Effect of preparation methods on hierarchical zeolites for cobalt-based Fischer–Tropsch synthesis
Yuelun Wang*a,
Yuan Jianga,
Jun Huanga,
Hui Wangb,
Zhuo Lib and
Jinhu Wub
aKey Laboratory of Coal Processing and Efficient Utilization, Ministry of Education, China University of Mining & Technology, Xuzhou, Jiangsu 221116, China. E-mail: wangyuelun@126.com
bKey Laboratory of Biofuel, Chinese Academy of Sciences, Qingdao Institute of Bioenergy and Bioprocess Technology, 266101, Qingdao, China
First published on 4th November 2016
Abstract
Hierarchical zeolites were prepared by a soft template method and alkali treatment. The effect of the physicochemical structures of these zeolites on the properties of cobalt catalysts for Fischer–Tropsch (FT) synthesis was investigated. It was found that the Co/MZ-ST catalyst prepared by the soft template method showed higher Co dispersion due to higher surface area, which resulted in higher FT activity. Meanwhile bimodal porous structures inside the zeolite were favored for the production of diesel fuels. While the Co/MZ-AT catalyst prepared by alkali treatment presented multiple porous structure with mesopores distributed on the outer layer of the zeolites, which showed higher selectivity to gasoline. Moreover, the Co/MZ-AT catalyst with a hierarchical and highly crystallized structure showed the lowest deactivation rate. The fastest deactivation rate was observed over the Co/MZ-ST catalyst resulting from cobalt aggregation due to the partial collapse of the lower crystal mesoporous structure under hydrothermal conditions.
1. Introduction
Fischer Tropsch (FT) synthesis is used for the production of liquid fuels and useful chemicals from syngas, in the presence of either supported or unsupported iron and cobalt metal catalysts.1–3 Co-based catalysts are excellent catalysts for low temperature FT synthesis due to their high stability, high conversion, and high selectivity to linear paraffins, low water-gas shift (WGS) activity and relatively low price.4The products of conventional FT catalysts generally follow the Anderson–Schulz–Flory (ASF) distribution, which is a wide range from CH4 to heavy hydrocarbons. The development of novel catalysts that can tune product selectivity would significantly improve FT technology. Catalytic performance in FT synthesis is correlated to the number of cobalt metallic particles and pore channels of supports. Hence, an ideal supported catalyst would have uniformly distributed cobalt species that form cobalt metallic nanoparticles at optimum sizes for objective products.5 Many researchers are interested in using ZSM-5,6,7 mordenite,8 MCM-22,9 β10 and ITQ11 etc. as the supports in FT synthesis. However, the micropores of these zeolites are not large enough to transfer larger molecules. The slow transportation of products in the micropores usually causes over-cracking, leading to high selectivity to undesirable light hydrocarbons. Recently, zeolites with hierarchically porous structures have been widely given much attention. Because the accessibility for molecular transport could improve via combining micropores with mesopores in a whole body compared with conventional zeolites.12–14 Many hierarchically structured zeolitic materials have been successfully synthesized and applied as novel supports in catalytic systems.15–18 Moreover, combination of mesoporous zeolites with FT active sites have been considered to be an effective way in tuning product selectivity. As acid sites are in a close vicinity to FT metal sites, olefinic hydrocarbons may crack or isomerize. Besides, the improved transport properties of hierarchically structured zeolites increase the selectivity toward liquid hydrocarbons.19–21
General preparation methods for hierarchical zeolites are dealumination, desilication or templating.22,23 Desilication by alkali treatment has been proven to be an effective and economical method to introduce additional mesopores into zeolites. It is reported that desilication by treatment in alkaline medium is a very suitable methodology to obtain mesoporous ZSM-5 with preserved structural integrity.24–26 However, it usually generated isolated mesopores by extracting Si atoms in the framework27–29 and easily resulted in irregular porous structure.30,31 Therefore the design of multimodal porous zeolite with ordered structure remained a great challenge. Soft templating is mainly used to create ordered mesopores within zeolitic materials, which is attributed to the meso scaled size of available soft templates. The use of soft templates that have strong interactions with silica based species has the greatest chance of fabricating ordered mesoporous zeolites at the relative high temperatures required for zeolite crystallization. Up to now, there have been several documents reporting catalytic performances in FT synthesis by using hierarchical zeolites prepared by desilication.20,21,32 However, limited knowledge is available for FT catalyst supported hierarchical zeolites prepared by soft templates, especially no comparison of such two kinds of hierarchical zeolites with catalytic performances in FT synthesis was reported. Recently, we synthesized mesoporous ZSM-5 using double-template method and applied it in FT synthesis.33 The results demonstrated that the catalysts with bimodal structure and moderate acidity showed higher C5–18 selectivity with relatively lower CH4 selectivity. Cobalt loaded on crystalline mesoporous ZSM-5 showed the lowest deactivated rate, which suggested that such bimodal structure was favor to the stabilization of cobalt particles. In this study, mesoporous ZSM-5 were prepared by alkaline treatment and soft template method, respectively. And then their catalytic properties of cobalt catalysts, especially their deactivation behaviors in FT synthesis, were further investigated. Additionally, cobalt supported ZSM-5 was also prepared for comparison.
2. Experimental
2.1 Sample preparation
Mesoporous ZSM-5 was prepared using soft templated method: first, 1 g of sodium aluminate were dissolved in 37.8 ml of tetrapropylammonium hydroxide (TPAOH) solution (25%), together with 65.5 ml of water and 0.191 g of NaOH, 81.9 ml of tetraethyl orthosilicate (TEOS) were added to this solution. The suspension obtained was maintained under mechanical agitation for 2 h. Cetyltrimethylammonium bromide solution (CTAB) was added after homogenization of mixtures at initial stage and continued to be agitated for 12 h. The mixture was heated at 413 K and kept at this temperature for 72 h in hydrothermal vessel. The obtained solid by centrifugation was washed with deionized water and then dried at 333 K for 12 h. The sample was then calcined at 823 K for 5 h. Ion exchange was performed using 1 M solution of NH4NO3 at 353 K for 4 h by two consecutive similar processes. After the ion exchange, the sample was filtered and washed with enough de-ionized water and after that dried in oven at 363 K overnight, NH4–zeolite was calcined in the static air at 773 K for 3 h to obtain mesoporous ZSM-5 named MZ-ST.ZSM-5 was prepared as follows: first, 1 g of sodium aluminate were dissolved in 37.8 ml of TPAOH solution (25%), together with 65.5 ml of water and 0.191 g of sodium hydroxide, 81.9 ml of TEOS were added to this solution. The suspension obtained was maintained under mechanical agitation for 2 h. The mixture was heated at 413 K and kept at this temperature for 72 h in hydrothermal vessel. The obtained solid by centrifugation was washed with deionized water and then dried at 333 K for 12 h. The sample was then calcined at 823 K for 5 h. Ion exchange was performed using 1 M solution of NH4NO3 at 353 K for 4 h by two consecutive similar processes. After the ion exchange, the zeolite was filtered and washed with enough de-ionized water, and then dried in oven at 363 K overnight. NH4–zeolite was calcined in the static air at 773 K for 3 h to obtain H-type ZSM-5.
Mesoporous ZSM-5 was prepared using an alkaline treatment method. Briefly, the experiment was carried out in a 250 cm3 round-baker adapted to a reflux-condenser filled with aqueous 0.15 M NaOH at 343 K for 30 min. ZSM-5 was added. After treatment for 3 h, the solid zeolite was recovered by filtration, washing with deionized water, drying, and calcination in air at 573 K for 5 h. The synthesized material was named MZ-AT.
The supported cobalt catalysts were prepared by initial wetness impregnation method. 3.0 g of different supports were completely wetted with deionized water to evaluate the corresponding volume. Then, cobalt nitrate was dissolved into the required deionized water to impregnate the supports with the loading of 15 wt%. The catalysts were then dried under temperature of 333 K and calcined at 673 K for 4 h. The obtained catalysts were named Co/MZ-ST catalyst, Co/MZ-AT catalyst, respectively. Co/ZSM-5 catalyst was also prepared for comparison.
2.2 Characterization
The textural properties of catalysts were determined by nitrogen adsorption, carried out at 77 K, in a Micromeritics equipment. The surface area was calculated using Brunauer–Emmett–Teller (BET) method. The pore size distribution was determined by NLDFT method.X-ray diffraction (XRD) patterns of the samples were recorded on a Bruker B5005 diffractometer using Cu Kα radiation. The mean Co3O4 crystallite sizes were deduced from the XRD data using the Scherrer equation. The metallic cobalt particle size was calculated from XRD: d(Co0) = 0.75 × d(Co3O4). While the cobalt dispersion calculated using the following equation: dispersion = 0.96/d.34 Before the characteristic of reduced catalysts by XRD, fresh catalysts were reduced for 10 h in H2 flow of 30 ml min−1, then they were sealed in liquid paraffin. The spent catalysts were refluxed with toluene for 6 h to remove the waxes on the catalyst surfaces, this entire wax extraction procedure was repeated 3 times, then they were characterized by XRD.
TPR was carried out in a U-tube quartz reactor at the ramp rate of 10 K min−1 in the 5% H2/Ar (vol) flow of 30 ml min−1. The H2 consumption was monitored with TCD using the reduction of CuO as the standard. The reduction percentage of the cobalt oxides at temperatures less than 673 K was calculated from TPR profiles. The ratio between the H2 consumption and the corresponding theoretical value, calculated for the full reduction of each catalyst (assuming all Co atoms to be initially in the form of Co3O4), was reported as the degree of reduction.
Temperature-programmed desorption of ammonia (NH3-TPD) was performed with a Chem TPR/TPD instrument (Quantachrome). Approximately 80.0 mg catalyst was activated for 1 h at 773 K heating with a ramp of 10 K min−1. After cooling to 473 K, pure ammonia was passed through the sample. The desorption of the ammonia was accomplished by purging helium with a flow of 20 ml min−1 and raising the temperature to 1073 K. Total acidity of the catalyst which may correspond to Brønsted and Lewis sites was measured by a thermal conductivity detector (TCD).
Si/Al ratio of zeolite samples was measured by ICP-OES (Spectro Ciros CCD ICP optical emission spectrometer with axial plasma viewing).
Scanning electron microscopy (SEM) was performed on a FEI QuantaTM 250. Samples were coated with a layer of gold using an Edwards S150A sputter coater, to make them conductive prior to imaging.
TEM micrographs were obtained from a Tecnai G20 (FEI-2012, LaB6) with an accelerating voltage of 200 kV. Each sample powder was dispersed in absolute ethanol, dropped on a carbon-coated copper grid, dried and transferred into TEM chamber.
Catalysts were evaluated in a pressured fixed-bed reactor at 2 MPa, 1000 h−1 with the H2/CO ratio of 2 after reduction at 673 K for 10 h. Wax was collected with a hot trap and the liquid products were collected in a cold trap after 24 h on-stream. The gas effluents were analyzed on-line by using Carbosieve-packed column with TCD. The gas hydrocarbons were analyzed on-line using Porapack-Q column with FID. Oil and wax were analyzed offline in OV-101 capillary columns. 5% N2 was added to syngas as an internal standard. The carbon balance and mass balance were 100 ± 5%.
3. Results and discussion
3.1 Characteristics of supports
The SEM micrographs of zeolites are shown in Fig. 1. It was found that all samples consisted of regular shaped crystals, and morphologies were almost homogeneous. The images of ZSM-5 exhibited hexagonal structure, typical for MFI phase. Average crystal size is about 3 μm in width and 6 μm in length. The alkali treatment resulted in no obviously detectable change in the morphology of particles and crystal sizes (see MZ-AT sample). But desilication happened on the surface of zeolites at the initial, large number of cavernous holes over the surface were observed. These cavities were interconnected with each other and extended to inner of zeolites, which eventually led to the formation of micro–mesopores at the outer layer.35 MZ-ST sample exhibited cubic prismatic structure with average particle size of 1.6 μm in width and 2 μm in length. The crystal size and morphology were different from that of MZ-AT sample indicating that different mechanisms of the formation were presented. Mesopores of MZ-ST sample were connected within the micropore network in the crystals. | ||
| Fig. 1 SEM profiles of the prepared supports. (1) and (2) MZ-ST; (3) and (4) ZSM-5; (5) and (6) MZ-AT. | ||
3.2 Characteristics of catalysts
The N2 adsorption/desorption isotherms of catalysts are shown in Fig. 2. For Co/ZSM-5 catalyst, a typical type I isotherm was observed, corresponding to a microporous zeolite. The isotherms for other two samples exhibited hysteresis loops at the range of P/P0 = 0.5–0.9, which usually were associated with the filling and emptying of mesopores by capillary condensation. This feature appears to be slightly more pronounced for Co/MZ-ST sample. Fig. 3 displays the pore size distribution of samples. It was observed that besides the microporosity around 0.8 nm, Co/MZ-AT sample showed multiple mesoporous distribution around 2.0 nm and 3.4 nm, respectively. Co/MZ-ST sample was observed to be a bimodal pore distribution with the microporosity around 0.8 nm and the mesoporosity around 3.4 nm. The pore size distribution of Co/ZSM-5 catalyst is also shown in Fig. 3, which mainly centered at 0.6 nm. The results of textural properties of catalysts are shown in Table 1. The BET surface areas of hierarchical zeolites increased compared with ZSM-5, and the increase is the greatest for MZ-ST sample due to the smallest crystal size. BET surface area of alkaline treated sample (MZ-AT) was larger than that of ZSM-5, which may be attributed to dissolution of the framework and mesopore formation.36 The total pore volumes increase for both MZ-AT sample and MZ-ST sample due to an increase in mesopore volume. After Co impregnation, BET surface area and total pore volume significantly decreased. This might result from a partial blockage of pores by cobalt oxide clusters and/or a partial collapse of mesoporous structure.| Samples | SBET/m2 g−1 | Vmicro/cm3 g−1 | Vmeso/cm3 g−1 | Co3O4 particle sizea/nm | Cobalt dispersionb/% | Reducibilityc/% | Si/Al ratio | Total acidityd/mmol g |
|---|---|---|---|---|---|---|---|---|
| a The average Co3O4 crystallite size was determined by XRD (Scherrer equation) at 2θ = 36.9°.b The cobalt dispersion calculated using the equation: dispersion = 0.96/d(Co0). The metallic cobalt particle size was calculated from XRD: d(Co0) = 0.75 × d(Co3O4).c The reducibility was obtained from TPR. The H2 consumption was monitored with TCD using the reduction of CuO as the standard.d Total acidity of the catalysts obtained from NH3-TPD. | ||||||||
| ZSM-5 | 412 | 0.25 | 0.09 | — | — | 29.6 | — | |
| MZ-AT | 546 | 0.24 | 0.19 | — | — | 23.8 | — | |
| MZ-ST | 614 | 0.26 | 0.17 | — | — | 30.4 | — | |
| Co/ZSM-5 | 342 | 0.22 | 0.06 | 19.6 | 6.5 | 69.3 | — | 0.29 |
| Co/MZ-AT | 462 | 0.21 | 0.16 | 18.2 | 7.0 | 74.6 | — | 0.38 |
| Co/MZ-ST | 526 | 0.24 | 0.14 | 14.4 | 8.9 | 78.6 | — | 0.28 |
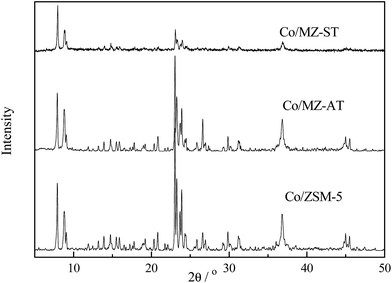
The XRD patterns of Co supported catalysts are presented in Fig. 4. All catalysts showed the characteristic reflection peak at 2θ = 36.8° for Co3O4 phase. The crystallite size of Co3O4 was calculated by using Scherrer equation and summarized in Table 1. Co/MZ-ST catalyst with the smallest cobalt particles was probably attributed to the largest surface area of zeolites. Co/ZSM-5 catalyst showed the largest cobalt crystal size. Strong characteristic peaks of MFI structures were observed over Co/ZSM-5 catalyst and Co/MZ-AT catalyst. The intensity was clearly lower over Co/MZ-ST catalyst suggesting that the process of crystallization became more difficult under the direction of meso-scaled soft template.
The reduction behaviors of catalysts were studied using H2-TPR. As shown in Fig. 5, two-step reduction were exhibited at 550–730 K and at 900–1073 K, respectively. The weight loss at 550–730 K was assigned to the stepwise reduction of Co3O4 to CoO and CoO to Co. The peaks in the 900–1073 K overlapped and extended over a wide temperature range, indicating several interactions occurring between cobalt species and supports, which led to the formation of unreductive cobalt aluminates or cobalt silicates.37 Lower reduction temperature for cobalt–support interaction indicated higher cobalt reducibility. In addition, smaller pore sizes of supports influenced H2 diffusion during TPR, partially leading to a higher reduction temperature. Contribution of the reduction peaks was thus pronounced in the case of Co/ZSM-5 catalyst, the lowest reducibility was observed due to its smaller microporosity and stronger cobalt–support interaction. Moreover, the overlap of reduction peaks of Co3O4 at 550–730 K for Co/ZSM-5 catalyst indicating that Co3O4 and CoO were located in different pore channels resulting in different reduction behaviors.38 The reduction degree was relatively expressed as the ratio of the amount of H2 consumption below 673 K to that of total H2 consumption. The values were 78.6, 74.6, 69.3% for Co/MZ-ST catalyst, Co/MZ-AT catalyst and Co/ZSM-5 catalyst, respectively, as shown in Table 1. Co/MZ-ST catalyst showed the highest reducibility due to the highest surface area resulting in higher cobalt dispersion and the lowest cobalt–support interaction.
The surface acidic properties of the catalysts were investigated by ICP-OES and NH3-TPD. In this study, the overall Si/Al ratios of samples determined with ICP-OES were 29.6, 23.8 and 30.4 for Co/ZSM-5 catalyst, Co/MZ-AT catalyst and Co/MZ-ST catalyst, respectively. The Si/Al ratio of Co/MZ-AT sample was found to be lower than that of other catalysts due to the partial removal of Si from the framework of zeolite during alkali treatment. Two NH3 desorption peaks were observed in Fig. 6. Lower temperature peak at 450–550 K could be assigned to the adsorption of ammonia over weak acid sites.31 Another broad peak at 600–750 K was attributed to the adsorption of ammonia over strong acid sites. The number of acid sites expressed as mmol NH3/g of catalysts with respect to only weak and strong acidic sites was 0.29 mmol g−1, 0.38 mmol g−1 and 0.28 mmol g−1 for Co/ZSM-5 catalyst, Co/MZ-AT catalyst and Co/MZ-ST catalyst, respectively. It was found that the number of acid sites increased appreciably after NaOH treatment, which was considered to be the result of partial desilication during the alkaline treatment. Moreover, the acid site distribution changed drastically after exchange of sodium cations with zeolitic protons. More Lewis acid sites were generated associating with extra framework aluminum,39 a decrease in the number of strong acid sites was observed while the total acid sites increased.
3.3 Catalytic performances
The experiments of FT synthesis were performed in a fixed bed at 473 K, 2 MPa and H2/CO = 2 feed. CO conversion as well as carbon selectivity to different products of catalysts is listed in Table 2. Conventional ZSM-5 supported Co catalyst exhibited 31.6% CO conversion. Hierarchical ZSM-5 supported cobalt catalyst, gave higher CO conversion. Moreover, Co/MZ-ST catalyst showed higher CO conversions than that of Co/MZ-AT catalyst. Obviously, CO conversion linearly increased with increasing the specific surface area of catalysts. Co/MZ-ST catalyst with the highest surface area tended to show the highest FT activity. Moreover, it was found that cobalt dispersion was correlated well with the surface area of the catalysts as shown in Table 1. The dispersion of cobalt was increased with the increase of specific surface areas, which confirmed that the catalyst with high specific surface area could efficiently raise the metal particle dispersive distribution and control metal particle size. This indicated that FT synthesis was the structure-sensitive reaction and depended on the number of exposed Co0 sites on catalyst surfaces. Co/MZ-ST catalyst showed higher cobalt dispersion. Thus, it is reasonable to expect to exhibit higher catalytic activity. These Co0 sites were further characterized by XRD shown in Fig. 7. The reduced catalysts exhibited a mixture of hexagonal (hcp) and face-centered cubic (fcc) cobalt with structural imperfections forming the metallic phase. More hexagonal Co (hcp) at 41.7° and less face-centered cubic Co (fcc) at 44.4° were observed over catalysts. Co/MZ-ST catalyst showed both distinct diffraction peaks of Co (hcp) and Co (fcc), which might be contributed to higher FT activity. Meanwhile the graphic of TPR showed the lowest cobalt–support interaction over Co/MZ-ST catalyst, thus the highest cobalt reducibility was obtained. The reducibility of these catalysts decreased in following order: Co/MZ-ST catalyst > Co/MZ-AT catalyst > Co/ZSM-5 catalyst, which was well corresponding to the activity. Co/ZSM-5 catalyst showed the lowest activity not only due to the lowest reducibility but also the lowest cobalt dispersion. Cobalt-time yields (CTY) estimated from FT reaction rates listed in Table 2, relative concentrations of cobalt metal phases were accordingly found to be higher for those catalysts with higher reducibility. The highest CTY was observed over Co/MZ-ST catalyst.| Sample | CO con. (%) | CTYb | αc | O/Pd | Ciso/Cne | Deactivation ratef | Selectivity (wt%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2–C4 | C5–C11 | C12–C18 | C18+ | |||||||
| a Reaction conditions: H2/CO = 2, GHSV = 1200 h−1, P = 2 MPa, T = 473 K, TOS = 24 h.b CTY = 10−5 mol CO per g Co per s.c Chain growth probability in the range C5–C18.d The ratio of olefins to paraffins in the range C5–C18.e The ratio of isoparaffins to n-paraffins in the range C5–C18.f Deactivation rate: (CO conv. at 24 h − CO conv. at 120 h)/(CO conv. at 24 h) × 100. | |||||||||||
| Co/ZSM-5 | 31.6 | 1.8 | 0.52 | 0.32 | 0.44 | 0.48 | 30.00 | 22.36 | 24.16 | 15.75 | 7.73 |
| Co/MZ-AT | 39.2 | 2.5 | 0.54 | 0.38 | 0.49 | 0.45 | 18.37 | 14.70 | 28.30 | 27.94 | 10.69 |
| Co/MZ-ST | 48.7 | 3.4 | 0.60 | 0.34 | 0.42 | 0.51 | 16.81 | 12.27 | 22.09 | 35.90 | 12.93 |
 | ||
| Fig. 7 XRD profiles of reduced cobalt catalysts and spent cobalt catalysts after support-pattern subtraction. | ||
The deactivation rate was calculated from CO conversions and compared to the stability of catalysts in Table 2. It was found that Co/MZ-ST catalyst deactivated the most rapidly while Co/MZ-AT catalyst exhibited the lowest deactivated rate. XRD results showed highly crystal structure over Co/MZ-AT catalyst in Fig. 4, which could stabilize cobalt active sites and prevent particles from migration to large catalyst grains.40,41 It should be noted that Co/ZSM-5 catalyst showed higher stability than that of Co/MZ-ST catalyst, which was contrary to our previous results.33 The reason might be that Co/MZ-ST catalyst showed lower crystal mesoporous structure in this study. To trace the origin of catalyst deactivation is difficult. It is usually a complex problem where several mechanisms interplaying contribute to the loss of activity, such as the destruction of porous structure, the coke formation, the aggregation of Co particles, oxidation/metal support compound formation, sintering, poisoning and cobalt reconstruction. The transmission electron microscopy (TEM) of fresh catalysts and spent catalysts shown in Fig. 8 was applied to supply the information of construction changes of catalysts before and after reaction. Compared to fresh Co/MZ-AT catalyst, cobalt particles with 10–50 nm were spheric granules located in the pores of Co/MZ-ST catalyst. Further, some Co crystallites were visibly tended to cluster on the support. While the dispersion of cobalt over Co/MZ-AT catalyst was completely different from that of Co/MZ-ST catalyst. Cobalt species showed diffuse distribution. Only a few obvious cobalt particles could be found. Images of spent catalysts were obtained after 120 h of catalytic testing. Cobalt particles of Co/MZ-AT were similarly dispersive state as that of the fresh sample, indicating the absence of cobalt aggregation during catalytic operation. While it was clearly illustrated that cobalt aggregation over spent Co/MZ-ST catalyst was more severe. These changes indicated a reconstruction of the cobalt particles during FT synthesis, which led to the loss of catalytic activity. XRD of the spent catalysts (Fig. 7) also showed that Co/MZ-ST catalyst presented larger Co3O4 particles than that of the fresh catalyst, further proved that cobalt particles had grown during the reaction and more easily aggregated. Small-angle XRD results in Fig. 9 revealed that meso-structure of Co/MZ-ST catalyst was originally less ordered. After reaction for 120 h, mesoporous structure totally collapsed due to an effect of water vapor produced during the reaction. Co particles had a more tendency to migrate to the external surface and aggregate to catalyst grains, then led to particle growth causing a loss of activity. While small-angle XRD patterns of Co/MZ-AT catalyst were almost unchanged before and after reaction for 120 h indicating that mesoporous structure was kept although it was observed long-range disordered structure. Additionally, carbon deposition over zeolite catalysts was unavoidable, which blocked active sites of this reaction.42–44 As the amount of carbon exceeded the coverage of the exposed metal surface resulting in deactivation of the catalyst by blocking. Zeolites with hierarchical structures could improve the accessibility for molecules within porosities, more effective mass transport can be offered, thus carbon deposition could be mitigated compared to ZSM-5.45 This effect was more pronounced for Co/MZ-AT catalyst by maintaining stable mesopore structures.
 | ||
| Fig. 8 TEM profiles of fresh cobalt catalysts and spent cobalt catalysts. (1) Fresh Co/MZ-ST, (2) spent Co/MZ-ST, (3) fresh Co/MZ-AT, (4) spent Co/MZ-AT. | ||
The different selectivity to products of FT synthesis was observed at the same reaction condition (Table 2). It is known that CO diffusion limitations increased effective H2/CO ratio in pore channels thus leading to higher chain termination probability.46 Thus, Co/ZSM-5 catalyst showed the highest CH4 selectivity. While for cobalt catalysts supported mesoporous ZSM-5, hierarchical structures with both microporosity and mesoporosity improved the diffusion rate of products, which restricted to the production of methane. Meanwhile, Co/MZ-ST catalyst showed higher diesel selectivity (C12–18) while higher gasoline selectivity (C5–12) was observed over Co/MZ-AT catalyst. These two catalysts presented different selectivity to liquid fuels. The reason might be attributed to their different porous structures and cobalt reducibility. It was observed that Co/MZ-AT sample showed multiple porous distribution and mesopores were in fact cavities that were only connected to the outer surface of the crystals via the micropore network. While bimodal porous structure was observed over Co/MZ-ST catalyst. Such mesopores with connecting to the whole body of zeolites were favored to the production of larger hydrocarbons (i.e. diesel fuels). Moreover, higher reducibility also promoted the selectivity for heavier hydrocarbons over Co/MZ-ST catalyst.
ASF plot of hydrocarbon distribution of catalysts are illustrated in Fig. 10. A curve for chain growth trend was observed over these catalysts. As we know that secondary reactions such as hydrocarbon cracking, isomerization and reinsertion of α-olefins in chain growth were mainly responsible for a non-ASF product distribution.47 Acidic sites presented good performance for secondary reactions. Co/ZSM-5 catalyst showed lower α than that of Co/MZ-AT catalyst indicating that diffusion limitation of micropores could also be contributed to non-ASF distribution due to longer residence time for larger hydrocarbons and then secondary reactions occurred. This implied that not only acid sides of zeolites could modify the chain growth probability but also the diffusion limitation of pore channels played a role for a deviation of ASF distribution. Co/MZ-ST catalyst exhibited the highest α due to its lower acid sites and larger mesopore size.
 | ||
| Fig. 10 ASF plot of the hydrocarbon distribution of cobalt catalysts supported ZSM-5 and mesoporous ZSM-5. | ||
In addition, the ratio of olefins to paraffins (denoted as O/P) was investigated in this study. α-Olefins re-adsorbed on the catalyst surface and underwent competitive reactions to produce n-paraffins, olefins or reinserted to initiate new growing chains to produce larger hydrocarbons, which all changed O/P ratio.48 It was reported that α-olefins were favored to adsorbing strongly over weak acid sites and then dehydrogenated to produce olefins, accordingly increasing O/P ratio.49 The results of NH3-TPD indicated that desilication increased the number of weak acid sites. Thus the highest O/P ratio was observed over Co/MZ-AT catalyst. While diffusion limitations for carbon monoxide in catalyst micropores increased H2/CO ratio then increased H2 partial pressure, which enhanced the probability of hydrogenation of olefins leading to a reducing O/P ratio.50 Then the lowest O/P ratio was observed over Co/ZSM-5 catalyst.
Acid sites were inclined to produce more isoparaffins due to hydrocracking and isomerization of primary products. Thus the ratio of isoparaffins to n-paraffins (denoted as Ciso/Cn) was also studied. Large number of acid sites led to an increase in Ciso/Cn ratio due to overcracking of heavy molecules over Co/MZ-AT catalyst. Co/MZ-ST catalyst showed lower Ciso/Cn ratio than that of Co/ZSM-5 catalyst due to the presence of mesoporosity that relieved diffusion constraints and hindered selectivity towards isoparaffins. Therefore, the highest Ciso/Cn ratio was obtained over Co/MZ-AT catalyst.
4. Conclusion
Two kinds of hierarchical zeolites were prepared by soft template method and alkali treatment, respectively. The influence of physicochemical properties of cobalt catalysts supported on these zeolites in FT synthesis was investigated. Co/MZ-ST catalyst with the highest surface area showed higher Co dispersion, thus higher activity was obtained. Meanwhile bimodal porous structure accompanying higher cobalt reducibility was favored to the production of diesel fuels. While Co/MZ-AT catalyst prepared by alkali treatment showed multiple porous structure with most mesopores distributing on the outer surface, which showed higher selectivity to gasoline. On the other hand, Co/MZ-AT catalyst exhibited higher acid sites especially increased Lewis acid sides, thus the highest O/P ratio and Ciso/Cn ratio were observed. Moreover, Co/MZ-AT catalyst with hierarchical and highly crystal structure showed the lowest deactivated rate, while the fastest deactivation rate was observed over Co/MZ-ST catalyst due to cobalt aggregation resulting from the collapse of lower crystal mesoporous structure in hydrothermal condition.Acknowledgements
The work was supported by the scientific research program for the frontier of subject funded by China University of Mining And Technology (2015XKMS044), National Nature Science Foundation of China (NSFC) and Shanxi Low Carbon Coal Foundation (U1510106), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- X. Li, K. Asami, M. Luo, K. Michiki, N. Tsubaki and K. Fujimoto, Catal. Today, 2003, 84, 59 CrossRef CAS.
- A. Martínez and C. López, Appl. Catal., A, 2005, 294, 251 CrossRef.
- Y. W. Chen, H. T. Tang and J. G. Goodwin Jr, J. Catal., 1983, 83, 415 CrossRef CAS.
- H. H. Nijs and P. A. Jacobs, J. Catal., 1980, 66, 401 CrossRef CAS.
- G. L. Bezemer, J. H. Bitter, H. P. C. E. Kuipers, H. Oosterbeek, J. E. Holewijn, X. Xu, F. Kapteijn, A. Jos van Dillen and K. P. de Jong, J. Am. Chem. Soc., 2006, 128, 3956 CrossRef CAS PubMed.
- V. Subramanian, V. L. Zholobenko, K. Cheng, C. Lancelot, S. Heyte, J. Thuriot, S. Paul, V. V. Ordomsky and A. Y. Khodakov, ChemCatChem, 2016, 8(2), 380 CrossRef CAS.
- X. Sun, S. Sartipi and F. Kapteijn, New J. Chem., 2016, 40(5), 4167 RSC.
- Q. H. Lin, G. H. Yang, X. N. Li, Y. Yoneyama, H. L. Wan and N. Tsubaki, ChemCatChem, 2016, 5(10), 3101 CrossRef.
- R. Ravishankar, M. M. Li and A. Borgna, Catal. Today, 2005, 106(1–4), 149 CrossRef CAS.
- C. Wang, X. Ma and Q. Ge, Catal. Sci. Technol., 2015, 5(3), 1847–1853 CAS.
- P. Concepcion, C. Lopez, A. Martınez and V. F. Puntes, J. Catal., 2004, 228, 321–332 CrossRef CAS.
- M. Hartmann, Angew. Chem., Int. Ed., 2004, 43, 5880 CrossRef CAS PubMed.
- J. Perez-Ramırez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- S. A. Lopez-Orozco, A. Inayat, A. Schwab, T. Selvam and W. Schwieger, Adv. Mater., 2011, 23, 2602 CrossRef CAS PubMed.
- J. J. Zhao, Z. Hua, Z. C. Liu, Y. S. Li, L. Guo, W. Bu, X. Z. Cui, M. L. Ruan, H. G. Chen and J. L. Shi, Chem. Commun., 2009, 48, 7578 RSC.
- M. B. Yue, L. B. Sun, T. T. Zhuang, X. Dong, Y. Chun and J. H. Zhu, J. Mater. Chem., 2008, 18, 2044 RSC.
- F. N. Gu, F. Wei, J. Yang, N. Lin, W. G. Lin, Y. Wang and J. H. Zhu, Chem. Mater., 2010, 22, 2442 CrossRef CAS.
- M. Choi, H. S. Cho, R. Srivastava, C. Venkatesan, D. H. Choi and R. Ryoo, Nat. Mater., 2006, 5, 718 CrossRef CAS PubMed.
- S. Fathi, M. Sohrabi and C. Falamaki, Fuel, 2014, 116, 529 CrossRef CAS.
- J. C. Kang, K. Cheng, L. Zhang, Q. H. Zhang, J. S. W. Q. D. Hua and Y. Wang, Angew. Chem., Int. Ed., 2011, 50, 5200 CrossRef CAS PubMed.
- S. Sartipi, K. Parashar and M. J. Valero-Romero, J. Catal., 2013, 305, 179 CrossRef CAS.
- K. P. de Jong, J. Zecevic, H. Friedrich, P. E. de Jongh, M. Bulut and S. van Donk, et al., Angew. Chem., Int. Ed., 2010, 49, 10074 CrossRef CAS PubMed.
- S. Fathi, M. Sohrabi and C. Falamaki, Fuel, 2014, 116, 529 CrossRef CAS.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. P. Crez-Ram Drez, Colloids Surf., A, 2004, 241, 53 CrossRef CAS.
- J. C. Groen, L. A. A. Peffer, J. A. Moulijn and J. P. Crez-RamDrez, Microporous Mesoporous Mater., 2004, 69, 29 CrossRef CAS.
- L. Su, L. Liu, J. Zhuang, H. Wang, Y. Li, W. Shen, Y. Xu and X. Bao, Catal. Lett., 2003, 91, 155 CrossRef CAS.
- L. Sommer, D. Mores, S. Svelle, M. Stöcker, B. M. Weckhuysen and U. Olsbye, Microporous Mesoporous Mater., 2010, 132, 384 CrossRef CAS.
- R. Chal, C. Gérardin, M. Bulut and S. van Donk, ChemCatChem, 2011, 3, 67 CrossRef CAS.
- J. Pérez-Rírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- B. Louis, F. Ocampo, H. S. Yun, J. P. Twssonnier and M. M. Pereira, Chem. Eng. J., 2010, 161, 397 CrossRef CAS.
- T. Tatsumi, M. Nakamura, S. Negishi and H. Tominaga, J. Chem. Soc., Chem. Commun., 1990, 6, 476 RSC.
- S. Sartipi, K. Parashar, M. Makkee, J. Gascon and F. Kapteijn, Catal. Sci. Technol., 2013, 3(3), 572 CAS.
- Y. L. Wang, Y. Jiang, J. Huang, J. Liang, H. Wang, Z. Li, J. Wu, M. Li, Y. P. Zhao and J. C. Niu, Fuel, 2016, 174, 17 CrossRef CAS.
- L. Wei, Y. Zhao, Y. Zhang, C. Liu, J. Hong, H. Xiong and J. L. Li, J. Catal., 2016, 340, 205 CrossRef CAS.
- Y.-Q. Song, Y.-L. Feng, F. Liu, C.-L. Kang, X.-L. Zhou, L.-Y. Xu and G.-X. Yu, J. Mol. Catal. A: Chem., 2009, 310, 130 CrossRef CAS.
- J. C. Groen, J. A. Moulijn and J. Perez-Ramirez, Microporous Mesoporous Mater., 2005, 87, 153 CrossRef CAS.
- E. Steen, G. S. Sewell and R. A. Makhothe, et al., J. Catal., 1996, 162(2), 220 CrossRef.
- H. X. Zhao, J. G. Chen and Y. H. Sun, Chin. J. Catal., 2003, 24(12), 933 CAS.
- D. Esquivel, A. J. Cruz-Cabeza, C. Jiménez-Sanchidrián and F. J. Romero-Salguero, Microporous Mesoporous Mater., 2011, 142, 672 CrossRef CAS.
- A. Y. Khodakov, Catal. Today, 2009, 144, 251 CrossRef CAS.
- P. Munnik, P. E. de Jongh and K. P. de Jong, J. Am. Chem. Soc., 2014, 136, 7333 CrossRef CAS PubMed.
- A. Martínez and C. López, Appl. Catal., A, 2005, 294, 251 CrossRef.
- L. Borkó, Z. E. Horváth, Z. Schay and L. Guczi, Stud. Surf. Sci. Catal., 2007, 167, 231 CrossRef.
- D. W. Goodman, R. D. Kelley, T. E. Madey and J. T. Yates Jr, J. Catal., 1980, 63, 226 CrossRef CAS.
- D. J. Moodley, J. van de Loosdrecht, A. M. Saib, M. J. Overett, A. K. Datye and J. W. Niemantsverdriet, Appl. Catal., A, 2009, 354, 102 CrossRef CAS.
- M. Lualdi, S. Lögdberg, G. Carlo, S. Jaras, M. Boutonnet, A. Venezia, E. Blekkanand and A. Holmen, Top. Catal., 2011, 54, 1175 CrossRef CAS.
- J. Kang, K. Cheng, L. Zhang, Q. Zhang, J. Ding, W. Hua, Y. Lou, Q. Zhai and Y. Wang, Angew. Chem., 2011, 123, 5306 CrossRef.
- E. W. Kuipers, I. H. Vinkenburg and H. Oosterbeek, J. Catal., 1995, 152, 137 CrossRef CAS.
- H. Lu, Catalytic Cracking, 1998, 17(2), 13 Search PubMed.
- F. Yan, W. Qian, Q. Sun, H. Zhang, W. Ying and D. Fang, React. Kinet., Mech. Catal., 2014, 113, 471 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |