Efficient near-infrared luminescence in a Na2CaTi2Ge3O12:Cr3+ garnet for light-emitting-diode applications†
Yi
Xu
 a,
Xuewan
Lin
a and
Jiyou
Zhong
a,
Xuewan
Lin
a and
Jiyou
Zhong
 *ab
*ab
aSchool of Physics and Optoelectronic Engineering, Guangdong University of Technology, Guangzhou, 510006, China. E-mail: zhongjiyou@126.com
bGuangdong Provincial Key Laboratory of Sensing Physics and System Integration Applications, Guangdong University of Technology, Guangzhou, 510006, China
First published on 15th April 2025
Abstract
Near-infrared phosphor-converted light-emitting diodes (NIR pc-LEDs) have emerged as ideal light sources for a variety of advanced spectroscopy applications, demonstrating the importance of developing efficient NIR phosphors. In this work, a Ti4+-containing garnet, Na2CaTi2Ge3O12, was selected as the host material to minimize the ionic size mismatch after Cr3+ substitution. Interestingly, this substitution generates charge self-balance, enabling excellent efficiency of the developed material. Under 465 nm excitation, it exhibits broadband emission covering the first NIR window (λem,max = 790 nm) with a full width at half maximum (FWHM) of 120 nm. The optimized sample achieved a photoluminescence quantum yield (PLQY) of 75%. Moreover, a NIR pc-LED device was fabricated by combining the optimized phosphor with a blue (λem = 455 nm) InGaN chip. Upon application of a driving current of 100 mA, the device demonstrated a NIR output power of 18.24 mW and a corresponding NIR photoelectronic conversion efficiency of 6.49% and exhibited excellent performance in biological tissue imaging and non-destructive testing. Therefore, this work provides an efficient NIR phosphor, which has promising potential in spectroscopy applications.
1. Introduction
Near-infrared (NIR) spectroscopy in the region of 700–1100 nm has a wide range of applications in fields, such as security monitoring, biological imaging, and medical diagnostics, due to its invisibility to the human eye and strong penetration capability in biological tissues of these wavelengths.1–7 However, conventional NIR light sources, including incandescent and halogen lamps, suffer from low efficiency, short lifetimes, large volumes, and poor spectral tunability, which cannot meet the requirements for intelligent applications of NIR light sources in portable devices.8–12 In contrast, NIR phosphor-converted light-emitting diodes (NIR pc-LEDs), manufactured by coating a NIR phosphor on a high-brightness blue InGaN LED chip, exhibit high efficiency, spectral tunability, small size, and low cost, making them ideal NIR light sources for multifunctional smart applications.13,14 As a crucial component, the NIR phosphor is the key material determining the output power and spectral coverage of a NIR pc-LED device. Therefore, there is a significant demand for developing efficient NIR phosphors.To date, various activators have been adopted to achieve NIR emission via directly doping inorganic compounds.15 Among them, Cr3+ ions, when introduced in a weak crystal field, exhibit broadband absorption in the blue light region and produce spin-allowed 4T2 → 4A2 transitions, realizing broadband NIR emission. Moreover, the emission peak wavelength can be tuned by adjusting the octahedral field strength, making the output spectrum of the device designable.16 However, as the 3d electrons of Cr3+ are exposed in the outer layer, the 3d–3d transitions are easily affected by external environments, including lattice vibrations and high-energy phonons, making it a challenge to develop efficient Cr3+-activated NIR phosphors with excellent thermal stability.
