Open Access Article
Open Access ArticleDeep eutectic solvents towards green polymeric materials
Udyani Aloka Weerasinghe ab,
Tingting Wud,
Pei Lin Cheea,
Pek Yin Michelle Yewac,
Hiang Kwee Lee
ab,
Tingting Wud,
Pei Lin Cheea,
Pek Yin Michelle Yewac,
Hiang Kwee Lee b,
Xian Jun Loh
b,
Xian Jun Loh *a and
Kai Dan
*a and
Kai Dan *abd
*abd
aInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, Singapore 138634, Republic of Singapore. E-mail: lohxj@imre.a-star.edu.sg; kaid@imre.a-star.edu.sg
bSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore 637371, Republic of Singapore
cDepartment of Biomedical Engineering, Faculty of Engineering, National University of Singapore, 117583, Singapore
dInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis, #08-03, Singapore 138634, Republic of Singapore
First published on 19th June 2024
Abstract
Solvents are essential for chemical synthesis and material preparation; however, traditional solvents face challenges in meeting safety and sustainability standards. Consequently, there is a growing focus on environmentally friendly solvent systems for polymeric materials, with deep eutectic solvents (DESs) standing out as promising alternatives owing to their eco-friendly attributes. In this review, we summarize studies on this novel solvent system in a broad range of applications, where DESs play multiple roles in polymer synthesis and corresponding material fabrication. In detail, we focus on the utilization of DESs in the synthesis of green polymers and related functional polymeric materials ranging from soft materials such as gels and nanofibers to membranes, films, and complex architectures generated from various techniques such as 3D printing, electrospinning, and self-assembly, together with their properties. Moreover, we discuss the green credentials through sustainability analysis and life-cycle assessment of DES-based polymeric materials. We believe this review will inspire more investigation into the use of DESs for functional green polymeric materials towards a sustainable future.
1. Introduction
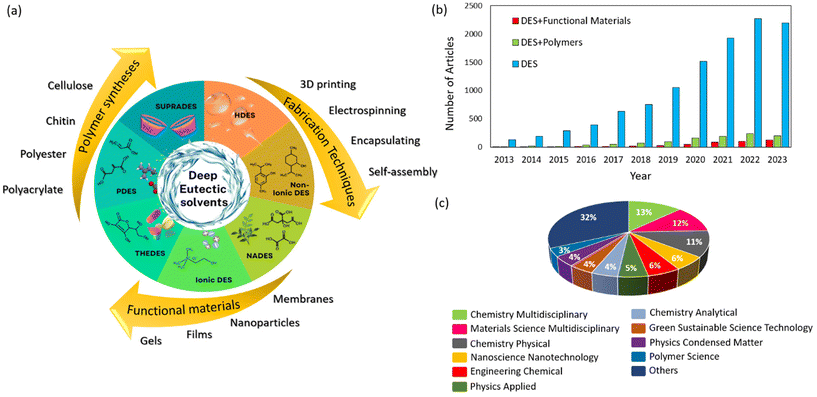
The shift towards green technologies and materials is crucial for mitigating adverse environmental effects, promoting economic growth, ensuring energy security, and safeguarding all life forms and the natural environment for the current and future generations. The twelve principles of green chemistry have been articulated since 1998 as a guideline for researchers to seek environmentally responsible pathways when designing products and processes. Polymer materials have attracted much attraction from their inception by virtue of their unique properties to support daily life. However, the excessive production and consumption of synthetic polymer materials has resulted in diverse global issues with severe impacts on ecosystems. In addressing both scenarios while acknowledging the wide demand for polymer materials due to their durability and adaptivity, researchers have focused on ways to cater to this requirement with the development of environmentally friendly approaches.The conventional synthesis approaches for polymer materials have various limitations due to the need for harsh conditions (e.g. concentrated solvents), high energy requirements (high heat, pressure), high cost, need to maintain a strict environment (anaerobic), use of toxic and/or volatile solvents and catalysts, and high waste generation coupled with an inability to reuse materials and difficulties in recycling. Deep eutectic solvents (DESs) come into play in this instance for generating a multitude of solvent systems for novel polymeric materials. DESs were first reported by Abbott et al. in 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and have been proven to have vast areas of applications in subsequent research. DESs are categorized as green solvents in the current era, whereby they can serve as valuable alternatives to conventional solvents due to their extremely low vapor pressure and non-flammability. Even though more than two decades have passed since the first report of this solvent class, there exist many possibilities for the exploration of DESs in polymer science owing to their low toxicity and natural degradability (Fig. 1). Apart from simply using DESs as a solvent replacement, their ability to act as a solvent as well as a monomer simultaneously generates more inroads towards green polymeric materials, even surpassing conventional polymer synthesis methods. Moreover, DESs present benefits in allowing for milder conditions to be used in polymer synthesis and lead to enhanced thermal and mechanical properties of the resulting polymeric materials. Besides their function in the synthesis of polymers and related materials, the use of DESs for biopolymer treatment and corresponding material engineering has been widely investigated, in which DESs can overcome the recalcitrance and structural complexity of biomass, facilitating subsequent functional biopolymer material engineering.
1 and have been proven to have vast areas of applications in subsequent research. DESs are categorized as green solvents in the current era, whereby they can serve as valuable alternatives to conventional solvents due to their extremely low vapor pressure and non-flammability. Even though more than two decades have passed since the first report of this solvent class, there exist many possibilities for the exploration of DESs in polymer science owing to their low toxicity and natural degradability (Fig. 1). Apart from simply using DESs as a solvent replacement, their ability to act as a solvent as well as a monomer simultaneously generates more inroads towards green polymeric materials, even surpassing conventional polymer synthesis methods. Moreover, DESs present benefits in allowing for milder conditions to be used in polymer synthesis and lead to enhanced thermal and mechanical properties of the resulting polymeric materials. Besides their function in the synthesis of polymers and related materials, the use of DESs for biopolymer treatment and corresponding material engineering has been widely investigated, in which DESs can overcome the recalcitrance and structural complexity of biomass, facilitating subsequent functional biopolymer material engineering.
This review aimed to identify the current trends in using DESs for green polymer synthesis and related functional polymeric materials, while outlining their green credentials and performing a sustainability evaluation of DES-based materials. Even though various applications of DESs have been discussed in previous studies, a compilation of the data and findings to address the needs of functional green polymeric materials has not been performed yet. Here, not only are the uses and advantages of DESs as a solvent or monomer discussed, but also the applications harnessing their unique properties are reported in emerging areas and technologies, such as efficient biomass extraction, stimuli-responsive materials, membrane science, nanofibers, and 3D printing, highlighting their future potential for helping drive society towards a green economy.
2. Background and properties of DESs
“Eutectic”, originating from the Greek language, is a term used to describe substances that are readily melted, which can be interpreted in another way by considering the melting point depression of two components. With the discovery of a preparation process for ChCl and urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
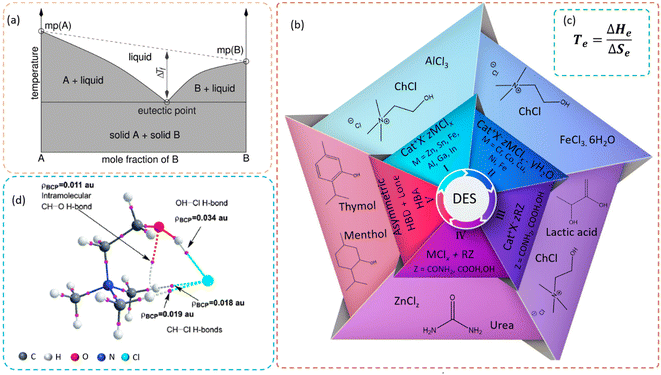
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) that could occur at ambient temperature by Abbott et al.,2 the aforementioned word gained greater significance in chemistry, and its use has increased over the past two decades. Deep eutectic solvents (DESs), as the name implies, represent the combination of two or more Lewis or Brønsted acids and bases with at least one solid, where the mixture exhibits melting point depression with respect to the ideal constituents of the same chemical substances at relatively low temperature levels. Here, the term “Deep” is mainly attributed to the negative deviations from the thermodynamic ideality, where the intermolecular interactions are stronger in the resultant solvent than in the liquid phases of the pure substances (Fig. 2a).3
2) that could occur at ambient temperature by Abbott et al.,2 the aforementioned word gained greater significance in chemistry, and its use has increased over the past two decades. Deep eutectic solvents (DESs), as the name implies, represent the combination of two or more Lewis or Brønsted acids and bases with at least one solid, where the mixture exhibits melting point depression with respect to the ideal constituents of the same chemical substances at relatively low temperature levels. Here, the term “Deep” is mainly attributed to the negative deviations from the thermodynamic ideality, where the intermolecular interactions are stronger in the resultant solvent than in the liquid phases of the pure substances (Fig. 2a).3
 | ||
| Fig. 2 (a) Two-component solid–liquid phase diagram showing the eutectic point.4 Copyright 2014, American Chemical Society. (b) Schematic illustration of the classification of DESs (Cat+ and X− represent ammonium, phosphonium or sulfonium cations, and Lewis base, respectively). (c) Gibbs equation. (d) Non-conventional intramolecular hydrogen bonds generated from H in the methyl groups (CH⋯Cl, CH⋯OH) with the bond critical points (BCP) calculated by quantum theory of atoms in molecules (QTAIM) molecular graphs.8 Copyright 2016, Royal Society of Chemistry. | ||
Some researchers consider this solvent category as a sub-class of ionic liquids, representing a class of ionic fluids generated at temperatures less than 100 °C.4 However, a clear distinction of this class with DESs was eventually generated that considers the chemical nature of the two starting materials, the synthesis methods, the bonding nature, and greenness. The main constituents of ionic liquids are anions (organic heterocyclic) and cations (organic or inorganic), whereas the emphasis in DESs is to the hydrogen-bond donor and acceptor abilities.5 This gives rise to differences in the intermolecular attractions, with ionic liquids having Coulomb forces as the main driving force for the ionic interactions with DESs having hydrogen bonds. Additionally, the preparation of ionic liquids involves several steps and reagents, apart from the main constituents for dilution of the compounds and purification to remove by-products, which can give rise to the generation of waste, while DESs only requires simple steps (mixing the starting materials and applying heat, until the DES forms a transparent and viscous liquid). As for the components (starting materials), the initial components of the DESs are often nontoxic, cheap, and environmentally friendly. Compared to DESs, the starting materials of ionic liquids cannot be regarded as green and sustainable, whereby the environmental impact of ionic liquid systems strongly depend on the anions and cations used as the components.6 However, when considering the low volatility, tunability, low melting temperature, and ability to dissolve organic and inorganic compounds, both ionic liquids and DESs have similarities.
The initial studies based on DESs were focused on the extensive hydrogen bonding between the constituents, which was used to categorize the components as a hydrogen-bond acceptor (HBA) or hydrogen-bond donor (HBD). Afterward, with further investigations on the thermodynamic aspects of eutectic mixtures, it was found that, apart from the hydrogen bonding, the effect of entropy in the system plays a pivotal role.7 This can be further explained by the high level of disorderness gained by the mixture over the initial components, where the melting point is inversely proportional to the entropy, resulting in a melting point depression. Additionally, it has been found that for most eutectic systems, the enthalpy change is much lower than the change in entropy. This can be derived by the Gibbs equation (Fig. 2c), where Te, ΔHe, and ΔSe represent the eutectic temperature, enthalpy change, and entropy change, respectively.
Over time, the utilization of molecular simulations further facilitated visualization of the atomic level interactions within DES systems, whereby new approaches for hydrogen bonds generated were observed. In one instance, this was termed as an “alphabet soup”, where novel noncovalent type interactions in the ChCl:urea system were observed, representing a deviation from the conventional hydrogen bonding definition (Fig. 2d).8 In this study, the HBD ability of C–H bonds was stated to be one of the stronger interactions than traditional N–H or O–H bonding, leading to a high number of hydrogen bonds within the system. However, even though, DESs were introduced a few decades ago, studies on their interactions are still in a nascent state. Also, novel ways of interpreting the bonding nature and reaction modes of different DES systems are still emerging, and it is extremely difficult to generalize the behavior of different systems owing to minute changes in their chemical environment.
2.1 Categories of DESs
The classification of DESs is mainly based on the HBDs and HBAs, whereby initially four main types of DESs were defined based on the ionic components4 (Fig. 2b). Here, type I DESs represent quaternary halides (with an ammonium, phosphonium, or sulfonium cation) and metal chloride (Lewis base) as the main constituents, which were first discovered in 2001.1 The scope of these was further improved with the incorporation of hydrated metal halides in type II, which results in a further reduction of the eutectic point compared to the analogous anhydrous compounds. Type III DESs were introduced after the discovery of the ChCl:urea system representing quaternary salts2 and HBDs, where a vast number of possible compounds were suggested afterward as potential HBDs. A combination of metal halides and HBDs led to type IV DESs. With the quest for the meeting the requirements for non-ionic applications and the availability of strong negative deviations, type V DES have been defined recently.9Great attention has been given to quaternary ammonium salts for the defined types I–III, and among these, the most commonly used HBA for type III DESs is considered to be choline chloride (ChCl) with the chemical formula [HOC2H4N+(CH3)3Cl−]. However, following the inception of DES systems, scientists came up with numerous types of HBAs to tune the desired qualities for different applications. Additionally, owing to the vast adjustability and the absence of metal ions, type III DESs have drawn greater attention among the stated categories.
Apart from the stated classification, various sub-categories have evolved too, namely polymerizable DESs (PDESs), natural DESs (NADESs), therapeutic DESs (THEDESs), hydrophobic DESs (HDESs), and supramolecular DESs (SUPRADESs), indicating the plethora of combinations of different chemical species with HBA and HBD properties (Fig. 1a). The NADESs comprise bio-derived compounds that use nature's building blocks to generate eutectic mixtures. This type has attracted great attention from scientists owing to their nontoxic, biodegradable, and sustainable aspects. The eutectic mixtures of this kind comprise bio-derived compounds, mainly sugars, amino acids, and organic acids.10 Further, the fine-tuning of the HBD and HBA towards biocompatible molecules can enable their pharmaceutical applications. This is where the THEDESs come into play, which highlights the biocompatibility, non-volatility, solubility, retention, and controlled release of therapeutic, bioactive compounds of interest with the use of DESs.11,12 The stability of supramolecular structures, such as cyclodextrins and DNA, has been demonstrated in the context of the formation of hydrogen bonds.13,14 This characteristic paves the way for extensive applications of these substances as SUPRADESs, which are explored in greater detail in subsequent sections.
2.2 Physicochemical properties of DESs
Identifying the properties of a DES plays a vital role in determining its functionality for specific applications. Even though the vast tunability of DES systems can be considered as an advantage, the availability of data related to their physicochemical properties is still in its infancy when it comes to the novel combinations. However, most of the properties of conventional DES systems have been studied and reported extensively by many researchers. For instance, Omar et al. set up a database of existing DESs in 2023 together with their physical properties during the period from 2003 to 2021, which may be remarkably beneficial for researchers looking to scale up the applications of these DESs.15A great number of explanations regarding the physicochemical properties of DESs have been given in past publications, such as hole theory to explain the interdiffusion and motion,16 the Kamlet–Taft equation representing the strength of noncovalent interactions,17 and the entropy of the system.7 Below, we discuss some of the core observations of the past studies conducted that could have an effect on the performance of polymeric materials generated using these systems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of trimethylsulfonium bis[trifluoromethylsulfonyl]imide
1 of trimethylsulfonium bis[trifluoromethylsulfonyl]imide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formamide and the highest (75
formamide and the highest (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mPa s at 40 °C) being a 1
000 mPa s at 40 °C) being a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of ChCl
2 molar ratio of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2.15 These values show the tunability of the viscosity in DES systems. This can be dependent on many factors, such as the molar ratio, temperature, interactions between the HBA/HBD, and the molar mass. According to hole theory, with the rise in temperature, the mobility of molecules increases by the generation of large voids, whereas at lower temperature, smaller voids are available, which, coupled with the larger sizes of ionic components, hinder molecular mobility, resulting in high viscosity, as represented by eqn (1), where r, k, T, and γ represent the radius of an average-sized void, the Boltzmann constant, absolute temperature, and the surface tension of the liquid, respectively.19
ZnCl2.15 These values show the tunability of the viscosity in DES systems. This can be dependent on many factors, such as the molar ratio, temperature, interactions between the HBA/HBD, and the molar mass. According to hole theory, with the rise in temperature, the mobility of molecules increases by the generation of large voids, whereas at lower temperature, smaller voids are available, which, coupled with the larger sizes of ionic components, hinder molecular mobility, resulting in high viscosity, as represented by eqn (1), where r, k, T, and γ represent the radius of an average-sized void, the Boltzmann constant, absolute temperature, and the surface tension of the liquid, respectively.19
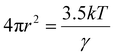 | (1) |
This is further evident from the Arrhenius equation, where an inverse relationship between viscosity and temperature is stated. Further, when considering the interactions between the HBDs and HBAs, the polar functional groups, anions of the HBA present (RCl:HBD < RBr:HBD < RI:HBD),15 and steric hindrance provided by longer chain alkyl groups in the system have a crucial impact. Also, the effect of the impurities should not be ignored as these can have an effect on the interactions. Additionally, for solvent-based applications, hydrophobic DESs with viscosities less than 20 mPa s are considered as attractive alternatives to conventional organic solvents (e.g., heptane, octane, nonane, hexyl benzene).20
The preliminary study of the physicochemical characteristics of DESs offers a deeper understanding of some innovative applications involving polymers. Therefore, comprehending the interconnectedness among the DESs’ properties becomes crucial for prioritizing specific attributes at the expense of others. From the aforementioned discussion, it is evident that numerous properties of DESs are intertwined, primarily from the noncovalent bonding tendencies of the constituent species.
3. DESs for green polymer synthesis
The use of DESs is considered a crucial requirement for polymer synthesis by virtue of the environmental concerns over hazardous solvents and their ease of processing. DESs can be used either as a solvent to facilitate the polymerization process, as the monomer participating in the final polymer synthesis, or both simultaneously. In whichever case, a basic understanding and careful selection of the constituents of the corresponding DES system is vital to generate the desired valuable outputs for the polymer synthesis. The understanding of the properties of DES systems, such as polarity of both the DES and polymer (using the Kamlet–Taft parameters) and the boiling point of the prepared DES, allows determining the maximum usable limit of the system.17 In this part, the function of DESs as a reaction medium is summarized based on different polymerization processes.3.1 DESs as a solvent for polymerization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) decanoic acid (1
decanoic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a template-based polymerization of aniline. This study revealed that both the selected DES and aniline had a significant inhibition effect on the catalytic activity of laccase. The authors were able to generate a yield of 84% polyaniline product with the addition of 10% DES in the system.25 Another study carried out by Khlupova et al. was able to generate oligomers with six monomer units of dihydroquercetin (Taxifolin) in a betanin:glycerol DES system. Here, the selection of betanin as a HBA was specifically considered as it would not inhibit the activity of the laccase enzyme, like ChCl. The resultant oligomers had an average molecular weight of 1800 g mol−1 with a polydispersity index of 1.09 and obtained a yield of 58 ± 7%.27 The same approach was utilized by another set of researchers with an aim to produce conductive polyanilines by polymerization and copolymerization. In both cases, the DES facilitated a favorable environment for the enzyme at a DES
1) in a template-based polymerization of aniline. This study revealed that both the selected DES and aniline had a significant inhibition effect on the catalytic activity of laccase. The authors were able to generate a yield of 84% polyaniline product with the addition of 10% DES in the system.25 Another study carried out by Khlupova et al. was able to generate oligomers with six monomer units of dihydroquercetin (Taxifolin) in a betanin:glycerol DES system. Here, the selection of betanin as a HBA was specifically considered as it would not inhibit the activity of the laccase enzyme, like ChCl. The resultant oligomers had an average molecular weight of 1800 g mol−1 with a polydispersity index of 1.09 and obtained a yield of 58 ± 7%.27 The same approach was utilized by another set of researchers with an aim to produce conductive polyanilines by polymerization and copolymerization. In both cases, the DES facilitated a favorable environment for the enzyme at a DES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) buffer 60
buffer 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio.26
40 ratio.26RAFT (Reversible Addition–Fragmentation Chain Transfer) and ATRP (Atom Transfer Radical Polymerization) can be considered as emerging radical-based polymerization approaches for achieving more controlled outputs. The polymerization of HEMA and block copolymerization with methyl methacrylate were studied by Kumar et al. using ChCl:urea.28 The study revealed that higher molecular weight polymers (up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1) could be synthesized using this DES, with a conversion percentage of up to 90%. Additionally, the team suggested this DES as a non-aqueous solvent system for the polymerization-induced self-assembly (PISA) of block copolymers. Another more convenient RAFT polymerization was revealed by Li and Yu, who proposed a polymerization environment under visible light and in the open air in the presence of DES.29 Here, the authors used a tetrabutylammonium chloride:ethylene glycol DES to prompt the polymerization of methyl methacrylate, methyl acrylate, dimethyl acrylamide, and styrene. During this study, they observed that the DES medium provided for photostability through a “protected radical effect” on the chain transfer agents used (trithiocarbonate) compared to DMSO. This enhanced stability of the chain transfer agents enabled better control over the polymerization kinetics, resulting in uniform chain lengths and end group functionalities in the final polymer product. Both these studies suggested that the high viscosity of the DES facilitated polymerization when carried out in confined spaces (mainly generated by long-chain RAFT agents), with high concentrations of monomers and radicals, leading to improved polymerization rates compared to conventional solvents.28,29 A study carried out by Mendonça et al. involved the use of ChCl:urea for ATRP for the generation of polymers and block copolymers using 2-hydroxyethyl acrylate, HEMA, and (3-acrylamidopropyl)trimethylammonium chloride with Na2S2O4. The study reported on the room-temperature polymerization (30 °C) with polymers having a polydispersity of less than 1.2 throughout the process.30 Another group compared three ChCl-based DES systems (glycerol/urea/ethylene glycol) for the generation of poly(methyl methacrylate) with ATRP.31 The team studied homogeneous and heterogeneous Cu(II) catalysis in DES media, which resulted in polymer synthesis with polydispersity index values of approximately 1.2 in both catalytic systems.
700 g mol−1) could be synthesized using this DES, with a conversion percentage of up to 90%. Additionally, the team suggested this DES as a non-aqueous solvent system for the polymerization-induced self-assembly (PISA) of block copolymers. Another more convenient RAFT polymerization was revealed by Li and Yu, who proposed a polymerization environment under visible light and in the open air in the presence of DES.29 Here, the authors used a tetrabutylammonium chloride:ethylene glycol DES to prompt the polymerization of methyl methacrylate, methyl acrylate, dimethyl acrylamide, and styrene. During this study, they observed that the DES medium provided for photostability through a “protected radical effect” on the chain transfer agents used (trithiocarbonate) compared to DMSO. This enhanced stability of the chain transfer agents enabled better control over the polymerization kinetics, resulting in uniform chain lengths and end group functionalities in the final polymer product. Both these studies suggested that the high viscosity of the DES facilitated polymerization when carried out in confined spaces (mainly generated by long-chain RAFT agents), with high concentrations of monomers and radicals, leading to improved polymerization rates compared to conventional solvents.28,29 A study carried out by Mendonça et al. involved the use of ChCl:urea for ATRP for the generation of polymers and block copolymers using 2-hydroxyethyl acrylate, HEMA, and (3-acrylamidopropyl)trimethylammonium chloride with Na2S2O4. The study reported on the room-temperature polymerization (30 °C) with polymers having a polydispersity of less than 1.2 throughout the process.30 Another group compared three ChCl-based DES systems (glycerol/urea/ethylene glycol) for the generation of poly(methyl methacrylate) with ATRP.31 The team studied homogeneous and heterogeneous Cu(II) catalysis in DES media, which resulted in polymer synthesis with polydispersity index values of approximately 1.2 in both catalytic systems.
Overall, DESs as polymerization solvents have been shown to facilitate faster polymerization rates, allow maintaining mild reaction conditions, and enable controlled polymerizations compared to conventional solvents, primarily attributed to improved monomer dissolution, interactions with propagating radicals, controlled terminations due to their high viscosity, and a radical protection effect on chain transfer agents, therefore optimizing their use. However, studies on the interactions between DESs and the monomers that lead to these properties are required for further exploration in this area to identify the effect of the DESs in radical polymerization approaches.
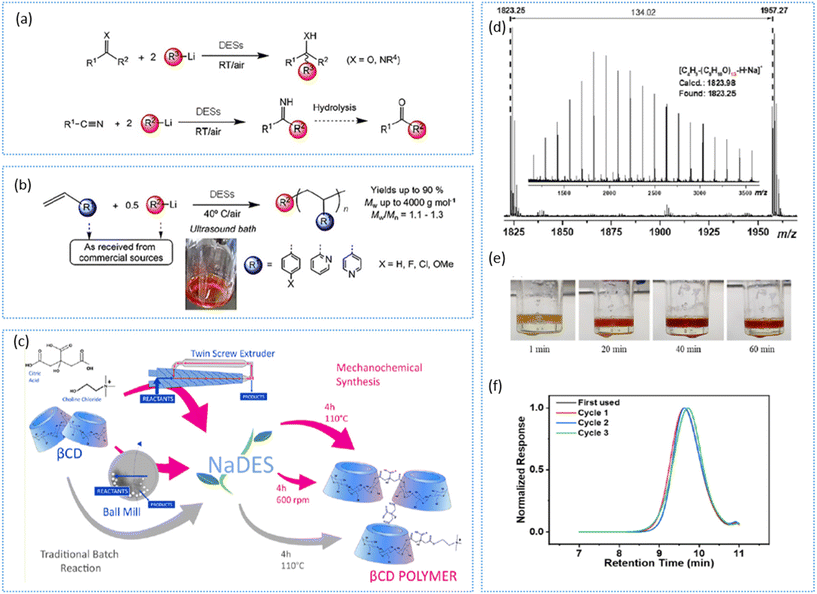 | ||
| Fig. 3 (a) Nucleophilic addition reaction of the organolithium compound (R–Li) to carbonyl compounds in DES media; (b) anionic polymerization of olefins promoted by organolithium compounds and different ChCl-based DESs.32 Copyright 2019, Wiley. (c) Polycondensation of β-cyclodextrin using NADES using a ball mill and twin-screw extruder.34 Copyright 2021, American Chemical Society. (d) Anionic polymerization showing the MALDI-TOF MS spectra of a polyisoprene homopolymer. (e) Colour change during copolymerization between styrene and isoprene in ChCl:glycerol. (f) Weight distribution of synthesized polystyrene with the use of a recovered DES for up to three cycles.33 Copyright 2023, Elsevier. | ||
A study carried out by Sanchez-Condado et al. investigated several DES systems for the synthesis of polymerization and copolymerization products (polystyrene, 4-aryl substituted polystyrenes, poly[2-vinylpyridine], poly[4-vinylpyridine], random copolymers, and block copolymers), and revealed that a DES system with ChCl:glycerol enabled the polymerization to proceed with greater yields of up to 90% and with low polydispersity indexes (1.1–1.3), with greater stability for the organolithium species compared to those obtained with conventional solvents (Fig. 3b).32 Recently, another team probed this study for the polymerization of styrene and its derivatives, isoprene, and 2-vinylpyridine using the same DES system in the presence of organolithum species.33 Here, the team highlighted the importance of the factors beyond the selection of the DES system, such as the reaction conditions, including the viscosity of the DES, the initiator concentration, and the duration of mechanical treatment (ultrasonication), all of which could significantly affect the resulting polymer properties (Fig. 3d–f).
Moving towards a sustainable pathway, Pedrazzo et al. studied the polycondensation reaction of β-cyclodextrin with the use of NADESs (ChCl:citric acid).34 The team utilized mechano-chemical approaches using a ball mill and twin-screw extruder for the synthesis of a water-soluble biopolymer and a crosslinked polymer (Fig. 3c). The authors pointed out there were differences in the polymer yield, noting that the inability to remove water from the system when using the ball mill resulted in a decreased product yield due to a shift in the equilibrium towards the product side.
3.2 DESs as monomers
Apart from acting as a medium for polymerization reactions to facilitate a favorable environment, the DES components (HBA or HBD) can actively participate in the reaction for the generation of polymer materials (also termed as polymerizable DESs or PDESs). The main requirements of the monomer component in PDESs are considered to be the ability to generate sufficient hydrogen bonding interactions between the selected DES components (HBD and HBA) to generate a eutectic mixture and the polymerizability. These limit the number of candidates applicable for PDESs compared to the vast number of conventional monomers available. Primarily, research in this area has focused on the incorporation of one or more vinyl groups as the HBD or HBA, which then leads to free radical polymerization.Among these, the majority of the studies have been conducted using AA or methacrylic acid. The traditional way of carrying out AA free radical polymerization requires the addition of inert solvents to dissolve the monomer and then eventual removal of the inert solvent. However, the utilization of PDES has demonstrated a better solubility of the selected monomers via hydrogen bonding with a higher polymer conversion rate and percentage over those of traditional solvents. In a recent study, Lin et al. investigated the effect of a PDES (consisting of quaternary ammonium salts as the HBAs and AA as the HBD) on the polymerization kinetics of the monomer component (AA) compared to the utilization of the inert solvents DMF, DMSO, and methanol.35 The authors reported a significantly high C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C conversion (∼99%) in PDES medium as opposed to with traditional solvents (less than 30% conversion), besides the high rate of conversion. The main reason for this was attributed to the increased C
C conversion (∼99%) in PDES medium as opposed to with traditional solvents (less than 30% conversion), besides the high rate of conversion. The main reason for this was attributed to the increased C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond activity of the AA monomer (which acted as the HBD) resulting from the hydrogen bonding with the quaternary ammonium salt-based HBAs. Additionally, a vast tunability of the physicomechanical properties of the PDES-based ionic gels was demonstrated by probing the hydrogen bonding levels between the different HBAs and the AA HBD. Here, one of the main advantage was the utilization of all the components of the DES system, even after the complete conversion of the monomers into the final polymer product, unlike in the traditional approach where the solvents are evaporated off. This reduces waste generation by eliminating the solvent evaporation step, while allowing for the tuning of the physicomechanical properties through varying the hydrogen-bond energies within the system.
C bond activity of the AA monomer (which acted as the HBD) resulting from the hydrogen bonding with the quaternary ammonium salt-based HBAs. Additionally, a vast tunability of the physicomechanical properties of the PDES-based ionic gels was demonstrated by probing the hydrogen bonding levels between the different HBAs and the AA HBD. Here, one of the main advantage was the utilization of all the components of the DES system, even after the complete conversion of the monomers into the final polymer product, unlike in the traditional approach where the solvents are evaporated off. This reduces waste generation by eliminating the solvent evaporation step, while allowing for the tuning of the physicomechanical properties through varying the hydrogen-bond energies within the system.
Following the first report by Mota-Morales et al. on polymerizable eutectics using AA and methacrylic acid, a great number of studies based on attractive applications have been published on this topic, mainly due to the fast polymerization and the ability to reduce chemicals in the process.36 Fazende et al. conducted a kinetic study comparing the roles of DESs as solvents, and the polymerizable components. The team utilized a polymerizable DES (ChCl as the HBA, and AA, methacrylic acid as the HBD monomers), comparing it with the polymerization of methyl methacrylate in the presence of a nonpolymerizable DES (ChCl:isobutyric acid).37 In this context, the authors proposed that an increase in rate could be observed in both systems, whether the monomer was a part of the DES or not. Even though increased viscosity is seen as an unfavorable property in certain applications, for radical polymerization processes several studies have indicated that it plays a vital role in increasing the rate of the reaction. However, for the rate of the reaction, other factors, such as light intensity, absorption coefficient, efficiency of initiation, diffusion of the reactants, and polarity of the DES, should not be neglected.36–38 Jablonský et al. highlighted an enhancement of the monomer reactivity in polar media compared to in nonpolar ones and the ability of the DESs (especially ChCl) to influence the rate constant of the initiator decomposition.17
Owing to the significant number of studies carried out based on AA monomer-based polymerizable DESs, Tolmachev et al. recently reported the molecular arrangement and viscosity properties of ChCl:AA through the use of both experimental and simulation studies. The team used different molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. A reduction in the viscosity of the DES with the increased AA concentration was attributed primarily to the size disparity between the choline ions and AA, as well as the balance of the interactions between the AA and Cl− ions. Excess AA, which remains unreacted with Cl− ions, forms pairs with other AA molecules, resulting in mixtures with higher diffusion coefficients.39 Further developments of such free radical polymerizations have been probed by modifying the additives included in the systems. Wang et al. suggested the use of liquid metal nanodroplets (specifically, a mixture of indium and gallium), which could act as a substitute for the initiators and crosslinkers in the ChCl:AA PDES.40 Here, the stabilization of the liquid metal nanodroplets by the viscosity of the PDES medium was reported. While AA served as the polymerizable component in the DES system, ChCl contributed to the system's non-covalent interactions, including hydrogen bonding and ionic bonding with Ga3+ ions, as well as its conductive properties. The polymerization demonstrated a fast curing (6 min), with high transparency (94.1%), and high stretchability (2600%), coupled with other desirable properties, such as ionic conductivity, self-healing, and resistance to freezing and drying.40 Another group generated a polymer matrix with antimicrobial properties by incorporating benzalkonium chloride as an HBA to AA and methacrylic acid-based HBDs.41
4. A reduction in the viscosity of the DES with the increased AA concentration was attributed primarily to the size disparity between the choline ions and AA, as well as the balance of the interactions between the AA and Cl− ions. Excess AA, which remains unreacted with Cl− ions, forms pairs with other AA molecules, resulting in mixtures with higher diffusion coefficients.39 Further developments of such free radical polymerizations have been probed by modifying the additives included in the systems. Wang et al. suggested the use of liquid metal nanodroplets (specifically, a mixture of indium and gallium), which could act as a substitute for the initiators and crosslinkers in the ChCl:AA PDES.40 Here, the stabilization of the liquid metal nanodroplets by the viscosity of the PDES medium was reported. While AA served as the polymerizable component in the DES system, ChCl contributed to the system's non-covalent interactions, including hydrogen bonding and ionic bonding with Ga3+ ions, as well as its conductive properties. The polymerization demonstrated a fast curing (6 min), with high transparency (94.1%), and high stretchability (2600%), coupled with other desirable properties, such as ionic conductivity, self-healing, and resistance to freezing and drying.40 Another group generated a polymer matrix with antimicrobial properties by incorporating benzalkonium chloride as an HBA to AA and methacrylic acid-based HBDs.41
In addition to the widely recognized AA and methacrylic acid-based HBDs, there is a growing interest in researching renewable monomers, such as itaconic acid and caffeic acid, and thermoresponsive monomers, such as N-isopropylacrylamide (NIPAM), as PDESs for specific applications. Additionally, in most of the stated polymerizations, higher reaction rates and crosslinking densities were achieved than in the synthesis in water.17,38,42–45 Further, the use of HBAs with vinyl groups in an organic salt unit has been reported as PDESs. HBAs with quaternary ammonium monomers, such as 2-cholinium bromide methacrylate and 3-acrylamidopropyl trimethylammonium chloride, have also been studied.17,42 Ajino et al. compared three quaternary ammonium monomers having methacrylate, acrylate, and diallyl units, namely, methacroylcholine chloride, [2-(acryloyloxy)ethyl]-trimethyl ammonium chloride, and diallyl-dimethyl ammonium chloride, respectively, with urea to probe the ion-conductive ability for lithium-ion battery applications. Here, the authors documented that the diallyl-type monomer generated the highest ionic conductivity (5.3 mS cm−1 at 25 °C under ambient humidity conditions).46
Various attempts to conduct polycondensation reactions using alcoholic HBDs, such as resorcinol,36 1,8-octanediol,36 ammonium tetraol,17 and ammonium triol,17 in the presence of citric acid or formaldehyde have been reported for the generation of polyesters.
Compared to conventional organic solvents, DESs offer a new platform for polymer synthesis, with the ability to act as a green solvent that can facilitate polymer syntheses under mild reaction conditions. DESs can engage actively in the synthesis process by involving monomers as HBD or HBA components within the system. Further investigations are required to overcome some limitations based on the hygroscopic nature of the system, and for optimizing the yield with a narrow polydispersity index.
4. DESs with biomass functional materials
Besides their use as a solvent or monomer in synthetic polymers, DESs are widely utilized as a solvent for biomass pretreatment or bioactive compound extraction, due to their ability to disrupt the noncovalent bonds within the biomass substructure. Recently, increasing studies have explored the potential of using these green solvents to convert biomass (such as lignocellulose, chitin, and starch) into functional materials (see Table 1).| Feedstock/resource | Materials generated | DES | Major treatment conditions | Features | Application demonstration | Ref. | |
|---|---|---|---|---|---|---|---|
| Yield | Size/Mw/PDI/CRI | ||||||
| Abbreviations: ChCl, choline chloride; LA, lactic acid; OA, oxalic acid; OAd, oxalic acid dihydrate; MA, malonic acid; FA, formic acid; CA, citric acid; EG, ethylene glycol; U, urea; CNC, cellulose nanocrystals; CNF, cellulose nanofibers; LCNC, lignin-containing cellulose nanocrystals; LCNF, lignin-containing cellulose nanofibers; LNP, lignin nanoparticles; Mw, molecular weight; CRI, crystallinity index; PDI, polydispersity index. | |||||||
| Bleached cotton fiber | CNC | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OAd, 1 OAd, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2/3 1/2/3 |
80 °C, 100 °C for 2 h, ultrasonic homogenization | — | Length 122.4–205.9 nm, height 4.7–9.6 nm | — | 47 |
| Bleached eucalyptus kraft pulp | CNC | OAd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeCl3·6H2O, 4 FeCl3·6H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
80 °C for 6 h | Over 90% | Diameter range of 5–20 nm and length of 50–300 nm | — | 48 |
| Grape pomace from red-wine making (cellulose after fermentation in grape pomace) | CNC | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 °C for 6 h | — | Length 241.5 ± 45.3 nm, diameter 22.0 ± 3.9 nm, CRI 95.2% | Self-healing nanocomposite hydrogels | 49 |
| Microcrystalline cellulose | CNC | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U (2.71 U (2.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.32 by weight) 2.32 by weight) |
110 °C for 2–4 days | 33–56% | 20 × 100 × 700 nm | Reinforcing filler/reinforcing fillers for chitosan films plasticized with DES | 50 |
| Bleached rice straw pulp | CNC | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA, 1 OA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 °C for 4 h | 55.1% | Average particle size of 69.3 ± 22.8 nm | — | 51 |
| Kraft pulp from poplar wood | CNC, CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OAd, 1 OAd, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with excess water (10–30%) 1 with excess water (10–30%) |
80 °C for 1 h, microwave, ultrasonic process | 80.8–88.8% | CNC: diameter ∼20 nm, CNF: diameter 20 nm, width 300–2000 nm | — | 52 |
| Microcrystalline cellulose | CNC, CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA, ChCl FA, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U, 1 U, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 °C for 2 h, ball mill for 8 h, 16 h | CNF urea: 85–89%, formic acid: 82–87%, CNC urea: 7–10%, formic acid: 7–13% | CRI urea: 67–69%, formic acid: 69–72%, CNC diameter 8–12 nm | Oil-in-water Pickering emulsion, plastic/rubber systems or advanced materials | 53 |
| Microcrystalline cellulose | CNC, CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA, 1 OA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with ultrapure water 1 with ultrapure water |
90 °C for 0.5–2.5 h, high rate shear dispersing emulsifier | 27.2% (0.5 h) to 65.2% (2.5 h) | Average diameter of 25.1–33.3 nm, length 281.3–404.2 nm | — | 54 |
| Wood cellulose pulp | CNF | Sulfamic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) U, 1 U, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4/3/2 4/3/2 |
150 °C for 30 min | — | Average width: 4.4 ± 1.6 nm | Rheology modifier, reinforcing additives | 55 |
| Hardwood bleached kraft pulp | CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA/LA/acetic/MA/OA/CA, 1 FA/LA/acetic/MA/OA/CA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/9/2/1/1/1 2/9/2/1/1/1 |
50–100 °C for 3 h, mechanical extrusion, colloid mill treatment | 72–88% | Diameters of mono-, di-, tri-substituted carboxylic acids: 15–30 nm, 20–50 nm, and 10–25 nm, respectively | CNF-strengthened polylactic acid (PLA) composites | 56 |
| Bleached softwood kraft pulp | CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anhydrous CA/CA monohydrate, 1 anhydrous CA/CA monohydrate, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 °C,90 °C, 100 °C for 2 h, high-pressure homogenizer | Highest: 87.3 ± 1.8% (citric acid monohydrate at 80 °C), lowest: 74.4 ± 0.9% (anhydrous citric acid at 100 °C) | Diameter highest: 42 ± 5 nm (citric acid monohydrate at 80 °C), lowest: 17 ± 2% (anhydrous citric acid at 100 °C) | Flexible cellulose nano paper | 57 |
| Needle bleached kraft pulp | CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG, 1 EG, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with 20–80% water, H2SO4 catalysis 1, with 20–80% water, H2SO4 catalysis |
120 °C for 2 h, screw extrusion, colloid mill | ∼80–100% | Mean diameter: 14–38 nm | — | 58 |
| Ramie fibers | CNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OAd, 1 OAd, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
100 °C for 4 h, ball mill for 6 h,12 h | — | Mean length 523.4 nm, width 14.3 nm, CRI 79.17% | Thin films | 59 |
| Bleached kraft poplar pulp | CNF | Sulfamic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mass ratio 1 glycerol mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
100 °C for 1–1.5 h, grinder (super mass collider) | 80.9% to 95.2% | Diameters from 10–25 nm, CRI 53–62% | UV-blocking materials | 60 |
| Thermomechanical pulp | LCNC | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA, 1 OA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ChCl 1, ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-toluenesulfonic acid, 2 p-toluenesulfonic acid, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 °C for 3 h | — | Width: ∼6.0 nm, thickness: ∼3.3 nm | Nano-composites reinforcement, cement additives, and packaging materials | 61 |
| Energy cane bagasse | LCNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
110 °C for 30 min, microwave assisted, ultrasonication | 45.2% | Average crystal size 3.14 ± 0.01 nm to 3.44 ± 0.02 nm | Reinforcing and UV absorbing agents in polyanionic cellulose (PAC) films, potential packaging material | 62 |
| Corncob | LCNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA/LA/acetic acid, 1 FA/LA/acetic acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/5/2 2/5/2 |
90 °C for 9 h, enzymatic hydrolysis, high-pressure homogenization | 36.3–82.2% | Average diameter 60–90 nm | Fluorescent, hydrophobic, thermally stable, redispersable LCNF | 63 |
| Hardwood bleached kraft pulp with varying amounts of lignin from poplar wood chips | LCNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
100 °C for 3 h, spiral-squeezing, colloid milling | 80–90% | Diameters 20 nm and 100 nm, CRI 71–78% | Reinforcing agents for PLA films | 64 |
| Pine-wood powder | LCNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3/5 1/3/5 |
Conventional heating 110 °C, 130 °C for 2 h, microwave-assisted heating (80–110 s), high-pressure homogenization | Conventional heating: 40–66%, microwave-assisted heating: 42–80% | Diameter 60–90 nm, conventional CRI 62–71%, microwave CRI 63–70% | Packaging applications | 65 |
| Abaca | LNP | EG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlCl3, 2 AlCl3, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
100–120 °C, 30 min | ∼5.6–32.4% | 30–70 nm, with relatively homogeneous morphology | Natural antioxidant | 66 |
| Corncob alkali lignin | LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanolamine, 1 ethanolamine, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
Ambient temp. (25 °C) 1 h, dialyze for 72 h | 62.7–70.4% | Average diameter 8.9–177.0 nm | Food and medical packaging materials with UV-blocking behavior | 67 |
| Rice straw | LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Microwave irradiation (680 W) 4 min | 63.4% high lignin purity (86.8%) | Average particle size 48–95 nm | — | 68 |
| Dealkaline lignin | LNP | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 30 wt% ultrapure water 2, 30 wt% ultrapure water |
Ambient temp. (25 °C) for 12 h, dialyze for 72 h | 74.8 ± 1.6% | Average size 57.7 ± 1.4 nm | Stabilizer in Pickering emulsions | 69 |
| Kraft lignin | LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanolamine, 1 ethanolamine, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
Ambient temp. (25 °C) for 2 h, dialyze for 72 h | — | Average diameter 123.6–140.7 nm | Nanocomposite gel beads using a sodium alginate (SA) matrix to remove methylene blue (MB) from aqueous solution | 70 |
| Corn cob | LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/5/10 1/5/10 |
100–150 °C for 6–18 h | Up to 85.6% (treatment with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 150 °C for 18 h) highest purity of 97.8% 10, 150 °C for 18 h) highest purity of 97.8% |
Hydrodynamic diameter 48.7 nm | — | 71 |
| Eucommia ulmoides wood | LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malic acid, 2 malic acid, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Hydrothermal pretreated fiber treatment with DES at 120 °C, 130 °C for 3 h | — | 60–110 nm, Mw 1130–8500 | Drug delivery, biomedical and tissue engineering applications | 72 |
| Poplar wood chips, pine wood shavings, moso bamboo powder, wheat straw, rice straw | Activated nano carbon, LCNF, LNP | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Hydrothermal pretreated fiber treatment with DES at 130 °C for 3 h | ANC: ∼1.0–2.7%, LCNF ∼40.5–65.7%, LNP ∼5.7–13.7% | ANC: specific surface area ∼2680 m2 g−1, LNP 200–600 nm, LCNF: aspect ratio ∼150, CRI 72.3–83.3% | — | 73 |
| Wheat straw | LNP | K2CO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol, 1 glycerol, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
100 °C for 16 h, pH 6, 4, 2 | 1.2–7.8% | 200–700 nm | — | 74 |
| Bamboo powder | LNP, LCNF, carbon quantum dots | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Microwave-assisted (maximum 300 W) 100–130 °C, 20 min | — | LNP: 30–75 nm, LCNF: average diameter 8.8–12.8 nm, carbon quantum dots: 9.8 nm | Drug delivery, water treatment, and nano-fillers | 75 |
| Rice straw | Activated nanocarbon (ANC), lignin nanosphere (LNS), LCNF | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA, 1 LA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 °C for 3 h, hydrothermal pretreatment | LNS: 31.8%, LCNF: 99.7% | LNS: 262 nm, LCNF CRI: 76.9%, Mw: ∼6500 g mol−1 | ANC: energy storage application LNS, LCNF: green agriculture, green nano-carrier for enhancing pesticide deposition and retention | 76 |
| Chitin | Chitin nanocrystals | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p-toluene sulfonic acid, 1 p-toluene sulfonic acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95 °C, 30 min | — | Width: ∼12–44 nm, length: ∼206–399 nm | Enzyme carrier | 77 |
| Chitin from crab shell | Chitin nanocrystals | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OAd/LA/MA/CA monohydrate/DL-malic acid, 1 OAd/LA/MA/CA monohydrate/DL-malic acid, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 °C, 1–3 h, ultrasonication | Highest 87.5% ChCl:LA 1 h, lowest 77.9%, ChCl:CA monohydrate 3 h | Average diameter: 42–49 nm, average length: 257–670 nm, mass yield ranging from 78–87.5% | Emulsifiers for Pickering emulsions | 78 |
| Alpha (α)-chitin from shrimp shells | Chitin nanocrystals | Betaine chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ferric chloride hexahydrate, 1 ferric chloride hexahydrate, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70–100 °C, 1–4 h, ultrasonication | 83.2–88.5% | Average diameter of 10 nm and length of 201–259 nm, CRI 89.2% | Emulsion stabilizers | 79 |
| Alpha (α)-chitin from shrimp shells | Chitin nanocrystals | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2, 1 ZnCl2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
90 °C, 3–6 h, ultrasonication | 53.8–97.4% | Diameter 20–80 nm, length 100–700 nm, CRI 84.5–89.8% | — | 80 |
| Waxy maize starch | Starch nanoparticles | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA, 1 OA, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 °C for 0.5, 1, 1.5, 2 h | Highest (0.5 h): 84.3 ± 2.7%, lowest (2 h): 14.1 ± 2.1% | Thickness of crystalline lamellae decreased from 6.4 to 5.6 nm (from 0 to 0.5 h treatment), Mw highest (0.5 h): 1.8 ± 0.4 Da, lowest (2 h): 0.3 ± 0.1 Da, CRI highest (0.5 h): 28.1 ± 0.4%, lowest (2 h): 25.2 ± 0.4% | Food and pharmaceutical industry; film enhancer, rheological modifier, Pickering emulsion stabilizer | 81 |
4.1 Lignocellulose
Lignocellulose biomass is the most abundant natural biopolymer and primarily comprises three sub-units: cellulose (35–83% of dry mass), lignin (0–43%), and hemicellulose (0–30%) combined in a complex structural arrangement.82 The composition of each may vary with the plant category, age, and position of the plant material depending on its designated purpose. Cellulose is a homopolymer containing D-glucose monomers linked by β-1,4-glycosidic bonds with strong hydrogen-bond attachments among the polymer chains, leading to crystalline and amorphous regions within the structure (Fig. 4a). Hemicellulose, on the other hand, is a heterogeneous polymer mainly consisting of xylose, pentose, and hexose. Lignin, being the most abundant natural aromatic feedstock, is composed of a complex arrangement of coniferyl, sinapyl, and p-hydroxyphenyl alcohol units. The availability of the aromatic groups (mainly linked with β-O-4 aryl-ether bonds) generates hydrophobic characteristics in lignin. Owing to the recalcitrant nature of these lignocellulose biomasses, pretreatment is essential to generate value-added functional polymers. The utilization of DESs for this purpose has emerged during the last two decades, mainly due to factors such as recyclability/low waste generation, ease of preparation, and non-toxicity. Vast numbers of studies have been conducted aiming for the isolation of lignin and cellulose biopolymers, whereas research on the isolation of hemicellulose is still in its infancy.83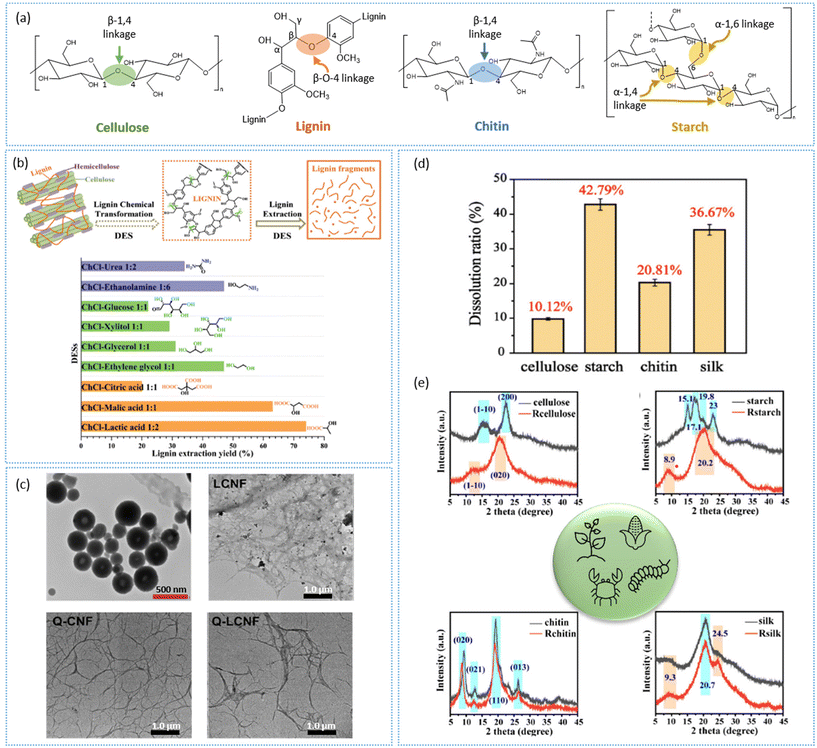 | ||
Fig. 4 (a) Main chemical linkages of cellulose, lignin, chitin, and starch. (b) Lignin fractionation using a DES and the extraction yield.88 Copyright 2020, Royal Society of Chemistry. (c) TEM image of lignin nanospheres, LCNF, quaternized CNF (Q-CNF), and LCNF (Q-LCNF).76 Copyright 2023, Elsevier. (d) Dissolution of biopolymers at room temperature using a DES, and (e) XRD of biopolymers and the regenerated materials at room temperature with ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid 1 formic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.98 Copyright 2023, Royal Society of Chemistry. 4.98 Copyright 2023, Royal Society of Chemistry. | ||
The fractionation efficiency of biomass using DESs depends on various factors, including for the DES itself (molar ratio, temperature, viscosity, acidity/basicity) and the bonding nature within the lignocellulose biomass (number of cleavable bonds available). DESs are widely popular for their specific ability to promote the dissolution of hemicellulose and lignin without disturbing cellulose, as first reported in 2012.84,85 The main reason for this was attributed to the strong cohesive energy existing within the cellulose structure preventing its dissociation in the presence of DESs. Further, the formation of robust hydrogen bonds between the DES and cellulose stabilizes the DES–cellulose system, simultaneously selectively disrupting other noncovalent interactions with lignin and hemicellulose.84 This is commonly referred to as the ‘delignification’ process, and a significant amount of studies have been published on this to date (Fig. 4b).
Apart from the fractionation of lignocellulose biomass, DESs can reduce the size of these natural polymeric materials towards the nanoscale, including cellulose nanocrystals (CNCs),48,51,52,54,85 cellulose nanofibers (CNFs),57,58,63 lignin nanoparticles (LNPs),66,69,70 and lignin-containing CNCs/CNFs61,62,64,86 (Fig. 4c). Even though it was stated that DESs can selectively dissociate the bonds of lignin to promote detachment from the lignocellulose biomass structure, the studies of the actions of DESs on cellulose as a starting material in the microscale have revealed an effect on the hydrogen-bond cleavage of cellulose structures to generate nanoscale materials (Table 1). In most cases, this has been achieved by the synergistic effects of DES treatment together with physicomechanical treatments (e.g. milling, high-pressure homogenization, screw extrusion, microwave, ultrasound) or chemical treatments (addition of catalytic amounts of strong acid/bases, Lewis acids).76,87–89 Luo et al. explored the use of different DES systems to develop stable and uniform LNPs, and incorporated these into a polyvinyl alcohol matrix as a nanocomposite material for medical and food-packaging applications.90 The addition of LNPs increased the tensile strength and thermal stability of the matrix, as well as introducing UV-blocking properties. A recent study carried out by Shen et al. revealed the utilization of hydrothermal pretreatment (with 0.5% H2SO4) coupled with ChCl:lactic acid on straw waste to generate three value-added nanomaterials, namely hemicellulose-derived activated nanocarbon, lignin nanospheres, and lignin containing CNFs (Fig. 4c).76
Wang et al. reported the generation of nanocellulose materials with a width of 3–90 nm and a crystallinity index (CRI) ranging from 44–96% using ChCl and carboxylic acid-based DESs.89 However, even though oxalic acid-based treatment can provide promising results in many instances, dimer formation owing to the dicarboxylic groups in oxalic acid cannot be overlooked since it can have an effect on the mobility of choline cations.91 Additionally, when considering scale-up operations, pulp charring and the release of CO2 at high temperatures have also been observed in this system.84 Other types of acids, such as lactic acid, citric acid, and acetic acid have also been utilized in the past years. However, the main challenge with these carboxylic acid-based DES treatments is their high viscosity level, which affects the yield. Even though the addition of water can result in promising outputs by reducing the viscosity, ionizing the H+, and delocalizing the Cl−, the optimization of this approach is still in the nascent stage.52,76,92,93
The effect of DES treatment on lignin and cellulose structures has been studied on several occasions. DES-treated lignin often exhibits high thermal stability (selective cleavage of abundant β-O-4 aryl-ether linkages of the lignin structure) and high reactivity (increased phenolic hydroxyl groups of the lignin structure due to β-O-4 bond cleavage and demethylation of the methoxy groups) compared to conventional technical lignin.74,92,94,95 Additionally, several studies have indicated that carboxylic acid-based DES treatments lead to the esterification of the γ-hydroxyl group of the lignin substructure and cellulose hydroxyl groups.88,89,96 The generation of cellulose carbamates with different nitrogen contents by urea-based DESs has also been reported as an alternative to more viscose processes, which can have adverse environmental effects and reduce the recylability.97 These modifications under tunable conditions offer pathways to disturb the recalcitrant nature of the lignocellulose biomass compared to the conventional chemical approaches that face several limitations, including the creation of significant waste, oxidation of the biomass, and the introduction of sulfur groups to the structure.
4.2 Chitin
Chitin is the second-most-abundant polysaccharide biopolymer consisting of N-acetyl-glucosamine and N-glucosamine units, and is mainly found in sources such as crustacean shells, insect exoskeletons, and some fungi and algae.99 The β-1-4 bonds make it comparable with the structure of cellulose and it exists mainly in three configurations, namely α-, β-, and γ-chitin, based on the molecular arrangement. The N-deacetylation process (at least 50%) leads to the generation of chitosan, which is considered an alternative biopolymer derived from chitin.100The extraction of chitin consists of two main steps, namely demineralization and deproteinization, where the former involves the removal of CaCO3 with the use of acidic media and the latter by alkali treatment. This procedure facilitates the efficient utilization of chitin, allowing it to be applied effectively in specialized uses within the biomedical industry and other consumer sectors. With the introduction of DESs, researchers have applied their knowledge of DESs to process this biomaterial, with the first attempt at this conducted in 2017.100 Since then, numerous studies have been published in this area over the past years owing to the ease of processing (one step) and the attractive properties of DESs. The highly organized nature of chitin demands strong DES systems mainly consisting of acidic HBDs.99–101 Here, the utilization of type III DESs with carboxylic acids as HBDs has enabled acylation of the hydroxyl groups of the chitin, which enhances the dispersibility (in water) of the regenerated polymer structures.101,102 Further, these acidic DESs have displayed a specific ability to reduce the particle size into the nanoscale. For instance, Yuan et al. highlighted the dual role of the aforementioned DES as a hydrolysation and acylation reagent.78 Among the different acidic systems used, ChCl:malonic acid has demonstrated remarkable outcomes in terms of both the purity and yield, with the effective removal of proteins and minerals.99–103
Li et al. reported the detailed mechanism of chitin extraction, with demineralization by the hydrogen ions of acidic DESs generating soluble calcium salts, water, and CO2. This leads to a loosely bound chitin–protein structure, whereby the DES molecules can penetrate in to the microstructure of the chitin to swell and disrupt the hydrogen bonding network.104 The yield of chitin can depend on various factors, such as temperature, acidic nature, and reaction time within the system and process.102 Additionally, numerous nanomaterials, including nanocrystals and nanofibers, have been isolated by chitin with the use of DESs coupled with mechanical treatments, which enable removing the amorphous zones in raw chitin.103 The synthesis of chitin nanomaterials has been reported with diameters ranging from 20–80, 12–44, and 42–49 nm with the use of ChCl:ZnCl2,80 ChCl:p-toluenesulfonic acid,77 and ChCl:carboxylic acids (oxalic acid dihydrate, lactic acid, malonic acid, citric acid monohydrate, DL-malic acid)78 based DESs respectively. In the latter study, when comparing acidic DESs is concerned, Yuan et al. observed O-acetylation in all the nanocrystals, while the ChCl:lactic acid-based system generated the optimum option, yielding the highest mass of 87.5 wt% along with a stable aqueous suspension.78
Owing to the compositional changes of chitosan, the main application of the DES in those systems was identified as the enhancement of the mechanical properties and plasticity of chitosan-based films.101 Additionally the chemical property variance between chitin and chitosan leads to different solubilities in the same DES system.103
4.3 Starch
Starch is another class of natural polymeric material with a complex assembly made out of amylose- and amylopectin-based polysaccharides with varying arrangements of α-1,4-linkages and α-1,6-branches.105 Various attempts have been carried out in the past to dissolve and isolate this complex polysaccharide with numerous solvents, such as water, DMSO, and pyridine.106 Owing to the attractive abilities of polysaccharide treatment as well as the green credentials, several researchers have tried to utilize DESs to unveil the structural transition of starch. The use of DES-based starch treatment can be categorized into two main parts: dissolution and plasticizisation.107The solubility of starch with the use of different DESs has been studied, and in many studies a maximum solubility up to 10% has been observed.108 A study carried out by Zdanowicz and Spychaj indicated that the treatment of a carboxylic acid-based DES on potato starch led to starch degradation. As a result, most researchers tend to probe ways to utilize this polysaccharide with the use of non-destructive constituents of DESs, mostly containing substituents such as imidazole, urea, and glycerol, with no chemical structure alterations realized in most instances.106,109 The dissolution process is mainly observed via microscopic observations and DSC analysis. Zdanowicz studied the effect of urea-based DESs (with polyols and monosugars) on potato starch and reported that the DES with glycerol displayed better performance over sorbitol, and also that the urea content of the system affected the dissolution temperature of starch.107 In the current stage where the synthesis of starch nanoparticles using DESs is still in its infancy, Xiao et al. successfully utilized ChCl:oxalic acid dihydrate treatment for the generation of waxy maize starch nanoparticles.81 The team observed a dissolution of the crystalline and amorphous regions of the starch, with DES treatment for 2 h generating aggregated nanoplatelets sized 38–117 nm (Fig. 5b). Further, esterification of the nanoparticles due to the effect of oxalic acid in the DES has been observed with increased treatment time.
 | ||
| Fig. 5 (a) Plasticization effect of ChCl:urea DES on dried potato starch at 80 °C, 100 °C, and 118 °C.109 Copyright 2018, Elsevier. (b) Illustration of the formation of starch nanoparticles: DES treatment (ChCl:oxalic acid dihydrate) on waxy maize starch for 1 h and 2 h.81 Copyright 2022, Elsevier. | ||
DESs are usually popular for their plasticizing ability when it comes to starch-based applications. The plasticization of starch takes place when the DES constituents disrupt the granular structure of starch via hydrogen bonding, resulting in swelling.108 The main factors affecting the plasticizing include the temperature, force, water content, and chemical constituents.108,109 Zdanowicz compared different DESs and ionic liquids with the conventional plasticizer glycerol to treat potato starch, and revealed that urea-based DESs and ionic liquids generated a better dissolving capacity while having lower viscosity values compared to glycerol, which is a beneficial property for thermoplastic starch processing (Fig. 5a).110
The addition of a plasticizer for starch processing plays a vital role owing to the glass transition temperature lying close to its degradation temperature. It has been found that the use of a DES can enhance the surface properties of starch when compared to conventional plasticizers, such as glycerol and urea.107 The effects of sugar-alcohol-based DESs were studied by Zdanowicz et al., who revealed that a sorbitol-based DES provided attractive mechanical properties (tensile strength of 8.6 MPa, 33% elongation at break).111 Another study carried out by Zdanowicz compared different DES combinations with urea-, polyol-, and sugar-based starch treatments, resulting in the production of flexible films with a highest elongation at break greater than 200% with a urea:glycerol system.107 The plasticizing ability of DESs can depend on several factors, such as temperature and force applied, as well as the technique used (e.g., extrusion, thermocompression).108 A study carried out by Deng et al. discussed the importance of the use of a ChCl:ethylene glycol DES on corn starch for non-destructive starch processing and also carried out a structural analysis.106
Utilizing renewable feedstocks (in accordance with the seventh principle of green chemistry) to produce value-added functional materials with the help of DESs can open an avenue for environmentally friendly alternative ways to synthesize materials. Here, the effect of hydrogen bonding in the dissociation of biomasses plays a prominent role, while the plasticizing effect of DESs on starch provides attractive physicomechanical properties in the subsequent composite materials. The utilization of DESs for the treatment of biomass materials discussed herein has the ability to generate nanomaterials with high purity levels compared to the harsh conditions needed with conventional approaches. The most common chemical modification arises with the acylation of the abundant hydroxyl groups of the biomasses, especially esterification with carboxylic acid-based DES treatment. This functionalization is deemed to be favorable owing to the improved dispersibility of the regenerated biopolymers and their high storage stability, which ensures their effective utilization for most applications.
5. Functional materials engineered utilizing DESs
In the above two chapters, we summarized how DESs facilitate the polymerization of synthetic polymers as monomers or solvents and the use of DESs in biomass pretreatment. All these DES adoptions aim to assist the engineering of DES-synthetic polymers and biomass into functional materials. Some of the specific properties of these functional materials include stimuli responsiveness, self-healing properties, electrical conductivity, and biodegradability, besides an improved mechanical strength. The versatility of DESs enable them to play an important role in this context as a green alternative to conventional approaches, thus they are the subject of increasing research as an emerging area of interest (Fig. 1b). In this section, we reveal the strategies and properties of the functional materials generated by the application of DESs in a range of categories.5.1 Gels
5.1.1.1 Eutectogels using PDESs. The application of DESs in gel materials has shown beneficial results over conventional hydrogels and ionic liquid gels mainly owing to their non-volatility, non-toxicity, and ease of preparation. The utilization of PDESs further enhances the properties through noncovalent ionic and hydrogen bonding within the DES components and polymer chain, besides from the covalent bonds with the polymer backbone of the HBD or HBA. This leads to enhanced mechanical properties of the system, together with high fatigue resistance.113 The vast tunability of DESs enables an effective dissolution of organic and inorganic additives, thereby enabling their use in diverse applications.114 The low or no water within the eutectogel system further overcomes the drawbacks associated with conventional hydrogels due to the evaporation of water at high temperatures and freezing at lower temperatures.114–116 The self-healing effect arising from the abundant hydrogen bonding is also another added advantage of DESs in gel-based systems, which enhances their robust nature. Collectively, these properties of DESs open up attractive avenues for the utilization of DES-based gel materials compared to traditional gel materials.
The utilization of the polymerizable, renewable itaconic acid for hydrogels was proposed by Bednarz et al., who generated a poly(itaconic acid-co-bisacrylamide) hydrogel using a ChCl:itaconic acid PDES, highlighting the faster copolymerization in the DES medium than in water.44 Another dual network eutectogel with more properties, including a visual strain-sensing ability, was developed recently by Liu et al.114 utilizing a ternary DES ChCl:glycerol:N-acryloyl glycinamide (NAGA) with a conductivity of 0.7405 mS cm−1 and temperature tolerance of −20 °C to 60 °C. Here, the incorporation of photonic crystals into the gel led to colour changes at different strains, while the polymerized NAGA provided mechanical stability. Most of the gel-based applications reported to date have used AA, acrylamide, and acrylic acid derivatives in a DES medium.117
In 2021, Wang et al. suggested a different approach to incorporate a PDES (ChCl:AA) into a silicone tube.115 This silicone tube was weavable on fabrics and could be used as a strain sensor to identify human motions. Additionally, this system was able to withstand a temperature range from −30 °C to 100 °C and C2Cl4, which is widely used for the dry cleaning of fabrics. The AA-based DES was initially formed, followed by photopolymerization after being inserted into the tube. Another study carried out by Lim et al.113 probed the use of a ChCl:AA PDES as a screen-printable conductive ink. In their study, they used a supramolecular assembly of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT:PSS) to support the system, which generated a conductivity of 1.3 mS cm−1 (sevenfold higher than the normal ChCl:AA gel). This conductivity was further tremendously improved to 130 mS cm−1 with the addition of H2SO4 in to the system. The authors attributed this observation to structural transformations of the supramolecules into a linearly ordered manner, which facilitated the conductivity, thereby improving the signal response rate. The prepared gel material showed self-healing, stretchable, and high fatigue-resistance properties. Another double network PDES with the same DES system was generated with robust mechanical properties (strain up to 1373%, stress up to 3.14 MPa) by Wang et al. utilizing a polyacrylamide hydrogel consisting of acrylamide and hyper-branched polyester H20P.116 Here the availability of a high number of hydrogen bonds as well as the covalent crosslinks enhanced the mechanical strength. The optimal conductivity of the hydrogel was reported to be approximately 0.21 mS cm−1.
5.1.1.2 DESs as a medium. The other approach of generating stimuli-responsive gels is the utilization of polymers (mainly AA and its derivatives) as a support for double network gels. A study carried out by Wu et al. investigated the effect of the self-assembly of biopolymer agar in the DES system of ChCl:urea:water with Al3+ crosslinked polyAA.120 A room temperature ionic conductivity of 2.1 mS cm−1 was achieved in this study, and it was also reported that the effect of Al3+ may adversely affect the conductivity owing to the high amount of dynamic crosslinking, even though it enhanced the mechanical strength (stretchability of up to 1595%) of the gel system.
A hydrophobic strain-sensing eutectogel was investigated by Gao et al. with a N,N-dimethylacrylamide (DMA) polymer base (Fig. 6b).119 Here, the traditional ChCl was not used as the HBA to facilitate the conductivity, instead methyltrioctylammonium chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl 4-hydroxybenzoate (1
ethyl 4-hydroxybenzoate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was chosen to enhance the hydrophobicity. A stretchability of 900%, toughness of 341.14 kJ m−3, and tensile strength of 97.58 kPa were achieved owing to the reversible hydrogen-bond interactions between the DES and the polymer in the gel with 30 wt% polymer scaffolds. The greatest strength was observed in the gel with 50 wt% polymer (tensile strength of 4092.63 kPa, toughness of 13
2) was chosen to enhance the hydrophobicity. A stretchability of 900%, toughness of 341.14 kJ m−3, and tensile strength of 97.58 kPa were achieved owing to the reversible hydrogen-bond interactions between the DES and the polymer in the gel with 30 wt% polymer scaffolds. The greatest strength was observed in the gel with 50 wt% polymer (tensile strength of 4092.63 kPa, toughness of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502.90 kJ m−3). Another interesting approach for strain sensing with the use of DESs was probed under harsh conditions for underwater communication.121 Here, the team used both hydrophilic and hydrophobic DESs as well as polymer systems to achieve the intended properties with a high level of interactions. ChCl:ethylene glycol was utilized as the hydrophilic DES system, whereas thymol:decanoic acid was its hydrophobic counterpart. For the polymers, AA and 2,2,2-trifluoroethyl methacrylate were copolymerized as the hydrophilic and hydrophobic ends, respectively. The team was able to generate stimuli-responsive eutectogels with 0.93 mS cm−1 conductivity along with a fast response time (1 s), and immunity to pH ranging from 3–9, together with low swelling and low drying properties.
502.90 kJ m−3). Another interesting approach for strain sensing with the use of DESs was probed under harsh conditions for underwater communication.121 Here, the team used both hydrophilic and hydrophobic DESs as well as polymer systems to achieve the intended properties with a high level of interactions. ChCl:ethylene glycol was utilized as the hydrophilic DES system, whereas thymol:decanoic acid was its hydrophobic counterpart. For the polymers, AA and 2,2,2-trifluoroethyl methacrylate were copolymerized as the hydrophilic and hydrophobic ends, respectively. The team was able to generate stimuli-responsive eutectogels with 0.93 mS cm−1 conductivity along with a fast response time (1 s), and immunity to pH ranging from 3–9, together with low swelling and low drying properties.
 | ||
| Fig. 6 (a) Use of gelatin based eutectogels for medical applications. (i) ECG comparison with a commercial medical electrode, (ii) time evolution EMG signals for thigh contraction/relaxation.118 Copyright 2022, Wiley. (b) Hydrophobic DES-based gel. (i) Schematic illustration of hydrophobic DES-based gel synthesis. (ii) Photographs of PDMA–DES gels with different polymer contents (20 to 50 wt%). (iii) Tensile stress–strain curves of PDMA–DES gels, with the highest tensile strength observed for the 50 wt% polymer loading (4092.63 kPa). (iv) Conductivity of the gel with 30 wt% polymer loading (PDMA/DES3) at different temperatures from −20 °C to 120 °C.119 Copyright 2022, Wiley. | ||
A supramolecular assembly for a D-gluconic acetal gelator was utilized for a N-(2-hydroxyethyl)acrylamide polymer-based dual network hydrogel with ChCl:monoethylene glycol.122 The results suggested that increasing the loading of the DES led to an increase in conductivity (up to 3.74 mS cm−1), accompanied with improved mechanical properties, including an elongation at break of over 4300%, tensile fracture strain of 0.21 MPa, and stability over the range of −20 °C to 100 °C. Additionally, with the strong intermolecular interactions of the gelator G18 a high level of adhesion on different substrates was observed. Apart from the strain- and pressure-sensing ability, this eutectogel showcased temperature-sensing properties as well, owing to the reduction in resistance at higher temperatures. Additionally, reversible noncovalent interactions within the double network system were demonstrated in this study by the overlapping front and rear hysteresis loops, indicating a rapid partial self-recovery.
Prasad et al. reported in 2018 a number of ionic gels based on polysaccharides that were responsive to pH, heat, solvent, and shear changes.123 In their work, the team investigated the use of self-polymerizable HEMA for pH-responsive drug release (indomethacin) in ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose (2
fructose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Another use of HEMA was presented in the same paper using ChCl
1). Another use of HEMA was presented in the same paper using ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) orcinol (1
orcinol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) for achieving a highly stretchable and good capacitance behavior. Fan et al. introduced a eutectogel through the polymerization of 1-viniylimidazole in ChCl:glycerol, which demonstrated high stretchability (2300% elongation at break), self-healability, and ionic conductivity in a wide temperature range (−30 °C to 60 °C).124 The generated eutectogel also demonstrated good adhesion to different substrates, with the highest adhesive strength of approximately 70 kPa with a glass substrate.
1.5) for achieving a highly stretchable and good capacitance behavior. Fan et al. introduced a eutectogel through the polymerization of 1-viniylimidazole in ChCl:glycerol, which demonstrated high stretchability (2300% elongation at break), self-healability, and ionic conductivity in a wide temperature range (−30 °C to 60 °C).124 The generated eutectogel also demonstrated good adhesion to different substrates, with the highest adhesive strength of approximately 70 kPa with a glass substrate.
5.1.1.3 Self-assembly within DESs for supramolecular eutectogels. Self-assembly in deep eutectic solvents represents an emerging frontier in materials science, offering a versatile platform for the creation of intricate supramolecular structures. Several systems, including PEDOT:PSS, cyclodextrins, DNA, and gelatin, have attracted significant attention in this context. Among these systems, gelatin has emerged as a particularly prominent candidate for the development of stimuli-responsive materials over the past few years, and is covered in detail in this section.
Gelatin is a widely produced protein-based natural biopolymer that has been ubiquitously applied in the food and pharmaceutical industry thanks to its nontoxic nature. The availability of polar groups within the structure makes gelatin a perfect candidate for the fabrication of eutectogels with greater stability than conventional hydrogels (loss of water content). Studies on the effects of DESs on the interaction of gelatin's triple helices have been performed by a number of research groups when fabricating sensors.
A study carried out by Qin et al. utilized gelatin as a 22 wt% loading for a ChCl:ethylene glycol DES to generate a nonvolatile transparent gel electrolyte for strain- and pressure-sensing applications. The prepared eutectogel showed a pressure-sensing ability around 1 kPa levels and the ability to withstand a strain of 320% (tensile stress of 67 kPa) with a conductivity of 2.5 mS cm−1 at room temperature.125 Here, both the ChCl and ethylene glycol played vital roles in the mechanical stability of the gel, where the kosmotropic nature coupled with high ion density of ChCl facilitated the assembly of gelatin chain bundles with higher intermolecular attractions (lowering the intramolecular interactions within the triple helices), while ethylene glycol promoted the formation of a fewer number of gelatin triple helices with larger diameters, thus providing greater flexibility.
Owyeung et al. studied the effect of HBDs on gelatin–DES-based hydrogels using three main HBDs: ethylene glycol, glycerol, and 1,2-propanediol.126 Interestingly, this study revealed that the careful addition of a small amount of water (5–6 wt%) to the gelatin supported (20 wt%)-DES system enabled an improvement not only in the conductivity but also in the toughness of the resultant eutectogel. The Young's modulus was also changed with respect to the HBDs, indicating a lowest value of 7 kPa with 1,2-propanediol to a highest value of 42 kPa for glycerol. The incorporation of water in to the ethylene glycol-based DES increased the toughness value by 195%, while also increasing the conductivity by 58% (to 5.2 mS cm−1). The authors highlighted the formation of shorter triple helices of gelatin with the presence of glycerol and ethylene glycol by virtue of the steric hindrance they generated compared to water molecules. The generated shorter helices facilitated a greater amount of crosslinks in a selected volume, resulting in a higher stretchability of the material. Additionally, the carefully added water molecules helped to build up interaction between non-helical inter-chain bonding, which then led to higher toughness values synergistically.
Another class of electron-conductive eutectogel was generated by Picchio et al. using a gelatin matrix with the addition of PEDOT:lignin sulfonate with gelatin and a DES (ChCl:glycerol). The resultant eutectogel showed high ionic and electronic conductivity values of 7.3 and 8.7 mS cm−1,118 respectively, which suggested this system could be a possible candidate for strain sensors as well as a bioelectrode for ECG and EMG recording (Fig. 6a).
The self-healability and recyclability of gelatin/polyvinyl alcohol/dialdehyde carboxymethyl cellulose (DCMC)-based composite eutectogels (ChCl:glycerin) were studied for potential applications requiring strain, humidity, and temperature sensitivity.127 Here, the self-healing and mechanical properties (tensile strength of ∼1.25 MPa, elongation at break of 1400%) were enhanced by synergistic effects gained through the imine and hydrogen bonds within the system. Each of the constituent played a vital role in the overall performance of the resultant material, whereby the DES contributed to the ion conductivity, self-healing properties, and durability over a wide range of operating temperatures, while polyvinyl alcohol/DCMC acted as water-capturing agents during humidity changes, and structural transition of the gelatin had an effect on the temperature-sensing ability.
Recently, Mercadal et al. developed a non-cytotoxic bio-based eutectogel with strain-sensing abilities and 3D printability through the use of ChCl:ethylene glycol, porcine gelatin.128 Here, tannic acid-coated CNC was utilized as a reinforcing agent at low concentrations (1–2 wt%) and the resultant gel revealed a strength of 30 kPa and elongation at break of 180%. A time-dependent adhesive performance was incorporated into the underwater adhesives from the silane-based PDES (lauric acid:diethylaminoethyl methacrylate:(3-amino-propyl)trimethoxysilane).129 Here the authors were able to introduce controllability of the gel adhesive remotely with the use of a laser beam.
The use of DESs as solvents has been primarily applied for phenol–formaldehyde polycondensations. Chen et al. reported a ChCl:urea-based DES system for CO2 capture using xerogels, with the DES system serving multiple functions, including acting as a structure-directing agent, and providing a source of nitrogen for the carbon, besides functioning as a solvent.130 Another use of a similar polymer system was generated for capacitor applications, where a ZnCl2 and urea DES was used to facilitate the generation of carbon-based aerogels with a high surface area (1238.81 m2 g−1).131 Additionally, an iron-based DES system for xerogel-based supercapacitor applications was studied by Chen et al., which delivered a capacitance of 209 F g−1 at a current density of 0.5 A g−1. Here, the authors highlighted the advantages gained by using DES-assisted resorcinol–formaldehyde polycondensation by eliminating the costly supercritical CO2 drying processes incorporated in traditional xerogel preparation.132 Itaconic acid133 and anilinium chloride134 have been used as PDESs for the generation of xerogels. Further the pretreatment of biomass has been studied for the separation of oil, organic molecules, water, and dyes. Glucose and ChCl DES-treated CNFs were utilized by Long et al. for the generation of carbon aerogel with a recycling ability of up to 100 cycles (Fig. 7b).135 Peng et al. documented a top-down approach utilizing cornstalk pith for aerogels with an oil/organic solvent sorption ability (Fig. 7a). Here, they used a DES for the selective delignification and removal of hemicellulose to generate a hydrophobic aerogel through further modifications.136 Recently, Zhu et al. generated a composite aerogel with DES (ChCl:lactic acid)-treated lignin from grapevine with chitosan for the removal of dyes (methylene blue, Congo red), catechin, and epicatechin, where the π orbit interactions of lignin and the samples played a prominent role.137
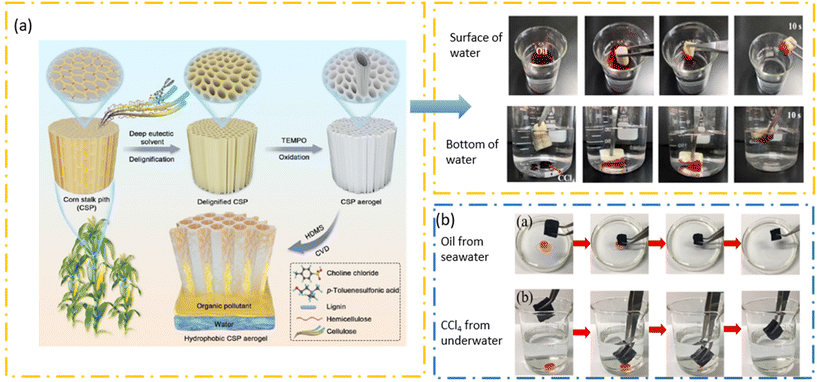 | ||
| Fig. 7 (a) Top-down approach for generating a hydrophobic aerogel from cornstalk pith that demonstrated a good oil/water separation capability.136 Copyright 2023, Elsevier. (b) Absorption of oil from sea water and CCl4 from underwater using a carbon aerogel derived from DES-modified CNFs.135 Copyright 2021, Elsevier. | ||
5.2 Fibers
The exploratory research efforts of DESs has opened up a myriad of possibilities for material fabrication. The use of these green alternative solvents would not only be an environmentally conscious approach but also cost effective. To further demonstrate the potential of DES materials, various research works have been done to exploit the potential of their usage in nanofibers. These nanofibers coupled with electrospinning techniques can be applied to diverse fields, such as biomedical and agriculture. Sousa et al. first reported the use of a DES as a solvent for electrospinning to produce agar nanofibers for potential biomedical applications.138 The alternative solvent for electrospinning was prepared with the DES ChCl:urea. Different blends of polyvinyl alcohol/agar and DES conditions resulted in varying the characteristics of the fibers, in terms of the surface roughness and diameter size, and all the blends exhibited good spinnability and mechanical resistance. Additionally, Mano et al. reported an approach for the fabrication of electrospun polyvinyl alcohol fibers with a NADES.139 The main advantage of NADES in the fabrication of polyvinyl alcohol biopolymers is the acceptable toxicity profile and biocompatibility. As DESs and NADESs are hygroscopic liquids, they prevent premature evaporation of the solvent during fiber fabrication. The benefits of DESs in terms of recyclability, lower cost, and suitable compatibility have put them in the spotlight as possible alternatives to regular volatile solvents and their more expensive counterparts, ionic liquids.However, there are some possible adverse effects from residual DES that could be detrimental to the integrity of the biopolymer fibers. A wet electrospinning strategy with the use of a ChCl:lactic acid DES was explored to prepare lignin fiber aerogels. The electrospun fibers were reported to coagulate in the water bath setup sufficiently enough to coagulate and remove the DES residues.140 The resulting lignin fiber aerogel was carbonized and exhibited excellent electrochemical stability as a supercapacitor electrode, as compared to the commercial counterpart. This demonstrated that DESs can be a greener solvent alternative to produce quality electrospun fibers to further build into aerogels. The compatibility of DESs as better solvents in electrospinning compared to volatile solvents, such as ethanol and DMF, extends to the production of protein-based electrospun nanofibers as well. Zein nanofibers are difficult to be electrospun directly under certain conditions, such as temperature and UV-light exposure.141 Mouro et al. fabricated nanofibers from electrospinning a polymeric blend of polyvinyl alcohol and regenerated wool keratin from wool waste in different DES mixtures of ChCl:urea and L-cysteine:lactic acid.142 The nanofibrous membrane exhibited good antioxidative and antimicrobial properties that could be valorized in other industrial applications. As the choice of solvents impacts the quality and outcome of the fibers, DESs can facilitate the fabrication of these nanofibers due to their non-volatility.
On the other hand, DESs can be incorporated directly into polymers for obtaining electrospun nanofibers. Sereshti et al. synthesized polyamide 6 (PA6)/[polymerized HEMA:1-tetradecanol]-based electrospun nanofibers to extract residual pesticides for cereal flour analysis.143 At a higher DES content, the increase in viscosity of the polymer blend becomes a hindrance, whereas too low a viscosity results in droplet formation. An optimal range of polymeric DES nanofibers was tested and resulted in a satisfactory recovery of pesticides, demonstrating their potential as a fast and green alternative for multiclass pesticide analysis. Furthermore, NADES-integrated poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) electrospun nanofibers were successfully fabricated for air filtration and resulted in a unique rugose morphology (Fig. 8a).144
 | ||
| Fig. 8 (a) Schematic diagram of sample preparation via electrospinning and its subsequent morphology for (i) pure PHBV on Al foil, (ii) PHBV/DES on polypropylene (PP), (iii) pure PHBV on PP, and (iv) PHBV/DES on PP.144 Copyright 2023, American Chemical Society. (b) Schematic diagram of the fabrication of polyvinyl alcohol–DES, polyvinyl alcohol–DES–honey, and polyvinyl alcohol–DES–ASA systems prepared by electrospinning, and their subsequent morphology in (i) pure polyvinyl alcohol nanofibers, (ii) polyvinyl alcohol–DES, (iii) polyvinyl alcohol–DES–honey and (iv) polyvinyl alcohol–DES–ASA.145 Copyright 2021, Royal Society of Chemistry. | ||
Electrospinning with DESs or DES-containing polymers can create unique nanofibers with promising opportunities for application in healthcare, particularly in drug-delivery systems. The DES ChCl:mannose was used to dissolve polyvinyl alcohol and then combined with honey or acetylsalicylic acid (ASA) to fabricate nanofibers through an electrospinning process (Fig. 8b).145 In different blends of polymeric nanofibers, the polyvinyl alcohol–DES demonstrated fast-release drug delivery, while the polyvinyl alcohol–DES–honey system exhibited excellent properties for wound healing, and the polyvinyl alcohol–DES–ASA can potentially be used for rapid drug release in oral mucosal systems.
Drug release can be optimized with the correct morphology, porosity, and interactions between the active agent and the polymeric nanofibers. Generally, electrospun drug systems can deliver small molecules, such as proteins or small drugs, by surface interaction with the nanofibers. The smooth surface characteristics of the nanofibers can be achieved with the help of DES additives as well. Polycaprolactone fibers containing DES1 (ChCl:acetic acid) and DES2 (ChCl:glycerol) were electrospun for the release of ibuprofen and were found to have hydrophilic behaviors that could affect the drug-release profile.146 The application of DESs in electrospinning techniques and polymeric nanofibers minimizes the use of hazardous, volatile solvents, together with providing an option to recycle the residual DES. The properties and morphologies of nanofibers derived from DESs can be tuned to the level of the DES in the system.
5.3 3D printing
With the improvement of fast-paced scientific advancements, additive manufacturing has attracted greater interest over the past few years. Researchers are continuously searching for ways to develop novel printable materials that can be used for a range of applications. DESs, being nonvolatile, environmentally friendly materials, have been proven to be able to cater this demand in several studies, and this area of research is evolving rapidly owing to the vast tunability of properties by fine-tuning the bonding nature with polymers and other constituents.Several applications of the use of gelatin for non-cytotoxic sensor-based applications were already discussed in Section 5.1.1.3 in detail.118,128 These studies also probed the ability of the gels for 3D printing applications via hot extrusion processes under mild temperature conditions (37 °C to 42 °C), where the printed articles were able to preserve the original shape and pattern. Sheikhi et al. suggested the use of DESs for a radically distinct approach for the 3D printing of jammed microgels.150 Here, the authors used a DES in the final step to coat a 3D printed jammed article to ensure the strength of the material, which ended up generating self-standing structures. The team used an L-arginine and glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)-based DES system for this purpose with a polymer network consisting of vinyl-functionalized gelatin, AA, and glycidyl methacrylate. The distinct role of the amino acids and 1,2-diols-based DES as an activator in covalent bond formation to make epoxy functional groups for nucleophilic reactions under ambient conditions was highlighted in this study.
2)-based DES system for this purpose with a polymer network consisting of vinyl-functionalized gelatin, AA, and glycidyl methacrylate. The distinct role of the amino acids and 1,2-diols-based DES as an activator in covalent bond formation to make epoxy functional groups for nucleophilic reactions under ambient conditions was highlighted in this study.
Aguzin et al. studied the use of PEDOT:PSS in DES systems to enhance the ionic electronic conductivity and observed a supramolecular assembly and gelification in the system accompanied by the DESs. The group studied several DES systems with differing HBDs, such as aliphatic polyols, and organic acids to aromatic compounds, and suggested the PEDOT:PSS/DES ratio of 30 wt% provided the desired gel-like textures. Here, p-toluenesulfonic acid- and pyrogallol-containing DESs presented the highest storage modulus values (160 Pa and 120 Pa, respectively) and the authors attributed this phenomenon to the π–π interactions between the phenolic moieties of PEDOT, PSS, and HBDs. Furthermore, a system with ChCl:lactic acid was used for a DIW application owing to its biocompatibility, with an annealing process at 50 °C for 16 h for the fabrication of conductive patterns.151
The generation of 3D printing ink using lactic acid and maleic acid-based DES systems in a one-pot strategy was suggested by Wang et al. with the addition of a glycidyl methacrylate polymer system and CNCs.152 In this research, the team observed a higher strength in the maleic acid-based DES system due to the presence of additional chemical crosslinks generated by the unsaturation present in the structure of maleic acid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylene glycol
ethylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA (1
AA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), which resulted in an enhanced stretchability (300%) and high sensitivity (gauge factor of 3.1) in the 3D printed structures.160
2), which resulted in an enhanced stretchability (300%) and high sensitivity (gauge factor of 3.1) in the 3D printed structures.160In addition to the commonly employed DIW printing method, other techniques, such as stereo lithography (SLA), digital light processing (DLP), and liquid crystal displays (LCDs), have also been explored in conjunction with DES systems. The rapid curing time is a significant factor in most of these printing approaches, surpassing the importance of viscosity adjustments. Up to the present time, researchers have investigated numerous combinations of PDESs to address this aspect.
Su et al. suggested the fabrication of transparent antistatic 3D printed materials using the SLA method. The group utilized a PDES system consisting of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA (1
AA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), which contributed to the enhancement of the antistatic property, and 2-hydroxyethyl acrylate for the strength requirements. The photopolymerization of this system indicated a fast curing speed (10 s) and the highest mechanical strength was observed at a 20% DES loading with a 90% polymer conversion rate.153 Cai et al. worked on a similar DES system with the SLA technique to fine-tune the printing structure as an interlocked pyramid type for pressure-sensing applications.154 A ternary PDES with acrylamide
2), which contributed to the enhancement of the antistatic property, and 2-hydroxyethyl acrylate for the strength requirements. The photopolymerization of this system indicated a fast curing speed (10 s) and the highest mechanical strength was observed at a 20% DES loading with a 90% polymer conversion rate.153 Cai et al. worked on a similar DES system with the SLA technique to fine-tune the printing structure as an interlocked pyramid type for pressure-sensing applications.154 A ternary PDES with acrylamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ChCl
ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) maleic acid (1
maleic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was studied with this printing technique, which resulted in highly transparent objects (95.6%) coupled with a faster curing time of 6 s (24 W UV light 410 nm), as reported by the same team (Fig. 9a).156 Here, the team suggested that the strength of the material was due to its structure comprising a soft monomer system (maleic acid/ChCl) and a hard system (acrylamide/ChCl), which resulted in the generation of a copolymer product of poly(acrylamide/ChCl-co-maleic acid/ChCl). Furthermore, they emphasized that increasing the photoinitiator concentration resulted in higher conductivity due to the decrease in molecular weights of the polymers. Conversely, an increase in crosslinking agents led to a reduction in conductivity. The same group investigated the application of this PDES system in a DLP-based printing technique and highlighted the temperature stability of the printed material over a wide temperature range (−70 °C to 120 °C) while maintaining its properties (conductive, compressible, and transparent) on the generation of capacitive ionic skins.159
2) was studied with this printing technique, which resulted in highly transparent objects (95.6%) coupled with a faster curing time of 6 s (24 W UV light 410 nm), as reported by the same team (Fig. 9a).156 Here, the team suggested that the strength of the material was due to its structure comprising a soft monomer system (maleic acid/ChCl) and a hard system (acrylamide/ChCl), which resulted in the generation of a copolymer product of poly(acrylamide/ChCl-co-maleic acid/ChCl). Furthermore, they emphasized that increasing the photoinitiator concentration resulted in higher conductivity due to the decrease in molecular weights of the polymers. Conversely, an increase in crosslinking agents led to a reduction in conductivity. The same group investigated the application of this PDES system in a DLP-based printing technique and highlighted the temperature stability of the printed material over a wide temperature range (−70 °C to 120 °C) while maintaining its properties (conductive, compressible, and transparent) on the generation of capacitive ionic skins.159
 | ||
Fig. 9 Photocurable resins generated by PDESs for 3D printing applications. (a) Schematic illustration of SLA printing of a PDES (acrylamide:ChCl:maleic acid) and 3D printed models for conductive elastomeric sensors.156 Copyright 2021, Elsevier. (b) LCD 3D printing of a bio-based PDES (ChCl:HEMA).163 Copyright 2023, American Chemical Society. (c) (i) Composition and hydrogen bonding in a PDES (ChCl:acrylamide:4-acryloylmorpholine). (ii) Conductivity variations with respect to humidity from 0–85%. (iii) Structures generated by the LCD 3D printing of PCR 1/4/1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acrylamide acrylamide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-acryloylmorpholine, 1 4-acryloylmorpholine, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (iv) Epoxy structures generated by 3D printed PCR 1/4/1 sacrificial molds.161 Copyright 2023, American Chemical Society. 1). (iv) Epoxy structures generated by 3D printed PCR 1/4/1 sacrificial molds.161 Copyright 2023, American Chemical Society. | ||
Lacalle et al. suggested a novel DES system by probing the interconnections between methacrylic and acrylic quaternary ammonium monomers with phenolic derivatives. Here, unlike conventional approaches, the authors used the polymerization of the HBA for the PDES for 3D printing based on the DLP method. This work highlighted the metal complexation ability, fast processability, and antibacterial properties of the suggested PDES, while providing more inputs on the adhesive properties on the pyrogallol and hydrocaffeic acid-derived ionic polymers, and an increase in strength due to the ultratough nature of the tannic acid- and gallic acid-derived polymers (strength ∼ 3 MPa).162
A study conducted by Zhu et al. utilized an LCD-based printing technique to generate printable materials with a ChCl:HEMA PDES (Fig. 9b). The addition of tannic acid (10–40 wt%) not only enhanced the antibacterial properties of the printed items but also improved the homogeneity of the generated inks by interconnecting via noncovalent bonds with the DES system.163 An interesting study on the 3D printing of a sacrificial mold coupled with a reprocessing study of the ink was conducted by Li and co-workers utilizing a ternary PDES (ChCl:acrylamide:4-acryloylmorpholine) with an LCD (Fig. 9c). The solubility parameters of the final products could be tuned by adjusting the ratios of the PDES components, and the authors highlighted that the 4-acryloylmorpholine monomer played a vital role in lowering the viscosity at ambient temperature. Studies conducted on the recycling of the printed material indicated a recovery efficiency exceeding 65% in tensile strength, strain at break, and Young's modulus.161
5.4 Membranes and films
The escalating challenges associated with plastic waste have spurred significant research into environmentally friendly and biodegradable solutions for membrane and thin film based applications. The transition in membrane science from traditional size separation to selective filtration and plastic-based thin films to functional polymeric materials from natural substitutes has been developed with a notable focus on the application of DESs due to their unique ability to interact via hydrogen bonding, serving both as modifiers for structural enhancement and as key functional materials.Pulyalina et al. investigated a polymer composite using polyamide-imide (Torlon) and a DES comprising zinc chloride and acetamide. The optimal DES loading of 50% resulted in the generation of pores within the polyamide-imide Torlon membrane, enhancing the surface hydrophilicity by 24.9%, and showcasing the role of the DES as a plasticizer (Fig. 10a).164 Non-ionic DESs were employed by Ismail et al. to dissolve PVDF polymer, producing three different morphologies of the resultant membranes with varying pore sizes and thickness values (Fig. 10b).165 The addition of polyvinylpyrrolidone resulted in a finger-like structure, while the membrane prepared with N-methylacetamide–acetamide and 2 wt% polyvinylpyrrolidone demonstrated superior performance, with a high water permeate flux (96.82 L m−2 h−1) and 96.32% bovine serum albumin separation. In the pursuit of sustainability, another study evaluated the performance of NADESs as solvents for polyvinylidene fluoride (PVDF) and poly(acrylonitrile) membranes, aiming to differentiate the structures of the polymer membranes for water-purification applications. The generated membranes exhibited diverse morphologies, pore sizes ranging from 0.03 ± 0.01 μm to 1.08 ± 0.01 μm, and an average porosity of 84 ± 1%, demonstrating potential in water-purification processes.166
 | ||
Fig. 10 Illustration of the generation of porous membranes and their SEM images. (a) Polyamide-imide membranes before and after incorporation of ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetamide.164 Copyright 2021, MDPI. (b) With non-ionic DES (N-methyl acetamide:N-methyl urea).165 Copyright 2022, Elsevier. (c) Schematic illustration of the plasticization effect of edible films generated by Averrhoa bilimbi pectins with a DES.175 Copyright 2020, Elsevier. acetamide.164 Copyright 2021, MDPI. (b) With non-ionic DES (N-methyl acetamide:N-methyl urea).165 Copyright 2022, Elsevier. (c) Schematic illustration of the plasticization effect of edible films generated by Averrhoa bilimbi pectins with a DES.175 Copyright 2020, Elsevier. | ||
The exploration of DESs for plasticizer applications began a decade ago, with the initial uses highlighting their potential as a novel plasticizer for cellulose acetate-based applications in 2013. The plasticizing ability of DESs, particularly ChCl-based DESs with urea, was attributed to their high electronegativity and charge delocalization, which could enhance the ionic conductivity of cellulose esters.167 Subsequent research has probed the plasticizing abilities of various DESs, using ChCl as the HBA and urea, citric acid, malonic acid, and glycerol as the primary HBDs.168
Biopolymer-based films, including chitin,101 chitosan,169–171 cellulose,172,173 lignin,174 pectin,175 agar, agarose,168 starch,176 and gelatin,177 have been extensively explored using DESs for film formation, serving as solvents for size reduction and homogenization. The tensile properties of chitosan-based films were found to be dependent on the degree of deacetylation (DDA), with higher DDA (around 95%) levels resulting in increased mechanical strength.101 Apart from the studies related to structural enhancement on the surface physicomechanical properties and antibacterial activities in chitosan films, the antioxidant activity of such films was reported by Jakubowska et al. with the inclusion of quercetin, which is a naturally obtainable plant polyphenol, for a storage application for rapeseed oil.171 Yu et al. studied the effect of chitosan-based films for food packaging with different combinations of DESs with ChCl and organic acids.170 Here, the team probed the antibacterial, antioxidant, as well as UV-barrier properties of the prepared films and found out that the one prepared with ChCl and acetylsalicylic acid had the best performance (DPPH scavenging activity of approximately 60%, inhibition of E. coli and S. aureus). Additionally, the team suggested that the enhancement of the antibacterial properties was due to the synergistic effects of the chitosan and chosen DESs.
Studies on plant-based derivatives, such as cellulose, lignin, and pectin, have been performed with an aim to achieve property enhancements in areas such as antioxidant and UV shielding.178 Composite films from cellulose and modified lignin revealed that the antioxidant and UV-shielding performances depended heavily on the lignin pretreatment methods. Cellulose-lignin-based films with enhanced UV protection properties were generated using ChCl:oxalic acid and ChCl:glycerol-based DES treatments.174 Xia et al. generated recyclable lignocellulosic bioplastic film with ∼128 MPa tensile strength and toughness of 2.8 MJ m−3 with a DES-treated (ChCl:oxalic acid) wood powder.179 Pectin-based edible films, utilizing different DES ratios of ChCl, and citric acid monohydrate, exhibited diverse structural transitions and film properties, with the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DES treatment showing superior tensile strength (7.32 ± 0.50 MPa), melting temperature, and barrier properties (relative humidity and water vapor transmission rate).175 The authors attributed this property to the entanglement of branches with a lower plasticizing effect (Fig. 10c).
1 DES treatment showing superior tensile strength (7.32 ± 0.50 MPa), melting temperature, and barrier properties (relative humidity and water vapor transmission rate).175 The authors attributed this property to the entanglement of branches with a lower plasticizing effect (Fig. 10c).
Hydrophobic DES systems, such as thymol:octanoic acid, were studied for their water barrier properties on chitosan/gelatin films.180 While the water contact angle (above 90%) and water vapor transmission rate were improved at higher DES loadings (6–15%), the tensile strength decreased. Nonetheless, the elongation at break increase (for DES systems with an above 6% loadings) was attributed to an increase in the free volume of the polymer matrix, making 9% DES-incorporated chitosan films recommended for food-packaging applications. NADESs have been explored for achieving self-healing properties in polymer-based films.169 An optimal loading of 67 wt% NADES (ChCl:citric acid) in chitosan films exhibited a 56% recovery of the elongation at break and 72% of the original strength after breaking. Additionally, NADESs, particularly ChCl:vanillyl alcohol:gelatin, demonstrated superior adhesive strength (approximately 135 kPa) with shear-thinning behavior for thin-film applications.181
Recent studies on sodium acetate trihydrate/urea-based DESs revealed enhanced thermal stability and crosslinking in gelatin/polyvinyl alcohol composite films.177 Higher DES loadings (60%) prevented undesired gelation of the gelatin at low temperatures, increased the elongation at break to 631%, and accelerated crosslinking without the need for additional catalysts.
All the aforementioned applications provide evidence for the application of DESs as solvents for a vast majority of polysaccharides and their use for the surface modification of films as mainly plasticizers and for achieving other modifications, such as endowing systems with hydrophobicity, UV-shielding, antioxidant, antibacterial, and barrier properties as well as a self-healing property. Additionally, the use of DES extractants for property enhancement represents a novel and sustainable pathway, allowing for the recycling and reuse of the extracted DESs in functionalized polymer film formation. Various studies have explored the extraction of bioactive compounds, including polyphenols and anthocyanins, using DESs for modifying polymer films. Notably, DES-extracted anthocyanins have exhibited superior antibacterial and antioxidant properties for edible films,182 while carotenoids extracted by DESs have shown enhanced plasticity and been used as a colorant antioxidant in corn starch-based films.173 Additionally, specialized applications, such as generating chitosan-based bioactive polymer films with improved mechanical and anti-plasticity properties, were achieved through SUPRADES (β-cyclodextrin as the HBA and maleic acid, lactic acid, and citric acid as HBDs) extraction of polyphenols from bayberry.183 Here, the effect of ultrasound on the extraction of polyphenols was assessed and it was found to improve the mechanical properties of the chitosan-based film, including its anti-plasticity ability as well as adhesion property, which could enable its use in animal-tissue-based applications.
All the above studies have demonstrated that DESs can be used as green solvents to develop various types of functional materials with different properties. DESs can also be integrated with advanced manufacturing methods, such as electrospinning and 3D printing. We believe that these studies can be further extended towards cutting-edge applications based on specialty and sustainable materials. The careful engineering of DESs by balancing the hydrophilic/hydrophobic, polar/nonpolar ratios can also play a vital role in the generation of advanced polymeric nanomaterials. Additionally, the stabilization achieved in organolithium species in anionic polymerizations32 and the metal coordination ability in DESs162 suggest the potential for them to be used as coordination polymer materials.
6. Green credentials and sustainability evaluation of DES-based materials
Sustainability has been increasingly discussed and emphasized, be it at the government level or at the scientific community level, as an important issue to be prioritized, likely due to the climatic change experienced in recent years.189–192 The concept of sustainability identified by the United Nations in the 2030 Agenda for Sustainable Development comprises several aspects. A few goals set by the United Nations are to “ensure availability and sustainable management of water and sanitation for all”, “ensure sustainable consumption and production patterns”, “ensure healthy lives and promote well-being for all at all ages”, and to “protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss”.184 In short, actions that can threaten human survivability and destroy nature in the long run are not sustainable.It is not uncommon for DESs to be considered as green alternatives to ionic liquids or other conventional solvents used for extraction and synthesis. In fact, this exact belief has fueled an increase in research on DESs185 (Fig. 1b and c). The basis of this confidence mainly comes from the idea that DES components are derived from natural sources. However, it is questionable whether being derived from natural sources is sufficient to view DESs as green alternatives without further assessment and proof.
A way to assess their green credentials is by using the concept of life-cycle assessment (LCA). LCA is a well-known tool that has largely been used to evaluate the environmental impact of a technology or a product from cradle to grave. This tool has the advantage of capturing all the impacts without having the burden shift from one environmental impact to another. It comprises four stages, starting from setting the scope and the goals, followed by identifying the inputs and outputs as well as the possible impacts. Lastly, interpretation can be made with all the information gathered in the earlier steps (Fig. 11).186 As such, the accuracy and effectiveness of LCA depend primarily on the completeness of the scope and knowledge for each step.
Under LCA guidelines, the inputs and outputs are one of the main components to evaluate the green credentials of DES-based materials. First, the acid used to prepare DESs is less harsh than those needed for acid hydrolysis. Choline chloride is a popular HBA, perhaps due to it being an additive for domestic animal feed as well as due to its biodegradable and regenerable nature, making it a sustainable choice. Hence, it is expected that the combination of both reagents would be sustainable and green compared to conventional solvents. Unfortunately, it is not as green as it appears to be upon a deeper understanding of its synthesis process. The production of 539 kg ChCl requires 157 kg of 30% (w/w) hydrochloric acid (HCl), 99 kg of 30% (w/w) trimethylamine (TMA), 206 kg of DI water, and 962 kg of 8% (w/w) ethylene oxide/steam mixture.187 Despite so, its use is still preferred over the use of strong acids, such as HCl. Using the example of nanochitin preparation, 3 M HCl aqueous solution (150 ml) was used for 5 g α-chitin powder.195 This is equivalent to 3.29 g HCl required for 1 g chitin. In contrary, 50 g DESs (molar ratio of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) organic acid is 1
organic acid is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was used for 2 g chitin powder to prepare chitin nanocrystals.78 In the scenario that lactic acid is used as the hydrogen-bond donor, 1.91 g HCl is needed for ChCl synthesis to use with 1 g chitin powder. If malic acid is used instead of lactic acid, the amount of HCl required for 1 g chitin powder is 1.50 g. This brief comparison shows that the use of DES is indeed a greener approach.
2) was used for 2 g chitin powder to prepare chitin nanocrystals.78 In the scenario that lactic acid is used as the hydrogen-bond donor, 1.91 g HCl is needed for ChCl synthesis to use with 1 g chitin powder. If malic acid is used instead of lactic acid, the amount of HCl required for 1 g chitin powder is 1.50 g. This brief comparison shows that the use of DES is indeed a greener approach.
Kyung et al. compared the potential environmental profiles of reline, a DES that is made up of ChCl and urea, to other common organic solvents, such as ethanol, methanol, ethyl acetate, and dichloromethane, for the process of synthesizing 0.2 kg acetophenone.187 For the seven examined areas (global warming potential, freshwater eutrophication potential, metal depletion potential, freshwater ecotoxicity potential, human toxicity potential, terrestrial acidification potential, and water depletion potential), reline performed comparably well with a relative impact below 50% for all the fields, except for metal depletion potential, which displayed a slightly poorer performance (∼60%). Among the tested solvents, ethyl acetate and dichloromethane fared the worst, as each topped the group in different areas.
In recent years, many studies have investigated recycling DES to enhance the sustainability of DES-based materials.188,193,194 It was discovered that the reactivity of a DES (i.e., choline chloride and oxalic acid) to deconstruct lignocellulosic material only dropped by ∼3.2% after 5 rounds of recycling.179 It was further estimated that the DES could be recycled up to nine times.179 These findings suggest that DESs are an even more attractive sustainable alternative compared to conventional solvents. While the idea is promising, the inputs and the outputs for DES recycling must be cautiously weighed to determine if it is indeed more environmentally friendly than the act of using fresh DES for the reaction. For instance, resources, such as electricity and solvents, would be necessary to recycle the DES. Would it be more sustainable to channel these invaluable resources to recycle the DES or to extract and mix fresh DES? Additionally, one needs to determine the emissions and to see from which reaction they are lower? In summary, DES-based materials seem to be relatively green but more study is needed.
7. Conclusion and outlook
This review provided an overall summary and an update on the current trends in the utilization of DESs in green polymer synthesis and the construction of functional polymer materials, with eco-friendly nontoxic constituents. Regarding the synthesis and preparation of polymer materials, DESs can function as solvents, monomers, and can be used for biomass pretreatment, whereby variable functional polymer materials can be realized in the form of gels, nanofibers, films, and membranes. The various characteristics of DESs, such as the utilization of renewable feedstocks (e.g. NADESs), vast tunability, recyclability, and non-toxicity, align well with the twelve principles of green chemistry. Their all-in-one system approaches and mild reaction conditions ensure 100% atom efficiency with a prevention of waste, demonstrating the capability of DESs for use in large-scale manufacturing in an environmentally sustainable manner. The unique hydrogen bonding existing within DESs coupled with their other properties, such as catalyst, plasticizer, pore generator, shear-thinning behavior, dispersant, and tunable hydrophilicity/hydrophobicity, provides numerous possibilities to probe more applications for polymeric materials in the future.However, given that different extents of hydrogen bonding can significantly affect the generation of functional polymeric materials by DESs, the mechanism of the influence of hydrogen bonding is still unclear. More extensive computational simulations could be utilized in this aspect to visualize the molecular interactions, in this case, the hydrogen bonding. This approach would help to understand the bonding nature of the DES systems more distinctively while reducing unnecessary lab trials. Another aspect of interest involves how to investigate DESs’ influences on the initiators employed in polymerization reactions. Moreover, it is essential to clearly differentiate between the presence of water molecules as an impurity, influenced by the hygroscopic properties of specific chemicals like ChCl, and the deliberate introduction of water into the system. The impact of water in DESs has been emphasized in various cases as influencing the efficiency of polymer synthesis, depolymerization, or functionalization processes, as well as cost reduction. Further studies on the reaction rates can provide deeper insights into identifying the influences of water within DES systems.
There is still a great potential to exploit this comparatively new solvent system to use as a top-down approach for the valorization of unpopular biomasses (e.g., marine biomass, industrial waste, and food waste). Despite the passage of two decades, ChCl continues to be emphasized as the HBA in most applications. It is now opportune to investigate alternative HBAs that offer comparable performance while overcoming the hygroscopicity of ChCl. Additionally, research based on renewable- or non-petroleum-derived HBDs and HBAs (e.g., itaconic acid, caffeic acid, menthol, thymol) with room temperature DES pretreatment could be more insightful.98,196 The application of DESs in crafting polymeric items, like 3D printing, can facilitate the creation of customized structures for functional polymers, resulting in reduced waste generation. However, further inroads in this technology could be probed with the advancements in 4D printing and smart materials, especially given the stimuli-responsive materials generated via polymeric DESs owing to their responsiveness to external stimuli, such as temperature, pH, humidity, and electricity.
Currently, there is extensive discussion on sensor-related applications of DES-based polymeric material technologies and the conductivity levels generated in DES media. Further exploration of these conductive DES-based polymer sensors could be expanded to the robotic field, incorporating the use of magnetic DESs, which have been identified recently as a new sub-class.197 We have summarized the multiple roles of DESs in different polymer materials. The integration of these multi-functions of DESs in one polymeric material is the most challenging part and worth more effort to investigate. Moreover, research on the longevity and LCA of these materials seems to be still in the initial phases. Specifically, we evaluated the green credentials and sustainability of DES-based polymer materials. The economic evaluation of the recyclability of DESs and further utilization of the extractives generated during biomass pretreatment could be another focus area in the future.
This comprehensive review offers a detailed examination of the present patterns in the utilization of DESs for the synthesis of functional polymeric materials, showcasing their versatility across a wide range of applications and highlighting possible future pathways. These applications extend from the efficient use of biomass resources to the development of advanced sensor-based technologies. By exploring the diverse functionalities of DESs, the review seeks to inspire innovative thinking and strategic planning for the integration of these solvents in emerging areas of research and technology.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This Research is supported by the RIE2025 MTC Individual Research Grants (M22K2c0085), administered by the Agency of Science, Technology and Research (A*STAR), Singapore. This research is also supported by National Research Foundation (NRF) Singapore under its NRF Investigatorship (NRF-NRFI07-2021-0003). This work was also supported by the National Medical Research Council (NMRC), Singapore, under its Clinician Scientist-Individual Research Grant (MOH-001357-00). The first author would like to acknowledge the Agency for Science, Technology and Research (A*STAR) for providing the sponsorship for her Ph.D. programme.References
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 Search PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 Search PubMed.
- D. O. Abranches and J. A. P. Coutinho, Curr. Opin. Green Sustainable Chem., 2022, 35, 100612 Search PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 Search PubMed.
- J. Płotka-Wasylka, M. De La Guardia, V. Andruch and M. Vilková, Microchem. J., 2020, 159, 105539 Search PubMed.
- C. W. Cho, T. P. T. Pham, Y. Zhao, S. Stolte and Y. S. Yun, Sci. Total Environ., 2021, 786, 147309 Search PubMed.
- D. Yu, Z. Xue and T. Mu, Chem. Soc. Rev., 2021, 50, 8596–8638 Search PubMed.
- C. R. Ashworth, R. P. Matthews, T. Welton and P. A. Hunt, Phys. Chem. Chem. Phys., 2016, 18, 18145–18160 Search PubMed.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2019, 55, 10253–10256 Search PubMed.
- R. E. Dazat, E. Vidal, A. S. Lorenzetti, C. D. García, C. Domini, M. F. Silva and F. J. V. Gomez, ChemistrySelect, 2022, 7, e202104362 Search PubMed.
- J. M. Silva, R. L. Reis, A. Paiva and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2018, 6, 10355–10363 Search PubMed.
- P. Pradeepkumar, A. Subbiah and M. Rajan, SN Appl. Sci., 2019, 1, 1–13 Search PubMed.
- J. Zhang, L. Yao, S. Li, S. Li, Y. Wu, Z. Li and H. Qiu, Green Chem., 2023, 25, 4180–4195 Search PubMed.
- J. A. Kist, H. Zhao, K. R. Mitchell-Koch and G. A. Baker, J. Mater. Chem. B, 2021, 9, 536–566 Search PubMed.
- K. A. Omar and R. Sadeghi, J. Mol. Liq., 2023, 384, 121899 Search PubMed.
- C. D'Agostino, RSC Adv., 2017, 7, 51864–51869 Search PubMed.
- M. Jablonský, A. Škulcová and J. Šima, Molecules, 2019, 24, 1–33 Search PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 Search PubMed.
- A. P. Abbott, ChemPhysChem, 2004, 5, 1242–1246 Search PubMed.
- M. H. Zainal-Abidin, M. Hayyan and W. F. Wong, J. Ind. Eng. Chem., 2021, 97, 142–162 Search PubMed.
- S. P. Ijardar, V. Singh and R. L. Gardas, Molecules, 2022, 27, 1368 Search PubMed.
- K. A. Omar and R. Sadeghi, J. Mol. Liq., 2022, 360, 119524 Search PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285 Search PubMed.
- N. Ndizeye, S. Suriyanarayanan and I. A. Nicholls, Polym. Chem., 2019, 10, 5289–5295 Search PubMed.
- A. Altundağ, A. E. Ünlü and S. Takaç, J. Chem. Technol. Biotechnol., 2021, 96, 1107–1115 Search PubMed.
- I. Vasil'eva, O. Morozova, G. Shumakovich and A. Yaropolov, Int. J. Mol. Sci., 2022, 23, 11409 Search PubMed.
- M. Khlupova, I. Vasil, G. Shumakovich, E. Zaitseva, V. Chertkov, A. Shestakova, O. Morozova and A. Yaropolov, Catalysts, 2021, 11, 1–15 Search PubMed.
- A. R. S. S. Kumar and N. K. Singha, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 2281–2286 Search PubMed.
- C. Y. Li and S. S. Yu, Macromolecules, 2021, 54, 9825–9836 Search PubMed.
- P. V. Mendonça, M. S. Lima, T. Guliashvili, A. C. Serra and J. F. J. Coelho, Polymer, 2017, 132, 114–121 Search PubMed.
- L. Quirós-Montes, G. A. Carriedo, J. García-Álvarez and A. Presa Soto, Green Chem., 2019, 21, 5865–5875 Search PubMed.
- A. Sanchez-Condado, G. A. Carriedo, A. P. Soto, M. J. Rodríguez-Alvarez, J. García-Alvarez and E. Hevia, ChemSusChem, 2019, 12, 3134–3143 Search PubMed.
- J. Li, M. Zhang, J. He and P. Ni, Eur. Polym. J., 2023, 191, 112044 Search PubMed.
- A. Rubin Pedrazzo, C. Cecone, F. Trotta and M. Zanetti, ACS Sustainable Chem. Eng., 2021, 9, 14881–14889 Search PubMed.
- L. Lin, R. Li, G. Chen, X. Wang, J. Cheng, J. Zhao, K. Zhao and M. He, Polym. Chem., 2024, 15, 783–795 Search PubMed.
- Y. Nahar and S. C. Thickett, Polymers, 2021, 13, 1–24 Search PubMed.
- K. F. Fazende, D. P. Gary, J. D. Mota-Morales and J. A. Pojman, Macromol. Chem. Phys., 2020, 221, 1900511 Search PubMed.
- Y. Nahar, J. Horne, V. Truong, A. C. Bissember and S. C. Thickett, Polym. Chem., 2021, 12, 254–264 Search PubMed.
- D. Tolmachev, V. Nazarychev, V. Fedotova, V. Vorobiov, N. Lukasheva, M. Smirnov and M. Karttunen, J. Mol. Liq., 2023, 370, 121030 Search PubMed.
- M. Wang, Z. Lai, J. Xiaolin, T. Sun, H. Liu and H. Qi, Adv. Funct. Mater., 2021, 31, 2101957 Search PubMed.
- J. Wang, J. Xue, X. Dong, Q. Yu, S. N. Baker, M. Wang and H. Huang, Int. J. Pharm., 2020, 575, 119005 Search PubMed.
- A. Roda, A. A. Matias, A. Paiva and A. R. C. Duarte, Polymers, 2019, 11, 1–22 Search PubMed.
- S. Bednarz, K. Półćwiartek, J. Wityk, B. Strachota, J. Kredatusová, H. Beneš, A. Wesołowska-Piętak and G. Kowalski, Eur. Polym. J., 2017, 95, 241–254 Search PubMed.
- S. Bednarz, M. Fluder, M. Galica, D. Bogdal and I. Maciejaszek, J. Appl. Polym. Sci., 2014, 131, 40608 Search PubMed.
- X. Li and K. H. Row, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1068, 56–63 Search PubMed.
- K. Ajino, A. Torii, H. Ogawa and H. Mori, Polymer, 2020, 204, 122803 Search PubMed.
- Z. Ling, J. V. Edwards, Z. Guo, N. T. Prevost, S. Nam, Q. Wu, A. D. French and F. Xu, Cellulose, 2019, 26, 861–876 Search PubMed.
- X. Yang, H. Xie, H. Du, X. Zhang, Z. Zou, Y. Zou, W. Liu, H. Lan, X. Zhang and C. Si, ACS Sustainable Chem. Eng., 2019, 7, 7200–7208 Search PubMed.
- Q. Fan, C. Jiang, W. Wang, L. Bai, H. Chen, H. Yang, D. Wei and L. Yang, Cellulose, 2020, 27, 2541–2553 Search PubMed.
- M. A. Smirnov, M. P. Sokolova, D. A. Tolmachev, V. K. Vorobiov, I. A. Kasatkin, N. N. Smirnov, A. V. Klaving, N. V. Bobrova, N. V. Lukasheva and A. V. Yakimansky, Cellulose, 2020, 27, 4305–4317 Search PubMed.
- W. L. Lim, A. A. N. Gunny, F. H. Kasim, S. C. B. Gopinath, N. H. I. Kamaludin and D. Arbain, Cellulose, 2021, 28, 6183–6199 Search PubMed.
- Y. Ma, Q. Xia, Y. Liu, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, ACS Omega, 2019, 4, 8539–8547 Search PubMed.
- M. Wu, K. Liao, C. Liu, G. Yu, M. Rahmaninia, H. Li and B. Li, Cellulose, 2021, 28, 9689–9703 Search PubMed.
- H. Zhang, Y. Wu, J. Zhang, Z. Wu and X. Zhan, Ind. Crops Prod., 2022, 189, 115781 Search PubMed.
- J. A. Sirviö, J. Ukkola and H. Liimatainen, Cellulose, 2019, 26, 2303–2316 Search PubMed.
- S. Liu, Q. Zhang, S. Gou, L. Zhang and Z. Wang, Carbohydr. Polym., 2021, 251, 117018 Search PubMed.
- W. Liu, H. Du, K. Liu, H. Liu, H. Xie, C. Si, B. Pang and X. Zhang, Carbohydr. Polym., 2021, 267, 118220 Search PubMed.
- M. Yan, C. Tian, T. Wu, X. Huang, Y. Zhong, P. Yang, L. Zhang, J. Ma, H. Lu and X. Zhou, Int. J. Biol. Macromol., 2021, 191, 422–431 Search PubMed.
- W. Yu, C. Wang, Y. Yi, H. Wang, Y. Yang, L. Zeng and Z. Tan, Cellulose, 2021, 28, 175–188 Search PubMed.
- W. Li, Y. Xue, M. He, J. Yan, L. A. Lucia, J. Chen, J. Yu and G. Yang, Nanomaterials, 2021, 11, 2778 Search PubMed.
- J. Jiang, N. C. Carrillo-Enríquez, H. Oguzlu, X. Han, R. Bi, M. Song, J. N. Saddler, R. C. Sun and F. Jiang, ACS Sustainable Chem. Eng., 2020, 8, 7182–7191 Search PubMed.
- C. Liu, M. C. Li, W. Chen, R. Huang, S. Hong, Q. Wu and C. Mei, Carbohydr. Polym., 2020, 246, 116548 Search PubMed.
- X. Li, C. Ning, L. Li, W. Liu, Q. Ren and Q. Hou, Carbohydr. Polym., 2021, 274, 118650 Search PubMed.
- Q. Zhang, R. Ma, L. Ma, L. Zhang, Y. Fan and Z. Wang, Ind. Crops Prod., 2021, 166, 113460 Search PubMed.
- G. J. Kwon, S. W. Cho, R. Bandi, B. S. Yang, R. Dadigala, S. Y. Han, S. Y. Ma, J. K. Kim, N. H. Kim and S. H. Lee, Cellulose, 2023, 30, 4277–4292 Search PubMed.
- C. Y. Ma, X. P. Peng, S. Sun, J. L. Wen and T. Q. Yuan, Int. J. Biol. Macromol., 2021, 192, 417–425 Search PubMed.
- T. Luo, C. Wang, X. Ji, G. Yang, J. Chen, C. G. Yoo, S. Janaswamy and G. Lyu, Int. J. Biol. Macromol., 2021, 183, 781–789 Search PubMed.
- Z. Yan, Z. Wang, Y. Chen, C. Liu, Y. Liu, R. Li, M. Si and Y. Shi, Biotechnol. Bioeng., 2023, 120, 1557–1568 Search PubMed.
- W. Zhang, J. Shen, P. Gao, Q. Jiang and W. Xia, Ind. Crops Prod., 2022, 188, 115651 Search PubMed.
- T. Luo, Y. Hao, C. Wang, W. Jiang, X. Ji, G. Yang, J. Chen, S. Janaswamy and G. Lyu, Nanomaterials, 2022, 12, 176 Search PubMed.
- H. Zhang, Y. Shi, M. Li, J. Chen, Y. Xin, L. Zhang, Z. Gu, J. Liu and R. Liu, Chem. Eng. Sci., 2022, 256, 117694 Search PubMed.
- W. H. Gong, C. Zhang, J. W. He, Y. Y. Gao, Y. J. Li, M. Q. Zhu and J. L. Wen, Int. J. Biol. Macromol., 2022, 209, 188–197 Search PubMed.
- D. Tian, F. Shen, J. Hu, M. Huang, L. Zhao, J. He, Q. Li, S. Zhang and F. Shen, Chem. Eng. J., 2022, 428, 131373 Search PubMed.
- X. Yue, T. Suopajärvi, S. Sun, O. Mankinen, A. Mikkelson, H. Huttunen, S. Komulainen, I. Romakkaniemi, J. Ahola, V. V. Telkki and H. Liimatainen, Bioresour. Technol., 2022, 360, 127570 Search PubMed.
- Y. Xu, S. C. Sun, C. Zhang, C. Y. Ma, J. L. Wen and T. Q. Yuan, Chem. Eng. J., 2023, 462, 142213 Search PubMed.
- F. Shen, C. He, Y. Wang, J. Hu, M. Huang, L. Zhao, S. Zhang, D. Tian and F. Shen, Chem. Eng. J., 2023, 467, 143376 Search PubMed.
- S. L. Cao, W. M. Gu, W. D. Ou-Yang, D. C. Chen, B. Y. Yang, L. H. Lai, Y. Da Wu, Y. J. Liu, J. Zhu, W. J. Chen, Z. Q. Gai, X. D. Hou, Y. Z. Ma and Y. X. An, Carbohydr. Polym., 2019, 213, 304–310 Search PubMed.
- Y. Yuan, S. Hong, H. Lian, K. Zhang and H. Liimatainen, Carbohydr. Polym., 2020, 236, 116095 Search PubMed.
- S. Hong, Y. Yuan, K. Zhang, H. Lian and H. Liimatainen, Nanomaterials, 2020, 10, 869 Search PubMed.
- S. Hong, Y. Yuan, Q. Yang, L. Chen, J. Deng, W. Chen, H. Lian, J. D. Mota-Morales and H. Liimatainen, Carbohydr. Polym., 2019, 220, 211–218 Search PubMed.
- Q. Xiao, M. Dai, H. Zhou, M. Huang, L. T. Lim and C. Zeng, Carbohydr. Polym., 2022, 282, 119105 Search PubMed.
- M. Przypis, A. Wawoczny and D. Gillner, Appl. Sci., 2023, 13, 1055 Search PubMed.
- W. Wang and D. J. Lee, Bioresour. Technol., 2021, 339, 125587 Search PubMed.
- A. R. Mankar, A. Pandey, A. Modak and K. K. Pant, Bioresour. Technol., 2021, 334, 125235 Search PubMed.
- J. Tong, W. Hu, Y. Qin and Y. Liu, Cellulose, 2023, 30, 4773–4792 Search PubMed.
- S. Zargar, J. Jiang, F. Jiang and Q. Tu, Biofuels, Bioprod. Biorefin., 2022, 16, 68–80 Search PubMed.
- S. Banu Jamaldheen, M. B. Kurade, B. Basak, C. G. Yoo, K. K. Oh, B. H. Jeon and T. H. Kim, Bioresour. Technol., 2022, 346, 126591 Search PubMed.
- S. Hong, X. J. Shen, Z. Xue, Z. Sun and T. Q. Yuan, Green Chem., 2020, 22, 7219–7232 Search PubMed.
- Y. Wang, H. Liu, X. Ji, Q. Wang, Z. Tian and P. Fatehi, Int. J. Biol. Macromol., 2023, 245, 125227 Search PubMed.
- T. Luo, C. Wang, X. Ji, G. Yang, J. Chen, S. Janaswamy and G. Lyu, Molecules, 2021, 26, 218 Search PubMed.
- C. D'Agostino, R. C. Harris, A. P. Abbott, L. F. Gladden and M. D. Mantle, Phys. Chem. Chem. Phys., 2011, 13, 21383–21391 Search PubMed.
- M. Zhou, O. A. Fakayode, A. E. G. Ahmed Yagoub, Q. Ji and C. Zhou, Renewable Sustainable Energy Rev., 2022, 156, 111986 Search PubMed.
- V. Sharma, M. L. Tsai, C. W. Chen, P. P. Sun, A. K. Patel, R. R. Singhania, P. Nargotra and C. Di Dong, Bioresour. Technol., 2022, 360, 127631 Search PubMed.
- S. Hong, X. J. Shen, B. Pang, Z. Xue, X. F. Cao, J. L. Wen, Z. H. Sun, S. S. Lam, T. Q. Yuan and R. C. Sun, Green Chem., 2020, 22, 1851–1858 Search PubMed.
- Z. Chen, A. Ragauskas and C. Wan, Ind. Crops Prod., 2020, 147, 112241 Search PubMed.
- E. S. Morais, A. M. Da Costa Lopes, M. G. Freire, C. S. R. Freire and A. J. D. Silvestre, ChemSusChem, 2021, 14, 686–698 Search PubMed.
- P. Willberg-Keyriläinen, J. Hiltunen and J. Ropponen, Cellulose, 2018, 25, 195–204 Search PubMed.
- Z. Tong, S. Zeng, H. Tang, W. Wang, Y. Sun, Q. Xia and H. Yu, Green Chem., 2023, 25, 5086–5096 Search PubMed.
- K. Morgan, C. Conway, S. Faherty and C. Quigley, Molecules, 2021, 26, 7603 Search PubMed.
- N. Özel and M. Elibol, Carbohydr. Polym., 2021, 262, 117942 Search PubMed.
- M. Khajavian, V. Vatanpour, R. Castro-Muñoz and G. Boczkaj, Carbohydr. Polym., 2022, 275, 118702 Search PubMed.
- K. Mohan, A. R. Ganesan, P. N. Ezhilarasi, K. K. Kondamareddy, D. K. Rajan, P. Sathishkumar, J. Rajarajeswaran and L. Conterno, Carbohydr. Polym., 2022, 287, 119349 Search PubMed.
- C. McReynolds, A. Adrien, N. Castejon and S. C. M. Fernandes, Green Chem. Lett. Rev., 2022, 15, 382–403 Search PubMed.
- Z. Li, C. Liu, S. Hong, H. Lian, C. Mei, J. Lee, Q. Wu, M. A. Hubbe and M. C. Li, Chem. Eng. J., 2022, 446, 136953 Search PubMed.
- E. Bertoft, Agronomy, 2017, 7, 56 Search PubMed.
- X. Deng, M. Zhang, K. Liu, M. Pu and X. Han, Starch/Stärke, 2022, 74, 2100278 Search PubMed.
- M. Zdanowicz, Int. J. Biol. Macromol., 2021, 176, 387–393 Search PubMed.
- D. Skowrońska and K. Wilpiszewska, Polymers, 2022, 14, 220 Search PubMed.
- M. Zdanowicz, K. Wilpiszewska and T. Spychaj, Carbohydr. Polym., 2018, 200, 361–380 Search PubMed.
- M. Zdanowicz, Carbohydr. Polym., 2020, 229, 115574 Search PubMed.
- M. Zdanowicz, P. Staciwa, R. Jedrzejewski and T. Spychaj, Polymers, 2019, 11, 1385 Search PubMed.
- D. Yu, Z. Xue and T. Mu, Cell Rep. Phys. Sci., 2022, 3, 1–23 Search PubMed.
- J. H. Lim, M. J. Kim, H. G. Yoon and S. W. Kim, Composites, Part B, 2022, 247, 110299 Search PubMed.
- Y. Liu, X. Zhang, B. Li, H. Chen, H. Li, J. Chen and H. Dong, Chem. Eng. J., 2023, 461, 141965 Search PubMed.
- X. Wang, G. Chen, L. Cai, R. Li and M. He, ACS Appl. Mater. Interfaces, 2021, 13, 8952–8959 Search PubMed.
- R. Wang, Y. Ma, P. Chen, L. Sun, Y. Liu and C. Gao, Colloids Surf., A, 2023, 656, 130349 Search PubMed.
- G. Sennakesavan, M. Mostakhdemin, L. K. Dkhar, A. Seyfoddin and S. J. Fatihhi, Polym. Degrad. Stab., 2020, 180, 109308 Search PubMed.
- M. L. Picchio, A. Gallastegui, N. Casado, N. Lopez-Larrea, B. Marchiori, I. del Agua, M. Criado-Gonzalez, D. Mantione, R. J. Minari and D. Mecerreyes, Adv. Mater. Technol., 2022, 7, 2101680 Search PubMed.
- Y. Gao, L. Wu, J. Zhou, X. Ma, Y. Fang, X. Fang and Q. Dou, J. Appl. Polym. Sci., 2023, 140, 53285 Search PubMed.
- L. Wu, J. Zhou, X. Bu, Y. Ge, Y. Gao and X. Ma, J. Electron. Mater., 2022, 51, 5074–5086 Search PubMed.
- C. Chai, L. Ma, Y. Chu, W. Li, Y. Qian and J. Hao, J. Colloid Interface Sci., 2023, 638, 439–448 Search PubMed.
- H. Sun, B. Zhang, L. Lu, Z. Chen, Y. Huo, W. Li, B. Zhang and J. Song, Chem. Eng. J., 2023, 451, 139051 Search PubMed.
- K. Prasad, D. Mondal, M. Sharma, M. G. Freire, C. Mukesh and J. Bhatt, Carbohydr. Polym., 2018, 180, 328–336 Search PubMed.
- K. Fan, W. Wei, Z. Zhang, B. Liu, W. Feng, Y. Ma and X. Zhang, Chem. Eng. J., 2022, 449, 137878 Search PubMed.
- H. Qin, R. E. Owyeung, S. R. Sonkusale and M. J. Panzer, J. Mater. Chem. C, 2019, 7, 601–608 Search PubMed.
- R. E. Owyeung, S. R. Sonkusale and M. J. Panzer, J. Phys. Chem. B, 2020, 124, 5986–5992 Search PubMed.
- Y. Wang, S. Fu, L. A. Lucia and H. Zhang, Compos. Sci. Technol., 2022, 229, 109696 Search PubMed.
- P. Mercadal, M. Romero, M. del Mar Montesinos, J. P. Real, M. Picchio and A. Gonzalez, ACS Appl. Electron. Mater., 2023, 5, 2184–2196 Search PubMed.
- K. Yang, Z. Ge, M. Zhang, C. Wang, K. Peng, H. Yang and Y. You, Chem. Eng. J., 2022, 439, 135646 Search PubMed.
- L. Chen, J. Deng, S. Hong and H. Lian, J. Sol-Gel Sci. Technol., 2018, 86, 795–806 Search PubMed.
- J. Deng, L. Chen, S. Hong and H. Lian, J. Porous Mater., 2020, 27, 789–800 Search PubMed.
- L. Chen, J. Deng, Y. Yuan, S. Hong, B. Yan, S. He and H. Lian, Diamond Relat. Mater., 2022, 121, 108781 Search PubMed.
- S. Bednarz, A. Wesołowska, M. Trątnowiecka and D. Bogdał, J. Renewable Mater., 2016, 4, 18–23 Search PubMed.
- R. J. Sánchez-Leija, N. López-Salas, J. L. G. Fierro, M. C. Gutiérrez, M. L. Ferrer, J. D. Mota-Morales, G. Luna-Bárcenas and F. del Monte, Carbon, 2019, 146, 813–826 Search PubMed.
- S. Long, Y. Feng, Y. Liu, L. Zheng, L. Gan, J. Liu, X. Zeng and M. Long, Sep. Purif. Technol., 2021, 254, 117577 Search PubMed.
- D. Peng, J. Zhao, X. Liang, X. Guo and H. Li, J. Hazard. Mater., 2023, 448, 130954 Search PubMed.
- Y. Zhu, B. K. Qi, H. N. Lv, Y. Gao, S. H. Zha, R. Y. An, Q. S. Zhao and B. Zhao, Int. J. Biol. Macromol., 2023, 247, 125761 Search PubMed.
- A. M. M. Sousa, H. K. S. Souza, J. Uknalis, S. C. Liu, M. P. Gonçalves and L. Liu, Int. J. Biol. Macromol., 2015, 80, 139–148 Search PubMed.
- F. Mano, I. M. Aroso, S. Barreiros, J. P. Borges, R. L. Reis, A. R. C. Duarte and A. Paiva, ACS Sustainable Chem. Eng., 2015, 3, 2504–2509 Search PubMed.
- K. Rong, J. Wei, Y. Wang, J. Liu, Z.-A. Qiao, Y. Fang and S. Dong, Green Chem., 2021, 23, 6065–6075 Search PubMed.
- C. Yao, X. Li and T. Song, J. Appl. Polym. Sci., 2007, 103, 380–385 Search PubMed.
- C. Mouro, R. Martins, A. P. Gomes and I. C. Gouveia, Gels, 2023, 9, 661 Search PubMed.
- H. Sereshti, Z. Mohammadi, S. Soltani and H. Najarzadekan, J. Mol. Liq., 2022, 364, 120077 Search PubMed.
- A. O. Basar, C. Prieto, M. Pardo-Figuerez and J. M. Lagaron, ACS Omega, 2023, 8, 3798–3811 Search PubMed.
- Q. Zhang, Z. Lin, W. Zhang, T. Huang, J. Jiang, Y. Ren, R. Zhang, W. Li, X. Zhang and Q. Tu, RSC Adv., 2021, 11, 1012–1021 Search PubMed.
- L. Marincaş, N. I. Farkas, L. Barbu-Tudoran, R. Barabás and M. I. Toşa, Mater. Chem. Phys., 2023, 304, 127862 Search PubMed.
- C. W. Lai and S. S. Yu, ACS Appl. Mater. Interfaces, 2020, 12, 34235–34244 Search PubMed.
- P. C. Lai, Z. F. Ren and S. S. Yu, ACS Appl. Polym. Mater., 2022, 4, 9221–9230 Search PubMed.
- S. Pal, Y. Z. Su, Y. W. Chen, C. H. Yu, C. W. Kung and S. S. Yu, ACS Appl. Mater. Interfaces, 2022, 14, 28247–28257 Search PubMed.
- M. Sheikhi, F. Rafiemanzelat, S. Ghodsi, L. Moroni and M. Setayeshmehr, Addit. Manuf., 2022, 58, 102997 Search PubMed.
- A. Aguzin, A. Dominguez-Alfaro, M. Criado-Gonzalez, S. Velasco-Bosom, M. L. Picchio, N. Casado, E. Mitoudi-Vagourdi, R. J. Minari, G. G. Malliaras and D. Mecerreyes, Mater. Horiz., 2023, 10, 2516–2524 Search PubMed.
- S. Wang, L. Zhang, R. Ma, J. Yu, X. Zhang, C. Shi, L. Ma, T. Li, Y. Huang, Y. Hu, Y. Fan and Z. Wang, Chem. Eng. J., 2023, 454, 140022 Search PubMed.
- J. Su, S. Li, Y. Chen, Y. Cui and M. He, Ind. Eng. Chem. Res., 2021, 60, 17797–17803 Search PubMed.
- L. Cai, G. Chen, J. Tian, B. Su and M. He, Chem. Mater., 2021, 33, 2072–2079 Search PubMed.
- M. A. Smirnov, V. S. Fedotova, M. P. Sokolova, A. L. Nikolaeva, V. Y. Elokhovsky and M. Karttunen, Polymers, 2021, 13, 3044 Search PubMed.
- L. Cai, G. Chen, B. Su and M. He, Chem. Eng. J., 2021, 426, 130545 Search PubMed.
- V. K. Vorobiov, M. P. Sokolova, N. V. Bobrova, V. Y. Elokhovsky and M. A. Smirnov, Carbohydr. Polym., 2022, 290, 119475 Search PubMed.
- A. P. Prosvirnina, A. N. Bugrov, A. V. Dobrodumov, E. N. Vlasova, V. S. Fedotova, A. L. Nikolaeva, V. K. Vorobiov, M. P. Sokolova and M. A. Smirnov, J. Mater. Sci., 2022, 57, 20543–20557 Search PubMed.
- Y. Wu, L. Cai, G. Chen, F. Yang and M. He, J. Mater. Chem. A, 2022, 10, 18218–18225 Search PubMed.
- T. H. Vo, P. K. Lam, Y.-J. Sheng and H.-K. Tsao, ACS Appl. Mater. Interfaces, 2023, 15, 33109–33118 Search PubMed.
- Y. Li, R. K. Kankala, L. Wu, A. Z. Chen and S. Bin Wang, ACS Appl. Polym. Mater., 2023, 5, 991–1001 Search PubMed.
- J. L. de Lacalle, A. Gallastegui, J. L. Olmedo-Martínez, M. Moya, N. Lopez-Larrea, M. L. Picchio and D. Mecerreyes, ACS Macro Lett., 2023, 12, 125–132 Search PubMed.
- G. Zhu, J. Zhang, J. Huang, X. Yu, J. Cheng, Q. Shang, Y. Hu, C. Liu, M. Zhang, L. Hu and Y. Zhou, ACS Sustainable Chem. Eng., 2022, 10, 7954–7964 Search PubMed.
- A. Pulyalina, V. Rostovtseva, I. Faykov, M. Tataurov, R. Dubovenko and S. Shugurov, Molecules, 2021, 26, 990 Search PubMed.
- N. Ismail, J. Pan, M. Rahmati, Q. Wang, D. Bouyer, M. Khayet, Z. Cui and N. Tavajohi, J. Membr. Sci., 2022, 646, 120238 Search PubMed.
- F. Russo, M. Tiecco, F. Galiano, R. Mancuso, B. Gabriele and A. Figoli, J. Membr. Sci., 2022, 649, 120387 Search PubMed.
- T. Mekonnen, P. Mussone, H. Khalil and D. Bressler, J. Mater. Chem. A, 2013, 1, 13379–13398 Search PubMed.
- M. Lončarić, L. Jakobek and M. Molnar, Croat. Chem. Acta, 2021, 94, 75–82 Search PubMed.
- M. A. Smirnov, A. L. Nikolaeva, N. V. Bobrova, V. K. Vorobiov, A. V. Smirnov, E. Lahderanta and M. P. Sokolova, Polym. Test., 2021, 97, 107156 Search PubMed.
- J. Yu, S. Xu, G. Goksen, C. Yi and P. Shao, Food Hydrocolloids, 2023, 135, 108196 Search PubMed.
- E. Jakubowska, M. Gierszewska, A. Szydłowska-Czerniak, J. Nowaczyk and E. Olewnik-Kruszkowska, Food Chem., 2023, 399, 133934 Search PubMed.
- M. Lakovaara, J. A. Sirviö, M. Y. Ismail, H. Liimatainen and R. Sliz, Cellulose, 2021, 28, 5433–5447 Search PubMed.
- J. Yu, X. Liu, S. Xu, P. Shao, J. Li, Z. Chen, X. Wang, Y. Lin and C. M. G. C. Renard, Compr. Rev. Food Sci. Food Saf., 2023, 22, 1030–1057 Search PubMed.
- D. Guo, Y. Guo, L. Sha, G. Lyu, J. Li, X. Zhang and B. Liu, Energy Fuels, 2020, 34, 8395–8402 Search PubMed.
- M. H. Shafie, R. Yusof, D. Samsudin and C. Y. Gan, Int. J. Biol. Macromol., 2020, 163, 1276–1282 Search PubMed.
- A. S. B. de Sousa, R. P. Lima, M. C. A. da Silva, D. das Neves Moreira, M. M. E. Pintado and S. de Melo Silva, Polymer, 2022, 259, 125314 Search PubMed.
- T. Wu, R. Dai, Z. Shan, H. Chen, M. W. Woo and J. Yi, Process Biochem., 2022, 118, 32–40 Search PubMed.
- Y. Guo, D. Tian, F. Shen, G. Yang, L. Long, J. He, C. Song, J. Zhang, Y. Zhu, C. Huang and S. Deng, Polymers, 2019, 11, 1455 Search PubMed.
- Q. Xia, C. Chen, Y. Yao, J. Li, S. He, Y. Zhou, T. Li, X. Pan, Y. Yao and L. Hu, Nat. Sustain., 2021, 4, 627–635 Search PubMed.
- H. Wen, D. Tang, Y. Lin, J. Zou, Z. Liu, P. Zhou and X. Wang, Carbohydr. Polym., 2023, 303, 120435 Search PubMed.
- M. L. Picchio, D. Minudri, D. Mantione, M. Criado-Gonzalez, G. Guzmán-González, R. Schmarsow, A. J. Müller, L. C. Tomé, R. J. Minari and D. Mecerreyes, ACS Sustainable Chem. Eng., 2022, 10, 8135–8142 Search PubMed.
- P. Velásquez, D. Bustos, G. Montenegro and A. Giordano, Molecules, 2021, 26, 984 Search PubMed.
- F. Shi, X. Hai, Y. Zhu, L. Ma, L. Wang, J. Yin, X. Li, Z. Yang, M. Yuan, H. Xiong and Y. Gao, Ultrason. Sonochem., 2023, 92, 106283 Search PubMed.
- S. Kamalam, Pondicherry J. Nurs., 2017, 10, 42–49 Search PubMed.
- D. V. Wagle, H. Zhao and G. A. Baker, Acc. Chem. Res., 2014, 47, 2299–2308 Search PubMed.
- S. Hellweg and L. Milà i Canals, Science, 2014, 344, 1109–1113 Search PubMed.
- Q. Zaib, M. J. Eckelman, Y. Yang and D. Kyung, Green Chem., 2022, 24, 7924–7930 Search PubMed.
- Y. Zhang, Z. Zhang, K. Guo and X. Liang, Bioresour. Technol., 2022, 365, 128175 Search PubMed.
- Y. Hu, Z. Wang, P. Liang, H. Zhu and Q. Liu, Sustainable Chem. Pharm., 2023, 33, 101099 Search PubMed.
- C. Fanali, V. Gallo, S. D. Posta, L. Dugo, L. Mazzeo, M. Cocchi, V. Piemonte and L. De Gara, Molecules, 2021, 26, 2652 Search PubMed.
- W. W. Yan, Z. M. Zong, Z. X. Li, J. Li, G. H. Liu, Z. H. Ma, Y. Y. Zhang, M. L. Xu, F. J. Liu and X. Y. Wei, ACS Sustainable Chem. Eng., 2020, 8, 9464–9471 Search PubMed.
- A. Fridrihsone, F. Romagnoli, V. Kirsanovs and U. Cabulis, J. Cleaner Prod., 2020, 266, 121403 Search PubMed.
- Y. Chen, K. Shen, Z. He, T. Wu, C. Huang, L. Liang and G. Fang, Cellulose, 2021, 28, 11503–11517 Search PubMed.
- X. Liang and Y. Guo, Bioresour. Technol., 2022, 362, 127805 Search PubMed.
- T. H. Tran, H. L. Nguyen, D. S. Hwang, J. Y. Lee, H. G. Cha, J. M. Koo, S. Y. Hwang, J. Park and D. X. Oh, Carbohydr. Polym., 2019, 205, 392–400 Search PubMed.
- J. A. Sirviö, I. Romakkaniemi, J. Ahola, S. Filonenko, J. P. Heiskanen and A. Ämmälä, Green Chem., 2024, 26, 287–294 Search PubMed.
- P. Makoś-Chełstowska, M. Kaykhaii, J. Płotka-Wasylka and M. De La Guardia, J. Mol. Liq., 2022, 365, 120158 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |