A layered basic zinc acetate coating for dendrite-free Zn anodes by interface environment regulation in aqueous zinc-ion batteries†
Qingsong
Cai
a,
Zhenyu
Guan
a,
Yue
Hu
a,
Jianmin
Zhang
*a,
Kai
Zhang
 *b and
Zongmin
Zheng
*b and
Zongmin
Zheng
 *a
*a
aNational Engineering Research Center for Intelligent Electrical Vehicle Power System, College of Mechanical and Electrical Engineering, Qingdao University, Qingdao, 266071, China. E-mail: zhangjm@qdu.edu.cn; zmzheng@qdu.edu.cn
bKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin 300071, China. E-mail: zhangkai_nk@nankai.edu.cn
First published on 15th January 2025
Abstract
Zinc metal is an excellent anode material due to its high theoretical capacity, low cost, good safety, and many other advantages. However, uncontrollable side reactions and dendrite growth remain significant challenges in developing aqueous zinc-ion batteries (ZIBs). Building artificial coatings is one of the most effective strategies for addressing these problems. Herein, porous layered basic zinc acetate nanosheets (LBZA) were innovatively constructed to protect zinc anodes (LBZA@Zn) against uncontrollable side reactions and dendrite growth in aqueous ZIBs. The hydrophobic and positively charged surface eventually constructs a local high-concentration region, thereby facilitating the dissociation and transport of Zn2+, as well as reducing the hydrogen evolution reaction and the by-product growth of the Zn anode. The LBZA@Zn∥LBZA@Zn symmetrical cell has a cycling stability of 1800 h at 2 mA cm−2 and 1 mA h cm−2. While the LBZA@Zn∥Cu cell maintains stable deposition of Zn2+ after 5000 cycles at 1 mA cm−2 and 0.5 mA h cm−2, with a high average coulombic efficiency of 99.91%. These findings provide new ideas for the construction of efficient artificial coatings.
1. Introduction
In the context of carbon neutrality, lithium-ion batteries (LIBs) consistently dominate the field of new energy batteries due to their light weight design and high energy density.1 With the rapid growth, LIBs have also exposed several issues, including high costs, poor safety, and sustainability concerns. These factors accelerate the exploration of more efficient, economical, and secure energy storage devices.2–4 Zinc-ion batteries (ZIBs) are considered one of the most promising next-generation energy storage systems due to their high theoretical specific capacity, low reduction potential, low cost, and abundant reserves.5–8 However, the exposed zinc metal as a negative electrode suffers from inherent defects and roughness, leading to uneven distribution of the electric field. This uneven distribution promotes Zn2+ to gather at protrusions, resulting in dendrite formation and potential short circuits within the battery.9 Moreover, direct contact between the zinc metal anode and the electrolyte exacerbates the hydrogen evolution reaction (HER).10,11 Hence, uncontrollable zinc dendrite growth, surface passivation, and the HER significantly impede the further advancement of ZIBs.12–14 To address these challenges, several mitigation methods have been proposed, including artificial coating construction,15 three-dimensional skeleton,16 membrane modifications,17 electrolyte additives,18 hydrogel electrolytes,19 and composite electrodes.20 Among these strategies, artificial coating construction has emerged as one of the most direct and effective approaches for controlling the electrode–electrolyte interface.21Presently, many types of materials have been investigated as surface modification layers for zinc metal anodes for ZIBs, including carbon-based materials, metal particles and alloys, and inorganic acid salts. For carbon-based material coatings, reduced graphene oxide (rGO),22 zinc-affinitive carbon nanotubes (CNTs),23 and porous carbon24 were used to improve the electrochemical performance and induce uniform deposition of Zn2+. However, when the deposition/stripping cycles are increased to a certain value, the effectiveness of the conductive coating is greatly reduced, thereby increasing the risk of dendrite formation. Metal nanoparticles25 and alloy coatings26 can serve as nucleation sites, inducing uniform deposition of Zn2+. However, alloy elements may exhibit uneven distribution, and an excessive or inappropriate application of metal nanoparticle coatings may impact the electrode's performance. Recently, a lot of research has been done in the field of hydrophilic coatings, including inorganic27–29 and organic coatings.30,31 These coatings with excellent hydrophilicity enhance the wettability of the electrode surface and speed up the migration speed of Zn2+. However, while hydrophilic coatings facilitate Zn2+ transport, they also tend to accumulate large amounts of water molecules and SO42−, which excessively participate in side reactions, intensifying hydrogen evolution and byproduct formation, thereby leading to dendrite growth. Additionally, during cycling, the residual Zn2+ within the coating may cause volume expansion and detachment of the coating, ultimately resulting in the failure of the interfacial layer. Therefore, it is crucial to design a multifunctional “zincphilic and hydrophobic” interfacial layer with a specific Zn2+ transport channel that allows for uniform and dense Zn deposition beneath the coating, while simultaneously suppressing the growth of zinc dendrites and byproducts.
Herein, we propose layered basic zinc acetate (LBZA) nanosheets to modify the zinc metal. The typical double-layer structure of LBZA contains two positively charged hydroxide layers and interlayer anions (Fig. S1†). Due to the 2D structure of the LBZA, the smooth and tightly packed coating can prevent direct contact between the Zn anode and the aqueous electrolyte. At the same time, the hydrophobic and positively charged surface finally builds a localized high-concentration region whose surface can greatly immobilize SO42−. It further prevents the entry of water molecules and anions and adjusts the interfacial solvation structure of Zn2+. Due to the greatly reduced number and activity of free water molecules, the hydrogen evolution reaction (HER) and the formation of by-products will be reduced on the Zn anode side. More importantly, the OAc− also provides zincphilic sites for the transport of Zn2+, leading to the flat deposition of Zn2+ (002), thereby effectively inhibiting the uncontrollable growth of dendrites.
2. Experimental section
2.1. Chemicals
MgO powder was purchased from XFNANO Materials Tech Co., Ltd. Zinc acetate (Zn(CH3COO)2·2H2O) and NaCl were purchased from Sinopharm Chemical Reagent Co., Ltd. Divanadium pentoxide (V2O5) was purchased from Macklin. Zinc sulfate (ZnSO4·7H2O) was purchased from Aladdin. The zinc foil (80 μm in thickness) was polished with 1200 mesh sandpaper to remove the surface oxide layer, then cleaned and dried as a bare Zn electrode with a diameter of 12 mm.2.2. Materials synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and an appropriate amount of N-methyl 2-pyrrolidone (NMP) solvent. The resulting slurry was coated on a graphite sheet and dried in a vacuum oven at 80 °C for 12 hours. After taking it out, it was cut it into a disc with a diameter of 12 mm as a positive electrode. The mass loading of active materials is controlled between 2–3 mg cm−2.
1, and an appropriate amount of N-methyl 2-pyrrolidone (NMP) solvent. The resulting slurry was coated on a graphite sheet and dried in a vacuum oven at 80 °C for 12 hours. After taking it out, it was cut it into a disc with a diameter of 12 mm as a positive electrode. The mass loading of active materials is controlled between 2–3 mg cm−2.
2.3. Materials characterization
A Fourier transform infrared spectrometer (FT-IR) was used to characterize the functional groups of the material. The powder X-ray diffraction (XRD) patterns of the material were collected on a Rigaku Ultima IV X-ray diffractometer with Cu Kα radiation. The contact angles between bare Zn and LBZA@Zn electrodes and the aqueous ZnSO4 electrolyte were tested using the Biolin contact angle measuring system (Theta Flex, Biolin, Sweden). Zeta potential testing was performed on the Brookhaven particle size analysis and Zeta potentiometer (NanoBrook 90 Plus Zeta, USA). Raman spectra were recorded using a Renishaw inVia a microscope with a wavelength of 532 nm. A high-resolution transmission electron microscope (HRTEM, JEOL JEM-2100Plus) and a field emission scanning electron microscope (FESEM, JEOL JSM-7800F) equipped with an energy-dispersive X-ray spectrometer (EDX, OXFORD X-Max) were used to examine the microstructure and Particle size. X-ray photoelectron spectroscopy (XPS) was tested using a K-Alpha electron spectrometer (PHI5000 Versaprobe III, Japan).2.4. Electrochemical measurements
In the experiment, glass fiber (Whatman GF/D) was used as the separator, and 2 M ZnSO4 solution was used as the electrolyte to assemble Zn∥Zn symmetric cells for electrochemical testing. Zn∥Cu asymmetric cells with Cu foil as the positive electrode were used. Linear sweep voltammetry (LSV), linear polarization (Tafel) curves, chrono-amperometry (CA), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were recorded on an electrochemical workstation (CHI 660E, S4 China). For LSV and Tafel curves a three-electrode system was used. In the 2 M ZnSO4 electrolyte, a bare Zn electrode was used as the working electrode, AgCl as the reference electrode, and a platinum electrode as the auxiliary electrode. The CA test was conducted with Zn∥Zn and LBZA@Zn∥LBZA@Zn symmetric cells under constant potential −150 mV. The CV curve of Zn∥Cu asymmetric cells is set with a scanning rate of 10 mV s−1 and a potential range of −0.2–0.8 V. The scan rate of the CV curve of the Zn∥NVO full cell is 0.5 mV s−1 and the frequency range of EIS is from 50 MHz to 0.01 Hz.The Zn2+ transfer number is tested by Zn∥Zn symmetric cells and calculated according to eqn (1).
 | (1) |
 | (2) |
 | (3) |
2.5. COMSOL simulations
In order to protect the protective effect of LBZA coating, a simplified two-dimensional model was established using COMSOL Multiphysics simulation software. Combined with the phase field model framework, a partial differential equation was established to simulate the growth of zinc dendrites in the LBZA coating simulation, and the ion field calculation domain was represented by a two-dimensional plane. The size of the entire two-dimensional simulation model is 6 × 8 μm. The upper part of the geometric model represents the nodes, and the opposite part is the topology. Assuming that the original nucleation point of the zinc dendrite is a small circular shape, multi-physics coupling simulation was realized.3. Results and discussion
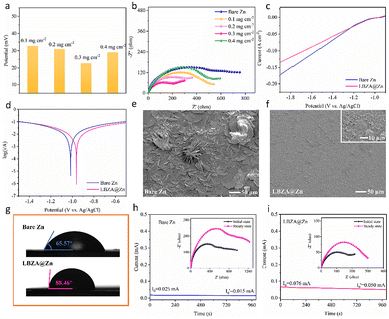
The LBZA@Zn electrode was prepared using a simple drop coating method (Fig. 1a). Firstly, the LBZA powder was synthesized by the phase converting between solid MgO nanoparticles and Zn(CH3COOH)2 (ZnOAc) salt. Then, ultrasonically dispersed LBZA suspension was coated on the surface of bare polished zinc foil. The mass loading can be easily controlled by adjusting the volume of the suspension. In Fig. 1b, the XRD (X-ray diffraction) pattern of LBZA is consistent with the zinc basic salts (PDF#56-0569).33,34 The diffraction peaks at 7.11°, 14.14°, and 33.07° can be indexed to (001), (002), and (100) crystal planes, respectively. Fig. 1c shows a typical TEM (transmission electron microscopy) image of the as-prepared LBZA powder, in which a band-like structure with obvious pores can be found. The pore size distribution is around 5 nm measured by the N2 adsorption–desorption analysis (Fig. S2†), consistent with the size statistics in Fig. S3.† The high-resolution TEM images in Fig. 1d and Fig. S4† show the interplanar spacing of 0.272 nm, corresponding to the (100) crystal plane.35 To further investigate the structure of LBZA, the FT-IR (Fourier transform infrared spectroscopy) spectrum in Fig. 1e represents the typical characteristic absorption bands of O–H (3589 cm−1), OAc− (1552, 1394 cm−1), and CH3 (1334, 1018 cm−1).36 There is an obvious peak position difference in OAc− shown in the data, which is mainly attributed to the symmetric stretches and asymmetric stretches of OAc− (Δa–s = Va (OAc−) − Vs(OAc−)).37 This peak position difference (≈158 cm−1) indicates that OAc− is in a state of near freedom between the LBZA double-layer structure.38 For the as-prepared electrodes, compared with the bare Zn (inevitable scratches and bulges on the surface), the surface of the LBZA@Zn electrode is evenly and densely coated with LBZA layers (Fig. 1f). And, the cross-sectional SEM image in Fig. 1g reveals a thickness of approximately 4 μm when the mass loading is 0.3 mg cm−2. To further assess the adhesion performance of the LBZA coating, a cross-cut tester was used. As shown in Fig. S5,† a small amount of coating material remained on the adhesive tape with no large-area peeling observed. Simultaneously, the energy-dispersive X-ray spectroscopy (EDS) analysis in Fig. S6† (top-section) and Fig. 1g (cross-section) all validate the uniform distribution of Zn, C, and O elements, and the formation of a relatively smooth and dense interfacial layer on the zinc metal surface. The flat and dense flake structure of LBZA coating can effectively weaken the “tip effect” of the electric field, induce the uniform deposition of Zn2+, and inhibit the uncontrollable growth of dendrites.To investigate the effect of the thickness of the LBZA coating on the physical and electrochemical properties of the electrodes, Zn∥Zn symmetric cells were assembled and tested. Compared to the bare Zn electrode, the various LBZA coatings all obviously prolong the cyclic life of the stripping/deposition process (Fig. S7†). When the mass loading is 0.3 mg cm−2, the LBZA@Zn electrode displays much lower stable overpotential (Fig. 2a) and charge transfer resistance (Rct) (Fig. 2b). The porous structure of the evenly coated LBZA layer contributes to the fast transfer of Zn2+ in the solid–liquid interface. Unless otherwise stated, 0.3 mg cm−2 was chosen for the next measurements and discussions. To evaluate the protective effect of LBZA coating, linear sweep voltammetry curves (LSV) and Tafel plots of the electrodes were studied in 2 M ZnSO4 solution using a three-electrode system. The results show that the hydrogen evolution current density of the LBZA@Zn electrode is always lower than that of the bare Zn electrode (Fig. 2c), indicating that the LBZA coating can effectively inhibit water-induced side reactions and unnecessary by-product accumulation. The corrosion resistance and corrosion rate of the zinc anode in the electrolyte are shown in Fig. 2d. The results show that compared to the bare Zn electrode, the corrosion potential of the LBZA@Zn electrode shifted positively (from −1.016 V to −0.962 V). To further confirm the corrosion resistance of the LBZA coating, bare Zn and LBZA@Zn electrodes were immersed in 2 M ZnSO4 electrolyte for 7 days. The digital photos in Fig. S8† show that a megascopic white coating gradually covers the polished bare Zn, while the LBZA@Zn electrode remains in the initial state. The SEM image in Fig. 2e further shows that hexagonal flakes by-products accumulate on the surface of the bare Zn electrode, indicating serious side reactions. For the LBZA@Zn electrode, there is no obvious corrosion phenomenon (Fig. 2f). The difference in the hydrophobic and hydrophilic performance of bare Zn and LBZA@Zn electrodes with the electrolyte was also investigated. As shown in Fig. 2g, the LBZA@Zn electrode exhibits a certain degree of hydrophobicity with a contact angle of 88.46°, higher than the bare Zn (65.57°). The hydrophobic surface caused by the methyl group in the OAc− is beneficial for isolating active H2O, inhibiting side reactions, and reducing the HER activity.39 To further prove the role of the LBZA hydrophobic film in the coin cells, the symmetrical cells were cycled for 100 h under the conditions of 0.5 mA cm−2 and 0.5 mA h cm−2. The Zn∥Zn symmetrical cell displays larger magnitudes in thickness after cycling than the LBZA@Zn∥LBZA@Zn cell (Fig. S9†). This is mainly because the bare Zn electrode undergoes serious side reactions with the aqueous solution during the cycles, producing a large amount of hydrogen (H2), which causes the coin cell to expand. Instead, the LBZA@Zn electrode effectively reduces the HER due to the hydrophobicity of the coating. Besides the effective protection, the transport property of Zn2+ through the interface layer is also crucial. The Zn2+ transference number (tZn2+) of the LBZA@Zn electrode is calculated to be 0.62, which is higher than that of bare Zn 0.34 (Fig. 2h and i). The above analysis shows that the hydrophobic interface prevents the Zn anode from directly contacting with active H2O, meanwhile, the porous LBZA layer provides multi-channel transmission of Zn2+ and promotes the migration of Zn2+.
Subsequently, the cycling and rate stability of Zn∥Zn symmetric cells were further evaluated. The LBZA@Zn∥LBZA@Zn cell exhibits an impressive lifetime of over 1100 h at 0.5 mA cm−2 and 0.5 mA h cm−2 (Fig. S10†). In contrast, there is a short-circuit after 90 h for the Zn∥Zn symmetrical cell. Even at a higher current density and areal capacity, the LBZA@Zn∥LBZA@Zn cells can remain stable over 1300 h (Fig. 3a, at 1 mA cm−2 and 1 mA h cm−2) and 1800 h (Fig. 3b, at 2 mA cm−2 and 1 mA h cm−2). The excellent stability of the LBZA-protected electrode is superior to the previous works (Table S1†). In addition, the rate performances of bare Zn and LBZA@Zn electrodes were compared at a capacity density of 1 mA h cm−2 and a current density ranging from 0.5 to 20 mA cm−2 (Fig. 3c). As the current density increases, the LBZA@Zn∥LBZA@Zn symmetrical cell always maintains a low and stable polarization voltage. After the high current shock, a short-circuit quickly occurs in the Zn∥Zn symmetrical cell, while the LBZA@Zn∥LBZA@Zn cell remains stable. By analyzing the relationship between the voltage hysteresis and the current density of these charge/discharge profiles, the exchange current densities for the zinc plating/stripping reactions in the symmetrical cell were found to be 9.81 mA cm−2 and 11.07 mA cm−2 respectively (Fig. S11†). This indicates that the LBZA coating significantly enhances the zinc deposition kinetics and has excellent transmission and protection capabilities, achieving rapid and stable plating/stripping behaviors.40
 | ||
| Fig. 3 Voltage-time curves of the Zn∥Zn and LBZA@Zn∥LBZA@Zn symmetric cells under the conditions of (a) 1 mA cm−2 and 1 mA h cm−2, (b) 2 mA cm−2 and 1 mA h cm−2, (c) various current densities. | ||
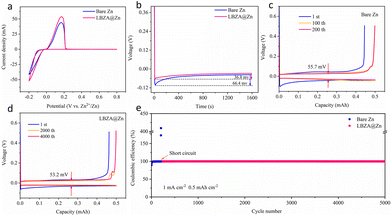
To evaluate the kinetics of the stripping/plating process, the Zn∥Cu asymmetric cells were further investigated using cyclic voltammetry (CV) and constant current charge–discharge methods. As shown by the CV curves in Fig. 4a, the redox current of the LBZA@Zn∥Cu asymmetric cell is notably higher, indicating an increased number of active sites on the LBZA coating surface and accelerated reaction kinetics. Additionally, the nucleation overpotential for the LBZA@Zn∥Cu cell at 1 mA cm−2 is 36.8 mV, significantly lower than that of Zn∥Cu cell (66.4 mV) (Fig. 4b). The long-time cycling was performed at a current density of 1 mA cm−2 with an electroplating capacity of 0.5 mA h cm−2 and a charging cut-off potential of 0.5 V (vs. Zn2+/Zn). The voltage hysteresis of the Zn∥Cu cell gradually increases over 200 cycles and reaches 55.7 mV (Fig. 4c). In contrast, the LBZA@Zn∥Cu cell exhibited a significantly lower voltage hysteresis of approximately 53.2 mV over 4000 cycles (Fig. 4d). Moreover, the LBZA@Zn∥Cu cell can reversibly cycle over 5000 cycles with an average coulombic efficiency of 99.91% (Fig. 4e), which is superior to most previous works (Table S2†). As a comparison, the Zn∥Cu cell experiences a short circuit only after 210 cycles. These results show that the LBZA coating can provide more zincphilic sites, effectively promoting the uniform deposition of Zn2+ and suppressing the zinc dendrite growth.
 | ||
| Fig. 4 (a) CV curves, (b) zinc deposition voltage-time curves, (c and d) voltage-capacity curves at different cycles and (e) the coulombic efficiency (CE) of Zn∥Cu and LBZA@Zn∥Cu asymmetric cells. | ||
To further analyze the plating/stripping mechanism of Zn2+, bare Zn and LBZA@Zn electrodes were assembled into symmetric beaker-type cells and coin cells respectively. In the symmetric beaker-type cells, as shown in Fig. S12,† the overpotential of the LBZA@Zn electrode is always lower than that of the bare Zn electrode discharged at 0.5 mA cm−2 for 20 h. In addition, the overpotential of the bare Zn electrode fluctuates during the test. This fluctuation may be due to severe dendrite growth and shedding on the surface of the bare Zn electrode.41 It can be seen from the digital photos (Fig. S13†) and SEM images (Fig. 5a) that disorderly growing zinc dendrites and by-products appeared on the surface of the bare Zn electrode. According to the EDS elemental mapping analysis in Fig. S14 and S15,† it is known that the surface sediments consist of spongy zinc dendrites and hexagonal by-products. However, under the same plating conditions, dense and uniform zinc deposits under the LBZA layer (Fig. 5b). On the stripping electrodes, numerous pores are observed on the bare Zn electrode (Fig. 5c), while the surface is smooth on the LBZA@Zn electrode (Fig. 5d). When the beaker-type cells are cycled at 0.5 mA cm−2 and 0.5 mA h cm−2 for 1 cycle, the morphology of the surface of the bare Zn electrode undergoes significant changes. Numerous hexagonal flake-like crystals are attached to the surface disorderly (Fig. 5e). At the scratched areas, due to the non-uniform distribution of the electric field, the deposition of Zn2+ and the aggregation of by-products are more pronounced. In contrast, the Zn2+ is uniformly deposited under the LBZA coating (Fig. 5f). The plating/stripping mechanism of Zn2+ was further investigated in coin symmetric cells by morphology, phase, and dynamical model analysis. After being cycled at 1 mA cm−2 and 1 mA h cm−2 for 200 h, the surface morphologies of different electrodes after 100 cycles were analyzed by SEM. The surface of the bare Zn electrode also exhibited a significant amount of dendritic growth and by-products (Fig. 5g). In contrast, the surface of the LBZA@Zn electrode showed dense and smooth deposition and large amounts of zinc tend to be deposited in a two-dimensional direction (Fig. 5h). Similarly, the XRD patterns in Fig. S16† show a significant difference in the crystallographic orientation of deposited Zn on the cycled bare Zn and LBZA@Zn electrodes. The (002) diffraction peak of the cycled LBZA@Zn electrode is greatly enhanced compared with the bare Zn electrode. These results suggest that the LBZA coating facilitates Zn deposition along the (002) crystal plane orientation while suppressing Zn deposition along the (100) and (101) crystal plane orientations. The interaction between functional groups in the LBZA coating and the Zn (002) plane reduces surface energy and induces Zn deposition along the (002) crystal plane. Furthermore, the LBZA coating inhibits the two-dimensional diffusion of Zn2+, ensuring a uniform zinc concentration distribution.42 X-ray photoelectron spectroscopy (XPS) analysis of post-cycling electrodes reveals that for the LBZA@Zn electrode, an increased proportion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the C 1s spectrum (Fig. S17a†) indicates the residual acetates on the electrode surface. Meanwhile, the increased ZnS content in the S 2p spectrum indicates effective anion immobilization and enhanced anion reduction at the interface (Fig. S17b†).43 In contrast, the bare Zn electrode generates significantly more by-products during cycling (Fig. S17c and S17d†).44,45 Moreover, the zinc deposition behavior was further studied using the chronoamperometry (CA) method.46 The results in Fig. 5i indicate that bare Zn exhibits a two-dimensional diffusion curve with lateral migration of Zn2+, leading to uneven nucleation and consequently uncontrolled growth of zinc dendrites.47 In contrast, the LBZA@Zn electrode exhibits a three-dimensional stable diffusion after a short period of two-dimensional diffusion, indicating that the LBZA coating can regulate the diffusion of Zn2+ and promote uniform deposition.48
O bonds in the C 1s spectrum (Fig. S17a†) indicates the residual acetates on the electrode surface. Meanwhile, the increased ZnS content in the S 2p spectrum indicates effective anion immobilization and enhanced anion reduction at the interface (Fig. S17b†).43 In contrast, the bare Zn electrode generates significantly more by-products during cycling (Fig. S17c and S17d†).44,45 Moreover, the zinc deposition behavior was further studied using the chronoamperometry (CA) method.46 The results in Fig. 5i indicate that bare Zn exhibits a two-dimensional diffusion curve with lateral migration of Zn2+, leading to uneven nucleation and consequently uncontrolled growth of zinc dendrites.47 In contrast, the LBZA@Zn electrode exhibits a three-dimensional stable diffusion after a short period of two-dimensional diffusion, indicating that the LBZA coating can regulate the diffusion of Zn2+ and promote uniform deposition.48
To deeply understand the mechanism of the LBZA coating, the surface charge effect of LBZA was analyzed. The zeta potential of original LBZA in pure water is 47.9 mV, while in the ZnSO4 solution, the zeta potential moves to −4.71 mV (Fig. 6a), indicating that LBZA can greatly adsorb SO42− on the surface in the electrolyte environment. The FT-IR spectra of LBZA powder before and after being soaked in ZnSO4 solution are shown in Fig. 6b. Among them, the stretching and contraction bands of CH3 are at (1336 cm−1) and (1016 cm−1).36 The strong and broad peak appearing near 1120 cm−1 in the spectrum after soaking indicates the presence of SO42−. In addition, the Raman spectra of the LBZA@Zn electrode before and after cycling were also tested. The existence of SO42− (970 cm−1) was also detected through the Raman spectrum of the LBZA@Zn electrode after cycling (Fig. S18†). Before cycling, the C–C stretching vibration band was at 940 cm−1. However, since the LBZA is unstable in an acidic environment and is prone to releasing acetic acid molecules, the coordination mode of the acetate group may change or even fall off, resulting in a significant shift in the stretching vibration frequency of the original C–C bond or a weakened signal.37 According to the classical Eigen-Tamm mechanism, the ionic binding of solvated ZnSO4 aqueous solution can be divided into solvent separation ion pairs (SSIPs, the ions coordinate with water, and SO42− is separated by solvent molecules) and contact ion pairs (CIPs, a certain amount of SO42− binds tightly to Zn2+ in the coordination sphere).49 A dynamic equilibrium model was established to visually reveal the condition of the water (Fig. S19†).50 As shown in Fig. 6c, as the concentration increases, the vibration of Zn–OH2 (≈390 cm−1) is inhibited, and more Zn–O vibrations evolve into the [Zn2+·OSO3]2− ligand pattern (≈260 cm−1).51 Meanwhile, a high-frequency shift occurs from SSIP to CIP regions according to the n-SO42− band around 981 cm−1 (Fig. 6d), indicating that more Zn2+ coordinates with the anion and the typical [Zn(H2O)6]2+ complex is significantly inhibited.52 It is also found that the tensile band [HOH–OH2] shifts to high frequency [HOH–OSO32−], indicating that the activity of free H2O molecules in the electrolyte is inhibited (Fig. 6e).53 To study the desolvation behavior of hydrated Zn2+ at the zinc deposition interface, the activation energy (Ea) of the Zn2+ desolvation process was first calculated using the Arrhenius equation. In Fig. S20,† the activation energy value when using the LBZA@Zn electrode (17.9 kJ mol−1) is smaller than that when using the bare Zn electrode (46.1 kJ mol−1). This is because the hydrophobic property of the LBZA interfacial layer and the electrostatic attraction between the positively charged LBZA surface and sulfate ions are conducive to promoting the desolvation of Zn2+.54Fig. 6f illustrates the mechanism of LBZA coating to affect the solvation structure of the electrolyte. The hydrophobic and positively charged surface eventually constructs a local high-concentration region, bringing abundant CIPs to facilitate the dissociation and transport of Zn2+. The COMSOL Multiphysics simulation software and phase-field principles were used to simulate the dendritic growth (Fig. 6g) and the distribution of the Zn2+ concentration field (Fig. 6h). The results reveal that multi-layered porous LBZA coating ensures uniform ion concentration and electric field on the electrode surface. This substantial suppression of uncontrolled dendritic growth guarantees the uniform upward growth of metallic zinc.
Thus, the mechanism of LBZA coating is gradually clear and summarized in Fig. 6i. For the bare Zn electrode, inevitable scratches, and protrusions on its surface result in uneven electric field distribution, and the “tip effect”.55 Additionally, there are other challenges like corrosion, generation of H2, and formation of alkali sulfate by-products, which seriously affect the cycling performance of the bare Zn anode. For the LBZA@Zn electrode, the LBZA coating has many positive effects in improving the existing problems of the bare Zn anode: (1) physical smooth coating significantly reduces the “tip-effect” of the electric field, resulting in uniform nucleation and deposition of Zn; (2) the hydrophobicity LBZA coating can effectively reduce the contact between the electrode and the isolate active H2O, thus inhibit the HER; (3) positive charged surface can greatly fix the SO42− anions and regulate the interfacial solvation structure of Zn2+. (4) The porous layered structure and the interlayer OAc− anions provide more channels and zincophilic sites for Zn2+ migration.
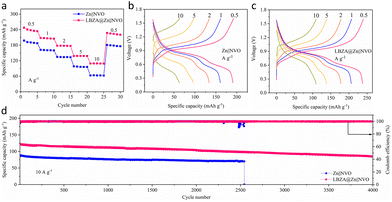
To verify the actual electrochemical properties of the LBZA@Zn electrode, the commercial NVO was chosen as the positive electrode material for assembling the full cells.56,57 In Fig. S21,† the LBZA@Zn∥NVO full cell displays two reduction/oxidation peaks in the cyclic voltammetry (CV) curves, which correspond to the valence changes of V5+/V4+ and V4+/V3+ respectively.58 In addition, it shows a smaller charge transfer resistance in the electrochemical impedance spectroscopy (EIS). Fig. 7a shows the rate properties of the Zn∥NVO and LBZA@Zn∥NVO full cells. The LBZA@Zn∥NVO cell obtains specific capacities of 237.8, 206.5, 177.8, 138.3, and 109.5 mA h g−1 at current densities of 0.5, 1, 2, 5 and 10 A g−1, respectively. At the same current density, the rate capacity of the Zn∥NVO cell is inferior. According to the charging–discharging curves in Fig. 7b and c, the LBZA@Zn∥NVO full cell still performs a relatively smooth charging–discharging platform at a high current density. The cyclic properties of the full cell were also tested at a current density of 10 A g−1 (Fig. 7d). The increased capacity in the early cycling process is attributed to the activation of the layered NVO.59,60 After 2550 cycles, the capacity of the Zn∥NVO full cell decreases significantly, while the LBZA@Zn∥NVO full cell remains relatively stable. For 4000 cycles, the LBZA@Zn∥NVO full cell still maintained a reversible capacity of 85.3 mA h g−1, far exceeding that of the Zn∥NVO full cell. Based on these results and discussions, the LBZA coating can effectively improve the utilization and stability of the zinc metal anode in the full cells.
4. Conclusions
In summary, we prepared the LBZA coating through a simple and effective method and verified the efficient reversible stability of the LBZA@Zn electrode. Combining theoretical simulation and experiment, the results show that the growth of dendrites on the surface of the LBZA@Zn electrode is significantly inhibited, effectively reducing the non-uniformity of electric field distribution. More importantly, the hydrophobicity of the LBZA coating can effectively reduce the contact between the electrode and active H2O, thereby inhibiting the HER, and its positively charged surface can greatly immobilize SO42− anions and adjust the interfacial solvation structure of Zn2+. In addition, the porous double-layer structure provides a channel for Zn2+ migration, and the free OAc− anions between the layered structures provide more zincophilic sites for Zn2+ migration, which is not only beneficial for the transport of Zn2+ but also shows excellent reversibility, properties and good cycling stability. Therefore, the LBZA@Zn∥LBZA@Zn symmetric cell demonstrated excellent cycling stability at 2 mA cm−2 and 1 mA h cm−2 (1800 h). The LBZA@Zn∥Cu cell exhibited an average coulombic efficiency of 99.91% over 5000 cycles at 1 mA cm−2 and 0.5 mA h cm−2. Furthermore, the LBZA@Zn∥NVO full cell retained a reversible capacity of 85.3 mA h g−1 after 4000 cycles at 10 A g−1, which is significantly higher than the Zn∥NVO full cell. This work highlights the remarkable role of the LBZA coating in protecting the zinc anode and provides new insights into the design of efficient protective coatings.Author contributions
Qingsong Cai: methodology, formal analysis, investigation, and writing – original draft. Zhenyu Guan: formal analysis and data curation. Yue Hu: formal analysis and data curation. Jianmin Zhang: writing – review & editing. Kai Zhang: writing – review & editing and funding acquisition. Zongmin Zheng: conceptualization, writing – review & editing, and funding acquisition.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the Key R&D Program of Shandong Province, China (2022TSGC1252 and 2023TSGC0754), the National Natural Science Foundation of China (22479080, 52072186, 92372203 and 92372001), and the Fundamental Research Funds for the Central Universities of Nankai University (63241206 and 9242000710).References
- J. Q. He, C. H. Lu, H. B. Jiang, F. Han, X. Shi, J. X. Wu, L. Y. Wang, T. Q. Chen, J. J. Wang, Y. Zhang, H. Yang, G. Q. Zhang, X. M. Sun, B. J. Wang, P. Chen, Y. G. Wang, Y. Y. Xia and H. S. Peng, Scalable production of high-performing woven lithium-ion fiber batteries, Nature, 2021, 597, 57–63 CrossRef CAS PubMed
.
- J. Liu, H. Yuan, H. Liu, C. Z. Zhao, Y. Lu, X. B. Cheng, J. Q. Huang and Q. Zhang, Unlocking the Failure Mechanism of Solid State Lithium Metal Batteries, Adv. Energy Mater., 2021, 12, 2100748 CrossRef
.
- Y. Guo, S. C. Wu, Y. B. He, F. Y. Kang, L. Q. Chen, H. Li and Q. H. Yang, Solid-state lithium batteries: Safety and prospects, eScience, 2022, 2, 138–163 CrossRef
.
- Y. Wei, Q. Yang, M. Q. Li, Q. W. Ran, W. Y. Sheng and Y. Xu, Electrochemical behavior and reaction mechanism of nano-BiOBr/rGO composite micro flower with strong interface coupling as potassium-ion battery anodes, J. Colloid Interface Sci., 2025, 679, 1–9 Search PubMed
.
- Y. Zong, H. W. He, Y. Z. Wang, M. H. Wu, X. C. Ren, Z. C. Bai, N. N. Wang, X. Ning and S. X. Dou, Functionalized Separator Strategies toward Advanced Aqueous Zinc–Ion Batteries, Adv. Energy Mater., 2023, 13, 2300403 CrossRef CAS
.
- H. B. Yan, S. M. Li, J. Y. Zhong and B. L. An, Electrochemical Perspective of Aqueous Zinc Metal Anode, Nano-Micro Lett., 2024, 16, 15 CrossRef CAS PubMed
.
- J. N. Hao, S. J. Zhang, H. Wu, L. Yuan, K. Davey and S. Z. Qiao, Advanced cathodes for aqueous Zn batteries beyond Zn2+ intercalation, Chem. Soc. Rev., 2024, 9, 4305 Search PubMed
.
- J. P. Yan, E. H. Ang, Y. Yang, Y. F. Zhang, M. H. Ye, W. C. Du and C. C. Li, High-Voltage Zinc-Ion Batteries: Design Strategies and Challenges, Adv. Funct. Mater., 2021, 31, 2010213 CrossRef CAS
.
- X. X. Guo and G. J. He, Opportunities and challenges of zinc anodes in rechargeable aqueous batteries, J. Mater. Chem. A, 2023, 11, 11987–12001 RSC
.
- M. D. Xue, J. Bai, M. C. Wu, Q. Q. He, Q. C. Zhang and L. Y. Chen, Carbon-assisted anodes and cathodes for zinc ion batteries: From basic science to specific applications, opportunities and challenges, Energy Storage Mater., 2023, 62, 102940 CrossRef
.
- Y. Li, Y. F. Guo, Z. X. Li, P. F. Wang, Y. Xie and T. F. Yi, Carbon-based nanomaterials for stabilizing zinc metal anodes towards high-performance aqueous zinc-ion batteries, Energy Storage Mater., 2024, 67, 103300 CrossRef
.
- Y. X. Gong, B. Wang, H. Z. Ren, D. Y. Li, D. L. Wang, H. K. Liu and S. X. Dou, Recent Advances in Structural Optimization and Surface Modification on Current Collectors for High-Performance Zinc Anode: Principles, Strategies, and Challenges, Nano-Micro Lett., 2023, 15, 208 CrossRef PubMed
.
- X. Y. Li, L. Wang, Y. H. Fu, H. Dang, D. H. Wang and F. Ran, Optimization strategies toward advanced aqueous zinc-ion batteries: From facing key issues to viable solutions, Nano Energy, 2023, 116, 108858 CrossRef CAS
.
- L. Tang, H. J. Peng, J. R. Kang, H. Chen, M. Y. Zhang, Y. Liu, D. H. Kim, Y. J. Liu and Z. Q. Lin, Zn-based batteries for sustainable energy storage: strategies and mechanisms, Chem. Soc. Rev., 2024, 53, 4877–4925 RSC
.
- F. Tao, Y. Liu, X. Y. Ren, J. Wang, Y. Z. Zhou, Y. J. Miao, F. Z. Ren, S. Z. Wei and J. M. Ma, Different surface modification methods and coating materials of zinc metal anode, J. Energy Chem., 2022, 66, 397–412 CrossRef CAS
.
- W. J. Deng, Z. Y. Huang, Z. Q. Zhou, C. Li, Y. Chen, Y. Zhou, C. Huang, J. Zhu, W. X. Zou, R. D. Zhu, Y. S. Xu and R. Li, A Near 0 V and Low-Strain Intercalative Anode for Aqueous Zinc-Ion Batteries, ACS Energy Lett., 2023, 8, 3171–3179 CrossRef CAS
.
- Z. Y. Zheng, S. J. Guo, M. Y. Yan, Y. Z. Luo and F. F. Cao, A Functional Janus Ag Nanowires/Bacterial Cellulose Separator for High-Performance Dendrite-Free Zinc Anode Under Harsh Conditions, Adv. Mater., 2023, 35, 2304667 CrossRef CAS PubMed
.
- S. C. Bai, Z. D. Huang, G. J. Liang, R. Yang, D. Liu, W. Wen, X. Jin, C. Y. Zhi and X. Q. Wang, Electrolyte Additives for Stable Zn Anodes, Adv. Sci., 2023, 11, 2304549 CrossRef PubMed
.
- S. G. Ji, H. Luo, S. Qin, X. Y. Zhang, Y. Y. Hu, W. W. Zhang, J. C. Sun, J. Xu, H. J. Xie, Z. H. Yan and K. Yang, Component Fluctuation Modulated Gelation Effect Enable Temperature Adaptability in Zinc–Ion Batteries, Adv. Energy Mater., 2024, 14, 2301835 Search PubMed
.
- C. H. Cao, K. Q. Zhou, W. C. Du, C. C. Li, M. H. Ye, Y. F. Zhang, Y. C. Tang and X. Q. Liu, Designing Soft Solid–like Viscoelastic Zinc Powder Anode toward High–Performance Aqueous Zinc–Ion Batteries, Adv. Energy Mater., 2023, 13, 2301835 CrossRef CAS
.
- K. Wu, J. Yi, X. Y. Liu, Y. Sun, J. Cui, Y. Xie, Y. Y. Liu, Y. Y. Xia and J. J. Zhang, Regulating Zn Deposition via an Artificial Solid-Electrolyte Interface with Aligned Dipoles for Long Life Zn Anode, Nano-Micro Lett., 2021, 13, 79 CrossRef PubMed
.
- Q. H. Wang, J. Q. Zhao, J. Zhang, X. L. Xue, M. Li, Z. Y. Sui, X. Zhang, W. Zhang and C. H. Lu, Dendrite–Free Zn/rGO@CC Composite Anodes Constructed by One–Step Co–Electrodeposition for Flexible and High–Performance Zn–Ion Batteries, Adv. Funct. Mater., 2023, 33, 2306346 CrossRef CAS
.
- Y. R. Zhou, J. J. Xia, J. T. Di, Z. J. Sun, L. M. Zhao, L. Li, Y. L. Wu, L. Z. Dong, X. N. Wang and Q. W. Li, Ultrahigh–Rate Zn Stripping and Plating by Capacitive Charge Carriers Enrichment Boosting Zn–Based Energy Storage, Adv. Energy Mater., 2023, 13, 2203165 CrossRef CAS
.
- Z. W. Li, D. H. Chen, Y. F. An, C. L. Chen, L. Y. Wu, Z. J. Chen, Y. Sun and X. G. Zhang, Flexible and anti-freezing quasi-solid-state zinc ion hybrid supercapacitors based on pencil shavings derived porous carbon, Energy Storage Mater., 2020, 28, 307–314 CrossRef
.
- Q. F. Huang, L. Y. Shao, X. Y. Shi, J. D. Guan, J. L. Xu, Y. X. Wu and Z. P. Sun, Na3V2O2(PO4)2F nanoparticles@reduced graphene oxide: a high-voltage polyanionic cathode with enhanced reaction kinetics for aqueous zinc-ion batteries, Chem. Eng. J., 2023, 468, 143738 CrossRef CAS
.
- W. Liu, Q. W. Zhao, H. M. Yu, H. Wang, S. Z. Huang, L. J. Zhou, W. F. Wei, Q. C. Zhang, X. B. Ji, Y. J. Chen and L. B. Chen, Metallic Particles–Induced Surface Reconstruction Enabling Highly Durable Zinc Metal Anode, Adv. Funct. Mater., 2023, 33, 2302661 CrossRef CAS
.
- K. L. Liu, Y. F. Li, T. Zhang, A. Q. Zhu, G. Q. Gan, D. W. Lin, K. Liu, C. H. Luan, S. Y. Bu, X. B. Zhang, Y. Yang, C. Yu, Y. Wu, G. Hong and W. J. Zhang, Hydrophilic and Insulative Interface Strategy Against Side Reactions for Dendrite–Free Zinc Metal Anodes, Adv. Funct. Mater., 2024, 2409251 Search PubMed
.
- N. Guo, Z. Peng, W. J. Huo, Y. H. Li, S. D. Liu, L. Kang, X. W. Wu, L. Dai, L. Wang, S. C. Jun and Z. X. He, Stabilizing Zn Metal Anode Through Regulation of Zn Ion Transfer and Interfacial Behavior with a Fast Ion Conductor Protective Layer, Small, 2023, 19, 2303963 CrossRef CAS PubMed
.
- C. Wang, D. D. Wang, D. Lv, H. L. Peng, X. X. Song, J. Yang and Y. T. Qian, Interface Engineering by Hydrophilic and Zincophilic Aluminum Hydroxide Fluoride for Anode–Free Zinc Metal Batteries at Low Temperature, Adv. Energy Mater., 2023, 13, 2204388 CrossRef CAS
.
- W. W. Zhang, W. T. Qi, K. Yang, Y. Y. Hu, F. Y. Jiang, W. B. Liu, L. Y. Du, Z. H. Yan and J. C. Sun, Boosting tough metal Zn anode by MOF layer for high-performance zinc-ion batteries, Energy Storage Mater., 2024, 71, 103616 CrossRef
.
- X. P. Zhang, Y. Y. Liu, P. F. Shen, L. Q. Ren, D. L. Han, M. Feng and H. G. Wang, Engineering an Artificial Coating Layer of Metal Porphyrin–Based Porous Organic Polymers Toward High Stable Aqueous Zinc–Ion Batteries, Adv. Funct. Mater., 2024, 34, 2400032 CrossRef CAS
.
- L. Y. Liu, H. Y. Lu, C. Han, X. F. Chen, S. C. Liu, J. K. Zhang, X. H. Chen, X. Y. Wang, R. Wang, J. T. Xu, H. K. Liu, S. X. Dou and W. J. Li, Salt Anion Amphiphilicity-Activated Electrolyte Cosolvent Selection Strategy toward Durable Zn Metal Anode, ACS Nano, 2023, 17, 23065 CrossRef CAS PubMed
.
- Z. B. Xia, J. Sha, Y. J. Fang, Y. T. Wan, Z. L. Wang and Y. W. Wang, Purposed Built ZnO/Zn5(OH)8Ac2·2H2O Architectures by Hydrothermal Synthesis, Cryst. Growth Des., 2010, 10, 2759–2765 CrossRef CAS
.
- J. Wojnarowicz, T. Chudoba, I. Koltsov, S. Gierlotka, S. Dworakowska and W. Lojkowski, Size control mechanism of ZnO nanoparticles obtained in microwave solvothermal synthesis, Nanotechnology, 2018, 29, 065601 CrossRef PubMed
.
- L. Poul, N. Jouini and F. Fiévet, Layered Hydroxide Metal Acetates (Metal = Zinc, Cobalt, and Nickel): Elaboration via Hydrolysis in Polyol Medium and Comparative Study, Chem. Mater., 2000, 12, 3123–3132 CrossRef CAS
.
- T. Shinagawa, M. Watanabe, T. Mori, J. I. Tani, M. Chigane and M. Izaki, Oriented Transformation from Layered Zinc Hydroxides to Nanoporous ZnO: A Comparative Study of Different Anion Types, Inorg. Chem., 2018, 57, 13137–13149 CrossRef CAS PubMed
.
- A. Moezzi, M. B. Cortie, R. Shimmon and A. M. McDonagh, On the Reactivity of Zinc Hydroxide Acetate Dihydrate in Ethanol, Eur. J. Inorg. Chem., 2013, 2013, 5133–5137 CrossRef CAS
.
- G. B. Deacon and R. J. Phillips, Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS
.
- L. S. Han, Y. M. Guo, F. H. Ning, X. Y. Liu, J. Yi, Q. Luo, B. H. Qu, J. L. Yue, Y. F. Lu and Q. Li, Lotus
Effect Inspired Hydrophobic Strategy for Stable Zn Metal Anodes, Adv. Mater., 2023, 36, 2308086 CrossRef PubMed
.
- Y. Li, X. Y. Peng, X. Li, H. Duan, S. Y. Xie, L. B. Dong and F. Y. Kang, Functional Ultrathin Separators Proactively Stabilizing Zinc Anodes for Zinc-Based Energy Storage, Adv. Mater., 2023, 35, e2300019 CrossRef PubMed
.
- H. J. Kim, S. Kim, K. Heo, J. H. Lim, H. Yashiro and S. T. Myung, Nature of Zinc–Derived Dendrite and Its Suppression in Mildly Acidic Aqueous Zinc–Ion Battery, Adv. Energy Mater., 2022, 13, 2203189 CrossRef
.
- X. Z. Zhou, B. Wen, Y. C. Cai, X. M. Chen, L. Li, Q. Zhao, S. L. Chou and F. J. Li, Interfacial Engineering for Oriented Crystal Growth toward Dendrite-Free Zn Anode for Aqueous Zinc Metal Battery, Angew. Chem., Int. Ed., 2024, 63, e202402342 CrossRef CAS PubMed
.
- G. Z. Guo, C. C. Ji, J. D. Lin, T. L. Wu, Y. L. Luo, C. R. Sun, M. J. Li, H. Y. Mi, L. X. Sun and H. J. Seifert, Interfacial Domino Effect Triggered by β-Alanine Cations Realized Highly Reversible Zinc-Metal Anodes, Angew. Chem., Int. Ed., 2024, 63, e202407417 CrossRef CAS PubMed
.
- L. T. Ma, Q. Li, Y. R. Ying, F. X. Ma, S. M. Chen, Y. Y. Li, H. T. Huang and C. Y. Zhi, Toward Practical High-Areal-Capacity Aqueous Zinc-Metal Batteries: Quantifying Hydrogen Evolution and a Solid-Ion Conductor for Stable Zinc Anodes, Adv. Mater., 2021, 33, e2007406 CrossRef PubMed
.
- X. X. Zeng, S. Zhang, T. Long, Q. Y. Zhao, H. R. Wang, W. Ling, X. W. Wu, A. P. Yu and Z. W. Chen, An Amphipathic Ionic Sieve Membrane for Durable and Dendrite-Free Zinc-Ion Batteries, Renewables, 2024, 2, 52–60 CrossRef
.
- C. J. Lan, C. Y. Lee and T. S. Chin, Tetra-alkyl ammonium hydroxides as inhibitors of Zn dendrite in Zn-based secondary batteries, Electrochim. Acta, 2007, 52, 5407–5416 CrossRef CAS
.
- N. Guo, W. J. Huo, X. Y. Dong, Z. F. Sun, Y. T. Lu, X. W. Wu, L. Dai, L. Wang, H. C. Lin, H. D. Liu, H. F. Liang, Z. X. He and Q. B. Zhang, A Review on 3D Zinc Anodes for Zinc Ion Batteries, Small Methods, 2022, 6, 2200597 CrossRef CAS PubMed
.
- H. M. Yu, D. P. Chen, Q. Y. Li, C. S. Yan, Z. H. Jiang, L. J. Zhou, W. F. Wei, J. M. Ma, X. B. Ji, Y. J. Chen and L. B. Chen, In Situ Construction of Anode–Molecule Interface via Lone–Pair Electrons in Trace Organic Molecules Additives to Achieve Stable Zinc Metal Anodes, Adv. Energy Mater., 2023, 13, 2300550 CrossRef CAS
.
- R. Zhao, J. J. Yang, X. M. Han, Y. H. Wang, Q. Ni, Z. F. Hu, C. Wu and Y. Bai, Stabilizing Zn Metal Anodes via Cation/Anion Regulation toward High Energy Density Zn–Ion Batteries, Adv. Energy Mater., 2023, 13, 2203542 CrossRef CAS
.
- H. J. Yang, Z. Chang, Y. Qiao, H. Deng, X. W. Mu, P. He and H. S. Zhou, Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries, Angew. Chem., Int. Ed., 2020, 59, 9377–9381 CrossRef CAS PubMed
.
- H. J. Yang, Y. Qiao, Z. Chang, H. Deng, P. He and H. S. Zhou, A Metal-Organic Framework as a Multifunctional Ionic Sieve Membrane for Long-Life Aqueous Zinc-Iodide Batteries, Adv. Mater., 2020, 32, 2004240 CrossRef CAS PubMed
.
- Y. Liu, Y. K. An, L. Wu, J. G. Sun, F. Y. Xiong, H. Tang, S. L. Chen, Y. Guo, L. Zhang, Q. Y. An and L. Q. Mai, Interfacial Chemistry Modulation via Amphoteric Glycine for a Highly Reversible Zinc Anode, ACS Nano, 2023, 17, 552–560 CrossRef CAS PubMed
.
- S. Chen, D. Ji, Q. W. Chen, J. Z. Ma, S. Q. Hou and J. T. Zhang, Coordination modulation of hydrated zinc ions to enhance redox reversibility of zinc batteries, Nat. Commun., 2023, 14, 3526 CrossRef CAS PubMed
.
- X. Y. Peng, Y. Li, F. L. Kang, X. Li, Z. Y. Zheng and L. B. Dong, Negatively Charged Hydrophobic Carbon Nano-Onion Interfacial Layer Enabling High-Rate and Ultralong-Life Zn-Based Energy Storage, Small, 2024, 20, e2305547 CrossRef PubMed
.
- H. M. Yu, Y. J. Chen, W. F. Wei, X. B. Ji and L. B. Chen, A Functional Organic Zinc-Chelate Formation with Nanoscaled Granular Structure Enabling Long-Term and Dendrite-Free Zn Anodes, ACS Nano, 2022, 16, 9736–9747 CrossRef CAS PubMed
.
- Y. Liu, Y. Liu, X. Wu and Y. R. Cho, General Carbon Modification Avenue to Construct Highly Stable V2O5 Electrodes for Aqueous Zinc-Ion Batteries, ACS Sustainable Chem. Eng., 2023, 11, 13298–13305 CrossRef CAS
.
- C. Guo, S. J. Yi, R. Si, B. J. Xi, X. G. An, J. Liu, J. F. Li and S. L. Xiong, Advances on Defect Engineering of Vanadium–Based Compounds for High–Energy Aqueous Zinc–Ion Batteries, Adv. Energy Mater., 2022, 12, 2202039 CrossRef CAS
.
- Z. Y. Miao, M. Du, H. Z. Li, F. Zhang, H. C. Jiang, Y. H. Sang, Q. F. Li, H. Liu and S. H. Wang, Constructing nano-channeled tin layer on metal zinc for high-performance zinc-ion batteries anode, EcoMat, 2021, 3, e12125 CrossRef CAS
.
- P. X. Sun, Z. J. Cao, Y. X. Zeng, W. W. Xie, N. W. Li, D. Luan, S. B. Yang, L. Yu and X. W. Lou, Formation of Super-Assembled TiOx/Zn/N-Doped Carbon Inverse Opal Towards Dendrite-Free Zn Anodes, Angew. Chem., Int. Ed., 2022, 61, 202115649 CrossRef PubMed
.
- J. Zhou, L. T. Shan, Z. X. Wu, X. Guo, G. Z. Fang and S. Q. Liang, Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode, ChemComm, 2018, 54, 4457–4460 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi02769h |
| This journal is © the Partner Organisations 2025 |