Metal-free synthesis of quinazolinones without any additives in water†
Ben-Quan Huab,
Jie Cuic,
Li-Xia Wang*b,
Ya-Lin Tang*b and
Luo Yang*a
aKey Laboratory for Environmental Friendly Chemistry and Application, Department of Chemistry, Xiangtan University, Hunan 411105, PR China. E-mail: yangluo@xtu.edu.cn
bBeijing National Laboratory for Molecular Sciences (BNLMS), Center for Molecular Sciences, State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, PR China. E-mail: wlx8825@iccas.ac.cn; tangyl@iccas.ac.cn
cCenter for Physicochemical Analysis and Measurement, Institute of Chemistry, Chinese Academy of Sciences (ICCAS), Beijing 100190, China
First published on 20th April 2016
Abstract
Here we report that an excess amount of aldehyde, in particular, aliphatic aldehyde, without any additives, efficiently facilitates the oxidation of aminal intermediates to quinazolinones in pure water.
In the last decade, there has been an increasing focus on developing green chemical processes for synthetic organic chemists. Developing atom economical reactions, minimizing the use of toxic reagents1 and adopting environmentally benign solvents2 have all been explored in an effort to achieve ideal chemical transformations and this shows that synthetic organic chemists have begun to rise to the green challenge.3 Quinazolinones represent a class of privileged scaffolds that occur in approximately 150 naturally occurring alkaloids, some of which exhibit a wide range of biological and pharmacological activities, such as rutaecarpine, luotonin A, luotonin F, sildenafil, bouchardatine and raltitrexed.4 Although numerous synthesis methods have been developed,5 the previously reported conditions required the synthesis to include metal catalysts, organic solvents and/or specific oxidants.
The most classical and general protocols for the synthesis of quinazolinones are still via the condensation reaction between o-aminobenzamides and aldehydes followed by the oxidation of the resulting aminal intermediates.6 However, these procedures suffer from the use of stoichiometric or excess amounts of toxic and/or hazardous oxidants, such as KMnO4,6a MnO2,6b CuCl,6c DDQ,6d I2,6e t-BuOOH6f and PhI(OAc)2,6g and the generation of a large amount of harmful by-products.7 In addition, the above procedures are generally performed in organic solvents, which may cause environmental pollution. In this communication, we report our initial results that indicate that an excess amount of aldehyde, in particular, aliphatic aldehydes, without any additives, efficiently facilitates the oxidation of aminal intermediates to quinazolinones in pure water.
Recently, we found that C–C (or C–H) bonds at the 2-position of 2,2-disubstituted-1,2,3,4-tetrahydroquinazolinone could be selectively cleaved by a Cu/air catalytic system, and the C–H bond was the most easily cleaved.8 In our ongoing research, we detected excess amounts of aldehyde by accident in the reaction system and found that this could favor the synthesis of quinazolinones (Table 1). The condensation reaction between o-aminobenzamide 1a and 3-phenylpropanal 2a (1 equiv., relative to the amount of 1a) could provide the cyclic aminal intermediate 3a easily with a yield of 92% and just a trace amount of quinazolinone 4a was detected (entry 1), when water was chosen as the solvent. While increasing the amount of 3-phenylpropanal 2a to 1.5 equiv. or even to 2.0 equiv. (relative to the amount of 1a), the yield of quinazolinone 4a was increased to 58% (entry 2) and 73% (entry 3), respectively, and of course the yields of the aminal intermediate 3a were correspondingly decreased. Control experiments under argon and dioxygen atmospheres both produced the quinazolinone 4a in lower yields (entries 4 and 5), which confirmed that the aromatization of the cyclic aminal intermediate was promoted by the excess aldehyde. When the model reactions were conducted in mixed solvents [water/ethanol (entry 6) or water/IPA (entry 7)], slightly lower yields of 4a were obtained compared with pure water. As with the solvent requirements of green chemistry, water was chosen as the best solvent for further study.
| Entry | 2a (equiv.) | Solvent | Yieldb [%] | |
|---|---|---|---|---|
| 3a | 4a | |||
| a Reaction conditions: 1a (0.3 mmol), H2O: 2 ml, 120–130 °C, 24 h.b Isolated yield.c Degassed before heating and reacted under argon.d Dioxygen atmosphere. | ||||
| 1 | 1.0 | H2O | 92 | Trace |
| 2 | 1.5 | H2O | 34 | 58 |
| 3 | 2.0 | H2O | 11 | 73 |
| 4c | 2.0 | H2O | 29 | 68 |
| 5d | 2.0 | H2O | 0 | 60 |
| 6 | 2.0 | H2O/EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
14 | 72 |
| 7 | 2.0 | H2O/i-PrOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
26 | 61 |
When investigating the quinazolinone synthesis in the presence of 2 equiv. of aldehyde (relative to the amount of 2-anthranilamide), we found that 2-anthranilamide 1a, treated with aliphatic aldehyde 2a–e in water at 120–130 °C, produced 2-substituted quinazolinones 4a–e as the major products with yields in the range of 41% to 73%. Aromatic aldehydes showed different results, benzaldehyde 2f and para-nitrobenzaldehyde 2h gave the cyclic aminal intermediates 3f in 86% yield and 3h in 63% yield, respectively. However, para-methoxylbenzaldehyde 2g and picolinaldehyde 2i produced 2-substituted quinazolinones as the major products, with isolated yields of 71% and 39%, respectively. 2-Anthranilamide 1a, 4-methyl-2-anthranilamide 1b and 4-nitro-2-anthranilamide 1c reacted with 3-phenylpropanal 2a, also produced 2-substituted quinazolinones as the major products in moderate to good yields.
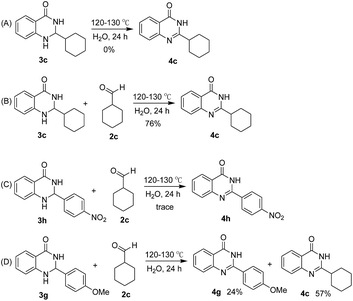
During the synthetic processes of quinazolinones investigated here, the oxidation of the cyclic aminal intermediate was very important. To elucidate the transformation process from the cyclic aminal intermediates 3 to 2-substituted quinazolinones 4, some control experiments were carried out. As shown in Scheme 1, cyclohexyl-substituted aminal intermediate 3c could convert into 2-cyclohexyl-quinazolinone 4c with a yield of 76%, in the presence of additional cyclohexanecarboxaldehyde 2c. These results indicated that the excess amount of aliphatic aldehyde might serve as an oxidant to convert the aminal intermediate 3 into the final product 4. Aryl-substituted aminal intermediates gave different results, which are shown in Table 2. We investigated the transformations from 3h and 3g to 4h and 4g, respectively, in the presence of cyclohexanecarboxaldehyde 2c, and we found the para-nitrophenyl substituted substrate 3h gave a trace amount of quinazolinone 4h, while the para-methoxylphenyl substituted substrate 3g gave two quinazolinone products 4g and 4c in yields of 24% and 57%, respectively. The difference in reactivity might be attributed to the increased stability of the para-nitrophenyl substituted aminal intermediate 3h.
| Entry | 1 | 2 (R2) | Yieldb (%) | |
|---|---|---|---|---|
| 3 | 4 | |||
| a Reaction conditions: 1 (0.3 mmol), 2 (0.6 mmol); H2O: 2 ml, 120–130 °C, 24 h.b Isolated yield.c 2d (1.2 mmol).d 48 h.e Not detected. | ||||
| 1 | 1a | Phenylethyl, 2a | 3a 11 | 4a 73 |
| 2 | 1a | H, 2b | —e | 4b 68 |
| 3 | 1a | Cyclohexyl, 2c | 3c 34 | 4c 62 |
| 4c | 1a | Ethyl, 2d | 3d 21 | 4d 52 |
| 5d | 1a | n-Hexyl, 2e | —e | 4e 41 |
| 6 | 1a | Phenyl, 2f | 3f 86 | 4f <5 |
| 7 | 1a | p-Methoxyphenyl, 2g | 3g 27 | 4g 71 |
| 8 | 1a | p-Nitrophenyl, 2h | 3h 63 | —e |
| 9 | 1a | 2-Pyridyl, 2i | —e | 4i 39 |
| 10 | 1b | 2a | —e | 4j 57 |
| 11 | 1c | 2a | 3k 22 | 4k 78 |
Based on the results above, we tried to provide a reasonable mechanism to elaborate the role of the excess amount of aldehyde, especially aliphatic aldehyde. As is known from Scheme 2, the cyclic aminal intermediate 3 was easily prepared from the condensation reaction between o-aminobenzamide and an aldehyde. As shown in Scheme 2, there will be a balance between the aminal intermediate i and the imine intermediate ii. In the presence of an additional aliphatic aldehyde, the aromatic imine intermediate ii will be transformed into the aliphatic imine intermediate iii to a certain extent, which would form the cyclic alkyl-substituted aminal intermediate iv readily. Subsequently, the nucleophilic addition of this aminal intermediate iv to the excess aliphatic aldehyde generates v, which then further dehydrates to produce an exo imine cation vi. Then, a [1,3] hydride shift takes place to transform this exo imine cation into the endo imine cation vii.9 The final hydrolysis of the endo imine cation vii would afford the quinazolinone product.
Besides the mechanism described above, there is one other possibility that the newly formed acid from the oxidation of aldehyde in this reaction system facilitated the transformation from the aminal intermediate to quinazolinone, if the excess amount of aldehyde doesn’t play the role of an oxidant. In order to clarify these two possibilities, the two control experiments shown in Scheme 3 were investigated. In the presence of cyclohexanecarboxylic acid instead of cyclohexanecarboxaldehyde 2c, 2-cyclohexyl-substituted aminal intermediate 3c could be converted into 2-cyclohexyl-quinazolinone 4c with a yield of 49%, the value of which was obviously lower than the result of cyclohexanecarboxaldehyde 2c. Moreover, the addition of 2.0 equiv. of the organic base triethylamine didn’t inhibit the transformation process from the aminal intermediate 3c to quinazolinone 4c aided by cyclohexanecarboxaldehyde 2c. Cyclohexanemethanol as a by-product was detected by GC-MS (Fig. S1†), indicating that the excess amount of aldehyde possibly served as an oxidant in this reaction.
Conclusions
In conclusion, excess amounts of aldehyde during the condensation reaction between 2-anthranilamide and aldehyde, in particular, aliphatic aldehyde, efficiently facilitate the oxidation of an aminal intermediate to quinazolinone in pure water. This protocol was simple and avoided the use of any additional oxidants and additives. To the best of our knowledge, the work reported herein provides the first example of the use of excess amounts of aldehyde as an oxidant.Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (Grant No. 21302188, 91027033) and Chinese Academy of Sciences (Grant No. KJCX2-EW-N06-01 and XDA09030307) for financial support.References
- (a) B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS PubMed; (b) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259–281 CrossRef CAS.
- (a) W. M. Nelson, Green Solvents for Chemistry: Perspectives and Practice, Oxford University Press, 2003 Search PubMed; (b) J. M. DeSimone, Science, 2002, 297, 799–803 CrossRef CAS PubMed.
- A. R. H. Narayan and R. Sarpong, Green Chem., 2010, 12, 1556–1559 RSC.
- For selected reviews, see: (a) S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787–9826 CrossRef CAS; (b) I. Khan, A. Ibrar, N. Abbas and A. Saeed, Eur. J. Med. Chem., 2014, 76, 193–244 CrossRef CAS PubMed.
- For selected examples, see: (a) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348–351 CrossRef CAS PubMed; (b) B. Ma, Y. Wang, J. Peng and Q. Zhu, J. Org. Chem., 2011, 76, 6362–6366 CrossRef CAS PubMed; (c) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846–3852 CrossRef CAS PubMed; (d) W. Xu, Y. Jin, H. Liu, H. Jiang and H. Fu, Org. Lett., 2011, 13, 1274–1277 CrossRef CAS PubMed; (e) L. Xu, Y. Jiang and D. Ma, Org. Lett., 2012, 14, 1150–1154 CrossRef CAS PubMed; (f) J. E. R. Sadig, R. Foster, F. Wakenhut and M. C. Willis, J. Org. Chem., 2012, 77, 9473–9486 CrossRef CAS PubMed; (g) Y. F. Wang, F. L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842–2845 CrossRef CAS PubMed; (h) X. F. Wu, L. He, H. Neumann and M. Beller, Chem.–Eur. J., 2013, 19, 12635–12638 CrossRef CAS PubMed; (i) X. Jiang, T. Tang, J. Wang, Z. Chen, Y. Zhu and S. Ji, J. Org. Chem., 2014, 79, 5082–5087 CrossRef CAS PubMed; (j) Y. F. Wang, F. L. Zhang and S. Chiba, Org. Lett., 2013, 15, 2842–2845 CrossRef CAS PubMed; (k) T. B. Nguyen, J. L. Bescont, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 6218–6221 CrossRef CAS PubMed; (l) X. Yang, G. Cheng, J. Shen, C. Kuai and X. Cui, Org. Chem. Front., 2015, 2, 366–368 RSC.
- (a) T. Hisano, M. Ichikawa, A. Nakagawa and M. Tsuji, Chem. Pharm. Bull., 1975, 23, 1910–1916 CrossRef CAS PubMed; (b) C. Balakumar, P. Lamba, D. P. Kishore, B. L. Narayana, K. V. Rao, K. Rajwinder, A. R. Rao, B. Shireesha and B. Narsaiah, Eur. J. Med. Chem., 2010, 45, 4904–4913 CrossRef CAS PubMed; (c) Y. Mitobe, S. Ito, T. Mizutani, T. Nagase, N. Sato and S. Tokita, Bioorg. Med. Chem. Lett., 2009, 19, 4075–4078 CrossRef CAS PubMed; (d) R. J. Abdel-Jalil, W. Voelter and M. Saeed, Tetrahedron Lett., 2004, 45, 3475–3476 CrossRef CAS; (e) K. Juvale and M. Wiese, Bioorg. Med. Chem. Lett., 2012, 22, 6766–6769 CrossRef CAS PubMed; (f) M. Sharif, J. Opalach, P. Langer, M. Beller and X. Wu, RSC Adv., 2014, 4, 8–17 RSC; (g) R. Cheng, T. Guo, D. Zhang-Negrerie, Y. Du and K. Zhao, Synthesis, 2013, 2998–3006 CAS.
- F. Li, L. Lu and J. Ma, Org. Chem. Front., 2015, 2, 1589–1597 RSC.
- (a) L. X. Wang, J. F. Xiang and Y. L. Tang, Eur. J. Org. Chem., 2014, 2682–2685 CrossRef CAS; (b) B. Q. Hu, L. X. Wang, L. Yang, J. F. Xiang and Y. L. Tang, Eur. J. Org. Chem., 2015, 4504–4509 CrossRef CAS.
- For recent reports on [1,3] hydride shift: (a) M. Tang, L. Tong, L. Ju, W. Zhai, Y. Hu and X. Yu, Org. Lett., 2015, 17, 5180–5183 CrossRef CAS PubMed; (b) W. Chenand and D. Seidel, Org. Lett., 2014, 16, 3158–3161 CrossRef PubMed , and references cited therein.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra05777b |
| This journal is © The Royal Society of Chemistry 2016 |