Integrated real-time optofluidic SERS via a liquid-core/liquid-cladding waveguide
Jeongan Choi†
,
Kang Soo Lee†,
Jin Ho Jung,
Hyung Jin Sung* and
Sang Soo Kim*
Department of Mechanical Engineering, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, Korea. E-mail: hjsung@kaist.ac.kr; sskim@kaist.ac.kr
First published on 25th November 2014
Abstract
This paper describes the realization of highly sensitive surface-enhanced Raman spectroscopy (SERS) via an integrated three-dimensional liquid-core/liquid-cladding waveguide. The cladding flow enclosed the core flow in both the horizontal and vertical directions within a single-layered microfluidic channel, and the laser beam was guided through the core stream. Deionized water was used to suspend SERS-active silver nanocolloids to form the core fluid, and 2,2,2-trifluoroethanol was used as the cladding fluid. Dipicolinic acid, commonly used as a biomarker for the detection of Bacillus anthracis, was employed as the target analyte. The sensitivity of the SERS signal was enhanced through this design because the signals backscattered from the analytes accumulated along the core stream and increased the SERS detection volume. The SERS data was evaluated in terms of the analyte concentration in the core fluid, the irradiated laser power, and the cross-sectional area of the core stream. The present data were compared with a conventional SERS detection approach in order to quantify the enhancement factor. The detection limit of concentration, 50 nM at an acquisition time of 6 s, was a factor of 35 smaller than that of conventional methods.
Introduction
Raman microspectroscopy is a robust vibrational analysis method with several advantages in applications to molecular detection or cellular analysis: the method is non-destructive, label-free, measures a sharply defined fingerprint, unlike fluorescent signals, and water does not interfere with the Raman measurement.1 The widespread use of Raman microspectroscopy, however, has been limited by the intrinsically weak signal intensity (on the order of 1 photon detected per 106 incident photons), which provides a low sensitivity and hinders real-time detection.2 This shortcoming has been addressed in surface-enhanced Raman spectroscopy (SERS) methods, which are currently in common use.3 SERS techniques improve the Raman signal dramatically by a factor of 1011 by taking advantage of plasmonic resonance effects in the presence of metal nanoparticles.3,4 The high sensitivity, rapid, and accurate detection capabilities of SERS render the technique suitable for real-time environmental analysis and national defense applications, for example, for the detection of pathogenic biomaterials.5 Several platforms have been adapted to real-time SERS applications, including SERS-active substrates, microfluidic channels, and optical fibers.6–8Optofluidics is a research field that integrates optical and fluidic components into a single system. Extensive studies have developed optofluidic systems over the last few decades toward applications such as optofluidic waveguides, particle/cell manipulation platforms, optical components, and bioreactors.9–11 Optofluidic platforms have a variety of advantages: small sample volumes may be assayed, the platforms yield a high sensitivity, manipulations may be readily controlled, and the platforms are easily integrated into larger systems.12 SERS may be adapted to microfluidic platforms by coating SERS-active nanoparticles onto the fluidic channel or mixing the nanoparticles with an analyte that is passed through the SERS detection region.5,13,14 The latter approach is advantageous because it does not require a coating step; however, the technique provides a low sensitivity due to the low probability of interactions between the nanoparticles and the analyte. This limitation may be addressed by optofluidic SERS devices, for example, that incorporate a photonic crystal fiber (PCF) to improve the SERS signal by increasing the detection volume through a waveguide.12 Several types of PCFs have been developed, including liquid-core PCFs (LCPCFs), hollow-core PCFs (HCPCFs), and solid-core PCFs (SCPCFs).15–19 A liquid-core anti-resonant reflecting optical waveguide (ARROW) system has been developed to integrate waveguides into commonly used microfluidic channels.6 Although these systems have improved the SERS intensity dramatically, several technical limitations prevent their more widespread adoption. The fabrication processes required of these PCFs are quite complex, and contamination of the analyte during the measurement can hinder their long-term use.
Liquid-core/liquid-cladding (L2) waveguide systems may be constructed in a simple manner from two fluids with different refractive indices.20,21 The interface between the core and cladding liquids is optically smooth, which minimizes optical losses, and the liquid systems offer flexibility in controlling the refractive index contrast between the core and cladding fluids or the size of the core fluid diameter. Because these systems utilize a liquid as a working fluid, they can be easily adapted to sensors for in situ detection of a target analyte mixed with the working fluid.
In this study, we describe the fabrication of a three-dimensional L2 optical waveguide in a single-layered microfluidic channel to enable highly sensitive SERS detection in real time. A Dean vortex, followed by the introduction of two sheath flows, implemented vertical and horizontal focusing of the core fluid, respectively. Numerical simulations were performed prior to the experiment to predict the optimal experimental conditions. Dipicolinic acid (DPA), a biomarker for the detection of pathogenic bacterium, Bacillus anthracis, was adopted as a target analyte and the performance of the present SERS measurement strategy was assessed by comparing with the results obtained with a conventional SERS detection technique.22,23 Highly efficient SERS detection through L2 waveguides shows tremendous promise for detection applications that require control over the core size and channel length (i.e., the detection volume).
Materials and methods
Preparation of SERS-active silver nanocolloids
SERS-active silver nanocolloids were synthesized according to the method Leopold and Lendl, which is a rapid and simple generation process at room temperature and yields an average particle size of 35–40 nm.5,24 5 mL of 0.03 M hydroxylamine hydrochloride (HONH2·HCl) and 3 mL of 0.1 M sodium hydroxide (NaOH) were dissolved in 82 mL deionized (DI) water to sustain an adequate pH condition at 7.0 after the silver reduction reaction.24 Thereafter, 10 mL of 0.02 M silver nitrate (AgNO3) was added into the solution and agitated until a milky gray-color appeared. All the compounds were purchased at Sigma Aldrich Co., USA.Experimental
Experimental setup
Fig. 1 shows a schematic diagram of the experimental setup and the microfluidic channel design. An Ar ion laser (λ = 514 nm) was introduced to and guided along the core flow. The SERS signal was accumulated through the guided liquid core stream, and backscattered SERS signals were collected using a commercial Raman microspectroscopy detector (LabRAM ARAMIS, Horiba Jobin Yvon, France). The measurements were conducted using a 10 mW laser power, a 300 μm pinhole, a 50 μm slit, a 1800 l mm−1 grating, and an 50 × objective lens (NA = 0.5), respectively. The polydimethylsiloxane (PDMS) microfluidic channel was fabricated using soft-lithography processes and included four inlets (one core and three cladding fluid inlets) and two outlets. For the visualization of the cross-sectional area, the geometry for the side window was placed in the channel design. The three-dimensional L2 waveguide was formed in a single-layered channel using the “microfluidic drifting” technique described in the right panel of Fig. 1.25 The end of the core fluids focused with Raman microspectroscopy. A colloidal suspension of SERS-active silver nanoparticles in deionized (DI) water (ncore = 1.33) and 2,2,2-trifluoroethanol (nclad = 1.29, Alfa Aesar) were used as the core and cladding fluids, respectively. These miscible fluids were selected to reduce fluid instabilities in the low flow rate regime, which tend to create undesirable artifacts, including droplet generation. Dipicolinic acid (DPA) was used as an analyte. The molecular structure of DPA is depicted in Fig. 2. It is a biomarker for the detection of pathogen bacterium, Bacillus anthracis, which has been considered as one of the hazardous substances as a biological weapon because its inhalation lethal dose for 50% kill (LD50) is an order of 100 ng.22 DPA signal at the SERS spectra was identified by a fingerprint at 1007 cm−1 corresponding to the pyridine ring “breathing” band.26Formation of L2 waveguide and SERS measurement
A schematic diagram of the experimental setup and the microfluidic channel design is portrayed in Fig. 1. The core and cladding fluids flowed separately at the entrance. As the two fluids came in contact at a point of great curvature, the fluids created a secondary flow, that is, a Dean vortex, through interactions between the parabolic velocity profiles under the pressure-driven Poiseuille flow and the centrifugal force induced at the curved geometry (region 1 in Fig. 1). The Dean vortex effectively focused the core fluid in the vertical direction (region 2 in Fig. 1). Subsequently, the core fluid was horizontally sandwiched between two cladding fluids to create a three-dimensional core stream (region 3 in Fig. 1). Two non-dimensional numbers were used as a measure the magnitude of the Dean vortex:27 the Reynolds number (Re = ρVD/μ, where ρ is the fluid density, V is the average flow velocity, D is the hydraulic diameter of the channel, and μ is the dynamic viscosity of the fluid) and the Dean number (Dn = Re (D/R)1/2, where R is the mean radius of the 90° curve). In this study, Re and Dn were 86 and 53, respectively, to ensure appropriate Dean vortex effects.The SERS detection region was located at the main channel with dimensions of 192 μm × 100 μm × 5 mm (depth × width × length). T-shaped endpoint with two outlets was employed to facilitate the laser alignment with ease. In order to quantify the performance of the system, the SERS signals obtained with the optofluidic L2 waveguide were compared to the data of conventional point measurement approach. In order to characterize the system, (1) DPA concentrations, (2) irradiated laser power, and (3) core flow rate were adjusted between (1) 100 nM–1 mM, (2) 1 mW–10 mW, and (3) 60–180 μL min−1, respectively. The limit of detection (LOD), reflecting the system performance, was estimated.
Results and discussion
Configuration of L2 waveguide
Fig. 3(a) shows the results of a computational fluid dynamics (CFD) simulation of the refractive index profile in region 3 shown in Fig. 1. The Navier–Stokes and diffusion equations were solved together in three dimensions using commercial CFD software (COMSOL Multiphysics 3.5). The flow rates of the core, sheath (for vertical focusing), and two subsequent sheath flows (for horizontal focusing) were 60, 450, and 300 μL min−1, respectively. Robust core flow focusing in three dimensions was predicted, and the calculated core diameter was approximately 30 μm. Fig. 3(b) shows the experimental results obtained through guiding a beam through the L2 waveguide system. The laser beam was confined in and guided along the core stream, which corresponded to the prediction of CFD simulation. The high flow velocity prevented rapid diffusion between the core and cladding fluids and delayed the reduction in contrast between the refractive indices of the two fluids along the longitudinal direction. Meanwhile, binary diffusion between the core and cladding fluids provided the present L2 waveguide with the characteristics of a graded-index (GRIN) waveguide by creating a smooth concentration gradient perpendicular to the flow direction.27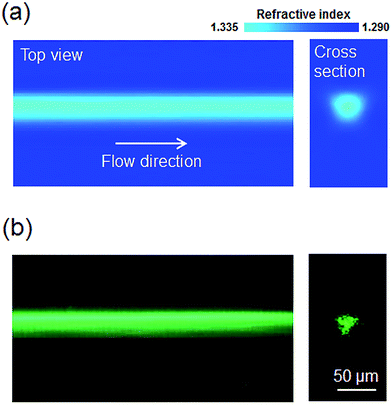 | ||
| Fig. 3 (a) CFD simulation analysis of the refractive index profile in region 3 of Fig. 1; (b) experimental results showing the laser beam guidance through the L2 waveguide. | ||
In this system, SERS signal could be enhanced according to the signal accumulation along the core fluid by the total internal reflection. Backscattered SERS signal was collected toward the objective lens and quantified by the Raman spectroscopy. The intensity of the Raman signal in L2 waveguide is calculated by:16,28
 | (1) |
| I = PκAσπ(NA)L, | (2) |
In addition to the advantages of the L2 waveguide as an optofluidic SERS application,21 the system prevented the memory effect that corresponded to the undesirable permanent SERS signals stemmed from a target analyte attached to a channel wall. The core fluid did not come into contact with the channel wall owing to the three-dimensional flow focusing and the system was not established with stagnated fluidic system, but in a flowing system. The system thoroughly expanded the SERS detection volume simply by increasing the core size and channel length. The core size can be controlled by adjusting the flow rate ratio between the core and cladding fluids, which secures the capability of control of SERS detection cross-sectional area. Moreover, various optical fibers with different core sizes could be easily integrated with less optical coupling loss (i.e., core-on-demand).
SERS detections with conventional and waveguide-based approaches
Fig. 4 shows the performances of two different measurement techniques. The inset describes the working principle underlying each method. The main difference between the two measurement methods stemmed from the detection volume. The conventional method acquired the SERS signal at the focused laser beam spot, whereas the L2 waveguide method accumulated the signal through the core flow via the total internal reflection-driven guided beam. In this study, backscattered SERS signals were collected in both experimental setups. In general, SERS signals may be enhanced by (1) the laser excitation intensity, (2) the Raman scattering cross-section, or (3) the number of analyte molecules present within the detection volume.12 For comparison, the laser power and DPA concentration were set to 10 mW and 1 mM, respectively. The elongation of the detection volume by the waveguide directly increased the number of molecules present in the SERS detection volume, leading to a dramatic enhancement in the signal. The fingerprint intensity obtained using the waveguide-based measurement was 35 times greater than the intensity obtained using conventional measurements. The increment of detection volume via L2 waveguide secures the economic and simple in fabrication aspect, compared to other designs, for example, photonic crystal fiber.30,31 Moreover, the waveguide-based approach is facile to be integrated with laser beam system, while the conventional method is quite sensitive to the laser alignment.SERS for different DPA concentrations
Fig. 5(a) shows the SERS spectra of the DPA solution obtained from the L2 waveguide system prepared with different DPA concentrations. The DPA concentration was adjusted incrementally to the values: 100 nM, 1 μM, 10 μM, 100 μM, 500 μM, and 1 mM. The baseline signal was subtracted from the raw data using an embedded LabSpec 5.0 software algorithm (Horiba Jobin Yvon). A distinct fingerprint was obtained at 1007 cm−1 in each spectrum. As the DPA concentration increased, the signal intensity increased accordingly. The method was evaluated quantitatively by exporting the fingerprint intensities at each DPA concentration, as shown in Fig. 5(b). A linear correlation was obtained within the concentration range of 100 nM–10 μM DPA (inset) because the binding between the DPA analytes and the silver nanoparticles was linearly dependent on the DPA concentration in this range. Thereafter, the slope decreased, indicating saturation as the DPA concentration approached 1 mM. This phenomenon could be explained in terms of the Freundlich adsorption isotherm and the Langmuir isotherm.6,32 The enhancement of Raman signal is mainly determined by the first adsorbed layer.33 As the analyte concentration in silver colloidal solution increased beyond a specific saturation point, the slope started to decay. The limit of detection (LOD), reflecting the system performance, was estimated to be 50 nM at the acquisition time of 6 s. This result is comparable to LODs of other types of optofluidic SERS systems, for example, ARROW, PCF, and liquid-core/solid-cladding waveguide (10–30 nM), whilst the system configuration became dramatically simpler and easier to be integrated to monolithic labs-on-a-chip system.6,16,19,34 Intuitively, a better sensitivity is expected for a larger channel length, and the concentration of silver nanoparticles in the core fluid being optimized.SERS for different incident laser powers
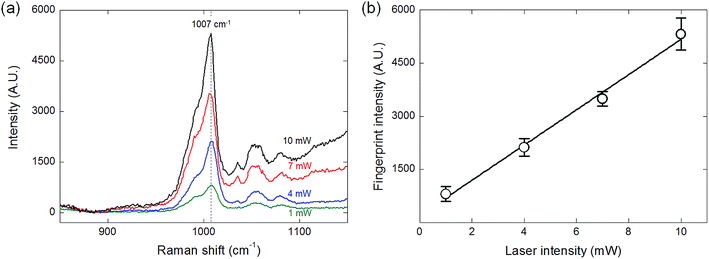
It could be foreseen intuitively that the intensity of the Raman signal is proportional to the laser power. SERS signals with four different laser powers (1, 4, 7, and 10 mW) were measured as shown in Fig. 6(a) and the fingerprint intensity of 1 mM DPA solution at each laser power is portrayed in Fig. 6(b). Bello et al. reported that there is a maximum laser power adaptable because the laser power above the specific value induces the photodamage on the sample analyte, resulting in the reduction of measurement efficiency and distortion to the spectra due to the undesirable shift of the fingerprint value.35 In this study, however, the fingerprint signal was clearly identified for every condition, and the laser power and the fingerprint intensity showed the linear correlation without the photo-induced damage.SERS for different cross-sectional area of the core fluid
One of the powerful strength of the optofluidic system is the flexibility in a system configuration. The cross-sectional area of the core fluid can be manipulated by simply adjusting the flow rate ratio between the core and cladding fluids. Fig. 7 shows the SERS spectra of the DPA solution by varying the core flow rates from 180 to 60 μL min−1. The corresponding core sizes were approximately 50 μm (180 μL min−1), 40 μm (120 μL min−1), and 30 μm (60 μL min−1), respectively. The fingerprint intensity was proportional to the cross-sectional area of the core fluid because the larger core size means the higher fluid flux in the SERS detection region, leading to the increased interplay between the light and the analyte. The optical loss during the coupling from the objective lens to the L2 waveguide could be diminished. Even though the higher optical loss during the coupling from the L2 waveguide to the objective lens and the reduced power density of the guided beam due to the enlarged cross-sectional area could play an adverse role in the SERS intensity perspective, afore-mentioned two positive effects led to the signal improvements.Conclusions
In conclusion, a three-dimensional liquid-core/liquid-cladding optical waveguide system was established and exploited for the detection of surface-enhanced Raman scattering. CFD simulations supported the experimental results, and two miscible fluids were used to create a graded-index waveguide. The proposed system configuration is suitable for applications requiring highly sensitive chemical detection in real time: The system minimizes the memory effects and optical losses, and increases the detection volume in a simple monolithic system configuration. The present system improved the SERS signal of the DPA by a factor of 35 relative to the value obtained using conventional methods. The calculated limit of detection was 50 nM. This system is expected to be useful for real-time environmental analysis and national defense applications, for example, as a detection/alert system for the identification of pathogenic biomaterials.Acknowledgements
This work was supported by the Creative Research Initiatives Program (no. 2014-001493) of the National Research Foundation of Korea (MSIP).Notes and references
- J. W. Chan, J. Biophotonics, 2013, 6, 36 CrossRef CAS PubMed.
- L. Chen and J. Choo, Electrophoresis, 2008, 29, 1815 CrossRef CAS PubMed.
- M. Fleischmann, P. J. Hendra and A. J. Mcquillan, Chem. Phys. Lett., 1974, 26, 163 CrossRef CAS.
- M. Moskovits, J. Chem. Phys., 1978, 69, 4159 CrossRef CAS PubMed.
- L. X. Quang, C. Lim, G. H. Seong, J. Choo, K. J. Do and S.-K. Yoo, Lab Chip, 2008, 8, 2214 RSC.
- P. Measor, L. Seballos, D. Yin, J. Z. Zhang, E. J. Lunt, A. R. Hawkins and H. Schmidt, Appl. Phys. Lett., 2007, 90, 211107 CrossRef PubMed.
- G. Wei, H. Zhou, Z. Liu and Z. Li, Appl. Surf. Sci., 2005, 240, 260 CrossRef CAS PubMed.
- Y. Guo, M. K. K. Oo, K. Reddy and X. Fan, ACS Nano, 2012, 6, 381 CrossRef CAS PubMed.
- D. Erickson, D. Sinton and D. Psaltis, Nat. Photonics, 2011, 5, 583 CrossRef CAS.
- K. S. Lee, S. Y. Yoon, K. H. Lee, S. B. Kim, H. J. Sung and S. S. Kim, Opt. Express, 2012, 20, 17348 CrossRef CAS PubMed.
- C. Monat, P. Domachuk and B. J. Eggleton, Nat. Photonics, 2007, 1, 106 CrossRef CAS.
- I. M. White, S. H. Yazdi and W. W. Yu, Microfluid. Nanofluid., 2012, 13, 205 CrossRef CAS.
- J. Leem, H. W. Kang, S. H. Ko and H. J. Sung, Nanoscale, 2014, 6, 2895 RSC.
- B.-B. Xu, Z.-C. Ma, L. Wang, R. Zhang, L.-G. Niu, Z. Yang, Y.-L. Zhang, W.-H. Zheng, B. Zhao, Y. Xu, Q.-D. Chen, H. Xia and H.-B. Sun, Lab Chip, 2011, 11, 3347 RSC.
- Y. Zhang, C. Shi, C. Gu, L. Seballos and J. Z. Zhang, Appl. Phys. Lett., 2007, 90, 193504 CrossRef PubMed.
- F. Eftekhari, J. Irizar, L. Hulbert and A. S. Helmy, J. Appl. Phys., 2011, 109, 113104 CrossRef PubMed.
- Y. Han, S. Tan, M. K. K. Oo, D. Pristinski, S. Sukhishvili and H. Du, Adv. Mater., 2010, 22, 2647 CrossRef CAS PubMed.
- M. K. K. Oo, Y. Han, R. Martini, S. Sukhishvili and H. Du, Opt. Lett., 2009, 34, 968 CrossRef CAS.
- X. Yang, Z. Tanaka, R. Newhouse, Q. Xu, B. Chen, S. Chen, J. Z. Zhang and C. Gu, Rev. Sci. Instrum., 2010, 81, 123103 CrossRef PubMed.
- D. B. Wolfe, R. S. Conroy, P. Garstecki, B. T. Mayers, M. A. Fischbach, K. E. Paul, M. Prentiss and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12434 CrossRef CAS PubMed.
- D. V. Vezenov, B. T. Mayers, D. B. Wolfe and G. M. Whitesides, Appl. Phys. Lett., 2005, 86, 041104 CrossRef PubMed.
- D. D. Evanoff, Jr, J. Heckel, T. P. Caldwell, K. A. Christensen and G. Chumanov, J. Am. Chem. Soc., 2006, 128, 12618 CrossRef PubMed.
- J. K. Daniels, T. P. Caldwell, K. A. Christensen and G. Chumanov, Anal. Chem., 2006, 78, 1724 CrossRef CAS PubMed.
- N. Leopold and B. Lendl, J. Phys. Chem. B, 2003, 107, 5723 CrossRef CAS.
- X. Mao, J. R. Waldeisen and T. J. Huang, Lab Chip, 2007, 7, 1260 RSC.
- A. A. Kolomenskii and H. A. Schuessler, Spectrochim. Acta, Part A, 2005, 61, 647 CrossRef CAS PubMed.
- K. S. Lee, S. B. Kim, K. H. Lee, H. J. Sung and S. S. Kim, Appl. Phys. Lett., 2010, 97, 021109 CrossRef PubMed.
- R. Altkorn, M. D. Malinsky, R. P. van Duyne and I. Koev, Appl. Spectrosc., 2001, 55, 373 CrossRef CAS.
- G. M. Whitesides and S. K. Y. Tang, Proc. SPIE, 2006, 6329, 63290A–63291A CrossRef PubMed.
- A. F. Chrimes, K. Khoshmanesh, P. R. Stoddart, A. Mitchell and K. K. Zadeh, Chem. Soc. Rev., 2013, 42, 5880 RSC.
- S. H. Yazdi and I. M. White, Biomicrofluidics, 2012, 6, 014105 CrossRef PubMed.
- W. Hüttner, K. Christou, A. Göhmann, V. Beushausen and H. Wackerbarth, Microfluid. Nanofluid., 2012, 12, 521 CrossRef.
- C. McLaughlin, D. Graham and W. E. Smith, J. Phys. Chem. B, 2002, 106, 5408 CrossRef CAS.
- W. Xu, S. Xu, Z. Lü, L. Chen, B. Zhao and Y. Ozaki, Appl. Spectrosc., 2004, 58, 414 CrossRef CAS.
- J. M. Bello and T. V. Dinh, Appl. Spectrosc., 1990, 44, 63 CrossRef CAS.
Footnote |
| † J. Choi and K.S. Lee contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |