-2 and Eq-4). ΣδGatom indices are higher for all the cation–anion ion-pairs in the NBA system. These data are all consistent with previous work that quantified an additional stabilisation in [1-NBA][BArF4] that was due to the solid-state environment (5.7 kcal mol−1, Fig. 2).16 However, what precisely is the driving force that creates this greater stability and what role does it play, if any, in determining the chemical stability of these crystalline solids?The presence of several propylene motifs in the cations, either from the propane ligand itself, the cyclohexyl substituents or, for the NBA ligand, a topologically related –{C3H4}– moiety, is a recurring pattern in all but the bottom ion-pairs. While this does not discriminate the [1-propane][BArF4] and [1-NBA][BArF4] systems (with their very different chemical stability) it may be that this propylene moiety acts as a privileged motif for the formation of σ-alkane complexes in the solid state, providing a robust environment such that these species are long-lived after the SC–SC transformations necessary for their formation. Other closely related NBA complexes, [(R2PCH2CH2PR2)Rh(NBA)][BArF4], that lack the rigid propylene motif of the cyclohexyl substituents show very different behaviours: with R = iBu8 the system is only stable for a few hours at room temperature whereas with R = iOPr9 the corresponding alkane complex cannot be isolated and the formation of the zwitterion [1-BArF4] is observed. The combination of the cyclohexyl substituents and the NBA ligand is clearly particularly favourable.
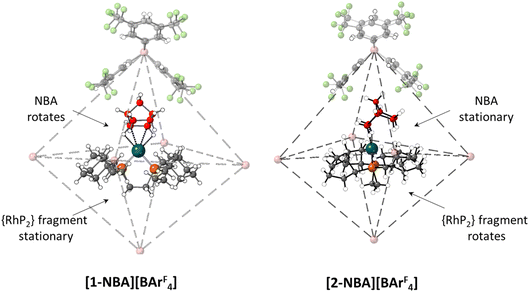
A further indication of the importance of the propylene motif is seen with the cyclopentyl (Cyp) analogue, [(Cyp2PCH2CH2PCyp2)Rh(NBA)][BArF4], [2-NBA][BArF4].44 With [1-NBA][BArF4] hydrogenation of the NBD precursor proceeds with rotation of the NBA ligand such that the plane of the C7 bridging methylene is perpendicular to the {PRhP} coordination plane (see Fig. 10 and Scheme 1). Remarkably, with [2-NBA][BArF4] the NBA ligand is stationary and it is the {Rh(Cyp2CH2CH2PCyp2)}+ fragment that rotates. Thus the excision of a single methylene group from each of the phosphine substituents significantly perturbs the behaviour of the system in the SC–SC transformation.
Returning to the [1-NBA][BArF4] system, in the top anion the NBA ligand shows enhanced C–H⋯F interactions compared to the propane structure. In addition, the more diffuse C–H⋯π interactions appear energetically more significant and the presence of the C7 methylene bridge in the NBA confers additional interactions that are not possible for propane. The NBA appears to be a particularly good fit to this pocket and its presence has knock-on effects around the anionic octahedral array. The enhanced contribution of C–H⋯F contacts has already been noted for Eq-2 and Eq-4, however, even for Eq-1 and Eq-3, where the % contributions of the Cyinner and Cyouter cyclohexyl substituents are very similar (and the alkane ligands play a negligible role, Table 2) the overall strength of interaction as gauged via the δGatom indices is greater for the NBA system. A range of geometrical parameters were assessed to account for this difference, however no coherent trend could be found. The overall dimensions of the anion octahedron may give some clue: as mentioned in the introduction the Rh cation sits lower down the axial direction in [1-NBA][BArF4] while the octahedron of anions itself is slightly compressed along the equatorial axes (B-1⋯B-3 = 19.26 Å cf. 19.94 Å in [1-propane][BArF4]; B-2⋯B-4 = 19.04 Å cf. 19.41 Å in [1-propane][BArF4]). One possibility is that the octahedral array of anions is more perturbed in [1-propane][BArF4] and evidence for this is seen in the wider range of C–H⋯F anion–anion contacts in this system (1.91–2.75 Å, Fig. 4) whereas these cluster around 2.40 Å in [1-NBA][BArF4]. Relating these differences to specific contributions is challenging, however, and most likely the differences in behaviour reflect an accumulation of small effects arising from the subtly different orientations of the cations within each octahedral array of anions, as well as the detailed shape of the octahedra themselves.
Computed BCP ρ(r) values and δGatom indices indicate that the C–H⋯F interactions provide the strongest non-covalent interactions in these systems and these two metrics also show a strong correlation (see Fig. S27†). By these measures the C–H⋯Caryl contacts are less significant; however in these systems such bond paths flag up more diffuse C–H⋯π interactions that incorporate several atom–atom contributions, the accumulative effect of which is much more significant. While this issue is well-known, and has been addressed in model systems such as the benzene dimer through comparison of QTAIM and NCI plots,45 the use of the IGMH isosurfaces and quantification via %δGatom contributions provide a particularly graphic illustration of these points. The contributions of the more directional C–H⋯F short contacts are less significant, although the presence of many more of these contacts in [1-NBA][BArF4] compared to [1-propane][BArF4] may still play a role in the greater stability of the former.
On this note, the recent synthesis of [1-NBA][S-BArF4] where S-BArF4 is the Mecking anion in which –SF5 groups replace the –CF3 substituents in [BArF4]− is particularly interesting.17 This species provides a robust σ-alkane complex that is geometrically analogous to [1-NBA][BArF4] in the solid state. Moreover, (unlike its [BArF4] congener) it is completely insoluble in alkane solvents, making it an interesting candidate for a heterogeneous SMOM catalyst. The greater number of C–H⋯F short contacts in [1-NBA][S-BArF4] make it an attractive system for comparison with the current study. This will be the topic of future work, along with the application of high-level quantum mechanical calculations to quantify non-covalent interactions in these systems and benchmark the IGMH approach.
3. Conclusions
Non-covalent interactions around the cationic Rh σ-alkane complexes within the solid state structures of [1-NBA][BArF4] and [1-propane][BArF4] have been compared. Geometric and QTAIM analyses highlight a greater number of C–H⋯F contacts around the [1-NBA]+ cation and the shortest of these correspond to the strongest individual atom–atom non-covalent interactions between the cation and anions in these systems. IGMH plots show the high directionality of these C–H⋯F interactions, and that these contrast with the more diffuse nature of C–H⋯π interactions. The accumulative nature of these C–H⋯π interactions that are distributed over many adjacent atom pairs results in these being more significant in the overall array of cation–anion non-covalent interactions. IGMH δGatom plots provide a particularly useful graphic guide to identifying key non-covalent interactions between the cation and anions. An important contribution from –{C3H6}– propylene motifs on both the alkane ligands and the phosphine cyclohexyl substituents is highlighted and these are more significant for [1-NBA][BArF4] than [1-propane][BArF4]. All measures point to greater non-covalent stabilisation between the cation and anions in [1-NBA][BArF4] and this follows the greater stability of this species in the solid state.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the EPSRC for funding via awards EP/W015498/1 and EP/W015552/1.References
- F. M. Chadwick, A. I. McKay, A. J. Martinez-Martinez, N. H. Rees, T. Krämer, S. A. Macgregor and A. S. Weller, Chem. Sci., 2017, 8, 6014–6029 RSC.
- S. D. Pike and A. S. Weller, Philos. Trans. R. Soc., A, 2015, 373, 20140187 CrossRef PubMed.
- K. A. Reid and D. C. Powers, Chem. Commun., 2021, 57, 4993–5003 RSC.
- R. H. Crabtree, Chem. Rev., 1985, 85, 245–269 CrossRef CAS.
- C. Hall and R. N. Perutz, Chem. Rev., 1996, 96, 3125–3146 CrossRef CAS PubMed.
- A. S. Weller, F. M. Chadwick and A. I. McKay, in Advances in Organometallic Chemistry, ed. P. J. Pérez, Academic Press, 2016, vol. 66, pp. 223–276 Search PubMed.
- For a recent example of the characterisation of σ-alkane complexes in solution see: J. D. Watson, L. D. Field and G. E. Ball, J. Am. Chem. Soc., 2022, 144, 17622–17629 CrossRef CAS PubMed.
- S. D. Pike, A. L. Thompson, A. G. Algarra, D. C. Apperley, S. A. Macgregor and A. S. Weller, Science, 2012, 337, 1648–1651 CrossRef CAS PubMed.
- S. D. Pike, F. M. Chadwick, N. H. Rees, M. P. Scott, A. S. Weller, T. Krämer and S. A. Macgregor, J. Am. Chem. Soc., 2015, 137, 820–833 CrossRef CAS PubMed.
- F. M. Chadwick, T. Krämer, T. Gutmann, N. H. Rees, A. L. Thompson, A. J. Edwards, G. Buntkowsky, S. A. Macgregor and A. S. Weller, J. Am. Chem. Soc., 2016, 138, 13369–13378 CrossRef CAS PubMed.
- A. J. Martínez-Martínez, B. E. Tegner, A. I. McKay, A. J. Bukvic, N. H. Rees, G. J. Tizzard, S. J. Coles, M. R. Warren, S. A. Macgregor and A. S. Weller, J. Am. Chem. Soc., 2018, 140, 14958–14970 CrossRef PubMed.
- A. I. McKay, A. J. Martínez-Martínez, H. J. Griffiths, N. H. Rees, J. B. Waters, A. S. Weller, T. Krämer and S. A. Macgregor, Organometallics, 2018, 37, 3524–3532 CrossRef CAS.
- S. K. Furfari, B. E. Tegner, A. L. Burnage, L. R. Doyle, A. J. Bukvic, S. A. Macgregor and A. S. Weller, Chem.–Eur. J., 2021, 27, 3177–3183 CrossRef CAS PubMed.
- F. M. Chadwick, N. H. Rees, A. S. Weller, T. Krämer, M. Iannuzzi and S. A. Macgregor, Angew. Chem., Int. Ed., 2016, 55, 3677–3681 CrossRef CAS PubMed.
- A. I. McKay, A. J. Bukvic, B. E. Tegner, A. L. Burnage, A. J. Martinez-Martinez, N. H. Rees, S. A. Macgregor and A. S. Weller, J. Am. Chem. Soc., 2019, 141, 11700–11712 CrossRef CAS PubMed.
- A. J. Bukvic, A. L. Burnage, G. J. Tizzard, A. J. Martínez-Martínez, A. I. McKay, N. H. Rees, B. E. Tegner, T. Krämer, H. Fish, M. R. Warren, S. J. Coles, S. A. Macgregor and A. S. Weller, J. Am. Chem. Soc., 2021, 143, 5106–5120 CrossRef CAS PubMed.
- L. R. Doyle, E. A. Thompson, A. L. Burnage, A. C. Whitwood, H. T. Jenkins, S. A. Macgregor and A. S. Weller, Dalton Trans., 2022, 51, 3661–3665 RSC.
- T. M. Boyd, B.E. Tegner, G. J. Tizzard, A. J. Martínez-Martínez, S. E. Neale, M. A. Hayward, S. J. Coles, S. A. Macgregor and A. S. Weller, Angew. Chem., Int. Ed., 2020, 59, 6177–6181 CrossRef CAS PubMed.
- A. G. Algarra, A. L. Burnage, M. Iannuzzi, T. Krämer, S. A. Macgregor, R. E. M. Pirie, B. Tegner and A. S. Weller, in 21st Century Challenges in Chemical Crystallography II: Structural Correlations and Data Interpretation, ed. D. M. P. Mingos and P. R. Raithby, Springer International Publishing, Cham, 2020, pp. 183–228 Search PubMed.
