Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical nitrate reduction to ammonia using laser-processed Nb2AlC: the role of effective Al etching†
Shaista
Nouseen
ab,
Sujit
Deshmukh
b,
Michal
Langer
c,
Michal
Otyepka
 cd and
Martin
Pumera
cd and
Martin
Pumera
 *abefg
*abefg
aQuantum Materials Laboratory, 3D Printing & Innovation Hub, Center for Nanorobotics and Machine Intelligence, Department of Chemistry and Biochemistry, Mendel University in Brno, Zemedelska 1, Brno 61300, Czech Republic. E-mail: pumera.research@gmail.com
bFuture Energy and Innovation Laboratory, Central European Institute of Technology, Brno University of Technology, Purkynova 123, Brno 61200, Czech Republic
cIT4Innovations, VŠB – Technical University of Ostrava, 17. listopadu 2172/15, Poruba 70800, Ostrava, Czech Republic
dCzech Advanced Technology and Research Institute (CATRIN), Regional Centre of Advanced Technologies and Materials, Palacký University Olomouc, Šlechtitelů 27, Olomouc, 77900, Czech Republic
eAdvanced Nanorobots and Multiscale Robotics Laboratory, Faculty of Electrical Engineering and Computer Science, VSB - Technical University of Ostrava, 17. listopadu 2172/15, Ostrava 70800, Czech Republic
fDepartment of Medical Research, China Medical University Hospital, China Medical University, No. 91 Hsueh-Shih Road, Taichung, Taiwan
gDepartment of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul, 03722, Korea
First published on 9th June 2025
Abstract
Electrocatalytic nitrate reduction reaction to ammonia (NH3) is a promising approach for generating NH3 compared with the widely used Haber–Bosch process. It offers the advantage of zero carbon emission and helps in recycling nitrate waste. However, the challenge remains in the synthesis and engineering of proficient electrocatalytic materials with high faradaic efficiency and yield rate. Herein, we report a unique laser-processed niobium oxide-graphene (NbOx-Gr) electrode attached to a conductive support of graphene for NH3 generation. The two-step fabrication process started with spatially and temporally controlled pulsed laser writing on an Nb2AlC-coated polymer surface followed by simple electrochemical etching to remove excess Al. Electrochemical analysis elucidated that the NbOx-Gr electrode exhibited improved activity for ammonia generation. Moreover, theoretical studies provide insights into the nitrate reduction reaction mechanism, confirming that the electrochemical active site was located on the Nb atom of the NbOx-Gr electrode. Laser processing is a cost-effective, less chemically hazardous, versatile, and efficient approach to utilize MAX phases for multiple energy storage and conversion applications.
1 Introduction
Ammonia (NH3) is an indispensable industrial-scale chemical feedstock used in the agriculture industry; it is a hydrogen-rich and carbon-free fuel and has environmentally friendly nature.1–3 The industrial-scale synthesis of NH3 is largely dependent on the Haber–Bosch process. While millions of tonnes of NH3 are produced annually through the Haber–Bosch process,2–8 it consumes 1–2% of global energy and contributes to 1.0% of global carbon emissions, elevating environmental hazards.9–11 Another conventional approach involves nitrogen (N2) reduction to NH3, where water molecules act as proton sources. Nevertheless, the intrinsic properties of the N2 molecule, like high N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond dissociation energy of 941 kJ mol−1 and low solubility in water, negatively impact the yield rate and selectivity of the N2 to NH3 conversion process.10–15 Thus, developing more sustainable approaches for NH3 production have recently gained significant research interest.
N bond dissociation energy of 941 kJ mol−1 and low solubility in water, negatively impact the yield rate and selectivity of the N2 to NH3 conversion process.10–15 Thus, developing more sustainable approaches for NH3 production have recently gained significant research interest.
In this direction, the electrocatalytic nitrate reduction reaction, NO3− + 6H2O + 8e− → NH3+ + 9OH−, has high potential as it offers a sustainable route and provides a plethora of advantages such as (i) less environmental impact and high feedstock availability, i.e., nitrate (NO3−) ions are produced as agricultural fertilizers and industrial waste;15–17 this waste can be utilized for NH3 generation, making this route environmentally friendly while simultaneously remedying nitrate waste, (ii) high energy efficiency: NO3− reduction to ammonia consumes less energy as the dissociation energy for N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the NO3− ion is approximately 204 kJ mol−1, which is lesser compared to other approaches, (iii) high solubility: the solubility of the NO3− ion (880 g L−1) in H2O is superior compared to of N2 (0.02 g L−1).15–20
O in the NO3− ion is approximately 204 kJ mol−1, which is lesser compared to other approaches, (iii) high solubility: the solubility of the NO3− ion (880 g L−1) in H2O is superior compared to of N2 (0.02 g L−1).15–20
Thus, the adsorption of the NO3− ions on the electrocatalyst surface is more efficient, leading to enhanced electrochemical performance.21,22 However, a major obstacle in producing ammonia from NO3− ions is the competing hydrogen evolution reaction (HER), leading to lower faradaic efficiency.23,24 Currently, noble and non-noble metal-based electrocatalysts,25 such as Pt–TiO2,26 copper-based catalysts,27 metal oxides,28 and 2D materials,29 have been explored as electroactive materials for the nitrate reduction reaction. Nevertheless, these electrocatalyst materials face some limitations, such as high cost, low abundance, susceptibility to leaching, and predominance of the HER at high potentials.30–32 Therefore, developing new electrocatalyst materials for the effective conversion of NO3− into NH3 is crucial.
In this context, ternary-layered MAX phases with their unique blend of metallic and ceramic properties, along with high thermal stability and a combination of covalent and metallic bonds, offer substantial potential for electrochemical energy conversion applications.33,34 Typically, MAX phases are employed to fabricate MXenes by removing aluminium layers using hazardous acids (HF).35 Thus, HF-free approaches are gaining significant attention.36 It is noteworthy here that recently MAX phases have gained significant attention for energy applications.37 However, MAX phases for electrocatalytic nitrate reduction reactions have not been reported. The major obstacles in this context are tuning their electrical properties and removing aluminium layers to fabricate a well-ordered architecture that utilizes the full potential of MAX phases.33–38 To resolve these issues, heterostructure engineering,39 interface engineering,40 and doping approach41 have been explored to enhance the electrochemical performance of MAX phases.42–44
Herein, we reported a versatile, single-step, cost-effective, less chemically hazardous laser processing approach for fabricating the heterostructures of MAX (Nb2AlC) with graphene.45 This involves a single-step laser processing of Nb2AlC decorated over graphene. Subsequently, to eradicate the aluminium content from the laser-processed Nb2AlC, an electrochemical etching approach is employed. The fabricated Nb matrix-loaded graphene (NbOx-Gr) electrodes were employed for electrocatalytic nitrate reduction reaction to generate NH3. This approach to fabricate NbOx-Gr electrode using Nb2AlC-coated PI, employing laser treatment followed by electrochemical etching enables the utilisation of MAX phases more efficiently without the use of hazardous HF and opens the door for exploring a broad range of different MAX phases and other 2D materials integrated with graphene for diverse applications beyond energy conversion and storage applications.
2 Results and discussion
2.1 Fabrication process
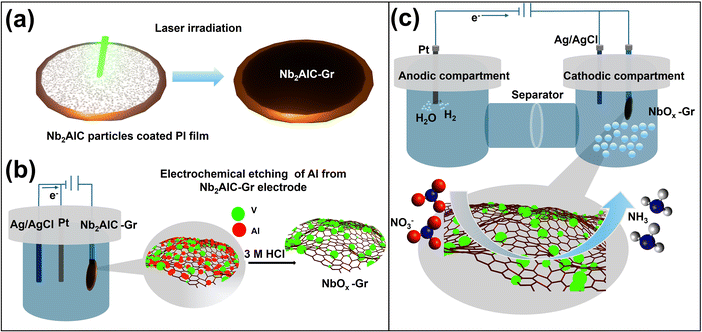
First Nb2AlC-coated polyimide sheets are subjected to laser irradiation using a diode-pumped Nd:YAG solid pulsed laser beam (532 nm), generating niobium-based spherical nanoparticle decorated over graphene thin films. The high-energy laser pulses create substantial mechanical stress and thermal shock on the stacked 2D Nb2AlC nanosheets, leading to the formation of small fragments of NbOx spherical nanoparticles. Concurrently, the infrared energy is absorbed by the PI sheet, generating a temperature exceeding 2000 K within a millisecond range, enabling the photothermal transformation of the polyimide into graphene (Fig. 1a).In the subsequent step, an electrochemical etching route is employed to eliminate the excess aluminium metal using the chronoamperometry method with 3 M HCl as an electrolyte in a three-electrode setup. The chloride ions (Cl−) ions in the HCl electrolyte react with the Al in the fragmented Nb2AlC and form aluminium chloride (AlCl3).46,47 Nonetheless, compared to the traditional approach, this process eliminates the reliance on toxic and hazardous HF-based compounds, while maximizing the usage of the MAX phase efficiently (Fig. 1b). The details of the fabrication process are outlined in the experimental section.
In the application context, the electrocatalytic nitrate reduction reaction to generate NH3 was carried out using a three-electrode set-up employing an H-type electrolytic cell with a frit separation. In the three-electrode setup, Gr, Nb2AlC-Gr, and NbOx-Gr are employed as the working electrode, Platinum (Pt) as the counter electrode and Ag/AgCl as the reference electrode utilizing 0.5 M Na2SO4 as the electrolyte and 0.1 M KNO3 as the nitrate source (Fig. 1c). We investigated the physical and chemical composition, followed by morphological analysis of these electrodes in the following sections.
2.2 Material characterization
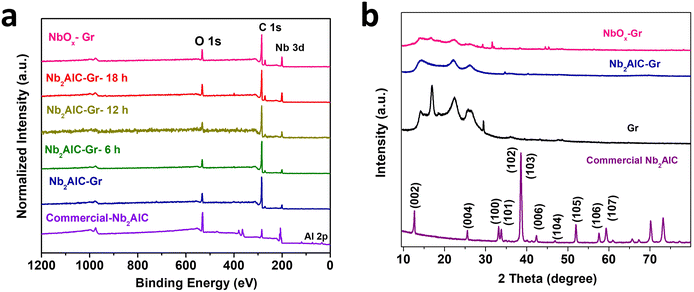
To investigate the material composition analysis and to select suitable electrodes after electrochemical etching for the electrochemical application, X-ray photoelectron spectrum (XPS) analysis was carried out. Wide survey spectrum analysis was employed to confirm the effectiveness of the etching process and to select the appropriate electrodes for the electrochemical analysis. To enable a comprehensive comparison of aluminium removal we varied the etching time (6, 12, 18, and 24 hours) during the electrochemical etching process. XPS analysis of each electrode was carried out to verify the efficiency of Al removal with varying etching times.Note that laser-treated electrochemically etched electrodes only displayed peaks for Nb 3d, C 1s, and O 1s, indicating the effective removal of aluminium from the electrode surface (Fig. 2a). The extracted elemental composition information in atomic percentages from XPS survey spectra is provided in Table 1, which shows that aluminium accounted for 8.66% in the commercial Nb2AlC sample, 0.39% in the Nb2AlC-Gr electrode, and was undetectable in the electrochemically etched electrodes. This validates the effectiveness of aluminium removal through the in situ electrochemical etching process.
| Samples | Nb (at%) | Al (at%) | C (at%) | O (at%) |
|---|---|---|---|---|
| Commercial-Nb2AlC | 10.31 | 8.66 | 40.35 | 40.69 |
| Nb2AlC-Gr | 1.09 | 0.39 | 88.50 | 10.02 |
| Nb2AlC-Gr-6 h | 1.03 | — | 90.83 | 8.14 |
| Nb2AlC-Gr-12 h | 1.61 | — | 89.88 | 8.52 |
| Nb2AlC-Gr-18 h | 2.84 | — | 84.18 | 12.98 |
| Nb2AlC-Gr-24 h/NbOx-Gr | 2.90 | — | 86.64 | 10.45 |
Before testing the electrodes for the electrochemical application, the selection of the appropriate electrochemically etched electrode for the nitrate reduction to ammonia is essential. Thus, we performed linear sweep voltammetry (LSV) measurements in a three-electrode setup employing 0.5 M Na2SO4 as the electrolyte, as illustrated in Fig. S1 in ESI.† We observed that the NbOx-Gr electrode subjected to the etching process for 24 h exhibited enhanced electrochemical performance compared to the Nb2AlC-Gr, and other etching time variants (6 h, 12 h, and 18 h). Thus, we selected the NbOx-Gr-24 h. electrode for further investigation, and we named the electrode NbOx-Gr throughout the manuscript.
X-ray diffraction (XRD) patterns were acquired to investigate the phase transition of the commercial-Nb2AlC, Gr, Nb2AlC-Gr, and NbOx-Gr electrodes as illustrated in Fig. 2b. The diffraction peaks detected for the commercial-Nb2AlC powder correspond closely to those previously reported Nb2AlC MAX phases, indicating the absence of impurities.48 The Gr demonstrated a characteristic graphene peak and additional peaks corresponding to the PI substrate.49 The (002) peak associated with Nb2AlC disappeared in the Nb2AlC-Gr electrode, indicating a phase transformation induced by laser treatment. Likewise, in the NbOx-Gr electrode, the (002) peak was absent, and it exhibited new peaks, potentially corresponding to the niobium oxide composite with graphene formed after the electrochemical etching treatment.50 The XRD pattern confirms the phase transformation of the Nb2AlC MAX phase.
We utilized Raman spectroscopy to correlate the phase transformation findings observed in the XRD measurements and to investigate the changes in the Gr, Nb2AlC-Gr, and NbOx-Gr electrodes. The outcome reveals that two Raman-active E2g and A1g optical modes of the Nb2AlC MAX phase are absent in the Nb2AlC-Gr and NbOx-Gr electrode samples, as illustrated in Fig. S2a–c.† According to the existing literature, the E2g optical mode (theoretical value ≈181.5 cm−1) corresponds to the Nb and C atoms' in-plane oscillations, coupled with minor contributions from Al atom oscillations. The observed suppression of the E2g mode could be attributed to the substitution of the Al atom with lighter O atoms. The absence of the A1g optical mode (theoretical value ≈262.8 cm−1) corresponds to the Nb and C atom's symmetric vibrations out-of-plane and could be associated with an expansion in layer spacing.51–53 In our case the A1g optical mode could undergo a shape transformation and downshift due to the laser and etching treatment. The suppression of the optical mode in the Nb2AlC-Gr and NbOx-Gr electrodes confirms the etching of Al and phase transformation, corroborated by the XRD measurements.51–53 Besides, after the laser treatment, the intensity of D bands does not have a significant difference in the Gr and Nb2AlC-Gr samples. However, the D band in the NbOx-Gr increased after electrochemical etching, while the intensity of the G band decreased, which indicates an increase in defects. We also determined the coherence length (La) of the graphene layered structure along ab planes (see details in the ESI†). The coherence lengths of NbOx-Gr, Nb2AlC-Gr, and Gr electrodes are 16.31 nm, 21.40 nm, and 22.97 nm, respectively. This trend in coherence length implies that crystallinity is crippled along the ab planes.54 Additionally, the Raman spectra of pristine polyimide sheets are provided,55 to confirm the successful conversion of polyimide sheets into graphene (Fig. S2b†).
XRD and Raman measurements display substantial alterations in the surface chemical compositions of Nb2AlC-Gr and NbOx-Gr electrodes following laser exposure and subsequent etching treatment. To validate these surface chemical composition modulations, high-resolution XPS spectra of laser treated and electrochemically etched electrodes both were recorded. To better analyze the chemical bonding environment and oxidation state of the elements and to reveal the chemical transformations in the chemical composition of the Nb2AlC-Gr electrode induced by the laser processing, individual XPS spectrum analysis for Nb 3d, Al 2p, C 1s, and O 1s was carried out. The Nb 3d high-resolution spectrum displays a doublet, with peaks at ≈207 eV corresponding to Nb–O 3d5/2 and ≈210 eV to Nb–O 3d3/2, confirming the presence of Nb2O5 and the Nb5+ oxidation state, as illustrated in Fig. 3a.56–59 Additionally, the chemical transformation of the Nb2AlC MAX phase is confirmed in the laser treated Nb2AlC-Gr electrode due to the absence of the metal-carbide bond.58,59 The detailed analysis of the C 1s spectrum illustrated in Fig. 3b shows the presence of distinct types of carbon bonds like the C–C sp2 bond at ≈284.0 eV, C–C sp3 bond at ≈285.0 eV, and C–O bond at ≈286.0 eV, like those in the Nb2AlC MAX phase.52,53,59 However, the absence of the Nb–C bond peak in the ≈282.3 eV region compared to the Nb2AlC MAX phase confirms a chemical composition transformation of the Nb2AlC phase induced by the laser processing.56–60
 | ||
| Fig. 3 Chemical composition analysis: high-resolution XPS analysis of Nb2AlC-Gr and NbOx-Gr electrodes. (a and d) Nb 3d, (b and e) C 1s, and (c and f) Al 2p spectra, respectively. | ||
The high-resolution Al 2p spectrum was recorded as illustrated in Fig. 3c for the Nb2AlC-Gr electrode. A peak is detected in the ≈75.0 eV region, indicating the presence of oxides of Al.52,53,59 Additionally, the O 1s spectrum is provided in Fig. S3a in the ESI,† confirming the presence of all requisite elements in the Nb2AlC-Gr electrode.56–60 For NbOx-Gr the Nb 3d core level spectrum exhibits doublet peaks, reflecting ≈207.0 eV for Nb–O 3d5/2 and ≈210.0 eV for Nb–O 3d3/2, indicating the existence of the Nb5+ oxidation state in Nb2O5 as shown in Fig. 3d.56–60 These results indicate that the Nb 3d chemical composition remains unchanged relative to the laser-processed Nb2AlC-Gr electrode.
The in-depth analysis of the carbon C 1s spectrum of the NbOx-Gr electrode as illustrated in Fig. 3e shows similar findings compared to the C 1s spectrum of the Nb2AlC-Gr electrode, the carbon bonds like the C–C sp2 bond at ≈284 eV, C–Csp3 bond at ≈285 eV, and C–O bond at ≈286.56–59 The core level Al 2p spectrum, shown in Fig. 2f, confirms the absence of oxides of Al; this indicates the effective removal of Al through the electrochemical etching process. Fig. S3b in the ESI† exhibits the O 1s high-resolution spectra of the NbOx-Gr electrode. The findings from the XPS measurements corroborate the results of the XRD and Raman measurements, suggesting that laser processing modifies the chemical composition of the Nb2AlC phase, and the laser processing with the in situ electrochemical etching successfully removes the excess of oxides of Al from the surface of the electrodes.
The scanning electron microscopy (SEM) micrographs of the Gr, Nb2AlC-Gr, and NbOx-Gr electrodes are displayed in Fig. 4, where the successful integration of the Nb-rich spherical nanoparticles onto the graphene network is visualized. In the case of the Nb2AlC-Gr film, Nb-rich spherical nanoparticles are evenly distributed onto the graphene with particle sizes spanning from the micrometre (μm) to the sub-nanometre (nm) scale (Fig. 4a and b). Even though the SEM images of the electrochemically etched sample NbOx-Gr elucidated a similar morphology to Nb2AlC-Gr, the particle size has decreased in NbOx-Gr as compared to Nb2AlC-Gr, as displayed in Fig. 4c and d, respectively. The SEM micrographs (low and high magnification) of bare Gr are provided in the ESI in Fig. S4a and b,† elucidating the successful formation of the graphene like structure from the polyimide sheet after laser processing.
To quantify the surface roughness of the Nb2AlC-Gr and NbOx-Gr electrodes, optical micrographs were acquired using a confocal laser scanning microscope (CLSM). The topographical variations are presented in Fig. 4e and h collectively with the 2D micrographs (Fig. 4f and i) along with their 3D false colour mapping micrographs (Fig. 4g and j), where the colour variations reflect the difference in height profiles. The average surface roughness (Sq) of the electrodes was investigated utilizing height profile analysis, yielding values of ≈9.79 μm and ≈7.30 μm for the Nb2AlC-Gr and NbOx-Gr electrodes, respectively. These findings correlate with the SEM morphological studies, which indicate that the particle size reduction in NbOx-Gr indeed reduces the surface roughness, causing the formation of a smoother electrode surface. Such surface modifications could potentially lead to boosting the electrochemical performance of the electrodes. STEM micrographs (Fig. S5 in ESI†) of NbOx-Gr electrodes further confirm the presence of Nb nanoparticles on the graphene sheet.
2.3 Electrochemical characterization for nitrate reduction reaction
Firstly, the electrochemical active surface area (ECSA) of each electrode was evaluated, as the ECSA plays an important role in the electrochemical activity of the material. The ECSA values obtained for Gr, Nb2AlC-Gr, and NbOx-Gr as working electrodes are 0.03 cm−2, 0.07 cm−2, and 0.33 cm−2, respectively. The detailed experimental procedures and calculations are provided in the ESI, Fig. S6a–f.† The two-fold increase in the ECSA after integrating the Nb2AlC-MAX phase into the graphene network, followed by the additional increment due to the electrochemical etching, is beneficial for electrochemical performance.The combination of simple laser treatment with electrochemical etching is responsible for the effective improvement of ECSA of the NbOx-Gr sample. Furthermore, the electrochemical activity of the electrocatalyst material for nitrate reduction to generate NH3 and by-product NO2− ions is evaluated employing a three-electrode setup in an H-type glass cell, separated via a frit in an ambient environment, as shown in Fig. 5a. The Gr, Nb2AlC-Gr, and NbOx-Gr pendulum-shaped electrodes were employed as the working electrodes as illustrated in ESI Fig. S7,† where Pt acted as the counter electrode and Ag/AgCl acted as the reference electrode.
First, the linear sweep voltammetry (LSV) curve was recorded for Gr, Nb2AlC-Gr and NbOx-Gr as working electrodes in the absence of NO3− ions (0.5 M Na2SO4 electrolyte) and the presence of NO3− ions (0.5 M Na2SO4 + 0.1 M KNO3 electrolyte) at a scan rate of 5 mV−1 as depicted in Fig. 5b. The LSV curves demonstrate that the Gr, Nb2AlC-Gr, and NbOx-Gr electrodes exhibit electrochemical activity for nitrate reduction as proven by the increment in the current density, in the presence of NO3− ions. The comparative LSV study further indicates that the NbOx-Gr as a working electrode is the most efficient for nitrate reduction reaction, demonstrating the highest increment in the current density among all the tested electrodes. This enhanced electrochemical performance towards the nitrate reduction reaction of NbOx-Gr aligns with the physical characterization findings, suggesting that the fabrication process involving laser treatment and in situ electrochemical etching of Al metal improves the electrochemical activity of the material.
To further confirm the electrochemical activity of Gr, Nb2AlC-Gr, and NbOx-Gr electrodes for nitrate reduction reaction, electrolysis was performed at selected potentials in the range of −0.20 to −1.10 V vs. RHE for 1-hour time duration, as illustrated in ESI Fig. S8a–c.† The electrolysis findings demonstrate an increase in the current density for the NbOx-Gr electrode compared to the Gr and Nb2AlC-Gr electrodes, aligning with the findings of LSV measurements. Next, the NH3 concentration in the electrolytic solutions was quantified by employing the well-known indophenol blue colorimetric approach. The electrolytic solution upon the reaction with the indophenol blue reagent changes its colour from yellow to dark green, suggesting the formation of higher concentration of NH3 for the electrolytic solution of the NbOx-Gr electrode sample as depicted in ESI Fig. S9.† This indicates that ammonia is generated in high concentration when the potential is increased. The UV-vis spectra were recorded as shown in ESI Fig. S10a–c,† displaying an increase in peak intensity at 655 nm, which corresponds to ammonia detection.
The UV-vis spectra reveal a progressive increase in the ammonia peak as the potential is increased for the Gr, Nb2AlC-Gr, and NbOx-Gr electrodes. The NbOx-Gr electrode shows the most efficient results for NH3 generation compared to Gr and Nb2AlC-Gr electrodes, corroborating with the results of LSV and electrochemical measurements. The faradaic efficiency (FE) and ammonia yield rate were calculated, utilizing the ammonia concentration acquired from the indophenol colorimetric approach utilizing eqn (1) and (2), illustrating a gradual enhancement when the potential is increased from −0.20 V to −1.1 V, as demonstrated in Fig. 5c and d. At −1.1 V vs. RHE, the FE and yield rates are ≈9.05% and ≈5.68 μg h−1 cm−2 for the Gr electrode, ≈45.47% and ≈71.50 μg h−1 cm−2 for the Nb2AlC-Gr electrode, and ≈46.50% and ≈194.79 μg h−1 cm−2 for the NbOx-Gr electrode, respectively. The obtained yield rate is comparable with those of the previously reported MXene-based electrocatalysts for nitrate reduction to ammonia. The comparative table is provided in ESI Table S1.†
In addition to ammonia generation, nitrite ions are a primary by-product formed during the nitrate reduction reaction electrolysis process. Thus, to evaluate NO2− ions in the electrolytic solution, UV spectra were recorded for Nb2AlC-Gr and NbOx-Gr electrodes, as shown in ESI Fig. S11a and b.† The observed peaks at 540 nm indicate the presence of NO2− ions. Consequently, FE and yield rate were calculated utilizing the nitrite ion concentration, using eqn (1) and (2) as demonstrated in Fig. 5e and f. The FE and the yield rate of NO2− ions are lower compared to those of NH3, which confirms that the electrocatalyst materials generate NO2− ions as a by-product. Furthermore, a potential of −0.8 V is selected for the stability measurements of the NbOx-Gr electrode. The chronoamperometry measurement and UV spectra measurement are shown in ESI in Fig. S12 and 13,† confirms the formation of NH3 at different times, as presented in Fig. 5g and h. Long-time electrolysis at a potential of −0.8 V was performed, which suggests that the NbOx-Gr electrode was able to generate ammonia for 24 hours as demonstrated in Fig. 5i. Furthermore, the UV spectra (Fig. S14†) confirm the presence of an ammonia peak at 655 nm, with a calculated ammonia yield rate of 194.04 μg h −1 cm−2. Moreover, to validate the conversion of NO3− ions into NH3 through electrolysis, we acquired the 1H NMR spectra of the electrolysis solution of the NbOx-Gr electrode in a15N-labeled NO3 + 0.5 M Na2SO4 electrolyte and a blank electrolyte containing 0.5 M Na2SO4. The obtained spectra demonstrate that the blank solution has no signal (Fig. S15†), while in the 15N-labeled NO3 + 0.5 M Na2SO4 electrolytic solution we observed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 doublet and a 1
1 doublet and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triplet peak, which could be related to NH4+ and its by-product.61 Furthermore to confirm the presence of requisite elements on the surface and structural stability of NbOx-Gr electrodes, SEM-EDS mapping micrographs were acquired before and after electrochemical analysis, which confirm the presence of elements like C, O, Nb, and Al on the surface of electrodes after electrochemical analysis, suggesting that the electrodes are stable as illustrated in Fig. S16 in the ESI.†
1 triplet peak, which could be related to NH4+ and its by-product.61 Furthermore to confirm the presence of requisite elements on the surface and structural stability of NbOx-Gr electrodes, SEM-EDS mapping micrographs were acquired before and after electrochemical analysis, which confirm the presence of elements like C, O, Nb, and Al on the surface of electrodes after electrochemical analysis, suggesting that the electrodes are stable as illustrated in Fig. S16 in the ESI.†
2.4 Theoretical calculations
Density functional theory (DFT) calculations were employed to investigate the mechanism of electrocatalytic conversion of NO3− to NH3 over NbOx-Gr. A model comprising a NbOx nanocluster supported on pristine graphene was used to examine the structural reorganization of NbOx upon adsorption on the graphene surface, construct the Gibbs free energy profile, and analyze charge density differences (CDD) and Bader charges.Our calculations revealed a negative binding energy of −2.99 eV, indicating thermodynamically favorable adsorption of the NbOx nanocluster on graphene (see details in the ESI†). Additionally, a geometry analysis showed that the NbOx model underwent no substantial structural reorganization after adsorption on the graphene surface (Fig. S17†). The most pronounced deviation was observed for the O16 atom (Table S2†), attributed to the interaction of the Nb2 atom with the graphene sheet (see Fig. S17† for atom labelling). The observed changes correspond to those occurring upon non-covalent interaction between nanoclusters and graphene. Furthermore, the optimized structure of the NbOx-Gr catalyst confirmed the absence of a covalent bond between the NbOx nanocluster and the graphene support (Fig. 6a).
 | ||
| Fig. 6 (a–h) DFT optimized structures of the reaction intermediates for nitrate reduction over NbOx-Gr. | ||
The Gibbs free energy profile displays the energy changes during nitrate reduction, which involves nine proton and eight electron transfers, following the sequence:62 NO3− → *NO3 → *NO2 → *NO → *N → *NH → *NH2 → *NH3 → NH3(g) (Fig. 6).
Furthermore, DFT-optimized structures of the reaction intermediates identified the Nb atom on the NbOx cluster surface as the active site. The reaction starts with the favourable adsorption of the NO3− anion, which is strongly bound, exhibiting a Gibbs free change (ΔG) of −1.91 eV for this reaction step. Furthermore, Gibbs free energy changes were negative for all examined reaction steps along the reduction pathway, except for the *NO → *N step, which may serve as the potential-determining step (PDS). As depicted in Fig. 7a, the ΔG for this step is 0.88 eV. The corresponding limiting potential (UL) of −0.44 V for the overall energy profile suggests that NbOx-Gr can act as an effective electrocatalyst for nitrate reduction.
The electronic structure of the intermediates was further probed, and the calculated CDDs revealed distinct charge accumulation patterns. For oxygen-containing intermediates (*NO3, *NO2, and *NO), charge accumulation primarily occurs on bonds connecting the active site (Nb atom) and oxygen atoms (Fig. 7c–e). In contrast, a high accumulation of charge on the N atom was observed for the *N intermediate (Fig. 7). This can explain the strongly negative ΔG of −2.53 eV associated with the *N → *NH reaction step. Similar charge accumulation on the nitrogen atoms was found for *NH2 and *NH3 intermediates (Fig. 7h–i). The facile hydrogenation steps in nitrate reduction on NbOx-Gr can also be inferred from Bader charge analysis, which reveals an increasing negative charge on nitrogen atoms as the reaction progresses (Fig. 7b).
3 Conclusion
In summary, we reported a new kind of electrode material for nitrate reduction to ammonia, which was fabricated by employing a single-step lasing process followed by the electrochemical etching technique. The lasing process concurrently modified the chemical composition of the Nb2AlC phase and converted the polyimide substrate into a graphene structure. Moreover, the in situ electrochemical etching technique etched the aluminium metal from the surface of the electrocatalyst electrode, enhancing its electrochemical activity. This fabricated heterostructure of NbOx-Gr electrode delivered a faradaic efficiency and ammonia yield rate of ≈46.50% and ≈194.79 μg h−1 cm−2, respectively, at −1.1 V vs. RHE. Additionally, theoretical calculations provided a deeper understanding of the reaction mechanism of the nitrate reduction to NH3, confirming that the electrochemical active site is present on the Nb atom of the NbOx-Gr electrode. In the future direction, the present work exhibits huge potential for the fabrication of cost-effective Nb2AlC electrocatalyst materials for nitrate reduction to ammonia, offering an advanced material fabrication strategy to achieve high faradaic efficiency and yield rate for ammonia generation through the introduction of suitable MAX phases.4 Materials and experimental methods
4.1 Materials
Nb2AlC powder was obtained from Laizhou Kai Ceramic Materials Co., Ltd, China. Polyimide sheets (PI, 0.005′′) were used as received from Fiedler Scientific Instruments, Czech Republic. Hydrochloric acid (35%) was obtained from Penta Chemicals Unlimited. Potassium nitrate was procured from Alfa Aesar. Sodium hypochlorite solution, phosphoric acid, salicylic acid, sodium hydroxide, citric acid, sodium nitroferricyanide, N-(1-naphthyl) ethylenediamine dihydrochloride, sulfanilamide, Na15NO3 and sodium sulfate were acquired from Merck. All the chemicals were used as procured and all the solutions were prepared using Millipore water with a resistivity of >18 MΩ cm.4.2 Laser treatment of Nb2AlC-Gr electrodes
The Gr, Nb2AlC-Gr, and NbOx-Gr electrodes were fabricated using a Laser dicer Oxford Lasers A-Series equipped with a diode-pumped solid-state Nd:YAG laser operating at 532 nm wavelength. The defocused laser writing technique was directly applied on polyimide sheets to fabricate the Gr electrode. In contrast, for the Nb2AlC-Gr electrode, firstly, Nb2AlC dispersed in ethanol was drop-cast onto the polyimide sheets before defocused laser writing. The laser parameters used in this study were as follows: a pulse frequency of 7 kHz, a scanning speed of 50 mm s−1, and a laser power of 3 W.4.3 In situ electrochemical etching
A three-electrode set-up, where Ag/AgCl acts as the reference electrode, Platinum (Pt) acts as the counter electrode, and Nb2AlC-Gr acts as the working electrode, was employed for the electrochemical etching process. A potential of 2 V vs. Ag/AgCl was applied for the chronoamperometry measurement in 3 M HCl as the electrolyte. The etching process was performed for different time durations like 6 h, 12 h, 18 h, and 24 h under room temperature conditions. After the etching the electrodes were rinsed with H2O, to remove any residual impurity and AlCl3 on the surface of the electrodes.4.4 Electrochemical measurement
All the electrochemical experiments were carried out employing a three-electrode setup. The linear sweep voltammetry profile was obtained in 0.5 M Na2SO4 and 0.5 M Na2SO4 + 0.1 M KNO3 (nitrate source) electrolyte with the scan rate of 5 mV−1 using a potentiostat instrument (PGSTAT 204 from Metrohm Autolab, Netherlands) that was operated through NOVA software version 2.1, connected with a computer. The chronoamperometry measurements were carried out using 0.5 M Na2SO4 + 0.1 M KNO3 (nitrate source) as the electrolyte, a Ag/AgCl electrode filled with the KCl gel electrolyte as the reference electrode, Platinum (Pt) as the counter electrode, and Gr, Nb2AlC-Gr, and NbOx-Gr as the working electrode in a similar setup.To investigate the nitrate reduction reaction activity. The electrolysis process to generate ammonia was conducted in an H-cell separated via a frit. To ensure a balanced distribution of electrolytes, 20 mL of electrolyte was added to both the anodic and cathodic compartments. The electrolysis process was carried out for one hour for each electrode like Gr, Nb2AlC-Gr, and NbOx-Gr with continuous stirring to investigate the faradaic efficiency (FE%) and the ammonia yield rate. During the electrolysis process, different potentials were applied in the range of −0.20 to −1.10 V vs. RHE. The potential was converted into a reversible hydrogen electrode (RHE) scale using the following equation: ERHE = EAg/AgCl + 0.0591 pH + 0.199.63
4.5 Colorimetric procedure for the quantification of NH3 and NO2− concentrations
A well-established colorimetric analysis procedure was employed to evaluate the NH3 and NO2− ion concentration after the electrolytic measurements. A UV-vis spectrophotometer was employed to identify the concentration of NH3 and NO2− ions. The detailed procedure is described in subsequent sections.4.6 Calculation to determine the performance of the working electrodes (electrocatalysts)
The faradaic efficiency (FE) and yield rate (YR) were obtained by the following equations:63| FE (%) = (n × F × cNH3/NO2− × V)/Q | (1) |
| YR = (cNH3/NO2− × V × M)/(t × A) | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), cNH3/NO2− is the concentration of NH3 or NO2−, V is the electrolytic solution volume (20 mL) at the cathodic compartment, Q is the total charge observed during the process of electrolysis, M is 17.03 for NH3 or 46.005 for NO2−, t is the electrolysis time, and A is the surface area of the working electrode.
485 C mol−1), cNH3/NO2− is the concentration of NH3 or NO2−, V is the electrolytic solution volume (20 mL) at the cathodic compartment, Q is the total charge observed during the process of electrolysis, M is 17.03 for NH3 or 46.005 for NO2−, t is the electrolysis time, and A is the surface area of the working electrode.
4.7 Evaluation of ammonia by the 1H NMR quantitative technique and isotopic labelling measurement
For the isotopic labelling analysis, the electrolysis was recorded in the 0.5 M Na2SO4 + 0.1 M Na15NO3 electrolyte solution. Following electrolysis, an aliquot of 5 mL catholyte was collected from the H-cell and 250 μL of concentrated H2SO4 was added to create an acidic environment, which is the standard for the quantification of NH4+ ions. After this, 0.002 g of maleic acid was added as an internal standard to the above solution. 500 μL of this solution was taken in the NMR tube and 50 μL deuterium oxide (D2O) was added for the NMR detection.634.8 Material characterization
SEM (FEI VERIOS 460 L) is used for the surface morphology investigation of the Gr, Nb2AlC-Gr, and NbOx-Gr electrodes. An Olympus Lext-OLS4100 confocal laser scanning microscope is utilized to obtain the optical images of the Nb2AlC-Gr and NbOx-Gr electrodes. Physical characterization of Nb2AlC-Gr and NbOx-Gr electrodes was performed using a Rigaku SmartLab 3 kW X-ray diffractometer for the X-ray diffraction (XRD) investigation. This XRD device was operated with a current of 30 mA and a voltage of 40 kV. The XRD investigation was carried out following the Bragg Brentano geometry with Cu Kα radiation (λ = 0.15418 nm). The chemical composition investigation was carried out using an X-ray photoelectron spectroscopy device (XPS, Kratos AXIS Supra). The XPS wide survey scan and core level spectra of the elements like Nb, C, O, and Al present in Gr, commercial Nb2AlC, Nb2AlC-Gr, and NbOx-Gr are acquired. The software Casa XPS is employed for the fitting of XPS spectra curves, and the calibration of the obtained spectra is done against the carbon C 1s peak i.e., 284.8 eV. A Witec Alpha 300R instrument is utilized to obtain the Raman spectra curve. The UV-visible absorption spectra studies are performed with a UV-Vis-spectrophotometer JASCO V-750.4.9 Theoretical calculations
Spin-polarized DFT calculations were carried out using the Vienna Ab initio Simulation Package (VASP), introducing the projector augmented wave (PAW) potentials with a plane-wave cutoff energy of 520 eV.64–67 The Perdew–Burke–Ernzerhof (PBE) parametrization of the generalized gradient approximation (GGA) was used to describe the electronic exchange–correlation energies, and the DFT-D3 method of Grimme with zero-damping was also introduced to describe the van der Waals interactions.68,69 All structures were fully optimized until forces acting on all atoms were reduced to 0.03 eV Å−1, and electronic degrees of freedom were relaxed until the change in total electronic energy between successive iteration steps was smaller than 10−5 eV. For structural optimization, the Brillouin zone was sampled using a 1 × 1 × 1 k-point grid based on the Monkhorst–Pack scheme, centred at Gamma. Since the nitrate reduction proceeds in an aqueous environment and involves H+ transfer, the solvation effects of water were considered by VASPsol.70 To eliminate the effects between two adjacent layers, a vacuum region of 15 Å was added in the z-direction. For electronic structure calculations, the Brillouin zone integrations were performed with a gamma-centred 3 × 3 × 1 Monkhorst–Pack k-point mesh grid.71 The computational hydrogen electrode (CHE) model was used to calculate the Gibbs free energy of reactions involving electron-proton transfer. The vibrational modes were calculated at 298.15 K to obtain the zero-point energy, entropy, and temperature corrections to enthalpy, and VASPKIT was used for the post-processing.72,73 To avoid calculating the charged molecule NO3−, the Gibbs free energy of aqueous NO3− was derived from those of gaseous HNO3 and H2. The Gibbs free energy for nitrate NO3− to adsorb on the NbOx-Gr model in aqueous solution forming *NO3 was calculated as follows:74,75where G*NO3, G*, G*HNO3(g), and GH2(g) are the Gibbs free energies of NO3− adsorbed on the NbOx-Gr model, the NbOx-Gr model itself, HNO3 and H2 molecules in the gas phase, respectively. The value of 0.392 is the correction of adsorption energy.76 Limiting potential UL (UL = −ΔGmax/e) was employed to describe the lowest bias requirement for the nitrate reduction.77
Data availability
Data for this article are available at ZENODO at https://zenodo.org/.Author contributions
S. N., S. D., and M. P. conceived the idea. S. N. carried out laser treatment, in situ etching of the Nb2AlC electrodes and their characterizations (such as SEM, CLSM, XPS, XRD, Raman, electrochemical characterization, and ammonia production), data acquisition, investigation and preparation of the first draft of the manuscript. S. D. carried out laser treatment, managed the project, and revised the manuscript. M. L. performed theoretical calculations and wrote the theoretical section. M. O. participated in the validation of the theoretical calculations, writing of the manuscript and funding acquisition. M. P. supervised the research, acquired funding, and revised the manuscript. All the data included in the manuscript were discussed and approved by all the authors.Conflicts of interest
The authors affirm no conflict of financial interest.Acknowledgements
S. N. would like to acknowledge Mendelu PhD talent award for funding and Josef Dadok National NMR Centre, CEITEC, Central European Institute of technology, Masaryk University for NMR measurements. The work was supported by the ERDF/ESF project TECHSCALE (No. CZ.02.01.01/00/22_008/0004587). The material characterization was done with the support of the CzechNanoLab project at CEITEC Nano Research Infrastructure MEYS CR (LM2023051). M. L. acknowledges VSB – Technical University of Ostrava, IT4Innovations National Supercomputing Center, Czech Republic, for awarding this project access to the LUMI supercomputer, owned by the EuroHPC Joint Undertaking, hosted by CSC (Finland) and the LUMI consortium through the Ministry of Education, Youth and Sports of the Czech Republic through the e-INFRA CZ (grant ID: 90254). M. P., M. L. and M. O. acknowledge that this research was co-funded by the European Union under the REFRESH-Research Excellence For Region Sustainability and High-tech Industries project number CZ.10.03.01/00/22_003/0000048 via the Operational Programme Just Transition.References
- M. Duca and M. T. M. Koper, Powering Denitrification: The Perspectives of Electrocatalytic Nitrate Reduction, Energy Environ. Sci., 2012, 5(12), 9726, 10.1039/c2ee23062c.
- P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Electrocatalytic Nitrate Reduction for Sustainable Ammonia Production, Joule, 2021, 5(2), 290–294, DOI:10.1016/j.joule.2020.12.025.
- L. Li, C. Tang, D. Yao, Y. Zheng and S.-Z. Qiao, Electrochemical Nitrogen Reduction: Identification and Elimination of Contamination in Electrolyte, ACS Energy Lett., 2019, 4(9), 2111–2116, DOI:10.1021/acsenergylett.9b01573.
- S. Li, X. Fu, J. K. Nørskov and I. Chorkendorff, Towards Sustainable Metal-Mediated Ammonia Electrosynthesis, Nat. Energy, 2024, 9(11), 1344–1349, DOI:10.1038/s41560-024-01622-7.
- S. Ye, Z. Chen, G. Zhang, W. Chen, C. Peng, X. Yang, L. Zheng, Y. Li, X. Ren, H. Cao, D. Xue, J. Qiu, Q. Zhang and J. Liu, Elucidating the Activity, Mechanism and Application of Selective Electrosynthesis of Ammonia from Nitrate on Cobalt Phosphide, Energy Environ. Sci., 2022, 15(2), 760–770, 10.1039/D1EE03097C.
- J. Wang, T. Feng, J. Chen, V. Ramalingam, Z. Li, D. M. Kabtamu, J.-H. He and X. Fang, Electrocatalytic Nitrate/Nitrite Reduction to Ammonia Synthesis Using Metal Nanocatalysts and Bio-Inspired Metalloenzymes, Nano Energy, 2021, 86, 106088, DOI:10.1016/j.nanoen.2021.106088.
- F. Haber and R. Le Rossignol, Über Die Technische Darstellung von Ammoniak Aus Den Elementen, Z. Elektrochem. Angew. Phys. Chem., 1913, 19(2), 53–72, DOI:10.1002/bbpc.19130190201.
- T. Kandemir, M. E. Schuster, A. Senyshyn, M. Behrens and R. Schlögl, The Haber–Bosch Process Revisited: On the Real Structure and Stability of “Ammonia Iron” under Working Conditions, Angew. Chem., Int. Ed., 2013, 52(48), 12723–12726, DOI:10.1002/anie.201305812.
- N. Hazari, Homogeneous Iron Complexes for the Conversion of Dinitrogen into Ammonia and Hydrazine, Chem. Soc. Rev., 2010, 39(11), 4044, 10.1039/b919680n.
- D. Ye and S. C. E. Tsang, Prospects and Challenges of Green Ammonia Synthesis, Nat. Synth., 2023, 2(7), 612–623, DOI:10.1038/s44160-023-00321-7.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, How a Century of Ammonia Synthesis Changed the World, Nat. Geosci., 2008, 1(10), 636–639, DOI:10.1038/ngeo325.
- G. Soloveichik, Electrochemical Synthesis of Ammonia as a Potential Alternative to the Haber–Bosch Process, Nat. Catal., 2019, 2(5), 377–380, DOI:10.1038/s41929-019-0280-0.
- B. H. R. Suryanto, H.-L. Du, D. Wang, J. Chen, A. N. Simonov and D. R. MacFarlane, Challenges and Prospects in the Catalysis of Electroreduction of Nitrogen to Ammonia, Nat. Catal., 2019, 2(4), 290–296, DOI:10.1038/s41929-019-0252-4.
- G. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C.-P. Chen, M. R. Smith and T. W. Hamann, Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia, Chem. Rev., 2020, 120(12), 5437–5516, DOI:10.1021/acs.chemrev.9b00659.
- D. Hao, Z. Chen, M. Figiela, I. Stepniak, W. Wei and B.-J. Ni, Emerging Alternative for Artificial Ammonia Synthesis through Catalytic Nitrate Reduction, J. Mater. Sci. Technol., 2021, 77, 163–168, DOI:10.1016/j.jmst.2020.10.056.
- Y. Wang, C. Wang, M. Li, Y. Yu and B. Zhang, Nitrate Electroreduction: Mechanism Insight, in Situ Characterization, Performance Evaluation, and Challenges, Chem. Soc. Rev., 2021, 50(12), 6720–6733, 10.1039/D1CS00116G.
- N. Hoang Truong, J.-S. Kim, J. Lim and H. Shin, Electrochemical Reduction of Nitrate to Ammonia: Recent Progress and Future Directions, Chem. Eng. J., 2024, 495, 153108, DOI:10.1016/j.cej.2024.153108.
- J. Wan, H. Zhang, J. Yang, J. Zheng, Z. Han, W. Yuan, B. Lan and X. Li, Synergy between Fe and Mo Single Atom Catalysts for Ammonia Electrosynthesis, Appl. Catal., B, 2024, 347, 123816, DOI:10.1016/j.apcatb.2024.123816.
- Y. Ren, S. You, Y. Wang, J. Yang and Y. Liu, Bioinspired Tandem Electrode for Selective Electrocatalytic Synthesis of Ammonia from Aqueous Nitrate, Environ. Sci. Technol., 2024, 58(4), 2144–2152, DOI:10.1021/acs.est.3c09759.
- G. Zhang, X. Li, K. Chen, Y. Guo, D. Ma and K. Chu, Tandem Electrocatalytic Nitrate Reduction to Ammonia on MBenes, Angew. Chem., Int. Ed., 2023, 62(13), e202300054, DOI:10.1002/anie.202300054.
- Y. Xiong, Y. Wang, J. Zhou, F. Liu, F. Hao and Z. Fan, Electrochemical Nitrate Reduction: Ammonia Synthesis and the Beyond, Adv. Mater., 2024, 36(17), 2304021, DOI:10.1002/adma.202304021.
- J. Lim, C.-Y. Liu, J. Park, Y.-H. Liu, T. P. Senftle, S. W. Lee and M. C. Hatzell, Structure Sensitivity of Pd Facets for Enhanced Electrochemical Nitrate Reduction to Ammonia, ACS Catal., 2021, 11(12), 7568–7577, DOI:10.1021/acscatal.1c01413.
- D. Liu, L. Qiao, S. Peng, H. Bai, C. Liu, W. F. Ip, K. H. Lo, H. Liu, K. W. Ng, S. Wang, X. Yang and H. Pan, Recent Advances in Electrocatalysts for Efficient Nitrate Reduction to Ammonia, Adv. Funct. Mater., 2023, 33(43), 2303480, DOI:10.1002/adfm.202303480.
- W. Chen, X. Yang, Z. Chen, Z. Ou, J. Hu, Y. Xu, Y. Li, X. Ren, S. Ye, J. Qiu, J. Liu and Q. Zhang, Emerging Applications, Developments, Prospects, and Challenges of Electrochemical Nitrate-to-Ammonia Conversion, Adv. Funct. Mater., 2023, 33(29), 2300512, DOI:10.1002/adfm.202300512.
- I. M. u. Hasan, N. Xu, Y. Liu, M. Z. Nawaz, H. Feng and J. Qiao, Noble and Non-Noble Metal Based Catalysts for Electrochemical Nitrate Reduction to Ammonia: Activity, Selectivity and Stability, Electrochem. Energy Rev., 2024, 7(1), 36, DOI:10.1007/s41918-024-00236-7.
- Z.-L. Xie, D. Wang and X.-Q. Gong, Theoretical Insights into Nitrate Reduction to Ammonia over Pt/TiO2 : Reaction Mechanism, Activity Regulation, and Catalyst Design, ACS Catal., 2022, 12(16), 9887–9896, DOI:10.1021/acscatal.2c01694.
- Y. Wang, W. Zhou, R. Jia, Y. Yu and B. Zhang, Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia, Angew. Chem., Int. Ed., 2020, 59(13), 5350–5354, DOI:10.1002/anie.201915992.
- F. Zhao, G. Hai, X. Li, Z. Jiang and H. Wang, Enhanced Electrocatalytic Nitrate Reduction to Ammonia on Cobalt Oxide Nanosheets via Multiscale Defect Modulation, Chem. Eng. J., 2023, 461, 141960, DOI:10.1016/j.cej.2023.141960.
- J. Choi, S. Im, J. Choi, S. Surendran, D. J. Moon, J. Y. Kim, J. K. Kim and U. Sim, Recent Advances in 2D Structured Materials with Defect-Exploiting Design Strategies for Electrocatalysis of Nitrate to Ammonia, Energy Mater., 2024, 4(2), 400020, DOI:10.20517/energymater.2023.67.
- X. Zhang, Y. Wang, C. Liu, Y. Yu, S. Lu and B. Zhang, Recent Advances in Non-Noble Metal Electrocatalysts for Nitrate Reduction, Chem. Eng. J., 2021, 403, 126269, DOI:10.1016/j.cej.2020.126269.
- H. Zhang, H. Wang, X. Cao, M. Chen, Y. Liu, Y. Zhou, M. Huang, L. Xia, Y. Wang, T. Li, D. Zheng, Y. Luo, S. Sun, X. Zhao and X. Sun, Unveiling Cutting-Edge Developments in Electrocatalytic Nitrate-to-Ammonia Conversion, Adv. Mater., 2024, 36(16), 2312746, DOI:10.1002/adma.202312746.
- H. Xu, Y. Ma, J. Chen, W. Zhang and J. Yang, Electrocatalytic Reduction of Nitrate – a Step towards a Sustainable Nitrogen Cycle, Chem. Soc. Rev., 2022, 51(7), 2710–2758, 10.1039/D1CS00857A.
- M. Sokol, V. Natu, S. Kota and M. W. Barsoum, On the Chemical Diversity of the MAX Phases, Trends Chem., 2019, 1(2), 210–223, DOI:10.1016/j.trechm.2019.02.016.
- Md. S. Alam, M. A. Chowdhury, T. Khandaker, M. S. Hossain, M. S. Islam, M. M. Islam and M. K. Hasan, Advancements in MAX Phase Materials: Structure, Properties, and Novel Applications, RSC Adv., 2024, 14(37), 26995–27041, 10.1039/D4RA03714F.
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Fundamentals of MXene Synthesis, Nat. Synth., 2022, 1(8), 601–614, DOI:10.1038/s44160-022-00104-6.
- S. Kumar, Fluorine-Free MXenes: Recent Advances, Synthesis Strategies, and Mechanisms, Small, 2024, 20(16), 2308225, DOI:10.1002/smll.202308225.
- S. Nouseen, S. Deshmukh and M. Pumera, Nanoarchitectonics of Laser Induced MAX 3D-Printed Electrode for Photo-Electrocatalysis and Energy Storage Application with Long Cyclic Durability of 100 000 Cycles, Adv. Funct. Mater., 2024, 34(45), 2407071, DOI:10.1002/adfm.202407071.
- Z. M. Sun, Progress in Research and Development on MAX Phases: A Family of Layered Ternary Compounds, Int. Mater. Rev., 2011, 56(3), 143–166, DOI:10.1179/1743280410Y.0000000001.
- S. Huang, Z. Wang, Y. Von Lim, Y. Wang, Y. Li, D. Zhang and H. Y. Yang, Recent Advances in Heterostructure Engineering for Lithium–Sulfur Batteries, Adv. Energy Mater., 2021, 11(10), 2003689, DOI:10.1002/aenm.202003689.
- P. Wang, K. Jiang, G. Wang, J. Yao and X. Huang, Phase and Interface Engineering of Platinum–Nickel Nanowires for Efficient Electrochemical Hydrogen Evolution, Angew. Chem., 2016, 128(41), 13051–13055, DOI:10.1002/ange.201606290.
- J. Lv, X. Sun, F. Wang, R. Yang, T. Zhang, T. Liang, W. Rong, Q. Yang, W. Xue, L. Wang, X. Xu and Y. Liu, Engineering Nickel Dopants in Atomically Thin Molybdenum Disulfide for Highly Efficient Nitrate Reduction to Ammonia, Adv. Funct. Mater., 2024, 34(49), 2411491, DOI:10.1002/adfm.202411491.
- J. Lin, Z. Peng, Y. Liu, F. Ruiz-Zepeda, R. Ye, E. L. G. Samuel, M. J. Yacaman, B. I. Yakobson and J. M. Tour, Laser-Induced Porous Graphene Films from Commercial Polymers, Nat. Commun., 2014, 5(1), 5714, DOI:10.1038/ncomms6714.
- L. X. Duy, Z. Peng, Y. Li, J. Zhang, Y. Ji and J. M. Tour, Laser-Induced Graphene Fibers, Carbon, 2018, 126, 472–479, DOI:10.1016/j.carbon.2017.10.036.
- R. Ye, D. K. James and J. M. Tour, Laser-Induced Graphene: From Discovery to Translation, Adv. Mater., 2019, 31(1), 1803621, DOI:10.1002/adma.201803621.
- R. Ye, D. K. James and J. M. Tour, Laser-Induced Graphene, Acc. Chem. Res., 2018, 51(7), 1609–1620, DOI:10.1021/acs.accounts.8b00084.
- J. Chen, M. Chen, W. Zhou, X. Xu, B. Liu, W. Zhang and C. Wong, Simplified Synthesis of Fluoride-Free Ti3C2Tx via Electrochemical Etching toward High-Performance Electrochemical Capacitors, ACS Nano, 2022, 16(2), 2461–2470, DOI:10.1021/acsnano.1c09004.
- C. Wang, H. Shou, S. Chen, S. Wei, Y. Lin, P. Zhang, Z. Liu, K. Zhu, X. Guo, X. Wu, P. M. Ajayan and L. Song, HCl-Based Hydrothermal Etching Strategy toward Fluoride-Free MXenes, Adv. Mater., 2021, 33(27), 2101015, DOI:10.1002/adma.202101015.
- Z. U. Din Babar, J. Fatheema, N. Arif, M. S. Anwar, S. Gul, M. Iqbal and S. Rizwan, Magnetic Phase Transition from Paramagnetic in Nb2 AlC-MAX to Superconductivity-like Diamagnetic in Nb2 C-MXene: An Experimental and Computational Analysis, RSC Adv., 2020, 10(43), 25669–25678, 10.1039/D0RA04568C.
- B. Sindhu, A. Kothuru, P. Sahatiya, S. Goel and S. Nandi, Laser-Induced Graphene Printed Wearable Flexible Antenna-Based Strain Sensor for Wireless Human Motion Monitoring, IEEE Trans. Electron Devices, 2021, 68(7), 3189–3194, DOI:10.1109/TED.2021.3067304.
- T. Li, G. Nam, K. Liu, J.-H. Wang, B. Zhao, Y. Ding, L. Soule, M. Avdeev, Z. Luo, W. Zhang, T. Yuan, P. Jing, M. G. Kim, Y. Song and M. Liu, A Niobium Oxide with a Shear Structure and Planar Defects for High-Power Lithium Ion Batteries, Energy Environ. Sci., 2022, 15(1), 254–264, 10.1039/D1EE02664J.
- L. Gao, H. Chen, F. Zhang, S. Mei, Y. Zhang, W. Bao, C. Ma, P. Yin, J. Guo, X. Jiang, S. Xu, W. Huang, X. Feng, F. Xu, S. Wei and H. Zhang, Ultrafast Relaxation Dynamics and Nonlinear Response of Few-Layer Niobium Carbide MXene, Small Methods, 2020, 4(8), 2000250, DOI:10.1002/smtd.202000250.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows, J. Am. Chem. Soc., 2017, 139(45), 16235–16247, DOI:10.1021/jacs.7b07818.
- N. J. Lane, M. Naguib, V. Presser, G. Hug, L. Hultman and M. W. Barsoum, First-order Raman Scattering of the MAX Phases Ta4AlC3, Nb4AlC3, Ti4AlN3, and Ta2AlC, J. Raman Spectrosc., 2012, 43(7), 954–958, DOI:10.1002/jrs.3101.
- W. Li, D. Peng, W. Huang, X. Zhang, Z. Hou, W. Zhang, B. Lin and Z. Xing, Adjusting Coherence Length of Expanded Graphite by Self-Activation and Its Electrochemical Implication in Potassium Ion Battery, Carbon, 2023, 204, 315–324, DOI:10.1016/j.carbon.2022.12.072.
- J. Wang, S. Yang, C. Xiao, Z. Yu, R. Ren and X. Xiong, Effects of Atmospheric Pressure Plasma Treatment and Its Aging Behaviors on Interfacial Strength of PI/PEEK Composite Film, J. Appl. Polym. Sci., 2025, 142(3), e56369, DOI:10.1002/app.56369.
- P. Arunkumar, A. G. Ashish, B. Babu, S. Sarang, A. Suresh, C. H. Sharma, M. Thalakulam and M. M. Shaijumon, Nb 2 O 5/Graphene Nanocomposites for Electrochemical Energy Storage, RSC Adv., 2015, 5(74), 59997–60004, 10.1039/C5RA07895D.
- X. Wu, W. Hu, J. Qiu, B. Geng, M. Du and Q. Zheng, Solvent-Assisted Self-Assembly to Fabricate a Ternary Flexible Free-Standing Polyaniline@MXene-CNTs Electrode for High-Performance Supercapacitors, J. Alloys Compd., 2022, 921, 166062, DOI:10.1016/j.jallcom.2022.166062.
- H. Dong, P. Xiao, N. Jin, B. Wang, Y. Liu and Z. Lin, Molten Salt Derived Nb2CTxMXene Anode for Li-ion Batteries, ChemElectroChem, 2021, 8(5), 957–962, DOI:10.1002/celc.202100142.
- M. Sanna, K. A. Novčić, S. Ng, M. Černý and M. Pumera, The Unexpected Photoelectrochemical Activity of MAX Phases: The Role of Oxide Impurities, J. Mater. Chem. A, 2023, 11(6), 3080–3090, 10.1039/D2TA06929F.
- X. Zhang, J. Wang, X. Wang, Y. Li, Y. Zhao, Z. Bakenov and G. Li, 3D Ordered Macroporous Amorphous Nb2O5 as Anode Material for High-Performance Sodium-Ion Batteries, Appl. Surf. Sci., 2021, 567, 150862, DOI:10.1016/j.apsusc.2021.150862.
- A. C. Nielander, J. M. McEnaney, J. A. Schwalbe, J. G. Baker, S. J. Blair, L. Wang, J. G. Pelton, S. Z. Andersen, K. Enemark-Rasmussen, V. Čolić, S. Yang, S. F. Bent, M. Cargnello, J. Kibsgaard, P. C. K. Vesborg, I. Chorkendorff and T. F. Jaramillo, A Versatile Method for Ammonia Detection in a Range of Relevant Electrolytes via Direct Nuclear Magnetic Resonance Techniques, ACS Catal., 2019, 9(7), 5797–5802, DOI:10.1021/acscatal.9b00358.
- T. Hu, M. Wang, C. Guo and C. M. Li, Functionalized MXenes for Efficient Electrocatalytic Nitrate Reduction to Ammonia, J. Mater. Chem. A, 2022, 10(16), 8923–8931, 10.1039/D2TA00470D.
- W. Gao, J. V. Perales-Rondon, J. Michalička and M. Pumera, Ultrathin Manganese Oxides Enhance the Electrocatalytic Properties of 3D Printed Carbon Catalysts for Electrochemical Nitrate Reduction to Ammonia, Appl. Catal., B, 2023, 330, 122632, DOI:10.1016/j.apcatb.2023.122632.
- G. Kresse and J. Furthmüller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 11169–11186, DOI:10.1103/PhysRevB.54.11169.
- G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Comput. Mater. Sci., 1996, 6(1), 15–50, DOI:10.1016/0927-0256(96)00008-0.
- P. E. Blöchl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953–17979, DOI:10.1103/PhysRevB.50.17953.
- G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(3), 1758–1775, DOI:10.1103/PhysRevB.59.1758.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865 ( Phys. Rev. Lett. , 1997 , 78 , 1396 ) CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu, J. Chem. Phys., 2010, 132(15), 154104, DOI:10.1063/1.3382344.
- K. Mathew, R. Sundararaman, K. Letchworth-Weaver, T. A. Arias and R. G. Hennig, Implicit Solvation Model for Density-Functional Study of Nanocrystal Surfaces and Reaction Pathways, J. Chem. Phys., 2014, 140(8), 084106, DOI:10.1063/1.4865107.
- H. J. Monkhorst and J. D. Pack, Special Points for Brillouin-Zone Integrations, Phys. Rev. B, 1976, 13(12), 5188–5192, DOI:10.1103/PhysRevB.13.5188.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode, J. Phys. Chem. B, 2004, 108(46), 17886–17892, DOI:10.1021/jp047349j.
- V. Wang, N. Xu, J.-C. Liu, G. Tang and W.-T. Geng, VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code, Comput. Phys. Commun., 2021, 267, 108033, DOI:10.1016/j.cpc.2021.108033.
- J.-X. Liu, D. Richards, N. Singh and B. R. Goldsmith, Activity and Selectivity Trends in Electrocatalytic Nitrate Reduction on Transition Metals, ACS Catal., 2019, 9(8), 7052–7064, DOI:10.1021/acscatal.9b02179.
- X. Gao and E. C. M. Tse, Unraveling the Performance Descriptors for Designing Single-Atom Catalysts on Defective MXenes for Exclusive Nitrate-To-Ammonia Electrocatalytic Upcycling, Small, 2024, 20(11), 2306311, DOI:10.1002/smll.202306311.
- J. Hu, S. Osella, E. A. dos Reis, A. B. da Silva, C. Ribeiro, L. H. Mascaro, J. Albero and H. Garcia, Mixed Fe-Mo Carbide Prepared by a Sonochemical Synthesis as Highly Efficient Nitrate Reduction Electrocatalyst, Appl. Catal., B, 2024, 357, 124247, DOI:10.1016/j.apcatb.2024.124247.
- L. Li, X. Wang, H. Guo, G. Yao, H. Yu, Z. Tian, B. Li and L. Chen, Theoretical Screening of Single Transition Metal Atoms Embedded in MXene Defects as Superior Electrocatalyst of Nitrogen Reduction Reaction, Small Methods, 2019, 3(11), 1900337, DOI:10.1002/smtd.201900337.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ta02418h |
| This journal is © The Royal Society of Chemistry 2025 |