(−)-Cytisine: Access to a stereochemically defined and functionally flexible piperidine scaffold†
Worawat
Niwetmarin
 ,
Hugo
Rego Campello
,
Hugo
Rego Campello
 ,
Hazel A.
Sparkes
,
Varinder K.
Aggarwal
,
Hazel A.
Sparkes
,
Varinder K.
Aggarwal
 and
Timothy
Gallagher
and
Timothy
Gallagher
 *
*
School of Chemistry, University of Bristol, Bristol BS8 1TS, UK. E-mail: t.gallagher@bristol.ac.uk
First published on 23rd July 2018
Abstract
N-Benzyl cytisine undergoes an efficient C(6)–N(7) cleavage via directed C(6) lithiation, borylation and oxidation to provide a “privileged” heterocyclic core unit comprising a highly functionalised, cis-3,5-disubstituted piperidine in enantiomerically pure form. The potential offered by this unit as a means to explore chemical space has been evaluated and methods have been defined (and illustrated) that allow for selective manipulation of N(1), C(3′), and the pyridone N. The pyridone core can also be diversified via bromination (at C(3′′) and C(5′′)) which is complementary to direct C–H activation based on Ir-catalyzed borylation to provide access to C(4′′). The use of a boronate-based 1,2-migration as an alternative trigger to mediate C(6)–N(7) cleavage of cytisine was evaluated but failed. However, the stability of the intermediate boronate opens a new pathway for the elaboration of cytisine itself using both Matteson homologation and Zweifel olefination.
Introduction
Contemporary “small molecule” drug discovery relies on a variety of strategies to both identify and develop “hits” into leads and then drug candidates. Clearly, the ability to define and explore novel “chemical space” plays a key role in terms of generating (and then protecting to enable further exploitation) intellectual property.1 For many years natural products provided a valued entry to enable structural variation but pharmaceutical industry interest in this area has, at points, waned in favor of, for example, combinatorial chemistry and related approaches as the preferred means by which to achieve molecular diversity.2 However, the enormous structural variation associated with natural products has, regardless, continued to provide a source of inspiration (and a guide) that has led to the development of, for example, diversity-oriented and “chemical genetics” approaches to lead discovery.3Natural products, which are often available in enantiomerically pure form and may contain multiply stereogenic centers, do provide one important starting point for a diversity-oriented approach to drug discovery. Several natural products or core components of natural products, such as 1-O-acetylbritannilactone4a and maslinic acid4b have found application in this area (Scheme 1).
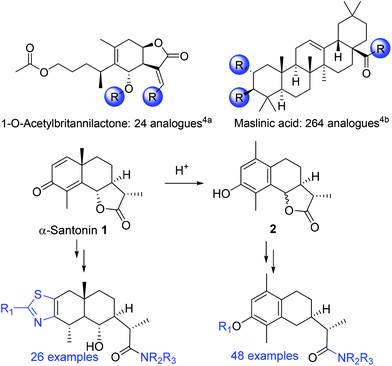 | ||
| Scheme 1 Top: Example of natural products providing a functionalised core scaffold. Bottom: Derivatisation of α-santonin 1. | ||
Taking this further, an ability to harness further the reactivity of a readily available natural product to widen the reach of the structural diversity available is well exemplified by Schwarz's work on α-santonin.5 Here, both the natural product 1 and a readily available variant 2 (available from 1 by acid-catalysed rearrangement) provided analogues of both of these related scaffolds that were explored as inhibitors of 5-lipoxygenase.
The role of “privileged scaffolds” serves as a further focus in this area, with many being based on or incorporating a heterocyclic core. Of these, piperidines are especially widely distributed and are found in a large number of bioactive and important pharmaceutical compounds.6 Our work has focussed on developing new and stereochemically-defined heterocyclic scaffolds based, in part, on nitrogen-based natural products that incorporate piperidine moieties.7 These include coerulescine 3, which led to the development of a range of stereochemically-defined spirocyclic bis(azacycles), such as the piperidine and the pyrrolidine-containing units 4 and 5.8 Each of these spirocyclic scaffolds (within which the individuals enantiomers are accessible) offers three discrete functionalisation sites: two secondary but readily differentiated amines (blue and purple), and a primary alcohol (red), all of which are distributed across a spatially distinctive spirocyclic core.
(−)-Cytisine, which is a partial agonist of the nicotinic acetylcholine receptor (the high affinity nicotine binding site in brain), is marketed (as Tabex®) within Eastern and Central Europe for smoking cessation.10,11 While that specific nicotinic profile may not be required (or desired), we recognised that cytisine 6 offered a readily available starting point from which to construct a series of structurally diverse libraries if, for example, the piperidine unit (coded blue) could be “released” from within the tricyclic heterocyclic core of 6.
Cytisine 6 has already inspired a diversity-oriented synthesis approach to novel inhibitors of Bcl-2 and there has been extensive studies associated with the substitution of cytisine itself, driven largely by attempts to develop novel nicotinic ligands.12 Of particular relevance to the work described here are the studies of Rouden and co-workers on the site-specific lithiation of N-acylated cytisines; this is illustrated by an N to C acyl transfer to functionalise at C(6) (Scheme 2).13 We had reasoned that using an appropriate electrophile, functionalisation of C(6) would allow for cleavage of the C(6)–N(7) link, and release of an intact piperidine unit. This was achieved as shown in Scheme 2 by C(6)-lithiation (via8) and (in situ) silylation of (−)-N-methylcytisine (caulophylline) 7 followed by C–Si → C–O oxidation followed by reduction. This sequence demonstrated the underlying concept by liberating the piperidine moiety (highlighted in blue) and provided a direct entry to the related lupin piperidine alkaloid (+)-kuraramine 11 in 18% overall yield from (−)-7.14
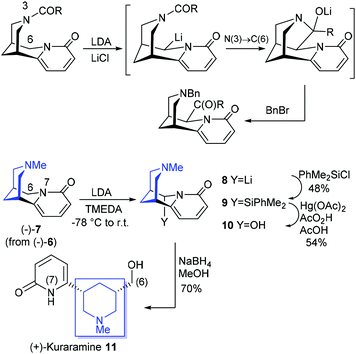 | ||
| Scheme 2 Top: C(6) lithiation triggering N(3) → C(6) acyl migration (Rouden et al.13). Bottom: C(6) oxidative functionalisation of N-methylcytisine 7; a biomimetic synthesis of (+)-kuraramine 11 (Gallagher et al.14). | ||
However, this initial solution involved the use of a silyl electrophile which necessitated a stoichiometric amount of a mercury-based oxidant to achieve the key Fleming–Tamao oxidative cleavage (9→ hemiaminal 10; overall 26% from 7); alternatives to Hg(II) were evaluated but failed (see below). This issue alone, notwithstanding the modest yields, presents a clear block to any more general use of this approach to access a versatile heterocyclic (piperidine) scaffold or in a fragment-based approach to drug discovery. Given the goal of exploiting an ability to fragment cytisine, alongside the recognised importance of the piperidine scaffold within medicinal chemistry and a consequential ability to access efficiently differentiated and enantiomerically-pure piperidine-based libraries, this key synthetic obstacle had to be addressed.
In this paper, we describe a much more user-friendly solution to the cleavage of the C(6)–N(7) bond of cytisine shown in Scheme 2, which also offers access to the piperidine core with flexibility around the secondary amine protecting group. Further, the chemistry we have now developed avoids any heavy metals and is significantly more efficient and scalable. We have not explicitly focussed on a specific medicinal chemistry target, rather we have aimed to exemplify how the piperidine core can be both accessed and manipulated at various sites to provide access to a wide range of functional variants. The scope of this chemistry is outlined, in terms of the sites that become accessible, by general structure A and the issues around accessing each of these sites serves as the focus of this paper. The positions immediately accessible for derivatisation include two differentiated nitrogen centres (N(1) and N(1′′) in the piperidine and the pyridone moieties respectively); an ability to manipulate the C(3′) methylene unit; further functionalisation of the pyridone moiety via either electrophilic bromination (which occurs predominantly at C(3′′)) or, and of more utility and novelty, Ir-catalysed C–H activation which selectively targets C(4′′).
This program has been driven in part by an increased awareness of the power and potential within medicinal chemistry associated with cost effective in silico assessment of ligands against protein targets of interest. Indeed, the goal would be to enable a full evaluation of the “effectiveness” of a ligand or family of ligands prior to embarking on an expensive program of chemical synthesis and evaluation by biological assay.15 As the dependability of these in silico methods increases so will their value as a reliable predictive tool. This would then enable the routine application of in silico prioritization of putative ligands as an early step in lead selection. It is likely that this, in turn, will lead to a rebalancing of the current reliance on existing library collections (i.e. physical samples) towards evaluating synthetic methodologies that offer opportunities to access as-yet-unexplored ligands.16 Those methodologies must, necessarily, encompass versatile, drug-like scaffolds such as A.
Results and discussion
To improve the efficiency of the fragmentation of cytisine, we have explored the chemistry illustrated in Scheme 2 in a number of ways: (i) the conditions for C(6) lithiation must avoid metalation at other sites, see below. (ii) The variation of the N(3) substituent that is possible; this was N-methyl earlier but this is less attractive for downstream synthetic utility. (iii) The choice of electrophile (ideally employed in situ during the deprotonation step) used to trap the C(6) lithiated species (i.e.8) in order facilitate C(6)–N(7) cleavage. (iv) The conditions needed to achieve that critical transformation that also avoid heavy metals such as Hg(II).Revisiting C(6) lithiation of N-substituted cytisines
The original conditions used to lithiate N-methycytisine 7 (which was trapped with Me2PhSiCl to give 9) had proceeded in 48% yield. Relatively straightforward variation of some of the basic reaction parameters allowed us to optimise this basic transformation to 83% in favour of C(6) lithiation and trapping.Although we did observe yields of 9 of up to 90%, 83% reflected the yield obtained when competing lithiation (and trapping) at C(10) was suppressed. The latter led to a C(10) silylation adduct 12 (and this was exacerbated in the absence of TMEDA) and we also observed a (known)13b dimerization product 13 (Scheme 3).
 | ||
| Scheme 3 Optimization of C(6) lithiation. Variables evaluated were substrate concentration, equivalents of LDA and Me2PhSiCl, and presence of TMEDA (key to a high yield of 9) Details are available in the ESI.† | ||
While LDA provided cleanly lithiation at C(6), Rouden observed C(10) (as well as competing C(9)) metalation and (in situ) silylation of N-methylcytisine 7 when a particularly bulky base (LiTMP) was used. We did see some level of C(10) lithiation with LDA and an analogous metalation (cf.12) within a simple pyridone (i.e. at C(4), pyridone numbering) has been reported by Katritzky.17 We evaluated N-Boc cytisine as a substrate for C(6) metalation but analysis of the crude reaction mixture (following reaction with LDA and silylation) indicated substitution at C(2) and/or C(4) had occurred which is associated with Boc-directed metalation.18
Use of N-benzyl cytisine 14 was more attractive both for downstream manipulations and because we did not observe competitive metalation (as 12) or dimer formation (as 13). Although Rouden has examined lithiation of 14, in our hands and using a more synthetically useful aryl-based chlorosilane (Me2PhSiCl), TMEDA (1 equiv.) did have an impact in terms of reaction efficiency and in particular suppressed a small amount of competing lithiation at C(10); 15 was isolated in 84% yield free of competing side products, with the mass balance accounted for by recovered 14 (Scheme 4).
Options around electrophilic component to facilitate C(6)–N(7) cleavage
Our original conditions for fragmentation of the cytisine tricycle to generate the piperidine unit associated with kuraramine 11 involved silylation and Fleming–Tamao oxidation (using peracid and stoichiometric Hg(OAc)2) followed by a reductive ring opening of hemiaminal 10 (Scheme 2). Closer inspection of this process indicated two competing reactions: desilylation of 9 to regenerate 7 and (ii) possible competing N-oxidation of the tertiary (N(3)) amine was suggested by MS analysis. These oxidation conditions were modified to give a small increase in yield of 11 (from 38% to 47% from 8) by using a combination of H2O2 and peracetic acid (rather than peracetic acid alone) but this transformation still required Hg(OAc)2. All attempts, however, to achieve the conversion of 9 to 10 by other means e.g. KBr, AcOOH/AcOH; HBF4, then H2O2; KH, tBuOOH then TBAF, thereby avoiding mercury salts, failed. This forced consideration of an electrophile that would offer an alternative cleavage mechanism. Lithiation followed by halogenation at C(6) was evaluated using a variety of halogen sources (Br2, I2, N-bromosuccinimide, BrCCl2CCl2Br, CBr4) proved unproductive. However, diphenyl disulfide (not shown, but see ESI†) and boron-based electrophiles were effective in situ traps for the C(6) lithio intermediate derived from 7 (Scheme 5). In the boron series, further optimisation using both N-methyl and N-benzylcytisine 7 and 14 respectively led to (OiPr)Bpin as the electrophile of choice, and adducts 16 and 17 were obtained in 93 and 89% yields respectively. While boronate esters 16 and 17 were relatively tolerant of chromatography, further purification was unnecessary. Direct oxidation of the crude boronates (using NaBO3) followed by reduction (NaBH4) gave the piperidine core structures, (+)-kuraramine 11 and the synthetically more flexible N-benzyl variant 18 in 32% and 52% overall yields (from 7 and 14) respectively. | ||
| Scheme 5 C(6) borylation and ring cleavage.‡ | ||
In summary, cytisine 6 is easily derivatised to the N-methyl and N-benzyl variants 7 and 14, each of which provides efficient access to the corresponding enantiomerically pure cis-3,5-disubstituted piperidines 11 and 18 respectively. The results reported here serve to improve significantly the synthesis of (+)-kuraramine 11 from N-methylcytisine and avoid heavy metal oxidants to mediate the key C(6)–N(7) bond cleavage. Use of a boron-based electrophile, and specifically (OiPr)Bpin to trap the C(6) lithiated intermediate, was applicable to both the N-methyl and N-benzyl series.
Elaboration of the piperidine scaffold; identification of protecting group arrays that enable selective site manipulation
Heterocyclic scaffolds, such as 11 and 18, offer significant potential with the N-Bn residue in particular cleavable under a variety of different reaction conditions. Consequently, the majority of the remainder of this paper is focused on developing the potential of the N-benzyl piperidine 18 as the basis of a flexible heterocyclic core structure. Our aim here is to demonstrate how the periphery of 18 can be functionalised in ways that would make this unit well suited to a general fragment-based drug discovery strategy, for the reasons articulated above. We have explored chemistry around four specific sites illustrated in general scaffold A: via the piperidine nitrogen (N(1)), at C(3′) (in 18, a primary alcohol), and in three areas of the pyridone moiety, at C(3′′) and C(4′′) and across the lactam unit (N(1′′) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The latter is especially important to protect (deactivate) when pursuing functionalization of C(3′) (see below). Given the array of functional groups present within 18, we have determined methods for differentiating at these different sites. One consequence is that a series of protecting group arrays have been assessed and this is illustrated in Scheme 6.
O). The latter is especially important to protect (deactivate) when pursuing functionalization of C(3′) (see below). Given the array of functional groups present within 18, we have determined methods for differentiating at these different sites. One consequence is that a series of protecting group arrays have been assessed and this is illustrated in Scheme 6.
Selective N- and O-Boc protection in combination with O-silylation provided a comprehensive set of options, which has allowed us to generate a number of differentially protected variants 19–24; this includes the fully deprotected variant 22. Additionally, the structure of the N,O-bis-Boc protected alcohol 20 was solved by X-ray crystallographic analysis (see ESI†). The pyridone lactam underwent O-acylation in the presence of an excess of Boc2O, a protecting group array that is then cleavable under basic/nucleophilic conditions, see below. Further, the ability to manipulate selectively the pyridone nitrogen N(1′′) is illustrated by N-methylation of 24 to give adduct 25.
Elaboration at C(3′); variation of C(3′) oxidation level
That there is a requirement to protect/deprotect easily the pyridone moiety is exemplified attempts to derivatise the C(3′) primary alcohol (Scheme 7). Reaction of N-Boc piperidine 19 with phthalimide under Mitsunobu conditions in an attempt to access the 3′ amino variant led cleanly (and in essentially quantitative yield) to N-Boc cytisine 26.Of course, cis-disubstituted piperidine 19 is well set up to undergo intramolecular alkylation, a possibility that was both recognized and exploited elegantly in one of the very early syntheses of cytisine carried out 60 years ago by van Tamelen and Baran,19a,b and subsequently used in related contexts by others.19c–e
We attempted to circumvent this issue via double mesylation of 19 to generate intermediate 27 (not isolated, but assignment of a double mesylate was based on MS). However, cyclisation still occurred with 26 being the only product observed on exposure of 27 to azide or phthalate. Clearly, the O-mesylate is labile in the presence of a good nucleophile but O-Boc protection solves this issue. Using intermediate 20, activation and phthalimide displacement at C(3′) followed by imide cleavage provided the C(3′) amino derivative 28 (Scheme 8). Here, hydrazine also cleaves the O-Boc residue liberating the pyridone unit, but this is presumed to occur after nucleophilic displacement at C(3′).
In addition, the scope of the C(3′) amino substituent can be extended using aldehyde 29 derived from 20 using Swern oxidation.
Aldehyde 29 was not routinely isolated (to minimize any risk of epimerization) but used directly for reductive amination or further oxidation (see below). Reductive amination using nucleophilic amines was generally accompanied by O-Boc cleavage under the reaction conditions (see 30a and 30b), and where (as in the case of an α-amino ester) this does not occur, O-Boc cleavage is then readily achieved using aqueous ammonia (leading to 30c). The latter is a generally applicable deprotection method20 and is illustrated further below. Alcohol 20 is also readily converted to the corresponding carboxylic acid 31 in excellent overall yield and acid 31 offers access to a representative library of C(3′) derivatives (Scheme 9).
Again, the pyridone unit is unmasked by O-Boc cleavage under nucleophilic conditions without disruption of other functionality to provide pyridones 32a–c. In addition, global deprotection of 31 is readily achieved to give the heterocyclic β-amino acid 33.
Acid 33 is related to (S)-nipecotic acid (the parent piperidine-based β-amino acid), where 5-aryl substituted nipecotic acid derivatives have attracted medicinal chemistry interest.21 We had recognized a risk associated with C(3) epimerization of e.g. aldehyde 29. For that reason we secured the X-ray crystal structure of acid 31 (see ESI†), which confirmed both the (intact) cis-configuration and the presence of the O-Boc protected pyridine moiety.
Elaboration at N(1)
The final target site for substitution on this piperidine scaffold was at N(1). This can be carried out in a number of ways, and we have simply illustrated the accessibility of this key position by N-debenzylation of 18 to give 22 (Scheme 6) as an isolable and versatile intermediate, followed by a representative reductive amination leading to the bis(piperidine) 34 (Scheme 10). This does, however, serve to show that protection elsewhere is not necessary in order to access N(1).Regioselective functionalisation of the pyridone moiety; electrophilic bromination and Ir-catalyzed borylation
The remaining region of the piperidine scaffold that was of interest is associated with the pyridone unit. Electrophilic halogenation of pyridones is well-established and leads predominately to 3-substitution, with the 5-halo isomer as (usually) the minor product. Using N-bromosuccinimide, this pattern was observed with pyridone 19 (Scheme 11).Bromides 35 and 36 are readily separated (and easily differentiated by 1H NMR) and provide an obvious and very useful handle at C(3′′) and C(5′′).§ Access to C(4′′) is also achievable using Ir-catalyzed borylation, as has been reported recently.22 In our hands, however, NH pyridones (e.g.19) are not viable substrates for this C–H activation method. N-Alkyl pyridones (e.g.7) are, though, generally reactive and with O-Boc protected pyridines (i.e.23) highly selective borylation at C(4′′) is observed.
The scope and potential of this chemistry is illustrated in Scheme 11, and this includes two representative transformations of the intermediate boronate esters: Suzuki coupling of adduct 37, followed by O-Boc cleavage (using basic ammonia) gave 38; Cu-mediated bromination and deprotection of adduct 39a gave 40, offering a reactant that complements the corresponding Bpin esters. It is useful to note that the low yield of 40 reflects the instability in solution of the intermediate bromide prior to O-Boc cleavage but this transformation has not been optimized.
The chemistry outlined above serves to demonstrate the ability to manipulate the piperidine scaffold aligned to general structure A. This scaffold can be manipulated in a variety of distinct locations, using different transformations, and the only major requirement is the engagement of a suitable protecting group array that is primarily associated with moderating the reactivity of the pyridone unit. That moderation, largely based on N- and O-(Boc) protection, is readily achieved. Thus, we believe that the ready availability of (−)-cytisine and the efficiency with which the piperidine core unit can be revealed and then differentially manipulated makes this a potentially attractive starting point for a fragment-based discovery approach.
Enhancing the complexity of (−)-cytisine via C(6) lithiation; Matteson homologation and Zweifel olefination
A key aspect of a fragment-based approach must be an ability to increase rapidly molecular complexity. We have also explored additional avenues to address that issue where the goal has been to identify methods to introduce additional (and novel) complexity to the cytisine-based (tricyclic) precursor prior to any fragmentation and further functionalisation. While these reactions do not contribute to extending the scope of the piperidine scaffold discussed earlier, they do provide access to novel cytisine variants that can be considered as alternative scaffold configurations and progenitors.Of particular relevance here was the potential associated with trapping a C(6) lithiated cytisine (see Scheme 2, intermediate 8) with a secondary boronate ester (e.g. EtBpin). Our goal here had been to trigger a 1,2-migration sequence that would also serve to fragment the tricycle of cytisine and simultaneously create an addition stereocenter(*) at a key position on the periphery. The concept, which draws on previous work done within the Aggarwal group,23 is illustrated in Scheme 12.
 | ||
| Scheme 12 Top: Attempt to couple 1,2-migration with tricycle cleavage. Bottom: C(6) alkenylation of N-benzyl cytisine 14. | ||
Using N-benzyl cytisine 14, lithiation and trapping with EtBpin appeared to generate the requisite borate intermediate 41 based on 11B NMR which showed a signal at 6.3 ppm. However, all attempts to achieve 1,2-migration using a range of Lewis acids failed. Given that Bpin esters are sterically demanding, we also evaluated Et3B as a less demanding electrophile. Again, “ate” formation (analogous to 41) occurred (as judged by 11B NMR) but no migration products could be detected.
The most plausible explanation for these outcomes is that the pyridone N is not a sufficiently good leaving group to drive the migration step. However, an appreciation of the stability of the pyridone moiety with respect to a 1,2-migration provided an opportunity to assess to other “ate”-based chemistries available using boronate ester 17.
Boronate 17 undergoes efficient Matteson homologation24 to give boronate 42 in 89% yield. Oxidation of 42 provided primary alcohol 43 and the structure of the corresponding 4-nitrobenzoate 44 confirmed the configuration at C(6) (see ESI†). A detailed 1H NMR analysis of 44 was also carried (see ESI†) which aided in the assignment of adduct 46 (see below). Zweifel olefination25 also involves generation of a stable borate intermediate and using boronate 17 proceeds very efficiently. Using a modified procedure developed in the Aggarwal group,25c exposure of 17 to vinyl Grignard (and this likely generates the triethenyl borate intermediate 45), followed by exposure to I2 in methanol gave the C(6) ethenyl adduct 46 in 94% yield (Scheme 12). The configuration at C(6) was confirmed by NOESY experiments (using 44 for comparison) and details of these studies are also available in the ESI.†
Conclusions
In summary, C(6) metalation of N-alkyl cytisine derivatives provides a direct and versatile method of functionalization that allows for ring fragmentation to provide a synthetically flexible, enantiomerically pure piperidine based heterocyclic scaffold. Given the privileged nature of the piperidine ring within medicinal chemistry, we suggest that this is an attractive unit for the development of more complex heterocyclic ligands within the context of a fragment-based approach to drug discovery. The potential of this functionalised piperidine scaffold has been exemplified using the N-benzylated variant 18. Critically it is possible to differentiate all key elements and, using C–H activation, to provide an additional functional handle within the pyridone unit. Attempts to induce a B-based 1,2-migration as a means to increase the molecular complexity within the piperidone scaffold failed, however, the relative stability of the key intermediate (identified by a lack of reactivity) can be exploited. Derivatives such as 43 and 46 that arise from this offer new opportunities for modification of the established nicotinic ligand profile of cytisine and studies in this area are underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Achieve Life Sciences and Allychem Co. Ltd for generous gifts of (−)-cytisine and bis(pinacolato)diboron respectively, and financial support from the Development and Promotion of Science and Technology (DPST) Thailand, the University of Bristol and EPSRC (EP/N024117/1) is acknowledged.Notes and references
- For leading discussions associated with small molecule drug discovery linked to “chemical space” and privileged scaffolds, see: (a) R. W. DeSimone, K. S. Currie, S. A. Mitchell, J. W. Darrow and D. A. Pippin, Comb. Chem. High Throughput Screening, 2004, 7, 473–493 CrossRef; (b) S. J. Haggarty, Curr. Opin. Chem. Biol., 2005, 9, 296–303 CrossRef PubMed; (c) L. Costantino and D. Barlocco, Curr. Med. Chem., 2006, 13, 65–85 CrossRef PubMed; (d) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef PubMed; (e) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347–361 CrossRef PubMed; (f) R. S. Bon and H. Waldmann, Acc. Chem. Res., 2010, 43, 1103–1114 CrossRef PubMed; (g) S. R. Langdon, N. Brown and J. Blagg, J. Chem. Inf. Model., 2011, 51, 2174–2185 CrossRef PubMed; (h) F. Lovering, MedChemComm, 2013, 4, 515–519 RSC.
- For recent overviews of the role (and pitfalls e.g. PAINS2n) of natural products within a drug discovery context and related combinatorial chemistry applications, see: (a) M. L. Lee and G. Schneider, J. Comb. Chem., 2001, 3, 284–289 CrossRef PubMed; (b) R. Breinbauer, M. Manger, M. Scheck and H. Waldmann, Curr. Med. Chem., 2002, 9, 2129–2145 CrossRef PubMed; (c) R. Breinbauer, I. R. Vetter and H. Waldmann, Angew. Chem., Int. Ed., 2002, 41, 2879–2890 Search PubMed; (d) M. S. Butler, Nat. Prod. Rep., 2005, 22, 162–195 RSC; (e) F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discovery, 2005, 4, 206–220 CrossRef PubMed; (f) I. Paterson and E. A. Anderson, Science, 2005, 310, 451–453 CrossRef PubMed; (g) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461–477 CrossRef PubMed; (h) D. J. Newman, J. Med. Chem., 2008, 51, 2589–2599 CrossRef PubMed; (i) J. W. H. Li and J. C. Vederas, Science, 2009, 325, 161–165 CrossRef PubMed; (j) D. Morton, S. Leach, C. Cordier, S. Warriner and A. Nelson, Angew. Chem., Int. Ed., 2009, 48, 104–109 CrossRef PubMed; (k) W. Wilk, H. Waldmann and M. Kaiser, Bioorg. Med. Chem., 2009, 17, 2304–2309 CrossRef PubMed; (l) K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2009, 48, 3224–3242 CrossRef PubMed; (m) E. E. Carlson, ACS Chem. Biol., 2010, 5, 639–653 CrossRef PubMed; (n) J. B. Baell and G. A. Holloway, J. Med. Chem., 2010, 53, 2719–2740 CrossRef PubMed; (o) S. Danishefsky, Nat. Prod. Rep., 2010, 27, 1114–1116 RSC; (p) A. L. Harvey, R. L. Clark, S. P. Mackay and B. F. Johnston, Expert Opin. Drug Discovery, 2010, 5, 559–568 CrossRef PubMed; (q) B. Over, S. Wetzel, C. Grutter, Y. Nakai, S. Renner, D. Rauh and H. Waldmann, Nat. Chem., 2013, 5, 21–28 CrossRef PubMed; (r) G. M. Cragg and D. J. Newman, Biochim. Biophys. Acta, 2013, 1830, 3670–3695 CrossRef PubMed; (s) E. C. Barnes, R. Kumar and R. A. Davis, Nat. Prod. Rep., 2016, 33, 372–381 RSC; (t) J. Bisson, J. B. McAlpine, J. B. Friesen, S. N. Chen, J. Graham and G. F. Pauli, J. Med. Chem., 2016, 59, 1671–1690 CrossRef PubMed; (u) T. Rodrigues, D. Reker, P. Schneider and G. Schneider, Nat. Chem., 2016, 8, 531–541 CrossRef PubMed.
- For overviews of diversity-oriented synthesis approaches, see: (a) M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46–58 CrossRef PubMed; (b) M. D. Burke, E. M. Berger and S. L. Schreiber, J. Am. Chem. Soc., 2004, 126, 14095–14104 CrossRef PubMed; (c) W. Galloway and D. R. Spring, Nature, 2011, 470, 43–43 Search PubMed; (d) K. M. G. O'Connell, M. Diaz-Gavilan, W. Galloway and D. R. Spring, Beilstein J. Org. Chem., 2012, 8, 850–860 CrossRef PubMed; (e) C. J. O'Connor, H. S. G. Beckmann and D. R. Spring, Chem. Soc. Rev., 2012, 41, 4444–4456 RSC; (f) A. Koutsoukas, S. Paricharak, W. Galloway, D. R. Spring, A. P. Ijzerman, R. C. Glen, D. Marcus and A. Bender, J. Chem. Inf. Model., 2014, 54, 230–242 CrossRef PubMed; (g) S. Collins, S. Bartlett, F. L. Nie, H. F. Sore and D. R. Spring, Synthesis, 2016, 1457–1473 Search PubMed; (h) F. L. Nie, D. L. Kunciw, D. Wilcke, J. E. Stokes, W. Galloway, S. Bartlett, H. F. Sore and D. R. Spring, Angew. Chem., Int. Ed., 2016, 55, 11139–11143 CrossRef PubMed. For chemical genetics approaches, see: (i) M. Bredel and E. Jacoby, Nat. Rev. Genet., 2004, 5, 262–275 CrossRef PubMed; (j) Chemogenomics in drug discovery: a medicinal chemistry perspective, ed. H. Kubinyi and G. Müller, Wiley, Weinheim, 2004 Search PubMed; (k) T. Klabunde, Br. J. Pharmacol., 2007, 152, 5–7 CrossRef PubMed; (l) B. R. Stockwell, Nat. Rev. Genet., 2000, 1, 116–125 CrossRef PubMed; (m) B. R. Stockwell, Trends Biotechnol., 2000, 18, 449–455 CrossRef PubMed; (n) C. J. O'Connor, L. Laraia and D. R. Spring, Chem. Soc. Rev., 2011, 40, 4332–4345 RSC; (o) H. van Hattum and H. Waldmann, J. Am. Chem. Soc., 2014, 136, 11853–11859 CrossRef PubMed.
- (a) S. Dong, J. J. Tang, C. C. Zhang, J. M. Tian, J. T. Guo, Q. Zhang, H. Li and J. M. Gao, Eur. J. Med. Chem., 2014, 80, 71–82 CrossRef PubMed; (b) A. Parra, S. Martin-Fonseca, F. Rivas, F. J. Reyes-Zurita, M. Medina-O'Donnell, E. E. Rufino-Palomares, A. Martinez, A. Garcia-Granados, J. A. Lupianez and F. Albericio, ACS Comb. Sci., 2014, 16, 428–447 CrossRef PubMed.
- O. Schwarz, S. Jakupovic, H. D. Ambrosi, L. O. Haustedt, C. Mang and L. Muller-Kuhrt, J. Comb. Chem., 2007, 9, 1104–1113 CrossRef PubMed.
- (a) M. G. P. Buffat, Tetrahedron, 2004, 60, 1701–1729 CrossRef; (b) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319 CrossRef PubMed; (c) J. C. D. Hartwieg, J. W. Priess, H. Schutz, D. Rufle, C. Valente, C. Bury, L. La Vecchia and E. Francotte, Org. Process Res. Dev., 2014, 18, 1120–1127 CrossRef; (d) R. Vardanyan, Piperidine-based Drug Discovery, in Heterocyclic Drug Discovery, Elsevier, Amsterdam, 2017 Search PubMed.
- (a) J. F. Bower, T. Riis-Johannessen, P. Szeto, A. J. Whitehead and T. Gallagher, Chem. Commun., 2007, 728–730 RSC; (b) J. F. Bower, P. Szeto and T. Gallagher, Org. Lett., 2007, 9, 4909–4912 CrossRef PubMed; (c) J. F. Bower, J. Rujirawanicha and T. Gallagher, Org. Biomol. Chem., 2010, 8, 1505–1519 RSC.
- (a) C. Hirschhaeuser, J. S. Parker, M. W. D. Perry, M. I. F. Haddow and T. Gallagher, Org. Lett., 2012, 14, 4846–4849 CrossRef PubMed; (b) K. Weinberg, A. Stoit, C. G. Kruse, M. F. Haddow and T. Gallagher, Tetrahedron, 2013, 69, 4694–4707 CrossRef; (c) A. A. Kirichok, I. Shton, M. Kliachyna, I. Pishel and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2017, 56, 8865–8869 CrossRef PubMed.
- J. Rouden, M. C. Lasne, J. Blanchet and J. Baudoux, Chem. Rev., 2014, 114, 712–778 CrossRef PubMed.
- Cytisine (marketed as Tabex) is widely available across eastern and central Europe as a smoking cessation aid: (a) J. F. Etter, R. J. Lukas, N. L. Benowitz, R. West and C. M. Dresler, Drug Alcohol Depend., 2008, 92, 3–8 CrossRef PubMed; (b) K. Cahill, N. Lindson-Hawley, K. H. Thomas, T. R. Fanshawe and T. Lancaster, Cochrane Database Syst. Rev., 2016, CD006103 Search PubMed. Recent clinical trials relating to the effectiveness of cytisine have also been reported, see: (c) N. Walker, C. Howe, M. Glover, H. McRobbie, J. Barnes, V. Nosa, V. Parag, B. Bassett and C. Bullen, N. Engl. J. Med., 2014, 371, 2353–2362 CrossRef PubMed; (d) R. West, W. Zatonski, M. Cedzynska, D. Lewandowska, J. Pazik, P. Aveyard and J. Stapleton, N. Engl. J. Med., 2011, 365, 1193–1200 CrossRef PubMed.
- For the key pharmacological features of cytisine, see: (a) M. Amar, P. Thomas, C. Johnson, G. G. Lunt and S. Wonnacott, FEBS Lett., 1993, 327, 284–288 CrossRef PubMed; (b) C. C. Boido and F. Sparatore, Farmaco, 1999, 54, 438–451 CrossRef; (c) H. Rollema, A. Shrikhande, K. M. Ward, J. W. Coe, E. Tseng, E. Q. Wang, M. De Vries, T. Cremers, S. Bertrand and D. Bertrand, Biochem. Pharmacol., 2009, 78, 918–919 CrossRef; (d) H. Rollema, A. Shrikhande, K. M. Ward, F. D. Tingley, J. W. Coe, B. T. O'Neill, E. Tseng, E. Q. Wang, R. J. Mather, R. S. Hurst, K. E. Williams, M. de Vries, T. Cremers, S. Bertrand and D. Bertrand, Br. J. Pharmacol., 2010, 160, 334–345 CrossRef PubMed; (e) C. Peng, C. Stokes, Y. S. Mineur, M. R. Picciotto, C. J. Tian, C. Eibl, I. Tomassoli, D. Guendisch and R. L. Papke, J. Pharmacol. Exp. Ther., 2013, 347, 424–437 CrossRef PubMed.
- Cytisine has found use in the generation of diazaadamantane frameworks: (a) A. V. Ivachtchenko, A. Khvat, S. E. Tkachenko, Y. B. Sandulenko and V. Y. Vvedensky, Tetrahedron Lett., 2004, 45, 6733–6736 CrossRef. As the basis of chiral ligands for Pd-catalysed processes: (b) I. Philipova, G. Stavrakov, N. Vassilev, R. Nikolova, B. Shivachev and V. Dimitrov, J. Organomet. Chem., 2015, 778, 10–20 CrossRef. To inspire pyrrolidine-based libraries to target Bcl-2: (c) L. A. Marcaurelle, C. Johannes, D. Yohannes, B. P. Tillotson and D. Mann, Bioorg. Med. Chem. Lett., 2009, 19, 2500–2503 CrossRef PubMed.
- C(6) lithiation and silylation (using TMSCl) of cytisine scaffold was described by Rouden: (a) J. Rouden, A. Ragot, S. Gouault, D. Cahard, J. C. Plaquevent and M. C. Lasne, Tetrahedron: Asymmetry, 2002, 13, 1299–1305 CrossRef; (b) N. Houllier, S. Gouault, M. C. Lasne and J. Rouden, Tetrahedron, 2006, 62, 11679–11686 CrossRef. This relates to an earlier observation by Joule on the C-metalation of N–Me 2-pyridone: (c) P. Meghani and J. A. Joule, J. Chem. Soc., Perkin Trans. 1, 1988, 1–8 RSC.
- F. Frigerio, C. A. Haseler and T. Gallagher, Synlett, 2010, 729–730 Search PubMed.
- (a) W. L. Jorgensen, Acc. Chem. Res., 2009, 42, 724–733 CrossRef PubMed; (b) J. Michel and J. W. Essex, J. Comput. Aided Mol. Des., 2010, 24, 639–658 CrossRef PubMed; (c) J. Michel, N. Foloppe and J. W. Essex, Mol. Inf., 2010, 29, 570–578 CrossRef PubMed; (d) R. E. Amaro and A. J. Mulholland, Nat. Rev. Chem., 2018, 2, 0148 CrossRef.
- D. M. Andrews, L. M. Broad, P. J. Edwards, D. N. A. Fox, T. Gallagher, S. L. Garland, R. Kidd and J. B. Sweeney, Chem. Sci., 2016, 7, 3869–3878 RSC.
- A. R. Katritzky, W. Q. Fan, A. E. Koziol and G. J. Palenik, Tetrahedron, 1987, 43, 2343–2348 CrossRef.
- (a) P. Beak and W. K. Lee, Tetrahedron Lett., 1989, 30, 1197–1200 CrossRef; (b) P. Beak and W. K. Lee, J. Org. Chem., 1990, 55, 2578–2580 CrossRef; (c) P. Beak and W. K. Lee, J. Org. Chem., 1993, 58, 1109–1117 CrossRef; (d) K. M. B. Gross and P. Beak, J. Am. Chem. Soc., 2001, 123, 315–321 CrossRef PubMed.
- (a) E. E. van Tamelen and J. S. Baran, J. Am. Chem. Soc., 1955, 77, 4944–4945 CrossRef; (b) E. E. van Tamelen and J. S. Baran, J. Am. Chem. Soc., 1958, 80, 4659–4670 CrossRef; (c) B. T. O'Neill, D. Yohannes, M. W. Bundesmann and E. P. Arnold, Org. Lett., 2000, 2, 4201–4204 CrossRef; (d) D. Gray and T. Gallagher, Angew. Chem., Int. Ed., 2006, 45, 2419–2423 CrossRef PubMed; (e) C. Hirschhaeuser, C. A. Haseler and T. Gallagher, Angew. Chem., Int. Ed., 2011, 50, 5162–5165 CrossRef PubMed.
- F. Effenberger and W. Brodt, Chem. Ber., 1985, 118, 468–482 CrossRef.
- N-Substituted 5-arylated nipecotic acid derivatives are of interest as inhibitors of Aurora A kinase, see: H. Binch, M. Hashimoto, M. Mortimore, M. Ohkubo and T. Sunami, PCT Int. Appl, WO2010111056, 2010 Search PubMed.
- (a) W. Miura, K. Hirano and M. Miura, Synthesis, 2017, 4745–4752 Search PubMed; (b) H. Rego Campello, A. Honraedt, C. Gotti and T. Gallagher, Synthesis, 2018 DOI:10.1055/s-0036-1591594.
- (a) J. L. Stymiest, G. Dutheuil, A. Mahmood and V. K. Aggarwal, Angew. Chem., Int. Ed., 2007, 46, 7491 CrossRef PubMed; (b) D. Leonori and V. K. Aggarwal, Acc. Chem. Res., 2014, 47, 3174 CrossRef PubMed; (c) S. Balieu, G. E. Hallett, M. Burns, T. Bootwicha, J. Studley and V. K. Aggarwal, J. Am. Chem. Soc., 2015, 137, 4398–4403 CrossRef PubMed.
- (a) K. M. Sadhu and D. S. Matteson, Organometallics, 1985, 4, 1687–1689 CrossRef; (b) H. C. Brown, S. M. Singh and M. V. Rangaishenvi, J. Org. Chem., 1986, 51, 3150–3155 CrossRef.
- (a) G. Zweifel, R. P. Fisher, J. T. Snow and C. C. Whitney, J. Am. Chem. Soc., 1971, 93, 6309–6311 CrossRef; (b) G. Zweifel, C. C. Whitney, J. T. Snow and R. P. Fisher, J. Am. Chem. Soc., 1972, 94, 6560–6561 CrossRef; (c) R. J. Armstrong, W. Niwetmarin and V. K. Aggarwal, Org. Lett., 2017, 19, 2762–2765 CrossRef PubMed; (d) R. J. Armstrong and V. K. Aggarwal, Synthesis, 2017, 3323–3336 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, compound characterisation and crystallographic data. CCDC 1840054–1840056. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ob01456f |
| ‡ When we applied a more conventional oxidation protocol (NaOH, H2O2) to the N-methyl boronate ester 16, we only observed decomposition. |
| § It is noteworthy that the corresponding O-Boc protected variant 23 was unreactive under these same conditions. |
| This journal is © The Royal Society of Chemistry 2018 |









