Open Access Article
Open Access ArticleHigh coercivity Pr2Fe14B magnetic nanoparticles by a mechanochemical method
Xiaoyun Shanga,
Haoran Tub,
Jingjing Zhang *a,
Bingying Nia,
Liying Wang
*a,
Bingying Nia,
Liying Wang a,
Minggang Wanga,
Chen Wua and
Zhankui Zhao*a
a,
Minggang Wanga,
Chen Wua and
Zhankui Zhao*a
aCollege of Material Science and Engineering, Key Laboratory of Advanced Structural Materials, Ministry of Education, Changchun University of Technology, Changchun 130012, China. E-mail: zhangjj@ccut.edu.cn
bKey Laboratory of Physics and Technology for Advanced Batteries (Ministry of Education), Department of Physics, Jilin University, Changchun 130012, China
First published on 29th March 2021
Abstract
Nd2Fe14B nanoparticles are widely used because of their outstanding hard magnetic properties. In fact, Pr2Fe14B has higher magneto-crystalline anisotropy than Nd2Fe14B, which makes Pr-Fe-B a promising magnetic material. However, the chemical synthesis route to Pr2Fe14B nanoparticles is challenging because of the higher reduction potential of Pr3+, as well as the complex annealing conditions. In this work, Pr2Fe14B nanoparticles were successfully synthesized via an efficient and green mechanochemical method consisting of high energy ball milling, annealing, and a washing process. Microstructural investigations revealed that the oxide precursors were uniformly wrapped by CaO and CaH2, which formed an embedded structure after ball milling. Then, Pr2Fe14B powder was synthesized via a time-saving annealing process. The impact of the Pr2O3 content and the preparation conditions was investigated. The coercivity of the as-annealed powder with 100 wt% Pr2O3 excess is 18.9 kOe. After magnetic alignment, the coercivity, remanence, and maximum energy product were: 9.8 kOe, 78.4 emu g−1, and 9.8 MGOe, respectively. The present work provides a promising strategy for preparing anisotropic Pr-Fe-B permanent magnetic materials.
Introduction
Magnetic materials have been broadly applied in many industrial fields, including information storage, electronic devices, aerospace, energy generation, hybrid vehicles, wind power generators and so on.1–3 Rare earth transition metal (RE-TM) permanent magnets, especially Nd2Fe14B, have gained considerable attention and are commonly applied because they are a high energy product.4–6 The Nd2Fe14B is one of the rare earth magnetic materials, found by Sagawa et al.7 Because of the higher magneto-crystalline anisotropy of Dy2Fe14B (HA = 158 kOe) and Tb2Fe14B (HA = 220 kOe) compared to that of Nd2Fe14B (HA = 67 kOe),3,8 partial substitution of Dy and Tb for Nd in Nd–Fe–B magnets has been proved to be a convenient method to raise coercivity (Hc).7–11 However, the addition of Dy and Tb will decrease the magnetization and magnetic energy product due to the ferrimagnetic coupling between the rare earth atom and Fe and increases the cost due to the scarcity of Dy/Tb resources. Therefore, developing a high-coercivity Nd2Fe14B magnetic material without Dy/Tb is becoming a hot topic in the permanent magnetic materials field. Compared to Nd2Fe14B, Pr2Fe14B with a higher magneto-crystalline anisotropy (HA = 87 kOe) is a promising hard magnetic material with high coercivity.12The majority of the research on the fabrication of the Nd–Fe–B magnets has centered on physical methods, including powder metallurgy sintering,13 HDDR,14 melt-spinning,15,16 high-energy ball-milling,17,18 and so on. Chemical methods, for example, microwave combustion,3,21 sol–gel19,20 and thermal decomposition have been applied in the preparation of Nd2Fe14B.19 Compared with physical methods, a chemical method has distinct advantages in regulating size and morphology, which have a significant effect on the magnetic properties. The chemical synthesis used oxides as the precursors, which were reduction-diffused by CaH2 or Ca to form Nd2Fe14B.20–22 It is reported that the holding time during the annealing of the chemical synthesis method is generally greater than 90 min, which increased the energy loss and prolonged the production cycle so that limited the application in industrial production.23–25 Recently, an efficient and green synthesis method, a mechanochemical method, has been used to synthesize Nd2Fe14B magnetic nanoparticles.26–30 The mechanistic method can promote the reaction between solids quickly and quantitatively, without adding solvent or adding only a nominal amount of solvent.
In this study, anisotropic Pr2Fe14B magnetic powder with a high coercivity is synthesized by the simple mechanochemical method for the first time.31–33 The reducing agent, CaH2, was selected to reduce the Pr2O3, Fe2O3, and B2O3. The precursor samples were mixed uniformly and wrapped with CaH2 after the high energy ball milling, which contributed to the completed reduction of the as-milled oxide in the process of annealing. In this work, the optimal ball milling time, Pr2O3 content as well as annealing conditions were determined by comparing the magnetic properties. Meanwhile, the transition of the phases and microstructure were systematically investigated to reveal the reaction process.
Experimental
Materials
The precursors were commercially available and used without further purification: Pr2O3 (99.9%, Adamas-beta), and B2O3 (99.9%, Aladdin) powders, Fe2O3 (99.9%, Aladdin), and CaH2 granules (99.9%, Aladdin), NH4Cl (99.9%, Sigma-Aldrich), and methanol (99.99%, Sigma-Aldrich).Synthesis
The fabrication process of the Pr2Fe14B magnetic materials is demonstrated in Scheme 1. After milling for 2–5 h, the as-milled samples were annealed at 850 °C for 5 min in a glove box under an argon (Ar) atmosphere. The relative contents of Pr2O3, Fe2O3, B2O3 and were determined from the equation:| Pr2O3 + 7Fe2O3 + 0.5B2O3 + 25.5CaH2 → Pr2Fe14B + 25.5CaO + 25.5H2↑ |
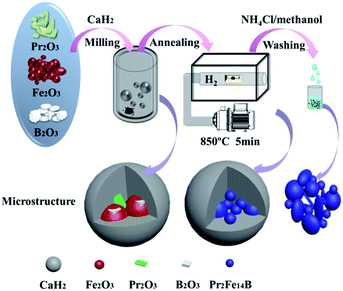 | ||
| Scheme 1 A schematic diagram of the fabrication process of Pr2Fe14B-based nanoparticles and their microstructure. | ||
The CaH2 acts as the reducing agent. The weight ratio of the preliminary reduction of Pr2O3 powders to CaH2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to ensure full reduction. A portion of the mixture (2 g) was added to a stable steel vial under an Ar atmosphere. Because of the evaporation losses, an excess of 50 wt%, 100 wt% and 150 wt% Pr2O3 were added (denoted as W50%, W100% and W150%). The ball milling was carried out using an 8000 M ball mill (SPEX Sample Prep) with a ball-to-powder ratio of 10
1.5 to ensure full reduction. A portion of the mixture (2 g) was added to a stable steel vial under an Ar atmosphere. Because of the evaporation losses, an excess of 50 wt%, 100 wt% and 150 wt% Pr2O3 were added (denoted as W50%, W100% and W150%). The ball milling was carried out using an 8000 M ball mill (SPEX Sample Prep) with a ball-to-powder ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, from 2 h to 5 h. The milling time was a key parameter and its influence on the phase transitions was investigated carefully. Subsequently the as-milled powders of 200 mg were annealed from room temperature to the target temperature in the RTP-1200 vacuum heating furnace. All the manipulations of the samples were performed in a LS800S glove box (Dellix, China) filled with a high purity Ar atmosphere. After agitating ultrasonically three times in a NH4Cl/methanol solution, the annealed samples were cleaned for 10 min. The NH4Cl/methanol solution was provided by dissolving 2 g of NH4Cl in 100 ml of methanol. The NH4Cl/methanol washing system was used, because CaO and NH4Cl are able to react to generate NH3 and CaCl2 which dissolve clearly in methanol solution with very little heat generation.26
1, from 2 h to 5 h. The milling time was a key parameter and its influence on the phase transitions was investigated carefully. Subsequently the as-milled powders of 200 mg were annealed from room temperature to the target temperature in the RTP-1200 vacuum heating furnace. All the manipulations of the samples were performed in a LS800S glove box (Dellix, China) filled with a high purity Ar atmosphere. After agitating ultrasonically three times in a NH4Cl/methanol solution, the annealed samples were cleaned for 10 min. The NH4Cl/methanol solution was provided by dissolving 2 g of NH4Cl in 100 ml of methanol. The NH4Cl/methanol washing system was used, because CaO and NH4Cl are able to react to generate NH3 and CaCl2 which dissolve clearly in methanol solution with very little heat generation.26
The sample components were determined by X-ray diffraction (XRD) on a D/max 2550 instrument (Rigaku) with Cu-Kα radiation. The elemental content and microstructure were determined using field emission scanning electron microscopy on a Supra 40/Gemini column instrument (Zeiss) with energy dispersive spectroscopy (EDS) detection and transmission electron microscopy (TEM) on a Talos F200S (FEI). The aligned sample was prepared as follows: the final powder was agitated ultrasonically in a methanol solvent and then placed in a 1 T magnetic field. After the methanol solvent had evaporated, the powder was solidified by addition of epoxy. Prior to magnetic measurement, a 9 T pulsed magnetic field was used to magnetize the sample to saturation. The final magnetic properties were measured by a vibrating sample magnetometer, VSM-7410 (VSM, Lake Shore Cryotronics) and the largest magnetic field was 2.8 T at 300 K.
The magnetization M and maximum energy product (BH)max of the powders were calculated using the density of a 100%-dense block material, and it was assumed that the magnets synthesized from the magnetic powders behaved similarly in the magnetic field. The sample density measured by the Archimedes principle was about 7.5 mg cm−1.32,34
Results and discussion
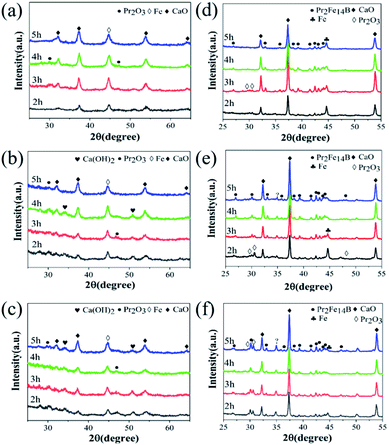
The XRD patterns of the as-milled samples with different Pr2O3 contents are shown in Fig. 1(a)–(c). The Fe2O3 vanished and CaO, Ca(OH)2 and α-Fe were observed, meaning that the Fe2O3 has been reduced to α-Fe by CaH2. The Pr2O3 exists, especially in a sample with short milling time, suggesting that it is hard to reduce Pr2O3. The reduction potential of Fe2O3 and Pr2O3 were Fe3+/Fe (−0.44 eV) and Pr3+/Pr (−2.47 eV).26,35 Therefore, Fe2O3 was reduced to α-Fe during high energy ball milling whereas Pr2O3 was seldom reduced. The Ca(OH)2 is observed in Fig. 1(a)–(c). The formation of Ca(OH)2 is due to the decomposition of CaH2 being initially excessive, and it can easily convert into Ca(OH)2 upon exposure to air.36After annealing, the α-Fe and Pr2O3 phases gradually decreased and Pr2Fe14B was observed (Fig. 1(d)–(f)), suggesting that the Pr2O3 was reduced during the annealing and then reacted with Fe and B to produce Pr2Fe14B. The B2O3 was reduced during the high energy ball milling, but it was difficult to observe in the XRD patterns due to the small amount. The reduction of Pr2O3 and the formation of Pr2Fe14B were promoted by the ball milling. For W50%, the relative content of α-Fe increased abnormally when milled for 5 h, which should be attributed to the insufficiency of Pr2O3 and hence, the Pr content, caused by the loss of Pr during the long time of ball milling. No α-Fe appeared in the samples with more Pr2O3 (W100% and W150%) and the long milling time, suggesting that the Pr2O3 content was sufficient.
The magnetization hysteresis loops of the as-milled powder (Fig. 2(a)) show the existence of abundant soft magnetic α-Fe phases, which is in agreement with the XRD results in Fig. 1(a). The VSM results in Fig. 2(a) show that the coercivity was about 500 Oe, which may be the reason for the existence of trace amounts of Pr2Fe14B during the ball milling process.
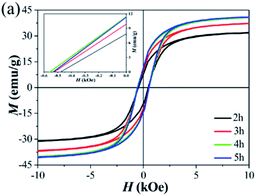 | ||
| Fig. 2 Magnetization hysteresis loops of (a) an as-milled Pr2O3 sample (W50%). The inset shows the corresponding magnified second quadrant of these M–H curves. | ||
The demagnetization curves of the as-annealed samples are presented in Fig. 3(a)–(c). With an increase of ball milling time, the permanent magnetic properties were first enhanced and then weakened, the magnetism peaked at 4 h. The corresponding coercivity and remanence of the annealed samples ball milled for 4 h were 14.2 kOe and 24.1 emu g−1, 18.9 kOe and 23.0 emu g−1, 20.4 kOe and 19.2 emu g−1, for W50%, W100%, and W150%, respectively, see Table 1.37 The poor permanent magnetic properties of the samples with short ball milling time was because of the existence of magnetically soft α-Fe, as proved by the XRD patterns. Whereas excessive ball milling might result in a grain size which was too small, which decreased the coercivity by the superparamagnetic effect from the magnetic properties shown in Fig. 3(d) and Table 1, an insufficient Pr2O3 content caused the appearance of α-Fe which decreased the coercivity, and an excessive Pr2O3 content decreased magnetization because of the incomplete reduction of no magnetic Pr2O3. Therefore, the optimum Pr2O3 content is W100%, and the optimal ball milling time is 4 h.
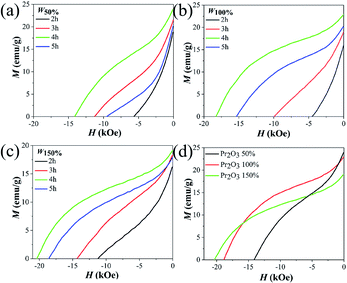 | ||
| Fig. 3 Demagnetization curves of annealed Pr2O3 ((a) W50%, (b) W100%, (c) W150%), and the demagnetization curves of samples annealed at the optimum ball milling time of 4 h (d). | ||
| Sample name | Ball milling time | ||||
|---|---|---|---|---|---|
| 2 h | 3 h | 4 h | 5 h | ||
| W50% | Hc (kOe) | 5.6 | 11.2 | 14.2 | 9.6 |
| Mr (emu g−1) | 18.7 | 21.5 | 24.1 | 20.1 | |
| W100% | Hc (kOe) | 4.5 | 9.9 | 18.9 | 15.4 |
| Mr (emu g−1) | 18.3 | 18.9 | 23.0 | 20.4 | |
| W150% | Hc (kOe) | 11.2 | 14.3 | 20.4 | 18.6 |
| Mr (emu g−1) | 16.3 | 18.3 | 19.2 | 17.9 | |
The formation mechanism of Pr2Fe14B in this mechanochemical method during annealing was further explored. The influence of the ball milling temperature was investigated based on Pr2O3 (W100%) ball milled for 4 h, as shown in Fig. 4. It was found that the diffraction peaks of Pr2Fe14B become more obvious with the increase of the annealing temperature. Whereas magnetically soft α-Fe phase was found when the temperature rose to 900 °C, which may be due to the loss of Pr. The demagnetization curves (Fig. 4(b)) show that the optimum temperature was 850 °C, at which point the optimum permanent magnetic properties were gained. According to Fig. 5(a) and (b), the optimum holding time was 5 min. Extending the holding time has the same effect as raising the temperature.
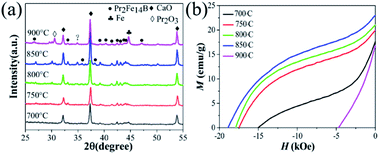 | ||
| Fig. 4 The XRD diffraction patterns (a) and demagnetization curves (b) of Pr2O3 (W100%) ball milled for 4 h and annealed at 700–900 °C. | ||
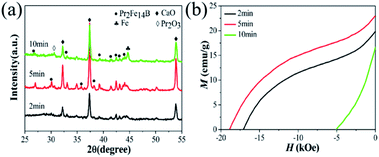 | ||
| Fig. 5 The XRD diffraction patterns (a) and demagnetization curves (b) of Pr2O3 (W100%) milled for 4 h and then annealed to 850 °C and held for 2 to 10 min. | ||
The XRD patterns of samples, of different Pr2O3 contents, washed with NH4Cl/methanol, with a ball milling time of 4 h are shown in Fig. 6(a). It can be observed that the Pr2Fe14B phase is the major phase with a small amount of an unidentified phase. The magnetically soft α-Fe phase is observed in washed Pr2O3 (W50%). The standard data of PDF#50-0678 (Pr2Fe14B) is also presented. Comparing the washed Pr2O3 with PDF#50-0678, the peaks shifted a little to the lower 2θ positions, which arose from the formation of a small amount of Pr2Fe14BHx during the washing process. From the magnetic hysteresis curves (Fig. 6(b)), after removing CaO, the Hc of W50%, W100% and W150% with a ball milling time of 4 h decreased to 6.8 kOe, 9.4 kOe and 11.7 kOe, respectively. The obvious decrease of coercivity may be attributed to an occurrence of an exothermic oxidation reaction, which lead to a partial Pr2Fe14B magnetic phase decomposition to soft magnetic phases. Accordingly, the magnetic properties of the final products were generally decreased, when the annealed samples were washed with an NH4Cl/methanol solution to remove the CaO.23,24,35,38–40 The Mr of Pr2O3 (W50%, W100% and W150%) at a ball milling time of 4 h increased to 53.3 emu g−1, 56.3 emu g−1 and 44.4 emu g−1 after the washing process, respectively, because of the elimination of nonmagnetic phases such as CaO, as well as Ca(OH)2. The maximum energy product of Pr2O3 (W50%, W100% and W150%) prepared at optimum conditions were 3.7 MGOe, 5.0 MGOe and 3.2 MGOe.
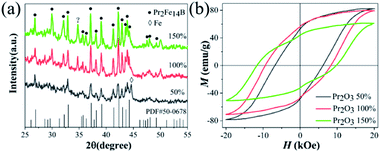 | ||
| Fig. 6 The XRD diffraction patterns (a) and the magnetic hysteresis curves (b) of washed samples with different Pr2O3 levels after annealing. | ||
Fig. 7 shows the hysteresis loops, parallel and perpendicular to the alignment direction, of magnetically aligned Pr2O3 (W100%). Two hysteresis loops show a distinct difference, meaning that the particle is a partial crystal and can be made into anisotropic magnets. With the original washed samples, the Mr value obviously increased, which demonstrated that most of the washed Pr2Fe14B powders were single domain particles.41 Fig. 7(b) shows the coercivities, remanence, saturation magnetization and maximum energy product of the magnetically aligned Pr2Fe14B particles of W50%, W100% and W150%. The Mr increased from 53.3 emu g−1, 56.3 emu g−1, 44.4 emu g−1 to 77.5 emu g−1, 78.4 emu g−1, 61.1 emu g−1, after alignment, respectively. The best permanent magnetic property, a remanence of 78.4 emu g−1, coercivity of 9.8 kOe, and a maximum energy product of 9.8 MGOe, was obtained in Pr2O3 (W100%).
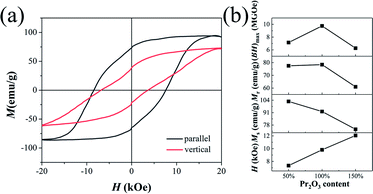 | ||
| Fig. 7 The magnetization hysteresis loops of magnetically aligned Pr2O3 (W100%) (a), and Hc, Ms, Mr, and (BH)max data for aligned samples measured along the direction of alignment (b). | ||
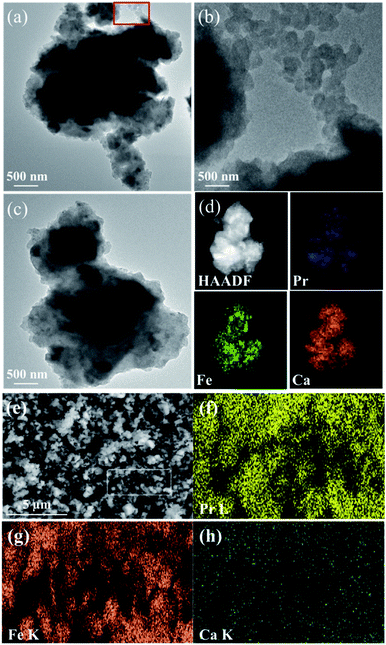
To explore the microstructural evolution, SEM and TEM measurements were performed. It can be seen in Fig. 8(a), that the particles were relatively large after a ball milling time of 4 h. The TEM images (Fig. 9(a) and (b)) display sheet-shaped structures of W100% after milling. Meanwhile, the HRTEM and HAADF images demonstrate that the as-milled powders have an embedded morphology, as shown in Fig. 9(c) and (d). The excessive amounts of CaH2 ensure that the Pr2O3, α-Fe and B2O3 were uniformly wrapped by CaO or CaH2 after high energy ball milling. This special structure may contribute to the fact that the reduction reaction happened in a short time. After annealing, the size of the particles did not change significantly, as shown in Fig. 8(b), because of the covering of CaO on the Pr2Fe14B particles. The existence of the CaO shell effectively inhibited the growth of the Pr2Fe14B particles. After removal of the external CaO covering with a NH4Cl/methanol solution, the irregular spherical or fine rod shaped particles with a smaller size were obtained, which is shown in Fig. 8(c).
 | ||
| Fig. 8 SEM images of powder with a Pr2O3 content of W100% ball milled for 4 h after (a) milling, (b) annealing, and (c) washing. | ||
Elements were uniformly distributed, as shown in Fig. 9(f) and (g) in the element mapping images. Due to the limitation of SEM, it was not possible to see the boron element. The atomic ratio between Pr and Fe was near to 0.21, which is similar to the best reactant ratio of Pr as well as the Fe precursors. The trace atomic ratio of Ca was 0.04% which means that the nonmagnetic phases as CaO and Ca(OH)2 were eliminated in the cleaning process.
Conclusions
In summary, Pr2Fe14B nanoparticles were prepared from Pr2O3, Fe2O3, B2O3, and CaH2 powder via applying a mechanochemical method. The powders were partially reduced during high-energy ball milling, leading to the formation of a Pr2Fe14B nucleus, which helped the as-milled powders to transform to Pr2Fe14B in a time-saving annealing process. The phase compositions and magnetic properties can be enhanced via fine-tuning the Pr/Fe ratio and preparation conditions. Coercivity of 18.9 kOe was obtained in the sample with 100 wt% Pr2O3 excess, ball milled for 4 h and annealed to 850 °C for 5 min. After washing and magnetic alignment, the coercivity, remanence, and maximum energy product were improved to 9.8 kOe, 78.4 emu g−1, and 9.8 MGOe, respectively. Via fine-tuning the particle size and microstructure, it is possible to further improve the magnetic performance of Pr2Fe14B materials.Conflicts of interest
There are no conflicts to declare.References
- X. Zhou, Y. L. Tian, H. Y. Yu, H. Zhang, X. C. Zhong and Z. W. Liu, Waste Manag., 2019, 87, 645–651 CrossRef CAS PubMed.
- H. Tu, Y. Yan, D. Cai, J. Wang, F. Li, F. Su and X. Du, J. Magn. Magn. Mater., 2019, 490, 165497 CrossRef CAS.
- X. Tan, H. Parmar, Y. Zhong, V. Chaudhary and R. V. Ramanujan, J. Magn. Magn. Mater., 2019, 471, 278–285 CrossRef CAS.
- D. B. D. Mooij and K. H. J. Buschow, J. Less-Common Met., 1988, 142, 349–357 CrossRef.
- D. Goll, M. Seeger and H. Kronmüller, J. Magn. Magn. Mater., 1998, 185, 49–60 CrossRef CAS.
- N. Cannesan, J. M. Lebreton, A. J. Williams and I. R. Harris, J. Magn. Magn Mater., 2002, 242, 1372–1374 CrossRef.
- M. Sagawa, S. Fujimura, N. Togawa, H. Yamamoto and Y. Matsuura, J. Appl. Phys., 1984, 55, 2083–2087 CrossRef CAS.
- S.-q. Hu, K. Peng and H. Chen, J. Magn. Magn. Mater., 2017, 426, 340–346 CrossRef CAS.
- M. Yue, X. Zhang and J. P. Liu, Nanoscale, 2017, 9, 3674–3697 RSC.
- D.-s. Wang, Z.-b. Li, F. Liu, Q. Ma, Y.-f. Li, Q. Zhao and X.-f. Zhang, Mater. Lett., 2018, 228, 509–512 CrossRef CAS.
- R. Goto, M. Matsuura, S. Sugimoto, N. Tezuka, Y. Une and M. Sagawa, J. Appl. Phys., 2012, 111, 2083 Search PubMed.
- W.-W. Yang, J. Luo, H.-J. Peng, Y.-L. Sui and X.-X. Gao, Rare Met., 2014, 33, 171–175 CrossRef CAS.
- J. Song, S. Guo, G. Ding, K. Chen, R. Chen, D. Lee and A. Yan, J. Magn. Magn. Mater., 2019, 469, 613–617 CrossRef CAS.
- J. J. Zhang, Y. Yan, H. M. Gao, H. M. Geng, X. Y. Feng, Z. P. Hou, H. D. Li, W. Q. Wang, F. Su and X. B. Du, J. Magn. Magn. Mater., 2015, 374, 317–320 CrossRef CAS.
- I. Shchetinin, P. Aggrey, I. Bordyuzhin, A. Savchenko, M. Gorshenkov, M. Zhelezniy, V. Menushenkov and P. Mogil’nikov, Metals, 2019, 9, 497 CrossRef CAS.
- X. Lin, Y. Luo, H.-j. Peng, Y.-f. Yang, Y.-k. Dou, Z.-l. Wang, K.-s. Xu, S.-l. Diao and D.-b. Yu, J. Magn. Magn. Mater., 2019, 490, 165454 CrossRef CAS.
- Z. Wang, X. Liu, Z. Chen, R. Zhu, W. Yang, Z. Zhang, W. Wang, Q. Wu, K. M. U. Rehman and M. Shezad, J. Supercond. Novel Magn., 2018, 32, 715–720 CrossRef.
- B. Z. Cui, W. F. Li and G. C. Hadjipanayis, Acta Mater., 2011, 59, 563–571 CrossRef CAS.
- L. Yu, C. Yang and Y. Hou, Nanoscale, 2014, 6, 10638–10642 RSC.
- H. Rahimi, A. Ghasemi, R. Mozaffarinia and M. Tavoosi, J. Magn. Magn. Mater., 2017, 444, 111–118 CrossRef CAS.
- L. Wu, A. Mendoza-Garcia, Q. Li and S. Sun, Chem. Rev., 2016, 116, 10473–10512 CrossRef CAS PubMed.
- Z. Ma, T. Zhang and C. Jiang, Chem. Eng. J., 2015, 264, 610–616 CrossRef CAS.
- P. K. Deheri, V. Swaminathan, S. D. Bhame, Z. Liu and R. V. Ramanujan, Chem. Mater., 2010, 22, 6509–6517 CrossRef CAS.
- C.-Q. Chen, D. Kim and C. Choi, J. Magn. Magn. Mater., 2014, 355, 180–183 CrossRef CAS.
- H. Rahimi, A. Ghasemi, R. Mozaffarinia and M. Tavoosi, J. Magn. Magn. Mater., 2017, 424, 199–206 CrossRef CAS.
- Y. Zhong, V. Chaudhary, X. Tan, H. Parmar and R. V. Ramanujan, Nanoscale, 2017, 9, 18651–18660 RSC.
- A. M. Gabay, X. C. Hu and G. C. Hadjipanayis, J. Alloys Compd., 2013, 574, 472–476 CrossRef CAS.
- A. Pal, A. Gabay and G. C. Hadjipanayis, J. Alloys Compd., 2012, 543, 31–33 CrossRef CAS.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- V. Chaudhary, Y. Zhong, H. Parmar, X. Tan and R. V. Ramanujan, Chemphyschem, 2018, 19, 2370–2379 CrossRef CAS.
- K. Filipecka, P. Pawlik, A. Kozdraś, J. Filipecki and K. Pawlik, Acta Phys. Pol., A, 2019, 135, 226–228 CrossRef CAS.
- P. Mingxiang, Z. Pengyue, G. Hongliang, Y. Hangfu and W. Qiong, J. Magn. Magn. Mater., 2012, 324, 2953–2957 CrossRef.
- O. Koylu-Alkan, J. M. Barandiaran and D. Salazar, et al. Submicron R2Fe14B particles, AIP Adv., 2016, 6(5), 056027 CrossRef.
- H. Tu, Y. Yan, D. Cai, J. Wang, X. Shang, H. Tian, F. Su and X. Du, Mater. Des., 2019, 183, 108140 CrossRef CAS.
- H. X. Ma, C. W. Kim, D. S. Kim, J. H. Jeong, I. H. Kim and Y. S. Kang, Nanoscale, 2015, 7, 8016–8022 RSC.
- A. M. Gabay, X. C. Hu and G. C. Hadjipanayis, J. Magn. Magn. Mater., 2014, 368, 75–81 CrossRef CAS.
- W. F. Li, A. M. Gabay, X. C. Hu, C. Ni and G. C. Hadjipanayis, J. Phys. Chem. C, 2013, 117, 10291–10295 CrossRef CAS.
- V. Swaminathan, P. K. Deheri, S. D. Bhame and R. V. Ramanujan, Nanoscale, 2013, 5, 2718–2725 RSC.
- J. H. Jeong, H. X. Ma, D. Kim, C. W. Kim, I. H. Kim, J. W. Ahn, D. S. Kim and Y. S. Kang, New J. Chem., 2016, 40, 10181–10186 RSC.
- Y. Wang, J. Ahn, D. Kim, W. J. Ren, W. Liu, Z. D. Zhang and C. J. Choi, J. Magn. Magn. Mater., 2017, 439, 91–94 CrossRef CAS.
- C. Li, Q. Wu, Z. Ma, H. Xu, L. Cong and M. Yue, J. Mater. Chem. C, 2018, 6, 8522–8527 RSC.
| This journal is © The Royal Society of Chemistry 2021 |