Recently, Cr3+-doped garnet NIR phosphors have attracted considerable attention due to their superior photoluminescence quantum yields (PLQY) and thermal robustness, as exemplified by Y3In2Ga3O12:Cr3+ (λem,max = 760 nm, PLQY = 91.6%, I423K/I298K = 100%), Ca3Sc2Si3O12:Cr3+ (λem,max = 770 nm, PLQY = 92.3%, I423K/I298K = 97.4%), SrLu2Al3ScSiO12:Cr3+ (λem,max = 785 nm, PLQY = 89.4%, I423K/I298K = 97.6%), Y3Ga3MgSiO12:Cr3+ (λem,max = 782 nm, PLQY = 79.9%, I423K/I298K = 84.4%), Gd3Al2Ga3O12:Cr3+ (λem,max = 730 nm, PLQY = 97.3%, I423K/I298K = 96.8%), and Gd3Mg0.5Al1.5Ga2.5Ge0.5O12:Cr3+ (λem,max = 763 nm, PLQY = 90%, I423K/I298K = 85.3%).9,17–21 However, garnets always have a strong crystal field, resulting in emission wavelengths shorter than 790 nm for most Cr3+-activated garnet phosphors. To achieve relatively long-wavelength NIR emission, a universal strategy is to weaken the crystal field strength by introducing cations with larger ionic size, such as Sc3+ (r6-coor. = 0.745 Å), In3+ (r6-coor. = 0.80 Å), Mg2+ (r6-coor. = 0.72 Å), and Zr4+ (r6-coor. = 0.72 Å) ions.22–29 However, the Ti4+ (r6-coor. = 0.605 Å) ion is rarely considered for incorporation into garnet phosphor materials, mainly due to its small radius, which is unlikely to generate a weak crystal field environment; moreover, the non-equivalent substitution by Cr3+ ions will lead to the formation of defects, which are detrimental to the luminescence efficiency. Notably, outside the garnet system, some Ti4+-containing phosphors exhibit excellent luminescence performance, such as Li2Mg3TiO6:Cr3+ with a PLQY as high as 72.1%.30 In fact, Ti4+ ions have an ionic radius very similar to that of Cr3+ (r6-coor. = 0.615 Å), which can minimize the ionic radius mismatch and lattice distortion caused by Cr3+ substitution.
Based on the above considerations, here, we attempted to introduce Ti4+ ions into the octahedral sites of a garnet. To suppress the formation of defects or Cr4+ caused by Cr3+ substitution, the dodecahedral site was set to be co-occupied by Na+ and Ca2+ ions to self-balance the charge. Consequently, the Na2CaTi2Ge3O12 garnet material was proposed as a host for Cr3+ substitution and was synthesized via a solid-state reaction method at a relatively low temperature. This material exhibits broadband emission centered at 790 nm, which has a full width at half maximum (FWHM) of 120 nm and effectively covers the NIR-I region. The optimized Na2CaTi1.94Ge3O12:0.06Cr3+ sample demonstrates a PLQY of 75.06% and an absorption efficiency (AE) of 34.07%. The fabricated NIR pc-LED device achieves a NIR output of 18.24 mW and efficiency of 6.49% under a driving current of 100 mA. This material shows promising potential for applications in biological tissue imaging and non-destructive testing.
2. Experimental
2.1. Synthesis
Na2CaTi2−xGe3O12:xCr3+ (x = 0, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1) samples were synthesized using the conventional high-temperature solid-state reaction method. The raw materials, including Cr2O3 (Aladdin, 99.99%), NaHCO3 (Aladdin, 99.95%), CaCO3 (Macklin, 99.9%), TiO2 (Aladdin, 99.99%), and GeO2 (Aladdin, 99.999%) were accurately weighed based on the chemical composition. To compensate for the loss of Na at high temperature during the synthesis process, an excess of 5% NaHCO3 was added. The powders were thoroughly ground in an agate mortar for 30 min. The mixtures were subsequently transferred to alumina crucibles and sintered in a tube furnace under an air atmosphere. The synthesis temperature was set to be 1000 °C and retained for 8 h. The cooled product was then removed from the crucible and ground into fine powder for subsequent characterization and analysis.2.2. Characterization
X’Pert3 PANalytical (Cu Kα, λ = 1.5406 Å) was used to record X-ray diffraction (XRD) patterns of the samples. Rietveld refinements of the XRD patterns were performed using the GSAS software to determine the precise crystal structures. VESTA software was used to visualize the crystal structure. Electron paramagnetic resonance (EPR) spectra were recorded at 77 K (liquid nitrogen) using a JESFA300 spectrometer. Photoluminescence (PL) spectra and fluorescence decay curves were acquired using the FLS-980 fluorescence spectrophotometer (Edinburgh Instruments). Diffuse reflectance (DR) spectra were recorded on a UV-3600 plus UV-NIR Spectrophotometer (Shimadzu, Japan). PLQY and AE were measured using a Quantaurus-QY Plus C13534-11 (Hamamatsu Photonics). NIR pc-LED devices were fabricated by mixing the optimized Na2CaTi1.94Ge3O12:0.06Cr3+ phosphor with UV photosensitive resin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio and coating the mixture on 455 nm LED chips. The NIR output power, photoelectric conversion efficiency, and electroluminescence spectra of the devices were recorded on the HAAS2000 High Accuracy Array Spectroradiometer photoelectric system (EVERFINE, China).
1 weight ratio and coating the mixture on 455 nm LED chips. The NIR output power, photoelectric conversion efficiency, and electroluminescence spectra of the devices were recorded on the HAAS2000 High Accuracy Array Spectroradiometer photoelectric system (EVERFINE, China).
3. Results and discussion
3.1. Crystal structure
The phase purity and crystal structure of the synthesized Na2CaTi2−xGe3O12:xCr3+ (x = 0, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.10) samples were identified using XRD patterns as shown in Fig. 1a. The diffraction peaks of all samples are in excellent agreement with the standard XRD pattern for Na2CaTi2Ge3O12 (ICSD no. 15418), being isostructural with our previously reported Na2CaZr2Ge3O12 garnet,25 confirming the successful synthesis of the target compounds with a single pure garnet phase. Notably, Cr3+ substitution even at a high concentration (x = 0.1) did not cause significant variations in the XRD patterns, indicating that the substitution did not change the garnet phase. In these garnets (Fig. 1b), the octahedral and tetrahedral sites were expected to be occupied by Ti4+ and Ge4+, respectively, forming [TiO6] and [GeO4] polyhedrons, while the dodecahedral site was expected to be filled with Na+ and Ca2+ ions with an atomic ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To confirm this hypothesis, Rietveld refinements were performed on the XRD pattern of the undoped Na2CaTi2Ge3O12 (Fig. 1c) and Na2CaTi1.94Ge3O12:0.06Cr3+ (Fig. 1d) samples. The refined structural parameters are provided in Table 1, and atomic positions are provided in Tables S1 and S2 (ESI†). The excellent residual factors validate the success of these refinements and confirm the crystal structure as expected.
1. To confirm this hypothesis, Rietveld refinements were performed on the XRD pattern of the undoped Na2CaTi2Ge3O12 (Fig. 1c) and Na2CaTi1.94Ge3O12:0.06Cr3+ (Fig. 1d) samples. The refined structural parameters are provided in Table 1, and atomic positions are provided in Tables S1 and S2 (ESI†). The excellent residual factors validate the success of these refinements and confirm the crystal structure as expected.
| Formula | Na2CaTi2Ge3O12 | Na2CaTi1.94Cr0.06Ge3O12 |
|---|---|---|
| Temperature (°C) | 25 | 25 |
| Radiation type; λ (Å) | X-ray; 1.5406 | X-ray; 1.5406 |
| 2θ range (deg) | 15–90 | 15–90 |
| Space group; Z |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d d |
Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d d |
| β (deg) | 90 | 90 |
| a = b = c (Å) | 12.268569 | 12.267690 |
| Unit cell volume (Å3) | 1846.64 | 1846.24 |
| Weighted profile R-factor, Rwp | 9.47% | 8.51% |
| Profile R-factor, Rp | 6.70% | 6.08% |
| χ 2 | 1.264 | 1.016 |
In these garnets, Cr3+ (r6-coor. = 0.615 Å) is expected to replace Ti4+ (r6-coor. = 0.605 Å) in the octahedral site, owing to their similar ionic radii. Generally, the slightly larger Cr3+ substituting smaller Ti4+ will, to some extent, induce lattice expansion. However, the lattice shrinkage was detected after Cr3+ substitution, deduced from the refined results as shown in Table 1. To verify this trend, the lattice parameters (a and V) of the Na2CaTi2−xGe3O12:xCr3+ (x = 0, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.1) samples (Fig. 1e and f) were determined by performing Rietveld refinements. A linear lattice shrinkage is clearly observed as the Cr3+ substitution ratio increases. This phenomenon seems abnormal when only Cr3+ substituting Ti4is considered. In fact, the same amount of smaller Ca2+ (r8-coor. = 1.12 Å) will substitute larger Na+ (r8-coor. = 1.18 Å), driven by the need to maintain charge self-balance. Therefore, it can be considered that co-substitution is happening, with a slightly smaller [Cr3+–Ca2+] unit replacing the slightly larger [Ti4+–Na+] unit, which should be the origin of the lattice shrinkage. This result confirmed that defects formed by Cr3+ substituting Ti4+ can be avoided via automatic adjustment of the Na+/Ca2+ ratio in the dodecahedral site, which demonstrates the beneficial effects of mixed site occupation in stabilizing the Cr3+ valence state and preventing the formation of defects.
To reveal the local structural environment of Cr3+ ions in these garnets, EPR spectra of Na2CaTi2−xGe3O12:xCr3+ (x = 0.04 and 0.08) samples were collected at 77 K as presented in Fig. 1g. The dominant resonance signal observed at g = 3.97 is attributed to isolated Cr3+ ions occupying octahedral sites, while the signal at g = 1.93 is derived from exchange-coupled Cr3+–Cr3+ ion pairs, suggesting most of the Cr3+ ions are isolated. Notably, no additional resonance signals corresponding to Cr3+ ions occupying other crystallographic sites can be observed in the EPR spectra, which further corroborates the incorporation of Cr3+ ions only into the octahedral sites of Na2CaTi2Ge3O12.
3.2. Photoluminescence
The band gap of the host material plays an important role in the photoluminescence properties of phosphors. To evaluate the bandgap of the Na2CaTi2Ge3O12 host, DR spectra were collected as shown in Fig. 2a. The DR spectrum of the host demonstrates strong absorption in the ultraviolet region of this garnet. The band gap can be estimated from the optical bandgap (Eg) calculated using the Kubelka–Munk function:31,32 | (1) |
 | (2) |
Upon 465 nm excitation, the photoluminescence (PL) spectra of the Na2CaTi2−xGe3O12:xCr3+ (x = 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, and 0.10) samples were collected, as shown in Fig. 2c. The normalized emission spectra as presented in Fig. 2d exhibit broadband emission covering 700 to 1100 nm with a FWHM of about 120 nm. A slight spectral red-shift (from 779 to 794 nm) can be observed with the increase of Cr3+-dopant concentration, which is likely to be caused by reabsorption due to a considerable overlap between the excitation and emission spectra as depicted in Fig. 2e. Moreover, a slight spectral broadening with the FWHM extended from 120 to 127 nm was observed. The spectral broadening should be due to the increase of the electron-lattice coupling strength.34,35 To verify this, the electron-lattice coupling energy (Sħω) was calculated as shown in Fig. 2f. The electron-lattice coupling energy clearly shows a gradual increase as the Cr3+ dopant concentration (x) increases, demonstrating the gradually enhanced electron-lattice coupling effect.
The optimal Cr3+ dopant concentration in this garnet is x = 0.06, which has the highest emission intensity (Fig. 3a). The PLQY and AE of this sample were measured under 465 nm excitation (Fig. 3b), yielding values of 75.06% and 34.07%, respectively. Compared to some other Ti4+-containing phosphor materials (Table 2), the Na2CaTi1.94Ge3O12:0.06Cr3+ phosphor exhibits excellent PLQY and demonstrates relatively long-wavelength NIR emission.
| Compound | λ em (nm) | PLQY (%) | AE (%) | I 373K/I298K (%) | Ref. |
|---|---|---|---|---|---|
| Na2CaTi2Ge3O12:Cr3+ | 790 | 75.06 | 34.07 | 42.13 | This work |
| Li2Mg3TiO6:Cr3+ | 790 | 72.1 | 28.1 | 68.2 | 30 |
| Ca3MgTiGe3O12:Cr3+ | 786 | 66.5 | 37 | ∼50 | 36 |
| Li2Mg2.99Ti0.99Ga0.02O6:Cr3+ | 707 | 73.1 | — | ∼70 | 37 |
| Ca3ZnTiGe3O12:Cr3+ | 785 | 39 | 29.6 | >70 | 29 |
| LiGaTiO4:Cr3+ | 717 | 70.22 | 23.2 | 52.7 | 38 |
This broadband NIR emission is attributed to the 4T2(4F) → 4A2 transition of Cr3+ ions in a weak octahedral crystal field. The strength of the crystal field can be approximately estimated from the ratio between the crystal field splitting (Dq) and the Racah parameters (B):39,40
 | (3) |
 | (4) |
 | (5) |
As there is only one kind of octahedral site in the garnet, it is expected that the NIR emission of this material should be derived from only one type of luminescence center. Thus, the emission spectra should be well fitted by a single Gaussian band. However, the fact is that the emission spectrum of the Na2CaTi1.94Ge3O12:0.06Cr3+ sample shows a slight deviation from a single Gaussian band (Fig. 3d). To further confirm this deviation, the decay curve of this sample was recorded, which shows slight deviation from a single exponential function as shown in Fig. 3e. In fact, this deviation can be seen in all the decay curves of the Na2CaTi2−xGe3O12:xCr3+ (x = 0.005–0.10) samples (Fig. 3f). Nevertheless, these decay curves cannot be fitted by a double exponential function, excluding the existence of a second type of luminescence center. The reason for this deviation should be ascribed to the differentiation of the octahedral site caused by the disordered distribution of Na+ and Ca2+ ions in the second coordination sphere, which would lead to coordination of Na–Cr–Na, Na–Cr–Ca, and Ca–Cr–Ca. In our previous work, the cationic disorder at the dodecahedral site of the garnet consistently proved advantageous for achieving relatively long-wavelength NIR emission. This material clearly possesses relatively long-wavelength NIR emission (λem = 790 nm) compared to many other garnets, such as Y3In2Ga3O12:Cr3+ (λem,max = 760 nm), Ca3Sc2Si3O12:Cr3+ (λem,max = 770 nm) and Gd3Mg0.5Al1.5Ga2.5Ge0.5O12:Cr3+ (λem,max = 763 nm),17,18,21 benefiting from the cationic disorder.34,41
The lifetime of each sample can be obtained by fitting the decay curves (Fig. S2, ESI†) with the following single exponential function:42,43
 | (6) |
3.3. Thermal stability
The temperature-dependent PL spectra of the Na2CaTi1.94Ge3O12:0.06Cr3+ sample were acquired under 465 nm excitation covering a temperature range of 298 to 473 K as depicted in Fig. 4a and Fig. S3 (ESI†). As the temperature increases, the emission intensity gradually decreases. At 373 K, the sample retains 42.13% of its initial intensity (at 298 K), as shown in Fig. 4b, suggesting a relatively poor thermal stability when compared with some other Ti4+-containing phosphors as listed in Table 2. Nevertheless, the almost linear decrease in the temperature range of 298–398 K makes it an excellent material to be applied in temperature sensing with a sensitivity of 0.67% K−1 (Fig. 4c).The thermal stability of the phosphor is generally determined by two mechanisms, namely, the cross-over process and thermal ionization. The cross-over process occurs when the excited electrons pass through the intersection of the ground state energy levels and excited state energy (Fig. S4, ESI†). The energy barrier is the activation energy (ΔEa), the energy difference between the lowest excited state and the crossing point, which can be calculated by fitting the Arrhenius equation:44
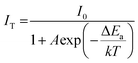 | (7) |
The normalized PL spectra (Fig. 4e) reveal a red-shift of approximately 16 nm for the emission peak wavelength (791 → 807 nm), accompanied by a peak width expansion from 126 nm to 139 nm. The red-shift of the emission spectra can possibly be attributed to the lattice expansion with increasing temperature, which leads to a consequent weakening of the crystal field.46 The broadening of the temperature-dependent PL spectra reflects enhanced electron-lattice interactions, which increase the vibrational amplitude of excited electrons with the assistance of the electron–phonon coupling (EPC) effect. The degree of the EPC effect can be represented by the Huang–Rhys factor (S) via fitting the following equation:47,48
 | (8) |
Considering the relatively weak EPC effect in this material, the influence of high-energy phonons on the excited electrons is weak, thereby minimizing the possibility of thermal quenching via a cross-over process. Consequently, the relatively poor thermal stability of this material can be primarily attributed to thermally induced ionization. This mechanism depends on the energy gap between the lowest excited state and the bottom of the conduction band, which is significantly determined by the band gap of the host material. Considering that the optical band gap of Na2CaTi2Ge3O12 is relatively narrow, it is likely that thermal ionization will happen at elevated temperatures.
