Thermal, mechanical and antibacterial properties of cyclophosphazene incorporated benzoxazine blended bismaleimide composites
Krishnamoorthy Krishnadevi,
Vaithilingam Selvaraj* and
Dakshinamoorthy Prasanna
Department of Chemistry, University College of Engineering-Villupuram (A Constituent College of Anna University, Chennai), Kakuppam, Villupuram-605 103, Tamil Nadu, India. E-mail: rajselva_77@yahoo.co.in; Fax: +91-04146-224500
First published on 14th November 2014
Abstract
Cyclophosphazene (Cp) incorporated benzoxazine (Bz) and bismaleimide (Bmi) blended (Cp–Bz–Bmi) composites were obtained through ring-opening polymerization with benzoxazine and bismaleimide polymerized via Michael addition with a phosphazene group and a Diels–Alder reaction with a polybenzoxazine group. The cyclophosphazene material was chosen as a filler to improve the thermal, mechanical, electrical resistance and antibacterial properties of Bz–Bmi composites. The results show that the addition of phosphazene can largely enhance the mechanical properties due to the strong chemical interaction between the Bz–Bmi and Cp, which was confirmed by Fourier transform infrared (FT-IR) spectroscopy. The fracture surfaces of the composites were determined by scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis. The mechanical, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) results show that with the increasing percentage of Cp (5, 10 and 15%) in the Bz–Bmi composites, they exhibit better thermal resistance and their corresponding char yield was improved. Broadband dielectric spectroscopy (BDS) studies proved that the Cp–Bz–Bmi composites can be used as electrically resistive materials. The antibacterial properties of the Cp incorporated Bz–Bmi composites also improved due to cyclophosphazene being a bioactive material. Hence, Cp–Bz–Bmi composites are potent materials for marine coating, aerospace and microelectronic applications.
Introduction
Composites have become important materials in terms of accessing new properties and exploiting unique synergism between materials. They have been used to fabricate many structural parts for engineering purposes. This is due to their many attractive characteristics such as low weight, high strength, high stiffness, good fatigue resistance and good corrosion resistance. Polybenzoxazine based composites have attracted the interest of researchers during the last decade due to the superior properties of polybenzoxazines over other polymers. Benzoxazines, a new type of phenolic resin, can be polymerized by a thermally induced ring-opening reaction.1 Polybenzoxazines are a versatile type of thermosetting resin which can effectively replace other resins in a number of applications because of their excellent mechanical and thermal properties with flame retardation, low water absorption, and remarkable molecular design flexibility with near-zero shrinkage.2–4After curing, polybenzoxazines display unique characteristics, such as heat resistance, superior electronic properties, low water absorption, small shrinkage upon curing, and low surface energy.5–11 Some of the disadvantages associated with polybenzoxazine based materials are high brittleness, requirement of high temperatures for curing and most of the monomers are solid. These properties limit their processability and make it difficult to prepare films or complex materials.12,13 To improve the mechanical properties and processability, several strategies have been adopted including the synthesis of benzoxazine monomers with additional functionality,14,15 incorporation of benzoxazine in polymer chains13,14,16–31 and synthesis of benzoxazine based composites.32–44 Another effective approach for the preparation of high performance materials was benzoxazines containing a curable group, such as an allyl,45 nitrile,46 ethynyl47 or propargyl group48, to increase the cross-linking density. In addition to aromatic biphenol based (conventional) benzoxazines, aromatic diamine based benzoxazines have been reported.49–51 The aromatic diamine based benzoxazines can be polymerized similarly to conventional aromatic biphenol based benzoxazines, which yield structures with higher Tg and better thermal stability.50 Bismaleimides offer excellent thermomechanical properties and can withstand high stress at high temperatures at which typical phenol and epoxy resins, as well as most high performance plastics, are no longer satisfactory. Thus, the maleimide structure has long been incorporated into many polymeric systems because it often yields thermally stable polymers with high Tg values and improved heat distortion temperatures due to the rigid imide ring.52–54 Recently, it was reported that incorporation of the maleimide group into a benzoxazine monomer can effectively improve the thermal properties of its thermosets.55–57
Bismaleimide systems are considered as an important class of polymeric system due to their high performance-to-cost ratio and relatively high temperature resistance. They possess superior thermal and oxidative stability, low propensity for moisture absorption and good flame retardance. Furthermore, they have excellent thermomechanical properties and can withstand high stress at high temperatures compared to typical phenolics, epoxies and most high performance plastics. Adverse effects on their high temperature performance have been observed when attempts have been made to reduce their brittleness by increasing flexibility through structural modification and toughening, etc.
In recent years, there has been considerable interest in the phosphazene based family of materials because they not only have a wide range of thermal and chemical stabilities, but can also provide improved thermal and flame retardant properties to polymers, and in turn, to their composites.58–63 Hexachlorocyclotriphosphazene is a versatile starting monomer for the synthesis of phosphazene based polymers. Cyclotriphosphazene is a ring compound consisting of alternating phosphorus and nitrogen atoms which exhibits unusual thermal properties such as flame retardancy and self-extinguishability.64,65 The mechanical properties and the rate of degradation of a polymer can be varied by a proper choice of chemical composition of the polymer backbone. The presence of –P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– structural units in the macromolecular backbone provides tremendous flexibility for functionalizing the materials through chemical modification for various applications. Phosphazene containing materials have been used as biomaterials,66–68 membranes,69,54 electrochromic materials,70 ceramics,71 hybrid materials72 and electrical materials.73
N– structural units in the macromolecular backbone provides tremendous flexibility for functionalizing the materials through chemical modification for various applications. Phosphazene containing materials have been used as biomaterials,66–68 membranes,69,54 electrochromic materials,70 ceramics,71 hybrid materials72 and electrical materials.73
The objective of the present investigation is to develop star-shaped cyclophosphazene incorporated bismaleimide based benzoxazine composites and to assess their structures by FTIR and XRD characterizations. The star-shaped material has been developed to increase the cross-linking density of the composite.74,75 The effect of chemical structure with the addition of Cp to Bz–Bmi and the morphology of the products were investigated by scanning electron microscopy (SEM). The thermal properties of the Cp–Bz–Bmi composites were characterized by differential scanning calorimetry and thermogravimetric analysis. Dielectric constants, dielectric loss and impedance were measured by broadband dielectric spectroscopy. The mechanical properties such as tensile strength, impact and hardness of the composites were analyzed and compared with those of pristine Bz–Bmi composites. Antimicrobial studies were carried out for the Cp–Bz–Bmi composites through in vitro studies.
Experimental
Materials
Hexachlorocyclotriphosphazene was purchased from Sigma–Aldrich, India. 4-Acetamido phenol, bisphenol A, triethyl amine, potassium carbonate, aniline, paraformaldehyde, 4,4′-diaminodiphenylmethane, maleic anhydride, nickel acetate and other solvents (AR grade) were purchased from SRL Chemicals, India.Synthesis of Cp–Bz–Bmi composites
The precursor (hexa(aminophenyl)cyclotriphosphazene) (Cp),76 bisphenol-A/aniline based benzoxazine (Bz)77 and N,N′-bismaleimido-4,4′-diphenylmethane (Bmi)78 were synthesized as per the reported procedures. The Cp reinforced Bz–Bmi composites were obtained by adding various weight percentages of Cp (5, 10 and 15 wt%) to the Bz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bmi (1
Bmi (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) in the presence of tetrahydrofuran (THF). Then, the reaction mixture was stirred for 10 hours at ambient temperature and degassed to remove entrapped air, and then poured into a mould. Further to this, the reaction mixture in the mould was thermally treated at 80, 100, 120, 140, 160, 180, 200 and 250 °C for 1 hour for each temperature. Light brown colored films were formed which were then utilized for further characterization. The proposed schematic representation of the Cp–Bz–Bmi composites is shown in Fig. 1.
1 ratio) in the presence of tetrahydrofuran (THF). Then, the reaction mixture was stirred for 10 hours at ambient temperature and degassed to remove entrapped air, and then poured into a mould. Further to this, the reaction mixture in the mould was thermally treated at 80, 100, 120, 140, 160, 180, 200 and 250 °C for 1 hour for each temperature. Light brown colored films were formed which were then utilized for further characterization. The proposed schematic representation of the Cp–Bz–Bmi composites is shown in Fig. 1.
Antimicrobial study of the Cp–Bz–Bmi composites
Common gram-positive and gram-negative pathogens like Staphylococcus aureus and Escherichia coli (Department of microbiology, St. Joseph’s college of arts & science, Cuddalore, Tamilnadu, India) were used to test the antibacterial activity of the Cp–Bz–Bmi composites. The test strain was subcultured and incubated for 1 h at 37 °C. After the incubation, the bacterial culture was swabbed on the previously prepared and solidified Muller Hinton Agar plates. The disc was placed on the Petri dish and each disc was inoculated with different Cp–Bz–Bmi composites. The antibacterial assessment was performed in triplicate and the average results were reported. The bacterial resistance of the Cp–Bz–Bmi samples was examined based on the zone of inhibition of the disc. The total diameter of the inhibition zone was measured.Characterization
FT-IR spectra were recorded on a Perkin Elmer 6X FT-IR spectrometer. About 100 mg of optical grade KBr was ground with a sufficient quantity of the sample for making KBr pellets.Thermogravimetric analysis (TGA) was performed on a Netzsch STA 409 thermogravimetric analyzer. The instrument was calibrated with calcium oxalate and aluminum supplied by Netzsch. The samples (about 10 mg) were heated from 25 °C to 800 °C under a continuous flow of nitrogen (20 mL min−1) at 10 °C min−1. Differential scanning calorimetry (DSC) analysis was performed on a Netzsch DSC-200. The instrument was calibrated with indium supplied by Netzsch. Measurements were performed under a continuous flow of nitrogen (20 mL min−1). The samples (about 10 mg in weight) were heated from ambient temperature to 300 °C.
The dielectric constant, dielectric loss and impedance of the samples were measured using a broadband dielectric spectrometer (BDS-NOVOCONTROL Technologies, Germany) at 35 °C in the frequency range from 1 Hz to 20 MHz. The surface morphology of the samples was examined by scanning electron microscopy (SEM; JEOL JSM Model 6360). The wide-angle X-ray diffraction spectra were obtained using a Rich Seifert (Model 3000) diffractometer (with Cu Kα radiation (λ = 0.15418 nm)) for the powder samples by measuring the diffraction angle at 2θ values of 10 to 80°.
The tensile properties were determined according to ASTM standard D 3039 using a universal testing machine (Model 6025; Instron, UK) at a crosshead speed of 2 mm min−1. The unnotched Izod impact strength of each sample was tested as per ASTM standard D 256-88. All samples were tested unnotched so that they would be more sensitive to the transition between ductility and brittleness. Specimens of 3.2 mm thickness, 10 mm cross-section and 64 mm length were clamped in the base of the pendulum testing machine so that they cantilevered upward. The pendulum was released and the force consumed in breaking the sample was calculated from the height the pendulum reached on the follow through. Three specimens were tested for each sample and average statistical data are listed in Tables 2 and 3
Results and discussion
Structural characterization
Fig. 2 shows the FT-IR spectra of Bz–Bmi and various percentages of Cp incorporated Bz–Bmi composites. The FT-IR spectrum of the cured composite shows the disappearance of the peak at 947 cm−1 corresponding to the oxazine ring and trisubstituted benzene ring. The tetrasubstituted aromatic ring peak appears at 1514 cm−1 and the peaks observed at 2925 and 2851 cm−1 correspond to –CH2 stretching vibrations. Furthermore, the disappearance of the peak at 693 cm−1 corresponds to the maleimide group. The peaks at 1708 and 1390 cm−1 confirm the presence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N in the polymer composites. The peak observed at 3300 cm−1 indicates the presence of OH which confirms the ring-opening reaction of polybenzoxazine. The peaks observed at 1250, 1101, 950 and 829 cm−1 correspond to P–O–Ph, P–N–P, P–O–C and P–N of the phosphazene ring, respectively. This confirms the presence of the phosphazene moiety in the Cp–Bz–Bmi composites. From the FT-IR results, it was concluded that Cp incorporated Bz–Bmi composites were obtained through ring-opening polymerization with benzoxazine and bismaleimide polymerized via a Michael addition reaction with the phosphazene group via a thermal cure method.
O and C–N in the polymer composites. The peak observed at 3300 cm−1 indicates the presence of OH which confirms the ring-opening reaction of polybenzoxazine. The peaks observed at 1250, 1101, 950 and 829 cm−1 correspond to P–O–Ph, P–N–P, P–O–C and P–N of the phosphazene ring, respectively. This confirms the presence of the phosphazene moiety in the Cp–Bz–Bmi composites. From the FT-IR results, it was concluded that Cp incorporated Bz–Bmi composites were obtained through ring-opening polymerization with benzoxazine and bismaleimide polymerized via a Michael addition reaction with the phosphazene group via a thermal cure method.
Surface morphology
Thermal properties
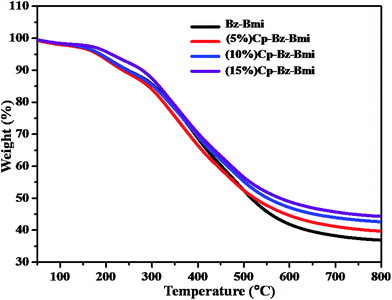
The thermal properties of pristine Bz–Bmi and the Cp (5, 10 & 15%) reinforced Bz–Bmi composites were characterized using DSC (Fig. 5) and TGA (Fig. 6). From an industrial application point of view, the Tg value of a material is considered to be a crucial parameter. Both chain rigidity and polarity are predominant factors in the glass transitions of polymers, and in turn, the degree of cross-linking density also affects the Tg values of polymers. The glass transition temperatures (Tg) of the neat Bz–Bmi and the 5 wt%, 10 wt% and 15 wt% Cp incorporated Bz–Bmi composites were studied by DSC analysis with the temperature ranging from ambient temperature to 250 °C. The resultant Tg values are presented in Table 1. The glass transition temperature is generally dependent on the segmental motion of the polymeric networks. The Tg is a measure of the degree of freedom for the segmental motion, cross-linking, entanglement constraints, and the packing density of the macromolecular segments.83 The data from the DSC analysis show that the values of Tg for neat Bz–Bmi and the 5 wt%, 10 wt% and 15 wt% Cp incorporated Bz–Bmi composites are 170, 202, 224 and 231 °C, respectively. The Cp incorporated Bz–Bmi composites possess higher values of Tg than neat Bz–Bmi. This is because incorporation of the cyclophosphazene group generates the star-like structure which restricts the movement of polymer chain segments due to steric hindrance. The star-like structure arises due to a high cross-linking density. Hence, it is reasonable to believe that the rotational hindrance restricts segmental motion and consequently results in an improvement in Tg of the thermosets.84| Sample | DSC (°C) | TGA (°C) | ||||
|---|---|---|---|---|---|---|
| Tg | Td (5%) | Td (20%) | Td (40%) | Td (max) | Char yield at 800 °C (%) | |
| Bz–Bmi | 170 | 236 | 338 | 443 | 516 | 34 |
| (5%) Cp–Bz–Bmi | 202 | 245 | 344 | 451 | 523 | 38 |
| (10%) Cp–Bz–Bmi | 224 | 250 | 357 | 464 | 552 | 43 |
| (15%) Cp–Bz–Bmi | 231 | 278 | 450 | 473 | 579 | 45 |
The thermal stability of neat Bz–Bmi and the Cp reinforced Bz–Bmi composites was investigated by TGA under a nitrogen atmosphere. The TGA thermograms of these thermosets are given in Fig. 6 and their corresponding data are summarized in Table 1. From the TGA thermograms, it was clearly observed that the Cp–Bz–Bmi composites exhibit a two-stage thermal degradation. The first stage of thermal degradation is caused by hydrocarbon segments of the thermoset, and the second stage is attributed to decomposition of the major backbones of the polymeric network due to their higher thermal stability. From the TGA results, it was inferred that the initial decomposition (5 wt% loss) temperatures of neat Bz–Bmi and 5 wt%, 10 wt% and 15 wt% Cp reinforced Bz–Bmi are 236, 245, 250 and 278 °C, respectively. The increases in initial degradation temperatures are due to the phosphazene ring present in the Cp–Bz–Bmi composite system. This is attributed to increases in steric hindrance and high cross-linking network formation between the polymer and the phosphazene group. These results imply that the phosphorus-rich residues may inhibit decomposition of the Bz–Bmi matrix.
Antibacterial properties
Fig. 7 shows the antibacterial effects of pristine Bz–Bmi and the Cp reinforced Bz–Bmi composites against Staphylococcus aureus and Escherichia coli. The presence of various concentrations of Cp reinforced Bz–Bmi shows an enhancement of the antibacterial activity against S. aureus and E. coli bacterial growth. However, the diameter of the zone formation around the well indicates bacterial inhibition. The diameter of the zone of inhibition formed around the disc diffusion of Bz–Bmi and the Cp incorporated Bz–Bmi composites against S. aureus and E. coli is shown in Fig. 8. Interesting results were observed from this study, indicating that the antibacterial activity is increased with respect to the phosphazene concentration (5, 10 & 15 wt%). The variation in the antibacterial activity of the Cp reinforced Bz–Bmi composites is mainly due to the presence of the phosphazene group. The enhanced antibacterial activity of Cp reinforced Bz–Bmi can be attributed to (i) abrasive surface texture due to surface defects, (ii) better dispersibility and subsequent compatibility exhibited by the Cp reinforced Bz–Bmi composites. From the results, it was concluded that Cp reinforced Bz–Bmi has a greater effect against bacteria. The bacterial effects may be due to the van der Waals force of interaction85 between the composites and the bacterial surface. Further cell death is also caused by the decomposition of the cell wall of the bacterium, which disturbs cell permeability and enhances the photocatalytic generation of hydrogen peroxide which subsequently leads to the leakage of minerals, proteins, genetic materials and consequently cell death.86 | ||
| Fig. 7 The zone of inhibition of antibacterial effect against S. aureus and E. coli for (a) pristine Bz–Bmi, (b) Cp (5%)–Bz–Bmi, (c) Cp (10%)–Bz–Bmi, (d) Cp (15%)–Bz–Bmi composites and (e) control. | ||
 | ||
| Fig. 8 Bar diagram of antibacterial activity against S. aureus and E. coli for pristine Bz–Bmi and the Cp (5, 10 and 15 wt%) Bz–Bmi composites. | ||
Mechanical properties
The tensile properties of pristine Bz–Bmi and the Cp reinforced Bz–Bmi composites are given in Tables 2 and 3. Improved mechanical properties were observed for the phosphazene incorporated Bz–Bmi composites. In the case of 50 wt% Bz and 50 wt% Bmi reinforced with 5, 10 and 15 wt% phosphazene, there is an increase in the values of tensile strength from 68.2 to 87.5. The introduction of phosphazene into the Bz–Bmi matrix gives a significant improvement in the values of tensile strength as the presence of Bmi along with Bz reduces the stress of the resin matrix during the curing process. The Bmi molecules are expected to suppress the stress concentration, which prevents the occurrence of micro cracking. Then, the phosphazene group improves the cross-linking density which also contributes to an enhanced value of tensile strength. The strong interfacial attraction between Bz–Bmi and Cp is responsible for the enhanced strength and energy absorption behaviour of the composites. To break the chemical bonds, a very high activation energy barrier must be overcome. By stretching a bond, the initial state increases its energy until thermal fluctuations are sufficient to overcome the activation barrier that indicates an increased tensile modulus value. The fracture surface of cured Cp–Bz–Bmi is not very smooth, indicating that cured Cp–Bz–Bmi exhibits improved toughness when compared with that of pristine Bz–Bmi. The fracture surfaces of the Cp–Bz–Bmi systems are relatively irregular; indicating that the fracture surfaces of the composites consume more energy which contributes to enhanced values of tensile strength and modulus.87 Tables 2 and 3 present the values of tensile strength, flexural strength, impact strength and hardness of pristine Bz–Bmi and phosphazene reinforced Bz–Bmi. The value of impact strength observed for Cp–Bz–Bmi is higher than that of the pristine Bz–Bmi matrix and is explained by the presence of the imide linkage between the phosphazene group and the bismaleimide skeleton which contributes to an enhanced toughness of the composites.| S. no | Composites | Tensile strength (Mpa) | Tensile modulus (Mpa) | ||
|---|---|---|---|---|---|
| 1 | Bz–Bmi | 68.2 | 68.2 | 2552 | 2553 |
| 68.4 | 2555 | ||||
| 68.3 | 2554 | ||||
| 2 | (5%) Cp–Bz–Bmi | 75.1 | 75.4 | 2873 | 2874 |
| 75.5 | 2875 | ||||
| 75.8 | 2874 | ||||
| 3 | (10%) Cp–Bz–Bmi | 80.3 | 80.3 | 3053 | 3053 |
| 80.5 | 3052 | ||||
| 80.2 | 3054 | ||||
| 4 | (15%) Cp–Bz–Bmi | 87.6 | 87.5 | 3542 | 3543 |
| 87.4 | 3544 | ||||
| 87.5 | 3545 | ||||
| S. no | Composites | Unnotched Izod impact strength (J m−1) | Hardness (HV) 1.2 | ||
|---|---|---|---|---|---|
| 1 | Bz–Bmi | 42.69 | 42.69 | 71 | 72 |
| 42.68 | 73 | ||||
| 42.72 | 72 | ||||
| 2 | (5%) Cp–Bz–Bmi | 53.82 | 53.82 | 74 | 75 |
| 53.81 | 76 | ||||
| 53.84 | 76 | ||||
| 3 | (10%) Cp–Bz–Bmi | 62.44 | 62.42 | 84 | 84 |
| 62.42 | 85 | ||||
| 62.42 | 84 | ||||
| 4 | (15%) Cp–Bz–Bmi | 75.23 | 75.22 | 92 | 93 |
| 75.22 | 95 | ||||
| 75.21 | 94 | ||||
Dielectric constant and loss
The rapid development of high-speed rail transportation, wind power and hydroelectric power generation have increasingly newer and higher requirements for high-performance electrical insulation materials with much better thermal resistance, lower dielectric constant and dielectric loss. Low-dielectric (k) films are one of the performance drivers for the continued scaling of integrated circuit devices. These films are required in microelectronic device interconnects to lower power consumption and minimize crosstalk between metal lines that “interconnect” transistors. Lowering the dielectric constant reduces propagation delays, RC constant (R = the resistance of the metal lines; C = the line capacitance) and metal crosstalk between wires. Hence, these materials should have a low dielectric constant and at the same time high thermal stability.32,33 The development of new low k materials can be carried out by two main approaches. The first method is to decrease the dipole strength by using materials with non-polar bonds. The other approach is to decrease the dipole density of the material itself, by introducing porosity or increasing the free volume. Enhanced cross-linking tends to restrict the mobility of polymer chains, and thus decreases the dielectric constant, while a larger free volume has the opposite effect on the dielectric properties.The values of the dielectric constant (Fig. 9) of pristine Bz–Bmi and cyclophosphazene (5, 10 and 15%) incorporated Bz–Bmi were analyzed at 20 MHz. The values of the dielectric constant are decreased with varying the percentage of Cp into the Bz–Bmi matrix system. From the values, it is ascertained that the Cp–Bz–Bmi systems exhibit low dielectric constant due to the presence of free volume and charge distribution and thus molecules are less closely aligned with each other. Furthermore, polarization of the molecules lowers the value of the dielectric constant and is due to the statistical thermal motion of the polar groups. The values of the dielectric constant of pristine Bz–Bmi and the cyclophosphazene (5, 10 and 15%) incorporated Bz–Bmi composites are 3.12, 2.9, 2.56 and 2.7 respectively at 30 °C. Similar trends were also observed in dielectric loss, when increasing the concentration of phosphazene in the Bz–Bmi composites. The dielectric loss was reduced due to the creation of free volume. The values of dielectric loss of pristine Bz–Bmi and the cyclophosphazene (5, 10 & 15%) incorporated Bz–Bmi composites are 0.15, 0.14, 0.13 and 0.10 respectively at 30 °C (Fig. 10).
Impedance measurements were also used to determine the electrical properties of the polymer composites. From the Nyquist plots, (Fig. 11) a typical capacitance response is observed as a semicircle for pristine Bz–Bmi and the Cp (5, 10 & 15%) incorporated Bz–Bmi composites. The large semicircle is related to charge transfer resistance (Rct) and constant phase element (CPE). The size of the semicircles decreases which indicates the lowering of electrical resistivity due to the creation of conductive paths. This technique is based on the consideration of a linear behaviour of the system under study. However, it has been demonstrated that polymeric composites can show a non-linear electrical behaviour.60,61 From this plot, a typical capacitance response is observed for pristine Bz–Bmi and the Cp (5, 10 and 15%) incorporated Bz–Bmi composites because there is no semicircle observed in the Nyquist plot. This indicates that the resultant Cp–Bz–Bmi composite act as electrical resistant materials. In general, when the size of the semicircle in the Nyquist plot increases, the electrical resistivity increases owing to a decrease in the conductive path, which in turn indicates suitability as an electrical resistance material.
 | ||
| Fig. 11 Nyquist plots of pristine Bz–Bmi and the Cp reinforced (5, 10 and 15 wt%) Bz–Bmi composites. | ||
Conclusion
Cyclophosphazene incorporated benzoxazine and bismaleimide blended (Cp–Bz–Bmi) composites were obtained through ring-opening polymerization with benzoxazine and bismaleimide polymerized via Michael addition with a phosphazene group and a Diels–Alder reaction with a polybenzoxazine group by solution polymerization via a thermal cure method. The resulting (5, 10 and 15%) Cp–Bz–Bmi composites acquire enhanced thermal properties and significant mechanical performance compared to the pristine Bz–Bmi composite. The resulting values of tensile strength, tensile modulus, impact strength and hardness observed for pristine Bz–Bmi were lower than for the phosphazene reinforced Cp–Bz–Bmi composites due to the presence of the imide linkage between the phosphazene group and the bismaleimide skeleton which contributes to the enhanced toughness of the composites. Data from TGA and DSC analyses indicate that the introduction of Cp can contribute to an improved value of Tg and thermal stability, as well as a higher percentage of char yield. SEM images show that increased Cp loading in the Bz–Bmi system resulted in the fracture surfaces being rough and irregular in appearance which showed good compatibility between the polymer matrix and the Cp phase. This may be due to the chemical reaction of Bz–Bmi with the phosphazene group and good miscibility and compatibility between the polymer matrix and phosphazene. The antibacterial properties of the Cp–Bz–Bmi composites exhibit a better performance against S. aureus and E. coli bacteria. Such properties are utilized to inhibit the bacteria for further use in microelectronic applications. The values of dielectric constant are decreased with increasing the percentage of Cp in the Bz–Bmi composites, which indicates that the Cp–Bz–Bmi systems exhibit electrical resistance activities. The uniform dispersion of Cp in the Bz–Bmi composites was confirmed by XRD. Data from different studies indicate that the Cp–Bz–Bmi composites developed in the present study can be used as potential candidates for high performance applications.References
- X. Ning and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1121 CrossRef CAS.
- T. Tsutomu, K. Takehiro and A. Tarek, Polym. J., 2008, 40, 1121 CrossRef.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344 CrossRef CAS PubMed.
- M. R. Vengatesan, S. Devaraju, K. Dinakaran, J. K. Song and M. Alagar, Polym. Int., 2012, 127, 62 Search PubMed.
- H. Ishida and D. J. Allen, J. Polym. Sci., Part B: Polym. Phys., 1996, 34, 1019 CrossRef CAS.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344 CrossRef CAS PubMed.
- H. Ishida and H. Y. Low, Macromolecules, 1997, 30, 1099 CrossRef CAS.
- Y. X. Wang and H. Ishida, Polymer, 1999, 40, 4563 CrossRef CAS.
- S. B. Shen and H. Ishida, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 3257 CrossRef CAS.
- J. A. Macko and H. Ishida, Polymer, 2001, 42, 227 CrossRef CAS.
- C. F. Wang, Y. C. Su, S. W. Kuo, C. F. Huang, Y. C. Sheen and F. C. Chang, Angew. Chem., Int. Ed., 2006, 45, 248 Search PubMed.
- N. N. Ghosh, B. Kiskan and Y. Yagci, Prog. Polym. Sci., 2007, 32, 1344 CrossRef CAS PubMed.
- Y. Yagci, B. Kiskan and N. N. Ghosh, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5565 CrossRef CAS.
- B. Kiskan and Y. Yagci, Polymer, 2005, 46, 11690 CrossRef CAS PubMed.
- C. H. Lin, S. L. Chang, H. H. Lee, H. C. Chang, K. Y. Hwang and A. P. Tu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4970 CrossRef CAS.
- B. Koz, B. Kiskan and Y. Yagci, Polym. Bull., 2010, 66, 165 CrossRef.
- M. Kukut, B. Kiskan and Y. Yagci, Des. Monomers Polym., 2009, 12, 167 CrossRef CAS PubMed.
- B. Kiskan, B. Aydogan and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 804 CrossRef CAS.
- B. Kiskan, Y. Yagci and H. Ishida, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 414 CrossRef CAS.
- B. Kiskan and Y. Yagci, Polymer, 2008, 49, 2455 CrossRef CAS PubMed.
- B. Kiskan, G. Demiray and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3512 CrossRef CAS.
- M. Ergin, B. Kiskan, B. Gacal and Y. Yagci, Macromolecules, 2007, 40, 4724 CrossRef CAS.
- A. Yildirim, B. Kiskan, A. L. Demirel and Y. Yagci, Eur. Polym. J., 2006, 42, 3006 CrossRef CAS PubMed.
- M. A. Tasdelen, B. Kiskan and Y. Yagci, Macromol. Rapid Commun., 2006, 27, 1539 CrossRef CAS.
- B. Kiskan, D. Colak, A. E. Muftuoglu, I. Cianga and Y. Yagci, Macromol. Rapid Commun., 2005, 26, 819 CrossRef CAS.
- M. Nakamura and H. Ishida, Polymer, 2009, 50, 2688 CrossRef CAS PubMed.
- A. Chernykh, T. Agag and H. Ishida, Polymer, 2009, 50, 382 CrossRef CAS PubMed.
- T. Agag and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1878 CrossRef CAS.
- A. Chernykh, J. P. Liu and H. Ishida, Polymer, 2006, 47, 7664 CrossRef CAS PubMed.
- T. Takeichi, T. Kano and T. Agag, Polymer, 2005, 46, 12172 CrossRef CAS PubMed.
- L. Wang and S. Zheng, Polymer, 2010, 51, 1124 CrossRef CAS PubMed.
- B. Kiskan, A. L. Demirel, O. Kamer and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6780 CrossRef CAS.
- J. M. Huang, S. W. Kuo, H. J. Huang, Y. X. Wang and Y. T. Chen, J. Appl. Polym. Sci., 2009, 111, 628 CAS.
- K. S. S. Kumar, C. P. R. Nair and K. N. Ninan, Polym. Adv. Technol., 2008, 19, 895 CrossRef CAS.
- H. Yeganeh and A. Jangi, Polym. Int., 2010, 59, 1375 CrossRef CAS.
- S. Tiptipakorn, S. Damrongsakkul, S. Ando, K. Hemvichian and S. Rimdusit, Polym. Degrad. Stab., 2007, 92, 1265 CrossRef CAS PubMed.
- T. Agag, V. Taepaisitphongse and T. Takeichi, Polym. Compos., 2007, 28, 680 CrossRef CAS.
- H. Y. Low and H. Ishida, Polym. Degrad. Stab., 2006, 91, 805 CrossRef CAS PubMed.
- Q. A. Chen, R. W. Xu and D. S. Yu, J. Appl. Polym. Sci., 2006, 100, 4741 CrossRef CAS.
- H. M. Huang and S. J. Yang, Polymer, 2005, 46, 8068 CrossRef PubMed.
- Q. Chen, R. W. Xu, J. Zhang and D. S. Yu, Macromol. Rapid Commun., 2005, 26, 1878 CrossRef CAS.
- T. Agag, H. Tsuchiya and T. Takeichi, Polymer, 2004, 45, 7903 CrossRef CAS PubMed.
- T. Takeichi and Y. Guo, J. Appl. Polym. Sci., 2003, 90, 4075 CrossRef CAS.
- Y. C. Su, S. W. Kuo, D. R. Yei, H. Y. Xu and F. C. Chang, Polymer, 2003, 44, 2187 CrossRef CAS.
- T. Agag and T. Takeichi, Macromolecules, 2003, 36, 6010 CrossRef CAS.
- Z. Brunovska and H. Ishida, J. Appl. Polym. Sci., 1999, 73, 2937 CrossRef CAS.
- H. J. Kim, Z. Brunovska and H. Ishida, Polymer, 1999, 40, 1815 CrossRef CAS.
- T. Agag and T. Takeichi, Macromolecules, 2001, 34, 7257 CrossRef CAS.
- S. Li and L. Wang, J. Appl. Polym. Sci., 2006, 99, 1359 CrossRef CAS.
- C. H. Lin, L. S. Chang, W. C. Hsieh and H. H. Lee, Polymer, 2008, 49, 1220 CrossRef CAS PubMed.
- R. Andereu, A. J. Reina and C. J. Ronda, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3353 CrossRef.
- T. Otsu, A. Matsumoto, T. Kubota and S. Mori, Polym. Bull., 1990, 23, 43 CrossRef CAS.
- C. P. R. Nair and T. Francis, J. Appl. Polym. Sci., 1999, 74, 3365 CrossRef CAS.
- S. Thamizharasi and B. S. R. Reddy, J. Appl. Polym. Sci., 2001, 80, 1870 CrossRef CAS.
- T. Agag and T. Takeichi, High Perform. Polym., 2001, 13, 327 CrossRef.
- Y. L. Liu, J. M Yu and C. I Chou, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5954 CrossRef CAS.
- T. Agag and T. Takeichi, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1424 CrossRef CAS.
- N. Lejeune, I. Dez, P. A. Jaffres, J. F. Lohier and P. J. Madec, Eur. J. Inorg. Chem., 2008, 1, 138 CrossRef.
- H. R. Allcock, in Inorganic and organometallic polymers II, ed. P. Wisian-Neilson, H. R. Allcock and K. L. Wynne, American Chemical Society, Washington, DC, 1994, ch 17, ACS Symposium Series 572 Search PubMed.
- D. Kumar, G. M. Fohlen and J. A. Parker, Macromolecules, 1983, 16, 1250 CrossRef CAS.
- C. J. Orme, J. R. Klaehn, M. K. Harrup, R. P. Lash and F. F. Stewart, J. Appl. Polym. Sci., 2005, 97, 939 CrossRef CAS.
- L. Zhu, Y. Zhu, Y. Pan, Y. W. Huang, X. B. Huang and X. Z. Tang, Macromol. React. Eng., 2007, 1, 45 CrossRef CAS.
- D. Kumar, M. Khullar and A. D. Gupta, Polymer, 1993, 34, 3025 CrossRef CAS.
- Advances in inorganic chemistry and radiochemistry, ed. S. S. Krishnamurthy, A. C. Sau and M. Woods, Academic Press, New York, 1978, p. 41 Search PubMed.
- C. W. Allen, Chem. Rev., 1991, 91, 119 CrossRef CAS.
- Y. E. Greish, J. D. Bender, S. Lakshmi, P. W. Brown, H. R. Allcock and C. T. Laurencin, Biomaterials, 2005, 26, 1 CrossRef CAS PubMed.
- J. K. Kim, U. S. Toti, R. Song and Y. S. Sohn, Bioorg. Med. Chem. Lett., 2005, 15, 3576 CrossRef CAS PubMed.
- M. Heyde, M. Moens, L. V. Vaeck, K. M. Shakesheff, M. C. Davies and E. H. Schacht, Biomacromolecules, 2007, 8, 1436 CrossRef CAS PubMed.
- C. J. Orme and F. F. Stewart, J. Membr. Sci., 2005, 253, 243 CrossRef CAS PubMed.
- E. Morales and L. Acostaj, Solid State Ionics, 1997, 96, 99 CrossRef CAS.
- H. R. Allcock, M. F. Welker and M. Parvez, Chem. Mater., 1992, 4, 296 CrossRef CAS.
- M. Guglielmi, G. Brusatin, G. Facchin and M. Gleria, Appl. Organomet. Chem., 1999, 13, 339 CrossRef CAS.
- H. R. Allcock, M. F Welker and M. Parvez, Chem. Mater., 1992, 4, 296 CrossRef CAS.
- J. Y. Chang, H. J. Ji, M. J. Han, S. B. Rhee, S. Cheong and M. Yoon, Macromolecules, 1994, 27, 1376 CrossRef CAS.
- Y. J. Cui, X. M. Ma, X. Z. Tang and Y. P. Luo, Eur. Polym. J., 2004, 40, 299 CrossRef CAS PubMed.
- K. Krishnadevi, A. Nirmala Grace, M. Alagar and V. Selvaraj, High Perform. Polym., 2014, 26, 89 CrossRef PubMed.
- M. R. Vengatesan, S. Devaraju, K. Dinakaran and M. Alagar, J. Mater. Chem., 2012, 22, 7559 RSC.
- J. V. Crivello, J. Polym. Sci., Part A: Polym. Chem., 1976, 14, 159 CrossRef CAS.
- A. Proctor, P. K Clark and C. A Pareker, J. Am. Oil Chem. Soc., 1995, 72, 459 CrossRef CAS.
- M. Rozainee, S. P. Ngo, A. A. SLEM, K. G. Tan, M. Ariffin and Z. N. Zainura, Bioresour. Technol., 2008, 99, 703 CrossRef CAS PubMed.
- A. Fina, D. Tabuani and A. Frache, Polymer, 2005, 46, 7855 CrossRef CAS PubMed.
- T. Ohno, S. Tagawa and H. Itoh, Mater. Chem. Phys., 2009, 113, 119 CrossRef CAS PubMed.
- Y. S. He, J. B. Zeng, S. L. Li and Y. Z. Wang, Thermochim. Acta, 2012, 529, 80 CrossRef CAS PubMed.
- L. Huan, W. Xiaodong and W. Dezhen, Polym. Degrad. Stab., 2013, 103, 96 Search PubMed.
- J. vandiver, D. Dean, N. Patel, C. Botelho, S. Best, J. D. Santos, M. A. lopes, W. Bondield and C. Ortiz, J. Biomed. Mater. Res., Part A, 2006, 78, 1619 Search PubMed.
- P. K. Stoimenov, R. L Klinger, G. L Marchi and K. J Klabunde, Langmuir, 2002, 18, 6679 CrossRef CAS.
- L. Yuan, A. Gu, G. Liang and Z. Zhang, Compos. Sci. Technol., 2008, 68, 2107 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |