Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Decoding the AlPO4 and LATP surface with a combined NMR-DFT approach†
Javier Valenzuela
Reina‡
 bd,
Vera M.
Barysch‡
bd,
Vera M.
Barysch‡
 ac,
Conrad
Szczuka
ac,
Conrad
Szczuka
 a,
Simone S.
Köcher
a,
Simone S.
Köcher
 *ab,
Josef
Granwehr
*ab,
Josef
Granwehr
 ac and
Christoph
Scheurer
ac and
Christoph
Scheurer
 ab
ab
aInstitute of Energy Technologies (IET-1), Forschungszentrum Jülich GmbH, 52425, Jülich, Germany. E-mail: s.koecher@fz-juelich.de
bFritz-Haber Institute of the Max Planck Society, 14195 Berlin, Germany
cInstitute of Technical and Macromolecular Chemistry, RWTH Aachen University, 52056 Aachen, Germany
dHumboldt Graduate School, Humboldt University of Berlin, 10099 Berlin, Germany
First published on 23rd June 2025
Abstract
A milestone in the development of next generation high-performance lithium ion batteries is the understanding and targeted engineering of hybrid electrolytes, consisting of a polymer and a ceramic component, and in particular their interfaces. Nuclear magnetic resonance (NMR) spectroscopy is a powerful non-destructive technique for unraveling the intricate interface structures and ion dynamics in these materials, yet data interpretation often relies on empirical rules that have been devised using data from the bulk of materials. By exploiting the synergies between advanced NMR experiments and density functional theory (DFT) simulations, AlPO4 is studied as a model for the surface of the well-known solid ion conductor Li1+xAlxTi2−x(PO4)3 with 0.3 ≤ x ≤ 0.5 (LATP), which is a promising candidate for the ceramic component of a hybrid electrolyte. By combining the multi-nuclear NMR techniques cross-polarization (CP) and transfer of populations in double resonance (TRAPDOR) on AlPO4 powder with DFT calculations of NMR observables for a variety of surface models, the surface structure of commercial AlPO4 is elucidated. It is shown that even after extended drying, the surface of AlPO4 is hydroxylated, exhibiting a TRAPDOR-estimated 1H–27Al quadrupolar coupling constant, CQ, of 5.8 ± 0.9 MHz. The joint theoretical-experimental approach also enables first insights into the bonding motifs of organic entities on functionalized AlPO4 surfaces as a model for LATP surfaces. Surface interactions and the presence of functional groups upon silanization of hydroxylated surfaces are confirmed both on AlPO4 and LATP. We demonstrate that observables, which are experimentally as well as theoretically accessible, provide information on interfacial bonding motifs, interatomic distances, and interactions, surpassing the capabilities of either NMR or DFT techniques alone.
1 Introduction
Even though lithium ion batteries (LIBs) have already been established industrially since the 1990s,1 they are still lacking in certain areas such as energy and power density, long cycle life, and safety.2,3 Hybrid electrolytes consisting of a composite of ion-conducting polymers and superionic ceramics have the potential to combine the mechanical flexibility and improved fabrication of the polymer with the superior ionic conductivity and improved chemical stability and aging behavior of the superionic ceramic.4–6 At room temperature, the ceramic usually exhibits the higher ionic conductivity, but with higher temperatures the ionic conductivity of the polymer increases.7 However, the interfacial resistance between the polymer and ceramic phases has turned out to be a major obstacle for the successful application of composite electrolytes.4 The complex interface behavior of polymers and ceramics has already been studied in relation to features such as the degree of polymer crystallization.8Experimental as well as theoretical studies have elucidated various atomistic processes and structure–property relationships for different superionic electrolytes, yet atomistic structure, ionic transport,9,10 and degradation processes11 even in pure solid electrolytes remain poorly understood. In comparison, hybrid materials such as ceramic–polymer composites and their interfaces are much more challenging and represent an active and controversial field of research.12–14 A systematic quantification of the different mobility contributions within and across the different phases and interfaces to the overall ionic conductivity is still lacking. Although, several hypotheses address the impact and mechanisms of ionic motion along or across the ceramic–polymer interface,4,12 it is generally unknown how the polymer and the ceramic are connected at the molecular level, and whether they are separated by a passivated or hydrated ceramic surface that either strongly enhances or blocks ionic conductivity.12 Nevertheless, a thorough understanding of ceramic–polymer interfaces is essential for targeted and efficient functionalization of ceramic nanoparticles and engineering of the interface in order to improve the interfacial resistance and make hybrid electrolytes industrially relevant. So far, this was mainly investigated for the electrolyte–electrode interface,15–20 but offers also potential for the ceramic–polymer interface. Surfaces were already successfully modified, as for example Kuhnert et al. demonstrated for lithium lanthanum zirconium oxide (LLZO)21 or Li et al.22 for LATP that was coated with ZnO via atomic layer deposition. Another approach was chosen by Scharf et al., who described the grafted electrolyte Al2O3_PCL with polycaprolactone.23
The ceramic material of interest in this work is Li1+xAlxTi2−x(PO4)3 with x = 0.5 (LATP) from the structural family of Na super ionic conductors (NASICON), which shows a high ionic conductivity (10−4–10−3 S cm−1) and an advantageous resistance to dendrite formation and growth over long cycling times.24 The properties of LATP can be significantly influenced by the synthesis route, such as by the sintering and annealing parameters25–27 as well as interfacial Mg2+ doping.28 Due to the structural complexity of LATP, AlPO4 can be used as a model system since it is structurally similar to LATP, but easier to compute by means of density functional theory (DFT). Even though LATP possesses more chemical species than AlPO4, structural similarities such as PO4 tetrahedral motifs exist.29 Moreover, AlPO4 is a well-studied material both experimentally30–32 and theoretically33 in all of its phases. Several phases of AlPO4 including berlinite, cristobalite, and tridymite, are structural analogs of the corresponding polymorphs of SiO2, while its hydrated form variscite (AlPO4·2H2O) represents a distinct phase.34–36 Amongst the anhydrous AlPO4 phases, the berlinite, which is isostructural with α-quartz, is known to be the most stable at room temperature.37 AlPO4 has been relevant in catalysis for several decades already.38,39 Both for catalysis and as a model system for interfaces in hybrid electrolytes, its surface properties are of interest.40,41
Modifications and functionalizations of ceramic surfaces such as zeolites in order to tune catalytic surface properties is an established procedure.42 One method of catalytic surface modification is the silanization of surface hydroxy groups, which leads to the assumption that silanization of solid electrolytes exhibiting hydroxy groups at the surface can also affect the interfacial properties, such as the interfacial resistance at the ceramic–polymer interface.
