Open Access Article
Open Access ArticleTowards circular fashion – transforming pulp mills into hubs for textile recycling†
Miguel Sanchis-Sebastiá ,
Vera Novy
,
Vera Novy ,
Lars Stigsson,
Mats Galbe
,
Lars Stigsson,
Mats Galbe and
Ola Wallberg
and
Ola Wallberg *
*
Department of Chemical Engineering, Lund University, P. O. Box 124, SE-221 00 Lund, Sweden. E-mail: miguel.sanchis_sebastia@chemeng.lth.se; vera.novy@t-online.de; novafiber@icloud.com; mats.galbe@chemeng.lth.se; ola.wallberg@chemeng.lth.se
First published on 29th March 2021
Abstract
Most waste textiles are currently incinerated or landfilled, which is becoming an increasing environmental problem due to the ever-increasing consumption of textiles in the world. New recycling processes are required to address this problem and, although textile-to-textile recycling would be preferable, many researchers have suggested implementing processes based on the depolymerization of the textile fibers. We suggest integrating textile recycling with pulp mills, which would reduce the cost of depolymerizing the textile fibers and, at the same time, would diversify the product portfolio of the pulp mill, transforming the facility into a true biorefinery. This integration would be based on using green liquor as the pretreatment agent in the textile recycling process, as well as energy integration between the two processes. Na2CO3 was used to identify the conditions under which this pretreatment should be performed. Temperature and residence time proved to be critical in the efficacy of the pretreatment, as suitable values were required to ensure partial solubilization of the waste textiles. The conditioning of the pretreated material also had an important effect on the process, as it ensured a suitable environment for the enzymatic depolymerization while maintaining the changes in the material caused by pretreatment. Pretreatment was then performed with industrial green liquor, showing that the efficiency of textile recycling was about 70% when integrated in a pulp mill.
1. Introduction
Waste textiles are becoming an increasing environmental problem as the fiber consumption per capita has almost tripled over the past six decades,1 and waste textiles are usually landfilled or, in the best case, incinerated for energy recovery.2 New textile recycling processes are needed to address the accumulation of waste textiles, and there are already some facilities recycling waste textiles on a small scale, such as the company Re:newcell in Sweden.3 The textile-to-textile recycling implemented by Re:newcell is preferable as it maintains the value of the fibers. However, several researchers have suggested combining textile-to-textile recycling with end-of-life technologies based on the depolymerization of the fibers to produce valuable fuels or chemicals.2,4,5 This could completely close the fashion loop and reduce the flow of waste textiles to landfills.Cotton fibers can be depolymerized enzymatically, and the resulting glucose solution could then be fermented into different products such as ethanol,6 hydrogen,7 or bacterial cellulose.8 However, enzymes cannot directly depolymerize cotton into glucose as this material has a high degree of polymerization and crystallinity, which makes the cellulose chains inaccessible to the enzymes.9 In fact, cotton fibers have one of the highest molecular weights and structural order among all plant fibers.10 Thus, in most of the studies reported in the literature, technologies based on pretreatment prior to enzymatic hydrolysis have been applied to overcome the high crystallinity of cotton,11–14 in a similar fashion to the techniques used to depolymerize lignocellulosic biomass.
Pretreatment with alkaline solutions is known to reduce the crystallinity of cellulose significantly, through the conversion of cellulose I into cellulose II.15 For this reason, several previous studies have been based on pretreatment with NaOH, usually at low temperatures, to improve the enzymatic digestibility of waste textiles.5,11,12 Although NaOH has been the most widely used alkali, as the pretreatment mechanism is based on the titration of the –OH groups in the cellulose chains,16 other alkaline compounds could be used to pretreat waste textiles. For example, it has been demonstrated that Na2CO3 can also enhance the enzymatic hydrolysis of waste textiles, although to a lesser extent than NaOH.17
Regardless of the alkali chosen, technologies based on pretreatment prior to enzymatic hydrolysis suffer from a fundamental problem when applied to waste textiles: namely, the material does not contain any lignin that can be incinerated to supply the energy required in the process, as in the case of lignocellulosic biomass.18 Thus, novel solutions will be required to reduce the cost of depolymerizing waste textiles in order to implement this technology on a commercial scale.
A possible solution to this problem would be to integrate textile recycling in pulp mills, since these facilities usually produce excess heat that is sold as low-value energy.19 This excess heat could instead be used to cover the energy needs of the textile recycling process, which would reduce the utility cost, and therefore improve the economics of textile recycling. Moreover, green liquor, one of the internal streams in a pulp mill, is rich in alkaline compounds such as Na2CO3, Na2S, and NaOH.20,21 At the beginning of the pulping process, the liquor contains only NaOH and Na2S (in kraft pulping) but Na2CO3, which is subsequently recaustized in order to bring the liquor back to its original form, is formed in the recovery boiler after the combustion of the black liquor. This means that integration could also be implemented in terms of material flows, as green liquor could be used as the alkaline solution for the pretreatment of waste textiles. This could lead to an attractive industrial symbiosis, as textile recycling would in turn help the pulp mill to diversify its portfolio of both feedstocks and products, thus promoting its transformation into a true biorefinery, accomplishing an industrial change that has been widely encouraged in the academic literature.22–24
The aim of this study was to investigate the viability of integrating textile recycling in pulp mills by evaluating the pretreatment of waste textiles with green liquor. Experiments were performed with Na2CO3, the most abundant component in green liquor, to identify the optimal conditions and possible constraints, and to evaluate the efficiency of the process. The Na2CO3 solution was then replaced by industrial green liquor to assess the actual performance of the proposed integration.
2. Materials and methods
2.1. Collection and preparation of waste textiles
The waste textiles used in this study were donated by the authors and other employees at the Department of Chemical Engineering at Lund University. Shirts and T-shirts labelled as 100% cotton were used in the experiments. Buttons, labels, and printed patterns were removed, and the remaining material was cut into pieces measuring approximately 5 × 5 cm. These were then thoroughly mixed to ensure representability of the samples used in the experiments and analyses.2.2. Pretreatment of waste textiles
The general procedure for pretreatment of waste textiles consisted of exposing 40 dry g waste textiles to 760 g of an alkaline solution at a certain temperature for a certain residence time, with occasional stirring during the treatment. The specific conditions at which the pretreatment was performed are described in the following sections, as they were varied differently in several series of experiments. Pretreatment was performed in a 2 L stirred tank reactor (Polyclave, Büchi AG, Switzerland), equipped with a stirrer unit (Cyclone 300, Büchi AG) and a thermostat (Unistat T305, Huber Kältemaschinenbau AG, Germany), and the resulting material was filtered in a filter press (Fischer, Germany). The solid fraction after filtration was used for the subsequent enzymatic hydrolysis experiments as well as for compositional analyses.The citrate buffers were prepared by mixing citric acid monohydrate (Merck, Germany) and sodium citrate dihydrate (VWR, Belgium) in water to final buffer concentrations of 0.1 M, 0.2 M, or 0.3 M, depending on the strategy being tested. After the addition of the citric acid and sodium citrate, small amounts of KOH or H2SO4 were added to the solution to adjust the final pH of the buffer to 5. In experiments were citrate buffer was not used, the pH was adjusted during enzymatic hydrolysis by adding H2SO4 to the reaction vessel, prior to the addition of enzymes, until a pH of 5 was reached.
The pretreated material was subjected to three washing steps in experiments where washing was included in the conditioning strategy. The material was washed with 60 times its weight of water, in the first and third washing steps, whereas the same amount of 0.05 M HCl was used in the second washing step. The washing liquid was removed by vacuum filtration between each washing step. In the experiments where drying was applied in combination with washing, the washed material was dried at room temperature for approximately 24 h.
Finally, experiments were conducted with industrial green liquor containing a combination of different alkaline compounds. The green liquor was sourced from the pulp mill operated by Södra in Mörrum, in Southern Sweden, and its composition was: 95.8 ± 2.5 g L−1 Na2CO3, 49.5 ± 4.3 g L−1 Na2S, and 12.0 ± 2.8 g L−1 NaOH. The composition of the green liquor was determined using the SCAN-N 30:85 methodology.25 The green liquor was diluted prior to use so that the concentration of Na2CO3 was similar to that in the experiments with model solutions.
2.3. Enzymatic hydrolysis
Enzymatic Hydrolysis (EH) experiments were performed at 50 °C for 96 h in 50 mL Falcon tubes with a working mass of 20 g. A solids loading of 5% and Cellic CTec 2 (Novozymes, Denmark) enzyme cocktail at a loading of 0.15 g enzymes per g solids (approximately 30 FPU per g solids) were used in these experiments, so that the yield from the enzymatic hydrolysis of untreated waste textiles (negative control) would be similar to those reported previously in the literature.11,12,17 The hydrolysis experiments were performed in a combi-H12 hybridization incubator (FinePCR, South Korea), which maintained the temperature and mixing during the experiment.Samples obtained from the enzymatic hydrolysis experiments were centrifuged in 2 mL Eppendorf tubes at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The supernatant was filtered through 0.2 μm syringe filters (GVS North America, Sanford, USA) and stored at −20 °C until HPLC analysis. Glucose was analyzed using a Shimadzu LC-20 AD HPLC system equipped with a Shimadzu RID 10A refractive index detector (Shimadzu Corporation, Kyoto, Japan). The chromatography column used was a CarboSep CHO 782 column (Concise Separations, San Jose, CA, USA) with a De-Ashing Bio-Rad Micro-Guard column at 80 °C, using reagent grade water as eluent at a flow rate of 0.6 mL min−1.
000 rpm for 5 min. The supernatant was filtered through 0.2 μm syringe filters (GVS North America, Sanford, USA) and stored at −20 °C until HPLC analysis. Glucose was analyzed using a Shimadzu LC-20 AD HPLC system equipped with a Shimadzu RID 10A refractive index detector (Shimadzu Corporation, Kyoto, Japan). The chromatography column used was a CarboSep CHO 782 column (Concise Separations, San Jose, CA, USA) with a De-Ashing Bio-Rad Micro-Guard column at 80 °C, using reagent grade water as eluent at a flow rate of 0.6 mL min−1.
2.4. Compositional analysis
Glucose and degradation by-products generated in the liquid samples during the cellulose analysis, were analyzed using the same HPLC system as described above. However, the chromatography column used was an Aminex HPX-87H, with a Cation-H Bio-Rad MicroGuard column (Bio-Rad Laboratories, Hercules, CA, USA) at 50 °C, using a 5 mM sulfuric acid solution as eluent at a flow rate of 0.5 mL min−1.
Glucose and degradation by-products generated in the liquid samples in these analyses were analyzed using the same HPLC system, chromatography column and conditions as those used for the analysis of raw textiles. These analyses were also performed in triplicate.
2.5. Enzyme adsorption measurements
The accessibility of pretreated waste textiles to the enzymes was estimated through enzyme adsorption tests. These tests were performed under the same conditions as the enzymatic hydrolysis experiments, but the preparations were incubated in an ice bath for 6 h, in constant agitation with a magnetic stirrer. After the incubation time, the concentration of unbound proteins was measured in the liquid after vacuum filtration of the preparation. The concentration of unbound proteins was measured by incubating the samples with ready-to-use Bradford reagent (Sigma Aldrich, USA) for 5 min, measuring the absorbance at 595 nm and determining the protein concentration against an external BSA standard.2.6. CLSM imaging
Confocal Laser Scanning Microscopy (CLSM) imaging was performed with a Nikon Ti-E/A1+ (Nikon Instruments Inc., USA) equipped with a 20× (air, N.A. 0.75) and a 60× (oil, N.A. 1.4) objective. For imaging, the fibers were mounted on the objective slide and covered in ∼10 μL of calcofluor solution (#18909, Sigma-Aldrich, USA). After 5 min incubation in the dark, a drop of 10% (w/v) KOH was added, and the cover slide sealed with nail polish. Imaging was performed with a 409 nm fixed wavelength laser with an emission collection range of 425 to 475 nm. The laser power was kept constant for all images, where the imaging settings (i.e., gain and offset) were adjusted to maximize the dynamic range of the detector. Each micrograph presented herein is a 2D maximum intensity projection of a 3D z-stack consisting of ∼10 (20×) or ∼20 (60×) optical slices. ImageJ (Fiji28) was used as processing software. 2 specimens were prepared for each fiber type and after manual screening of each specimen, 2 to 4 images were taken. Overall, the fibers were homogenous, and the micrographs shown herein are representative of the entire specimen.2.7. Yield calculations
Cellulose losses resulting from alkaline pretreatment were calculated based on the amount of cellulose remaining in the solid material recovered after the pretreatment and the amount of cellulose available in the starting material. The enzymatic hydrolysis yield was based on the amount of glucose available in the pretreated textiles, which corresponds to 1.11 times the amount of cellulose contained in the material, due to the addition of water during the hydrolysis reaction.3. Results and discussion
3.1. Composition of the waste textiles
The waste textiles contained 93.0% cellulose (standard deviation 2.0%) and 4.4% non-cellulosic residue (standard deviation 1.2%), prior to alkaline pretreatment. This result is reasonable, given that virgin cotton usually has a cellulose content between 88% and 96%; 94% being the most typical value.29 Although non-cellulosic components present in virgin cotton, such as proteins, waxes, or pectins, might be removed during its processing,30 it has been shown that non-cellulosic components nevertheless account for approximately 4% of processed cotton fibers.31 It was therefore considered that the composition determined here was reliable for the determination of cellulose losses in the pretreatment step, as well as the yield from enzymatic hydrolysis.The composition of the waste textiles after pretreatment can be found in ESI File 1 (Tables 1–5†), but no further discussion is included here since alkaline pretreatment causes mainly structural changes in cellulose, rather than changes in its chemical composition.15 The discussion in the following sections thus concerns the yield from enzymatic hydrolysis obtained after each set of pretreatment conditions, which provides an indication of the accessibility of the cellulose to the enzymes and can therefore be interpreted as a measure of the pretreatment efficacy.
3.2. Conditioning of pretreated textiles
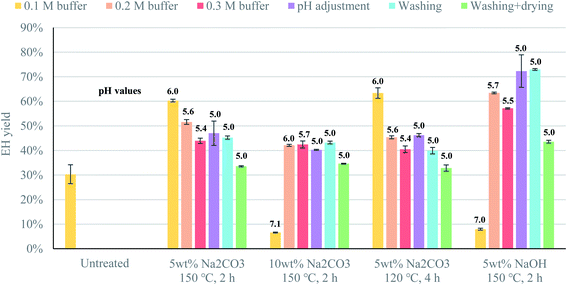
Conditioning of the pretreated material prior to enzymatic hydrolysis had a strong effect on the efficiency of enzymatic saccharification (Fig. 1). For example, drying the material had a marked negative effect on enzymatic hydrolysis, as the materials that were washed and then dried yielded the lowest glucose production in all cases (Fig. 1). In its hydrated form, cellulose II is an inflated structure, due to water molecules intercalated between the cellulose chains, but when the material is dried, the structure shrinks due to the removal of the water molecules, reducing the accessibility of the cellulose chains to the enzymes.32,33 In spite of this, the material was dried as part of the conditioning of the pretreated textiles in some previous studies.11,12 The yields reported in those studies may, therefore, be underestimated, and greater attention should be paid to the effect of conditioning on the enzymatic depolymerization process.Washing the pretreated material without subsequent drying resulted in a similar enzymatic hydrolysis yield to that when adjusting the pH prior to the addition of the enzymes (Fig. 1). This means that similar and higher yields could be achieved without washing by adjusting the pH prior to enzyme additions. Thus, the goal of conditioning is to establish favorable conditions for the enzymes to act, rather than to remove residual compounds from the alkaline solution used in the pretreatment.
Adjusting the pH prior to the addition of the enzymes seemed to have a different effect depending on the strength of the alkaline solution. For the strongest bases (10% Na2CO3 and 5% NaOH), the best yield was obtained when enzymatic hydrolysis was performed at the optimal pH of the enzymes, that is, a pH of 5 (Fig. 1). However, for the weakest base (5% Na2CO3), higher yields were obtained when enzymatic hydrolysis was performed at a pH of 6 (Fig. 1). The reason for this might be that the strong acid used for pH adjustment neutralizes the titration effect of the weak base, reversing the structural changes that took place during pretreatment, thus reducing the accessibility of the cellulose chains to the enzymes, even though the high ionic strength of the solution might also play a role in the efficiency of the saccharification.
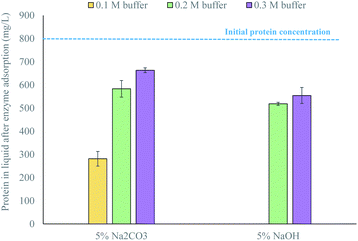
Additional enzyme adsorption tests showed that enzymes adsorbed differently to waste textiles pretreated with 5% Na2CO3 depending on the citrate buffer used to perform the enzymatic hydrolysis, while they adsorbed approximately the same to waste textiles pretreated with 5% NaOH (Fig. 2). This indicates that the conditioning of the pretreated material can revert the effect of pretreatment, but only when a weak alkali is used. Thus, if green liquor were to be used as a pretreatment agent, the accessibility of the material to the enzymes would depend on the conditions of pretreatment as well as the conditioning of the pretreated material prior to enzymatic hydrolysis.
Although adjusting the pH might reduce the accessibility of the cellulose in some cases, it would not be possible to carry out enzymatic hydrolysis without adjusting the pH to some extent, as the enzymes lost their activity at pH 7 (Fig. 1). This means that, if green liquor were to be used as the pretreatment agent, it is likely that setting the pH at a suboptimal value might benefit enzymatic hydrolysis, since there is a trade-off between high enzyme activity and maintaining the structural changes in the cotton fibers resulting from pretreatment. For this reason, it was decided to perform all subsequent experiments using 0.1 M citrate buffer, in order to ensure a pH of approximately 6 during enzymatic hydrolysis.
3.3. Influence of pretreatment conditions
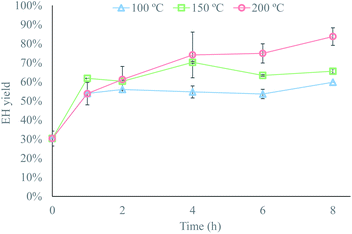
Once the best conditioning strategy had been established, the effects of temperature and residence time were investigated to determine the conditions that provided a material susceptible to enzymatic hydrolysis. The highest enzymatic hydrolysis yield achieved at 100 and 150 °C was about 60–65%, while a yield of over 80% was achieved at 200 °C (Fig. 3). The improved enzymatic hydrolysis yield compared to untreated textiles (30% EH yield) arise probably from organizational changes at the macrofibril level, caused by the titration of the hydroxyl groups in cellulose.16 These organizational changes likely reduced the degree of organization of microfibrils in the microfibril, and of the microfibril in the visible fiber. As a result, a less ordered arrangement was created, which probably increased the cellulose accessibility to enzymes and therefore aided the enzymatic hydrolysis of the pretreated fibers.The reason for the higher yield observed at 200 °C might be that, in an alkaline environment, degradation reactions can only take place above 170 °C.34 These reactions would generate random scission of the glycosidic bonds, releasing short-chain molecules into the solution, which would create new attack sites for the enzymes and therefore improve the accessibility to the enzymes.34 Moreover, fiber fragmentation, induced by degradation of the more reactive paracrystalline cellulose regions,33 leads to higher surface-to-bulk-ratios that, together with the increase in attack sites, aid the enzymatic hydrolysis. In spite of this, the fact that the best yield was obtained at the highest temperature and longest residence time would limit the size of the textile recycling plant, since the amount of excess heat available in the pulp mill is limited.
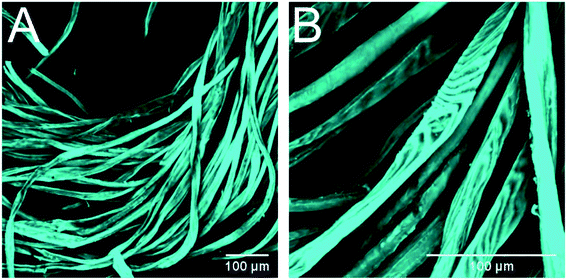
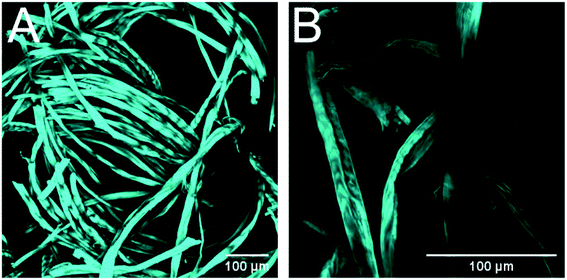
CLSM imagining was used to confirm the morphological and organizational changes caused by alkaline pretreatment. It was observed that the raw fibers were very smooth, with blunt ends, and were arranged tightly with each other (Fig. 4). On the contrary, the fibers treated with Na2CO3 exhibited a less ordered arrangement, with a larger distance between the fibers, and shorter fiber fragments were observed in the material as well as interfibrillar damages that allowed to observe macrofibrils at the end of the fibers (Fig. 5). These images confirm that the material treated with Na2CO3 presents many more attack sites for the enzymes, leading to an increased enzyme accessibility and therefore a higher enzymatic hydrolysis yield.
These degradation reactions had another effect on the pretreatment, which would have negative effects on the textile recycling process. Part of the cellulose contained in the waste textiles solubilized during pretreatment, which would be accounted for as a cellulose loss in the recycling process. For example, cellulose losses of about 3.5–6% were observed at 100 and 150 °C, which could be attributed to the mass losses due to experimental error, while cellulose losses of 15% and 20% were observed following pretreatment at 200 °C for 6 and 8 h, respectively. These losses confirm partial solubilization of the material during pretreatment, but they also indicate that the degradation reactions only take place after a contact time between the waste textiles and the alkaline solution of at least 6 h, as the cellulose losses at 200 °C were negligible for shorter residence times.
In spite of the cellulose losses, the overall yield of the process was higher at 200 °C than at 100 °C or 150 °C. The highest overall yield achieved at 200 °C was approximately 70%, while the overall yield at 100 or 150 °C, which is the same as the enzymatic hydrolysis yield since there were no cellulose losses at these conditions, never exceeded 65%. This shows that degradation reactions during pretreatment are beneficial to the process, to some extent, as the improved accessibility of the material to the enzymes compensated for the cellulose losses. Thus, the design of the alkaline pretreatment step consists of identifying the conditions under which a suitable degree of degradation reactions takes place, so that the improved accessibility to the enzymes counterbalances the cellulose losses.
3.4. Pretreatment with several alkaline species
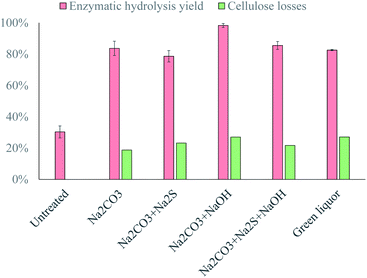
The addition of sodium sulfide to the alkaline solution had a negative impact on the process, as the yield following enzymatic hydrolysis decreased from 84% to 79% (Fig. 6). The problem associated with sodium sulfide is that, although the protonation of S2− is almost immediate,25 HS− is a very weak base, which would contribute little to the titration of the cellulose chains. Although HS− makes a small contribution to the titration, a second Na+ ion is added to the solution because of it, which has a negative effect on the pretreatment due to the formation of cellulose–Na complexes,15 which have been reported to cause the gelation of cellulose solutions,16 and would thus reduce the accessibility of the material to the enzymes. It therefore appears that the negative effect of introducing additional Na+ outweighs the increased titration capacity when Na2S is added to the alkaline solution.In contrast, the addition of NaOH increased the enzymatic hydrolysis yield from 84% to 98% (Fig. 6), which again demonstrates the important role of the strength of the base in the alkaline pretreatment. The OH− ions were the strongest base added to the alkaline solution, which explains the improvement in the pretreatment efficacy, as these ions would significantly increase the extent to which the –OH groups in the cellulose chains are titrated.
The simultaneous addition of Na2S and NaOH resulted in a similar enzymatic hydrolysis yield to that obtained with pure Na2CO3 (Fig. 6). This implies that the negative effect of Na2S was compensated for by the positive effect of NaOH, and it could therefore be expected that the efficacy of the pretreatment would be approximately the same with green liquor as with a pure Na2CO3 solution. However, the amount of Na2S added to the alkaline solution was more than 4 times the amount of NaOH added, which shows that NaOH had a greater effect on the pretreatment than Na2S.
Pretreatment with the green liquor resulted in a similar yield to that obtained with pure Na2CO3 (Fig. 6), as was expected from the experiments with model solutions, but the cellulose losses were slightly higher than expected (27% instead of 21%). The higher cellulose losses could be attributed to the higher concentration of NaOH in the green liquor, which would increase the extent of the degradation reactions during pretreatment. Nevertheless, the overall yield was approximately the same as with pure Na2CO3; thus, the efficiency of textile recycling would be about 70% when this process is integrated with a pulp mill.
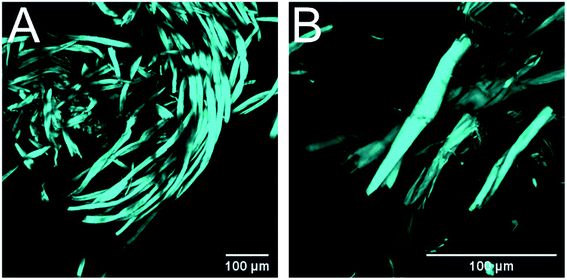
The material pretreated with green liquor was examined with CLSM and similar effects to those exerted by Na2CO3 pretreatment were observed: shorter fiber fragments, peeling reactions (dethreading) and increased surface to bulk ratio (Fig. 7). This proves that green liquor modifies the textile fibers in a similar fashion than pure Na2CO3, although to a larger extent. It seems that the images display increased extent of the degradation reactions during pretreatment with green liquor, which would explain the higher cellulose losses registered in this treatment.
The composition of the green liquor was changed during pretreatment although the concentrations of Na2S and Na2CO3 remained the same (49.3 ± 1.1 g L−1 Na2S and 93.1 ± 0.6 g L−1 Na2CO3 after pretreatment). However, the concentration of NaOH in the solution was negligible, which means that this component was completely consumed during pretreatment. This, together with the fact that some liquid was retained in the pretreated textiles, resulted in a sodium loss of 15% in pretreatment, which means that less sodium would be returned to the pulp mill. It would thus be necessary to compare the economic impact of this sodium loss with the revenue from textile recycling to ascertain the economic viability of the proposed integration.
The sodium loss estimated based on the titration of the green liquor might be an overestimate of the actual sodium loss during pretreatment as an additional inflexion point was observed in the titration curve when analyzing the green liquor after pretreatment (Fig. 8). This inflexion point suggests the presence of an additional extremely weak base (weaker than HS−) in the solution, which could be the short-chain molecules generated in the degradation reactions. Since these molecules could establish ionic bonds with sodium, the presence of this base would reclaim sodium from the pretreatment, as the charge balance in the solution must be maintained, which implies that a higher amount of sodium would be returned to the pulp mill. Thus, the partial solubilization of the waste textiles during pretreatment, which translated into a cellulose loss that affected textile recycling negatively, would have a positive impact on the operation of the pulp mill, as more sodium is maintained in the alkaline solution.
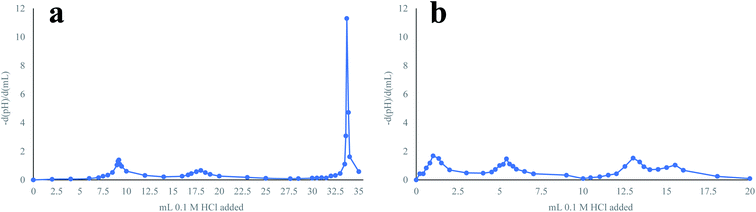 | ||
| Fig. 8 Titration curves for the green liquor before (a) and after (b) pretreatment. The titration curves are presented as the first derivatives of the pH. | ||
4. Conclusions
This study shows that it would be possible to integrate textile recycling with pulp mills, by utilizing green liquor as the alkaline solution in the pretreatment of the textiles. An efficiency of about 70% could be expected in the textile recycling process, while sodium losses in the pulping process would be below 15%.It was observed that the conditioning of the pretreated textiles had a significant impact on the efficiency of the process. Some conditioning strategies might reverse the changes that the material underwent during pretreatment, and it was concluded that, when green liquor is used as the alkaline solution, it might be beneficial to maintain the pH as high as the enzymes allow. The temperature and residence time also played an important role; temperatures of at least 200 °C and residence times longer than 6 h were required to partially dissolve the textiles through degradation reactions, which was necessary to achieve high enzymatic hydrolysis yields.
The effects of Na2S and NaOH in green liquor counterbalance each other, and other impurities from the pulping process did not interfere with the pretreatment of the textiles. Thus, the same efficacy could be expected from a pretreatment process with green liquor, as from a pure Na2CO3 solution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the employees at the Department of Chemical Engineering, Lund University for their contribution to the collection of raw material for this study. This research did not receive any funding from external sources.References
- Food and Agriculture Organization of the United Nations (FAO) and International Cotton Advisory Committee (ICAC), A summary of the world apparel fiber consumption survey 2005–2008, 2011 Search PubMed.
- N. Pensupa, S.-Y. Leu, Y. Hu, C. Du, H. Liu, H. Jing, H. Wang and L. Carol Sze Ki, in Chemistry and Chemical Technologies in Waste Valorization, ed. C. S. K. Lin, Springer International Publishing, Cham, 2018, pp. 189–228, DOI:10.1007/978-3-319-90653-9_7.
- Re:newcell AB, Re:newcell. We make fashion sustainable, https://renewcell.com/, accessed July 2020 Search PubMed.
- G. M. Peters, G. Sandin and B. Spak, ACS Sustainable Chem. Eng., 2019, 7, 11682–11690 CrossRef CAS.
- A. Palme, A. Peterson, H. de la Motte, H. Theliander and H. Brelid, Text. Cloth. Sustain., 2017, 3, 4 CrossRef.
- A. Jeihanipour, K. Karimi, C. Niklasson and M. J. Taherzadeh, Waste Manag., 2010, 30, 2504–2509 CrossRef CAS PubMed.
- C.-Y. Chu, S.-Y. Wu, C.-Y. Tsai and C.-Y. Lin, Int. J. Hydrogen Energy, 2011, 36, 8743–8750 CrossRef CAS.
- F. Hong, X. Guo, S. Zhang, S.-f. Han, G. Yang and L. J. Jönsson, Bioresour. Technol., 2012, 104, 503–508 CrossRef CAS.
- T. Jeoh, C. I. Ishizawa, M. F. Davis, M. E. Himmel, W. S. Adney and D. K. Johnson, Biotechnol. Bioeng., 2007, 98, 112–122 CrossRef CAS PubMed.
- Y. L. Hsieh, in Cotton, ed. S. Gordon and Y. L. Hsieh, Woodhead Publishing, 2007, pp. 3–34, DOI:10.1533/9781845692483.1.3.
- A. Jeihanipour and M. J. Taherzadeh, Bioresour. Technol., 2009, 100, 1007–1010 CrossRef CAS PubMed.
- E. Gholamzad, K. Karimi and M. Masoomi, Chem. Eng. J., 2014, 253, 40–45 CrossRef CAS.
- Y. Ma, B. Zeng, X. Wang and N. Byrne, ACS Sustainable Chem. Eng., 2019, 7, 11937–11943 CAS.
- C.-H. Kuo, P.-J. Lin and C.-K. Lee, J. Chem. Technol. Biotechnol., 2010, 85, 1346–1352 CrossRef CAS.
- E. Sjöström, Wood chemistry: fundamentals and applications, Academic Press, 2nd edn, 1993 Search PubMed.
- M. Gubitosi, P. Nosrati, M. Koder Hamid, S. Kuczera, M. A. Behrens, E. G. Johansson and U. Olsson, R. Soc. Open Sci., 2017, 4, 170487 CrossRef PubMed.
- E. Hasanzadeh, S. Mirmohamadsadeghi and K. Karimi, Fuel, 2018, 218, 41–48 CrossRef CAS.
- E. Joelsson, D. Dienes, K. Kovacs, M. Galbe and O. Wallberg, Sustainable Chem. Processes, 2016, 4, 14 CrossRef.
- A. Erik, R. O. Marcus and B. Thore, Nord. Pulp Pap. Res. J., 2006, 21, 466–475 CrossRef.
- A. Wensley and P. Champagne, presented in part at the CORROSION 99, San Antonio, Texas, 1999 Search PubMed.
- B.-H. Um and G. P. van Walsum, Appl. Biochem. Biotechnol., 2009, 153, 127–138 CrossRef CAS PubMed.
- S. González-García, A. Hospido, R. Agnemo, P. Svensson, E. Selling, M. T. Moreira and G. Feijoo, J. Ind. Ecol., 2011, 15, 568–583 CrossRef.
- R. Fornell, T. Berntsson and A. Åsblad, Energy, 2013, 50, 83–92 CrossRef CAS.
- M. Moshkelani, M. Marinova, M. Perrier and J. Paris, Appl. Therm. Eng., 2013, 50, 1427–1436 CrossRef CAS.
- Scandinavian Pulp Paper and Board Testing Comittee, Total, active and effective alkali, Stockholm, Sweden, 1985 Search PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton D. Crocker and C. O. Golden, Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory, 2008 Search PubMed.
- International Organization for Standardization (ISO), Textiles – Quantitative chemical analysis – Part 1: General principles of testing (ISO 1833-1:2006, including Cor 1:2009), 2010 Search PubMed.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J. Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Nat. Methods, 2012, 9, 676–682 CrossRef CAS PubMed.
- E. R. McCall and J. F. Jurgens, Text. Res. J., 1951, 21, 19–21 CrossRef CAS.
- R. Freytag and J.-J. Donzé, Chemical Processing of Fibres and Fabrics, Fundamentals and Preparation, Part A, Handbook of Fiber Science and Technology, Marcel Dekker, NY, 1983, vol. 1 Search PubMed.
- J. Buchert, J. Pere, L. S. Johansson and J. M. Campbell, Text. Res. J., 2001, 71, 626–629 CrossRef CAS.
- M. Wada, M. Ike and K. Tokuyasu, Polym. Degrad. Stab., 2010, 95, 543–548 CrossRef CAS.
- V. Novy, K. Aïssa, F. Nielsen, S. K. Straus, P. Ciesielski, C. G. Hunt and J. Saddler, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 22545–22551 CrossRef CAS.
- P. J. Wakelyn, N. R. Bertoniere, A. D. French, D. P. Thibodeaux, B. A. Triplett, M.-A. Rousselle, W. R. Goynes, J. V. Edwards, L. Hunter, D. D. McAlister and G. R. Gamble, in Handbook of Fiber Chemistry, ed. M. Lewin, CRC Press, Boca Raton, FL, USA, 2006, pp. 607–608 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00168j |
| This journal is © The Royal Society of Chemistry 2021 |