45S5 Bioglass analogue reinforced akermanite ceramic favorable for additive manufacturing mechanically strong scaffolds
Xiaoqing Wanga,
Lei Zhanga,
Xiurong Kea,
Juncheng Wanga,
Guojing Yang*a,
Xianyan Yangb,
Dongshuang Heb,
Huifeng Shaoc,
Yong Hec,
Jianzhong Fuc,
Sanzhong Xud and
Zhongru Gou*b
aRui’an People’s Hospital & the 3rd Affiliated Hospital to Wenzhou Medical University, Rui’an 325200, China. E-mail: Ygj.1008@163.com
bZhejiang-California International Nanosystems Institute, Zhejiang University, Hangzhou 310058, China. E-mail: zhrgou@zju.edu.cn; Tel: +86 571 8820 8353
cInstitute of Advanced Manufacturing Engineering, Department of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China
dThe First Affiliated Hospital, School of Medicine of Zhejiang University, Hangzhou 310003, China
First published on 23rd November 2015
Abstract
Calcium–magnesium silicate bioceramics have attracted increased interest in the development of porous scaffolds for bone tissue engineering applications, mainly due to their excellent bioactivity and ability to bond to hard tissue. However, the shaping of these bioceramics into complex porous constructs is challenging, and, especially, conventional high temperature pressureless sintering is not always an effective method to improve their mechanical properties without further compromising their biologically relevant performances. Here we developed a low melting-point bioactive glass (BG)-assisted sintering approach to improve the mechanical properties of akermanite ceramics with and without intentionally manufacturing macroporous structures. The experimental results indicated that the 4 wt% B2O3-containing 45S5 BG analogue could readily reinforce akermanite ceramics at a 20–40 wt% content, and material extrusion 3D-printing followed by a pressureless sintering process could be employed to fabricate high-strength porous scaffolds with compressive strength (∼36 MPa) ten times higher than those of pure akermanite porous ceramics. Moreover, the composite porous ceramics showed slower biodegradation in Tris buffer in vitro and this did not heavily affect the strength of their porous formulation over a long time period (6 weeks). It is proposed that 3D printing followed by an NCS-B-assisted sintering process represents an effective alternative for developing high strength bioceramic scaffolds potentially for the repair of load-bearing segmental bone defects.
1. Introduction
For bone defect repair, favorable mechanical strength, excellent bioactivity and the controlled degradation of porous biomaterials are needed to meet different clinical requirements. Calcium phosphates (CaPs) such as hydroxyapatite (HA) and biphasic calcium phosphate (BCP) from HA/β-tricalcium phosphate (β-TCP), have been widely used in synthetic bone replacement due to their chemical similarity to bone mineral. In vivo and in vitro studies have reported that CaPs, no matter which form (bulk, coating, or porous) or phase (crystalline or amorphous) that they are in, consistently support the attachment, proliferation, and differentiation of osteoblasts. However, clinical investigation has demonstrated that porous BCP and HA are virtually inert, remaining in the body for 3 to 7 years post-implantation.1–3 This should make BCP and HA less favored as scaffold materials for use in bone tissue engineering.The bioactivity of bioglass (BG) and glass-ceramics (GCs) is attributed to the formation of a bone-like carbonated hydroxyapatite (CHA) layer on their surface. BGs and GCs of specific compositions have been studied for over four decades since the milestone formulation of Hench’s 45S5 Bioglass®, which was shown to directly bond to bone mineral.4 The ability of 45S5 BG to enhance bone growth has been widely assessed, due to a possible effect on the gene expression of osteoblastic cells.5 Some attempts have also confirmed that BGs can be used as sintering aids to improve the mechanical and biological performances of bioceramics. Winkel et al.6 developed 13-93 BG reinforced HA ceramic sintering at a low temperature, and obtained oriented pore composite scaffolds. Lin et al. prepared 45S5 BG-reinforced wollastonite porous ceramics which showed appreciable compressive strength (∼109 MPa) at nearly 50% of open porosity when sintering at 1100 °C.7,8 However, the high strength of the latter was sacrificed for the porosity, and the low biodegradation nature of the former also limits its applications. In particular, in vivo studies have shown that the overfast degradation of wollastonite scaffolds may not match with new bone ingrowth during bone defect repair.9
Calcium–magnesium silicate ceramics have received increased interest due to their desirable bioactivity.10 Studies show that akermanite (Ca2MgSi2O7) ceramic is bioactive,11 and in vitro studies12 and in vivo evaluations in animal models13 have displayed better proliferation and osteogenesis activities on akermanite ceramic than on the clinically available β-TCP. Hence, it can be assumed that a suitable combination of akermanite with an appropriate BG to produce a porous formulation of their composites might render greater mechanical and biological functions compared to them alone.
On the other hand, the shaping of bioceramics into complex structures is challenging, especially for developing porous scaffolds for bone tissue engineering. Traditional methods such as direct foaming and sacrificial templates have limited control over the shape and dimension of the individual pores,14 thus the simultaneous control of shape and properties is difficult. In contrast, additive manufacturing technology (AMT) offers the potential to precisely replicate a geometry directly from a bone defect by adding material in a layer-by-layer approach. AMT is particularly useful for the shaping of complex and porous structures for bone repair.15 In the ceramic ink-based material extrusion 3D-printing system, the binder pre-mixed with ceramic ink is extruded through a micro-nozzle to form a filament and spread on the substrate, thus inscribing in the layer the corresponding cross-section of a 3D-model of the object to be built. Although this technique is simple and intriguing, the direct ceramic ink writing method is either confined to pressureless compacting and/or pressureless sintering, which impairs the densification and strength of the pore struts.16
Recently, we have designed a new low-melting-point BG, which showed high bioactivity and appreciable strength after sintering below 900 °C.17 The parent BG of this biomaterial was based on a composition of 45S5 Bioglass® with ∼4% B2O3 added to form a Na2O–2CaO–3SiO2-based BG (named as ‘NCS-B’). Thus, such a 45S5 BG analogue would be a promising co-firer for reinforcing bioceramics. Based on this hypothesis, the objective of this study is to investigate the effect of NCS-B on the sintering, structural, mechanical, and in vitro biological properties of akermanite/NCS-B (porous) ceramics with varying biphasic percent ratio (hereafter denoted as AKx/NCS-By; x = 100, 80, 60, 40; y = 0, 20, 40, 60). The experimental results confirmed that the AK60/NCS-B40 porous ceramic possessed high strength and slower biodissolution in vitro in comparison with pure akermanite, which may provide an insight into design rules for new porous bioceramics for large-area segmental bone defect repair.
2. Materials and methods
2.1 Preparation of akermanite and NCS-B powders
The NCS-B (CaO–SiO2–Na2O–P2O5–B2O3) was prepared using a melt-quenching method as described in our previous study.17 Akermanite powders were prepared using a sol–gel process using tetraethyl orthosilicate ((C2H5O)4Si, TEOS), Mg(NO3)2·6H2O, and Ca(NO3)2·4H2O as starting materials.11 The powders were planetary milled for 4 h to form superfine particles (<2.0 μm). Thermogravimetric and differential thermal analysis (TG/DTA) were carried out on the NCS-B powder using a TG/DTA6200 from TA Instruments with a 10 °C min−1 heating rate under an air atmosphere.2.2 Preparation of AKx/NCS-By GC composite samples
Cylindrical (∅ 6 × 2 mm, ∅ 8 × 10 mm, ∅ 25 × 4 mm) and cubic (45 × 8 × 6 mm) AKx/NCS-By compacts with 0%, 20%, 40%, and 60% NCS-B were prepared with a pressure of 10 MPa (see Table 1). Specifically, a polyvinyl alcohol (PVA; ∼6 kDa) solution (1.2% w/v) was prepared under magnetic stirring. Then the PVA solution was used to disperse the milled powders with a L/S mass ratio of 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pastes were kept at 60 °C for 8 h. The compact samples were prepared using uniaxial pressure in stainless steel moulds. Finally, the green compacts were sintered at 1000–1150 °C for 3 h in a muffle furnace with a heating rate of 3 °C min−1, followed by cooling naturally. The phase of the ceramics was examined using a Rigaku D/max-rA (Geigerflex) X-ray diffractometer (XRD) with a scanning rate of 0.02° min−1.
1. The pastes were kept at 60 °C for 8 h. The compact samples were prepared using uniaxial pressure in stainless steel moulds. Finally, the green compacts were sintered at 1000–1150 °C for 3 h in a muffle furnace with a heating rate of 3 °C min−1, followed by cooling naturally. The phase of the ceramics was examined using a Rigaku D/max-rA (Geigerflex) X-ray diffractometer (XRD) with a scanning rate of 0.02° min−1.
| Samples | Composition | Porosity (%) | ||||
|---|---|---|---|---|---|---|
| AK | NCS-B | 1000 °C | 1050 °C | 1100 °C | 1150 °C | |
| AK100/NCS-B0 | 100 | 0 | 10.8 ± 2.7 | 7.4 ± 2.1 | 5.6 ± 2.7 | 5.1 ± 1.3 |
| AK80/NCS-B20 | 80 | 20 | 6.5 ± 1.6 | 2.7 ± 0.8 | 2.2 ± 1.1 | 2.5 ± 1.7 |
| AK60/NCS-B40 | 60 | 40 | 5.7 ± 1.9 | 2.2 ± 1.4 | 1.8 ± 1.3 | 3.4 ± 2.4 |
| AK40/NCS-B60 | 40 | 60 | 3.4 ± 1.2 | 3.0 ± 2.4 | 3.5 ± 2.6 | 4.6 ± 2.6 |
2.3 Porosity test
Various heat treatment temperatures were considered and the densification of the ceramics was monitored by measuring the open pore porosity. The porosity was measured using a mercury porosimeter (AutoPore IV 9510). The Hg intrusion volume during the high pressure loading phase was determined in cm3 per unit sample weight.2.4 Evaluation of mechanical properties
The compressive and flexural strength of the ceramics (n = 8) were measured using a universal testing machine (Instron, Canton, MA) at a constant crosshead speed of 0.5 mm min−1. The mechanical tests followed the guidelines set in ASTM D5024-95a.2.5 In vitro bioactivity assessment in SBF
The 1100 °C-sintered samples (∅ 6 × 2 mm) were immersed in 8.0 mL SBF at 37 °C and the formation of CHA was monitored on the discs. After soaking for 1–7 d, the discs were washed with ethanol and observed using scanning electric microscopy (SEM; JEM-6700F, Japan), and a local chemical analysis was carried out using face-scanning energy dispersive X-ray spectroscopy (EDX). Prior to examination, the samples were coated with a thin layer of gold. All the immersion media were saved for inductively coupled plasma (ICP; Thermo) analysis of Mg, Si, B, Ca and P to measure the ionic concentrations.2.6 In vitro biodissolution test
In order to evaluate the in vitro biodissolution (weight loss) of the ceramics, the discs (W0; ∅ 25 × 4 mm) sintered at 1100 °C were immersed in 200 mL of Tris buffer, with an initial pH of 7.4 at 37 °C, simulating the body’s pH as a function of immersion time (up to 56 d). After immersing, every 3 d, 20% of the supernatant was extracted for ICP-OES measurements and an equal volume of fresh buffer was added. The disc samples were rinsed with ethanol, and then dried until a constant weight (Wt) was reached before weighing. The weight decrease was expressed using the following equation: weight loss = Wt/W0 × 100%.2.7 Material extrusion 3D printing of AKx/NCS-By BGC scaffolds
Taking into account the aggregation of the superfine AKx/NCS-By powders in PVA solution, the homogenous AKx/NCS-By ink was prepared by mixing 4 g of powder with 6.5(±0.2) g of aqueous carboxyl methyl cellulose solution (0.9 wt%). The printing device was based on a home-made three-axis positioning system. For the layer-by-layer (LbL) ceramic ink writing of the scaffolds (∼10 × 10 × 10 mm), the ceramic ink was added to a 5 mL syringe and extruded through a conical nozzle (400 μm) by the movement of a piston rod. A porous scaffold model with 3D periodic porous architecture was designed using software. The scaffolds used an initial distance between green filaments of ∼350 μm. The dosing pressure to the syringe pump was 0.95 bar and the moving speed of the dispensing unit was set to 3 mm s−1. The obtained scaffolds were dried at 65 °C, and then sintered respectively at 1050 °C and 1100 °C for 3 h using a heating rate of 2 °C min−1, and finally were cooled naturally.2.8 Statistical analysis
All data were expressed as mean ± standard deviation (SD) and analyzed with the one-way ANOVA. In all cases the results were considered statistically significant with a p-value less than 0.05.3. Results
3.1 Thermal analysis of the NCS-B powder
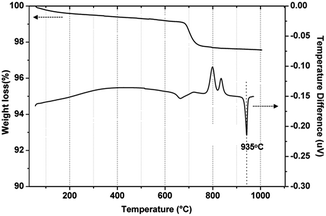
According to the TG-DTA analysis (Fig. 1), the principal characteristic was the presence of an endothermic melting peak (Tm) at ∼935 °C for the NCS-B powder. The intensities of exothermal peaks for this BG were very high at 790–860 °C, which could be attributed to the crystalline products of Na2Ca2Si3O9 and CaB2O4. Evidently, the former was consistent with the well-known crystalline phase of pure 45S5 BG.3.2 Phase analysis of the AKx/NCS-By ceramics
XRD analysis results for the AKx/NCS-By compacts after sintering at 1150 °C are shown in Fig. 2. The XRD patterns reveal information about the different crystalline phases with increasing NCS-B content. All compacts showed the presence of akermanite (PDF #74-0990), while the composites of AKx/NCS-By (y = 20, 40, 60) showed one additional crystalline phase which is attributed to Na2Ca2Si3O9 (PDF #22-1455). With the composite proportion varying in an orderly manner, the relative intensity of the characteristic XRD peaks of akermanite decreased and simultaneously that of Na2Ca2Si3O9 increased. Moreover, it should be mentioned that no phosphate crystalline phases or calcium borate could be detected in the ceramic composition using XRD, mainly due to their low content in the glass matrix. | ||
| Fig. 2 XRD patterns of the AKx/NCS-By compacts sintered at 1150 °C. (a) AK100/NCS-B0; (b) AK80/NCS-B20; (c) AK60/NCS-B40; (d) AK40/NCS-B60. | ||
3.3 Surface and fracture morphologies of AKx/NCS-By ceramics
The surface morphology and fracture microstructure of the AKx/NCS-By compacts were then investigated systematically using SEM observation. Fig. 3 shows the SEM images of typical AKx/NCS-By samples after sintering at 1150 °C. For the AK100/NCS-B0, the shape of particles in the surface layer almost remained unchanged. However, the composite samples showed grain growth, possibly due to the fluidizing of melted glass particles with increasing NCS-B content from 20% to 60%, and thus the loosely bonded grains could not be seen in the surface layer of AK40/NCS-B60.The effects of chemical composition on the fracture microstructure of the AKx/NCS-By compacts can be observed in Fig. 4. The presence of widespread closed pores with the increase of NCS-B content in the composites was observed. High densities of closed pores were present in the cross-section of the AK40/NCS-B60 (1050, 1100, 1150 °C), and the pore size was very large, ranging from submicrons to several microns. All of the NCS-B-rich ceramics presented a modified densification and the fractures were predominantly transgranular. In particular, the AK80/NCS-B20 and AK60/NCS-B40 (1050, 1100 °C) showed a denser structure with only very limited closed pores. In contrast, the AK100/NCS-B0 discs showed a high density of irregular open pores in the fracture structure, probably due to under-sintering.
 | ||
| Fig. 4 SEM micrographs of the cross-sectional microstructures of the AKx/NCS-By compacts after a bending strength test. The bar represents 20 μm (short) and 5 μm (long). | ||
3.4 Mechanical strength of AKx/NCS-By compacts
The porosities of the AKx/NCS-By compacts are summarized in Table 1. Evidently, AK100/NCS-B0 (≤1150 °C) showed a significantly high amount of open pores (from 10.8 ± 2.7% at 1000 °C to 5.1 ± 1.3% at 1150 °C), while the porosities of AK80/NCS-B20 and AK60/NCS-B40 (1050 °C, 1100 °C) were below 3.0%, implying markedly high densification for the latter two. Interestingly, the porosity of AK40/NCS-B60 increased with the increase in sintering temperature (1050–1150 °C). This result suggests that an overly high content of NCS-B in the composites would lead to significant liquid-phase sintering, and thus negatively affect the densification of the AKx/NCS-By composites.Fig. 5 shows the compressive and flexural strength of the AKx/NCS-By ceramics sintered at 1000–1150 °C. It can be seen that AK80/NCS-B20 (1050 °C) and AK60/NCS-B40 (1100 °C) showed 1.5 times higher compressive strength (>220 MPa) than the pure akermanite (i.e. AK100/NCS-B0; Fig. 5a). However, AK40/NCS-B60 (1150 °C) showed a marked strength decay (∼47 MPa), and the average compressive strengths (160–170 MPa) of AK80/NCS-B20 and AK60/NCS-B40 (1150 °C) were nearly 5 times higher than that of AK100/NCS-B0 (∼36 MPa). As for the flexural strength (Fig. 5b), the maximum strength was nearly 100 MPa for AK80/NCS-B20 (1100 °C). This value was nearly ten times higher than AK100/NCS-B0 sintered at the same temperature. Meanwhile, AK80/NCS-B20 and AK60/NCS-B4 showed an increase in flexural strength when sintered at 1000–1100 °C. AK80/NCS-B20 (1150 °C) exhibited considerably high flexural strength, which was three-fold higher than AK100/NCS-B0 (1150 °C). In contrast, AK100/NCS-B0 exhibited very limited flexural strength (<35 MPa). These mechanical data suggest that an appropriate amount of NCS-B (e.g. 20–40%) may readily reinforce akermanite-based dense bioceramics when sintering at 1050–1100 °C.
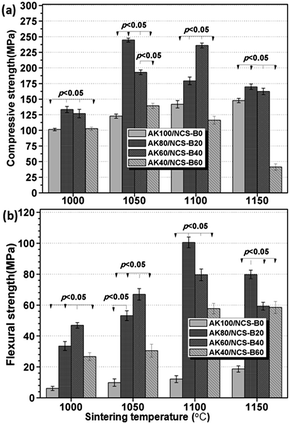 | ||
| Fig. 5 The compressive and flexural strength of AKx/NCS-By compacts sintered at different temperatures. | ||
3.5 CHA formation ability on the ceramic composites
Fig. 6 shows SEM micrographs of the AKx/NCS-By discs after immersion in SBF. After immersion for 1 and 4 d, it was occasionally observed that some globular structures precipitate on the surface of the samples containing ≤40% NCS-B, which might be identified as the first Ca-phosphate nuclei. All of the sample surface was completely covered by a continuous coating layer with the typical CHA morphology after immersion in SBF for 7 d. EDX spectra (Fig. 6, inset) for AK100/NCS-B0 and AK40/NCS-B60 showed that the chemical composition of the surface layer slightly differed in Ca/P ratio after 4 and 7 d of immersion in SBF, and the Ca/P ratio at 7 d (∼1.43–1.66) was similar to that in calcium-deficient CHA.Fig. 7 shows the change in Ca, P, Si, and Mg concentrations in SBF during the soaking of the AKx/NCS-By discs. The B concentration changed very slowly but was consistent with the boron content in the composites (not shown). Appreciable differences in Ca and P concentrations were observed for the four groups within 48 h, and there followed a similar trend in ion concentrations. For AK100/NCS-B0, the P and Si concentrations in SBF increased rapidly within 6 h and then P decreased abruptly. As for AK40/NCS-B60, the Ca, Si and Mg concentrations gradually increased and then stabilized at a certain value, but the P concentration experienced an increase and then decreased rapidly within 168 h, implying fast CHA deposition during the early stage of immersion in SBF. For AK80/NCS-B20 and AK60/NCS-B40, the P concentration had a similar trend showing a high increase within 12 h, followed by a moderate decrease by 168 h. Moreover, the Ca concentration also increased firstly and then changed mildly.
 | ||
| Fig. 7 Changes in Ca (a), P (b), Si (c), and Mg (d) concentration in SBF during soaking the AKx/NCS-By compacts sintered at 1100 °C. | ||
3.6 Biodissolution in vitro
Fig. 8 shows that the weight losses of the AKx/NCS-By ceramics sintered at 1100 °C were similar to each other after 7 d (∼2.4%), 28 d (∼5.2%), and 56 d (∼7.6%) of immersion time, whereas the weight loss of AK100/NCS-B0 in Tris buffer increased significantly with increasing time. The pure akermanite showed a weight loss of 6.6%, and ∼11% after immersion for 28 and 56 d, respectively. Meanwhile, the biodissolution rate of all AKx/NCS-By groups sintered at 1050 °C also showed a similar tendency but only displayed a slightly faster weight loss (not shown), probably due to looser structures in the ceramics. | ||
| Fig. 8 Weight loss of the AKx/NCS-By compacts sintered at 1100 °C after immersion in Tris buffers for different time periods. | ||
3.7 Structural characteristics of 3D printed AKx/NCS-By scaffolds
According to the quantitative analyses of the scaffold structure parameters, it was measured that the strut thickness, pore size and porosity were slightly decreased with increasing NCS-B content and sintering temperature from 1050 °C to 1100 °C (not shown). Specifically, AK80/NCS-B20 and AK60/NCS-B40 (1100 °C) exhibited a significantly appreciable pore size (∼327–340 μm) and open porosity (62–65%) compared to those of AK40/NCS-B60 sintered at the same temperature. These quantitative analyses suggest the higher the NCS-B content, the higher the shrinkage of the ceramic scaffolds during the sintering process.Fig. 9a and b shows optical images of the as-sintered scaffolds. The ceramic scaffolds had a uniform structure with a regular pore morphology. There were no marked changes in the microstructure along the length of the ceramic ink filaments. So each fracture surface would, in general, be representative of the whole construct. It is seen from the SEM images (Fig. 9c–f, inset) that the sintering process only improved the densification of the filament while avoiding the deformation of the pore structure. Instead of nearly rectangular pores in the AK100/NCS-B0 scaffolds, the incorporation of NCS-B resulted in a rectangular-to-round corner transformation around the pores with increasing sintering temperature, implying liquid-phase sintering in the ceramics. At 1100 °C, the shape of particles in AK100/NCS-B0 remained almost unchanged (Fig. 9c). In comparison, the micrographs of other ceramic particles show softening and the beginnings of bonding to each other (Fig. 9d and e). With increasing NCS-B up to 60% (e.g. AK40/NCS-B60; Fig. 9f), it appeared that the small pores or pits in the ceramic strut surface were hardly noticeable when sintering at 1100 °C.
3.8 Mechanical properties of 3D printed AKx/NCS-By scaffolds
The effect of sintering temperature and NCS-B content on the compressive strength and porosity of the macroporous scaffolds is shown in Fig. 10. Overall, the strength (in the direction parallel to the pore channel) increased from AK100/NCS-B0 to AK60/NCS-B40, and then decreased for AK40/NCS-B60. The AK80/NCS-B20 and AK60/NCS-B40 scaffolds sintered at 1050 °C showed more appreciable strength (19–36 MPa) than those sintered at 1100 °C. Furthermore, these strength values for the scaffolds sintered at 1050 °C were respectively nearly 6 and 10 times higher than that of AK100/NCS-B0 (1050 °C), though the open porosity of the latter was only ∼2.8% higher than those of the AK80/NCS-B20 and AK60/NCS-B40 composite scaffolds. Moreover, it should be mentioned that the compressive strength and porosity of the porous scaffolds slowly decreased and increased, respectively, with the prolongation of the immersion time. The AK60/NCS-B40 scaffolds (1050 °C) still had a compressive strength of ∼25 MPa after 4 weeks. This suggests that the 3D-printed macroporous scaffolds possess excellent mechanical stability. | ||
| Fig. 10 Changes in compressive strength and porosity of the 3D printed AKx/NCS-By scaffolds sintered at 1050 °C (a) and 1100 °C (b) before and after immersion in SBF. | ||
4. Discussion
The biphasic hybrid process, especially low melting-point phase-assisted sintering, is a widely applied technological process in ceramic science that involves incorporating a secondary phase of appropriate composition with a low melting-point into the substrate to yield hybrid materials with desirable properties and functions.18 For inorganic bioactive ceramics and glass-ceramics, secondary phase-assisted sintering is of fundamental importance in stabilizing a specific crystallographic phase, modifying mechanical properties, and modulating sintering behavior as well as tuning biological performances. In this study we described a BG-reinforced akermanite, to give simultaneous control over the biodegradation and micro-structures of the (macroporous) ceramics. We showed that the phase component (Ca2MgSi2O7–Na2Ca2Si3O9) and mechanical parameters of akermanite ceramic could be rationally tuned through the usage of highly bioactive, low melting-point NCS-B over a defined concentration range.Previous studies have shown that the flexural strength of akermanite ceramic was <40 MPa with pressureless sintering at below 1200 °C.19 In this study, it is seen that the NCS-B-assisted sintering approach can significantly enhance the flexural strength (over 8 times) of akermanite ceramics at a very low sintering temperature level (e.g. 1100 °C). The flexural strength (85–100 MPa) of AK80/NCS-B20 (1100 °C) is significantly optimized (Fig. 5b), which better matches with bone tissue (flexural strength: 50–150 MPa) compared to other low-temperature sintered Ca–Mg-silicate ceramics.20 Meanwhile, the AK80/NCS-B20 and AK60/NCS-B40 ceramics (1050, 1100 °C) show extremely high compressive strength (Fig. 5a), superior to the lower limit reported for cortical bone, and especially their strength value in compression for the porous formulation is over twice that of other stoichiometric Ca–Mg silicate porous ceramics21–23 and calcium phosphates,24,25 and ten and seven times higher than the pure akermanite scaffolds fabricated by ceramic ink writing in this study and laser sintering in ref. 26, respectively. Shuai’s group developed some laser-sintered Ca–/Ca–Mg silicate-based scaffolds via the incorporation of a secondary phase such as boron nitride, nano-titania, silicon carbide, and graphene.21,22,27,28 Unfortunately, the potential long-term effects in the living body of these bio-inert additives is still unknown. In comparison, this study provides a reliable strategy in that the addition of highly biocompatible and bioactive low-melt NCS-B is beneficial in improving mechanical properties with respect to the as-known inert phase or foreign ion doping routes.
According to previous studies, with a decrease in MgO content, the mechanical strengths of stoichiometric monticellite, akermanite, and merwinite ceramics decreased.29 It is indicated that Mg plays an important role in affecting the mechanical properties of bioceramics in Ca–Mg silicates, and the mechanical properties of the bioceramics in this system may be controlled by adjusting the Mg content. On the other hand, additions of BG, alone and with other metal oxides in various combinations, have been used to promote the densification of structural bioceramics.6–8,30,31 The presence of specific BG additives in ceramic powder compacts is believed to provide a liquid phase at sintering temperatures by a reaction with other components from the ceramic grain surfaces.32,33 The presence of BG causes obvious signs of discontinuous grain growth which accounts for the decrease in residual porosity and the relatively high strength.
In general, pure akermanite ceramic should be sintered at above 1300 °C to obtain appropriate mechanical strength.19 In fact, laser sintered akermanite scaffolds also showed very poor strength even if high temperature sintering was carried out.22 These investigation indicate that, therefore, the optimal sintering temperature for the AKx/NCS-By (porous) ceramics does not only satisfy the presence of a liquid phase in the composites, but the temperature is also helpful for the densification of the akermanite phase. In this regard, we found that the NCS-B aid could significantly reinforce the mechanical strengths of the dense or porous AKx/NCS-By ceramics at 1050–1100 °C (Fig. 4). This is evidently attributed to the part liquid-phase sintering in the composite ceramics. To our knowledge, our study is an important attempt to confirm the effect of the incorporation of low-melt BG on the mechanical evolution of a Ca–Mg silicate bioceramic system. It is known that borate BG has a lower melting point than silicate BG.34 In view of this, it can be seen from the presented results that the appropriate amount of NCS-B occupies the interface of the akermanite grains in the dense compacts or pore struts, with the ceramics exhibiting a more dense structure and higher strength. Accordingly, the NCS-B in akermanite readily improves the sintering behavior, structural densification, and mechanical strength of the (porous) ceramics.
On the other hand, because the grain growth and phase conversion of low-melt BG are very sensitive to sintering temperature, liquid-phase sintering of low melting-point BG-rich products usually occurs at temperatures below 1200 °C. Abnormal grain growth in low melting-point BG would occur in the final stage of densification if the sintering temperature is too high, which has a great influence on the flexural strength with increasing grain size. Pure akermanite ceramic has poor sinterability and it is difficult to obtain a dense sintering body below 1200 °C, which leads to very poor flexural strength (<50 MPa). In contrast, the microstructural properties of AK80/NCS-B20 and AK60/NCS-B40 ceramics sintered at 1050–1100 °C have demonstrated that the small amount of NCS-B (≤40%) readily contributes to assisting sintering. The densification of AKx/NCS-By ceramics is apparently influenced by the sintering temperature and NCS-B, and especially sintering densification is easily achieved in the AKx/NCS-By ceramics. This is confirmed by the ultrahigh relative density (>97%) of AK80/NCS-B20 and AK60/NCS-B40 (1050, 1100 °C) and the appreciable value (>96%) for AK40/NCS-B60 (1050, 1100 °C). As a result, the compressive strength and flexural strength of AKx/NCS-By ceramics were both significantly enhanced with the incorporation of NCS-B up to 20–40% (Fig. 5). In general, the relative density and average crystal size of ceramics significantly affect the mechanical properties.35 The high sintering temperatures of low-melting point BG often result in extreme grain coarsening and flawed structures.36 This is also evidenced in AK40/NCS-B60 (1050, 1100 °C), where the ceramic fracture surface shows significant difference. An increase in sintering temperature up to 1150 °C causes the small pores to disappear as a result of crystal-boundary migration and grain growth, which may explain the decrease in the mechanical strength of (porous) AK40/NCS-B60. Moreover, the densification of the composite bioceramics is also affected by some other factors including grain morphology, grain size and the thermal expansion coefficient of biphasic ceramics, and these factors probably influence the flexural strength and compressive strength. It is reasonable to assume that the porosity mainly affects the compressive strength, but the grain morphology and size contribute to the flexural strength. Therefore, it is difficult to obtain optimized sintering temperature conditions to endow the highest compressive strength and flexural strength. Indeed, to get better mechanical strength, the optimum sintering temperature for AK80/NCS-B20 and AK60/NCS-B40 specimens is 1050–1100 °C.
Previous studies have demonstrated that the CHA plays an essential role in the maintenance of the tissue–biomaterial interface, and this apatite layer could be reproduced in SBF.37 In this study, the decrease in P concentration in SBF after reaching maximum values indicates that such ions could be incorporated in the layer formed on the surface of the ceramics. However, the increasing trend of Ca concentration as well as the decrease in P concentration in SBF within 4 d suggest that the solubility and apatite-formation ability of AKx/NCS-By ceramics increase with increasing NCS-B content. The retardation in apatite formation on the pure akermanite ceramics could be attributed to the influence of no P releasing to the solution, decreasing the rate of formation of certain CaPs, as already reported.38 Our results obtained using SEM-EDX analysis showed that a calcium-deficient apatite layer fully covered the AK40/NCS-B60 surfaces after immersion for 4 d, but the AK100/NCS-B0 ceramics displayed a slight difference in apatite-formation ability. According to the mechanisms of nucleation and growth of biomimetic CHA on the inorganic biomaterials, the rate of apatite formation increased with the increase in material dissolution.39 Hence, although there was a slight decrease in the solubility of AKx/NCS-By ceramics with adding NCS-B (Fig. 7), the existence of the highly bioactive Na2Ca2Si3O9, which is the crystalline phase of 45S5 BG-derived GC, readily promotes the apatite mineralization rate (Fig. 6). Conversely, this is substantially favorable for overcoming the relatively fast dissolution rate of porous bioceramics that could compromise their mechanical strength in vitro and in vivo.40,41
As demonstrated in our previous studies,17 NCS-B exhibits good low temperature sintering ability, and the higher NCS-B content in AKx/NCS-By results in increased densification, which leads to a slower ion leaching rate from the surface layer of the ceramics. Therefore, AK40/NCS-B60 should undergo less biodissolution in aqueous solution and the leaching ion rate should be lower (Fig. 7). Hence, with the increase in NCS-B content, the degradation rate decreased with the increase in the grain bonding from pure akermanite to AK40/NCS-B60 ceramic, resulting in slower Ca ion release. This is also in agreement with the experimental results of the weight loss of the biomaterials when immersing in Tris buffer (Fig. 8).
In addition, the biocompatibility of akermanite has been validated by some previous studies.42 Meanwhile, the 45S5 BG and some borate-containing 45S5 GC analogues have also demonstrated good biocompatibility and osteoconductive activity in vivo.43–45 Moreover, long-term intravenous administration studies have demonstrated that CaO–SiO2–P2O5–B2O3 GC showed no toxic effects on rats of either sex.46 In this regard, it is reasonable to consider that the AKx/NCS-By bioceramics would be highly cytocompatible due to their retarded ion release acceleration in vitro in comparison with pure akermanite (Fig. 7). Also, the in vivo biological response of such mechanically strong porous constructs is expected since the evaluation of the spinal fusion animal model is under investigation and promises to yield more intriguing experimental results in the near future.
5. Conclusion
In summary, this investigation demonstrates that a low-melt BG reinforcing approach can be used to modulate the mechanical and biological properties of akermanite-based dense and porous bioceramics using the conventional pressureless sintering technique. In vitro SBF immersion experiments revealed that the appropriate amount (e.g. 40%) of NCS-B addition showed appreciable apatite formation ability similar to pure akermanite, and the highly biocompatible BG reinforcement substantially contributed to the structural and strength reliability in such a medium, which is particularly beneficial for enhancing osteogenic cell activity and bone regeneration. This finding suggests that our AK60/NCS-B40 porous scaffold is well suited for use in bone repair—for example, in in situ bone regeneration or in tissue engineering.Acknowledgements
This work is supported by the National Science Foundation of China (81271956, 81301326, 51372218), the Zhejiang Provincial Natural Science Foundation of China (LZ14E020001, LQ14H060003), and the Science and Technology Department of Zhejiang Province Foundation (2015C33119, 2014C33202).References
- P. Weiss, P. Layrolle, L. P. Clergeau, B. Enckel, P. Pilet, Y. Amouriq, G. Daculsi and B. Giumelli, Biomaterials, 2007, 28, 3295–3305 CrossRef CAS PubMed.
- M. Marcacci, E. Kon, V. Moukhachev, A. Lavroukov, S. Kutepov, R. Quarto, M. Mastrogiacomo and R. Cancedda, Tissue Eng., 2007, 13, 947–955 CrossRef CAS PubMed.
- R. Quarto, M. Mastrogiacomo, R. Cancedda, S. M. Kutepov, V. Mukhache, A. Lavroukov, E. Kon and M. Marcacci, N. Engl. J. Med., 2001, 344, 385–386 CrossRef CAS PubMed.
- L. L. Hench, J. Am. Ceram. Soc., 1991, 74, 1487–1510 CrossRef CAS.
- L. L. Hench, J. Eur. Ceram. Soc., 2009, 29, 1257–1265 CrossRef CAS.
- A. Winkel, R. Meszaros, S. Reinsch, R. Muller, N. Travitzky, T. Fey, P. Greil and L. Wondraczek, J. Am. Ceram. Soc., 2012, 95, 3387–3393 CrossRef CAS.
- K. L. Lin, J. Chang, Z. W. Liu, Y. Zeng and R. X. Shen, J. Eur. Ceram. Soc., 2009, 29, 2937–2943 CrossRef CAS.
- K. L. Lin, S. Y. Ni, J. Chang, W. Y. Zhai and W. M. Gu, Key Eng. Mater., 2007, 330–332, 181–184 CrossRef CAS.
- S. Xu, K. Lin, Z. Wang, J. Chang, L. Wang, J. Lu and C. Ning, Biomaterials, 2008, 29, 2588–2596 CrossRef CAS PubMed.
- C. Wu and J. Chang, J. Biomed. Mater. Res., Part B, 2007, 83, 153–160 CrossRef PubMed.
- C. T. Wu and J. Chang, J. Biomater. Appl., 2006, 21, 119–129 CrossRef CAS PubMed.
- H. L. Sun, C. T. Wu, K. R. Dai, J. Chang and T. T. Tang, Biomaterials, 2006, 27, 5651–5657 CrossRef CAS PubMed.
- Y. Huang, X. G. Jin, X. L. Zhang, H. L. Sun, J. W. Tu, T. T. Tang, J. Chang and K. R. Dai, Biomaterials, 2009, 30, 5041–5048 CrossRef CAS PubMed.
- D. Lin, Q. Nian, B. W. Deng, S. Y. Jin, Y. W. Hu, W. Q. Wang and G. J. Cheng, ACS Nano, 2014, 8, 9710–9715 CrossRef CAS PubMed.
- S. Bose, S. Vahabzadeh and A. Bandyopadhyay, Mater. Today, 2013, 16, 496–504 CrossRef CAS.
- N. Travitzky, A. Bonet, B. Dermeik, T. Fey, I. Filbert-Demut, L. Schlier, T. Schlordt and P. Greil, Adv. Eng. Mater., 2014, 16, 729–745 CrossRef CAS.
- K. Xie, L. Zhang, X. Yang, G. Yang, X. Wang, L. Zhang, H. Shao, Y. He, J. Fu and Z. Gou, Biomedical Glasses, 2015, 1, 81–93 CrossRef.
- J. R. Jones, Acta Biomater., 2013, 9, 4457–4486 CrossRef CAS PubMed.
- C. T. Wu and J. Chang, J. Biomater. Appl., 2006, 21, 119–129 CrossRef CAS PubMed.
- M. Diba, O. M. Goudouri, F. Tapia and A. R. Boccaccini, Curr. Opin. Solid State Mater. Sci., 2014, 18, 147–167 CrossRef CAS.
- C. J. Shuai, C. D. Gao, P. Feng and S. P. Peng, RSC Adv., 2014, 4, 12782–12788 RSC.
- P. Feng, C. D. Gao, C. J. Shuai and S. P. Peng, RSC Adv., 2015, 5, 3498–3507 RSC.
- C. T. Wu, J. A. Chang, W. Y. Zhai, S. Y. Ni and J. Y. Wang, J. Biomed. Mater. Res., Part B, 2006, 78, 47–55 CrossRef PubMed.
- G. A. Fielding, A. Bandyopadhyay and S. Bose, Dent. Mater., 2011, 28, 113–122 CrossRef PubMed.
- D. M. Liu, Ceram. Int., 1997, 23, 135–139 CrossRef CAS.
- Z. Han, P. Feng, C. Gao, Y. Shen, C. Shuai and S. Peng, Bio-Med. Mater. Eng., 2014, 24, 2073–2080 CAS.
- C. Shuai, Z. Han, P. Feng, C. Gao, T. Xiao and S. Peng, J. Mater. Sci.: Mater. Med., 2015, 26, 188 CrossRef PubMed.
- Z. K. Han, C. D. Gao, P. Feng, Y. Shen, C. J. Shuai and S. P. Peng, RSC Adv., 2014, 4, 36868–36874 RSC.
- X. C. Chen, J. Ou, Y. Wei, Z. B. Huang, Y. Q. Kang and G. F. Yin, J. Mater. Sci.: Mater. Med., 2010, 21, 1463–1471 CrossRef CAS PubMed.
- M. A. Lopes, J. D. Santos, F. J. Monteiro and J. C. Knowles, J. Biomed. Mater. Res., 1998, 39, 244–251 CrossRef CAS PubMed.
- C. Bergmann, M. Lindner, W. Zhang, K. Koczur, A. Kirsten, R. Telle and H. Fischer, J. Eur. Ceram. Soc., 2010, 30, 2563–2567 CrossRef CAS.
- C. Y. Huang, Z. T. Zhang and Z. L. Tang, J. Mater. Sci. Lett., 1999, 18, 1815–1816 CrossRef CAS.
- X. Yu, S. Cai, G. Xu, W. Zhou and D. Wang, J. Mater. Sci.: Mater. Med., 2009, 20, 2025–2034 CrossRef CAS PubMed.
- H. Segawa, T. Igarashi, T. Sakamoto, K. Mizuno, T. Konishi and S. Inoue, Int. J. Appl. Glass Sci., 2010, 1, 378–387 CrossRef CAS.
- R. W. Rice, J. Mater. Sci., 1996, 31, 1969–1983 CrossRef CAS.
- L. Lin, L. Zhang, J. Wang, K. Xie, X. Yang, X. Chen, G. Yang, C. Gao and Z. Gou, Mater. Lett., 2014, 126, 154–1458 CrossRef CAS.
- T. Kokubo, H. Kushitani, S. Saka, T. Kitsgi, T. Kitsugi and T. Yamamuro, J. Biomed. Mater. Res., 1990, 24, 721–734 CrossRef CAS PubMed.
- P. Ducheyne, S. Radin and L. King, J. Biomed. Mater. Res., 1993, 27, 25–34 CrossRef CAS PubMed.
- L. L. Hench and K. J. West, Life Chem. Rep., 1996, 13, 187–241 CAS.
- C. Wu, J. Chang, W. Zhai, S. Ni and J. Wang, J. Biomed. Mater. Res., Part B, 2006, 78, 47–55 CrossRef PubMed.
- J. H. Lee, H.-S. Ryu, J.-H. Seo, D.-Y. Lee, B.-S. Chang and C.-K. Lee, Clinics in Orthopedic Surgery, 2014, 6, 87–95 CrossRef PubMed.
- C. Chen, P. Watkins-Curry, M. Smoak, K. Hogan, S. Deese, G. T. McCandless, J. Y. Chan and D. J. Hayes, ACS Biomater. Sci. Eng., 2015, 1, 94–102 CrossRef CAS.
- M. Hamadouche, A. Meunier, D. C. Greenspan, C. Blanchat, J. P. Zhong, G. P. La Torre and L. Sedel, J. Biomed. Mater. Res., 2001, 54, 560–566 CrossRef CAS PubMed.
- Z. Xie, X. Liu, W. Jia, C. Zhang, W. Huang and J. Wang, J. Controlled Release, 2009, 139, 118–126 CrossRef CAS PubMed.
- J. H. Lee, C.-K. Lee, B.-S. Chang, H.-S. Ryu, J.-H. Seo, K. S. Hong and H. Kim, J. Biomed. Mater. Res., Part A, 2006, 77, 362–569 CrossRef PubMed.
- J. H. Lee, H.-S. Ryu, J.-H. Seo, B.-S. Chang and C.-K. Lee, Drug Chem. Toxicol., 2010, 33, 38–47 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |