Cu(I)-catalyzed microwave-assisted synthesis of 1,2,3-triazole linked with 4-thiazolidinones: a one-pot sequential approach†
Yogesh Kumarab,
Akanksha Mattaa,
Prashant Kumara,
Virinder S. Parmarab,
Erik V. Van der Eyckenb and
Brajendra K. Singh*a
aBioorganic Laboratory, Department of Chemistry, University of Delhi, Delhi 110 007, India. E-mail: singhbk@chemistry.du.ac.in
bLaboratory for Organic & Microwave-Assisted Chemistry (LOMAC), Department of Chemistry, University of Leuven (KU Leuven), Celestijnenlaan 200F, B-3001 Leuven, Belgium
First published on 24th November 2014
Abstract
A novel copper(I) catalyzed, microwave-assisted one-pot, four-component sequential reaction between a propargyloxybenzaldehyde, a substituted phenyl azide, a substituted aniline and thioglycolic acid has been developed for the synthesis of 3-phenyl-2-[4-{(1-phenyl-1H-1,2,3-triazol-4-yl)methoxy}phenyl]thiazolidin-4-ones.
Introduction
Thiazolidinones and 1,2,3-triazoles represent important classes of drugs in medicinal chemistry. They are among the most extensively investigated compounds by biochemists and medicinal chemists.1 Thiazolidinones in particular show interesting anticancer,2 anti-HIV,3 antimalarial,4 tuberculostatic,5 antihistaminic,6 anticonvulsant,7 antibacterial8 and antiarrythmic9 activities. Similarly, various triazole derivatives possess antifungal,10 anticancer,11 antituberculosis12 and antimicrobial13 activities.So called hybrid molecules have been shown to be highly active and effective in medicinal chemistry. Synergistic effects are obtained via hybridization of two different bioactive moieties with complementary pharmacophoric functions, or with different modes of action.14 The confirmation of this hypothesis has been well established in previous studies of 4-thiazolidinones coupled with other heterocyclic fragments,15 i.e. resulting in high antitumor activity. As a result, we have planned the synthesis of linked thiazolidinone–triazole hybrid molecules. 4-Thiazolidinones have been conveniently synthesized by a three-component condensation of a primary amine, an aldehyde, and either a mercaptoacetic or mercaptopropanoic acid.15 This cyclocondensation could be accelerated with N,N′-dicyclohexylcarbodiimide (DCC),16 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU),17 γ-ferrite,18 ZnCl2,19 sodium sulfate,20 [bmim][PF6],21 and activated fly ash.22 The use of microwave irradiation23 and polymer supported systems24 has also been reported. However, the main bottleneck of these protocols is mostly harsh reaction conditions, prolonged heating, and the need for simultaneous removal of water to accelerate the cyclocondensation. On the other hand, the synthesis of 1,2,3-triazoles has been reported by various methods,25 like copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction26 and microwave-assisted one-pot reaction of an alkyl halide, sodium azide and an alkyne.27 We have recently reported the formation of 1,2,3-triazoles using D-glucose as a reducing agent for the copper catalyst.28 Very recently, metal free synthesis of 1,2,3-triazoles has been also developed.29
Microwave irradiation is an alternative heating technique based on the transformation of electromagnetic energy into heat. Often this method increases the rate of chemical reactions30 and results in higher yields.
In recent years, multicomponent reactions (MCRs)28b,31 have received increasing attention due to their simplicity, efficiency, atom economy, shortened reaction times, and the possibility for diversity-oriented synthesis. The combination of MCRs with transition metal-catalysis gives access to complex molecules in few steps as compared to traditional multistep processes. We have investigated the application of microwave irradiation for the synthesis of our hybrid molecules.
Results and discussion
In view of the useful applications of 4-thiazolidinones and 1,2,3-triazoles, we have developed a new microwave-assisted Cu(I)-catalyzed one-pot, two step sequential synthesis via reaction of an propargyloxybenzaldehyde, an aryl azide, an aniline and thioglycolic acid under microwave irradiation. This synthesis could be achieved by three different pathways A, B and C (Scheme 1). The simple one could be a two-step synthesis where either triazole ring formation takes place, followed by the thiazolidinone ring (Path A) or vice versa, i.e. formation of the thiazolidinone ring followed by the triazole ring (Path B). However, alternatively, one-pot, two-step synthesis could be employed, where initially 4-(prop-2-yn-1-yloxy)benzaldehyde and an aryl azide are reacted followed by addition of the aniline and thioglycolic acid, to form the desired hybrid molecule (Path C). Interesting to mention here that the second step where aniline and thioglycolic acid react with triazolyated intermediate didn't proceed under conventional heating. Moreover, attempt to succeed this reaction in a single step, one-pot manner was not feasible even under microwave irradiation in contrast to the sequential addition of the reactants as shown in Path C (Scheme 1).Our initial investigation was focused on the screening of various solvents to achieve the best yield under microwave irradiation (Table 1). The reaction was unsuccessful when carried out in polar aprotic solvents like tetrahydrofuran (THF), acetonitrile (MeCN), 1,4-dioxane and non polar aprotic solvent like toluene, while the reaction went to completion when carried out in polar protic solvents, like ethanol (EtOH) and methanol (MeOH) resulting in the formation of the desired product in 33% and 29% yield, respectively (Table 1, entries 5 and 6). To our delight, when the reaction was carried out in an aqueous mixture of toluene or THF, decent amounts of compound 8a were obtained (Table 1, entries 7 and 8). The reaction did not run to completion when it was carried out in chloroform or dioxane mixed with water (Table 1, entries 9 and 10). Among the screened aqueous solvent, THF–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) appeared to be the most effective (Table 1, entry 8).
1) appeared to be the most effective (Table 1, entry 8).
| Entry | Solvent | Temp. (°C) | Time (min) Ist step +2nd step | Yieldb (%) |
|---|---|---|---|---|
| a The first step involves the reaction of 4-(prop-2-yn-1-yloxy)benzaldehyde (3) (1.8 mmol), phenyl azide (5a) (2.2 mmol), CuSO4·5H2O (0.35 mmol) and D-glucose (0.75 mmol) in different solvents under microwave irradiation for the indicated time and temperature at 100 W maximum power, followed by the addition of aniline (7a) (2.2 mmol) and thioglycolic acid (6) (2.2 mmol) in the second step.b Isolated yields, c = no reaction. | ||||
| 1 | Toluene | 110 | 20 + 35 | c |
| 2 | THF | 70 | 20 + 25 | c |
| 3 | MeCN | 100 | 20 + 30 | c |
| 4 | 1,4-Dioxane | 100 | 5 + 10 | c |
| 5 | EtOH | 85 | 15 + 55 | 33 |
| 6 | MeOH | 80 | 5 + 40 | 29 |
| 7 | Toluene–H2O, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
90 | 5 + 35 | 50 |
| 8 | THF–H2O, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
70 | 15 + 40 | 75 |
| 9 | CHCl3–H2O, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 10 + 30 | c |
| 10 | Dioxane–H2O, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 10 + 30 | c |
| 11 | MeCN–H2O, (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
75 | 15 + 70 | 17 |
To investigate the scope and limitations, various phenyl azides 5a–c and anilines 7a–i were evaluated employing the optimized conditions. All the screened azides and anilines reacted smoothly with propargyloxybenzaldehyde (3) and thioglycolic acid (6) and afforded the expected desired products 8a–d′ (Table 2) in good yields. Anilines bearing either an electron donating group or electron withdrawing group were well tolerated.
| Entry | Compd | R1 | R2 | Time (min) (Ist step)b + IInd step | Yieldc (%) |
|---|---|---|---|---|---|
| a The first step involves the reaction of 4-(prop-2-yn-1-yloxy)benzaldehyde (3) (1.8 mmol), phenyl azide (5a–c) (2.2 mmol), CuSO4·5H2O (0.35 mmol) and D-glucose (0.75 mmol) in THF–H2O solvent under microwave irradiation for an appropriate time and temperature at 100 W maximum power, followed by the addition of aniline (7a–i) (2.2 mmol) and thioglycollic acid (6) (2.2 mmol).b 15 min.c Isolated yields. | |||||
| 1 | 8a | H | H | 40 | 75 |
| 2 | 8b | H | 4-COCH3 | 50 | 82 |
| 3 | 8c | H | 3-COCH3 | 45 | 76 |
| 4 | 8d | H | 4-CH3 | 40 | 75 |
| 5 | 8e | H | 3-CH3 | 40 | 84 |
| 6 | 8f | H | 4-OCH3 | 40 | 82 |
| 7 | 8g | H | 2-OCH3 | 40 | 76 |
| 8 | 8h | H | 4-Br | 45 | 62 |
| 9 | 8i | H | 3-F | 40 | 71 |
| 10 | 8j | CH3 | H | 45 | 85 |
| 11 | 8k | CH3 | 4-COCH3 | 50 | 69 |
| 12 | 8l | CH3 | 3-COCH3 | 40 | 74 |
| 13 | 8m | CH3 | 4-CH3 | 40 | 87 |
| 14 | 8n | CH3 | 3-CH3 | 45 | 80 |
| 15 | 8o | CH3 | 4-OCH3 | 40 | 9 |
| 16 | 8p | CH3 | 2-OCH3 | 40 | 70 |
| 17 | 8q | CH3 | 4-Br | 40 | 68 |
| 18 | 8r | CH3 | 3-F | 40 | 76 |
| 19 | 8s | OCH3 | H | 45 | 76 |
| 20 | 8t | OCH3 | 4-COCH3 | 40 | 76 |
| 21 | 8u | OCH3 | 3-COCH3 | 40 | 83 |
| 22 | 8v | OCH3 | 4-CH3 | 45 | 79 |
| 23 | 8w | OCH3 | 3-CH3 | 50 | 73 |
| 24 | 8x | OCH3 | 4-OCH3 | 40 | 87 |
| 25 | 8y | OCH3 | 2-OCH3 | 40 | 80 |
| 26 | 8z | OCH3 | 4-Br | 40 | 74 |
| 27 | 8a′ | OCH3 | 3-F | 40 | 75 |
| 28 | 8b′ | OCH3 | 4-F | 40 | 78 |
| 29 | 8c′ | OCH3 | 4-Cl | 50 | 85 |
| 30 | 8d′ | OCH3 | 2-Cl | 50 | 77 |
Conclusions
In summary, we have developed a convenient route for the synthesis of thaizolidinones linked triazoles through a Cu(I)-catalyzed one-pot sequential approach. The products could be isolated in good yields. Work is ongoing to investigate the biological properties of these novel heterocyclic compounds.Experimental section
General information
All microwave assisted experiments were run in a closed vial applying a dedicated CEM-Discover monomode microwave apparatus operating at a frequency of 2.45 GHz with continuous irradiation power from 0 to 300 W (CEM Corporation, P.O. Box 200, Matthews, NC 28106). Analytical TLCs were performed on Merck silica gel 60F254 plates. All liquid column chromatographic separations were performed on column chromatography. IR spectra were recorded on a Perkin-Elmer 2000 FT-IR spectrometer at Department of Chemistry, University of Delhi. The 1H and 13C NMR spectra (in CDCl3) were recorded on a JEOL ECX-400P NMR/Bruker Avance at 400 MHz/300 MHz and 100 MHz/75 MHz, respectively at USIC, University of Delhi/Katholiek University Leuven, TMS was used as internal standard. The NMR spectra were processed by JEOL Delta™ NMR data processing software, the chemical shift values are on a δ scale and coupling constants (J) are in ppm and Hz respectively. Abbreviations used are: s (singlet), d (doublet), t (triplet), dd (double doublet) and m (multiplet). The high-resolution mass spectral data was obtained using a JEOL JMS-SX-102A spectrometer at Institute for Chemistry and Biochemistry, Free University Berlin, Germany. Melting points were recorded on a Buchi M-560 melting point apparatus and are uncorrected. All the chemicals and reagents like phenol, aniline, thioglycolic acid and propargyl bromide were purchased from commercial sources and used as received unless otherwise indicated.General procedure for the synthesis32 of 4-(prop-2-yn-1-yloxy) benzaldehyde or propargyloxybenzaldehyde (3)
A mixture of 4-hydroxy benzaldehyde (1 mmol) and propargyl bromide (1.2 mmol) in DMF (2 mL) as solvent was stirred with K2CO3 at r.t. for 24 h. The progress of the reaction was monitored on TLC [ethyl acetate–petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)]. After completion of the reaction, the mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was directly used in the next step without any further purification.
4)]. After completion of the reaction, the mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was directly used in the next step without any further purification.
General procedure for the synthesis33 of azidobenzene and its derivatives (5a–c)
A mixture of appropriate aniline 4a–c (1 mmol) in HCl (17%, 5 mL) was stirred at 0 °C. Sodium nitrite (1.5 equiv., dissolved in 5 mL of water) was added dropwise and stirring continued at 0 °C. After 15 min, sodium azide (1.5 equiv., dissolved in 5 mL of water) was added dropwise at 0 °C and the mixture was stirred for 3–4 h. The progress of the reaction was monitored by TLC [ethyl acetate–petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)]. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was directly used in the next step without any further purification.
5)]. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was directly used in the next step without any further purification.
Synthesis of 3-phenyl-2-(4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)thiazolidin-4-one and its derivatives (8a–d′)
A mixture of the alkyne (1 mmol), the appropriate azide (1.2 mmol), CuSO4·5H2O (0.2 equiv.) and D-glucose were taken in THF–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in microwave transparent glass vial equipped with a small magnetic stirring bar, and the vial was tightly sealed with a Teflon crimp cap. The mixture was then irradiated for 15 min at 70 °C and 100 W maximum power. The reaction mixture was cooled to r.t. and aniline (1.2 mmol) and thioglycolic acid (1.2 mmol) were added. The reaction mixture was irradiated again for appropriate time (40–50 min) at 70 °C and 100 W maximum power. The progress of the reaction was monitored by TLC [ethyl acetate–petroleum ether (1
1) in microwave transparent glass vial equipped with a small magnetic stirring bar, and the vial was tightly sealed with a Teflon crimp cap. The mixture was then irradiated for 15 min at 70 °C and 100 W maximum power. The reaction mixture was cooled to r.t. and aniline (1.2 mmol) and thioglycolic acid (1.2 mmol) were added. The reaction mixture was irradiated again for appropriate time (40–50 min) at 70 °C and 100 W maximum power. The progress of the reaction was monitored by TLC [ethyl acetate–petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)]. After completion of the reaction, the mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography over silica gel to yield the desired products 8a–d′.
2)]. After completion of the reaction, the mixture was extracted with ethyl acetate (3 × 50 mL). The combined ethyl acetate layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography over silica gel to yield the desired products 8a–d′.
It was obtained as white solid having m.p. 169–171 °C in 75% yield. IR (KBr) νmax (cm−1) = 2931 (C–H, Ar), 1689 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1226 (C–O), 765 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.00 (s, 1H, H-5), 7.72 (dd, J = 1.5, 6.5 Hz, 2H, Ar), 7.53–7.45 (m, 3H, Ar), 7.29–7.23 (m, 4H, Ar), 7.17–7.12 (m, 3H, Ar), 6.92 (dd, J = 2.1, 6.5 Hz, 2H, Ar), 6.07 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.98 (dd, J = 1.5, 16.1 Hz, 1H, H-9′′), 3.87 (d, J = 16.0 Hz, 1H, H-9′′); 13C NMR (75 MHz, CDCl3) δ 170.918 (C
O), 1226 (C–O), 765 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.00 (s, 1H, H-5), 7.72 (dd, J = 1.5, 6.5 Hz, 2H, Ar), 7.53–7.45 (m, 3H, Ar), 7.29–7.23 (m, 4H, Ar), 7.17–7.12 (m, 3H, Ar), 6.92 (dd, J = 2.1, 6.5 Hz, 2H, Ar), 6.07 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.98 (dd, J = 1.5, 16.1 Hz, 1H, H-9′′), 3.87 (d, J = 16.0 Hz, 1H, H-9′′); 13C NMR (75 MHz, CDCl3) δ 170.918 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.51 (C-1′′), 144.56, 137.45, 136.90, 131.89, 129.80, 129.09, 128.95, 128.64, 127.12, 125.89, 120.95, 120.61, 115.01, 65.31 (C-7′′), 61.99 (–OCH2), 33.58 (C-9′′); HRMS calcd for C24H20N4O2SH: 429.5141; found [M + H]+: 429.5215.
O), 158.51 (C-1′′), 144.56, 137.45, 136.90, 131.89, 129.80, 129.09, 128.95, 128.64, 127.12, 125.89, 120.95, 120.61, 115.01, 65.31 (C-7′′), 61.99 (–OCH2), 33.58 (C-9′′); HRMS calcd for C24H20N4O2SH: 429.5141; found [M + H]+: 429.5215.It was obtained as a white solid with a m.p. of 123–125 °C in 82% yield. IR (KBr) νmax (cm−1) = 2923 (–C–H, Ar), 1679 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1599 (C
O), 1599 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1267 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.86 (d, J = 7.5 Hz, 2H, Ar), 7.72 (d, J = 7.32 Hz, 2H, Ar), 7.54–7.42 (m, 3H, Ar), 7.34–7.22 (m, 4H, Ar), 7.31 (d, J = 8.05 Hz, 2H, Ar), 7.25–7.21 (m, 2H, Ar), 6.93 (d, J = 8.02 Hz, 2H, Ar), 6.19 (s, 1H, H-7′′), 5.27 (s, 2H, –OCH2), 3.96 (d, J = 16.2 Hz, 1H, H-9′′), 3.86 (d, J = 16.2 Hz, 1H, H-9′′), 2.52 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.00 (–COCH3), 171.03 (C-10′′), 158.59 (C-1′′), 144.39, 141.70, 136.81, 134.80, 131.18, 129.78, 129.11, 128.98, 128.34, 124.63, 121.02, 120.56, 115.15, 64.67 (C-7′′), 61.90 (–OCH2), 33.59 (C-9′′), 26.49 (–COCH3); HRMS calcd for C26H22N4O3SH: 471.5508; found [M + H]+: 471.5514.
O), 1267 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.86 (d, J = 7.5 Hz, 2H, Ar), 7.72 (d, J = 7.32 Hz, 2H, Ar), 7.54–7.42 (m, 3H, Ar), 7.34–7.22 (m, 4H, Ar), 7.31 (d, J = 8.05 Hz, 2H, Ar), 7.25–7.21 (m, 2H, Ar), 6.93 (d, J = 8.02 Hz, 2H, Ar), 6.19 (s, 1H, H-7′′), 5.27 (s, 2H, –OCH2), 3.96 (d, J = 16.2 Hz, 1H, H-9′′), 3.86 (d, J = 16.2 Hz, 1H, H-9′′), 2.52 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.00 (–COCH3), 171.03 (C-10′′), 158.59 (C-1′′), 144.39, 141.70, 136.81, 134.80, 131.18, 129.78, 129.11, 128.98, 128.34, 124.63, 121.02, 120.56, 115.15, 64.67 (C-7′′), 61.90 (–OCH2), 33.59 (C-9′′), 26.49 (–COCH3); HRMS calcd for C26H22N4O3SH: 471.5508; found [M + H]+: 471.5514.It was obtained as a brown semi solid in 76% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1685 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1240 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.74–7.70 (m, 4H, Ar), 7.55–7.44 (m, 5H, Ar) 7.38–7.36 (m, 2H, Ar), 6.92 (dd, J = 2.2, 6.5 Hz, 2H, Ar), 6.14 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.98 (dd, J = 1.7, 14.6 Hz, 1H, H-9′′), 3.88 (d, J = 15.6 Hz, 1H, H-9′′), 2.50 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.08 (–COCH3), 171.12 (C-10′′), 158.59 (C-1′′), 144.38, 137.84, 137.76, 136.89, 131.16, 130.47, 129.77, 129.32, 128.98, 128.73, 126.89, 125.23, 121.02, 120.56, 115.06, 65.06 (C-7′′), 61.83 (–OCH2), 33.54 (C-9′′), 26.56 (–COCH3); HRMS calcd for C26H22N4O3SH: 471.5508; found [M + H]+: 471.5521.
