Crystallization induces thermally activated delayed fluorescence of Ag14 nanoclusters†
Jin-Sen
Yang
a,
Lu-Yao
Xiao
b,
Fan
Liu
b,
Jun
Xu
 a,
Xi-Yan
Dong
a,
Xi-Yan
Dong
 *ab,
Jia-Hua
Hu
*b,
Jing
Li
*c and
Shuang-Quan
Zang
*ab,
Jia-Hua
Hu
*b,
Jing
Li
*c and
Shuang-Quan
Zang
 b
b
aCollege of Chemistry and Chemical Engineering, Henan Polytechnic University, 454000 Jiaozuo, China. E-mail: dongxiyan0720@hpu.edu.cn
bCollege of Chemistry, Zhengzhou University, 450001 Zhengzhou, China. E-mail: zangsqzg@zzu.edu.cn; jhhu@zzu.edu.cn
cSchool of Science, Xuchang University, 461000, Xuchang, China. E-mail: lijing42@xcu.edu.cn
First published on 10th January 2025
Abstract
Studying the differences in the excitonic dynamic processes between the dispersed state and crystalline state is important for understanding crystallization-induced emission enhancement (CIEE). In this work, we characterized the photophysical processes of Ag14 nanoclusters both in solution and in the crystalline state using photoluminescence spectra combined with transient absorption spectra. The nanoclusters exhibit fluorescence (Fl)–phosphorescence (Ph) co-dominant emission in solution, while thermally activated delayed fluorescence (TADF) is observed in the crystals. From solution to crystals, the photoluminescence quantum yield (PLQY) of the cluster improves from below 0.1% to 33% due to the boosting of TADF combined with the restriction of intramolecular motion (RIM) and the aggregation-induced barrier to oxygen (AIBO). We reveal that the enhanced TADF of crystalline samples of Ag14-dcbdt can be attributed to inter-cluster electron orbital coupling, which decreases the singlet–triplet splitting energy. These findings provide new insights into the CIEE of cluster-based aggregates and can be used to guide the synthesis of high-performance cluster-based luminescent materials.
Introduction
The development of solid-state luminescent materials with high emission efficiency is important for the preparation of light-emitting devices.1–3 However, conventional luminescent molecules are plagued by aggregation-caused luminescence quenching (ACQ).4,5 Intriguingly, most ultrasmall metal nanoclusters (NCs) are free from ACQ due to their unique geometric and electronic structures, which enable their application in lighting devices.6 Luminescent metal NCs, due to high spin–orbit coupling (SOC) induced by the metal core, usually exhibit fast intersystem crossing (ISC).6–8 This enables excited electrons to quickly populate the excited triplet state.9,10 Consequently, the luminescence performance of metal NCs is mainly dominated by the radiative transition efficiency of triplet excitons.11,12 However, the triplet excitons are easily quenched by temperature and oxygen, which reduces the luminescence efficiency.13Recently, researchers have discovered that metal NCs show crystallization-induced emission (CIE) or crystallization-induced enhanced emission (CIEE).14–17 For instance, Zhu et al. reported that Au4Ag13 NC exhibits the CIEE phenomenon. The emission enhancement is attributed to the inter-cluster C–H⋯π interactions, which restrain the intramolecular rotations and vibrations.14 Zang et al. found that the aggregation or crystallization of Cu NCs forms a barrier to oxygen, thus significantly enhancing the emission.11 The restriction of intramolecular rotation and vibration (RIM) and the aggregation-induced barrier to oxygen (AIBO) greatly reduce the non-radiative quenching of triplet excitons, resulting an enhanced emission.5 Triplet excitons have two radiative transition pathways: phosphorescence (Ph) and thermally activated delayed fluorescence (TADF).18,19 However, few studies have reported whether the enhanced emission of metal NCs stems from Ph or TADF and what the underlying mechanism of the CIE/CIEE of metal NCs is. Filling this research gap demands comparative studies on the differences in excited-state electronic dynamics processes between condensed and dispersed metal NCs.
Here, we report a metal nanocluster Ag14(dcbdt)6(TTPP)8 (Ag14-dcbdt) (where dcbdt is 4,5-dicyanobenzene-1,2-dithiolate and TTPP is tri-p-tolylphosphane). The emission of the cluster in solution is scarcely perceptible to the naked eye, and its photoluminescence quantum yield (PLQY) is below 0.1%. Surprisingly, it displays strong luminescence in the crystalline state, with a PLQY of 33%. From solution to crystals, the PLQY increases hundreds of times because of the activation of TADF in combination with RIM and AIBO induced by crystallization. By combining the photoluminescence spectra with transient absorption spectra, we discovered that the boosting of TADF in the crystalline state is due to the formation of ordered cluster assemblies. The emission mode switches from co-dominant fluorescence (Fl)–phosphorescence (Ph) emission to TADF-dominant emission caused by inter-cluster electron orbital coupling. This work elucidates the effect of crystallization on the emission mode of metal NCs and specifies the mechanism of CIEE. These results are expected to guide the design and synthesis of high-performance solid luminescent materials.
Results and discussion
Crystal structure of Ag14-dcbdt
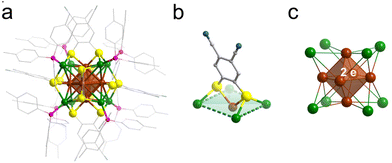
Single-crystal X-ray diffraction (SCXRD) analysis revealed that Ag14-dcbdt crystallizes in the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group (Table S1†). The unit cell comprises three cluster molecules (Z = 3), and no counter ions were found. The molecular formula of the cluster was determined as Ag14(dcbdt)6(TTPP)8. One Ag14-dcbdt cluster contains a pseudo-cubic Ag14 core, with six face-capping bidentate dcbdt ligands and eight vertex-capping TTPP ligands (Fig. 1a–c). This cluster is a two electron superatomic cluster. The calculated highest occupied molecular orbital (HOMO−7) exhibits a clear S-symmetric character, and the lowest unoccupied molecular orbitals (LUMO+4, LUMO+7 and LUMO+8) show a strong P-symmetric character (Fig. S1 and S2†). The formula and phase purity of Ag14-dcbdt were verified by electrospray ionization mass spectrometry (ESI-MS), Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS) and powder X-ray diffraction (PXRD) (Fig. S3–S6†).
space group (Table S1†). The unit cell comprises three cluster molecules (Z = 3), and no counter ions were found. The molecular formula of the cluster was determined as Ag14(dcbdt)6(TTPP)8. One Ag14-dcbdt cluster contains a pseudo-cubic Ag14 core, with six face-capping bidentate dcbdt ligands and eight vertex-capping TTPP ligands (Fig. 1a–c). This cluster is a two electron superatomic cluster. The calculated highest occupied molecular orbital (HOMO−7) exhibits a clear S-symmetric character, and the lowest unoccupied molecular orbitals (LUMO+4, LUMO+7 and LUMO+8) show a strong P-symmetric character (Fig. S1 and S2†). The formula and phase purity of Ag14-dcbdt were verified by electrospray ionization mass spectrometry (ESI-MS), Fourier transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS) and powder X-ray diffraction (PXRD) (Fig. S3–S6†).
Optical properties and theoretical calculations
Fig. 2a depicts the absorption spectrum of Ag14-dcbdt in CH2Cl2. Two shoulder band absorptions at 314 nm and 385 nm were observed. The optical energy gap was calculated to be 2.6 eV (Fig. S7†). Time-dependent density functional theory (TD-DFT) calculations were performed to assign the transitions (Table S2†). The simulated absorption spectrum for the cluster correlated well with the experimental results. In the calculated spectrum, the peak α at around 320 nm arises mainly from HOMO−7 → LUMO+8 (41.1%) and HOMO−8 → LUMO+6 (15.6%) transitions, which are assigned to a superatomic S orbital to P orbital transition (Fig. 2b). The shoulder band β at about 397 nm is primarily formed by a HOMO−5 → LUMO+4 (64.7%) transition, which involves the transitions from S 2p and Ag 3d atomic orbitals to a superatomic P orbital (Fig. 2c). The edge absorption originating from the HOMO → LUMO transition is dominated by the dcbdt ligand-centered transition (S 2p → π*) and the charge transfer transition from Ag 3d to π* of dcbdt ligands (Fig. 2d). Ag14-dcbdt does not show light absorption in the near-infrared (NIR) region (Fig. S7†), which is different from that of Ag14-bdt (bdt = 1,2-benzene dithiol) with a similar structure reported by Pradeep et al.20 Ag14-bdt has a distinct absorption at 860 nm.The difference in absorption is due to the fact that the –CN substituent of the dcbdt ligand significantly influences the frontier orbitals of Ag14 nanoclusters.21
The luminescence properties of Ag14-dcbdt in CH2Cl2 are shown in Fig. 3. Ag14-dcbdt exhibits an emission at about 690 nm that was almost imperceptible to the naked eye, with a PLQY below 0.1%. Purging with argon significantly enhanced the emission, indicating that the emission of Ag14-dcbdt involves an excited triplet-state (Fig. 3a).22 The trace of PL decay at 690 nm suggests two radiation pathways. We ascribe the processes of 5.2 ns and 23.7 μs to Fl and Ph, respectively (Fig. 3b and c). We further explored the origin of the luminescence of Ag14-dcbdt in solution by using time-resolved emission spectroscopy (TRES). Two emission peaks at 696 and 800 nm, which originated from the radiative transitions of S1 and T1, respectively, were observed (Fig. S8†). The energy difference between S1 and T1 (ΔE(S1 − T1)) is estimated to be 0.23 eV. Since the ΔE(S1 − T1) is distinctly above 0.13 eV, efficient thermal activation with fast reverse intersystem crossing (RISC) is not expected to occur.23 Therefore, the emission at 696 nm is dominated by Fl, and the emission at 800 nm is dominated by Ph. Removing oxygen can prolong the emission of Ag14-dcbdt (Fig. 3c), which is in line with the triplet-state emission characteristics.24 The Fl–Ph co-dominant emission characteristics are not present in the reported Ag14(SC6H4X)12(PPh3)8 (Ag-mt, X = F, Cl, Br),25,26 implying that the dcbdt ligand makes a significant contribution to PL.
To further understand the radiative transition process of dispersed Ag14-dcbdt NCs, the luminescence properties of Ag14-dcbdt NCs in a poly(methyl methacrylate) (PMMA) film were also studied. The emission characteristics of Ag14-dcbdt in a PMMA film and in CH2Cl2 are similar. The film exhibits an emission at 665 nm, with a PLQY of 1.1% and a decay lifetime of 19.6 μs under ambient conditions (Fig. S10 and S11†). The film serves as a good barrier to oxygen, which reduces the quenching of luminescence by oxygen (Fig. 4a). The enhancement of the emission in the film compared to that in CH2Cl2 can be attributed to AIBO and RIM.12,27 Temperature-dependent PL decay spectra show that the emission lifetimes decrease linearly with increasing temperature. The PL decay spectral features suggest that the emission of the film at 665 nm is dominant by Ph.
Crystalline Ag14-dcbdt exhibits intense emission centred at 665 nm under ambient conditions, with a PLQY of 33% (Fig. S13†). The average emission lifetime (τave) is 4.8 μs, and we hardly observe a nanosecond-scale decay process (Fig. S14 and S15†). To investigate the origin of the emission of crystalline Ag14-dcbdt, we measured its temperature-dependent emission spectra in the range of 83 to 283 K. Unlike the emission of Ag14-dcbdt in the PMMA film, the emission intensity of Ag14-dcbdt crystals shows a slight decrease as the temperature rises from 83 K to 283 K (Fig. 5a). A process of luminescence enhancement as the temperature rises from 123 K to 203 K was observed. When the temperature is increased from 83 K to 283 K, the emission maximum of Ag14-dcbdt blue-shifts from 673 nm to 665 nm (Fig. S16†). The blue shift of the emission and the luminescence enhancement at a higher temperature (123–203 K) suggest a TADF mechanism for this temperature-dependent process.28 The temperature-dependent PL lifetimes of Ag14-dcbdt were fitted using modified Boltzmann equation (eqn (1)).29
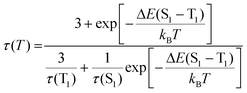 | (1) |
 | ||
| Fig. 5 PL properties of Ag14-dcbdt crystals. (a) Temperature-dependent emission upon excitation at 370 nm. (b) PL decay at 665 nm measured at different temperatures (excited by a 355 nm microsecond laser). (c) Plot of the emission decay lifetimes at 665 nm against temperature; the blue line represents the fit result according to the TADF equation (eqn (1)). (d) Comparison of the emission spectra under ambient and vacuum conditions. | ||
in which τ(T) is the measured average lifetime, T is the absolute temperature, and kB is Boltzmann constant. τ(S1) and τ(T1) are the lifetimes of the singlet (S1) and triplet (T1) states, and ΔE(S1 − T1) is the energy difference between S1 and T1. Three parameters, namely the activation energy of ΔE(S1 − T1) = 0.078 eV, τ(S1) = 94.3 ns and τ(T1) = 153.3 μs, were extracted (Fig. 4d). The value of ΔE(S1 − T1) (0.078 eV) is close to the result (0.08 eV) estimated from the TRES spectra (Fig. S17†). The very small ΔE(S1 − T1) supports the TADF mechanism. The emission of crystalline Ag14-dcbdt under ambient and vacuum conditions was also measured. The results show that oxygen slightly reduces the emission intensity and lifetime of crystalline Ag14-dcbdt (Fig. 5d and Fig. S16†), indicating an AIBO phenomenon.11
Actually, the PLQY of Ag14-dcbdt can be significantly improved through AIBO and RIM mechanisms, as verified by the fact that the PLQY of the cluster increased from below 0.1% in CH2Cl2 to 1.1% in a restricted dispersion state (film). However, the enhancement of PLQY from the film (1.1%) to crystal (33%) is mainly attributed to the boosting of TADF induced by crystallization, which prompts us to further investigate the difference in the excited-state electron dynamics of Ag14-dcbdt in different phase states.
Ultrafast excited-state dynamics
We performed femtosecond transient absorption (TA) spectroscopy to disclose the differences of ultrafast electron dynamics of Ag14-dcbdt between the CH2Cl2 solution and the single crystal. In CH2Cl2, the recorded TA spectra comprise a positive band centered at 400 nm and a broad positive band across 470–580 nm, which arises from the excited state absorption (ESA). The overlying ground-state bleaching (GSB) signals and ESA signals lead to a dip centred at 450 nm (Fig. 6a). Global analyses extract three evolution-associated spectra (EAS) (Fig. 6b). The very fast process (2.1 ps) can be assigned to the internal conversion (IC) from the Sn to S1 state. The process of 3.8 ns can be ascribed to the ISC from S1 to T1 coupled with Fl and nonradiative decay processes (Fig. 3b). The slow ISC gives rise to Fl–Ph co-dominant emission. The long lifetime (>7 ns) is associated with the Ph process. TA spectra of the single crystal of Ag14-dcbdt exhibit significant excited-state absorption (ESA) signals throughout the entire detection range. The GSB signals are covered by strong excited-state absorption. Global analyses extract a 4.6 ns time constant and a long lifetime dynamics process (>7 ns). The time constant of 4.6 ns is related to prompt fluorescence, nonradiative decay and ISC processes. The long lifetime dynamics process may be related to TADF coupled with the RISC process (Fig. 6d). EAS indicates that the signal intensities of the S1 state and the T1 state of crystalline Ag14-dcbdt are similar, which suggests that most singlet excitons populate to the excited triplet state via the ISC process. However, for the cluster in CH2Cl2, the signals of the T1 state are much smaller than those of the S1 state, indicating a low ISC efficiency (ϕISC) (Fig. 6). The ratios of ϕcrystalISC/ϕsolutionISC and kcrystalISC/ksolutionISC can be roughly estimated through the following eqn (2)–(5):| I(S1 → Sn) = N(S1)·σs(S1 → Sn) | (2) |
| I(T1 → Tn) = N(T1)·σT(T1 → Tn) | (3) |
 | (4) |
 | (5) |
in which, I represents excited state absorption intensity, σ is excited state absorption cross section, and N is the number of excitons. kISC, kFl and knr are the rates of intersystem crossing (ISC), prompt fluorescence (Fl) and non-radiative transition. In this work, I is the absorption intensity at 560 nm (Fig. S18†). The τ(S1) of Ag14-dcbdt in CH2Cl2 and crystalline state are 3.8 ns and 4.6 ns, respectively. Assuming that the ratio of σS/σT does not change with the physical form, the ratios of kcrystalISC/ksolutionISC and ϕcrystalISC/ϕsolutionISC were estimated to be 1.9 and 2.3, respectively. Hence, the ISC rate of Ag14-dcbdt in the crystals is faster than that in CH2Cl2 (Fig. S18†). Furthermore, the photoluminescence decay process of the Ag14-dcbdt crystals was re-measured by full-time-domain time-resolved spectroscopy. The test results showed no obvious fluorescence process (Fig. S19†), indicating that the emission of the cluster originates from the excited triplet state, benefiting from the fast ISC process. According to the Fermi golden rule approximation in combination with a Marcus formalism,30,31kISC can be expressed as:
 | (6) |
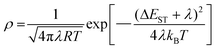 | (7) |
in which kB represents the Boltzmann constant, ħ is Planck's constant divided by 2π, T is the absolute temperature, Ψ(S) and Ψ(T) are the electronic wave functions of the singlet and triplet states, HSOC is the SOC Hamiltonian, λ is Marcus reorganization energy, ΔEST is the singlet–triplet splitting energy and Ψ(S)|HSOC|Ψ(T)2 represents the spin–orbit coupling (SOC) constant. The results of TA spectra indicate that Ag14-dcbdt has a larger SOC constant or a smaller (ΔEST + λ) in the crystalline state compared with that in CH2Cl2. Because the structure of Ag14-dcbdt is almost identical in CH2Cl2 and in the crystalline state, the SOC terms are very similar.9 Therefore, the smaller value of (ΔEST + λ) in the crystalline state is consistent with the calculated result (Fig. S20†).
Discussion on PL mechanisms
Due to the slow ISC process, Ag14-dcbdt exhibits Fl–Ph co-dominant emission. The Fl emission may originate from the locally excited transitions (LE) (d → sp) of the metal core.25 The Ph might correspond to the transition of ligand to metal charge transfer (LMCT).32 After crystallization, the emission of Ag14-dcbdt is dominated by the TADF process, which indicates that a reverse intersystem crossing (RISC) process occurs in the crystalline state. The RISC rate (kRISC) follows an Arrhenius formula:33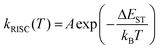 | (8) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C hydrogen bonding interactions (2.73–2.807 Å, Fig. S22†).34–40 The inter-cluster electron orbital coupling may further decrease the ΔEST, causing the TADF of Ag14-dcbdt crystals through thermally assisted RISC. Additionally, the effects of RIM and AIBO induced by crystallization can also facilitate TADF by suppressing non-radiative transitions of triplet excitons.
C hydrogen bonding interactions (2.73–2.807 Å, Fig. S22†).34–40 The inter-cluster electron orbital coupling may further decrease the ΔEST, causing the TADF of Ag14-dcbdt crystals through thermally assisted RISC. Additionally, the effects of RIM and AIBO induced by crystallization can also facilitate TADF by suppressing non-radiative transitions of triplet excitons.
Conclusions
In summary, we report a metal nanocluster (Ag14-dcbdt) with crystallization-induced enhanced thermally activated delayed fluorescence (TADF). The nanocluster exhibits fluorescence (Fl)–phosphorescence (Ph) co-dominant emission and TADF-dominant emission in the crystals. A photophysical study reveals that the emission mode switches from Fl–Ph co-dominant to TADF. This is caused by inter-cluster electron orbital coupling. This work presents the crystallization events for tuning the optical properties of metal nanoclusters and proposes a new perspective for understanding the crystallization induced emission enhancement (CIEE).Author contributions
S. Q. Z. designed the project. J. S. Y. and F. L. performed the synthesis, structure, optical absorption and photoluminescence studies. J. H. H. and L. Y. X. conducted all TA spectral studies. J. L. performed the studies of DFT calculations. S. Q. Z. and J. S. Y. analysed the experimental results. J. S. Y. prepared the first draft of the manuscript including the figures, which was then revised and adapted upon contribution from all authors. The overall project was supervised by S. Q. Z. and X. Y. D.Data availability
The data supporting the findings of this study are provided in the ESI† and are available from the corresponding author on request. The X-ray crystallographic coordinates for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) 2400571.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U21A20277 and 22103072), the Excellent Youth Foundation of Henan Scientific Committee (232300421022), Natural Science Foundation of Henan (242300421631), Science and Technology Key Project in the University of Henan province (24B150037), and the Program for Innovative Research Team (in Science and Technology) in the University of Henan province (IRTSTHN and 25IRTSTHN001).References
- S. Tong, J. Dai, J. Sun, Y. Liu, X. Ma, Z. Liu, T. Ma, J. Tan, Z. Yao, S. Wang, H. Zheng, K. Wang, F. Hong, X. Yu, C. Gao and X. Gu, Fluorescence-based monitoring of the pressure-induced aggregation microenvironment evolution for an AIEgen under multiple excitation channels, Nat. Commun., 2022, 13, 5234 CrossRef CAS PubMed.
- K. Pei, S. Ji, M. Zhao, C. Li, P. Luo, L. Jiang, Y. Zhao and T. Zhai, 2D metal-organic complex luminescent crystals, Adv. Funct. Mater., 2021, 31, 2106160 CrossRef CAS.
- W.-M. He, J.-H. Hu, Y.-J. Cui, J. Li, Y.-B. Si, S.-B. Wang, Y.-J. Zhao, Z. Zhou, L.-F. Ma and S.-Q. Zang, Filling the gaps in icosahedral superatomic metal clusters, Natl. Sci. Rev., 2024, 11, nwae174 CrossRef.
- Y. Wang, J. Nie, W. Fang, L. Yang, Q. Hu, Z. Wang, J. Z. Sun and B. Z. Tang, Sugar-Based Aggregation-induced emission luminogens: Design, structures, and applications, Chem. Rev., 2020, 120, 4534–4577 CrossRef CAS.
- X.-Y. Lou and Y.-W. Yang, Aggregation-induced emission systems involving supramolecular assembly, Aggregate, 2020, 1, 19–30 CrossRef.
- X. Kang and M. Zhu, Tailoring the photoluminescence of atomically precise nanoclusters, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- J. Kong, W. Zhang, Y. Wu and M. Zhou, Optical properties of gold nanoclusters constructed from Au13 units, Aggregate, 2022, 3, e207 CrossRef CAS.
- L. Luo, Z. Liu, X. Du and R. Jin, Near-infrared dual emission from the Au42(SR)32 nanocluster and tailoring of intersystem crossing, J. Am. Chem. Soc., 2022, 144, 19243–19247 CrossRef CAS PubMed.
- W.-Q. Shi, L. Zeng, R.-L. He, X.-S. Han, Z.-J. Guan, M. Zhou and Q.-M. Wang, Near-unity NIR phosphorescent quantum yield from a room-temperature solvated metal nanocluster, Science, 2024, 383, 326–330 CrossRef CAS PubMed.
- W.-D. Si, C. Zhang, M. Zhou, W.-D. Tian, Z. Wang, Q. Hu, K.-P. Song, L. Feng, X.-Q. Huang, Z.-Y. Gao, C.-H. Tung and D. Sun, Two triplet emitting states in one emitter: Near-infrared dual-phosphorescent Au20 nanocluster, Sci. Adv., 2023, 9, eadg3587 CrossRef CAS PubMed.
- Y. Jin, Q.-C. Peng, S. Li, H.-F. Su, P. Luo, M. Yang, X. Zhang, K. Li, S.-Q. Zang, B. Z. Tang and T. C. W. Mak, Aggregation-induced barrier to oxygen—a new AIE mechanism for metal clusters with phosphorescence, Natl. Sci. Rev., 2021, 9, nwab216 CrossRef PubMed.
- L. Luo, Z. Liu, A. Mazumder and R. Jin, Raising near-infrared photoluminescence quantum yield of Au42 quantum rod to 50% in solutions and 75% in films, J. Am. Chem. Soc., 2024, 146, 27993–27997 CAS.
- X.-Y. Dong, Y. Si, J.-S. Yang, C. Zhang, Z. Han, P. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Ligand engineering to achieve enhanced ratiometric oxygen sensing in a silver cluster-based metal-organic framework, Nat. Commun., 2020, 11, 3678 CrossRef CAS PubMed.
- T. Chen, S. Yang, J. Chai, Y. Song, J. Fan, B. Rao, H. Sheng, H. Yu and M. Zhu, Crystallization-induced emission enhancement: A novel fluorescent Au-Ag bimetallic nanocluster with precise atomic structure, Sci. Adv., 2017, 3, e1700956 CrossRef PubMed.
- D. Liu, G. Gao, Y. Zhang, Q. Li, S. Yang, J. Chai, H. Yu and M. Zhu, [Au14(2-SAdm)9(Dppe)2]+: a gold nanocluster with a crystallization-induced emission enhancement phenomenon, Chem. Commun., 2024, 60, 1337–1340 RSC.
- Y. Saito, A. Suda, M. Sakai, S. Nakajima, Y. Shichibu, H. Kanai, Y. Ishida and K. Konishi, Controlled nanocrystallization of gold nanoclusters within surfactant envelopes: enhancing aggregation-induced emission in solution, Chem. Sci., 2024, 15, 11775–11782 RSC.
- M. Sugiuchi, J. Maeba, N. Okubo, M. Iwamura, K. Nozaki and K. Konishi, Aggregation-induced fluorescence-to-phosphorescence switching of molecular gold clusters, J. Am. Chem. Soc., 2017, 139, 17731–17734 CrossRef CAS PubMed.
- P. Pander, A. V. Zaytsev, A. Sil, G. V. Baryshnikov, F. Siddique, J. A. G. Williams, F. B. Dias and V. N. Kozhevnikov, Thermally activated delayed fluorescence in a deep red dinuclear iridium(III) complex: a hidden mechanism for short luminescence lifetimes, Chem. Sci., 2023, 14, 13934–13943 RSC.
- W. Zhang, S. Li, Y. Gong, J. Zhang, Y. Zhou, J. Kong, H. Fu and M. Zhou, Aggregation enhanced thermally activated delayed fluorescence through spin-orbit coupling regulation, Angew. Chem., Int. Ed., 2024, 63, e202404978 CrossRef CAS.
- M. Bodiuzzaman, E. Khatun, K. S. Sugi, G. Paramasivam, W. A. Dar, S. Antharjanam and T. Pradeep, Dithiol-induced contraction in Ag14 clusters and its manifestation in electronic structures, J. Phys. Chem. C, 2020, 124, 23426–23432 CrossRef CAS.
- G. Deng, S. Malola, P. Yuan, X. Liu, B. K. Teo, H. Häkkinen and N. Zheng, Enhanced surface ligands reactivity of metal clusters by bulky ligands for controlling optical and chiral properties, Angew. Chem., Int. Ed., 2021, 60, 12897–12903 CrossRef CAS PubMed.
- W.-D. Si, C. Zhang, M. Zhou, Z. Wang, L. Feng, C.-H. Tung and D. Sun, Arylgold nanoclusters: Phenyl-stabilized Au44 with thermal-controlled NIR single/dual-channel phosphorescence, Sci. Adv., 2024, 10, eadm6928 CrossRef CAS.
- H. Yersin, R. Czerwieniec, M. Z. Shafikov and A. F. Suleymanova, TADF material design: photophysical background and case studies focusing on CuI and AgI complexes, ChemPhysChem, 2017, 18, 3508–3535 CrossRef CAS PubMed.
- J.-S. Yang, Y.-J. Zhao, X.-M. Li, X.-Y. Dong, Y.-B. Si, L.-Y. Xiao, J.-H. Hu, Z. Yu and S.-Q. Zang, Staggered assembly of a dimeric Au13 cluster: impacts on coupling of geometric isomerism, Angew. Chem., Int. Ed., 2024, 63, e202318030 CrossRef CAS PubMed.
- A. K. Das, R. Mekkat, S. Maity, A. S. Nair, S. Bhandary, R. Bhowal, A. Patra, B. Pathak, D. Chopra and S. Mandal, Role of ligand on photophysical properties of nanoclusters with fcc kernel: A case study of Ag14(SC6H4X)12(PPh3)8 (X = F, Cl, Br), Inorg. Chem., 2021, 60, 19270–19277 CrossRef CAS.
- H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Crystal structure of a luminescent thiolated Ag nanocluster with an octahedral Ag64+ core, Chem. Commun., 2013, 49, 300–302 RSC.
- X.-H. Ma, Y. Si, J.-H. Hu, X.-Y. Dong, G. Xie, F. Pan, Y.-L. Wei, S.-Q. Zang and Y. Zhao, High-efficiency pure blue circularly polarized phosphorescence from chiral N-heterocyclic-carbene-stabilized copper(I) clusters, J. Am. Chem. Soc., 2023, 145, 25874–25886 CrossRef CAS PubMed.
- X.-S. Han, X. Luan, H.-F. Su, J.-J. Li, S.-F. Yuan, Z. Lei, Y. Pei and Q.-M. Wang, Structure determination of alkynyl-protected gold nanocluster Au22(tBuC
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)18 and its thermochromic luminescence, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS.
C)18 and its thermochromic luminescence, Angew. Chem., Int. Ed., 2020, 59, 2309–2312 CrossRef CAS. - H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS.
- V. Lawetz, G. Orlandi and W. Siebrand, Theory of intersystem crossing in aromatic hydrocarbons, J. Chem. Phys., 1972, 56, 4058–4072 CrossRef CAS.
- R. A. Marcus and N. Sutin, Electron transfers in chemistry and biology, Biochim. Biophys. Acta, Bioenerg., 1985, 811, 265–322 CrossRef CAS.
- Y. Zeng, S. Havenridge, M. Gharib, A. Baksi, K. L. D. M. Weerawardene, A. R. Ziefuß, C. Strelow, C. Rehbock, A. Mews, S. Barcikowski, M. M. Kappes, W. J. Parak, C. M. Aikens and I. Chakraborty, Impact of ligands on structural and optical properties of Ag29 nanoclusters, J. Am. Chem. Soc., 2021, 143, 9405–9414 CrossRef CAS PubMed.
- Y. Wang, Z. Liu, A. Mazumder, C. G. Gianopoulos, K. Kirschbaum, L. A. Peteanu and R. Jin, Tailoring carbon tails of ligands on Au52(SR)32 nanoclusters enhances the near-Infrared photoluminescence quantum yield from 3.8 to 18.3%, J. Am. Chem. Soc., 2023, 145, 26328–26338 CrossRef CAS PubMed.
- G. J. O. Beran, Modeling polymorphic molecular crystals with electronic structure theory, Chem. Rev., 2016, 116, 5567–5613 CrossRef CAS PubMed.
- S. Monaco, R. P. Baer, R. P. Giernacky, M. E. Villalba, T. M. Garcia, C. Mora-Perez, S. E. Brady, K. D. Erlitz, C. Kunkel, S. R. Jezowski, H. Oberhofer, C. Lange and B. Schatschneider, Electronic property trends of single-component organic molecular crystals containing C, N, O, and H, Comput. Mater. Sci., 2021, 197, 110510 CrossRef CAS.
- Z. Liu, H. Tan, B. Li, Z. Hu, D.-E. Jiang, Q. Yao, L. Wang and J. Xie, Ligand effect on switching the rate-determining step of water oxidation in atomically precise metal nanoclusters, Nat. Commun., 2023, 14, 3374 CrossRef CAS.
- S. Rabaça, I. C. Santos, G. Lopes, V. Gama, L. F. Veiros and M. Almeida, C–H⋯N
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C hydrogen bonding in cyanobenzene-ethylenedithio-tetrathiafulvalene compounds, CrystEngComm, 2022, 24, 1145–1155 RSC.
C hydrogen bonding in cyanobenzene-ethylenedithio-tetrathiafulvalene compounds, CrystEngComm, 2022, 24, 1145–1155 RSC. - S. Cao, H. Yuan and J. Zhang, A mechanistic investigation into N-heterocyclic carbene (NHC) catalyzed umpolung of ketones and benzonitriles: is the cyano group better than the classical carbonyl group for the addition of NHC?, Org. Chem. Front., 2019, 6, 523–531 RSC.
- K. M. Lambert, Z. D. Stempel, K. B. Wiberg and W. F. Bailey, Experimental demonstration of a sizeable nonclassical CH⋯G hydrogen bond in cyclohexane derivatives: stabilization of an axial cyano group, Org. Lett., 2017, 19, 6408–6411 CrossRef CAS.
- M. Mamada, H. Katagiri and C. Adachi, Effect of hydrogen bonding on thermally activated delayed fluorescence behaviors based on a study of hydrate crystals, J. Phys. Chem. Lett., 2023, 14, 5221–5225 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2400571. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02920h |
| This journal is © the Partner Organisations 2025 |