3.4. Application of a NIR pc-LED device
To assess the practical performance of the Na2CaTi1.94Ge3O12:0.06Cr3+ phosphor, the optimized sample was mixed with UV photosensitive resin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio and then coated on a 455 nm blue InGaN chip (inset of Fig. 5a) to prepare a NIR pc-LED device. Fig. 5a shows the electroluminescence (EL) spectra of the manufactured devices at different driving currents. Fig. 5b and Table S3 (ESI†) show the NIR power and the photoelectric conversion efficiency corresponding to each driving current. The NIR output power exhibits an increase from 5.33 to 49.27 mW as the current is gradually increased from 25 to 450 mA, while the NIR photoelectric conversion efficiency decreases from 7.43% to 3.4%. Notably, at a driving current of 100 mA, the NIR output and NIR photoelectric conversion efficiency reached 18.24 mW and 6.49%, respectively, with a maximum emission peak at 790 nm. These output parameters suggest great potential of this device for spectroscopic applications.
1 mass ratio and then coated on a 455 nm blue InGaN chip (inset of Fig. 5a) to prepare a NIR pc-LED device. Fig. 5a shows the electroluminescence (EL) spectra of the manufactured devices at different driving currents. Fig. 5b and Table S3 (ESI†) show the NIR power and the photoelectric conversion efficiency corresponding to each driving current. The NIR output power exhibits an increase from 5.33 to 49.27 mW as the current is gradually increased from 25 to 450 mA, while the NIR photoelectric conversion efficiency decreases from 7.43% to 3.4%. Notably, at a driving current of 100 mA, the NIR output and NIR photoelectric conversion efficiency reached 18.24 mW and 6.49%, respectively, with a maximum emission peak at 790 nm. These output parameters suggest great potential of this device for spectroscopic applications.
To evaluate the practical application of this device, a scheme of non-invasive and non-destructive NIR imaging was constructed, as demonstrated in Fig. 5c, via capturing photographs under NIR light generated by the fabricated NIR pc-LED device. Fig. 5d demonstrates the photographs of a hand captured under natural light and NIR light. Under the exposure of NIR light, the blood vessels in the palm can be clearly identified, and the subtle skeletal contours were visible, demonstrating the potential of the device for non-invasive biological imaging applications.
This NIR pc-LED device was also employed for the detection of cable breakpoints. Fig. 5e demonstrates a broken cable with an intact protective layer under the exposure of visible and NIR light. The protective layer of the cable exhibits high transmittance under the NIR light, and the captured NIR image effectively revealed the cable breakpoint. Extracting the intensity distribution from the NIR image as grayscale values and plotting a pseudo-color map generates a longitudinal sectional intensity profile of the cable breakpoint (Fig. 5f and g), enabling better observation of the damage point. These applications highlight the immense potential of this work in biological tissue imaging and industrial non-destructive testing.
4. Conclusion
In summary, we successfully designed and synthesized efficient Na2CaTi2Ge3O12:Cr3+ garnet phosphors. The phosphors exhibit a broad NIR emission ranging from 700 to 1100 nm, with a peak centered at 790 nm and a FWHM of 120 nm under 465 nm excitation. The optimized PLQY and AE reach 75.06% and 34.07%, respectively, for the sample with x = 0.06, demonstrating the material's excellent efficiency. This excellent efficiency possibly benefits from the suppression of defect formation and Cr4+via charge self-balance. Although the thermal stability of this material is relatively poor, an unexpected nearly linear decrease of the emission intensity as a function of temperature up to 400 K demonstrates its excellent temperature sensing properties. Finally, a NIR pc-LED device was constructed using the Na2CaTi1.94Ge3O12:0.06Cr3+ phosphor. The device achieves a NIR output power of 18.24 mW and photoelectric conversion efficiency of 6.49% at 100 mA. The device's practical applications in biological tissue imaging and industrial non-destructive testing have been successfully demonstrated.Data availability
Data underlying the results presented in this paper can be obtained from the authors upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (52372139) and the Guangzhou Basic and Applied Basic Research Project (2025A04J5106).References
- J. Zhou, X. Liu, R. Sun and L. Sun, Food Anal. Methods, 2022, 15, 2575–2593 CrossRef.
- H. Suo, Y. Wang, X. Zhao, X. Zhang, L. Li, K. Guan, W. Ding, P. Li, Z. Wang and F. Wang, Laser Photonics Rev., 2022, 16, 2200012 CrossRef CAS.
- H. Yu, J. Chen, R. Mi, J. Yang and Y. Liu, Chem. Eng. J., 2021, 417, 129271 CrossRef CAS.
- R. J. Xie, Light: Sci. Appl., 2020, 9, 155 CrossRef PubMed.
- Z. Hu, C. Fang, B. Li, Z. Zhang, C. Cao, M. Cai, S. Su, X. Sun, X. Shi, C. Li, T. Zhou, Y. Zhang, C. Chi, P. He, X. Xia, Y. Chen, S. S. Gambhir, Z. Cheng and J. Tian, Nat. Biomed. Eng., 2020, 4, 259–271 CrossRef PubMed.
- B. Geng, J. Hu, Y. Li, S. Feng, D. Pan, L. Feng and L. Shen, Nat. Commun., 2022, 13, 5735 CrossRef CAS.
- S. Yakunin, B. M. Benin, Y. Shynkarenko, O. Nazarenko, M. I. Bodnarchuk, D. N. Dirin, C. Hofer, S. Cattaneo and M. V. Kovalenko, Nat. Mater., 2019, 18, 846–852 CrossRef CAS.
- S. Pradhan, F. Di Stasio, Y. Bi, S. Gupta, S. Christodoulou, A. Stavrinadis and G. Konstantatos, Nat. Nanotechnol., 2019, 14, 72–79 CrossRef CAS.
- H. Jiang, L. Chen, G. Zheng, Z. Luo, X. Wu, Z. Liu, R. Li, Y. Liu, P. Sun and J. Jiang, Adv. Opt. Mater., 2022, 10, 2102741 CrossRef CAS.
- S. Pradhan, M. Dalmases, A. B. Baspinar and G. Konstantatos, Adv. Funct. Mater., 2020, 30, 2004445 CrossRef CAS.
- M. Zhao, S. Liu, H. Cai, F. Zhao, Z. Song and Q. Liu, Inorg. Chem. Front., 2022, 9, 4602–4607 RSC.
- Y. Zhang, S. Miao, Y. Liang, C. Liang, D. Chen, X. Shan, K. Sun and X. J. Wang, Light: Sci. Appl., 2022, 11, 136 CrossRef CAS.
- J. Wang, L. Jiang, R. Pang, S. Zhang, D. Li, K. Li, C. Li and H. Zhang, Inorg. Chem. Front., 2022, 9, 425–444 Search PubMed.
- Q. Zhang, D. Liu, P. Dang, H. Lian, G. Li and J. Lin, Laser Photonics Rev., 2022, 16, 2100459 CrossRef CAS.
- Y. Xiao, W. Xiao, D. Wu, L. Guan, M. Luo and L. D. Sun, Adv. Funct. Mater., 2022, 32, 2109618 CrossRef CAS.
- M. Sójka, J. Zhong and J. Brgoch, ACS Appl. Opt. Mater., 2023, 1, 1138–1149 CrossRef.
- C. Li and J. Zhong, Chem. Mater., 2022, 34, 8418–8426 CrossRef CAS.
- Z. Jia, C. Yuan, Y. Liu, X. J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light: Sci. Appl., 2020, 9, 86 CrossRef CAS.
- X. Chen and X. Huang, Laser Photonics Rev., 2025, 2402226 CrossRef.
- L. Jiang, X. Jiang, L. Zhang, Q. Liu, X. Mi, Z. Yu, G. Lv and Y. Su, Inorg. Chem., 2023, 62, 4220–4226 CrossRef CAS.
- L. Jiang, X. Jiang, C. Wang, P. Liu, Y. Zhang, G. Lv, T. Lookman and Y. Su, ACS Appl. Mater. Interfaces, 2022, 14, 52124–52133 CrossRef CAS.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang and J. Jiang, RSC Adv., 2018, 8, 12035–12042 RSC.
- L. Cao, P. Li, J. Cui, X. Wang, Y. Yao, M. Zhang, M. Zheng, Z. Yang, H. Suo and Z. Wang, RSC Adv., 2021, 11, 10043–10053 RSC.
- B. Malysa, A. Meijerink and T. Jüstel, J. Lumin., 2018, 202, 523–531 CrossRef CAS.
- Z. Liao, M. Sójka, J. Zhong and J. Brgoch, Chem. Mater., 2024, 36, 4654–4663 CrossRef CAS.
- C. Li and J. Zhong, Adv. Opt. Mater., 2023, 11, 2202323 CrossRef CAS.
- L. Jiang, X. Jiang, J. Xie, T. Zheng, G. Lv and Y. Su, J. Lumin., 2022, 247, 118911 CrossRef CAS.
- J. Zhang, L. Zhang, F. Liu, H. Wu, H. Wu, G. Pan, Y. Luo, Z. Hao and J. Zhang, Laser Photonics Rev., 2023, 17, 2200586 CrossRef CAS.
- C. Li, Y. Li, Y. Xu and J. Zhong, Ceram. Int., 2024, 50, 53970–53975 CrossRef CAS.
- T. Tan, S. Wang, J. Su, W. Yuan, H. Wu, R. Pang, J. Wang, C. Li and H. Zhang, ACS Sustainable Chem. Eng., 2022, 10, 3839–3850 CrossRef CAS.
- A. Mondal, S. Das and J. Manam, RSC Adv., 2016, 6, 82484–82495 RSC.
- P. Makuła, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef.
- X. Xu, Q. Shao, L. Yao, Y. Dong and J. Jiang, Chem. Eng. J., 2020, 383, 123108 CrossRef CAS.
- C. Li and J. Zhong, Chem. Commun., 2024, 60, 9270–9273 RSC.
- M. H. Fang, K. C. Chen, N. Majewska, T. Leśniewski, S. Mahlik, G. Leniec, S. M. Kaczmarek, C. W. Yang, K. M. Lu, H. S. Sheu and R. S. Liu, ACS Energy Lett., 2021, 6, 109–114 CrossRef CAS.
- M. He, S. Yi, Z. Hu, W. Zhao and Y. Wang, J. Lumin., 2024, 266, 120304 CrossRef CAS.
- Y. Ma, S. Li, J. Wei, W. Liao, B. Quan, M. S. Molokeev, M. Cheng, X. Chen, Z. Zhou and M. Xia, Mater. Adv., 2023, 4, 5808–5816 RSC.
- R. Ma, K. Cheng, J. Long, X. Liu, B. Li, C. Yang, X. Gong, C. Deng and W. Huang, Mater. Sci. Semicond. Process., 2024, 179, 108527 CrossRef CAS.
- B. Struve and G. Huber, Appl. Phys. B, 1985, 36, 195–201 CrossRef.
- A. Trueba, P. Garcia-Fernandez, J. M. García-Lastra, J. A. Aramburu, M. T. Barriuso and M. Moreno, J. Phys. Chem. A, 2011, 115, 1423–1432 CrossRef CAS.
- Y. Li, C. Li, C. Li and J. Zhong, Opt. Lett., 2024, 49, 5834 CrossRef CAS.
- J. Xie, J. Tian and W. Zhuang, Inorg. Chem., 2023, 62, 10772–10779 CrossRef CAS.
- Y. Yan, M. Shang, S. Huang, Y. Wang, Y. Sun, P. Dang and J. Lin, ACS Appl. Mater. Interfaces, 2022, 14, 8179–8190 CrossRef CAS.
- X. Ji, J. Zhang, Y. Li, S. Liao, X. Zhang, Z. Yang, Z. Wang, Z. Qiu, W. Zhou, L. Yu and S. Lian, Chem. Mater., 2018, 30, 5137–5147 CrossRef CAS.
- D. Huang, H. Zhu, Z. Deng, H. Yang, J. Hu, S. Liang, D. Chen, E. Ma and W. Guo, J. Mater. Chem. C, 2021, 9, 164–172 RSC.
- J. Zhong, C. Li, W. Zhao, S. You and J. Brgoch, Chem. Mater., 2022, 34, 337–344 CrossRef CAS.
- J. H. Ryu, H. S. Won, Y. G. Park, S. H. Kim, W. Y. Song, H. Suzuki and C. Yoon, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 747–752 CrossRef CAS.
- C. Liu, Z. Qi, C. G. Ma, P. Dorenbos, D. Hou, S. Zhang, X. Kuang, J. Zhang and H. Liang, Chem. Mater., 2014, 26, 3709–3715 CrossRef CAS.
- H. Zhang, J. Zhong, F. Du, L. Chen, X. Zhang, Z. Mu and W. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 11663–11671 CrossRef CAS.
- J. Zhong, Y. Zhuo, F. Du, H. Zhang, W. Zhao, S. You and J. Brgoch, Adv. Opt. Mater., 2022, 10, 2101800 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5tc01230a |
| This journal is © The Royal Society of Chemistry 2025 |