- S. A. Macgregor, T. Krämer, F. M. Chadwick and A. S. Weller, Helv. Chim. Acta, 2022, 2023(106), e202200154 Search PubMed.
- Q. Lu, F. Neese and G. Bistoni, Phys. Chem. Chem. Phys., 2019, 21, 11569–11577 RSC.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625–632 CrossRef PubMed.
- T. Lu and Q. Chen, J. Comput. Chem., 2022, 43, 539–555 CrossRef CAS PubMed.
- M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka and M. A. Spackman, CrystalExplorer17, University of Western Australia, 2017, https://hirshfeldsurface.net/ Search PubMed.
- M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 4, 378–392 RSC.
- S. Alvarez, Dalton Trans., 2013, 42, 8617–8636 RSC.
- R. F. W. Bader, Atoms in Molecules, Oxford University Press, 1990 Search PubMed.
- T. A. Keith, AIMAll, 2009–2017, http://aim.tkgristmill.com Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- E. D’Oria and J. J. Novoa, CrystEngComm, 2008, 10, 423–436 RSC.
- V. R. Thalladi, H. C. Weiss, D. Blaser, R. Boese, A. Nangia and G. R. Desiraju, J. Am. Chem. Soc., 1998, 120, 8702–8710 CrossRef CAS.
- P. A. Deck, M. J. Lane, J. L. Montgomery, C. Slebodnick and F. R. Fronczek, Organometallics, 2000, 19, 1013–1024 CrossRef CAS.
- R. Shukla and D. Chopra, CrystEngComm, 2015, 17, 3596–3609 RSC.
- P. Panini, R. G. Gonnade and D. Chopra, New J. Chem., 2016, 40, 4981–5001 RSC.
- H. Omorodion, B. Twamley, J. A. Platts and R. J. Baker, Cryst. Growth Des., 2015, 15, 2835–2841 CrossRef CAS.
- L. J. L. Häller, M. J. Page, S. A. Macgregor, M. F. Mahon and M. K. Whittlesey, J. Am. Chem. Soc., 2009, 131, 4604–4605 CrossRef PubMed.
- J. Ran and M. W. Wong, J. Phys. Chem. A, 2006, 110, 9702–9709 CrossRef CAS PubMed.
- M. Nishio, Phys. Chem. Chem. Phys., 2011, 13, 13873–13900 RSC.
- R. Gopi, N. Ramanathan and K. Sundararajan, Spectrochim. Acta, Part A, 2017, 181, 137–147 CrossRef CAS PubMed.
- R. M. Kumar, M. Elango, R. Parthasarathi, D. Vijay and V. Subramanian, J. Chem. Sci., 2012, 124, 193–202 CrossRef CAS.
- C. Lefebvre, G. Rubez, H. Khartabil, J.-C. Boisson, J. Contreras-García and E. Hénon, Phys. Chem. Chem. Phys., 2017, 19, 17928–17936 RSC.
- C. Lefebvre, H. Khartabil, J.-C. Boisson, J. Contreras-García, J.-P. Piquemal and E. Hénon, ChemPhysChem, 2018, 19, 724–735 CrossRef CAS PubMed.
- A. I. McKay, T. Krämer, N. H. Rees, A. L. Thompson, K. E. Christensen, S. A. Macgregor and A. S. Weller, Organometallics, 2017, 36, 22–25 CrossRef CAS.
- C. Narth, Z. Maroun, R. A. Boto, R. Chaudret, M.-L. Bonnet, J.-P. Piquemal and J. Contreras-García, in Applications of Topological Methods in Molecular Chemistry, ed. E. Alikhani, R. Chauvin, C. Lepetit and B. Silvi, Springer, 2016, vol. 22, pp. 491–527 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full computational details and references, computed Cartesian coordinates, tables and images for QTAIM and IGMH calculations. See DOI: https://doi.org/10.1039/d3fd00009e |
| This journal is © The Royal Society of Chemistry 2023 |