O), 1240 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.74–7.70 (m, 4H, Ar), 7.55–7.44 (m, 5H, Ar) 7.38–7.36 (m, 2H, Ar), 6.92 (dd, J = 2.2, 6.5 Hz, 2H, Ar), 6.14 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.98 (dd, J = 1.7, 14.6 Hz, 1H, H-9′′), 3.88 (d, J = 15.6 Hz, 1H, H-9′′), 2.50 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.08 (–COCH3), 171.12 (C-10′′), 158.59 (C-1′′), 144.38, 137.84, 137.76, 136.89, 131.16, 130.47, 129.77, 129.32, 128.98, 128.73, 126.89, 125.23, 121.02, 120.56, 115.06, 65.06 (C-7′′), 61.83 (–OCH2), 33.54 (C-9′′), 26.56 (–COCH3); HRMS calcd for C26H22N4O3SH: 471.5508; found [M + H]+: 471.5521.It was obtained as a yellowish solid with a m.p. of 166.0–168.0 °C in 75% yield. IR (KBr) νmax (cm−1) = 2924 (C–H, Ar), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1239 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.70 (d, J = 8.05 Hz, 2H, Ar), 7.49 (d, J = 7.32 Hz, 2H, Ar) 7.45–7.41 (m, 1H, Ar), 7.24–7.21 (m, 2H, Ar), 7.04 (d, J = 7.32 Hz, 2H, Ar), 6.97 (d, J = 8.05 Hz, 2H, Ar), 6.90 (d, J = 8.05 Hz, 2H, Ar), 6.00 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.95 (d, J = 16.1 Hz, 1H, H-9′′), 3.85 (d, J = 16.1 Hz, 1H, H-9′′), 2.22 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.12 (C-10′′), 158.42 (C-1′′), 144.45, 137.07, 136.78, 134.62, 131.92, 129.70, 128.89, 128.64, 125.84, 120.99, 120.52, 114.89, 65.35 (C-7′′), 61.88 (–OCH2), 33.46 (C-9′′), 20.93 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5414.
O), 1239 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.70 (d, J = 8.05 Hz, 2H, Ar), 7.49 (d, J = 7.32 Hz, 2H, Ar) 7.45–7.41 (m, 1H, Ar), 7.24–7.21 (m, 2H, Ar), 7.04 (d, J = 7.32 Hz, 2H, Ar), 6.97 (d, J = 8.05 Hz, 2H, Ar), 6.90 (d, J = 8.05 Hz, 2H, Ar), 6.00 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.95 (d, J = 16.1 Hz, 1H, H-9′′), 3.85 (d, J = 16.1 Hz, 1H, H-9′′), 2.22 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.12 (C-10′′), 158.42 (C-1′′), 144.45, 137.07, 136.78, 134.62, 131.92, 129.70, 128.89, 128.64, 125.84, 120.99, 120.52, 114.89, 65.35 (C-7′′), 61.88 (–OCH2), 33.46 (C-9′′), 20.93 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5414.It was obtained as a yellowish solid with a m.p. of 181–183 °C in 84% yield. IR (KBr) νmax (cm−1) = 2924 (C–H, Ar), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1238 (C–O), 759 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.70 (d, J = 7.32 Hz, 2H, Ar), 7.52–7.49 (m, 2H, Ar), 7.45–7.41 (m, 1H, Ar), 7.24–7.21 (m, 2H, Ar), 7.13–7.09 (m, 1H, Ar), 6.95–6.85 (m, 5H, Ar), 6.02 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95 (d, J = 1.46, 16.11 Hz, 1H, H-9′′), 3.84 (d, J = 16.11 Hz, 1H, H-9′′), 2.23 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.96 (C-10′′), 158.43 (C-1′′), 144.50, 139.00, 137.24, 136.82, 131.96, 129.75, 128.93, 128.81, 128.60, 128.05, 126.66, 122.93, 120.96, 120.56, 114.91, 65.36 (C-7′′), 61.90 (–OCH2), 33.52 (C-9′′), 21.29 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5419.
O), 1238 (C–O), 759 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.70 (d, J = 7.32 Hz, 2H, Ar), 7.52–7.49 (m, 2H, Ar), 7.45–7.41 (m, 1H, Ar), 7.24–7.21 (m, 2H, Ar), 7.13–7.09 (m, 1H, Ar), 6.95–6.85 (m, 5H, Ar), 6.02 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95 (d, J = 1.46, 16.11 Hz, 1H, H-9′′), 3.84 (d, J = 16.11 Hz, 1H, H-9′′), 2.23 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.96 (C-10′′), 158.43 (C-1′′), 144.50, 139.00, 137.24, 136.82, 131.96, 129.75, 128.93, 128.81, 128.60, 128.05, 126.66, 122.93, 120.96, 120.56, 114.91, 65.36 (C-7′′), 61.90 (–OCH2), 33.52 (C-9′′), 21.29 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5419.It was obtained as a brown semi solid in 82% yield. IR (KBr) νmax (cm−1) = 2926 (C–H, Ar), 1676 (C
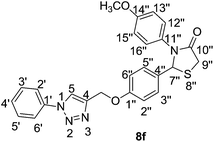
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1215 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.72 (dd, J = 2.0, 7.6 Hz, 2H, Ar), 7.55–7.44 (m, 3H, Ar), 7.26–7.22 (m, 2H, Ar), 7.00–6.91 (m, 4H, Ar), 6.79–6.76 (m, 2H, Ar), 5.96 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.97 (dd, J = 1.7, 16.11 Hz, 1H, H-9′′), 3.86 (d, J = 16.0 Hz, 1H, H-9′′) 3.71 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.14 (C-10′′), 158.52 (C-1′′), 144.51, 136.84, 131.91, 129.90, 129.77, 128.95, 128.83, 127.62, 120.97, 120.58, 114.91, 114.42, 65.67 (C-7′′), 61.89 (–OCH2), 55.28 (–OCH3), 33.40 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5417.
O), 1215 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.02 (s, 1H, H-5), 7.72 (dd, J = 2.0, 7.6 Hz, 2H, Ar), 7.55–7.44 (m, 3H, Ar), 7.26–7.22 (m, 2H, Ar), 7.00–6.91 (m, 4H, Ar), 6.79–6.76 (m, 2H, Ar), 5.96 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.97 (dd, J = 1.7, 16.11 Hz, 1H, H-9′′), 3.86 (d, J = 16.0 Hz, 1H, H-9′′) 3.71 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.14 (C-10′′), 158.52 (C-1′′), 144.51, 136.84, 131.91, 129.90, 129.77, 128.95, 128.83, 127.62, 120.97, 120.58, 114.91, 114.42, 65.67 (C-7′′), 61.89 (–OCH2), 55.28 (–OCH3), 33.40 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5417.It was obtained as a white solid with a m.p. of 168.0–170.0 °C in 76% yield. IR (KBr) νmax (cm−1) = 2926 (C–H, Ar), 1686 (C
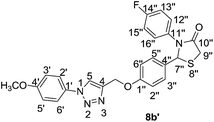
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1236 (C–O), 761 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.00 (s, 1H, H-5), 7.69 (d, J = 8.79 Hz, 2H, Ar), 7.52–7.41 (m, 3H, Ar), 7.26–7.24 (m, 2H, Ar), 6.87–6.82 (m, 4H, Ar), 6.77–6.74 (m, 1H, Ar), 6.05 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 3.90–3.80 (m, 2H, H-9′′), 3.79 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.52 (C-10′′), 158.45 (C-1′′), 154.74, 144.50, 136.78, 131.32, 130.29, 129.74, 129.49, 129.39, 128.94, 125.24, 120.94, 120.71, 120.54, 114.53, 111.81, 64.35 (C-7′′), 61.79 (–OCH2), 55.59 (–OCH3), 33.24 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5409.
O), 1236 (C–O), 761 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 8.00 (s, 1H, H-5), 7.69 (d, J = 8.79 Hz, 2H, Ar), 7.52–7.41 (m, 3H, Ar), 7.26–7.24 (m, 2H, Ar), 6.87–6.82 (m, 4H, Ar), 6.77–6.74 (m, 1H, Ar), 6.05 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 3.90–3.80 (m, 2H, H-9′′), 3.79 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.52 (C-10′′), 158.45 (C-1′′), 154.74, 144.50, 136.78, 131.32, 130.29, 129.74, 129.49, 129.39, 128.94, 125.24, 120.94, 120.71, 120.54, 114.53, 111.81, 64.35 (C-7′′), 61.79 (–OCH2), 55.59 (–OCH3), 33.24 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5409.It was obtained as a yellow solid with a m.p. of 87.0–89.0 °C in 62% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1685 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1216 (C–O), 758 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H, H-5), 7.72 (d, J = 8.05 Hz, 2H, Ar), 7.53–7.43 (m, 3H, Ar), 7.38 (d, J = 8.79 Hz, 2H, Ar), 7.22 (d, J = 8.05 Hz, 2H, Ar), 7.02 (d, J = 8.05 Hz, 2H, Ar), 6.93 (d, J = 8.05 Hz, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.25 (s, 2H, –OCH2), 3.95 (d, J = 16.11 Hz, 1H, H-9′′), 3.85 (d, J = 15.38 Hz, 1H, H-9′′); 13C NMR (100 MHz, CDCl3) δ 170.90 (C-10′′), 158.62 (C-1′′), 144.49, 136.85, 136.45, 132.18, 131.32, 129.79, 128.99, 128.60, 127.23, 121.00, 120.59, 115.11, 65.05 (C-7′′), 61.94 (–OCH2), 33.48 (C-9′′); HRMS calcd for C24H19BrN4O2SH: 508.4102; found [M + H]+: 508.4110.
O), 1216 (C–O), 758 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.03 (s, 1H, H-5), 7.72 (d, J = 8.05 Hz, 2H, Ar), 7.53–7.43 (m, 3H, Ar), 7.38 (d, J = 8.79 Hz, 2H, Ar), 7.22 (d, J = 8.05 Hz, 2H, Ar), 7.02 (d, J = 8.05 Hz, 2H, Ar), 6.93 (d, J = 8.05 Hz, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.25 (s, 2H, –OCH2), 3.95 (d, J = 16.11 Hz, 1H, H-9′′), 3.85 (d, J = 15.38 Hz, 1H, H-9′′); 13C NMR (100 MHz, CDCl3) δ 170.90 (C-10′′), 158.62 (C-1′′), 144.49, 136.85, 136.45, 132.18, 131.32, 129.79, 128.99, 128.60, 127.23, 121.00, 120.59, 115.11, 65.05 (C-7′′), 61.94 (–OCH2), 33.48 (C-9′′); HRMS calcd for C24H19BrN4O2SH: 508.4102; found [M + H]+: 508.4110.It was obtained as a white solid with a m.p. of 156.0–158.0 °C in 71% yield. IR (KBr) νmax (cm−1) = 2926 (C–H, Ar), 1675 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1243 (C–O), 760 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.72 (dd, J = 1.09, 8.01 Hz, 2H, Ar), 7.54–7.51 (m, 2H, Ar), 7.47–7.43 (m, 1H, Ar), 7.25–7.19 (m, 3H, Ar), 6.98–6.82 (m, 5H, Ar), 6.07 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.95 (dd, J = 1.6, 16.1 Hz, 1H, H-9′′), 3.85 (d, J = 16.1 Hz, 1H, H-9′′); 13C NMR (100 MHz, CDCl3) δ 170.94 (C-10′′), 158.60 (C-1′′), 144.47, 131.44, 129.79, 128.96, 128.43, 120.93, 120.60, 115.12, 114.01, 113.84, 113.08, 112.85, 64.99 (C-7′′), 61.97 (–OCH2), 33.48 (C-9′′); HRMS calcd for C24H19FN4O2SH: 447.1291; found [M + H]+: 447.1296.
O), 1243 (C–O), 760 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.01 (s, 1H, H-5), 7.72 (dd, J = 1.09, 8.01 Hz, 2H, Ar), 7.54–7.51 (m, 2H, Ar), 7.47–7.43 (m, 1H, Ar), 7.25–7.19 (m, 3H, Ar), 6.98–6.82 (m, 5H, Ar), 6.07 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.95 (dd, J = 1.6, 16.1 Hz, 1H, H-9′′), 3.85 (d, J = 16.1 Hz, 1H, H-9′′); 13C NMR (100 MHz, CDCl3) δ 170.94 (C-10′′), 158.60 (C-1′′), 144.47, 131.44, 129.79, 128.96, 128.43, 120.93, 120.60, 115.12, 114.01, 113.84, 113.08, 112.85, 64.99 (C-7′′), 61.97 (–OCH2), 33.48 (C-9′′); HRMS calcd for C24H19FN4O2SH: 447.1291; found [M + H]+: 447.1296.It was obtained as a dark brown solid with a m.p. of 152.0–154.0 °C in 85% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1701 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1228 (C–O), 826 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H, H-5), 7.72 (d, J = 8.05 Hz, 2H, Ar), 7.54–7.43 (m, 4H, Ar), 7.26–7.23 (m, 1H, Ar), 7.06 (d, J = 7.32 Hz, 2H, Ar), 6.99 (d, J = 8.05 Hz, 2H, Ar), 6.92 (d, J = 8.05 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.97 (d, J = 15.38 Hz, 1H, H-9′′), 3.87 (d, J = 16.11 Hz, 1H, H-9′′), 2.24 (s, 2H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.48 (C-10′′), 158.93 (C-1′′), 144.97, 137.59, 137.29, 135.14, 132.44, 130.22, 129.41, 129.16, 126.35, 121.50, 121.03, 115.40, 65.87 (C-7′′), 62.40 (–OCH2), 33.97 (C-9′′), 21.45 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5401.
O), 1228 (C–O), 826 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H, H-5), 7.72 (d, J = 8.05 Hz, 2H, Ar), 7.54–7.43 (m, 4H, Ar), 7.26–7.23 (m, 1H, Ar), 7.06 (d, J = 7.32 Hz, 2H, Ar), 6.99 (d, J = 8.05 Hz, 2H, Ar), 6.92 (d, J = 8.05 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.97 (d, J = 15.38 Hz, 1H, H-9′′), 3.87 (d, J = 16.11 Hz, 1H, H-9′′), 2.24 (s, 2H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.48 (C-10′′), 158.93 (C-1′′), 144.97, 137.59, 137.29, 135.14, 132.44, 130.22, 129.41, 129.16, 126.35, 121.50, 121.03, 115.40, 65.87 (C-7′′), 62.40 (–OCH2), 33.97 (C-9′′), 21.45 (–CH3); HRMS calcd for C25H22N4O2SH: 443.5407; found [M + H]+: 443.5401.It was obtained as a white solid with a m.p. of 137.0–139.0 °C in 69% yield. IR (KBr) νmax (cm−1) = 2917 (C–H, Ar), 1683 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1227 (C–O), 750 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.86 (d, J = 8.6 Hz, 2H, Ar), 7.58 (d, J = 8.6 Hz, 2H, Ar), 7.33–7.29 (m, 4H, Ar), 7.23 (d, J = 8.6 Hz, 1H, Ar), 7.21 (m, 1H, Ar), 6.93 (d, J = 8.00 Hz, 2H, Ar), 6.18 (s, 1H, H-7), 5.22 (s, 2H, –OCH2), 3.95 (d, J = 16.8 Hz, 1H, H-9′′), 3.87 (d, J = 16.8 Hz, 1H, H-9′′), 2.51 (s, 3H, –COCH3), 2.41 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 197.05 (–COCH3), 170.97 (C-10′′), 158.49 (C-1′′), 144.31, 141.67, 139.21, 134.85, 134.57, 131.14, 130.26, 129.13, 128.32, 124.62, 120.98, 120.47, 115.17, 64.91 (C-7′′), 61.69 (–OCH2), 33.60 (C-9′′), 26.53 (–COCH3), 21.08 (–CH3); HRMS calcd for C27H24N4O3SH: 485.5774; found [M + H]+: 485.5770.
O), 1227 (C–O), 750 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.86 (d, J = 8.6 Hz, 2H, Ar), 7.58 (d, J = 8.6 Hz, 2H, Ar), 7.33–7.29 (m, 4H, Ar), 7.23 (d, J = 8.6 Hz, 1H, Ar), 7.21 (m, 1H, Ar), 6.93 (d, J = 8.00 Hz, 2H, Ar), 6.18 (s, 1H, H-7), 5.22 (s, 2H, –OCH2), 3.95 (d, J = 16.8 Hz, 1H, H-9′′), 3.87 (d, J = 16.8 Hz, 1H, H-9′′), 2.51 (s, 3H, –COCH3), 2.41 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 197.05 (–COCH3), 170.97 (C-10′′), 158.49 (C-1′′), 144.31, 141.67, 139.21, 134.85, 134.57, 131.14, 130.26, 129.13, 128.32, 124.62, 120.98, 120.47, 115.17, 64.91 (C-7′′), 61.69 (–OCH2), 33.60 (C-9′′), 26.53 (–COCH3), 21.08 (–CH3); HRMS calcd for C27H24N4O3SH: 485.5774; found [M + H]+: 485.5770.It was obtained as a dark brown semi solid in 74% yield. IR (KBr) νmax (cm−1) = 2923 (C–H, Ar), 1682 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1240 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.73–7.70 (m, 2H, Ar), 7.57 (d, J = 8.72 Hz, 2H, Ar), 7.36–7.35 (m, 2H, Ar), 7.30–7.26 (m, 4H, Ar), 6.91 (d, J = 8.70 Hz, 2H, Ar), 6.13 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 3.97 (d, J = 16.03 Hz, 1H, H-9′′), 3.88 (d, J = 16.03 Hz, 1H, H-9′′), 2.49 (s, 3H, –COCH3), 2.40 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 197.01 (–COCH3), 171.06 (C-10′′), 158.55 (C-1′′), 144.19, 138.89, 137.76, 134.48, 131.03, 130.44, 130.21, 129.28, 128.69, 126.84, 125.21, 120.98, 120.43, 115.03, 65.02 (C-7′′), 61.84 (–OCH2), 33.52 (C-9′′), 26.53 (–COCH3), 21.04 (–CH3); HRMS calcd for C27H24N4O3SH: 485.5774; found [M + H]+: 485.5779.
O), 1240 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.73–7.70 (m, 2H, Ar), 7.57 (d, J = 8.72 Hz, 2H, Ar), 7.36–7.35 (m, 2H, Ar), 7.30–7.26 (m, 4H, Ar), 6.91 (d, J = 8.70 Hz, 2H, Ar), 6.13 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 3.97 (d, J = 16.03 Hz, 1H, H-9′′), 3.88 (d, J = 16.03 Hz, 1H, H-9′′), 2.49 (s, 3H, –COCH3), 2.40 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 197.01 (–COCH3), 171.06 (C-10′′), 158.55 (C-1′′), 144.19, 138.89, 137.76, 134.48, 131.03, 130.44, 130.21, 129.28, 128.69, 126.84, 125.21, 120.98, 120.43, 115.03, 65.02 (C-7′′), 61.84 (–OCH2), 33.52 (C-9′′), 26.53 (–COCH3), 21.04 (–CH3); HRMS calcd for C27H24N4O3SH: 485.5774; found [M + H]+: 485.5779.It was obtained as a white solid with a m.p. of 159.0–161.0 °C in 87% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1690 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1232 (C–O), 819 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.59 (d, J = 2.48, 6.4 Hz, 2H, Ar), 7.31 (d, J = 8.4 Hz, 2H, Ar), 7.25–7.23 (m, 2H, Ar), 7.06 (d, J = 8.4 Hz, 2H, Ar), 7.04–6.97 (m, 2H, Ar), 6.93 (dd, J = 2.4, 6.8 Hz, 2H, Ar), 6.01 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.97 (dd, J = 1.6, 15.6 Hz, 1H, H-9′′), 3.86 (d, J = 15.6 Hz, 1H, H-9′′), 2.42 (s, 3H, –CH3), 2.24 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.02 (C-10′′), 158.47 (C-1′′), 144.30, 139.08, 137.12, 134.63, 134.53, 131.90, 130.22, 129.73, 128.66, 125.87, 120.94, 120.45, 114.90, 65.39 (C-7′′), 61.87 (–OCH2), 33.48 (C-9′′), 21.05 (–CH3), 20.96 (–CH3); HRMS calcd for C26H24N4O2SH: 457.5673; found [M + H]+: 457.5679.
O), 1232 (C–O), 819 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.59 (d, J = 2.48, 6.4 Hz, 2H, Ar), 7.31 (d, J = 8.4 Hz, 2H, Ar), 7.25–7.23 (m, 2H, Ar), 7.06 (d, J = 8.4 Hz, 2H, Ar), 7.04–6.97 (m, 2H, Ar), 6.93 (dd, J = 2.4, 6.8 Hz, 2H, Ar), 6.01 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.97 (dd, J = 1.6, 15.6 Hz, 1H, H-9′′), 3.86 (d, J = 15.6 Hz, 1H, H-9′′), 2.42 (s, 3H, –CH3), 2.24 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.02 (C-10′′), 158.47 (C-1′′), 144.30, 139.08, 137.12, 134.63, 134.53, 131.90, 130.22, 129.73, 128.66, 125.87, 120.94, 120.45, 114.90, 65.39 (C-7′′), 61.87 (–OCH2), 33.48 (C-9′′), 21.05 (–CH3), 20.96 (–CH3); HRMS calcd for C26H24N4O2SH: 457.5673; found [M + H]+: 457.5679.It was obtained as a white solid with a m.p. of 227.0–229.0 °C in 80% yield. IR (KBr) νmax (cm−1) = 2926 (C–H, Ar), 1676 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1237 (C–O), 824 (C–S–C); NMR (300 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.58 (dd, J = 2.4, 8.4 Hz, 2H, Ar), 7.32–7.21 (m, 4H, Ar), 7.16–7.1028 (m, 1H, Ar), 7.97–6.86 (m, 5H, Ar), 6.04 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.97 (dd, J = 2.4, 21.2 Hz, 2H, H-9′′), 3.85 (d, J = 15.3 Hz, 1H, H-9′′), 2.41 (s, 3H, –CH3), 2.21 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.50 (C-10′′), 158.95 (C-1′′), 144.78, 139.57, 137.60, 135.12, 135.01, 132.38, 130.70, 130.22, 129.15, 126.35, 121.42, 120.94, 115.38, 65.88 (C-7′′), 62.36 (–OCH2), 33.96 (C-9′′), 21.53 (–CH3), 21.44 (–CH3); HRMS calcd for C26H24N4O2SH: 457.5673; found [M + H]+: 457.5670.
O), 1237 (C–O), 824 (C–S–C); NMR (300 MHz, CDCl3) δ 7.97 (s, 1H, H-5), 7.58 (dd, J = 2.4, 8.4 Hz, 2H, Ar), 7.32–7.21 (m, 4H, Ar), 7.16–7.1028 (m, 1H, Ar), 7.97–6.86 (m, 5H, Ar), 6.04 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.97 (dd, J = 2.4, 21.2 Hz, 2H, H-9′′), 3.85 (d, J = 15.3 Hz, 1H, H-9′′), 2.41 (s, 3H, –CH3), 2.21 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.50 (C-10′′), 158.95 (C-1′′), 144.78, 139.57, 137.60, 135.12, 135.01, 132.38, 130.70, 130.22, 129.15, 126.35, 121.42, 120.94, 115.38, 65.88 (C-7′′), 62.36 (–OCH2), 33.96 (C-9′′), 21.53 (–CH3), 21.44 (–CH3); HRMS calcd for C26H24N4O2SH: 457.5673; found [M + H]+: 457.5670.It was obtained as a yellowish solid with a m.p. of 123.0–125.0 °C in 69% yield. IR (KBr) νmax (cm−1) = 2923 (C–H, Ar), 1678 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1246 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.60 (d, J = 8.4 Hz, 2H, Ar), 7.32 (d, J = 8.0 Hz, 2H, Ar), 7.26–7.23 (m, 2H, Ar), 7.00 (dd, J = 2.0, 6.8 Hz, 2H, Ar), 6.94 (d, J = 8.4 Hz, 2H, Ar), 6.79 (dd, J = 2.7, 7.3 Hz, 2H, Ar), 5.96 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.98 (dd, J = 1.6, 15.6 Hz, 1H, H-9′′), 3.87 (d, J = 16.0 Hz, 1H, H-9′′), 3.73 (s, 3H, –OCH3), 2.43 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.04 (C-10′′), 158.53 (C-1′′), 144.30, 139.12, 134.50, 131.90, 130.26, 129.93, 128.82, 127.62, 120.98, 120.48, 114.90, 114.41, 65.62 (C-7′′), 61.90 (–OCH2), 55.28 (–OCH3), 33.40 (C-9′′), 21.07 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5660.
O), 1246 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.60 (d, J = 8.4 Hz, 2H, Ar), 7.32 (d, J = 8.0 Hz, 2H, Ar), 7.26–7.23 (m, 2H, Ar), 7.00 (dd, J = 2.0, 6.8 Hz, 2H, Ar), 6.94 (d, J = 8.4 Hz, 2H, Ar), 6.79 (dd, J = 2.7, 7.3 Hz, 2H, Ar), 5.96 (s, 1H, H-7′′), 5.24 (s, 2H, –OCH2), 3.98 (dd, J = 1.6, 15.6 Hz, 1H, H-9′′), 3.87 (d, J = 16.0 Hz, 1H, H-9′′), 3.73 (s, 3H, –OCH3), 2.43 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.04 (C-10′′), 158.53 (C-1′′), 144.30, 139.12, 134.50, 131.90, 130.26, 129.93, 128.82, 127.62, 120.98, 120.48, 114.90, 114.41, 65.62 (C-7′′), 61.90 (–OCH2), 55.28 (–OCH3), 33.40 (C-9′′), 21.07 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5660.It was obtained as a white solid with a m.p. of 237.0–239.0 °C in 70% yield. IR (KBr) νmax (cm−1) = 2922 (C–H, Ar), 1672 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1271 (C–O), 757 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.95 (s, 1H, H-5), 7.59 (d, J = 8.8 Hz, 2H, Ar), 7.32–7.14 (m, 5H, Ar), 6.90–6.75–7.20 (m, 5H, Ar), 6.06 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.90 (s, 2H, H-9′′), 3.82 (s, 3H, –OCH3), 2.42 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.10 (C-10′′), 159.99 (C-12′′), 158.45 (C-1′′), 141.18, 137.18, 134.58, 131.92, 129.76, 128.69, 125.90, 122.25, 121.22, 120.03, 114.91, 114.78, 65.44 (C-7′′), 61.82 (–OCH2), 55.61 (–OCH3), 33.50 (C-9′′), 20.99 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5662.
O), 1271 (C–O), 757 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.95 (s, 1H, H-5), 7.59 (d, J = 8.8 Hz, 2H, Ar), 7.32–7.14 (m, 5H, Ar), 6.90–6.75–7.20 (m, 5H, Ar), 6.06 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.90 (s, 2H, H-9′′), 3.82 (s, 3H, –OCH3), 2.42 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.10 (C-10′′), 159.99 (C-12′′), 158.45 (C-1′′), 141.18, 137.18, 134.58, 131.92, 129.76, 128.69, 125.90, 122.25, 121.22, 120.03, 114.91, 114.78, 65.44 (C-7′′), 61.82 (–OCH2), 55.61 (–OCH3), 33.50 (C-9′′), 20.99 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5662.It was obtained as a white solid with a m.p. of 180.0–182.0 °C in 68% yield. IR (KBr) νmax (cm−1) = 2926 (C–H, Ar), 1684 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1231 (C–O), 823 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.59 (d, J = 8.4 Hz, 2H, Ar), 7.40–7.36 (m, 2H, Ar), 7.31 (d, J = 8.4 Hz, 2H, Ar), 7.24–7.20 (m, 2H, Ar), 7.03 (d, J = 8.8 Hz, 2H, Ar), 6.94 (d, J = 8.8 Hz, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95 (dd, J = 1.2, 15.6 Hz, 1H, H-9′′), 3.85 (d, J = 15.6 Hz, 1H, H-9′′), 2.42 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.87 (C-10′′), 158.64 (C-1′′), 144.26, 139.10, 136.44, 134.55, 132.17, 131.26, 130.26, 128.57, 127.22, 120.97, 120.58, 120.46, 115.09, 64.98 (C-7′′), 61.95 (–OCH2), 33.47 (C-9′′), 21.07 (–CH3); HRMS calcd for C25H21BrN4O2SH: 522.4368; found [M + H]+: 522.4360.
O), 1231 (C–O), 823 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.59 (d, J = 8.4 Hz, 2H, Ar), 7.40–7.36 (m, 2H, Ar), 7.31 (d, J = 8.4 Hz, 2H, Ar), 7.24–7.20 (m, 2H, Ar), 7.03 (d, J = 8.8 Hz, 2H, Ar), 6.94 (d, J = 8.8 Hz, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95 (dd, J = 1.2, 15.6 Hz, 1H, H-9′′), 3.85 (d, J = 15.6 Hz, 1H, H-9′′), 2.42 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.87 (C-10′′), 158.64 (C-1′′), 144.26, 139.10, 136.44, 134.55, 132.17, 131.26, 130.26, 128.57, 127.22, 120.97, 120.58, 120.46, 115.09, 64.98 (C-7′′), 61.95 (–OCH2), 33.47 (C-9′′), 21.07 (–CH3); HRMS calcd for C25H21BrN4O2SH: 522.4368; found [M + H]+: 522.4360.It was obtained as a white solid with a m.p. of 276–278 °C in 76% yield. IR (KBr) νmax (cm−1) = 2928 (C–H, Ar), 1638 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1255 (C–O), 825 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H, H-5), 7.41 (d, J = 8.05 Hz, 2H, Ar), 7.54 (t, J = 7.32 Hz, 2H, Ar), 7.49–7.45 (m, 1H, Ar), 7.26 (d, J = 8.05 Hz, 2H, Ar), 7.08 (d, J = 7.32 Hz, 2H, Ar), 7.01 (d, J = 8.05 Hz, 2H, Ar), 6.94 (d, J = 8.05 Hz, 1H, Ar), 6.04 (s, 1H, H-7′′), 5.26 (s, 2H, –OCH2), 3.98 (d, J = 16.11 Hz, 1H, H-9′′), 3.88 (d, J = 16.11 Hz, 1H, H-9′′), 2.26 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.04 (C-10′′), 157.66 (C-1′′), 143.11, 138.07, 133.64, 130.45, 129.37, 127.69, 120.84, 120.19, 119.47, 114.14, 112.86, 112.66, 112.19, 111.95, 63.74 (C-7′′), 60.75 (–OCH2), 32.56 (C-9′′), 20.17 (–CH3); HRMS calcd for C25H21FN4O2SH: 461.5312; found [M + H]+: 461.5318.
O), 1255 (C–O), 825 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 8.05 (s, 1H, H-5), 7.41 (d, J = 8.05 Hz, 2H, Ar), 7.54 (t, J = 7.32 Hz, 2H, Ar), 7.49–7.45 (m, 1H, Ar), 7.26 (d, J = 8.05 Hz, 2H, Ar), 7.08 (d, J = 7.32 Hz, 2H, Ar), 7.01 (d, J = 8.05 Hz, 2H, Ar), 6.94 (d, J = 8.05 Hz, 1H, Ar), 6.04 (s, 1H, H-7′′), 5.26 (s, 2H, –OCH2), 3.98 (d, J = 16.11 Hz, 1H, H-9′′), 3.88 (d, J = 16.11 Hz, 1H, H-9′′), 2.26 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 170.04 (C-10′′), 157.66 (C-1′′), 143.11, 138.07, 133.64, 130.45, 129.37, 127.69, 120.84, 120.19, 119.47, 114.14, 112.86, 112.66, 112.19, 111.95, 63.74 (C-7′′), 60.75 (–OCH2), 32.56 (C-9′′), 20.17 (–CH3); HRMS calcd for C25H21FN4O2SH: 461.5312; found [M + H]+: 461.5318.It was obtained as a yellowish solid with a m.p. of 170.0–172.0 °C in 76% yield. IR (KBr) νmax (cm−1) = 2924 (C–H, Ar), 1683 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1248 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H, H-5), 7.64–7.60 (m, 2H, Ar), 7.29–7.23 (m, 4H, Ar), 7.18–7.12 (m, 2H, Ar), 7.04–7.01 (m, 2H, Ar), 6.92 (d, J = 8.05 Hz, 2H, Ar), 6.07 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 4.00–3.85 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.93 (C-10′′), 159.88 (C-4′), 158.49 (C-1′′), 144.23, 137.36, 131.75, 130.26, 129.04, 128.59, 127.09, 125.85, 122.20, 121.11, 114.93, 114.73, 65.28 (C-7′′), 61.91 (–OCH2), 55.58 (–OCH3), 33.52 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5407.
O), 1248 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.92 (s, 1H, H-5), 7.64–7.60 (m, 2H, Ar), 7.29–7.23 (m, 4H, Ar), 7.18–7.12 (m, 2H, Ar), 7.04–7.01 (m, 2H, Ar), 6.92 (d, J = 8.05 Hz, 2H, Ar), 6.07 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 4.00–3.85 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.93 (C-10′′), 159.88 (C-4′), 158.49 (C-1′′), 144.23, 137.36, 131.75, 130.26, 129.04, 128.59, 127.09, 125.85, 122.20, 121.11, 114.93, 114.73, 65.28 (C-7′′), 61.91 (–OCH2), 55.58 (–OCH3), 33.52 (C-9′′); HRMS calcd for C25H22N4O3SH: 459.5401; found [M + H]+: 459.5407.It was obtained as a yellow solid with a m.p. of 131.0–133.0 °C in 76% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1685 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1230 (C–O), 837 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.86 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.60 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.32 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.03–6.99 (m, 2H, Ar), 6.94–6.91 (m, 2H, Ar), 6.18 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.83 (m, 5H, H-9′′ & –OCH3), 2.52 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.37 (–COCH3), 176.27 (C-10′′), 171.16, 159.61 (C-4′), 158.57 (C-1′′), 141.78, 134.72, 131.13, 129.14, 128.35, 124.68, 122.24, 115.15, 114.80, 64.70 (C-7′′), 61.80 (–OCH2), 55.61 (–OCH3), 33.60 (C-9′′), 26.50 (–COCH3); HRMS calcd for C27H24N4O4SH: 501.5768; found [M + H]+: 501.5760.
O), 1230 (C–O), 837 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.86 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.60 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.32 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.03–6.99 (m, 2H, Ar), 6.94–6.91 (m, 2H, Ar), 6.18 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.83 (m, 5H, H-9′′ & –OCH3), 2.52 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.37 (–COCH3), 176.27 (C-10′′), 171.16, 159.61 (C-4′), 158.57 (C-1′′), 141.78, 134.72, 131.13, 129.14, 128.35, 124.68, 122.24, 115.15, 114.80, 64.70 (C-7′′), 61.80 (–OCH2), 55.61 (–OCH3), 33.60 (C-9′′), 26.50 (–COCH3); HRMS calcd for C27H24N4O4SH: 501.5768; found [M + H]+: 501.5760.It was obtained as a brown semi solid in 83% yield. IR (KBr) νmax (cm−1) = 2922 (C–H, Ar), 1683 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1247 (C–O), 759 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.74–7.71 (m, 2H, Ar), 7.62–7.58 (m, 2H, Ar), 7.38–7.36 (m, 1H, Ar), 7.27–7.24 (m, 2H, Ar), 7.02–6.99 (m, 2H, Ar), 6.93–6.90 (m, 2H, Ar), 6.11 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 4.01–3.86 (m, 5H, H-9′′ & –OCH3), 2.50 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.12 (–COCH3), 171.17 (C-10′′), 159.94 (C-4′), 159.60 (C-1′′), 144.09, 131.08, 130.48, 130.15, 129.31, 128.72, 126.91, 125.25, 122.21, 121.22, 115.04, 114.75, 65.08 (C-7′′), 61.79 (–OCH2), 55.59 (–OCH3), 33.54 (C-9′′), 26.55 (–COCH3); HRMS calcd for C27H24N4O4SH: 501.5768; found [M + H]+: 501.5762.
O), 1247 (C–O), 759 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.74–7.71 (m, 2H, Ar), 7.62–7.58 (m, 2H, Ar), 7.38–7.36 (m, 1H, Ar), 7.27–7.24 (m, 2H, Ar), 7.02–6.99 (m, 2H, Ar), 6.93–6.90 (m, 2H, Ar), 6.11 (s, 1H, H-7′′), 5.20 (s, 2H, –OCH2), 4.01–3.86 (m, 5H, H-9′′ & –OCH3), 2.50 (s, 3H, –COCH3); 13C NMR (100 MHz, CDCl3) δ 197.12 (–COCH3), 171.17 (C-10′′), 159.94 (C-4′), 159.60 (C-1′′), 144.09, 131.08, 130.48, 130.15, 129.31, 128.72, 126.91, 125.25, 122.21, 121.22, 115.04, 114.75, 65.08 (C-7′′), 61.79 (–OCH2), 55.59 (–OCH3), 33.54 (C-9′′), 26.55 (–COCH3); HRMS calcd for C27H24N4O4SH: 501.5768; found [M + H]+: 501.5762.It was obtained as a white solid with a m.p. of 100–102 °C in 79% yield. IR (KBr) νmax (cm−1) = 2923 (C–H, Ar), 1683 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1238 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.07–6.97 (m, 6H, Ar), 6.92 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.83 (m, 5H, H-9′′ & –OCH3), 2.24 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.10 (C-10′′), 159.98 (C-4′), 158.45 (C-1′′), 144.18, 137.18, 134.45, 131.92, 129.76, 128.69, 125.90, 122.25, 121.22, 114.91, 114.78, 65.44 (C-7′′), 61.82 (–OCH2), 55.61 (–OCH3), 33.50 (C-9′′), 20.99 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5662.
O), 1238 (C–O), 758 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.07–6.97 (m, 6H, Ar), 6.92 (dd, J = 2.8, 8.8 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.83 (m, 5H, H-9′′ & –OCH3), 2.24 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 171.10 (C-10′′), 159.98 (C-4′), 158.45 (C-1′′), 144.18, 137.18, 134.45, 131.92, 129.76, 128.69, 125.90, 122.25, 121.22, 114.91, 114.78, 65.44 (C-7′′), 61.82 (–OCH2), 55.61 (–OCH3), 33.50 (C-9′′), 20.99 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5662.It was obtained as a white solid with a m.p. of 158.0–160.0 °C in 73% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1677 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1241 (C–O), 833 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.60 (dd, J = 3.2, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.16–7.10 (m, 1H, Ar), 7.02–6.86 (m, 7H, Ar), 6.04 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.82 (m, 5H, H-9′′ & –OCH3), 2.25 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 173.56 (C-10′′), 171.09, 159.92 (C-4′), 158.40 (C-1′′), 144.15, 138.98, 137.13, 131.83, 130.13, 128.78, 128.59, 128.07, 126.65, 122.93, 122.18, 121.20, 114.88, 114.72, 65.39 (C-7′′), 61.79 (–OCH2), 55.56 (–OCH3), 33.49 (C-9′′), 21.25 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5665.
O), 1241 (C–O), 833 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.60 (dd, J = 3.2, 8.8 Hz, 2H, Ar), 7.26–7.22 (m, 2H, Ar), 7.16–7.10 (m, 1H, Ar), 7.02–6.86 (m, 7H, Ar), 6.04 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.99–3.82 (m, 5H, H-9′′ & –OCH3), 2.25 (s, 3H, –CH3); 13C NMR (100 MHz, CDCl3) δ 173.56 (C-10′′), 171.09, 159.92 (C-4′), 158.40 (C-1′′), 144.15, 138.98, 137.13, 131.83, 130.13, 128.78, 128.59, 128.07, 126.65, 122.93, 122.18, 121.20, 114.88, 114.72, 65.39 (C-7′′), 61.79 (–OCH2), 55.56 (–OCH3), 33.49 (C-9′′), 21.25 (–CH3); HRMS calcd for C26H24N4O3SH: 473.5667; found [M + H]+: 473.5665.It was obtained as a white solid with a m.p. of 164.0–166.0 °C in 87% yield. IR (KBr) νmax (cm−1) = 2923 (C–H, Ar), 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1248 (C–O), 759 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (d, J = 8.8 Hz, 2H, Ar), 7.24 (d, J = 8.8 Hz, 2H, Ar), 7.02–6.96 (m, 4H, Ar), 6.92 (d, J = 8.4 Hz, 2H, Ar), 6.77 (d, J = 8.8 Hz, 2H, Ar), 5.95 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.98–3.84 (m, 5H, H-9′′ & –OCH3), 3.71 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.00 (C-10′′), 159.98, 158.41 (C-1′′), 144.17, 131.95, 130.17, 129.94, 128.81, 127.60, 122.25, 121.28, 114.91, 114.78, 114.40, 65.58 (C-7′′), 61.89 (–OCH2), 55.60 (–OCH3), 55.28 (–OCH3), 33.39 (C-9′′); HRMS calcd for C26H24N4O4SH: 489.5661; found [M + H]+: 489.5658.
O), 1248 (C–O), 759 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (d, J = 8.8 Hz, 2H, Ar), 7.24 (d, J = 8.8 Hz, 2H, Ar), 7.02–6.96 (m, 4H, Ar), 6.92 (d, J = 8.4 Hz, 2H, Ar), 6.77 (d, J = 8.8 Hz, 2H, Ar), 5.95 (s, 1H, H-7′′), 5.22 (s, 2H, –OCH2), 3.98–3.84 (m, 5H, H-9′′ & –OCH3), 3.71 (s, 3H, –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.00 (C-10′′), 159.98, 158.41 (C-1′′), 144.17, 131.95, 130.17, 129.94, 128.81, 127.60, 122.25, 121.28, 114.91, 114.78, 114.40, 65.58 (C-7′′), 61.89 (–OCH2), 55.60 (–OCH3), 55.28 (–OCH3), 33.39 (C-9′′); HRMS calcd for C26H24N4O4SH: 489.5661; found [M + H]+: 489.5658.It was obtained as a yellow solid with a m.p. of 156.0–158.0 °C in 80% yield. IR (KBr) νmax (cm−1) = 2929 (C–H, Ar), 1682 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1255 (C–O), 753 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.91 (s, 1H, H-5), 7.61 (dd, J = 3.2, 8.8 Hz, 2H, Ar), 7.28–7.25 (m, 2H, Ar), 7.20–7.14 (m, 1H, Ar), 7.03–6.99 (m, 2H, Ar), 6.90–6.78 (m, 5H, Ar), 6.06 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.91–3.82 (m, 8H, H-9′′, 2× –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.32 (C-10′′), 159.86 (C-4′), 158.48 (C-12′′), 154.76 (C-1′′), 144.22, 131.34, 130.32, 130.23, 129.44, 129.35, 125.32, 122.17, 121.10, 120.70, 114.71, 114.51, 111.80, 64.27 (C-7′′), 61.84 (–OCH2), 55.58 (–OCH3), 33.22 (C-9′′); HRMS calcd for C26H24N4O4SH: 489.5661; found [M + H]+: 489.5665.
O), 1255 (C–O), 753 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.91 (s, 1H, H-5), 7.61 (dd, J = 3.2, 8.8 Hz, 2H, Ar), 7.28–7.25 (m, 2H, Ar), 7.20–7.14 (m, 1H, Ar), 7.03–6.99 (m, 2H, Ar), 6.90–6.78 (m, 5H, Ar), 6.06 (s, 1H, H-7′′), 5.21 (s, 2H, –OCH2), 3.91–3.82 (m, 8H, H-9′′, 2× –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.32 (C-10′′), 159.86 (C-4′), 158.48 (C-12′′), 154.76 (C-1′′), 144.22, 131.34, 130.32, 130.23, 129.44, 129.35, 125.32, 122.17, 121.10, 120.70, 114.71, 114.51, 111.80, 64.27 (C-7′′), 61.84 (–OCH2), 55.58 (–OCH3), 33.22 (C-9′′); HRMS calcd for C26H24N4O4SH: 489.5661; found [M + H]+: 489.5665.It was obtained as a yellow solid with a m.p. of 104.0–106.0 °C in 74% yield. IR (KBr) νmax (cm−1) = 2957 (C–H, Ar), 1637 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1249 (C–O), 756 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H, H-5), 7.59 (d, J = 8.79 Hz, 2H, Ar), 7.36 (d, J = 8.79 Hz, 2H, Ar), 7.20 (d, J = 9.15 Hz, 2H, Ar), 7.00 (d, J = 8.79 Hz, 4H, Ar), 6.91 (d, J = 8.05 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95–3.82 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.11 (C-10′′), 160.05 (C-4′), 158.59 (C-1′′), 136.67, 132.19, 131.33, 130.17, 128.61, 127.24, 122.27, 121.26, 120.60, 115.10, 114.83, 65.00 (C-7′′), 61.82 (OCH2), 55.64 (–OCH3), 33.49 (C-9′′); HRMS calcd for C25H21BrN4O3SH: 538.4362; found [M + H]+: 538.4368.
O), 1249 (C–O), 756 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.95 (s, 1H, H-5), 7.59 (d, J = 8.79 Hz, 2H, Ar), 7.36 (d, J = 8.79 Hz, 2H, Ar), 7.20 (d, J = 9.15 Hz, 2H, Ar), 7.00 (d, J = 8.79 Hz, 4H, Ar), 6.91 (d, J = 8.05 Hz, 2H, Ar), 6.02 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.95–3.82 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 171.11 (C-10′′), 160.05 (C-4′), 158.59 (C-1′′), 136.67, 132.19, 131.33, 130.17, 128.61, 127.24, 122.27, 121.26, 120.60, 115.10, 114.83, 65.00 (C-7′′), 61.82 (OCH2), 55.64 (–OCH3), 33.49 (C-9′′); HRMS calcd for C25H21BrN4O3SH: 538.4362; found [M + H]+: 538.4368.It was obtained as a yellowish solid with a m.p. of 167.0–169.0 °C in 75% yield. IR (KBr) νmax (cm−1) = 2922 (C–H, Ar), 1689 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1255 (C–O), 756 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.92 (s, 1H, H-5), 6.97 (d, J = 8.8 Hz, 2H, Ar), 6.62–6.52 (m, 3H, Ar), 6.39–6.28 (m, 6H, Ar), 6.24–6.19 (m, 1H, Ar), 5.43 (s, 1H, H-7′′), 4.59 (s, 2H, –OCH2), 3.33–3.19 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.95 (C-10′′), 160.02 (C-4′), 158.56 (C-1′′), 144.09, 139.04, 131.44, 130.21, 128.43, 122.30, 115.15, 114.81, 114.06, 113.09, 65.01 (C-7′′), 62.02 (–OCH2), 55.63 (–OCH3), 33.49 (C-9′′); HRMS calcd for C25H21FN4O3SH: 477.5306; found [M + H]+: 477.5300.
O), 1255 (C–O), 756 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.92 (s, 1H, H-5), 6.97 (d, J = 8.8 Hz, 2H, Ar), 6.62–6.52 (m, 3H, Ar), 6.39–6.28 (m, 6H, Ar), 6.24–6.19 (m, 1H, Ar), 5.43 (s, 1H, H-7′′), 4.59 (s, 2H, –OCH2), 3.33–3.19 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.95 (C-10′′), 160.02 (C-4′), 158.56 (C-1′′), 144.09, 139.04, 131.44, 130.21, 128.43, 122.30, 115.15, 114.81, 114.06, 113.09, 65.01 (C-7′′), 62.02 (–OCH2), 55.63 (–OCH3), 33.49 (C-9′′); HRMS calcd for C25H21FN4O3SH: 477.5306; found [M + H]+: 477.5300.It was obtained as a brown solid with a m.p. of 76.0–78.0 °C in 78% yield. IR (KBr) νmax (cm−1) = 2925 (C–H, Ar), 1682 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1237 (C–O), 757 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (dd, J = 2.4, 8.8 Hz, 2H, Ar), 7.24–7.21 (m, 1H, Ar), 7.10–6.92 (m, 8H, Ar), 6.00 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.99–3.84 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.01 (C-10′′), 160.03 (C-4′), 158.47 (C-1′′), 144.23, 131.42, 128.86, 128.03, 122.24, 121.13, 116.20, 115.98, 115.04, 114.79, 65.38 (C-7′′), 61.89 (–OCH2), 55.68 (–OCH3), 33.42; HRMS calcd for C25H21FN4O3SH: 477.5306; found [M + H]+: 477.5302.
O), 1237 (C–O), 757 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (dd, J = 2.4, 8.8 Hz, 2H, Ar), 7.24–7.21 (m, 1H, Ar), 7.10–6.92 (m, 8H, Ar), 6.00 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.99–3.84 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 170.01 (C-10′′), 160.03 (C-4′), 158.47 (C-1′′), 144.23, 131.42, 128.86, 128.03, 122.24, 121.13, 116.20, 115.98, 115.04, 114.79, 65.38 (C-7′′), 61.89 (–OCH2), 55.68 (–OCH3), 33.42; HRMS calcd for C25H21FN4O3SH: 477.5306; found [M + H]+: 477.5302.It was obtained as a yellowish solid with a m.p. of. 84.0–86.0 °C in 85% yield. IR (KBr) νmax (cm−1) = 2923 (C–H, Ar), 1688 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1249 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.61 (d, J = 8.79 Hz, 2H, Ar), 7.26–7.21 (m, 4H, Ar), 7.07 (d, J = 8.79 Hz, 2H, Ar), 7.02 (d, J = 8.79 Hz, 2H, Ar), 6.93 (d, J = 8.05 Hz, 2H, Ar), 6.03 (s, 1H, H-7′′), 5.25 (s, 2H, –OCH2), 3.97–3.84 (m, 5H, H-9′′ & –OCH3),; 13C NMR (100 MHz, CDCl3) δ 171.01 (C-10′′), 160.13 (C-4′), 158.59 (C-1′′), 135.92, 132.65, 132.04, 131.37, 129.24, 128.63, 126.99, 122.28, 115.10, 114.85, 65.09 (C-7′′), 61.83 (–OCH2), 55.63 (–OCH3), 33.47 (C-9′′); HRMS calcd for C25H21ClN4O3SH: 493.1101; found [M + H]+: 493.1107.
O), 1249 (C–O), 757 (C–S–C); 1H NMR (400 MHz, CDCl3) δ 7.98 (s, 1H, H-5), 7.61 (d, J = 8.79 Hz, 2H, Ar), 7.26–7.21 (m, 4H, Ar), 7.07 (d, J = 8.79 Hz, 2H, Ar), 7.02 (d, J = 8.79 Hz, 2H, Ar), 6.93 (d, J = 8.05 Hz, 2H, Ar), 6.03 (s, 1H, H-7′′), 5.25 (s, 2H, –OCH2), 3.97–3.84 (m, 5H, H-9′′ & –OCH3),; 13C NMR (100 MHz, CDCl3) δ 171.01 (C-10′′), 160.13 (C-4′), 158.59 (C-1′′), 135.92, 132.65, 132.04, 131.37, 129.24, 128.63, 126.99, 122.28, 115.10, 114.85, 65.09 (C-7′′), 61.83 (–OCH2), 55.63 (–OCH3), 33.47 (C-9′′); HRMS calcd for C25H21ClN4O3SH: 493.1101; found [M + H]+: 493.1107.It was obtained as a light brown solid with a m.p. of 103.0–105.0 °C in 77% yield. IR (KBr) νmax (cm−1) = 2930 (C–H, Ar), 1702 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1256 (C–O), 830 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (d, J = 8.79 Hz, 2H, Ar), 7.42–7.38 (m, 1H, Ar), 7.32–7.29 (m, 2H, Ar), 7.23–7.07 (m, 3H, Ar), 7.03–7.00 (m, 2H, Ar), 6.93–6.90 (m, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.94–3.81 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 176.47 (C-10′′), 159.98 (C-4′), 158.33 (C-1′′), 144.37, 130.69, 130.28, 129.52, 122.28, 121.24, 115.00, 114.80, 62.14 (C-7′′), 55.63 (–OCH2), 51.54 (–OCH3), 33.42 (C-9′′); HRMS calcd for C25H21ClN4O3SH: 493.1101; found [M + H]+: 493.1112.
O), 1256 (C–O), 830 (C–S–C); 1H NMR (300 MHz, CDCl3) δ 7.93 (s, 1H, H-5), 7.61 (d, J = 8.79 Hz, 2H, Ar), 7.42–7.38 (m, 1H, Ar), 7.32–7.29 (m, 2H, Ar), 7.23–7.07 (m, 3H, Ar), 7.03–7.00 (m, 2H, Ar), 6.93–6.90 (m, 2H, Ar), 6.04 (s, 1H, H-7′′), 5.23 (s, 2H, –OCH2), 3.94–3.81 (m, 5H, H-9′′ & –OCH3); 13C NMR (100 MHz, CDCl3) δ 176.47 (C-10′′), 159.98 (C-4′), 158.33 (C-1′′), 144.37, 130.69, 130.28, 129.52, 122.28, 121.24, 115.00, 114.80, 62.14 (C-7′′), 55.63 (–OCH2), 51.54 (–OCH3), 33.42 (C-9′′); HRMS calcd for C25H21ClN4O3SH: 493.1101; found [M + H]+: 493.1112.Acknowledgements
The authors acknowledge financial assistance from the University of Delhi under the Strengthening R & D Doctoral Research Programme. Y.K. is thankful to the University Grants Commission (UGC, Delhi, India) for providing a JRF (Junior Research Fellowship), SRF (Senior Research Fellowship) and also thankful to EMA-2 Experts III (Erasmus Mundus Action 2: Expert III) for providing a doctoral exchange scholarship.References
- (a) A. K. Jain, A. Vaidya, V. Ravichandran, S. K. Kashaw and R. K. Agrawal, Bioorg. Med. Chem., 2012, 20, 3378 CrossRef CAS PubMed; (b) A. Verma and S. Saraf, Eur. J. Med. Chem., 2008, 43, 897 CrossRef CAS PubMed.
- Z. Hongyu, S. Wu, S. Zhai, A. Liu, Y. Sun, R. Li, Y. Zhang, S. Ekins, P. W. Swaan, B. Fang, B. Zhangand and B. Yan, J. Med. Chem., 2008, 51, 1242 CrossRef PubMed.
- (a) M. L. Barreca, J. Balzarini, A. Chimirri, E. De Clercq, L. De Luca, H. D. Höltje, M. Höltje, A. M. Monforte, P. Monforte, C. Pannecouque, A. Rao and M. Zapalla, J. Med. Chem., 2002, 45, 5410 CrossRef CAS PubMed; (b) A. Rao, J. Balzarini, A. Carbone, A. Chimirri, E. De Clercq, A. M. Monforte, P. Monforte, C. Pannecouque and M. Zapallà, Antiviral Res., 2004, 63, 79 CrossRef CAS PubMed; (c) R. K. Rawal, R. Tripathi, S. B. Katti, C. Pannecouque and E. De Clercq, Bioorg. Med. Chem., 2007, 15, 3134 CrossRef CAS PubMed.
- V. R. Solomon, W. Haq, K. Srivastava, S. K. Puri and S. B. Katti, J. Med. Chem., 2007, 50, 394 CrossRef CAS PubMed.
- G. C. Kucukguzel, J. R. Shchullek, A. Kaocatepe, E. De Clercq, F. Sahinv and M. Gulluce, Eur. J. Med. Chem., 2006, 41, 353 CrossRef PubMed.
- M. V. Diurno, O. Mazzoni, P. E. Calignano, F. Giorodano and A. Bolognese, J. Med. Chem., 1992, 35, 2910 CrossRef CAS.
- (a) T. Archana, V. K. Srivastava and A. Kumar, Eur. J. Med. Chem., 2002, 37, 873 CrossRef; (b) C. Dwivedi, S. S. Gupta and S. S. Parmar, J. Med. Chem., 1972, 15, 553 CrossRef CAS.
- K. G. Desai and K. R. Desai, J. Sulfur Chem., 2006, 27, 315 CrossRef CAS.
- C. M. Jackson, B. Blass, K. Coburn, L. Djandjighian, G. Fadayel, A. J. Fluxe, S. J. Hodson, J. M. Janusz, M. Murawsky, J. M. Ridgeway, R. E. White and S. Wu, Bioorg. Med. Chem. Lett., 2007, 17, 282 CrossRef CAS PubMed.
- V. S. Pore, M. A. Jagtap, S. G. Agalave, A. K. Pandey, M. I. Siddiqi, V. Kumar and P. K. Shukla, Med. Chem. Commun., 2012, 3, 484 RSC.
- N. Pokhodylo, O. Shyyka and V. Matiychuk, Med. Chem. Res., 2014, 23, 2426 CrossRef CAS.
- M. S. Costa, N. Boechat, E. A. Rangel, F. C. Silva, A. M. T. de Souza, C. R. Rodrigues, H. C. Castro, I. N. Junior, M. C. S. Lourenc, M. S. V. Wardell and V. F. Ferreira, Bioorg. Med. Chem., 2006, 14, 8644 CrossRef CAS PubMed.
- B. S. Holla, M. Mahalinga, M. S. Karthikeyan, B. Poojary, P. M. Akberali and N. S. Kumari, Eur. J. Med. Chem., 2005, 40, 1173 CrossRef CAS PubMed.
- (a) V. R. Solomon, C. Hu and H. Lee, Bioorg. Med. Chem., 2009, 17, 7585 CrossRef CAS PubMed; (b) B. Meunier, Acc. Chem. Res., 2008, 41, 69 CrossRef CAS PubMed.
- (a) D. Havrylyuk, L. Mosula, B. Zimenkovsky, O. Vasylenko, A. Gzella and R. Lesyk, Eur. J. Med. Chem., 2010, 45, 5012 CrossRef CAS PubMed; (b) R. Lesyk, O. Vladzimirska, S. Holota, L. Zaprutko and A. Gzella, Eur. J. Med. Chem., 2007, 42, 641 CrossRef CAS PubMed; (c) D. Havrylyuk, N. Kovach, B. Zimenkovsky, O. Vasylenko and R. Lesyk, Arch. Pharm. Chem. Life Sci., 2011, 344, 514 CrossRef CAS PubMed; (d) L. Mosula, B. Zimenkovsky, D. Havrylyuk, A. V. Missir, I. C. Chirita and R. Lesyk, FARMACIA, 2009, 57, 321 CAS; (e) D. Kaminskyy, O. Vasylenko, D. Atamanyuk, A. Gzella and R. Lesyk, Synlett, 2011, 10, 1385 Search PubMed.
- T. Srivastava, W. Haq and S. B. Katti, Tetrahedron, 2002, 58, 7619 CrossRef CAS.
- R. K. Rawal, T. Srivastava, W. Haq and S. B. Katti, J. Chem. Res., 2004, 5, 368 CrossRef.
- C. T. Sadashivaa, J. N. Narendra, S. Chandraa, C. V. Kavithaa, A. Thimmegowdab, M. N. Subhashc and K. S. Rangappaa, Eur. J. Med. Chem., 2009, 44, 4848 CrossRef PubMed.
- S. K. Srivastava and S. L. Srivastava, J. Indian Chem. Soc., 2002, 77, 104 Search PubMed.
- R. C. Kumar and D. Kumar, J. Indian Chem. Soc., 2002, 77, 492 Search PubMed.
- X. Zhang, X. Li, D. Li, G. Qu, J. Wang, P. M. Loiseau and X. Fan, Bioorg. Med. Chem. Lett., 2009, 19, 6280 CrossRef CAS PubMed.
- V. Kanagarajana, J. Thanusua and M. Gopalakrishnana, Green Chem. Lett. Rev., 2009, 2, 161 CrossRef.
- V. Gududuru, V. Nguyen, J. T. Dalton and D. D. Miller, Synlett, 2004, 2357 CAS.
- E. Stephanie, A. Justus, J. C. Hodges and M. W. Wilson, Biotechnol. Bioeng., 2000, 61, 17 Search PubMed.
- (a) Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless and M. G. Finn, J. Am. Chem. Soc., 2003, 125, 3192 CrossRef CAS PubMed; (b) A. E. Speers, G. C. Adam and B. F. Cravatt, J. Am. Chem. Soc., 2003, 125, 4686 CrossRef CAS PubMed; (c) J. C. Anderson and P. G. Schultz, J. Am. Chem. Soc., 2003, 125, 11782 CrossRef PubMed; (d) A. J. Link and D. A. Tirrell, J. Am. Chem. Soc., 2003, 125, 11164 CrossRef CAS PubMed; (e) J. P. Collman, N. K. Devaraj and C. E. D. Chidsey, Langmuir, 2004, 20, 1051 CrossRef CAS; (f) P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Frechet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928 CrossRef CAS PubMed; (g) A. Michael, J. Prakt. Chem., 1893, 48, 94 CrossRef; (h) A. Bertho and F. Holder, J. Prakt. Chem., 1928, 119, 189 CrossRef CAS; (i) T. Curtius and K. Raschig, J. Prakt. Chem., 1930, 125, 466 CrossRef CAS.
- E. J. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302 RSC.
- P. Appukkuttan, W. Dehaen, V. V. Valery, V. Fokin and E. V. Van der Eycken, Org. Lett., 2004, 6, 4223 CrossRef CAS PubMed.
- (a) Y. Kumar, V. Bahadur, A. K. Singh, V. S. Parmar and B. K. Singh, J. Indian Chem. Soc., 2013, 90, 1893 CAS; (b) Y. Kumar, V. Bahadur, A. K. Singh, V. S. Parmar, E. V. Van der Eycken and B. K. Singh, Beilstein J. Org. Chem., 2014, 10, 1413 CrossRef CAS PubMed.
- J. Thomas, J. John, N. Parekh and W. Dehaen, Angew. Chem., Int. Ed., 2014, 53, 10155 CrossRef CAS PubMed.
- (a) O. C. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef PubMed; (b) C. O. Kappe and D. Dallinger, Nat. Rev. Drug Discovery, 2006, 5, 51 CrossRef CAS PubMed; (c) A. K. Nagariya, A. K. Meena, K. Kiran, A. K. Yadav, U. S. Niranjan, A. K. Pathak, B. Singh and M. M. Rao, J. Pharmacol. Res., 2010, 3, 575 CAS; (d) K. S. Jignasa, T. S. Ketan, S. P. Bhumika and K. G. Anuradha, Pharma Chem., 2010, 2, 342 Search PubMed; (e) A. Loupy and L. Perreux, Tetrahedron, 2001, 57, 9199 CrossRef; (f) A. de la Hoz, A. Loupy, N. E. Leadbeater, J. R. Schmink and T. A. Hamlin, Microwaves in Organic Synthesis, Willey-VCH, 3rd edn, 2013, vol. 1 Search PubMed.
- (a) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (b) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (c) M. Shriri, Chem. Rev., 2012, 112, 3508 CrossRef PubMed; (d) D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed; (e) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS PubMed; (f) L. ElKaïm and L. Grimaud, Mol. Diversity, 2010, 14, 855 CrossRef PubMed; (g) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS PubMed; (h) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed.
- M. Dabiri, P. Salehi, M. Bahramnejad, F. Sherafat and M. Bararjanian, Synth. Commun., 2012, 42, 3117 CrossRef CAS.
- V. Haridas, S. Sahu and P. P. P. Kumar, Tetrahedron Lett., 2011, 52, 6930 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12592d |
| This journal is © The Royal Society of Chemistry 2015 |