To obtain structural information of the bulk of a solid material, magic angle spinning (MAS) nuclear magnetic resonance (NMR) is a standard technique, as anisotropic interactions can be (partially) averaged out, resulting in narrower signals in the NMR spectra. This technique already provided various insights for solid electrolytes, such as the impact of the LATP stoichiometry on the presence of the side phase AlPO4,43 the Li mobility and cation location in other NASICON materials,44 or the dopant site occupancy of Al and Ga in the LLZO lattice.45
The transfer of populations in double resonance (TRAPDOR) NMR technique enables the surface-sensitive detection of “invisible” Al species experiencing large quadrupolar coupling by exploiting dipolar recoupling of these nuclei through radio frequency (RF) irradiation during the dipolar evolution phase. This effect is induced by rotationally induced level crossings, and is measured using the spin-echo difference method.46,47 Using the TRAPDOR techniques in combination with multi-quantum (MQ) NMR techniques, materials such as amorphous lithium phosphorus oxynitrides (LiPON),48 zeolites,49 or aluminophosphate frameworks50 have already been investigated. TRAPDOR experiments can be correlated to theoretical atomistic models by comparing the experimental and calculated quadrupolar coupling constant. The first principles simulation of quadrupolar coupling constants with plane wave DFT is well established and has been shown to provide reliable, quantitative predictions in particular for 27Al.51–54
In this work, a combination of DFT simulations and NMR techniques is applied to investigate AlPO4, which is studied as a model system for LATP. First, the bulk properties are investigated by comparing experimental and simulated chemical shift differences as well as cross-polarization magic angle spinning (CPMAS) experiments. Then the surface is analyzed with respect to the molecular structure, involving experimental TRAPDOR NMR experiments. The quadrupolar coupling constant obtained from the TRAPDOR experiments is compared to the computed quadrupolar coupling constants of hydrated and hydroxylated surface models. Finally, the surface is modified using silanization to set the stage for more complex ceramic–polymer interfaces as they exist in hybrid electrolytes.
2 Materials and methods
Additional details on Materials and methods55–61 can be found in the ESI,† Section S1.2.1 Sample preparation
AlPO4 powder was procured from Sigma-Aldrich (99.99% trace metal basis), in the following referred to as commercial sample. The commercial sample was dried under vacuum at 120 °C for 63 h using a pressure of 100 mbar, in the following referred to as dried sample. Commercial (non-dried, stored under ambient atmosphere) and dried AlPO4 were packed into MAS NMR rotors (3.2 mm in diameter). The CPMAS and TRAPDOR experiments were conducted on the dried AlPO4 sample.For surface silanization, both AlPO4 (99.99% trace metal basis, Sigma-Aldrich, dried for 17 h at 120 °C) and LATP62 (stored under argon atmosphere) were dispersed in toluene. Trimethoxy-(2-phenylethyl)silane (TMPES, Sigma-Aldrich) was added. The product was centrifuged and washed with toluene, cyclohexane, and ethanol. It was dried at 60 °C and 100 mbar overnight. The samples were packed into MAS rotors (3.2 mm in diameter) under argon atmosphere.
2.2 NMR
1H, 31P, and 27Al measurements were conducted on a Bruker Avance III HD spectrometer with a 9.4 T magnet. A 3.2 mm triple resonance H/X/Y CPMAS probe was used for all samples. All measurements took place with a probe temperature of 20 °C. The working frequencies were 400.2 MHz for 1H, 162.0 MHz for 31P, and 104.3 MHz for 27Al. No decoupling was used in the pulse sequence. The samples were measured at a spinning frequency of 20 kHz if not stated differently. Pseudo-Voigt fits of the 1H and 27Al MAS NMR spectra were conducted using OriginPro 2021b (OriginLab Corporation, Northampton, MA, USA).31P{1H} as well as 27Al{1H} CPMAS NMR experiments were conducted using different contact times.
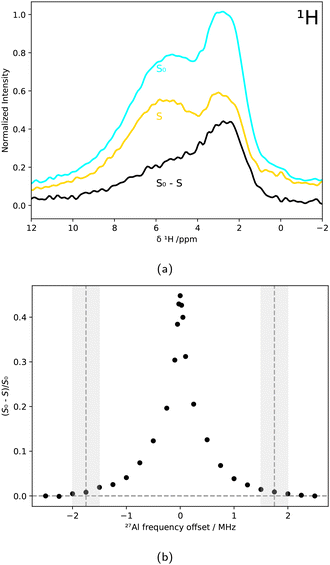
For the TRAPDOR experiments, first a rotor-synchronized 1H spin-echo sequence (90°–nτr–180°–nτr) as depicted in Fig. 1a was applied, with τr being one rotor period and n representing an integer number. Then the procedure was repeated with irradiating the 27Al nuclei in between the two 1H pulses, as shown in Fig. 1b. Due to the 27Al irradiation, refocusing of the 1H spins is prevented if they are coupled to 27Al nuclei. Rotationally induced level crossings between quadrupolar satellite transitions and the RF frequency perturb the spin states synchronously with the spinning frequency, enabling dipolar recoupling.47,6327Al{1H} TRAPDOR experiments were conducted by measuring 1H MAS NMR spectra (MAS spinning rate of 5 kHz) both with and without 27Al irradiation for different 27Al frequency offsets. The signal ratio between S0 (no 27Al irradiation) and S (irradiation of 27Al) was calculated based on the spinning side bands within the integration interval ranging from −93 ppm to 105 ppm. The cut-off frequency 2νQ, for which the TRAPDOR effect is no longer observable, determines the quadrupolar coupling constant by47,64
 | (1) |
 | ||
| Fig. 1 Scheme of the TRAPDOR pulse sequences without 27Al irradiation (a) and with 27Al irradiation (b). | ||
2.3 SEM-EDX
The specimen holders were prepared with silanized AlPO4 (TMPES) and silanized LATP (TMPES). The measurements were conducted on a Quanta FEG 650 scanning electron microscope (FEI), equipped with additional energy-dispersive X-ray spectroscopy equipment (SEM-EDX). An Everhard Thornley detector was used to detect the secondary electrons.2.4 Computational methods
NMR observables were calculated with the GIPAW method67 as implemented in CASTEP65 v.23 with on-the-fly generated GIPAW pseudopotentials. The CASTEP calculations yield the isotropic shielding σiso, which is converted into chemical shift δ, i.e. shielding difference, by
| δ = σisoref − σisosample | (2) |
The interactions between the buckled-berlinite surface and water molecules is divided between hydroxylation and hydration. The first describes the removal of an oxygen atom from the surface and the addition of two –OH groups to two P atoms ((P-OH)2-berlinite, cf.Fig. 2), two Al atoms ((Al-OH)2-berlinite), or one on both atoms ((Al-OH)(P-OH)-berlinite). The latter is stabilized by hydrogen bonds between water and different Al atoms (cf. ESI,† Fig. S3), either the Al in the protruding part of the buckle motif (Alhigh(H2O)-berlinite), in the sunken part of the surface (Allow(H2O)-berlinite) or at the surface level (Almiddle(H2O)-berlinite).
3 Results and discussion
3.1 AlPO4
The bulk structure of the AlPO4 powder samples is scrutinized by combining conventional 1D MAS solid state NMR spectroscopy of 1H, 27Al, and 31P nuclei with DFT simulations. The viability of the computational NMR techniques employed in this study is evaluated by a benchmarking analysis, comparing experimental36,37 and calculated NMR observables of berlinite and variscite as reliable models for 4- and 6-fold coordinated AlPO4 as well as other AlPO4 phases. The benchmarking yields quantitative results for quadrupolar coupling constants CQ and qualitatively correct assignment of the 27Al chemical shielding based on the coordination environments as shown in literature before (details in the ESI†).51–54The 4-fold coordinated Al environment is in agreement with the crystal structure of berlinite. In contrast to that, the 5- and 6-fold coordinated Al cannot be correlated with any structural motifs in pure crystalline AlPO4, neither berlinite, nor the less stable phases tridymite or cristobalite, which contain exclusively tetrahedrally coordinated Al nuclei. Hence, the signals from 20 to −40 ppm can only be attributed to either amorphous structures,74 zeolite-like motifs,75 or partially hydrated AlPO4 phases.
DFT simulated isotropic 27Al shieldings of berlinite (4-fold coordinated Al), variscite (6-fold coordinated Al), and UiO-769 (4-, 5- and 6-fold coordinated Al) confirm the assignment of the local Al environments and reproduce the δ(27Al) within ±5 ppm when aligning the calculated berlinite σiso with the δ(27Al) signal maximum of dried AlPO4 at 36 ppm (cf. next section). Thus, the DFT results can reproduce the experimental 27Al NMR spectrum of commercial AlPO4 with hydrous AlPO4 phases. In order to further investigate the mixture of AlPO4 hydrates and anhydrous AlPO4 in commercial AlPO4 powder, the presence of AlPO4 hydrates in the sample was studied in more detail using 1H MAS NMR spectroscopy.
Fig. 4 reveals a broad 1H signal centered at 5.4 ppm for commercial AlPO4. Moreover, there is an additional upfield shoulder at 3–5 ppm that indicates the existence of a second, different type of 1H local environment.
To assign the overlapping signals, the DFT simulated 1H shieldings in variscite (orange) and UiO-7 (blue) were analyzed, as depicted in Fig. 4. The 1H chemical shift of the 6-fold coordinated Al species in UiO-7 involving H2O is referenced to the broad experimental signal centered at 5.4 ppm, which is in the δ range for water molecules. The distribution of DFT simulated δ appears to be shifted with respect to the experimental signal, since the structural degrees of freedom of the rather mobile protons are not sampled sufficiently by DFT and the alignment with experiment is not unambiguous. But the simulated chemical shift range of about 9 ppm matches the experimental signal range and suggests that the sample also contains hydrated species with a mixture of 5- and 6-fold coordinated Al. There is no clear distinction of 1H shifts of H2O attached to a 6-fold coordinated Al versus a 5-fold coordinated Al, since the simulated 1H shifts of variscite and UiO-7 overlap. The distribution of signals is likely dominated by hydrogen bonds of different strengths between the coordinated H2O and its environment.
The combination of 27Al and 1H MAS NMR spectra indicates clearly that the commercial AlPO4 sample contains a mixture of anhydrous and hydrous AlPO4, presumably in different phases. In order to remove water and dehydrate the hydrous AlPO4 phases, the sample was dried as described in Section 2.
Analogously to the 27Al MAS NMR spectra, also the 1H MAS NMR spectra were measured at different stages of drying of the AlPO4 sample. The broad signal at 5.4 ppm decayed upon drying, which emphasized the signal at 3.0 ppm causing the shoulder in the spectrum of the non-dried sample (Fig. 4).
Using a pseudo-Voigt fit for the signal at 3.0 ppm, it was revealed that its intensity did not change systematically during the drying process (cf. ESI,† Fig. S6). In contrast, after drying the sample for 63 h, the broad signal attributed to water decreased by 81% compared to the commercial sample. The DFT calculated distribution of σiso showed that the removed signal corresponds best to H2O in the 6-fold coordinated Al environment in UiO-7, characterized by the absence of hydrogen bonds, yet also H2O in a 5-fold coordinated Al environments as well as some of the downfield 1H resonances of H2O in variscite are in the vicinity. Hence, the decaying signal is attributed to the removal of crystal water upon drying as expected and previously reported for variscite,34 which is in agreement with the development of the signals in the 27Al MAS NMR spectra. However, the constant signal at δ(1H) = 3.0 ppm indicates that some type of more strongly bound H2O or OH entities are remaining.
In Fig. 5, the 31P NMR spectrum of the commercial AlPO4 sample shows only one broad signal centered at −25.2 ppm, corresponding to 4-fold coordinated P in an amorphous environment.73 Upon drying, the signal in the 31P NMR spectrum becomes narrower, which is presumably due to the more ordered, less heterogeneous crystal structure as well as reduced relaxation owing to the removal of the mobile proton species. Moreover, its center is shifted from −25.2 ppm to about −26.3 ppm, indicating a reduction in the variety of P coordination environments and a larger average Al–O–P bond angle.76,77
The DFT simulated 31P shielding is referenced by aligning σiso of berlinite with the signal maximum of dried AlPO4 at a chemical shift of −26.3 ppm, as shown in Fig. 5. The calculation of the 31P shieldings in berlinite and variscite predicted a chemical shift difference between both systems of about 1.3 ppm that agrees well with the shift observed in the spectrum during the drying process. For UiO-7, considerably lower chemical shifts for 31P were predicted by DFT, probably due to the differently coordinated neighbouring Al nuclei. The average 31P chemical shift of −36.9 ppm is still within the experimental spectrum.
Further investigations were done by conducting CPMAS experiments, exploring the spatial proximity of H and P nuclei. Fig. 6 shows that 31P{1H} CPMAS measurements of the dried sample probing the interatomic distance of H and P result in an upfield shift of the signal towards lower chemical shift values for longer contact times tcontact.
The location of the maximum intensity decreased from −22.4 ppm (tcontact = 1 ms) to −24.9 ppm (tcontact = 7 ms), indicating that 31P nuclei closer to 1H nuclei have higher chemical shift values, since short contact times are sufficient to obtain polarization transfer from 1H to 31P. In contrast, P nuclei that are located farther away from H atoms need longer contact times for polarization enhancement. Thus, it is assumed that hydrated phases such as variscite at higher chemical shift values already contribute to the CPMAS signal at short contact times due to the short P–H distances. For longer contact times, also berlinite-like P nuclei coupling to either surface hydrogen or neighbouring hydrated phases can contribute to the CPMAS signal. This is in agreement with the 31P MAS NMR spectra shown in Fig. 5.
In contrast to 31P{1H} CPMAS, the 27Al{1H} CPMAS of dried AlPO4 did not provide any signal, contradicting a spatial proximity of 1H and 27Al nuclei. However, the large quadrupolar coupling of 27Al species can give rise to a signal too broad to be observed in this type of experiment due to second-order quadrupolar coupling that is not completely averaged out using MAS.78,79 Detection sensitivity is further reduced due to fast quadrupol relaxation accelerated by the loss of local symmetry.80
To sum up, both experimental 1H, 27Al, and 31P MAS NMR as well as DFT simulations of different hydrous and anhydrous AlPO4 models confirmed that the commercial AlPO4 sample consists of a mixture of anhydrous AlPO4 with 4-fold coordinated Al as in berlinite, and different hydrous phases with 5- and 6-fold coordinated Al in amorphous environments. Upon drying, the 6-fold coordinated hydrous Al species (δ(27Al) = −13 ppm) and its corresponding H2O contribution (δ(1H) = 5.4 ppm) were largely removed from the sample. However, a small contribution of about 11–12% of presumably 5-fold coordinated Al as well as a H2O or OH signal at δ(1H) = 3 ppm remained and was not notably affected by the drying procedure. 31P{1H} and 27Al{1H} CPMAS experiments confirmed the spatial proximity of 1H and 31P but not of 1H and 27Al. Nevertheless, the NMR study of the bulk AlPO4 indicates that berlinite is a reliable model for the predominant local atomistic motifs in the non-crystalline bulk of the dried AlPO4 sample. The CPMAS results are not conclusive with regard to the hydrogen configuration in dried AlPO4, but 1H and 27Al MAS NMR indicates that the drying procedure predominantly removes weakly coordinated crystal water while some more strongly bound 1H species remain. Hence, additional structure models of undercoordinated Al and P on AlPO4 surfaces, capable of forming strong interactions with either H2O or OH, were developed (Section 3.1.1). They are going to be correlated to TRAPDOR experiments in the following, probing the interaction between 27Al and 1H (Section 3.2).
The quartz-berlinite and buckled-berlinite surfaces were also studied, and their energies were determined to be 0.05 and 0.1 eV per atom lower than 001, respectively, which is in agreement with previous studies41 (cf. ESI†). Hence, the buckled-berlinite surface was set as the initial model for studying the interactions between AlPO4 and water or organic groups.
3.2 Water on AlPO4 surface
Conventional MAS NMR spectroscopy is a bulk method, and distinguishing minor contributions such as particular minority phases or surfaces is challenging. Thus, selective techniques such as TRAPDOR are required to tackle the structure elucidation of the AlPO4 surface.The 27Al{1H} TRAPDOR experiments shown in Fig. 7a, which exploit the reduction of 1H signal intensity due to dipolar coupling with nearby 27Al nuclei, directly confirmed the spatial proximity between H and Al nuclei. The broad signal centered around δ(1H) = 3 ppm was particularly affected by 27Al irradiation, as seen in the difference spectrum (black).
By analyzing (S0 − S)/S0 for different 27Al frequency offsets O2, the quadrupolar coupling constant was calculated from the cutoff frequency at 1.75 ± 0.25 MHz, marked in Fig. 7b. Using eqn 1, a CQ of 5.8 ± 0.9 MHz was obtained.
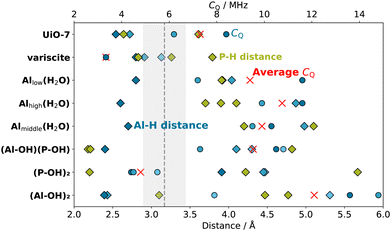
To determine the molecular structure that caused the TRAPDOR effect with CQ = 5.8 ± 0.9 MHz, computational models of hydrated and hydroxylated AlPO4 surfaces were evaluated for comparison. According to the computational study from Swang et al., the most energetically favourable interaction between the berlinite surface and water is the one represented in the (P-OH)2-berlinite model, where two P atoms are coordinated with –OH groups, allowing the formation of an Al–P dative bond that stabilizes the system (Fig. 2). In this structure, both H atoms are closer to P (2.2 Å) than to Al (3.9 Å), which is consistent with the CPMAS results. Our DFT calculations predict a CQ of 4.4, 4.7, and 5.5 MHz for the three closest Al atoms to the OH groups. Since the OH group can rotate, the effective CQ is expected to be an average of the closest Al nuclei and is in good agreement with the CQ deduced from the TRAPDOR experiments. Furthermore, the 1H chemical shift of the surface OH groups corresponds well with the signal at δ(1H) = 3 ppm, which remains at a constant intensity despite drying in Fig. 4 (yellow line).
The other hydrated and hydroxylated surface models, besides being energetically less stable, fail to provide the geometrical properties and NMR parameters observed experimentally as depicted in Fig. 8. In particular, the surface hydration on Al as described by the Ali(H2O)-berlinite (i = high, middle, low) models, whose stability rely solely on the formation of hydrogen bonds, result in a notable increase of the 27Al CQ to beyond 10 MHz for the closest Al and at least 7 MHz for the other Al in close proximity due to the breaking of the tetrahedral geometry of the electric field gradient tensor on the Al atom. Moreover, the distance between the H atoms and the closest P atoms on the surface rises to more than 4 Å, which is expected to render the distance-sensitive cross polarization ineffective for anything but the longest contact times. Since a CP signal was also obtained for a rather short contact time of 1 ms, it is concluded that the hydrated surface Al structures are not a main component of the sample.
The 5-fold coordinated Al (δ(27Al) = 10 ppm, Fig. 3) as well as the H species (δ(1H) = 3 ppm, Fig. 4) that remain unaffected by the drying of AlPO4 can be interpreted as either water on a 5-fold coordinated Al in bulk modelled by UiO-7 or as hydroxylated surface P simulated by (P-OH)2-berlinite, where the quantitative agreement of NMR observables as well as interatomic distances is even better for the latter than the former.
3.3 Organic functionalization of AlPO4 and LATP surface
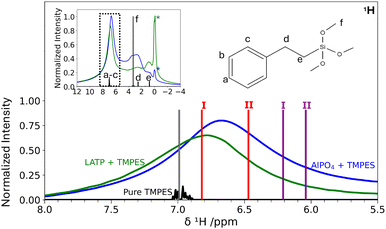
The surface models of AlPO4 do not only facilitate the elucidation of structural motifs for remnant H contributions, but also serve as starting point for understanding functionalized surfaces of hybrid electrolytes. Using the silanization procedure as described in Section 2, AlPO4 powder, as well as LATP powder were silanized with TMPES. SEM-EDX revealed the presence of Si at the powder surfaces, with an EDX-determined Si mass fraction of only <1% (cf. ESI,† Fig. S9 and S10). In addition, for each sample several one-point measurements were conducted. All points from within the sample exhibit a small silicon signal at around 1.75 keV, which is in agreement with other silanization processes from literature.81 Thus, it is expected that the distribution of silicon at the inorganic surface is rather homogeneous.By comparing the 1H MAS NMR spectra in Fig. 10, the presence of TMPES in the ceramic powders is verified by the aromatic phenyl signal a–c, also present for the silanized ceramics. In comparison to the aromatic signal a–c, signal f at 3.3 ppm resulting from the methoxy groups exhibits a lower intensity in the silanized ceramic samples, indicating that the methoxy groups are partially removed during the silanization process on the ceramic surface. Nevertheless, the spectra of the silanized ceramics still feature a significant broad signal in the range of 1.5 ppm to 4.2 ppm, which is more pronounced for the silanized AlPO4 than for LATP. Even though disentangling the individual contributions of the signals of the methylene group (d, 2.5 ppm) and potentially residual TMPES methoxy groups (f, 3.3 ppm) overlapping with the 1H signal of dried AlPO4 (cf.Fig. 4) at 3.0 ppm is not possible, the broad signal supports the SEM-EDX findings that the surface silanization is incomplete and that free OH groups remain.
Fig. 10 shows that the chemical shift of the phenyl protons shifts from about 7.0 ppm to 6.7 ppm for the AlPO4 sample upon silanization and to 6.8 ppm for the LATP sample. The changes in aromatic chemical shifts upon interaction with the ceramic can be compared to the calculated chemical shifts of exemplary physisorbed and chemisorbed structures of TMPES on the (P-OH)2-berlinite surface as described in Section 2 (Fig. 9). The comparison between the calculated NMR spectrum of the models with TMPES on the AlPO4 surface and the isolated TMPES molecule reveals an upfield shift of the average aromatic signals by 0.79 ppm and 0.96 ppm for the chemisorbed (purple I and II) and by 0.18 ppm and 0.53 ppm for the two physisorbed (red I and II) TMPES models.
The comparison, which should only be considered a reasonable approximation, suggests that TMPES is rather physisorbed than chemisorbed on both the LATP and the AlPO4 surfaces. The smaller change in the aromatic signal upon silanization of LATP in comparison to AlPO4 suggests a weaker physisorption interaction.
Anything beyond this qualitative, basic evaluation requires a thorough sampling of the configuration space of TMPES on AlPO4 with molecular dynamics, which is beyond the scope of this work.
Despite the proof of qualitative silanization, the silanization was incomplete for all investigated samples. One reason for the small yield is the fact that TMPES is apolar and thus it is unfavorable for it to come into contact with the hydroxy groups at the surface of LATP or AlPO4. Moreover, trimethoxy silanes have the tendency to form films on surfaces instead of binding as single molecules.82 To further investigate the silanized ceramic surface using NMR experiments, such as TRAPDOR, and DFT simulations, a more quantitative silanization of the ceramic surface is required.
4 Conclusion
By simulating 1H, 27Al, and 31P NMR spectra of berlinite (AlPO4), variscite (AlPO4·2H2O), and a zeolite-based UiO-7 structure of hydrous AlPO4 (Al32P32O128·62H2O) using DFT and comparing them with experimental MAS NMR spectra, commercially available AlPO4 powder is identified as a mixture of anhydrous and hydrous phases with 4-, 5-, and 6-fold coordinated Al atoms. The NMR experiments reveal that upon drying of the AlPO4 powder the 6-fold coordinated Al environment largely transforms to a 4-fold coordination environment due to a removal of crystal water, while about 12% of the Al remain in a 5-fold coordinated environment. Based on DFT simulations, the remnant 5-fold coordinated Al as well as the constant proton signal at δ(1H) = 3 ppm in dried AlPO4 are mapped to 5-fold coordinated Al motifs from UiO-7 as well as hydroxylated P on the AlPO4 surface. TRAPDOR NMR experiments yielding a 27Al CQ of 5.8 ± 0.9 MHz substantiate the model of hydroxylated P on the surface, agreeing well with the simulated CQ of 4.4 to 5.5 MHz.Aiming for functionalizing the surface hydroxy groups, AlPO4 and LATP were silanized with TMPES, which was verified with 1H MAS NMR measurements and corresponding simulated spectra, as well as with SEM/EDX measurements. The TMPES–AlPO4 bonding interaction is discussed qualitatively by comparison to different DFT models comparing chemisorbed and physisorbed bonding motifs. The TMPES–AlPO4 bonding interaction is studied by different DFT models comparing chemisorbed and physisorbed bonding motifs. The qualitative comparison of experimental and simulated NMR confirms the SEM-EDX result of incomplete, low-percentage silanization of the AlPO4 and suggests a physisorbed interaction between TMPES and AlPO4 rather than a chemical bond.
The results highlight the importance of combining theoretical and experimental efforts, as this allows structural elucidation not only of bulk material but also of complex interfaces, including their modification. Transferring the knowledge from the model system AlPO4 towards the solid electrolyte material LATP and hybrid solid-state electrolytes holds future potential to understand and finally improve the relevant interfaces by reducing interfacial resistance, which is crucial for efficient, long-range lithium ion transport.
Future investigations regarding the ionic conductivity of the silanized samples compared to non-silanized samples can be conducted, for example, using exchange spectroscopy (EXSY) or diffusion NMR measurements. Prior to these measurements and corresponding simulations, a more quantitative surface silanization needs to be achieved.
Author contributions
Javier Valenzuela Reina: investigation, data curation, methodology, visualization, writing – original draft. Vera M. Barysch: investigation, data curation, formal analysis, visualization, writing – original draft. Conrad Szczuka: conceptualization, investigation, writing – review editing. Simone S. Köcher: project administration, supervision, writing – original draft, writing – review & editing. Josef Granwehr: conceptualization, funding acquisition, project administration, supervision, writing – review & editing. Christoph Scheurer: conceptualization, funding acquisition, project administration, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Data availability
Data for this article, including NMR measurements, structure models, and DFT results will be made available on JülichDATA at https://doi.org/10.26165/JUELICH-DATA/MZPCAS following the publication of the paper.Acknowledgements
All the authors would like to thank Prof. Dr Rüdiger-A. Eichel for his support, Dr Martin Ihrig for providing the LATP samples, Dr Elisabeth Robens for conducting the SEM-EDX measurements, Viviana Piccinni for contributing to the DFT calculations, and Dr Emmanouil Veroutis, Dr Anna Windmüller, and Johannes Spiegel for facilitating the study. JVR, VB, CSz, JG, and ChS acknowledge funding by the Bundesministerium für Bildung und Forschung (BMBF) within the Verbundprojekt AdamBatt (FKZ: 13XP0305A and 03XP0305D) and AdamBatt-2 (FKZ: 13XP0558A and 03XP0558D). ChS acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the cluster of excellence EXC 2089: e-conversion, project number 390776260. All computational resources were provided by the Max Planck Computing and Data Facility (MPCDF).References
- M. V. Reddy, A. Mauger, C. M. Julien, A. Paolella and K. Zaghib, Materials, 2020, 13, 1884 CrossRef CAS.
- T. Rahman and T. Alharbi, Batteries, 2024, 10, 220 Search PubMed.
- F. Ghani, K. An and D. Lee, Batteries, 2024, 10, 340 CrossRef CAS.
- M. Keller, A. Varzi and S. Passerini, J. Power Sources, 2018, 392, 206–225 CrossRef CAS.
- A. Manthiram, X. Yu and S. Wang, Nat. Rev. Mater., 2017, 2, 1–16 Search PubMed.
- J. G. Kim, B. Son, S. Mukherjee, N. Schuppert, A. Bates, O. Kwon, M. J. Choi, H. Y. Chung and S. Park, J. Power Sources, 2015, 282, 299–322 CrossRef CAS.
- J. Zhang, X. Zang, H. Wen, T. Dong, J. Chai, Y. Li, B. Chen, J. Zhao, S. Dong, J. Ma, L. Yue, Z. Liu, X. Guo, G. Cui and L. Chen, J. Mater. Chem. A, 2017, 5, 4940–4948 RSC.
- E. Trevisanello, T. Ates, S. Passerini, F. H. Richter and J. Janek, J. Electrochem. Soc., 2022, 169, 110547 CrossRef CAS.
- H. S. Dhattarwal, R. Somni and R. C. Remsing, Nat. Commun., 2024, 15, 121 CrossRef CAS.
- M. Monchak, T. Hupfer, A. Senyshyn, H. Boysen, D. Chernyshov, T. Hansen, K. G. Schell, E. C. Bucharsky, M. J. Hoffmann and H. Ehrenberg, Inorg. Chem., 2016, 55, 2941–2945 CrossRef CAS.
- S. Hao, S. R. Daemi, T. M. Heenan, W. Du, C. Tan, M. Storm, C. Rau, D. J. Brett and P. R. Shearing, Nano Energy, 2021, 82, 105744 Search PubMed.
- S. C. Sand, J. L. Rupp and B. Yildiz, Chem. Soc. Rev., 2025, 54, 178–200 Search PubMed.
- Y. Horowitz, M. Lifshitz, A. Greenbaum, Y. Feldman, S. Greenbaum, A. P. Sokolov and D. Golodnitsky, J. Electrochem. Soc., 2020, 167, 160514 Search PubMed.
- J. Peng, Y. Xiao, D. A. Clarkson, S. G. Greenbaum, T. A. Zawodzinski and X. C. Chen, ACS Appl. Polym. Mater., 2020, 2, 1180–1189 Search PubMed.
- N. Tolganbek, A. Serikkazyyeva, S. Kalybekkyzy, M. Sarsembina, K. Kanamura, Z. Bakenov and A. Mentbayeva, Mater. Adv., 2022, 3, 3055–3069 RSC.
- H.-K. Tian, R. Jalem, B. Gao, Y. Yamamoto, S. Muto, M. Sakakura, Y. Iriyama and Y. Tateyama, ACS Appl. Mater. Interfaces, 2020, 12, 54752–54762 CrossRef CAS.
- Y. Liu, Q. Sun, Y. Zhao, B. Wang, P. Kaghazchi, K. R. Adair, R. Li, C. Zhang, J. Liu, L.-Y. Kuo, Y. Hu, T.-K. Sham, L. Zhang, R. Yang, S. Lu, X. Song and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 31240–31248 CrossRef CAS.
- F. Ichihara, K. Niitsu, Y. Tanaka, Y. Niwa, K. Mitsuishi, S. Miyoshi, T. Ohno and T. Masuda, J. Phys. Chem. C, 2023, 127, 15043–15050 CrossRef CAS.
- S. Stegmaier, R. Schierholz, I. Povstugar, J. Barthel, S. P. Rittmeyer, S. Yu, S. Wengert, S. Rostami, H. Kungl, K. Reuter, R.-A. Eichel and C. Scheurer, Adv. Energy Mater., 2021, 11, 2100707 CrossRef CAS.
- L. Wang, Z. Xie, X. Wu, Y. Xiao, H. Hu and Y. Liang, Small, 2025, 2411658 CrossRef CAS.
- E. Kuhnert, C. Slugovc, G. Trimmel, M. Wilkening and D. Rettenwander, ECS Meet. Abstr., 2020, MA2020-02, 962 CrossRef.
- C.-F. Li, R. Muruganantham, W.-C. Hsu, M. Ihrig, C.-T. Hsieh, C.-C. Wang and W.-R. Liu, J. Taiwan Inst. Chem. Eng., 2023, 144, 104681 CrossRef CAS.
- F. Scharf, A. Krude, P. Lennartz, M. Clausnitzer, G. Shukla, A. Buchheit, F. Kempe, D. Diddens, P. Glomb, M. M. Mitchell, T. Danner, A. Heuer, A. Latz, M. Winter and G. Brunklaus, Small, 2024, 20, 2404537 CrossRef CAS.
- R. DeWees and H. Wang, ChemSusChem, 2019, 12, 3713–3725 CrossRef CAS.
- E. Winter, P. Seipel, T. Zinkevich, S. Indris, B. Davaasuren, F. Tietz and M. Vogel, Z. Phys. Chem., 2022, 236, 817–837 Search PubMed.
- V. Siller, A. Morata, M. N. Eroles, R. Arenal, J. C. Gonzalez-Rosillo, J. M. L. del Amo and A. Tarancón, J. Mater. Chem. A, 2021, 9, 17760–17769 RSC.
- C. V. Chandran, S. Pristat, E. Witt, F. Tietz and P. Heitjans, J. Phys. Chem. C, 2016, 120, 8436–8442 CrossRef CAS.
- S. Stegmaier, K. Reuter and C. Scheurer, Nanomaterials, 2022, 12, 2912 CrossRef CAS.
- G. Redhammer, D. Rettenwander, S. Pristat, E. Dashjav, C. Kumar, D. Topa and F. Tietz, Solid State Sci., 2016, 60, 99–107 Search PubMed.
- S. Al-Omari, F. Afaneh, R. Elsad, Y. Rammah and Z. Khattari, Radiat. Phys. Chem., 2024, 215, 111377 CrossRef CAS.
- P. J. Byrne, J. E. Warren, R. E. Morris and S. E. Ashbrook, Solid State Sci., 2009, 11, 1001–1006 Search PubMed.
- D. H. Brouwer, J.-M. Chézeau and C. A. Fyfe, Microporous Mesoporous Mater., 2006, 88, 163–169 CrossRef CAS.
- N. Li, H. Hu, F. Guo and H. Tao, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2021, 36, 248–254 CrossRef CAS.
- M. Ardit, B. L. Phillips and D. L. Bish, Am. Mineral., 2022, 107, 1385–1395 CrossRef.
- P. Goj, B. Handke and P. Stoch, Sci. Rep., 2022, 12, 17495 CrossRef CAS.
- D. Müller, E. Jahn, G. Ladwig and U. Haubenreisser, Chem. Phys. Lett., 1984, 109, 332–336 Search PubMed.
- K. Druppel, A. Hosch and G. Franz, Am. Mineral., 2007, 92, 1695–1703 CrossRef.
- M. A. Harmer and A. J. Vega, Solid State Nucl. Magn. Reson., 1995, 5, 35–49 CrossRef CAS.
- J. M. Thomas, Angew. Chem., Int. Ed. Engl., 1994, 33, 913–937 CrossRef.
- A. Jhunjhunwala, J. F. Vetelino and J. C. Field, J. Appl. Phys., 1977, 48, 887–892 Search PubMed.
- E. Sagvolden, M. F. Sunding and O. Swang, J. Phys. Chem. C, 2020, 124, 6683–6688 CrossRef CAS.
- E. Pérez-Botella, S. Valencia and F. Rey, Chem. Rev., 2022, 122, 17647–17695 CrossRef.
- A. Best, M. Forsyth and D. R. Macfarlane, Solid State Ion., 2000, 136, 339–344 CrossRef.
- K. Arbi, W. Bucheli, R. Jiménez and J. Sanz, J. Eur. Ceram. Soc., 2015, 35, 1477–1484 CrossRef CAS.
- S. Vema, A. H. Berge, S. Nagendran and C. P. Grey, Chem. Mater., 2023, 35, 9632–9646 CrossRef CAS.
- Z. Gan, J. Magn. Reson., 2019, 306, 86–90 CrossRef CAS.
- C. P. Grey and A. J. Vega, J. Am. Chem. Soc., 1995, 117, 8232–8242 Search PubMed.
- R. Bayzou, J. Trébosc, A.-K. Landry, R. B. Nuernberg, B. Pecquenard-Le Cras, F. Le Cras, F. Pourpoint and O. Lafon, J. Magn. Reson., 2023, 354, 107530 CrossRef CAS.
- A. Abraham, R. Prins, J. A. van Bokhoven, E. R. van Eck and A. P. Kentgens, Solid State Nucl. Magn. Reson., 2009, 35, 61–66 CrossRef CAS.
- D. H. Brouwer, J.-M. Chézeau and C. A. Fyfe, Microporous Mesoporous Mater., 2006, 88, 163–169 CrossRef CAS.
- R. Lizárraga, E. Holmström, S. C. Parker and C. Arrouvel, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 094201 Search PubMed.
- P. Blaha, K. Schwarz, F. Tran, R. Laskowski, G. K. H. Madsen and L. D. Marks, J. Chem. Phys., 2020, 152, 074101 Search PubMed.
- J. V. Reina, F. Civaia, A. F. Harper, C. Scheurer and S. S. Köcher, Faraday Discuss., 2024, 255, 266–287 RSC.
- A. F. Harper, S. P. Emge, P. C. Magusin, C. P. Grey and A. J. Morris, Chem. Sci., 2023, 14, 1155–1167 RSC.
- S. Hayashi and K. Hayamizu, Bull. Chem. Soc. Jpn., 1989, 62, 2429–2430 CrossRef CAS.
- A. Grünert, W. Schmidt and F. Schüth, Catal. Lett., 2020, 150, 2951–2958 CrossRef.
- S. Dong, W. Shi, J. Zhang and S. Bi, ACS Earth Space Chem., 2019, 3, 1353–1361 CrossRef CAS.
- S. Sturniolo, T. F. Green, R. M. Hanson, M. Zilka, K. Refson, P. Hodgkinson, S. P. Brown and J. R. Yates, Solid State Nucl. Magn. Reson., 2016, 78, 64–70 CrossRef CAS.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- A. H. Larsen, J. J. Mortensen, J. Blomqvist, I. E. Castelli, R. Christensen, M. Dułak, J. Friis, M. N. Groves, B. Hammer, C. Hargus, E. D. Hermes, P. C. Jennings, P. B. Jensen, J. Kermode, J. R. Kitchin, E. L. Kolsbjerg, J. Kubal, K. Kaasbjerg, S. Lysgaard, J. B. Maronsson, T. Maxson, T. Olsen, L. Pastewka, A. Peterson, C. Rostgaard, J. Schiøtz, O. Schütt, M. Strange, K. S. Thygesen, T. Vegge, L. Vilhelmsen, M. Walter, Z. Zeng and K. W. Jacobsen, J. Phys.: Condens. Matter, 2017, 29, 273002 CrossRef.
- L. L. C. Schrödinger, The PyMOL Molecular Graphics System, 2025, https://www.pymol.org/ Search PubMed.
- B. Davaasuren and F. Tietz, Solid State Ion., 2019, 338, 144–152 CrossRef CAS.
- J. G. Longstaffe, B. Chen and Y. Huang, Microporous Mesoporous Mater., 2007, 98, 21–28 CrossRef CAS.
- S. Prasad, H.-T. Kwak, T. Clark and P. J. Grandinetti, J. Am. Chem. Soc., 2002, 124, 4964–4965 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr. – Cryst. Mater., 2005, 220, 567–570 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- C. J. Pickard and F. Mauri, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 245101 CrossRef.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- H. Fjellvåg, D. E. Akporiaye, E. Halvorsen, A. Karlsson, K. Kongshaug and K. Lillerud, Solid State Sci., 2001, 3, 603–611 CrossRef.
- J. Neugebauer and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 16067–16080 CrossRef CAS.
- M. Stöcker, Stud. Surf. Sci. Catal., 1994, 85, 429–507 CrossRef.
- R. Lookman, P. Grobet, R. Merckx and K. Vlassak, Eur. J. Soil Sci., 1994, 45, 37–44 CrossRef CAS.
- J. Klinowski, Molecular Sieves: Crystalline Systems, John Wiley & Sons, Ltd, 2007 Search PubMed.
- V. Sarou-Kanian, A. N. Gleizes, P. Florian, D. Samélor, D. Massiot and C. Vahlas, J. Phys. Chem. C, 2013, 117, 21965–21971 Search PubMed.
- F. Ji, C. Zhang and Y. Mi, Chem. Pap., 2023, 78, 1217–1226 Search PubMed.
- R. H. Meinhold and N. J. Tapp, J. Chem. Soc., Chem. Commun., 1990, 219–220 RSC.
- D. Müller, E. Jahn, G. Ladwig and U. Haubenreisser, Chem. Phys. Lett., 1984, 109, 332–336 CrossRef.
- D. Freude and J. Haase, in Quadrupole Effects in Solid-State Nuclear Magnetic Resonance, ed. H. Pfeifer and P. Barker, Springer Berlin Heidelberg, Berlin, Heidelberg, 1993, pp. 1–90 Search PubMed.
- F. Deng, Y. Yue and C. Ye, Solid State Nucl. Magn. Reson., 1998, 10, 151–160 Search PubMed.
- S. Berger, J. Nolde, T. Yüksel, W. Tremel and M. Mondeshki, Molecules, 2018, 23, 808 Search PubMed.
- O. G. Cisneros-Pineda, W. H. Kao, M. I. Loría-Bastarrachea, Y. Veranes-Pantoja, J. V. Cauich-Rodríguez and J. M. Cervantes-Uc, Mater. Sci. Eng., C, 2014, 40, 157–163 Search PubMed.
- G. T. Hermanson, Silane Coupling Agents, Academic Press, Boston, 3rd edn, 2013, ch. 13, pp. 535–548 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ya00079c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |