Open Access Article
Open Access ArticleThe untold story of starch as a catalyst for organic reactions†
Masoud Sadeghi
Department of Organic Chemistry, Faculty of Chemistry, University of Kashan, P.O. Box: 87317-51167, Kashan, Iran. E-mail: masoud.sadeghii@yahoo.com
First published on 19th April 2024
Abstract
Starch is one of the members of the polysaccharide family. This biopolymer has shown many potential applications in different fields such as catalytic reactions, water treatment, packaging, and food industries. In recent years, using starch as a catalyst has attracted much attention. From a catalytic point of view, starch can be used in organic chemistry reactions as a catalyst or catalyst support. Reports show that as a catalyst, simple starch can promote many heterocyclic compound reactions. On the other hand, functionalized starch is not only capable of advancing the synthesis of heterocycles but also is a good candidate catalyst for other reactions including oxidation and coupling reactions. This review tries to provide a fair survey of published organic reactions which include using starch as a catalyst or a part of the main catalyst. Therefore, the other types of starch applications are not the subject of this review.
1. A little bit about starch
Starch or amylum is the second most abundant natural polysaccharide on earth in terms of quantity after cellulose.1 Traditionally, this biopolymer has been used in the food industry, however, thanks to the advancement of technology and increasing research, starch is not limited only to the food industry anymore, and there are many other industries like textiles and paper, chemicals, agriculture, medicine, and engineering industries that benefit starch and its applications.2As the most common carbohydrate in the human diet3 and the main storing carbohydrate in many plants like vascular plants,4 this polymeric polyol can be found in many natural sources including grains (wheat, corn, rice, rye, barley, beans, peas, and lentil), fruit (banana peel, apple pulp, kiwi seeds, avocado, pineapple, and mango), plants (sorghum, cassava, potato, sweet potato, taro, amaranth, sago palm), and many other natural sources.1 Despite the numerous natural sources of starch, about 80% of the global starch production comes from Zea mays (corn).2
From a chemical point of view, starch (C6H10O5) is a white and tasteless powder that is insoluble in cold water, but if the water is heated beyond the boiling point in a closed container, it can be dissolved.5 This hydrocarbon polymer consists of α-D-glucopyranose units as monomers joined together through α-1,4-glycosidic bonds. Although the α-1,4-glycosidic bonds are the main way of connecting monomeric units and making the chain, there are other common bonds like α-1,6-glycosidic bonds which are responsible for making the connection between chains and creating branches.6
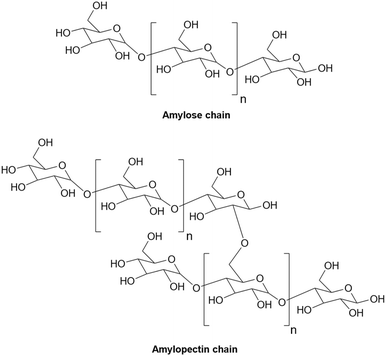
Starch is mainly made of two different types of molecules, i.e., amylose (linear and helical structure) and amylopectin (branched structure) (Fig. 1)7 that are responsible for about 20–30% and 70–80% of the natural starch composition, respectively.8 The chemical structure of starch makes this low-cost natural polymer a wonderful compound with many significant features including biodegradability, safe and convenient usage, annual renewability, and ease of availability.8 As a polyol, starch has many hydroxyl groups in its structure which can create a lot of intra and intermolecular hydrogen bonds. The presence of many hydroxyl groups makes starch suitable for many purposes. For example, starch can be converted into many valuable derivatives. Literature review9–11 shows that starch can undergo many reactions similar to alcohols including esterification,12,13 etherification,14,15 oxidation,16,17 cross-linking,18,19 sulfonation,20,21 and so on (Fig. 2). Also, the presence of glycosidic bonds in the structure of starch makes it susceptible to become hydrolyzed under alkaline, acidic, or enzymatic conditions.22–24
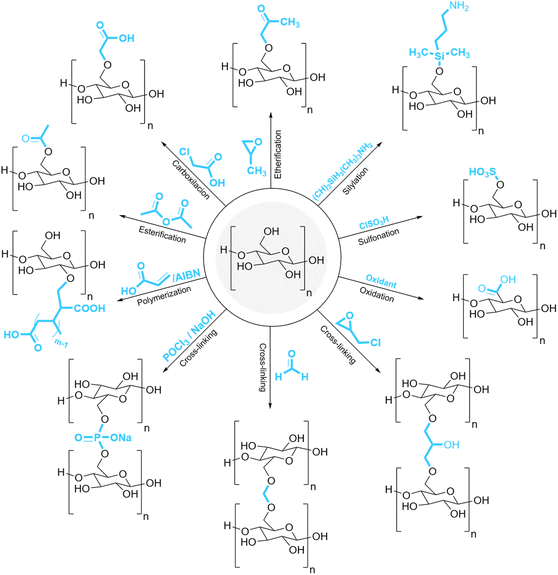 | ||
| Fig. 2 Some of the common modification reactions for starch.1,50 | ||
In addition to transformations such as depolymerization and modification of the hydroxyl groups on the surface of starch, this polysaccharide can undergo more drastic changes to produce many diverse products (Fig. 3). For instance, the thermal dehydration of starch opens the doors to many carbon-based materials such as porous carbon materials, composite carbon materials, and carbon nanomaterials. Also, starch can be a substrate for many small organic molecules especially industrially manufactured products including 5-hydroxymethylfurfural, levulinic acid, lactic acid, acetone, butanol, ethanol, etc.7
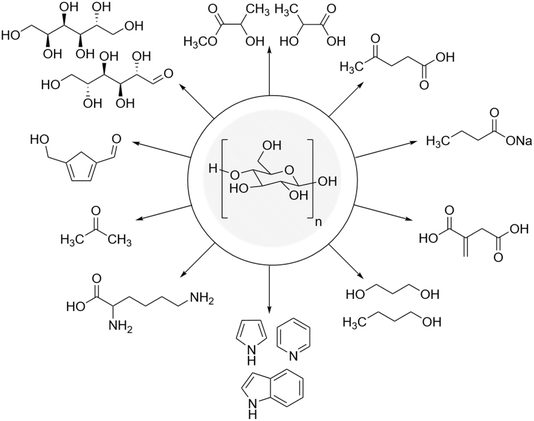 | ||
| Fig. 3 Some of the important compounds made from starch.7 | ||
Apart from the importance of starch as a substrate for the preparation of valuable products, this natural polymer or its slightly modified derivatives can be used as great tools for other purposes.25–31 For example, starch with many hydroxyl groups can act like a molecular octopus with many tentacles to remove environmental pollutants such as heavy metals,32 dyes,33 pesticides,34 anti-biotics,35 and oils.36 Also, there are many reports about starch-based material application as biodegradable and renewable alternatives for current widely used non-renewable resins.37
Besides the application of starch and starch-based materials in the mentioned fields, using starch or modified starch as catalysts has attracted growing attention.38–40 Regarding the increasing number of published reports on the use of starch as a catalyst, it is necessary to write a review about the application of starch in organic reactions. To the best of my knowledge, there is no related review about the role of starch as a catalyst in organic reactions and this one would be the first of its kind. This review aims to comprehensively examine organic reactions that have been carried out using starch or its derivatives as catalysts and categorize them based on the type and structure of the employed starch.
2. Starch as catalyst for organic reactions
Depending on the type and application of the catalyst, there are different mentioned properties for a suitable catalyst in scientific literature. However, for a heterogenous catalyst, we can point out properties like cost, toxicity, stability and durability, volumetric efficiency (good surface area and available active sites), activity (promoting the desired reaction with good yield at a sensible temperature and pressure), selectivity, recoverability and reusability.41 As a heterogeneous candidate catalyst, starch can fulfill many properties expected of a suitable catalyst. For example, it is cheap, non-toxic, environmentally friendly, reusable, and easy to access and apply, making this biopolymer worth exploring for different organic reactions. Also, with many hydroxyl groups on the surface of this member of the carbohydrate family, it is possible to convert starch to desired functionalized derivatives that can even widen the application of starch as a catalyst.Regarding the fact that there is no such thing as the perfect catalyst, it is not a big surprise that in addition to the many advantages of starch as a heterogeneous catalyst, there are also some disadvantages. Starch is a hygroscopic substance by nature, so it can absorb up to 17% of moisture.42 This means that at least naive starch can not be a good catalyst candidate for water-sensitive reactions. Moreover, naive or simple starch is not capable of coping with some conditions like high temperature, a wide range of pH, or freeze–thaw cycles.42 Starch swells when heated in water. As the temperature of heated starch in water reaches the “gelatinization temperature” which depends on the characteristics of its source, swollen starch loses its semi-crystalline structure. When the increasing temperature goes beyond that temperature, the structural integrity decreases. Eventually, this leads the starch structure to break down into its constituent molecules, including amylose, amylopectin, and some other minor components.43 These limitations combined with a natural tendency of naive starch for decomposition and brittleness make it not to be desirable for every reaction condition. However, chemical modification can overcome some of the limitations of simple starch and make it possible to use this inexpensive and environmentally friendly catalyst in reactions with different conditions.
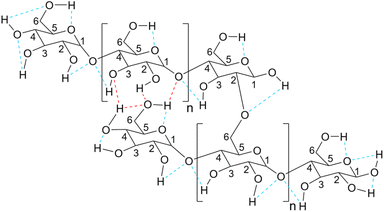
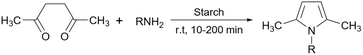
As mentioned before, starch in simple or chemically modified forms can be used for the desired reaction as a catalyst or catalyst support. To describe the mechanism of the action of starch as a catalyst in organic transformations we should resort to non-covalent interactions (NCIs).44 For the simple starch, the structural hydrogen bonding interactions are the major NCIs that can promote the conversion of starting materials to the desired products. As a polyhydroxy compound, each ring in the structure of starch has three hydroxyl groups (at C2, C4, and C6) that can make hydrogen bondings with active sites on substrate molecules and facilitate their transformations to the related products (Fig. 4).45 For example, Arabpourian and Behbahani proposed an overall reaction mechanism for the synthesis of pyrrole using starch as a heterogeneous catalyst (Fig. 5).46 The authors have shown how starch hydrogen bonding guides the starting materials to the desired product. In the first step, the diketone's carbonyl group is activated by starch's OH-groups to obtain intermediate 1. This hydrogen bonding makes the carbonyl group ready to be attacked by an amine as a nucleophile to produce intermediate 2. The second carbonyl activated by starch hydrogen bonding experiences an intramolecular amine nucleophilic attack which results in intermediate 3. Finally, the desired product 4 is obtained after the dehydration of intermediate 3.
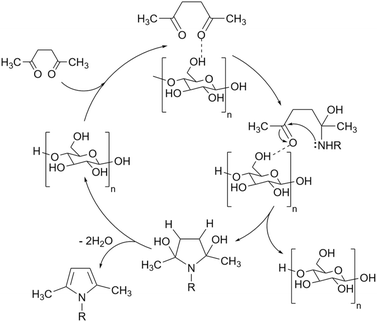 | ||
| Fig. 5 The proposed mechanism for the synthesis of N-substituted pyrroles using starch as a catalyst.46 | ||
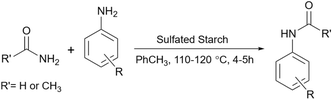
Similar to other polysaccharides,47 the hydroxyl groups on the surface of simple starch can be modified and attached to other functional groups to increase the ability of starch and accelerate organic reactions.48 For example, simple starch can be sulfonated using sulfonating agents (sulfuric acid, chlorosulfonic acid, and sulfur trioxide) to convert starch to a suitable brøsnted acid.21 As another instance, attaching amine groups to these hydroxyl groups by appropriate linkers to prepare related Schiff bases makes starch capable of being like molecular pincers holding metal ions as Lewis acid catalysts.49 Depending on the type of chemical functionalization on the surface of starch, the way that these modified starch can promote the reaction will be different but the important point is that the hydrogen bonds of starch with substrates are still considerable. This means when we have an acidic/basic or metal-loaded starch, even if the main driving force from the catalyst is coming from added parts (acid, base, or metal) to the starch, the effect of starch itself to promote the reaction still exists.
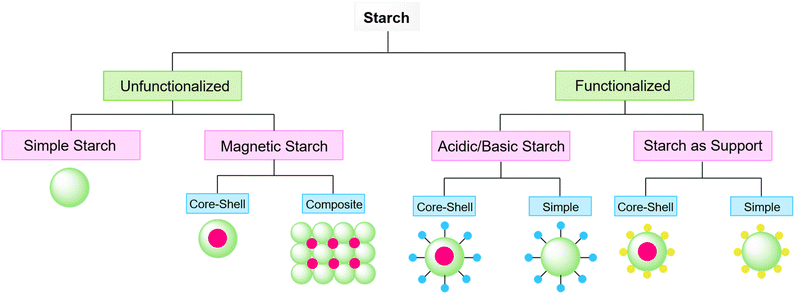
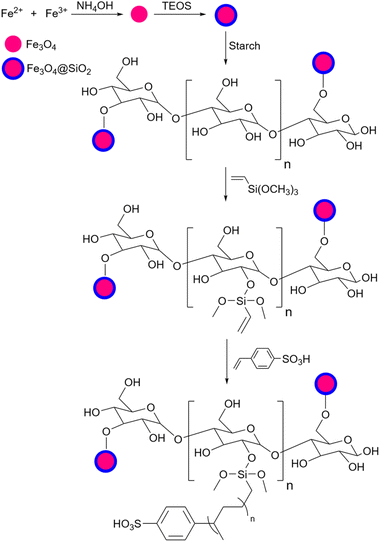
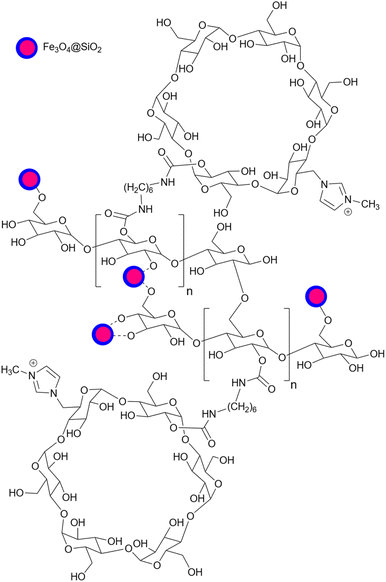
Based on published papers about using starch or modified starch as a catalyst for organic transformations, they can be placed in two categories: (1) unfunctionalized and (2) functionalized starch. The first type of starch is related to the ones with no surface functionalization which includes either simple starch or magnetic/composite starch. The second one indicates catalysts with chemical surface functionalization including acidic/basic, and metal-loaded starch. From the structural point of view, these two types of starch can have a simple or complex structure like a core–shell structure where starch is wrapped around a specific core usually a magnetic one, functionalized with desired groups (Fig. 6). In the following sections, this review deals with the reactions that employed simple and functionalized starch as a catalyst or catalyst support.
2.1 Simple starch
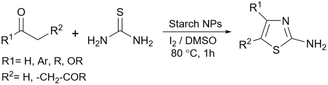
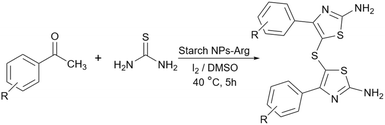
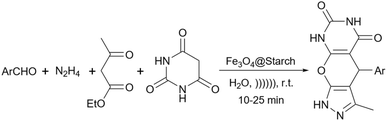
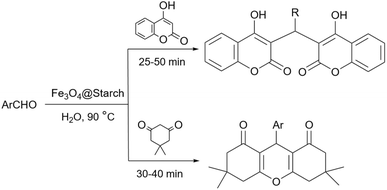
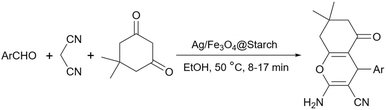
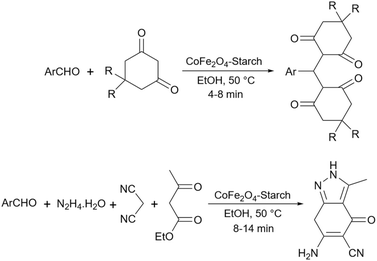
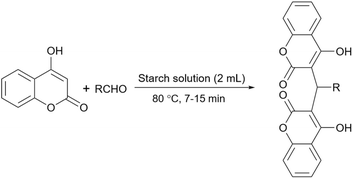
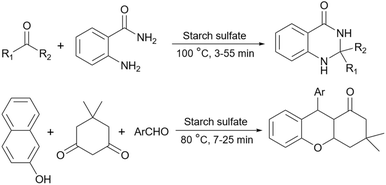
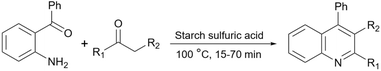
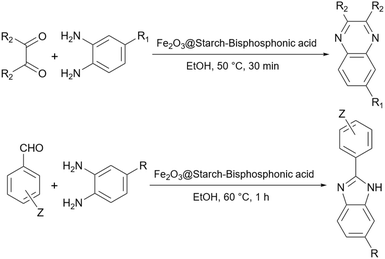
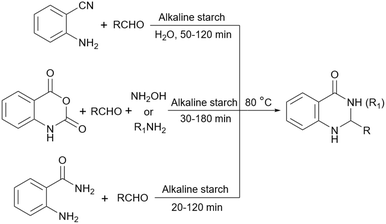
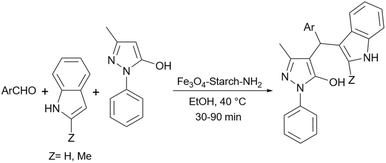
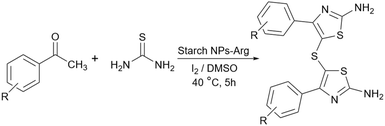
Simple or raw starch without any chemical modification could be used directly as a catalyst for the synthesis of many heterocyclic compounds. Published reports show that this cheap polysaccharide has been successfully used as a catalyst for the preparation of pyrrole, 2-aminothiazole, dihydropyranopyrazole, tetrahydrobenzopyran, and dihydropyranochromene derivatives. In the following, these reactions will be discussed.In search for new capabilities of starch as catalyst in the preparation of 2-aminothiazole derivatives, two years later our group succeeded in finding a similar protocol to produce some diheteroaryl thioether derivatives containing 2-aminothiazole units (Scheme 3).60 Compared to our previous protocol for the synthesis of 2-aminothiazoles, this one proceeded at a much slower speed and a lower temperature.
2.2 Magnetic starch
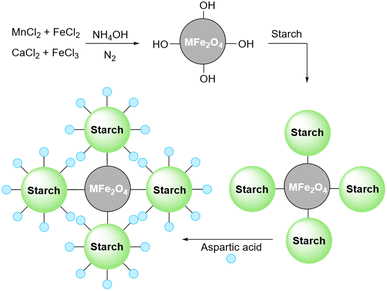
The recovery of the catalyst after the end of the reaction is one of the important problems with using simple starch as a catalyst in organic reactions. However, there are some solutions to fix this weakness. One of them is magnetizing the simple starch using iron oxide particles. The papers that used this technique will be presented, in the next sections.2.3 Acidic starch
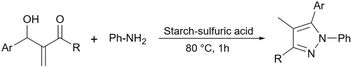
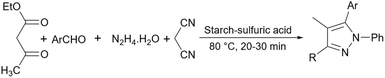
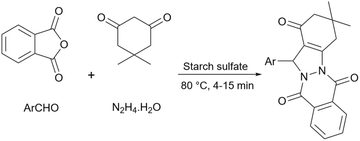
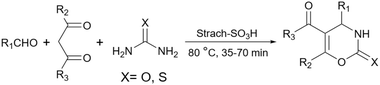
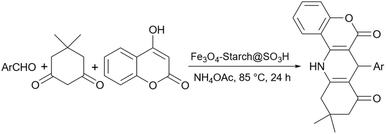
Simple starch has many hydroxyl groups and is capable of helping the reaction process through hydrogen bondings with substrates and intermediates, but sometimes these groups are not effective enough to help the reaction. Therefore, the functionalization of simple starch by acidic groups is used to increase the ability of starch to create more hydrogen bonds and the activation of substrates. The related papers will be discussed in the coming parts.To prepare bis-coumarins commonly known as coumarin derivatives (Scheme 11), Rezaei and Sheikhi used starch-sulfuric acid as a catalyst. Instead of modifying starch with linkers and attaching the sulfonate group to the linker,79 they just synthesized starch-sulfuric acid by adding chlorosulfonic acid to the starch in n-hexane as solvent at 0 °C (Scheme 12).80 In their provided protocol, the transformation of aldehydes and coumarins as substrates to the corresponding bis-coumarin derivatives involves a Knoevenagel reaction followed by a Michael addition which both reactions can be facilitated by Brønsted acids like sulfuric acid.
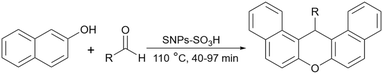
In 2017, our team at the University of Kashan proposed an effective procedure for the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes using sulfonated starch nanoparticles as a promising heterogeneous catalyst (Scheme 17).90 The reaction proceeded in a solvent-free condition at 110 °C with satisfactory yields for a wide range of substrates. To prepare starch nanoparticles, the bulk starch went through a two-step process. In the first step starch was treated with acid and then base under an argon atmosphere and rigorous stirring. Then, potassium persulfate was added to the resulting mixture, and in the end, the starch nanoparticle was separated from the solution using an ice bath.
2.4 Basic starch
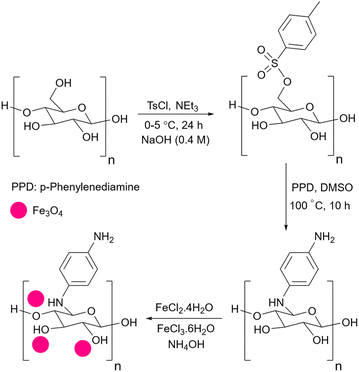
Although simple starch can act like a Brønsted acid to advance the reaction this is not very helpful for those reactions that need basic catalysts. To use starch as a catalyst for these types of reactions, researchers have used basic agents including NaOH, and p-phenylenediamine (PPD) to give basic properties to the starch. The papers in which basic starch has been used as a catalyst will be presented in the next sections.2.5 Starch as support
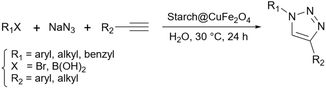
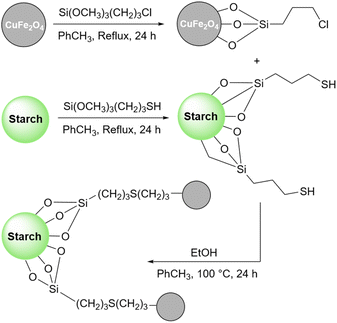
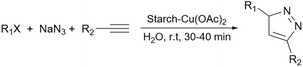
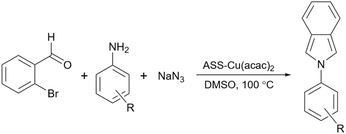
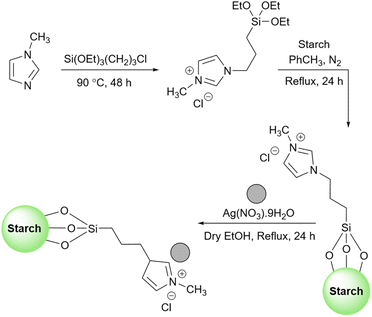
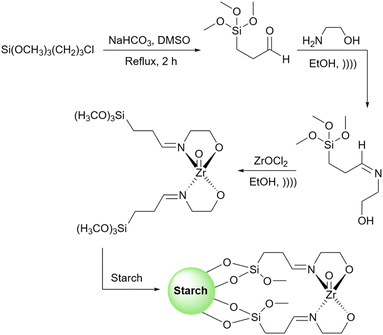
As mentioned in previous sections, simple starch can be used for many organic reactions, however, there are still some reactions including coupling, reduction, and oxidation, and some heterocyclic reactions that simple starch can not cope with. For this reaction, researchers have used starch as catalyst support to help the catalyst increase the reaction yield of the reaction. These catalytic systems will be introduced in the coming parts.In 2018, Bonyasi et al. proposed starch@CuFe2O4 as a magnetic reusable catalyst for the synthesis of 1,2,3-triazoles from benzyl bromide, sodium azide, and phenylacetylene (Scheme 32).124 To attach the prepared CuFe2O4 nanoparticles to the surface of starch, they functionalized the nanoparticles and starch using (3-chloropropyl)triethoxysilane and (3-mercaptopropyl)triethoxysilane, respectively (Scheme 33).
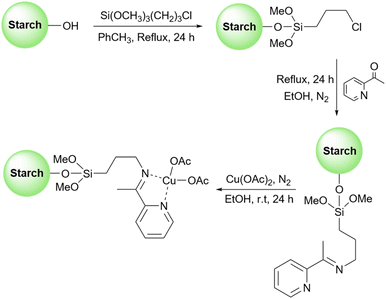
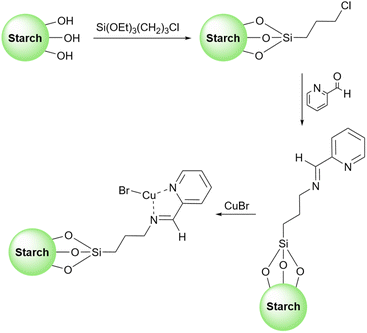
Inspired by previous work, very recently, Gupta et al. used surface functionalized starch with copper(II) acetate as a heterogeneous catalyst to promote the synthesis of 1,4-disubstituted-1,2,3-triazoles (Scheme 34).125 To stabilize the copper salt on the surface of the starch, they modified the surface of the starch with a Schiff base. To do this, first, they attached a 3-aminopropyl(trimethoxy)silane molecule to the starch and then converted the amine group on this molecule to the corresponding imine by adding 2-acetylpyridine to the prepared 3-aminopropyl starch (Scheme 35). Based on the proposed mechanism by authors for the synthesis of triazoles, the key step in this transformation is the reduction of Cu(II) to Cu(I) which facilitates the connection between an azide and terminal alkyne substrates to produce the related triazole derivatives.
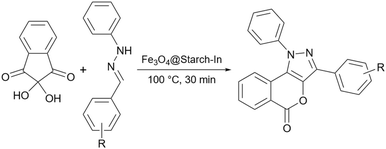
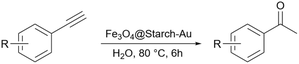
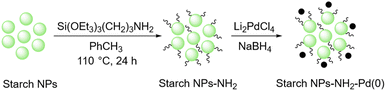
In 2019, Gholinejad and colleagues prepared starch support containing gold nanoparticles for the reduction of nitroarenes to their corresponding amines using NaBH4 as the reducing agent.146 To stabilize the gold nanoparticle on the surface of starch they modified the surface of starch with (3-chloropropyl)triethoxysilane so that it can act as a linker and hold the gold nanoparticles.
As another example of converting of nitroaromatic compounds to the corresponding amines, Nasiri et al. prepared and employed nanocomposites containing Fe3O4-starch-Cu nanoparticles as catalysts.147 The authors also tested their catalyst for the reduction of some dyes such as Congo red, rhodamine B, and methylene blue and the results were promising. Recently, Heravi's research group published another protocol for the hydrogenation of nitroaromatics using sulfur-doped graphitic carbon nitride decorated with starch, Fe3O4, and Ag.148 They managed to proceed with the reaction in aqueous media with excellent yield for many different nitrobenzene derivatives. Very recently, Lan et al. showed the potential catalytic activity of kaolin@chitosan-starch-Au for the reduction of nitrobenzene derivatives under mild conditions using sodium borohydride as a reductant.149
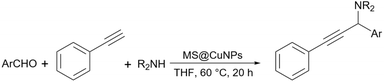
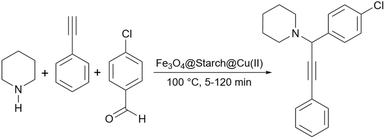
In the second report about using starch as support for the synthesis of propargylamines, Saadati et al. presented a modified starch with a linker to stabilize copper nanoparticles and use this copper-loaded support as a catalyst for the synthesis of propargylamine derivatives.152 Very recently, Tajbakhsh and co-workers provided a protocol for the synthesis of propargylamines through a one-pot coupling reaction using copper(II)-immobilized on starch-coated nanomagnetite (Scheme 43).153 Based on the optimization data reported by the authors, the simple starch can proceed with this reaction with 70% yield, an efficiency even better than simple Fe3O4. However, when Fe3O4@starch-Acr@Cu(II) was used as the catalyst, the yield of the reaction increased to 99% and the consuming time for the reaction to be complete decreased to about half an hour. The authors proposed a plausible mechanism in which Cu(II) is first coordinated with π electrons of acetylene to produce the acetylide-Cu(II) complex. In this condition, copper promotes the attack of acetylide on the reactive iminium intermediate to obtain the desired propargylamine.
As mentioned earlier, 5-hydroxymethylfurfural (HMF) can be used as a precursor for the synthesis of 2,5-diformylfuran. However, the literature review shows using HMF as substrates is not always the case and there is a good number of reports about preparing HMF as the final product.157,158 One of these reports is about preparing 5-hydroxymethylfurfural using a starch-based catalyst. In 2017, Matharu et al. published a paper about the conversion of fructose to HMF using expanded starch@ionic liquid-FeCl3 as a catalyst.159 To expand the surface of starch, the authors used N,N′-disuccinimidyl carbonate and to attach iron chloride to the surface of expanded starch, they modified the surface of expanded starch with N-(3-aminopropyl), N-aryl imidazolium chloride.
Biphenyl derivatives are one the most common products of coupling reactions. There are many synthetic methods for the synthesis of biphenyl compounds because of their synthetic and biological importance.187 Simple biphenyls are pretty non-reactive because they don't have any active functional group. However, their derivatives with active functional groups are more reactive, and because of this higher reactivity, they have a wide range of applications in different fields.188 Among synthetic protocols for coupling reactions, the Suzuki reaction is one of the famous methods for the synthesis of biphenyl compounds.189
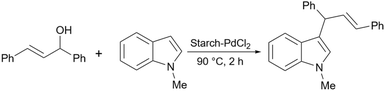
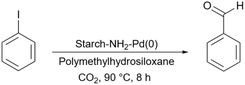
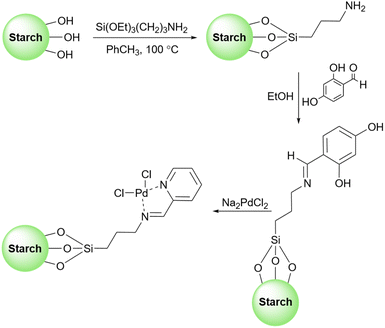
In 2017, Baran introduced a starch-based palladium catalyst for the Suzuki coupling reactions (Scheme 58).190 His protocol includes making the connection between the surface of starch and 3-aminopropyltriethoxysilane (APTES) as an amine linker. Then, 2,4-dihydroxybenzaldehyde as an aldehyde agent can be added to the prepared starch-APTES to reach the desired Schiff base. Finally, adding Na2PdCl4 to the prepared Schiff base from the last step leads to the starch-PdCl2 catalyst (Scheme 59).
In 2018, Baran's group disclosed another method for the palladium-catalyzed synthesis of biphenyls through a Suzuki-cross-coupling reaction (Scheme 60).191 They prepared a mixed composition of starch and chitosan by adding starch to the acidic solution containing chitosan. Then, the Na2PdCl4 was added dropwise to a mixture containing the prepared composite of starch/chitosan, and the solution was stirred for the next 3 hours. Finally, NaNBH4 was added to the solution to reduce the Pd(II) to Pd(0) and palladium nanoparticles precipitated on the surface of the mixed carbohydrate composite.
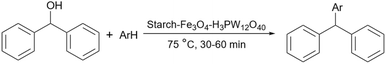
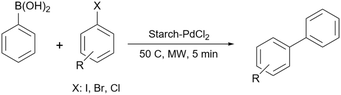
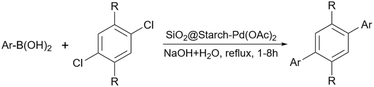
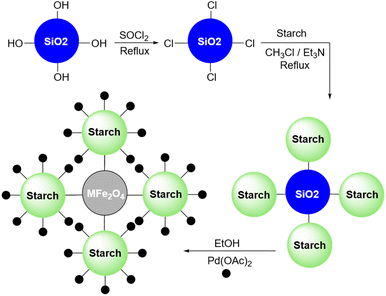
In 2012, Khalafi-Nezhad and Panahi reported a silica starch loaded with palladium nanoparticles as an efficient heterogeneous catalyst for the synthesis of p-teraryls using the Suzuki reaction (Scheme 61).192 They attached the starch to the silica chloride which was prepared by the reaction between silicon dioxide (silica gel) and thionyl chloride. Then, they added the palladium acetate to the achieved silica starch from the previous step to obtain the palladium nanoparticles-loaded silica starch (Scheme 62).
Five years later, this research group used the same catalyst for the Pd-catalyzed Buchwald–Hartwig C–N cross-coupling reaction (Scheme 63).193 They showed that Pd nanoparticles on silica-starch were powerful enough to promote the C–N cross-coupling reaction between aryl halides and amines almost as good as the carbon–carbon coupling reaction that they reported 5 years ago.
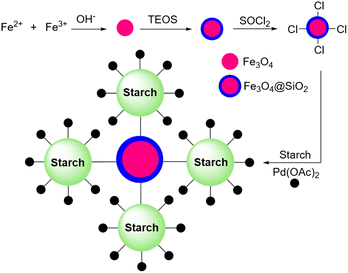
In 2018, Khalafi and Panahi reported another application for their Pd nanoparticles on the silica-starch catalyst (Scheme 64).194 This time the authors used a magnetic version of their catalyst by replacing simple silica with magnetic silica (Fe3O4@SiO2) (Scheme 65). They used this magnetic version of the palladium nanoparticles-loaded silica-starch as a catalyst for the Heck reaction between iodobenzene and styrene.
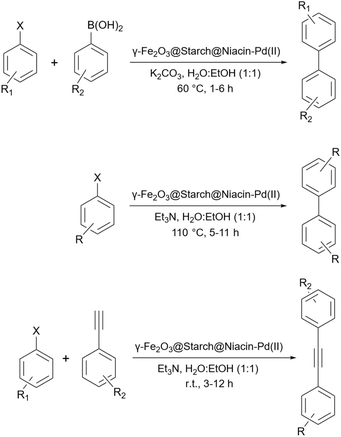
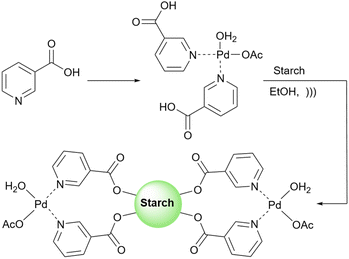
Rezapour et al. reported another palladium-loaded starch catalyst for the cross-coupling reaction (Scheme 66).195 This research group used a palladium niacin complex immobilized on starch-coated maghemite nanoparticles (γ-Fe2O3@starch@niacin-Pd(II)) for some of the homo- and cross-coupling reactions (Suzuki, Ullmann, and Sonogashira reactions (Scheme 67)).
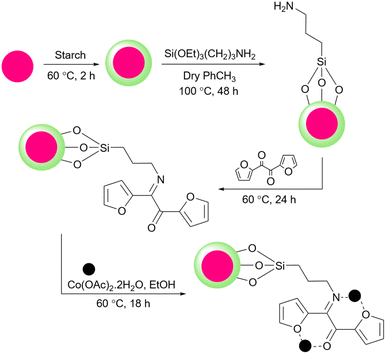
Arghan and colleagues reported a magnetic starch loaded with cobalt nanoparticles as a catalyst for the Mizoroki–Heck and the Suzuki–Miyaura reactions (Scheme 68).196 To prepare the catalyst they coated the Fe3O4 nanoparticles with starch and then modified the surface of starch with APTES. Using 2,2′-furil, they managed to achieve the related Schiff base. Finally, they added cobalt salt as Co(OAc)2 ·4H2O to the solution containing the Schiff base and prepared the desired catalyst (Scheme 69).
Veisi et al. presented another starch-based palladium catalyst for the coupling reaction. They prepared a Fe3O4@chitosan-starch core–shell decorated with palladium nanoparticles as a versatile for the Suzuki–Miyaura coupling and also reduction of nitrophenol to aminophenol reactions under ultrasonic conditions (Scheme 70).197 Based on the mechanism proposed by the authors, the key role in these transformations is on the palladium's shoulder which can be changed from Pd(0) to Pd(II) and make the coupling and reduction reactions possible.
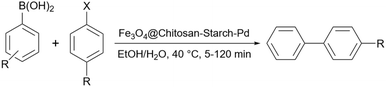 | ||
| Scheme 70 Suzuki reaction of 4-methylbromobenzene with phenyl boronic acid using Fe3O4@Chitosan-starch/Pd. | ||
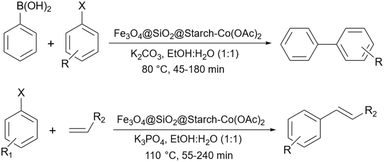
Recently, Koukabi and Arghan used a little bit different magnetic catalyst compared to their previous work196 to promote Mizoroki–Heck and Suzuki–Miyaura reactions.198 They prepared the Fe3O4@starch core–shell like before, however, instead of modifying it with a linker they just added Co(OAc)2·4H2O to the solution containing this magnetic structure to prepare their intended Fe3O4@starch-Co(II) catalyst. As we expect and also the authors proposed in their paper, Co(II) can change to Co(IV), to disconnect the Ar–X bond and help the coupling reaction to occur. Very recently, Li et al. another starch Schiff base as a support for palladium for the Suzuki coupling reactions (Scheme 71).49 They used almost the same technique that we have seen before for the preparation of starch Schiff base190 using APTES as a linker and 2-aminobenzaldehyde as a required carbonyl group.
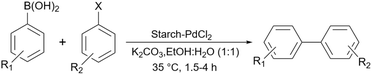 | ||
| Scheme 71 Suzuki–Miyaura coupling reactions of aryl halides with arylboronic acids using starch-PdCl2 as a catalyst. | ||
In addition to the mentioned examples of published work about using starch as support for coupling reactions, there is more work in this field that we can talk about. These protocols are gathered in Table 1.
| Entry | Year | Reaction type | Catalyst | Refs. |
|---|---|---|---|---|
| 1 | 2005 | Suzuki, Heck, and Sonogashira | Starch@Schiff base-Pd(OAc)2 | 199 |
| 2 | 2008 | Suzuki, Heck, and Sonogashira | Mesoporous starch-Pd(OAc)2 | 200 |
| 3 | 2010 | Miyaura–Suzuki | Starch-PdCl2 | 201 |
| 4 | 2011 | Sonogashira | SiO2@starch@Pd(OAc)2 | 202 |
| 5 | 2013 | Miyaura–Heck | Starch@ethylene diamine-PdCl2 | 203 |
| 6 | 2017 | Suzuki–Miyaura | Starch@polyethylenimine@Pd(0) | 204 |
| 7 | 2020 | Heck and Tsuji-Trost | Fe3O4@starch-PdCl2 | 205 |
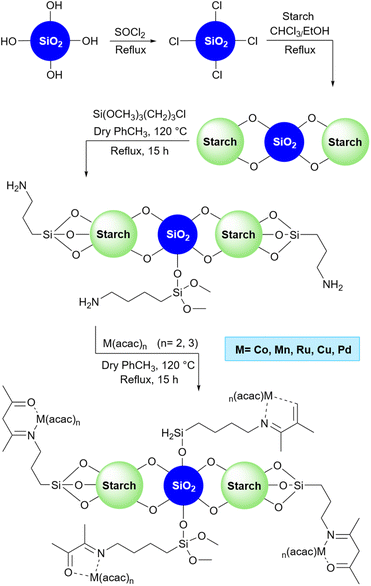
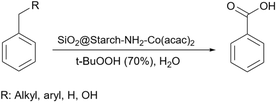
2.5.19.1 Oxidation of alkanes. Because of the presence of the carbonyl group, aryl carbonyls can be used as synthetic precursors to reach many products.206 There are many reports about the synthesis of aryl carbonyl from aryl alkanes207–209 including our previous work.210 In 2018, Sodhi and Paul prepared a starch-based catalyst to convert aryl alkane substrates to related aryl carbonyls. To do the job, they prepared and used a Co(acac)2 supported amine-functionalized silica/starch as a catalyst alongside t-BuOOH as an oxidant agent (Scheme 72).211
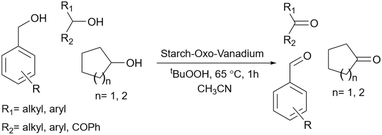
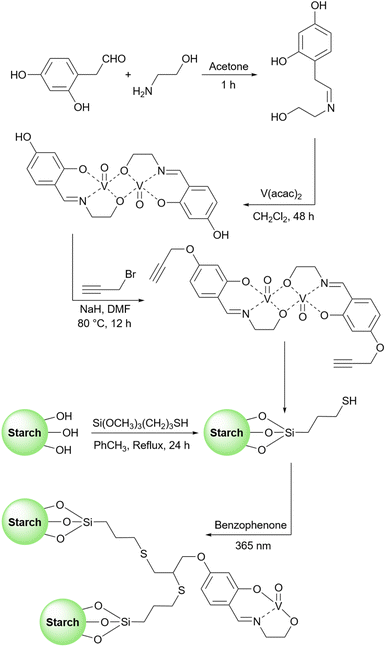
2.5.19.2 Oxidation of alcohols. The oxidation of alcohol is one of the most popular and key reactions in organic chemistry and many related reports are published every year.212–216 The literature review shows there are some published research papers about using starch as catalyst support for the oxidation reactions of alcohol.217 For example, one could point out the work that has been done by Yang's research group. This group introduced a novel method for the preparation of the starch-Schiff base Co(II) complex and used this complex as a catalyst to oxidize cyclohexanes.218 Another starch-based catalyst for the oxidation of alcohols emerged in 2013. Verma and colleagues published a research paper about the vanadium-catalyzed oxidation reaction of alcohols (Scheme 73).219 They used an oxo-vanadium Schiff base grafted on the surface of a functionalized starch as support. To prepare the catalyst, the authors functionalized the hydroxyl groups of the corn starch with 3-mercaptopropyltrimethoxysilane. Then, they prepared a Schiff base from amino ethanol and 2,4-dihydroxy benzaldehyde and added Vo(acac)2 to reach the related vanadium complex. After modifying the prepared vanadium complex with propargyl bromide, they connect this modified vanadium complex to the modified starch prepared in the first step (Scheme 74). In the same year, this group provided another catalyst for the oxidation reaction of alcohols. They prepared palladium nanoparticles grafted on nanocrystalline starch to convert primary and secondary alcohols to the related aldehydes and ketones (Scheme 75).220
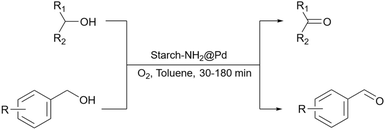
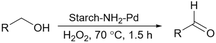
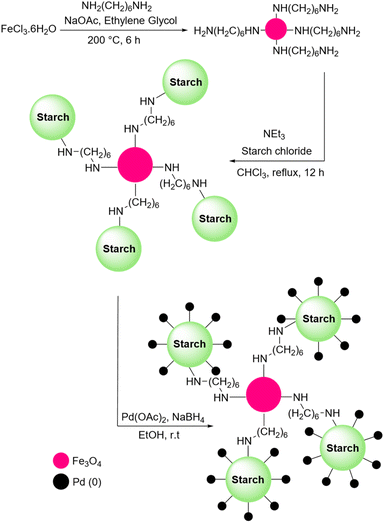
Sharma et al. proposed another method to produce grafted metallic palladium on the surface of starch and used this structure as a catalyst for the oxidation of alcohols (Scheme 76).221 First, they prepared Fe3O4 particles and then modified their surface using 1,6-hexanediamine. Next, they coated the amine-functionalized magnetic nanoparticles from the last step with starch chloride. Finally, Pd(OAc)2 was added to the solution containing starch-coated Fe3O4 particles and used NaBH4 to reduce Pd(II) to Pd(0). The authors also used the prepared catalyst for other reactions including reduction of nitroarenes and synthesis of functionalized amines from aldehydes at room temperature (Scheme 77).
Again in 2013, another method based on using starch as support was proposed, this time by the research group of Ebitani.222 They prepared some kind of platinum/gold alloy nanoparticles-supported hydrotalcite and used this catalyst to oxidize polyols at room temperature. The results showed the different percentages of Pt/Au can affect the amount of conversion of glycerol substrate to its oxidized forms like glyceric acid, tartronic acid, glycolic acid, and oxalic acid.
2.5.19.3 Oxidation of aldehydes. In 2019, Li's research group proposed a novel way for the synthesis of benzoic acid derivatives based on using starch as support (Scheme 78).223 They added HAuCl4·4H2O to the solution containing starch to reach a gold nanoparticle-supported starch and used that as a catalyst alongside H2O2 as an oxidant to convert benzaldehydes to their corresponding benzoic acids. According to the authors, in this catalytic system, starch doesn't have any specific direct influence on the outcome but works as a ligand and reducing agent for gold nanoparticles which play the main role as a catalyst.
Very recently, Ravichandran et al. introduced another starch-based catalyst to convert benzaldehyde to corresponding carboxylic acid.224 They prepared Fe3O4@starch@Ag via the grinding method and used this nanocomposite as a catalyst for the oxidation of benzaldehyde. Also, the published results indicate that the catalyst is capable of decreasing some common dyes including Methylene blue and Methyl red.
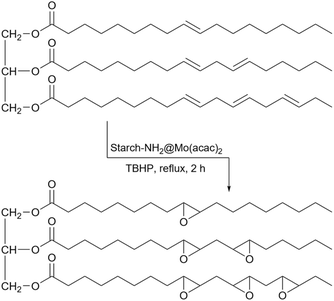
2.5.19.4 Epoxidation of alkenes. Epoxides can be very common intermediates in many organic reactions and alkenes are one of the major sources to reach epoxide.225,226 Vegetable oils are one of those compounds that contain double bounds and from a commercial point of view, the epoxidation of these bonds is very important. The reason is that epoxidized vegetable oils have many applications for the synthesis of polyurethane foams, synthetic detergents, coatings agents, and lubricant compounds.227 In 2015, Pan's research group presented a starch-Schiff base-Mo(acac)2 catalyst for the epoxidation of stillingia oil as a vegetable oil using tert-butyl hydroperoxide (TBHP) as an oxidizing agent (Scheme 79).228
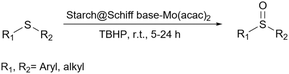
2.5.19.5 Oxidation of sulfoxides. Sulfoxides are valuable targets in synthetic and medicinal chemistry and the oxidation of sulfide is one of the easiest ways to prepare these compounds. In 2017, Hong and Yan prepared a starch@Schiff base-Mo(acac)2 as a catalyst to convert sulfides to sulfoxide using t-BuOOH as an oxidant (Scheme 80).229
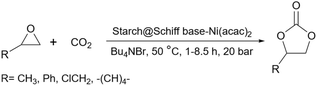
2.5.19.6 Synthesis of cyclic carbonates. There are many reports about using carbon dioxide as an undesirable molecule to prepare much more precious compounds. One of these reactions is converting epoxides to their related cyclic carbonate.230–233 In 2012, Kumar et al. published prepared and employed a starch-based catalyst to convert substituted epoxides to cyclic carbonates (Scheme 81). To do that, they prepared a starch Schiff base and attached Ni(acac)2 to the surface of the Schiff base.234
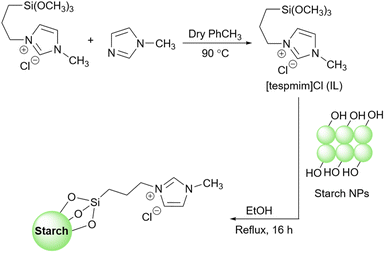
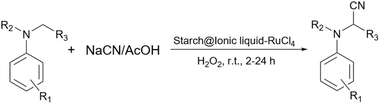
2.5.19.7 Oxidative cyanation of tertiary amines. The oxidative cyanation of tertiary amines is an interesting method for the synthesis of a-aminonitriles.235–237 In 2011, Verma et al. reported a ruthenium-catalyzed cyanation of aromatic tertiary amines (Scheme 82).238 They prepared a starch@ionic liquid-RuCl4 as a novel catalyst for this transformation. To prepare the ionic liquid layer around the starch, the authors took advantage of the reaction between 1-methylimidazole and 3-trimethoxysilylpropyl chloride. Then, they attached the prepared 1-methyl-3-(silylpropyl)imidazolium chloride from the last step to the surface of starch under reflux conditions in toluene and used the achieved starch@ionic liquid to reach the intended starch@ionic liquid-RuCl4.
3. Conclusion
In this review, the applications of starch as catalyst and catalyst support have been discussed. Literature review shows that starch has been used in different forms for a wide range of organic reactions. The application of starch in these reactions as a catalyst can be summarised based on the type of starch, i.e., unfunctionalized and functionalized starch. Unfunctionalized starch could be used as simple or attached to magnetic particles and functionalized starch could be employed with simple acidic/basic modification of the surface of starch or anchored to a complex structure using an appropriate linker. In either case, starch can have a simple or core–shell structure. Based on what is presented here, simple starch like raw starch or starch nanoparticles are good candidates to promote acid–base reactions like multi-component organic transformations. Also, modified starch with suitable functional groups like acidic or basic starch can do the same job even with greater efficiency in some cases. However, to do more difficult reactions including coupling and oxidation reactions that the presence of metals is almost necessary, starch or its modified versions can be used as efficient support to hold the desired metal or metal oxide like molecular pincers and facilitate the process. Like any other catalyst starch has its disadvantages including low tolerance to a wide range of temperatures, and its tendency to absorb and hold water. However, properties such as being cheap, environmentally friendly, and easy to prepare can help balance the situation. All in all, regardless of whether we use starch, as a catalyst or catalyst support, this biodegradable and environmentally friendly biopolymer is a very good option for accelerating many organic reactions.Conflicts of interest
There are no conflicts to declare.References
- B.-E. Channab, A. El Idrissi, M. Zahouily, Y. Essamlali and J. C. White, Int. J. Biol. Macromol., 2023, 238, 124075 CrossRef CAS PubMed.
- M. Emeje, Chemical Properties of Starch, IntechOpen, London, 2020 Search PubMed.
- K. Korompokis, K. Verbeke and J. A. Delcour, Compr. Rev. Food Sci. Food Saf., 2021, 20, 5965–5991 CrossRef CAS PubMed.
- A. Bahaji, J. Li, Á. M. Sánchez-López, E. Baroja-Fernández, F. J. Muñoz, M. Ovecka, G. Almagro, M. Montero, I. Ezquer, E. Etxeberria and J. Pozueta-Romero, Biotechnol. Adv., 2014, 32, 87–106 CrossRef CAS PubMed.
- S. Kim, A. Biswas, M. Singh, S. C. Peterson and S. Liu, J. Cereal Sci., 2012, 56, 720–725 CrossRef CAS.
- A. Zarski, K. Bajer and J. Kapuśniak, Polymers, 2021, 13, 832 CrossRef CAS PubMed.
- V. K. Khlestkin, S. E. Peltek and N. A. Kolchanov, Carbohydr. Polym., 2018, 181, 460–476 CrossRef CAS PubMed.
- N. A. Abd El-Ghany, M. H. A. Elella, H. M. Abdallah, M. S. Mostafa and M. Samy, J. Polym. Environ., 2023, 31, 2792–2825 CrossRef CAS.
- N. Masina, Y. E. Choonara, P. Kumar, L. C. Du Toit, M. Govender, S. Indermun and V. Pillay, Carbohydr. Polym., 2017, 157, 1226–1236 CrossRef CAS PubMed.
- M. Haroon, L. Wang, H. Yu, N. M. Abbasi, Z.-A. Zain-ul-Abdin, M. Saleem, R. U. Khan, R. S. Ullah, Q. Chen and J. Wu, RSC Adv., 2016, 6, 78264–78285 RSC.
- J. Watcharakitti, E. E. Win, J. Nimnuan and S. M. Smith, Polymers, 2022, 14, 2023 CrossRef CAS PubMed.
- Y. F. Zuo, J. Gu, Z. Qiao, H. Tan, J. Cao and Y. Zhang, Int. J. Biol. Macromol., 2015, 72, 391–402 CrossRef CAS PubMed.
- A. Gilet, C. Quettier, V. Wiatz, H. Bricout, M. Ferreira, C. Rousseau, E. Monflier and S. Tilloy, Green Chem., 2018, 20, 1152–1168 RSC.
- H. Bakouri and K. Guemra, Int. J. Biol. Macromol., 2019, 125, 1118–1127 CrossRef CAS PubMed.
- S. H. Clasen, C. M. O. Müller, A. L. Parize and A. T. N. Pires, Carbohydr. Polym., 2018, 180, 348–353 CrossRef CAS PubMed.
- B. Klein, N. L. Vanier, K. Moomand, V. Z. Pinto, R. Colussi, E. Da Rosa Zavareze and A. R. G. Dias, Food Chem., 2014, 155, 167–173 CrossRef CAS PubMed.
- Y.-R. Zhang, X.-L. Wang, G.-M. Zhao and Y.-Z. Wang, Carbohydr. Polym., 2012, 87, 2554–2562 CrossRef CAS.
- A. S. Ayoub and S. S. H. Rizvi, J. Plast. Film Sheeting, 2009, 25, 25–45 CrossRef CAS.
- H.-S. Kim, D.-K. Hwang, B.-Y. Kim and M.-Y. Baik, Food Chem., 2012, 130, 977–980 CrossRef CAS.
- D. Cui, M. Liu, L. Wu and Y. Bi, Int. J. Biol. Macromol., 2009, 44, 294–299 CrossRef CAS PubMed.
- F. Akman, A. S. Kazachenko, N. Y. Vasilyeva and Y. N. Malyar, J. Mol. Struct., 2020, 1208, 127899 CrossRef CAS.
- S. Dhital, G. Dolan, J. R. Stokes and M. J. Gidley, Food Funct., 2014, 5, 579–586 RSC.
- Y. Qin, H. Zhang, Y. Dai, H. Hou and H. Dong, Materials, 2019, 12, 1705 CrossRef CAS PubMed.
- S. Wang and L. Copeland, Crit. Rev. Food Sci. Nutr., 2015, 55, 1081–1097 CrossRef CAS PubMed.
- H. Cheng, L. Chen, D. J. McClements, T. Yang, Z. Zhang, F. Ren, M. Miao, Y. Tian and Z. Jin, Trends Food Sci. Technol., 2021, 114, 70–82 CrossRef CAS.
- A. Gamage, A. Liyanapathiranage, A. Manamperi, C. Gunathilake, S. Mani, O. Merah and T. Madhujith, Sustainability, 2022, 14, 6085 CrossRef CAS.
- X. Hou, H. Wang, Y. Shi and Z. Yue, Carbohydr. Polym., 2023, 302, 120392 CrossRef CAS PubMed.
- D. Le Corre and H. Angellier-Coussy, React. Funct. Polym., 2014, 85, 97–120 CrossRef CAS.
- S. Mohd Asharuddin, N. Othman, W. A. H. Altowayti, N. Abu Bakar and A. Hassan, Environ. Technol. Innovation, 2021, 23, 101637 CrossRef CAS.
- A. Rodrigues and M. Emeje, Carbohydr. Polym., 2012, 87, 987–994 CrossRef CAS.
- O. O. Ayodele and F. A. Dawodu, Energy Technol., 2014, 2, 912–920 CrossRef CAS.
- D. Soto, J. Urdaneta, K. Pernía, O. León, A. Muñoz-Bonilla and M. Fernandez-García, Starch, 2016, 68, 37–46 CrossRef CAS.
- I. Ihsanullah, M. Bilal and A. Jamal, Chem. Rec., 2022, 22, e202100312 CrossRef CAS PubMed.
- J. Tang, Q. Zhang, J. Zhou, H. Fang, H. Yang and F. Wang, Food Chem., 2021, 365, 130448 CrossRef CAS PubMed.
- S. Kang, W. Liu, Y. Wang, Y. Wang, S. Wu, S. Chen, B. Yan and X. Lan, J. Taiwan Inst. Chem. Eng., 2022, 135, 104383 CrossRef CAS.
- F. Wang, R. Ma and Y. Tian, Carbohydr. Polym., 2022, 287, 119297 CrossRef CAS PubMed.
- L. García-Guzmán, G. Cabrera-Barjas, C. G. Soria-Hernández, J. Castaño, A. Y. Guadarrama-Lezama and S. Rodríguez Llamazares, Polysaccharides, 2022, 3, 136–177 CrossRef.
- W.-Y. Lou, M.-H. Zong and Z.-Q. Duan, Bioresour. Technol., 2008, 99, 8752–8758 CrossRef CAS PubMed.
- J. Clohessy and W. Kwapinski, Appl. Sci., 2020, 10, 918 CrossRef CAS.
- S. H. Y. S. Abdullah, N. H. M. Hanapi, A. Azid, R. Umar, H. Juahir, H. Khatoon and A. Endut, Renew. Sustainable Energy Rev., 2017, 70, 1040–1051 CrossRef CAS.
- I. Melián-Cabrera, Ind. Eng. Chem. Res., 2021, 60, 18545–18559 CrossRef.
- O. O. Kunle, Starch source and its impact on pharmaceutical applications, in Chemical Properties of Starch, ed. M. Emeje, IntechOpen, 2020 Search PubMed.
- B. O. Fraser-Reid, K. Tatsuta and J. Thiem, Glycoscience: Chemistry and Chemical Biology, Springer-Verlag, Berlin, Heidelberg, 2008 Search PubMed.
- C. C. J. Loh, Nat. Rev. Chem., 2021, 5, 792–815 CrossRef CAS PubMed.
- M. Jaymand, Int. J. Biol. Macromol., 2022, 197, 111–120 CrossRef CAS PubMed.
- K. Arabpourian and F. K. Behbahani, Application of starch in the synthesis of N-substituted pyrroles by a simple and green route, in Greener Synthesis of Organic Compounds, Drugs and Natural Products, ed. A. Nag, CRC Press, Boca Raton, 2022, pp. 191–194 Search PubMed.
- J. Safari, Z. Zarnegar, M. Sadeghi and F. Azizi, Curr. Org. Chem., 2016, 20, 2926–2932 CrossRef CAS.
- C. Shi, Q. Huang, R. Zhang, X. Liang, F. Wang, Z. Liu, M. Liu, H. Hu and Y. Yin, RSC Adv., 2021, 11, 39758–39767 RSC.
- X. Li, Z. Zhou, Y. Wang, J. Dong, X. Jia, Z. Hu, Q. Wei, W. Zhang, Y. Jiang, J. Zhang and Y. Dong, Int. J. Biol. Macromol., 2023, 233, 123596 CrossRef CAS PubMed.
- A. D. Gupta, K. P. Rawat, V. Bhadauria and H. Singh, Carbohydr. Polym., 2021, 269, 117763 CrossRef CAS PubMed.
- S. C. Philkhana, F. O. Badmus, I. C. Dos Reis and R. Kartika, Synthesis, 2021, 53, 1531–1555 CrossRef CAS PubMed.
- S. S. Gholap, Eur. J. Med. Chem., 2016, 110, 13–31 CrossRef CAS PubMed.
- J. Safari and M. Sadeghi, Res. Chem. Intermed., 2016, 42, 8175–8183 CrossRef CAS.
- J. Safari, Z. Abedi-Jazini, Z. Zarnegar and M. Sadeghi, J. Nanopart. Res., 2015, 17, 495 CrossRef.
- Z. Zarnegar, M. Sadeghi, R. Alizadeh and J. Safai, J. Mol. Liq., 2018, 255, 76–79 CrossRef CAS.
- B. N. Ravi, J. Keshavayya, N. M. Mallikarjuna, V. Kumar and S. Kandgal, J. Mol. Struct., 2020, 1204, 127493 CrossRef CAS.
- M. Farouk Elsadek, B. Mohamed Ahmed and M. Fawzi Farahat, Molecules, 2021, 26, 1449 CrossRef PubMed.
- M. E. Khalifa, Acta Chim. Slov., 2018, 65, 1–22 CrossRef CAS PubMed.
- J. Safari and M. Sadeghi, Monatsh. Chem., 2017, 148, 745–749 CrossRef CAS.
- H. Rostami Monjezi, Z. Zarnegar and J. Safari, J. Saudi Chem. Soc., 2019, 23, 973–979 CrossRef CAS.
- M. Ahmadzadeh, M. Sadeghi and J. Safari, J. Chem., 2021, 2021, 1–11 CrossRef.
- M. Kangani, N. Hazeri and M.-T. Maghsoodlou, Iran J. Org. Chem., 2015, 7, 1591–1595 Search PubMed.
- A. A. Mohammadi, M. R. Asghariganjeh and A. Hadadzahmatkesh, Arab. J. Chem., 2017, 10, S2213–S2216 CrossRef CAS.
- Z. Benzekri, S. Sibous, H. Anahmadi, F. El hajri, D. E. Mekkaoui, R. Hsissou, A. Ouasri, A. Souizi, A. Rhandour and S. Boukhris, J. Mol. Struct., 2023, 1281, 135064 CrossRef CAS.
- N. Hazeri, M. T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani and E. Molashahi, Chin. J. Catal., 2014, 35, 391–395 CrossRef CAS.
- S. Agarwal, A. Sethiya, J. Soni, N. Sahiba and P. Teli, Appl. Organometal. Chem., 2022, 36, e6604 CrossRef CAS.
- D. Tejedor, S. Delgado-Hernández, R. Diana-Rivero, A. Díaz-Díaz and F. García-Tellado, Molecules, 2019, 24, 2904 CrossRef PubMed.
- S. Mal and M. Jana, ChemistrySelect, 2022, 7, e202103525 CrossRef CAS.
- M. Kamalzare, M. Bayat and A. Maleki, R. Soc. Open Sci., 2020, 7, 200385 CrossRef CAS PubMed.
- D. S. Aher, K. R. Khillare, L. D. Chavan and S. G. Shankarwar, Monatsh. Chem., 2022, 153, 79–85 CrossRef CAS.
- S. V. Akolkar, N. D. Kharat, A. A. Nagargoje, D. D. Subhedar and B. B. Shingate, Catal. Lett., 2020, 150, 450–460 CrossRef CAS.
- D. Dharmendra, P. Chundawat, Y. Vyas and C. Ameta, J. Chem. Sci., 2022, 134, 47 CrossRef CAS.
- S. Razikazemi, K. Rad-Moghadam and S. Toorchi-Roudsari, New J. Chem., 2018, 42, 12476–12485 RSC.
- R. M. Ward, Q. Gao, H. de Bruyn, R. G. Gilbert and M. A. Fitzgerald, Biomacromolecules, 2006, 7, 866–876 CrossRef CAS PubMed.
- A. Mohammadzadeh, A. P. Marjani and A. Zamani, S. Afr. J. Chem., 2020, 73, 55–63 CrossRef CAS.
- F. Karimi Rad and F. K. Behbahani, Curr. Org. Synth., 2016, 14, 22–39 CrossRef.
- F. M. Moghaddam, S. Aghili, M. Daneshfar, H. Moghimi and Z. Daneshfar, Res. Chem. Intermed., 2023, 49, 1507–1543 CrossRef CAS.
- A. Bouhaoui, M. Eddahmi, M. Dib, M. Khouili, A. Aires, M. Catto and L. Bouissane, ChemistrySelect, 2021, 6, 5848–5870 CrossRef CAS.
- S. Doi, J. H. Clark, D. J. Macquarrie and K. Milkowski, Chem. Commun., 2002, 2632–2633 RSC.
- R. Rezaei and M. R. Sheikhi, Res. Chem. Intermed., 2015, 41, 1283–1292 CrossRef CAS.
- N. Q. H. Doan, N. T. K. Nguyen, V. B. Duong, H. T. T. Nguyen, L. B. Vong, D. N. Duong, N.-T. T. Nguyen, T. L. T. Nguyen, T. T. H. Do and T. N. Truong, ACS Omega, 2022, 7, 33963–33984 CrossRef CAS PubMed.
- F. Hatamjafari, Helv. Chim. Acta, 2013, 96, 1560–1563 CrossRef CAS.
- R. H. Vekariya, K. D. Patel and H. D. Patel, Iran J. Org. Chem., 2015, 7, 1581–1589 Search PubMed.
- J. Sangshetti, S. K. Pathan, R. Patil, S. Akber Ansari, S. Chhajed, R. Arote and D. B. Shinde, Bioorg. Med. Chem., 2019, 27, 3979–3997 CrossRef CAS PubMed.
- H. R. Shaterian and F. Rigi, Starch, 2011, 63, 340–346 CrossRef CAS.
- M. Ahmad, T. A. King, D.-K. Ko, B. H. Cha and J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473–1476 CrossRef CAS.
- A. N. Dadhania, V. K. Patel and D. K. Raval, C. R. Chim., 2012, 15, 378–383 CrossRef CAS.
- L. G. Wang, I. Munhenzva, M. Sibrian-Vazquez, J. O. Escobedo, C. H. Kitts, F. R. Fronczek and R. M. Strongin, J. Org. Chem., 2019, 84, 2585–2595 CrossRef CAS PubMed.
- H. R. Shaterian and F. Rigi, Res. Chem. Intermed., 2015, 41, 721–738 CrossRef CAS.
- J. Safari, P. Aftabi, M. Ahmadzadeh, M. Sadeghi and Z. Zarnegar, J. Mol. Struct., 2017, 1142, 33–39 CrossRef CAS.
- P. Biginelli and P. Gazz, Chim. Ital., 1893, 23, 360–416 Search PubMed.
- L. H. S. Matos, F. T. Masson, L. A. Simeoni and M. Homem-de-Mello, Eur. J. Med. Chem., 2018, 143, 1779–1789 CrossRef CAS PubMed.
- R. Rezaei, S. Malek, M. R. Sheikhi and M. K. Mohammadi, Chem. J. Mold., 2013, 8, 101–106 CAS.
- S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463–24476 RSC.
- A. Shaabani, A. Rahmati and Z. Badri, Catal. Commun., 2008, 9, 13–16 CrossRef CAS.
- L. Biesen and T. J. J. Müller, Adv. Synth. Catal., 2021, 363, 980–1006 CrossRef CAS.
- J. A. Pereira, A. M. Pessoa, M. N. D. S. Cordeiro, R. Fernandes, C. Prudêncio, J. P. Noronha and M. Vieira, Eur. J. Med. Chem., 2015, 97, 664–672 CrossRef CAS PubMed.
- R. S. Keri, V. Adimule, P. Kendrekar and B. S. Sasidhar, Top. Catal., 2022 DOI:10.1007/s11244-022-01562-0.
- V. A. S. Pardeshi, N. S. Chundawat, S. I. Pathan, P. Sukhwal, T. P. S. Chundawat and G. P. Singh, Synth. Commun., 2021, 51, 485–513 CrossRef CAS.
- A. Farrokhi, M. Jafarpour and F. Feizpour, ChemistrySelect, 2018, 3, 1234–1241 CrossRef CAS.
- F. Cai, B. Hou, S. Zhang, H. Chen, S. Ji, X.-C. Shen and H. Liang, J. Mater. Chem. B, 2019, 7, 2493–2498 RSC.
- M. H. Sayahi, F. Shamkhani, M. Mahdavi and S. Bahadorikhalili, Starch, 2021, 73, 2000257 CrossRef CAS.
- R. M. Lanigan, P. Starkov and T. D. Sheppard, J. Org. Chem., 2013, 78, 4512–4523 CrossRef CAS PubMed.
- P. Nagaraaj and V. Vijayakumar, Org. Chem. Front., 2019, 6, 2570–2599 RSC.
- M. Kamal, T. Jawaid, U. A. Dar and S. A. Shah, Amide as a potential pharmacophore for drug designing of novel anticonvulsant compounds, in Chemistry of Biologically Potent Natural Products and Synthetic Compounds, ed. S.-U. Islam and J. A. Banday, Wiley, 2021, pp. 319–342 Search PubMed.
- W. Ma, B. Xu, R. Sun, Y.-J. Xu and J.-F. Ge, J. Mater. Chem. B, 2021, 9, 2524–2531 RSC.
- Y. Ma, J. Li, M. Tian, Y. Liu and A. Wei, Ind. Crops Prod., 2020, 155, 112770 CrossRef CAS.
- K. Yan, J. Wang, Z. Wang and L. Yuan, Chem. Commun., 2023, 59, 382–400 RSC.
- R. Wani and H. K. Chaudhari, Org. Prep. Proced. Int., 2023, 1–12 CrossRef.
- A. G. Al-kaf, Quinazolinone and Quinazoline Derivatives, IntechOpen, London, 2020 Search PubMed.
- D. Wang and F. Gao, Chem. Cent. J., 2013, 7, 95 CrossRef PubMed.
- H. R. Lobo, B. S. Singh and G. S. Shankarling, Catal. Commun., 2012, 27, 179–183 CrossRef CAS.
- F. Tamaddon and M. KazemiVarnamkhasti, Carbohydr. Res., 2017, 437, 9–15 CrossRef CAS PubMed.
- F. Tamaddon and M. Varnamkhasti, Curr. Catal., 2017, 6, 57–66 CrossRef CAS.
- X. Bao, X. Wang, J.-M. Tian, X. Ye, B. Wang and H. Wang, Org. Biomol. Chem., 2022, 20, 2370–2386 RSC.
- G. Mustafa, M. Zia-ur-Rehman, S. H. Sumrra, M. Ashfaq, W. Zafar and M. Ashfaq, J. Mol. Struct., 2022, 1262, 133044 CrossRef CAS.
- Z. Zhao, X. Dai, C. Li, X. Wang, J. Tian, Y. Feng, J. Xie, C. Ma, Z. Nie, P. Fan, M. Qian, X. He, S. Wu, Y. Zhang and X. Zheng, Eur. J. Med. Chem., 2020, 186, 111893 CrossRef CAS PubMed.
- A. Ramshini, S. M. Nezhad, S. A. Pourmousavi, E. Nazarzadeh Zare, M. Pourjafar and E. Sharifi, Catalysts, 2023, 13, 908 CrossRef CAS.
- T. Khosousi, M. Ahmadzadeh, N. Afrouz and M. Sadeghi, Results Chem., 2023, 6, 101063 CrossRef CAS.
- Z. Zarnegar, H. R. Monjezi and J. Safari, J. Mol. Struct., 2019, 1193, 14–23 CrossRef CAS.
- J. Dai, S. Tian, X. Yang and Z. Liu, Front. Chem., 2022, 10, 891484 CrossRef CAS PubMed.
- M. M. Matin, P. Matin, M. R. Rahman, T. Ben Hadda, F. A. Almalki, S. Mahmud, M. M. Ghoneim, M. Alruwaily and S. Alshehri, Front. Mol. Biosci., 2022, 9, 864286 CrossRef CAS PubMed.
- A. Pourjavadi, A. Motamedi, S. H. Hosseini and M. Nazari, RSC Adv., 2016, 6, 19128–19135 RSC.
- R. Bonyasi, M. Gholinejad, F. Saadati and C. Nájera, New J. Chem., 2018, 42, 3078–3086 RSC.
- P. Gupta, P. Kumar, B. Syal and T. Shamim, Res. Chem. Intermed., 2022, 48, 4601–4615 CrossRef CAS.
- A. V. Dolzhenko, Heterocycles, 2017, 94, 1819 CrossRef CAS.
- A. Nasiri, M. A. Khalilzadeh and D. Zareyee, J. Coord. Chem., 2022, 75, 256–279 CrossRef CAS.
- S. Kumar and Ritika, Futur. J. Pharm. Sci., 2020, 6, 121 CrossRef.
- V. Sharma, P. Kumar and D. Pathak, J. Heterocyclic Chem., 2010 Search PubMed.
- D. F. Taber and P. K. Tirunahari, Tetrahedron, 2011, 67, 7195–7210 CrossRef CAS PubMed.
- M.-Z. Zhang, Q. Chen and G.-F. Yang, Eur. J. Med. Chem., 2015, 89, 421–441 CrossRef CAS PubMed.
- A. A. Atia and M. Kimura, Tetrahedron, 2021, 90, 132213 CrossRef CAS.
- G. N. Vaidya, S. K. Lokhande, S. D. Shinde, D. P. Satpute, G. Narang and D. Kumar, Green Chem., 2022, 24, 4921–4927 RSC.
- S. Mallick, P. Mukhi, P. Kumari, K. R. Mahato, S. K. Verma and D. Das, Catal. Lett., 2019, 149, 3501–3507 CrossRef CAS.
- F. C. Teixeira, H. Ramos, I. F. Antunes, M. J. M. Curto, M. T. Duarte and I. Bento, Molecules, 2006, 11, 867–889 CrossRef CAS PubMed.
- S.-G. Zhang, C.-G. Liang and W.-H. Zhang, Molecules, 2018, 23 Search PubMed.
- R. K. Sodhi, A. Changotra and S. Paul, Catal. Lett., 2014, 144, 1819–1831 CrossRef CAS.
- T. Gessner and U. Mayer, Triarylmethane and diarylmethane dyes, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- R. Kshatriya, V. P. Jejurkar and S. Saha, Eur. J. Org Chem., 2019, 2019, 3818–3841 CrossRef CAS.
- X. Liu, X. Wu, L. Zhang, X. Lin and D. Huang, Synthesis, 2020, 52, 2311–2329 CrossRef CAS.
- E. Rafiee and M. Khodayari, J. Mol. Catal. A: Chem., 2015, 398, 336–343 CrossRef CAS.
- J. Safari, Z. Zarnegar, M. Sadeghi and A. Enayati-Najafabadi, J. Mol. Struct., 2016, 1125, 772–776 CrossRef CAS.
- H.-W. Liu, K. Huang, J.-D. Mi, Y. Jia, M.-Y. Huang and Y.-Y. Jiang, Polym. Adv. Technol., 2003, 14, 355–359 CrossRef CAS.
- S. Chairam, W. Konkamdee and R. Parakhun, J. Saudi Chem. Soc., 2017, 21, 656–663 CrossRef CAS.
- L. Qi, Z. Luo and X. Lu, Green Chem., 2018, 20, 1538–1550 RSC.
- M. Gholinejad, N. Dasvarz, M. Shojafar and J. M. Sansano, Inorg. Chim. Acta, 2019, 495, 118965 CrossRef CAS.
- A. Nasiri, M. A. Khalilzadeh and D. Zareyee, Inorg. Nano-Met. Chem., 2021, 1–15 Search PubMed.
- S. Taheri, M. M. Heravi and P. Mohammadi, Diamond Relat. Mater., 2022, 126, 109078 CrossRef CAS.
- Y. Lan, H. Veisi, F. Ebrahimi, S. Hemmati and B. Karmakar, Inorg. Chem. Commun., 2023, 156, 111136 CrossRef CAS.
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS PubMed.
- M. Gholinejad, B. Karimi, A. Aminianfar and M. Khorasani, ChemPlusChem, 2015, 80, 1573–1579 CrossRef CAS PubMed.
- F. Saadati, M. Gholinejad, H. Janmohammadi and S. Shaybanizadeh, Lett. Org. Chem., 2018, 15, 79–86 CrossRef CAS.
- M. Tajbakhsh, F. Mazhari and M. Mavvaji, Org. Prep. Proced. Int., 2022, 1–14 Search PubMed.
- Q. N. B. Nguyen, H. A. N. Le, P. D. Ly, H. B. Phan and P. H. Tran, Chem. Commun., 2020, 56, 13005–13008 RSC.
- M. Hong, J. Min, S. Wu, J. Li, J. Wang, L. Wei, Z. Ling, K. Li and S. Wang, Appl. Organomet. Chem., 2020, 34, e5411 CrossRef CAS.
- M. Hong, S. Wu, J. Li, J. Wang, L. Wei and K. Li, Catal. Commun., 2020, 136, 105909 CrossRef CAS.
- M. A. A. B. Abdul Rani, N. A. Karim and S. K. Kamarudin, Int. J. Energy Res., 2022, 46, 18996–19050 CrossRef CAS.
- B. Agarwal, K. Kailasam, R. S. Sangwan and S. Elumalai, Renew. Sustainable Energy Rev., 2018, 82, 2408–2425 CrossRef CAS.
- A. S. Matharu, S. Ahmed, B. Almonthery, D. J. Macquarrie, Y.-S. Lee and Y. Kim, ChemSusChem, 2018, 11, 716–725 CrossRef CAS PubMed.
- S. Askin, H. Tahtaci, C. Türkeş, Y. Demir, A. Ece, G. Akalın Çiftçi and Ş. Beydemir, Bioorg. Chem., 2021, 113, 105009 CrossRef CAS PubMed.
- S. Cascioferro, G. L. Petri, B. Parrino, D. Carbone, N. Funel, C. Bergonzini, G. Mantini, H. Dekker, D. Geerke, G. J. Peters, G. Cirrincione, E. Giovannetti and P. Diana, Eur. J. Med. Chem., 2020, 189, 112088 CrossRef PubMed.
- A. Cristina, D. Leonte, L. Vlase, L. C. Bencze, S. Imre, G. Marc, B. Apan, C. Mogoșan and V. Zaharia, Molecules, 2018, 23, 2425 CrossRef PubMed.
- S. Bahadorikhalili, S. Ansari, H. Hamedifar and M. Mahdavi, Int. J. Biol. Macromol., 2019, 135, 453–461 CrossRef CAS PubMed.
- J. Kim, H. Kim and S. B. Park, J. Am. Chem. Soc., 2014, 136, 14629–14638 CrossRef CAS PubMed.
- F. Sánchez-Sancho, M. Escolano, D. Gaviña, A. G. Csáky, M. Sánchez-Roselló, S. Díaz-Oltra and C. Del Pozo, Pharmaceuticals, 2022, 15, 948 CrossRef PubMed.
- R. Kaur, S. Chaudhary, K. Kumar, M. K. Gupta and R. K. Rawal, Eur. J. Med. Chem., 2017, 132, 108–134 CrossRef CAS PubMed.
- Z. Moutaoukil, C. Ronco and R. Benhida, J. Saudi Chem. Soc., 2022, 26, 101398 CrossRef CAS.
- N. Afradi, N. Foroughifar, H. Pasdar and M. Qomi, Res. Chem. Intermed., 2019, 45, 3251–3271 CrossRef CAS.
- M. Jafarpour and A. Rezaeifard, Transition Met. Chem., 2016, 41, 205–211 CrossRef CAS.
- A. Saeed, Eur. J. Med. Chem., 2016, 116, 290–317 CrossRef CAS PubMed.
- A. Marandi, E. Nasiri, N. Koukabi and F. Seidi, Int. J. Biol. Macromol., 2021, 190, 61–71 CrossRef CAS PubMed.
- Z.-W. Chen, D.-N. Ye, Y.-P. Qian, M. Ye and L.-X. Liu, Tetrahedron, 2013, 69, 6116–6120 CrossRef CAS.
- S. Hou, H. Yang, B. Cheng, H. Zhai and Y. Li, Chem. Commun., 2017, 53, 6926–6929 RSC.
- S. B. Ötvös, Z. Szécsényi and F. Fülöp, ACS Sustainable Chem. Eng., 2019, 7, 13286–13293 CrossRef.
- S. A. Mousavi-Mashhadi and A. Shiri, J. Iran. Chem. Soc., 2022, 19, 4523–4534 CrossRef CAS.
- M. D. Garba, M. Usman, S. Khan, F. Shehzad, A. Galadima, M. F. Ehsan, A. S. Ghanem and M. Humayun, J. Environ. Chem. Eng., 2021, 9, 104756 CrossRef CAS.
- V. Aomchad, À. Cristòfol, F. Della Monica, B. Limburg, V. D'Elia and A. W. Kleij, Green Chem., 2021, 23, 1077–1113 RSC.
- R. Dalpozzo, N. Della Ca’, B. Gabriele and R. Mancuso, Catalysts, 2019, 9, 511 CrossRef.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- S. W. Knowlden and B. V. Popp, Organometallics, 2022, 41, 1883–1891 CrossRef CAS.
- T. M. Perrone, A. S. Gregory, S. W. Knowlden, N. R. Ziemer, R. N. Alsulami, J. L. Petersen and B. V. Popp, ChemCatChem, 2019, 11, 5814–5820 CrossRef CAS.
- S. Kumar, S. Verma and S. L. Jain, Tetrahedron Lett., 2015, 56, 2430–2433 CrossRef CAS.
- M. Cherif, M. Horchani, Y. O. Al-Ghamdi, S. G. Almalki, Y. E. Alqurashi, H. Ben Jannet and A. Romdhane, J. Mol. Struct., 2020, 1220, 128685 CrossRef CAS.
- A. Amininia, K. Pourshamsian and B. Sadeghi, Russ. J. Org. Chem., 2020, 56, 1279–1288 CrossRef CAS.
- N. Kaur and G. Kaur, Mater. Today Proc., 2022, 48, 1283–1300 CrossRef CAS.
- L.-C. Campeau and N. Hazari, Organometallics, 2019, 38, 3–35 CrossRef CAS PubMed.
- Z. J. Jain, P. S. Gide and R. S. Kankate, Arab. J. Chem., 2017, 10, S2051–S2066 CrossRef CAS.
- J.-A. García-López and M. F. Greaney, Chem. Soc. Rev., 2016, 45, 6766–6798 RSC.
- I. Maluenda and O. Navarro, Molecules, 2015, 20, 7528–7557 CrossRef CAS PubMed.
- T. Baran, J. Colloid Interface Sci., 2017, 496, 446–455 CrossRef CAS PubMed.
- T. Baran, N. Yılmaz Baran and A. Menteş, Appl. Organomet. Chem., 2018, 32, e4075 CrossRef.
- A. Khalafi-Nezhad and F. Panahi, J. Organomet. Chem., 2012, 717, 141–146 CrossRef CAS.
- F. Panahi, F. Daneshgar, F. Haghighi and A. Khalafi-Nezhad, J. Organomet. Chem., 2017, 851, 210–217 CrossRef CAS.
- M. Tukhani, F. Panahi and A. Khalafi-Nezhad, ACS Sustainable Chem. Eng., 2018, 6, 1456–1467 CrossRef CAS.
- E. Rezapour, M. Jafarpour and A. Rezaeifard, Catal. Lett., 2018, 148, 3165–3177 CrossRef CAS.
- M. Arghan, N. Koukabi and E. Kolvari, Appl. Organomet. Chem., 2019, e5075 CrossRef.
- H. Veisi, Z. Joshani, B. Karmakar, T. Tamoradi, M. M. Heravi and J. Gholami, Int. J. Biol. Macromol., 2021, 172, 104–113 CrossRef CAS PubMed.
- N. Koukabi and M. Arghan, Res. Chem. Intermed., 2022, 48, 4553–4577 CrossRef CAS.
- M. J. Gronnow, R. Luque, D. J. Macquarrie and J. H. Clark, Green Chem., 2005, 7, 552 RSC.
- V. L. Budarin, J. H. Clark, R. Luque, D. J. Macquarrie and R. J. White, Green Chem., 2008, 10, 382–387 RSC.
- S. Liu, Q. Zhou and H. Jiang, Chin. J. Chem., 2010, 28, 589–593 CrossRef CAS.
- A. Khalafi-Nezhad and F. Panahi, Green Chem., 2011, 13, 2408 RSC.
- S. Verma, J. Le Bras, S. L. Jain and J. Muzart, Dalton Trans., 2013, 42, 14454–14459 RSC.
- L. Fu, W. Deng, L. Liu and Y. Peng, Appl. Organomet. Chem., 2017, 31, e3853 CrossRef.
- D. Patra, S. Panja and A. Saha, Eur. J. Org Chem., 2020, 2020, 878–883 CrossRef CAS.
- Modern Carbonyl Chemistry, ed. J. Otera, Wiley-VCH, Weinheim, 1st edn, 2008 Search PubMed.
- K. Moriyama, M. Takemura and H. Togo, Org. Lett., 2012, 14, 2414–2417 CrossRef CAS PubMed.
- L. Tanwar, J. Börgel and T. Ritter, J. Am. Chem. Soc., 2019, 141, 17983–17988 CrossRef CAS PubMed.
- G. Urgoitia, R. SanMartin, M. Herrero and E. Domínguez, Catalysts, 2018, 8, 640 CrossRef.
- A. Heydari, M. Sheykhan, M. Sadeghi and I. Radfar, Inorg. Nano-Met. Chem., 2017, 47, 248–255 CrossRef CAS.
- R. K. Sodhi and S. Paul, Curr. Organocatal., 2018, 4, 196–208 CrossRef.
- L. Chen, J. Tang, L.-N. Song, P. Chen, J. He, C.-T. Au and S.-F. Yin, Appl. Catal., B, 2019, 242, 379–388 CrossRef CAS.
- M. N. Kopylovich, A. P. Ribeiro, E. C. Alegria, N. M. Martins, L. M. Martins and A. J. Pombeiro, Catalytic oxidation of alcohols, in Advances in Organometallic Chemistry, 2015, pp. 91–174 Search PubMed.
- S. Najafishirtari, K. Friedel Ortega, M. Douthwaite, S. Pattisson, G. J. Hutchings, C. J. Bondue, K. Tschulik, D. Waffel, B. Peng, M. Deitermann, G. W. Busser, M. Muhler and M. Behrens, Chemistry, 2021, 27, 16809–16833 CrossRef CAS PubMed.
- H. Puetz, E. Puchľová, K. Vranková and F. Hollmann, Catalysts, 2020, 10, 952 CrossRef CAS.
- A. S. Sharma, H. Kaur and D. Shah, RSC Adv., 2016, 6, 28688–28727 RSC.
- Y. Zhao, X. Yang, L. Zhan, S. Ou and J. Tian, J. Mater. Chem., 2011, 21, 4257 RSC.
- D. Yang, L. Gao and W. Zhao, Catal. Lett., 2008, 126, 84–88 CrossRef CAS.
- S. Verma, J. Le Bras, S. L. Jain and J. Muzart, Appl. Catal., A, 2013, 468, 334–340 CrossRef CAS.
- S. Verma, D. Tripathi, P. Gupta, R. Singh, G. M. Bahuguna, L. N. Shivakumar K, R. K. Chauhan, S. Saran and S. L. Jain, Dalton Trans., 2013, 42, 11522–11527 RSC.
- H. Sharma, M. Bhardwaj, M. Kour and S. Paul, Mol. Catal., 2017, 435, 58–68 CrossRef CAS.
- D. Tongsakul, S. Nishimura and K. Ebitani, ACS Catal., 2013, 3, 2199–2207 CrossRef CAS.
- H. Chong, G. Gao and G. Li, Chin. J. Chem. Phys., 2019, 32, 747–752 CrossRef CAS.
- R. Ravichandran, K. Annamalai, A. Annamalai and S. Elumalai, Colloids Surf., A, 2023, 664, 131117 CrossRef CAS.
- I. Ahmad, Shagufta and S. Rehman, Tetrahedron, 2022, 104, 132604 CrossRef CAS.
- J. Mielby and S. Kegnæs, Catal. Lett., 2013, 143, 1162–1165 CrossRef CAS.
- S. M. Danov, O. A. Kazantsev, A. L. Esipovich, A. S. Belousov, A. E. Rogozhin and E. A. Kanakov, Catal. Sci. Technol., 2017, 7, 3659–3675 RSC.
- M. Hong, M.-Y. Yao and H. Pan, RSC Adv., 2015, 5, 91558–91563 RSC.
- M. Hong and J.-M. Yan, Phosphorus, Sulfur Silicon Relat. Elem., 2017, 192, 985–988 CrossRef CAS.
- H. Büttner, L. Longwitz, J. Steinbauer, C. Wulf and T. Werner, Recent developments in the synthesis of cyclic carbonates from epoxides and CO2, in, Chemical Transformations of Carbon Dioxide, ed. X.-F. Wu and M. Beller, Springer International Publishing, Cham, 2018, pp. 89–144 Search PubMed.
- J. W. Comerford, I. D. V. Ingram, M. North and X. Wu, Green Chem., 2015, 17, 1966–1987 RSC.
- A. A. Marciniak, K. J. Lamb, L. P. Ozorio, C. J. Mota and M. North, Curr. Opin. Green Sustainable Chem., 2020, 26, 100365 CrossRef.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514 RSC.
- S. Kumar, S. L. Jain and B. Sain, Catal. Today, 2012, 198, 204–208 CrossRef CAS.
- V. Panwar, P. Kumar, A. Bansal, S. S. Ray and S. L. Jain, Appl. Catal., A, 2015, 498, 25–31 CrossRef CAS.
- S. Saranya, M. Neetha, T. Aneeja and G. Anilkumar, Adv. Synth. Catal., 2020, 362, 4543–4551 CrossRef CAS.
- H. Shen, X. Zhang, Q. Liu, J. Pan, W. Hu, Y. Xiong and X. Zhu, Tetrahedron Lett., 2015, 56, 5628–5631 CrossRef CAS.
- S. Verma, S. L. Jain and B. Sain, ChemCatChem, 2011, 3, 1329–1332 CrossRef CAS.
Footnote |
| † This review is dedicated to the late Prof. Dr Javad Safari for his passion and devotion to educating and leading a new generation of Iranian chemists. |
| This journal is © The Royal Society of Chemistry 2024 |