Plasmonic metamaterials for chiral sensing applications
Yoon Young
Lee
,
Ryeong Myeong
Kim
,
Sang Won
Im
,
Mani
Balamurugan
 and
Ki Tae
Nam
and
Ki Tae
Nam
 *
*
Department of Materials Science and Engineering, Seoul National University, Seoul 08826, Republic of Korea. E-mail: nkitae@snu.ac.kr
First published on 27th November 2019
Abstract
Plasmonic metamaterials are artificially designed materials which exhibit optical properties that cannot be found in nature. They have unique and special abilities related to electromagnetic wave control, including strong field enhancement in the vicinity of the surfaces. Over the years, scientists have succeeded in dramatically improving the detection limit of molecular chirality utilizing a variety of plasmonic metamaterial platforms. In this mini-review, we will discuss the principles of most recent issues in chiral sensing applications of plasmonic metamaterials, including suggested formulas for signal enhancement of chiroptical plasmonic sensors, and studies on various platforms that employ different sensing mechanisms.
1. Introduction
Plasmonic metamaterials are artificially designed materials utilizing plasmons, collective oscillations of free electrons, which exhibit optical properties that cannot be found in nature.1 When the plasmons are bound to the metal/dielectric interface, the materials show vivid colors which are not observable in the bulk. In addition, they can generate a locally amplified field. The unconventional optical properties of plasmonic metamaterials, such as electric permittivity and magnetic permeability, strongly depend on their geometrical features. From this perspective, design and fabrication of these materials present a new paradigm in manipulating electromagnetic waves over a wide range of frequencies, and various review articles have been reported recently.2–5Chirality, which refers to structures with broken mirror symmetry, is ubiquitous in nature.6 Physically, chiroptical objects respond in a different way to right and left circularly polarized light (CPL), and the difference in absorption of CPL is referred to as circular dichroism (CD).7 Chiral sensing techniques including CD have been extensively applied to acquire information on biomolecular structures, arrangements and conformations. Although CD offers non-invasive detection and simple and low-cost measurement, the observed chiroptical signals of biomolecules are usually very weak (10−6 to 10−2 of absorption), so the accurate sensing of chiral analytes is always a challenging task.8
The development in nanofabrication of plasmonic metamaterials enhances the versatility towards control of electromagnetic waves, which can be applied to enhance the sensitivity of chirality detection.9 A variety of platform structures for chiral plasmonic sensors have been theoretically modeled and experimentally fabricated.10–13 Few review articles are devoted to the theoretical issues in plasmonic chirality, fabrication of platform structures for chiral sensors and chirality-based biomedical applications of plasmonic metamaterials.9–16
This mini-review will cover the general concepts and principles in chiral biosensing and the most recent issues in fabrication of plasmonic metamaterial platforms. Also, the following section will overview the general chiroptical spectroscopic methods such as CD and optical rotatory dispersion (ORD) followed by which the recently developed concepts such as helical dichroism (HD) and Raman optical activity (ROA) will be discussed. We will also summarize the chiroptical signal enhancement principles in the presence of a plasmonic metamaterial platform studied in the last two years. Various studies on different platforms using distinct detection mechanisms, including chiral and racemic plasmonic structures, will also be reviewed.
2. Principles and methods for chiral plasmonic sensors
Upon interaction of the light with a chiral material, it can be rotated either clockwise or counterclockwise based on the enantiomers. This phenomenon can be utilized for the applications of ultrasensitive molecular sensing with different chiroptical spectroscopic methods. Also, attempts have been made to combine fabrication of plasmonic metamaterials to result in greater signal amplification. The following section would discuss various types of chiroptical spectroscopy methods and principles of signal enhancement reported recently.2.1. Chiroptical spectroscopic methods
Chiroptical spectroscopy unveils the difference in response of chiral objects to circularly polarized light. It is a very useful method for the stereochemical description of organic and inorganic biomolecules. Since most of the biomolecules including enzymes and drugs are chiral, the applications of chiroptical spectroscopy vary from biochemistry, pharmacy and medicine to advanced nanoscience. This section will discuss the conceptual principles of conventional chiroptical spectroscopic methods and the recently proposed approaches.Optical rotation is the first observed chiroptical phenomenon and was discovered by a French physicist Arago in 1811.17 Optical rotation is a kind of optical activity in which a polarization plane rotates the linearly polarized (LP) light when it passes through a sample, and the rotation angle is proportional to the concentration and thickness of the analyte.18 Optical rotatory dispersion (ORD) is another phenomenon discovered by Biot in 1815, which means that the optical rotatory power of an optically active material changes with the wavelength of the incident beam.19 ORD can be plotted as a curve with the specific rotatory power on the y-axis and the wavelength on the x-axis, because the polarizability of a molecule changes with the wavelength of the incident light. The plot is observed as a positive curve when the light rotates clockwise whereas a negative curve is observed when the light rotates counterclockwise. Circular dichroism (CD) is measured using circularly polarized light (CPL) rather than linearly polarized (LP) light.20,21 CPL is a polarized light in which the end of a vibration vector rotates. When the direction of the light wave is viewed from the observer's viewpoint, right-handed circularly polarized (RCP) light is used for clockwise rotation, and left-handed circularly polarized (LCP) light is used for counterclockwise rotation. CD spectra can be obtained by measuring the difference in absorption between RCP and LCP light with respect to wavelength, after passing through the sample. The differences in absorption of the RCP and LCP light at certain wavelengths are due to the differences in extinction coefficients of the polarized states. Thus, ORD and CD have been widely used as basic methods to measure the handedness of the molecules.22
In addition to CD, the concept of helical dichroism (HD) has also been attracting attention recently.23 RCP and LCP light observed in CD represent two possible spin angular momenta of states for photons; thus, CD is also referred to as dichroism for spin angular momentum. Here the optical spin angular momentum (SAM) is assumed to be s = ±1, with only two opposite sign values. Chiral molecules with different handedness can respond differently towards the two optical SAMs, which exhibit different CD spectra. On the other hand, the sign of optical orbital angular momentum (OAM) can be a value that reaches infinity, depending on the handedness of the helical wavefronts.24 HD is the simplest chirality-specific technique using an OAM beam, which measures the difference in absorption between +1 angular momentum and −1 angular momentum. Laguerre-Gaussian beam is a characteristic light with a well-defined optical OAM, is used for HD measurements and also has a helical wavefront that rotates around the axis of propagation. The electric quadrupoles contribute dominantly in the HD measurement, whereas the interaction between the electric and magnetic dipoles mainly contributes in CD. Thus, the unique dependence on electric quadrupole contribution in HD demonstrates that the magnetic dipole contribution is not required to resolve the molecular handedness, which is the basis of CD. Moreover, combining CD with HD can empower us to unravel the chiral dipole and chiral quadrupole contributions to the total chiroptical response. Verbiest and coworkers demonstrated the difference between two enantiomers of phenylalanine molecules using the HD results as a function of OAM value.23
To enhance the signal sensitivity, attempts have been made to combine the chiral spectroscopic methods such as CD and ORD with other existing spectroscopic techniques. Raman Optical Activity (ROA) is a recent technique, which combines the variability of scattering experiments with the structural sensitivity of polarization spectroscopy techniques.25 ROA observes the difference in Raman scattering intensity between the RCP and LCP light.26 Although the low background measurement is advantageous, the intrinsic signal intensity is extremely weak. This is because of the very low cross section of parent Raman scattering (10−30 cm2 and 10−16 cm2 for fluorescence); consequently, ROA also exhibits three orders of magnitude lower cross sections. Therefore, many difficulties exist in actual ROA measurement of the chiral analyte. To overcome such difficulties, the concept of surface-enhanced Raman optical activity (SEROA) using near-field enhancement of plasmonic nanostructures has been proposed.27 SEROA is based on the principle of Surface-enhanced Raman Spectroscopy (SERS), in which the Raman scattering signal of a molecule is strongly amplified in the vicinity of the plasmonic nanostructures.28–32 Blanch and coworkers observed experimental proof of the SEROA concept by observing D- and L-enantiomers of the ribose molecules.30 They have employed the hydrophilic polyacrylic acid polymer as a stabilizing medium of silver colloids because of the very weak ROA spectrum exhibited by the polymer. So, the mirror image bands of the two enantiomers have been successfully obtained from the SEROA results with reproducibility and reliability. Kneipp and coworkers also demonstrated the SEROA measurements of biologically relevant molecules in the vicinity of silver nanoparticles.28 They have acquired the signals of adenosine and cytosine with shorter data acquisition times, lower laser excitation intensities and lower concentrations of target molecules compared to ROA.
The various chiroptical spectroscopic methods introduced in this section can be useful in chiral sensing studies using plasmonic metamaterial platforms. They can be not only used to distinguish molecular handedness but also have distinct advantages, including superior sensitivity and a low background signal when compared with conventional extinction spectroscopy-based sensing. These will be covered in the following sections.
2.2. Near-field optical chirality and enhanced chiroptical response
The chiroptical spectroscopic methods described in the previous section exhibit limitations such as low signal intensities and limited wavelength ranges. To overcome these limitations, plasmonic metamaterial platforms have attracted attention and few studies have been reported recently.10,16 This section will cover the principles of signal enhancement and amplifying the molecular chiroptical response using plasmonic metamaterial platforms that have been introduced by recent reports.Initially, the mathematical and theoretical studies on the enhancement of chiroptical response by chiral plasmonic metamaterial platforms are discussed. Circular dichroism (CD) is defined as the absorption difference of two polarized states, RCP and LCP light. If the transmitted power for RCP light is IR and the transmitted power for LCP light is IL, CD can be expressed as follows:33
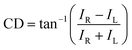 | (1) |
A chiral medium is a type of bi-isotropic medium in which the electric field and the magnetic field interact with each other. The following relation exists in the electromagnetic response.34
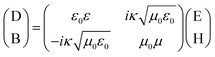 | (2) |
| CD = −2k0wIm{κ} | (3) |
As shown in eqn (3), the CD of the chiral molecular layer is proportional to the imaginary part of the chirality parameter κ of the chiral medium. However, when the chiral material is placed on the plasmonic metamaterial platform, the complex interactions of the incident plane wave and the local field enhancement from the nanostructure have to be considered to approximate the resultant CD. Altug and coworkers proposed a modified formula to obtain the resultant CD by introducing a local intensity enhancement factor and a local chirality enhancement factor, which are derived from the platform structures where the platform and chiral molecular layer coexist.35 They tried to calibrate the formula by assuming the near field intensity enhancement factor as |E|near2 − |E|far2 and near field optical chirality enhancement factor as Im{E·H}near/Im{E·H}far. In this case, the near field zone is defined as a space having the same volume as the chiral sample existing on the platform.
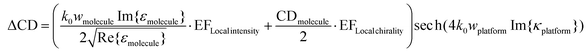 | (4) |
Eqn (4) is a revised version of the resultant CD formula proposed by Altug and coworkers, only for the increased amount of CD in the presence of the chiral platform. Here the enhancement factor for near field intensity (EFLocal intensity) and enhancement factor for near field chirality (EFLocal chirality) are added, and the role of the local chirality enhancement factor is emphasized to increase the sensitivity of enantiomer detection. This is because the EFLocal chirality in this equation belongs to the term in which the chirality of the molecule is reflected, not the permittivity or refractive index of the molecule. Additionally, what this local chirality enhancement factor means is related to the concept of optical chirality, introduced by Lipkin in 1964 and recently revised.34,36–38 Optical chirality is a local property, in which the chiral properties of the electromagnetic field can be changed with respect to the different spatial location. Thus, plasmonic metamaterial structures with the characteristics of an evanescent wave in the near-field region can provide a new method of generating strong chiral electromagnetic fields. Kadodwala and coworkers reported numerically measured optical chirality enhancement in plasmonic gammadion structures and also discussed the optimization of the optical chirality by observing the combinations of electric and magnetic fields with two nanoslit structures.39 Altug and coworkers suggested a very thin dielectric nanostructure as an optimal platform structure for detecting molecular handedness, which is achiral as a whole; however, there exists an increase in near-field optical chirality.35 Alù and coworkers have introduced a chirality detection platform based on plasmonic ‘twisted’ metamaterials.40 A pair of metamaterials with twist angles of +60° and −60°, respectively, were fabricated using electron beam lithography and they showed opposite CD signals. Comparing the cumulative CD spectra of this ‘enantiomeric pair’ of metamaterials for (S)-(+)- and (R)-(−)-1,2-propanediol, the handedness of the molecules could be distinguished. Very recently, the necessity of considering the interference effect of chiral molecules and optical chirality dissipation as well as the magnitude of optical chirality of the platform structures has been suggested.41 Upcoming section 3.2 will introduce the recent studies on plasmonic platforms to improve the sensitivity employing structures that maximize the optical chirality.
3. Metamaterial platforms for chiral plasmonic sensors
Metamaterial platforms have been applied as chiral plasmonic sensors with different enhancement mechanisms. Here, we will describe three types of platforms based on which target molecules were observed using chiroptical response measurements.3.1. Detection of changes in surrounding media
Nanobiosensors based on localized surface plasmon resonance (LSPR) phenomena track the shifts of resonance peaks in the extinction spectra with respect to the change of the surrounding medium near the plasmonic nanostructure.42,43 For enhanced LSPR sensitivity, a number of approaches which utilize shape-engineered materials composed of noble metals such as Au and Ag have been developed and recently several ideas employing chiral metamaterial platforms have been proposed.Although the studies mentioned in this section do not include a distinction of molecular handedness, they are classified in the ‘chiral plasmonic sensors’ category because they use a chiral structure as a sensing platform and a chiroptical spectroscopic measurement, and there are clear advantages compared to the conventional extinction spectroscopy-based sensing methods.
Fischer and coworkers reported significant refractive index sensitivities by employing shape-engineered chiral plasmonic nanostructures (Fig. 1a).44 They introduced theoretical concepts, such as full width at half maximum (FWHM) and figure of merit (FOM) for improving the sensitivities of LSPR sensors with chiral platforms and illustrated the roles of chirality and properties of the materials. When theoretically obtaining the sensitivity (Sn) and FOM (Sn/FWHM) at the point where the CD value is zero, the term of ‘chiral shape factor’ is introduced. A higher FOM value could be obtained by applying the ‘chiral shape factor’ with increased magnitude. Also it has been reported that the sensitivity can be improved by controlling the alloy composition with the metal having lower wavelength dependency of the dielectric constant. A molecular LSPR sensor using chiral magnesium nanoparticles with notable chiroptical effects in the ultraviolet (UV) region was also reported.45 The change in the refractive index of dimethyl sulfoxide (DMSO) solution is caused by the change in concentration as a function of the wavelength (dn/dc), which is larger in the UV region than in the visible region. Fig. 1b (upper panel) shows the CD result of chiral Mg nanoparticles in DMSO–water mixtures ranging from 0% to 30%. As shown in Fig. 1b (lower panel), the wavelength shift in the UV region is larger than the shift in the visible region. The results show that UV plasmonics is promising for enhanced LSPR sensors due to the dispersion of the medium. Liu and coworkers demonstrated a hydrogen sensing platform by employing chiral palladium nanomaterials with helical structures.46 When exposed to hydrogen, Pd metal goes through a reversible phase transition to a metal hydride form depending on the concentration. It is well known that the chiroptical properties of Pd nanostructures vary with the hydrogen uptake and depend on the concentration. As shown in Fig. 1c, CD and extinction intensities are decreased in response to the increasing hydrogen concentrations. When the hydrogen concentration increased to 1.6%, the CD signal change was about 40%, while the extinction signal change was only 10%. In addition, compared with the extinction spectra, the signal-to-noise ratio of CD was higher and the CD signal change upon hydrogen uptake was observed in a broad wavelength range from ultraviolet to near infrared.
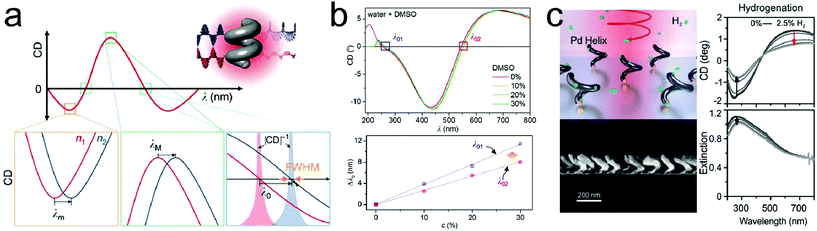 | ||
| Fig. 1 Representative examples of platforms for detection of changes in surrounding media: (a) a schematic diagram of circular dichroism sensing using chiral plasmonic nanohelices. 3 bottom panels illustrate the resonance shift in the circular dichroism (CD) spectra at minimum, maximum and zero crossing wavelengths where the refractive indices are changed between n1 and n2. (b) CD spectra of chiral magnesium nanoparticles in 4 different DMSO–water mixtures (red: 0%, orange: 10%, yellow: 20% and green: 30%) (upper panel). Wavelength shift of the λ01 and λ02 as a function of DMSO concentration (lower panel). (c) A schematic diagram of a chiral plasmonic hydrogen sensor using Pd nanohelices (left, upper panel). Electron microscopy image of the cross section of Pd nanohelices (left, lower panel). CD and extinction spectra during hydrogenation and dehydrogenation (right panel). Arrows indicate the evolutions of the spectra with the hydrogen uptake. (a) Reprinted from ref. 44 with permission. Copyright 2016, Nature Publishing Group. (b) Reprinted from ref. 45 with permission. Copyright 2016, Royal Society of Chemistry. (c) Reprinted from ref. 46 with permission. Copyright 2018, Wiley. | ||
3.2. Detection of molecular handedness
Metamaterials with chiral morphologies fabricated in various ways have been utilized for chiral enantiomer detection. The strong enhancement of near-field optical chirality in the vicinity of the metamaterial platforms can induce the sensitivity amplification.Fig. 2a shows a SEM image of a planar chiral metamaterial (PCM) named ‘gammadion’. The array structure with a gold gammadion of about 400 nm showed a strong CD signal at around 800 nm.39 PCMs were fabricated as left-handed and right-handed configurations and their CD spectra were observed as mirror images. It has been found that the enhancement of a large and uniform local optical chirality maximizes the molecular signal of the plasmonic gammadion structures. For example, in the presence of a protein tertiary structure (beta-lactoglobulin), a difference in resonance peak shifts between RCP and LCP light was observed, allowing detection at the pictogram level. In the case of an achiral molecule (ethanol), the difference of peak shifts was zero. Fig. 2b shows a SEM image of a Shuriken-shaped plasmonic metafilm.47 The left-handed or right-handed Shuriken structures have been reported to be suitable as probes for analysis of biointerfaces. Chiral near fields generated near this Shuriken structure are utilized to study the level of structural order. The chiroptical responses of the metamaterials are varied by the presence of a biomolecular protein layer and the magnitude of asymmetry depends on the structural order. Consequently, specifying a protein–protein interaction based on the level of structural anisotropy becomes available.48,49 Large-area arrays of chiral plasmonic nanocrescents were fabricated using a colloidal lithography technique by adjusting the mutual deposition angle of gold and silica.50 It was observed that the simulated optical chirality enhancement was maximized at the sharp tips of the plasmonic nanocrescents. Okamoto and coworkers prepared a two-dimensional lightning-bolt-like gold nanostructure consisting of two displaced rectangles and observed an enhancement in dissymmetry of circularly polarized photoluminescence (Fig. 2c).51 The structures fabricated with both left-handedness and right-handedness were used to provide a circularly polarized luminescence source, and a strong photoluminescence enhancement was observed in the 800 nm wavelength range.
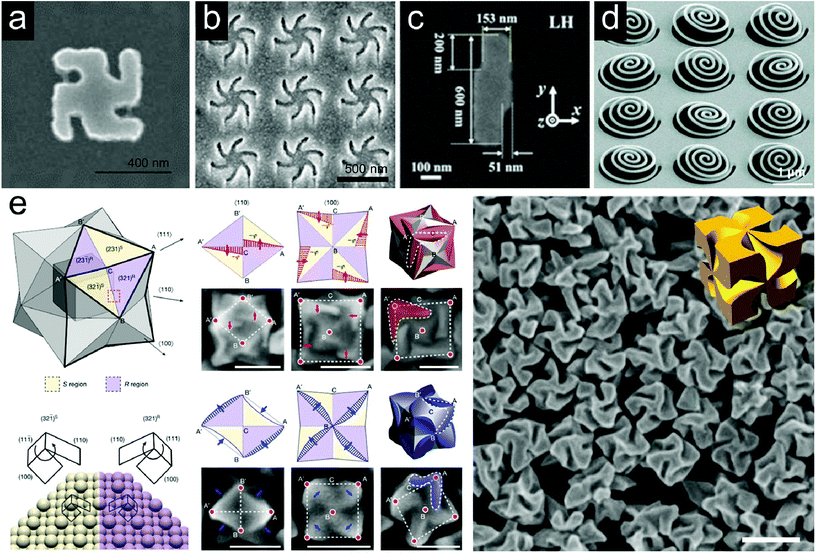 | ||
Fig. 2 Representative examples of chiral metamaterial platforms for detection of molecular handedness: (a) a SEM image of the gold gammadion nanostructure, (b) a SEM image of the gold Shuriken nanostructure, (c) a SEM image of the left-handed enantiomer of the lightning-bolt like gold nanostructure, (d) a tilted SEM image of the as-fabricated chiral metasurface consisting of arrays of 3 dimensional Archimedean spirals, and (e) evolution of chiral morphologies by the interplay between the enantioselective binding of thiol containing biomolecules and the asymmetric development of high-index facets. (i) A schematic diagram of a stellated octahedron differentiated by high-index facets of {321}S (yellow color) and {321}R (purple color) configurations. Vertices of the [111], [100] and [110] directions are marked as A, B and C. A′ and B′ indicate the symmetric points of A and B. (ii) Atomic arrangement of the (321)R and (32![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) )S Au surfaces for the region marked by a red dotted box in (i). The conformation at the ‘kink’ is determined by the rotational direction of microfacets in the (111) → (100) → (110) sequence: clockwise, {321}R; anti-clockwise, {321}S. (iii) Schematic diagrams (upper panel) and SEM images (lower panel) of R–S pairs describing the shape development in the presence of L-Cys as viewed along the [110] direction. (iv) Schematic diagrams (upper panel) and SEM images (lower panel) of R–S pairs describing the shape development of 432 helicoid I as viewed along the [100] direction. New boundaries are marked as a red patterned region with arrows; each vertex is indicated on the corresponding microscope image. (v) 3D model (upper panel) and SEM image (lower panel) of the final morphology of the chiral nanostructure. The newly developed area is marked in red color and the chiral element is marked as a dashed outline. The red shaded area in (v) is corresponding to the red patterned regions in (iii) and (iv). (vi) and (vii) Shape development in the presence of L-GSH. (viii) The corresponding resultant morphology. New boundaries and the chiral elements of the morphology are marked in blue color. Scale bars in (iii)–(viii): 100 nm. (ix) SEM image of 432 helicoid III and corresponding 3D model. Scale bar: 200 nm. (a) Reprinted from ref. 39 with permission. Copyright 2010, Nature Publishing Group. (b) Reprinted from ref. 47 with permission. Copyright 2018, American Chemical Society. (c) Reprinted from ref. 51 with permission. Copyright 2018, American Chemical Society. (d) Reprinted from ref. 66 with permission. Copyright 2019, Wiley. (e) Reprinted from ref. 67 with permission. Copyright 2018, Nature Publishing Group. )S Au surfaces for the region marked by a red dotted box in (i). The conformation at the ‘kink’ is determined by the rotational direction of microfacets in the (111) → (100) → (110) sequence: clockwise, {321}R; anti-clockwise, {321}S. (iii) Schematic diagrams (upper panel) and SEM images (lower panel) of R–S pairs describing the shape development in the presence of L-Cys as viewed along the [110] direction. (iv) Schematic diagrams (upper panel) and SEM images (lower panel) of R–S pairs describing the shape development of 432 helicoid I as viewed along the [100] direction. New boundaries are marked as a red patterned region with arrows; each vertex is indicated on the corresponding microscope image. (v) 3D model (upper panel) and SEM image (lower panel) of the final morphology of the chiral nanostructure. The newly developed area is marked in red color and the chiral element is marked as a dashed outline. The red shaded area in (v) is corresponding to the red patterned regions in (iii) and (iv). (vi) and (vii) Shape development in the presence of L-GSH. (viii) The corresponding resultant morphology. New boundaries and the chiral elements of the morphology are marked in blue color. Scale bars in (iii)–(viii): 100 nm. (ix) SEM image of 432 helicoid III and corresponding 3D model. Scale bar: 200 nm. (a) Reprinted from ref. 39 with permission. Copyright 2010, Nature Publishing Group. (b) Reprinted from ref. 47 with permission. Copyright 2018, American Chemical Society. (c) Reprinted from ref. 51 with permission. Copyright 2018, American Chemical Society. (d) Reprinted from ref. 66 with permission. Copyright 2019, Wiley. (e) Reprinted from ref. 67 with permission. Copyright 2018, Nature Publishing Group. | ||
In addition to the two-dimensional plasmonic asymmetric nanostructures described so far, examples of planar chiral metamaterials such as structures with tunable chirality52–54 and structures with both plasmonic Fano resonance effects and strong optical chirality were also reported.55–58 Cao and coworkers have introduced a gold chiral array of disc-double split ring resonators with a dipole–octupole Fano resonance.55 Using the distinct total transverse force and potential well from this structure, an effective enantioselective separation of sub-10 nm chiral particles can be achievable. Fang and coworkers fabricated 6-fold symmetric chiral heptamers and systematically studied the chiroptical response with changing the inter-particle rotation angles and separation distance.58,59 The authors noted that the response greatly depends on the plasmonic Fano resonance effect. Later, further studies on this structure were conducted to study chirality at the subnanoscale using cathodoluminescence microscopy and spectroscopy, which can carry the polarization information.60–64
Recently, efforts have been devoted to implementing 3-dimensional plasmonic chiral metamaterial platforms. Compared to planar materials, 3D chiral metamaterials have the advantage of more uniform and broadband near-field optical chirality enhancement.34 Accordingly, enantiomer-specific molecular sensing with more uniform, sensitive, and wider wavelength ranges can be expected using 3D chiral metamaterials. However, it is still a challenging topic with many technical barriers in practical applications. Giessen and coworkers proposed a design rule applicable to the 3D chiral metamaterial platform for biomolecular detection.65 They have demonstrated the optical chirality enhancement of chiral plasmonic oligomer structures. The arrangement of gold disks as building blocks of the oligomers was designed by mimicking a left-handed helix. This structure showed high circular dichroism activity in the infrared region around 900 nm wavelength. Also, the difference in optical chirality of RCP and LCP light was largest in the gap between the two disks. Fig. 2d shows a recently reported chiral metasurface consisting of nanospirals.66 The nanowires were cut and stretched by utilizing a focused ion beam to form a 3D Archimedean spiral. The authors noted that more uniform optical chirality enhancement was confined in the spiral structures than the simple 2D-planar array structures, suggesting that it could be applied to broadband chiral sensing. A new chiral plasmonic 3D nanostructure fabricated with a bottom-up approach is shown in Fig. 2e.67 We have succeeded in transferring the inherent chirality of organic materials to inorganic structures by using biomolecules such as amino acids and peptides in the growth process of gold nanoparticles. The high-Miller index chiral surfaces in the growth process of nanoparticles led to the final helicoidal morphology, by forming enantioselective interaction with biomolecules. Three kinds of nanoparticles with different shapes and anisotropy factors were fabricated depending on the seed particle morphologies and biomolecule types. The 432 helicoid III nanoparticle synthesized with octahedron seed particles and glutathione biomolecules showed high chirality with a dissymmetry factor of 0.2 and was used for polarization-dependent color generation.
Including the planar and 3D metamaterials and geometrically chiral plasmonic platforms can exhibit far-field signals that are larger than those of natural chiral molecules, which can be a stumbling block detecting the chiroptical signal of the analyte molecules. The concept of ‘plasmonic racemates’ which combines the advantage of strong near-field optical response of chiral platforms with zero far-field optical response of achiral systems was proposed.34 Here each individual structure is chiral, while the whole array is an achiral platform in the presence of enantiomers. Quidant and coworkers reported a racemic nanoplasmonic array for the enantiomer detection (Fig. 3a).68 Racemic gold nanoarrays consisting of gammadion structures were fabricated using e-beam lithography. The designed sensor showed zero intrinsic CD, high optical chirality and electric field enhancement in the near-field. It has been utilized for distinguishing D-, L- and racemic forms of phenylalanine molecules. Valev and coworkers proposed a racemic metamaterial platform, consisting of equivalent quantities of opposite chiral unit cells (Fig. 3b).69 They reported numerical simulation results for diffraction circular intensity spectroscopy, yielding an intensity difference of up to 15%, and suggested the potential use of racemic nanogratings in hyper-sensitive chiral molecular detection.
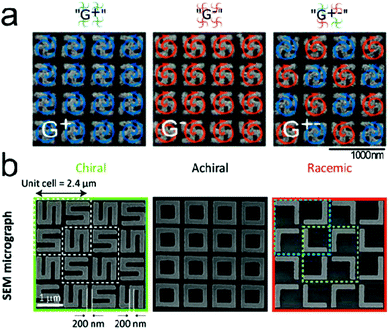 | ||
| Fig. 3 Representative examples of racemic metamaterial platforms for detection of molecular handedness. Racemic metamaterial platforms can maintain large values of optical chirality and near-field enhancement and at the same time far-field CD intensity can be suppressed. (a) SEM images of handed (G+, G−) and racemic (G±) gold gammadion arrays. (b) SEM image of an S-shaped chiral chip is shown in the green box and the achiral chip design (black box) is made of square-rings and the racemic chip (orange box) consists of L-shaped structures. The cyan and green lined-boxes in the racemic sample indicate the two enantiomeric unit cells. (a) Reprinted from ref. 68 with permission. Copyright 2018, American Chemical Society. (b) Reprinted from ref. 69 with permission. Copyright 2019, The Royal Society of Chemistry. | ||
3.3. Detection of molecules inducing self-assembled chiral structures
In addition to using optical chirality derived from the geometry of the plasmonic metamaterials, the utilization of chiral assemblies of the plasmonic nanostructures in the presence of the target molecules has also been reported.70,71 Biomolecules can induce chiral arrangement of plasmonic nanostructures, giving rise to intense chiroptical responses in the plasmon resonance wavelength region.A chiroplasmonic bioanalysis study using a gold–silver nanoparticle heterodimer is shown in Fig. 4a.72 When bridged by biomolecules, the nanoparticle dimers formed a scissor-like geometry with long axes at an angle of 9 degrees and its CD response is generated in the visible region. This heterodimer structure can be used to detect biomarker molecules such as prostate-specific antigen. A DNA-based self-assembled core–satellite structure was also used to detect Ochratoxin A molecules (Fig. 4b).73 First, an aptamer of Ochratoxin A and a complementary sequence are coupled to the surfaces of gold nanoshells respectively. In the absence of Ochratoxin A, the core–satellite structure showed a strong CD response, while in the presence of Ochratoxin A, the coupling phenomenon of the nanostructures was decreased and the CD intensity was weakened as the concentration of the biomolecule was increased. Fig. 4c shows a study of biomarker detection of Parkinson's disease by observing the chiral arrangement of plasmonic nanostructures.74 The authors employed Au nanorods to react in a different way to the stable monomeric proteins and the infectious fibrils. In the presence of monomeric proteins, Au nanorods did not show any chiroptical response. However, in the presence of amyloid fibrils, their helical properties caused the nanorods to form a 3D chiral arrangement and exhibit CD response at the resonance wavelength. They noted that this detection method could also be applied to the human brain samples.
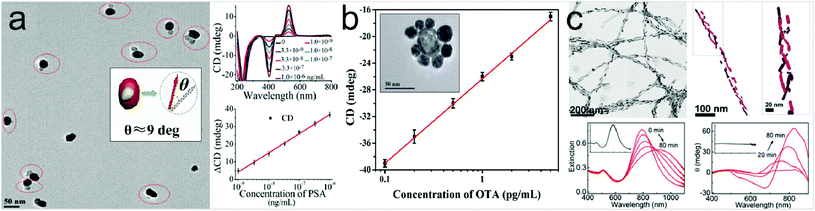 | ||
| Fig. 4 Representative examples of platforms for detection of molecules inducing self-assembled chiral structures. (a) SEM image of gold–silver heterodimers bridged by an immunocomplex (left panel). When bridged by an antigen and antibody, the nanoparticle dimers display a scissor-like conformation with long axes at an angle of 9 degrees (inset in the left panel). Results of detection of PSA with NP heterodimers (right panel). CD spectra with increasing concentrations of PSA solution (right, upper panel). CD calibration curves for PSA detection, where ΔCD is CD530 nm − CD403 nm, as a function of logarithmic PSA concentrations (right, lower panel). (b) Plot of CD (at 521 nm) of shell core–Au satellite superstructures versus the different concentrations of ochratoxin A (0.1, 0.2, 0.5, 1, 2, or 5 pg mL−1). Inset: A SEM image of a gold shell core–silver satellite assembly to detect mycotoxin ochratoxin A. (c) Chiral sensing for Parkinson's disease using arrangement of gold nanorods. TEM and cryo-TEM tomography images of the gold nanorod–protein fiber composite (upper panel) and extinction and circular dichroism spectral changes after addition of fibrillar proteins. Insets: corresponding spectra when monomeric proteins are added (lower panel). (a) Reprinted from ref. 72 with permission. Copyright 2013, American Chemical Society. (b) Reprinted from ref. 73 with permission. Copyright 2018, Wiley. (c) Reprinted from ref. 74 with permission. Copyright 2018, National Academy of Sciences. | ||
4. Conclusions
In this mini review, we discussed the most recent issues in chiral sensing applications of plasmonic metamaterials, including basic principles, suggested formulas for signal enhancement of chiroptical plasmonic sensors, and studies on various platform materials that utilize different sensing mechanisms. Plasmonic metamaterials have unique and special abilities related to electromagnetic wave control, including strong field enhancement in the vicinity of the surfaces. Over the years, a number of researchers have succeeded in dramatically improving the detection limit of molecular chirality using various plasmonic metamaterial platforms. However, the research field of chiral plasmonic sensors still has challenges to overcome. Representatively, selective sensing and discrimination of chiral molecules has presented chronic and major drawbacks in metamaterial based chirality sensors. To address this problem, we envision that techniques to functionalize metamaterials or filter target molecules near sensors should be developed. In addition to the problems in utilizing metamaterials as a sensor, fundamental problems, such as, hardness in processing and mass production of metamaterials, should be resolved for further development of this research field to practical and commercial fields. In spite of some drawbacks, we thought potential application and continuously increasing abilities of metamaterial based sensors are still enough to give a driving force to many researchers. When the three dimensional chiral nanostructures are practically applied to biomolecular chirality detection, enhanced molecular chirality detection by strong optical chirality enhancement near the structures can be expected. In addition, by optimizing the substrate fabrication based on the concept of ‘plasmonic racemates’, signal enhancement could be maximized. As studies on plasmonic metamaterial fabrication are continuously expanding and studies combining various biomolecules with metasurfaces are progressing deeply, highly sensitive molecular chirality detection is expected to be possible in the near future by exceeding the existing limit.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (NRF-2017M3D1A1039377), National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2017R1A2B3012003), and Interdisciplinary Research Initiatives Program by College of Engineering and College of Medicine, Seoul National University (2018).References
- K. Yao and Y. Liu, Nanotechnol. Rev., 2014, 3, 177–210 CAS.
- T. Lee, J. Jang, H. Jeong and J. Rho, Nano Convergence, 2018, 5, 1 CrossRef CAS PubMed.
- M. Keshavarz Hedayati, F. Faupel and M. Elbahri, Materials, 2014, 7, 1221–1248 CrossRef PubMed.
- F. Monticone and A. Alù, Rep. Prog. Phys., 2017, 80, 036401 CrossRef PubMed.
- K.-H. Kim and Y.-S. No, Nano Convergence, 2017, 4, 32 CrossRef PubMed.
- A. B. Harris, R. D. Kamien and T. C. Lubensky, Rev. Mod. Phys., 1999, 71, 1745–1757 CrossRef CAS.
- L. D. Barron, Molecular Light Scattering and Optical Activity, Cambridge University Press, 2004 Search PubMed.
- S. Yoo and Q. H. Park, Sci. Rep., 2015, 5, 14463 CrossRef CAS PubMed.
- M. Hentschel, M. Schäferling, X. Duan, H. Giessen and N. Liu, Sci. Adv., 2017, 3, e1602735 CrossRef PubMed.
- V. E. Bochenkov and T. I. Shabatina, Biosensors, 2018, 8, 120 CrossRef CAS PubMed.
- V. K. Valev, J. J. Baumberg, C. Sibilia and T. Verbiest, Adv. Mater., 2013, 25, 2517–2534 CrossRef CAS PubMed.
- J. T. Collins, C. Kuppe, D. C. Hooper, C. Sibilia, M. Centini and V. K. Valev, Adv. Opt. Mater., 2017, 5, 1700182 CrossRef.
- W. Ma, L. Xu, L. Wang, C. Xu and H. Kuang, Adv. Funct. Mater., 2019, 29, 1805512 CrossRef.
- B. Wang, J. Zhou, T. Koschny, M. Kafesaki and C. M. Soukoulis, J. Opt. A: Pure Appl. Opt., 2009, 11, 114003 CrossRef.
- S. S. Oh and O. Hess, Nano Convergence, 2015, 2, 24 CrossRef PubMed.
- S. Yoo and Q.-H. Park, Nanophotonics, 2019, 8, 249 Search PubMed.
- B. Kahr and O. Arteaga, ChemPhysChem, 2012, 13, 79 CrossRef CAS PubMed.
- P. L. Polavarapu, Chirality, 2002, 14, 768 CrossRef CAS PubMed.
- S. Chandrasekhar, Proc. R. Soc. London, Ser. A, 1961, 259, 1299 Search PubMed.
- J. T. Yang, in A Laboratory Manual of Analytical Methods of Protein Chemistry, 1969, pp. 23–92 Search PubMed.
- G. Snatzke, Angew. Chem., Int. Ed., 1968, 7, 1 CrossRef.
- P. Crabbe, ORD and CD in Chemistry and Biochemistry, Academic Press, 1972 Search PubMed.
- W. Brullot, M. K. Vanbel, T. Swusten and T. Verbiest, Sci. Adv., 2016, 2, e1501349 CrossRef PubMed.
- K. A. Forbes and D. L. Andrews, Opt. Lett., 2018, 43, 435–438 CrossRef CAS PubMed.
- L. D. Barron and A. D. Buckingham, Mol. Phys., 1971, 20, 1111–1119 CrossRef CAS.
- W. Hug, Chem. Phys., 2001, 264, 53–69 CrossRef CAS.
- S. Abdali and E. W. Blanch, Chem. Soc. Rev., 2008, 37, 980–992 RSC.
- H. Kneipp and K. Kneipp, Curr. Phys. Chem., 2013, 3, 136–139 CrossRef CAS.
- T. Wu, X. Zhang, R. Wang and X. Zhang, J. Phys. Chem. C, 2016, 120, 14795–14804 CrossRef CAS.
- S. O. Pour, S. E. J. Bell and E. W. Blanch, Chem. Commun., 2011, 47, 4754–4756 RSC.
- S. Efrima, Chem. Phys. Lett., 1983, 102, 79–82 CrossRef CAS.
- C. Johannessen, P. C. White and S. Abdali, J. Phys. Chem. A, 2007, 111, 7771–7776 CrossRef CAS PubMed.
- N. Berova, K. Nakanishi and R. W. Woody, Circular Dichroism: Principles and Applications, John Wiley & Sons, 2000 Search PubMed.
- M. Schäferling, Chiral nanophotonics, Springer, 2017 Search PubMed.
- E. Mohammadi, K. L. Tsakmakidis, A. N. Askarpour, P. Dehkhoda, A. Tavakoli and H. Altug, ACS Photonics, 2018, 5, 7 CrossRef.
- D. M. Lipkin, J. Math. Phys., 1964, 5, 696 CrossRef.
- Y. Tang and A. E. Cohen, Phys. Rev. Lett., 2010, 104, 163901 CrossRef PubMed.
- Y. Tang and A. E. Cohen, Science, 2011, 332, 333–336 CrossRef CAS PubMed.
- E. Hendry, T. Carpy, J. Johnston, M. Popland, R. V. Mikhaylovskiy, A. J. Lapthorn, S. M. Kelly, L. D. Barron, N. Gadegaard and M. Kadodwala, Nat. Nanotechnol., 2010, 5, 783 CrossRef CAS PubMed.
- Y. Zhao, A. N. Askarpour, L. Sun, J. Shi, X. Li and A. Alù, Nat. Commun., 2017, 8, 14180 CrossRef CAS PubMed.
- C. Gilroy, S. Hashiyada, K. Endo, A. S. Karimullah, L. D. Barron, H. Okamoto, Y. Togawa and M. Kadodwala, J. Phys. Chem. C, 2019, 123, 15195–15203 CrossRef CAS.
- S. Unser, I. Bruzas, J. He and L. Sagle, Sensors, 2015, 15, 15684 CrossRef PubMed.
- K. A. Willets and R. P. V. Duyne, Annu. Rev. Phys. Chem., 2007, 58, 267–297 CrossRef CAS PubMed.
- H.-H. Jeong, A. G. Mark, M. Alarcón-Correa, I. Kim, P. Oswald, T.-C. Lee and P. Fischer, Nat. Commun., 2016, 7, 11331 CrossRef PubMed.
- H.-H. Jeong, A. G. Marka and P. Fischer, Chem. Commun., 2016, 52, 12179–12182 RSC.
- M. Matuschek, D. P. Singh, H. H. Jeong, M. Nesterov, T. Weiss, P. Fischer, F. Neubrech and N. Liu, Small, 2018, 14, 1702990 CrossRef PubMed.
- C. Kelly, R. Tullius, A. J. Lapthorn, N. Gadegaard, G. Cooke, L. D. Barron, A. S. Karimullah, V. M. Rotello and M. Kadodwala, J. Am. Chem. Soc., 2018, 140, 8509–8517 CrossRef CAS PubMed.
- M. Rodier, C. Keijzer, J. Milner, A. S. Karimullah, L. D. Barron, N. Gadegaard, A. J. Lapthorn and M. Kadodwala, J. Phys. Chem. Lett., 2019, 10, 6105–6111 CrossRef CAS PubMed.
- R. Tullius, G. W. Platt, L. Khosravi Khorashad, N. Gadegaard, A. J. Lapthorn, V. M. Rotello, G. Cooke, L. D. Barron, A. O. Govorov, A. S. Karimullah and M. Kadodwala, ACS Nano, 2017, 11, 12049–12056 CrossRef CAS PubMed.
- V. E. Bochenkov and D. S. Sutherland, Opt. Express, 2018, 26, 27101–27108 CrossRef CAS PubMed.
- K. Q. Le, S. Hashiyada, M. Kondo and H. Okamoto, J. Phys. Chem. C, 2018, 122, 24924–24932 CrossRef CAS.
- T. Cao, Y. Li, C.-W. Wei and Y.-M. Qiu, Opt. Express, 2017, 25, 9911–9925 CrossRef CAS PubMed.
- T. Cao, Y. Li, X. Zhang and Y. Zou, Photonics Res., 2017, 5, 441–449 CrossRef CAS.
- T. Cao, C.-W. Wei, L.-B. Mao and S. Wang, Opt. Express, 2015, 23, 18620–18629 CrossRef CAS PubMed.
- T. Cao, L. Mao, Y. Qiu, L. Lu, A. Banas, K. Banas, R. E. Simpson and H. C. Chui, Adv. Opt. Mater., 2019, 7, 1801172 CrossRef.
- T. Cao and Y. Qiu, Nanoscale, 2018, 10, 566–574 RSC.
- X. Tian, Y. Fang and B. Zhang, ACS Photonics, 2014, 1, 1156–1164 CrossRef CAS.
- S. Zu, Y. Bao and Z. Fang, Nanoscale, 2016, 8, 3900–3905 RSC.
- Y. Luo, C. Chi, M. Jiang, R. Li, S. Zu, Y. Li and Z. Fang, Adv. Opt. Mater., 2017, 5, 1700040 CrossRef.
- T. Han, S. Zu, Z. Li, M. Jiang, X. Zhu and Z. Fang, Nano Lett., 2018, 18, 567–572 CrossRef CAS PubMed.
- C. I. Osorio, T. Coenen, B. J. M. Brenny, A. Polman and A. F. Koenderink, ACS Photonics, 2016, 3, 147–154 CrossRef CAS.
- S. Zu, T. Han, M. Jiang, Z. Liu, Q. Jiang, F. Lin, X. Zhu and Z. Fang, Nano Lett., 2019, 19, 775–780 CrossRef PubMed.
- S. Zu, T. Han, M. Jiang, F. Lin, X. Zhu and Z. Fang, ACS Nano, 2018, 12, 3908–3916 CrossRef CAS PubMed.
- Z. Liu, M. Jiang, Y. Hu, F. Lin, B. Shen, X. Zhu and Z. Fang, Opto-Electron. Adv., 2018, 1, 180007 Search PubMed.
- M. Schäferling, D. Dregely, M. Hentschel and H. Giessen, Phys. Rev. X, 2012, 2, 031010 Search PubMed.
- M. L. Tseng, Z. H. Lin, H. Y. Kuo, T. T. Huang, Y. T. Huang, T. L. Chung, C. H. Chu, J. S. Huang and D. P. Tsai, Adv. Opt. Mater., 2019, 1900617 CrossRef.
- H.-E. Lee, H.-Y. Ahn, J. Mun, Y. Y. Lee, M. Kim, N. H. Cho, K. Chang, W. S. Kim, J. Rho and K. T. Nam, Nature, 2018, 556, 360–365 CrossRef CAS PubMed.
- J. García-Guirado, M. Svedendahl, J. Puigdollers and R. Quidant, Nano Lett., 2018, 18, 6279–6285 CrossRef PubMed.
- C. Kuppe, X. Zheng, C. Williams, A. W. A. Murphy, J. T. Collins, S. N. Gordeev, G. A. E. Vandenbosch and V. K. Valev, Nanoscale Horiz., 2019, 4, 1056–1062 RSC.
- H. E. Lee, H. Y. Ahn, J. Lee and K. T. Nam, ChemNanoMat, 2017, 3, 685–697 CrossRef CAS.
- Y. Zhao, L. Xu, W. Ma, L. Wang, H. Kuang, C. Xu and N. A. Kotov, Nano Lett., 2014, 14, 3908–3913 CrossRef CAS PubMed.
- X. Wu, L. Xu, L. Liu, W. Ma, H. Yin, H. Kuang, L. Wang, C. Xu and N. A. Kotov, J. Am. Chem. Soc., 2013, 135, 18629–18636 CrossRef CAS PubMed.
- J. Cai, C. Hao, M. Sun, W. Ma, C. Xu and H. Kuang, Small, 2018, 14, 1703931 CrossRef PubMed.
- J. Kumar, H. Eraña, E. López-Martínez, N. Claes, V. F. Martín, D. M. Solís, S. Bals, A. L. Cortajarena, J. Castilla and L. M. Liz-Marzán, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 3225–3230 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |
